Abstract
Purpose:
Alveolar soft part sarcoma (ASPS) is a rare, highly vascular tumor with few treatment options. We designed a phase II randomized trial to determine the activity and tolerability of single-agent cediranib or sunitinib in patients with advanced metastatic ASPS.
Patients and Methods:
Patients 16 years of age and older were randomized to receive cediranib (30 mg) or sunitinib (37.5 mg) in 28-day cycles. Patients could cross over to the other treatment arm at disease progression. The primary endpoint was to measure the objective response rate (ORR) for each agent. Median progression-free survival (mPFS) for the two arms was also determined.
Results:
Twenty-nine of 34 enrolled patients were evaluable for response. One patient on each of the initial two treatment arms had a partial response (ORR: 6.7% and 7.1% for cediranib and sunitinib, respectively). Twenty-four patients had a best response of stable disease (86.7% and 78.6% for cediranib and sunitinib, respectively). There were no significant differences in mPFS for the two treatment arms. Clinical benefit (i.e., objective response or stable disease for a minimum of four or six cycles of therapy) on the first-line tyrosine kinase inhibitor (TKI) therapy did not predict benefit on the second-line TKI. Both drugs were well tolerated. As of August 2021, 1 patient (unevaluable for ORR) remains on study.
Conclusions:
The study did not meet its endpoints for ORR. Although both TKIs provided clinical benefit, the outcomes may have been attenuated in patients who had progressed ≤6 months before enrollment, potentially accounting for the low response rates.
Translational Relevance.
Alveolar soft part sarcoma (ASPS) is a rare, highly vascular tumor with few treatment options. Prior research suggested treatment with a tyrosine kinase inhibitor (TKI) of VEGFRs could benefit patients with ASPS. We conducted a randomized clinical trial of potent VEGFR TKIs cediranib and sunitinib in aggressive ASPS. The drugs were well tolerated, and the overall response rate was 6.7% and 7.1% for cediranib and sunitinib, respectively. Most patients had a best response of stable disease (86.7% and 78.6% for cediranib and sunitinib, respectively). The selection of patients with aggressive disease that progressed in the 6 months preceding enrollment may account for the lower objective response rates compared with previous reports. Our data indicate that prior TKI therapy does not preclude clinical benefit from subsequent TKI treatments, suggesting that sequential TKI therapy may benefit patients with ASPS.
Introduction
Alveolar soft part sarcoma (ASPS) is an extremely rare, highly vascular tumor accounting for less than 1% of soft-tissue sarcomas, predominantly affecting adolescents and young adults (1–3). ASPS has a variable growth pattern, and frequently metastasizes to the lungs, brain, and bones. The historical 5-year survival rate for patients with unresectable metastatic disease is 20% (4). Cytotoxic chemotherapy is ineffective in the treatment of ASPS (5).
ASPS tumors present with a characteristic unbalanced nonreciprocal chromosomal translocation t(X,17)(p11;q25) resulting in the creation of ASPSCR1-TFE3 fusion protein, an aberrant transcription factor associated with mesenchymal epithelial transition (MET)-related signal transduction. Gene expression profiling studies have revealed upregulation of transcripts associated with proliferation, metastasis, myogenic differentiation, and angiogenesis, including VEGF (6, 7).
Cediranib is a small-molecule inhibitor of all three VEGFR (VEGFR-1, -2, and -3) tyrosine kinases. In a phase II trial, single-agent cediranib produced an objective response rate (ORR) of 35% and a disease control rate of 84% at 24 weeks in 43 patients with metastatic, unresectable ASPS (8). Other clinical results support targeting VEGFR in this tumor, including a trial of the multikinase inhibitor sunitinib which demonstrated five partial responses (PR) in 9 patients with metastatic ASPS in the context of a compassionate use program (9). In addition, retrospective (10) and prospective (11, 12) studies of sunitinib, alone or in combination, indicate that this agent may have clinical activity in several sarcomas, including soft-tissue sarcomas. Therefore, we conducted a phase II randomized trial of single-agent cediranib or sunitinib to determine whether sunitinib or cediranib could be associated with a modestly high rate of clinical response in patients with ASPS.
Patients and Methods
Eligibility criteria
Patients 16 years of age or older with pathologically confirmed metastatic ASPS not curable by surgery were eligible. For patients previously treated with surgery, radiation, and/or systemic therapy, evidence of objective disease progression per RECIST 1 (13) on two CT scans within the 3-month period immediately preceding enrollment was required; this criterion was later amended to two scans within the 6-month period immediately preceding enrollment. Prior treatment with any VEGFR tyrosine kinase inhibitor (TKI) was not allowed; prior treatment with bevacizumab was allowed. Prior treatment with systemic therapy, radiotherapy, and surgery must have been completed at least 4 weeks prior to enrollment. Patients with previously untreated, unresectable, metastatic ASPS who showed clinical evidence of disease progression (including history and increasing physical symptoms) were also eligible and were assessed in a separate cohort through an amendment in 2014.
All patients were required to have measurable disease, defined as at least one lesion that could be accurately measured in at least one dimension (longest diameter to be recorded) as ≥20 mm with conventional techniques or as ≥10 mm with spiral CT scan (13). Patients ages 16–17 years were eligible only if they had a BSA ≥ 1.7 m2 or weighed ≥60 kg. Patients with history of serious ventricular arrhythmias or prolonged QTc were excluded. An Eastern Cooperative Oncology Group (ECOG) performance status of ≤2 and adequate organ and marrow function defined as an absolute neutrophil count ≥1,500/μL, platelets ≥100,000/μL, total bilirubin within normal institutional limits, aspartate aminotransferase (AST) and/or alanine aminotransferase (ALT) ≤2.5× institutional upper limit of normal, creatinine within normal institutional limits or creatine clearance ≥60 mL/minute for patients with creatinine levels above institutional normal were required.
Trial design
The trial enrolled patients from July 2011 to September 2019. This was an open-label, multicenter, randomized phase II study of cediranib or sunitinib in patients with metastatic ASPS, with crossover to the other treatment arm at disease progression and after a 2-week washout period. Two sets of patients were enrolled on each arm: one set consisted of patients previously treated with either surgery, radiation, and/or systemic therapy, and the other set was treatment-naïve patients (Table 1). The study was conducted using an optimal two-stage design to rule out an unacceptably low 15% clinical response rate [PR + complete response (CR)] in favor of a modestly high response rate of 40%. Randomization to single-agent cediranib or single-agent sunitinib was performed using a predetermined, balanced list based on unique subject identifiers. Depending on treatment allocation, patients received either cediranib 30 mg orally once a day or sunitinib 37.5 mg orally once a day in 28-day cycles. Patients were asked to maintain a diary documenting when drugs were taken and any associated side effects. Cediranib and sunitinib were supplied by the Division of Cancer Treatment and Diagnosis, NCI, under collaborative agreements with AstraZeneca and Pfizer, respectively.
Table 1.
Patient characteristics.
| Characteristic | No. of patients |
|---|---|
| Number of patients enrolled/evaluable | 34/29 |
| Median age, years (range) | 26 (16–68) |
| Female/male | 7/27 |
| Race | |
| White | 16 |
| Black | 10 |
| Asian | 6 |
| Native Hawaiian or other Pacific Islander | 2 |
| ECOG performance status | |
| 0 | 11 |
| 1 | 21 |
| 2 | 2 |
| Newly diagnosed/previously treated | 20/14 |
| Number with prior chemotherapy alone | 3 |
| Number with prior radiotherapy alone | 7 |
| Number with chemo and radiation | 4 |
| Number with a prior surgery | 10 |
Blood for pharmacokinetic analysis was collected from patients receiving cediranib only into a 4 mL EDTA tube at baseline and prior to drug administration on cycle 1 day 15 and on day 1 of cycles 2, 3, and 4.
Adverse events were graded according to NCI Common Toxicity Criteria version 4.0. Doses were reduced for grade ≥3 non-hematologic toxicities (except electrolyte abnormalities that were correctable within 48 hours and tumor pain responsive to supportive measures) and grade 4 hematologic toxicities (except lymphopenia and anemia). Toxicities were required to have resolved to ≤grade 2 prior to continuing treatment. Up to 14 days were allowed for resolution of toxicity; otherwise, patients were taken off treatment. Radiographic evaluation was performed at baseline and every two cycles to assess tumor response based on the RECIST version 1 (13).
This trial was conducted under an NCI-sponsored Investigational New Drug Application with Institutional Review Board (IRB) approval at each participating site. All patients provided written informed consent before participation and protocol design and the study was conducted in accordance with recognized ethical guidelines (U.S. Common Rule) and approved by the NIH Institutional Review Board (ClinicalTrials.gov Identifier: NCT01391962).
Statistical considerations
The study design initially required evidence of objective disease progression on two scans within the 3-month period immediately preceding enrollment; to increase the number of eligible patients and improve accrual, in August 2013 the protocol was amended to allow assessment within 6 months preceding enrollment. For statistical purposes, the first 13 patients enrolled prior to the protocol amendment were assessed separately for median progression-free survival (mPFS).
Patients were randomized between the two arms using unstratified randomization with variable block sizes to prevent bias in patient selection. There was no formal comparison of the response rates obtained on the two arms as this was not the purpose of the study and the trial lacked sufficient power to detect any reasonable difference between the arms. No “pick the winner” design was intended; at the conclusion of the trial, all information obtained on outcomes and toxicity was to be evaluated.
For previously treated patients in each of the two treatment arms, the study was conducted as an optimal two-stage phase II trial (14) to rule out an unacceptably low 15% clinical response rate (PR+CR; p0 = 0.15) in favor of a modestly high response rate of 40% (p1 = 0.40). With alpha = 0.10 and beta = 0.10, the study would initially enroll 10 evaluable patients in each treatment arm. Accrual would continue to 22 patients if 2 or more patients responded, but the arm would close (including to crossover of patients progressing on the other arm) if ≤1 of the first 10 patients responded. If 6 or more patients had a response among the 22, the agent would be considered sufficiently interesting for further study in later trials with similar patients.
The study was initially designed for patients with refractory or relapsed metastatic ASPS but was later amended to include patients with newly diagnosed, previously untreated (i.e., no prior radiation, chemotherapy, or surgery) ASPS. Newly diagnosed patients with extensive metastatic disease at time of diagnosis were assessed in a separate cohort. For newly diagnosed, previously untreated patients in each of the two treatment arms, the study was conducted as a Minimax two-stage phase II trial (14) to rule out an unacceptably low 15% clinical response rate (PR+CR; p0 = 0.15) in favor of a modestly high response rate of 45% (p1 = 0.45). With alpha = 0.10 and beta = 0.20, the study would initially enroll 8 evaluable patients in each treatment arm. If 2 or more of these first 8 patients responded, accrual would continue to a total of 11 patients. If ≤1 of the 8 patients responded, that arm would close and there would be no crossover of patients who progressed on the other agent to this arm. If there were 4 or more patients with a response out of 11, the agent would be considered sufficiently interesting for further study in later trials with similar patients.
We also performed an exploratory analysis to investigate whether patients who achieved clinical benefit [defined as ORR or stable disease (SD) for minimum four cycles, or minimum six cycles of therapy) from the first line of TKI therapy also experienced clinical benefit from the second line of therapy. To do so, we reported the fraction of paired times which agreed on clinical benefit for the two outcomes (with/without clinical benefit on first and second therapy) and tested whether the outcomes agreed by a McNemar test, recognizing the small number of subjects and the test's limited power in the current setting.
Data availability
The data generated in this study are available upon request from the corresponding author.
Results
Demographics
Thirty-four patients were enrolled (Table 1) between July 2011 and April 2019; 7 patients had received prior chemotherapy, 11 had received prior radiotherapy, and 10 had prior surgery. Twenty patients were newly diagnosed, unresectable, and treatment-naïve. Twenty-seven of the patients were male (79%). The study enrolled patients 16 years of age and older (range, 16–68 years); 29 of the enrolled patients (85.3%) were adolescents and young adults (16–39 years old). The cohort comprised a diverse group of patients including 47% White, 29.4% Black, 17.6% Asian, and 5.8% Native Hawaiian or other Pacific Islander. Patients were enrolled through multiple sites.
Clinical activity
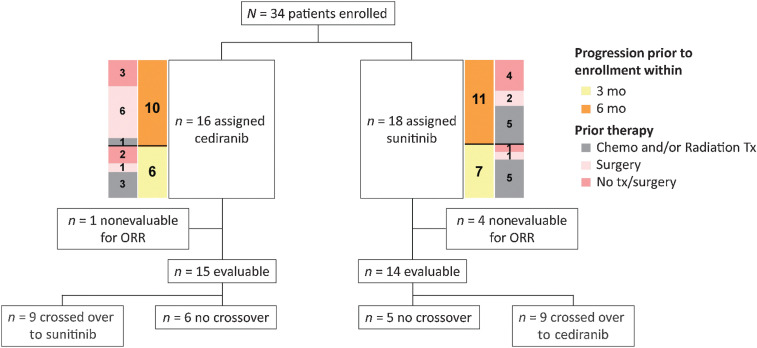
Sixteen patients received cediranib as initial therapy and 18 received sunitinib; 29 patients were evaluable for response, of whom 15 received cediranib as initial therapy and 14 of whom received sunitinib (Fig. 1).
Figure 1.
CONSORT diagram of the two-arm randomized trial. Patients were randomly assigned to either the cediranib arm or the sunitinib arm. Crossover was allowed at disease progression.
Cediranib arm
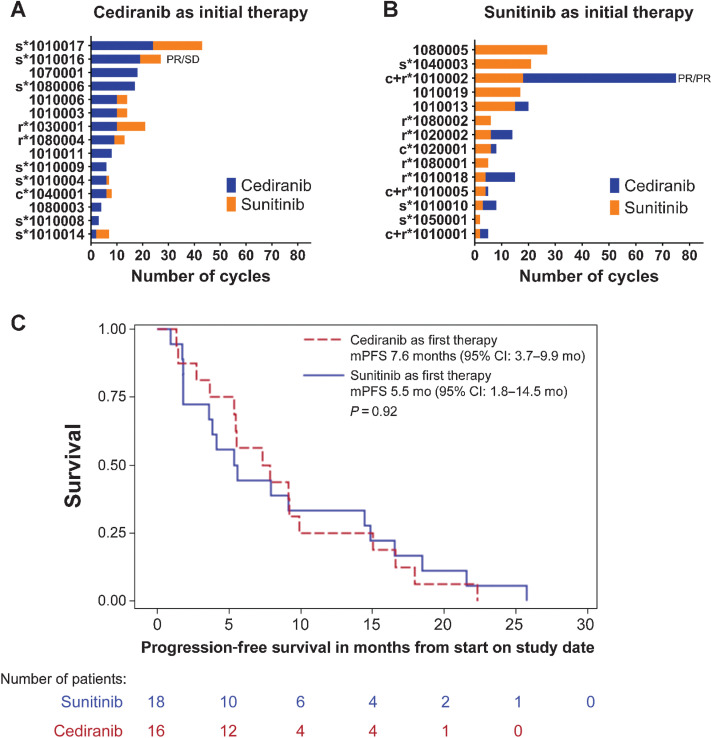
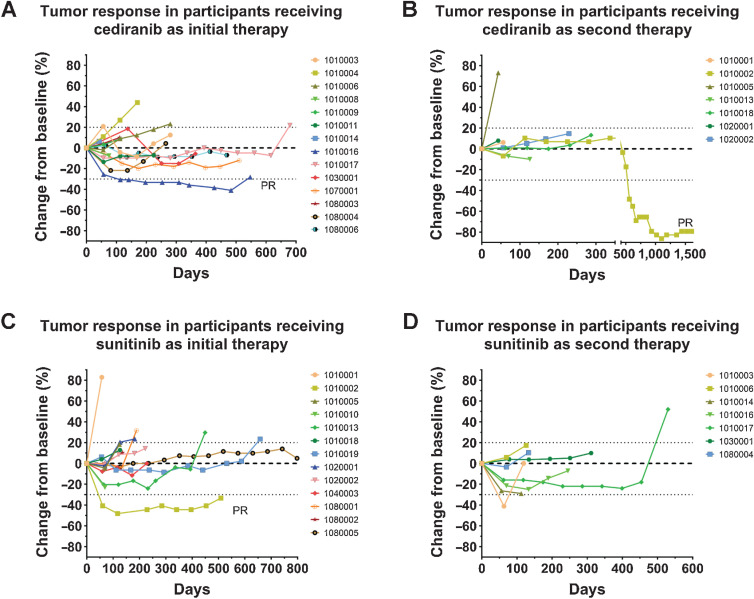
The median number of treatment cycles completed for patients in the cediranib arm was 9 (range, 2–24; Fig. 2A); 13 of 15 patients (86.7%) had a best response of SD. One patient, who was treatment-naïve except for surgical resection, had a PR (6.7% response rate) by cycle 4 and remained on cediranib for 19 cycles before experiencing disease progression and then crossing over to the sunitinib arm, where the patient achieved a best response of SD for eight cycles.
Figure 2.
Number of cycles of therapy for each of the evaluable 29 patients on the study based on their initial therapy: cediranib (A) or sunitinib (B). Prior therapy is indicated next to patient ID: s, surgery; c, chemotherapy; r, radiation. Two patients achieved a PR in at least one arm of the study, before/and after crossover. C, Median PFS for all 34 patients based on initial therapy arm assignment.
A total of 9 evaluable patients crossed over to cediranib from sunitinib at progression (median number of cycles = 5; range, 1–57; Fig. 2B) of whom 5 had SD (55.5%). One patient (who achieved a PR on sunitinib) had a PR (11.1% response rate) on cediranib. One patient (unevaluable for ORR) who crossed over from the sunitinib arm remained on study, completing 46 cycles on cediranib, by the cutoff of August 2021; the patient is still on study as of August 2022.
The mPFS for all 16 patients who received initial therapy with cediranib was 7.6 months [95% confidence interval (CI): 3.7–9.9 months; Fig. 2C]; the PFS probability at 24 weeks was 62.5% (95% CI: 29.5–76.2).
Seven of the 16 patients (44%) assigned to the cediranib arm had prior surgery (including the patient who had a PR; Fig. 3A). The mPFS was 5.6 months (95% CI: 1.3–17.9 months) for the patients who had prior surgery and 7.9 months (95% CI: 1.4–9.9 months) for those without prior surgery (P = 0.41). Four of the 16 patients assigned to the cediranib arm had prior chemotherapy and/or radiotherapy. The mPFS was 6.7 months (95% CI: 1.4–9.9 months) for the patients receiving prior treatment, and 8.3 months (95% CI: 2.7–16.6 months) for those who were treatment-naïve (P = 0.35). Five patients had received no prior therapy.
Figure 3.
Percentage change from baseline in tumor size over time for patients with ASPS receiving cediranib as initial therapy (A), cediranib as second therapy (B), sunitinib as initial therapy (C), and sunitinib as second therapy (D). PR, partial response. Dotted lines represent the thresholds for PR and progressive disease according to RECIST 1 guidelines.
There was no difference in mPFS between the first 6 patients enrolled to the cediranib arm (with disease progression documented ≤3 months before enrollment) and the subsequent 10 patients enrolled to the same arm (with an extended, 6-month, window for documenting disease progression prior to enrollment): 7.4 months (95% CI: 1.4–9.9 months) versus 7.6 months (95% CI: 1.3–16.6 months), respectively (P = 0.35).
Sunitinib arm
The median number of treatment cycles completed for patients on the sunitinib arm was 6 (range, 2–27; Fig. 2B); 11 of 14 evaluable patients (78.6%) had a best response of SD, and 1 patient had a PR (7.1% response rate) by cycle 2 and eventually received 18 cycles of therapy. After crossing over to cediranib, this patient had a PR and remained on study for 57 additional cycles (Fig. 3B and C).
Nine patients crossed over to sunitinib (median number of crossover cycles = 5; range, 1–19; Fig. 2A), of whom 7 (77.8%) had SD (Fig. 3D).
The mPFS for all 18 patients who received sunitinib initially was 5.5 months (95% CI: 1.8–14.5 months; Fig. 2C); the PFS probability at 24 weeks was 50% (95% CI: 25.9–70.1).
Only 3 patients in the sunitinib arm had been treated previously with surgery, precluding any statistical comparison. Ten of the 18 patients had received prior chemotherapy and/or radiotherapy in the sunitinib arm (including the patient who had a PR; Fig. 3C). The mPFS was 4.8 months (95% CI: 0.9–7.9 months) for previously treated patients, and 14.7 months (95% CI: 1.8–21.6 months) for those who were treatment-naïve (P = 0.058). Five patients had no prior therapy.
The PFS for the initial 7 patients (with disease progression documented ≤3 months before enrollment) receiving sunitinib compared with the subsequent 11 patients (with a 6-month window for documenting disease progression prior to enrollment) was 1.8 months (95% CI: 1.8–5.4 months) versus 9.2 months (95% CI: 3.9–18.5 months; P = 0.035).
Response in patients who progressed 3 and 6 months prior to treatment
The first 13 patients accrued to this study were required to demonstrate evidence of objective disease progression within a 3-month window immediately preceding enrollment. Six patients received cediranib as their initial treatment and 7 received sunitinib. The mPFS was 7.4 months for the 6 patients on the cediranib arm (95% CI: 1.4–9.9 months) and 1.8 months (95% CI: 1.8–5.4 months) for the 7 patients on the sunitinib treatment arm (P = 0.45). To increase accrual, subsequent patients were considered eligible if they had disease progression within 6 months preceding enrollment. mPFS for the 21 patients accrued after the change in eligibility was 7.6 months (95% CI: 1.3–16.6 months) for the 10 patients receiving cediranib and 9.2 months (95% CI: 3.9–18.9 months) for the 11 patients receiving sunitinib (P = 0.58).
Clinical benefit of first- and second-line TKI therapy
Of 29 evaluable patients, 16 crossed over at the time of progression and 15 of 16 were evaluable for response following crossover.
When clinical benefit was defined as objective response or SD for a minimum of four treatment cycles, 12 of 15 (80%) evaluable crossover patients achieved benefit on first-line therapy and 12 of 15 (80%) had clinical benefit on second-line therapy. Ten of 15 patients (66.7%) experienced clinical benefit on both first-line and second-line therapy. One of 15 patients (6.7%) had no clinical benefit on either TKI treatment. Two patients (13.3%) had no benefit from the first TKI therapy but benefited from the second therapy; 2 patients (13.3%) experienced clinical benefit from the first TKI therapy but did not benefit from the second-line therapy (McNemar test, P = 1.00)
When clinical benefit was defined as objective response or SD for a minimum of six treatment cycles, 10 of 15 (66.7%) crossover-evaluable patients achieved clinical benefit on the first-line therapy and 6 of 15 (40%) had clinical benefit on the second-line therapy. Five of the 15 patients (33%) experienced clinical benefit on both first-line and second-line therapy. Four of 15 (26.7%) had no clinical benefit from either treatment. One patient (6.7%) had no benefit from the first-line therapy but benefitted from the second-line therapy; 5 patients (33.3%) experienced benefit from the first-line therapy but had no benefit from the second TKI therapy (McNemar test, P = 0.22).
Unevaluable patients
Five patients were not evaluable for response. Treatment was discontinued for 1 patient receiving sunitinib during cycle 1 due to toxicity (grade 4 ALT increase, not resolved within 14 days); the patient crossed over to the cediranib arm and completed 12 cycles before progressing. One patient withdrew after 1 week of cediranib to obtain radiotherapy for a preexisting bone lesion. One patient died during cycle 1 from a previously undiagnosed brain lesion causing seizures, an event attributed to disease. An IRB special exemption was granted for a patient with Gilbert syndrome whose total bilirubin exceeded the inclusion criteria limit at screening—this patient had two cycles of sunitinib before crossing over to the cediranib arm but progressed after two cycles. Finally, a treatment-naïve patient was determined to be unevaluable after beginning treatment with sunitinib. Although baseline CT demonstrated a right posterior orbital tumor and measurable liver lesions, review of an MRI performed at the end of cycle 1 characterized the liver lesions as typical hemangiomas and probable iron deposits. Despite not meeting the inclusion criteria, he continued to receive sunitinib for 16 cycles before progressing and crossing over to the cediranib arm for a further 46 cycles of therapy. This patient remains on study with SD.
Arm closure
After interim analysis, the cediranib arm of the newly diagnosed ASPS cohort was closed because of inadequate clinical response, and no future crossover to the cediranib arm was permitted. The other arms and/or cohorts did not complete accrual due to lack of response. Insufficient pharmacokinetic data were obtained to draw meaningful conclusions (data not shown).
Toxicity
Study drugs were generally well tolerated. The most common grade ≥2 adverse events that were considered related to cediranib were hypertension, diarrhea, lymphopenia, weight loss and/or anorexia, and hypothyroidism. The most common grade ≥2 adverse events reported to be related to sunitinib were hypertension, neutropenia, lymphopenia, diarrhea, fatigue, proteinuria, and palmar-plantar erythrodysesthesia (Table 2). Overall, 43.7% of patients treated with cediranib and 77.8% patients treated with sunitinib as initial treatment experienced grade ≥3 adverse events that were considered at least possibly related to study drug.
Table 2.
Adverse events by patient on each study drug.
| Cediranib (N = 16) | Sunitinib (N = 18) | ||||
|---|---|---|---|---|---|
| Adverse event | Gr 2 | Gr 3 | Gr 2 | Gr 3 | Gr 4 |
| Blood and lymphatic system | |||||
| Neutropenia | 4 (25%) | 1 (6.3%) | 16 (88.9%) | 9 (50%) | 1 (5.6%) |
| Hypertension | 13 (81.3%) | 8 (50%) | 12 (66.7%) | 3 (16.7%) | 0 |
| Leukopenia | 0 | 0 | 11 (61.1%) | 1 (5.6%) | 0 |
| Lymphocytopenia | 2 (12.5%) | 0 | 3 (16.7%) | 1 (5.6%) | 1 (5.6%) |
| Thrombocytopenia | 1 (6.3%) | 0 | 2 (11.1%) | 0 | 0 |
| Anemia | 0 | 0 | 1 (5.6%) | 1 (5.6%) | 0 |
| Endocrine | |||||
| Hypothyroidism | 4 (25%) | 0 | 3 (16.7%) | 0 | 0 |
| Gastrointestinal | |||||
| Mucositis oral | 1 (6.3%) | 0 | 4 (22.2%) | 0 | 0 |
| Diarrhea | 4 (25%) | 3 (18.8%) | 3 (16.7%) | 1 (5.6%) | 0 |
| Dyspepsia | 0 | 0 | 2 (11.1%) | 0 | 0 |
| Dysgeusia | 0 | 0 | 1 (5.6%) | 0 | 0 |
| Vomiting | 1 (6.3%) | 1 (6.3%) | 1 (5.6%) | 0 | 0 |
| Abdominal pain | 2 (12.5%) | 0 | 0 | 0 | 0 |
| Nausea | 0 | 1 (6.3%) | 0 | 0 | 0 |
| Anal/perianal abscess | 0 | 0 | 1 (5.6%) | 2 (11.1%) | 0 |
| Hepatobiliary | |||||
| AST increased | 0 | 0 | 1 (5.6%) | 1 (5.6%) | 0 |
| ALT increased | 1 (6.3%) | 0 | 0 | 1 (5.6%) | 1 (5.6%) |
| Blood bilirubin increased | 2 (12.5%) | 0 | 0 | 0 | 0 |
| Immune system | |||||
| Allergic reaction | 0 | 0 | 2 (11.1%) | 0 | 0 |
| Investigations | |||||
| Hypophosphatemia | 2 (12.5%) | 1 (6.3%) | 4 (22.2%) | 2 (11.1%) | 0 |
| Metabolism and nutrition | |||||
| Weight loss/anorexia | 5 (31.3%) | 0 | 0 | 0 | 0 |
| Nervous system | |||||
| Fatigue | 0 | 0 | 4 (22.2%) | 0 | 0 |
| Intracranial hemorrhage | 0 | 0 | 0 | 0 | 1 (5.6%) |
| Neoplasm | |||||
| Tumor pain | 2 (12.5%) | 0 | 0 | 1 (5.6%) | 0 |
| Renal and urinary | |||||
| Proteinuria | 2 (12.5%) | 0 | 1 (5.6%) | 1 (5.6%) | 0 |
| Skin and subcutaneous tissue | |||||
| Palmar-plantar erythrodysesthesia | 3 (18.8%) | 0 | 4 (22.2%) | 2 (11.1%) | 0 |
| Skin hypopigmentation | 0 | 0 | 3 (16.7%) | 0 | 0 |
Note: Grade ≥ 2 events occurring in at least 10% of patients (or one or more patients for ≥grade 3 events) that were considered possibly, probably, or definitely related to each study drug over the course of the trial are shown for each patient (N = 34 total patients).
Abbreviation: Gr, grade.
Among patients receiving cediranib as initial or crossover therapy, 11 (28.5%) had dose reductions, and 1 (3.5%) discontinued treatment due to adverse events at least possibly related to study drug. Among patients receiving sunitinib as initial or crossover treatment 11 (29.6%) had dose reductions and 5 (18.5%) discontinued treatment due to adverse events at least possibly related to study drug.
There was one death on study. A patient who received sunitinib had a grade 4 intracranial hemorrhage attributable to disease; this patient was taken off treatment during cycle 1 due to disease progression from previously undiagnosed brain metastases and died during follow-up while receiving treatment for an intracranial hemorrhage.
Discussion
ASPS is a highly vascular tumor associated with upregulation of VEGF. The ultrarare status of ASPS makes it difficult for clinicians to conduct well-powered, prospective trials in this population, but multiple clinical studies have suggested antitumor activity of VEGF-targeted therapy in ASPS. Our current study of cediranib or sunitinib in patients with metastatic, unresectable ASPS did not meet its primary endpoint for ORR but suggests these TKIs have activity in this disease. The cediranib arm was closed due to inadequate clinical response at its interim analysis, and the sunitinib arm subsequently did not complete accrual due to lack of response according to the statistical plan. One patient in each arm had a confirmed PR with cediranib (ORR, 6.7%) or sunitinib (ORR, 7.1%) as initial treatment. The latter patient eventually crossed over to cediranib treatment and experienced a PR on cediranib as well; none of the other patients who crossed over from one agent to the other experienced an objective response.
The trial enrolled an ethnically diverse cohort that consisted predominantly of adolescents and young adults. A similarly diverse cohort was accrued during our previously reported ASPS study (ref. 8; NCT00942877). Our cohort of 34 patients mostly recapitulated the expected racial demographics as reported previously (15) with 47% White, 29.4% Black, 17.6% Asian, and 5.8% Native Hawaiian or other Pacific Islander. Compared with the racial distribution of the U.S. population (16) African Americans (13.4% of U.S. population vs. 29.4% of this ASPS population), Asians (5.9% vs. 17.6%) and Native Hawaiian or other Pacific Islanders (0.2% vs. 5.8%) are overrepresented while Whites (76.3% vs. 47%) are underrepresented. This diversity does not affect the interpretation of activity in this disease as there currently are no suggestions of ethnicity-based differences in the natural history of ASPS.
In this study, cediranib yielded a lower ORR and shorter PFS compared with other prospective cediranib studies in ASPS, including a single-arm phase II trial we reported previously (8). While the mPFS was 7.6 months in the cediranib arm of the study described herein, our previous single-arm phase II trial of patients with metastatic unresectable ASPS had an ORR of 35% and a disease control rate of 84% at 24 weeks in the adult cohort (8). In the pediatric cohort of that NCI trial, there were no objective responses, but 5 of 7 patients had SD for ≥14 months (17). In cediranib in alveolar soft part sarcoma (CASPS), a double-blind, placebo-controlled, phase II trial in Europe and Australia, an ORR of 19% and mPFS of 10.1 months was observed in the 32 patients with metastatic, unresectable ASPS who were randomized to cediranib compared with an ORR of 0% and mPFS of 4.9 months in the 16 patients randomized to placebo; this difference in PFS was not statistically significant (18). The primary endpoint in the CASPS study, the percentage change in sum of target marker lesion diameters between baseline and week 24 or progression if sooner, was significantly different between the two groups, −8.3% [interquartile range (IQR), −26.5 to 5.9] for patients treated with cediranib versus +13.4% (IQR, 1.1–21.3) for those receiving placebo (one-sided P = 0.0010; ref. 18). Likewise, in the majority of our patients treated with cediranib, we saw target marker lesion diameter reductions, though they did not meet the threshold for PR by RECIST 1.
There have been no prospective sunitinib trials specifically for patients with ASPS. In our review of retrospective studies, sunitinib demonstrated PRs in 19 of 46 patients (41%) with mPFS ranging from 17 to 41 months (2, 9, 19–22). Our trial constitutes the first published prospective study of sunitinib in patients with ASPS and reports an ORR of 7.1% and a mPFS of 5.5 months. Treatment-naïve patients may derive some benefit from sunitinib therapy (mPFS of 14.7 months for treatment-naïve vs. 4.8 months for prior chemotherapy and/or radiotherapy; P = 0.058), but the limited numbers of patients in our treatment group preclude any definitive conclusions.
Our survey of published literature did not identify any reports of an association between previous TKI or antiangiogenesis therapy and the ORR or PFS of patients who received subsequent cediranib or sunitinib therapy. In our study, based on a limited number of patients, we found some evidence that prior clinical benefit from TKI therapy is associated with benefit from subsequent TKI therapy; note that this association is dependent on how clinical benefit was defined. When clinical benefit was defined as ORR or SD for a minimum of four cycles of therapy, 10 of 12 patients who achieved benefit on the first therapy, went on to benefit from the subsequent TKI treatment. If clinical benefit was defined as ORR or SD for minimum six cycles, 5 of 10 patients who improved or did not progress on the first therapy benefitted from the subsequent TKI treatment as well. Therefore, response to prior TKI therapy may be an indicator that subsequent TKIs should be considered. More data are required to establish sequencing order and selection criteria for TKI therapy in ASPS.
Our current study evaluated response according to RECIST 1 based on target lesion measurement size, which may underestimate the response rate to TKI inhibitors in sarcomas (23). Using alternative response criteria, such as decreased tumor density regardless of tumor size as defined by the Choi criteria (24), may be a consideration for future studies. Additional limitations of our study are that it was not set up to evaluate different radiographic methods or powered to carry out any comparisons.
Metastatic ASPS is typically characterized by indolent growth, with occasional spontaneous regression and spontaneous stabilization. Our requirement that patients have progressive disease per RECIST on two scans within the 3 months—later amended to 6 months—immediately preceding enrollment, was meant to select for patients with more aggressive disease. This selection may account for the lower ORR and shorter PFS in our trial compared with previous reports. CASPS was the only other study of cediranib treatment of ASPS that required evidence of progression per RECIST in the 6 months preceding enrollment, and that study also reported a lower ORR and shorter PFS when compared with other studies. However, the limited sample sizes of these ASPS studies, as well as the absence of blinding and a placebo control in our current study, preclude definitive conclusions about the differences in clinical outcomes between trials. It is unclear whether the durable disease control observed in some patients is indicative of intrinsic indolent disease, particular sensitivity to inhibition of angiogenesis, or another mechanism, such as antitumor immune response resulting from VEGF promotion of a protumor microenvironment (25).
Overall, treatment with cediranib or sunitinib showed acceptable toxicity in our randomized phase II trial. Earlier reports suggested that VEGFR inhibition has clinical activity in ASPS, and it is possible that the inferior activity seen in our study is attributable to patient selection criteria enriching for more aggressive disease. Some patients have achieved long-lasting control of disease. Therefore, future investigations should include exploration of biomarkers that may select for patients more likely to benefit from VEGFR inhibition. Given the role of VEGF signaling in promoting a protumor immune microenvironment, future therapies combining immune checkpoint blockade and VEGFR targeting are worth exploring for patients suffering from ASPS. Indeed, initial investigations of sequential or combination TKI and ICI therapy are demonstrating positive and durable responses (5, 12, 26–29). We are currently assessing the clinical activity of the immune checkpoint inhibitor (ICI) atezolizumab with and without the anti-VEGF agent bevacizumab in a phase II study of patients with ASPS (ref. 30; NCT03141684). Importantly, this atezolizumab ± bevacizumab trial has enrolled patients who were previously enrolled on the currently reported study of cediranib or sunitinib in ASPS and may provide further insight as to the therapeutic value of sequential TKI and ICI therapy for patients with ASPS.
Acknowledgments
This project has been funded with federal funds from the NCI, NIH, under contract no. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
The publication costs of this article were defrayed in part by the payment of publication fees. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This article is featured in Highlights of This Issue, p. 1161
Authors' Disclosures
S. Kummar reports other support from SpringWorks Therapeutics, Bayer, Gilead, Mundibiopharma, Genome & Company, Harbour Biomed, Arxeon, PathomIQ, Cadila Pharmaceuticals, Mirati, and Boehringer Ingelheim outside the submitted work. S.P. Chawla reports research funding from Amgen, Roche, GSK, Ignyta, Tracon Pharma, Karyopharm Therapeutics, SARC, Janssen, Inhibrix, Immix, Aadi, Ayala, Immune Design, Lilly, NanoCarrier, Novartis, Philogen, Plexxikon, SpringWorks, Threshold, and Trillium and stocks/stock options in AADi, Cellestia Biotech, CounterPoint, and Immix BioPharma. S. George reports grants from NCI during the conduct of the study as well as personal fees and other support from Blueprint Medicines and Deciphera; other support from Daiichi Sankyo, SpringWorks, BioAtla, Tracon, Merck, Eisai, Theseus, and IDRX; and personal fees from Kayothera, WCG, Immunicum, and UpToDate outside the submitted work; in addition, S. George has served as Interim Group Chair, Alliance for Clinical Trials in Oncology, and President, Alliance Foundation. S.R. Patel reports personal fees from Deciphera, Daiichi Sanyo, Adaptimmune, and GSK; grants and personal fees from Rain Therapeutics; and grants from Blueprint Medicines outside the submitted work. S. Movva reports grants from Hutchinson Medipharma, Ascentage Pharma, and Trillium/Pfizer and nonfinancial support from Merck, Clovis Oncology, and BMS during the conduct of the study. L. Juwara reports other support from NIH/NCI during the conduct of the study. L. Kuhlmann reports other support from NCI during the conduct of the study. A.P. Chen reports nonfinancial support from AstraZeneca during the conduct of the study. No disclosures were reported by the other authors.
Authors' Contributions
J. Nguyen: Investigation, writing–original draft, writing–review and editing. N. Takebe: Supervision, investigation, writing–original draft, writing–review and editing. S. Kummar: Supervision, investigation, writing–review and editing. A. Razak: Investigation, writing–review and editing, principal investigator (PI) at study center. S.P. Chawla: Investigation, writing–review and editing, PI at study center. S. George: Investigation, writing–review and editing, PI at trial center. S.R. Patel: Investigation, writing–review and editing, PI at trial center. M.L. Keohan: Investigation, writing–review and editing, PI at trial center. S. Movva: Investigation, writing–review and editing, PI at trial center. G. O'Sullivan Coyne: Supervision, writing–original draft, writing–review and editing. K. Do: Investigation, writing–review and editing. L. Juwara: Investigation, writing–review and editing. B. Augustine: Data curation, investigation, writing–review and editing. S.M. Steinberg: Formal analysis, writing–review and editing. L. Kuhlmann: Data curation, writing–original draft, writing–review and editing. S.P. Ivy: Investigation, writing–review and editing. J.H. Doroshow: Supervision, investigation, writing–review and editing. A.P. Chen: Supervision, investigation, writing–review and editing.
References
- 1. Flores RJ, Harrison DJ, Federman NC, Furman WL, Huh WW, Broaddus EG, et al. Alveolar soft part sarcoma in children and young adults: a report of 69 cases. Pediatr Blood Cancer 2018;65:e26953. [DOI] [PubMed] [Google Scholar]
- 2. Paoluzzi L, Maki RG. Diagnosis, prognosis, and treatment of alveolar soft-part sarcoma: a review. JAMA Oncol 2019;5:254–60. [DOI] [PubMed] [Google Scholar]
- 3. Stacchiotti S, Frezza AM, Blay JY, Baldini EH, Bonvalot S, Bovee J, et al. Ultra-rare sarcomas: a consensus paper from the Connective Tissue Oncology Society community of experts on the incidence threshold and the list of entities. Cancer 2021;127:2934–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Portera CA Jr, Ho V, Patel SR, Hunt KK, Feig BW, Respondek PM, et al. Alveolar soft part sarcoma: clinical course and patterns of metastasis in 70 patients treated at a single institution. Cancer 2001;91:585–91. [DOI] [PubMed] [Google Scholar]
- 5. Groisberg R, Roszik J, Conley AP, Lazar AJ, Portal DE, Hong DS, et al. Genomics, morphoproteomics, and treatment patterns of patients with alveolar soft part sarcoma and response to multiple experimental therapies. Mol Cancer Ther 2020;19:1165–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stockwin LH, Vistica DT, Kenney S, Schrump DS, Butcher DO, Raffeld M, et al. Gene expression profiling of alveolar soft-part sarcoma (ASPS). BMC Cancer 2009;9:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lazar AJ, Das P, Tuvin D, Korchin B, Zhu Q, Jin Z, et al. Angiogenesis-promoting gene patterns in alveolar soft part sarcoma. Clin Cancer Res 2007;13:7314–21. [DOI] [PubMed] [Google Scholar]
- 8. Kummar S, Allen D, Monks A, Polley EC, Hose CD, Ivy SP, et al. Cediranib for metastatic alveolar soft part sarcoma. J Clin Oncol 2013;31:2296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stacchiotti S, Negri T, Zaffaroni N, Palassini E, Morosi C, Brich S, et al. Sunitinib in advanced alveolar soft part sarcoma: evidence of a direct antitumor effect. Ann Oncol 2011;22:1682–90. [DOI] [PubMed] [Google Scholar]
- 10. Smrke A, Frezza AM, Giani C, Somaiah N, Brahmi M, Czarnecka AM, et al. Systemic treatment of advanced clear cell sarcoma: results from a retrospective international series from the world sarcoma network. ESMO Open 2022;7:100522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jakob J, Simeonova A, Kasper B, Ronellenfitsch U, Rauch G, Wenz F, et al. Combined sunitinib and radiation therapy for preoperative treatment of soft tissue sarcoma: results of a phase I trial of the German interdisciplinary sarcoma group (GISG-03). Radiat Oncol 2016;11:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Martin-Broto J, Hindi N, Grignani G, Martinez-Trufero J, Redondo A, Valverde C, et al. Nivolumab and sunitinib combination in advanced soft tissue sarcomas: a multicenter, single-arm, phase Ib/II trial. J Immunother Cancer 2020;8:e001561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205–16. [DOI] [PubMed] [Google Scholar]
- 14. Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials 1989;10:1–10. [DOI] [PubMed] [Google Scholar]
- 15. Sankaran H, O'Sullivan G, Chen A, Best A. A SEER based analysis of alveolar soft part sarcoma from 1975 2018: incidence, patterns of presentation, and trends in survival. Connective Tissue Oncology Society; 2021 [Google Scholar]
- 16. US Census Bureau; 2022
- 17. Cohen JW, Widemann BC, Derdak J, Dombi E, Goodwin A, Dompierre J, et al. Cediranib phase-II study in children with metastatic alveolar soft-part sarcoma (ASPS). Pediatr Blood Cancer 2019;66:e27987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Judson I, Morden JP, Kilburn L, Leahy M, Benson C, Bhadri V, et al. Cediranib in patients with alveolar soft-part sarcoma (CASPS): a double-blind, placebo-controlled, randomised, phase 2 trial. Lancet Oncol 2019;20:1023–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stacchiotti S, Tamborini E, Marrari A, Brich S, Rota SA, Orsenigo M, et al. Response to sunitinib malate in advanced alveolar soft part sarcoma. Clin Cancer Res 2009;15:1096–104. [DOI] [PubMed] [Google Scholar]
- 20. Orbach D, Brennan B, Casanova M, Bergeron C, Mosseri V, Francotte N, et al. Paediatric and adolescent alveolar soft part sarcoma: a joint series from European cooperative groups. Pediatr Blood Cancer 2013;60:1826–32. [DOI] [PubMed] [Google Scholar]
- 21. Li T, Wang L, Wang H, Zhang S, Atikan K, Zhang X, et al. A retrospective analysis of 14 consecutive Chinese patients with unresectable or metastatic alveolar soft part sarcoma treated with sunitinib. Invest New Drugs 2016;34:701–06. [DOI] [PubMed] [Google Scholar]
- 22. Jagodzinska-Mucha P, Switaj T, Kozak K, Kosela-Paterczyk H, Klimczak A, Lugowska I, et al. Long-term results of therapy with sunitinib in metastatic alveolar soft part sarcoma. Tumori 2017;103:231–5. [DOI] [PubMed] [Google Scholar]
- 23. Desai J. Response assessment in gastrointestinal stromal tumor. Int J Cancer 2011;128:1251–8. [DOI] [PubMed] [Google Scholar]
- 24. Choi H, Charnsangavej C, de Castro Faria S, Tamm EP, Benjamin RS, Johnson MM, et al. CT evaluation of the response of gastrointestinal stromal tumors after imatinib mesylate treatment: a quantitative analysis correlated with FDG PET findings. AJR Am J Roentgenol 2004;183:1619–28. [DOI] [PubMed] [Google Scholar]
- 25. Fukumura D, Kloepper J, Amoozgar Z, Duda DG, Jain RK. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol 2018;15:325–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Conley AP, Trinh VA, Zobniw CM, Posey K, Martinez JD, Arrieta OG, et al. Positive tumor response to combined checkpoint inhibitors in a patient with refractory alveolar soft part sarcoma: a case report. J Glob Oncol 2018;4:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lewin J, Davidson S, Anderson ND, Lau BY, Kelly J, Tabori U, et al. Response to immune checkpoint inhibition in two patients with alveolar soft-part sarcoma. Cancer Immunol Res 2018;6:1001–7. [DOI] [PubMed] [Google Scholar]
- 28. Wilky BA, Trucco MM, Subhawong TK, Florou V, Park W, Kwon D, et al. Axitinib plus pembrolizumab in patients with advanced sarcomas including alveolar soft-part sarcoma: a single-centre, single-arm, phase 2 trial. Lancet Oncol 2019;20:837–48. [DOI] [PubMed] [Google Scholar]
- 29. Shi Y, Cai Q, Jiang Y, Huang G, Bi M, Wang B, et al. Activity and safety of geptanolimab (GB226) for patients with unresectable, recurrent, or metastatic alveolar soft part sarcoma: a phase II, single-arm study. Clin Cancer Res 2020;26:6445–52. [DOI] [PubMed] [Google Scholar]
- 30. Naqash AR, O'Sullivan Coyne GH, Moore N, Sharon E, Takebe N, Fino KK, et al. Phase II study of atezolizumab in advanced alveolar soft part sarcoma (ASPS). J Clin Oncol 2021;39:11519. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data generated in this study are available upon request from the corresponding author.