Abstract
Background:
Head and neck cancer (HNC) mortality differs by race, ethnicity, and socioeconomic status (SES). However, it is unclear whether the relationship between race/ethnicity and HNC-specific mortality varies according to the residence-level SES.
Methods:
Data from the Surveillance Epidemiology and End Results database included participants with primary HNC between 2006 and 2017 (followed through 2018) to assess the joint association of race/ethnicity and census-tract level SES Yost-index groups (quintiles) with all-cause and HNC-specific mortalities. Relative survival rates at 1, 5, and 10 years were calculated. Multivariable Cox proportional hazard regression models estimated hazard-ratios and 95% confidence intervals for all-cause mortality, and Fine-Gray subdistribution hazard models for HNC-specific mortality. Cumulative incidence curves for HNC-specific deaths were estimated.
Results:
76,095 patients were included in the analysis: 63.2% were <65 years, 73.4% male, and 11.3% non-Hispanic (NH) Black. Most patients (58.3%) were diagnosed at regional or distant stages and 20.6% died of HNC. The five-year relative survival rate increased with SES group, with 51.6% in the lowest SES group, and 74.1% in the highest SES group. NH-Black patients had higher risk of all-cause and HNC-specific mortality than NH-White patients, regardless of the SES group. NH-Asian/Pacific Islander and Hispanic patients had higher risk of HNC-specific mortality in some SES groups.
Conclusions:
NH-Black patients of all SES strata had significantly worse outcomes. Other factors, such as healthcare quality, may be associated with persistent disparities.
Impact:
The study highlights the persistence of significant racial disparities in HNC survival across socioeconomic categories. There is need to consider additional factors underlying these disparities.
Introduction
Cancers of the head and neck (HNC) account for ∼4% of all primary cancers in the United States (1). In 2022, approximately 66,000 new cases and 15,000 HNC-related deaths are estimated in the United States (2). The overall survival outcomes among HNC have improved in the past decades. The 5-year relative survival rate increased from 54.7% in 1992 to 1996 to 65.9% in 2002 to 2006 (3), largely due to improved diagnostic techniques, advancements in treatment modalities, and involvement of multidisciplinary teams in cancer care (3–6). However, not all patients appear to benefit equally from advanced treatments. For example, compared with the non-Hispanic (NH) White patients, racial and ethnic minorities are more likely to be diagnosed with late-stage cancers, experience inadequate care, and lack treatment adherence (7). White patients with HNC are more likely to receive life-saving treatments, such as surgery plus radiation and/or chemotherapy. In contrast, Black patients are more likely to be treated with only radiation and/or chemotherapy (8). Thus, differences in HNC outcomes may be attributed to a combination of multiple factors that result in higher mortality among racial/ethnic minorities (8, 9).
Racial and ethnic disparities for many cancers are widened by individual and residential area-level socioeconomic status (SES; refs. 10, 11). Specifically, individual-level factors such as low income, lack of education, cultural barriers, and lack of insurance are major contributing factors to unequal access to care, amongst minority populations (12). In a large study that examined SES and racial and ethnic disparities in all cancers, combined, and major cancers from 1950 to 2014, survival was significantly lower in more deprived neighborhoods and among most ethnic-minority groups (13).
In the past few decades, few studies have investigated the association between SES and HNC survival (14–17). Area-level measures of SES include neighborhood disadvantages (e.g., poverty, local health-related businesses, household crowding, etc.), which have been shown to reduce access to care for treatment and screening services (18, 19), ultimately leading to poorer health outcomes (20–22).
An important first step towards achieving cancer survivorship equity is to better understand how race/ethnicity and SES status interact. To address this, we used data from the Surveillance Epidemiology and End Results (SEER) Cancer Registry database to evaluate the joint association of race/ethnicity and census-tract-level SES with relative survival rates and all-cause and HNC-specific mortality. We hypothesized that census-tract-based socioeconomic factors play a significant role in persistent racial and ethnic disparities in patients with HNC. Our study may help inform clinicians and health policy officials of the need to increase access to and quality of healthcare for patients from racial and ethnic minorities and low-SES areas to improve survival.
Materials and Methods
Data source and study population
The data for this study were obtained from the publicly available SEER database of the NCI. We downloaded SEER Datasets and Software (RRID:SCR_006902; ref. 23) version 8.4.0 (http://seer.cancer.gov/seerstat/) and acquired specialized SEER Research Plus Data (Specialized with Census Tract SES/Rurality), 18 Registries (excl AK), Nov 2020 Sub (2006–2018), which covers ∼28% of the U.S. population (24). Patients with HNC were defined as adults aged ≥20 years diagnosed between 2006 and 2017 (followed through 2018) with primary cancer of the oral cavity, pharynx (hypopharynx, nasopharynx, oropharynx), salivary glands, nasal cavity, middle ear, and larynx. Although cases diagnosed in 2018 were available, they were not included to allow at least one year of survival. We excluded patients (n = 389) who were identified as American Indian/Alaska Native due to small sample size, those with missing Yost Index (n = 5,834). In addition, we excluded patients with a follow-up time of <12 months (n = 15,652). The characteristics of 15,652 excluded patients are shown in Supplementary Table S1 and the characteristics of patients with missing Yost Index in Supplementary Table S2. We defined HNC cases using the International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3) site (25). This study was exempted by the Institutional Review Board of the University of Florida because the analysis was based on publicly available data. Data were abstracted from the SEER*Stat version 8.4.0.
Demographic variables and clinical characteristics were obtained from the SEER database (24). Self-reported race/ethnicity were categorized into NH [NH-White, NH-Black, NH-Asian Pacific Islander (Asian/PI), and Hispanic (all races)]. Hereafter, we will exclude the NH prefix when referring to racial groups. Demographic variables included age at diagnosis (20–49, 50–64, 65–79, and ≥ 80 years) and sex (female/male). The tumor stage was classified according to the SEER Summary stage as localized, regional [regional by direct extension, regional lymph nodes, regional by both direct extension and lymph node involvement, regional (not otherwise specified)], distant [distant site(s)/node(s) involved], and unstaged/unknown (26). The histology type was categorized according to the histology recode broad groupings, into squamous cell carcinoma (8050–8089), adenomas/adenocarcinomas (8140–8389), and other histologic types (8000–8009, 8010–8049, 8090–8139, 8390- 9589; ref. 24). Other demographic variables included marital status (married/partnered vs. never married/divorced/separated/widowed/unknown), and treatment received, which was defined as receipt of surgery (yes/no), receipt of radiotherapy (yes/no), and receipt of chemotherapy (yes/no/unknown). The years of HNC diagnosis were categorized as 2006 to 2009, 2010 to 2013, and 2014 to 2017.
Census-tract SES
We used the Census-tract SES Yost index as the SES measure, as defined by Yost and colleagues (2001), with higher scores corresponding to higher SES (27). The index is based on seven measures: education index, median household income, percent unemployed, percent below 150% of the poverty line, percent working class, median house value, and median rent. The Yost index scores were categorized into quintiles with roughly equal proportions of the population as group 1 (lowest SES), group 2 (low-middle), group 3 (middle), group 4 (high-middle), and group 5 (highest SES). SES classification was then assigned to all cancer cases residing in the census tract at the time of cancer diagnosis (27).
HNC sites
HNC was defined by the primary sites and categorized into the oral cavity (codes C00.0-C00.9, C02.0-C02.3, C02.8, C02.9, C03.0-C06.9, and C07.9- C08.9), pharyngeal (codes C01.9, C02.4, C09.0- C10.9, C11.0- C11.9, C12.9-C13.9, C14.0-C14.8), sinonasal (C30.0-C31.9), and larynx (C32.0-C32.9; ref. 25).
Outcome
The study had two main outcomes: all-cause death and HNC-specific death through December 31, 2018. We used two variables to identify death: vital status and SEER cause-specific death classification, which were combined to provide: (i) a single dichotomous variable for all-cause death and (ii) a 3-level variable each for HNC-specific death (live/censored, HNC-specific death, and competing causes of death). The follow-up time was defined as the interval from cancer diagnosis to the date of death or December 31, 2018, whichever occurred first. The patients were censored if they were alive at the end of the study period.
Statistical analysis
All statistical analyses were performed using SAS version 9.4. Patient characteristics were summarized according to the four race/ethnic groups. Among each census-tract SES Yost index group, 1-, 5-, and 10-year relative survival rates per 100,000 (with standard errors) were computed for HNC cases using the SEER*Stat (Version 8.4.0) for race/ethnicity. The relative survival rate represents the ratio of two survival rates: the survival of the population of patients with cancer (which is the observed rate) divided by the expected survival of the general population with a similar distribution of age, sex, and race during the same period using the actuarial method (28). General population mortality data used are from the National Center for Health Statistics and accessed through SEER, which is used to generate expected survival using the Ederer II method (28, 29). Multivariable Cox regression models were used to examine the joint association between race/ethnicity and SES index and the risk of all-cause mortality to estimate HRs and 95% confidence intervals (CI), evaluating each SES group in a separate model adjusted for age, sex, marital status, stage at diagnosis, treatment (surgery, chemotherapy, and radiotherapy), histology type, and year of diagnosis. The Cox model proportionality assumption was examined using the Schoenfeld residual method (30). Patients who died from other non-HNC causes were considered competing risks. Several adjusted multivariable competing risk hazard models (Fine and Gray's sub-distribution) were used to estimate the subdistribution HR (sHR) with a 95% CI for HNC-specific mortality. Statistical significance was set at P < 0.05, and the Holm Bonferroni procedure (31) was used to preserve the family-wise type-1 error rate for multiple comparisons within each set of analyses (15 hypothesis tests in each). In addition, we examined the joint association of the SES index [reference: Group 1(lowest SES)] and race/ethnicity and the risk of all-cause and HNC-specific mortality evaluating each race/ethnicity group in separate models adjusted for the same covariates mentioned above.
Adjusted cumulative incidence function curves with 95% CIs were generated to describe the incidence over time of death (32) for HNC-specific death with a 'baseline' statement to predict the cumulative incidence for male patients, 60 to 64 years at diagnosis, married, surgically treated for localized stage HNC cancer, and regional-stage cancer separately. This analysis aimed to compare racial and ethnic groups across SES groups, using similar baseline variables.
Subgroup analyses were conducted to further explore and examine the interaction of race/ethnicity and SES for each cancer site-specific mortality to estimate the HR and 95% CIs.
Data availability
The data analyzed in this study are available from the SEER database, which is available from the NCI (https://seer.cancer.gov/data-software/).
Results
The final study sample included 76,095 individuals aged 20 years and older who had survived for ≥1 year with a median [interquartile range (IQR)] follow-up time of 4.3 (2.5–7.5) years (Supplementary Fig. S1). Table 1 presents the demographic and clinical distribution of primary HNC cases diagnosed between 2006 and 2017. Almost 60% were aged <65 years at the time of diagnosis; approximately two thirds (73.4%) were male, and the majority were White (75.3%), followed by Black (11.3%), Hispanic (6.8%), and Asian/PI (6.7%). The pharyngeal (39.3%) and oral cavities (33.8%) were the most common HNC subsites. After stratifying by race, approximately half of the Black patients (49.7% vs. 13.5%) lived in lowest SES regions and were more likely to be younger (<65 years) at diagnosis (70.9% vs. 61.4%), had laryngeal cancer (34.7% vs. 21.8%), and more likely to be diagnosed with a distant stage (22.6% vs. 13.7%), received radiotherapy (77.6% vs. 69.5%) and chemotherapy (49.9% vs. 42.9%), and were less likely to undergo surgery (43.5% vs. 55.2%) than White patients. Asian/PI patients lived in the highest SES regions (41.1%) and were more likely to be diagnosed with pharyngeal cancer (44.4% vs. 39.8%) than White patients. Our main analysis excluded patients who survived for <12 months. The overall characteristics of these excluded patients showed that 4,261 (27.2%) belonged to the lowest-SES group, and of these, 1,642 (38.5%) were Black (Supplementary Table S1). In addition, we examined the demographic and clinical characteristics by SES groups, comparing lowest versus highest SES groups, distant stage was prevalence was 18.8% versus 13.0% and were less likely to receive surgery (47.4% vs. 58.1%; Supplementary Table S3).
Table 1.
Demographic and clinical characteristics of patients with primary HNC by race/ethnicity.
| Characteristic N (%) | Overall N = 76,095 | NH-Black 8,595 (11.3) | Hispanic 5,136 (6.8) | NH-Asian/PI 5,087 (6.7) | NH-White 57,277 (75.3) |
|---|---|---|---|---|---|
| Age groups, year | |||||
| 20–49 years | 12,281 (16.1) | 1,566 (18.2) | 1,179 (23.0) | 1,471 (28.9) | 8,065 (14.1) |
| 50–64 years | 35,814 (47.1) | 4,531 (52.7) | 2,234 (43.5) | 1,959 (38.5) | 27,090 (47.3) |
| 65–79 years | 22,494 (29.6) | 2,166 (25.2) | 1,404 (27.3) | 1,294 (25.4) | 17,630 (30.8) |
| 80+ years | 5,506 (7.2) | 332 (3.9) | 319 (6.2) | 363 (7.1) | 4,492 (7.8) |
| Sex | |||||
| Female | 20,280 (26.6) | 2,429 (28.3) | 1,475 (28.7) | 1,663 (32.7) | 14,713 (25.7) |
| Male | 55,815 (73.4) | 6,166 (71.7) | 3,661 (71.3) | 3,424 (67.3) | 42,564 (74.3) |
| Marital status | |||||
| Married or Partnered | 41,337 (54.3) | 2,900 (33.7) | 2,614 (50.9) | 3,456 (67.9) | 32,367 (56.5) |
| Other | 34,758 (45.7) | 5,695 (66.3) | 2,522 (49.1) | 1,631 (32.1) | 24,910 (43.5) |
| SES index (quintiles) | |||||
| Group 1 (lowest) | 13,558 (17.8) | 4,270 (49.7) | 1,199 (23.4) | 362 (7.6) | 7,727 (13.5) |
| Group 2 (low-middle) | 13,128 (17.3) | 1,751 (20.4) | 1,102 (21.5) | 527 (10.4) | 9,748 (17.0) |
| Group 3 (middle) | 13,621 (17.9) | 1,083 (12.6) | 959 (18.7) | 781 (15.4) | 10,798 (18.9) |
| Group 4 (high-middle) | 16,129 (21.2) | 919 (10.7) | 946 (18.4) | 1,328 (26.1) | 12,936 (22.6) |
| Group 5 (highest) | 19,659 (25.8) | 572 (6.7) | 930 (18.1) | 2,089 (41.1) | 16,068 (28.1) |
| Primary site | |||||
| Oral cavity | 25,782 (33.8) | 2,181 (25.3) | 1,880 (36.6) | 2,024 (39.8) | 19,697 (34.4) |
| Pharyngeal | 29,901 (39.3) | 3,083 (35.9) | 1,764 (34.3) | 2,259 (44.4) | 22,795 (39.8) |
| Sinonasal | 3,173 (4.2) | 353 (4.1) | 307 (6.0) | 250 (4.9) | 2,263 (4.0) |
| Larynx | 17,239 (22.7) | 2,978 (34.7) | 1,185 (23.1) | 554 (10.9) | 12,522 (21.8) |
| Stage | |||||
| Localized | 29,147 (38.3) | 2,861 (33.3) | 1,972 (38.4) | 1,804 (35.5) | 22,510 (39.3) |
| Regional | 32,648 (42.9) | 3,545 (41.2) | 2,065 (40.2) | 2,031 (39.9) | 25,007 (43.7) |
| Distant | 11,733 (15.4) | 1,938 (22.6) | 876 (17.1) | 1,066 (21.0) | 7,853 (13.7) |
| Unknown | 2,567 (3.4) | 251 (2.9) | 223 (4.8) | 186 (3.7) | 1,907 (3.3) |
| Histology type | |||||
| Squamous | 64,884 (85.3) | 7,203 (83.8) | 4,065 (79.2) | 3,481 (68.4) | 50,135 (87.6) |
| Adenoma/Adeno | 2,823 (3.7) | 348 (4.1) | 277 (5.4) | 285 (5.6) | 1,913 (3.3) |
| Other type | 8,388 (11.0) | 1,044 (12.1) | 794 (15.4) | 1,321 (26.0) | 5,229 (9.1) |
| Surgery | |||||
| Yes | 40,970 (53.8) | 3,741 (43.5) | 2,961 (57.7) | 2,665 (52.4) | 31,603 (55.2) |
| No | 35,125 (46.2) | 4,854 (56.5) | 2,175 (42.4) | 2,422 (47.6) | 25,674 (44.8) |
| Radiotherapy | |||||
| Yes | 53,506 (70.3) | 6,667 (77.6) | 3,478 (67.7) | 3,542 (69.6) | 39,819 (69.5) |
| No | 22,589 (29.7) | 1,928 (22.4) | 1,658 (32.3) | 1,545 (30.4) | 17,458 (30.5) |
| Chemotherapy | |||||
| Yes | 33,424 (43.9) | 4,285 (49.9) | 2,160 (42.1) | 2,385 (46.9) | 24,594 (42.9) |
| No/unknown | 42,671 (56.1) | 4,310 (50.1) | 2,976 (57.9) | 2,702 (53.1) | 32,683 (57.1) |
| Year of diagnosis | |||||
| 2006–2010 | 23,234 (30.5) | 2,705 (31.4) | 1,537 (29.9) | 1,520 (29.9) | 17,472 (30.5) |
| 2011–2013 | 25,796 (33.9) | 2,886 (33.6) | 1,756 (34.2) | 1,702 (33.5) | 19,452 (34.0) |
| 2014–2017 | 27,065 (35.6) | 3,004 (35.0) | 1,843 (35.9) | 1,865 (36.7) | 20,353 (35.5) |
| Years of follow-up | |||||
| Median (IQR) | 4.3 (2.5–7.5) | 3.6 (1.8–6.8) | 4.2 (2.0–7.3) | 4.3 (2.2–7.6) | 4.4 (2.3–7.5) |
Oral cavity, oral cavity, and salivary gland tumors; pharyngeal, oropharyngeal, nasopharynx, and hypopharynx; sinonasal, nasal cavity, and paranasal sinus; marital status other; never married/divorced/separated/widowed/unknown.
Abbreviation: PI, Pacific Islander.
One-, 5-, and 10-year, relative cumulative survival rates are presented according to race/ethnicity and census-tract SES groups in Table 2. The overall 1-, 5-, and 10-year survival rates on SES showed an increasing pattern from group 1 (lowest) to group 5 (highest) SES. All racial groups mostly followed a similar pattern of highest relative survival rate in the high SES group compared with the low SES group, but the rates were consistently lowest among Black patients.
Table 2.
One-, 5-, and 10-year relative survival rates (%) by SES and race/ethnicity.
| One-year relative survival rates (%, SE) | |||||
|---|---|---|---|---|---|
| SES index (quintiles)a | Overall | NH-Black | Hispanic | NH-Asian/PI | NH-White |
| Group 1 (lowest) | 77.2 (0.3) | 73.1 (0.6) | 79.3 (0.8) | 79.3 (1.6) | 78.8 (0.4) |
| Group 2 (low-middle) | 82.4 (0.3) | 77.6 (0.8) | 82.5 (0.8) | 83.7 (1.2) | 83.1 (0.3) |
| Group 3 (middle) | 84.1 (0.3) | 79.0 (1.0) | 83.3 (0.9) | 84.8 (1.0) | 84.6 (0.3) |
| Group 4 (high-middle) | 86.4 (0.2) | 82.7 (1.1) | 85.5 (0.8) | 86.6 (0.8) | 86.7 (0.3) |
| Group 5 (highest) | 89.9 (0.2) | 85.1 (1.3) | 88.7 (0.8) | 91.0 (0.5) | 90.0 (0.2) |
| Five-year relative survival rates (%, SE) | |||||
| SES index (quintiles)a | Overall | NH-Black | Hispanic | NH-Asian/PI | NH-White |
| Group 1 (lowest) | 51.6 (0.4) | 43.4 (0.7) | 55.5 (1.2) | 52.0 (2.2) | 55.0 (0.5) |
| Group 2 (low-middle) | 59.6 (0.4) | 50.8 (1.2) | 57.2 (1.2) | 59.5 (1.9) | 61.4 (0.5) |
| Group 3 (middle) | 63.6 (0.4) | 56.0 (1.5) | 65.4 (1.3) | 62.3 (1.5) | 64.2 (0.5) |
| Group 4 (high-middle) | 67.3 (0.4) | 61.4 (1.6) | 64.2 (1.3) | 67.1 (1.2) | 68.0 (0.4) |
| Group 5 (highest) | 74.1 (0.3) | 66.1 (2.0) | 70.5 (1.4) | 72.5 (1.0) | 74.9 (0.4) |
| Ten-year relative survival rates (%, SE) | |||||
| SES index (quintiles)a | Overall | NH-Black | Hispanic | NH-Asian/PI | NH-White |
| Group 1 (lowest SES) | 40.5 (0.5) | 32.2 (1.0) | 46.2 (1.3) | 43.5 (2.4) | 43.0 (0.8) |
| Group 2 (low-middle) | 49.1 (0.5) | 42.1 (1.5) | 49.7 (1.7) | 50.0 (2.1) | 50.0 (0.6) |
| Group 3 (middle) | 52.5 (0.5) | 45.9 (1.8) | 54.6 (2.0) | 57.4 (1.9) | 52.5 (0.6) |
| Group 4 (high-middle) | 58.2 (0.2) | 50.6 (2.3) | 56.8 (2.1) | 61.5 (1.7) | 58.5 (0.6) |
| Group 5 (highest) | 67.2 (0.5) | 57.3 (3.0) | 63.8 (2.6) | 65.4 (1.6) | 67.9 (0.6) |
aPI, Pacific Islanders.
Survival analysis
Table 3 presents the joint associations of race/ethnicity and SES with all-cause and HNC-specific mortalities. The all-cause death rate in group 1 (lowest SES) was 92.9/1,000 person-years, which was 44% higher than that in group 5 (highest SES) (52.0/1,000 person-years). Similarly, the HNC-specific death rate was highest in group 1 (56.9/1,000 person-years), which was 45% higher than that in group 5 (31.3/1,000 person-years).
Table 3.
Joint association between Race/Ethnicity and SES and Mortality.
| No. deaths | Person-years | Deaths/1,000 person-years (95% CI) | NH-Black vs. NH-White | NH-Asian/PI vs. NH-White | Hispanic vs. NH-White | |
|---|---|---|---|---|---|---|
| All-cause mortality among patients who survived ≥ 1 year | aHR and 95% CI | |||||
| SES index (quintiles) | ||||||
| Group 1 (lowest) | 5,846 | 62,914 | 92.9 (90.6–95.3) | 1.19 (1.12–1.26)* | 0.87 (0.73–1.04) | 0.88 (0.80–0.97) |
| Group 2 (low-middle) | 5,028 | 64,129 | 78.4 (76.3–80.6) | 1.19 (1.10–1.29)* | 0.99 (0.85–1.16) | 1.05 (0.94–1.16) |
| Group 3 (middle) | 4,749 | 68,817 | 69.0 (67.1–71.0) | 1.16 (1.05–1.29) | 0.87 (0.76–0.99) | 0.95 (0.85–1.07) |
| Group 4 (high-middle) | 5,232 | 83,510 | 62.7 (60.9–64.4) | 1.21 (1.08–1.35)* | 0.94 (0.85–1.05) | 1.14 (1.02–1.28) |
| Group 5 (highest) | 5,470 | 105,202 | 52.0 (50.6–53.4) | 1.25 (1.08–1.44) | 1.01 (0.92–1.11) | 0.95 (0.83–1.08) |
| P interaction = 0.018 | ||||||
| HNC-specific mortality among patients who survived ≥ 1 year | sHR and 95% CI | |||||
| Group 1 (lowest) | 3581 | 62914 | 56.9 (55.1–58.8) | 1.32 (1.23–1.43)* | 1.10 (0.89–1.36) | 1.10 (0.97–1.25) |
| Group 2 (low-middle) | 2972 | 64129 | 46.3 (44.7–48.0) | 1.30 (1.18–1.45)* | 1.20 (1.00–1.45) | 1.23 (1.09–1.40)* |
| Group 3 (middle) | 2713 | 68817 | 39.4 (38.0–40.9) | 1.19 (1.04–1.36) | 1.01 (0.85–1.20) | 1.09 (0.94–1.27) |
| Group 4 (high-middle) | 3099 | 83510 | 37.1 (35.8–38.4) | 1.34 (1.16–1.54)* | 1.14 (1.00–1.30) | 1.36 (1.18–1.57)* |
| Group 5 (highest) | 3295 | 105202 | 31.3 (30.3–32.4) | 1.37 (1.14–1.62)* | 1.18 (1.05–1.30) | 1.10 (0.94–1.30) |
| P interaction = 0.241 | ||||||
Fine–Gray models were adjusted for age, sex, marital status, tumor site, stage, surgery, radiotherapy, chemotherapy, histology, and year of diagnosis.
*Significant when multiple comparison corrections for P values were applied.
*Statistical significance was determined by post hoc Holm-Bonferroni correction for multiple comparisons.
Compared with White patients, Black patients had an increased risk of all-cause mortality among all SES groups (but only groups 1, 2, and 4 were significant at P < 0.003 when adjusted for multiple comparisons). In contrast, Asian/PI patients had a lower risk of all-cause mortality, while Hispanic patients had a higher risk of mortality in the SES group 4 [adjusted HR (aHR), 1.14; 95% CI, 1.02–1.28]. The joint association between SES and race/ethnicity was significant (P = 0.018).
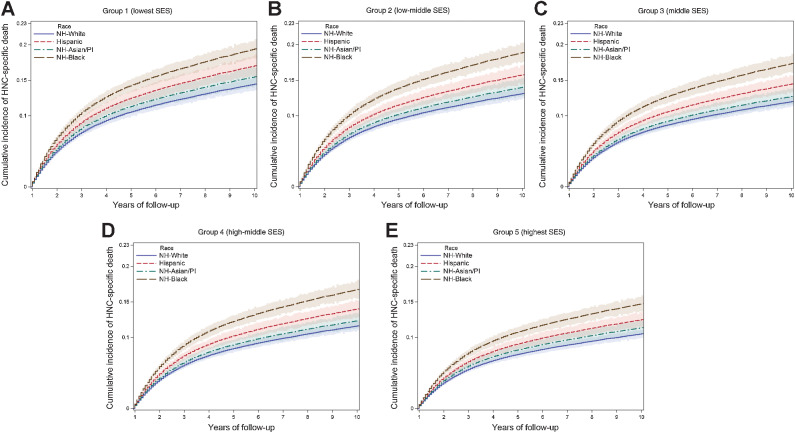
In the adjusted Fine-Gray hazard models for HNC-specific mortality (Table 3), there was no observed joint association (P = 0.241); however, Black patients had ∼30% increased mortality among four SES groups compared with White patients. The adjusted sHR for group 1 (lowest SES) was 1.32 (95% CI, 1.23–1.42) and 1.37 (95% CI, 1.14–1.62) for group 5 (highest SES). Asian/PI and Hispanic patients had higher HNC-specific mortality than White patients, but the associations were not significant, except for Hispanic patients in groups 2 (low-middle) and 4 (high-middle). These results are illustrated in the cumulative incidence curves for the lowest and highest SES groups by race/ethnicity in Fig. 1. The predicted curves in younger males (60–64 years) with localized HNC showed an increased incidence of death among Black followed by Hispanic patients, followed by Asian/PI, as compared with White patients in all SES groups. Using the same baseline variables as the regional stage HNC, the cumulative survival curves showed an increased risk, but similar patterns in all SES groups (Supplementary Fig. S2). The Supplementary Table S4 presents cancer mortality HRs for each race/ethnic subgroup. There was a dose–response effect seen with lower HRs with an increase in each SES group compared with group 1 (lowest SES) in all race/ethnic subgroups except the Hispanic.
Figure 1.
Cumulative incidence curves for the HNC-specific death by race/ethnicity and SES. The predicted curves were based on male participants who are 60 to 64 years old at diagnosis, married, surgically treated for localized stage HNC cancer, and survived for at least 12 months. Abbreviations: PI, Pacific Islander. A, Cumulative incidence curves for the HNC-specific death by Race/Ethnicity and Group 1(lowest SES); B, Cumulative incidence curves for the HNC-specific death by Race/Ethnicity and Group 2 (low-middle SES); C, Cumulative incidence curves for the HNC-specific death by Race/Ethnicity and Group 3 (middle SES); D, Cumulative incidence curves for the HNC-specific death by Race/Ethnicity and Group 4 (high-middle SES); E, Cumulative incidence curves for the HNC-specific death by Race/Ethnicity and Group 5 (highest SES).
Survival analysis by HNC subsites
Furthermore, we examined the four cancer subtypes individually (Supplementary Tables S5–S6). Similar to the results stated above, the all-cause and HNC-specific death rates were higher in group 1 and lower in the higher SES groups. The disparity in pharyngeal cancer death rate was widest, 67.6 deaths/person-years in the lowest SES group versus 28.6/person-years in the highest SES group. There was increased mortality due to all-cause and pharyngeal-specific deaths (Supplementary Table S5b) among four SES groups in Black patients and the lower-middle SES group among Hispanic patients, compared with their White counterparts.
Discussion
Using the population-based SEER dataset, this study intended to examine the role of area-level SES in racial disparities in mortality among individuals diagnosed with HNC. We found that the survival rates increased with each SES quintile. Compared with White patients, Black patients across all SES levels have lower relative survival rates and higher risk of mortality, while Hispanic and Asian/PI patients have a higher risk of cause-specific mortality. This study contributes to the literature on survival disparities by considering the joint association between race/ethnicity and SES groups. Although a total of 76,095 individuals included in the study had survived for at least 1 year after HNC diagnosis, racial, and SES disparities persisted and increased over time.
It is well documented that minority populations are at a higher risk of all-cause and cause-specific death compared with White individuals (7, 33–35). Proffered reasons for racial disparities include differences in the advanced stage of diagnosis, suboptimal surgical care, access to health care, quality of medical care, biologic/genetic factors, comorbid conditions, exposure to carcinogens, diet, and cultural beliefs (35, 36, 37). However, we observed that Black patients had poorer outcomes in most SES groups, indicating other possible etiologic pathways. The 10-year cumulative incidence curves of HNC-specific mortality showed that disparity gaps increased dramatically after 3 years of survival in Black and Hispanic patients, and to a lesser extent in Asian/PI, compared with the White patients in all SES groups. To the best of our knowledge, this has not been demonstrated in previous research.
In the current study, all-cause and HNC-specific mortality were largely driven by pharyngeal cancers. Black patients had an increased mortality risk in most SES groups compared with White patients. In a study by Rotsides and colleagues, the authors reported that Black versus White patients (HR = 1.22; 95% CI, 1.11–1.34) and low versus high SES status (income <$38,000 vs. >$63,000; HR = 1.58; 95% CI, 1.45–1.72) were associated with worse overall survival in oropharyngeal cancer (38). However, similar to other studies (39–41), the joint association of race and SES status was not evaluated in that study. Our analysis indicates that compared with White patients, Hispanic patients had a higher risk of pharyngeal-specific mortality in the low-middle SES group, oral cavity and sinonasal-specific mortality in the high-middle SES group, and laryngeal-specific among the highest SES group. This contradicts the Hispanic Paradox of lower mortality rates compared with the White population reported in prior research (7, 39–41). By examining the census-tract level SES, we observed higher mortality among Hispanic patients, and to our knowledge, this has not been reported previously.
The current study evaluated the role of area-level SES using the Yost index, which can be moderately approximated to individual socioeconomic measures and access to healthcare (42). To our knowledge, this is the first study to use the Yost index as a measure of SES in a large number of patients to evaluate HNC-specific mortality among the racially diverse population-based SEER database. Our analysis illustrated that lower SES neighborhoods were associated with decreased survival rates in a dose-dependent manner, clearly demonstrating a socioeconomic gradient, with each increasing SES quintile associated with longer survival across all racial and ethnic groups. Prior research by Reitzel and colleagues showed an increased risk of mortality among oropharyngeal cancer patients living in the most deprived neighborhoods (17). Patients living in lower SES neighborhoods lack adequate healthcare facilities, may have less access to cancer screening, have insufficient knowledge of the signs and symptoms of cancer, and have higher levels of stress leading to poorer cancer outcomes (43–45). Notably, our analysis examining cumulative incidence curves demonstrated a consistent pattern of disparities and a higher risk of HNC-specific mortality in Black patients, followed by Hispanic and Asian/PI patients who had survived for one year or more following diagnosis. Possible explanations for disparities after treatment are associated with factors such as lack of abstinence from smoking, alcohol use, treatment-related toxicities, existing comorbidities, and inadequate survivorship care (46, 47). Massa and colleagues reported that approximately one in three head and neck squamous cell carcinoma patients died from competing causes linked to tobacco use (48).
Currently, there is a lack of evidence from real-world data concerning the optimal surveillance period for HNC survivors. HNC survivors are at high risk of second primary cancers and other smoking-related comorbidities because 70% to 80% of HNC cases are associated with prior tobacco use (49). In a recently published secondary analysis of the National Lung Screening Trial study participants, the risk of second primary lung cancer was 2.5 times higher among HNC survivors compared with participants without a history of HNC (50). Hence, adequate tobacco cessation and surveillance, targeted interventions, and dismantling of structural racism may hold the key to reducing disparities (48, 51, 52). We were unable to estimate these aspects in our current analysis due to the lack of data in the SEER database, however, we believe the influence of residential SES on access to quality healthcare before, during, and after treatment is demonstrated by substandard survivorship care (53) and inferior cancer outcomes (54). Approximately 50% of Black patients belonged to low SES, which highlights the importance of addressing policies on financial hardship due to cancer treatment as a key to promoting cancer health equity. For instance, one study demonstrated that Medicaid expansion in Louisiana improved access to care and potential clinical courses for Louisiana residents diagnosed with HNC (55). Other dimensions of access are also relevant for HNC quality survivorship care, including accessibility (distance and transportation), availability (density of oncologists or specialists, hospital quality), and acceptability (trust and cultural competency), and a better understanding of these dimensions can help identify interventional opportunities to reduce disparities (56).
Strengths and limitations
The strengths of this study include the large sample size from a population-based database that enabled the examination of differences by race/ethnicity and SES groups in the United States. In addition, the large sample size allowed for stratification by HNC subtype, and the use of SEER data ensured that all variables were standardized. However, our study has certain limitations. First, although census-tract SES has been validated as a solid measure of neighborhood SES, we were unable to account for individual-level variation, which could lead to a potential misclassification using area-based measures (22). Also, the census tract SES measures were taken at the time of diagnosis, and these measures can change between diagnosis and mortality (11). In addition, the classification of race/ethnicity was based on a race recode variable with a combined group for NH-Asian Americans and NH-Pacific Islanders (24), and the aggregation may mask variation in poorer health outcomes, predominantly amongst NH-Pacific Islanders (57, 58). Furthermore, key variables such as insurance, comorbidities, tobacco use, HPV status, and alcohol use were not available. Despite these limitations, our study provides information on SES and its relationship with survival using a composite measure rather than a single measure of SES.
Conclusion
This is the first U.S. population-based study to characterize the joint association between census-tract-based SES and race/ethnicity in HNC mortality. Compared with other race/ethnic groups, Black patients had significantly worse survival outcomes across all SES strata compared with NH-White patients. By restricting the sample to patients who survived at least 1 year after diagnosis, the current study highlights the critical importance of long-term surveillance and cancer care for patients diagnosed with HNC. Our study emphasizes the importance of using an "intersectional lens" to design multi-level interventions and implement policies to improve HNC screening, diagnosis, treatment, and specifically survivorship for racial and ethnic minorities and lowest-SES residential areas.
Supplementary Material
Figure S1 shows flowchart of study participant selection.
Figure S2 shows cumulative incidence curves by socioeconomic status for the HNC-specific death by race/ethnicity.
Table S1 shows descriptive statistics of primary head and neck cancer patients with survival time <12 Months by race/ethnicity.
Table S2 shows demographic and clinical characteristics of patients with primary head and neck cancer by race/ethnicity with missing socioeconomic status.
Table S3 shows demographic and clinical characteristics of patients with primary head and neck cancer by socioeconomic status.
Table S4 shows the joint association between race/ethnicity and socioeconomic status and mortality.
Table S5a shows the Cox proportional hazards model for oral cavity cancer, stratified by race/ethnicity and socioeconomic status. Table S5b shows the Cox proportional hazards model for pharyngeal cancers, stratified by race/ethnicity and socioeconomic status.
Table S6a shows the Cox proportional hazards model for laryngeal cancer, stratified by race/ethnicity and socioeconomic status. Table S6b shows the Cox proportional hazards model for sinonasal cancers, stratified by race/ethnicity and socioeconomic status.
Acknowledgments
D. Braithwaite received NIH R01CA249506. T. Akinyemiju received NIH R37CA233777. K.M. Fredenburg received DOD CA210915 and NIH U54CA233465.
The authors acknowledge the helpful assistance provided by the SEER team. The study was internally funded through the University of Florida Health Cancer Center.
The publication costs of this article were defrayed in part by the payment of publication fees. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This article is featured in Highlights of This Issue, p. 463
Footnotes
Note: Supplementary data for this article are available at Cancer Epidemiology, Biomarkers & Prevention Online (http://cebp.aacrjournals.org/).
Authors' Disclosures
No disclosures were reported.
Authors' Contributions
S.D. Karanth: Conceptualization, formal analysis, methodology, writing–original draft, writing–review and editing. T. Akinyemiju: Conceptualization, supervision, methodology, writing–review and editing. C.J. Walker: Formal analysis, writing–review and editing. D. Yang: Formal analysis, writing–review and editing. C.A. Migliorati: Writing–review and editing. H.S. Yoon: Writing–review and editing. Y.R. Hong: Writing–review and editing. C.J. Washington: Writing–original draft, writing–review and editing. C. Lattimore: Writing–review and editing. K.M. Fredenburg: Writing–review and editing. D. Braithwaite: Conceptualization, supervision, funding acquisition, writing–review and editing.
References
- 1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin 2021;71:7–33. [DOI] [PubMed] [Google Scholar]
- 2. Head and Neck Cancer: Statistics 2022. Available from:https://www.cancer.net/cancer-types/head-and-neck-cancer/statistics.
- 3. Pulte D, Brenner H. Changes in survival in head and neck cancers in the late 20th and early 21st century: a period analysis. Oncologist 2010;15:994–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shang C, Feng L, Gu Y, Hong H, Hong L, Hou J. Impact of multidisciplinary team management on the survival rate of head and neck cancer patients: a cohort study meta-analysis. Front Oncol 2021;11:630906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Calais G, Alfonsi M, Bardet E, Sire C, Germain T, Bergerot P, et al. Randomized trial of radiation therapy versus concomitant chemotherapy and radiation therapy for advanced-stage oropharynx carcinoma. J Natl Cancer Inst 1999;91:2081–6. [DOI] [PubMed] [Google Scholar]
- 6. Budach W, Hehr T, Budach V, Belka C, Dietz K. A meta-analysis of hyperfractionated and accelerated radiotherapy and combined chemotherapy and radiotherapy regimens in unresected locally advanced squamous cell carcinoma of the head and neck. BMC Cancer 2006;6:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Taylor DB, Osazuwa-Peters OL, Okafor SI, Boakye EA, Kuziez D, Perera C, et al. Differential Outcomes among survivors of head and neck cancer belonging to racial and ethnic minority groups. JAMA Otolaryngol Head Neck Surg 2022;148:119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ragin CC, Langevin SM, Marzouk M, Grandis J, Taioli E. Determinants of head and neck cancer survival by race. Head Neck 2011;33:1092–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Woods LM, Rachet B, Coleman MP. Origins of socioeconomic inequalities in cancer survival: a review. Ann Oncol 2006;17:5–19. [DOI] [PubMed] [Google Scholar]
- 10. Von Behren J, Abrahão R, Goldberg D, Gomez SL, Setiawan VW, Cheng I. The influence of neighborhood socioeconomic status and ethnic enclave on endometrial cancer mortality among Hispanics and Asian Americans/Pacific Islanders in California. Cancer Causes Control 2018;29:875–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sangaramoorthy M, Shariff-Marco S, Conroy SM, Yang J, Inamdar PP, Wu AH, et al. Joint associations of race, ethnicity, and socioeconomic status with mortality in the multiethnic cohort study. JAMA Netw Open 2022;5:e226370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chornokur G, Amankwah EK, Schildkraut JM, Phelan CM. Global ovarian cancer health disparities. Gynecol Oncol 2013;129:258–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Singh GK, Jemal A. Socioeconomic and racial/ethnic disparities in cancer mortality, incidence, and survival in the United States, 1950–2014: over six decades of changing patterns and widening inequalities. J Environ Public Health 2017;2017:2819372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Weizman B, Golan N, Ronen O. Effect of socioeconomic status on survival in patients with head and neck cancer. Head Neck 2021;43:3001–9. [DOI] [PubMed] [Google Scholar]
- 15. McDonald JT, Johnson-Obaseki S, Hwang E, Connell C, Corsten M. The relationship between survival and socioeconomic status for head and neck cancer in Canada. J Otolaryngol Head Neck Surg 2014;43:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Megwalu UC. Impact of county-level socioeconomic status on oropharyngeal cancer survival in the United States. Otolaryngol Head Neck Surg 2017;156:665–70. [DOI] [PubMed] [Google Scholar]
- 17. Reitzel LR, Nguyen N, Zafereo ME, Li G, Wei Q, Sturgis EM. Neighborhood deprivation and clinical outcomes among head and neck cancer patients. Health Place 2012;18:861–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kirby JB, Kaneda T. Neighborhood socioeconomic disadvantage and access to health care. J Health Soc Behav 2005;46:15–31. [DOI] [PubMed] [Google Scholar]
- 19. Eibner C, Sturm R. U.S.-based indices of area-level deprivation: results from HealthCare for Communities. Soc Sci Med Jan 2006;62:348–59. [DOI] [PubMed] [Google Scholar]
- 20. Brewer KC, Peterson CE, Davis FG, Hoskins K, Pauls H, Joslin CE. The influence of neighborhood socioeconomic status and race on survival from ovarian cancer: a population-based analysis of cook county, Illinois. Ann Epidemiol 2015;25:556–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Peterson CE, Rauscher GH, Johnson TP, Kirschner CV, Freels S, Barrett RE, et al. The effect of neighborhood disadvantage on the racial disparity in ovarian cancer-specific survival in a large hospital-based study in Cook County, Illinois. Front Public Health 2015;3:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kish JK, Yu M, Percy-Laurry A, Altekruse SF. Racial and ethnic disparities in cancer survival by neighborhood socioeconomic status in Surveillance, Epidemiology, and End Results (SEER) registries. J Natl Cancer Inst Monogr 2014;2014:236–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. re3data.org: surveillance epidemiology and end results; editing status 2022–06–15; re3data.org - registry of research data repositories. Available from: 10.17616/R3VK6P [DOI]
- 24. Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER Research Data, 18 Registries (2000–2018), National Cancer Institute, DCCPS, Surveillance Research Program, released April 2021, based on the November 2020 submission. [Google Scholar]
- 25. Fritz APC, Jack A, Shanmugaratnam K, Sobin L, Parkin DM, S. W. International classification of diseases for oncology. 3rd ed.World Health Organization; 2000. [Google Scholar]
- 26. Young JL. SEER summary staging manual 2000: codes and coding instructions . National Cancer Institute, National Institutes of Health; 2001. [Google Scholar]
- 27. Yost K, Perkins C, Cohen R, Morris C, Wright W. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control 2001;12:703–11. [DOI] [PubMed] [Google Scholar]
- 28. SEER*Stat Survival Exercise 1: Relative Survival . National Cancer Institute; 2022. https://seer.cancer.gov/seerstat/tutorials/survival1/webprint/ [Google Scholar]
- 29. Howlader N, Ries LA, Mariotto AB, Reichman ME, Ruhl J, Cronin KA. Improved estimates of cancer-specific survival rates from population-based data. J Natl Cancer Inst 2010;102:1584–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika 1982;69:239–41. [Google Scholar]
- 31. Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat 1979;6:65–70. [Google Scholar]
- 32. Zhang MJ, Zhang X, Scheike TH. Modeling cumulative incidence function for competing risks data. Expert Rev Clin Pharmacol 2008;1:391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Thomas GR. Racial disparity in head and neck cancer. Cancer 2021;127:2612–3. [DOI] [PubMed] [Google Scholar]
- 34. Gourin CG, Podolsky RH. Racial disparities in patients with head and neck squamous cell carcinoma. Laryngoscope 2006;116:1093–106. [DOI] [PubMed] [Google Scholar]
- 35. Goodwin WJ, Thomas GR, Parker DF, Joseph D, Levis S, Franzmann E, et al. Unequal burden of head and neck cancer in the United States. Head Neck 2008;30:358–71. [DOI] [PubMed] [Google Scholar]
- 36. Haider AH, Scott VK, Rehman KA, Velopulos C, Bentley JM, Cornwell EE 3rd, et al. Racial disparities in surgical care and outcomes in the United States: a comprehensive review of patient, provider, and systemic factors. J Am Coll Surg. 2013;216:482–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vyfhuis MAL, Suzuki I, Bentzen SM, Cullen KJ, Goloubeva OG. Adherence to guideline-concordant care and its effect on survival in black patients with head and neck cancers: a SEER-Medicare analysis. Oncologist 2021;26:579–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rotsides JM, Oliver JR, Moses LE, Tam M, Li Z, Schreiber D, et al. Socioeconomic and racial disparities and survival of human papillomavirus-associated oropharyngeal squamous cell carcinoma. Otolaryngol Head Neck Surg 2021;164:131–8. [DOI] [PubMed] [Google Scholar]
- 39. Parasher AK, Abramowitz M, Weed D, Franzmann E, Goodwin J, Hu J, et al. Ethnicity and clinical outcomes in head and neck cancer: an analysis of the SEER database. J Racial Ethn Health Disparities 2014;1:267–74. [Google Scholar]
- 40. Cruz GD, Salazar CR, Morse DE. Oral and pharyngeal cancer incidence and mortality among Hispanics, 1996–2002: the need for ethnoregional studies in cancer research. Am J Public Health 2006;96:2194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shin JY, Yoon JK, Shin AK, Blumenfeld P, Mai M, Diaz AZ. Association of insurance and community-level socioeconomic status with treatment and outcome of squamous cell carcinoma of the pharynx. JAMA Otolaryngol Head Neck Surg 2017;143:899–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Moss JL, Johnson NJ, Yu M, Altekruse SF, Cronin KA. Comparisons of individual- and area-level socioeconomic status as proxies for individual-level measures: evidence from the mortality disparities in American communities study. Population Health Metrics 2021;19:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sorice KA, Fang CY, Wiese D, Ortiz A, Chen Y, Henry KA, et al. Systematic review of neighborhood socioeconomic indices studied across the cancer control continuum. Cancer Med 2022;11:2125–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ravaghi V, Durkan C, Jones K, Girdler R, Mair-Jenkins J, Davies G, et al. Area-level deprivation and oral cancer in England 2012–2016. Cancer Epidemiol 2020;69:101840. [DOI] [PubMed] [Google Scholar]
- 45. Fong AJ, Lafaro K, Ituarte PHG, Fong Y. Association of living in urban food deserts with mortality from breast and colorectal cancer. Ann Surg Oncol 2021;28:1311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zavala VA, Bracci PM, Carethers JM, Carvajal-Carmona L, Coggins NB, Cruz-Correa MR, et al. Cancer health disparities in racial/ethnic minorities in the United States. Br J Cancer 2021;124:315–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ellis L, Canchola AJ, Spiegel D, Ladabaum U, Haile R, Gomez SL. Racial and ethnic disparities in cancer survival: the contribution of tumor, sociodemographic, institutional, and neighborhood characteristics. J Clin Oncol 2018;36:25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Massa ST, Osazuwa-Peters N, Christopher KM, Arnold LD, Schootman M, Walker RJ, et al. Competing causes of death in the head and neck cancer population. Oral Oncol 2017;65:8–15. [DOI] [PubMed] [Google Scholar]
- 49. Cohen EEW, LaMonte SJ, Erb NL, Beckman KL, Sadeghi N, Hutcheson KA, et al. American Cancer Society head and neck cancer survivorship care guideline. CA Cancer J Clin 2016;66:203–39. [DOI] [PubMed] [Google Scholar]
- 50. Cramer JD, Grauer J, Sukari A, Nagasaka M. Incidence of second primary lung cancer after low-dose computed tomography vs chest radiography screening in survivors of head and neck cancer: a secondary analysis of a randomized clinical trial. JAMA Otolaryngol Head Neck Surg 2021;147:1071–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bailey ZD, Feldman JM, Bassett MT. How structural racism works — racist policies as a root cause of U.S. racial health inequities. N Engl J Med 2020;384:768–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bailey ZD, Krieger N, Agénor M, Graves J, Linos N, Bassett MT. Structural racism and health inequities in the USA: evidence and interventions. Lancet North Am Ed 2017;389:1453–63. [DOI] [PubMed] [Google Scholar]
- 53. Massa ST, Pipkorn P, Jackson RS, Zevallos JP, Mazul AL. Access to a regular medical provider among head and neck cancer survivors. Head Neck 2020;42:2267–76. [DOI] [PubMed] [Google Scholar]
- 54. Levesque JF, Harris MF, Russell G. Patient-centred access to health care: conceptualizing access at the interface of health systems and populations. Int J Equity Health Mar 11 2013;12:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Noonan M, Pang J, Li T, Bhuiyan MAN, Nathan CAO, Yim MT. Access to care for head and neck cancer patients: the influence of expanded Medicaid in Louisiana. I Int J Radiat Oncol Biol Phys 2022;112:e28–9. [Google Scholar]
- 56. Karanth S, Fowler ME, Mao X, Wilson LE, Huang B, Pisu M, et al. Race, Socioeconomic status, and healthcare access disparities in ovarian cancer treatment and mortality: systematic review and meta-analysis. JNCI Cancer Spectr 2019;3:pkz084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bhakta S. Data disaggregation: the case of Asian and Pacific Islander data and the role of health sciences librarians. J Med Libr Assoc 2022;110:133–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wang Q, Xie H, Li Y, Theodoropoulos N, Zhang Y, Jiang C, et al. Racial and ethnic disparities in nasopharyngeal cancer with an emphasis among Asian Americans. Int J Cancer 2022;151:1291–303. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 shows flowchart of study participant selection.
Figure S2 shows cumulative incidence curves by socioeconomic status for the HNC-specific death by race/ethnicity.
Table S1 shows descriptive statistics of primary head and neck cancer patients with survival time <12 Months by race/ethnicity.
Table S2 shows demographic and clinical characteristics of patients with primary head and neck cancer by race/ethnicity with missing socioeconomic status.
Table S3 shows demographic and clinical characteristics of patients with primary head and neck cancer by socioeconomic status.
Table S4 shows the joint association between race/ethnicity and socioeconomic status and mortality.
Table S5a shows the Cox proportional hazards model for oral cavity cancer, stratified by race/ethnicity and socioeconomic status. Table S5b shows the Cox proportional hazards model for pharyngeal cancers, stratified by race/ethnicity and socioeconomic status.
Table S6a shows the Cox proportional hazards model for laryngeal cancer, stratified by race/ethnicity and socioeconomic status. Table S6b shows the Cox proportional hazards model for sinonasal cancers, stratified by race/ethnicity and socioeconomic status.
Data Availability Statement
The data analyzed in this study are available from the SEER database, which is available from the NCI (https://seer.cancer.gov/data-software/).