This safety run-in study cohort suggests that PVX2 immunotherapy is well tolerated in the target population and is sufficiently safe to warrant further clinical testing in a randomized study. The combined vaccines may facilitate higher-than-expected rate of human papillomavirus type 16 viral clearance 6 and 12 months after treatment, although this requires validation.
Abstract
Patients with human papillomavirus type 16 (HPV16) infection and low-grade cervical dysplasia [low-grade squamous intraepithelial lesion (LSIL)/CIN1] or atypical squamous cells [atypical squamous cells of undetermined significance (ASC-US)/atypical squamous cells- cannot exclude high-grade squamous intraepithelial lesion (ASC-H)] require active surveillance for disease progression. A safe and effective immunotherapy to clear HPV16 is an unmet medical need. The safety run-in cohort of a randomized double-blind, placebo-controlled phase II trial of PVX2 [vaccination twice with HPV16-targeting pNGVL4a-Sig/E7(detox)/HSP70 plasmid and once with the HPV16 L2E7E6 fusion protein “TA-CIN”] as immunotherapy for patients with HPV16+ ASC-US, ASC-H, or LSIL/CIN1 (NCT03911076) was recently completed. The primary objective of this cohort was to determine the safety and tolerability of PVX2 vaccination. Subjects were confirmed to have HPV16 infection and LSIL/CIN1, ASC-US, or ASC-H. Adverse events were evaluated using Common Terminology Criteria for Adverse Events v5.0. HPV typing by HPV16 18/45 Aptima Assay was done at baseline, month 6, and month 12, with simultaneous cytology analysis. Cervical biopsies and endocervical curettage were performed at baseline and month 6. In the safety run-in cohort 12 eligible patients were enrolled. Each received three monthly immunizations. One was lost to follow-up after week 12. There were no serious adverse events. A total of five adverse events were noted by 4 patients; 4 were considered not vaccine-related, and one ‘unlikely related’ by the investigator. At month 6, 45% (5/11) of participants converted to HPV16-negative and 2 others developed CIN2+ and received a loop electrosurgical excision procedure. At month 12, 64% (7/11) were HPV16-negative, including those HPV16-negative at month 6. In conclusion, PVX2 immunotherapy was well tolerated and associated with viral regression, supporting further testing.
Prevention Relevance:
This safety run-in study cohort suggests that PVX2 immunotherapy is well tolerated in the target population and is sufficiently safe to warrant further clinical testing in a randomized study. The combined vaccines may facilitate higher-than-expected rate of human papillomavirus type 16 viral clearance 6 and 12 months after treatment, although this requires validation.
Introduction
Globally, human papillomavirus (HPV) causes 10% of malignancies in women, and it is a necessary cause of cervical cancer. Cervical cancer is the fourth leading cancer in women worldwide, disproportionately afflicting low-resource and rural populations (1). Current primary therapies for cervical cancer include surgery and chemoradiation. For recurrent cervical cancer, despite a combination of radical surgery (including pelvic exenteration), radiation, and biologic/chemo-therapy, overall 5-year survival remains a dismal 53% (2). This situation emphasizes the need for, and promise of, cervical cancer prevention.
The effectiveness of prophylactic HPV vaccination notwithstanding, many women lack access to these vaccines or have elected not to receive them (3, 4). Cytologic screening has been highly effective in identifying cervical dysplasia or precancers (5), including CIN2/3 that are treated by surgery/ablation [e.g., loop electrosurgical excision procedure or (LEEP)], albeit with inherent risks for recurrence, major bleeding, infection, and obstetric morbidity (6). However, there are challenges in establishing national programs including the need for repeated delivery and acceptability to patients.
HPV type 16 (HPV16) alone accounts for over half of all cases of cervical malignancy, and is therefore the most important target for treatment (7). There are currently no treatments for < CIN2 lesions or < HSIL cytologic abnormalities [i.e., low-grade squamous intraepithelial lesion (LSIL), atypical squamous cells of undetermined significance (ASC-US), or atypical squamous cells of undetermined significance (ASC-US)/atypical squamous cells- cannot exclude high-grade squamous intraepithelial lesion (ASC-H)] associated with HPV16. These patients are recalled for repeat cytologic evaluation and HPV testing, and consequently suffer psychosexual stress due to frequent colposcopies, inconvenience and healthcare costs from active surveillance, and constant high risk for the development of CIN2+ and/or infecting other sites or partners (8).
Although prophylactic HPV vaccines have been available since 2006, due to its limited global use, high-risk HPV (hrHPV) infections including HPV16 remain highly prevalent (4, 9). Fortunately, most genital infections clear spontaneously without sequelae; indeed 50% of HPV16 infections in young women become undetectable within a year (10). However, a significant fraction of HPV16 infections persist, progress and manifest as intraepithelial lesions throughout the lower anogenital tract, predominantly the cervix (11). LSIL represents productive infections (12). While most such lesions will regress spontaneously, 30% to 40% of HPV16 infections will progress to high-grade squamous intraepithelial lesions (HSIL) within 2 years, a rate 5-fold greater than that of other hrHPV infections (5). Surgical resection of vaginal, vulvar and anal intraepithelial lesions, most often driven by HPV16, is similarly associated with significant morbidity and high recurrence rates (13, 14).
A third of HSILs progress to invasive disease absent intervention (15, 16). Progression is associated with integration of the viral genome, with concomitant deletion or inactivation of E2, a down regulator of E6 and E7 expression (17, 18). The HPV E6 and E7 gene products inactivate p53 and retinoblastoma proteins (pRb), respectively, and their expression is required to maintain the transformed phenotype. This consistent and obligatory expression of the non-self E6 and E7 suggests their potential as targets for immunotherapy. By contrast, the late antigens such as L2 are expressed only in the productive low-grade lesions and are targets for protective antibodies. Therefore, immunotherapeutic strategies directed toward the E6, E7, and L2 antigens have the potential to treat low-grade HPV16-associated cervical dysplasia for cancer prevention.
Mild cervical cytologic abnormalities are common, including LSIL, ASC-US, or ASC-H. Management of these diagnoses is governed by risk-based strategies impacted by age, prior HPV vaccination, and, increasingly, the presence and type of hrHPV detected (especially HPV16 and/or HPV18). Thus, in the absence of CIN2+, women with HPV16+ ASC-US, ASC-H, or LSIL endure frequent active monitoring for CIN2+, the psychologic stress from regular procedures, and high risk of progression as well as having an active oncogenic sexually transmitted infection without any curative treatment (19).
The naked DNA vaccine pNGVL4a-Sig/E7(detox)/HSP70 (pBI-1) expresses HPV16 E7 with inactivating mutations and linked to Mycobacterium tuberculosis heat shock protein 70 (HSP70) and signal peptide (Sig) to enhanced CD8 T-cell immune responses (20). pNGVL4a-Sig/E7(detox)/HSP70 vaccination in otherwise healthy women with HPV16+ CIN3 is well tolerated. A homologous DNA-DNA-DNA prime-boost regimen was assessed at three dose levels: 0.5 mg (3 patients), 1.0 mg (3 patients), and 3.0 mg (9 patients), with each dose given intramuscularly a total of 3 times at 4-week intervals. In the highest dose cohort (n = 9), 3 subjects had complete histologic regression of disease (although 2 would have been expected based upon prior studies of spontaneous regression rates). No dose-limiting or severe adverse events were observed. The most common side effect was transient, minimal injection site discomfort (20). Patients only reported grade 1 adverse events: 4 with vaccine site tenderness, 3 with malaise and flu-like symptoms, 5 with fatigue, and 2 with vaginal discomfort and discharge.
TA-CIN is a single fusion protein comprised of HPV16 L2, E7, and E6 proteins produced in bacteria. While vaccination with TA-CIN can induce L2-specific protective antibodies, it is designed to trigger HPV16-specific therapeutic immunity targeting the E6, E7, and L2. A placebo-controlled, double-blinded phase I dose-escalation study provided preliminary evidence that serial vaccination with up to 533 μg of TA-CIN for a total of three doses in the absence of an adjuvant is safe, well tolerated, and immunogenic in healthy volunteers (21). However, vaccination of patients produced only low titers of L2-specific cross-neutralizing antibodies and weak E6/E7-specific IFNγ and proliferative T-cell responses following a TA-CIN dose response (21, 22).
Heterologous prime with DNA vaccine followed by boosting with recombinant protein represents another effective strategy in eliciting CD8+ and CD4+ T-cell responses as well as antibody responses. Several recent preclinical animal studies in mice and nonhuman primates have shown that vaccination of DNA prime followed by recombinant protein boost can elicit more potent cell-mediated and humoral immune responses against a number of infectious diseases, including malaria (23) and HIV (24–26), compared with DNA vaccination alone. In preclinical experiments, intramuscular administration of pNGVL4a-Sig/E7(detox)/HSP70 DNA vaccine twice followed by boosting with TA-CIN generated significantly more HPV-specific CD8+ T-cells in mice carrying TC-1 tumor as compared with either the DNA or protein-based vaccine alone (27), and is hereafter referred to as PVX2 immunotherapy. Neither pNGVL4a-Sig/E7(detox)/HSP70 nor TA-CIN (21) demonstrated dose-limiting toxicities (DLT) in when administered individually to patients in three monthly doses (20). This first-in-human trial of the PVX2 regimen examines its safety and tolerability as well as an exploratory endpoint of efficacy in clearing HPV16 infection of the cervix in 12 patients with ASC-US, ASC-H, or LSIL.
Materials and Methods
Study design
This phase II safety run-in of clinical trial NCT03911076 was conducted at five sites in the USA and followed CONSORT guidelines. The study was performed per the moral, ethical, and scientific principles governing clinical research as set out in the Declaration of Helsinki, and the guidelines on Good Clinical Practice. The protocol was reviewed and approved by the Advarra Institutional Review Board (Columbia, MD). Study monitoring was provided by Parexel International (Newton, MA). Written informed consent was obtained from all patients.
Patients with a cytologic diagnosis of ASC-US, ASC-H, or LSIL who met the enrollment criteria were prescreened within 8 weeks of their diagnosis at which they underwent an initial informed consent process to obtain a cervical cytology sample and Aptima HPV 16 18/45 Genotype Assay testing for hrHPV and HPV16 and HPV18. A colposcopic exam with required endocervical curettage (ECC) and cervical biopsy, as clinically indicated, was obtained at the screening visit. Eligibility inclusion criteria included ability to give consent, HPV16-positive, HIV/HBV/HCV-negative, age 25 to 70, adequate organ functioning, nonpregnant status, willingness to use contraception for 6 months, and histologic diagnosis of ≤ CIN1. Exclusion criteria included pregnancy or desiring pregnancy within 6 months, breastfeeding, immunodeficiency or receiving systemic immunosuppressive therapies, receiving any blood product within 3 months, receiving any licensed vaccine within 2 weeks of treatment, administration of any investigational compound or device within 30 days, undergoing surgery, chemotherapy, radiation, or biological cancer therapy within 28 days, history of seizures, cancer history within the past 5 years, uncontrolled intercurrent illness, active autoimmune disease, prior cervical conization, LEEP or ablative procedure, or total hysterectomy.
The patients were evaluated at enrollment with vital signs, medical history, demographic data, Eastern Cooperative Oncology Group (ECOG), serum human chorionic gonadotropin, and blood draws for chemistry and disease screening. Once all eligibility criteria were met and informed consent obtained, subjects were vaccinated in the upper outer quadrant of the right buttock muscle with the PVX2 immunotherapy comprising 3-mg pNGVL4a-Sig/E7(detox)/HSP70 DNA in 1-mL PBS (Waisman Biomanufacturing, Madison, WI) at weeks 0 and 4, and 0.1-mg TA-CIN vialed in 0.53-mL phosphate/glycine/cysteine (PGC) buffer (Waisman Biomanufacturing, Madison, WI) at week 8, with vitals monitored in the hour postimmunization and a diary card provided each time.
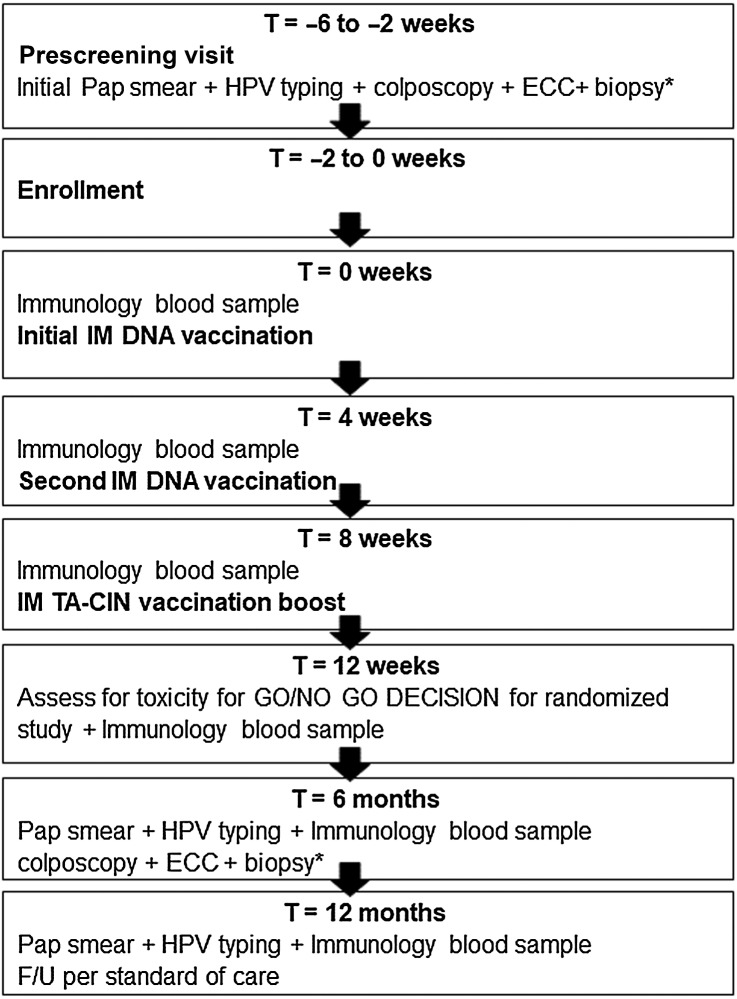
Subject follow-ups were conducted at week 12, month 6, and month 12. At each visit vital signs, adverse events, medical issue updates, blood samples, and urine samples were collected. A cervical sample was obtained for cytologic examination and HPV testing at months 6 and 12. A colposcopic exam with required ECC and cervical biopsy, as clinically indicated, was obtained at month 6. Immunology blood samples were obtained at weeks 0, 4, 8 (each time prior to vaccine administration), week 12, month 6, and month 12 visits. Subjects were monitored throughout the study with physical assessments, including self-assessment for local and systemic symptoms by diary cards after each vaccination. The study design is summarized in Fig. 1.
Figure 1.
Schematic of study design. IM, intramuscular. If less than 2 individuals (0/12 or 1/12) developed vaccine attributable adverse event (AE) of grade 3 and none experience vaccine attributable grade 4 toxicities, proceed to randomized placebo-controlled study. If ≥ 2/12 developed vaccine attributable AE of grade 3 or any experience vaccine attributable grade 4 toxicities, then DSMB determines whether to stop, and/or whether to move to the randomized placebo-controlled study. *ECC required. Biopsy/biopsies as clinically indicated and prophylactic antibiotics given.
Statistical methods
For the safety run-in, a sample size of 12 patients was selected. The sample size was selected such that the study stopping rule (2 or more DLTs) would be met with high probability if the true toxicity rate was about 20% and that progression to the next cohort would occur with high probability if the true toxicity rate was less than 3.5%. With the planned 12 vaccine recipients evaluable for the toxicity endpoint, if the true rate of toxicity is less than 3.5%, then there is at least a 0.94 probability that randomized study enrollment would occur. If the true rate of toxicity is 10%, 15%, 20%, or 30%, respectively, then there are probabilities of 0.34, 0.56, 0.73, and 0.91, respectively, to stop the randomized study enrollment.
All safety parameters were summarized and presented in tables based on this safety population. Adverse event data were presented in frequency tables (overall and by intensity) by body system. In tables showing the overall incidence of adverse events, patients who experienced the same event on more than one occasion were counted only once in the calculation of the event frequency. Laboratory data were presented as summary statistics for each sampling time point using both shift and frequency tables. All adverse events and abnormal laboratory variables are assessed according to the NCI Common Terminology Criteria for Adverse Events (CTCAE) v5.0 grading system.
Data availability
Data were generated by the authors and included in the article.
Results
Study population demographics
A total of 28 eligible female patients were screened, and 12 participants enrolled for vaccination from 5 active sites, with one participant lost to follow-up after week 12. As cervical cancer afflicts adult women, only females ≥ 18 years of age were enrolled. The study enrolled women regardless of race and gender. Enrollment occurred between June 25, 2019 and November 22, 2019, and was delayed by the COVID-19 pandemic likely due to drops in screening rates and concerns about physician visits. The primary reasons for exclusion were absence of HPV16 mRNA (n = 11), presence of HSIL, > CIN2 (n = 3), or AGUS (n = 1). The mean age of study subjects was 43.5 (Table 1). One participant (8%) was 21 to 30 years old, 6 participants were 31 to 40 years old (50%), 1 participant (8%) was 41 to 50 years old, and 4 participants (34%) were 50 to 60 years old. Eleven participants (92%) of the study were white, and 1 participant (8%) was Asian, with 5 participants (42%) identifying as Hispanic/Latino. The mean weight was 69.10 kg (min 51.5, max 94.8), body mass index 25.7 (min 18.5, max 31.8), and all subjects were ECOG status 0. Vital signs including diastolic blood pressure (Supplementary Table S1), systolic blood pressure (Supplementary Table S2), heart rate (Supplementary Table S3), respiratory rate (Supplementary Table S4) as well as blood counts and chemistry (Supplementary Table S5) are presented. The safety run-in study was a non-blinded, non-randomized single arm cohort. All enrolled subjects received all three injections, and vital signs monitored immediately before and 30 minutes after, as well as other visits (Supplementary Tables S1–S4). One subject was lost to follow-up at the 6-month mark for cytologic/histologic analyses and HPV typing, and a second subject was lost to follow-up at the 12-month mark for histologic analysis and HPV typing. Both subjects were included in the safety data analysis.
Table 1.
Baseline patient characteristics.
| Patient number | Age | Race | Ethnicity |
|---|---|---|---|
| 1002–002 | 53 | White | Not Hispanic or Latino |
| 1002–003 | 39 | White | Hispanic or Latino |
| 1003–001 | 39 | White | Not Hispanic or Latino |
| 1003–002 | 58 | White | Hispanic or Latino |
| 1003–006 | 57 | White | Not Hispanic or Latino |
| 1003–007 | 36 | White | Not Hispanic or Latino |
| 1003–008 | 48 | White | Not Hispanic or Latino |
| 1003–009 | 39 | White | Hispanic or Latino |
| 1003–015 | 32 | White | Hispanic or Latino |
| 1004–001 | 39 | Asian | Not Hispanic or Latino |
| 1004–002 | 53 | White | Not Hispanic or Latino |
| 1004–003 | 29 | White | Hispanic or Latino |
Safety and tolerability of PVX2
The first primary objective of this study was to evaluate the safety and tolerability of the PVX2 immunization regimen. No serious adverse events or toxicities were observed associated with this PVX2 immunotherapy. There were 5 adverse events during the safety run-in trial, and all were categorized as CTCAEv5.0 grade 1 or 2 (Table 2). Four subjects reported at least one adverse event. Four of the five total adverse events were determined to be not related to PVX2 vaccination (vaginal irritation, rash, back pain, and yeast infection). The remaining adverse event was determined by the investigator to be ‘unlikely related’ to PVX2 vaccination (influenza). All adverse events were managed per standard clinical practice and were resolved by the conclusion of the study.
Table 2.
New adverse events.
| Adverse event | Number reporting by grade | Relationship to study treatment | Outcome | |||
|---|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |||
| Infections and infestations | ||||||
| Influenza | 1 | 0 | 0 | 0 | Unlikely related | Recovered/Resolved |
| Yeast Infection | 1 | 0 | 0 | 0 | Not related | Recovered/Resolved |
| Reproductive system and breast disorders | ||||||
| Vulvovaginal discomfort | 1 | 0 | 0 | 0 | Not related | Recovered/Resolved |
| Musculoskeletal and connective tissue disorders | ||||||
| Back pain | 1 | 0 | 0 | 0 | Not related | Recovered/Resolved |
| Skin and subcutaneous tissue disorders | ||||||
| Dermatitis contact | 1 | 0 | 0 | 0 | Not related | Recovered/Resolved |
Changes in HPV16 infection
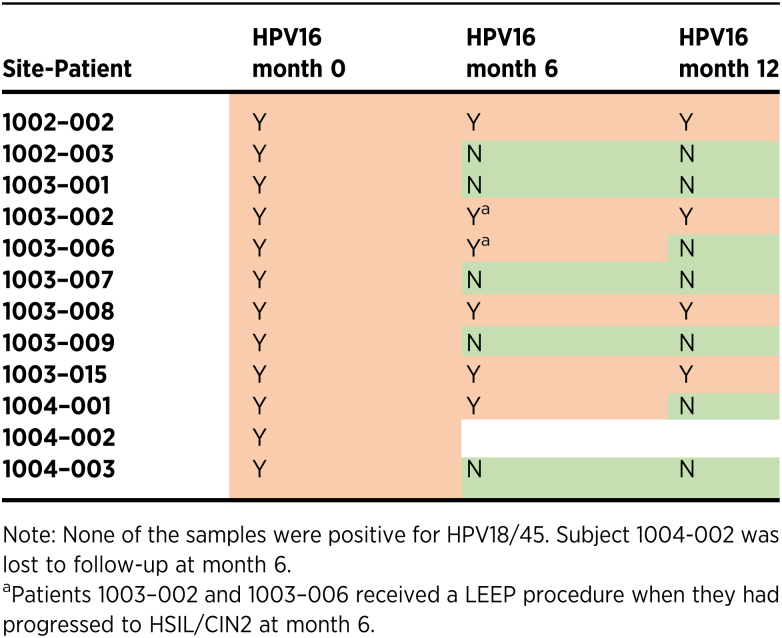
An exploratory objective of this cohort was to evaluate the efficacy of dual IM pNGVL4a-Sig/E7(detox)/HSP70 DNA and single IM TA-CIN immunization regimen on HPV16 clearance by month 6. HPV typing using the Aptima HPV 16 18/45 Genotype Assay was conducted at week 0, month 6, and month 12. At week 0, all 12 enrolled patients tested positive for HPV16. Each patient then received PVX2 immunotherapy on weeks 0, 4, and 8. Upon completion of immunotherapy, all enrolled patients were tested for HPV16 at month 6. One of the 12 subjects was lost to follow-up at month 6. Five (45%) of the remaining 11 subjects tested negative for HPV16 at month 6 (Table 3), while the other 6 subjects remained HPV-positive. Two of these 11 subjects (1003–002 and 1003–006) developed HSIL (CIN2) and both subjects underwent LEEP per standard of care.
Table 3.
HPV16 testing data.
The 11 subjects who completed PVX2 immunotherapy and HPV16 typing at months 0 and 6 were subsequently tested at month 12 (Table 3). At month 12, 7 (64%) of the 11 subjects tested HPV16-negative, including one who received LEEP at month 6. All of the 5 subjects who tested negative for HPV16 at month 6 remained HPV16-negative at month 12. Four (36%) of the 11 subjects remained HPV16-positive at month 12, including 1 who received LEEP at month 6. None of the subjects tested positive for either HPV18 or HPV45 at any time point, but positivity for another HPV was not confirmed nor excluded.
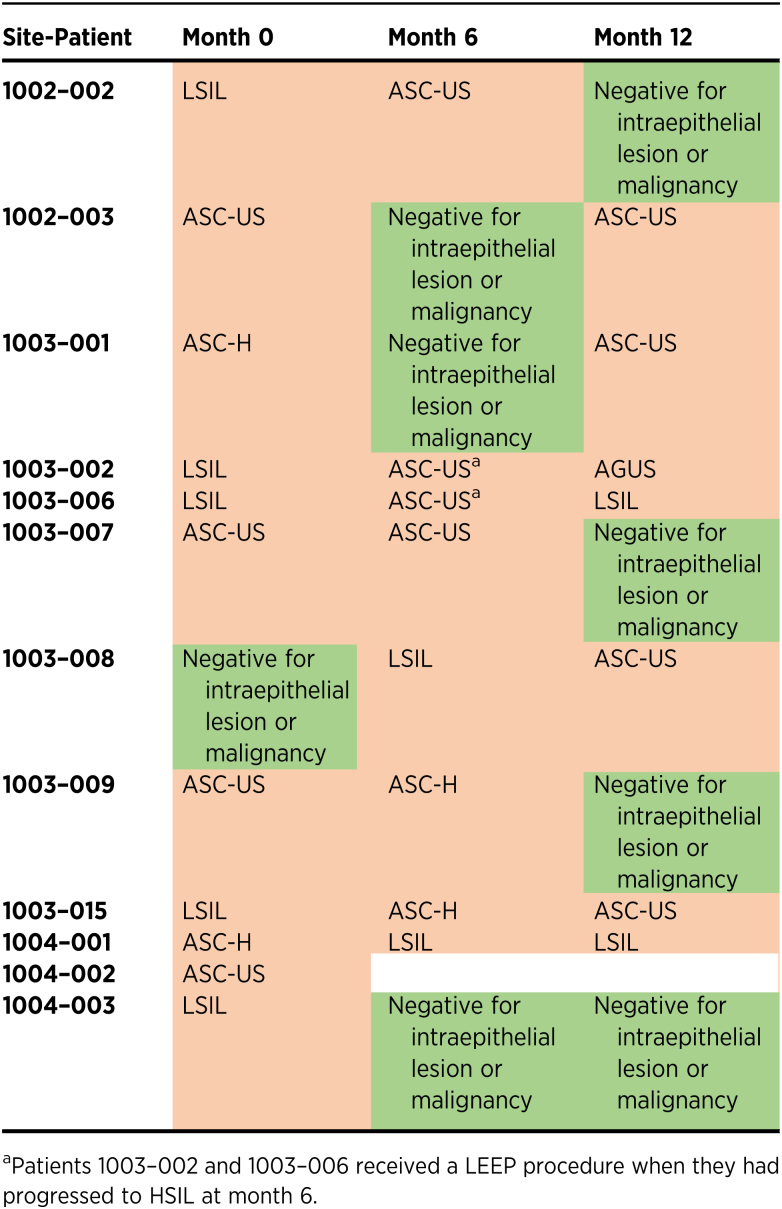
Changes in cervical cytology
Following PVX2 immunotherapy, cervical cytology of all subjects was assessed at months 6 and 12. By month 6, 3 (27%) of 11 subjects went from abnormal cytology to normal cytology (negative for intraepithelial lesion or malignancy, a.k.a. NILM), and the observed cytologic regression correlated with conversion of HPV16 status [i.e., all these 3 subjects whose cytology reverted to normal at month 6 (Table 4) tested HPV-negative at month 6 (Table 3)]. The other 2 subjects who tested HPV-negative at month 6 still had abnormal cytology at month 6, but then reverted to normal cytology at month 12. Overall at month 12, 4 (36%) of 11 subjects had normal cytology (Table 4), and 3 of these 4 subjects tested HPV16-negative with the 1 remaining subject still testing HPV16-positive at month 12 despite her normal cytology. On the other hand, 4 subjects who tested HPV16-negative at month 12 still had mild abnormal cytologic abnormalities. Of the 3 subjects who had normal cytology at month 6, 2 had ASC-US cytology, and 1 still had normal cytology (Table 4).
Table 4.
Cytologic testing data.
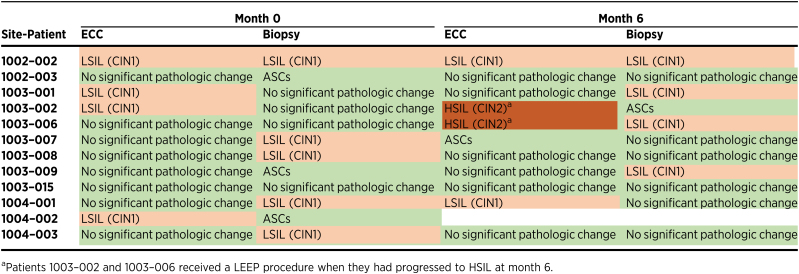
Changes in cervical histology
After PVX2 vaccination at month 6, subjects underwent histologic assessment with an ECC and colposcopically directed cervical biopsy that was compared with the same obtained at the time of enrollment (month 0) by a central panel of gynecologic pathologists. At month 6, 2 (18%) of the 11 evaluable subjects demonstrated histologic progression from normal or CIN1 to CIN2 in their ECC specimens (1003–002 and 1003–006; Table 5). Both of these subjects were HPV16-positive at month 6 (Table 3) and were treated with a LEEP. Neither of the 2 subjects with progression to CIN2 in ECC showed CIN2 in their cervical biopsies, suggesting that their dysplastic lesions were located in the endocervix (Table 5). Another subject (1002–002) had LSIL/CIN1 on ECC and biopsy at months 0 and 6 (Table 5), and was HPV16-positive at both time points (Table 3).
Table 5.
Histologic data.
Of the remaining 8 subjects with ECC samples at months 0 and 6, 5 did not show significant histopathologic changes at either time point (1002–003, 1003–008, 1003–009, 1003–015 and 1004–003). One subject's ECC evaluation changed from no pathologic change at month 0 to presence of atypical squamous cells (ASC) at month 6 (1003–007), but the biological significance of such ASCs is poorly defined. One patient showed no significant pathologic change in ECC but demonstrated LSIL/CIN1 in biopsy at month 0 (1004–001). The patient developed LSIL/CIN 1 in ECC at month 6. The remaining 1 patient showed ECC histologic regression from LSIL/CIN1 at month 0 to no significant pathologic changes at month 6 but her biopsy demonstrated LSIL/CIN1 (1003–001).
In addition to ECC, subjects’ cervical biopsies at months 0 and 6 were examined and compared. Three subjects (27%) of the 11 subjects had normal cervical biopsy and normal ECC at month 6 after having CIN1 (1003–008 and 1004–003) or ASCs (1002–003) in either ECC or biopsy specimen type at month 0 (Table 5). Two of these 3 subjects (1002–003 and 1004–003) tested HPV16-negative at month 6 (Table 3). Interestingly, 1 subject who was originally negative in ECC with cervical biopsy showing ASCs at month 0 later displayed CIN1 in cervical biopsy (1003–009) and tested HPV16-negative at month 6 suggesting infection with another type. Of the 7 subjects who had no significant pathologic changes or ASCs in cervical biopsies at month 0, 2 later displayed normal histology (1002–003 and 1003–015) and 1 had ASCs (1003–002), and 3 CIN1 in cervical biopsy at month 6 (1003–001, 1003–006 and 1003–009), and the other was lost to follow-up (1004–002).
Of the 6 subjects who were HPV16-positive at month 6, the highest histopathologic diagnosis was HSIL/CIN2 in 2 subjects, LSIL/CIN1 in 2 subjects and no significant pathologic findings were noted in the other 2 subjects. However, these latter 2 subjects were found to still have abnormal cytology [LSIL (1003–008) and ASC-H (1003–015); Table 4]. Five of the subjects became HPV16-negative at month 6; of these, 2 had no significant pathologic findings on either ECC or biopsy, and 1 only had ASCs. Two others had LSIL/CIN1 at month 6 suggesting a possible infection with a non-16/18/45 HPV type.
Discussion
PVX2 is a heterologous prime-boost immunotherapy regimen as it induces therapeutic HPV16-specific cellular immune responses in a mouse model of cervical cancer (27) and therefore has promise for the treatment of HPV16 infections and low-grade disease. Important for this otherwise generally healthy patient population, in this small safety run-in cohort, PVX2 was well tolerated by all 12 study subjects with only 5 mild likely non–vaccine-related adverse events reported.
An important exploratory endpoint for this cohort was to evaluate the clearance of HPV16 infection by month 6 (i.e., 4 months after the last vaccination). For women with HPV16 infection and normal cervical cytology, approximately 34% of cases will spontaneously resolve by month 6 (11). However, for women with HPV16 infection and abnormal cervical cytology, such as those in our study cohort, the rate of spontaneous viral clearance is significantly lower; in a large prior study, women with HPV16 infection and LSIL/ASC-US/ASC-H showed a spontaneous viral clearance rate of only 9% at 6 months after initial diagnosis (11). In the current study, of the 11 evaluable subjects with abnormal cervical cytology and HPV16 infection at month 0, 5 (45%) had cleared their HPV16 infection within 6 months of initiating PVX2 immunotherapy (Table 3). Importantly all 5 of these subjects remained HPV16-negative at month 12, suggesting a durable immunity. Further, by month 12, 2 more immunized subjects had cleared their HPV16 infection (Table 3), although 1 also received LEEP.
Two major limitations of our report are the lack of a control/placebo cohort and the small number of tested subjects, both of which will be addressed in an upcoming randomized double-blinded cohort. The attribution of adverse events is a subjective assessment highly dependent upon specialized training, and this is limitation of our single arm study. In addition, it is possible that the biopsies taken during the trial could be considered an intervention leading to increased clearance rates. Another limitation of the study is the limited of racial/ethnic variability of the participants, although we did seek diversity in participants via selection of our geographically distinct and large clinical sites in areas with high rates of cervical cancer. Patients were screened and enrolled regardless of race/ethnicity and consent forms translated into Spanish. The limited diversity likely reflects a small sample size. The separate randomized study will be performed at different sites to remediate this issue.
Although HPV infection clearance, particularly of HPV16, can be an important marker of disease regression, we also assessed cervical histology over the course of 6 months via colposcopic exam with required ECC and relevant biopsy. If low-grade lesions progress to high-grade lesions, approximately a third of patients will progress to more serious malignancy (16). By month 6, the majority of subjects available for analysis (9 of 11), demonstrated no histologic progression upon ECC (Table 5). However, 2 of these subjects, who remained HPV16+, progressed from LSIL or lack of significant lesions to HSIL/CIN2 by month 6 (Table 5). This histologic progression may be due a number of factors in addition to persistent HPV16 including the subject being infected with multiple hrHPV types, i.e., if a different hrHPV type was present it could have driven the observed lesion progression. Indeed, of the 2 HPV16+ subjects with CIN2 treated with LEEP at month 6, 1 developed LSIL at month 12 despite being HPV16-negative, suggestive of infection with another type. The other subject who received a LEEP at month 6 remained HPV16-positive at month 12.
A challenge in interpreting mild atypia and longitudinal changes in low-grade histology and cytology is interobserver variation. Interpretation of patient cervical slides can vary depending on the investigator examining the samples, although here we utilized a consensus pathology review panel to mitigate this issue for interpretation of exploratory endpoints of this cohort evaluating regression to typical cervical cytology by month 6 (Table 4) and to normal histology (Table 5).
Although 2 subjects experienced lesion progression to HSIL/CIN2 in ECC at month 6 (Table 5) in association with HPV16 infection, the unexpectedly high regression rate in HPV16 infection is a promising indication that PVX2 may nevertheless assist in overall preventing HPV16-associated lesion progression. Indeed, none of the subjects who cleared HPV16 by month 6 progressed to HSIL by month 12. However, only 3 of the 7 subjects who experienced HPV16 clearance reverted to normal cytology; 2 had ASC-US and 2 had LSIL suggesting the possibility of co-infections with types other than HPV16/18/45. Thus, an absence of individual typing for HPV other than HPV16/18/45 is another limitation of this study.
Supplementary Material
Supplementary Table S1 shows Diastolic blood pressure of study participants at each study visit, including immediately pre- and 30 minutes post-vaccination
Supplementary Table S2 shows Systolic blood pressure of study participants at each visit, including immediately pre- and 30 minutes post-vaccination
Supplementary Table S3 shows Heart rate of study participants at each visit, including immediately pre- and 30 minutes post-vaccination
Supplementary Table S4 shows respiratory rate of study participants at each visit, including immediately pre- and 30 minutes post-vaccination
Supplementary Table S5 shows the baseline blood counts and chemistry of study participants
Acknowledgments
The study was funded by PapiVax Biotech, Inc.
We are very grateful to the volunteers for their participation in this study, and greatly appreciate the work of the personnel at each clinical site. Development of the vaccines was supported by awards to Drs. R.B.S. Roden from the PREVENT/Division of Cancer Prevention program and T.C. Wu from the NeXT (RAID) program of the NCI. The study was funded by Papivax Biotech Inc.
The publication costs of this article were defrayed in part by the payment of publication fees. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Footnotes
Note: Supplementary data for this article are available at Cancer Prevention Research Online (http://cancerprevres.aacrjournals.org/).
Authors' Disclosures
M.H. Einstein reports M.H. Einstein has advised or participated in educational speaking activities, but does not receive an honorarium from any companies. In specific cases, his employer, Rutgers University, has received payment for his time spent for these activities from Papivax, Merck, BD, and PDS Biotechnologies. If travel required for meetings with industry, the company pays for Dr. Einstein's travel expenses. Rutgers has received grant funding for research-related costs of clinical trials that he has been the overall or local PI within the past 12 months from Johnson & Johnson, Pfizer, Inovio, AstraZeneca, Imvax, Iovance, PDS Biotechnologies, and BD. Dr. Einstein is a consultant to the World Health Organization. Payment for these efforts go to Rutgers. M.H. Einstein has leadership positions in ASCCP, SGO. R.B.S. Roden reports other support from Papivax LLC; and other support from Papivax Biotech Inc. during the conduct of the study. T.C. Wu reports other support from PapiVax Biotech Inc. during the conduct of the study; other support from PapiVax LLC outside the submitted work; in addition, T.C. Wu has a patent for Fusion of Heat shock protein 70 to antigens enhance the potency of DNA vaccines issued and licensed to PapiVax Biotech Inc, a patent for Chimeric Immunogenicity Compositions and Nucleic Acids Encoding Them issued and licensed to Papivax Biotech Inc, a patent for Chimeric Immunogenicity Compositions and Nucleic Acids Encoding Them issued and licensed to Papivax Biotech Inc, a patent for Molecular Vaccine Linking an Endoplasmic Reticulum Chaperone Polypeptide to an Antigen issued and licensed to Papivax Biotech Inc, a patent for Fusion of Calreticulin (CRT) to Antigen issued and licensed to PapiVax Biotech Inc, a patent for Molecular Vaccine Linking an Endoplasmic Reticulum Chaperone Polypeptide to an Antigen issued and licensed to PapiVax Biotech Inc, a patent for Molecular Vaccine Linking an Endoplasmic Reticulum Chaperon Polypeptide to an Antigen issued and licensed to PapiVax Biotech Inc, a patent for Chimeric Immunogenic Compositions and Nucleic Acids Encoding Them issued and licensed to PapiVax Biotech Inc, and a patent for Molecular Vaccine Linking an Endoplasmic Reticulum Chaperon Polypeptide to an Antigen issued and licensed to PapiVax Biotech Inc. Y.N. Chang reports other support from Papivax Biotech Inc. during the conduct of the study; other support from Papivax LLC outside the submitted work. No disclosures were reported by the other authors.
Authors' Contributions
M.H. Einstein: Conceptualization, resources, data curation, formal analysis, supervision, validation, investigation, writing–original draft, project administration, writing–review and editing. R.B.S. Roden: Conceptualization, resources, methodology, writing–review and editing. L. Ferrall: Conceptualization, writing–original draft, writing–review and editing. M. Akin: Resources, supervision, validation, investigation, visualization, methodology, writing–review and editing. A. Blomer: Data curation, supervision, validation, investigation, visualization, methodology, writing–review and editing. T.C. Wu: Conceptualization, resources, data curation, supervision, visualization, writing–original draft, writing–review and editing. Y.-N. Chang: Conceptualization, resources, data curation, formal analysis, supervision, funding acquisition, validation, investigation, visualization, methodology, writing–original draft, project administration, writing–review and editing.
References
- 1. American Cancer Society. Cancer Facts & Figures. 3rd Ed.Available from:https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2015/cancer-facts-and-figures-2015.pdf. Atlanta: American Cancer Society; 2015. [Google Scholar]
- 2. Quinn MA, Benedet JL, Odicino F, Maisonneuve P, Beller U, Creasman WT, et al. Carcinoma of the cervix uteri. FIGO 26th annual report on the results of treatment in gynecological cancer. Int J Gynaecol Obstet 2006;95:S43–103. [DOI] [PubMed] [Google Scholar]
- 3. Bruni L, Saura-Lazaro A, Montoliu A, Brotons M, Alemany L, Diallo MS, et al. HPV vaccination introduction worldwide and WHO and UNICEF estimates of national HPV immunization coverage 2010–2019. Prev Med 2021;144:106399. [DOI] [PubMed] [Google Scholar]
- 4. Rosenblum HG, Lewis RM, Gargano JW, Querec TD, Unger ER, Markowitz LE. Declines in prevalence of human papillomavirus vaccine-type infection among females after introduction of vaccine - United States, 2003–2018. MMWR Morb Mortal Wkly Rep 2021;70:415–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Castle PE, Solomon D, Schiffman M, Wheeler CM. Human papillomavirus type 16 infections and 2-year absolute risk of cervical precancer in women with equivocal or mild cytologic abnormalities. J Natl Cancer Inst 2005;97:1066–71. [DOI] [PubMed] [Google Scholar]
- 6. Santesso N, Mustafa RA, Wiercioch W, Kehar R, Gandhi S, Chen Y, et al. Systematic reviews and meta-analyses of benefits and harms of cryotherapy, LEEP, and cold knife conization to treat cervical intraepithelial neoplasia. Int J Gynaecol Obstet 2016;132:266–71. [DOI] [PubMed] [Google Scholar]
- 7. Bosch FX, Manos MM, Munoz N, Sherman M, Jansen AM, Peto J, et al. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International biological study on cervical cancer (IBSCC) study group. J Natl Cancer Inst 1995;87:796–802. [DOI] [PubMed] [Google Scholar]
- 8. Perkins RB, Guido RS, Castle PE, Chelmow D, Einstein MH, Garcia F, et al. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis 2020;24:102–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kreisel KM, Spicknall IH, Gargano JW, Lewis FMT, Lewis RM, Markowitz LE, et al. Sexually Transmitted infections among us women and men: prevalence and incidence estimates, 2018. Sex Transm Dis 2021;48:208–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Evander M, Edlund K, Gustafsson A, Jonsson M, Karlsson R, Rylander E, et al. Human papillomavirus infection is transient in young women: a population-based cohort study. J Infect Dis 1995;171:1026–30. [DOI] [PubMed] [Google Scholar]
- 11. Bulkmans NW, Berkhof J, Bulk S, Bleeker MC, van Kemenade FJ, Rozendaal L, et al. High-risk HPV type–specific clearance rates in cervical screening. Br J Cancer 2007;96:1419–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lin Z, Yemelyanova AV, Gambhira R, Jagu S, Meyers C, Kirnbauer R, et al. Expression pattern and subcellular localization of human papillomavirus minor capsid protein L2. Am J Pathol 2009;174:136–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cardosi RJ, Bomalaski JJ, Hoffman MS. Diagnosis and management of vulvar and vaginal intraepithelial neoplasia. Obstet Gynecol Clin North Am 2001;28:685–702. [DOI] [PubMed] [Google Scholar]
- 14. Abbasakoor F, Boulos PB. Anal intraepithelial neoplasia. Br J Surg 2005;92:277–90. [DOI] [PubMed] [Google Scholar]
- 15. McCredie MR, Sharples KJ, Paul C, Baranyai J, Medley G, Jones RW, et al. Natural history of cervical neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: a retrospective cohort study. Lancet Oncol 2008;9:425–34. [DOI] [PubMed] [Google Scholar]
- 16. Nasiell K, Nasiell M, Vaclavinkova V. Behavior of moderate cervical dysplasia during long-term follow-up. Obstet Gynecol 1983;61:609–14. [PubMed] [Google Scholar]
- 17. Hudson JB, Bedell MA, McCance DJ, Laiminis LA. Immortalization and altered differentiation of human keratinocytes in vitro by the E6 and E7 open reading frames of human papillomavirus type 18. J Virol 1990;64:519–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Werness BA, Levine AJ, Howley PM. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science 1990;248:76–9. [DOI] [PubMed] [Google Scholar]
- 19. Bennett KF, Waller J, McBride E, Forster AS, Di Gessa G, Kitchener H, et al. Psychosexual distress following routine primary human papillomavirus testing: a longitudinal evaluation within the English Cervical screening program. BJOG 2021;128:745–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Trimble CL, Peng S, Kos F, Gravitt P, Viscidi R, Sugar E, et al. A phase I trial of a human papillomavirus DNA vaccine for HPV16+ cervical intraepithelial neoplasia 2/3. Clin Cancer Res 2009;15:361–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. de Jong A, O'Neill T, Khan AY, Kwappenberg KM, Chisholm SE, Whittle NR, et al. Enhancement of human papillomavirus (HPV) type 16 E6 and E7-specific T-cell immunity in healthy volunteers through vaccination with TA-CIN, an HPV16 L2E7E6 fusion protein vaccine. Vaccine 2002;20:3456–64. [DOI] [PubMed] [Google Scholar]
- 22. Gambhira R, Gravitt PE, Bossis I, Stern PL, Viscidi RP, Roden RB. Vaccination of healthy volunteers with human papillomavirus type 16 L2E7E6 fusion protein induces serum antibody that neutralizes across papillomavirus species. Cancer Res 2006;66:11120–4. [DOI] [PubMed] [Google Scholar]
- 23. Jones TR, Narum DL, Gozalo AS, Aguiar J, Fuhrmann SR, Liang H, et al. Protection of Aotus monkeys by plasmodium falciparum EBA-175 region II DNA prime-protein boost immunization regimen. J Infect Dis 2001;183:303–12. [DOI] [PubMed] [Google Scholar]
- 24. Pal R, Kalyanaraman VS, Nair BC, Whitney S, Keen T, Hocker L, et al. Immunization of rhesus macaques with a polyvalent DNA prime/protein boost human immunodeficiency virus type 1 vaccine elicits protective antibody response against simian human immunodeficiency virus of R5 phenotype. Virology 2006;348:341–53. [DOI] [PubMed] [Google Scholar]
- 25. Pal R, Wang S, Kalyanaraman VS, Nair BC, Whitney S, Keen T, et al. Polyvalent DNA prime and envelope protein boost HIV-1 vaccine elicits humoral and cellular responses and controls plasma viremia in rhesus macaques following rectal challenge with an R5 SHIV isolate. J Med Primatol 2005;34:226–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cristillo AD, Wang S, Caskey MS, Unangst T, Hocker L, He L, et al. Preclinical evaluation of cellular immune responses elicited by a polyvalent DNA prime/protein boost HIV-1 vaccine. Virology 2006;346:151–68. [DOI] [PubMed] [Google Scholar]
- 27. Peng S, Qiu J, Yang A, Yang B, Jeang J, Wang JW, et al. Optimization of heterologous DNA-prime, protein boost regimens and site of vaccination to enhance therapeutic immunity against human papillomavirus–associated disease. Cell Biosci 2016;6:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1 shows Diastolic blood pressure of study participants at each study visit, including immediately pre- and 30 minutes post-vaccination
Supplementary Table S2 shows Systolic blood pressure of study participants at each visit, including immediately pre- and 30 minutes post-vaccination
Supplementary Table S3 shows Heart rate of study participants at each visit, including immediately pre- and 30 minutes post-vaccination
Supplementary Table S4 shows respiratory rate of study participants at each visit, including immediately pre- and 30 minutes post-vaccination
Supplementary Table S5 shows the baseline blood counts and chemistry of study participants
Data Availability Statement
Data were generated by the authors and included in the article.