Abstract
Background
An objective of the Severe Heterogeneous Asthma Registry, Patient-centered (SHARP) is to produce real-world evidence on a pan-European scale by linking nonstandardised, patient-level registry data. Mepolizumab has shown clinical efficacy in randomised controlled trials and prospective real-world studies and could therefore serve as a proof of principle for this novel approach. The aim of the present study was to harmonise data from 10 national severe asthma registries and characterise patients receiving mepolizumab, assess its effectiveness on annual exacerbations and maintenance oral glucocorticoid (OCS) use, and evaluate treatment patterns.
Methods
In this observational cohort study, registry data (5871 patients) were extracted for harmonisation. Where harmonisation was possible, patients who initiated mepolizumab between 1 January 2016 and 31 December 2021 were examined. Changes of a 12-month (range 11–18 months) period in frequent (two or more) exacerbations, maintenance OCS use and dose were analysed in a privacy-preserving manner using meta-analysis of generalised estimating equation parameters. Periods before and during the coronavirus disease 2019 pandemic were analysed separately.
Results
In 912 patients who fulfilled selection criteria, mepolizumab significantly reduced frequent exacerbations (OR 0.18, 95% CI 0.13–0.25), maintenance OCS use (OR 0.75, 95% CI 0.61–0.92) and dose (mean −3.93 mg·day−1, 95% CI −5.24–2.62 mg·day−1) in the pre-pandemic group, with similar trends in the pandemic group. Marked heterogeneity was observed between registries in patient characteristics and mepolizumab treatment patterns.
Conclusions
By harmonising patient-level registry data and applying federated analysis, SHARP demonstrated the real-world effectiveness of mepolizumab on asthma exacerbations and maintenance OCS use in severe asthma patients across Europe, consistent with previous evidence. This paves the way for future pan-European real-world severe asthma studies using patient-level data in a privacy-proof manner.
Short abstract
This proof-of-principle study using the SHARP federated analysis platform confirms the real-world effectiveness of mepolizumab in 10 European countries. This paves the way for future studies involving thousands of severe asthma patients across Europe. https://bit.ly/3GPsg3T
Introduction
Meta-analyses of randomised controlled trials (RCTs) have shown that biological therapies targeting interleukin (IL)-5 are effective in patients with severe eosinophilic asthma and lead to significant improvements in exacerbation rates, oral corticosteroid (OCS) use, asthma control and health-related quality of life [1–3]. However, these RCTs are performed in highly selected populations under standardised and fully controlled conditions typically different from those in clinical practice [4, 5]. Therefore, real-world evidence is an indispensable complement to RCTs as it helps to elucidate which patients are prescribed these therapies and how effective they are in terms of relevant clinical end-points [6].
Many European countries have established registries of patients with severe asthma in order to collect real-world data on the impact of novel biological treatments [7, 8]. Unfortunately, each single country usually has a limited number of included patients, restricting the ability to deliver generalisable evidence and answer important research questions. Therefore, combining patient data from multiple countries and institutes is required in order to generate more robust and meaningful outcomes by increasing sample size and statistical power.
The European Respiratory Society (ERS) clinical research collaboration SHARP (Severe Heterogeneous Asthma Registry, Patient-centered) was set up to harmonise severe asthma management across Europe and unravel heterogeneity in a patient-centred way [9]. An objective of SHARP is to produce real-world evidence on a pan-European scale by linking together all available data from the national severe asthma registries that are part of the SHARP network. However, linking data from pre-existing registries is challenging, due to unavoidable discrepancies between the data collection models and limitations on the transfer of privacy-sensitive data [10]. For that reason, SHARP leveraged a federated analytics platform that enabled privacy-preserving analysis of distributed datasets and could deliver accurate results without revealing sensitive data [11–13].
The first research question that SHARP aimed to answer using this federated analytics platform, and which would also serve as proof of principle for this novel approach, was to assess the real-world effectiveness of mepolizumab in patients with severe asthma in Europe. Mepolizumab was the first anti-IL-5 biologic for severe eosinophilic asthma available in most European countries [14]. Its clinical efficacy has been demonstrated in multiple randomised, double-blind clinical trials showing roughly a halving of the rate of severe asthma exacerbations, a significant reduction in maintenance oral glucocorticoid use and improved health-related quality of life [15–18]. Other prospective and closely monitored studies also showed that unselected patients with severe eosinophilic asthma who were prescribed mepolizumab in a real-life setting showed similar improved outcomes [19, 20].
The present study was designed to evaluate the real-world use of mepolizumab in patients with severe asthma who had been included and followed up in national disease registries of several European countries since the introduction of mepolizumab. The specific aims of the study were to characterise patients receiving mepolizumab, evaluate the number or patients switching or discontinuing mepolizumab treatment, and assess the effectiveness of mepolizumab in patients with severe asthma across Europe using a federated analysis approach. We hypothesised that mepolizumab treatment would 1) reduce frequent (two or more per year) severe exacerbations and 2) lower maintenance use and daily dose of OCS after 1 year (range 11–18 months).
Methods
Study population and design
This was a real-world observational study involving the extraction and analysis of patient-level data from nonstandardised severe asthma registries from 10 countries in Europe. Most European registries included patients who fulfilled the severe asthma criteria according to the ERS/American Thoracic Society (ATS) guidelines [21], or national asthma guidelines, and in some cases other patients who attended specialist asthma centres were included as well based on clinical judgement of the treating specialist. The final results were obtained from adult severe asthma patients who initiated mepolizumab between 1 January 2016 and 31 December 2021 and had a follow-up visit available at 1 year (range 11–18 months) after mepolizumab initiation, or at the time of stopping mepolizumab, if sooner. Patients were excluded if they had received another biological treatment for severe asthma in the 12 months prior to inclusion, in order to remove the potential confounding effect of other biological treatments.
We distinguished two separate study periods, as it was likely that the coronavirus disease 2019 (COVID-19) pandemic would have influenced the treatment of patients with mepolizumab in terms of initiation, modification and discontinuation of concomitant treatments, as well as outcomes such as the number of asthma exacerbations [22]. The first period was pre-pandemic and was defined by the initiation and follow-up of mepolizumab treatment between 1 January 2016 and 31 March 2020. The second period spanned the pandemic and was defined by initiation and/or follow-up of mepolizumab treatment between 1 April 2020 and 31 December 2021.
Patients’ informed consent for using their data for international research purposes was collected at registry enrolment; the respective national medical ethics committees approved the study protocol.
Data source
10 individual SHARP registries agreed to participate in the study and to have their data used for federated analyses. The database field names of each national registry were harmonised to concepts via the Observational Medical Outcomes Partnership Common Data Model developed by the Observational Health Data Sciences and Informatics community [12, 23]. A federated analysis information technology platform (FAP) was then developed and implemented by SHARP in order to generate summary statistics from the harmonised registries, ensuring that individual patient data would remain at the local sites.
The process of, and experiences with, the registry mapping, development and implementation of the FAP has been described separately [24].
Study outcomes
Two time points were considered: initiation of mepolizumab (baseline) and 11–18 months after baseline (follow-up). Clinical comparisons were drawn between the baseline and follow-up.
Primary outcomes
Co-primary study outcomes included 1) change in frequent (two or more per year) severe exacerbations; and 2) change in maintenance use and daily dose of OCS after 1 year (range 11–18 months) of mepolizumab treatment.
Secondary outcomes
Secondary outcomes included the description of 1) characteristics of patients prescribed mepolizumab in the 10 registries; and 2) rates of discontinuation of mepolizumab and/or switching to another biologic.
Study variables and definitions
Study variables included demographics, pulmonary function (forced expiratory volume in 1 s), comorbidities (nasal polyposis), inflammatory markers (blood eosinophils, fraction of exhaled nitric oxide, total immunoglobulin (Ig)E), exacerbation rate, OCS use, OCS maintenance dose and rates of patients switching or discontinuing mepolizumab treatment.
Severe asthma exacerbations were defined by at least one of the following criteria: 1) patient-reported use of OCS courses (if not on maintenance OCS); 2) patient-reported doubling of maintenance dose of OCS for ≥3 days; and 3) patient-reported unscheduled emergency visits or hospitalisation for asthma. Frequent exacerbations were defined as two or more exacerbations per year. This categorical variable was chosen to maximise the availability of this outcome variable due to different methods of recording annual exacerbation rate across registries.
Maintenance OCS dose was expressed as the prednisolone-equivalent daily maintenance dose of OCS (mg·day−1).
Statistical analysis
Continuous variables were expressed as mean±sd or median (interquartile range) as appropriate, and categorical variables as n (%). Generalised estimating equations (GEE) were used to derive a parameter estimate for the difference between time windows (baseline and follow-up). The outcomes of interest were regressed upon time-window (pre-/post-), and a sandwich estimator was used to correct the standard errors for within-person correlation, where present. It is noted that there was a mixture of paired (baseline plus follow-up) observations and unpaired (baseline or follow-up) observations. An inverse variance weighted, fixed-effects meta-analysis was used to combine results and estimate effect sizes across participating registries, and results were presented in a forest plot. The pre-pandemic and pandemic groups were analysed separately. To describe patients switching or discontinuing mepolizumab treatment, a tabular summary was generated, by registry. The discontinuation date was set at 28 days after the last known prescription. Discontinuation was also considered to have occurred if there was a break of ≥90 days between prescriptions; the 90 days were measured from the end of the last known prescription plus 28 days. All statistical analyses were performed using R Statistical Software (version 4.1.2) [25].
Results
Patients
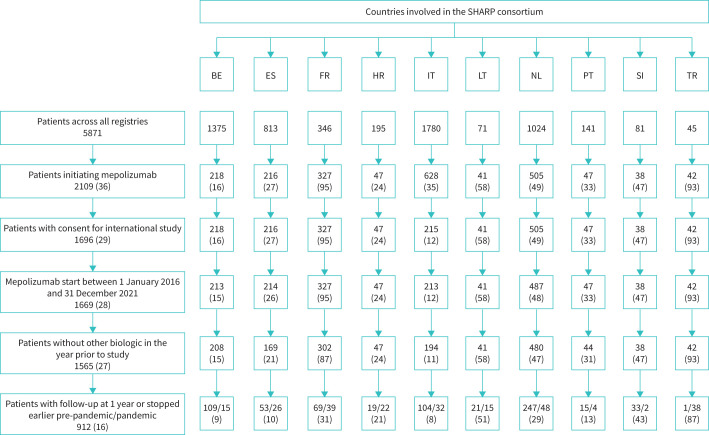
Figure 1 shows the flow chart of inclusion in the study. Out of 5871 patients with severe asthma included in the 10 national registries, 2109 initiated mepolizumab treatment. A total of 912 patients met the additional inclusion criteria of consent for an international study; initiation of treatment between 1 January 2016 and 31 December 2021 and no biological treatment for severe asthma for 1 year prior. 671 patients initiated mepolizumab and had follow-up data before the COVID-19 pandemic (pre-pandemic group) and 241 patients either initiated mepolizumab or were followed-up during the pandemic (pandemic group) (figure 1).
FIGURE 1.
Flow chart of selected patients. Data are presented as n or n (%). SHARP: Severe Heterogeneous Asthma Registry, Patient-centred; BE: Belgium; ES: Spain; FR: France; HR: Croatia; IT: Italy; LT: Lithuania; NL: the Netherlands; PT: Portugal; SI: Slovenia; TR: Turkey.
Tables 1 (pre-pandemic) and 2 (pandemic) summarise the characteristics of the study patients from each national registry at initiation of mepolizumab treatment for the two time periods. The majority of patients had at least two exacerbations per year and the use of maintenance OCS ranged from 26.3% to 80% in the pre-pandemic group and from 10% to 50% in the pandemic group. Although no patient had received biological treatment for severe asthma in the year prior to inclusion in this analysis, six patients in the pre-pandemic group and one in the pandemic group had earlier received another biologic for severe asthma.
TABLE 1.
Baseline characteristics per country, pre-pandemic
| Belgium | Spain | France | Croatia | Italy | Lithuania | The Netherlands | Portugal | Slovenia | Turkey | |
| Patients | 109 | 53 | 69 | 19 | 104 | 21 | 247 | 15 | 33 | 1 |
| Age at index (years) | 55.39±15.92 | 58.3±13.63 | 52.55±13.25 | 54.32±13.84 | 56.15±11.34 | 55.86±12.66 | 54.84±14.78 | 56.33±15.23 | 57.85±11.38 | |
| Female | 62 (56.9) | 40 (75.5) | 44 (63.8) | 15 (78.9) | 67 (64.4) | 13 (61.9) | 128 (51.8) | 10 (66.7) | 23 (69.7) | |
| BMI (kg·m−2) | 27.04±5.37 | 28.6±5.36 | 26.08±4.25 | 25.92±5.2 | 24.98±4.45 | 27.86±5.21 | 28.14±5.59 | 27.85±4.3 | 25.62±4.66 | |
| Age of onset (years) | 34.65±18.97 | 36.32±17.88 | 37.43±16.48 | 36.05±21.3 | 37.96±20.08 | 33.33±21.89 | 40.53±16.99 | |||
| Adult onset | 53 (81.5) | 16 (84.2) | 92 (88.5) | 17 (81) | 180 (77.6) | 9 (60) | 28 (87.5) | |||
| Current smoker | 3 (2.8) | 1 (2) | 4 (6) | 1 (5.3) | 3 (2.9) | 0 (0) | 1 (0.4) | 0 (0) | 0 (0) | |
| Previous smoker | 37 (33.9) | 18 (36) | 27 (40.3) | 5 (26.3) | 23 (22.1) | 4 (19) | 107 (43.3) | 2 (13.3) | 10 (30.3) | |
| Pack-years | 15.5 (8–30) | 8 (3–30) | 11.25 (5–18) | 22.5 (12–37) | 10.5 (5.4–22.5) | 12 (3.25–22.5) | 10 (5–20) | 12.5 (5–20) | 15 (7.5–20) | |
| Blood eosinophil count (×109 cells·L−1) | 0.59 (0.36–1) | 0.52 (0.4–0.8) | 0.31 (0.12–0.51) | 0.5 (0.15–1.01) | 0.59 (0.3–0.8) | 0.41 (0.35–0.56) | 0.39 (0.2–0.63) | 0.37 (0.22–0.61) | ||
| Total IgE (kU·L−1) | 111 (60–207) | 71 (65–262) | 93.7 (43–224) | 271 (8.3–287) | 99.8 (48.2–181) | 36.6 (19–103) | 110 (50–246) | 17 (15–51) | 150 (47–247) | |
| Frequent exacerbations (≥2 per year) | 54 (79.4) | 11 (68.8) | 45 (53.6) | 19 (95) | 114 (65.9) | 23 (74.2) | ||||
| OCS maintenance | 22 (35.5) | 8 (80) | 12 (29.3) | 47 (52.2) | 5 (26.3) | 73 (60.3) | 3 (60) | 12 (42.9) | ||
| Daily OCS dose (mg·day−1) | 7.98±3.05 | 9.12±8.69 | 16.36±8.97 | 19.12±14.41 | 19±7.42 | 14.37±14.31 | 10.25±7.53 | |||
| FEV1 pre-BD (% pred) | 58.29±18.86 | 57.69±18.55 | 49.43±17.94 | 59.88±23.19 | 76.51±21.54 | 69.7±34.79 | 75.16±21.85 | 74.81±31.73 | 66.74±21.77 | |
| FEV1 post-BD (% pred) | 60.82±18.98 | 57±12.55 | 48.62±19.33 | 83.14±17.89 | 62.33±22.01 | 80.84±21.28 | 66.9±15.72 | 77.88±10.51 | ||
| FENO (ppb) | 46 (23–68) | 21 (19.2–27.3) | 30 (10.82–80) | 29.5 (23.5–66) | 44 (30–73) | 40 (27–72) | 36 (23–64) | 89.5 (60–101) | ||
| Nasal polyps | 56 (51.4) | 27 (50.9) | 22 (31.9) | 9 (47.4) | 62 (59.6) | 6 (28.6) | 115 (46.6) | 4 (26.7) | 17 (51.5) |
Data are presented as n, mean±sd, n (%) or median (interquartile range). BMI: body mass index; Ig: immunoglobulin; OCS: oral corticosteroids (prednisone equivalents); FEV1: forced expiratory volume in 1 s; BD: bronchodilator; FENO: fractional exhaled nitric oxide.
TABLE 2.
Baseline characteristics per country, during the coronavirus disease 2019 pandemic
| Belgium | Spain | France | Croatia | Italy | Lithuania | The Netherlands | Portugal | Slovenia | Turkey | |
| Patients | 15 | 26 | 39 | 22 | 32 | 15 | 48 | 4 | 2 | 38 |
| Age at index (years) | 58.87±13.26 | 58.19±11.28 | 51.82±16.66 | 56.32±13.76 | 56.94±10.3 | 61.13±12.52 | 60.27±12.17 | 48.34±10.89 | ||
| Female | 8 (53.3) | 17 (65.4) | 23 (59) | 19 (86.4) | 24 (75) | 8 (53.3) | 27 (56.2) | 19 (50) | ||
| BMI (kg·m−2) | 27.53±4.02 | 28.77±4.16 | 26.36±3.97 | 27.46±7 | 25.67±4.24 | 29.74±4.17 | 28.83±5.74 | 26.87±3.89 | ||
| Age of onset (years) | 39.44±18.59 | 39.55±16.96 | 37.47±14.81 | 43.67±19.45 | 38.32±23.9 | 36.16±12.4 | ||||
| Adult onset | 32 (82.1) | 21 (95.5) | 28 (87.5) | 13 (86.7) | 35 (74.5) | 36 (94.7) | ||||
| Current smoker | 2 (13.3) | 1 (4.2) | 1 (2.6) | 1 (4.5) | 0 (0) | 1 (6.7) | 0 (0) | 1 (2.6) | ||
| Previous smoker | 6 (40) | 6 (25) | 12 (31.6) | 6 (27.3) | 10 (31.2) | 5 (33.3) | 27 (56.2) | 10 (26.3) | ||
| Pack-years | 15 (10–30) | 10.5 (3.8–20.3) | 19.5 (10–37.5) | 25 (15–40) | 8.75 (2.5–15) | 16.5 (5–30) | 11 (5–15) | 5.5 (4–7) | ||
| Blood eosinophil count (×109 cells·L−1) | 1 (0.61–1.25) | 0.5 (0.33–0.65) | 0.31 (0.1–0.52) | 0.7 (0.28–0.88) | 0.6 (0.39–0.76) | 0.64 (0.4–0.76) | 0.31 (0.2–0.52) | 0.7 (0.4–1.2) | ||
| Total IgE (kU·L−1) | 216 (78–317) | 83.5 (47–133.5) | 157 (101–233) | 111 (63.9–264) | 131.1 (51.9–208) | 160 (42.8–220) | 118 (57–221) | 168 (94.7–292) | ||
| Frequent exacerbations (≥2 per year) | 5 (62.5) | 5 (83.3) | 12 (66.7) | 15 (65.2) | 13 (92.9) | 25 (61) | 30 (81.1) | |||
| OCS maintenance | 1 (16.7) | 4 (44.4) | 6 (20.7) | 2 (50) | 14 (45.2) | 1 (10) | 12 (50) | 18 (50) | ||
| Daily OCS dose (mg·day−1) | 5 | 12.43±15.05 | 17.76±18.54 | 12.5±4.95 | 25.68±14.52 | 40 | 11.82±8.59 | 16.56±10.69 | ||
| FEV1 pre-BD (% pred) | 62.83±23.88 | 82.72±20.43 | 70.33±22.24 | 64.52±21.19 | 74.89±20.25 | 54.25±12.72 | 79.51±23.74 | 72.12±22.77 | ||
| FEV1 post-BD (% pred) | 84.81±23.51 | 83.24±15.81 | 66.84±18.38 | 91.33±27.86 | 63±20.4 | 90.95±23.96 | 68.17±19.23 | |||
| FENO (ppb) | 44.5 (21.5–91.5) | 67.85 (62–91) | 85 (20.7–89) | 30.5 (14.5–50) | 23 (21–80) | 41.5 (30–61) | 34.5 (14–48) | 22 (16–25) | ||
| Nasal polyps | 10 (66.7) | 9 (34.6) | 17 (43.6) | 8 (36.4) | 16 (50) | 5 (33.3) | 14 (29.2) | 24 (63.2) |
Data are presented as n, mean±sd, n (%) or median (interquartile range). BMI: body mass index; Ig: immunoglobulin; OCS: oral corticosteroids (prednisone equivalents); FEV1: forced expiratory volume in 1 s; BD: bronchodilator; FENO: fractional exhaled nitric oxide.
Exacerbation rate
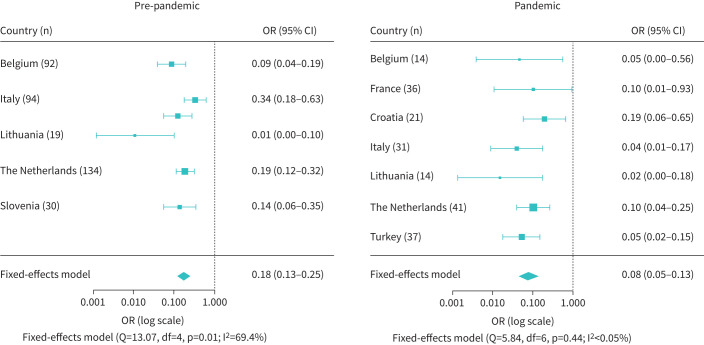
Annual exacerbation rate data were available from 369 patients in the pre-pandemic group and 194 patients in the pandemic group (figure 2). The odds of having experienced two or more exacerbations per year after mepolizumab initiation was significantly reduced for both the pre-pandemic group (OR 0.18, 95% CI 0.13–0.25; p<0.001) and the pandemic group (OR 0.08, 95% CI 0.05–0.13; p<0.001). Heterogeneity was clearly present in the pre-pandemic group (I2=69.4%), but not in the pandemic group (I2<0.05%).
FIGURE 2.
Forest plot of the odds of having experienced two or more exacerbations per year after mepolizumab initiation compared to the year before initiating mepolizumab for the individual countries and the countries combined. The odds were statistically significantly reduced for both the pre-pandemic group (n=369) and the pandemic group (n=194).
Maintenance OCS use
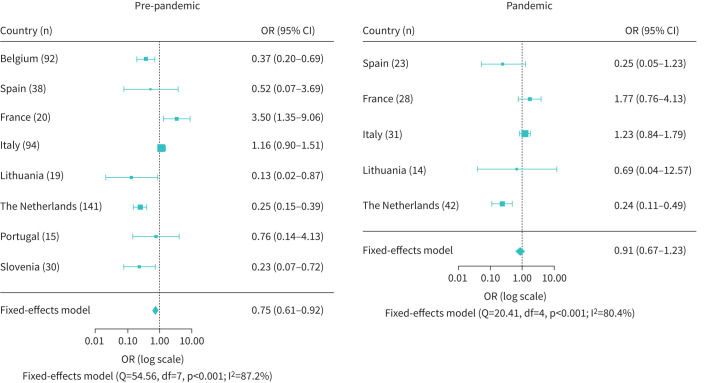
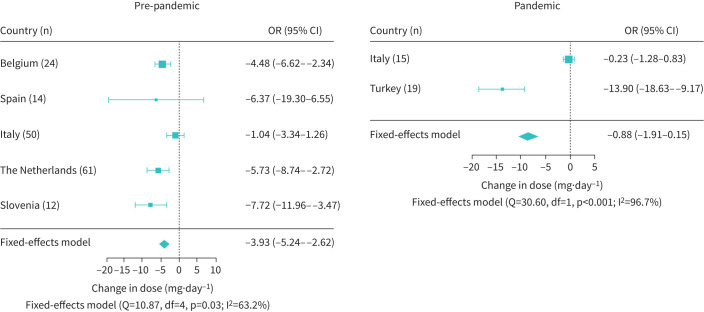
Figure 3 shows the odds of receiving maintenance OCS therapy at follow-up. Data on maintenance OCS use were available for 449 patients in the pre-pandemic group and 138 patients in the pandemic group. For the pre-pandemic group, the odds of patients receiving maintenance OCS at follow-up was significantly reduced from baseline (OR 0.75, 95% CI 0.61–0.92; p=0.005), whereas for the pandemic group the effect was not statistically significant (OR 0.91, 95% CI 0.67–1.23; p=0.527). Heterogeneity was present in both analyses (I2>80%). The reduction in maintenance OCS dose is shown in figure 4. Data on daily OCS maintenance dose were available for 161 patients in the pre-pandemic group. The maintenance OCS dose was significantly different from baseline (mean −3.93 mg·day−1, 95% CI −5.24−2.62; p<0.001). For the pandemic group, the dose of maintenance OCS at follow-up was not significantly different from baseline (mean −0.88 mg·day−1, 95% CI −1.91–0.15; p=0.096). However, the available data were extremely limited, with only two countries contributing (Italy n=15, Turkey n=19). Again, heterogeneity was observed in both meta-analyses, with I2>60%.
FIGURE 3.
Forest plot of the odds of receiving maintenance oral corticosteroid treatment after mepolizumab initiation for the individual countries and the countries combined. The odds were statistically significantly reduced from baseline for the pre-pandemic group (n=449), but not for the pandemic group (n=138).
FIGURE 4.
Forest plot of the reduction in mean maintenance oral corticosteroid dose after mepolizumab initiation. for the individual countries and the countries combined. The dose was statistically significantly reduced from baseline for the pre-pandemic group (n=161), but not for the pandemic group (n=34).
Characteristics of patients prescribed mepolizumab
The baseline characteristics of patients in the analysis set are shown graphically by country in supplementary figure S1. These figures show marked heterogeneity in characteristics, both within individual registries between the periods before and during the pandemic, and between registries. For example, the proportion of previous smokers ranges from 13.3% to 43.3% in the pre-pandemic group and from 25% to 56.2% in the pandemic group. Similarly, body mass index, age and total IgE show marked differences between periods and between registries.
Rates of patients switching or discontinuing mepolizumab
475 (71%) patients in the pre-pandemic group continued mepolizumab treatment at follow-up, while 291 (91%) patients in the pandemic group continued mepolizumab treatment (tables 3 and 4, respectively). There were marked differences between countries in the number of patients who stopped biological treatment or switched to another biologic. One-third of patients in France stopped biological therapy in the pre-pandemic period and patients in the French and Dutch registries most frequently switched to another anti-IL-5-targeting biologic (23% and 26%, respectively, in the pre-pandemic period).
TABLE 3.
Rates of patients switching or discontinuing mepolizumab treatment in pre-pandemic period
| All | Belgium | Spain | France | Croatia | Italy | Lithuania | The Netherlands | Portugal | Slovenia | Turkey | |
| Patients | 671 | 109 | 53 | 69 | 19 | 104 | 21 | 247 | 15 | 33 | 1 |
| Patients who continued using mepolizumab at follow-up | 475 (71) | 92 (84) | 40 (76) | 27 (39) | 15 (79) | 96 (92) | 19 (90) | 141 (57) | 15 (100) | 30 (91) | |
| Patients who stopped all biological therapy during follow-up | 94 (14) | 14 (13) | 8 (15) | 23 (33) | 4 (21) | 6 (6) | 1 (5) | 35 (14) | 0 (0) | 3 (9) | |
| Patients who switched to another biologic during follow-up | 101 (15) | 3 (3) | 5 (1) | 19 (28) | 0 (0) | 2 (2) | 1 (5) | 71 (29) | 0 (0) | 0 (0) | |
| Omalizumab | 6 | 0 | 0 | 2 | 1 | 0 | 3 | ||||
| Reslizumab | 37 | 0 | 2 | 0 | 0 | 0 | 35 | ||||
| Benralizumab | 52 | 3 | 3 | 16 | 1 | 1 | 28 | ||||
| Dupilumab | 6 | 0 | 0 | 1 | 0 | 0 | 5 | ||||
| Months of mepolizumab therapy in patients who stopped or switched during follow-up | # | 6 (5.2–9.6) |
4.9 (3.4–9.2) |
6 (5–9.9) |
4.8 (3.9–5) |
7.2 (3.2–8.2) |
5.6 (4.2–7) |
5.5 (3.6–8.4) |
11.1 (7.4–11.5) |
Data are presented as n, n (%) or median (interquartile range). #: not calculable from medians extracted.
TABLE 4.
Rates of patients switching or discontinuing mepolizumab treatment in the pandemic period
| All | Belgium | Spain | France | Croatia | Italy | Lithuania | The Netherlands | Portugal | Slovenia | Turkey | |
| Patients | 241 | 15 | 26 | 39 | 22 | 32 | 15 | 48 | 4 | 2 | 38 |
| Patients who continued using mepolizumab at follow-up | 219 (91) | 14 (93) | 23 (88) | 36 (92) | 21 (95) | 31 (97) | 14 (93) | 42 (88) | 38 (100) | ||
| Patients who stopped all biological therapy during follow-up | 13 (5) | 1 (7) | 3 (12) | 0 (0) | 1 (5) | 1 (3) | 1 (7) | 6 (12) | 0 (0) | ||
| Patients who switched to another biologic during follow-up | 3 (1) | 0 (0) | 0 (0) | 3 (8) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Omalizumab | 0 | 0 | |||||||||
| Reslizumab | 0 | 0 | |||||||||
| Benralizumab | 1 | 1 | |||||||||
| Dupilumab | 2 | 2 | |||||||||
| Months of mepolizumab therapy in patients who stopped or switched during follow-up | # | 5.5 (5.5–5.5) |
3.8 (2–4.8) |
8.8 (8–9.4) |
11.7 (11.7–11.7) |
2.7 (2.7–2.7) |
2.8 (2.8–2.8) |
5.3 (2–6) |
Data are presented as n, n (%) or median (interquartile range). #: not calculable from medians extracted.
Discussion
In this study, SHARP succeeded in linking existing patient-level data from 10 different national registries for severe asthma to evaluate the use of mepolizumab therapy across Europe. With the use of a federated analysis approach, the study shows that treatment with mepolizumab significantly reduces severe exacerbations, as well as maintenance OCS use in our sample of patients with severe asthma. In addition, the results show substantial heterogeneity among patients initiating mepolizumab, and rates of switching and discontinuing mepolizumab treatments across European countries. The registry data used for this study were pre-existing, and turned out to be far from complete for current purposes. This fact, in combination with strict requirement for defined entry and outcome data to be available to enable the federated analysis to be undertaken, resulted in a high analysis dropout rate. Thus, the results of this study, while in line with those of RCTs, should be interpreted with these limitations in mind.
The present real-world study supports and complements the results from RCTs and previous real-world studies on the effectiveness of mepolizumab therapy [5, 15–18, 26–29]. While the RCTs involve large numbers of patients from around the world, most real-world studies were conducted with data from a single institution or national registry. In addition, these studies often included small numbers of patients [30]. We are aware of only one real-world study that used data from patients with severe asthma from different countries [19]. In contrast to our study, patients in this study were prospectively followed over time, capturing data from clinical practice and recorded in a standardised way. Our study, which made use of data already collected by clinicians in 10 different European countries, not only complements the findings from the previously published studies, but also reflects the effectiveness of mepolizumab in daily clinical practice. Registries have been recognised as an important tool to provide real-world evidence [8]. By connecting several registries, our study further seeks to generate real-world evidence in large populations.
In our study, we used a relatively new approach to link privacy-sensitive data from clinical disease registries. That process was not without its challenges. The complexity and labour-intensive nature of harmonising privacy-sensitive data from different sources has been extensively described by Biedermann et al. [23]. In their study, three registries of pulmonary hypertension patients were linked and the data analysed in a federated manner. The harmonisation process in our study was even more complex, as all registries had a different data model. This makes our study the first to have harmonised and used nonstandardised real-world disease registries to obtain real-world evidence. The harmonisation process has been completed and the present proof-of-principle study has demonstrated that this federated approach can produce valid results. The platform can now be further used to obtain real-world evidence to help guide better treatment and care to the many thousands of patients with severe asthma in Europe.
In addition to the exceptional method by which we have linked registries in a privacy-protective way, our research is unique in several respects. Successfully analysing patient-data that have been collected in clinical practice and entered into local registries by clinicians from 10 different countries without the involvement or monitoring of contract research organisations including pharmaceutical companies is unprecedented to date. This is probably the best method to get as close as possible to daily clinical practice and to compare treatment practices across countries. Another strength of our study is that our large-scale approach has allowed us to analyse data from the time periods before and during the pandemic separately, so that we could avoid bias due to the alterations in circulating pathogens and changed circumstances of care for severe asthma patients.
Nevertheless, this study has its limitations. First, there are the limitations inherent to conducting real-world studies, for example lack of a control group. Due to the lack of a control group, it is not certain that the observed effectiveness of mepolizumab is caused solely by mepolizumab treatment or by other contributing factors. Second, many patients’ data could not be included in the analysis because data were missing or incomplete, or because the moment of data collection was not recorded. In addition, some patients had not given informed consent to use their data for research outside the institution in which they were treated. This feature of real-world data might have led to a selection bias between countries and may limit the generalisability of our study results. Nevertheless, we observe that the health outcomes of well-designed clinical trials can also be detected in a highly imperfect real-world setting. Third, treatment outcomes could only be evaluated for patients still on mepolizumab after 1 year of follow-up, which may have led to a selection bias, potentially overestimating our results and limiting the generalisability of our results. Furthermore, a potential limitation of the analysis is that patients considered at baseline overlapped with, but were not exactly the same as those considered at follow-up. Where paired data was available, the correlation was appropriately accounted for in the analysis. Due to reasons of statistical power, the GEE analyses were not adjusted for possible confounders. Future larger studies might be able to repeat the analyses and include covariates. Finally, there was the effect of the COVID-19 pandemic. This led to many hurdles in data collection and forced us to split the analysis into two periods. Although this reduced the statistical power of the study, we still were able to demonstrate the effect of mepolizumab in reducing exacerbations in both the pre-pandemic and pandemic time periods.
Our study shows that mepolizumab treatment leads to a reduction in the number of exacerbations and in the use of maintenance OCS in a real-world population. While this outcome was expected, it was reassuring to observe this finding in a setting alternative to RCTs or prospective observational studies, as patients included in an RCT are strictly selected, and may differ significantly from real-world populations. Our results imply that physicians should not be concerned that the effect of mepolizumab in their patients with severe eosinophilic asthma will be below expectations, even if they are less rigorously selected in terms of factors that were exclusion criteria in the RCTs. Of note was the consistent reduction in exacerbations across the countries despite the heterogeneity in the patient populations, with respect to baseline demographics.
The characteristics of real-world patients not only differ from those in the RCTs, but our study also found that patients prescribed mepolizumab differed considerably between the different European countries. This heterogeneity may be due to differences in reimbursement practices for biological therapies, to recommendations in national guidelines and therefore the eligibility criteria for biologics, or to preferences and choices of individual physicians. The differences in patient characteristics treated with mepolizumab before and during the pandemic may have influenced the extent to which some patients could or could not cope with remote care [22]. The heterogeneity of our population also illustrates that the definition of “severe asthma” does not appear to be used unambiguously. A revision of the international ERS/ATS definition of severe asthma may therefore be required. Interestingly, we also observed differences in OCS tapering strategies, and this important medical practice also requires greater attention and harmonisation. Fortunately, an important first step in the right direction was recently taken with the PONENTE study [31, 32].
A remarkable finding of our study is the difference between countries in the proportion of patients who continue or discontinue mepolizumab, or switch to another biological treatment. These switchers are likely to be patients who have partially responded to mepolizumab treatment, but have not yet shown full normalisation of all outcome parameters [33]. The fact that such a switch has not been observed in all countries probably reflects different availability of alternative biologics and contrasting practices and levels of acceptance of what is a beneficial outcome. These findings define an area in which greater insight is needed. Until now the best treatment with biologics could only be found based on physician knowledge and experience. It would be less burdensome for all parties if switching of biologics were not necessary and we had good predictors of response. By continuing to use the federated analysis approach and by further optimising national databases and enriching them with biological samples, finding reliable predictors will very likely become possible in the future. More so, if we apply artificial intelligence, machine learning and federated learning to clinical outcome data. The SHARP federated analysis platform is optimally suited for this.
In this study, SHARP demonstrated the real-world effectiveness of mepolizumab in patients with severe asthma from 10 different European countries. Mepolizumab reduced asthma exacerbations and OCS use consistent with evidence generated by RCTs. We observed heterogeneity in characteristics of patients receiving mepolizumab and in rates of switching or discontinuing treatment across countries, signalling the need for further alignment of asthma management across European countries. Our study can be seen as a successful proof of principle as to whether a federated analysis approach can be used to link privacy-sensitive data from different sources. It can thus serve as an example for other clinical research collaborations with a similar ambition. While there is still some room for improvement regarding completeness and quality of data, the SHARP federated analysis platform has great potential for future pan-European real-world severe asthma studies using patient-level data in a privacy-protected way.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00745-2022.SUPPLEMENT (145.5KB, pdf)
FIGURE S1. Baseline characteristics shown graphically by country. This figure (Panels A-P) shows the baseline characteristics of patients in the analysis set graphically by country for all patients initiating mepolizumab, the Pre-Pandemic group and the Pandemic group. These figures show marked heterogeneity in characteristics, both within individual registries between the periods before and during the pandemic, and between registries. 00745-2022.FIGURES1 (47.6KB, pdf)
Acknowledgements
This study was conducted on behalf of the Severe Heterogeneous Asthma Registry, Patient-centred (SHARP) (a full contributor acknowledgement list is presented in the supplementary material).
Provenance: Submitted article, peer reviewed.
Support statement: This study was supported by GlaxoSmithKline (study identifier: 212821). Funding information for this article has been deposited with the Crossref Funder Registry.
Conflict of interest: Z. Csoma reports lecture honoraria from AstraZenca, Sanofi, Teva and GSK outside the submitted work. A. ten Brinke reports grants and payment for expert testimony from GlaxoSmithKline, AstraZeneca, TEVA and Sanofi-Genzyme Regeneron outside the submitted work. B. Gemicioglu reports support for the present work from GSK; lecture honoraria from GSK, Novartis, Deva, Chiesi, Abdi İbrahim and Sandoz; travel support from GSK, AstraZeneca and Novartis; and leadership positions with SHARP (Turkey Coordinator), GARD (Turkey Coordinator) and Turkish Board of Pulmonology (Chair), outside the submitted work. C.C. Loureiro reports grants from GSK; consulting fees from AstraZeneca, GSK, Novartis and Sanofi; lecture honoraria from AstraZeneca, GSK, Novartis, Sanofi and Teva; and travel support from AstraZeneca, Novartis and Sanofi, outside the submitted work. D. Ramos-Barbón reports honoraria and institutional research funding from GSK, outside the submitted work. E.H. Bel reports grants from GlaxoSmithKline and TEVA; lecture honoraria from GlaxoSmithKline, TEVA, AstraZeneca, Sanofi-Genzyme, Regeneron, Chiesi and Sterna, outside the submitted work. J.A. Kroes reports grants from AstraZeneca, outside the submitted work. K. Samitas reports lecture honoraria from MSD, GSK, Chiesi, Novartis, AstraZeneca, ELPEN, Menarini, BMS, Specialty Therapeutics, Boehringer and Rontis; travel support from Boehringer; advisory board participation with AstraZeneca, GSK and Specialty Therapeutics; and a leadership position with the Hellenic Thoracic Society, outside the submitted work. L. Pérez de Llano reports support for the present work from AstraZeneca; grants from AstraZeneca, Faes, TEVA and Sanofi; lecture honoraria from AstraZeneca, TEVA, Sanofi, MSD, Leo Pharma, Gebro, GSK, Novartis, Chiesi, Techdow Pharma and Gilead; patents from AstraZeneca, Novartis, Faes, TEVA, GSK, Sanofi and Chiesi; and advisory board participation with AstraZeneca, outside the submitted work. R.W. Jakes reports support for the present work from GSK, as an employee and shareholder. S. Škrgat reports honoraria for lectures and educational events, supported by AstraZeneca, Sanofi, Chiesi, Pliva Teva and Medis; and participation on advisory boards of AstraZeneca, Chiesi and Sanofi, outside the submitted work. A. Bourdin reports support for the present work from GSK; grants from AstraZeneca, GSK and Boehringer Ingelheim; lecture honoraria, nonfinancial support and other support from AstraZeneca, GSK, Boehringer Ingelheim, Novartis, TEVA, Chiesi Farmaceuticals, Actelion, Gilead, Roche and Regeneron, outside the submitted work. B. Dahlén reports grants from Novartis and GSK; and consulting fees from AstraZeneca, Teva and Sanofi, outside the submitted work. E. Zervas reports advisory board fees from Astra, Chiesi, Elpen, GSK, Menarini, MSD and Novartis; honoraria and fees for lectures from Astra, Boehringer, Bristol-Myers, Chiesi, Elpen, GSK, Menarini, MSD and Novartis; travel accommodations and meeting expenses from Astra, Boehringer, Chiezi, Galenica, GSK, Elpen, MSD, Novartis and Roche; and holds a leadership role as Secretary General of Hellenic Thoracic Society, outside the submitted work. E. Heffler reports consulting fees and lecture honoraria from AstraZeneca, Sanofi, Regeneron, Novartis, GSK, Stallergenes-Greer and Circassia outside the submitted work. F. Schleich reports grants from GSK and AstraZeneca; and consulting fees and lecture honoraria from GSK, AstraZeneca and Chiesi, outside the submitted work. K. Eger reports that TEVA sponsored printing their PhD thesis, outside the submitted work. K. Bieksiene reports lecture honoraria from AstraZeneca and Berlin Chemie, outside the submitted work. M. Paula Rezelj reports lecture honoraria from AstraZeneca, Novartis, GSK and Stallergenes, outside the submitted work. M. Masoli reports an investigator-led nonpromotional grant from GlakoSmithKline and an advisory board fee from AstraZeneca outside the submitted work. N. Kwon reports support for the present work from GSK, as an employee and shareholder. P. Howarth reports support for the present work from GSK, as an employee and shareholder. P. Kopač reports lecture honoraria and advisory board participation from AstraZeneca, GSK and Berlin-Chemie outside the submitted work. R. Alfonso-Cristancho reports support for the present work from GSK, as an employee and shareholder. G. Celik reports consulting fees and lecture honoraria from Novartis and GSK outside the submitted work. J.K. Sont reports grants from AstraZeneca; outside the submitted work. All other authors have nothing to disclose.
References
- 1.Pavord ID, Bel EH, Bourdin A, et al. From DREAM to REALITI-A and beyond: mepolizumab for the treatment of eosinophil-driven diseases. Allergy 2022; 77: 778–797. doi: 10.1111/all.15056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li J, Wang F, Lin C, et al. The efficacy and safety of reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: a systematic review and meta-analysis. J Asthma 2017; 54: 300–307. doi: 10.1080/02770903.2016.1212371 [DOI] [PubMed] [Google Scholar]
- 3.Tian BP, Zhang GS, Lou J, et al. Efficacy and safety of benralizumab for eosinophilic asthma: a systematic review and meta-analysis of randomized controlled trials. J Asthma 2018; 55: 956–965. doi: 10.1080/02770903.2017.1379534 [DOI] [PubMed] [Google Scholar]
- 4.Brown T, Jones T, Gove K, et al. Randomised controlled trials in severe asthma: selection by phenotype or stereotype. Eur Respir J 2018; 52: 1801444. doi: 10.1183/13993003.01444-2018 [DOI] [PubMed] [Google Scholar]
- 5.Richards LB, van Bragt JJMH, Aarab R, et al. Treatment eligibility of real-life mepolizumab-treated severe asthma patients. J Allergy Clin Immunol Pract 2020; 8: 2999–3008. doi: 10.1016/j.jaip.2020.04.029 [DOI] [PubMed] [Google Scholar]
- 6.Sherman RE, Anderson SA, Dal Pan GJ, et al. Real-world evidence – what is it and what can it tell us? N Engl J Med 2016; 375: 2293–2297. doi: 10.1056/NEJMsb1609216 [DOI] [PubMed] [Google Scholar]
- 7.van Bragt JJMH, Adcock IM, Bel EHD, et al. Characteristics and treatment regimens across ERS SHARP severe asthma registries. Eur Respir J 2020; 55: 1901163. doi: 10.1183/13993003.01163-2019 [DOI] [PubMed] [Google Scholar]
- 8.Paoletti G, Pepys J, Casini M, et al. Biologics in severe asthma: the role of real-world evidence from registries. Eur Respir Rev 2022; 31: 210278. doi: 10.1183/16000617.0278-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Djukanovic R, Adcock IM, Anderson G, et al. The Severe Heterogeneous Asthma Research Collaboration, Patient-centred (SHARP) ERS clinical research collaboration: a new dawn in asthma research. Eur Respir J 2018; 52: 1801671. doi: 10.1183/13993003.01671-2018 [DOI] [PubMed] [Google Scholar]
- 10.Bentzen HB, Høstmælingen N. Balancing protection and free movement of personal data: the new European Union general data protection regulation. Ann Intern Med 2019; 170: 335–337. doi: 10.7326/M18-2782 [DOI] [PubMed] [Google Scholar]
- 11.Wirth FN, Meurers T, Johns M, et al. Privacy-preserving data sharing infrastructures for medical research: systematization and comparison. BMC Med Inform Decis Mak 2021; 21: 242. doi: 10.1186/s12911-021-01602-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Observational Health Data Sciences and Informatics . Standardized Data: the OMOP Common Data Model. 2022. www.ohdsi.org/data-standardization/the-common-data-model/. Date last accessed: 24 January 2022.
- 13.Kent S, Burn E, Dawoud D, et al. Common problems, common data model solutions: evidence generation for health technology assessment. Pharmacoeconomics 2021; 39: 275–285. doi: 10.1007/s40273-020-00981-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brusselle GG, Koppelman GH. Biologic therapies for severe asthma. N Engl J Med 2022; 386: 157–171. doi: 10.1056/NEJMra2032506 [DOI] [PubMed] [Google Scholar]
- 15.Pavord ID, Korn S, Howarth P, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet 2012; 380: 651–659. doi: 10.1016/S0140-6736(12)60988-X [DOI] [PubMed] [Google Scholar]
- 16.Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med 2014; 371: 1198–1207. doi: 10.1056/NEJMoa1403290 [DOI] [PubMed] [Google Scholar]
- 17.Chupp GL, Bradford ES, Albers FC, et al. Efficacy of mepolizumab add-on therapy on health-related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): a randomised, double-blind, placebo-controlled, parallel-group, multicentre, phase 3b trial. Lancet Respir Med 2017; 5: 390–400. doi: 10.1016/S2213-2600(17)30125-X [DOI] [PubMed] [Google Scholar]
- 18.Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med 2014; 371: 1189–1197. doi: 10.1056/NEJMoa1403291 [DOI] [PubMed] [Google Scholar]
- 19.Harrison T, Canonica GW, Chupp G, et al. Real-world mepolizumab in the prospective severe asthma REALITI-A study: initial analysis. Eur Respir J 2020; 56: 2000151. doi: 10.1183/13993003.00151-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schleich F, Graff S, Nekoee H, et al. Real-world experience with mepolizumab: does it deliver what it has promised? Clin Exp Allergy 2020; 50: 687–695. doi: 10.1111/cea.13601 [DOI] [PubMed] [Google Scholar]
- 21.Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 2014; 43: 343–373. doi: 10.1183/09031936.00202013 [DOI] [PubMed] [Google Scholar]
- 22.Eger K, Paroczai D, Bacon A, et al. The effect of the COVID-19 pandemic on severe asthma care in Europe: will care change for good? ERJ Open Res 2022; 8: 00065-2022. doi: 10.1183/23120541.00065-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Biedermann P, Ong R, Davydov A, et al. Standardizing registry data to the OMOP common data model: experience from three pulmonary hypertension databases. BMC Med Res Methodol 2021; 21: 238. doi: 10.1186/s12874-021-01434-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kroes JA, Bansal AT, Berret E, et al. Blueprint for harmonizing unstandardized disease registries to allow federated data analysis: prepare for the future. ERJ Open Res 2022; 8: 00168-2022. doi: 10.1183/23120541.00168-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.R Core Team . R: A Language and Environment for Statistical Computing. Vienna, R Foundation for Statistical Computing, 2021. www.R-project.org/. Date last accessed: 25 May 2022. [Google Scholar]
- 26.Thomas D, Harvey ES, McDonald VM, et al. Mepolizumab and oral corticosteroid stewardship: data from the Australian Mepolizumab Registry. J Allergy Clin Immunol Pract 2021; 9: 2715–2724. doi: 10.1016/j.jaip.2021.01.028 [DOI] [PubMed] [Google Scholar]
- 27.Silver J, Bogart M, Packnett E, et al. Real-world reductions in oral corticosteroid use in the USA following mepolizumab therapy for severe asthma. J Asthma Allergy 2020; 13: 689–699. doi: 10.2147/JAA.S275944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elsey L, Pantin T, Holmes LJ, et al. Outcomes over the first two years of treatment with mepolizumab in severe asthma. Eur Respir J 2021; 58: 2101313. doi: 10.1183/13993003.01313-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pilette C, Canonica GW, Chaudhuri R, et al. REALITI – a study: real-world oral corticosteroid-sparing effect of mepolizumab in severe asthma. J Allergy Clin Immunol Pract 2022; 10: 2646-2656. doi: 10.1016/j.jaip.2022.05.042 [DOI] [PubMed] [Google Scholar]
- 30.Li H, Zhang Q, Wang J, et al. Real-world effectiveness of mepolizumab in severe eosinophilic asthma: a systematic review and meta-analysis. Clin Ther 2021; 43: e192–e208. doi: 10.1016/j.clinthera.2021.03.023 [DOI] [PubMed] [Google Scholar]
- 31.Menzies-Gow A, Gurnell M, Heaney LG, et al. Oral corticosteroid elimination via a personalised reduction algorithm in adults with severe, eosinophilic asthma treated with benralizumab (PONENTE): a multicentre, open-label, single-arm study. Lancet Respir Med 2022; 10: 47–58. doi: 10.1016/S2213-2600(21)00352-0 [DOI] [PubMed] [Google Scholar]
- 32.Korn S, Howarth P, Smith SG, et al. Development of methodology for assessing steroid-tapering in clinical trials for biologics in asthma. Respir Res 2022; 23: 45. doi: 10.1186/s12931-022-01959-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eger K, Kroes JA, Ten Brinke A, et al. Long-term therapy response to anti-IL-5 biologics in severe asthma – a real-life evaluation. J Allergy Clin Immunol Pract 2021; 9: 1194–1200. doi: 10.1016/j.jaip.2020.10.010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00745-2022.SUPPLEMENT (145.5KB, pdf)
FIGURE S1. Baseline characteristics shown graphically by country. This figure (Panels A-P) shows the baseline characteristics of patients in the analysis set graphically by country for all patients initiating mepolizumab, the Pre-Pandemic group and the Pandemic group. These figures show marked heterogeneity in characteristics, both within individual registries between the periods before and during the pandemic, and between registries. 00745-2022.FIGURES1 (47.6KB, pdf)