Abstract
Not all older adults with dementia-related neuropathology in their brains experience cognitive decline or impairment. Instead, some people maintain relatively normal cognitive functioning despite neuropathologic burden, a phenomenon called cognitive resilience. Using a longitudinal, epidemiological, clinical-pathologic cohort study of older adults in the United States (N = 348), the present research investigated associations between well-being and cognitive resilience. Consistent with preregistered hypotheses, results showed that higher eudaimonic well-being (measured via the Ryff Psychological Well-Being Scale) and higher hedonic well-being (measured via the Satisfaction with Life Scale) were associated with better-than-expected cognitive functioning relative to one’s neuropathological burden (i.e., beta-amyloid, neurofibrillary tangles, Lewy bodies, vascular pathologies, hippocampal sclerosis, and TDP-43). The association of eudaimonic well-being in particular was present above and beyond known cognitive resilience factors (i.e., socioeconomic status, education, cognitive activity, low neuroticism, low depression) and dementia risk factors (i.e., apolipoprotein E [ApoE] genotype, medical comorbidities). This research highlights the importance of considering eudaimonic well-being in efforts to prevent dementia.
Keywords: Alzheimer’s disease, cognitive resilience, dementia, eudaimonic, hedonic, life satisfaction, psychological well-being, subjective well-being, preregistered
Alzheimer’s disease and related dementias (ADRD)—a set of syndromes that destroy memory and thinking skills—are a leading cause of disability and death, affecting an estimated 55 million people globally (James et al., 2014; National Institute on Aging, 2021; World Health Organization, 2021). Treatments that target the neuropathology underlying ADRD are limited. However, increasing evidence suggests that ADRD-related neuropathology does not lead to cognitive impairment and dementia in all people (Bennett, Schneider, Arvanitakis, et al., 2006). Instead, some people tolerate high levels of ADRD-related neuropathologies without experiencing cognitive decline or developing dementia, a phenomenon known as cognitive resilience (James & Bennett, 2019; Negash et al., 2013). Identifying the factors that contribute to cognitive resilience is a promising avenue for dementia prevention. Well-being—defined broadly as living in a way that is consistent with one’s potential and experiencing one’s life as enjoyable and satisfying—is a key focus of resilience-based approaches to preventing a number of diseases, given its effects on health behaviors and physiological processes (Cross et al., 2018; Ryff et al., 2004). Resilience approaches to dementia prevention may also benefit from a focus on well-being given that well-being has been associated with less cognitive decline (e.g., Dewitte et al., 2021; Gerstorf et al., 2007; Hittner et al., 2020; G. Kim et al., 2019; Wingo et al., 2020) and lower incidence of cognitive impairment and dementia (e.g., Boyle et al., 2010; Peitsch et al., 2016; Sutin et al., 2018).
Links between well-being, cognitive decline, and dementia provide initial evidence that well-being may be a key protective factor. Specifically, well-being may promote cognitive resilience by improving the functioning of physiological systems or by influencing health behaviors (e.g., E. S. Kim et al., 2020; Ryff et al., 2004) that in turn support more efficient or more flexible neural systems. However, very little research has directly examined well-being as a promoter of cognitive resilience. One notable exception is an investigation using a subsample of the participants included in the present research, which found that higher levels of sense of purpose moderated the effects of neuropathology on cognition and cognitive decline (Boyle et al., 2012). These findings provide initial evidence that at least one component of well-being, sense of purpose, may serve as a source of cognitive resilience. However, well-being is a multifaceted construct (Gallagher et al., 2009), and different types of well-being have been shown to have differential associations with health outcomes, including dementia (Sutin et al., 2018), as well as differential responses to interventions (Bolier et al., 2013). Thus, it is important to identify the types of well-being that are associated with cognitive resilience. This will set the foundation for improving our understanding of potential mechanisms linking well-being to cognitive resilience and will inform future intervention efforts aimed at increasing well-being and in turn promoting cognitive resilience.
A key distinction in the well-being literature is between eudaimonic well-being (i.e., living in a way that is consistent with one’s potential) and hedonic well-being (i.e., experiencing life as enjoyable and satisfying; Ryff et al., 2021). A prominent theoretically derived model of eudaimonic well-being consists of six components that together characterize “challenged thriving”: autonomy, environmental mastery, personal growth, sense of purpose, positive relations with other people, and self-acceptance (Ryff, 1989). In contrast, hedonic well-being includes positive and negative affect and evaluations of one’s life satisfaction (Diener et al., 1985). Eudaimonic and hedonic well-being are related but distinct aspects of what it means to be psychologically well, yet relatively little research has directly compared their effects on health outcomes (Ryff et al., 2021). In the context of cognitive health outcomes, we are aware of one study that directly compared associations between different components of eudaimonic and hedonic well-being and dementia risk. Components of both types of well-being were associated with dementia risk, but only sense of purpose (a component of eudaimonic well-being) was uniquely associated with dementia risk after analyses adjusted for depressive symptoms and other risk factors (Sutin et al., 2018). Thus, initial evidence suggests that different types of well-being may have differential associations with dementia-related outcomes, but more research is needed particularly with regard to cognitive resilience.
Statement of Relevance.
Alzheimer’s disease and related dementias (ADRD)—a set of syndromes that destroy memory and thinking skills—are a leading cause of disability and death worldwide. Treatments are limited, and prevention is essential for reducing the adverse public health impact of the diseases. The present research found that people with higher levels of well-being can tolerate higher levels of ADRD-related neuropathology without experiencing memory and thinking impairments (i.e., greater cognitive resilience). Importantly, the association of well-being with cognitive resilience was observed even when analyses accounted for other known cognitive resilience factors and was among the strongest and most consistent associations compared with these other factors. This research suggests that well-being should be a focus of resilience-based prevention models and may be a powerful target of interventions aimed at preventing or delaying the onset of dementia.
The present research investigated the preregistered hypothesis that greater eudaimonic and hedonic well-being would both be associated with greater cognitive resilience to dementia-related neuropathology. To investigate the robustness of effects and to rule out potential third-variable confounds, we also examined the unique effects of both types of well-being above and beyond sociodemographic characteristics (i.e., sex, age at death), known cognitive resilience factors (i.e., socioeconomic status, education, cognitive activity, low neuroticism, low depression; Bennett et al., 2003; Graham et al., 2021; James & Bennett, 2019; Wilson et al., 2002, 2014), and dementia risk factors (i.e., apoliprotein E [ApoE] genotype, medical comorbidities; James & Bennett, 2019; Liu et al., 2013). Given prior research on sense of purpose (Boyle et al., 2012), we additionally examined the extent to which eudaimonic well-being associations could be explained by sense of purpose (a component of eudaimonic well-being). Together, the present research provided a strong test of associations between well-being and cognitive resilience to dementia-related neuropathology.
Open Practices
Analysis scripts have been made publicly available on OSF (https://osf.io/7yfqt). The data used in this study can be requested through the Rush Alzheimer’s Disease Center (RADC) Resource Sharing Hub (https://www.radc.rush.edu). Study hypotheses and analysis plans were preregistered prior to data analysis (https://osf.io/4bz62/).
Method
Participants and procedure
The present research used data from the Rush Memory and Aging Project (MAP), a longitudinal, epidemiological, clinical-pathologic cohort study of aging and dementia (Bennett et al., 2012, 2018). Older adults were recruited from retirement communities, subsidized senior housing facilities, and individual homes throughout Chicago and northeastern Illinois. Participants had no known dementia at study entry. Participants completed annual clinical evaluations that included assessments of global cognition. The study was approved by an institutional review board of Rush University Medical Center. All participants provided informed consent, an Anatomical Gift Act, and repository consent to allow their data to be shared. Enrollment began in 1997 and is ongoing (i.e., the study has rolling admission), with new participants recruited each year (total MAP N at time of analysis = 2,193).
The present investigation was limited to participants who participated in the MAP after well-being assessment was introduced in 2008. Further, because neuropathology data were collected after death, all participants in the present investigation were deceased. Because the MAP has rolling recruitment, our final analytic sample of deceased participants included those who died shortly after study entry as well as those who lived well past the average adult life span (age at death ranged from 71 to 104). Among those MAP participants who met these basic inclusion criteria, participants had to have at least one measurement occasion of well-being, at least one measurement occasion of global cognition, and complete neuropathology data, and they could not have had dementia at analytic baseline to be included in the final sample (analytic N = 348).1 See Figure 1 for a flowchart of participant exclusions, starting with the complete sample of MAP participants.
Fig. 1.
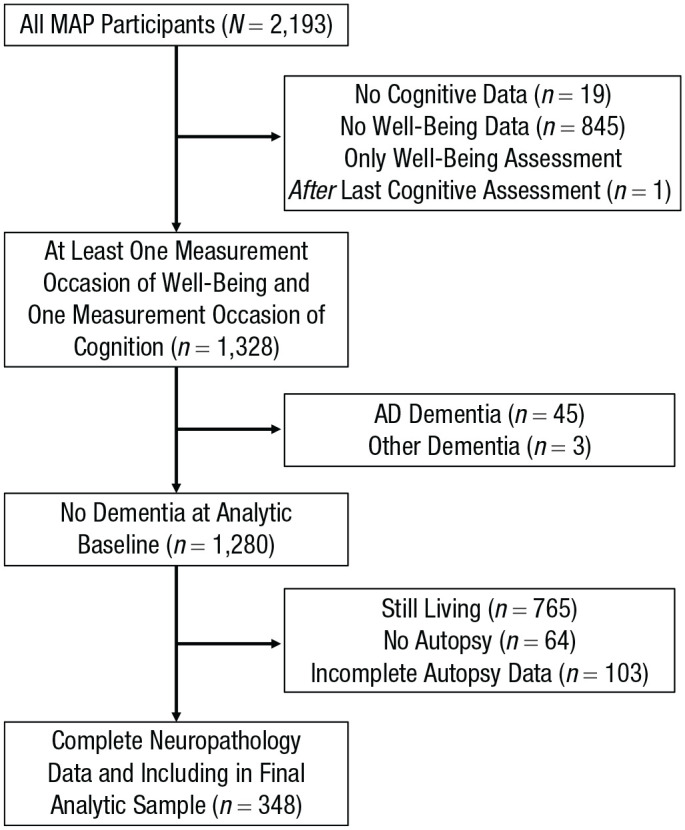
Flowchart of participant exclusions. MAP = Rush Memory and Aging Project; AD = Alzheimer’s disease.
Because this project involved secondary analyses of existing data, sample size was predetermined on the basis of the number of participants who met inclusion criteria. However, sensitivity power analyses suggested that we had adequate statistical power to detect small effects. Specifically, the final analytic sample size of 348 provided 80% statistical power to detect small associations between well-being and cognitive resilience of .15 or larger, given our α level of .05. The final analytic sample was predominately White, mostly female, and highly educated and experienced high longevity (see Table 1 for participant characteristics).
Table 1.
Descriptive Statistics for Predictor Variables
| Predictor | Value | Range | Correlation (r) with global cognition | Correlation (r) with cognitive resilience |
|---|---|---|---|---|
| Eudaimonic well-being | M = 5.34, SD = 0.57 | 3.39–6.78 | .21 | .24 |
| Hedonic well-being | M = 5.50, SD = 0.97 | 2.00–7.00 | .04 | .13 |
| Age at death (years) | M = 91.21, SD = 5.82 | 71–104 | −.21 | −.09 |
| Sexa (male) | n = 98, 28.2% | .05 | −.02 | |
| Early life SES (standardized composite) | M = 0.06, SD = 0.72 | −2.60 to 2.16 | .20 | .10 |
| Education (years) | M = 14.98, SD = 2.93 | 5–25 | .19 | .15 |
| Baseline cognitive activity | M = 3.17, SD = 0.50 | 1.84–4.41 | .07 | .13 |
| Neuroticism | M = 14.91, SD = 6.55 | 0–33 | −.14 | −.11 |
| Depressive symptoms | M = 1.20, SD = 1.69 | 0–10 | −.15 | −.17 |
| ApoE presentb | n = 67, 19.3% | −.15 | −.03 | |
| Medical comorbidities | M = 1.60, SD = 1.06 | 0–5 | .10 | .04 |
Note: Correlations with 95% confidence intervals that do not include zero are given in boldface. Associations with the last global cognition measurement before death are shown because this was the variable used in the primary operationalization of cognitive resilience. SES = socioeconomic status.
Sex was coded 0 for female and 1 for male. bApoliprotein E [ApoE] genotype was dichotomized by the presence of the e4 allele (0 = no, 1 = yes).
Measures
Well-being
Participants completed well-being assessments annually beginning in 2008. In the present research, we used the first available measurement occasion of well-being for each participant. To assess eudaimonic well-being, we asked participants to complete the Ryff Psychological Well-Being Scale (Ryff & Keyes, 1995). Participants were asked to rate the extent to which they agreed with a series of 18 items on a scale from 1 (strongly agree) to 7 (strongly disagree). The 18 items reflect six components of psychological well-being: self-acceptance (e.g., “I like most parts of my personality”), autonomy (e.g., “I have confidence in my own opinions, even if they are different from those of others”), environmental mastery (e.g., “I am good at managing the responsibilities of daily life”), sense of purpose (e.g., “Some people wander aimlessly through life, but I am not one of them”), positive relations with other people (e.g., “People would describe me as a giving person, willing to share my time with others”), and personal growth (e.g., “For me, life has been a continuous process of learning, changing, and growing”). When necessary, the 18 items were reverse-scored so that higher values indicated greater eudaimonic well-being, and then all items were averaged together to form a mean composite. The resulting eudaimonic well-being measure had a Cronbach’s α of .78.
To assess hedonic well-being, we asked participants to complete the Satisfaction With Life Scale (Diener et al., 1985). Participants were asked to rate the extent to which they agreed with a series of statements (e.g., “I am satisfied with my life”) on a scale from 1 (strongly agree) to 7 (strongly disagree). The five items were reverse scored so that higher values indicated greater life satisfaction and then averaged together to form a mean composite. The resulting hedonic well-being measure had a Cronbach’s α of .78.
The time lag between well-being assessment and death ranged from 0.15 to 11.84 years (M = 5.30, SD = 2.69).2 The time lag between well-being assessment and final measurement of cognition before death ranged from 0.00 to 11.13 years (M = 4.34, SD = 2.64).3
Diagnosis of dementia
A clinical diagnosis of cognitive status was made at each annual assessment on the basis of a three-stage process including computer scoring of cognitive tests, clinical judgment by a neuropsychologist, and diagnostic classification by a clinician (Bennett, Schneider, Aggarwal, et al., 2006). Participants with a clinical diagnosis of dementia at analytic baseline were excluded from analyses.
Global cognition
Global cognition was assessed annually across the entire study period using 19 cognitive performance tests across five cognitive domains (episodic memory, semantic memory, working memory, perceptual orientation, and perceptual speed; Wilson et al., 2015). Scores from each test were z-scored, averaged together, then restandardized, resulting in a single global cognition score for each annual assessment. This approach results in a single global cognition score per measurement occasion that encompasses a broad range of cognitive abilities.
In primary analyses, we used the participants’ final clinical assessment prior to death to obtain a measure of global cognition as close to the measurement of neuropathology as possible. The time lag between final cognitive assessment and death was on average 1 year (M = 0.97, SD = 0.96, range = 0.03–6.66).4 In exploratory analyses, we used all available annual clinical assessments after the initial well-being assessment to calculate trajectories of cognitive decline.
Neuropathology
Ten common age-related pathologies were assessed by examining participants’ brains after autopsy. These pathologies included abnormal accumulations of proteins, neuronal cell loss, and vascular abnormalities.
Specifically, postmortem neuropathological evaluation was conducted after autopsy to obtain quantitative and semiquantitative indices of common-age related pathologies. All neuropathologic indices were collected by researchers naive to clinical data and reviewed by experienced neuropathologists. The following measures of neuropathology were used in the present research: cortical beta-amyloid load, neuronal neurofibrillary tangles density, Lewy body pathology, vascular pathologies (chronic macro- and microscopic infarcts, atherosclerosis, cerebral amyloid angiopathy, and arteriolosclerosis), hippocampal sclerosis, and TDP-43.
Beta-amyloid protein was identified by molecularly-specific immunohistochemistry and quantified by image analysis. A continuous measure indicating the percentage area of the cortex occupied by beta-amyloid was used (Bennett et al., 2018). Neuronal neurofibrillary tangles were identified by molecularly specific immunohistochemistry (antibodies to abnormally phosphorylated tau protein, AT8). Quantification of tangle density was performed by stereology microscopy. A continuous measure with higher scores indicating greater tangle burden was used (Bennett et al., 2018). For both beta-amyloid and neurofibrillary tangles, composite measures were obtained by computing mean scores across eight regions: hippocampus, entorhinal cortex, midfrontal cortex, inferior temporal, angular gyrus, calcarine cortex, anterior cingulate cortex, and superior frontal cortex. Pathologic presence (coded 1) or absence (coded 0) of Lewy body pathology was identified with antibodies to phosphorylated α-synuclein across multiple brain regions, including amygdala, midbrain, limbic, and neocortical regions.
The presence (coded 1) or absence (coded 0) of chronic macro- and microinfarcts were reviewed by a board-certified neuropathologist (Schneider et al., 2005). A scale from 0 (none) to 3 (severe) was used to quantify cerebral amyloid angiopathy, atherosclerosis, and arteriolosclerosis (Arvanitakis et al., 2016; Boyle et al., 2015; Buchman et al., 2011). Hippocampal sclerosis was evaluated as severe neuronal loss and gliosis in the CA1/subiculum subregion of the hippocampus on a hematoxylin and eosin stain (Nag et al., 2015). TDP-43 immunohistochemistry was performed to identify TDP-43 cytoplasmic inclusions in neurons and glia. TDP-43 was quantified as four stages (0 = none, 1 = present in amygdala, 2 = present in amygdala and limbic regions, 3 = present in amygdala, limbic, and neocortical regions).
Covariates
When computing the cognitive resilience scores, we adjusted for time between final cognitive assessment and death by subtracting the date of the final cognitive assessment from the date of death.
We conducted subsequent analyses with and without adjusting for the following covariates: sociodemographic characteristics (age at death, sex), known cognitive resilience factors (early life socioeconomic status, education, baseline cognitive activity, low neuroticism, low depressive symptoms), and dementia risk factors (ApoE genotype, medical comorbidities).
Age at death was assessed as date of death minus date of birth, divided by 365 (days per year). Sex was reported by the participant and coded 0 for female and 1 for male. Early life socioeconomic status was assessed as a composite of fathers’ education, mothers’ education, and the number of children in the household. First, all three indicators were z-scored and the number of children was multiplied by −1. Then, the mean of the three z scores was computed to form the early life socioeconomic status variable.
Education was assessed in years. Baseline cognitive activity was assessed by asking participants to rate the typical time spent doing common activities that involve intellectual processing: watching television; listening to the radio; reading newspapers, magazine, or books; playing puzzle or strategy games; and going to museums. Responses were averaged into a composite measure of cognitive activity.
Neuroticism was assessed with 12 items from the NEO Five-Factor Inventory (Costa & McCrae, 1989). Participants were asked to rate the extent to which they agreed with a series of statements assessing neuroticism (e.g., “I often feel tense and jittery”) on a scale from 1 (strongly disagree) to 7 (strongly agree). The 12 neuroticism items were summed to create a total trait score. Depressive symptoms were assessed with a modified, 10-item version of the Center for Epidemiologic Studies–Depression (CES-D) scale (Kohout et al., 1993). Participants were asked whether or not they experienced each of 10 symptoms much of the time in the past week, and a sum score indicating the total number of symptoms experienced was computed.
To assess ApoE genotype, we extracted DNA from peripheral blood or frozen postmortem brain tissue (Yu et al., 2017). Genotyping was performed at Polymorphic DNA Technologies (Alameda, CA) by investigators naive to all clinical and pathologic data. ApoE genotype was dichotomized by the presence of the e4 allele (0 = no, 1 = yes). Medical comorbidities were assessed as a sum score of the following seven conditions: head trauma, hypertension, heart conditions, hypothyroidism, stroke, cancer, and diabetes (Wilson et al., 2002).
Analytic approach
All analyses were performed in R (Version 4.0; R Core Team, 2020).
Computing cognitive resilience scores
To compute cognitive resilience scores, we used the same approach as Negash et al. (2013) and Graham et al. (2021). Specifically, we regressed the final measurement occasion of global cognition onto the 10 pathology indicators (i.e., beta-amyloid, neurofibrillary tangles, Lewy Body Disease, chronic macroscopic infarctions, microscopic infarctions, cerebral amyloid angiopathy, cerebral atherosclerosis, arteriolosclerosis, hippocampal sclerosis, TDP-43), adjusting for time between final cognitive assessment and death. This provided two pieces of information: first, the association between cognitive score and pathology (the adjusted R2 from the model was .40) and, second, the residual, which we then used as our index of cognitive resilience. More positive values indicate better-than-expected cognitive functioning for a given level of neuropathology. More negative values indicate worse-than-expected cognitive functioning for a given level of neuropathology. These residuals were stored and used as the dependent variable for our primary analyses.
In a second set of exploratory analyses, we calculated an alternative operationalization of cognitive resilience that considered global cognitive decline rather than global cognition level. First, we used a random-intercept, random-slope growth curve model to predict global cognition from follow-up time. We used all available measurement occasions of global cognition after the baseline assessment of well-being. The fixed effect from this model indicated that on average, participants’ global cognition declined 0.16 standard-deviation units per year. Next, we stored the random slopes around this fixed effect, which reflect individuals’ cognitive decline slopes. We then regressed individual cognitive decline slopes onto the 10 pathology indicators, adjusting for time between final cognitive assessment and death. Like our primary operationalization of cognitive resilience, this provided two pieces of information: first, the association between cognitive decline and pathology (the adjusted R2 from the model was .29) and, second, the residual, which we then used as our alternative index of cognitive resilience. More positive values indicate less-than-expected cognitive decline for a given level of neuropathology. More negative values indicate more-than-expected cognitive decline for a given level of neuropathology. These residuals were used as the dependent variable for our exploratory analyses. Associations between our primary and alternative operationalization of cognitive resilience were correlated at .76 with one another.
Preliminary analyses
In preliminary analyses, we computed simple correlations between all predictor variables, global cognition at the final time point before death, and cognitive resilience. We additionally computed simple correlations between both types of well-being and the 10 pathology indicators.
Testing associations between well-being and cognitive resilience
To test whether well-being is associated with cognitive resilience, we used two sets of linear regression models to predict cognitive resilience scores from eudaimonic well-being and hedonic well-being, respectively. Continuous variables were z-standardized, and categorical variables were dummy-coded to aid in interpretation. We tested well-being associations with cognitive resilience both with and without adjusting for covariates, to ascertain the robustness of the effects once covariates were added. In Model 1, we did not include any covariates. In Model 2, we included only sociodemographic covariates (including early life socioeconomic status and education, which are known cognitive resilience factors). In Model 3, we included sociodemographic covariates, past cognitive activity, ApoE genotype, and medical comorbidities. Given conceptual and empirical overlap between well-being and neuroticism (Anglim et al., 2020) and between well-being and depressive symptoms (Wood & Joseph, 2010), we added these covariates in the final two stages. Specifically, in Model 4, we included demographic covariates, past cognitive activity, ApoE genotype, medical comorbidities, and neuroticism. Finally, in Model 5 (which was not preregistered), we included demographic covariates, past cognitive activity, ApoE genotype, medical comorbidities, neuroticism, and depressive symptoms.
We conducted two sets of exploratory analyses. First, because prior research has focused on sense of purpose associations with cognitive resilience and other dementia-related outcomes and given that sense of purpose is a component of eudaimonic well-being, we additionally examined the extent to which the eudaimonic well-being association could be explained by sense of purpose. We used the same 10-item measure of sense of purpose used in prior research (Boyle et al., 2012). Second, we repeated these analyses using the alternative operationalization of cognitive resilience, in which cognitive decline rather than cognition level was regressed onto the neuropathology indicators.
Results
Preliminary results
Table 1 displays descriptive statistics and simple correlations between all predictor variables, global cognition at the final time point before death, and cognitive resilience. Associations with the last global cognition measurement before death are shown because this is the variable used in the primary operationalization of cognitive resilience. Eudaimonic well-being was moderately positively correlated with global cognition. In contrast, the 95% confidence interval (CI) around the association between hedonic well-being and global cognition included zero. All of the covariates except for sex and cognitive activity were also correlated with global cognition in the expected directions. Both types of well-being as well as the known cognitive resilience factors (i.e., socioeconomic status, education, cognitive activity, neuroticism, and depressive symptoms) were correlated with cognitive resilience in the expected directions.
Table 2 displays simple correlations between well-being variables and individual pathology indicators. Eudaimonic and hedonic well-being were moderately positively correlated with one another. Correlations between well-being and neuropathology were largely null, with two exceptions. Greater eudaimonic well-being was associated with less beta-amyloid burden, and hedonic well-being was associated with more microscopic infarctions.
Table 2.
Associations Between Well-Being and Pathology Variables
| Variable | 1 | 2 |
|---|---|---|
| 1. Eudaimonic well-being | — | |
| 2. Hedonic well-being | .38 | — |
| 3. Beta-amyloid | −.16 | −.07 |
| 4. Neurofibrillary tangles | .00 | .07 |
| 5. Lewy body disease | −.03 | −.04 |
| 6. Chronic macroscopic infarctions | −.05 | .01 |
| 7. Microscopic infarctions | .07 | .19 |
| 8. Cerebral amyloid angiopathy | −.03 | .05 |
| 9. Cerebral atherosclerosis | −.09 | .07 |
| 10. Arteriolosclerosis | −.02 | .02 |
| 11. Hippocampal sclerosis | −.03 | −.02 |
| 12. TDP-43 | .02 | .08 |
Note: Correlations with 95% confidence intervals that do not include zero are given in boldface.
Primary analyses
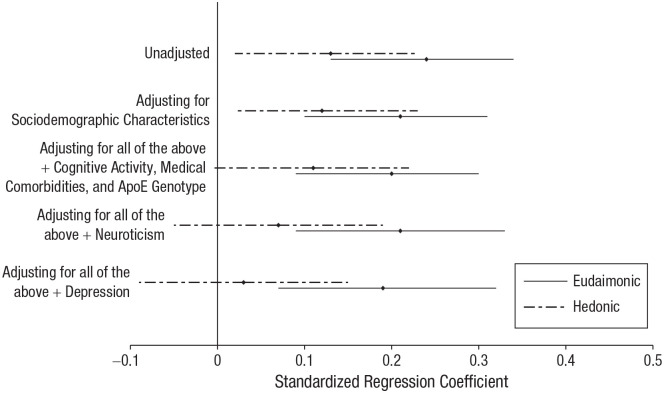
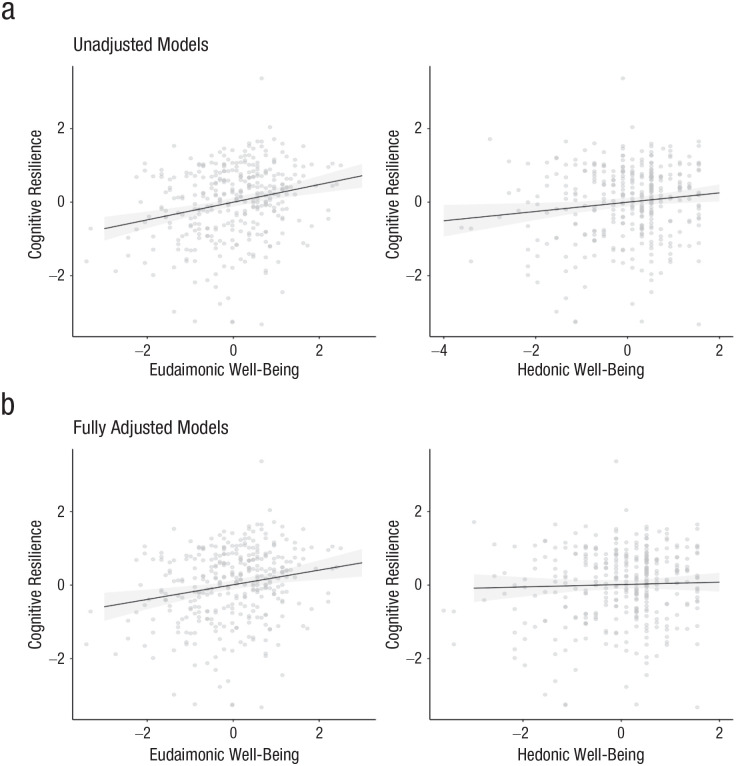
Table 3 displays coefficients and 95% CIs for all models testing the association of eudaimonic well-being with cognitive resilience. Table 4 displays coefficients and 95% CIs for all models testing the association of hedonic well-being with cognitive resilience. Figure 2 depicts associations between both well-being types and cognitive resilience across model specifications. Figure 3 depicts scatterplots of associations between both well-being types and cognitive resilience.
Table 3.
Results of Regression Analyses Testing the Associations Between Eudaimonic Well-Being and Cognitive Resilience
| Predictor | Model 1a (primary) | Model 2a (sensitivity) | Model 3a (sensitivity) | Model 4a (sensitivity) | Model 5a (sensitivity) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| β | 95% CI | β | 95% CI | β | 95% CI | β | 95% CI | β | 95% CI | |
| Intercept | 0.00 | [−0.10, 0.10] | 0.03 | [−0.10, 0.15] | 0.08 | [−0.05, 0.22] | 0.09 | [−0.05, 0.22] | 0.10 | [−0.04, 0.23] |
| Eudaimonic well-being | 0.24 | [0.14, 0.34] | 0.21 | [0.11, 0.32] | 0.20 | [0.09, 0.31] | 0.21 | [0.09, 0.34] | 0.20 | [0.08, 0.32] |
| Age at death | −0.06 | [−0.16, 0.05] | −0.05 | [−0.16, 0.06] | −0.04 | [−0.15, 0.07] | −0.04 | [−0.15, 0.07] | ||
| Sex (male) | −0.10 | [−0.33, 0.13] | −0.14 | [−0.38, 0.09] | −0.14 | [−0.37, 0.10] | −0.17 | [−0.41, 0.07] | ||
| Early life SES | 0.05 | [−0.07, 0.16] | 0.04 | [−0.08, 0.15] | 0.05 | [−0.08, 0.17] | 0.03 | [−0.09, 0.16] | ||
| Education | 0.10 | [−0.01, 0.21] | 0.08 | [−0.04, 0.20] | 0.07 | [−0.05, 0.19] | 0.07 | [−0.05, 0.19] | ||
| Baseline cognitive activity | 0.09 | [−0.02, 0.20] | 0.07 | [−0.05, 0.19] | 0.07 | [−0.05, 0.18] | ||||
| ApoE present | −0.16 | [−0.42, 0.10] | −0.20 | [−0.47, 0.06] | −0.21 | [−0.47, 0.05] | ||||
| Medical comorbidities | 0.06 | [−0.05, 0.17] | 0.07 | [−0.04, 0.18] | 0.08 | [−0.03, 0.19] | ||||
| Neuroticism | 0.01 | [−0.11, 0.13] | 0.05 | [−0.08, 0.17] | ||||||
| Depressive symptoms | −0.13 | [−0.25, −0.01] | ||||||||
Note: CI = confidence interval; SES = socioeconomic status; ApoE = apolipoprotein E. Standardized coefficients with 95% confidence intervals that do not include zero are given in boldface.
Table 4.
Results of Regression Analyses Testing the Associations Between Hedonic Well-Being and Cognitive Resilience
| Predictor | Model 1b
(Primary) |
Model 2b (Sensitivity) | Model 3b (Sensitivity) | Model 4b (Sensitivity) | Model 5b (Sensitivity) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| β | 95% CI | β | 95% CI | β | 95% CI | β | 95% CI | β | 95% CI | |
| Intercept | 0.00 | [−0.10, 0.10] | 0.03 | [−0.09, 0.16] | 0.09 | [−0.04, 0.23] | 0.10 | [−0.03, 0.24] | 0.11 | [−0.03, 0.25] |
| Hedonic well-being | 0.13 | [0.02, 0.23] | 0.12 | [0.02, 0.23] | 0.11 | [−0.004, 0.22] | 0.07 | [−0.05, 0.19] | 0.03 | [−0.09, 0.15] |
| Age at death | −0.09 | [−0.20, 0.02] | −0.08 | [−0.19, 0.02] | −0.07 | [−0.18, 0.04] | −0.06 | [−0.17, 0.05] | ||
| Sex (male) | −0.13 | [−0.36, 0.11] | −0.17 | [−0.41, 0.07] | −0.17 | [−0.41, 0.07] | −0.20 | [−0.44, 0.04] | ||
| Early life SES | 0.03 | [−0.08, 0.15] | 0.02 | [−0.09, 0.14] | 0.02 | [−0.11, 0.14] | 0.00 | [−0.12, 0.13] | ||
| Education | 0.14 | [0.03, 0.26] | 0.11 | [−0.005, 0.23] | 0.11 | [−0.02, 0.23] | 0.10 | [−0.02, 0.22] | ||
| Baseline cognitive activity | 0.11 | [−0.01, 0.22] | 0.09 | [−0.03, 0.20] | 0.09 | [−0.03, 0.21] | ||||
| ApoE present | −0.18 | [−0.44, 0.09] | −0.21 | [−0.48, 0.06] | −0.21 | [−0.47, 0.06] | ||||
| Medical comorbidities | 0.05 | [−0.06, 0.16] | 0.07 | [−0.05, 0.18] | 0.08 | [−0.04, 0.19] | ||||
| Neuroticism | −0.05 | [−0.17, 0.06] | −0.02 | [−0.14, 0.10] | ||||||
| Depressive symptoms | −0.14 | [−0.27, −0.02] | ||||||||
Note: CI = confidence interval; SES = socioeconomic status; ApoE = apolipoprotein E. Standardized coefficients with 95% confidence intervals that do not include zero are given in boldface.
Fig. 2.
Associations between both types of well-being and cognitive resilience, separately for each model specification. Diamonds depict means, and error bars depict 95% confidence intervals.
Fig. 3.
Scatterplots showing the associations between cognitive resilience and eudaimonic well-being (left column) and hedonic well-being (right column), separately for (a) the unadjusted models and (b) the fully adjusted models. Solid lines indicate best-fitting regressions, the shaded region is the 95% confidence band.
Higher eudaimonic well-being was associated with greater cognitive resilience in the unadjusted model and in all sensitivity analyses. The standardized effect size, which ranged from 0.20 in the fully adjusted model to 0.24 in the unadjusted model, was moderate compared with other effect sizes in psychological science (Funder & Ozer, 2019) and was larger than previously observed associations between cognitive resilience and other psychosocial factors, such as education (Bennett et al., 2003), sense of purpose (Boyle et al., 2012), and personality (Graham et al., 2021). The association of eudaimonic well-being with cognitive resilience could not be explained by demographic characteristics (i.e., age at death, sex, early life socioeconomic status, and education), concurrent cognitive activity, concurrent medical comorbidities, ApoE genotype, concurrent neuroticism, or concurrent depressive symptoms.
Higher hedonic well-being was also associated with greater cognitive resilience in the unadjusted model, but the 95% CI included zero when we adjusted for covariates in sensitivity analyses. Moreover, the standardized effect size was very small to small, ranging from 0.03 in the fully adjusted model to 0.13 in the unadjusted model. When including eudaimonic and hedonic well-being in the same model,5 we found that a moderate association between eudaimonic well-being and cognitive resilience remained, β = 0.22, 95% CI = [0.11, 0.34], whereas the 95% CI around the association of hedonic well-being with cognitive resilience included zero, β = 0.04, 95% CI = [−0.07, 0.15].
Exploratory analyses
In the first set of exploratory analyses, we examined the extent to which the eudaimonic well-being association could be explained by sense of purpose alone. Higher sense of purpose was associated with greater cognitive resilience in an unadjusted model, β = 0.23, 95% CI = [0.12, 0.33], and the effect size was similar to the association of the full eudaimonic well-being composite. However, in contrast to eudaimonic well-being, the sense of purpose association weakened when we adjusted for covariates, and the 95% CI included zero in the fully adjusted model, β = 0.11, 95% CI = [−0.01, 0.24]. Moreover, when including both eudaimonic well-being (minus the sense-of-purpose subscale) and sense of purpose in the same fully adjusted model, we found that a moderate association between eudaimonic well-being and cognitive resilience remained, β = 0.17, 95% CI = [0.04, 0.30], whereas the 95% CI around the association of sense of purpose with cognitive resilience included zero, β = 0.06, 95% CI = [−0.07, 0.19].
Second, we repeated primary analyses using the alternative operationalization of cognitive resilience, in which cognitive decline rather than cognition level was regressed onto the neuropathology indicators. Tables S1 and S2 in the Supplemental Material available online display results of these exploratory analyses. Figure S1 in the Supplemental Material depicts scatterplots of associations between both well-being types and the alternative operationalization of cognitive resilience. Results for eudaimonic well-being were largely the same. However, the 95% CI around the association of hedonic well-being with this alternative operationalization of cognitive resilience included zero in all models.
Discussion
The present research found that older adults with higher levels of well-being experienced better-than-expected cognitive functioning for a given level of dementia-related neuropathology. These findings provide strong evidence for eudaimonic well-being in particular as a key protective factor against cognitive decline and dementia.
The association between eudaimonic well-being and cognitive resilience was remarkably consistent across sensitivity analyses. The size of the effect was not meaningfully reduced when we adjusted for sociodemographic characteristics (i.e., age at death, sex), known cognitive resilience factors (i.e., socioeconomic status, education, cognitive activity, low neuroticism, low depressive symptoms), and known dementia risk factors (i.e., ApoE genotype, medical comorbidities). Depressive symptoms was the only other robust predictor when we adjusted for covariates, suggesting that both positive and negative aspects of psychological functioning contribute to resilience. Importantly, the absence of robust associations for the other factors in the fully adjusted models does not mean that they were not associated with cognitive resilience. Indeed, all of the known cognitive resilience factors were correlated with greater cognitive resilience in unadjusted models (See Table 1). However, these factors were not associated with cognitive resilience above and beyond eudaimonic well-being and other covariates. In sum, these results suggest that eudaimonic well-being is robustly associated with cognitive resilience above and beyond other known cognitive resilience and dementia risk factors.
In addition to the eudaimonic well-being findings described above, the present study also directly compared eudaimonic well-being with hedonic well-being. Although both types of well-being were associated with greater cognitive resilience in primary models, only eudaimonic well-being was uniquely associated with cognitive resilience when we adjusted for other resilience and risk factors. Although the 95% CIs for associations between eudaimonic and hedonic well-being overlapped with one another, only eudaimonic well-being was uniquely associated with cognitive resilience when we included both types of well-being in the same model, and only eudaimonic well-being was associated with the alternative operationalization of cognitive resilience, providing further evidence that associations between eudaimonic well-being and cognitive resilience may be more robust than associations between hedonic well-being and cognitive resilience. These findings are consistent with those of prior research, which found that although components of both eudaimonic and hedonic well-being are associated with dementia risk, only sense of purpose (a component of eudaimonic well-being) was uniquely associated with dementia risk when models accounted for covariates (Sutin et al., 2018). Taken together, these findings provide converging evidence that associations between eudaimonic well-being and dementia-related outcomes may be more robust than associations between hedonic well-being and dementia-related outcomes, and observed links between hedonic well-being and dementia outcomes may be due in part to third-variable confounds. However, this conclusion should be considered tentative given the small number of studies that have compared eudaimonic and hedonic well-being. Importantly, some prior research focusing on hedonic well-being alone has found robust links between hedonic well-being and dementia-related outcomes even when adjusting for covariates (Hittner et al., 2020; Peitsch et al., 2016). Thus, more research is needed to understand when and for whom hedonic well-being is uniquely associated with reduced dementia risk.
Given the strength and robustness of the association of eudaimonic well-being with cognitive resilience, eudaimonic well-being may be an effective target of intervention efforts aimed at preventing dementia. Because the present study was observational, interventional research is needed to test this possibility. Positive psychological interventions, which involve building skills such as emotion regulation, goal setting, and practices such as kindness and gratitude, have been shown to effectively increase well-being (Bolier et al., 2013; Weiss et al., 2016); however, more research is needed to understand which types of interventions are most effective and whether effects persist for long enough to impact long-term outcomes such as cognitive resilience. Moreover, in addition to individual-level interventions, policy-level interventions may be particularly useful for increasing population well-being (Diener et al., 2009). This type of intervention research is particularly valuable, given that increasing individual and population well-being are important goals in and of themselves.
Future researchers should also seek to explain why eudaimonic well-being is related to cognitive resilience. Our conception of cognitive resilience here is defined only by the disconnect between observed cognitive abilities and what would be expected on the basis of a given person’s level of neuropathologic burden (i.e., the residual) and is agnostic to mechanism. The reason for such resilience may be due to biological differences between people in their brains’ ability to tolerate pathological damage—that is, cognitive or neural reserve capacity: cellular, synaptic, and biochemical mechanisms that allow the brain to function despite damage from disease, perhaps by enabling more efficient use of brain networks or through the recruitment of alternate brain networks (Arnold et al., 2013; James & Bennett, 2019; Stern, 2002; Stern et al., 2019). Prior theory and research suggest a number of potential avenues through which well-being may promote cognitive reserve capacity. For example, higher eudaimonic well-being may improve the functioning of physiological systems or influence health behaviors including sleep and physical, social, and cognitive activity (e.g., E. S. Kim et al., 2020; Ryff et al., 2004). In turn, these physiological and behavioral mechanisms may support more efficient neural systems, greater cognitive flexibility, and greater ability to recruit compensatory processes. Importantly, the present research does not rule out the resilience and risk factors included as covariates as potential mediators of the link between eudaimonic well-being and cognitive resilience. Covariates were assessed concurrently with well-being to rule them out as potential confounds (i.e., variables that cause both well-being and cognitive resilience). However, later instances of these same constructs may serve as mediators (i.e., variables caused by well-being that in turn cause cognitive resilience). For example, it is possible that higher well-being promotes continued cognitive engagement later in life, which in turn promotes cognitive resilience.
Overall, the present research provided a strong test of the associations between well-being (both eudaimonic and hedonic) and cognitive resilience. However, the following limitations should be considered when evaluating the strength of this evidence. First, the six eudaimonic well-being subscales were assessed by only three items each and tend to have low reliability on their own. Thus, we could not examine the individual associations between each of the eudaimonic well-being components and cognitive resilience. One important exception is sense of purpose. We used a reliable, 10-item measure of sense of purpose to rule out the possibility that the eudaimonic well-being associations observed here could be fully explained by sense of purpose. Second, we examined only the cognitive component of hedonic well-being (i.e., life satisfaction). Prior research suggests that the affective components (i.e., positive and negative affect) may also be related to cognitive decline and dementia risk (e.g., Hittner et al., 2020; Wilson et al., 2014). Third, our approach to operationalizing cognitive resilience as the residual from models predicting cognition level/cognitive decline from neuropathology means that our indices of cognitive resilience encompass both resilience and measurement error. This likely contributed to underestimates of effect sizes. Fourth, the sample used in the present research was predominately White (98%), mostly female (72%), and highly educated (M = 15 years of education) and experienced high longevity (M = 91 years old at death). Further research is needed to investigate the extent to which these findings generalize to other groups, given that cognitive resilience factors observed in one sociocultural group do not always generalize to other sociocultural groups (Avila et al., 2021). To investigate the generalizability of the present findings, more longitudinal studies of aging and dementia need to include participants from historically underrepresented and excluded sociocultural groups, as well as measures of well-being and neuropathology.
Concluding Comment
Eudaimonic well-being was robustly associated with cognitive resilience to dementia-related neuropathology above and beyond known cognitive resilience and dementia risk factors. Moreover, the association of eudaimonic well-being was among the largest and most consistent of the factors examined. This research suggests that eudaimonic well-being should be incorporated into resilience-based prevention models and may be a powerful target of interventions aimed at preventing or delaying the onset of dementia.
Supplemental Material
Supplemental material, sj-docx-1-pss-10.1177_09567976221119828 for Well-Being and Cognitive Resilience to Dementia-Related Neuropathology by Emily C. Willroth, Bryan D. James, Eileen K. Graham, Alifiya Kapasi, David A. Bennett and Daniel K. Mroczek in Psychological Science
When we excluded 27 participants whose final cognitive assessment was concurrent with their only well-being assessment, the results of the primary and sensitivity analyses remained the same, with the following exception: The 95% confidence interval (CI) around the unadjusted association between hedonic well-being and cognitive resilience included zero, β = 0.11, 95% CI = [−0.005, 0.23].
Results of primary analyses remained the same when we adjusted for time between well-being assessment and death, and the relationships between well-being and cognitive resilience were not moderated by time between well-being assessment and death.
Results of primary analyses remained the same when we adjusted for time between well-being assessment and cognition assessment, and the relationships between well-being and cognitive resilience were not moderated by time between well-being assessment and cognitive assessment.
Results of primary analyses remained the same when we adjusted for time between final cognitive assessment and death, and the relationships between well-being and cognitive resilience were not moderated by time between final cognitive assessment and death.
This analysis was not preregistered.
Footnotes
ORCID iDs: Emily C. Willroth  https://orcid.org/0000-0002-4780-6883
https://orcid.org/0000-0002-4780-6883
Bryan D. James  https://orcid.org/0000-0003-1932-151X
https://orcid.org/0000-0003-1932-151X
Eileen K. Graham  https://orcid.org/0000-0003-3095-4625
https://orcid.org/0000-0003-3095-4625
Supplemental Material: Additional supporting information can be found at http://journals.sagepub.com/doi/suppl/10.1177/09567976221119828
Transparency
Action Editor: Karen Rodrigue
Editor: Patricia J. Bauer
Author Contributions
E. C. Willroth: conceptualization, formal analysis, writing – original draft, writing – review and editing, visualization
B. D. James: conceptualization, data curation, writing – review and editing
E. K. Graham conceptualization, writing – review and editing, visualization
A. Kapasi: data curation, writing – review and editing
D. A. Bennett: data curation, resources, writing – review and editing
D. K. Mroczek: conceptualization, writing – review and editing, supervision
The author(s) declared that there were no conflicts of interest with respect to the authorship or the publication of this article.
Funding: The Rush Memory and Aging Project (MAP) is supported by the National Institute on Aging (Grant Nos. R01AG17917 and R01AG24490). Preparation of this manuscript was supported by a National Institute on Aging Career Development Award to E. C. Willroth (No. K99AG07183).

References
- Anglim J., Horwood S., Smillie L. D., Marrero R. J., Wood J. K. (2020). Predicting psychological and subjective well-being from personality: A meta-analysis. Psychological Bulletin, 146(4), 279. [DOI] [PubMed] [Google Scholar]
- Arnold S. E., Louneva N., Cao K., Wang L. S., Han L. Y., Wolk D. A., Negash S., Leurgans S. E., Schneider J. A., Buchman A. S., Wilson R. S., Bennett D. A. (2013). Cellular, synaptic, and biochemical features of resilient cognition in Alzheimer’s disease. Neurobiology of Aging, 34(1), 157–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvanitakis Z., Capuano A. W., Leurgans S. E., Bennett D. A., Schneider J. A. (2016). Relation of cerebral vessel disease to Alzheimer’s disease dementia and cognitive function in elderly people: a cross-sectional study. The Lancet Neurology, 15(9), 934–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila J. F., Rentería M. A., Jones R. N., Vonk J. M., Turney I., Sol K., Seblova D., Arias F., Hill-Jarrett T., Levy S., Meyer O., Racine A. M., Tom S. E., Melrose R. J., Deters K., Medina L. D., Carrion C. I., Diaz-Santos M., Byrd D. R., . . .Manly J. J. (2021). Education differentially contributes to cognitive reserve across racial/ethnic groups. Alzheimer’s & Dementia, 17(1), 70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett D. A., Buchman A. S., Boyle P. A., Barnes L. L., Wilson R. S., Schneider J. A. (2018). Religious orders study and rush memory and aging project. Journal of Alzheimer’s Disease, 64, S161–S189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett D. A., Schneider J. A., Aggarwal N. T., Arvanitakis Z., Shah R. C., Kelly J. F., Fox J. H., Cochran E. J., Arends D., Treinkman A. D., Wilson R. S. (2006). Decision rules guiding the clinical diagnosis of Alzheimer’s disease in two community-based cohort studies compared to standard practice in a clinic-based cohort study. Neuroepidemiology, 27(3), 169–176. [DOI] [PubMed] [Google Scholar]
- Bennett D. A., Schneider J. A., Arvanitakis Z., Kelly J. F., Aggarwal N. T., Shah R. C., Wilson R. S. (2006). Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology, 66, 1837–1844. [DOI] [PubMed] [Google Scholar]
- Bennett D. A., Schneider J. A., Buchman A. S., Barnes L. L., Boyle P. A., Wilson R. S. (2012). Overview and findings from the Rush Memory and Aging Project. Current Alzheimer Research, 9, 646–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett D. A., Wilson R. S., Schneider J. A., Evans D. A., Mendes de Leon C. F., Arnold S. E., Barnes L. L., Bienias J. L. (2003). Education modifies the relation of AD pathology to level of cognitive function in older persons. Neurology, 60, 1909–1915. [DOI] [PubMed] [Google Scholar]
- Bolier L., Haverman M., Westerhof G. J., Riper H., Smit F., Bohlmeijer E. (2013). Positive psychology interventions: A meta-analysis of randomized controlled studies. BMC Public Health, 13, Article 119. 10.1186/1471-2458-13-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle P. A., Buchman A. S., Barnes L. L., Bennett D. A., Boyle P. A., Buchman A. S., Barnes L. L., Bennett D. A. (2010). Effect of a purpose in life on risk of incident Alzheimer disease and mild cognitive impairment in community-dwelling older persons. Archives of General Psychiatry, 67, 304–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle P. A., Buchman A. S., Wilson R. S., Yu L., Schneider J. A., Bennett D. A. (2012). Effect of purpose in life on the relation between Alzheimer disease pathologic changes on cognitive function in advanced age. Archives of General Psychiatry, 69, 499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle P. A., Yu L., Nag S., Leurgans S., Wilson R. S., Bennett D. A., Schneider J. A. (2015). Cerebral amyloid angiopathy and cognitive outcomes in community-based older persons. Neurology, 85, 1930–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman A. S., Leurgans S. E., Nag S., Bennett D. A., Schneider J. A. (2011). Cerebrovascular disease pathology and parkinsonian signs in old age. Stroke, 42, 3183–3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa P., McCrae R. (1989). NEO five-factor inventory (NEO-FFI). Psychological Assessment Resources. [Google Scholar]
- Cross M. P., Hofschneider L., Grimm M., Pressman S. D. (2018). Subjective well-being and physical health. In Diener E., Oishi S., Tay L. (Eds.), Handbook of well-being. DEF Publishers. https://www.nobascholar.com/chapters/64 [Google Scholar]
- Dewitte L., Lewis N. A., Payne B. R., Turiano N. A., Hill P. L. (2021). Cross-lagged relationships between sense of purpose in life, memory performance, and subjective memory beliefs in adulthood over a 9-year interval. Aging & Mental Health, 25(11), 2018–2027. [DOI] [PubMed] [Google Scholar]
- Diener E. D., Emmons R. A., Larsen R. J., Griffin S. (1985). The Satisfaction With Life Scale. Journal of Personality Assessment, 49, 71–75. [DOI] [PubMed] [Google Scholar]
- Diener E. D., Lucas R., Helliwell J. F., Schimmack U., Helliwell J. (2009). Well-being for public policy. Oxford Positive Psychology. [Google Scholar]
- Funder D. C., Ozer D. J. (2019). Evaluating effect size in psychological research: Sense and nonsense. Advances in Methods and Practices in Psychological Science, 2, 156–168. [Google Scholar]
- Gallagher M. W., Lopez S. J., Preacher K. J. (2009). The hierarchical structure of well-being. Journal of Personality, 77(4), 1025–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstorf D., Lövdén M., Röcke C., Smith J., Lindenberger U. (2007). Well-being affects changes in perceptual speed in advanced old age: Longitudinal evidence for a dynamic link. Developmental Psychology, 43, 705–718. [DOI] [PubMed] [Google Scholar]
- Graham E. K., James B. D., Jackson K. L., Willroth E. C., Boyle P., Wilson R., Bennett D. A., Mroczek D. K. (2021). Associations between personality traits and cognitive resilience in older adults. The Journals of Gerontology: Series B, 76, 6–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hittner E. F., Stephens J. E., Turiano N. A., Gerstorf D., Lachman M. E., Haase C. M. (2020). Positive affect is associated with less memory decline: Evidence from a 9-year longitudinal study. Psychological Science, 31, 1386–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James B. D., Bennett D. A. (2019). Causes and patterns of dementia: An update in the era of redefining Alzheimer’s disease. Annual Review of Public Health, 40, 65–84. [DOI] [PubMed] [Google Scholar]
- James B. D., Leurgans S. E., Hebert L. E., Scherr P. A., Yaffe K., Bennett D. A. (2014). Contribution of Alzheimer disease to mortality in the United States. Neurology, 82, 1045–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E. S., Shiba K., Boehm J. K., Kubzansky L. D. (2020). Sense of purpose in life and five health behaviors in older adults. Preventive Medicine, 139, Article 106172. 10.1016/j.ypmed.2020.106172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G., Shin S. H., Scicolone M. A., Parmelee P. (2019). Purpose in life protects against cognitive decline among older adults. The American Journal of Geriatric Psychiatry, 27, 593–601. [DOI] [PubMed] [Google Scholar]
- Kohout F. J., Berkman L. F., Evans D. A., Cornoni-Huntley J. (1993). Two shorter forms of the CES-D depression symptoms index. Journal of Aging and Health, 5, 179–193. [DOI] [PubMed] [Google Scholar]
- Liu C. C., Kanekiyo T., Xu H., Bu G. (2013). Apolipoprotein E and Alzheimer disease: Risk, mechanisms and therapy. Nature Reviews Neurology, 9, 106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nag S., Yu L., Capuano A. W., Wilson R. S., Leurgans S. E., Bennett D. A., Schneider J. A. (2015). Hippocampal sclerosis and TDP-43 pathology in aging and Alzheimer disease. Annals of Neurology, 77, 942–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Aging. (2021). Alzheimer’s disease fact sheet. https://www.nia.nih.gov/health/alzheimers-disease-fact-sheet
- Negash S., Wilson R. S., Leurgans S. E., Wolk D. A., Schneider J. A., Buchman A. S., Bennett D. A., Arnold S. E. (2013). Resilient brain aging: Characterization of discordance between Alzheimer’s disease pathology and cognition. Current Alzheimer Research, 10, 844–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peitsch L., Tyas S. L., Menec V. H., St John P. D. (2016). General life satisfaction predicts dementia in community living older adults: A prospective cohort study. International Psychogeriatrics, 28, 1101–1109. [DOI] [PubMed] [Google Scholar]
- R Core Team. (2020). R: A language and environment for statistical computing (Version 4.0) [Computer software]. http://www.R-project.org
- Ryff C. D. (1989). Happiness is everything, or is it? Explorations on the meaning of psychological well-being. Journal of Personality and Social Psychology, 57(6), 1069–1081. [Google Scholar]
- Ryff C. D., Boylan J. M., Kirsch J. A. (2021). Eudaimonic and hedonic well-being: An integrative perspective with linkages to sociodemographic factors and health. In Lee M. T., Kubzansky L. D., VanderWeele T. J. (Eds.), Measuring well-being (pp. 92–135). Oxford University Press. [Google Scholar]
- Ryff C. D., Keyes C. L. (1995). The structure of psychological well-being revisited. Journal of Personality and Social Psychology, 69, 719–757. [DOI] [PubMed] [Google Scholar]
- Ryff C. D., Singer B. H., Dienberg Love G. (2004). Positive health: Connecting well-being with biology. Philosophical Transactions of the Royal Society of London, Series B: Biological Sciences, 359, 1383–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider J. A., Bienias J. L., Wilson R. S., Berry-Kravis E., Evans D. A., Bennett D. A. (2005). The apolipoprotein E ε4 allele increases the odds of chronic cerebral infarction detected at autopsy in older persons. Stroke, 36, 954–959. [DOI] [PubMed] [Google Scholar]
- Stern Y. (2002). What is cognitive reserve? Theory and research application of the reserve concept. Journal of the International Neuropsychological Society, 8, 448–460. [PubMed] [Google Scholar]
- Stern Y., Barnes C. A., Grady C., Jones R. N., Raz N. (2019). Brain reserve, cognitive reserve, compensation, and maintenance: Operationalization, validity, and mechanisms of cognitive resilience. Neurobiology of Aging, 83, 124–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutin A. R., Stephan Y., Terracciano A. (2018). Psychological well-being and risk of dementia. International Journal of Geriatric Psychiatry, 33, 743–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss L. A., Westerhof G. J., Bohlmeijer E. T. (2016). Can we increase psychological well-being? The effects of interventions on psychological well-being: A meta-analysis of randomized controlled trials. PLOS ONE, 11(6), Article e0158092. 10.1371/journal.pone.0158092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R. S., Boyle P. A., Yu L., Barnes L. L., Sytsma J., Buchman A. S., Bennett D. A., Schneider J. A. (2015). Temporal course and pathologic basis of unawareness of memory loss in dementia. Neurology, 85, 984–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R. S., Capuano A. W., Boyle P. A., Hoganson G. M., Hizel L. P., Shah R. C., Nag S., Schneider J. A., Arnold S. E., Bennett D. A. (2014). Clinical-pathologic study of depressive symptoms and cognitive decline in old age. Neurology, 83(8), 702–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R. S., Mendes de Leon C. F., Barnes L. L., Schneider J. A., Bienias J. L., Evans D. A., Bennett D. A. (2002). Participation in cognitively stimulating activities and risk of incident Alzheimer disease. Journal of the American Medical Association, 287, 742–748. [DOI] [PubMed] [Google Scholar]
- Wingo A. P., Wingo T. S., Fan W., Bergquist S., Alonso A., Marcus M., Levey A. I., Lah J. J. (2020). Purpose in life is a robust protective factor of reported cognitive decline among late middle-aged adults: The Emory Healthy Aging Study. Journal of Affective Disorders, 263, 310–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood A. M., Joseph S. (2010). The absence of positive psychological (eudemonic) well-being as a risk factor for depression: A ten year cohort study. Journal of Affective Disorders, 122(3), 213–217. [DOI] [PubMed] [Google Scholar]
- World Health Organization. (2021). Dementia. https://www.who.int/news-room/fact-sheets/detail/dementia
- Yu L., Lutz M. W., Wilson R. S., Burns D. K., Roses A. D., Saunders A. M., Gaiteri C., De Jager P. L., Barnes L. L., Bennett D. A. (2017). TOMM40’ 523 variant and cognitive decline in older persons with APOE ε3/3 genotype. Neurology, 88(7), 661–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-pss-10.1177_09567976221119828 for Well-Being and Cognitive Resilience to Dementia-Related Neuropathology by Emily C. Willroth, Bryan D. James, Eileen K. Graham, Alifiya Kapasi, David A. Bennett and Daniel K. Mroczek in Psychological Science