Abstract
Precision cardiology aims to implement personalized health care and precise medical decisions based on the specific characteristics of individuals. Metabolic remodeling plays a causal role in the pathogenesis of heart failure (HF). Changes in metabolic pathways such as substrate preference, high-energy phosphate metabolism and amino acid metabolism, are involved in pathological structural remodeling and functional impairment. These metabolic alterations are usually not restricted in the cardiac tissue, but also manifest in circulation. In clinical practice, blood sample is routinely used for HF screening. Metabolomics is an emerging omics technology that provides an efficient way to acquire dynamic metabolic profiles in circulation. An increasing number of metabolic biomarkers have been implicated in disease progression, making it possible to fight HF in a more effective and precise way. This review summarizes the modern analytical techniques in metabolomics as well as emerging circulating metabolites during the pathogenesis of HF, aiming to provide new insights into the prevention, diagnosis and treatment of HF in the era of precision medicine.
Keywords: heart failure, precision medicine, metabolomics, lipids, glucose, ketone bodies
This review summarizes the modern analytical techniques in metabolomics as well as emerging circulating metabolites during the pathogenesis of heart failure, to provide new insights into the prevention, diagnosis and treatment of the disease in the era of precision medicine.
Introduction
Heart failure (HF) occurs when the heart cannot pump blood properly and does not send enough blood through the body. The most common risk factors for HF are high blood pressure, coronary artery disease, diabetes, obesity, smoking and genetics.1 The 2021 American Heart Association Statistical Update estimates that HF affects more than 60 million individuals globally.2 Studies of the epidemiology of HF over the past ten years have revealed that the incidence of HF is primarily stable or dropping but the burden of death and hospitalization remains largely unabated.3
There are different HF classifications. Acute heart failure (AHF) occurs suddenly and is defined as new-onset HF or worsening symptoms and signs of HF, whereas chronic heart failure (CHF) occurs over time.4,5 The stages (A-D) of HF proposed by the American Heart Association American College of Cardiology guidelines emphasize the evolution and progression of the disease, and the New York Heart Association (NYHA) class I-IV categorizes the relationship between symptoms of dyspnea and physical activity.6 In addition, HF with preserved ejection fraction (HFpEF) is defined as an ejection fraction (EF) ≥ 50%, while its counterpart with reduced ejection fraction (HFrEF) refers to that < 40%. The 2016 European Society of Cardiology heart failure guidelines encourage research in EF range and define heart failure with mid-range ejection fraction (HFmrEF) as an EF of 40%-49%.7 The heterogeneity of HF syndrome has become widely recognized and the disease characterizations toned to be well addressed for the sake of developing, implementing, and evaluating strategies for precise management.
The pathophysiology of HF has been demonstrated to be closely related to cardiometabolic risk in humans. The Human Metabolome Database (HMDB, hmdb.ca) has recorded 220 945 metabolite entries, including carbohydrates, lipids, amino acids and ketone bodies. Metabolic inefficiency is detrimental to systolic function, since the heart consumes a large amount of ATP to sustain cardiac contraction, and high ATP demand is fulfilled by utilizing various metabolic substrates.8 Thus, changes in metabolites and related enzymes are reflective of the pathological process of HF.9,10 Notably, changes in the circulating metabolic landscape, rather than a single specific metabolite, are more representative in depicting the characterization of HF.11
Metabolomics is a discovery-based science that depicts metabolic fingerprint to establish predictive models and search for novel targets for intervention.12 Circulating metabolomics is the study of the identification and quantification of metabolic profile in blood, providing clinic workers with the opportunity to trace circulating metabolic signatures in different stages of HF. Conventional routine blood examinations exhibit general effectiveness in the diagnosis of HF, but lack specificity and personality.13 One potential application of circulating metabolomics in clinical practice is to explain individual variability based on various environmental exposures, such as dietary habits, tobacco, chronic stress and microbiota.14 What also deserves expectation is its combination with other omics technologies, and its integration into bioinformatics.15,16 Moreover, metabolic alterations in different pathological pathways could advance our insight into the potential mechanism behind the phenotype of HF.17 In this review, we will summarize the approaches and technologies suitable for detecting circulating metabolites; the characterizations of circulating metabolites and their roles in the pathogenesis of HF; and finally, the implications of metabolic changes in precision medicine.
Approaches and techniques for metabolomics analysis
Untargeted metabolomics and targeted metabolomics
The approaches of metabolomics include untargeted metabolomics and targeted metabolomics. Untargeted metabolomics is an unbiased analysis that aims to identify as many metabolites as possible. This approach is applied to analyze both known and unknown metabolites, discover novel biomarkers, propose a new hypothesis and establish comprehensive and systematic knowledge for pathological processes. Untargeted metabolomics has a huge demand for data tools, as the volume and complexity of data can scale by multiple orders of magnitude, especially in the study of a large population.14 However, many major pertinent challenges remain. The biggest drawback is that the majority of peaks in the profile are not identifiable, leading to poor compound identification. Partial and incomplete metabolomes persist due to factors such as limitations in mass spectrometry data acquisition speeds, and a wide range of metabolite concentrations that confound the understanding of metabolite perturbations.18,19 Untargeted metabolomics is suitable for qualitative and semi-quantitative research independent of reference standards, which is beneficial to cost reduction.
In contrast, targeted metabolomics is used for quantifying a comparatively smaller group of metabolites that are biochemically significant and relevant, with higher sensitivity and selectivity.20 This approach exists for interrogating pathophysiological processes of interest and focuses on the already verified functions and pathways. Internal standards with stable isotope-labeled metabolites, or external standards with calibration curves are indispensable for absolute quantification, thus leading to the relatively high cost of targeted metabolomics.
Techniques
Various techniques have been applied in metabolomics. In this review, we specifically elaborate on the technologies that are suited for measuring circulating metabolites in humans. We intend to highlight the advantages and disadvantages of these technologies and introduce their application in precision clinical medicine (Table 1).
Table 1.
Comparison between the analytical techniques in metabolomics.
| Advantages | Disadvantages | |
|---|---|---|
| NMR | Non-destructive; | Relatively poor sensitivity; |
| High throughput; | Limited metabolites coverage | |
| Unbiased sample analysis | ||
| GC-MS | High sensitivity and specificity; | Requirement for derivatization |
| Reproducibility; | Biased sample analysis (gasified and stable metabolites); | |
| Public database/libraries | Longer sample preparation time | |
| LC-MS/MS | High sensitivity and specificity; | Sample needs to be ionized; |
| Detecting semipolar, polar and high weight metabolites; | Poor for unknown metabolites identification; | |
| Reproducibility; | Local database dependence | |
| Nonessentials for derivatization; | ||
| Wide metabolites coverage |
NMR, nuclear magnetic resonance spectroscopy; GC-MS, gas chromatography-mass spectrometry; LC-MS/MS, liquid chromatography-tandem mass spectrometry.
Nuclear magnetic resonance spectroscopy (NMR) is nondestructive and has advantages at times when the volume of clinical samples is limited. The ability to detect all metabolites within detectable concentrations in a single test dramatically reduces the experimental period. Furthermore, NMR spectroscopy is ideal for measuring certain metabolites for which the mass spectrum is unsuitable, such as protein-bound metabolites, metal ions, and H+ ions. Molecular information at the atomic level could be released through NMR detection.21 However, the major limitations of NMR lie in sensitivity and quantification.22 There are thousands of metabolites in body fluids, but only hundreds of them can be detected by NMR, while the low-abundance metabolites are filtered.21 Notably, metabolites of lower concentration sometimes play a more critical role in clinical diagnostics.
Gas chromatography-mass spectrometry (GC-MS) is applied to detect metabolites that are easily gasified, or have stable properties, with high chromatographic efficiency and reproducibility. For detecting polar, thermolabile, nonvolatile metabolites, chemical derivatization is required and prolongs the processing time for sample preparation.20 There are public databases/libraries for subsequent analysis, making it the method of choice for many analysts.20,23
Liquid chromatography-tandem mass spectrometry (LC-MS/MS) is extensively applied in precision medicine, such as biomarker discovery, disease diagnosis and treatment.12 This approach is suitable for analyzing semipolar, polar and high weight metabolites, with excellent reproducibility, specificity and sensitivity, and does not require chemical derivatization. Although LC-MS identifies a wide range of metabolites, it is limited to detecting compounds that readily ionize.19 The greatest issue that LC-MS-based metabolomics confronts is the identification of unknown metabolites.19 In addition, qualitative identification of metabolites largely depends on local databases since the data derived from different instruments are not always compatible.
Circulating metabolic changes in HF
Circulating metabolites provide the heart with energy substrates and building blocks. Dysregulation of circulating metabolites emerges as a valuable indicator/biomarker in the pathogenesis of cardiovascular diseases. Previous studies have identified plenty of metabolites that are changed along with the development of HF, such as lipids, glucose, ketone bodies, amino acids and lactate (Table 2).
Table 2.
Major findings from clinical studies.
| Disease | Metabolites | Major findings | Study design and size | Reference |
|---|---|---|---|---|
| AF | FFA | FFA levels were associated with the development of AF. | Prospective: 182 patients | Jung, Y., et al.30 |
| Diabetes | NEFA | Plasma NEFA was positively association with incident diabetes. | Prospective: 3740 Cardiovascular Health Study participants | Djousse, L., et al.31 |
| CHD | FFA | High FFA might identify patients with stable CHD with worse prognoses. | Prospective: 1206 participants in 3-weeks inpatient rehabilitation programs | Breitling, L.P., et al.32 |
| HF | FFA | Plasma FFA was associated with a higher risk of HF in older adults. | Prospective: 4248 men and women free of HF at baseline and > 65 years old | Djousse, L., et al.33 |
| HF | FFA | A long-term reduction in FFAs with acipimox did not change cardiac function in patients with HF. | Randomized, double-blind and crossover: 24 HF patients with ischemic heart disease | Halbirk, M., et al.35 |
| Heart failure caused by IDCM | FFA | Acutely decreased serum FFA depressed cardiac work. | Randomized and controlled: 18 fasting nondiabetic patients and 8 matched healthy controls | Tuunanen, H., et al.36 |
| Obese HFpEF | UFA | Consumption of UFA was linked to better cardiorespiratory fitness. | Randomized: 23 obese HFpEF patients | Carbone, S., et al.39 |
| Incident congestive heart failure | LCMUFA | LCMUFAs had possible cardiotoxicity in humans. | Retrospective: 3694 older adults and 3577 middle-aged adults | Imamura, F., et al.40 |
| Non-ST‐segmentelevation‐acute coronary syndrome | ω‐3 PUFA | Plasma long‐chain ω3‐PUFAs were inversely associated with lower odds of sudden cardiac death. | Randomized and controlled: 203 patients with cardiovascular death, 325 with myocardial infarction, 271 with ventricular tachycardia, and 161 with atrial fibrillation, and a random sample of 1612 event‐free subjects as controls | Zelniker, T.A., et al.42 |
| CVD | ω‐3 PUFA | Higher levels of blood ω-3 PUFAs were associated with a lower risk of cardiovascular disease death. | Prospective: 42 466 individuals | Harris, W.S., et al.43 |
| CVD | ω‐3 PUFA | ω-3 PUFAs could be predictors of cardiovascular risk. | Randomized and controlled: 356 individuals who are 30 to 74 years, with at least one cardiovascular risk factor, and no previous cardiovascular events | Goncalinho, G.H.F., et al.44 |
| HF | EPA | Higher plasma EPA was associated with reduced risk for HF. | Prospective: a total of 6562 participants | Block, R.C., et al.46 |
| Cardiomyopathy | DHA, EPA | Decreased DHA and EPA levels were associated with reduced LVEF. | Randomized and controlled: 30 patients | Alter, P., et al.47 |
| ADHF | TyG | The elevated plasma TyG was independently associated with poor prognosis in patients with ADHF. | Retrospective: a total of 932 hospitalized patients with ADHF | Huang, R., et al.49 |
| ADHF | ω‐6 PUFA | Lower ω-6 PUFA levels might be useful for identifying higher risk ADHF patients. | Prospective: 685 consecutive ADHF patients | Nagai, T., et al.51 |
| ADHF | AA | The AA score predicted 1-year death in patients with ADHF. | Retrospective: discovery cohort n = 419; validation cohort n = 386 | Ma, K., et al.52 |
| HF | EPA, AA | The ratio of EPA to AA was an independent predictor of cardiac mortality in patients with HF. | Randomized and controlled: a total of 577 consecutive patients | Watanabe, S., et al.53 |
| Congestive heart failure | SCFA | Altered SCFA concentrations were additional intestinal dysfunctions in patients with congestive heart failure. | Dual stable tracer: 14 clinical patients | Kirschner, S.K., et al.57 |
| HF | LCAC | Increased circulating LCACs were associated with adverse effects of HF. | Randomized and controlled: 72 control subjects and 68 HF patients | Ruiz, M., et al.65 |
| HF | LCAC | The plasma LCACs were substantially higher as the HF developed. | Randomized and controlled: 515 participants | Cheng, M.L., et al.66 |
| HFpEF, HFrEF | LCAC | LCACs were independently associated with HF and differentially elevated in HFpEF and HFrEF. | Randomized and controlled: HFpEF cases (n = 282), HFrEF controls (n = 279), no-HF controls (n = 191) | |
| Hunter, W.G., et al.67 | ||||
| HFpEF, HFrEF | AC, FC | The ratio of AC/FC could identify high risk HF patients, especially those with HFpEF. | Prospective: 168 HF patients | Yoshihisa, A., et al.68 |
| CHF | LCAC | Increased LCACs were independently associated with adverse clinical outcomes and decreased after circulatory support. | Randomized and controlled: 453 chronic systolic HF patients | Ahmad, T., et al.69 |
| HF | Amino acids, LCAC | Circulating amino acids and LCACs were associated with progression of HF. | Randomized and controlled: a total of 96 HF cases and 97 controls | Liu, C., et al.70 |
| HF, DM | LCAC | LCACs were differentially associated with clinical outcomes in HF patients with or without DM. | Randomized and controlled: 664 participants | Truby, L.K., et al.71 |
| T2DM | LCAC | Elevated LCACs were associated with CVD risk in T2DM. | Cross-sectional: 741 patients with T2DM | Zhao, S., et al.73 |
| HF | Triglycerides | Stepwise higher concentrations of nonfasting triglycerides were associated with stepwise higher risk of heart failure. | Prospective: 113 554 individuals | Varbo, A. and B.G.77 |
| CAD, ACS | Ceramides | Ceramides were significant predictors of CV death both in patients with stable CAD and ACS. | Prospective: 160 stable CAD patients | Laaksonen, R., et al.79 |
| Major adverse cardiovascular events | Ceramides | Ceramides were associated with the risk of major adverse cardiovascular events. | Prospective: 8101 apparently healthy individuals. | Havulinna, A.S., et al.81 |
| HF | Ceramide, Sphingomyelins | Plasma levels of ceramide and sphingomyelins were associated with risk of heart failure. | Prospective: 1179 cases of incident heart failure | Lemaitre, R.N., et al.85 |
| HF | VLSFA | Higher levels of circulating VLSFAs were associated with lower risk of incident HF in older adults. | Prospective: 1304 incident heart failure events | Lemaitre, R.N., et al.88 |
| HF | SFA | Plasma phospholipid SFAs were not associated with HF. | Prospective nested matched case–control: 788 cases of incident HF and 788 controls | Matsumoto, C., et al.90 |
| HF | Glucose | Admission glucose levels had no significant association with mortality in patients hospitalized with other cardiovascular conditions. | Retrospective: 50 532 elderly patients | Kosiborod, M., et al.93 |
| HF | FPG | FPG was positively, continuously, and independently associated with risk for HF. | Prospective: 1740 men aged 42–61 years | Khan, H., et al.95 |
| HF, diabetes | FPG | Impaired FPG and diabetes were predictors of death in patients with HF. | Prospective: 1791 military veterans | Gotsman, I., et al.101 |
| Congestive heart failure | Ketone bodies | Blood ketone bodies were increased in accordance with the severity of cardiac dysfunction in CHF. | Randomized and controlled: 45 patients with chronic CHF and 14 control subjects free of CHF | Lommi, J., et al.114 |
| HFrEF | 3-OHB | High plasma concentrations of 3-OHB were associated with an increased risk of HFrEF, particularly in women. | Prospective: 227 subjects (137 with HFrEF, 90 with HFpEF) | Flores-Guerrero, J.L., et al.115 |
| CHF | 3-OHB | Increased 3-OHB after treatment with empagliflozin had negative effects on vascular function in patients with stable CHF ketone bodies. | Prospective, double blind, placebo controlled and parallel-group single centre: 75 patients | Pietschner, R., et al.119 |
| HF | 277 metabolites | Comprehensive quantification of fuel use by the failing and nonfailing human heart | Randomized and controlled: 110 patients | Murashige, D., et al.124 |
| T2DM | BCAAs | Circulating levels of BCAAs were positively associated with incident HF in patients with T2DM. | Prospective: 2139 T2D patients free of cardiovascular-renal diseases | Lim, L.L., et al.126 |
| STEMI with AHF | BCAAs | Increased plasma BCAA levels were associated with long-term adverse cardiovascular events in patients with STEMI and AHF. | Prospective: 138 patients with STEMI and AHF | Du, X., et al.127 |
| HF | Leucine, valine and acylcarnitines | A profile that consisted of leucine, valine and acylcarnitines was a predictor of mortality. | Randomized and controlled: 1032 HFrEF patients | Lanfear, D.E., et al.128 |
| Asymptomatic left ventricular diastolic dysfunction | BCAAs | Higher BCAAs could maintain diastolic left ventricular function in individuals without structural heart disease. | Prospective: 570 randomly recruited people | Zhang, Z.-Y., et al.132 |
| HF | Two metabolic panels | The profile of metabolites provided better prognostic value compared to conventional biomarkers. | Randomized and controlled: 515 participants | Cheng, M.L., et al.66 |
| HF | Phenylalanine | Phenylalanine was a novel predictor for incident heart failure hospitalization in the elderly | Double-blind, randomised, placebo-controlled trial: the Elderly (n = 12 671) | Delles, C., et al.133 |
| HFpEF, HFrEF | Amino acids, phospholipids and acylcarnitines | Higher levels of hydroxyproline and symmetric dimethyl arginine, alanine, cystine, and kynurenine, and lower levels of serine and arginine were found in the plasma of HFpEF patients compared to HFrEF patients. | Randomized and controlled: HFpEF (n = 46) and HFrEF (n = 75) patients | Hage, C., et al.134 |
| HF and reduced muscle endurance | Kynurenine | kynurenine might be a potential biomarker for patients with HF and reduced muscle endurance. | Prospective: 18 HFrEF, 17 HFpEF, and 20 healthy controls | Bekfani, T., et al.135 |
| AHF | Lactate | Infusion of half-molar sodium lactate improved cardiac performance in AHF patients without any detrimental effects on organ function. | Prospective, randomized, controlled and open-label: 40 patients | Nalos, M., et al.139 |
| AHF | Lactate | An elevated blood lactate on admission was common in AHF patients. | Prospective: 237 patients with AHF | Zymlinski, R., et al.142 |
AF, Atrial fibrillation; FFA, Free fatty acid; NEFA, Nonesterified fatty acids; CHD, Coronary heart disease; HF, Heart failure; IDCM, Idiopathic dilated cardiomyopathy; HFpEF, Heart failure with preserved ejection fraction; UFA, Unsaturated fatty acid; LCMUFA, Long-chain monounsaturated fatty acid; ω-3 PUFA, Omega-3 polyunsaturated fatty acid; EPA, Eicosapentaenoic acid; DHA, Docosahexaenoic acid; ADHF, Acute decompensated heart failure; TyG, The triglyceride glucose; ω-6 PUFA, Omega-6 polyunsaturated fatty acid; AA, Arachidonic acid; SCFA, Short-chain fatty acid; LCAC, Long-chain acylcarnitine; AC, Acylcarnitine; FC, Free carnitine; HFrEF, Heart failure with preserved ejection fraction; DCM, Dilated cardiomyopathy; VHD, valvular heart disease; DM, Diabetes mellitus; T2DM, Type 2 diabetes mellitus; CAD, Coronary artery disease; ACS, Acute coronary syndromes; VLSFA, Very-long-chain saturated fatty acid; SFA, Saturated fatty acids; FPG, Fasting plasma glucose; 3-OHB, 3-hydroxybutyrate; CHF, Chronic heart failure; BCAAs, Branched-chain amino acids; STEMI, ST-segment elevation myocardial infarction; AHF, Acute heart failure.
Circulating lipids in HF
Lipid metabolism
The LIPID MAPS consortium has divided lipids into eight principal categories: fatty acids (FFAs), glycerolipids, glycerophospholipids, sphingolipids, sterol lipids, prenol lipids, saccharolipids and polyketides.24 The heart primarily relies on the oxidation of fatty acids to meet the high ATP demand of contractible function.8 In HF, the changes in the metabolic pattern of fatty acids are not conclusive and can either be diminished,25,26 enhanced27 or unchanged28 in cardiac tissue on condition of different disease severities. However, pathological metabolic remodeling extends beyond simple changes in substrate preference for ATP production and comprises altered contents of acyl-esters and derivatives in cardiac tissue and circulation.29 Herein, we summarize the changes in different circulating lipids in the pathogenesis of HF, hoping to provide referential significance in clinical management.
FFAs
Many HF risk factors, including atrial fibrillation,30 diabetes31 and coronary heart disease,32 are related to the levels of plasma FFAs. According to a prospective study, every 0.2 mEq/L rise in total FFAs was associated with a 12% higher risk of HF.33 Elevated circulating FFAs may lead to increased FFAs uptake within cardiomyocytes, and result in left ventricular dysfunction.33,34 Interestingly, acute reduction of serum FFAs seems to exert no benefits on cardiac function in patients with HF. In a randomized double-blind crossover study of 24 individuals with HF, a long-term reduction in circulating FFAs with acipimox did not change cardiac function.35 In patients with heart failure caused by idiopathic dilated cardiomyopathy, acute FFA withdrawal rapidly decreased cardiac work and efficiency.36
The type of fatty acids should be taken into consideration when discussing the associations between HF and circulating fatty acids. The major categories are saturated fatty acids (SFAs) and unsaturated fatty acids (UFAs), and the latter can be further subdivided into monounsaturated and polyunsaturated acids (MUFAs and PUFAs).37 Among 4249 older adults free of HF at baseline, palmitic acid (PA; C16:0) was significantly correlated with incident HF, while myristic acid (MA; C14:0) and stearic acid (SA; C18:0) were not associated with it.38 Regarding UFAs, dietary UFAs consumption was proved to be associated with better cardiorespiratory fitness in HFpEF patients.39 However, the specific role of MUFAs and PUFAs should not be generalized. In two independent cohorts, circulating levels of phospholipid long-chain MUFAs including erucic acid (C22:1) and nervonic acid (C24:1), were robustly and consistently associated with incident congestive HF, while gadoleic acid (C20:1) was not related to incident HF.40 A basic experimental study indicated that oleate (C18:1) might serve as a beneficial energy substrate in decompensated hearts.41 In the case of PUFAs, long-chain ω-3 PUFAs, including eicosapentaenoic acid (EPA, C20:5 ω-3), docosapentaenoic acid (DPA, C22:5 ω-3), and docosahexaenoic acid (DHA, C22:6 ω-3), are considered to be important in regulating cardiovascular health.42–44 A meta-analysis of 13 studies demonstrated that a higher dietary intake of LC ω-3 PUFAs was associated with a lower risk of HF.45 In a multi-ethnic study of 6562 participants with atherosclerosis, higher plasma EPA was significantly associated with a reduced risk for HF, with both HFpEF and HFrEF.46 In individuals with suspected cardiomyopathy, DHA and EPA levels were associated with reduced left ventricular ejection fraction (LVEF).47 The level of plasma PUFAs also indicates different clinical outcomes. A large RCT among patients with CHF reported that long-term ω-3 PUFAs supplementation decreased plasma triglycerides, which was reported to be independently associated with poor prognosis in HF, and reduced all-cause mortality and cardiovascular-related hospitalizations or death.48,49 However, the beneficial effect of ω-3 PUFAs cannot extend to patients with cardiovascular risk factors, such as diabetes, obesity, hypertension and hypercholesterolemia, since ω-3 PUFAs supplementation exerts no influence on mortality or morbidity, as shown in a randomized controlled trial.50 In addition, lower ω-6 PUFAs levels on admission were related to worse clinical outcomes in acute decompensated HF patients.51 A machine learning-based arachidonic acid (AA, ω-6 PUFA) metabolite score could predict 1-year death in patients with acute decompensated HF.52 The ratio of EPA to AA was regarded as an independent predictor of cardiac mortality in patients with HF.53
A further aspect to consider for circulating fatty acids is chain length. Carboxylic acids within 5 carbons are defined as short-chain fatty acids (SCFAs) and include acetate (2 carbons), propionate (3 carbons), and butyrate (4 carbons), which are primarily generated by gut microbial fermentation.54 The gut microbiota profile of CHF patients is characterized by the decreased abundance of SCFA-producing bacteria.55,56 In a study of 14 CHF outpatients, propionate, butyrate, and isovalerate concentrations were lower in CHF patients, while acetate and valerate concentrations did not differ.57 A recent study has proven that butyrate, as an alternative for impaired LCFA oxidation, was the preferred energy source over ketone bodies in HF. The enhanced butyrate oxidation in HF was associated with an increase in synthetase medium chain family member (ACSM) 3, a mitochondrial enzyme activating butyrate to butyryl CoA.58 Interventions through diet, probiotics, antibiotics and fecal transplantation have demonstrated protective effects on cardiovascular pathology and function, which were correlated with SCFA-producing bacteria.59–62 Notably, a stable isotope study quantifying the fraction of colonic administered SCFAs that could enter the circulation reported that the systemic availability of acetate, propionate and butyrate was 36%, 9% and 2%, respectively.63 This finding suggests that the efficacy of interventions on colonic-derived SCFAs is worthy of consideration in clinical practice.
LCACs
Long-chain acylcarnitines (LCACs) are long-chain fatty acids esterified to carnitine through which fatty acids are transferred into mitochondria for ß-oxidation. LCACs accumulate in states of inefficient fatty acid oxidation (FAO).64 In two cohorts, increased circulating LCACs were associated with adverse effects of HF.65 Furthermore, mass spectrometry-based profiling of plasma metabolites was performed in 515 participants and demonstrated that plasma LCACs were significantly higher in the worsening stages of HF.66 Targeted metabolomic profiling in a large cohort of HF patients with controls revealed that LCAC metabolites were highest in HFrEF, intermediate in HFpEF, and lowest in controls.67 In 168 patients with HF, the ratio of plasma acylcarnitine to free carnitine was recognized as a predictor of cardiac events with regard to HFpEF.68 In a study randomizing 453 chronic systolic HF patients to exercise training versus usual care, circulating LCACs were independently associated with increased risks of mortality and hospitality, and decreased after receiving mechanical circulatory support with a left ventricular assist device.69 Additionally, LCACs might characterize HF patients with diverse accompanying diseases, such as coronary heart disease, dilated cardiomyopathy, valvular heart disease, diabetes and pulmonary hypertension.70–73 In addition, medicines exert different influences on plasma LCACs. Long-term meldonium treatment could lower L-carnitine and LCAC contents in plasma, inducing cardioprotective effects.74 Among the 234 participants of Dapagliflozin Effects on Biomarkers, Symptoms and Functional Status in Patients with HF with Reduced Ejection Fraction (DEFINE-HF), dapagliflozin increased short-chain acylcarnitine and medium-chain acylcarnitine, but elicited no effects on LCACs.75 In the Diastolic Heart Failure with Preserved Ejection Fraction (RELAX) clinical trial, sildenafil increased LCACs and short-chain dicarboxylacylcarnitines with a potential negative mechanism of mitochondrial dysfunction and endothelin-1 signaling.76
Other lipids
FAs can be incorporated into carrier molecules and are acylated to triglycerides, sphingolipids, glycerolipids and glycerophospholipids. Two cohort studies of 113 554 individuals demonstrated that stepwise higher non-fasting triglycerides were associated with stepwise higher heart failure risk.77 Ceramide is a biologically active sphingolipid and serum ceramides are proven to be biomarkers for cardiovascular disease.78–81 Lipidomic analysis revealed increased long-chain and very long-chain ceramides (with 23 or more carbon atoms) in the serum of patients with advanced HF.82 Whether ceramides are deleterious or benign remains elusive. Trials have shown the associations between higher plasma levels of ceramide (C16:0, C18:0) and sphingomyelins (C16:0) with an increased risk of HF.83–85 However, there were mixed views on ceramides with longer carbon chains. A prospective cohort of older adults demonstrated that ceramide (C22:0) and sphingomyelins (C20:0, C22:0, C24:0) were thought to be associated with a decreased risk of heart failure.85 However, another cohort substantiated that there was no association between ceramide (C24:0) and adverse outcomes (admission or death) of HF.84 A cohort study of women with HFpEF and obesity suggested that plasma ceramide concentrations were associated with gastric bypass surgery-induced weight reduction, but the change in plasma lipidomic might not be critical to the improvement of cardiac function.86 Therefore, ceramides may play diverse roles in patients of different ages, sexes, disease stages and therapies.
A study concerning plasma phospholipids reported that each standard deviation increase in plasma cis palmitoleic acid was associated with 17% higher odds of heart failure.87 A cohort study enrolled 1304 incident heart failure events and found that higher levels of circulating phospholipids (C20:0, C22:0 and C24:0) were associated with a lower risk of HFpHF and HFrEF.88 In contrast, earlier studies did not show significant associations between phospholipids (C14:0, C15:0, C17:0, C18:0, C20:0, or C22:0) and HF.89,90 A study based on 216 targeted lipids identified that phosphatidylcholine (C32:0) was significantly associated with HF risk, thus serving as a potential biomarker of HF risk.83 The lipidomes of erythrocytes indicated that the levels of lysophospholipids, ceramides, and oxysterols, such as oxidized cholesterol 7-ketocholesterol (7KCh), were higher in the HF erythrocytes than in the control erythrocytes, while the levels of phospholipids were decreased in the HF erythrocytes. Among these lipids, 7KCh was best at discriminating between HF and controls, and might serve as an early biomarker for HF.91
Circulating glucose in HF
Glucose metabolism
Because glucose can generate ATP through both aerobic and anaerobic glycolysis, it is the most efficient carbon source. Glucose contributes to 20%–30% of total carbon combustion in heart.27 There have been limited studies on the associations between blood glucose and HF. An observational cohort demonstrated that elevated blood glucose could estimate the prognosis of 30-day mortality in AHF patients.92 However, an earlier study reported that glucose levels at admission had no significant correlation with mortality in a cohort of 50 532 patients hospitalized with HF.93
Prediabetes/diabetes and HF
Prediabetes and diabetes are common abnormal blood glucose in clinical practice. Impaired fasting plasma glucose (FPG) and diabetes are risk factors for HF.94,95 HF remains the major cause of death in patients with diabetes.96 The triglyceride-glucose index, calculated as ln[fasting triglyceride (mg/dL) × fasting glucose (mg/dL)/2], was regarded as a surrogate marker of insulin resistance and was proven to be positively associated with the risk of HF in several researches.97–99 In a post-hoc test of patients with HFrEF, 39% of those without a history of type 2 diabetes mellitus (T2DM) had prediabetes, and 21% had undiagnosed T2DM based on hemoglobin A1c (HbA1c) levels.100 Moreover, impaired FPG and diabetes are predictive of adverse outcomes in patients with HF. In patients with HF as coded at a health maintenance organization in Jerusalem, diabetes and IFG were similar predictors of reduced survival and increased cardiac hospitalizations.101 Similarly, in a multinational cohort of 6926 hospitalized patients, the presence of diabetes was independently associated with an increased risk of in-hospital mortality, 1-year all-cause mortality, and 1-year re-hospitalizations for HF.102 However, it is noteworthy that diabetes is a complex syndrome, and confounding factors such as vasculopathy should be excluded when discussing the causality between glucose abnormalities and HF. Although glucose abnormalities confer risks in patients with HF, there is insufficient evidence to treat HF with antihyperglycemic medications.103 Studies have demonstrated that intensive glucose therapy does not improve cardiovascular mortality or even potentially increases its risk.92,104–106
Circulating ketone bodies in HF
Ketone bodies
Ketone metabolism is upregulated in the failing heart, serving as an anaplerotic fuel source that has a higher phosphate to oxygen (P/O) ratio (2.5) than fatty acid oxidation (2.3). Ketone bodies, including acetoacetate, 3-hydroxybutyrate (3-OHB) and acetone, are primarily formed from FA-derived acyl-CoA and transported to extrahepatic tissues. Acetoacetate and 3-OHB could be transferred to cardiac tissue through systemic circulation, while the breakdown product acetone is eliminated through urine or expiration (Fig. 1).107
Figure 1.
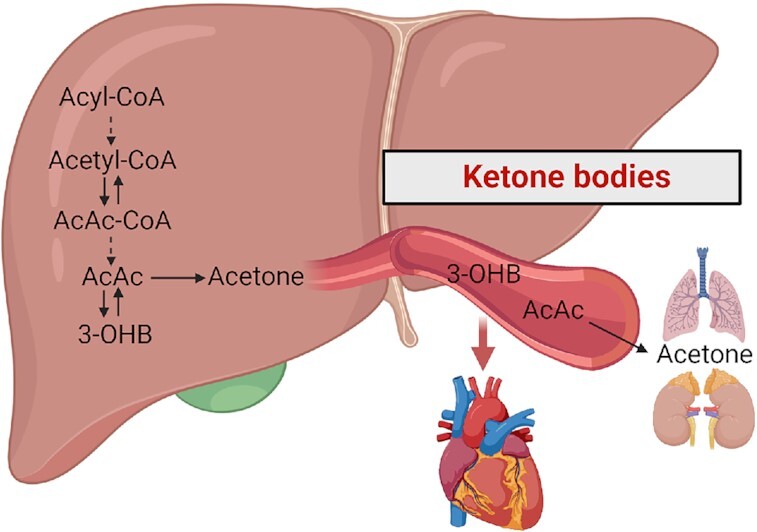
Metabolism of ketone bodies. Ketone bodies are predominantly produced from Acyl-CoA in liver and they are transported to extrahepatic tissue for utilization or elimination. AcAc-CoA, acetoacetyl Coenzyme A; AcAc, acetoacetate; 3-OHB, 3-hydroxybutyrate.
Exhaled breath acetone
Researchers have found that measuring exhaled breath acetone (EBA) might contribute to HF diagnosis, and prognosis.108 Additionally, elevated EBA in the setting of HF is correlated with disease severity.109–111 In 102 non-ischemic HF patients with NYHA class I-III, the EBA level was proportional to the NYHA class, showing a comparable diagnostic efficiency to B-type natriuretic peptide (BNP), a conventional biomarker for predicting HF.112 Another study suggested that EBA might be linked to poor prognosis of patients with HFrEF.113
3-OHB
In the heart, the most extensively utilized ketone body is 3-OHB. In an early study of 45 patients with CHF, blood ketone bodies were mildly increased in patients with HF and were negatively correlated with cardiac function.114 In a Dutch population cohort, circulating 3-OHB alteration in HF was sex dimorphic. High levels of 3-OHB were associated with an increased risk of HFrEF in women, while the association was absent in men.115 Notably, the observation of increased ketone bodies in HF patients does not indicate their vicious role in disease progression. In fact, the increased ketone bodies might be compensatory and beneficial to cardiac energetics. An investigation collecting blood samples from arterial circulation and coronary sinus demonstrated that cardiac tissue uptake more ketone bodies in HFrEF patients than in controls.28 In a basic study, increasing circulating 3-OHB abundance ameliorated HFpEF phenotypes.116 Furthermore, in a study of 19 HFrEF patients and 9 controls, oral supplementation with ketone ester enhanced 3-OHB uptake in patients with HFrEF, which had a negative correlation with LVEF and a positive correlation with LV mass and LV diameter.117 A randomized crossover study also showed that the infusion of 3-OHB in HFrEF patients exerted positive inotropic and chronotropic effects, improving LVEF, cardiac output, and decreasing systemic vascular resistance.118 These studies support increased oxidation of ketone bodies in HF and the potential therapeutic role of exogenous ketone bodies.
Impact of HF strategies on circulating ketone bodies
In addition, the improvements in cardiac function in HF patients after drug administration or exercise are accompanied by increased circulating ketone bodies. In the DEFINE-HF trial, dapagliflozin induced a modest increase in systemic ketones in HFrEF patients, although a few participants experienced ketosis (3-OHB > 500 μM).75 A clinical trial proposed negative effects of increased serum 3-OHB induced by empagliflozin on vascular function in patients with CHF.119 Exercise training-based cardiac rehabilitation (ET-CR) is an effective HF therapy.120 In an observational study, ET-CR induced an increase in circulating 3-OHB levels in HFpEF patients.121 However, it is unclear whether the elevation of ketone bodies in HF therapeutics is a mechanism of benefit. Overall, studying the alterations of circulating ketone bodies in different sexes, disease stages and therapeutics will lead to stratified and personalized management for HF patients.
Circulating amino acids in HF
Branched-chain amino acids and derivates
Mounting evidence has shown the critical role of altered metabolism of amino acids in cardiovascular disease.122,123 Branched-chain amino acids (BCAAs) have garnered significant attention in heart metabolism. Although BCAAs, including leucine, isoleucine and valine, contribute to < 5% of total carbon combustion, the failing heart shows remarkable changes in BCAA metabolism.27,124 Failing hearts are defective in BCAA catabolism since the expression of BCAA catabolic genes is downregulated.125 These BCAAs, which cannot be combusted in cardiac tissue, are released into the bloodstream and are predictive of adverse outcomes. In a prospective observational study of patients with T2DM, circulating BCAAs were positively correlated with incident HF.126 Likewise, in 138 patients with ST-elevation myocardial infarction and AHF, the elevations of BCAAs in serum exhibited better prognostic value and Kaplan–Meier curves than N-terminal pro-B-type natriuretic peptide (NT-proBNP), and predicted adverse cardiovascular events. More importantly, the combination of BCAAs and NT-proBNP yielded a stronger predictive value.127 In a study with 1032 HFrEF patients, a plasma metabolite profile that consisted of leucine, valine and acylcarnitines was a predictor of mortality.128 Some risk factors for HF, such as higher blood pressure and arrythmia, are also associated with elevated plasma BCAAs.125,129 Notably, the increase in circulating BCAAs is not a shared feature in HF but depends on specific pathological status. For example, an increased level of circulating α-keto-β-methylvalerate (the catabolite of isoleucine) but not α-ketoisocaproate (the catabolite of leucine), α-ketoisovalerate (the catabolite of valine), or BCAAs was observed in a human cohort of DCM with HFrEF.130 In a cross-sectional study, patients with HFrEF had higher plasma BCAAs than those who had HFpEF or no HF, while there were no distinctions in BCAA levels between HFrEF and no-HF controls. In some cases, the elevations of BCAAs in plasma might be beneficial. A study of HFrEF patients after cardiac resynchronization therapy showed that the improvement of plasma BCAAs indicated better LVF.131 Moreover, higher plasma BCAAs were associated with more performant diastolic LV function in individuals without structural heart disease.132
Other amino acids and derivates
Several other amino acids and their derivates in plasma also have diagnostic and prognostic value in HF. Researchers have found that a metabolic panel including histidine, phenylalanine and spermidine contributed to the diagnostic value similar to BNP.66 In this same cohort, the panel comprised of the asymmetric methylarginine/arginine ratio (a sign of endothelial dysfunction), butyrylcarnitine (a sign of abnormal lipid and energy metabolism), spermidine (a sign of compensation or toxicity to cardiomyocytes), and total essential amino acids (a sign of malnutrition), had a prognostic value better than BNP.66 A prospective study showed that phenylalanine was a predictor of incident heart failure hospitalization in the elderly.133 A metabolomic profile comparing HFrEF and HFpEF displayed higher levels of hydroxyproline and symmetric dimethyl arginine, alanine, cystine, and kynurenine, and lower levels of serine and arginine in the plasma of HFpEF patients.134 Kynurenine, the degradation product of tryptophan, was proven to be a potential marker for reduced muscle endurance in HF patients in a prospective observational study.135 Circulating 5-oxoproline, a member of glutamyl cycle, has diagnostic and prognostic potential in HF. Plasma 5-oxoproline was increased in AHF patients compared to healthy controls, which was associated with higher incidence of atrial fibrillation, higher NT-proBNP and cardiac remodeling.136
Circulating lactate in HF
Lactate metabolism
Lactate serves as an important energy substrate that can be converted into pyruvate via the enzyme lactate dehydrogenase (LDH) and thereby feed the tricarboxylic acid (TCA) cycle to produce ATP. Indeed, glucose feeds the TCA cycle by circulating lactate.137 Since lactate clearance (320 mmol/h) in the liver far exceeds the normal rate of lactate production, increasing peripheral lactate alone rarely leads to lactic acidosis in patients with normal hepatic metabolism.138 The result is consistent with a prospective, randomized, controlled, open-label, pilot clinical trial in which the infusion of half-molar sodium lactate improves cardiac performance in AHF patients without any detrimental effects on organ function.139
In conditions of tissue hypoxia or enhanced aerobic glycolysis, as seen in sepsis, cancer and liver disease, there is an excess of lactate production compared to its elimination, resulting in hyperlactatemia. The major causes of lactic acidosis are divided into 2 categories, type A and type B. Type A lactic acidosis is due to hypoperfusion or ischemia while Type B is under adequate tissue perfusion.140 Hyperlactatemia in HF could be of both hypoxic and nonhypoxic origin.141 In a study of AHF patients, elevated blood lactate on admission was associated with markers of organ dysfunction/damage and a worse prognosis, and the clinical evidence of hypoperfusion was not obvious.142 In CHF, lactate was lower in HFpEF patients than in patients with HFrEF.134 Notably, in a cohort of patients with advanced heart failure, lactate levels were normal in about 75% of patients,141 indicating that tissue hypoperfusion was not prevalent in HF.
Impact of HF strategies on circulating lactate
Some treatments for heart failure also cause changes in circulating lactate. A retrospective study of 215 HF patients treated with extracorporeal life support (ECLS) demonstrated that a low lactate level before receiving ECLS was a surrogate marker for successful weaning and discharge from the hospital.143 Metformin, the first-line medication for T2DM patients, increases the risk of lactic acidosis, especially in patients with renal or hepatic insufficiency. One mechanism relates to the inhibition of mitochondrial oxidation in tissues responsible for lactate removal.144
Perspectives
Although considerable progress has been made in understanding the metabolic alterations and pathophysiology of HF, personalized management in clinical practice is unsatisfactory. Cardiac biopsy is rarely performed in the diagnosis and treatment of HF due to ethical issues, while blood testing is a routine procedure. If blood metabolomics can be used to understand the mechanism and progression of HF, or even to characterize the individual differences in the occurrence of the disease, it will be of great benefit to precision cardiology. Hundreds of metabolites are detected and constitute a complex metabolic network. Thus, the application of data tools is of particular importance in biomarker discovery and hypothesis generation related to HF. Pathway and enrichment analysis using public databases (KEGG, BiGG Models, HumanCyc, etc.) and visualization tools (iPATH, Paintomics, Metscape, etc.) narrow the gap between raw data and the pathogenic mechanism of HF. In addition, the integration of metabolomics with genomics, transcriptomics or proteomics may provide a more personalized and comprehensive perspective to understand the progression of HF. In particular, circulating metabolomics has already been successfully used to determine biomarkers, though only a very limited number are employed in clinical practice. We need to understand that there is still a long way to go before metabolites are truly used as diagnostic and therapeutic criteria. The metabolomic profile is affected by confounding factors such as sex, age, body weight and dietary habits. How to find specific triggers to the disease throughout these confounding factors remains a challenge. One possible solution is the use of electronic medical records to combine metabolic information with clinical data. The use of cell phones and wearable electronic devices to collect data on personal information, such as the number of steps taken, heart rate and sleep quality, is also helpful. In addition, therapies including caloric restriction, medication, ET-CR, mechanical circulatory support and heart transplantation trigger changes in systematic metabolism.145–148 We hope that circulating metabolomics will strengthen therapeutic management and supervision, and promote rational and personalized treatment regimens. Moreover, since metabolites are not species-specific, it is reasonable to establish animal models to conduct research on disease mechanisms. Understanding the pathophysiological changes in patients will provide reasonable hypotheses for laboratory experiments and research directions with clinical significance. In turn, conclusions drawn from basic experiments can be verified by circulating metabolomics from patients. This positive feedback provides a better understanding of the pathophysiological basis of HF, facilitating the translation of research findings into clinical strategies. Overall, the detection of circulating metabolites is a promising approach to improve the quality of life and to extend the lifespan of HF patients via personalized therapies (Fig. 2).
Figure 2.
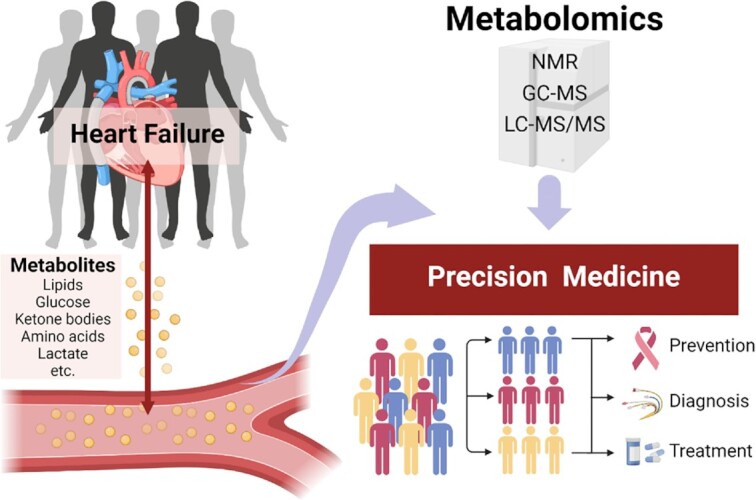
Circulating metabolic signatures of heart failure in precision cardiology.
Acknowledgement
This study was supported in part by grants from the National Natural Science Foundation of China (Grant No. 81970715), the Key Research and Development Program of Sichuan Province (Grant No. 2022YFS0132), and the Innovation Spark Project of Sichuan University (Grant No. 2018SCUH0065). All figures are created with BioRender.com.
Contributor Information
Huijing Xie, Department of Anesthesiology, National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University, Chengdu 610041, China; Laboratory of Mitochondria and Metabolism, National-Local Joint Engineering Research Centre of Translational Medicine of Anesthesiology, West China Hospital, Sichuan University, Chengdu 610041, China.
Bowen Zhang, Department of Anesthesiology, National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University, Chengdu 610041, China; Laboratory of Mitochondria and Metabolism, National-Local Joint Engineering Research Centre of Translational Medicine of Anesthesiology, West China Hospital, Sichuan University, Chengdu 610041, China.
Maodi Xie, Department of Anesthesiology, National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University, Chengdu 610041, China; Laboratory of Mitochondria and Metabolism, National-Local Joint Engineering Research Centre of Translational Medicine of Anesthesiology, West China Hospital, Sichuan University, Chengdu 610041, China.
Tao Li, Department of Anesthesiology, National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University, Chengdu 610041, China; Laboratory of Mitochondria and Metabolism, National-Local Joint Engineering Research Centre of Translational Medicine of Anesthesiology, West China Hospital, Sichuan University, Chengdu 610041, China.
Conflict of interest
None declared.
References
- 1. Baman JR, Ahmad FS. Heart failure. JAMA. 2020;324(10):1015. doi: 10.1001/jama.2020.13310. [DOI] [PubMed] [Google Scholar]
- 2. Becher PM, Lund LH, Coats AJSet al. An update on global epidemiology in heart failure. Eur Heart J. 2022;43(32):3005–7.. doi: 10.1093/eurheartj/ehac248. [DOI] [PubMed] [Google Scholar]
- 3. Roger VL. Epidemiology of heart failure: A contemporary perspective. Circ Res. 2021;128(10):1421–34.. doi: 10.1161/CIRCRESAHA.121.318172. [DOI] [PubMed] [Google Scholar]
- 4. Arrigo M, Jessup M, Mullens Wet al. Acute heart failure. Nat Rev Dis Primers. 2020;6(1):16. doi: 10.1038/s41572-020-0151-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ramani GV, Uber PA, Mehra MR. Chronic heart failure: contemporary diagnosis and management. Mayo Clin Proc. 2010;85(2):180–95.. doi: 10.4065/mcp.2009.0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hunt SA, Baker DW, Chin MHet al. ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult: executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to revise the 1995 Guidelines for the Evaluation and Management of Heart Failure). J Am Coll Cardiol. 2001;38(7):2101–13.. doi: 10.1016/s0735-1097(01)01683-7. [DOI] [PubMed] [Google Scholar]
- 7. Savarese G, Stolfo D, Sinagra Get al. Heart failure with mid-range or mildly reduced ejection fraction. Nat Rev Cardiol. 2022;19(2):100–16.. doi: 10.1038/s41569-021-00605-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lopaschuk GD, Karwi QG, Tian Ret al. Cardiac energy metabolism in heart failure. Circ Res. 2021;128(10):1487–513.. doi: 10.1161/CIRCRESAHA.121.318241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Müller OJ, Heckmann MB, Ding Let al. Comprehensive plasma and tissue profiling reveals systemic metabolic alterations in cardiac hypertrophy and failure. Cardiovasc Res. 2019;115(8):1296–305.. doi: 10.1093/cvr/cvy274. [DOI] [PubMed] [Google Scholar]
- 10. Liu X, Zhang YB, Deng Yet al. Mitochondrial protein hyperacetylation underpins heart failure with preserved ejection fraction in mice. J Mol Cell Cardiol. 2022;165:76–85.. doi: 10.1016/j.yjmcc.2021.12.015. [DOI] [PubMed] [Google Scholar]
- 11. McGarrah RW, Crown SB, Zhang GFet al. Cardiovascular metabolomics. Circ Res. 2018;122(9):1238–58.. doi: 10.1161/CIRCRESAHA.117.311002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jacob M, Lopata AL, Dasouki Met al. Metabolomics toward personalized medicine. Mass Spectrom Rev. 2019;38(3):221–38.. doi: 10.1002/mas.21548. [DOI] [PubMed] [Google Scholar]
- 13. Ibrahim NE, Januzzi JL Jr. Established and emerging roles of biomarkers in heart failure. Circ Res. 2018;123(5):614–29.. doi: 10.1161/CIRCRESAHA.118.312706. [DOI] [PubMed] [Google Scholar]
- 14. Cheng SS, Shah SH, Corwin EJet al. Potential impact and study considerations of metabolomics in cardiovascular health and disease: A Scientific Statement from the American Heart Association. Circ Cardiovasc Genet. 2017;10(2):e000032. doi: 10.1161/HCG.0000000000000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Karczewski KJ, Snyder MP. Integrative omics for health and disease. Nat Rev Genet. 2018;19(5):299–310.. doi: 10.1038/nrg.2018.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bayes-Genis A, Liu PP, Lanfear DEet al. Omics phenotyping in heart failure: the next frontier. Eur Heart J. 2020;41(36):3477–84.. doi: 10.1093/eurheartj/ehaa270. [DOI] [PubMed] [Google Scholar]
- 17. Kimball TH, Vondriska TM. Metabolism, epigenetics, and causal inference in heart failure. Trends Endocrinol Metab. 2020;31(3):181–91.. doi: 10.1016/j.tem.2019.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Azad RK, Shulaev V. Metabolomics technology and bioinformatics for precision medicine. Brief Bioinform. 2019;20(6):1957–71.. doi: 10.1093/bib/bbx170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cui L, Lu HT, Lee YH. Challenges and emergent solutions for LC-MS/MS based untargeted metabolomics in diseases. Mass Spectrom Rev. 2018;37(6):772–92.. doi: 10.1002/mas.21562. [DOI] [PubMed] [Google Scholar]
- 20. Beale DJ, Pinu FR, Kouremenos KAet al. Review of recent developments in GC-MS approaches to metabolomics-based research. Metabolomics. 2018;14(11):152. doi: 10.1007/s11306-018-1449-2. [DOI] [PubMed] [Google Scholar]
- 21. Emwas AH, Roy R, McKay RTet al. NMR spectroscopy for metabolomics research. Metabolites. 2019;9(7):E123. doi: 10.3390/metabo9070123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wishart DS, Cheng LL, Copié Vet al. NMR and metabolomics—A roadmap for the future. Metabolites. 2022;12(8):678. doi: 10.3390/metabo12080678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zeki ÖC, Eylem CC, Reçber Tet al. Integration of GC-MS and LC-MS for untargeted metabolomics profiling. J Pharm Biomed Anal. 2020;190:113509. doi: 10.1016/j.jpba.2020.113509. [DOI] [PubMed] [Google Scholar]
- 24. Wu ZJ, Bagarolo GI, Thoröe-Boveleth Set al. “Lipidomics”: Mass spectrometric and chemometric analyses of lipids. Adv Drug Deliv Rev. 2020;159:294–307.. doi: 10.1016/j.addr.2020.06.009. [DOI] [PubMed] [Google Scholar]
- 25. Kato T, Niizuma S, Inuzuka Yet al. Analysis of metabolic remodeling in compensated left ventricular hypertrophy and heart failure. Circ Heart Fail. 2010;3(3):420–30.. doi: 10.1161/CIRCHEARTFAILURE.109.888479. [DOI] [PubMed] [Google Scholar]
- 26. Neglia D, De Caterina A, Marraccini Pet al. Impaired myocardial metabolic reserve and substrate selection flexibility during stress in patients with idiopathic dilated cardiomyopathy. Am J Physiol Heart Circ Physiol. 2007;293(6):H3270–8.. doi: 10.1152/ajpheart.00887.2007. [DOI] [PubMed] [Google Scholar]
- 27. Gibb AA, Hill BG. Metabolic coordination of physiological and pathological cardiac remodeling. Circ Res. 2018;123(1):107–28.. doi: 10.1161/CIRCRESAHA.118.312017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Voros G, Ector J, Garweg Cet al. Increased cardiac uptake of ketone bodies and free fatty acids in human heart failure and hypertrophic left ventricular remodeling. Circ Heart Fail. 2018;11(12):e004953. doi: 10.1161/CIRCHEARTFAILURE.118.004953. [DOI] [PubMed] [Google Scholar]
- 29. Doenst T, Nguyen TD, Abel ED. Cardiac metabolism in heart failure: implications beyond ATP production. Circ Res. 2013;113(6):709–24.. doi: 10.1161/CIRCRESAHA.113.300376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jung Y, Cho Y, Kim Net al. Lipidomic profiling reveals free fatty acid alterations in plasma from patients with atrial fibrillation. PLoS One. 2018;13(5):e0196709. doi: 10.1371/journal.pone.0196709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Djoussé L, Khawaja O, Bartz TMet al. Plasma fatty acid-binding protein 4, nonesterified fatty acids, and incident diabetes in older adults. Diabetes Care. 2012;35(8):1701–7.. doi: 10.2337/dc11-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Breitling LP, Rothenbacher D, Grandi NCet al. Prognostic usefulness of free fatty acids in patients with stable coronary heart disease. Am J Cardiol. 2011;108(4):08–13.. doi: 10.1016/j.amjcard.2011.03.076. [DOI] [PubMed] [Google Scholar]
- 33. Djoussé L, Benkeser D, Arnold Aet al. Plasma free fatty acids and risk of heart failure: the cardiovascular health study. Circ Heart Fail. 2013;6(5):964–9.. doi: 10.1161/CIRCHEARTFAILURE.113.000521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. van de Weijer T, Schrauwen-Hinderling VB, Schrauwen P. Lipotoxicity in type 2 diabetic cardiomyopathy. Cardiovasc Res. 2011;92(1):10–8.. doi: 10.1093/cvr/cvr212. [DOI] [PubMed] [Google Scholar]
- 35. Halbirk M, Nørrelund H, Møller Net al. Suppression of circulating free fatty acids with acipimox in chronic heart failure patients changes whole body metabolism but does not affect cardiac function. Am J Physiol Heart Circ Physiol. 2010;299(4):H1220–5.. doi: 10.1152/ajpheart.00475.2010. [DOI] [PubMed] [Google Scholar]
- 36. Tuunanen H, Engblom E, Naum Aet al. Free fatty acid depletion acutely decreases cardiac work and efficiency in cardiomyopathic heart failure. Circulation. 2006;114(20):2130–7.. doi: 10.1161/CIRCULATIONAHA.106.645184. [DOI] [PubMed] [Google Scholar]
- 37. Sobczak AIS, Blindauer CA, Stewart AJ. Changes in plasma free fatty acids associated with type-2 diabetes. Nutrients. 2019;11(9):E2022. doi: 10.3390/nu11092022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lee YJ, Lai HTM, de Oliveira Otto MCet al. Serial biomarkers of de novo lipogenesis fatty acids and incident heart failure in older adults: the cardiovascular health study. J Am Heart Assoc. 2020;9(4):e014119. doi: 10.1161/JAHA.119.014119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Carbone S, Canada JM, Buckley LFet al. Dietary fat, sugar consumption, and cardiorespiratory fitness in patients with heart failure with preserved ejection fraction. JACC Basic Transl Sci. 2017;2(5):513–25.. doi: 10.1016/j.jacbts.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Imamura F, Lemaitre RN, King IBet al. Long-chain monounsaturated fatty acids and incidence of congestive heart failure in 2 prospective cohorts. Circulation. 2013;127(14):1512–21.. doi: 10.1161/CIRCULATIONAHA.112.001197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lahey R, Wang XR, Carley ANet al. Dietary fat supply to failing hearts determines dynamic lipid signaling for nuclear receptor activation and oxidation of stored triglyceride. Circulation. 2014;130(20):1790–9.. doi: 10.1161/CIRCULATIONAHA.114.011687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zelniker TA, Morrow DA, Scirica BMet al. Plasma omega-3 fatty acids and the risk of cardiovascular events in patients after an acute coronary syndrome in MERLIN-TIMI 36. J Am Heart Assoc. 2021;10(8):e017401. doi: 10.1161/JAHA.120.017401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Harris WS, Tintle NL, Imamura Fet al. Blood n-3 fatty acid levels and total and cause-specific mortality from 17 prospective studies. Nat Commun. 2021;12(1):2329. doi: 10.1038/s41467-021-22370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gonçalinho GHF, Sampaio GR, Soares-Freitas RAMet al. Omega-3 fatty acids in erythrocyte membranes as predictors of lower cardiovascular risk in adults without previous cardiovascular events. Nutrients. 2021;13(6):20406223221081616. doi: 10.3390/nu13061919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zheng SL, Qiu M, Wu JHYet al. Long-chain omega-3 polyunsaturated fatty acids and the risk of heart failure. Ther Adv Chronic Dis. 2022;13:20406223221081616. doi: 10.1177/20406223221081616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Block RC, Liu LX, Herrington DMet al. Predicting risk for incident heart failure with omega-3 fatty acids: from MESA. JACC Heart Fail. 2019;7(8):651–61.. doi: 10.1016/j.jchf.2019.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Alter P, Glück T, Figiel JHet al. From heart failure to highly unsaturated fatty acid deficiency and vice versa: bidirectional heart and liver interactions. Can J Cardiol. 2016;32(2):217–25.. doi: 10.1016/j.cjca.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 48. Tavazzi L, Maggioni AP, Marchioli Ret al. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372(9645):1223–30.. doi: 10.1016/S0140-6736(08)61239-8. [DOI] [PubMed] [Google Scholar]
- 49. Huang R, Wang ZY, Chen JZet al. Prognostic value of triglyceride glucose (TyG) index in patients with acute decompensated heart failure. Cardiovasc Diabetol. 2022;21(1):88. doi: 10.1186/s12933-022-01507-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Risk and Prevention Study Collaborative Group, Roncaglioni MC, Tombesi Met al. N-3 fatty acids in patients with multiple cardiovascular risk factors. N Engl J Med. 2013;368(19):1800–8.. doi: 10.1056/NEJMoa1205409. [DOI] [PubMed] [Google Scholar]
- 51. Nagai T, Honda Y, Sugano Yet al. Circulating omega-6, but not omega-3 polyunsaturated fatty acids, are associated with clinical outcomes in patients with acute decompensated heart failure. PLoS One. 2016;11(11):e0165841. doi: 10.1371/journal.pone.0165841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ma K, Yang J, Shao YHet al. Therapeutic and prognostic significance of arachidonic acid in heart failure. Circ Res. 2022;130(7):1056–71.. doi: 10.1161/CIRCRESAHA.121.320548. [DOI] [PubMed] [Google Scholar]
- 53. Watanabe S, Yoshihisa A, Kanno Yet al. Associations with eicosapentaenoic acid to arachidonic acid ratio and mortality in hospitalized heart failure patients. J Card Fail. 2016;22(12):962–9.. doi: 10.1016/j.cardfail.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 54. Challa AA, Lewandowski ED. Short-chain carbon sources: Exploiting pleiotropic effects for heart failure therapy. JACC Basic Transl Sci. 2022;7(7):730–42.. doi: 10.1016/j.jacbts.2021.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sun WJ, Du DB, Fu TZet al. Alterations of the gut microbiota in patients with severe chronic heart failure. Front Microbiol. 2021;12:813289. doi: 10.3389/fmicb.2021.813289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cui X, Ye L, Li Jet al. Metagenomic and metabolomic analyses unveil dysbiosis of gut microbiota in chronic heart failure patients. Sci Rep. 2018;8(1):635. doi: 10.1038/s41598-017-18756-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kirschner SK, Deutz NEP, Rijnaarts Iet al. Impaired intestinal function is associated with lower muscle and cognitive health and well-being in patients with congestive heart failure. JPEN J Parenter Enteral Nutr. 2022;46(3):660–70.. doi: 10.1002/jpen.2193. [DOI] [PubMed] [Google Scholar]
- 58. Carley AN, Maurya SK, Fasano Met al. Short-chain fatty acids outpace ketone oxidation in the failing heart. Circulation. 2021;143(18):1797–808.. doi: 10.1161/CIRCULATIONAHA.120.052671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Vijay A, Astbury S, Panayiotis Let al. Dietary interventions reduce traditional and novel cardiovascular risk markers by altering the gut microbiome and their metabolites. Front Cardiovasc Med. 2021;8:691564. doi: 10.3389/fcvm.2021.691564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chen L, He FJ, Dong YBet al. Modest sodium reduction increases circulating short-chain fatty acids in untreated hypertensives: a randomized, double-blind, placebo-controlled trial. Hypertension. 2020;76(1):73–9.. doi: 10.1161/HYPERTENSIONAHA.120.14800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pakhomov N, Baugh JA. The role of diet-derived short-chain fatty acids in regulating cardiac pressure overload. Am J Physiol Heart Circ Physiol. 2021;320(2):H475–86.. doi: 10.1152/ajpheart.00573.2020. [DOI] [PubMed] [Google Scholar]
- 62. Jia QJ, Li H, Zhou Het al. Role and effective therapeutic target of gut microbiota in heart failure. Cardiovasc Ther. 2019;2019:5164298. doi: 10.1155/2019/5164298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Boets E, Gomand SV, Deroover Let al. Systemic availability and metabolism of colonic-derived short-chain fatty acids in healthy subjects: a stable isotope study. J Physiol. 2017;595(2):541–55.. doi: 10.1113/JP272613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. McCoin CS, Knotts TA, Adams SH. Acylcarnitines—old actors auditioning for new roles in metabolic physiology. Nat Rev Endocrinol. 2015;11(10):617–25.. doi: 10.1038/nrendo.2015.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ruiz M, Labarthe F, Fortier Aet al. Circulating acylcarnitine profile in human heart failure: a surrogate of fatty acid metabolic dysregulation in mitochondria and beyond. Am J Physiol Heart Circ Physiol. 2017;313(4):H768–81.. doi: 10.1152/ajpheart.00820.2016. [DOI] [PubMed] [Google Scholar]
- 66. Cheng ML, Wang CH, Shiao MSet al. Metabolic disturbances identified in plasma are associated with outcomes in patients with heart failure: diagnostic and prognostic value of metabolomics. J Am Coll Cardiol. 2015;65(15):1509–20.. doi: 10.1016/j.jacc.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 67. Hunter WG, Kelly JP, McGarrahRW 3rd, et al. Metabolomic profiling identifies novel circulating biomarkers of mitochondrial dysfunction differentially elevated in heart failure with preserved versus reduced ejection fraction: evidence for shared metabolic impairments in clinical heart failure. J Am Heart Assoc. 2016;5(8):e003190. doi: 10.1161/JAHA.115.003190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Yoshihisa A, Watanabe S, Yokokawa Tet al. Associations between acylcarnitine to free carnitine ratio and adverse prognosis in heart failure patients with reduced or preserved ejection fraction. ESC Heart Fail. 2017;4(3):360–4.. doi: 10.1002/ehf2.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ahmad T, Kelly JP, McGarrah RWet al. Prognostic implications of long-chain acylcarnitines in heart failure and reversibility with mechanical circulatory support. J Am Coll Cardiol. 2016;67(3):291–9.. doi: 10.1016/j.jacc.2015.10.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Liu C, Li RH, Liu Yet al. Characteristics of blood metabolic profile in coronary heart disease, dilated cardiomyopathy and valvular heart disease induced heart failure. Front Cardiovasc Med. 2020;7:622236. doi: 10.3389/fcvm.2020.622236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Truby LK, Regan JA, Giamberardino SNet al. Circulating long chain acylcarnitines and outcomes in diabetic heart failure: an HF-ACTION clinical trial substudy. Cardiovasc Diabetol. 2021;20(1):161. doi: 10.1186/s12933-021-01353-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Tremblay-Gravel M, Fortier A, Baron Cet al. Long-chain acylcarnitines and monounsaturated fatty acids discriminate heart failure patients according to pulmonary hypertension status. Metabolites. 2021;11(4):196. doi: 10.3390/metabo11040196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zhao S, Feng XF, Huang Tet al. The association between acylcarnitine metabolites and cardiovascular disease in Chinese patients with type 2 diabetes mellitus. Front Endocrinol (Lausanne). 2020;11:212. doi: 10.3389/fendo.2020.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Dambrova M, Makrecka-Kuka M, Vilskersts Ret al. Pharmacological effects of meldonium: Biochemical mechanisms and biomarkers of cardiometabolic activity. Pharmacol Res. 2016;113:771–80.. doi: 10.1016/j.phrs.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 75. Selvaraj S, Fu ZX, Jones Pet al. Metabolomic profiling of the effects of dapagliflozin in heart failure with reduced ejection fraction: DEFINE-HF. Circulation. 2022;146(11):808–18.. doi: 10.1161/CIRCULATIONAHA.122.060402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wang H, Anstrom K, Ilkayeva Oet al. Sildenafil treatment in heart failure with preserved ejection fraction: targeted metabolomic profiling in the RELAX trial. JAMA Cardiol. 2017;2(8):896–901.. doi: 10.1001/jamacardio.2017.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Varbo A, Nordestgaard BG. Nonfasting triglycerides, low-density lipoprotein cholesterol, and heart failure risk: Two cohort studies of 113 554 individuals. Arterioscler Thromb Vasc Biol. 2018;38(2):464–72.. doi: 10.1161/ATVBAHA.117.310269. [DOI] [PubMed] [Google Scholar]
- 78. Choi RH, Tatum SM, Symons JDet al. Ceramides and other sphingolipids as drivers of cardiovascular disease. Nat Rev Cardiol. 2021;18(10):701–11.. doi: 10.1038/s41569-021-00536-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Laaksonen R, Ekroos K, Sysi-Aho Met al. Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL-cholesterol. Eur Heart J. 2016;37(25):1967–76.. doi: 10.1093/eurheartj/ehw148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Anroedh S, Hilvo M, Akkerhuis KMet al. Plasma concentrations of molecular lipid species predict long-term clinical outcome in coronary artery disease patients. J Lipid Res. 2018;59(9):1729–37.. doi: 10.1194/jlr.P081281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Havulinna AS, Sysi-Aho M, Hilvo Met al. Circulating ceramides predict cardiovascular outcomes in the population-based FINRISK 2002 cohort. Arterioscler Thromb Vasc Biol. 2016;36(12):2424–30.. doi: 10.1161/ATVBAHA.116.307497. [DOI] [PubMed] [Google Scholar]
- 82. Ji RP, Akashi H, Drosatos Ket al. Increased de novo ceramide synthesis and accumulation in failing myocardium. JCI Insight. 2017;2(9):e82922. doi: 10.1172/jci.insight.82922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wittenbecher C, Eichelmann F, Toledo Eet al. Lipid profiles and heart failure risk: results from two prospective studies. Circ Res. 2021;128(3):309–20.. doi: 10.1161/CIRCRESAHA.120.317883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Javaheri A, Allegood JC, Cowart LAet al. Circulating ceramide 16:0 in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2020;75(17):2273–5.. doi: 10.1016/j.jacc.2020.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Lemaitre RN, Jensen PN, Hoofnagle Aet al. Plasma ceramides and sphingomyelins in relation to heart failure risk. Circ Heart Fail. 2019;12(7):e005708. doi: 10.1161/CIRCHEARTFAILURE.118.005708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Mikhalkova D, Holman SR, Jiang Het al. Bariatric surgery-induced cardiac and lipidomic changes in obesity-related heart failure with preserved ejection fraction. Obesity (Silver Spring). 2018;26(2):284–90.. doi: 10.1002/oby.22038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Djoussé L, Weir NL, Hanson NQet al. Plasma phospholipid concentration of cis-palmitoleic acid and risk of heart failure. Circ Heart Fail. 2012;5(6):703–9.. doi: 10.1161/CIRCHEARTFAILURE.112.967802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lemaitre RN, McKnight B, Sotoodehnia Net al. Circulating very long-chain saturated fatty acids and heart failure: the cardiovascular health study. J Am Heart Assoc. 2018;7(21):e010019. doi: 10.1161/JAHA.118.010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Yamagishi K, Iso H, Yatsuya Het al. Dietary intake of saturated fatty acids and mortality from cardiovascular disease in Japanese: the Japan Collaborative Cohort Study for Evaluation of Cancer Risk (JACC) Study. Am J Clin Nutr. 2010;92(4):759–65.. doi: 10.3945/ajcn.2009.29146. [DOI] [PubMed] [Google Scholar]
- 90. Matsumoto C, Hanson NQ, Tsai MYet al. Plasma phospholipid saturated fatty acids and heart failure risk in the Physicians' Health Study. Clin Nutr. 2013;32(5):819–23.. doi: 10.1016/j.clnu.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Tang HY, Wang CH, Ho HYet al. Lipidomics reveals accumulation of the oxidized cholesterol in erythrocytes of heart failure patients. Redox Biol. 2018;14:499–508.. doi: 10.1016/j.redox.2017.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Chen YY, Chen Y, Liang SMet al. Prognostic impact of fasting plasma glucose on mortality and re-hospitalization in patients with acute heart failure. Chin Med J (Engl). 2018;131(17):2032–40.. doi: 10.4103/0366-6999.239310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kosiborod M, Inzucchi SE, Spertus JAet al. Elevated admission glucose and mortality in elderly patients hospitalized with heart failure. Circulation. 2009;119(14):1899–907.. doi: 10.1161/CIRCULATIONAHA.108.821843. [DOI] [PubMed] [Google Scholar]
- 94. Sinha A, Ning HY, Ahmad FSet al. Association of fasting glucose with lifetime risk of incident heart failure: the Lifetime Risk Pooling Project. Cardiovasc Diabetol. 2021;20(1):66. doi: 10.1186/s12933-021-01265-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Khan H, Kunutsor SK, Kauhanen Jet al. Fasting plasma glucose and incident heart failure risk: a population-based cohort study and new meta-analysis. J Card Fail. 2014;20(8):584–92.. doi: 10.1016/j.cardfail.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 96. Kenny HC, Abel ED. Heart failure in type 2 diabetes mellitus. Circ Res. 2019;124(1):121–41.. doi: 10.1161/CIRCRESAHA.118.311371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Xu LL, Wu MY, Chen SHet al. Triglyceride-glucose index associates with incident heart failure: A cohort study. Diabetes Metab. 2022;48(6):101365. doi: 10.1016/j.diabet.2022.101365. [DOI] [PubMed] [Google Scholar]
- 98. Huang RH, Lin YF, Ye XMet al. Triglyceride-glucose index in the development of heart failure and left ventricular dysfunction: analysis of the ARIC study. Eur J Prev Cardiol. 2022;2022:zwac058. doi: 10.1093/eurjpc/zwac058. [DOI] [PubMed] [Google Scholar]
- 99. Li XT, Chan JSK, Guan Bet al. Triglyceride-glucose index and the risk of heart failure: Evidence from two large cohorts and a mendelian randomization analysis. Cardiovasc Diabetol. 2022;21(1):229. doi: 10.1186/s12933-022-01658-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Kristensen SL, Preiss D, Jhund PSet al. Risk related to pre-diabetes mellitus and diabetes mellitus in heart failure with reduced ejection fraction: insights from prospective comparison of ARNI with ACEI to determine impact on global mortality and morbidity in heart failure trial. Circ Heart Fail. 2016;9(1):e002560. doi: 10.1161/CIRCHEARTFAILURE.115.002560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Gotsman I, Shauer A, Lotan Cet al. Impaired fasting glucose: a predictor of reduced survival in patients with heart failure. Eur J Heart Fail. 2014;16(11):1190–8.. doi: 10.1002/ejhf.146. [DOI] [PubMed] [Google Scholar]
- 102. Targher G, Dauriz M, Laroche Cet al. In-hospital and 1-year mortality associated with diabetes in patients with acute heart failure: results from the ESC-HFA Heart Failure Long-Term Registry. Eur J Heart Fail. 2017;19(1):54–65.. doi: 10.1002/ejhf.679. [DOI] [PubMed] [Google Scholar]
- 103. Testani JM, Inzucchi SE, Voors AA. Treating heart failure with antihyperglycemic medications: Is now the right time?. Circulation. 2019;139(21):2383–5.. doi: 10.1161/CIRCULATIONAHA.119.038854. [DOI] [PubMed] [Google Scholar]
- 104. Eshaghian S, Horwich TB, Fonarow GC. An unexpected inverse relationship between HbA1c levels and mortality in patients with diabetes and advanced systolic heart failure. Am Heart J. 2006;151(1):91. doi: 10.1016/j.ahj.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 105. Duckworth W, Abraira C, Moritz Tet al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129–39.. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 106. Nassif M, Kosiborod M. Effect of glucose-lowering therapies on heart failure. Nat Rev Cardiol. 2018;15(5):282–91.. doi: 10.1038/nrcardio.2017.211. [DOI] [PubMed] [Google Scholar]
- 107. Selvaraj S, Kelly DP, Margulies KB. Implications of altered ketone metabolism and therapeutic ketosis in heart failure. Circulation. 2020;141(22):1800–12.. doi: 10.1161/CIRCULATIONAHA.119.045033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Gouzi F, Ayache D, Hédon Cet al. Breath acetone concentration: too heterogeneous to constitute a diagnosis or prognosis biomarker in heart failure? A systematic review and meta-analysis. J Breath Res. 2021;16(1):016001. doi: 10.1088/1752-7163/ac356d. [DOI] [PubMed] [Google Scholar]
- 109. Biagini D, Lomonaco T, Ghimenti Set al. Determination of volatile organic compounds in exhaled breath of heart failure patients by needle trap micro-extraction coupled with gas chromatography-tandem mass spectrometry. J Breath Res. 2017;11(4):047110. doi: 10.1088/1752-7163/aa94e7. [DOI] [PubMed] [Google Scholar]
- 110. Samara MA, Wilson Tang WH, CikachF Jr, et al. Single exhaled breath metabolomic analysis identifies unique breathprint in patients with acute decompensated heart failure. J Am Coll Cardiol. 2013;61(13):1463–4.. doi: 10.1016/j.jacc.2012.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Yokokawa T, Sato T, Suzuki Set al. Change of exhaled acetone concentration levels in patients with acute decompensated heart failure. Int Heart J. 2018;59(4):808–12.. doi: 10.1536/ihj.17-482. [DOI] [PubMed] [Google Scholar]
- 112. Yokokawa T, Sugano Y, Shimouchi Aet al. Exhaled acetone concentration is related to hemodynamic severity in patients with non-ischemic chronic heart failure. Circ J. 2016;80(5):1178–86.. doi: 10.1253/circj.CJ-16-0011. [DOI] [PubMed] [Google Scholar]
- 113. Marcondes-Braga FG, Batista GL, Gutz IGet al. Impact of exhaled breath acetone in the prognosis of patients with heart failure with reduced ejection fraction (HFrEF). One year of clinical follow-up. PLoS One. 2016;11(12):e0168790. doi: 10.1371/journal.pone.0168790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Lommi J, Kupari M, Koskinen Pet al. Blood ketone bodies in congestive heart failure. J Am Coll Cardiol. 1996;28(3):665–72.. doi: 10.1016/0735-1097(96)00214-8. [DOI] [PubMed] [Google Scholar]
- 115. Flores-Guerrero JL, Westenbrink BD, Connelly MAet al. Association of beta-hydroxybutyrate with development of heart failure: Sex differences in a Dutch population cohort. Eur J Clin Invest. 2021;51(5):e13468. doi: 10.1111/eci.13468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Wang YQ, Huang KY, Liu FCet al. Association of circulating branched-chain amino acids with risk of cardiovascular disease: A systematic review and meta-analysis. Atherosclerosis. 2022;350:90–6.. doi: 10.1016/j.atherosclerosis.2022.04.026. [DOI] [PubMed] [Google Scholar]
- 117. Monzo L, Sedlacek K, Hromanikova Ket al. Myocardial ketone body utilization in patients with heart failure: The impact of oral ketone ester. Metabolism. 2021;115:154452. doi: 10.1016/j.metabol.2020.154452. [DOI] [PubMed] [Google Scholar]
- 118. Nielsen R, Møller N, Gormsen LCet al. Cardiovascular effects of treatment with the ketone body 3-hydroxybutyrate in chronic heart failure patients. Circulation. 2019;139(18):2129–41.. doi: 10.1161/CIRCULATIONAHA.118.036459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Pietschner R, Kolwelter J, Bosch Aet al. Effect of empagliflozin on ketone bodies in patients with stable chronic heart failure. Cardiovasc Diabetol. 2021;20(1):219. doi: 10.1186/s12933-021-01410-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Wheat HL, Fedson S, Bozkurt Bet al. Cardiac rehabilitation in heart failure: Indications for exercise training based on heart failure phenotype. Prog Cardiovasc Dis. 2022;70:16–21.. doi: 10.1016/j.pcad.2021.10.003. [DOI] [PubMed] [Google Scholar]
- 121. Corbi G, Conti V, Troisi Jet al. Cardiac rehabilitation increases SIRT1 activity and beta-hydroxybutyrate levels and decreases oxidative stress in patients with hf with preserved ejection fraction. Oxid Med Cell Longev. 2019;2019:7049237. doi: 10.1155/2019/7049237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. McGarrah RW, White PJ. Branched-chain amino acids in cardiovascular disease. Nat Rev Cardiol. 2023;20(2):77–89.. doi: 10.1038/s41569-022-00760-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Li T, Zhang Z, Kolwicz SCet al. Defective branched-chain amino acid catabolism disrupts glucose metabolism and sensitizes the heart to ischemia-reperfusion injury. Cell Metab. 2017;25(2):374–85.. doi: 10.1016/j.cmet.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Murashige D, Jang C, Neinast Met al. Comprehensive quantification of fuel use by the failing and nonfailing human heart. Science. 2020;370(6514):364–8.. doi: 10.1126/science.abc8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Murashige D, Jung JW, Neinast MDet al. Extra-cardiac BCAA catabolism lowers blood pressure and protects from heart failure. Cell Metab. 2022;34(11):1749–64.. doi: 10.1016/j.cmet.2022.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Lim LL, Lau ESH, Fung Eet al. Circulating branched-chain amino acids and incident heart failure in type 2 diabetes: The Hong Kong Diabetes Register. Diabetes Metab Res Rev. 2020;36(3):e3253. doi: 10.1002/dmrr.3253. [DOI] [PubMed] [Google Scholar]
- 127. Du XY, Li YL, Wang Yet al. Increased branched-chain amino acid levels are associated with long-term adverse cardiovascular events in patients with STEMI and acute heart failure. Life Sci. 2018;209:167–72.. doi: 10.1016/j.lfs.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 128. Lanfear DE, Gibbs JJ, Li Jet al. Targeted metabolomic profiling of plasma and survival in heart failure patients. JACC Heart Fail. 2017;5(11):823–32.. doi: 10.1016/j.jchf.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Portero V, Nicol T, Podliesna Set al. Chronically elevated branched chain amino acid levels are pro-arrhythmic. Cardiovasc Res. 2022;118(7):1742–57.. doi: 10.1093/cvr/cvab207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Sun H, Olson KC, Gao Cet al. Catabolic defect of branched-chain amino acids promotes heart failure. Circulation. 2016;133(21):2038–49.. doi: 10.1161/CIRCULATIONAHA.115.020226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Nemutlu E, Zhang S, Xu YZet al. Cardiac resynchronization therapy induces adaptive metabolic transitions in the metabolomic profile of heart failure. J Card Fail. 2015;21(6):460–9.. doi: 10.1016/j.cardfail.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Zhang ZY, Marrachelli VG, Yang WYet al. Diastolic left ventricular function in relation to circulating metabolic biomarkers in a population study. Eur J Prev Cardiol. 2018;26(1):22–32.. doi: 10.1177/2047487318797395. [DOI] [PubMed] [Google Scholar]
- 133. Delles C, Rankin NJ, Boachie Cet al. Nuclear magnetic resonance-based metabolomics identifies phenylalanine as a novel predictor of incident heart failure hospitalisation: results from PROSPER and FINRISK 1997. Eur J Heart Fail. 2018;20(4):663–73.. doi: 10.1002/ejhf.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Hage C, Löfgren L, Michopoulos Fet al. Metabolomic profile in HFpEF vs HFrEF patients. J Card Fail. 2020;26(12):1050–9.. doi: 10.1016/j.cardfail.2020.07.010. [DOI] [PubMed] [Google Scholar]
- 135. Bekfani T, Bekhite M, Neugebauer Set al. Metabolomic profiling in patients with heart failure and exercise intolerance: kynurenine as a potential biomarker. Cells. 2022;11(10):1674. doi: 10.3390/cells11101674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. van der Pol A, Gil A, Silljé HHWet al. Accumulation of 5-oxoproline in myocardial dysfunction and the protective effects of OPLAH. Sci Transl Med. 2017;9(415):eaam8574. doi: 10.1126/scitranslmed.aam8574. [DOI] [PubMed] [Google Scholar]
- 137. Hui S, Ghergurovich JM, Morscher RJet al. Glucose feeds the TCA cycle via circulating lactate. Nature. 2017;551(7678):115–8.. doi: 10.1038/nature24057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. DeFronzo R, Fleming GA, Chen Ket al. Metformin-associated lactic acidosis: Current perspectives on causes and risk. Metabolism. 2016;65(2):20–9.. doi: 10.1016/j.metabol.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 139. Nalos M, Leverve X, Huang Set al. Half-molar sodium lactate infusion improves cardiac performance in acute heart failure: a pilot randomised controlled clinical trial. Crit Care. 2014;18(2):R48. doi: 10.1186/cc13793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Kraut JA, Madias NE. Lactic acidosis. N Engl J Med. 2014;371(24):2309–19.. doi: 10.1056/NEJMra1309483. [DOI] [PubMed] [Google Scholar]
- 141. Adamo L, Nassif ME, Novak Eet al. Prevalence of lactic acidaemia in patients with advanced heart failure and depressed cardiac output. Eur J Heart Fail. 2017;19(8):1027–33.. doi: 10.1002/ejhf.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Zymliński R, Biegus J, Sokolski Met al. Increased blood lactate is prevalent and identifies poor prognosis in patients with acute heart failure without overt peripheral hypoperfusion. Eur J Heart Fail. 2018;20(6):1011–8.. doi: 10.1002/ejhf.1156. [DOI] [PubMed] [Google Scholar]
- 143. Lehle K, Lubnow M, Philipp Aet al. Prevalence of hemolysis and metabolic acidosis in patients with circulatory failure supported with extracorporeal life support: a marker for survival?. Eur J Heart Fail. 2017;19(Suppl 2):110–6.. doi: 10.1002/ejhf.854. [DOI] [PubMed] [Google Scholar]
- 144. Protti A, Russo R, Tagliabue Pet al. Oxygen consumption is depressed in patients with lactic acidosis due to biguanide intoxication. Crit Care. 2010;14(1):R22. doi: 10.1186/cc8885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Pang L, Jiang X, Lian Xet al. Caloric restriction-mimetics for the reduction of heart failure risk in aging heart: with consideration of gender-related differences. Mil Med Res. 2022;9(1):33. doi: 10.1186/s40779-022-00389-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Lavie CJ, Arena R, Swift DLet al. Exercise and the cardiovascular system: clinical science and cardiovascular outcomes. Circ Res. 2015;117(2):207–19.. doi: 10.1161/CIRCRESAHA.117.305205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Badolia R, Ramadurai DK, Taleb Iet al. The role of nonglycolytic glucose metabolism in myocardial recovery upon mechanical unloading and circulatory support in chronic heart failure. Circulation. 2020;142(3):259–74.. doi: 10.1161/CIRCULATIONAHA.119.044452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Newman JD, Schlendorf KH, Cox ZLet al. Post-transplant diabetes mellitus following heart transplantation. J Heart Lung Transplant. 2022;41(11):1537–46.. doi: 10.1016/j.healun.2022.07.011. [DOI] [PubMed] [Google Scholar]


