Abstract
Objective
Evaluate the in vivo efficacy and resistance prevention of cefiderocol in combination with ceftazidime/avibactam, ampicillin/sulbactam and meropenem using human-simulated regimens (HSR) in the murine infection model.
Methods
In total, 15 clinical A. baumannii were assessed: cefiderocol MICs, 2 mg/L (previously developed resistance on therapy), n = 3; 8 mg/L, n = 2; ≥32 mg/L, n = 10 (including VEB and PER-harbouring isolates). Mice received inactive control, cefiderocol, cefiderocol + ceftazidime/avibactam (C-CZA), cefiderocol + ampicillin/sulbactam (C-SAM) or cefiderocol + meropenem (C-MEM) HSRs. The mean change in log10 cfu/thigh compared with starting inoculum was assessed. Resistance development on treatment was a >4-fold increase in MIC relative control animals. In vitro activities of combinations were assessed by disc stacking.
Results
Against cefiderocol-non-susceptible isolates, combinations produced significant kill with C-CZA −3.75 ± 0.37 reduction in log10 cfu/thigh, C-SAM produced −3.55 ± 0.50 and C-MEM produced −2.18 ± 1.75 relative to baseline. Elevated MICs in cefiderocol treated animals occurred in three out of three isolates with MICs of 2 mg/L. Of these isolates, one developed elevated MICs with C-MEM compared with none treated with C-CZA or C-SAM. Disc stacking with C-CZA or C-SAM returned all isolates to at least the CLSI intermediate breakpoint, which may correlate with in vivo efficacy.
Conclusions
Against cefiderocol-non-susceptible isolates, cefiderocol + ceftazidime/avibactam or ampicillin/sulbactam HSR produced in vivo kill against all 12 cefiderocol-non-susceptible isolates. Cefiderocol with ceftazidime/avibactam or ampicillin/sulbactam prevented the development of resistance during treatment against cefiderocol-high-end-susceptible isolates with a propensity for resistance on therapy. These data support the clinical evaluation of cefiderocol with ceftazidime/avibactam or ampicillin/sulbactam against A. baumannii, including multi-drug-resistant isolates.
Background
Carbapenem-resistant Acinetobacter baumannii (CRAB) remains a clinical challenge as more than 57 000 deaths worldwide were attributed to this pathogen in 2019.1 Infection that is multi-drug-resistant and with CRAB has been associated with higher inpatient mortality in patients with A. baumannii infections compared to those with infections caused by susceptible isolates necessitating novel therapeutic strategies.2 Combination therapy for A. baumannii has been studied in numerous clinical trials although results have been inconclusive because of the heterogeneous populations and combinations studied largely consisted of polymyxin-based regimens that are plagued by high toxicity and a poor correlation with efficacy.3–5 Contemporary guidance recommend the combination of two active antimicrobials for moderate to severe infections caused by CRAB.6 Unfortunately, resistance to carbapenems is accompanied by cross-resistance to other β-lactams as well as agents from different antimicrobial classes necessitating novel agents and combinations.7
Due to its novel siderophore mechanism of cell entry and stability against acquired/intrinsic β-lactamases, cefiderocol is active in vitro against A. baumannii including CRAB.8 Indeed, cefiderocol inhibited 90% of A. baumannii isolates at an MIC of ≤1 mg/L despite meropenem-non-susceptibility.9 We previously determined the in vivo efficacy of humanized cefiderocol exposures against clinical A baumannii where >1 log10 kill was observed at 72 h in five out of seven isolates with MICs up to 2 mg/L.10 Development of resistance on therapy was observed although rare.10 A randomized controlled trial in nosocomial pneumonia has determined the efficacy of cefiderocol against A. baumannii including carbapenem-resistant strains, although a numeric difference in all-cause mortality was noted in the CREDIBLE-CR study in patients with CRAB infections where mortality was 49% in cefiderocol treated patients compared with 18% in best available therapy warranting further investigation.11,12 Despite the potent in vitro and in vivo activity, isolates with elevated MICs have been described, notably A. baumannii harbouring PER and VEB-β-lactamases, further reducing the available treatment options.13 The addition of β-lactamase inhibitors (i.e. avibactam) to cefiderocol have reduced cefiderocol MICs against cefiderocol-non-susceptible A. baumannii including isolates with and without acquired β-lactamases (i.e. VEB and PER).13,14 The novel mechanism and in vitro potency make cefiderocol an attractive component of combination therapy warranting investigation.
In vitro modelling has demonstrated synergy of cefiderocol when administered with ceftazidime/avibactam, ampicillin/sulbactam, meropenem or amikacin against A. baumannii.15 The present study aimed to evaluate the in vivo efficacy of cefiderocol in combination with ceftazidime/avibactam, ampicillin/sulbactam and meropenem at humanized exposures over 72 h against cefiderocol-non-susceptible (MIC 8–>32 mg/L) A. baumannii. The same combinations were evaluated against cefiderocol-high-end-susceptible isolates (MIC 2 mg/L) to evaluate whether combination therapy prevented the development of in vivo resistance. In vivo activity in the model may translate to clinical efficacy as the combinations evaluated are at clinically relevant exposures. An evaluation of practical in vitro testing using stacking of disc diffusion to correlate with the in vivo efficacy may help guide therapeutic selections of these combinations in the clinic against A. baumannii isolates where treatment options are currently limited.
Materials and methods
Ethics
The present study was reviewed and approved by the Institutional Animal Care and Use Committee of Hartford Hospital. All experiments were conducted in alignment with the National Research Council of the National Academy of Sciences standards.
Antimicrobial test agents
Commercially available cefiderocol (Shionogi, Japan), ceftazidime (Sandoz, IL, USA; Astral Steri Tech Pvt Ltd, India), ampicillin/sulbactam (Meitheal Pharmaceuticals, IL, USA) and meropenem (Aurobindo Pharma Limited-Hyderabad, India) were used for all in vivo experiments. Analytical grade avibactam (MedChemExpress, NJ, USA) was used in the ceftazidime/avibactam human simulating regimen (HSR).16 Analytical standard powders were used for the preparation of broth microdilution MIC trays (Cefiderocol: Shionogi, Japan; ceftazidime: MedChemExpress, NJ, USA; avibactam: MedChemExpress, NJ, USA; meropenem: Sigma-Aldrich, WY, USA; ampicillin: MedChemExpress, NJ, USA; sulbactam: United States Pharmacopeial Convention, MD, USA).
Isolates
Fifteen A. baumannii clinical isolates were tested in the murine model. Table 1 describes the modal MICs for each isolate to cefiderocol, ceftazidime/avibactam, ampicillin/sulbactam (MIC reported as sulbactam component) and meropenem in triplicate. Available genotypic data are presented in Table 1. Three cefiderocol-high-end-susceptible isolates (MIC = 2 mg/L) were chosen to evaluate the use of combination therapy to prevent the development of resistance as they have been previously found to have increased MICs in the model post-exposure.10,17 The remaining 12 isolates were selected due to baseline cefiderocol-non-susceptibility to evaluate the in vivo pharmacodynamics of the combinations against highly resistant isolates.
Table 1.
Clinical A. baumannii isolates included the in vivo model
| Isolate | Genotype | CFDC MIC | CZA MICa | MEM MIC | SAM MIC |
|---|---|---|---|---|---|
| AB 147 | OXA-23-like (PCR) | 2 | >64 | 64 | 32 |
| AB 230 | ADC-33, OXA-82 | 2 | >64 | 32 | 4 |
| AB 237 | ADC, OXA-58-like | 2 | >64 | 16 | 8 |
| AB 97 | PER-1, OXA-58, ADC-76, OXA-68 | 8 | >64 | 4 | 16 |
| AB 319 | ADC-Type, OXA-829, OXA-24 | 8 | >16 | >16 | 8 |
| AB 318 | ADC-33 (V317G variant), OXA-23, OXA-82 | 32 | >16 | >16 | 16 |
| AB 320 | ADC-33 (V317G variant); OXA-23, OXA-82 | 32 | >16 | >16 | 8 |
| AB 313 | PER-1, ADC-25, OXA-23, OXA-66 | >32 | 64 | 64 | 32 |
| AB 314 | PER-1, ADC-11, OXA-66, OXA-72 | >32 | 64 | 64 | 32 |
| AB 316 | ADC-25-like, OXA-66, OXA-72, PER-1, TEM-1D | >32 | 32 | >64 | 32 |
| AB 323 | ADC-25-like; OXA-172 | >32 | >64 | 16 | 16 |
| AB 324 | ADC-11; OXA-66; OXA-72; PER-1; TEM-1D | >32 | >16 | >16 | 64 |
| AB 325 | ADC-25-like; OXA-9; OXA-24; OXA-51-like; TEM-1A; VEB-9 | >32 | 16 | >64 | 32 |
| AB 326 | ADC-25-like; OXA-82 | >32 | >64 | 16 | 2 |
| AB 327 | ADC-25-like; OXA-66; OXA-72; PER-13 | >32 | >64 | 64 | 8 |
CFDC = cefiderocol, CZA = ceftazidime/avibactam, MEM = meropenem, SAM = ampicillin/sulbactam
Avibactam concentration fixed at 4 mg/L.
In vitro MIC testing
Pre- and post-exposure MICs were conducted for cefiderocol in iron-depleted CAMHB per CLSI Standards as previously described.10,18,19
To evaluate a clinically implementable in vitro combination testing, disc diffusion using conventional antimicrobial susceptibility discs stacked one agent on top of the other were assessed for each combination. Briefly, inoculua were prepared per CLSI standards and the bacterial suspension was lawned onto Muller–Hinton Agar plates (Becton Dickenson, Franklin Lakes, NJ, USA). Cefiderocol discs (Hardy Diagnostics, Santa Maria, CA, USA) were then placed on the agar. The second agent disc was placed on top of the cefiderocol disc and 30 µL of saline was placed on top of the second disc. Plates were incubated per CLSI guidance and the zone of inhibition was read by qualified personnel. Combination MICs were conducted in iron-depleted CAMHB using the following combinations: cefiderocol with ceftazidime/avibactam (1:1 ratio for cefiderocol to ceftazidime, avibactam fixed at 4 mg/L), cefiderocol with ampicillin/sulbactam (1:2:1 ratio cefiderocol, ampicillin, sulbactam) and cefiderocol with meropenem (1:1 ratio). Inoculua were prepared and trays incubated per CLSI standards for A. baumannii.18,19 The 1:1 ratio method was used since disc diffusion is validated against standard broth microdilution. As commercially available discs were used for ease of implementation clinically, the ratio of drugs in the disc is not altered, thus we compared the disc stacking to the MIC determined with an increasing ratio of the antibiotic, to which individual discs would be compared when tested alone because there is no gold standard methodology for in vitro combination testing.
Animals
Specific-pathogen-free, CD-1 mice (female, 20–22 g) were used for all in vivo experiments (Charles River Laboratories, Inc., Raleigh, NC, USA). Mice were acclimatized and housed as previously described.20
Neutropenic murine thigh infection model
Before all in vivo experiments, mice were pretreated with cyclophosphamide (150 mg/kg on day −4, and 100 mg/kg on day −1) and uranyl nitrate (5 mg/kg on day −3) via intraperitoneal injection. On the day of the experiment, one thigh per mouse was inoculated with a 0.1 mL injection intramuscularly of ∼1 × 107 cfu/mL bacterial suspension. Antibiotic dosing commenced 2 h post-inoculation to allow the bacteria to reach log-phase growth.
Previously defined human-simulated doses of cefiderocol, ceftazidime/avibactam and meropenem were administered as 0.1 mL of subcutaneous injections.16,21 The murine sulbactam (administered with ampicillin) HSR was developed for the neutropenic murine thigh infection model. The free-sulbactam plasma concentration in the mice mimicked the plasma pharmacodynamic profile (free-time above MIC, free Cmax and free-AUC) achieved in healthy volunteers treated with 3 g IV q8h as a 4 h infusion using previously established pharmacokinetic parameters from mice and humans.6,22,23 Once defined, the murine free plasma profile of the HSR was reassessed. Confirmatory pharmacokinetic studies after the administration of sulbactam (with ampicillin) at 10, 12 and 7.5 mg/kg at 0, 1.5 and 3 h, respectively (repeated ever 8 h), produced observed concentrations similar to the murine predicted and human-simulated profiles (Table S1, Figure S1, available as Supplementary data at JAC Online).
In vivo efficacy studies
Groups of six mice were randomized to the following groups for each isolate: 0 h control (baseline bacterial burden), 72 h control (growth control), cefiderocol HSR, cefiderocol with ceftazidime/avibactam HSR, cefiderocol with ampicillin/sulbactam HSR and cefiderocol with meropenem HSR. The 72 h control and each antibiotic treatment group were administered for the 72 h experiment and aseptically harvested at 72 h for cfu enumeration. If the animal was moribund (i.e. unable to right themselves) they were sacrificed. Any animal sacrificed or that had succumbed to infection before the end of the 72 h study had their infected thigh harvested for cfu enumeration at that time.
In vivo efficacy was assessed as log10 change in cfu/thigh in 72 h control and treatment groups from the 0-h control (baseline bacterial burden). Efficacy was assessed using the translational endpoint of >1-log10 bacterial kill from baseline.24 Statistical analysis was conducted using a one-way ANOVA with Tukey’s post hoc test to determine between-group differences. As a proof of concept, five isolates were selected to assess the in vivo effects of meropenem, ceftazidime/avibactam and ampicillin/sulbactam to isolates with various MICs to establish the individual agents were active against susceptible isolates.
Post-exposure development of resistance was assessed in the cefiderocol-high-end-susceptible isolates. Development of resistance was defined as a >4× MIC dilution increase in the post-exposure MIC in a treatment group compared with the untreated 72-h controls.
Results
In vivo efficacy studies
All test isolates adequately established infection in the model with a mean baseline bacterial burden of 5.67 ± 0.68 log10 cfu/thigh across the 15 isolates that increased by a mean of 3.34 ± 0.62 log10 cfu/thigh in the untreated 72-h control groups.
As a proof of concept, five isolates were assessed in vivo over 72 h of treatment with sulbactam (with ampicillin) HSR, ceftazidime/avibactam HSR and meropenem HSR. The sulbactam HSR resulted in bacterial kill consistent with the MIC and exposure of the high-dose, extended-infusion HSR. Ceftazidime/avibactam resulted in variable activity while meropenem HSR activity was consistent with the in vitro MIC and both agents lacked appreciable activity when the MIC was elevated (Figure S2).
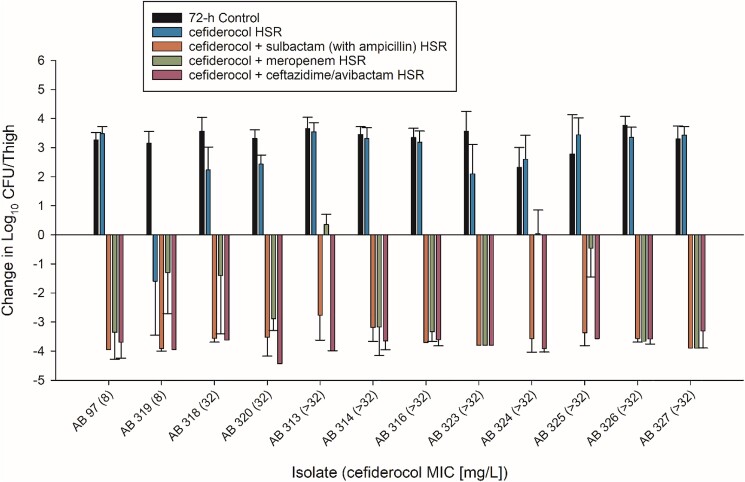
Twelve cefiderocol-non-susceptible isolates were assessed in vivo (cefiderocol MICs ranged 8–>32 mg/L). As predicted by the in vitro MICs, cefiderocol HSR treatment resulted in multilog bacterial growth in 11/12 isolates while kill was observed in one isolate at an MIC of 8 mg/L. On treatment with cefiderocol in combination with ceftazidime/avibactam or sulbactam (with ampicillin), 12/12 cefiderocol-non-susceptible isolates resulted in greater than 1-log10 kill (Figure 1) with a range of mean change in log10 cfu/thigh of −3.30 to −4.43 and −2.77 to −3.95 for each combination, respectively (Table S2). Conversely, cefiderocol in combination with meropenem resulted in 1-log10 kill in 9/12 isolates. Indeed, one isolate reached 1-log10 kill but was not significantly improved activity over cefiderocol alone (AB 319).
Figure 1.
In vivo efficacy of humanized regimens of cefiderocol alone and in combination with ceftazidime/avibactam, ampicillin/sulbactam and meropenem against cefiderocol non-susceptible A. baumannii after 72 h of treatment.
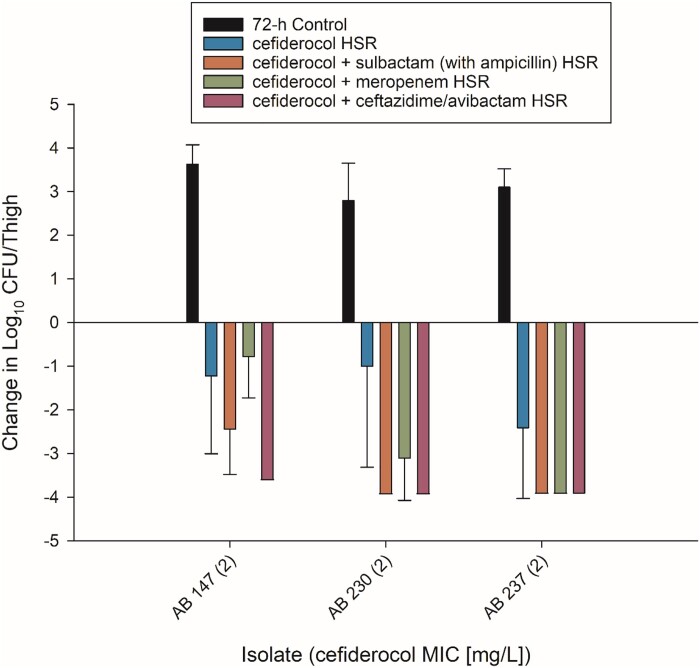
Three cefiderocol-high-end-susceptible isolates were tested to assess the development of resistance in vivo. Considering the pharmacodynamic study, cefiderocol HSR resulted in bacterial kill against all three isolates with a range of mean kill of −1.00 to −2.41 log10 cfu/thigh relative to baseline. Combination therapy significantly increased bacterial kill in three out of three isolates for cefiderocol plus ceftazidime/avibactam or ampicillin/sulbactam and two out of three isolates for cefiderocol plus meropenem (Figure 2).
Figure 2.
In vivo efficacy of humanized regimens of cefiderocol alone and in combination with ceftazidime/avibactam, ampicillin/sulbactam and meropenem against cefiderocol-susceptible A. baumannii after 72 h of treatment.
Post-exposure development of resistance
In cefiderocol HSR treated mice, post-exposure resistance developed in 8, 33 and 11% of thighs for AB 147, AB 230 and AB 237, respectively. All three combinations prevented the development of resistance for isolates AB 237 and AB 230 as no thighs exhibited resistance development to cefiderocol during combination therapy. For AB 147, 8% of thighs treated with cefiderocol and meropenem HSR developed elevated MICs on treatment while cefiderocol plus ceftazidime/avibactam or sulbactam (with ampicillin) resulted in no samples with elevated MICs.
In vitro combination testing
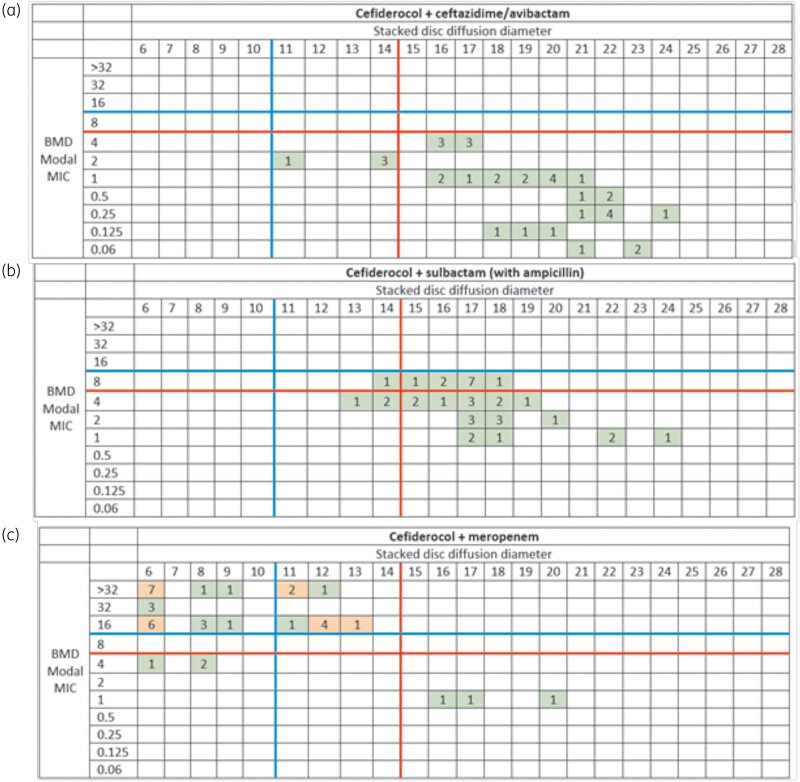
Broth microdilution modal MICs for cefiderocol with ceftazidime/avibactam, ampicillin/sulbactam and meropenem with a range of MICs of 0.06–4, 1–8 and 1–>32 mg/L for each agent, respectively. Tables S3 and S4 describe the modal broth microdilution MIC and the zone of inhibition for all replicates of the disc diffusion with each agent alone (Table S3) and stacked discs (Table S4). For the cefiderocol alone, seven isolates evaluated had microcolonies within the zone of inhibition for all replicates of the disc diffusion. When comparing the zone of inhibition of the stacked discs to the current cefiderocol susceptibility breakpoints per CLSI, 11/12 isolates tested with cefiderocol and ceftazidime/avibactam resulted in all zones of inhibition returning to the susceptible range with the single isolate not meeting these criteria returning to the intermediate range. For ampicillin/sulbactam, 9/12 reached the susceptible range while the remaining three reached at least the intermediate range for both cefiderocol and ampicillin/sulbactam. Interestingly, in the isolates that had microcolonies for cefiderocol alone, microcolonies within the zone of inhibition were dramatically reduced in the stacked disc experiments occurring in one replicate of one isolate for cefiderocol with ceftazidime/avibactam and six replicates for four different isolates for cefiderocol with ampicillin/sulbactam (one replicate for n = 3 isolates and all three replicates for n = 1 isolate). Similar to the in vivo findings, meropenem did not consistently return the zone of inhibition to either of the criteria with 9/12 isolates remaining resistant in the disc stacking experiments.
To evaluate cut-offs for the observed in vivo efficacy of the combinations, Figure 3 categorizes isolates by the modal combination MIC, the zone of inhibition of stacked discs (relative to current CLSI cefiderocol breakpoints) and the demonstration of >1-log10 kill. For both cefiderocol with ceftazidime/avibactam and cefiderocol with ampicillin/sulbactam, all 12 isolates had the zone of inhibition returned to at least intermediate range and resulted in >1-log10 kill.
Figure 3.
Distribution of the zone diameter of disc stacking of (a) cefiderocol + ceftazidime/avibactam, (b) cefiderocol + ampicillin/sulbactam and (c) cefiderocol + meropenem compared to the modal broth microdilution MIC of each respective combination. All disc diffusion zones are in at least three replicates per isolate. The red line corresponds to cefiderocol susceptibility per CLSI (BMD ≤ 4 mg/L, DD ≥ 15 mm). The blue line signifies cefiderocol intermediate per CLSI (BMD = 8 mg/L, DD = 11–14 mm). Green boxes indicate that >1-log10 reduction in cfu/thigh was observed in all isolates in each category. An orange box indicates at least one isolate in the category did not demonstrate >1-log10 reduction in cfu/thigh or was no better than cefiderocol alone. BMD = broth microdilution, DD = disc diffusion.
For meropenem, there was no clear relationship between zone size of the stacked disc experiment and in vivo efficacy but notably, all three isolates that failed to reach >1-log10 kill had stacked disc zone of inhibitions that remained in the resistant range. Based on the in vitro and in vivo findings a cut-off of returning the zone of inhibition using disc stacking to the intermediate range for cefiderocol with ceftazidime/avibactam or ampicillin/sulbactam may be a potential surrogate for in vivo efficacy.
Discussion
Although combination therapy is advocated for the treatment of carbapenem-resistant A. baumannii, the clinical evidence for this recommendation is controversial. The present study provides pre-clinical evidence of the microbiologic activity of cefiderocol in combination with ceftazidime/avibactam and sulbactam (with ampicillin) against clinical A. baumannii isolates with high-end-susceptible (MIC 2 mg/L) or non-susceptible (MIC 8–>32 mg/L) cefiderocol MICs using pharmacokinetic exposures similar to those seen in humans. Conversely, in vivo efficacy of the cefiderocol with meropenem combination was more variable. By using exposures of each agent that mimicked those seen in humans receiving each agent, these data provide a translational bridge to support the clinical evaluation of these rationally designed combinations.
Twelve clinical cefiderocol-non-susceptible A. baumannii isolates were tested to assess the in vivo efficacy of each combination. All isolates were meropenem-non-susceptible and 11 were sulbactam-non-susceptible, thus representing challenging clinical isolates. Consistent with the in vitro MICs, cefiderocol HSR monotherapy lead to in vivo growth in 11/12 isolates tested as the clinical exposure unlikely to meet its pharmacodynamic targets at such MICs. One isolate, AB 319 with an MIC of 8 mg/L, exhibited bactericidal activity over the 72-h experiment, which is not unexpected as the clinical dose of cefiderocol may achieve its pharmacodynamic target because the fT > MIC of 8 mg/L for the murine HSR was 80%.21 Combination of cefiderocol with ceftazidime/avibactam or sulbactam (with ampicillin) resulted in marked bactericidal activity across all 12 test isolates. The activity of cefiderocol with ceftazidime/avibactam is expected as previous in vitro assays have determined the MICs of cefiderocol with avibactam reduced the MICs including in cefiderocol-non-susceptible A. baumannii.10,13,14 Similar to the in vitro data, in vivo efficacy was seen in isolates with acquired serine β-lactamases that may be inhibited by avibactam (i.e. VEB and PER).13,14 Decreases in MIC have been observed with avibactam against isolates that only harboured intrinsic oxacillinases and cephalosporinases, these in vivo data suggest that inhibition of cephalosporinases by avibactam may also enhance the activity of cefiderocol.13,14 Cefiderocol in combination with sulbactam produced similarly impressive in vivo activity in the present study. Indeed, the present study used a murine HSR that mimicked the free plasma profile of high-dose, extended-infusion sulbactam (administered with ampicillin) that is advocated for in the IDSA guidance.6 The mechanism behind the potent in vivo activity of the combination is unclear, however; in addition to sulbactam’s antibacterial activity against A. baumannii, sulbactam can inhibit PER-type β-lactamases that may contribute to the efficacy for such isolates.25 Sulbactam has been described to weakly inhibit ADCs intrinsic to A. baumannii, thus the high exposure of sulbactam may provide activity via ADC inhibition.26 Some isolates assessed tested susceptible to sulbactam, thus activity of this agent alone at high doses and extended-infusion administration may contribute to the efficacy although most isolates tested were sulbactam-non-susceptible, which is consistent with <25% of carbapenem-resistant A. baumannii testing susceptible to sulbactam in vitro.7 Meropenem combinations failed to consistently produce significant bacterial kill in the present study. Previous assessments of dual carbapenem therapy for A. baumannii postulated that one may act as a suicide inhibitor of carbapenemases produced by the organism.27 This may have not led to clinically relevant bactericidal activity in vivo due to the diversity of enzymes produced as well as the other non-enzymatic resistance mechanisms likely present in this collection of highly resistant isolates.
Previous in vivo studies have demonstrated, although rare, post-exposure resistance to cefiderocol in A. baumannii has been observed.10 Similar to our previous data, development of resistance was seen in both AB 237 and AB 230 with the latter occurring more frequently, showing consistency across the model.10 Frequency of resistance for both isolates has been previously determined and was 4 × 10−7 and 1 × 10−6 for AB 230 and 237, respectively.10 The present study added to these findings as for both isolates combination therapy with each test agent increased the in vivo activity and prevented the emergence of resistance in cefiderocol-susceptible isolates with MICs of 2 mg/L. For AB 147, cefiderocol with ceftazidime/avibactam and sulbactam (with ampicillin) also prevented resistance emergence on therapy. Conversely, the combination with meropenem observed one thigh that had elevated MICs to cefiderocol post-exposure. This finding is consistent with the pharmacodynamic profile as AB 147 was the only cefiderocol-susceptible isolate where the cefiderocol and meropenem combination therapy resulted in similar reductions in log10 cfu/thigh compared with cefiderocol alone. The use of human-simulated exposure and a longer 72 h period allows for a translational assessment of not only in vivo efficacy, but also treatment emergent resistance, and may be a useful strategy to evaluate more target MDR organisms with other antibacterial combinations. These data provide foundational in vivo and microbiologic data to design rational combinations for clinical validation; however, practical in vitro assessments are needed to identify clinical isolates that are likely to respond to such combinations.
Various methods have been advocated to assess in vitro activity including checkerboard, time-kill, crossing of gradient diffusion strips and disc diffusion studies.28 An advantage of the gradient diffusion strips and disc diffusion-based methods is such procedures use materials commonly stocked in clinical microbiology laboratories. In the present study, we assessed in vitro activity using stacked discs as these materials are readily available for cefiderocol and all combination agents. Similar to previous data, the broth microdilution and disc stacking of ceftazidime/avibactam with cefiderocol resulted in the most prominent decrease in MIC and increase in zone of inhibition.10,13 Similar findings were noted with ampicillin/sulbactam where, for both combinations, all zones of inhibition were returned to at least intermediate. This translated to in vivo activity using the human-simulated exposures for all test isolates. Indeed, the influence of both test agents must be considered as the treatments were administered in the clinically available formulations, and thus the return of MICs and zones of inhibition to the sulbactam intermediate zone in the presence of cefiderocol probably contributed to the in vivo efficacy. A practical approach for clinical isolates may be the achievement of at least a zone of inhibition that is interpreted as intermediate with stacked discs, which may indicate an isolate that would benefit from combination therapy. Similar to the in vivo data, the in vitro activity of cefiderocol with meropenem was variable. The lack of in vitro and in vivo correlation may discourage the viability of this combination as, despite the fact most isolates respond in vivo, we were unable to find an in vitro correlation to guide therapy. While data derived from the disc stacking method is encouraging, no standardized methods for disc stacking are endorsed by CLSI or EUCAST. Assessment of disc stacking in a larger cohort of clinical A. baumannii should be evaluated to better understand the in vitro response to the studied combinations as well as the effect of these combinations on clinical outcomes.
An advantage of the present analysis was the use of clinical, highly resistant isolates that may be encountered and leave clinicians no viable treatment options. Although a limitation may be that only 15 isolates were assessed, these isolates had various genotypic and phenotypic profiles, thus these findings are likely generalizable to larger cohorts of MDR A. baumannii. Additionally, we did not assess isolates with cefiderocol MICs ≤1 mg/L (cefiderocol MIC90 in CRAB)9 because previous studies failed to find increases in potency when avibactam was added to cefiderocol for such isolates,12 and previous in vivo studies have found cefiderocol monotherapy highly active.10,21 Therefore, combination therapy may not provide an added benefit for highly susceptible isolates.
The present study is not without limitations. First, the discs used for combination in vitro testing contained fixed concentrations and ratios of each agent that may not have reflected the clinical exposures of the agents produced by the clinical doses in humans or the HSRs in mice. With that said, the in vitro findings provide an assessment of biologic plausibility that may be useful to identify isolates more likely to respond to each combination therapy in vivo. Indeed, the fixed concentration of antibiotics may under call synergy (as seen in the meropenem based experiments); this is preferable from a safety perspective as it reduces the risk of an in vitro test suggesting activity where it was not found in vivo. Second, not all monotherapies were assessed in vivo. Indeed, the five isolates tested against each monotherapy demonstrated that for isolates with elevated MICs to each agent (e.g. meropenem ≥8 mg/L, ceftazidime/avibactam >16 mg/L or sulbactam ≥8 mg/L) the combination with cefiderocol produced significantly better in vivo activity. The remaining cefiderocol-NS isolates assessed all had MICs within this range for each combination agent thus represent challenging clinical isolates. Combination of cefiderocol with ceftazidime/avibactam and sulbactam produced significant in vivo activity (e.g. > 1-log10 kill from baseline) against all isolates tested, suggesting these may represent rational combination therapy for highly resistant A. baumannii. These foundational in vivo data using human-simulated exposures require clinical validation to assess the effect of cefiderocol plus ceftazidime/avibactam or ampicillin/sulbactam on clinical outcomes for patients infected with A. baumannii.
In conclusion, human-simulated exposures of cefiderocol in combination with ceftazidime/avibactam or sulbactam (with ampicillin) resulted in potent in vivo activity against 15 carbapenem-non-susceptible A. baumannii including cefiderocol-non-susceptible isolates. Against three cefiderocol-high-end-susceptible isolates, these combinations also prevented the emergence of resistance in vivo. Cefiderocol in combination with meropenem resulted in more variable activity. Combination disc diffusion through disc stacking may be a feasible method to assess isolates that may respond to combination therapy. Disc stacking of cefiderocol and ceftazidime/avibactam or ampicillin/sulbactam, which results in a zone of inhibition that is indicative of the intermediate breakpoint, may indicate the isolate will respond in vivo at the studied clinical doses. Our findings are hypothesis generating, however, clinical validations of cefiderocol plus ceftazidime/avibactam or ampicillin/sulbactam therapy for A. baumannii and the impact on clinical outcomes are warranted.
Supplementary Material
Acknowledgements
This study was presented in part at IDWeek 2022, Washington DC, USA. We would all like to thank the staff from the Center for Anti-Infective Research and Development for their vital assistance in the conduct of this study.
Contributor Information
Christian M Gill, Center for Anti-Infective Research and Development, Hartford Hospital, 80 Seymour Street, Hartford, CT 06102, USA.
Debora Santini, Center for Anti-Infective Research and Development, Hartford Hospital, 80 Seymour Street, Hartford, CT 06102, USA.
Miki Takemura, Research Planning Department, Shionogi & Co., Ltd, 3-1-1, Futaba-cho, Toyonaka,Osaka 561-0825, Japan.
Christopher Longshaw, Scientific Affairs, Shionogi B.V., 33 Kingsway, London, WC2B 6UF, UK.
Yoshinori Yamano, Research Planning Department, Shionogi & Co., Ltd, 3-1-1, Futaba-cho, Toyonaka,Osaka 561-0825, Japan.
Roger Echols, Infectious Disease Drug Development Consulting, LLC, 753 Westport Road, Easton, CT, USA.
David P Nicolau, Center for Anti-Infective Research and Development, Hartford Hospital, 80 Seymour Street, Hartford, CT 06102, USA; Division of Infectious Diseases, Hartford Hospital, 80 Seymour Street, Hartford, CT 06102, USA.
Funding
This project was funded by Shionogi & Co., Ltd, Japan. The funder provided financial support and did not exercise control over the conduct of or reporting of the research.
Transparency declarations
C.M.G. has received research grants from Cepheid, Everest Medicines and Shionogi. M.T., C.L. and Y.Y. are employees of Shionogi & Co., Ltd, Japan. R.E. is a consultant with Shionogi & Co., Ltd, Japan. D.P.N. is a consultant, speaker bureau member and has received other research grants from Abbvie, Cepheid, Merck, Paratek, Pfizer, Wockhardt, Shionogi and Tetraphase. D.S. has none to declare.
Supplementary data
Figures S1 and S2 and Tables S1 to S4 are available as Supplementary data at JAC online.
References
- 1. Antimicrobial Resistance Collaborators . Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 2022; 399: 629–55. 10.1016/S0140-6736(21)02724-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Appaneal HJ, Lopes VV, LaPlante KLet al. Treatment, clinical outcomes, and predictors of mortality among a national cohort of admitted patients with Acinetobacter baumannii infection. Antimicrob Agents Chemother 2022; 66: e0197521. 10.1128/aac.01975-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Isler B, Doi Y, Bonomo RAet al. New treatment options against carbapenem-resistant Acinetobacter baumannii infections. Antimicrob Agents Chemother 2018; 63: e01110-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Paul M, Daikos GL, Durante-Mangoni Eet al. Colistin alone versus colistin plus meropenem for treatment of severe infections caused by carbapenem-resistant gram-negative bacteria: an open-label, randomised controlled trial. Lancet Infect Dis 2018; 18: 391–400. 10.1016/S1473-3099(18)30099-9 [DOI] [PubMed] [Google Scholar]
- 5. Kaye KS, Marchaim D, Tamlikitkul Vet al. Results from the OVERCOME trial: colistin monotherapy versus combination therapy for the treatment of pneumonia or bloodstream infection due to extensively drug-resistant Gram-negative bacilli. Presented at ECCMID 2021 #1773.
- 6. Tamma PD, Aitken SL, Bonomo RAet al. Infectious diseases society of America guidance on the treatment of AmpC β-lactamase-producing Enterobacterales, carbapenem-resistant Acinetobacter baumannii, and Stenotrophomonas maltophilia infections. Clin Infect Dis 2022; 74: 2089–2114. 10.1093/cid/ciab1013 [DOI] [PubMed] [Google Scholar]
- 7. McKay SL, Vlachos N, Daniels JBet al. Molecular epidemiology of carbapenem-resistant Acinetobacter baumannii in the United States, 2013-2017. Microb Drug Resist 2022; 28: 645–653. 10.1089/mdr.2021.0352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yamano Y. In vitro activity of cefiderocol against a broad range of clinically important gram-negative bacteria. Clin Infect Dis 2019; 69(Suppl 7):S544–S51. 10.1093/cid/ciz827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kazmierczak KM, Tsuji M, Wise MGet al. In vitro activity of cefiderocol, a siderophore cephalosporin, against a recent collection of clinically relevant carbapenem-non-susceptible Gram-negative bacilli, including serine carbapenemase- and metallo-β-lactamase-producing isolates (SIDERO-WT-2014 study). Int J Antimicrob Agents 2019; 53: 177–84. [DOI] [PubMed] [Google Scholar]
- 10. Gill CM, Abdelraouf K, Oota Met al. Assessment of sustained efficacy and resistance emergence under human-simulated exposure of cefiderocol against Acinetobacter baumannii using in vitro chemostat and in vivo murine infection models. JAC Antimicrob Resist 2022; 4: dlac047. 10.1093/jacamr/dlac047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wunderink RG, Matsunaga Y, Ariyasu Met al. Cefiderocol versus high-dose, extended-infusion meropenem for the treatment of gram-negative nosocomial pneumonia (APEKS-NP): a randomised, double-blind, phase 3, non-inferiority trial. Lancet Infect Dis 2021; 21: 213–25. 10.1016/S1473-3099(20)30731-3 [DOI] [PubMed] [Google Scholar]
- 12. Bassetti M, Echols R, Matsunaga Yet al. Efficacy and safety of cefiderocol or best available therapy for the treatment of serious infections caused by carbapenem-resistant Gram-negative bacteria (CREDIBLE-CR): a randomised, open-label, multicentre, pathogen-focused, descriptive, phase 3 trial. Lancet Infect Dis 2021; 21: 226–40. 10.1016/S1473-3099(20)30796-9 [DOI] [PubMed] [Google Scholar]
- 13. Kohira N, Hackel MA, Ishioka Yet al. Reduced susceptibility mechanism to cefiderocol, a siderophore cephalosporin, among clinical isolates from a global surveillance programme (SIDERO-WT-2014). J Glob Antimicrob Resist 2020; 22: 738–41. 10.1016/j.jgar.2020.07.009 [DOI] [PubMed] [Google Scholar]
- 14. Yamano Y, Ishibashi N, Kuroiwa Met al. Characterisation of cefiderocol-non-susceptible Acinetobacter baumannii isolates from Taiwan. J Glob Antimicrob Resist 2022; 28: 120–4. 10.1016/j.jgar.2021.12.017 [DOI] [PubMed] [Google Scholar]
- 15. Yamano Y, Takemura M, Anan Net al. 1626. Synergistic effect of cefiderocol with other antibiotics against PER-producing Acinetobacter baumannii isolates from the multinational SIDERO-WT studies. Open Forum Infect Dis 2020; 7(Suppl 1):S805. 10.1093/ofid/ofaa439.1806 [DOI] [Google Scholar]
- 16. Gill CM, Oliver A, Fraile-Ribot PAet al. In vivo translational assessment of the GES genotype on the killing profile of ceftazidime, ceftazidime/avibactam and meropenem against Pseudomonas aeruginosa. J Antimicrob Chemother 2022; 77: 2803–8. 10.1093/jac/dkac232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stainton SM, Monogue ML, Tsuji Met al. Efficacy of humanized cefiderocol exposures over 72 hours against a diverse group of gram-negative isolates in the neutropenic murine thigh infection model. Antimicrob Agents Chemother 2019; 63: e01040-18. 10.1128/AAC.01040-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. CLSI . Performance Standards for Antimicrobial Susceptibility Testing—Thirty-Second Edition: M100. 2022. [Google Scholar]
- 19. CLSI . Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically—Eleventh Edition: M07. 2018. [Google Scholar]
- 20. Gill CM, Abdelraouf K, Oota Met al. Discrepancy in sustained efficacy and resistance emergence under human-simulated exposure of cefiderocol against Stenotrophomonas maltophilia between in vitro chemostat and in vivo murine infection models. J Antimicrob Chemother 2021; 76: 2615–21. 10.1093/jac/dkab221 [DOI] [PubMed] [Google Scholar]
- 21. Monogue ML, Tsuji M, Yamano Yet al. Efficacy of humanized exposures of cefiderocol (S-649266) against a diverse population of gram-negative bacteria in a murine thigh infection model. Antimicrob Agents Chemother 2017; 61: e01022-17. 10.1128/AAC.01022-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Meyers BR, Wilkinson P, Mendelson MHet al. Pharmacokinetics of ampicillin-sulbactam in healthy elderly and young volunteers. Antimicrob Agents Chemother 1991; 35: 2098–101. 10.1128/AAC.35.10.2098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Abouelhassan Y, Kuti JL, Nicolau DP, et al. Sulbactam against Acinetobacter baumannii Pneumonia: Pharmacokinetic/Pharmacodynamic Appraisal of Current Dosing Recommendations. IDWeek 2022. October 19-23, 2022.
- 24. Bulitta JB, Hope WW, Eakin AEet al. Generating robust and informative nonclinical in vitro and in vivo bacterial infection model efficacy data to support translation to humans. Antimicrob Agents Chemother 2019; 63: e02307-18. 10.1128/AAC.02307-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nordmann P, Ronco E, Naas Tet al. Characterization of a novel extended-spectrum beta-lactamase from Pseudomonas aeruginosa. Antimicrob Agents Chemother 1993; 37: 962–9. 10.1128/AAC.37.5.962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shapiro AB. Kinetics of sulbactam hydrolysis by β-lactamases, and kinetics of β-lactamase inhibition by sulbactam. Antimicrob Agents Chemother 2017; 61: e01612-17. 10.1128/AAC.01612-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cebrero-Cangueiro T, Nordmann P, Carretero-Ledesma Met al. Efficacy of dual carbapenem treatment in a murine sepsis model of infection due to carbapenemase-producing Acinetobacter baumannii. J Antimicrob Chemother 2021; 76: 680–3. 10.1093/jac/dkaa487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Doern CD. When does 2 plus 2 equal 5? A review of antimicrobial synergy testing. J Clin Microbiol 2014; 52: 4124–8. 10.1128/JCM.01121-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.