Abstract
Background
The role of piperacillin/tazobactam for treatment of serious infections due to AmpC-producing organisms remains debatable, particularly in immunocompromised patients.
Methods
This was a retrospective cohort study in immunocompromised patients that investigated the effect of definitive treatment with either piperacillin/tazobactam versus cefepime or carbapenems for bacteraemia caused by cefoxitin-non-susceptible Enterobacterales. The primary endpoint was a composite of clinical and microbiological failure. A logistic regression model was constructed to assess the impact of definitive treatment choice on the primary endpoint.
Results
A total of 81 immunocompromised patients with blood cultures positive for cefoxitin-non-susceptible Enterobacterales were included for analysis. There was more microbiological failure in the piperacillin/tazobactam arm compared with the cefepime/carbapenem arm (11.4% versus 0.0%, P = 0.019). Definitive treatment with cefepime or a carbapenem was associated with a decreased odds of clinical or microbiological failure (OR 0.303, 95% CI 0.093–0.991, P = 0.048) when controlling for baseline characteristics.
Conclusions
In immunocompromised patients with bacteraemia due to cefoxitin-non-susceptible Enterobacterales, definitive treatment with piperacillin/tazobactam was associated with an increased risk of microbiological failure and higher odds of clinical or microbiological failure compared with cefepime or carbapenems.
Background
Several members of the Enterobacterales order (such as Enterobacter cloacae, Klebsiella aerogenes, Citrobacter freundii, Serratia marcescens and Morganella morganii) can produce AmpC β-lactamases that cause antibiotic resistance.1–4 Cefepime and carbapenems are often preferred to treat serious infections due to AmpC-producing organisms; however, the role of piperacillin/tazobactam is controversial due to its limited ability to inhibit AmpC β-lactamases.1,4,5
Guidance from the IDSA suggests ‘caution if prescribing piperacillin-tazobactam for serious infections caused by organisms at high risk of significant AmpC production’ and that the preferred antibiotic should be cefepime or a carbapenem.6 A recent pilot, randomized trial, albeit limited by lack of power, identified no difference in clinical failure, but a signal of increased microbiological failure with piperacillin/tazobactam when compared with meropenem.7 Observational studies have been mixed, with some identifying increased mortality with piperacillin/tazobactam when compared with carbapenems8,9 and others finding no difference in clinical outcomes.10,11
Immunocompromised patients have an increased risk of developing Gram-negative bacteraemia and worse outcomes compared with non-immunocompromised hosts.12–16 There are insufficient data to describe treatment outcomes of bacteraemia due to AmpC-producing organisms in this vulnerable population. The purpose of this study was to compare clinical and microbiological outcomes in significantly immunocompromised patients with cefoxitin-non-susceptible Enterobacterales bacteraemia when definitively treated with piperacillin/tazobactam versus cefepime or a carbapenem.
Methods
Ethics
This study did not include factors necessitating patient consent. Individual informed consent was waived. This study protocol was approved by the Stanford University review board (#58441).
Study population and design
This was a retrospective, single-center study conducted from January 2016 to December 2021 at Stanford Health Care. Immunocompromised patients ≥18 years old with a blood culture growing cefoxitin-non-susceptible Enterobacterales that was phenotypically susceptible to ceftriaxone, piperacillin/tazobactam, cefepime and carbapenems were included. The first positive blood culture for each patient within the study timeframe meeting these criteria was defined as the index culture. Patients included were definitively treated with either piperacillin/tazobactam, cefepime or a carbapenem, defined as the predominant antibiotic used within seven calendar days after the index culture. Immunocompromised was defined as: receipt of chemotherapy or an anti-TNF or anti-CD20 monoclonal antibody within 90 days, treatment with high-dose corticosteroids (20 mg daily prednisone or equivalent for ≥14 days), treatment with an immunosuppressive agent (i.e. tacrolimus, methotrexate, cyclosporine, mycophenolate), severe neutropenia (absolute neutrophil count <500 cells/mm3), CD4 < 200 cells/mm3 or an AIDS-defining condition, or history of leukaemia, lymphoma, solid organ transplant or bone marrow transplant. Patients were excluded if they had polymicrobial bacteraemia (excluding coagulase-negative staphylococci), died within 72 h of the index culture, received antibiotics active against the index culture at an outside facility, or were pregnant or incarcerated. Antibiotic susceptibility data were determined directly from the bottle by the Vitek 2 System (bioMérieux) or MicroScan WalkAway plus system (Beckman Coulter, Brea, CA, USA) per standard operating procedures.17
Endpoints
The primary endpoint was a composite of clinical and microbiological failure, defined by having at least one of the following: in-hospital 30-day mortality, a WBC count >12 000 cells/mm3 on days 5–7, a maximum temperature >38°C on days 5–7, microbiological failure on days 3–5 (blood culture with the organism identified on index culture) or microbiological relapse on days 5–30 (growth from any sterile site with the organism identified on index culture). Secondary endpoints included hospital length of stay, ICU length of stay and development of Clostridioides difficile infection.
Statistical analysis
Descriptive analyses were performed for the piperacillin/tazobactam and cefepime/carbapenem arms. Demographics and endpoints were compared using independent t-test for continuous data and chi-square test for categorical data. Logistic regression was used to evaluate impact of definitive treatment on the primary endpoint and was followed by an a priori model based on previously published factors associated with outcomes (sex, age, Charlson comorbidity index, Pitt bacteraemia score, and index culture with an organism at moderate-to-high risk of clinically significant AmpC production). Results from the regression were presented as the OR with its corresponding 95% CI. P values <0.05 were considered statistically significant. All analyses were performed using Stata 15 SE (StatCorp, LLC, College Station, TX, USA).
Results
Demographics
A total of 97 immunocompromised patients with blood cultures positive for cefoxitin-non-susceptible Enterobacterales were identified during the study period and 81 patients were included for analysis (piperacillin/tazobactam, n = 35; cefepime/carbapenem, n = 46) (Supplementary Figure S1; available as Supplementary data at JAC Online). Baseline characteristics are listed in Table 1. The cefepime/carbapenem arm had higher mean Pitt bacteraemia scores (2.2 versus 0.9, P = 0.042) and rates of severe neutropenia (32.6% versus 8.6%, P = 0.010) compared with the piperacillin/tazobactam arm. Otherwise, demographics between the two arms were well balanced (Table 1).
Table 1.
Demographics and endpoints
| Characteristic | Piperacillin-tazobactam (n = 35) | Cefepime or carbapenem (n = 46) | P value |
|---|---|---|---|
| Male, n (%) | 24 (68.6) | 35 (76.1) | 0.451 |
| Age (years), mean (SD) | 61.7 (12.9) | 64.9 (13.8) | 0.277 |
| BMI (kg/m2), mean (SD) | 25.7 (4.5) | 26.2 (5.8) | 0.637 |
| CrCl (mg/dL), mean (SD) | 97.7 (50.7) | 97.6 (77.4) | 0.993 |
| β-Lactam allergy, n (%) | 3 (8.6) | 11 (23.9) | 0.07 |
| ICU admission, n (%) | 9 (25.7) | 16 (34.8) | 0.381 |
| Renal replacement therapya, n (%) | 2 (5.7) | 1 (2.2) | 0.403 |
| History of MDROb, n (%) | 4 (11.4) | 1 (2.2) | 0.086 |
| Charlson comorbidity index, mean (SD) | 6.4 (3.0) | 5.7 (2.3) | 0.304 |
| Intravenous vasopressora, n (%) | 5 (14.3) | 13 (28.3) | 0.134 |
| Mechanical ventilationa, n (%) | 4 (11.4) | 8 (17.4) | 0.454 |
| Pitt bacteraemia score, mean (SD) | 0.9 (1.9) | 2.2 (3.3) | 0.042 |
| Infectious diseases consult, n (%) | 17 (48.6) | 25 (54.4) | 0.606 |
| Immunocompromised criteria, n (%) | |||
| ȃChemotherapy | 17 (48.6) | 23 (50.0) | 0.899 |
| ȃHigh-dose corticosteroidsc | 1 (2.9) | 1 (2.2) | 0.844 |
| ȃImmunosuppressive drugd | 9 (22.9) | 10 (22.2) | 0.946 |
| ȃSevere neutropeniae | 3 (8.6) | 15 (32.6) | 0.010 |
| ȃSolid organ transplant | 4 (11.4) | 3 (6.5) | 0.436 |
| ȃBone marrow transplant | 1 (2.9) | 7 (15.2) | 0.065 |
| ȃLeukaemia/lymphoma | 7 (20.0) | 17 (37.0) | 0.098 |
| Organism, n (%) | 0.641 | ||
| ȃModerate-high risk | 26 (74.3) | 32 (69.6) | |
| ȃȃEnterobacter cloacae | 19 (54.3) | 27 (58.7) | |
| ȃȃCitrobacter freundii | 2 (5.7) | 3 (6.5) | |
| ȃȃKlebsiella aerogenes | 5 (14.3) | 2 (4.4) | |
| ȃLow risk | 9 (25.7) | 14 (30.4) | |
| ȃȃSerratia marcescens | 9 (25.7) | 13 (28.3) | |
| ȃȃMorganella morganii | 0 (0.0) | 1 (2.2) | |
| Bacteraemia source, n (%) | 0.43 | ||
| ȃUrinary tract infection | 7 (20.0) | 3 (6.5) | |
| ȃIntra-abdominal | 16 (45.7) | 23 (50.0) | |
| ȃVascular catheter-related | 2 (5.7) | 3 (6.5) | |
| ȃSurgical site | 2 (5.7) | 1 (2.2) | |
| ȃPneumonia | 3 (8.6) | 2 (4.4) | |
| ȃMucositis/neutropenia | 2 (5.7) | 9 (19.6) | |
| ȃMusculoskeletal | 0 (0) | 1 (2.2) | |
| ȃSkin/soft tissue infection | 2 (5.7) | 2 (4.4) | |
| ȃUnknown | 1 (2.9) | 2 (4.4) | |
| Source control achieved, n (%) | 0.288 | ||
| ȃYes/not applicable | 28 (80.0) | 32 (69.6) | |
| ȃNo | 7 (20.0) | 14 (30.4) | |
| Appropriate initial antibiotic, n (%) | 33 (94.2) | 44 (95.7) | 0.779 |
| Outcomes | |||
| Clinical or microbiological failure, n (%) | 17 (48.6) | 17 (37.0) | 0.294 |
| ȃIn-hospital 30-day mortality | 2 (5.7) | 3 (6.5) | 0.881 |
| ȃWBC >12 × 109/L on days 5–7 | 8 (22.9) | 10 (21.7) | 0.905 |
| ȃTmax ≥38°C on days 5–7 | 6 (17.1) | 10 (21.7) | 0.607 |
| ȃMicrobiological failure on days 3–5f | 4 (11.4) | 0 (0.0) | 0.019 |
| ȃMicrobiological relapse on days 5–30g | 1 (2.9) | 2 (4.4) | 0.725 |
| Hospital length of stay (days), median (IQR) | 12.4 (6.1–22.2) | 13.2 (5.5–25.1) | 0.26 |
| ICU length of stay (days), median (IQR) | 2.4 (1.0–3.3) | 10.1 (2.8–14.6) | 0.855 |
| Clostridioides difficile infection, n (%) | 0 (0.0) | 2 (4.4) | 0.212 |
CrCl, creatinine clearance; MDRO, multidrug resistant organism; Tmax, maximum temperature.
Within 24 h before to 24 h after index blood culture.
Defined as history of infection due to MRSA, VRE, ESBL-producing or carbapenem-resistant Enterobacterales, Pseudomonas aeruginosa or Acinetobacter spp.
Defined as 20 mg daily of prednisone or equivalent for ≥14 days.
Defined as a non-chemotherapy immunosuppressive drug, such as tacrolimus, methotrexate, cyclosporine or mycophenolate.
Defined as an absolute neutrophil count of <500 cells/mm3.
Defined as a positive blood culture with the same species as the index culture.
Defined as growth from any sterile site with the same organism as the index culture.
Endpoints
Seventeen (48.6%) patients in the piperacillin/tazobactam arm had clinical or microbiological failure compared with 17 (37.0%) within the cefepime/carbapenem arm (P = 0.294) (Table 1). There were no differences in components of the composite primary endpoint, except for microbiological failure, which was higher in the piperacillin/tazobactam arm compared with the cefepime/carbapenem arm (11.4% versus 0.0%, P = 0.019). Details for patients with microbiological failure are in Supplementary Tables S1 and S2.
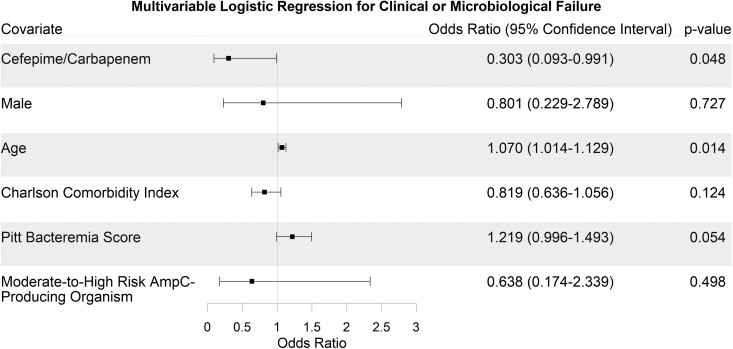
The logistic regression showed that definitive treatment with cefepime or a carbapenem was associated with a decreased odds of clinical or microbiological failure (OR 0.303, 95% CI 0.093–0.991, P = 0.048) when controlling for baseline characteristics. Age was associated with increased odds of clinical or microbiological failure (OR 1.070, 95% CI 1.014–1.129, P = 0.014). The other covariates in the analysis were not significantly associated with the primary endpoint (Figure 1).
Figure 1.
Forest plot depicting the a priori logistic regression for the primary endpoint of clinical or microbiological failure.
There were no differences reported in the secondary endpoints (Table 1).
Discussion
In this study of piperacillin/tazobactam versus cefepime or carbapenems for cefoxitin-non-susceptible Enterobacterales bacteraemia in immunocompromised patients, we found an association between definitive piperacillin/tazobactam treatment and poorer clinical and microbiological outcomes, when compared with treatment with cefepime or carbapenems. This association was driven primarily by increased microbiological failure. These observations align with those of the MERINO 2 trial, which found that piperacillin/tazobactam was associated with a higher risk of microbiological failure.7 Paradoxically, although patients in the cefepime/carbapenem arm had higher mean Pitt bacteraemia scores and rates of severe neutropenia, there were still lower rates of microbiological failure in this cohort, potentially strengthening these conclusions.
Whether this increased risk of microbiological failure has a clinical impact is debatable. Persistent Gram-negative bacteraemia has been associated with increased mortality,18,19 and two observational studies have identified increased mortality with piperacillin/tazobactam treatment for organisms with inducible AmpC.8,9 The only prospective randomized trial in this space, MERINO 2, albeit underpowered, was unable to detect clinical differences, including mortality, between piperacillin/tazobactam and meropenem. Our study was unable to detect a difference between treatment arms for any of the clinical endpoints, including mortality, but may have been underpowered to do so.
Notably, although S. marcescens has much lower AmpC induction potential than other organisms like E. cloacae and K. aerogenes,20 it comprised 50% of microbiological failure cases and 67% of microbiological relapse cases, despite being only 27% of the overall cohort. (Supplementary Figure S2). Although the number of observations is too small to make formal assertions, it is a concerning signal.
To our knowledge, this is the first study to focus on outcomes of infections due to AmpC-producing organisms specifically in immunocompromised patients. These patients are at high risk of infections from MDR organisms, but data are lacking to guide appropriate therapy.21 Prior studies have included immunocompromised patients in their analyses, but these patients were a minority of the study population.7,22 Immunocompromised patients may have decreased capabilities to clear infections, which may have potentiated the differences in microbiological failure rates we reported.23
There are several limitations that deserve discussion. First, our study was retrospective and observational, thus an a priori multivariable regression model was used based on previously published associations with clinical and microbiological outcomes. However, we acknowledge that the impact of known and unknown imbalances between groups cannot be fully controlled with these designs. Second, we included only immunocompromised patients, which limited population heterogeneity. Nevertheless, this design provides a more focused perspective on an understudied population. Third, although our observed differences in primary outcomes were driven by microbiological failure, not all patients had follow-up blood cultures. However, follow-up blood cultures are typically not needed for Gram-negative bacteraemia24,25 and may be a surrogate for clinical worsening. Fourth, our study may have been underpowered for the primary endpoint. A post hoc power analysis indicated that 283 patients were needed to achieve 80% power, based on the observed risk difference of 12% for the primary endpoint. That said, to our knowledge this is the largest investigation of outcomes for treatment of potential AmpC-producing organisms specifically in immunocompromised patients. Fifth, cefoxitin-non-susceptibility was used as a surrogate for AmpC production. Although a genetic methodology may be more accurate, cefoxitin-non-susceptibility is a fairly sensitive, specific and practical surrogate for AmpC production.26
Conclusion
In immunocompromised patients with bacteraemia due to AmpC-producing organisms, piperacillin/tazobactam may be associated with increased microbiological failure compared with cefepime or carbapenems. Given the retrospective, observational nature of this study and limited sample size, further prospective trials are needed.
Supplementary Material
Contributor Information
Brian Lu, Department of Pharmacy, Stanford Health Care, 300 Pasteur Drive, Stanford, CA 94305, USA.
Miranda Wong, Department of Pharmacy, Stanford Health Care, 300 Pasteur Drive, Stanford, CA 94305, USA.
David Ha, Department of Quality, Patient Safety and Effectiveness, Stanford Health Care, Stanford, CA, USA; Division of Infectious Diseases and Geographic Medicine, Stanford University School of Medicine, Stanford, CA, USA.
Mark Bounthavong, Division of Clinical Pharmacy, UCSD Skaggs School of Pharmacy & Pharmaceutical Sciences, La Jolla, CA, USA.
Niaz Banaei, Division of Infectious Diseases and Geographic Medicine, Stanford University School of Medicine, Stanford, CA, USA; Division of Clinical Pathology, Stanford University School of Medicine, Stanford, CA, USA.
Stanley Deresinski, Department of Quality, Patient Safety and Effectiveness, Stanford Health Care, Stanford, CA, USA; Division of Infectious Diseases and Geographic Medicine, Stanford University School of Medicine, Stanford, CA, USA.
Calvin Diep, Department of Pharmacy, Stanford Health Care, 300 Pasteur Drive, Stanford, CA 94305, USA.
Funding
This study was carried out as part of our routine work.
Transparency declarations
There are no conflicts of interest to declare.
Author contributions
BL, MW, DH and CD led the development of the project, contributed to and reviewed the manuscript, and collected and analysed data. MB contributed to the study design, analysed data, and contributed to and reviewed the manuscript. NB and SD contributed to the study design and contributed to and reviewed the manuscript.
Supplementary data
Figures S1 and S2 and Tables S1 and S2 are available as Supplementary data at JAC Online.
References
- 1. Tamma PD, Doi Y, Bonomo RAet al. A primer on AmpC β-lactamases: necessary knowledge for an increasingly multidrug-resistant world. Clin Infect Dis 2019; 69: 1446–55. 10.1093/cid/ciz173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bush K, Jacoby GA. Updated functional classification of β-lactamases. Antimicrob Agents Chemother 2010; 54: 969–76. 10.1128/AAC.01009-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nelson EC, Elisha BG. Molecular basis of AmpC hyperproduction in clinical isolates of Escherichia coli. Antimicrob Agents Chemother 1999; 43: 957–9. 10.1128/AAC.43.4.957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. MacDougall C. Beyond susceptible and resistant. Part I: treatment of infections due to gram-negative organisms with inducible β-lactamases. J Pediatr Pharmacol Ther 2011; 16: 23–30. [PMC free article] [PubMed] [Google Scholar]
- 5. Papp-Wallace KM, Bethel CR, Caillon Jet al. Beyond piperacillin-tazobactam: cefepime and AAI101 as a potent β-lactam−β-lactamase inhibitor combination. Antimicrob Agents Chemother 2019; 63: e00105–19. 10.1128/AAC.00105-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tamma PD, Aitken SL, Bonomo RAet al. Infectious Diseases Society of America guidance on the treatment of AmpC β-lactamase–producing enterobacterales, carbapenem-resistant Acinetobacter baumannii, and Stenotrophomonas maltophilia infections. Clin Infect Dis 2022; 74: 2089–114. 10.1093/cid/ciab1013 [DOI] [PubMed] [Google Scholar]
- 7. Stewart AG, Paterson DL, Young Bet al. Meropenem versus piperacillin-tazobactam for definitive treatment of bloodstream infections caused by AmpC β-lactamase–producing Enterobacter spp, Citrobacter freundii, Morganella morganii, Providencia spp, or Serratia marcescens: a pilot multicenter randomized controlled trial (MERINO-2). Open Forum Infect Dis 2021; 8: ofab387. 10.1093/ofid/ofab387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cheng L, Nelson BC, Mehta Met al. Piperacillin-tazobactam versus other antibacterial agents for treatment of bloodstream infections due to AmpC β-lactamase-producing Enterobacteriaceae. Antimicrob Agents Chemother 2017; 61: e00276–17. 10.1128/AAC.00276-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chaubey VP, Pitout JDD, Dalton Bet al. Clinical and microbiological characteristics of bloodstream infections due to AmpC β-lactamase producing Enterobacteriaceae: an active surveillance cohort in a large centralized Canadian region. BMC Infect Dis 2014; 14: 647. 10.1186/s12879-014-0647-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cheng MP, Lee RS, Cheng APet al. Beta-lactam/beta-lactamase inhibitor therapy for potential AmpC-producing organisms: a systematic review and meta-analysis. Open Forum Infect Dis 2019; 6: ofz248. 10.1093/ofid/ofz248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moy S, Sharma R. Treatment outcomes in infections caused by “SPICE” (Serratia, Pseudomonas, indole-positive Proteus, Citrobacter, and Enterobacter) organisms: carbapenem versus noncarbapenem regimens. Clin Ther 2017; 39: 170–6. 10.1016/j.clinthera.2016.11.025 [DOI] [PubMed] [Google Scholar]
- 12. Vidal F. Bacteraemia in adults due to glucose non-fermentative Gram-negative bacilli other than P. aeruginosa. QJM 2003; 96: 227–34. 10.1093/qjmed/hcg031 [DOI] [PubMed] [Google Scholar]
- 13. Kutob LF, Justo JA, Bookstaver PBet al. Effectiveness of oral antibiotics for definitive therapy of Gram-negative bloodstream infections. Int J Antimicrob Agents 2016; 48: 498–503. 10.1016/j.ijantimicag.2016.07.013 [DOI] [PubMed] [Google Scholar]
- 14. Chotiprasitsakul D, Han JH, Cosgrove SEet al. Comparing the outcomes of adults with Enterobacteriaceae bacteremia receiving short-course versus prolonged-course antibiotic therapy in a multicenter, propensity score–matched cohort. Clin Infect Dis 2018; 66: 172–7. 10.1093/cid/cix767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Harris PNA, Tambyah PA, Lye DCet al. Effect of piperacillin-tazobactam vs meropenem on 30-day mortality for patients with E coli or Klebsiella pneumoniae bloodstream infection and ceftriaxone resistance: a randomized clinical trial. JAMA 2018; 320: 984–94. 10.1001/jama.2018.12163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Porat Y, Nashashibi J, Poran Iet al. Predictors of readmission following discharge of patients with gram-negative bacteremia: a retrospective cohort study. Open Forum Infect Dis 2021; 8: ofab373. 10.1093/ofid/ofab373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hogan C, Watz N, Budvytiene Iet al. Rapid antimicrobial susceptibility testing by VITEK®2 directly from blood cultures in patients with gram-negative rod bacteremia. Diagn Microbiol Infect Dis 2019; 94: 116–21. 10.1016/j.diagmicrobio.2019.01.001 [DOI] [PubMed] [Google Scholar]
- 18. Harris JA, Cobbs CG. Persistent gram-negative bacteremia. Am J Surg 1973; 125: 705–17. 10.1016/0002-9610(73)90169-4 [DOI] [PubMed] [Google Scholar]
- 19. Maskarinec SA, Park LP, Ruffin Fet al. Positive follow-up blood cultures identify high mortality risk among patients with Gram negative bacteremia. Clin Microbiol Infect 2020; 26: 904–10. 10.1016/j.cmi.2020.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kohlmann R, Bähr T, Gatermann SG. Species-specific mutation rates for ampC derepression in Enterobacterales with chromosomally encoded inducible AmpC β-lactamase. J Antimicrob Chemother 2018; 73: 1530–6. 10.1093/jac/dky084 [DOI] [PubMed] [Google Scholar]
- 21. Abbo LM, Ariza-Heredia EJ. Antimicrobial stewardship in immunocompromised hosts. Infect Dis Clin North Am 2014; 28: 263–79. 10.1016/j.idc.2014.01.008 [DOI] [PubMed] [Google Scholar]
- 22. Jeon M, Huh K, Ko JHet al. Difference in the clinical outcome of bloodstream infections caused by Klebsiella aerogenes and Enterobacter cloacae complex. Open Forum Infect Dis 2021; 8: ofab390. 10.1093/ofid/ofab390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dropulic LK, Lederman HM. Overview of infections in the immunocompromised host. Microbiol Spectr 2016; 4: 4.4.43. 10.1128/microbiolspec.DMIH2-0026-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wiggers JB, Xiong W, Daneman N. Sending repeat cultures: is there a role in the management of bacteremic episodes? (SCRIBE study). BMC Infect Dis 2016; 16: 286. 10.1186/s12879-016-1622-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Canzoneri CN, Akhavan BJ, Tosur Zet al. Follow-up blood cultures in Gram-negative bacteremia: are they needed? Clin Infect Dis 2017; 65: 1776–9. 10.1093/cid/cix648 [DOI] [PubMed] [Google Scholar]
- 26. Polsfuss S, Bloemberg GV, Giger Jet al. Practical approach for reliable detection of AmpC beta-lactamase-producing Enterobacteriaceae. J Clin Microbiol 2011; 49: 2798–803. 10.1128/JCM.00404-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.