Abstract
Background/purpose
Head and neck squamous cell carcinoma (HNSCC) is a serious disease endangering the health of patients, and the application of immunotherapy in HNSCC is gradually emerging. However, there is no bibliometric analysis in this research field. This study aims to provide a comprehensive overview of the knowledge structure and research hotspots of immunotherapy for HNSCC.
Materials and methods
Publications related to immunotherapy for HNSCC from 2002 to 2021 were searched in the Web of Science Core Collection database. The software VOSviewers, CiteSpace, and the R package ‘bibliometrix’ were used to perform this bibliometric analysis.
Results
A total of 1297 publications were from 63 countries, led by the USA and China. The number of publications related to immunotherapy for HNSCC has increased rapidly from 2015. University of Pittsburgh and The University of Texas M.D. Anderson Cancer Center are the main research institutions. Oral Oncology is the most popular journal in this field, and the Journal of Clinical Oncology is the most highly co-cited journal. These publications were from 7569 authors, with Robert L. Ferris publishing the most papers and being the most frequently co-cited. Clinical trials related to nivolumab and pembrolizumab have attracted wide attention. ‘Immune checkpoint inhibitors’, ‘human papillomavirus’, ‘programmed cell death-ligand 1’, and ‘programmed cell death protein 1’ are the main keywords of emerging research hotspots.
Conclusion
This study presents a comprehensive summary of the trends and development of immunotherapy for HNSCC, identifies the research frontier and hotspot direction, and could provide a valuable reference for researchers in this field.
Keywords: Head and neck squamous cell carcinoma, Immunotherapy, Bibliometric, Immune checkpoint inhibitors, Programmed cell death-ligand 1
Introduction
As the seventh most common type of cancer worldwide, the incidence and mortality of head and neck cancer have been gradually increasing, with more than 930,000 new head and neck cancer cases, and 460,000 new deaths reported in 2020.1,2 Squamous cell carcinoma was the most common histopathological type of head and neck cancers.3 Tobacco smoking, alcohol drinking, betel nut chewing, and human papillomavirus infection were common risk factors for head and neck squamous cell carcinoma (HNSCC).4 Although the survival of HNSCC has improved slightly over the past few decades, the prognosis of advanced disease remains poor because of recurrence and metastasis.4,5
The main radical treatment modalities for local HNSCC are surgery and neck dissection, radiotherapy, and chemotherapy.4,6 Cisplatin and cetuximab are recommended for patients with local advanced HNSCC.4 The cytotoxic chemotherapeutic agent cisplatin increased overall survival, but the use of high-dose cisplatin has serious side effects.6 The anti-epidermal growth factor receptor (EGFR) treatment agent cetuximab was approved by the Food and Drug Administration for the treatment of multiple HNSCC, however, replacing cisplatin with cetuximab may reduce survival for cases with HPV-positive HNSCC.4,6 Given the intra- and inter-tumor molecular heterogeneity, the development of targeted therapy for HNSCC is difficult.6,7
In 2016, the Food and Drug Administration approved immunotherapy using the anti-programmed cell death protein 1 (PD-1) immune checkpoint inhibitors nivolumab and pembrolizumab.8 The European Commission began approving the use of these monoclonal antibodies in 2017.8 In the following years, the application range of PD-1 inhibitors gradually increased, and the combined positive score for programmed cell death-ligand 1 (PD-L1) was established as the standard of application.8 PD-1 is expressed on activated T cells, B cells, natural killer cells, and other cells, while PD-L1 is expressed by tumor cells in the tumor microenvironment. After PD-1 interaction with its ligand PD-L1, immune responses are reduced.9 The interaction of tumor PD-L1 and PD-1 on tumor-infiltrating lymphocytes has been suggested to be the main mechanism of tumor immune escape.9 Clinical trials with the PD-1 antibodies nivolumab and pembrolizumab have demonstrated anti-tumor activity towards HNSCC.10, 11, 12
Bibliometric analysis is a useful way to statistically assess trends in research work. It provides an objective assessment of academic contributions through a comprehensive analysis of selected literature by authors, institutions, countries, journals, citations, and keywords, and provides an understanding of research hotspots and trends. There is currently no relevant bibliometric analysis for HNSCC. To better grasp the current situation and trend of research of immunotherapy for HNSCC, this study conducted a bibliometric analysis of publications related to immunotherapy for HNSCC from 2000 to 2021 (22 years), to understand its distribution, explore research hotspots, predict potential research trends, and contribute to future research.
Materials and methods
Data collection
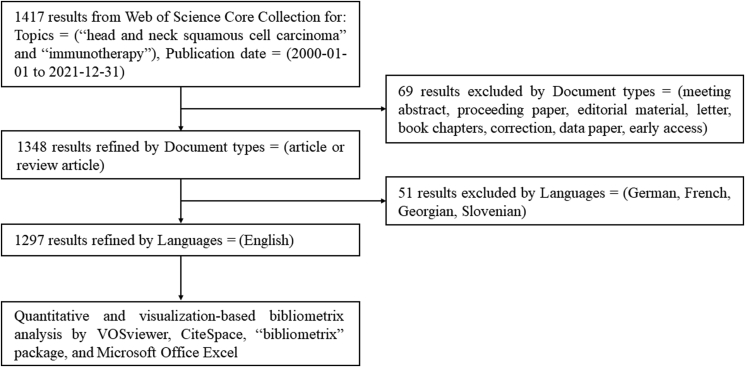
The bibliometrics investigation was conducted in the database of Web of Science Core Collection (https://www.webofscience.com/wos/alldb/basic-search). The procedure for data retrieval and collection is shown in Fig. 1. The search strategy was ‘head and neck squamous cell carcinoma’ and ‘immunotherapy’ covering 2000 to 2021. Retrieved papers were saved as a ‘plain text file’ and exported as ‘full record and cited references’.
Figure 1.
Publications screening flowchart.
Data analysis
The VOSviewer (version 1.6.18) software tool was used for constructing and visualizing bibliometric networks. VOSviewer offers text mining functionality used to construct and visualize co-occurrence networks of important terms extracted from a body of scientific literature.13 In this study, the software mainly completed the following: country and institution, journal and co-cited journal, author and co-cited author, and keyword co-occurrence analysis. CiteSpace (version 6.1.3) is a tool for progressive knowledge domain visualization, and focuses on finding critical points in the development of a field or a domain, especially intellectual turning points and pivotal points.14 In this study, CiteSpace was applied to map the dual-map overlay of journals and to analyze reference with Citation Bursts. The R package ‘bibliometrix’ (version 4.0.1) is an open-source tool for quantitative research in scientometrics and bibliometrics that includes all the main bibliometric methods of analysis, including the instrument to pursue a complete bibliometric analysis.15 In this study, ‘bibliometrix’ was applied for a thematic evolution analysis and to construct a global distribution network of publications. Microsoft Office Excel 2016 was used to conduct the global trend in publication outputs.
Results
Global trend in publication outputs
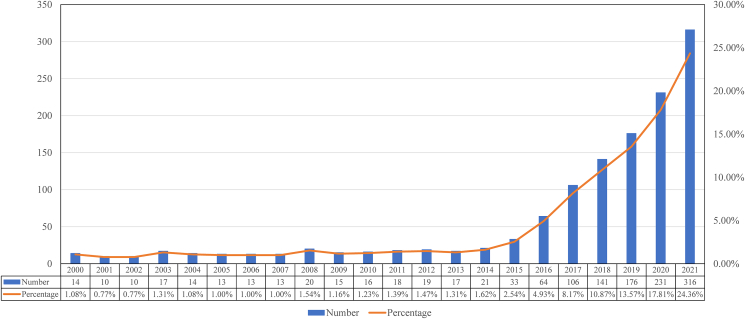
This study obtained 1297 articles published in the Web of Science Core Collection on immunotherapy for HNSCC. Fig. 2 shows the worldwide trend in publications from 2000 to 2021. Immunotherapy for HNSCC advanced rapidly in the last two years, accounting for 42% of all publications. The number of global articles yearly increased from 14 in 2000 to 316 in 2021, with a 7.5% annual growth rate. Only a quarter of publications were released before 2016. After 2017, there was a noticeable increase in the annual publications. In 2021, the number of publications of immunotherapy for HNSCC was 316.
Figure 2.
Worldwide trend in publications of immunotherapy for head and neck squamous cell carcinoma.
Distribution of countries and institutions
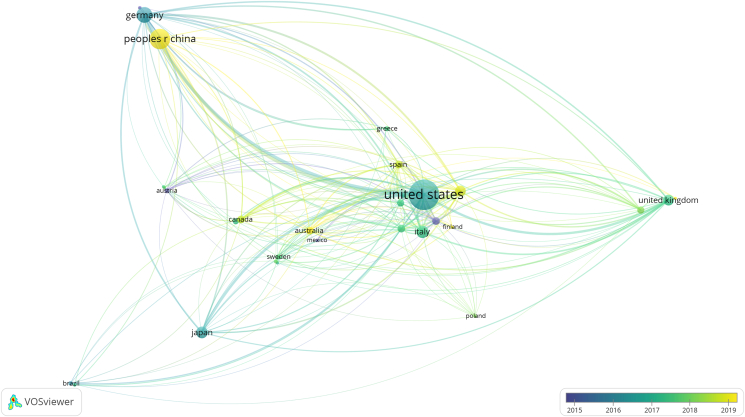
The 1297 publications came from 63 countries and 1837 institutions. The top ten countries were distributed in North America, Asia, and Europe (Table 1). The country with the largest number of publications was the USA (n = 587), followed by China (n = 256), and Germany (n = 162). The combined number of publications from the USA and China accounted for over 60% of the total. Subsequently, we filtered and visualized 30 countries based on the number of publications ≥ five, and constructed a collaborative network based on the number and relationship of publications in each country (Fig. 3). Notably, there was considerable active cooperation between certain countries. For example, the USA had close cooperation with China, Germany, Japan, and the UK.
Table 1.
Top 10 countries and institutions on research of immunotherapy for head and neck squamous cell carcinoma.
| Rank | Country | Number | Institution | Number |
|---|---|---|---|---|
| 1 | USA (North America) | 587 | University of Pittsburgh (USA) | 73 |
| 2 | China (Asia) | 256 | The University of Texas M.D. Anderson Cancer Center (USA) | 41 |
| 3 | Germany (Europe) | 162 | University of Michigan (USA) | 40 |
| 4 | Italy (Europe) | 94 | Sun Yat-sen University (China) | 37 |
| 5 | Japan (Asia) | 91 | Wuhan University (China) | 34 |
| 6 | France (Europe) | 73 | Johns Hopkins University (USA) | 33 |
| 7 | UK (Europe) | 73 | Dana-Farber Cancer Institute (USA) | 29 |
| 8 | Spain (Europe) | 39 | Harvard Medical School (USA) | 27 |
| 9 | Netherlands (Europe) | 37 | University of California San Diego (USA) | 24 |
| 10 | Canada (North America) | 37 | National Cancer Institute (USA) | 24 |
Figure 3.
The geographical distribution on research of immunotherapy for head and neck squamous cell carcinoma.
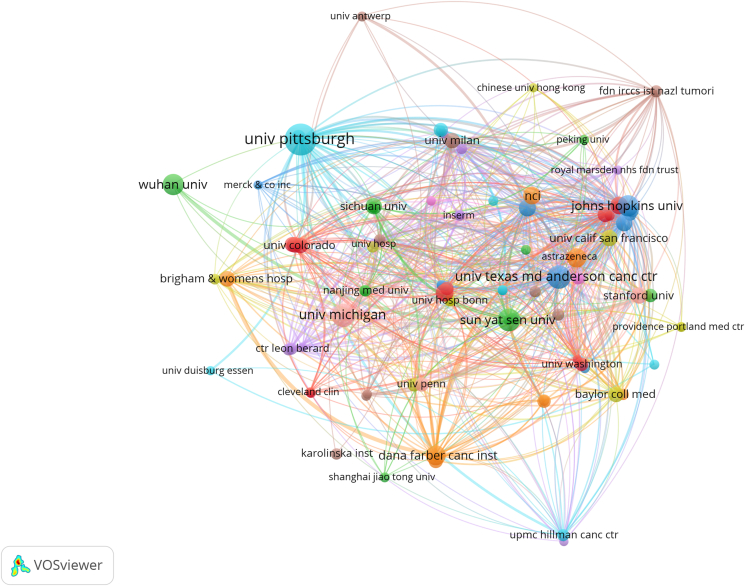
The top 10 institutions were located in two countries, with eight in the USA and two in China. The three institutions that published the most relevant papers were: University of Pittsburgh (n = 73), The University of Texas M.D. Anderson Cancer Center (n = 41), and University of Michigan (n = 40). Subsequently, we selected 97 institutions based on the minimum number of publications equal to seven for visualization, and constructed a collaborative network based on the number and relationship of publications of each institution (Fig. 4). The cooperation between the University of Pittsburgh, The University of Texas M.D. Anderson Cancer Center, and Johns Hopkins University was close, and there was active cooperation between the Dana-Farber Cancer Institute and Harvard Medical School. In addition, we note that two Chinese research institutions ranked in the top 10 did not work together.
Figure 4.
The visualization of institutions on research of immunotherapy for head and neck squamous cell carcinoma.
Journals and co-cited journals
Publications related to immunotherapy for HNSCC were published in 372 journals. Oral Oncology published the most papers (n = 82), followed by Cancers (n = 54), and Frontiers in oncology (n = 52). Among the top 10 journals, the journal with the highest impact factor (IF) was Clinical Cancer Research (IF = 13.801), followed by the Journal for Immunotherapy of Cancer (IF = 12.485). Subsequently, we screened 41 journals based on the minimum number of relevant publications equal to seven and mapped the journal network. Supplementary Fig. S1A shows that Oral Oncology had active citation relationships with Frontiers in Oncology, Journal for Immunotherapy of Cancer, and Clinical Cancer Research journals.
As shown in Table 2, among 4209 co-cited journals, four journals were cited more than 2800 times, and the Journal of Clinical Oncology (Co-citation = 4074) was the most cited journal, followed by Clinical Cancer Research (Co-citation = 3241), New England Journal of Medicine (Co-citation = 2978), and Cancer Research (Co-citation = 2811). The impact factor of five journals was more than 50. The impact factor of the New England Journal of Medicine was the highest (IF = 176.082), followed by Nature (IF = 69.504). Forty-seven journals with the minimum co-citation equal to 300 were filtered to map the co-citation network (Supplementary Fig. S1B). Journal of Clinical Oncology had positive co-citation relationships with Lancet Oncology, Annals of Oncology, Clinical Cancer Research, Cancer Research, and Oral Oncology journals.
Table 2.
Top 10 journals and co-cited journals for research of immunotherapy for head and neck squamous cell carcinoma.
| Rank | Journal | Number | IF (2021) | JCR (2021) | Co-cited Journal | Co-citations | IF (2021) | JCR (2021) |
|---|---|---|---|---|---|---|---|---|
| 1 | Oral Oncology | 82 | 5.972 | Q1 | Journal of Clinical Oncology | 4074 | 50.739 | Q1 |
| 2 | Cancers | 54 | 6.575 | Q1 | Clinical Cancer Research | 3241 | 13.801 | Q1 |
| 3 | Frontiers in Oncology | 52 | 5.738 | Q2 | New England Journal of Medicine | 2978 | 176.082 | Q1 |
| 4 | Head and Neck | 43 | 3.827 | Q1 | Cancer Research | 2811 | 13.312 | Q1 |
| 5 | Journal for Immunotherapy of Cancer | 32 | 12.485 | Q1 | Oral Oncology | 1766 | 5.972 | Q1 |
| 6 | Clinical Cancer Research | 31 | 13.801 | Q1 | Annals of Oncology | 1555 | 51.769 | Q1 |
| 7 | Oncoimmunology | 30 | 7.723 | Q1 | Lancet Oncology | 1374 | 54.433 | Q1 |
| 8 | Cancer Immunology Immunotherapy | 29 | 6.63 | Q1 | Nature | 1295 | 69.504 | Q1 |
| 9 | Frontiers in Immunology | 28 | 8.787 | Q1 | Head and Neck | 1285 | 3.827 | Q1 |
| 10 | International Journal of Molecular Sciences | 18 | 6.208 | Q1 | Journal of Immunology | 1234 | 5.43 | Q2 |
IF: impact factor, JCR: journal citation reports, Q1: first quartile (highest-ranked).
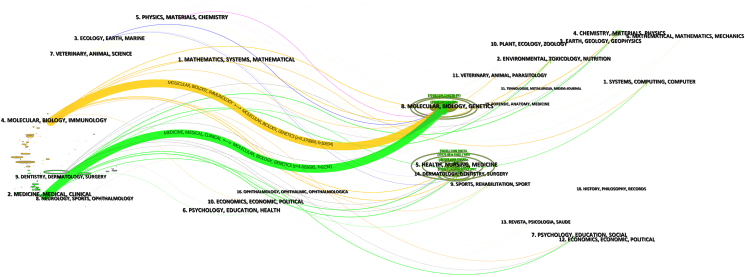
The dual-map overlay of journals shows the citation relationships between citing journals (left) and co-cited journals (right) (Fig. 5). The orange and green paths are the two main citation paths, representing research published in Molecular/Biology/Genetics journals mainly cited by literature in Molecular/Biology/Immunology and Medicine/Medical/Clinical journals (see Fig. 5).
Figure 5.
The dual-map overlay of journals on research of immunotherapy for head and neck squamous cell carcinoma.
Authors and co-cited authors
A total of 7569 authors participated in research of immunotherapy for HNSCC. Among the top ten authors, Robert L. Ferris published 49 papers while others published less than 20 papers (Table 3). We built a collaborative network based on authors whose number of published papers was ≥ six (Supplementary Fig. S2A). Robert L. Ferris had the largest node because he published the most related publications. Close collaboration was observed among multiple authors. For example, Nabil F. Saba had active cooperation with Robert L. Ferris, Wen-Feng Zhang and Liang Mao had close cooperation with Zhi-Jun Sun.
Table 3.
Top 10 authors and co-cited authors on research of immunotherapy for head and neck squamous cell carcinoma.
| Rank | Authors | Publications | Co-cited authors | Citations |
|---|---|---|---|---|
| 1 | Robert L. Ferris | 46 | Robert L. Ferris | 1048 |
| 2 | Zhi-Jun Sun | 19 | Tanguy Y. Seiwert | 465 |
| 3 | Wen-Feng Zhang | 16 | Jan B. Vermorken | 463 |
| 4 | Liang Mao | 15 | Ezra E W Cohen | 351 |
| 5 | Nabil F. Saba | 15 | Barbara Burtness | 295 |
| 6 | Lin-Lin Bu | 14 | Maura L. Gillison | 264 |
| 7 | Wei-Wei Deng | 14 | K Kian Ang | 245 |
| 8 | Ravindra Uppaluri | 14 | Theresa L. Whiteside | 210 |
| 9 | Guang-Tao Yu | 12 | Athanassios Argiris | 200 |
| 10 | Lisa Licitra | 12 | Michael S. Lawrence | 194 |
Among the 27,990 co-cited authors, four authors were co-cited more than 300 times (Table 3). The most co-cited author was Robert L. Ferris (n = 1048), followed by Tanguy Y. Seiwert (n = 465) and Jan B. Vermorken (n = 463). Authors with minimum co-citations equal to 50 were filtered to map co-citation network graphs (Supplementary Fig. S2B). There were active collaborations among different co-cited authors, such as Robert L. Ferris and Tanguy Y. Seiwert, Ezra E W Cohen and Jan B. Vermorken.
Co-cited references
There were 40,719 co-cited references on research of immunotherapy for HNSCC over the past 22 years. In the top 10 co-cited references (Table 4), all references were co-cited at least 130 times, and one reference was co-cited 472 times. Three papers were published in the New England Journal of Medicine, and Robert Ferris contributed two papers. Twenty-six references with co-citations ≥70 were used for construction of the co-citation network map (Supplementary Fig. S3). According to the figure, ‘Ferris RL, 2016, New Engl J med’ shows active co-cited relationships with ‘Seiwert TY, 2016, Lancet Oncol’, ‘Burtness B, 2019, Lancet’ and ‘Bauml J, 2017, J Clin Oncol’.
Table 4.
Top 10 co-cited references on research of immunotherapy for head and neck squamous cell carcinoma.
| Rank | Title | Journal | First author | Year | Ciitations |
|---|---|---|---|---|---|
| 1 | Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck | New England journal of medicine | Ferris, Robert L | 2016 | 472 |
| 2 | Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial | Lancet. Oncology | Seiwert, Tanguy Y | 2016 | 290 |
| 3 | Platinum-based chemotherapy plus cetuximab in head and neck cancer | New England journal of medicine | Vermorken, Jan B | 2008 | 224 |
| 4 | Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study | Lancet | Burtness, Barbara | 2019 | 193 |
| 5 | Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study | Lancet | Cohen, Ezra E W | 2019 | 173 |
| 6 | Comprehensive genomic characterization of head and neck squamous cell carcinomas | Nature | Lawrence, Michael S | 2015 | 166 |
| 7 | Immunology and Immunotherapy of Head and Neck Cancer | Journal of clinical oncology | Ferris, Robert L | 2015 | 161 |
| 8 | Human papillomavirus and survival of patients with oropharyngeal cancer | New England journal of medicine | Ang, K Kian | 2010 | 160 |
| 9 | Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries | CA: a cancer journal for clinicians | Bray, Freddie | 2018 | 140 |
| 10 | Pembrolizumab for Platinum- and Cetuximab-Refractory Head and Neck Cancer: Results From a Single-Arm, Phase II Study | Journal of clinical oncology | Bauml, Joshua | 2017 | 130 |
Reference with citation bursts
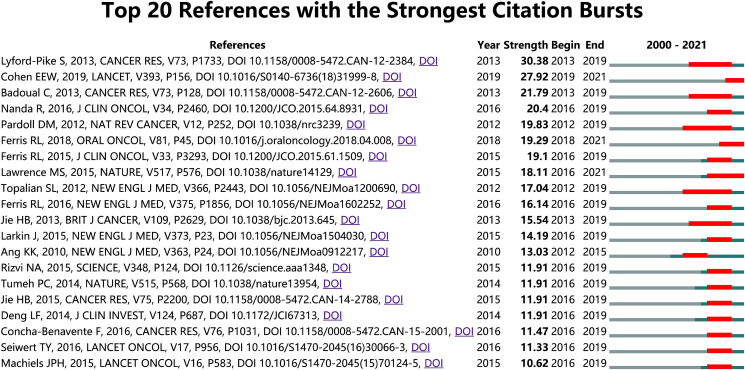
Reference with citation bursts refers to those references that are frequently cited by scholars in a certain field over a period of time. In this study, 20 references with strong citation bursts were identified by CiteSpace. As shown in Fig. 6, the red bar represents strong citation bursts. Citation bursts for references appeared as early as 2012 and as late as 2021. The reference with the strongest citation burst (strength = 30.38) was titled ‘Evidence for a Role of the PD-1: PD-L1 Pathway in Immune Resistance of HPV-Associated Head and Neck Squamous Cell Carcinoma’, authored by Sofia Lyford-Pike et al., with citation bursts from 2013 to 2019. The reference with the second strongest citation burst (strength = 27.92) was titled ‘Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study’, published in Lancet by E. W. Cohen Ezra et al., with citation bursts from 2019 to 2021. Overall, the burst strength of these 20 references ranged from 10.62 to 30.38, and endurance strength was from 2 to 7 years.
Figure 6.
Top 20 references with strong citation bursts. A red bar indicates high citations in that year.
Hotspots and frontiers
Co-occurrence analysis of keywords captured research hotspots in the field. Table 5 shows the top 20 high-frequency keywords in research of immunotherapy for HNSCC. Among 2214 keywords, immune checkpoint inhibitors appeared more than 100 times, followed by human papillomavirus (n = 99), PD-L1 (n = 86), and PD-1 (n = 74), representing the main research direction of immunotherapy for HNSCC.
Table 5.
Top 20 keywords on research of immunotherapy for head and neck squamous cell carcinoma.
| Rank | Keywords | Number | Rank | Keywords | Number |
|---|---|---|---|---|---|
| 1 | Head and neck squamous cell carcinoma | 687 | 11 | Chemotherapy | 50 |
| 2 | Immunotherapy | 506 | 12 | Oral squamous cell carcinoma | 50 |
| 3 | Immune checkpoint inhibitors | 104 | 13 | Tumor microenvironment | 49 |
| 4 | Human papillomavirus | 99 | 14 | Immune checkpoints | 35 |
| 5 | PD-L1 | 86 | 15 | Cancer | 35 |
| 6 | PD-1 | 74 | 16 | Head and neck | 34 |
| 7 | Prognosis | 65 | 17 | Cytokines | 31 |
| 8 | Radiotherapy | 56 | 18 | Cetuximab | 30 |
| 9 | Biomarkers | 55 | 19 | Pembrolizumab | 30 |
| 10 | Nivolumab | 51 | 20 | Clinical trials | 27 |
PD-L1: programmed cell death-ligand 1, PD1: programmed cell death protein 1.
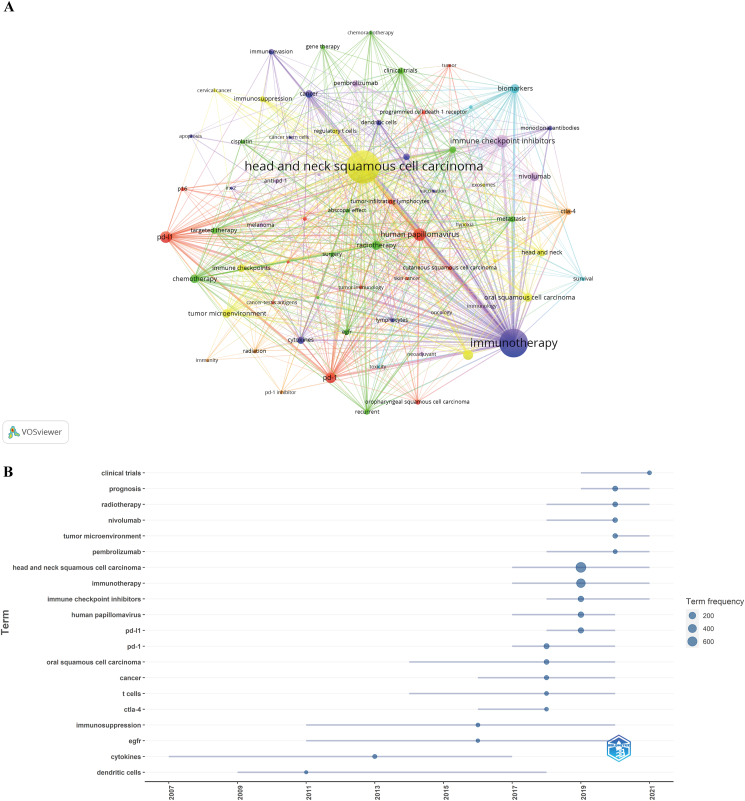
We filtered 69 keywords with the number of occurrences ≥7 and performed cluster analysis through VOSviewer (Fig. 7A). We obtained several clusters representing different research directions, as shown in Fig. 7. The keywords in yellow clusters consist of head and neck squamous cell carcinoma, tumor environment, and prognosis. The keywords in blue clusters consist of immunotherapy, immune evasion, and cytokines. The keywords in purple clusters consist of immune checkpoint inhibitors, nivolumab, and pembrolizumab. The keywords in red clusters consist of PD-L1, PD-1, human papillomavirus, and immunohistochemistry. The keywords in green clusters consist of radiotherapy, chemotherapy, chemoradiotherapy, and clinical trials.
Figure 7.
Keyword cluster analysis (A) and trend topic analysis (B) on research of immunotherapy for head and neck squamous cell carcinoma.
Trend topic analysis of keywords (Fig. 7B) showed that from 2000 to 2016, research in this period mainly focused on cytokines and immune cells, and that the main keywords were cytokines, dendritic cells, EGFR, immunosuppression, T cells, oral squamous cell carcinoma, CTLA-4, and cancer. Since 2017, research began to actively explore immunotherapy for HNSCC, and the main keywords were PD-1, PD-L1, human papillomavirus, immune checkpoint inhibitors, immunotherapy, and head and neck squamous cell carcinoma. For 2020–2021, the keywords pembrolizumab, tumor microenvironment, nivolumab, radiotherapy, prognosis, and clinical trials appeared frequently, and were very likely to represent current research hotspots of immunotherapy for HNSCC.
Discussion
This study obtained 1297 publications on immunotherapy for HNSCC from 2000 to 2021 by searching the Web of Science Core Collection. Annual publications increased rapidly after 2015 and were markedly increased in the last two years. The USA was the highest academic contribution driver for immunotherapy for HNSCC in the past 22 years, reflected in the number of publications, and eight of the top 10 institutions were located in the USA. The second largest contributor was China, and two of the top 10 institutions were located in China. European countries, such as Germany, have also contributed in this field. Extensive collaborative research has been conducted between countries and institutions. Because of the prevalence of betel nut chewing, the incidence of HNSCC is high in Central and South Asia,1 but research in the direction of immunotherapy for HNSCC is limited, which may need to be addressed in the future.
Among the top 10 journals, Oral Oncology, Cancers, and Frontiers in Oncology have published more than 50 papers. The most common co-cited journals were the Journal of Clinical Oncology, Clinical Cancer Research, and New England Journal of Medicine. Oral Oncology and Head and Neck are two professional journals in the field of head and neck diseases, simultaneously entering the 10 journals and top 10 co-cited journals, possibly representing their preference for immunotherapy for HNSCC. Research of immunotherapy for HNSCC was mainly published in molecular/biology/immunology, and medicine/medical/clinical journals, indicating that current research has made good progress in both basic research and clinical applications.
From the perspective of authors, Robert L. Ferris from the University of Pittsburgh was the most published author, with more than 40 publications, and the most co-cited author, with more than 1000 cited times. He is one of the pioneers of immunotherapy for HNSCC and his research interests include HNSCC immunology and immunotherapy, tumor antigen specific monoclonal antibodies, tumor microenvironment and immune evasion, and role of human papillomavirus in HNSCC.6,8,12,16, 17, 18, 19 His team conducted several phase I, II, and III immunotherapy trials, such as cetuximab and nivolumab trials.12,18,19 Tanguy Y. Seiwert, Y and Jan B. Vermorken were the second and third ranked co-cited authors, who also conducted clinical trials of pembrolizumab and cetuximab, respectively.20,21
Co-cited references refer to references cited together by multiple other publications, and therefore co-cited references can be regarded as a research basis and hotspot in a specific field. This bibliometric study selected the top 10 co-referenced references. From a review of the literature, these references were divided into three categories, including seven papers of clinical trials for HNSCC,11,12,20, 21, 22, 23, 24 one paper of a comprehensive genomics analysis of HNSCC,7 one paper presenting an immunology review of HNSCC,17 and one paper of global cancer statistics.25 Clinical trials included four papers of clinical trials related to pembrolizumab,11,21, 22, 23 one paper of a clinical trial related to nivolumab,12 one paper of a clinical trial related to cetuximab,20 and one paper of the prognosis in human papillomavirus-related oropharyngeal cancer.24 Both nivolumab and pembrolizumab are inhibitors of immune checkpoint PD-1 and are used as immunotherapeutic agents in the clinical management of HNSCC.8 According to the Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of HNSCC,8 pembrolizumab was applied as first- and second-line therapy for HNSCC, while nivolumab was applied as second-line therapy, possibly reflecting the co-citated differences between the two PD-1 inhibitors in this study. The paper reporting a clinical trial related to cetuximab was published in 2008.6 Cetuximab, a monoclonal antibody targeting EGFR, and provided the main first-line chemotherapeutic agent for HNSCC before PD-1 inhibitors were approved for treatment.6 The clinical trial on human papillomavirus and survival of patients with oropharyngeal cancer published by Ang et al. set the tone for a better prognosis in human papillomavirus-related oropharyngeal cancer.24 References with strongest citation bursts represent emerging topics in specific research areas, because these references have been frequently cited by investigators in the years examined. As of 2021, three publications have strong citation bursts, representing clinical trials related to nivolumab,19 and pembrolizumab,22 as well as the genomic characterization of HNSCC,7 respectively.
Keywords can quickly capture the distribution and evolution of hotspots in the research field of immunotherapy for HNSCC. Immune checkpoint inhibitors, human papillomavirus, PD-L1, and PD-1 represented the main research direction of immunotherapy for HNSCC. Cluster analysis of the keywords revealed the following main categories: tumor environment-related cluster, immunotherapy-related cluster, immune checkpoint inhibitors-related cluster, PD-L1 and PD-1-related cluster, and clinical trials-related cluster. From the research trends, immunotherapy for HNSCC focused on immune cells, such as dendritic and T cells, and their products, cytokines, as well as alterations in specific genes, such as EGFR. However, with the approval of PD-1 inhibitors for immunotherapy targeting HNSCC, the relevant studies on HNSCC have gradually focused on the immune checkpoint inhibitors, PD-1 and PD-L1. After a comprehensive genome analysis of HNSCC,7 research targeting a single biomarker gradually tended to be a global study of the tumor microenvironment. Although oral squamous cell carcinoma was the most common subsite in a HNSCC study,2 the significant correlation in prognosis between human papillomavirus and oropharyngeal squamous cell carcinoma,24 lead to obvious attention by researchers. Two approved monoclonal antibodies for HNSCC, nivolumab and pembrolizumab, led researchers to conduct hot clinical trials over the past few years, further promoting the application of PD-1 inhibitors in HNSCC immunotherapy.8
This study has its limitations. Data were collected from a single source (Web of Science Core Collection), leading to the omission of publications from other sources. Only English papers were entered in this study, which may also lead to source bias. Furthermore, papers published in the year of 2022 were not included in the statistics, and this may affect the research trends.
To the best of our knowledge, this study is the first bibliometric analysis of immunotherapy for HNSCC in the past 22 years. The rapid growth of publications indicates that HNSCC immunotherapy is gaining increasing attention worldwide. The USA and China have made significant contributions to progress in this field by publishing a high number of important articles and maintaining high quality research. Extensive cooperation has been conducted between different countries and institutions. PD-1 inhibitors are currently used in clinical immunotherapy for HNSCC. The development of multiple clinical trials has facilitated the application of nivolumab and pembrolizumab for treatment of HNSCC. In summary, the field of immunotherapy for HNSCC exhibits good trends in both basic research and clinical transformation.
Declaration of competing interest
The authors have no conflicts of interest relevant to this article.
Acknowledgement
This work was supported by research grants from the National Nature Science Foundation of China (81671006, 81300894), CAMS Innovation Fund for Medical Sciences (2019-I2M-5-038), National Clinical Key Discipline Construction Project (PKUSSNKP-202102).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jds.2023.02.007.
Contributor Information
He-Yu Zhang, Email: zhangheyu1983@sina.cn.
Jian-Yun Zhang, Email: jianyunz0509@aliyun.com.
Tie-Jun Li, Email: litiejun22@vip.sina.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2022. CA A Cancer J Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 3.Mody M.D., Rocco J.W., Yom S.S., Haddad R.I., Saba N.F. Head and neck cancer. Lancet. 2021;398:2289–2299. doi: 10.1016/S0140-6736(21)01550-6. [DOI] [PubMed] [Google Scholar]
- 4.Johnson D.E., Burtness B., Leemans C.R., Lui V.W.Y., Bauman J.E., Grandis J.R. Head and neck squamous cell carcinoma. Nat Rev Dis Prim. 2020;6:92. doi: 10.1038/s41572-020-00224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Magnes T., Wagner S., Kiem D., et al. Prognostic and predictive factors in advanced head and neck squamous cell carcinoma. Int J Mol Sci. 2021;22:4981. doi: 10.3390/ijms22094981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cramer J.D., Burtness B., Le Q.T., Ferris R.L. The changing therapeutic landscape of head and neck cancer. Nat Rev Clin Oncol. 2019;16:669–683. doi: 10.1038/s41571-019-0227-z. [DOI] [PubMed] [Google Scholar]
- 7.Cancer Genome Atlas N. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517:576–582. doi: 10.1038/nature14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen E.E.W., Bell R.B., Bifulco C.B., et al. The society for immunotherapy of cancer consensus statement on immunotherapy for the treatment of squamous cell carcinoma of the head and neck (hnscc) J Immunother Cancer. 2019;7:184. doi: 10.1186/s40425-019-0662-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morad G., Helmink B.A., Sharma P., Wargo J.A. Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell. 2021;184:5309–5337. doi: 10.1016/j.cell.2021.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harrington K.J., Burtness B., Greil R., et al. Pembrolizumab with or without chemotherapy in recurrent or metastatic head and neck squamous cell carcinoma: updated results of the phase III keynote-048 study. J Clin Oncol. 2022;41:790–802. doi: 10.1200/JCO.21.02508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burtness B., Harrington K.J., Greil R., et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (keynote-048): a randomised, open-label, phase 3 study. Lancet. 2019;394:1915–1928. doi: 10.1016/S0140-6736(19)32591-7. [DOI] [PubMed] [Google Scholar]
- 12.Ferris R.L., Blumenschein G., Jr., Fayette J., et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375:1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perianes-Rodriguez A., Waltman L., van Eck N.J. Constructing bibliometric networks: a comparison between full and fractional counting. J Informetr. 2016;10:1178–1195. [Google Scholar]
- 14.Chen C. Searching for intellectual turning points: progressive knowledge domain visualization. Proc Natl Acad Sci US A. 2004;101:5303–5310. doi: 10.1073/pnas.0307513100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aria M., Cuccurullo C. bibliometrix: an r-tool for comprehensive science mapping analysis. J Informetr. 2017;11:959–975. [Google Scholar]
- 16.Cillo A.R., Kurten C.H.L., Tabib T., et al. Immune landscape of viral- and carcinogen-driven head and neck cancer. Immunity. 2020;52:183–199. doi: 10.1016/j.immuni.2019.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferris R.L. Immunology and immunotherapy of head and neck cancer. J Clin Oncol. 2015;33:3293–3304. doi: 10.1200/JCO.2015.61.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferris R.L., Moskovitz J., Kunning S., et al. Phase I trial of cetuximab, radiotherapy, and ipilimumab in locally advanced head and neck cancer. Clin Cancer Res. 2022;28:1335–1344. doi: 10.1158/1078-0432.CCR-21-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferris R.L., Blumenschein G., Jr., Fayette J., et al. Nivolumab vs investigator's choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of checkmate 141 with analyses by tumor PD-L1 expression. Oral Oncol. 2018;81:45–51. doi: 10.1016/j.oraloncology.2018.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vermorken J.B., Mesia R., Rivera F., et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359:1116–1127. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 21.Seiwert T.Y., Burtness B., Mehra R., et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (keynote-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. 2016;17:956–965. doi: 10.1016/S1470-2045(16)30066-3. [DOI] [PubMed] [Google Scholar]
- 22.Cohen E.E.W., Soulieres D., Le Tourneau C., et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (keynote-040): a randomised, open-label, phase 3 study. Lancet. 2019;393:156–167. doi: 10.1016/S0140-6736(18)31999-8. [DOI] [PubMed] [Google Scholar]
- 23.Bauml J., Seiwert T.Y., Pfister D.G., et al. Pembrolizumab for platinum- and cetuximab-refractory head and neck cancer: results from a single-arm, phase II study. J Clin Oncol. 2017;35:1542–1549. doi: 10.1200/JCO.2016.70.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ang K.K., Harris J., Wheeler R., et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.