Abstract
Background:
Small for gestational age (SGA) birth is associated with poor long-term health outcomes. It is unclear whether maternal antihypertensive medication increases risk of SGA independently of maternal hypertension.
Methods:
We analyzed associations between maternal hypertension and antihypertensive medication use and SGA among non-malformed singleton controls in the National Birth Defects Prevention Study. We defined SGA as birthweight<10th percentile for a given gestational age, sex, race/ethnicity, and parity. We included 1,045 SGA and 10,019 non-SGA births. We used logistic regression to calculate adjusted odds ratios (AORs) and 95% confidence intervals (CIs). We assessed interaction between hypertension, antihypertensive medication use, and maternal race/ethnicity and age.
Results:
Overall, 122 (11.7%) SGA and 892 (8.9%) non-SGA mothers reported hypertension and 21 (2.0%) SGA and 154 (1.5%) non-SGA mothers reported antihypertensive medication use. The most commonly reported medications were centrally-acting antiadrenergics, β-blockers, calcium channel blockers, and diuretics. Compared to normotensive pregnancies, maternal hypertension, regardless of treatment (AOR, 1.49 [95% CI, 1.20, 1.86]), and untreated maternal hypertension (AOR, 1.46 [95% CI, 1.15, 1.86]) were associated with SGA. We observed a positive, but not significant, association between antihypertensive medication use and SGA. SGA risk varied by maternal race/ethnicity, being highest among Hispanic mothers, and age, being highest among mothers ≥35 years, but statistical tests for interaction were not significant.
Conclusions:
Consistent with the literature, our findings suggest that maternal hypertension slightly increases SGA risk. We did not find an appreciably increased SGA risk associated with antihypertensive medication use beyond that of the underlying maternal hypertension.
Keywords: antihypertensive medication, hypertension, National Birth Defects Prevention Study, pregnancy, small for gestational age
Introduction
Approximately 5-10% of pregnancies are complicated by hypertension, with some evidence that prevalence is increasing in the US (Bateman, Bansil, et al., 2012; Roberts et al., 2011; Wallis, Saftlas, Hsia, & Atrash, 2008). Distinct hypertensive disorders that complicate pregnancy include chronic hypertension, diagnosed before pregnancy or before 20 weeks gestation, and pregnancy-related hypertension, including gestational hypertension and preeclampsia, diagnosed after 20 weeks gestation with resolution after delivery (Hutcheon, Lisonkova, & Joseph, 2011). Hypertension in pregnancy is consistently associated with adverse maternal and perinatal outcomes, including fetal growth restriction and small for gestational age (SGA) birth (Ananth, Peedicayil, & Savitz, 1995; L. M. McCowan, Buist, North, & Gamble, 1996; Rey & Couturier, 1994). However, less is known about the risks associated with antihypertensive medication use during pregnancy.
SGA is often used as a clinical indicator of infant morbidity and mortality, developmental delays, and potential long-term cardiovascular risk (McCormick, 1985; Oken & Gillman, 2003). Several observational studies reported positive associations between antihypertensive medication use, specifically β-blockers, and SGA, but were unable to control for potential confounding by the underlying indication (Lydakis, Lip, Beevers, & Beevers, 1999; Meidahl Petersen et al., 2012; Nakhai-Pour, Rey, & Berard, 2010; Orbach et al., 2013; Su et al., 2013; Xie et al., 2014). A recent systematic review of clinical trials did not report an association between SGA and antihypertensive medication use overall, but did identify a potential association between SGA and β-blocker use (Abalos, Duley, & Steyn, 2014). The authors cautioned, however, that the available evidence was “moderate to poor.”
The proportion of pregnant women in the US using antihypertensive medications increased over the past decade to approximately 3-5% of all pregnancies (Andrade et al., 2008; Bateman, Hernandez-Diaz, et al., 2012). Antihypertensive medications can cross the placenta and may impact growth by directly influencing fetal hemodynamics, or they may reduce the flow of nutrients to the fetus by affecting placental perfusion (Khedun, Maharaj, & Moodley, 2000; von Dadelszen et al., 2000). Thus, it is important to clarify whether treatment introduces additional harm to the fetus, beyond that of the hypertension itself. To date, most large-scale studies on this topic have been registry-based studies from Europe, Canada, and Asia, and their conclusions on the risks associated with antihypertensive medication use are mixed (Meidahl Petersen et al., 2012; Nakhai-Pour et al., 2010; Orbach et al., 2013; Su et al., 2013; Xie et al., 2014). A population-based survey of a racially/ethnically diverse American population, the National Birth Defects Prevention Study (NBDPS) offers the opportunity to expand the existing body of literature. In our study, we assessed the risk of SGA birth associated with maternal self-reports of hypertension and antihypertensive medication use among non-malformed singleton controls in the NBDPS.
Methods
The NBDPS was a multi-site, population-based case-control study to investigate risk factors for more than 30 major birth defects (Reefhuis et al., 2015). We analyzed data on NBDPS singleton controls born during October 1997-December 2011. Controls were non-malformed live births randomly selected from birth certificates or hospital discharge records in 10 study sites: Arkansas, California, Georgia, Iowa, Massachusetts, New Jersey, New York, North Carolina, Texas, and Utah. A mother was eligible for the NBDPS if she had legal custody of her child, had not participated in the study with a previous pregnancy, was not incarcerated, and could complete the interview in English or Spanish. Each study site and the Centers for Disease Control and Prevention obtained Institutional Review Board approval for the study and participants provided informed consent.
Trained interviewers collected data via telephone interview within 24 months of the infant’s birth; the interview participation rate was 65% among eligible control mothers (Reefhuis et al., 2015). The interview included questions on maternal demographics, pregnancy history, behaviors, and medication use during the three months before pregnancy until delivery. Specifically, interviewers asked about diagnosis, timing, and treatment of “high blood pressure” for mothers with 1997-2005 births and “high blood pressure, toxemia, pre-eclampsia or eclampsia” for mothers with 2006-2011 births. Mothers with 2006-2011 births also reported the type of hypertension experienced (pregnancy-related, chronic hypertension, or both). Mothers reported the name, timing, and frequency of antihypertensive medication used during the three months before pregnancy until delivery. If the mother could not recall the medication name, the interviewer read a list of common antihypertensive medications to her.
We considered a mother exposed to hypertension if she reported hypertension, regardless of type, during the index pregnancy. We considered a mother exposed to antihypertensive medication if she reported use any time during the month before pregnancy until delivery. We categorized medication exposure into three non-exclusive time periods: first trimester (one month before pregnancy through gestational month three), second trimester, and/or third trimester. As a proxy for duration of use and indication, we also analyzed medication use by timing of the first pregnancy exposure (first trimester vs. second or third trimester). We coded medications using the Slone Epidemiology Center Drug Dictionary (Boston, MA). We categorized medications into drug classes based on mechanism of action: antiadrenergic agents (centrally- or peripherally-acting), α-blockers, β-blockers (selective or non-selective), angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers, calcium channel blockers (dihydropyridine or non-dihydropyridine), diuretics (thiazide and thiazide-like, potassium-sparing, loop, or carbonic anhydrase inhibitor), and direct vasodilators (Supplementary Table 1).
We assigned dichotomous SGA values, defined as birthweight less than the 10th percentile for a given gestational age in weeks, sex, race/ethnicity, and parity, based on published population growth references for singleton infants born at 25-42 weeks gestation (Overpeck, Hediger, Zhang, Trumble, & Klebanoff, 1999; Zhang & Bowes, 1995). For the SGA calculation, we categorized parity as primipara or multipara and race/ethnicity as non-Hispanic white or other, non-Hispanic black, or Hispanic. We used the non-Hispanic white reference population for infants categorized as “other”. Race/ethnicity and parity were self-reported; infant sex and gestational age were obtained from the infant’s clinical record.
Due to a well-established association with large for gestational age birth (Lapolla, Dalfra, & Fedele, 2008), we excluded mothers who reported preexisting diabetes (n=82). We also excluded women who were missing SGA or hypertension information (n=122 and n=223, respectively). Finally, because we were primarily concerned with measuring the effects of antihypertensive medication use among hypertensive women, we excluded from the main analyses mothers who reported use of an antihypertensive medication drug class without a report of hypertension during the pregnancy (n=49). After exclusions, we included interview data from 11,064 mothers.
We used unconditional logistic regression (SAS 9.3 [Cary, NC]) to estimate crude and adjusted odds ratios (AORs) and 95% confidence intervals (CIs) for the relative risk of SGA among mothers with untreated maternal hypertension and among mothers using antihypertensive medication, overall and by timing of use. For all regression analyses, the non-exposed group was normotensive mothers who did not report taking an antihypertensive medication during pregnancy. To attempt to control for the effect of underlying hypertension on risk of SGA, we conducted a sub-analysis using untreated hypertensive mothers as the non-exposed group.
We constructed a full logistic regression model based on bivariate analyses and narrowed the list of covariates using a backward selection approach, retaining only those covariates that altered the effect estimate by more than 10% when removed. For exposure categories with at least five exposed cases, we adjusted for: maternal race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, other); maternal age in years at delivery (<20, 20-34, ≥35); maternal prepregnancy body mass index (BMI) calculated as weight (in kilograms) divided by height (in meters) squared (underweight: <18.5, normal: 18.5-24, overweight: 25-29, obese: ≥30); folic acid-containing supplement use during the first trimester (yes/no); maternal cigarette smoking after the first trimester (yes/no); and study site. For groups with 3 or 4 exposed SGA births, we estimated crude ORs and exact 95% CIs.
We conducted a sub-analysis of mothers with 2006-2011 births who reported type of hypertension. We estimated crude and adjusted ORs for the associations between untreated hypertension and SGA and between antihypertensive medication use and SGA, by type of hypertension (chronic or pregnancy-related). In another sub-analysis, we estimated crude and adjusted ORs for the association between SGA and antihypertensive medication use among mothers who did not report hypertension.
We assessed interaction between maternal age at delivery and both antihypertensive medication use and untreated hypertension, as well as between maternal race/ethnicity and both antihypertensive medication use and untreated hypertension. We calculated the relative excess risk due to interaction (RERI) to assess additive interaction (Hosmer & Lemeshow, 1992), and used a product term in our logistic regression model to assess multiplicative interaction.
Results
We included interview data from 1,045 SGA births and 10,019 non-SGA births in our main analyses. Compared to mothers of non-SGA infants, mothers of SGA infants were more likely to be <20 years or ≥35 years at delivery; be categorized as Hispanic or “other” race/ethnicity; have a lower prepregnancy BMI; and have smoked cigarettes after the first trimester. SGA mothers were also less likely to have taken a folic acid-containing supplement during the first trimester (Table 1).
Table 1.
Selected characteristics of SGA and non-SGA births, National Birth Defects Prevention Study, 1997-2011. Values are n (%).
| Characteristic | SGA (n=1,045) |
Non-SGA (n=10,019) |
P-value |
|---|---|---|---|
| Maternal Age Group | 0.029 | ||
| <20 years | 117 (11.2) | 977 (9.8) | |
| 20-34 years | 764 (73.1) | 7,693 (76.8) | |
| ≥35 years | 164 (15.7) | 1,349 (13.5) | |
| Race/Ethnicity | <0.001 | ||
| Non-Hispanic White | 575 (55.0) | 5,847 (58.4) | |
| Non-Hispanic Black | 83 (7.9) | 1,114 (11.1) | |
| Hispanic | 297 (28.4) | 2,433 (24.3) | |
| Other | 90 (8.6) | 625 (6.2) | |
| Parity | 0.141 | ||
| 0 | 396 (37.9) | 3,954 (39.5) | |
| 1 | 370 (35.4) | 3,246 (32.4) | |
| ≥2 | 279 (26.7) | 2,819 (28.1) | |
| Annual Household Income | <0.001 | ||
| <$10,000 | 229 (23.9) | 1,681 (18.3) | |
| $10,000-$50,000 | 445 (46.4) | 4,113 (44.8) | |
| >$50,000 | 285 (29.7) | 3,390 (36.9) | |
| Maternal Education | <0.001 | ||
| < High school | 218 (21.1) | 1,587 (16.0) | |
| High school | 277 (26.8) | 2,333 (23.6) | |
| Some college | 276 (26.7) | 2,673 (27.0) | |
| College degree | 261 (25.3) | 3,308 (33.4) | |
| Prepregnancy BMI | <0.001 | ||
| Underweight | 90 (9.1) | 478 (5.0) | |
| Normal | 576 (58.0) | 5,139 (53.4) | |
| Overweight | 194 (19.5) | 2,212 (23.0) | |
| Obese | 134 (13.5) | 1,792 (18.6) | |
| Gestational Diabetes | 47 (4.6) | 448 (4.6) | 0.973 |
| Late Pregnancy Smoking | 149 (14.4) | 818 (8.2) | <0.001 |
| First Trimester Alcohol Use | 352 (34.2) | 3,731 (37.7) | 0.031 |
| First Trimester Folic Acid Supplement Use | 853 (90.0) | 8,630 (93.0) | <0.001 |
Overall, 122 (11.7%) SGA mothers and 892 (8.9%) non-SGA mothers reported hypertension during pregnancy (Table 2). Of these, 21 (2.0% overall; 17.2% of hypertensive mothers) SGA and 154 (1.5% overall; 17.3% of hypertensive mothers) non-SGA mothers reported antihypertensive medication use during pregnancy. The total number of SGA and non-SGA mothers reporting antihypertensive medication use increased from 101 in the first trimester to 123 in the second trimester to 145 in the third trimester. Most (83.0%) mothers who began using an antihypertensive medication before or during the first trimester continued use in the second trimester or later (data not shown). The most commonly reported medication classes were centrally-acting antiadrenergics (SGA=8, non-SGA=59) and β-blockers (SGA=5, non-SGA=49).
Table 2.
Antihypertensive medication use during pregnancy, among women who reported hypertension during the index pregnancy, and risk of SGA birth, National Birth Defects Prevention Study, 1997-2011. The reference group for all logistic regression models is normotensive women who did not report taking an antihypertensive medication during pregnancy. Values for SGA and Non-SGA columns are n (%).
| Exposure Group | SGA (n=1,045) |
Non-SGA (n=10,019) |
OR (95% CI)a | AOR (95% CI)b |
|---|---|---|---|---|
| Hypertension, regardless of treatment | 122 (11.7) | 892 (8.9) | 1.35 (1.11, 1.65) | 1.49 (1.20, 1.86) |
| Any antihypertensive medication use, any timec | 21 (2.0) | 154 (1.5) | 1.35 (0.85, 2.14) | 1.66 (0.99, 2.76) |
| Antihypertensive medication use, by trimesterc | ||||
| 1st trimester use | 13 (1.2) | 88 (0.8) | 1.46 (0.81, 2.63) | 1.66 (0.87, 3.19) |
| 2nd trimester use | 14 (1.3) | 109 (1.1) | 1.27 (0.73, 2.23) | 1.49 (0.80, 2.77) |
| 3rd trimester use | 16 (1.5) | 129 (1.3) | 1.23 (0.73, 2.07) | 1.54 (0.87, 2.73) |
| Antihypertensive medication use, by class | ||||
| Centrally-acting antiadrenergic | 8 (0.8) | 59 (0.6) | 1.34 (0.64, 2.82) | 1.72 (0.77, 3.85) |
| β-blocker | 5 (0.5) | 49 (0.5) | 1.01 (0.31, 2.53) | 1.29 (0.50, 3.29) |
| ACE inhibitor | 2 (0.2) | 8 (<0.1) | - | - |
| Angiotensin II receptor blocker | 0 (0.0) | 2 (<0.1) | - | - |
| Calcium channel blocker | 3 (0.3) | 15 (0.1) | 1.98 (0.37, 7.01) | - |
| Diuretic | 3 (0.3) | 12 (0.1) | 2.47 (0.45, 9.18) | - |
| Direct vasodilator | 2 (0.2) | 6 (<0.1) | - | - |
| Unspecified antihypertensive | 3 (0.3) | 34 (0.3) | 0.87 (0.17, 2.78) | - |
| Untreated hypertension | 101 (9.7) | 738 (7.4) | 1.35 (1.09, 1.69) | 1.46 (1.15, 1.86) |
| No hypertension (reference) | 923 (88.3) | 9,127 (91.1) | 1.00 | 1.00 |
For groups with <5 exposed SGA births, we calculated the exact 95% CI.
Adjusted for maternal race/ethnicity, maternal age at delivery, maternal prepregnancy BMI, late pregnancy smoking, early pregnancy folic acid supplement use, and study site.
Two non-SGA mothers reported antihypertensive medication use during pregnancy, but did not recall the specific timing of use. These mothers are excluded from the analysis by trimester.
Hypertension, regardless of treatment, was significantly associated with increased risk of SGA (AOR 1.49 [95% CI 1.20, 1.86]), as was untreated hypertension (AOR 1.46 [95% CI 1.15, 1.86]). Antihypertensive medication use, during any of the time periods analyzed, was also associated with increased risk of SGA, but AORs were not statistically significant (any antihypertensive medication use, any trimester AOR 1.66 [95% CI 0.99, 2.76]). This was true when we analyzed timing of medication initiation, as well; mothers who began taking an antihypertensive medication after the first trimester (SGA=8 non-SGA=64) had an AOR of 1.66 [95% CI 0.74, 3.71] (data not shown). Among each medication class with at least 3 exposed SGA births (centrally-acting antiadrenergics, β-blockers, calcium channel blockers, and diuretics), risk of SGA was increased but all CIs intersected the null. In our sub-analysis restricted to women with hypertension, the AOR for SGA in antihypertensive medication users (any use during any trimester) was 1.05 (95% CI 0.59-1.89) when compared to untreated hypertensive mothers; all other AORs for risk of SGA associated with specific medication classes or timing of use were also null (data not shown).
Data for our sub-analysis by type of hypertension were available for 505 SGA and 4,122 non-SGA births (41.8% of the total analytic sample) (Table 3). Among mothers with untreated hypertension, 97.8% (n=44) of SGA mothers and 94.4% (n=252) of non-SGA mothers reported pregnancy-related hypertension. We observed a marginally significantly increased risk of SGA associated with untreated pregnancy-related hypertension (AOR 1.45 [95% CI 1.00, 2.09]). Among mothers using antihypertensive medication to treat pregnancy-related hypertension, there was an increased, but not statistically significant, risk of SGA (AOR 2.19 [95% CI 0.81, 5.92]).
Table 3.
Antihypertensive medication use during pregnancy, among women who reported hypertension during the index pregnancy, and risk of SGA, by type of hypertension, National Birth Defects Prevention Study, 2006-2011. The reference group for all logistic regression models is normotensive women who did not report taking an antihypertensive medication during pregnancy. Values for SGA and Non-SGA columns are n (%).
| Exposure Groupa | SGA (n=505) |
Non-SGA (n=4,122) |
OR (95% CI) b | AOR (95% CI)c |
|---|---|---|---|---|
| Any antihypertensive medication use | ||||
| Among women with pregnancy related hypertension | 5 (1.0) | 28 (0.7) | 1.51 (0.58, 3.93) | 2.19 (0.81, 5.92) |
| Among women with chronic hypertension | 5 (1.0) | 38 (0.9) | 1.11 (0.44, 2.84) | 0.84 (0.25, 2.83) |
| Untreated hypertension | ||||
| Among women with pregnancy related hypertension | 44 (8.7) | 252 (6.1) | 1.47 (1.05, 2.06) | 1.45 (1.00, 2.09) |
| Among women with chronic hypertension | 1 (0.2) | 15 (0.4) | - | - |
| No hypertension (reference) | 450 (89.1) | 3,789 (91.9) | 1.00 | 1.00 |
Excludes n=12 women (3 SGA, 9 non-SGA) who reported both chronic and pregnancy related hypertension during the index pregnancy.
For groups with <5 exposed SGA births, we calculated the exact 95% CI.
Adjusted for maternal race/ethnicity, maternal age at delivery, maternal prepregnancy BMI, late pregnancy smoking, early pregnancy folic acid supplement use, and study site.
Among mothers who reported taking an antihypertensive medication but did not report hypertension, the crude risk of SGA was more than double that of untreated normotensive mothers (OR 2.28 [95% CI 1.10, 4.72]) (Table 4). This effect was slightly attenuated in the fully-adjusted model and no longer statistically significant (AOR 2.19 [95% CI 0.99, 4.82]). However among mothers taking β-blockers, specifically, for another indication, the AOR for SGA remained statistically significantly increased, at 3.81 [95% CI 1.20, 12.14]. Interview data on these alternative indications were limited (data not shown). Among non-SGA mothers who reported β-blocker use, one reported use for migraine headaches, while three others reported heart disease or cardiac dysrhythmia. No SGA mother reported a specific indication for off-label β-blocker use. Overall, normotensive β-blocker users were more likely to begin use before the second trimester (72.2% overall; SGA=5, non-SGA=8) than those who used other medication classes (26.7%% overall; SGA=1, non-SGA=7); calcium channel blockers were initiated almost exclusively in late pregnancy (90.4% overall; SGA=3, non-SGA=16).
Table 4.
Antihypertensive medication use for another indication during pregnancy and risk of SGA, National Birth Defects Prevention Study, 1997-2011. The reference group for all logistic regression models is normotensive women who did not report taking an antihypertensive medication during pregnancy. Values for SGA and Non-SGA columns are n (%).
| Exposure group | SGA (n=932) |
Non-SGA (n=9,161) |
OR (95% CI)a | AOR (95% CI)b |
|---|---|---|---|---|
| Any antihypertensive medication | 9 (0.97) | 39 (0.43) | 2.28 (1.10, 4.72) | 2.19 (0.99, 4.82) |
| β-blocker | 5 (0.54) | 13 (0.14) | 3.81 (1.35, 10.69) | 3.81 (1.20, 12.14) |
| Calcium channel blocker | 3 (0.32) | 18 (0.20) | 1.65 (0.48, 5.60) | - |
| Diuretic | 0 (0) | 6 (0.07) | - | - |
| α-blocker | 1 (0.11) | 1 (0.01) | - | - |
| Direct vasodilator | 0 (0) | 1 (0.01) | - | - |
| No hypertension (reference) | 923 (99.0) | 9,122 (99.6) | 1.00 | 1.00 |
For groups with <5 exposed SGA births, we calculated the exact 95% CI.
Adjusted for maternal race/ethnicity, maternal age at delivery, maternal prepregnancy BMI, late pregnancy smoking, early pregnancy folic acid supplement use, and study site.
Stratified analyses indicated that risk of SGA varied by race/ethnicity, both among mothers using an antihypertensive medication and among those with untreated hypertension (Table 5). Within each exposure strata, Hispanic mothers had higher risk of SGA than non-Hispanic white or non-Hispanic black mothers. Among non-Hispanic black mothers, risk of SGA was similar between normotensive mothers and those with untreated hypertension (AOR 1.04 [95% CI 0.78, 1.40] and AOR 0.99 [95% CI 0.40, 2.49], respectively), but was increased among hypertensive non-Hispanic black mothers using an antihypertensive medication (AOR 1.74 [95% CI 0.61, 4.98]). However, 95% CIs were wide and overlapped between groups, and neither multiplicative nor additive interaction tests were statistically significant.
Table 5.
Hypertension and antihypertensive medication use and risk of SGA, by maternal race/ethnicity, National Birth Defects Prevention Study, 1997-2011.a
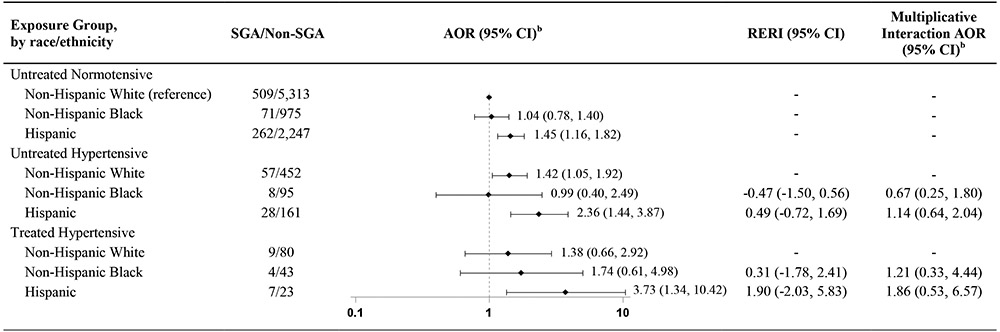
|
Table includes results of two separate comparisons: 1. Untreated hypertensive mothers vs. untreated normotensive mothers, by race/ethnicity; and 2. Treated hypertensive mothers vs. untreated normotensive mothers, by race/ethnicity. Reference group for all comparisons is non-Hispanic white untreated normotensive mothers.
Adjusted for maternal age at delivery, maternal prepregnancy BMI, late pregnancy smoking, early pregnancy folic acid supplement use, and study site.
We observed that risk of SGA also varied by maternal age at delivery (Table 6). Mothers over age 35 years appeared to have the highest risk of SGA, regardless of hypertension status. Among hypertensive older mothers, point estimates were similar whether their hypertension was treated or untreated (AOR 2.36 [95% CI 1.04, 5.37] or AOR 2.55 [95% CI 1.51, 4.30]). Sample size was limited for mothers under age 20 years, but whereas very young age may have a slightly protective effect against SGA among normotensive mothers, untreated hypertensive mothers under 20 years had higher risk of SGA than mothers ages 20-34 years (AOR 1.60 [95% CI 0.86, 3.00] vs. AOR 1.31 [95% CI 0.98, 1.76]). Nevertheless, as with the stratified results for maternal race/ethnicity, these point estimates had wide, overlapping confidence intervals, and we did not observe statistically significant additive or multiplicative interactions.
Table 6.
Hypertension and antihypertensive medication use and risk of SGA, by maternal age, National Birth Defects Prevention Study, 1997-2011.a
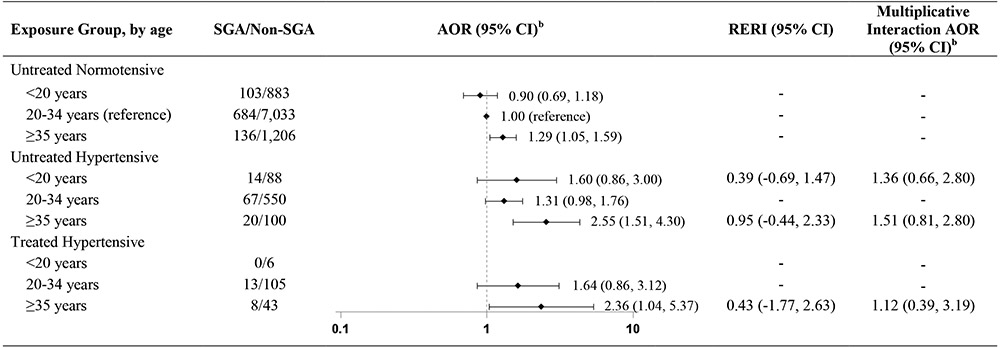
|
Table includes results of two separate comparisons: 1. Untreated hypertensive mothers vs. untreated normotensive mothers, by age; and 2. Treated hypertensive mothers vs. untreated normotensive mothers, by age. Reference group for all comparisons is untreated normotensive mothers ages 20-34 years.
Adjusted for maternal race/ethnicity, maternal prepregnancy BMI, late pregnancy smoking, early pregnancy folic acid supplement use, and study site.
Discussion
We observed that maternal hypertension during pregnancy was associated with an almost 50% increase in risk of SGA birth. This finding is consistent with the literature, in both direction and magnitude of effect (Ananth et al., 1995; Catov, Nohr, Olsen, & Ness, 2008; Su et al., 2013). Like several previously published epidemiologic studies (Lydakis et al., 1999; Meidahl Petersen et al., 2012; Nakhai-Pour et al., 2010; Su et al., 2013; Xie et al., 2014), we also observed an increase in SGA birth associated with antihypertensive medication use, but our findings were not statistically significant. Although our study was large, antihypertensive medication use was rare, limiting our ability to detect odds ratios less than 2.0 at 80% statistical power.
We found similar effect estimates across different time periods of antihypertensive medication use, different medication classes, and different treatment statuses. As some other researchers have concluded (Orbach et al., 2013), this may indicate that any observed effect is truly due to the underlying hypertension, as we would expect differently-acting medications to affect fetal growth differently. If the risk of SGA appears slightly higher among mothers using antihypertensive medications than among mothers with untreated hypertension, this may be because mothers requiring treatment generally had more severe hypertension. When compared to untreated hypertensive mothers, we did not find increased risk of SGA among mothers who reported antihypertensive medication use, further suggesting that hypertension itself is driving the observed association between antihypertensive medication use and SGA birth.
Several studies have reported that β-blockers increase risk of SGA (Abalos et al., 2014; Lydakis et al., 1999; Meidahl Petersen et al., 2012; Xie et al., 2014). β-blockers primarily lower blood pressure by reducing cardiac output; it is plausible that concomitant reduction in placental perfusion could impair fetal growth (Easterling, 2014). Among hypertensive mothers, we did not find a statistically significant association between β-blocker use and SGA. However, we did find increased risk of SGA among mothers who used β-blockers for an indication other than hypertension. In fact, this was the highest odds ratio of all comparisons we analyzed, although the confidence interval was wide. Not all studies of antihypertensive medication use restrict their study populations to hypertensive mothers, which may partially explain their more significant results (Meidahl Petersen et al., 2012; Nakhai-Pour et al., 2010). Common indications for off-label β-blocker use, in our study and others, include cardiac arrhythmias and other cardiovascular disease, as well as migraine headaches (Lin, Phan, & Lin, 2006). Maternal cardiovascular disease, in particular, may also be associated with SGA (Hameed et al., 2001), so unmeasured confounding by indication may still play a role in this association. However, we cannot draw conclusions about differences in the indication for off-label β-blocker use among SGA and non-SGA births because less than 25% of subjects provided this information.
Our sub-analysis of risk of SGA by type of hypertension indicated that pregnancy-related hypertension may be driving the observed association between maternal hypertension and SGA. This finding is supported by previous studies that found that preeclampsia and eclampsia are associated with higher risk of SGA than chronic hypertension (Ananth et al., 1995; Sibai et al., 1998). Among this subset of mothers, almost all mothers with chronic hypertension (72.8%) reported taking an antihypertensive medication. Mothers using medication for chronic hypertension did not have an increased risk of SGA. The opposite was true among mothers who reported pregnancy-related hypertension; only 10% of mothers with pregnancy-related hypertension reported antihypertensive medication use, but this group also had the highest risk of SGA. This may support the hypothesis that mothers taking an antihypertensive medication have more severe disease, thus explaining their increased risk of SGA.
Few studies have analyzed risk of SGA due to hypertension or antihypertensive medication use by race/ethnicity, particularly among Hispanic mothers. Our findings support limited evidence that hypertension may have less of an effect on fetal growth among non-Hispanic black mothers than among non-Hispanic white mothers (Jain, Ferre, & Vidyasagar, 1998). Contrary to a study of births in North Carolina (Miranda et al., 2010), we found that this may also be true compared to hypertensive Hispanic mothers, who had the highest risk of SGA across all strata. We hypothesize that these patterns may reflect differences in type of hypertension across racial/ethnic groups. A larger proportion of hypertensive non-Hispanic black women experience chronic hypertension during pregnancy than hypertensive Hispanic women, among whom pregnancy-related hypertension is more prevalent (Martin, Hamilton, Osterman, Curtin, & Matthews, 2015). If our findings that pregnancy-related hypertension is driving the association between hypertension and SGA are true, then this may partially explain differences by race/ethnicity. These results deserve further investigation.
Finally, we found that mothers ages 35 years and older had higher risk of SGA than younger mothers, regardless of whether they had hypertension or whether that hypertension was treated. To our knowledge, the relationship between maternal age, hypertension, antihypertensive medication use, and risk of SGA has not been evaluated in other studies. However, our findings align with existing conventional knowledge that older maternal age is associated with increased SGA risk (L. McCowan & Horgan, 2009). Although not significant, we also found that hypertension may disproportionately increase risk of SGA among mothers younger than 20 years. However, our sample size for mothers in this age group was small, particularly among antihypertensive medication users. Further targeted analyses are needed to better understand these findings.
Our study has a number of strengths. The NBDPS is a well-established population-based study with a standardized interview protocol. Trained interviewers collected detailed information on a broad range of potential confounders, as well as specific medications used and timing of use. However, the NBDPS did not collect detailed information on type of hypertension, medication dosage, or clinical blood pressure measurements, thus limiting our ability to control for confounding by indication. That our sub-analysis using untreated hypertensive mothers as the comparison group affirmed our interpretation of the main results should relieve at least some of those concerns. Because all exposures are based on self-report up to 24 months after delivery, maternal recall bias is another potential limitation. However, maternal recall of hypertensive disorders in pregnancy has been found to be “moderately” valid, with generally high sensitivity and specificity for reported antihypertensive medication use (Dietz et al., 2014; van Gelder, van Rooij, de Walle, Roeleveld, & Bakker, 2013). Additionally, given that our study population was originally selected as the control group for the NBDPS, not based on SGA status, we think that differential recall between cases and controls is likely minimal.
In conclusion, our study adds valuable new evidence to the continuing debate about whether to treat mild to moderate hypertension in pregnancy, both the risks and benefits of which remain unclear (Magee, Singer, & von Dadelszen, 2015). Our study found that, overall, antihypertensive medication use does not confer appreciably increased risk of SGA, above and beyond the risk associated with underlying maternal hypertension. Nor did we find any compelling evidence that one treatment regimen is notably safer or riskier than another with respect to SGA. These results should be reassuring to hypertensive pregnant women and their physicians, in cases where antihypertensive medication is considered clinically necessary. However, further research that can adequately adjust for confounding by indication will provide much-needed clarity on the relative risks and benefits of different antihypertensive medications during pregnancy.
Supplementary Material
Supplementary Table 1. Reported antihypertensive medications, by class and sub-class, National Birth Defects Prevention Study, 1997-2011.
Significance:
We observed an increased risk of small for gestational age (SGA) birth associated with maternal hypertension. There was little evidence of additional risk of SGA due to antihypertensive medication use during pregnancy. Our large, population-based sample of SGA and non-SGA births improves upon those used in most other previous reports, being more representative of pregnant women in the US. Our exploration of variation in risk by maternal race/ethnicity and age offers new directions for further research about the relationship between hypertension, antihypertensive medication use, and SGA.
Acknowledgements
We thank the participating families, scientists, and staff from all of the NBDPS sites. Drug information in the NBDPS is coded using the Slone Epidemiology Center Drug Dictionary, under license from the Slone Epidemiology Center at Boston University. This study was supported by a cooperative agreement from the Centers for Disease Control and Prevention (Cooperative Agreement U01DD001032). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. This study was presented, in part, as a poster at the 28th Annual Meeting of the Society for Pediatric and Perinatal Epidemiologic Research (SPER) in Denver, Colorado, USA, June 15-16, 2015. We thank Cristian Pantea for replicating the analyses.
Footnotes
Disclosure statement: The authors report no conflict of interest.
References
- Abalos E, Duley L, & Steyn DW (2014). Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database Syst Rev, 2, CD002252. doi: 10.1002/14651858.CD002252.pub3 [DOI] [PubMed] [Google Scholar]
- Ananth CV, Peedicayil A, & Savitz DA (1995). Effect of hypertensive diseases in pregnancy on birthweight, gestational duration, and small-for-gestational-age births. Epidemiology, 6(4), 391–395. [DOI] [PubMed] [Google Scholar]
- Andrade SE, Raebel MA, Brown J, Lane K, Livingston J, Boudreau D, … Platt R (2008). Outpatient use of cardiovascular drugs during pregnancy. Pharmacoepidemiol Drug Saf, 17(3), 240–247. doi: 10.1002/pds.1550 [DOI] [PubMed] [Google Scholar]
- Bateman BT, Bansil P, Hernandez-Diaz S, Mhyre JM, Callaghan WM, & Kuklina EV (2012). Prevalence, trends, and outcomes of chronic hypertension: a nationwide sample of delivery admissions. Am J Obstet Gynecol, 206(2), 134.e131–138. doi: 10.1016/j.ajog.2011.10.878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman BT, Hernandez-Diaz S, Huybrechts KF, Palmsten K, Mogun H, Ecker JL, & Fischer MA (2012). Patterns of outpatient antihypertensive medication use during pregnancy in a Medicaid population. Hypertension, 60(4), 913–920. doi: 10.1161/hypertensionaha.112.197095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catov JM, Nohr EA, Olsen J, & Ness RB (2008). Chronic hypertension related to risk for preterm and term small for gestational age births. Obstet Gynecol, 112(2 Pt 1), 290–296. doi: 10.1097/AOG.0b013e31817f589b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz P, Bombard J, Mulready-Ward C, Gauthier J, Sackoff J, Brozicevic P, … Taylor A (2014). Validation of self-reported maternal and infant health indicators in the Pregnancy Risk Assessment Monitoring System. Matern Child Health J, 18(10), 2489–2498. doi: 10.1007/s10995-014-1487-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easterling TR (2014). Pharmacological management of hypertension in pregnancy. Semin Perinatol, 38(8), 487–495. doi: 10.1053/j.semperi.2014.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hameed A, Karaalp IS, Tummala PP, Wani OR, Canetti M, Akhter MW, … Elkayam U (2001). The effect of valvular heart disease on maternal and fetal outcome of pregnancy. J Am Coll Cardiol, 37(3), 893–899. [DOI] [PubMed] [Google Scholar]
- Hosmer DW, & Lemeshow S (1992). Confidence interval estimation of interaction. Epidemiology, 3(5), 452–456. [DOI] [PubMed] [Google Scholar]
- Hutcheon JA, Lisonkova S, & Joseph KS (2011). Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol, 25(4), 391–403. doi: 10.1016/j.bpobgyn.2011.01.006 [DOI] [PubMed] [Google Scholar]
- Jain L, Ferre C, & Vidyasagar D (1998). Racial differences in outcome of pregnancies complicated by hypertension. J Matern Fetal Med, 7(1), 23–27. doi: [DOI] [PubMed] [Google Scholar]
- Khedun SM, Maharaj B, & Moodley J (2000). Effects of antihypertensive drugs on the unborn child: what is known, and how should this influence prescribing? Paediatr Drugs, 2(6), 419–436. [DOI] [PubMed] [Google Scholar]
- Lapolla A, Dalfra MG, & Fedele D (2008). Pregnancy complicated by type 2 diabetes: an emerging problem. Diabetes Res Clin Pract, 80(1), 2–7. doi: 10.1016/j.diabres.2007.11.009 [DOI] [PubMed] [Google Scholar]
- Lin HW, Phan K, & Lin SJ (2006). Trends in off-label beta-blocker use: a secondary data analysis. Clin Ther, 28(10), 1736–1746; discussion 1710-1731. doi: 10.1016/j.clinthera.2006.10.015 [DOI] [PubMed] [Google Scholar]
- Lydakis C, Lip GY, Beevers M, & Beevers DG (1999). Atenolol and fetal growth in pregnancies complicated by hypertension. Am J Hypertens, 12(6), 541–547. [DOI] [PubMed] [Google Scholar]
- Magee LA, Singer J, & von Dadelszen P (2015). Less-tight versus tight control of hypertension in pregnancy. N Engl J Med, 372(24), 2367–2368. doi: 10.1056/NEJMc1503870 [DOI] [PubMed] [Google Scholar]
- Martin JA, Hamilton BE, Osterman MJ, Curtin SC, & Matthews TJ (2015). Births: final data for 2013. Natl Vital Stat Rep, 64(1), 1–65. [PubMed] [Google Scholar]
- McCormick MC (1985). The contribution of low birth weight to infant mortality and childhood morbidity. N Engl J Med, 312(2), 82–90. doi: 10.1056/nejm198501103120204 [DOI] [PubMed] [Google Scholar]
- McCowan L, & Horgan RP (2009). Risk factors for small for gestational age infants. Best Pract Res Clin Obstet Gynaecol, 23(6), 779–793. doi: 10.1016/j.bpobgyn.2009.06.003 [DOI] [PubMed] [Google Scholar]
- McCowan LM, Buist RG, North RA, & Gamble G (1996). Perinatal morbidity in chronic hypertension. Br J Obstet Gynaecol, 103(2), 123–129. [DOI] [PubMed] [Google Scholar]
- Meidahl Petersen K, Jimenez-Solem E, Andersen JT, Petersen M, Brodbaek K, Kober L, … Poulsen HE (2012). beta-Blocker treatment during pregnancy and adverse pregnancy outcomes: a nationwide population-based cohort study. BMJ Open, 2(4). doi: 10.1136/bmjopen-2012-001185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda ML, Swamy GK, Edwards S, Maxson P, Gelfand A, & James S (2010). Disparities in maternal hypertension and pregnancy outcomes: evidence from North Carolina, 1994-2003. Public Health Rep, 125(4), 579–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhai-Pour HR, Rey E, & Berard A (2010). Antihypertensive medication use during pregnancy and the risk of major congenital malformations or small-for-gestational-age newborns. Birth Defects Res B Dev Reprod Toxicol, 89(2), 147–154. doi: 10.1002/bdrb.20238 [DOI] [PubMed] [Google Scholar]
- Oken E, & Gillman MW (2003). Fetal origins of obesity. Obes Res, 11(4), 496–506. doi: 10.1038/oby.2003.69 [DOI] [PubMed] [Google Scholar]
- Orbach H, Matok I, Gorodischer R, Sheiner E, Daniel S, Wiznitzer A, … Levy A (2013). Hypertension and antihypertensive drugs in pregnancy and perinatal outcomes. Am J Obstet Gynecol, 208(4), 301.e301–306. doi: 10.1016/j.ajog.2012.11.011 [DOI] [PubMed] [Google Scholar]
- Overpeck MD, Hediger ML, Zhang J, Trumble AC, & Klebanoff MA (1999). Birth weight for gestational age of Mexican American infants born in the United States. Obstet Gynecol, 93(6), 943–947. [DOI] [PubMed] [Google Scholar]
- Reefhuis J, Gilboa SM, Anderka M, Browne ML, Feldkamp ML, Hobbs CA, … National Birth Defects Prevention, Study. (2015). The National Birth Defects Prevention Study: A review of the methods. Birth Defects Res A Clin Mol Teratol, 103(8), 656–669. doi: 10.1002/bdra.23384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey E, & Couturier A (1994). The prognosis of pregnancy in women with chronic hypertension. Am J Obstet Gynecol, 171(2), 410–416. [DOI] [PubMed] [Google Scholar]
- Roberts CL, Ford JB, Algert CS, Antonsen S, Chalmers J, Cnattingius S, … Weir CJ (2011). Population-based trends in pregnancy hypertension and pre-eclampsia: an international comparative study. BMJ Open, 1(1), e000101. doi: 10.1136/bmjopen-2011-000101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibai BM, Lindheimer M, Hauth J, Caritis S, VanDorsten P, Klebanoff M, … Dombrowski M (1998). Risk factors for preeclampsia, abruptio placentae, and adverse neonatal outcomes among women with chronic hypertension. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med, 339(10), 667–671. doi: 10.1056/nejm199809033391004 [DOI] [PubMed] [Google Scholar]
- Su CY, Lin HC, Cheng HC, Yen AM, Chen YH, & Kao S (2013). Pregnancy outcomes of anti-hypertensives for women with chronic hypertension: a population-based study. PLoS One, 8(2), e53844. doi: 10.1371/journal.pone.0053844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gelder MM, van Rooij IA, de Walle HE, Roeleveld N, & Bakker MK (2013). Maternal recall of prescription medication use during pregnancy using a paper-based questionnaire: a validation study in the Netherlands. Drug Saf, 36(1), 43–54. doi: 10.1007/s40264-012-0004-8 [DOI] [PubMed] [Google Scholar]
- von Dadelszen P, Ornstein MP, Bull SB, Logan AG, Koren G, & Magee LA (2000). Fall in mean arterial pressure and fetal growth restriction in pregnancy hypertension: a meta-analysis. Lancet, 355(9198), 87–92. [DOI] [PubMed] [Google Scholar]
- Wallis AB, Saftlas AF, Hsia J, & Atrash HK (2008). Secular trends in the rates of preeclampsia, eclampsia, and gestational hypertension, United States, 1987-2004. Am J Hypertens, 21(5), 521–526. doi: 10.1038/ajh.2008.20 [DOI] [PubMed] [Google Scholar]
- Xie RH, Guo Y, Krewski D, Mattison D, Walker MC, Nerenberg K, & Wen SW (2014). Beta-blockers increase the risk of being born small for gestational age or of being institutionalised during infancy. Bjog, 121(9), 1090–1096. doi: 10.1111/1471-0528.12678 [DOI] [PubMed] [Google Scholar]
- Zhang J, & Bowes WA Jr. (1995). Birth-weight-for-gestational-age patterns by race, sex, and parity in the United States population. Obstet Gynecol, 86(2), 200–208. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Reported antihypertensive medications, by class and sub-class, National Birth Defects Prevention Study, 1997-2011.


