Abstract
Background
Real-life evidence on prevalence and management of severe asthma is limited. Nationwide population registries across the Nordic countries provide unique opportunities to describe prevalence and management patterns of severe asthma at population level. In nationwide register data from Sweden, Norway and Finland, we examined the prevalence of severe asthma and the proportion of severe asthma patients being managed in specialist care.
Methods
This is a cross-sectional study based on the Nordic Dataset for Asthma Research (NORDSTAR) research collaboration platform. We identified patients with severe asthma in adults (aged ≥18 years) and in children (aged 6–17 years) in 2018 according to the European Respiratory Society/American Thoracic Society definition. Patients managed in specialist care were those with an asthma-related specialist outpatient contact (only available in Sweden and Finland).
Results
Overall, we identified 598 242 patients with current asthma in Sweden, Norway and Finland in 2018. Among those, the prevalence of severe asthma was 3.5%, 5.4% and 5.2% in adults and 0.4%, 1.0%, and 0.3% in children in Sweden, Norway and Finland, respectively. In Sweden and Finland, 37% and 40% of adult patients with severe asthma and two or more exacerbations, respectively, were managed in specialist care; in children the numbers were 56% and 41%, respectively.
Conclusion
In three Nordic countries, population-based nationwide data demonstrated similar prevalence of severe asthma. In children, severe asthma was a rare condition. Notably, a large proportion of patients with severe asthma were not managed by a respiratory specialist, suggesting the need for increased recognition of severe asthma in primary care.
Short abstract
In nationwide cohorts of all asthma patients from three Nordic countries, the prevalence of severe asthma was comparable: 3.5–5.4% in adults and 0.3–1% in children. Many patients with severe asthma are not managed in specialist care. https://bit.ly/3vM1kMg
Introduction
Globally, asthma is one of the most common chronic diseases. Although most asthma patients are well controlled on the available inhaled therapy, a minor group has severe disease that is associated with substantial burdens due to increased risk of exacerbations, high healthcare costs and risk of serious side-effects to oral corticosteroids (OCS) [1–5].
Insights into the prevalence of severe asthma and the management of severe asthma patients are important for many reasons. The health and economic benefits derived from successful management of severe uncontrolled asthma in specialist care are well documented [6, 7] and generally recommended by guidelines [8–10]. Nevertheless, evidence suggests that severe asthma remains under-recognised and often managed only in primary care, where the risk of not achieving asthma control is high [11–14]. With the emergence of monoclonal antibodies targeting specific inflammatory pathway in patients with severe asthma, there is an urgent need to better understand the current management strategies for these patients. Lastly, from a health economic and political perspective, prevalence estimates may support decision-makers in the allocation of scarce healthcare resources in the most appropriate and cost-effective way [15].
Available studies have estimated that the prevalence of severe asthma ranges from 2% to 10% of asthma patients [5, 11–20]. However, prior studies are generally limited by the use of different methodologies and the lack of unified definitions of severe asthma. Furthermore, most of the existing literature is based on smaller population studies [16] or registries containing only selected patient groups from single countries, limiting the comparability of results between different studies [15, 21–23]. In particular, large nationwide registry studies that investigate severe asthma in children and adolescents are lacking [24]. Updated data on severe asthma and its morbidity using unified definitions on severe asthma in large, unselected paediatric and adult asthma populations from several countries are highly warranted.
The Nordic Dataset for Asthma Research (NORDSTAR) research platform is unique in terms of having complete nationwide data from all asthma patients in the Nordic European countries [25]. The database contains detailed information on medical diagnosis, medication use, use of healthcare resources and costs, sociodemographics and mortality, thereby enabling us to investigate potential inter-country differences in the prevalence of severe asthma, its morbidity and management strategies of severe asthma without the risk of selection bias.
The aim of this study was to estimate the prevalence and management strategies of severe asthma in children and adults in Sweden, Norway and Finland.
Methods
Study design and data sources
This was a cross-sectional study based on data from NORDSTAR. For this study, NORDSTAR data from 2018 were extracted from nationwide registries available in Sweden, Norway and Finland. Data from Denmark were not available for 2018 and were consequently not included in the present study. Specifically, data were obtained from the national patient registries and the drug registries for Sweden and Finland, while for patients in Norway, only prescription data were available (supplementary table S1). These nationwide registers are based on routinely collected administrative data on all residents in each country. Within each country, information from the population registers was used to link individual-level information between the registries using the unique social security number that enables the linking of data collected throughout an individual's lifetime from birth (or immigration) to death (or emigration).
Study population
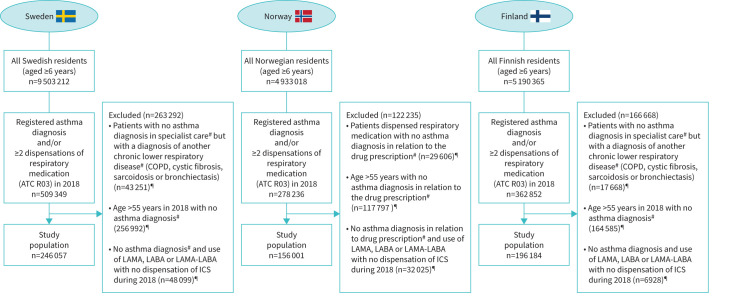
The study population was identified in a stepwise manner as illustrated in figure 1. The source population included all citizens in Sweden, Norway and Finland who were aged ≥6 years in 2018. From the source population, asthma patients were identified as those with either at least one asthma International Classification of Diseases, 10th revision (ICD-10) diagnosis (J45–J46) registered in specialist care during 2018 and/or dispensation with an Anatomical Therapeutic Chemical Classification (ATC) code R03 (except for omalizumab) on at least two occasions in 2018. In Norway, information about diagnoses was available from the prescription registry, where indications for treatment are registered. The applied exclusion criteria attempted to eliminate likely nonasthmatic patients from the analyses (figure 1), but only pertained to patients with no ICD-10 asthma diagnosis, e.g. if a patient had an asthma ICD-10 diagnosis, they were included regardless of comorbidities or age >55 years.
FIGURE 1.
Flowchart illustrating selection of the study population with inclusion and exclusion criteria. ATC: Anatomical Therapeutic Chemical Classification; LAMA: long-acting muscarinic antagonist; LABA: long-acting β2-agonist; ICS: inhaled corticosteroid. #: during 2018 or the preceding 5 years; ¶: not mutually exclusive.
Definition of severe asthma
Severe asthma was defined according to the European Respiratory Society (ERS)/American Thoracic Society (ATS) guidelines [9]. For patients aged ≥12 years, it included high-dose inhaled corticosteroids (ICS) (corresponding to dispensing ICS with an average daily dose of ≥1600 μg budesonide or equivalent) and at least one dispensed prescription with a second controller including long-acting β2-agonists (LABA), long-acting muscarinic antagonists (LAMA), xanthines or leukotriene receptor antagonists (LTRA) and/or a dispensed biological therapy (anti-IgE, anti-interleukin (IL)-5/IL-5r) and/or high OCS exposure in 2018. To reflect a significant steroid exposure comparable to daily use of OCS, high OCS exposure was defined as filled OCS prescriptions corresponding to an average of ≥5 mg per day during 2018 corresponding to ≥1825 mg total dispensed OCS [26]. For patients aged <12 years, those with severe asthma were defined as those requiring high-dose ICS (corresponding to dispensing ICS prescriptions of an average daily dose of ≥800 µg budesonide or equivalent) and at least one dispensed prescription of a second controller and/or dispensed biological therapy (anti-IgE, anti-IL5) in 2018. Patients aged 6–12 years were not included in the severe group based on high OCS exposure.
Additional study definitions
Several different definitions were used to characterise patients and study outcomes (supplementary table S2). Exacerbations were defined as either a dispensation with burst OCS (ATC code H02AB) or an asthma-related emergency department (ED) visit in 2018 or as an asthma-related hospital admission in 2018. High short-acting β-agonist (SABA) use was defined as dispensations of ≥600 doses (puffs) of SABA in 2018 [11, 12, 27]. Uncontrolled asthma was a composite measure defined as either 1) two or more OCS dispensations and/or asthma-related ED visits and/or 2) one or more asthma-related admission and/or 3) high SABA use (of ≥600 doses (puffs)) in 2018.
Asthma management in specialist care was defined as a specialist visit (not an ED visit), where asthma ICD-10 codes J45–J46 were the primary or secondary diagnosis. The patients, who did not fulfil the criteria for specialist asthma management, were considered to have been treated in primary care. Asthma management in specialist care was only reported for patient data derived from Finland and Sweden, as no data on primary versus specialist care were available for Norway.
Other measures included comorbidities, use of antibiotics and hospital admissions for pneumonia (refer to supplementary tables S3–S5 for definitions).
Analyses
The prevalence of asthma in 2018 was estimated in the populations aged ≥6 years in Sweden, Norway and Finland, as the proportion of severe asthma in the current asthma populations in each country. The prevalence measures were calculated separately in the paediatric populations (aged 6–17 years) and in the adult populations (aged ≥18 years). The study was descriptive in nature with calculation of frequencies and proportions (n, %), mean±sd and median (interquartile ranges). All study outcomes were evaluated separately in subsamples of patients with mild-to-moderate asthma and severe asthma.
No hypothesis tests analysing differences between the countries were conducted, as the data from each country were derived from separate nationwide registries, and despite the application of uniformed study definitions, the differences in the data registration could vary between the countries. Statistical analyses were performed using R (version 4.1.0; R Foundation for Statistical Computing, Vienna, Austria).
All data in this study were pseudo-anonymised and contained no direct identifiable patient information. Patient consent is not required in the Nordic countries for registry-based studies.
Results
The prevalence of severe asthma in Sweden, Norway and Finland
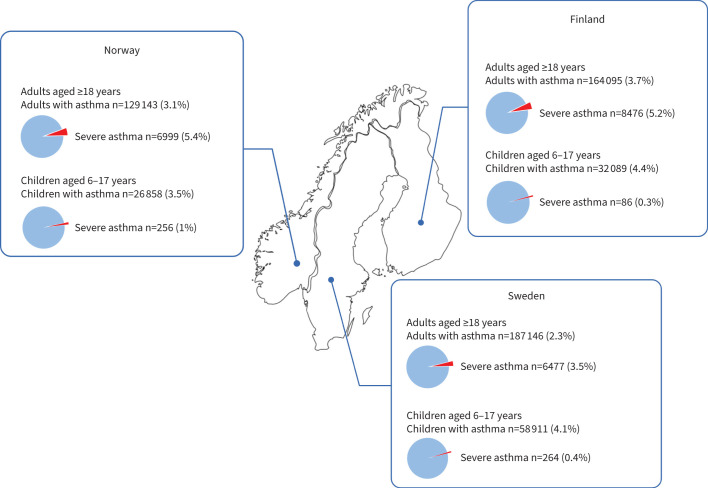
In total, the study populations consisted of 598 242 children and adults with asthma (Sweden n=246 057, Norway n=156 001, Finland n=196 184) (figure 1). When stratified by age, the prevalence of asthma in patients aged ≥18 years was 2.3% in Sweden, 3.1% in Norway and 3.7% in Finland. Of the prevalent asthma cases, 3.5%, 5.4% and 5.2%, respectively, had severe asthma, according to the ERS/ATS definition (figure 2, table 1). For children aged 6–17 years, the prevalence of asthma was 4.1% in Sweden, 3.5% in Norway and 4.4% in Finland, of whom 0.4%, 1.0% and 0.3%, respectively, had severe asthma (figure 2, table 2). In adults (age ≥18 years), the prevalence of both overall asthma and severe asthma was higher among females than males, whereas in children aged 6–17 years, asthma and severe asthma were more prevalent in males (tables 1 and 2). The mean±sd age of mild-to-moderate adult asthma patients ranged from 44±15 to 48±17 years, whereas the mean±sd age of severe asthma patients ranged from 56±16 to 60±16 years between countries.
FIGURE 2.
Prevalence of asthma and severe asthma in children and adults in Sweden, Norway and Finland.
TABLE 1.
Characteristics of asthma patients aged ≥18 years in Sweden, Norway and Finland in 2018
| Sweden | Norway | Finland | ||||
| Mild-to-moderate | Severe | Mild-to-moderate | Severe | Mild-to-moderate | Severe | |
| Current asthma population | 187 146 (2.3)§§ | 129 143 (3.1)§§ | 164 095 (3.7)§§ | |||
| 180 669 (96.5)ƒƒ | 6477 (3.5)ƒƒ | 122 144 (94.6)ƒƒ | 6999 (5.4)ƒƒ | 155 619 (94.8)ƒƒ | 8476 (5.2)ƒƒ | |
| Demographics | ||||||
| Age, years | 43.7±14.6 | 56.3±16.1 | 47.9±16.7 | 59.7±15.8 | 45.3±16.0 | 59.1±16.4 |
| Age group | ||||||
| 18–44 years | 91 676 (49) | 1464 (23) | 50 715 (42) | 1205 (17) | 77 730 (50) | 1681 (20) |
| ≥45 years | 88 993 (51) | 5013 (77) | 71 429 (58) | 5794 (83) | 77 889 (50) | 6795 (80) |
| Female | 106 234 (59) | 3991 (62) | 69 523 (57) | 4108 (59) | 94 458 (61) | 5433 (64) |
| Comorbidities# | ||||||
| Allergy¶,+ | 56 600 (31) | 3095 (48) | 33 814 (22) | 2819 (33) | ||
| Rhinitis§ | 37 362 (21) | 2484 (38) | 28 269 (18) | 2653 (31) | ||
| Nasal polyposisƒ | 3552 (2) | 478 (7) | 2281 (1) | 321 (4) | ||
| Asthma medications | ||||||
| SABAi | 125 255 (69) | 4670 (72) | 79 510 (65) | 5296 (76) | 110 258 (71) | 6472 (76) |
| ICS + ≥1 controllers | 98 345 (54) | 5621 (87) | 80 820 (66) | 5925 (84) | 83 419 (54) | 11 065 (88) |
| ICS + ≥2 controllers | 25 569 (14) | 3125 (38) | 21 248 (17) | 3521 (50) | 20 126 (13) | 3743 (45) |
| ICS + ≥3 controllers | 2614 (1) | 839 (13) | 2458 (2) | 863 (12) | 1194 (1) | 644 (8) |
| Daily budesonide equivalent dose, μg | 382±341 | 1365±960 | 361±349 | 1030±902 | 411±378 | 1314±875 |
| LABA | 94 564 (52) | 5499 (85) | 80 727 (66) | 6000 (86) | 77 037 (50) | 6988 (82) |
| LAMA | 9217 (5) | 1635 (25) | 12 848 (11) | 2665 (38) | 2121 (1) | 479 (6) |
| XANT | 298 (0.2) | 184 (3) | 541 (0) | 304 (4) | 1482 (1) | 749 (9) |
| LTRA | 26 927 (15) | 2516 (39) | 17 258 (14) | 1990 (28) | 28 329 (18) | 3762 (44) |
| Any OCS## | 39 316 (22) | 5085 (79) | 21 236 (17) | 5513 (79) | 29 867 (19) | 6703 (79) |
| High OCS exposure¶¶ | 0 (0) | 3431 (53) | 0 (0) | 4604 (66) | 0 (0) | 5360 (63) |
| Anti-IgE++ | 0 (0) | 800 (11) | ||||
| Anti-IL-5++ | 0 (0) | 551 (8) | ||||
Data are presented as n (%) or mean±sd. SABAi: inhaled short-acting β-agonist; ICS: inhaled corticosteroid; LABA: long-acting β2-agonist, LAMA: long-acting muscarinic antagonist; XANT: xanthines; LTRA: leukotriene receptor antagonist; OCS: oral corticosteroid; IL: interleukin. #: data not available in Norway; ¶: Anatomical Therapeutic Chemical Classification (ATC) code S01G cannot be captured in Finnish data, which may underestimate the prevalence of allergy; +: defined as at least two prescriptions with R06 and/or S01G or an International Classification of Diseases, 10th revision (ICD-10) diagnosis J45.0 or J3; §: defined as at least two prescriptions with R01AD or an ICD-10 diagnosis with J30-J32; ƒ: defined as an ICD-10 diagnosis with J33; ##: at least one prescription with OCS (ATC code H02AB); ¶¶: filled OCS prescriptions corresponding to an average of ≥5 mg per day (∼≥1825 mg total dispensed OCS); ++: data not available in Sweden and Finland; §§: in relation to the overall nationwide population; ƒƒ: in relation to the overall asthma population.
TABLE 2.
Characteristics of asthma patients aged 6–17 years in Sweden, Norway and Finland in 2018
| Sweden | Norway | Finland | ||||
| Mild-to-moderate | Severe | Mild-to-moderate | Severe | Mild-to-moderate | Severe | |
| Current asthma population | 58 911 (4.1)§§ | 26 858 (3.5)§§ | 32 089 (4.4)§§ | |||
| 58 647 (99.6)ƒƒ | 264 (0.4)ƒƒ | 26 602 (99.1)ƒƒ | 256 (1.0)ƒƒ | 32 003 (99.7)ƒƒ | 86 (0.3)ƒƒ | |
| Demographics | ||||||
| Age, years | 11.1±3.4 | 11.6±3.3 | 11.5±3.4 | 11.4±3.3 | 11.3±3.4 | 13.2±2.7 |
| Female | 24 445 (42) | 105 (40) | 11 324 (43) | 100 (39) | 13 143 (41) | 38 (44) |
| Comorbidities# | ||||||
| Allergy¶,+ | 23 015 (39) | 157 (59) | 9413 (29) | 31 (36) | ||
| Rhinitis§ | 10 209 (17) | 83 (31) | 5681 (18) | 23 (27) | ||
| Nasal polyposisƒ | 61 (0.1) | <5 | 16 (0.05) | <5 | ||
| Asthma medications | ||||||
| SABAi | 48 006 (82) | 231 (88) | 21 847 (82) | 220 (86) | 24 919 (78) | 65 (76) |
| ICS + ≥1 controllers | 18 822 (32) | 214 (81) | 9906 (37) | 211 (83) | 11 426 (35) | 70 (81) |
| ICS + ≥2 controllers | 4668 (8) | 108 (41) | 1659 (6) | 94 (37) | 3045 (9) | 25 (29) |
| ICS + ≥3 controllers | 81 (0.1) | 8 (3) | 16 (0.1) | 5 (2) | 5 (0.02) | <5 |
| Daily budesonide equivalent dose, μg | 184±182 | 758±565 | 174±198 | 830±656 | 169±172 | 1128±1075 |
| LABA | 13 353 (23) | 180 (68) | 8567 (32) | 184 (72) | 8651 (27) | 67 (78) |
| LAMA | 212 (0.4) | 12 (5) | 42 (0.2) | 16 (6) | 8 (0.02) | <5 |
| XANT | 9 (0.02) | <5 | <5 | <5 | 8 (0.02) | <5 |
| LTRA | 11 800 (20) | 149 (56) | 3667 (14) | 119 (46) | 7099 (22) | 29 (34) |
| Any OCS## | 6104 (10) | 137 (52) | 993 (4) | 88 (34) | 1533 (5) | 44 (51) |
| High OCS exposure¶¶ | 27 (0.05) | 71 (27) | 16 (0) | 56 (22) | 12 (0.04) | 39 (45) |
| Anti-IgE++ | <5 | 66 (26) | ||||
| Anti-IL-5++ | <5 | 65 (25) | ||||
Data are presented as n (%) or mean±sd. SABAi: inhaled short-acting β-agonist; ICS: inhaled corticosteroid; LABA: long-acting β2-agonist, LAMA: long-acting muscarinic antagonist; XANT: xanthines; LTRA: leukotriene receptor antagonist; OCS: oral corticosteroid; IL: interleukin. #: data not available in Norway; ¶: Anatomical Therapeutic Chemical Classification (ATC) code S01G cannot be captured in Finnish data, which may underestimate the prevalence of allergy; +: defined as at least two prescriptions with R06 and/or S01G or an International Classification of Diseases, 10th revision (ICD-10) diagnosis J45.0 or J3; §: defined as at least two prescriptions with R01AD or an ICD-10 diagnosis with J30-J32; ƒ: defined as an ICD-10 diagnosis with J33; ##: at least one prescription with OCS (ATC code H02AB); ¶¶: filled OCS prescriptions corresponding to an average of ≥5 mg per day (∼≥1825 mg total dispensed OCS); ++: data not available in Sweden and Finland; §§: in relation to the overall nationwide population; ƒƒ: in relation to the overall asthma population.
Asthma medication use
In adult patients fulfilling the definition of severe asthma, the mean±sd daily budesonide equivalent was 1375±960 µg in Sweden, 1314±875 µg in Finland and 1030±902 µg in Norway (table 1). In all countries, most adult patients with severe asthma were prescribed LABA as the second controller (82–86%). Larger proportions of severe asthma patients with dispensations of LAMA were found in Norway (38%) and Sweden (25%) compared to Finland (6%). Furthermore, 53% of patients with severe asthma in Sweden, 66% in Norway and 63% in Finland had high OCS exposure. Lastly, in Norway, 11% and 8% of severe asthma patients were on anti-IgE or anti-IL-5 therapies, respectively. Since information about hospital-administered medication was not available in the registries in Sweden and Finland, dispensations of biologicals are not presented in these countries.
In children with severe asthma, the controllers most frequently dispensed, apart from ICS, were LABA and LTRA (table 2). In Norway, 34% of children with severe asthma filled at least one OCS prescription; in Sweden and Finland, the numbers were 52% and 51%, respectively. Moreover, in Norway, 26% of children with severe asthma were treated with anti-IgE and 25% used anti-IL-5 therapies.
Asthma control outcomes according to asthma severity
In adult patients with severe asthma, the proportions of those patients who had two or more exacerbations were 67%, 61% and 63% in Sweden, Norway and Finland, respectively (table 3). Furthermore, 7–10% of these patients had one or more one ED visits, and 8–11% had at least one asthma-related hospital admission. The proportion of children with severe asthma who had two or more exacerbations varied from 14% in Norway to 37% in Sweden and Finland (table 4). Furthermore, in children with severe asthma, 2–10% and 4–5% had asthma-related ED visits and admissions, respectively (table 4).
TABLE 3.
Asthma control outcomes in asthma patients aged ≥18 years in Sweden, Norway and Finland in 2018
| Sweden | Norway | Finland | ||||
| Mild-to-moderate | Severe | Mild-to-moderate | Severe | Mild-to-moderate | Severe | |
| Patients | 180 669 | 6477 | 122 144 | 6999 | 155 619 | 8476 |
| Recurrent exacerbations# (≥2 exacerbations) | 14 185 (8) | 4335 (67) | 6557 (5) | 4285 (61) | 8167 (5) | 5370 (63) |
| Asthma-related ED visit | 5616 (3) | 480 (7) | 3767 (2) | 825 (10) | ||
| Asthma-related hospital admission | 2404 (1) | 517 (8) | 2642 (2) | 925 (11) | ||
| High SABA use | 33 103 (18) | 2457 (38) | 24 877 (20) | 3292 (47) | 23 227 (15) | 3458 (41) |
| Uncontrolled asthma¶ | 43 985 (24) | 5167 (80) | 29 120 (24) | 5395 (77) | 30 070 (19) | 6587 (78) |
| Antibiotics | 28 029 (16) | 2220 (34) | 26 145 (21) | 3348 (48) | 57 642 (37) | 4882 (58) |
| Hospital attendance with pneumonia | 2197 (1) | 455 (7) | 3337 (2) | 1002 (12) | ||
Data are presented as n or n (%). ED: emergency department; SABA: short-acting β-agonist. #: defined as either a dispensation with burst oral corticosteroid (OCS) (Anatomical Therapeutic Chemical Classification code H02AB), or an asthma-related ED visit in 2018, or as an asthma-related hospital admission; ¶: defined as at least two OCS dispensations and/or asthma-related ED visits and/or at least one asthma-related admission and/or high SABA use (of ≥600 doses (puffs)).
TABLE 4.
Asthma control outcomes in asthma patients aged 6–17 years in Sweden, Norway and Finland in 2018
| Sweden | Norway | Finland | ||||
| Mild-to-moderate | Severe | Mild-to-moderate | Severe | Mild-to-moderate | Severe | |
| Patients | 58 647 | 264 | 26 602 | 256 | 32 003 | 86 |
| Recurrent exacerbations#(≥2 exacerbations) | 1969 (3) | 98 (37) | 111 (0) | 36 (14) | 244 (8) | 32 (37) |
| Asthma-related ED visit | 2762 (5) | 26 (10) | 706 (2) | <5 | ||
| Asthma-related hospital admission | 308 (0.5) | 11 (4) | 234 (1) | <5 | ||
| High SABA use | 12 634 (22) | 125 (47) | 5915 (22) | 121 (47) | 3600 (11) | 18 (21) |
| Uncontrolled asthma¶ | 14 091 (24) | 186 (70) | 5998 (23) | 142 (55) | 3902 (12) | 46 (53) |
| Antibiotics | 8205 (14) | 70 (27) | 3337 (13) | 81 (32) | 10 643 (33) | 40 (47) |
| Hospital attendance with pneumonia | 375 (0.6) | 12 (5) | 275 (1) | <5 | ||
Data are presented as n or n (%). ED: emergency department; SABA: short-acting β-agonist. #: defined as either a dispensation with burst oral corticosteroid (OCS) (Anatomical Therapeutic Chemical Classification code H02AB), or an asthma-related ED visit in 2018, or as an asthma-related hospital admission; ¶: defined as at least two OCS dispensations and/or asthma-related ED visits and/or at least one asthma-related admission and/or high SABA use (of ≥600 doses (puffs)).
Overall, uncontrolled asthma (defined as at least two OCS dispensations/asthma-related ED visits and/or having an asthma-related admission and/or high SABA use) was observed for the majority of adult patients with severe asthma, i.e. 80% in Sweden, 78% in Finland and 77% in Norway (table 3). The corresponding proportions for children with severe asthma were smaller in all countries, i.e. 70% in Sweden, 53% in Finland and 55% in Norway (table 4). In patients with mild-to-moderate asthma, uncontrolled asthma was in the range 19–24% in adults, and 12–24% in children.
Patients with severe asthma were more likely to have been prescribed antibiotics against respiratory infections in all three countries in both children and adults when compared to mild-to-moderate asthma (tables 3 and 4). Admissions with pneumonia were similarly more prevalent in severe asthma patients compared to patients with mild-to-moderate asthma in both adults and in children (tables 3 and 4).
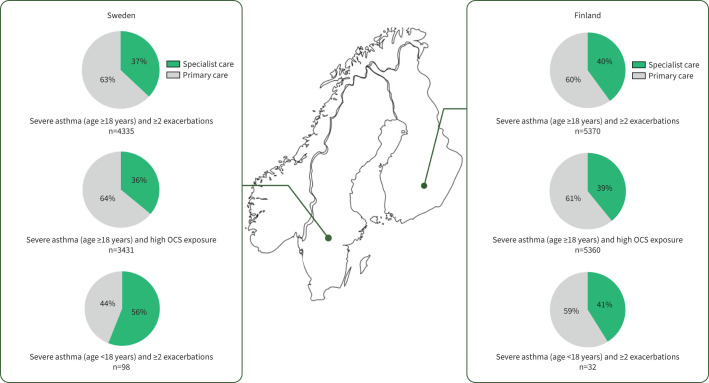
Management of severe asthma in specialist care
In adults with severe asthma and two or more exacerbations, 37% and 40% were seen by a specialist in secondary care in Sweden and Finland, respectively (figure 3). Furthermore, among those with high OCS exposure, 36% and 39% were managed in specialist care. In children with severe asthma and two or more exacerbations, the proportions of patients with a specialist care contact were 56% and 41% in Sweden and Finland, respectively.
FIGURE 3.
Management of severe asthma and recurrent exacerbations or high oral corticosteroid (OCS) exposure in children and adults in Sweden and Finland.
Discussion
In this study, utilising nationwide registry data in NORDSTAR, the prevalence of severe asthma in adult asthma patients ranged between 3.5% and 5.4% in Sweden, Norway and Finland. Severe asthma in children was a rare condition, with the prevalence in asthma patients ranging from 0.3% to 1.0%. Moreover, severe asthma patients frequently had indicators of poor asthma control, use of antibiotics against respiratory infections and pneumonia-related admissions. Notably, 12–24% of patients with mild-to-moderate asthma in both age groups had indicators of recurrent exacerbations, indicating potential undertreatment in this group. Lastly, in both children and adults, a large proportion of patients with severe asthma who experienced recurrent exacerbations were not currently managed by a respiratory specialist.
A strength of the present study was the consistent application of study definitions and methodology to assess the prevalence of severe asthma across three countries, ensuring comparability of the results. Another strength is the sample size of our study, which included nearly 600 000 patients with asthma in 2018. This is one of the largest asthma populations studied so far. Furthermore, by using nationwide population data, we minimised potential responder and selection biases, which limit epidemiologic studies based on self-reported questionnaires or registries that only cover selected patient groups. Conversely, the use of registry data can also be perceived as a limitation due to the lack of clinical information, such as measures of daily symptoms, smoking history and lung function. Therefore, the outcomes of our study represent proxies for uncontrolled asthma.
Our prevalence estimates of severe asthma ranging from 3.5% to 5.4% align well with previous population-based studies that have reported prevalence estimates of severe asthma in adults of 2% to 10% [5, 11–20]. Despite this agreement, we cannot exclude that we have overestimated the prevalence of severe asthma, since our study was register-based and therefore was limited by no possibility to distinguish between difficult-to-treat and true severe asthma. However, a common cause of difficult-to-treat asthma is poor adherence [28, 29]. In the Nordic countries, asthma inhalation medication is only partly reimbursed, and we therefore assume that patients who fill their prescriptions also adhere to their treatment. In contrast to the prevalence of severe asthma estimates, our findings regarding the prevalence of asthma in adults (2–4%) are lower than estimates from other population studies that have indicated a prevalence of approximately 6–10% [30–33]. This may be explained by our exclusion of patients aged >55 years without a diagnosis in secondary care. At the same time, the total adult population did not have an upper limit, which naturally has skewed the overall prevalence proportion towards a lower number in adults. In children, we found that severe asthma was a rare condition. Only a few studies have previously assessed the prevalence of severe asthma in children with estimates of 2.1% [19] and 4.5% [34] in two birth cohort studies, and estimates from 2% to 18% in a study using different European healthcare databases [5] However, compared to the present study, less strict definitions for severe asthma were used in these studies, which could explain the discrepancies.
Although the prevalence of severe asthma was similar across the countries, some noteworthy differences were also observed with respect to treatment patterns in adults that appeared to vary in Norway compared to Sweden and Finland. These differences may be real, but they may also be explained by a difference in the Norwegian data compared to the Finnish and Swedish data. Notably, we observed a lower mean ICS dose dispensed in adult severe asthma patients in Norway. Several reasons could explain this. Firstly, information about hospital-administered biological treatment was available in Norway, whereas no such data were available for Sweden and Finland. In Norway, nearly 20% of patients with severe asthma were treated with either anti-IgE or anti-IL-5 therapies, thereby being included as severe asthma with no requirement of high-dose ICS. Conversely, in Norway, information about diagnoses was only available as indications for treatment and not as separate ICD-10 codes from patient registries. Consequently, we speculate that in Norway, some patients with, for example, COPD and high OCS exposure might be misclassified as having severe asthma. This speculation is further supported by the observation of higher LAMA use in Norway (38%), compared to the other countries (25% in Sweden and 6% in Finland), which could indicate some degree of misclassification of COPD patients as severe asthma patients. In turn, such a possibility could also explain why a lower median ICS dose was observed in Norway.
The use of daily OCS is an important criterion in most of the definitions of severe asthma [8, 9]. Unfortunately, identifying daily OCS use is generally challenging, when using registry-based prescription data. Consequently, to estimate a cumulated steroid burden reflecting a daily use of OCS, adult patients with high-dose OCS exposure (with dispensed OCS prescriptions corresponding to an average daily dose of ≥5 mg per day) were classified as having severe asthma. In all three countries, a high proportion of high-dose OCS exposure was seen in adults with severe asthma, which may partly be explained by our inclusion of high-dose OCS users in our severe asthma definition. Likewise, as exacerbations were mostly driven by OCS prescriptions, the high proportion of exacerbations in patients with severe asthma could be, to some extent, caused by high-exposure OCS as a key inclusion criterion in adults with severe asthma. Furthermore, we cannot exclude that OCS were prescribed for other indications than asthma, such as inflammatory diseases, which could have led to some misclassification. However, although not all patients with severe asthma used ICS ≥1600 µg budesonide equivalent daily, few patients with high-dose OCS exposure had not been dispensed any ICS at all.
Finally, we found that patients with severe asthma and frequent exacerbations or high OCS exposure were often not treated in specialist care. This is a challenge also identified by other studies from the United Kingdom [13, 35], Denmark [11, 12] and Sweden [14] and is a great cause for concern. Since the benefits of management of severe asthma in specialist care are well documented [6], it should be a key priority to secure timely and appropriate referral of symptomatic asthma patients to specialist care in any healthcare system. Furthermore, with the emergence of new biological treatments and their availability in hospital settings, patients managed in primary care or by a private respiratory specialist will only have limited access to these treatments. However, it should be noted that the register data used, and the definitions applied in this study may have led to some degree of misclassification of level of management. For instance, registry data do not capture patients treated by respiratory specialists outside the hospitals, which could explain part of the gap, especially in the paediatric population.
In conclusion, to our knowledge, this is one of the largest studies to date having examined the prevalence of severe asthma in unselected population samples of nearly 600 000 asthma patients using complete nationwide registry data from multiple countries. The prevalence of severe asthma in asthma patients was comparable between three Nordic countries with variations from 3.5% to 5.4% in adults and 0.3% to 1.0% in children. Indicators of uncontrolled asthma were prevalent in patients with severe asthma in both children and adults, and many patients with severe asthma and recurrent exacerbations were currently only managed in primary care. Since the benefits from systematic assessment by respiratory specialist of severe asthma are well documented and effective biological therapies for severe asthma are only available in hospital settings, our findings highlight the need for increased focus on identifying patients with severe asthma in primary care.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00687-2022.supplement (199KB, pdf)
Footnotes
Provenance: Submitted article, peer reviewed.
Support statement: This study is presented on the behalf of the NORDSTAR study group. NORDSTAR is a pan-Nordic multiparty research collaboration platform governed by the Nordic Severe Asthma Network (NSAN). Data management and analyses are conducted by Quantify Research. NORDSTAR is financially supported by Novartis and Sanofi & Regeneron Pharmaceuticals. Funding information for this article has been deposited with the Crossref Funder Registry.
Conflict of interest: The work was financially supported by Novartis and Sanofi & Regeneron Pharmaceuticals. S. Hansen reports no conflicts of interest. A. von Bülow reports consulting fees from Novartis, speaker fees from Novartis, GSK and AstraZeneca, travel grants from AstraZeneca, and participation in advisory boards with AstraZeneca and Novartis. P. Sandin has no conflicts of interest. O. Ernstsson has no conflicts of interest. C. Janson reports speaker fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Novartis, Orion Pharma and Sanofi, and expert testimony payments from AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Novartis, Orion Pharma and Sanofi. L. Lehtimäki reports consulting fees from AstraZeneca and GSK, and speaker fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Novartis, Orion Pharma and Sanofi. H. Kankaanranta reports consulting fees from AstraZeneca, GSK, Chiesi, MSD, Orion Pharma, Novartis and Sanofi Genzyme, and speaker fees from AstraZeneca, GSK, Chiesi, Mundipharma, Orion Pharma and Sanofi Genzyme. C. Ulrik reports grants from Sanofi, Boehringer Ingelheim, AstraZeneca and Novartis, consulting fees from Orion Pharma, AstraZeneca and Teva, and participation in advisory boards with Novartis, GSK, AstraZeneca, Sanofi, Chiesi and Boehringer Ingelheim. B.B. Aarli reports consulting fees from GSK and AstraZeneca, lecture fees from GSK, AstraZeneca, Sanofi-Aventis Norge, Novartis and Boehringer Ingelheim, and participation in advisory boards with GSK, Astra Zeneca and Sanofi-Aventis Norge. H. Fues Wahl has no conflicts of interest. K. Geale is a board member of Quantify Research AB, Quantify Research ApS, Quantify Research AS and Quantify HEOR Private Limited, is CEO of Quantify Research AB, Quantify Research ApS and Quantify Research AS, and has stock and stock options in Quantify Research AB. S.T. Tang is an employee at Sanofi and holds stocks in Sanofi. M. Wolf is an employee at Novartis Finland. T. Larsen is an employee at Novartis Norway. A. Altraja reports consulting fees from AstraZeneca, Boehringer Ingelheim, GSK and Sanofi, speaker fees from AstraZeneca, Berlin-Chemie Menarini, Boehringer Ingelheim, Norameda (Chiesi), GSK, Sanofi, Teva and Zentiva, expert testimony for AstraZeneca, Boehringer Ingelheim, GSK and Sanofi, travel grants from AstraZeneca, Berlin-Chemie Menarini, Boehringer Ingelheim and Norameda (Chiesi), participation in advisory boards with AstraZeneca, Boehringer Ingelheim, GSK and Sanofi, and receipt of equipment from Berlin-Chemie Menarini. H. Backman reports speaker fees from AstraZeneca, Boehringer Ingelheim, GSK and Sanofi. M. Kilpeläinen reports no conflicts of interest. A. Viinanen reports consulting fees from GSK, speaker fees from AstraZeneca, ALK, GSK, Boehringer Ingelheim and Chiesi, and travel grants from AstraZeneca, Sanofi and Boehringer Ingelheim. D. Ludviksdottir reports travel grants from GSK and AstraZeneca. P. Kauppi reports no conflicts of interest. A. Sverrild reports grants from AstraZeneca, consulting fees from Novartis, speaker fees from AstraZeneca and Chiesi, travel grants from Teva, and participation in advisory boards with Chiesi and Sanofi-Genzyme. S. Lehmann reports no conflicts of interest. V. Backer reports no conflicts of interest. V. Yasinska reports lecture fees from GSK and Sanofi, and participation in advisory boards with AstraZeneca, Chiesi and GSK. T. Skjold reports no conflicts of interest. J. Karjalainen reports consulting fees from AstraZeneca, GSK, MSD and Novartis, and lecture fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, MSD, MundiPharma, Novartis and Orion Pharma. A. Bossios reports lecture fees from GSK, AstraZeneca, Teva and Novartis, travel grants from AstraZeneca and Novartis, and participation in advisory boards with GSK, AstraZeneca, Teva, Novartis and Sanofi, and is a member of the steering committee of SHARP, Secretary of Assembly 5 (Airway diseases, asthma, COPD and chronic cough) of the European Respiratory Society and the vice-president of the Nordic Severe Asthma Network. C. Porsbjerg reports grants from AstraZeneca, GSK, Novartis, Teva, Sanofi, Chiesi and ALK, consulting fees from AZ, GSK, Novartis, TEVA, Sanofi, Chiesi and ALK, lecture fees from AZ, GSK, Novartis, TEVA, Sanofi, Chiesi and ALK, and participation in advisory boards with AstraZeneca, Novartis, TEVA, Sanofi and ALK.
References
- 1.Montella S, Baraldi E, Cazzato S, et al. . Severe asthma features in children: a case–control online survey. Ital J Pediatr 2016; 42: 9. doi: 10.1186/s13052-016-0217-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Teague WG, Phillips BR, Fahy JV, et al. . Baseline features of the Severe Asthma Research Program (SARP III) cohort: differences with age. J Allergy Clin Immunol Pract 2018; 6: 545–554. doi: 10.1016/j.jaip.2017.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Neill S, Sweeney J, Patterson CC, et al. . The cost of treating severe refractory asthma in the UK: an economic analysis from the British Thoracic Society Difficult Asthma Registry. Thorax 2015; 70: 376–378. doi: 10.1136/thoraxjnl-2013-204114 [DOI] [PubMed] [Google Scholar]
- 4.Moore WC, Bleecker ER, Curran-Everett D, et al. . Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute's Severe Asthma Research Program. J Allergy Clin Immunol 2007; 119: 405–413. doi: 10.1016/j.jaci.2006.11.639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engelkes M, Baan EJ, Ridder MAJ, et al. . Incidence, risk factors and re-exacerbation rate of severe asthma exacerbations in a multinational, multidatabase pediatric cohort study. Pediatr Allergy Immunol 2020; 31: 496–505. doi: 10.1111/pai.13237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Denton E, Lee J, Tay T, et al. . Systematic assessment for difficult and severe asthma improves outcomes and halves oral corticosteroid burden independent of monoclonal biologic use. J Allergy Clin Immunol Pract 2020; 8: 1616–1624. doi: 10.1016/j.jaip.2019.12.037 [DOI] [PubMed] [Google Scholar]
- 7.McDonald VM, Clark VL, Cordova-Rivera L, et al. . Targeting treatable traits in severe asthma: a randomised controlled trial. Eur Respir J 2020; 55: 1901509. doi: 10.1183/13993003.01509-2019 [DOI] [PubMed] [Google Scholar]
- 8.Reddel HK, Bacharier LB, Bateman ED, et al. . Global Initiative for Asthma Strategy 2021: executive summary and rationale for key changes. Eur Respir J 2021; 59: 2102730. doi: 10.1183/13993003.02730-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung KF, Wenzel SE, Brozek JL, et al. . International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 2014; 43: 343–373. doi: 10.1183/09031936.00202013 [DOI] [PubMed] [Google Scholar]
- 10.Bel EH, Sousa A, Fleming L, et al. . Diagnosis and definition of severe refractory asthma: an international consensus statement from the Innovative Medicine Initiative (IMI). Thorax 2011; 66: 910–917. doi: 10.1136/thx.2010.153643 [DOI] [PubMed] [Google Scholar]
- 11.von Bülow A, Kriegbaum M, Backer V, et al. . The prevalence of severe asthma and low asthma control among Danish adults. J Allergy Clin Immunol Pract 2014; 2: 759–767. doi: 10.1016/j.jaip.2014.05.005 [DOI] [PubMed] [Google Scholar]
- 12.Håkansson KEJ, Backer V, Ulrik CS. Socioeconomic biases in asthma control and specialist referral of possible severe asthma. Eur Respir J 2021; 58: 2100741. doi: 10.1183/13993003.00741-2021 [DOI] [PubMed] [Google Scholar]
- 13.Ryan D, Heatley H, Heaney LG, et al. . Potential severe asthma hidden in UK primary care. J Allergy Clin Immunol Pract 2021; 9: 1612–1623. doi: 10.1016/j.jaip.2020.11.053 [DOI] [PubMed] [Google Scholar]
- 14.Larsson K, Ställberg B, Lisspers K, et al. . Prevalence and management of severe asthma in primary care: an observational cohort study in Sweden (PACEHR). Respir Res 2018; 19: 12. doi: 10.1186/s12931-018-0719-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hekking PW, Wener RR, Amelink M, et al. . The prevalence of severe refractory asthma. J Allergy Clin Immunol 2015; 135: 896–902. doi: 10.1016/j.jaci.2014.08.042 [DOI] [PubMed] [Google Scholar]
- 16.Backman H, Jansson S, Stridsman C, et al. . Severe asthma – a population study perspective. Clin Exp Allergy 2019; 49: 819–828. doi: 10.1111/cea.13378 [DOI] [PubMed] [Google Scholar]
- 17.Ilmarinen P, Tuomisto LE, Niemelä O, et al. . Prevalence of patients eligible for anti-IL-5 treatment in a cohort of adult-onset asthma. J Allergy Clin Immunol Pract 2019; 7: 165–174. doi: 10.1016/j.jaip.2018.05.032 [DOI] [PubMed] [Google Scholar]
- 18.Perez AV, Pol AB, Hernandez ER, et al. . Characterization of severe asthma in the pediatric population. Allergol Immunopathol 2021; 49: 60–65. doi: 10.15586/aei.v49i2.65 [DOI] [PubMed] [Google Scholar]
- 19.Nordlund B, Melén E, Schultz ES, et al. . Prevalence of severe childhood asthma according to the WHO. Respir Med 2014; 108: 1234–1237. doi: 10.1016/j.rmed.2014.05.015 [DOI] [PubMed] [Google Scholar]
- 20.Rönnebjerg L, Axelsson M, Kankaanranta H, et al. . Severe asthma in a general population study: prevalence and clinical characteristics. J Asthma Allergy 2021; 14: 1105–1115. doi: 10.2147/JAA.S327659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaw DE, Sousa AR, Fowler SJ, et al. . Clinical and inflammatory characteristics of the European U-BIOPRED adult severe asthma cohort. Eur Respir J 2015; 46: 1308–1321. doi: 10.1183/13993003.00779-2015 [DOI] [PubMed] [Google Scholar]
- 22.Bourdin A, Fabry-Vendrand C, Ostinelli J, et al. . The burden of severe asthma in France: a case–control study using a medical claims database. J Allergy Clin Immunol Pract 2019; 7: 1477–1487. doi: 10.1016/j.jaip.2018.12.029 [DOI] [PubMed] [Google Scholar]
- 23.Engelkes M, Janssens HM, de Jongste JC, et al. . Medication adherence and the risk of severe asthma exacerbations: a systematic review. Eur Respir J 2015; 45: 396–407. doi: 10.1183/09031936.00075614 [DOI] [PubMed] [Google Scholar]
- 24.Rusconi F, Fernandes RM, Pijnenburg MWH, et al. . The Severe Paediatric Asthma Collaborative in Europe (SPACE) ERS Clinical Research Collaboration: enhancing participation of children with asthma in therapeutic trials of new biologics and receptor blockers. Eur Respir J 2018; 52: 1801665. doi: 10.1183/13993003.01665-2018 [DOI] [PubMed] [Google Scholar]
- 25.Geale K, Darabi H, Lindh M, et al. . NORDSTAR: paving the way for a new era in asthma research. Eur Respir J 2020; 55: 1902476. doi: 10.1183/13993003.02476-2019 [DOI] [PubMed] [Google Scholar]
- 26.Ekström M, Nwaru BI, Hasvold P, et al. . Oral corticosteroid use, morbidity and mortality in asthma: a nationwide prospective cohort study in Sweden. Allergy 2019; 74: 2181–2190. doi: 10.1111/all.13874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schatz M, Zeiger RS, Vollmer WM, et al. . Validation of a β-agonist long-term asthma control scale derived from computerized pharmacy data. J Allergy Clin Immunol 2006; 117: 995–1000. doi: 10.1016/j.jaci.2006.01.053 [DOI] [PubMed] [Google Scholar]
- 28.Murphy AC, Proeschal A, Brightling CE, et al. . The relationship between clinical outcomes and medication adherence in difficult-to-control asthma. Thorax 2012; 67: 751–753. doi: 10.1136/thoraxjnl-2011-201096 [DOI] [PubMed] [Google Scholar]
- 29.von Bülow A, Backer V, Bodtger U, et al. . Differentiation of adult severe asthma from difficult-to-treat asthma – outcomes of a systematic assessment protocol. Respir Med 2018; 145: 41–47. doi: 10.1016/j.rmed.2018.10.020 [DOI] [PubMed] [Google Scholar]
- 30.Borna E, Nwaru BI, Bjerg A, et al. . Changes in the prevalence of asthma and respiratory symptoms in western Sweden between 2008 and 2016. Allergy 2019; 74: 1703–1715. doi: 10.1111/all.13840 [DOI] [PubMed] [Google Scholar]
- 31.Backman H, Hedman L, Jansson S-A, et al. . Prevalence trends in respiratory symptoms and asthma in relation to smoking – two cross-sectional studies ten years apart among adults in northern Sweden. World Allergy Organ J 2014; 7: 1. doi: 10.1186/1939-4551-7-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kainu A, Pallasaho P, Piirilä P, et al. . Increase in prevalence of physician-diagnosed asthma in Helsinki during the Finnish Asthma Programme: improved recognition of asthma in primary care? A cross-sectional cohort study. Prim Care Respir J 2013; 22: 64–71. doi: 10.4104/pcrj.2013.00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bloom CI, Saglani S, Feary J, et al. . Changing prevalence of current asthma and inhaled corticosteroid treatment in the UK: population-based cohort 2006–2016. Eur Respir J 2019; 53: 1802130. doi: 10.1183/13993003.02130-2018 [DOI] [PubMed] [Google Scholar]
- 34.Lang A, Carlsen KH, Haaland G, et al. . Severe asthma in childhood: assessed in 10 year olds in a birth cohort study. Allergy 2008; 63: 1054–1060. doi: 10.1111/j.1398-9995.2008.01672.x [DOI] [PubMed] [Google Scholar]
- 35.Bloom CI, Walker S, Quint JK. Inadequate specialist care referrals for high-risk asthma patients in the UK: an adult population-based cohort 2006–2017. J Asthma 2021; 58: 19–25. doi: 10.1080/02770903.2019.1672723 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00687-2022.supplement (199KB, pdf)