Abstract
Background
Nacubactam, a new β-lactamase inhibitor with antibacterial activity, is being developed as a single drug to be co-administered with cefepime or aztreonam. However, determining pharmacokinetics/pharmacodynamics (PK/PD) parameters in β-lactam/β-lactamase inhibitor combinations remains challenging. We aimed to establish a practical PK/PD analysis method for aztreonam/nacubactam that incorporates instantaneous MIC (MICi).
Methods
Based on chequerboard MIC measurements, MICi of aztreonam against carbapenemase-producing Klebsiella pneumoniae in the presence of nacubactam was simulated.
Results
The mean change in the bacterial count of thigh-infected mice in an in vivo PD study was plotted based on %fT>MICi and analysed using the inhibitory effect sigmoid Imax model. fT>MICi calculated from the PK experiments showed a high correlation with the in vivo bactericidal effect, suggesting that fT>MICi is the optimal PK/PD parameter for aztreonam/nacubactam. The target values of fT>MICi achieving growth inhibition, 1 log10 kill and 2 log10 kill, were 22, 38% and 75%, respectively.
Conclusions
The PK/PD analysis method proposed in this study is promising for determining practical PK/PD parameters in combination therapy. In addition, this is the first report of aztreonam/nacubactam showing a potent in vivo therapeutic effect against NDM-producing K. pneumoniae.
Introduction
Since the late 1900s, the development of many antimicrobial agents has greatly improved human health and welfare. However, the increase in drug-resistant bacteria due to the inappropriate use of antimicrobials has become a problem worldwide. Recently, the Antimicrobial Resistance Collaborators1 reported that in 2019, 4.95 million deaths were associated with drug-resistant bacteria, of which 1.27 million were directly attributable to drug-resistant bacteria, strongly indicating that overcoming drug resistance is an important global healthcare challenge. The O’Neill report2,3 estimates that 10 million people will die annually from drug-resistant bacterial infections in 2050 if drug resistance increases at its current rate. In particular, Gram-negative bacteria that have acquired resistance by producing β-lactamases that hydrolyse β-lactam antibiotics are positioned as a serious threat.4 To treat patients with β-lactamase-producing Gram-negative bacterial infections, the combination therapy of β-lactams and β-lactamase inhibitors, which inhibit the enzymatic inactivation of β-lactams by β-lactamases, has been the cornerstone of treatment of β-lactamase-producing Gram-negative bacterial infections in modern medical care.
In the clinical use of antimicrobials, pharmacokinetics/pharmacodynamics (PK/PD) parameters calculated using mouse infection models have contributed to determining evidence-based clinical doses of antimicrobials.5,6,7 In general, it is desirable to set the PK/PD parameters using specific MICs and pharmacokinetics parameters that can directly reflect the antimicrobial dose to be generalized to a wide variety of bacteria and doses. The PK/PD parameters for most antimicrobial monotherapies in clinical use are based on the percentage of free time above MIC (%fT>MIC) or fAUC/MIC and free maximum concentration (fCmax)/MIC to set the clinical dose.7
In contrast, the PK/PD parameters proposed for β-lactam/β-lactamase inhibitors are limited to fT>CT.8 The CT value, meaning ‘the lowest concentration of β-lactamase inhibitor required to inhibit β-lactamase when used with a given dose of β-lactam’,9 is based on the fixed values of factors such as MIC of the bacterial strain, β-lactam administration method, β-lactamase genotype and gene expression levels. This makes it impossible to analyse drug efficacy with flexibility for bacteria with different MICs and concentrations of both drugs using fT>CT, thus preventing comprehensive clinical efficacy prediction. It is difficult to determine the PK/PD parameter that reflects the instantaneous variation of MICs in the presence of fluctuating blood concentrations of β-lactams/β-lactamase inhibitors over time, given the interdependency in the efficacy of the β-lactams/β-lactamase inhibitors. Therefore, PK/PD parameters for β-lactam/β-lactamase inhibitors using fT>CT values are not practical. Hence, the establishment of new PK/PD parameters incorporating the concept of MIC is desired.
We considered that using the instantaneous MIC (MICi) for PK/PD analysis of β-lactams/β-lactamase inhibitors could overcome this challenge. MICi is a mathematical modelling and simulation assessment concept that depends on β-lactamase inhibitor concentration and the sensitivity for β-lactams that varies over time. This concept was first proposed by Bhagunde et al.10 in the imipenem/relebactam in vitro hollow-fibre infection model. The utility of MICi has since been demonstrated for several β-lactams/β-lactamase inhibitors.11–14 Therefore, applying the MICi concept to PK/PD analysis in a murine infection model allows for flexible clinical dosing design, considering the interdependence of β-lactams/β-lactamase inhibitors. However, no in vivo studies have proven the value of PK/PD analyses with MICi.
Recently, nacubactam (OP0595), a diazabicyclooctane (DBO)-type new β-lactamase inhibitor with antibacterial activity, is being developed as a single drug to be co-administered with cefepime or aztreonam. Our study aims to establish a PK/PD analysis approach for β-lactam/β-lactamase inhibitors using MICi and investigate their superiority to fT>CT. For this purpose, we used nacubactam, a novel β-lactamase inhibitor currently under development, and aztreonam, a β-lactam antibiotic. Furthermore, we used the PK/PD analysis method established in this study to explore the practical PK/PD parameters, and their target values of aztreonam/nacubactam against β-lactamase-producing Gram-negative bacteria.
Materials and methods
Antimicrobials
Nacubactam monohydrate was provided by Meiji Seika Pharma Co., Ltd. (Tokyo, Japan). Aztreonam powder (Tokyo Chemical Industry Co., Ltd., Tokyo, Japan) and aztreonam vials (Eisai Co., Ltd., Tokyo, Japan) were used for in vitro and in vivo studies, respectively.
Bacterial strains
Escherichia coli ATCC® 25922 was used as the quality control strain for susceptibility testing. Three strains of β-lactamase-producing Klebsiella pneumoniae listed in Table 1 were used in this study. E. coli ATCC 25922 and K. pneumoniae ATCC® BAA-2473 were obtained from ATCC®. Clinical isolates (K. pneumoniae MSC 21664 and MSC 21444) were provided by Meiji Seika Pharma.
Table 1.
Characteristics of the strains used
| MIC (mg/L)a | ||||
|---|---|---|---|---|
| Strain | β-Lactamase | Aztreonam | Nacubactam | Aztreonam/nacubactamb |
| K. pneumoniae | ||||
| ȃATCC BAA-2473 | NDM-1 | 256 | 256 | 1 |
| ȃMSC 21664 | IMP-6, CTX-M-2 | 32 | 16 | 0.016 |
| ȃMSC 21444 | OXA-48 | 512 | 2 | 1 |
MIC value is the median of technical replicates (n = 3).
MIC of nacubactam measured with a fixed aztreonam concentration of 4 mg/L.
Chequerboard assay
According to CLSI guidelines,15,16 chequerboard MICs of aztreonam/nacubactam were determined by broth microdilution using CAMHB (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) (n = 3). A 2-fold step dilution series of each drug in the concentration range of 0 and 0.001 to 1024 mg/L was added to the wells of a 96-well plate in a 1/1 volume of aztreonam/nacubactam. Bacteria were added to the plate to a final concentration of approximately 2–8 × 105 cfu/mL. After incubating at 37°C for 20 h, the lowest concentration of aztreonam with each nacubactam concentration, at which no bacterial growth was observed, was defined as the MIC. The MIC values for each drug alone and nacubactam when combined with 4 mg/L of aztreonam are shown in Table 1. MIC values were taken as the median of technical replicates (n = 3).
Effect model analysis
The results obtained by chequerboard assay for each strain were plotted and analysed using the inhibitory effect sigmoid Imax model (Equation 1) to draw an approximate curve representing the dose–response relationship of the aztreonam MIC to nacubactam concentration.
| (1) |
where MIC0 is the MIC of aztreonam alone, CNAC is the concentration of nacubactam (mg/L), Imax is the maximum inhibitory effect, IC50 is the concentration of nacubactam at which 50% of the maximum inhibition occurs and ɤ is the sigmoid coefficient.
Animals
Male ICR mice (Sankyo Labo Service Corporation, Inc., Shizuoka, Japan) were used for all animal experiments. The mice were acclimatized for 1 week under a 12 h light/dark cycle with feeding and watering ad libitum and used for the experiment at 5 weeks of age. The mice were intraperitoneally injected with cyclophosphamide (Shionogi & Co., Ltd., Osaka, Japan) for 4 days (150 mg/kg) and the day (100 mg/kg) before the experiment to decrease their neutrophils. Protocols for all animal experiments were approved by the Institutional Animal Care and Use Committee of Keio University (No. 19037) and the Animal Experiment Management Committee, Pharmaceutical Research Center, Meiji Seika Pharma.
PK analysis
Aztreonam/nacubactam was subcutaneously administered to neutropenic mice. Blood samples were collected through cardiac puncture under anaesthesia at 5, 15, 30, 60, 120, 240 and 300 min after administration (n = 3 per timepoint). Immediately after blood sampling, the mice were euthanized by cervical dislocation. The blood samples were centrifuged to separate the plasma and stored at −80°C. PK parameters were calculated by compartmental model analysis of free plasma nacubactam and aztreonam concentrations using Phoenix® WinNonlinTM (v. 8.0, Certara, NJ, USA).
LC-MS/MS analysis
The concentration of antibiotics in each plasma sample was measured using a validated LC-MS/MS method. Detailed measurement conditions are summarized in Table S1. The protein binding rates of nacubactam and aztreonam in mouse plasma were determined by the ultrafiltration method. In short, plasma containing nacubactam and aztreonam at concentrations of 1, 10 and 400 mg/L (n = 3 per concentration) was incubated at 37°C for 30 min and then set into Centrifree® 4104 Ultrafiltration Centrifugal Filters (Millipore, Bedford, MA, USA). The filtrate was obtained by centrifugation at 1500× g for 5 min. The concentrations of the antibiotics in the initial sample and the filtrate were measured using LC-MS/MS, and the protein binding rate was calculated.
PD study
Neutropenic mice were infected intramuscularly with 100 µL of inoculum bacterial suspension at a concentration of 1 × 106–7 cfu/mL in the left thigh 2 h before drug administration. A combination of nacubactam (0, 1.2, 3.6, 12, 36, 120, 360, 1200 mg/kg/day) and aztreonam (0, 1200, 2400, 4800 mg/kg/day) were subcutaneously administered from 2 to 26 h after inoculation (n = 3 per dose). In the dose-fractionation study, aztreonam was administered q2h. In contrast, nacubactam was administered at a variable interval schedule (q2h, q4h, and q8h) from 2 to 26 h after inoculation (Figure S1). In the dose-ranging study, both drugs were administered q2h from 2 to 26 h after inoculation (Figure S1). The control mice were euthanized by cervical dislocation at 0 h (2 h after inoculation). Other mice were euthanized 24 h after initial drug dosing (26 h after inoculation). The thigh was then aseptically collected and homogenized using Multi-Beads Shocker (Yasui Kikai Corp., Osaka, Japan). Serial dilution series of each homogenate were prepared, and an aliquot of each suspension was applied to Mueller–Hinton agar plates. After incubation at 37°C for about 20 h, the number of colonies that grew was measured. The lowest detection limit was 2.20 log10 cfu/thigh.
PK/PD analysis with fT>MICi
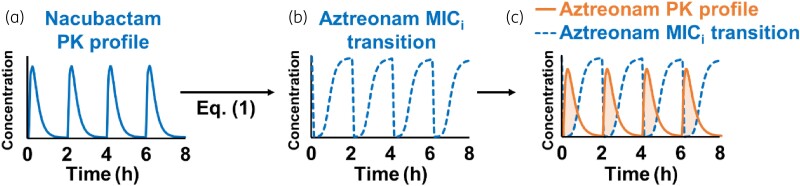
The dose–response curve (Equation 1) obtained by the model analysis of the chequerboard assay represents the MIC of aztreonam, which varies depending on the nacubactam concentration. Since the nacubactam concentration is constantly changing in vivo, the MIC of aztreonam is also constantly changing accordingly. This instantaneous MIC of aztreonam was taken as MICi. Therefore, by applying the time course of free nacubactam plasma concentration (Figure 1a) to the dose–response relational equation Equation 1, the time course of aztreonam MICi following nacubactam administration was calculated (Figure 1b). The percentage of time that the free aztreonam plasma concentration exceeded the MICi was defined as %fT>MICi (Figure 1c). The mean change in the bacterial count of each group obtained by in vivo PD study was plotted based on %fT>MICi and analysed using the inhibitory effect sigmoid Imax model (Equation 2).
Figure 1.
Estimation of fT>MICi. (a) Simulated free plasma nacubactam PK profile; (b) calculated aztreonam MICi transition after administration of nacubactam based on the dose–response curve; (c) free plasma aztreonam PK profile (solid orange line) and aztreonam MICi transition (blue dashed line) were superimposed. The %fT>MICi is the percentage of time that the free plasma aztreonam concentration exceeded the MICi. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
| (2) |
where E is the change in the bacterial count, E0 is bacterial count at fT>MICi = 0%, Imax is the maximum inhibitory effect, IC50 is the fT>MICi at which 50% of the maximum inhibition occurs and ɤ is the sigmoid coefficient.
PK/PD analysis with CT
fT>CT, fAUC and fCmax of β-lactamase inhibitors were used as PK/PD indices.17–19 Following their lead, the results of the in vivo PD study against fT>CT, fAUC and fCmax of nacubactam were analysed. The percentage of time that the free nacubactam plasma concentration at each dose exceeded the CT value (0.125, 0.25, 0.5, 1, 2 and 2.5 mg/L) was calculated as %fT>CT. The CT value was set with reference to past reports.17,18,19 The mean change in bacterial counts of each group obtained from the in vivo PD study was plotted based on % fT>CT and analysed using Equation 2 for each strain and dose of aztreonam used in combination to determine the CT value with the highest R2 value.
Results
Chequerboard MIC and effect model analysis
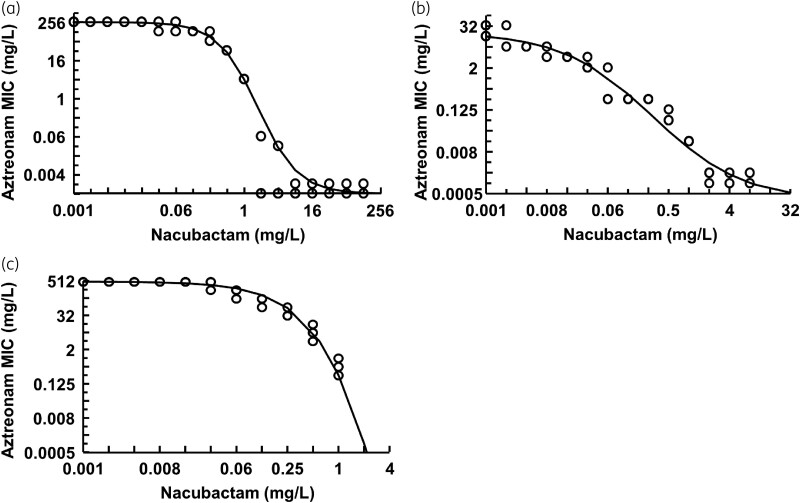
The β-lactamase genotype of each bacterial strain and the MIC values of aztreonam/nacubactam alone and nacubactam in the presence of 4 mg/L aztreonam for each bacterial strain are shown in Table 1. Figure 2 shows the dose–response relationship of aztreonam MIC to nacubactam concentration for each strain analysed by Equation 1. All strains were resistant to aztreonam alone, but the combination of aztreonam and nacubactam decreased MIC in a nacubactam concentration-dependent manner. Interestingly, nacubactam alone also showed slight antibacterial activity against all bacterial strains.
Figure 2.
MIC curve between the aztreonam MIC and the nacubactam concentration (n = 3). (a) NDM-1-positive K. pneumoniae ATCC BAA-2473; (b) IMP-6-positive K. pneumoniae MSC 21664; (c) OXA-48-positive K. pneumoniae MSC 21444.
PK analysis
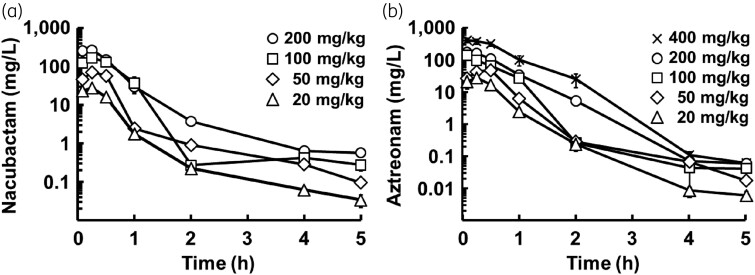
The PK profiles of nacubactam and aztreonam in neutropenic mice are shown in Figure 3. The plasma concentration curve for nacubactam and aztreonam best fitted the two-compartment model. The PK parameters of each drug analysed from the plasma concentration curve are listed in Table S2. Cmax and AUC of each drug showed good linearity within the dose range tested (Figure S2). The plasma protein binding rates of nacubactam and aztreonam were similar among the three concentrations (Table S3), with averages of 3.87% and 57.1% for nacubactam and aztreonam, respectively. The average values were used for PK/PD analysis.
Figure 3.
PK profiles of nacubactam (a) and aztreonam (b) in neutropenic mice (mean ± SD, n = 3).
PD study
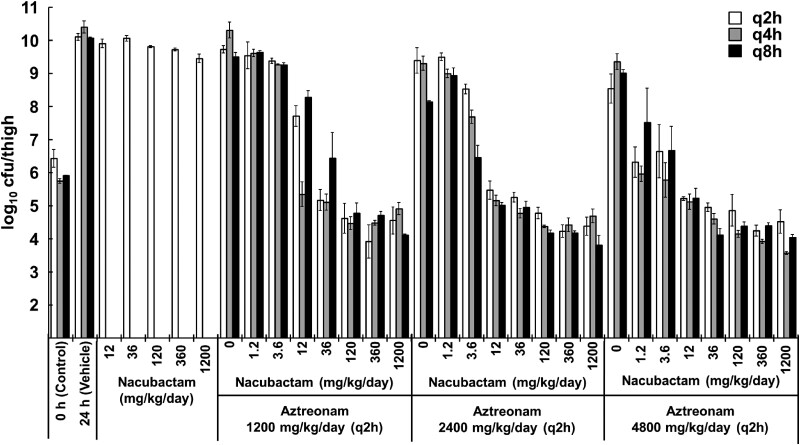
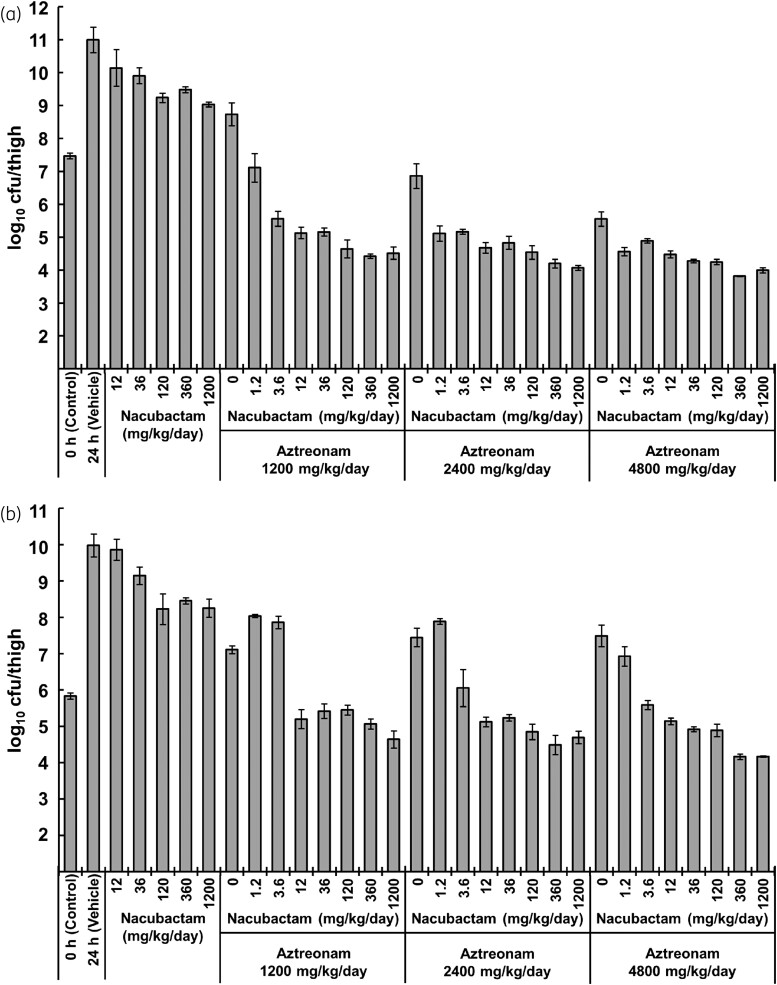
An in vivo dose-fractionation study in neutropenic mice thigh-infected with K. pneumoniae ATCC® BAA-2473 showed that aztreonam and nacubactam alone hardly reduced bacterial counts. However, the aztreonam/nacubactam combination showed a nacubactam dose-dependent bacterial reduction and a strong bactericidal effect of up to 2 log10 cfu/thigh compared with the control group (Figure 4).
Figure 4.
In vivo dose-fractionation study of aztreonam/nacubactam against a neutropenic murine model of thigh infection with NDM-1-positive K. pneumoniae ATCC BAA-2473 (mean ± SD, n = 3). Aztreonam was administered every 2 h, and nacubactam was administered with variable frequency: every 2 h, every 4 h and every 8 h.
The in vivo dose-ranging study of aztreonam/nacubactam was assessed in neutropenic thigh-infected mice with K. pneumoniae MSC 21664 and MSC 21444, administering aztreonam/nacubactam at various doses q2h (Figure 5). In both strains, nacubactam and aztreonam alone showed a slight bacterial reduction, but the combination of both antibiotics provided a potent bactericidal effect. The maximum bactericidal effect in mice infected with K. pneumoniae MSC 21664 and MSC 21444 was about 3 log10 and 1 log10 cfu/thigh, respectively.
Figure 5.
In vivo dose-ranging study of aztreonam/nacubactam against a neutropenic murine model of thigh infection with IMP-6-positive K. pneumoniae MSC 21664 (a) and OXA-48-positive K. pneumoniae MSC 21444 (b) (mean ± SD, n = 3). Both nacubactam and aztreonam were administered every 2 h.
PK/PD analysis with fT>MICi
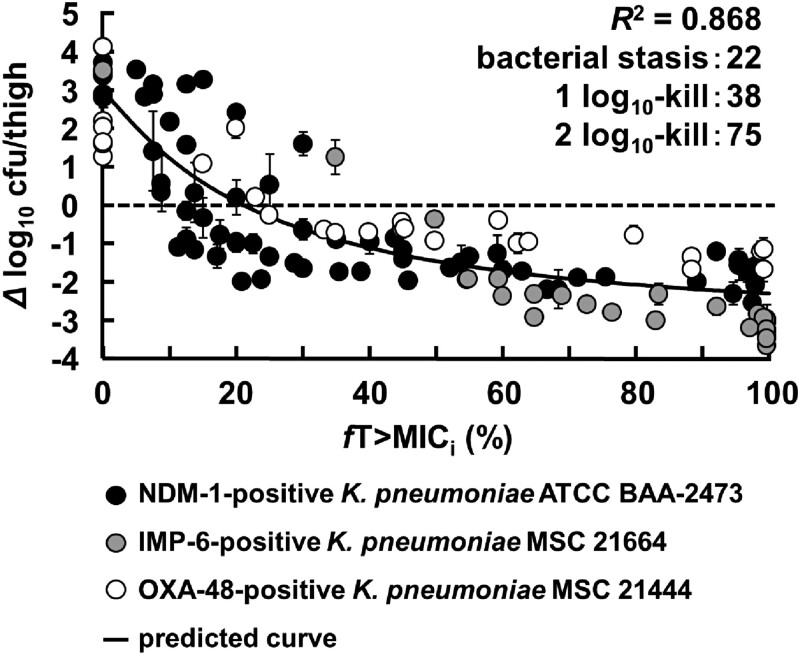
The values of fT>MICi in each dose group calculated from the PK data were plotted against the change in bacterial counts obtained in the PD study (Figure 6). The results showed a high correlation (R2 = 0.868) between the change in viable bacterial counts and fT>MICi following aztreonam/nacubactam administration. The growth inhibition and bactericidal effect target values were further analysed by Equation 2. The target values of fT>MICi required to achieve growth inhibition, 1 log10 kill and 2 log10 kill were 22%, 38% and 75%, respectively.
Figure 6.
PK/PD analysis and target fT>MICi values of aztreonam with/without nacubactam.
PK/PD analysis with CT
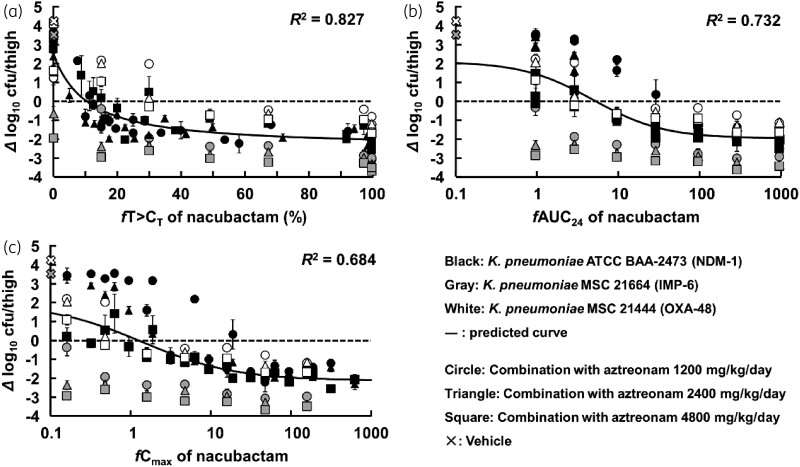
The values of fT>CT in each dose group calculated from the PK data were plotted against the change in bacterial counts obtained in the PD study. The R2 value in each CT of nacubactam was analysed by Equation 2 (Figures S3–S5). Figures S3–S5 summarize the relationship between CT of nacubactam and R2 values. The CT values with the highest R2 value for K. pneumoniae ATCC® BAA-2473 when combined with aztreonam 1200, 2400 and 4800 mg/kg/day were 2, 1 and 0.125 mg/L, respectively (Figure S3). For K. pneumoniae MSC 21664 and MSC 21444, the highest R2 values were obtained at CT = 0.125 mg/L for all doses (Figures S4 and S5). The optimum CT value was set for each strain and the dose of aztreonam in combination, and an analysis was performed collectively, and the R2 value was 0.827 (Figure 7). Analysis of the relationship between the fAUC or fCmax of nacubactam and bacterial counts revealed that the R2 value was lower than fT>CT (Figure 7). The R2 value for these parameters using only nacubactam exposure are inferior to those of the fT>MICi analysis, meaning that an integrated PK/PD approach is needed.
Figure 7.
PK/PD analysis based on fT>CT with optimal CT values of nacubactam (a), fAUC24 of nacubactam (b), and fCmax of nacubactam (c).
Discussion
PK/PD parameters of β-lactams/β-lactamase inhibitors are challenging to determine. This is because some β-lactamase inhibitors have antimicrobial activity in addition to protecting β-lactams from β-lactamases, and the antimicrobial effects of various combinations are interdependent. There is a need to establish accurate analytical methods to determine the PK/PD parameters of β-lactams/β-lactamase inhibitors because inappropriate analytical methods or models with low predictive accuracy risks creating a dissociation between susceptibility data in vitro and actual clinical efficacy. The current research shows how the MICi concept may be used in conventional PK/PD analysis to produce PK/PD parameters that are more practical, versatile and accurate.
The first-generation β-lactamase inhibitor combination piperacillin/tazobactam has been tested for the MIC of piperacillin in a fixed concentration of tazobactam of 4 mg/L, at a dose ratio of 8:1. On the other hand, amoxicillin/clavulanate is tested for susceptibility at a fixed 2:1 ratio, although several formulations with ratios of 2:1, 4:1 and 7:1 are used and may not correlate with efficacy. This in vitro and in vivo difference in combination ratios may be due to the fluctuating susceptibility of β-lactams in response to changing inhibitor concentrations over time being ignored during in vivo analysis. Optimization of fT>CT assumes that the susceptibility of β-lactams is constant in the presence of β-lactamase inhibitors. However, MICs for clinical strains are not incorporated into the dosing design and do not adequately reflect changes in β-lactam susceptibility caused by different β-lactamase inhibitor concentrations.
In the present study, the MIC curve for aztreonam varied significantly in the therapeutic concentration range of nacubactam (approximately 0.1–10 mg/L) (Figure 2). In evaluating the PK/PD properties of β-lactams/β-lactamase inhibitors, this result supports the importance of considering changes in sensitivity under different β-lactamase inhibitor concentration conditions. On the other hand, fT>MICi can reflect changes in β-lactam susceptibility and changes in β-lactamase inhibitor concentration. Comparing the PK/PD analysis results from the two approaches, the R2 values were higher for fT>MICi than for fT>CT, although curves of similar shape were drawn (Figures 6 and 7). The low R2 value for fT>CT may be due to underestimating the PK/PD predictions based on fT>CT. Higher aztreonam concentrations are clinically effective even when nacubactam concentrations are below the CT value. Therefore, fT>MICi is considered a more useful PK/PD parameter than fT>CT.
The antibacterial activity of interdependent concomitant drugs is affected by factors such as bacterial species, enzyme type, enzyme expression level, antibacterial activity and inhibitory activity, and concentration-dependent additive/synergistic effects. However, in the clinical treatment of infectious diseases, the data available for designing the administration of antibiotics are limited. In addition, carbapenemase-producing clinical isolates that co-produce multiple β-lactamases are not uncommon in practice, and there is little significance in setting PK/PD targets for each strain. Therefore, it would be desirable to define a single PK/PD target that can accommodate a wide variety of clinical isolates, allowing optimal dosing design based on limited MIC data. PK/PD analysis with fT>CT confirmed that the CT value of nacubactam differed depending on the strain and the dose of concomitant aztreonam (Figures S3–S5). Therefore, it lacks versatility according to individual patients, such as strains that produce multiple types of β-lactamases and patients with renal dysfunction that affects pharmacokinetics. On the other hand, it is worth noting that dosing design based on fT>MICi does not need to consider these factors and therefore has good practical versatility. The chequerboard MIC curves reflect enzyme type, inhibitory activity, antimicrobial activity, and the additive and synergistic effects of antimicrobial agents. By simulating based on these chequerboard MIC curves, it is considered that a single PK/PD target value can be obtained even with different genotypes and different inhibitory activities. Indeed, the analysis by fT>MICi showed a high correlation when analysed for each strain (Figure S6), but a good correlation (R2 = 0.868) was also obtained when the results of three β-lactamase-producing strains with different genotypes were analysed together (Figure 6).
The novel DBO-based β-lactamase inhibitor, nacubactam, is a potent inhibitor of Ambler class A (e.g. KPC and ESBL) and C (AmpC) β-lactamases but weakly inhibits class D (OXA) β-lactamases.20,21 In addition, nacubactam exhibits antibacterial activity through binding to the bacterial PBP-2. Furthermore, unlike first-generation β-lactamase inhibitors (e.g. sulbactam, clavulanate, tazobactam), second-generation DBO-based β-lactamase inhibitors, including nacubactam, do not have a β-lactam structure and are not subject to β-lactamase degradation. Therefore, they are expected to be a new treatment option for carbapenemase (MBL)-producing bacteria, including class B (NDM and IMP),22,23 because no other established treatment is available. In the current chequerboard assay, aztreonam/nacubactam showed good combination efficacy against class B and D β-lactamase-producing bacteria such as NDM-1, IMP-6 and OXA-48 β-lactamase-producing K. pneumoniae (Table 1). Furthermore, the MIC of nacubactam against all bacterial species in the presence of 4 mg/L aztreonam reflects the minimum nacubactam concentration to achieve the microbiological breakpoint,16 and can be easily achieved in clinical practice, indicating aztreonam/nacubactam in vivo potent antimicrobial effect is obtainable. Furthermore, aztreonam/nacubactam combination therapy was effective in the murine model against NDM-1-, IMP-6- and OXA-48-producing K. pneumoniae in a nacubactam dose-dependent manner, despite aztreonam monotherapy being ineffective. Thus, the present study provides the first evidence of a satisfactory therapeutic effect of nacubactam against refractory β-lactamase-producing K. pneumoniae, particularly the in vivo antibacterial effect of aztreonam/nacubactam against NDM-1-producing bacteria.
There are two drawbacks to this research. The first is that all PD experiments only measured activity against fixed inoculum levels (approximately 106 cfu/mL). The effect of greater inoculum levels on aztreonam/nacubactam drug efficacy is unknown. Due to the inoculum effect, several antimicrobials have lower antimicrobial effectiveness at higher initial inoculum levels.24 Some β-lactam/β-lactamase inhibitors, on the other hand, have been less influenced by the inoculum effect. Ceftazidime/tazobactam, for example, showed decreased action at high bacterial levels, but ceftazidime/avibactam was said to be unaffected by inoculum levels.14 The causes for this are unknown. However, the inoculum effect could be related to avibactam’s reversible inhibitory action and resistance to enzymatic hydrolysis. Because nacubactam inhibits in the same way that avibactam does, it may be less vulnerable to the inoculum effect. On this point, more evidence is required. Second, the site of action of the antimicrobials was the femoral tissue, but plasma drug concentrations were used for PK. Although evaluation of drug concentration at the infection site can improve the accuracy of predicting therapeutic efficacy, quantification of the drug in the interstitial fluid of murine thigh tissue is difficult. Therefore, PK/PD studies using murine thigh-infection models are usually analysed based on free drug blood concentrations, assuming equal free drug concentrations in blood and thigh tissue. Further studies using models capable of assessing drug concentrations at the infection site, such as pneumonia models, are needed to obtain a more accurate exposure–response relationship. Finally, adopting MICi, a comprehensive concept, a new PK/PD analysis approach for β-lactam/β-lactamase inhibitor combination therapy was developed. In comparison with existing PK/PD parameters (fT>CT), fT>MICi and their target values in this newly designed PK/PD analysis approach were more practical, generic and accurate.
Supplementary Material
Acknowledgements
This work was supported by The Keio University Doctorate Student Grant-in-Aid Program from Ushioda Memorial Fund and Meiji Seika Pharma Co., Ltd., Japan. We appreciate the support from Meiji Seika Pharma Co., Ltd., Japan in supplying nacubactam hydrate and clinical isolates.
Contributor Information
Yuki Igarashi, Division of Pharmacodynamics, Keio University Faculty of Pharmacy, 1-5-30 Shibakoen, Minato-ku, Tokyo 105-8512, Japan.
Wataru Takemura, Division of Pharmacodynamics, Keio University Faculty of Pharmacy, 1-5-30 Shibakoen, Minato-ku, Tokyo 105-8512, Japan.
Xiaoxi Liu, Division of Pharmacodynamics, Keio University Faculty of Pharmacy, 1-5-30 Shibakoen, Minato-ku, Tokyo 105-8512, Japan.
Nana Kojima, Division of Pharmacodynamics, Keio University Faculty of Pharmacy, 1-5-30 Shibakoen, Minato-ku, Tokyo 105-8512, Japan.
Takumi Morita, Division of Pharmacodynamics, Keio University Faculty of Pharmacy, 1-5-30 Shibakoen, Minato-ku, Tokyo 105-8512, Japan.
Victor Tuan Giam Chuang, Discipline of Pharmacy, Curtin Medical School, Faculty of Health Sciences, Curtin University, GPO Box U1987, Perth, Western Australia 6845, Australia.
Yuki Enoki, Division of Pharmacodynamics, Keio University Faculty of Pharmacy, 1-5-30 Shibakoen, Minato-ku, Tokyo 105-8512, Japan.
Kazuaki Taguchi, Division of Pharmacodynamics, Keio University Faculty of Pharmacy, 1-5-30 Shibakoen, Minato-ku, Tokyo 105-8512, Japan.
Kazuaki Matsumoto, Division of Pharmacodynamics, Keio University Faculty of Pharmacy, 1-5-30 Shibakoen, Minato-ku, Tokyo 105-8512, Japan.
Funding
Japan Agency for Medical Research and Development (grant number JP18pc0101028) supported this work.
Transparency declarations
K.M. received grant support funding from Meiji Seika Pharma Co., Ltd., Sumitomo Pharma Co., Ltd., and Shionogi & Co., Ltd., and speaker honoraria from Meiji Seika Pharma Co., Ltd. The other authors have no conflicts of interest to declare.
Supplementary data
Figures S1 to S6 and Tables S1 to S3 are available as Supplementary data at JAC Online.
References
- 1. Antimicrobial Resistance Collaborators . Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 2022; 399: 629–55. 10.1016/S0140-6736(21)02724-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. O’Neill J. Tackling drug-resistant infections globally: final report and recommendations. The review on antimicrobial resistance. 2016.https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf.
- 3. O’Neill J. Antimicrobial resistance: tackling a crisis for the health and wealth of nations. The review on antimicrobial resistance. 2014.https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf.
- 4. Jacoby GA, Munoz-price LS. The new β-lactamases. N Engl J Med 2005; 352: 380–91. 10.1056/NEJMra041359 [DOI] [PubMed] [Google Scholar]
- 5. Craig WA. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis 1998; 26: 1–12. 10.1086/516284 [DOI] [PubMed] [Google Scholar]
- 6. Andes D, Craig WA. Animal model pharmacokinetics and pharmacodynamics: a critical review. Int J Antimicrob Agents 2002; 19: 261–8. 10.1016/S0924-8579(02)00022-5 [DOI] [PubMed] [Google Scholar]
- 7. Ambrose PG, Bhavnani SM, Rubino CMet al. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: it’s not just for mice anymore. Clin Infect Dis 2006; 44: 79–86. 10.1086/510079 [DOI] [PubMed] [Google Scholar]
- 8. Coleman K, Levasseur P, Girard AMet al. Activities of ceftazidime and avibactam against β-lactamase-producing Enterobacteriaceae in a hollow-fiber pharmacodynamic model. Antimicrob Agents Chemother 2014; 58: 3366–72. 10.1128/AAC.00080-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Crass RL, Pai MP. Pharmacokinetics and pharmacodynamics of β-lactamase inhibitors. Pharmacotherapy 2019; 39: 182–95. 10.1002/phar.2210 [DOI] [PubMed] [Google Scholar]
- 10. Bhagunde P, Chang KT, Hirsch EBet al. Novel modeling framework to guide design of optimal dosing strategies for β-lactamase inhibitors. Antimicrob Agents Chemother 2012; 56: 2237–40. 10.1128/AAC.06113-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wu J, Racine F, Wismer MKet al. Exploring the pharmacokinetic/pharmacodynamic relationship of relebactam (MK-7655) in combination with imipenem in a hollow-fiber infection model. Antimicrob Agents Chemother 2018; 62: e02323-17. 10.1128/AAC.02323-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Abodakpi H, Chang KT, Zhou Jet al. A novel framework to compare the effectiveness of β-lactamase inhibitors against extended-spectrum β-lactamase-producing Enterobacteriaceae. Clin Microbiol Infect 2019; 25: 1154.e9–14. 10.1016/j.cmi.2019.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Abodakpi H, Chang KT, Gao Set al. Optimal piperacillin-tazobactam dosing strategies against extended-spectrum-β-lactamase-producing Enterobacteriaceae. Antimicrob Agents Chemother 2019; 63: e01906-18. 10.1128/AAC.01906-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tam VH, Abodakpi H, Wang Wet al. Optimizing pharmacokinetics/pharmacodynamics of β-lactam/β-lactamase inhibitor combinations against high inocula of ESBL-producing bacteria. J Antimicrob Chemother 2021; 76: 179–83. 10.1093/jac/dkaa412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. CLSI . Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically—Eleventh Edition: M07. 2018. [Google Scholar]
- 16. CLSI . Performance Standards for Antimicrobial Susceptibility Testing—Thirty-First Edition: M100. 2021. [Google Scholar]
- 17. Melchers MJ, Van Mil AC, Lagarde Cet al. Pharmacodynamics of ceftolozane combined with tazobactam against Enterobacteriaceae in a neutropenic mouse thigh model. Antimicrob Agents Chemother 2017; 61: 7272–9. 10.1128/AAC.00267-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Berkhout J, Melchers MJ, Van Mil ACet al. Pharmacodynamics of ceftazidime and avibactam in neutropenic mice with thigh or lung infection. Antimicrob Agents Chemother 2016; 60: 368–75. 10.1128/AAC.01269-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rodriguez CA, Agudelo M, Zuluaga AFet al. In vivo pharmacodynamics of piperacillin/tazobactam: implications for antimicrobial efficacy and resistance suppression with innovator and generic products. Int J Antimicrob Agents 2017; 49: 189–97. 10.1016/j.ijantimicag.2016.10.011 [DOI] [PubMed] [Google Scholar]
- 20. Morinaka A, Tsutsumi Y, Yamada Met al. OP0595, a new diazabicyclooctane: mode of action as a serine β-lactamase inhibitor, antibiotic and β-lactam “enhancer.”. J Antimicrob Chemother 2015; 70: 2779–86. 10.1093/jac/dkv166 [DOI] [PubMed] [Google Scholar]
- 21. Morinaka A, Tsutsumi Y, Yamada Ket al. In vitro and in vivo activities of OP0595, a new diazabicyclooctane, against CTX-M-15-positive Escherichia coli and KPC-positive Klebsiella pneumoniae. Antimicrob Agents Chemother 2016; 60: 3001–6. 10.1128/AAC.02704-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Livermore DM, Mushtaq S, Warner Met al. Activity of OP0595/β-lactam combinations against Gram-negative bacteria with extended-spectrum, AmpC and carbapenem-hydrolysing β-lactamases. J Antimicrob Chemother 2015; 70: 3032–41. 10.1093/jac/dkv239 [DOI] [PubMed] [Google Scholar]
- 23. Mushtaq S, Vickers A, Woodford Net al. Activity of nacubactam (RG6080/OP0595) combinations against MBL-producing Enterobacteriaceae. J Antimicrob Chemother 2018; 74: 953–60. 10.1093/jac/dky522 [DOI] [PubMed] [Google Scholar]
- 24. Brook I. Inoculum effect. Rev Infect Dis 1989; 11: 361–8. 10.1093/clinids/11.3.361 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.