Abstract
Rationale
COPD is the third leading cause of death in the United States. Sphingolipids, structural membrane constituents that play a role in cellular stress and apoptosis signalling, may be involved in lung function.
Methods
In the Cardiovascular Health Study, a prospective cohort of older adults, we cross-sectionally examined the association of plasma levels of 17 sphingolipid species with lung function and COPD. Multivariable linear regression and logistic regression were used to evaluate associations of sphingolipid concentrations with forced expiratory volume in 1 s (FEV1) and odds of COPD, respectively.
Results
Of the 17 sphingolipids evaluated, ceramide-18 (Cer-18) and sphingomyelin-18 (SM-18) were associated with lower FEV1 values (–0.061 L per two-fold higher Cer-18, p=0.001; −0.092 L per two-fold higher SM-18, p=0.002) after correction for multiple testing. Several other associations were significant at a 0.05 level, but did not reach statistical significance after correction for multiple testing. Specifically, Cer-18 and SM-18 were associated with higher odds of COPD (odds ratio per two-fold higher Cer-18 1.29, p=0.03 and SM-18 1.73, p=0.008). Additionally, Cer-16 and SM-16 were associated with lower FEV1 values, and Cer-14, SM-14 and SM-16 with a higher odds of COPD.
Conclusion
In this large cross-sectional study, specific ceramides and sphingomyelins were associated with reduced lung function in a population-based study. Future studies are needed to examine whether these biomarkers are associated with longitudinal change in FEV1 within individuals or with incident COPD.
Short abstract
Several ceramide and sphingomyelin species are associated with lower FEV1 and higher odds of COPD https://bit.ly/3uDLXVq
Introduction
COPD, which affects an estimated 30 million Americans, is the third leading cause of death in the United States [1]. A number of COPD risk factors, such as smoking (including secondhand smoke inhalation), air pollution and occupational hazards, have been identified [2]. Lesser known are the biochemical mechanisms that lead to the characteristic inflammation and tissue injury in the lungs.
Sphingolipids, a class of lipids comprised of a sphingosine backbone with an attached fatty acid and various head groups, perform various roles within the cell, from serving as structural membrane constituents to ligands in intracellular signalling [3]. One family of sphingolipids, the ceramides [4], has been shown to play a role in cellular stress and apoptosis signalling pathways [5]. Another class of sphingolipids, sphingomyelins, are cell membrane components that can also be metabolised to ceramides. Ceramides and sphingomyelins of different fatty acyl chain lengths may exhibit different biological activities [6] and have been implicated in pathways leading to inflammation, oxidative stress and cell death [3].
Limited prior research suggests that sphingolipids may play a role in the pathophysiology of COPD and its risk factors. A report within the Multi-Ethnic Study of Atherosclerosis (MESA) cohort showed that higher total plasma sphingomyelin concentrations were associated with higher risk of emphysema [7]. In this study, we examined 17 sphingolipid species for their association with lung function and COPD in a large biracial population-based cohort, the Cardiovascular Health Study (CHS).
Methods
Study design and data collection
The CHS is a prospective cohort study of risk factors for cardiovascular disease in older adults (aged ≥65 years) selected randomly from Medicare beneficiary lists. Participants were recruited from four field centres; institutional review boards at each of the four field centres approved the CHS, and all study participants provided written, informed consent. Between 1989 and 1999, participants underwent annual study examinations that included personal interviews, physical examinations, laboratory assessments and diagnostic tests. These included height, weight and blood pressure measurement, questions about tobacco and alcohol use, and medical history. A more detailed description of the study design and procedures can be found elsewhere [8]. The study examination from which sphingolipids were measured (1994–1995) formed the study baseline for this analysis, and participant characteristics were drawn from that examination.
Sphingolipid measurement
Ceramide and sphingomyelin species were measured at the 1994–1995 examination, using fasting EDTA-plasma samples; samples were stored in a freezer at −70°C until they were extracted. Sphingolipids were then quantified by liquid chromatography–tandem mass spectrometry. A complete description of sphingolipid measurement methods and quality control, including details of lipid extraction, sample plating, instrumentation, internal standards and normalisation, has been described previously [9]. In total, 17 sphingolipid species were examined: six ceramide species (with acylated fatty acid chain lengths of 14, 16, 18, 20, 22 and 24 carbons), six sphingomyelin species (of chain lengths 14, 16, 18, 20, 22 and 24), three hexosylceramide species (of chain lengths 16, 22 and 24), and two lactosylceramide species (of chain lengths 16 and 24) were assayed. Specifically, we measured ceramide (Cer) and sphingomyelin (SM) with myristic acid (Cer-14 and SM-14), with palmitic acid (Cer-16 and SM-16), with stearic acid (Cer-18 and SM-18), with arachidic acid (Cer-20 and SM-20), with behenic acid (Cer-22 and SM-22) and with lignoceric acid (Cer-24 and SM-24), with Cer-24 being computed as the sum of two ceramide species with distinct “d181” and “d182” sphingoid backbones. Three hexosylceramide (HexCer) species (HexCer-16, HexCer-22 and HexCer-24), and two lactosylceramide (LacCer) species (LacCer-16 and LacCer-24) were also measured as byproducts of the assay. Absolute sphingolipid concentrations are listed in supplementary table S1.
Spirometry measurement and COPD definition
Forced expiratory volume in 1 s (FEV1) and forced vital capacity (FVC) were measured by spirometry performed by trained technicians using a standard spirometry system meeting or exceeding the American Thoracic Society (ATS) spirometer recommendations during the 1996–1997 examinations, as described previously [10]. COPD was defined as a FEV1/FVC ratio of <0.7 and FEV1 <80% of the predicted value [11].
Statistical analyses
For this study, only CHS participants who had sphingolipid measurements at year 7 of the study (1994–1995 examination) and spirometry measurements at year 9 (1996–1997) were included in analyses (n=2675 participants). Linear regression models with robust standard error estimates were used to assess the association between sphingolipid levels and FEV1 values. Logistic regression models with robust standard error estimates were used to assess association between sphingolipid levels and COPD. All sphingolipid concentrations were log base 2 transformed to reduce spread.
For each of the FEV1 and COPD analyses, we adjusted the regression models for age, race, sex, clinic site, height, weight, low-density lipoprotein (LDL) and high-density lipoprotein (HDL) cholesterol, weekly alcohol intake, diabetic status, smoker status and pack-years of smoking. Missing LDL values (n=128), missing HDL values (n=78) and missing weight measurements (n=13) were multiply imputed using information about age, race, sex, height, weight, alcohol intake, diabetic status and smoker status. 20 chained imputations were used for each analysis. To account for multiple tests examining 17 lipid species, we used a Bonferroni correction to set a statistical significance threshold of 0.003 (0.05/17), and nominal significance was set as a threshold of 0.05.
In secondary analyses, we tested for an interaction of several factors (smoker status, age, race and sex) on the association of sphingolipids with lung function by inclusion of multiplicative interaction terms in the respective regression models.
Institutional review board approval
Each field centre's institutional review board approved the CHS study, and at the time of enrolment all participants provided informed written consent to participate in the CHS study and for their blood specimen to be used in future studies. The use of specimens and data from CHS is covered by a University of Washington Human Subject Committee approval (STUDY00000109). The present study was conducted on de-identified blood specimens and data and not human subjects.
Results
Participant characteristics
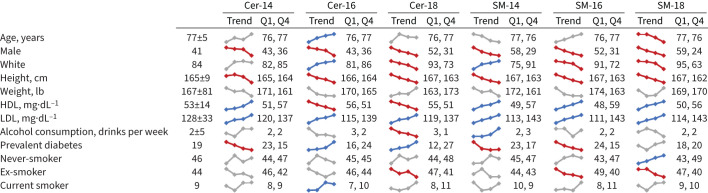
The CHS participants examined (n=2675) were older adults (mean age 77 years), mostly white (84%) and female (59%), and few (9%) were current smokers (figure 1). The study participants had a median FEV1 1.84 L (range 0.39–3.89 L); 317 (12%) met criteria for COPD, of whom 63 (20%) had been previously diagnosed with asthma. Higher concentrations of LDL cholesterol were associated with higher plasma levels of all 17 sphingolipid species in univariate analysis, whereas association with age, sex, race and other clinical factors differed by sphingolipid species type (figure 1 and supplementary tables S2–S4).
FIGURE 1.
Baseline characteristics of study participants are shown as quartile trends for six sphingolipid species. A blue line indicates a statistically significant positive trend in univariate analyses. A red line indicates a statistically significant negative trend in univariate analyses. A grey line indicates no significant trend. Data are presented as mean±sd or %, unless otherwise stated. HDL: high-density lipoprotein; LDL: low-density lipoprotein; Cer: ceramide; SM: sphingomyelin.
Association with FEV1 and odds of COPD
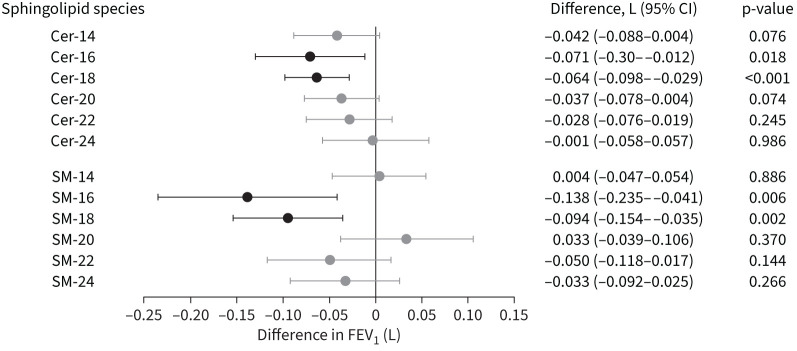
Of the 17 species examined, Cer-18 and SM-18 were significantly associated with lung function, with each doubling of the respective sphingolipid species associated with lower FEV1 values (–0.061 L for Cer-18, p=0.001; and −0.092 L for SM-18, p=0.002) (figure 2 and supplementary table S5). Cer-16 and SM-16 were nominally associated with lower FEV1 values (figure 2). The remaining 13 ceramides, sphingomyelins, hexosylceramides and lactosylceramide species were not associated with FEV1 (supplementary table S5).
FIGURE 2.
Ceramide (Cer) and sphingomyelin (SM) associations with forced expiratory volume in 1 s (FEV1). A forest plot of 12 sphingolipid concentrations (six ceramides and six sphingomyelins) on the vertical axis are plotted against the corresponding difference in absolute FEV1 value measured in litres. Differences in FEV1 (L) are shown for a two-fold change for each sphingolipid species. Associations with p<0.05 are shown in black.
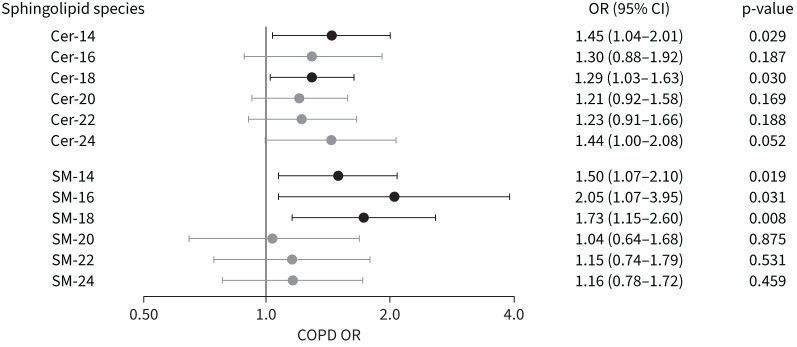
Ceramides Cer-14 and Cer-18 and sphingomyelins SM-14, SM-16 and SM-18 were all nominally associated with higher odds of COPD (figure 3 and supplementary table S6), although no association remained significant after adjustment for multiple testing. A two-fold increase in the plasma concentration of these five sphingolipids was nominally associated with 1.28–2.03 times higher odds of having COPD, depending on the sphingolipid examined (figure 3).
FIGURE 3.
Ceramide (Cer) and sphingomyelin (SM) associations with odds of COPD. A forest plot of 12 sphingolipid concentrations (six ceramides and six sphingomyelins) on the vertical axis are plotted against the corresponding odds ratio for COPD. Odds ratios are shown for a two-fold change for each sphingolipid species. Associations with p<0.05 are shown in black.
In secondary analysis, we found no significant interaction of smoker status, age, race or sex on the association between any sphingolipid and either FEV1 or COPD (supplementary tables S8 and S9).
The two sphingolipids significantly associated with lung function, Cer-18 and SM-18, were highly correlated with each other (r=0.7) (supplementary figure S1). Inclusion of both lipid species in the same regression model attenuated the point estimate for both species, suggesting an association of both with lung function (supplementary table S7). Moreover, SM-20, a sphingomyelin highly correlated with SM-18 (r=0.8), showed no evidence of association with either FEV1 or COPD, further suggesting that individual sphingolipids play distinct roles in COPD pathophysiology.
Discussion
In this study, sphingolipids with 18 carbons, Cer-18 and SM-18, were associated with worse lung function as assessed by lower FEV1 values, and nominally associated with higher odds of COPD. Several other ceramides (Cer-14 and Cer-16) and sphingomyelins (SM-14 and SM-16) were also nominally associated with FEV1 or obstructive lung disease.
Our findings, which are consistent with basic and population data, suggest that sphingolipids are involved in pathological mechanisms of obstructive lung disease. In mice and human studies, ceramides of shorter chain lengths (<20-carbon fatty acid chain) have been observed to be present at increased levels in patients with inflammatory obstructive lung disease, cystic fibrosis, especially in the epithelial lining of the lower airways [12]. These findings suggest that increased levels of specific ceramides in the lung are associated with destructive airway disease.
Previous studies have also reported on the association of plasma sphingomyelins with COPD. A study in the MESA cohort showed that each 1-sd higher total plasma sphingomyelin was associated with a 0.12% higher risk of emphysema per year of the study [7]. Another study has shown that levels of blood serum sphingomyelins are higher among COPD patients than among those of pneumonia patients [13]. However, these studies did not differentiate between sphingomyelin species with various acylated fatty acids.
The ceramides Cer-16 and Cer-24 have been found in mice lungs and human lung cells in culture, and in both systems, Cer-16 shows evidence of lung toxicity, while Cer-24 may be protective [14, 15]. In mice, inhibition of CerS2, the ceramide synthase mostly responsible for the production of Cer-24, results in decrease in lung Cer-24, but also a compensatory increase in Cer-16, resulting in airway inflammation and increased lung volumes [14]. Furthermore, CerS2−/− mice are also more susceptible to cigarette smoke toxicity than wild-type mice [15]. In cultured human lung cells, it was found that Cer-16 produced from SM-16 by sphingomyelinase amplified mitochondrial damage from cigarette smoking [15]. While smoking was uncommon in CHS, our findings of an association of both Cer-16 and SM-16 with lower FEV1, and SM-16 with COPD, generally agrees with these basic experiments.
Smoking and inhalation of other respiratory irritants, known risk factors for lung damage and COPD, have been associated with increased levels of neutral sphingomyelinase (NSMase) activation in bronchial epithelial cells [16], an enzyme (along with other members of the sphingomyelinase family) that converts sphingomyelin into ceramide. The fact that elevated NSMase concentration leads to an increased level of ceramides in bronchial epithelial cells [1] suggests a potential link between upregulation of ceramides, as observed in our study, and inflammatory/obstructive respiratory diseases such as COPD as reported in a murine model [17]. Interestingly, N-acetyl cysteine, an antioxidant, has been shown to reduce NSMase levels [18] and its use may reduce acute exacerbations in patients with COPD [19]. Taken together, our results identify specific sphingolipids such Cer-18 and SM-18 as putative therapeutic targets for reducing the burden of obstructive lung disease.
Interestingly, our findings do not agree with a report from the COPDGene study where plasma SM-14 and SM-16 were inversely associated with emphysema severity among 129 current and former smokers [20]. Differences in age, smoking and population characteristics may explain differences in findings.
Our study was cross-sectional and we cannot determine the directionality of the sphingolipid–COPD associations. We and others have shown associations of Cer-16 and SM-16 with higher risk of cardiovascular outcomes and mortality [21–24]. Given the known association of COPD with higher risk of heart disease, it will be interesting to investigate whether Cer-16 and SM-16 mediate the risk associated with COPD.
Our study has a number of strengths. First, this is the largest study to date examining the relationship between sphingolipid species and lung function. Second, our study was conducted in a large biracial population-based cohort, reducing the concern of selection bias. Third, individual sphingolipids were assayed, providing us with the opportunity to identify specific species associated with impaired lung function.
Several weaknesses deserve consideration. The biracial population (majority white with an African American minority) of older adults does not fully capture racial nor age diversity and findings may not be generalisable to other ethnic populations or younger individuals. Measurement error in covariates may have resulted in incomplete adjustment. Additionally, there was no replication cohort to validate our findings. We defined COPD based on commonly accepted criteria of reduced FEV1 and presence of airflow obstruction, which did not include subjects with preserved ratio impaired spirometry. Finally, this is an observational study examining the cross-sectional association between sphingolipids and lung function; underlying causal roles cannot be inferred.
In conclusion, this large study identified several distinct sphingolipid species linked to reduced lung function and elevated odds of COPD. Future studies are needed to examine whether these molecules are associated with longitudinal change in FEV1 and incident COPD, investigate the mechanisms elevated Cer-18 and SM-18 levels lead to impaired pulmonary function, and explore the consequences of modulating these sphingolipid species as a potential therapeutic in obstructive lung disease.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00346-2022.supplement (2.5MB, pdf)
Acknowledgements
We thank the study participants and the Cardiovascular Health Study (CHS) Coordinating Center. A full list of principal CHS investigators and institutions can be found at https://CHS-NHLBI.org.
Provenance: Submitted article, peer reviewed.
Conflict of interest: The authors have nothing to disclose.
Support statement: This research was supported by contracts 75N92021D00006, HHSN268201200036C, HHSN268200800007C, HHSN268201800001C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083 and N01HC85086, and grants U01HL080295, U01HL130114 and HL128575 from the National Heart, Lung, and Blood Institute, with additional contribution from the National Institute of Neurological Disorders and Stroke. Additional support was provided by R01AG023629 from the National Institute on Aging, and DK103657 and P30 DK035816 from the National Institute of Diabetes and Digestive and Kidney Diseases. Funding for the sphingolipid assays was provided by NHLBI R01 HL146499. N. Sotoodehnia was supported by the Laughlin Family. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Chakinala RC, Khatri A, Gupta K, et al. Sphingolipids in COPD. Eur Respir Rev 2019; 28: 190047. doi: 10.1183/16000617.0047-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet 2007; 370: 765–773. doi: 10.1016/S0140-6736(07)61380-4 [DOI] [PubMed] [Google Scholar]
- 3.Yang Y, Uhlig S. The role of sphingolipids in respiratory disease. Ther Adv Respir Dis 2011; 5: 325–344. doi: 10.1177/1753465811406772 [DOI] [PubMed] [Google Scholar]
- 4.Pinto SN, Silva LC, Futerman AH, et al. Effect of ceramide structure on membrane biophysical properties: the role of acyl chain length and unsaturation. Biochim Biophys Acta 2011; 1808: 2753–2760. doi: 10.1016/j.bbamem.2011.07.023 [DOI] [PubMed] [Google Scholar]
- 5.Parveen F, Bender D, Law S-H, et al. Role of ceramidases in sphingolipid metabolism and human diseases. Cells 2019; 8: 1573. doi: 10.3390/cells8121573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turpin-Nolan SM, Brüning JC. The role of ceramides in metabolic disorders: when size and localization matters. Nat Rev Endocrinol 2020; 16: 224–233. doi: 10.1038/s41574-020-0320-5 [DOI] [PubMed] [Google Scholar]
- 7.Ahmed FS, Jiang X-c, Schwartz JE, et al. Plasma sphingomyelin and longitudinal change in percent emphysema on CT. The MESA lung study. Biomarkers 2014; 19: 207–213. doi: 10.3109/1354750X.2014.896414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol 1991; 1: 263–276. doi: 10.1016/1047-2797(91)90005-W [DOI] [PubMed] [Google Scholar]
- 9.Lemaitre RN, Yu C, Hoofnagle A, et al. Circulating sphingolipids, insulin, HOMA-IR, and HOMA-B: the Strong Heart Family Study. Diabetes 2018; 67: 1663–1672. doi: 10.2337/db17-1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Enright PL, Kronmal RA, Higgins M, et al. Spirometry reference values for women and men 65 to 85 years of age: Cardiovascular Health Study. Am Rev Respir Dis 1993; 147: 125–133. doi: 10.1164/ajrccm/147.1.125 [DOI] [PubMed] [Google Scholar]
- 11.Pauwels RA, Buist AS, Calverley PMA, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med 2001; 163: 1256–1276. doi: 10.1164/ajrccm.163.5.2101039 [DOI] [PubMed] [Google Scholar]
- 12.Grösch S, Schiffmann S, Geisslinger G. Chain length-specific properties of ceramides. Prog Lipid Res 2012; 51: 50–62. doi: 10.1016/j.plipres.2011.11.001 [DOI] [PubMed] [Google Scholar]
- 13.Baumgartner T, Zurauskaite G, Steuer C, et al. Association of serum sphingomyelin profile with clinical outcomes in patients with lower respiratory tract infections: results of an observational, prospective 6-year follow-up study. Clin Chem Lab Med 2019; 57: 679–689. doi: 10.1515/cclm-2018-0509 [DOI] [PubMed] [Google Scholar]
- 14.Petrache I, Kamocki K, Poirier C, et al. Ceramide synthases expression and role of ceramide synthase-2 in the lung: insight from human lung cells and mouse models. PLoS One 2013; 8: e62968. doi: 10.1371/journal.pone.0062968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mizumura K, Justice MJ, Schweitzer KS, et al. Sphingolipid regulation of lung epithelial cell mitophagy and necroptosis during cigarette smoke exposure. FASEB J 2018; 32: 1880–1890. doi: 10.1096/fj.201700571R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levy M, Khan E, Careaga M, et al. Neutral sphingomyelinase 2 is activated by cigarette smoke to augment ceramide-induced apoptosis in lung cell death. Am J Physiol Lung Cell Mol Physiol 2009; 297: L125–L133. doi: 10.1152/ajplung.00031.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petrache I, Natarajan V, Zhen L, et al. Ceramide upregulation causes pulmonary cell apoptosis and emphysema-like disease in mice. Nat Med 2005; 11: 491–498. doi: 10.1038/nm1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Filosto S, Castillo S, Danielson A, et al. Neutral sphingomyelinase 2: a novel target in cigarette smoke-induced apoptosis and lung injury. Am J Respir Cell Mol Biol 2011; 44: 350–360. doi: 10.1165/rcmb.2009-0422OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng J-P, Wen F-Q, Bai C-X, et al. Twice daily N-acetylcysteine 600 mg for exacerbations of chronic obstructive pulmonary disease (PANTHEON): a randomised, double-blind placebo-controlled trial. Lancet Respir Med 2014; 2: 187–194. doi: 10.1016/S2213-2600(13)70286-8 [DOI] [PubMed] [Google Scholar]
- 20.Bowler RP, Jacobson S, Cruickshank C, et al. Plasma sphingolipids associated with chronic obstructive pulmonary disease phenotypes. Am J Respir Crit Care Med 2015; 191: 275–284. doi: 10.1164/rccm.201410-1771OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meeusen JW, Donato LJ, Bryant SC, et al. Plasma ceramides. Arterioscler Thromb Vasc Biol 2018; 38: 1933–1939. doi: 10.1161/ATVBAHA.118.311199 [DOI] [PubMed] [Google Scholar]
- 22.Fretts AM, Jensen PN, Hoofnagle AN, et al. Circulating ceramides and sphingomyelins and risk of mortality: the Cardiovascular Health Study. Clin Chem 2021; 67: 1650–1659. doi: 10.1093/clinchem/hvab182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jensen PN, Fretts AM, Hoofnagle AN, et al. Plasma ceramides and sphingomyelins in relation to atrial fibrillation risk: the Cardiovascular Health Study. J Am Heart Assoc 2020; 9: e012853. doi: 10.1161/JAHA.119.012853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lemaitre RN, Jensen PN, Hoofnagle A, et al. Plasma ceramides and sphingomyelins in relation to heart failure risk. Circ Heart Fail 2019; 12: e005708. doi: 10.1161/CIRCHEARTFAILURE.118.005708 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00346-2022.supplement (2.5MB, pdf)