Abstract
Background/purpose
Toothpaste plays an important role in brushing teeth to maintain oral hygiene and health. The purpose of this study was to develop a new toothpaste containing surface nanocrystal-rich dicalcium phosphate anhydrous (DCPA) powder and to investigate its effect on tooth samples.
Materials and methods
The innovative toothpaste (REALCaP®/Group R) was compared with two commercial toothpastes (BioRepair®/Group B and Sensodyne®/Group S). Brushing cycle tests were performed on bovine tooth slices coated with individual toothpaste and a control group without toothpaste (Group C). Microhardness, roughness, surface structure observation, and X-ray diffraction (XRD) were performed on cycle days 4, 7, and 14 to analyze the impact of the toothpastes on tooth samples.
Reults
Microhardness in the Group R was higher than that of the other groups regardless of the cycle days. Roughness in the Group R increased on days 4 and 7 but decreased on day 14. Roughness in the groups S and B increased with days. Microstructural observation revealed that most exposed dentinal tubules had been sealed in the Group R on day 14. Overlay thickness in the Group R was significantly higher than that in the groups S and B on days 4, 7, and 14. XRD analysis showed no hydroxyapatite (HA) peak in the Group S. The HA peak in the Group R was higher than that in the Group B on day 14.
Conclusion
The innovative toothpaste has better properties than the commercially available products in terms of microhardness, roughness, and effectiveness in sealing dentinal tubules.
Keywords: Anti-sensitivity, Dicalcium phosphate anhydrous (DCPA), Remineralization, Toothpaste
Introduction
Teeth are very important for masticatory and digestive function, and brushing teeth is an essential step in maintaining oral hygiene and health. Proper tooth brushing combined with appropriate oral cleaner can maintain healthy teeth, which in turn aids the masticatory and digestive systems as well as facial aesthetics, promoting physical and mental health and social confidence. Toothpaste is a daily oral cleanser that contains many ingredients in addition to promoting oral hygiene, for example it contains an abrasive that removes plaque and food from the teeth. Each manufacturer has different additives touting various benefits, such as promoting periodontal health, strengthening enamel, alleviating tooth sensitivity, and whitening. The vast market demand has led to a lot of research and development to facilitate the advertising and product sales of various brands of toothpaste.
Calcium phosphate bioceramics are often used to make various biomedical materials. Calcium phosphates can phase transform their products into hydroxyapatite (HA) through the precipitation of calcium and phosphorus ions in an ion-rich environment under biomimetic conditions. Human hard tissues are rich in a large amount of HA, for example, alveolar bone and teeth in the body are mainly composed of HA.1, 2, 3, 4 In addition, calcium phosphate compounds have excellent biocompatibility and plenty of applications in dental restoration. Literature review in vitro or in vivo studies suggested that the addition of calcium phosphate biomedical ceramics can release calcium and phosphorus ions, thereby promoting the remineralization of tooth enamel.4, 5, 6
Our research team has developed surface-modified dicalcium phosphate anhydrous (DCPA) particles with nanocrystals on the surface.7, 8, 9 DCPA particles are coated with a layer of nanocrystals to make the particles form whisker-like crystals, which are used as resin fillers to compound dental resins. The whisker-like shape of the surface may increase the physical bond, and the hydroxyl group (-OH) of the calcium phosphate can be used to enhance the chemical bond between the resin and the tooth. Resin fillings can aid in the remineralization of damaged enamel through the spontaneous release of calcium and phosphate ions, which penetrate deeply into the dentinal tubules and form a strong bond between the resin and the tooth.
Studies have proposed that particles with calcium and phosphate are often used as ingredients of toothpaste, and toothpaste containing HA has a significant remineralization effect on teeth.10,11 Our previous study used surface nanocrystal-rich DCPA as an ingredient in toothpaste to explore whether it penetrates deep into the dentinal tubules like resin filler particles, and helps seal the dentinal tubules. Previous studies12, 13, 14, 15 have analyzed the efficacy of toothpaste in sealing dentinal tubules, but few studies have compared and explored the company's own toothpaste with commercially available products.
The purpose of this study was to develop a novel toothpaste with nanocrystal-enriched DCPA and to investigate its effect on the remineralization coverage of dentinal tubules. The samples of bovine tooth slices were subjected to brushing cycle tests, twice a day, and soaked in artificial saliva to simulate people's daily oral environment. The comparative effects of the innovative toothpaste and commercially available products on tooth samples were analyzed by microhardness, roughness, surface structure, and crystal phase. We look forward to the further application of this innovative toothpaste in daily oral health care and clinical treatment.
Materials and methods
Sample preparation
In this study, slices of bovine teeth were used as experimental samples. A slow-speed diamond cutter was used to create 84 samples with a length and width of 4 mm and a thickness of 2 ± 0.5 mm. The tooth samples must have good integrity, especially intact surface condition. Samples with decay or white spots on the surfaces were excluded.
After screening, the samples were embedded in epoxy resin (EPOXY601, Nan Pao Resins Chemical Co., Ltd, Tainan, Taiwan). Sandpapers with different roughness values (grit sizes: 800, 1200, 2000), alumina powders (0.1, 0.3, 1.0 μm) and polishing flannelettes were used to grind and polish the sample surfaces. After polishing, the sample surfaces were etched with citric acid (pH 2.2) for 2 min to expose the dentinal tubules.
The status of the tooth surfaces were examined with an optical microscope (Fig. 1). After completion, the samples were washed with distilled water in an ultrasonic oscillator for 3 min, and kept dry in a cool place for later use.
Fig. 1.
Optical microscope examination of bovine tooth specimens revealed the openings of dentinal tubules.
Experimental groups
The descriptions of the four groups in this study are shown in Table 1. The four groups included a control group in which the cleaner used was deionized water, a group of innovative toothpaste (REALCaP®, REALBONE Technology, Kaohsiung, Taiwan), and two groups of commercially available anti-sensitivity toothpaste (BioRepair®, Coswell S.p.A., Funo di Argelato, Italy) (Sensodyne®, Neocosmed, Ladlumkaew, Thailand).
Table 1.
Description of the four groups in this study.
| Group | Toothpaste | Manufacturer | Main ingredient |
|---|---|---|---|
| C | Control group | n/a | Deionized water |
| R | REALCaP® | REALBONE Technology, Kaohsiung, Taiwan | Dicalcium phosphate with nanocrystals |
| B | BioRepair® | Coswell S.p.A., Funo di Argelato, Italy | Zinc hydroxyapatite |
| S | Sensodyne® | Neocosmed, Ladlumkaew, Thailand | Calcium sodium phosphosilicate, Sodium fluoride |
Solution preparation
To simulate an acidic environment, citric acid powder was dissolved in deionized water, and an ion concentration analyzer (SP-2500, SUNTEX, New Taipei City, Taiwan) was used to monitor the pH value till the solution demonstrated a pH of 2.2. Artificial saliva was adjusted to pH 7.0 with 1 M sodium hydroxide (NaOH) alkaline solution.
Toothpaste slurry preparation
In the study, the toothpastes were individually mixed with deionized water to prepare the toothpaste slurries.16
Brushing cycle test
A tooth brushing machine was utilized to simulate human brushing action (Fig. 2). The brushing was set at 150 g normal force, and the samples were brushed with soft-bristled toothbrushes (Colgate Extra clean professional, Colgate-Palmolive Company, Binh Duong, Vietnam). Three periods (4, 7, 14 days) of brushing trials were carried out. The samples were etched with pH 2.2 citric acid solution for 2 min every day before brushing tests to simulate the acidic environment generated when people eat.
Fig. 2.
Equipment setup for the brushing cycle test.
The toothpaste slurries were individually applied to brushes and processed the samples for 3 min (brushing for 15 s with 10 times back and forth movement, and soaking for 165 s), twice a day. The solution was replaced daily with fresh artificial saliva (pH 7.0) to prevent saliva from starving of mineral ions. After brushing and soaking for different days, the samples were cleaned in an ultrasonic oscillator for 3 min and then stored in a saturated humidity tank (37 °C) until testing.
Microhardness analysis
A microhardness tester (HMV-2, Shimadzu, Kyoto, Japan) was utilized in this study. A Vickers pyramid was used as the indenter and a load of 100 g was applied for 10 s. The hardness value of the material was obtained by measuring the contour of diagonal line created by the indenter. The values at six different positions were recorded, and the average value was calculated as the microhardness of the sample.
Roughness analysis
The tooth samples were pre-recorded for roughness before brushing. Roughness analysis was done after processing the brushing test for 4, 7, and 14 days. A contact roughness analyzer (SJ-301, Mitutoyo Ltd., Kawasaki, Japan) was used to measure the surface roughness, and the distance between each scratch was set to be 100 μm. The values at six different positions were recorded, and average value was calculated as surface roughness of the sample.
Scanning electron microscope
A scanning electron microscope (SEM, Hitachi S3000 N, Tokyo, Japan) was used to observe the surface morphology of the tooth samples before and after brushing. The different magnifications were observed.
X-ray diffraction evaluation of overlays
The surface crystal phases of samples with various toothpastes were analyzed with an X-ray diffractometer (XRD-6000, Shimadzu, Kyoto, Japan). The setting condition was 40 kV and 30 mA, using Ni-filter Cu target Kα. The experiment adopted low angle X-ray diffraction mode with the incident angles (ω) of 1°, 2.5°, and 5.0° between X-ray and the samples. The scanning angle (2θ) was from 25° to 40°, and the scanning rate was 2°/min. Crystal phase analysis was compared to the Joint Committee on Powder Diffraction Standards (JCPDS) database.
Statistical analysis
The microhardness, roughness, and overlay thickness among different groups were compared using analysis of variance (ANOVA) and Tukey HSD in a statistical software package (JMP® 10.0.0, JMP Statistical Discovery LLC, Cary, NC, USA).
Results
Microhardness
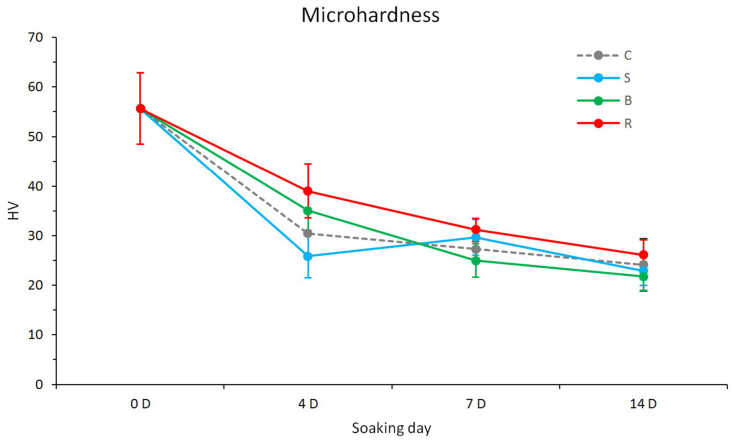
The results of the microhardness test are shown in Fig. 3 and Table 2. Before the brushing test, the microhardness of the bovine teeth was 56.67 ± 7.22 HV. After the brushing test, the microhardness values of all groups were lower than those before the test.
Fig. 3.
Microhardness of the samples processed with and without individual toothpaste. C: Control group (without toothpaste), S: Sensodyne®, B: BioRepair®, R: REALCaP® (n = 18).
Table 2.
Differences in microhardness, roughness, and overlay thickness between groups after 4, 7, and 14 days of immersion.
| Group |
||||||
|---|---|---|---|---|---|---|
| C |
S |
B |
R |
|||
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | P value | Tukey's pairwise comparison | |
| Microhardness | ||||||
| 4 days | 30.48 ± 4.33 | 25.88 ± 4.35 | 35.11 ± 4.06 | 39.00 ± 5.41 | <.0001∗ | R = B > C > S |
| 7 days | 27.28 ± 2.59 | 29.66 ± 3.64 | 24.98 ± 3.37 | 31.16 ± 2.34 | <.0001∗ | R>C = B, R = S, S = C, S > B |
| 14 days | 24.13 ± 5.31 | 22.88 ± 2.96 | 21.77 ± 2.81 | 26.16 ± 2.98 | 0.0045 | R = C, R > S > B, C = S = B |
| Roughness | ||||||
| 4 days | 1.60 ± 0.25 | 1.37 ± 0.21 | 1.43 ± 0.19 | 1.54 ± 0.11 | 0.0641 | NS |
| 7 days | 0.87 ± 0.57 | 1.46 ± 0.34 | 2.17 ± 0.41 | 1.92 ± 0.51 | <.0001∗ | R = B, R = S, B > S > C, R > C |
| 14 days | 1.57 ± 0.28 | 3.07 ± 0.52 | 3.69 ± 1.13 | 1.50 ± 0.36 | <.0001∗ | B = S > R = C |
| Overlay thickness | ||||||
| 4 days | 0.80 ± 0.12 | 0.65 ± 0.09 | 1.04 ± 0.17 | <.0001∗ | R>S > B | |
| 7 days | 2.29 ± 0.28 | 2.47 ± 0.67 | 2.86 ± 0.42 | 0.0402 | R = B, S = B, R > S | |
| 14 days | 2.92 ± 0.36 | 2.97 ± 0.29 | 3.55 ± 0.44 | 0.0022 | R > S = B | |
C, Control group; S, Sensodyne®; B, BioRepair®; R, REALCaP®; SD, standard deviation; ∗, statistical significance (P < 0.05); NS, no significant difference.
There were significant differences between the groups on days 4, 7, and 14 (P < 0.05). In general, the microhardness values of the Group R were significantly higher than those of the other groups (P < 0.05), regardless of the days of the brushing test.
Roughness
The results of the roughness analysis are depicted in Fig. 4 and Table 2. The roughness of the bovine teeth before the brushing test was 1.07 ± 0.63 μm. After the brushing test, the roughness values of the Group C demonstrated a trend of up and down in comparison to increased values of the other groups. In the Group C, roughness increased slightly to 1.60 ± 0.25 μm on day 4, decreased to 0.87 ± 0.57 μm on day 7, and raised to 1.57 ± 0.28 μm on day 14.
Fig. 4.
Roughness of the samples processed with and without individual toothpaste. C: Control group (without toothpaste), S: Sensodyne®, B: BioRepair®, R: REALCaP® (n = 10).
Statistical analysis revealed no significant differences in roughness between the groups on day 4 (P = 0.0641). However, there were significant differences in roughness between the groups on days 7 and 14 (P < 0.05). Tukey's pairwise comparison showed no significant differences between the Group R and the other groups on day 4. No significant differences were found between the Group R and the Group S (P = 0.1019) as well as the Group R and the Group B (P = 0.0707) on day 7. There was a significant difference between the Group R and the Group C (P < 0.0001) on day 7. Significant differences existed between the Group R and the commercial groups (P < 0.0001) on day 14. There was no significant difference between the Group R and the Group C (P = 0.9953) on day 14.
Scanning electron microscope
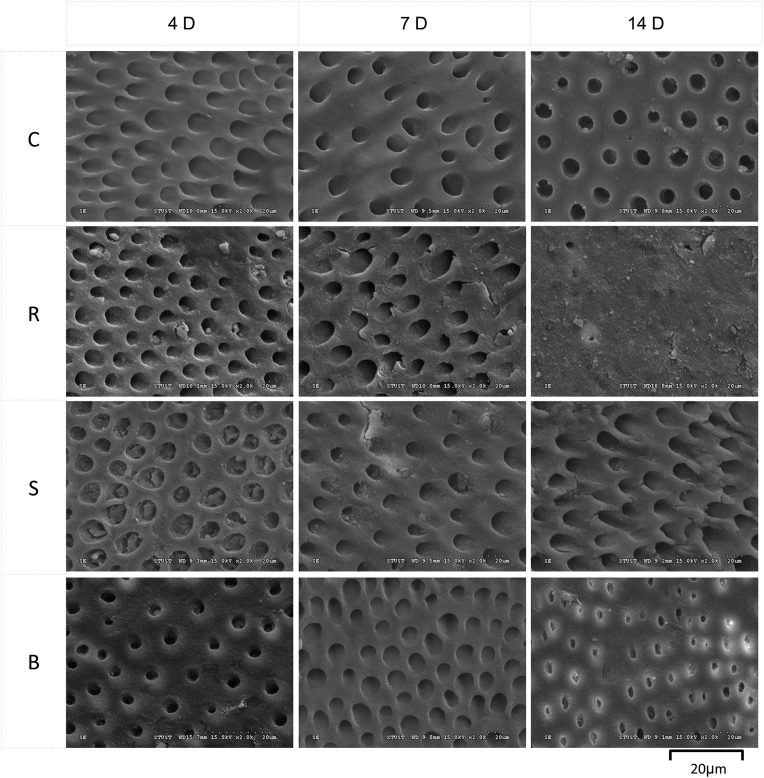
A scanning electron microscope was used to observe the surface of the samples (Fig. 5, Fig. 6). On days 4 and 7, numerous dentinal tubule openings were seen in the samples of the groups R, S, and B. However, most of the exposed dentinal tubules in the Group R had been sealed on day 14 in comparison to the two commercial toothpaste products.
Fig. 5.
Observation of the sample surfaces on days 4, 7, and 14 with a scanning electron microscope. C: Control group (without toothpaste), S: Sensodyne®, B: BioRepair®, R: REALCaP®.
Fig. 6.
Observation of the sample surfaces on day 14 with a scanning electron microscope under high magnification (magnification: 1000 × ). C: Control group (without toothpaste), S: Sensodyne®, B: BioRepair®, R: REALCaP®.
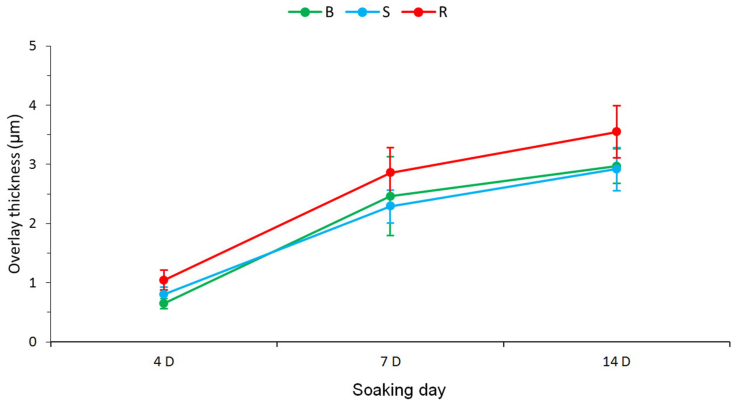
The overlay thickness on the tooth surfaces was scrutinized through examining cross section of the samples and the results are summarized in Fig. 7 and Table 2. On day 4, the overlay thickness in the Group R was 1.04 ± 0.17 μm, which was significant thicker than 0.80 ± 0.12 μm in the Group S (P = 0.0007) and 0.65 ± 0.09 μm in the Group B (P = 0.0001). The overlay thickness in the Group R on day 7 was 2.86 ± 0.42 μm, which was significant thicker than 2.29 ± 0.28 μm in the Group S (P = 0.0357) but not significant thicker than 2.47 ± 0.67 μm in the Group B (P = 0.1860). On day 14, the overlay thickness in the Group R was significant thicker than that of Group S (P = 0.0034) and that of Group B (P = 0.0070).
Fig. 7.
Overlay thickness of sample cross sections measured with a scanning electron microscope. B: BioRepair®, S: Sensodyne®, R: REALCaP® (n = 10).
X-ray diffraction
The surface crystals were analyzed using X-ray diffraction, as shown in Fig. 8. According to JCPDS database (Card No. 74–0566), the highest HA peak is 2θ = 31.77. An incident angle of 1° was the surface of the toothpaste, 2.5° was the surface of the tooth, and 5° was the interior of the tooth.
Fig. 8.
X-ray diffraction analysis of the crystal composition of tooth surfaces on days 4, 7, and 14. S: Sensodyne®, B: BioRepair®, R: REALCaP®, C: Control group (without toothpaste).
The peak of HA in the Group S was not visible on days 4, 7, and 14, whereas the peak of HA in the Group C was detected earlier on day 4. Up to day 7, HA peaks were visible in the groups R and B. On day 7, the peak intensity of HA in the Group R was lower than that in the Group B, while the peak intensity of HA in the Group R was higher than that in the Group B on day 14.
Discussion
The Vickers microhardness of the samples in this study before brushing test was 56.67 HV, which was similar to the research by Fonseca et al.17 All of the four groups decreased in microhardness as the days of the experiment increased. It is hypothesized that during the brushing cycle test, the surface erosion of the acid decalcified the dentin, thereby reducing the surface microhardness. In addition to the influence of acid erosion during the brushing test, extra layers of various toothpaste coating on the surfaces might reduce the microhardness of the samples. Observed under an electron microscope, the toothpaste deposition increased with the number of days, further contributed to the surface coating and led to a decrease in microhardness.
Although the microhardness decreased in all groups, the Group R demonstrated higher microhardness than that of other groups. One of the possible reasons may be the addition of DCPA powder with surface rich in nanocrystals, even in an acidic environment, the nanocrystals on DCPA will be converted into HA.7, 8, 9 Furthermore, the crystals may grow into a whisker status, which increases bonding in dentinal tubules.
Hossein et al.18 proposed that DCPA can biologically interact with hard tissues in the oral cavity through releasing Ca2+ and PO43− ions, thereby increase the bonding strength. In addition, Xu et al.19 indicated that when the added powder is whisker-shaped fillers, it can maintain good mechanical strength. The above reasons may cause the microhardness of the Group R to be higher than other groups.
The mean roughness of bovine tooth sample after grinding and polishing was 1.07 μm. The roughness in the Group C was below 1.60 μm after 14 days of brushing test. It was worth noting the roughness in the groups S and B increased with days and higher than Group C on days 7 and 14. The overlay thickness showed thickening during the test days, but on day 14, there were still many exposed dentinal tubules in groups S and B. This phenomenon might increase the height differences on the tooth surface, and could be a possible reason for the increased roughness.
Investigation of the roughness in the Group R showed a maximal value of 1.92 μm on day 7 and declined to 1.50 μm on day 14. Dentinal tubule openings were still visible microscopically on day 7, although toothpaste coating had increased. However, the dentinal tubule openings were almost sealed on day 14 in the Group R. This might explain the increase in roughness on days 4 and 7 but decreased on day 14.
Microscopic examination showed that the overlay in the Group R nearly sealed the openings of the dentinal tubules on day 14. A previous study found that the innovative surface nanocrystal-rich DCPA could release Ca2+ and PO43− ions after soaking then produce deposition on the resin surface.7 Another related study composited the surface nanocrystal-rich DCPA with dental resin to fill cavities in bovine teeth and found that the bonding strength was superior to commercially available resins.9 The above reasons might explain the better sealing effect of dentinal tubules in the Group R than other groups.
Diffraction patterns can reveal various peaks for different compositions and phase transitions. The HA peak was undetectable in the Group S due to the main component was stannous fluoride (SnF2). Because the Group C did not apply toothpaste, the HA peak could be seen on day 4. A higher HA peak in the Group B than that in the Group R on day 7 probably because its main ingredient was zinc hydroxyapatite. It is speculated that since the main component of the Group R is modified DCPA, the surface is rich in nanocrystals, which might be slowly converted to HA, resulting in a higher peak on day 14 than that of the Group B. Carino et al.20 proposed a pathway for apatite formation under a thermodynamic kinetic model in which the nucleation stage is dominated by dicalcium phosphate dihydrate (DCPD) or DCPA under ion-rich conditions. Whereas the phase transition in the rapid growth phase is regulated by the diffusion of calcium ions that limit the driving force of octacalcium phosphate or HA solubility.
Modern dental anti-sensitivity treatments mostly use the sealing of dentinal tubules to reduce the permeability of dentin, thereby reducing the stimulation of teeth by changes in external ambient temperature or osmotic pressure. The results of this study showed that the microhardness and sealing effect of dentinal tubules in the Group R were better than those in other groups. It was concluded that the toothpaste in this study containing modified DCPA may help remineralization and reduce tooth sensitivity.
Declaration of competing interest
The authors have no conflicts of interest relevant to this article.
Acknowledgments
This study was multi-supported by Kaohsiung Municipal Ta-Tung Hospital plan (grant number kmtth-109-040), by the Ministry of Science and Technology, Taiwan (grant numbers MOST 108-2745-8-035-003; 109-2622-E-035-014-CC2; 110-2221-E-035-013), and by the Special Research Project of the Southern Taiwan Science Park Precision Health Cluster of the National Science and Technology Council (BX-03-11-15-111). The authors express their gratitude to the staff at REALBONE Technology Co., LTD, Kaohsiung, Taiwan for generously providing the raw materials in this study.
Contributor Information
Wen-Cheng Chen, Email: wencchen@mail.fcu.edu.tw.
Chun-Cheng Hung, Email: chuchh@kmu.edu.tw.
References
- 1.Xu H.H.K., Sun L., Weir M.D., et al. Nano DCPA-whisker composites with high strength and Ca and PO4 Release. J Dent Res. 2006;85:722–727. doi: 10.1177/154405910608500807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu H.H.K., Weir M.D., Sun L. Nanocomposites with Ca and PO4 release: effects of reinforcement, dicalcium phosphate particle size and silanization. Dent Mater. 2007;23:1482–1491. doi: 10.1016/j.dental.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu H.H.K., Weir M.D., Sun L., Takagi S., Chow L.C. Effects of calcium phosphate nanoparticles on Ca-PO4 composite. J Dent Res. 2007;86:378–383. doi: 10.1177/154405910708600415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu H.H.K., Weir M.D., Sun L. Calcium and phosphate ion releasing composite: effect of pH on release and mechanical properties. Dent Mater. 2009;25:535–542. doi: 10.1016/j.dental.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen M.H. Update on dental nanocomposites. J Dent Res. 2010;89:549–560. doi: 10.1177/0022034510363765. [DOI] [PubMed] [Google Scholar]
- 6.Xu H.H.K., Moreau J.L., Sun L., Chow L.C. Strength and fluoride release characteristics of a calcium fluoride based dental nanocomposite. Biomaterials. 2008;29:4261–4267. doi: 10.1016/j.biomaterials.2008.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen W.C., Wu H.Y., Chen H.S. Evaluation of reinforced strength and remineralized potential of resins with nanocrystallites and silica modified filler surfaces. Mater Sci Eng C Mater Biol Appl. 2013;33:1143–1151. doi: 10.1016/j.msec.2012.12.022. [DOI] [PubMed] [Google Scholar]
- 8.Chen W.C., Chang K.C., Wu H.Y., Ko C.L., Huang C.L. Thermal cycling effect of dicalcium phosphate-reinforced composites on auto-mineralized dental resin. Mater Sci Eng C Mater Biol Appl. 2014;45:359–368. doi: 10.1016/j.msec.2014.09.032. [DOI] [PubMed] [Google Scholar]
- 9.Chen W.C., Ko C.L., Wu H.Y., Lai P.L., Shih C.J. Thermal cycling effects on adhesion of resin-bovine enamel junction among different composite resins. J Mech Behav Biomed Mater. 2014;38:105–113. doi: 10.1016/j.jmbbm.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Tschoppe P., Zandim D.L., Martus P., Kielbassa A.M. Enamel and dentine remineralization by nano-hydroxyapatite toothpastes. J Dent. 2011;39:430–437. doi: 10.1016/j.jdent.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 11.Najibfard K., Ramalingam K., Chedjieu I., Amaechi B.T. Remineralization of early caries by a nano-hydroxyapatite dentifrice. J Clin Dent. 2011;22:139–143. [PubMed] [Google Scholar]
- 12.Creeth J., Maclure R., Seong J., et al. Three randomized studies of dentine hypersensitivity reduction after short-term SnF2 toothpaste use. J Clin Periodontol. 2019;46:1105–1115. doi: 10.1111/jcpe.13175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hiller K.A., Buchalla W., Grillmeier I., Neubauer C., Schmalz G. In vitro effects of hydroxyapatite containing toothpastes on dentin permeability after multiple applications and ageing. Sci Rep. 2018;8:4888. doi: 10.1038/s41598-018-22764-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kar P.P., Shaikh Z.A., Hiremath A.M., Vikneshan M. Comparison of the effectiveness of three different desensitizing toothpastes in reducing dentin hypersensitivity: a 4-week clinical study. J Conserv Dent. 2019;22:181–184. doi: 10.4103/JCD.JCD_304_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xia Y., Yang Z.Y., Li Y.H., Zhou Z. The effects of a toothpaste containing the active ingredients of galla chinensis and sodium fluoride on dentin hypersensitivity and sealing of dentinal tubules: an in vitro study and an eight-week clinical study in 98 patients. Med Sci Mon Int Med J Exp Clin Res. 2020;26 doi: 10.12659/MSM.920776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Itthagarun A., Thaveesangpanich P., King N.M., Tay F.R., Wefel J.S. Effects of different amounts of a low fluoride toothpaste on primary enamel lesion progression: a preliminary study using in vitro pH-cycling system. Eur Arch Paediatr Dent. 2007;8:69–73. doi: 10.1007/BF03262573. [DOI] [PubMed] [Google Scholar]
- 17.Fonseca R.B., Haiter-Neto F., Carlo H.L., et al. Radiodensity and hardness of enamel and dentin of human and bovine teeth, varying bovine teeth age. Arch Oral Biol. 2008;53:1023–1029. doi: 10.1016/j.archoralbio.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 18.Hossein E.H., Reza H.M., Mohammad C., Abbas Y. Preparation of anhydrous dicalcium phosphate, DCPA, through sol–gel process, identification and phase transformation evaluation. J Non-Cryst Solids. 2008;354:3854–3857. [Google Scholar]
- 19.Xu H.H.K., Eichmiller F.C., Smith D.T., Schumacher G.E., Giuseppetti A.A., Antonucci J.M. Effect of thermal cycling on whisker-reinforced dental resin composites. J Mater Sci Mater Med. 2002;13:875–883. doi: 10.1023/a:1016504530133. [DOI] [PubMed] [Google Scholar]
- 20.Carino A., Ludwig C., Cervellino A., Müller E., Testino A. Formation and transformation of calcium phosphate phases under biologically relevant conditions: experiments and modelling. Acta Biomater. 2018;74:478–488. doi: 10.1016/j.actbio.2018.05.027. [DOI] [PubMed] [Google Scholar]