Abstract
We present guidelines by the European Association of Nuclear Medicine (EANM) for routine quality control (QC) of PET-CT and PET-MR systems. These guidelines are partially based on the current EANM guidelines for routine quality control of Nuclear Medicine instrumentation but focus more on the inherent multimodal aspect of the current, state-of-the-art PET-CT and PET-MR scanners. We briefly discuss the regulatory context put forward by the International Electrotechnical Commission (IEC) and European Commission (EC) and consider relevant guidelines and recommendations by other societies and professional organizations. As such, a comprehensive overview of recommended quality control procedures is provided to ensure the optimal operational status of a PET system, integrated with either a CT or MR system. In doing so, we also discuss the rationale of the different tests, advice on the frequency of each test and present the relevant MR and CT tests for an integrated system. In addition, we recommend a scheme of preventive actions to avoid QC tests from drifting out of the predefined range of acceptable performance values such that an optimal performance of the PET system is maintained for routine clinical use.
Keywords: Guidelines, Quality control, PET-CT, PET-MR
Preamble
The European Association of Nuclear Medicine (EANM) is a professional non-profit medical association that facilitates communication worldwide among individuals pursuing clinical and research excellence in nuclear medicine. The EANM was founded in 1985.
These guidelines are intended to assist practitioners in providing appropriate nuclear medicine care for patients. They are not inflexible rules or requirements of practice and are not intended, nor should they be used, to establish a legal standard of care.
The ultimate judgment regarding the propriety of any specific procedure or course of action must be made by medical professionals considering the unique circumstances of each case. Thus, there is no implication that an approach differing from the guidelines, standing alone, is below the standard of care. To the contrary, a conscientious practitioner may responsibly adopt a course of action different from that set out in the guidelines when, in the reasonable judgment of the practitioner, such course of action is indicated by the condition of the patient, limitations of available resources or advances in knowledge or technology after publication of the guidelines.
The practice of medicine involves not only the science but also the art of dealing with the prevention, diagnosis, alleviation, and treatment of disease.
The variety and complexity of human conditions make it impossible to always reach the most appropriate diagnosis or to predict with certainty a particular response to treatment. Therefore, it should be recognised that adherence to these guidelines will not ensure an accurate diagnosis or a successful outcome.
All that should be expected is that the practitioner will follow a reasonable course of action based on current knowledge, available resources, and the needs of the patient to deliver effective and safe medical care. The sole purpose of these guidelines is to assist practitioners in achieving this objective.
Background
According to the IEC (International Electrotechnical Commission) 61223 standard [1] and the ‘Radioprotection-162’ publication (RP-162) by the European Commission (EC) [2], routine quality control (QC) should be an inherent part of the quality assurance of a medical device during its full lifecycle, starting with its purchase and going from commissioning and acceptance over routine clinical use to decommissioning. To achieve compliance with these quality management regulations, it is necessary to define a QC program to support the clinical use of a Positron Emission Tomography (PET) system integrated with either a Computed Tomography (CT) or Magnetic Resonance (MR) system. Moreover, PET imaging is inherently quantitative as it measures activity concentrations. Therefore, routine QC of a PET-CT or PET-MR system should go beyond monitoring of the PET detector response and cover PET system calibration and specific aspects of the integrated CT-or MR-component as well, especially since a CT-or MR-based attenuation map is needed for quantitative PET imaging.
Recommendations for routine QC of PET-CT systems have already been presented and discussed extensively by professional bodies such as IAEA (International Atomic Energy Agency) [3], AAPM (American Association of Physicists in Medicine) [4], [5], and IPEM (Institute of Physics and Engineering in Medicine) [6], amongst others. In addition, the physics committee of the EANM (European Association of Nuclear Medicine) listed a comprehensive overview of the recommended testing for routine QC of nuclear medicine instrumentation in general and PET-CT specifically [7], together with the recommended frequency of the different tests.
The aim of these guidelines is to provide a comprehensive overview of recommended routine QC tests for PET-CT and PET-MR. However, it should be noted that these guidelines are recommendations, and a routine PET QC program remains the responsibility of each individual nuclear medicine department and the physicists working with the equipment. In addition, these guidelines do not present the recommended tests in a detailed, manual-like manner, but focus on the rationale and frequency of each test. CT- and MR-testing is also discussed but only in the context of PET imaging to give medical physicists working in the nuclear medicine field, adequate background on the relevant QC of these modalities. For more elaborated discussions on CT- and MR-QC, we refer to guidelines presented by other professional bodies, respectively.
While this is not the focus of these guidelines, it should be stressed that acceptance testing is essential for initiating routine QC testing, as acceptance testing provides baseline performance values as a reference for routine QC. Therefore, acceptance tests should be performed on a fully installed and calibrated system which is considered ready for clinical use. Apart from varying local regulations for a survey by an external radiation protection expert to be performed and passed, currently there are no legal provisions on further requirements for acceptance testing and placing into service of a PET-CT or PET-MR system. Therefore, the most appropriate strategy is to agree with the manufacturer on a series of acceptance tests and a range of measured values to be considered acceptable for adequate system performance. For this purpose, the NEMA (National Electrical Manufacturers Association) and IEC standards [8], [9] provide a solid basis for defining appropriate measurements for PET acceptance testing. These acceptance measurements include resolution measurements using point sources acquired at different positions in the PET Field of View (FOV) as well as sensitivity and count rate measurements using dedicated NEMA phantoms. Manufacturers have largely adopted these NEMA standards to specify PET system characteristics so that NEMA measurements can validate PET system performance claims and provide reference data for future QC testing. However, it should be noted that acceptance testing aims at validating PET system performance claims by the manufacturer and/or comparing the performance of different PET systems. As such, acceptance tests focus on measuring the PET system performance determined by the detector design (f.e. crystal size), geometry (f.e. axial Field of View), electronics, and specific acquisition parameters (f.e. timing window). These systems and acquisition parameters are expected to remain stable unless substantial hardware or software changes are made to the PET system configuration and settings which could impact sensitivity and noise equivalent count rate (NECR). In addition, limited access to the dedicated hardware phantoms and the 1 to 2 days of full scanner access required for acceptance testing makes the feasibility of regular PET acceptance testing much more challenging on busy clinical systems. Therefore, it is not formally recommended to repeat acceptance testing on a regular basis unless major upgrades and hard- or software changes have been made to the PET detector and acquisition system.
Once the PET-CT or PET-MR system has been accepted, routine QC testing should be initiated with the primary goal of monitoring the operational level of the PET-CT or PET-MR system and classifying the system performance according to the following performance levels [2]:
-
•
Satisfactory level:
The PET-CT or PET-MR system meets all performance and safety criteria with QC testing within the prescribed range of values.
-
•
Remedial Level:
The PET-CT or PET-MR system performs unsatisfactory but sufficiently close to satisfactory performance such that it will not reduce clinical effectiveness or safety. However, remedial action is to be initiated to restore satisfactory performance.
-
•
Suspension Level:
The PET-CT or PET-MR system needs to be immediately suspended from clinical use while the cause of the unsatisfactory performance is being investigated and remedial action is initiated to restore satisfactory performance. Alternatively, the suspended system may be considered for limited clinical use following a documented risk assessment, clearly describing the appropriate circumstances for this limited use.
These performance levels define the appropriate clinical use of the PET-CT or PET-MR system and as such, allow for an informed decision on the administration of radiopharmaceuticals to patients and exposure of patients to other scan-related radiation while the minimum required diagnostic image quality is assured. Next to routine QC, we also discuss additional preventive actions to maintain the operational status of the PET-CT or PET-MR system at a satisfactory level of performance.
Routine QC testing
As a part of a QC program, the daily QC comprises the most essential tests to closely monitor the operational status of a PET-CT or PET-MR system. In this sense, daily testing doesn’t necessarily mean on a daily basis, but on the day the system will be used for scanning patients. On these days, the daily QC should be performed prior to any administration of radiopharmaceuticals to patients. Having a clear indication of system performance prior to patient scanning avoids unnecessary radiation exposure to patients in case of faulty system performance. Moreover, close monitoring of the detector response can determine whether service or other actions are needed to resolve potential future issues before system performance and image quality become compromised.
In the next sections, the QC will be discussed for a PET, CT and MR system, as well as the recommended tests for an integrated PET-CT and PET-MR system. Next to the daily QC, additional QC testing is mandatory on a quarterly and yearly basis to monitor PET-system performance and confirm appropriate operational status for scanning patients. For these tests, we base our recommendations on the EARL accreditation program (resEARch4Life) for state-of-the-art FDG (fluorodeoxyglucose) PET-CT imaging which has been initiated by the EANM and meanwhile has been endorsed by the European Organisation for Research and Treatment of Cancer (EORTC) Imaging Group [10], [11], [12].
PET QC
Daily PET QC
In general, the daily QC of the PET system monitors the response of the detection system and all detector elements. This can be done by measuring a dedicated source which is positioned within the gantry at one reproducible, fixed position which is exactly known. Usually, a 68Ge-source (Germanium) is used, although some vendors make use of a 22Na point source while older PET systems may still apply a rotating rod source which covers multiple positions. Meanwhile, more recent PET systems also use the inherent radioactivity of the crystals to measure the detector response on a daily basis. The sinogram of this measurement is visually inspected for unusual patterns or obvious artifacts, such as dark diagonal streaks. In addition, the daily QC acquisition analysis checks whether signal gain, energy calibrations, coincidence timings as well as singles and coincidence rates of the individual detector elements and modules are within predefined and acceptable limits.
We recommend scanning a uniform cylindrical 68Ge-reference source as part of daily QC and especially with a PET-CT system where a CT-based attenuation map is used to reconstruct the measured activity concentration. Although this measurement results in additional scanning time, potentially higher costs related to a dedicated source and a slight increase in radiation exposure for personnel, it allows a daily visual assessment of the image quality of a reconstructed PET image before patient scanning is performed while it can also be used to check PET-CT alignment, bed movement, PET reconstruction and PET system calibration based on the quantitative consistency between the measurements and the activity concentration of the uniform source.
Quarterly PET QC
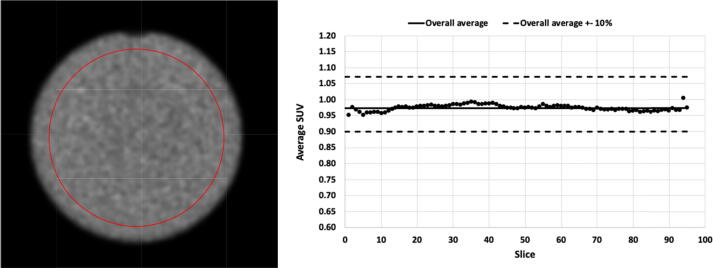
In line with this program, we recommend a quarterly check of the uniformity and SUV (Standardized Uptake Value) of PET data acquired with a uniform, cylindrical phantom. Here, the SUV is calculated as the measured activity concentration divided by the expected activity concentration as determined by the radionuclide calibrator and phantom volume [13]. For this test, a cylindrical phantom, which can span the entire axial PET FOV, is filled with a uniformly distributed activity concentration of a radioligand labelled with 18F (Fluorine) in an aqueous solution. For an acquisition in ‘step and shoot’ mode, a PET scan with one or preferably two bed positions should be acquired to mimic a clinical scan protocol as much as possible while for an acquisition with continuous bed motion, a scanning range is preferred such that bed motion is required. Activity concentration in the phantom and acquisition time per bed position or bed motion should be in line with clinical protocols. In addition, a dedicated CT scan should be acquired in case of a PET-CT system, to generate a CT-based attenuation map for appropriate attenuation and scatter correction. For a PET-MR system, the attenuation map generally is based on a predefined attenuation template of the cylindrical phantom which is registered to the PET emission data to support an accurate attenuation and scatter correction [14]. Next, PET images can be reconstructed using the same reconstruction protocols as used in clinical routine. A circular region of interest (ROI) with a diameter equal to 85% of the diameter of the activity distribution in the cylinder is defined for each plane except for the three at both ends of the cylindrical activity distribution (see Fig. 1).
Figure 1.
Visual assessment of a circular region for in-plane SUV statistics of a cylindrical phantom with uniform activity distribution together with the in-plane SUV compared to the overall average SUV ± 10%.
To determine how accurately a known and uniformly distributed activity concentration can be reconstructed with the PET system, the average activity concentration in the volume determined by all circular ROIs is calculated (see Fig. 1). If the cross-calibration between the radionuclide calibrator and PET system is done properly, a SUV of 1 should be obtained with a deviation of no more than 10%. In addition, a deviation of no more than 5% should be considered achievable as the accuracy of radionuclide calibrators is expected to be within 5% for PET radionuclides [15], [16].
The measured activity concentrations in these ROIs are also used to determine whether a uniform source also provides a uniform PET image. Therefore, the PET image of the uniform, cylindrical source should be visually checked for non-uniformities. Additionally, the average and standard deviation of activity concentration in each circular ROI should be calculated as well as the average overall ROIs. For the relative difference between the average activity concentration per slice and the global average, one should consider a range of ± 10% as acceptable and ± 5% as achievable. In addition, it is recommended to check whether intensity gradients or asymmetries are present in the reconstructed PET images. These can be caused by a faulty attenuation map [18] or by using inaccurate Time of Flight (TOF) information during reconstruction because of systematic inaccuracies in the timing offset calibration of detector elements. An accurate timing calibration of all detector elements is needed to accurately map TOF differences between two detector elements to specific locations along the line of response (LOR) [17]. Therefore, it is useful to compare both TOF and non-TOF reconstructions to determine the impact of TOF on the PET image quality.
The necessary imaging processing and data analysis for the quarterly PET QC can be performed in a vendor-neutral way using standard spreadsheet software and freeware packages for basic image processing such as AMIDE [18].
Given that PET systems are usually calibrated in terms of measured activity to radionuclide calibrators and/or well-counters and blood sampling systems for tracer kinetic modelling, it is recommended to use this quarterly QC acquisition to evaluate and, if necessary, recalibrate these systems to ensure accurate cross-calibration. This can be done by measuring the activity for filling the uniform phantom with the different radionuclide calibrators and by taking aliquots after the PET measurements to check the activity concentration with well-counter or blood sampling devices.
Yearly PET QC
The quarterly calibration and uniformity test is generally performed with 18F (Fluorine), as this is the most available PET isotope because of its very frequent clinical use. However, on a annual basis, it is recommended to perform this test also for other PET isotopes which are clinically used, but not that frequent. This way, the uniformity and calibration of the PET system can be confirmed for all clinically relevant PET isotopes.
In addition to this additional calibration and uniformity test, it is recommended to run a yearly image quality test with 18F as PET isotope to evaluate the image quality of clinical whole-body PET scanning. This test uses the NEMA IEC Body Phantom which consists essentially of 6 hollow spheres with different diameters and a background compartment. This way, this phantom mimics tumoral lesions of different sizes with a predetermined tumour to background ratio. If the PET system operates in step and shoot mode, one or preferably two bed positions should be acquired to mimic a clinical protocol as close as possible while in case of continuous bed motion, a scan range is preferred such that the bed is moving during scanning. This way, the impact of bed motion or multiple bed positions on the PET image quality can be assessed. In terms of reconstructions, the acquired PET data should be reconstructed with representative clinical reconstruction protocols. The image quality of the reconstructed PET data is evaluated by the recovered tumour to background ratios for the different sphere sizes as well as background variability represented by the standard deviation or coefficient of variation measured across the background regions. In addition, the residual PET signal in the cold lung compartment should be used as a measure for the accuracy of the scatter and attenuation correction. Referring to the EARL procedure for the image quality test [19], one can consider filling the two largest spheres with the same activity concentration as the other, smaller spheres, instead of using a cold solution such that contrast recovery can be better estimated for a wider range of lesion sizes. In addition, the activity concentration in the spheres doesn’t need to match a specific sphere to background ratio if this ratio is accurately determined beforehand, although a sphere to background activity concentration ratio of 10:1 is advised to stay in line with the EARL procedure and have a more standardized measurement. Once image quality measures have been obtained, these results should be compared to previous measurements to confirm that image quality is maintained within the predefined range and at a clinically acceptable level [11].
Since this test closely resembles the NEMA image quality acceptance test, the same processing software can be used although this generally means the data analysis will be dependent on software provided by the manufacturer. On the other hand, a vendor-neutral assessment of the image quality can be obtained as part of the EARL accreditation program.
CT QC
As with the PET detector, it is essential to ascertain that the CT system is working to predefined specifications every day before injecting a patient with a radiopharmaceutical. It is important to determine that the reconstructed CT image is free of artifacts and has consistent Hounsfield Units (HU) to ensure that the attenuation map that is derived from the CT is accurate.
For daily CT QC, the first task to perform at the start of each working day is a tube warm up, which will gradually bring the tube to a standard operating temperature to avoid damage caused by a sudden high load being exerted onto the x-ray anode. During this time all CT “x-ray on” entry warning lights should be checked to ensure the safety of staff and patients around the area. This is followed by an air calibration scan to assess the response with no material in the x-ray beamline. The calibration will be performed for a series of tube voltages, tube current, focus geometries, beam collimations, and detector configurations, respectively. It is therefore essential to check that nothing is present in the CT bore during this process. The acquisition and associated analysis are usually combined in the manufacturer’s protocol and typically take 5 to 10 minutes to automatically complete.
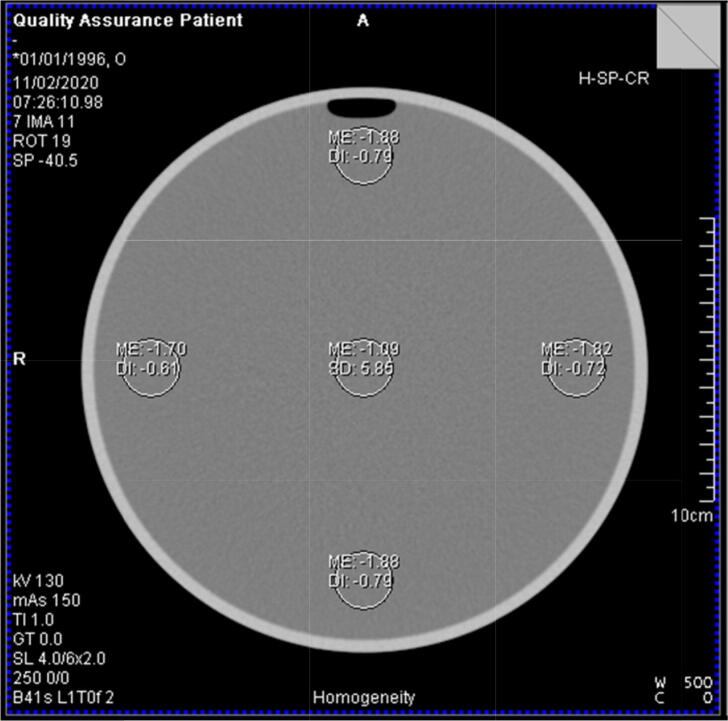
Once the tube is warmed up and the air calibration has been performed, it is recommended to scan a water phantom daily and assess the phantom/table position, image noise as well as uniformity of Hounsfield Units for a range of tube voltages, tube current and detector configurations [20]. Analysis is usually automated by the manufacturer’s software (see Fig. 2).
Figure 2.
Analysis of a uniform cylindrical water phantom as part of a daily CT QC procedure.
For an integrated PET-CT system, spatial alignment between the PET- and CT-component is critical for the CT-based attenuation correction and the accurate correlation of CT-based anatomical and PET-based functional information. While the quarterly PET uniformity test is already sensitive to PET-CT alignment errors because of the CT-based attenuation map, it is mandatory to verify the PET-CT alignment with a dedicated, vendor-specific alignment phantom and to reduce potential alignment errors in order to achieve submillimetre PET-CT alignment. This dedicated PET-CT alignment check should be performed at least once a year or after maintenance with corrective interventions involving a software recalibration of table position or table motion and should be ideally part of the planned preventive maintenance by the manufacturer.
While the PET-CT alignment needs close follow-up from a nuclear medicine perspective, other regular and mandatory CT tests should be performed under the supervision of a medical physicist with the appropriate certification for CT. These tests include regular uniformity and contrast checks as well as a more extensive, yearly test [20]. Some of these tests can be done as part of preventive maintenance as they require a manufacturer supplied phantom. Note that for radiotherapy applications, specialist external lasers are typically installed on a PET-CT system. If this external laser system is regularly used for patient care, it is worthwhile to have these tested on a regular basis as part of the CT-QC procedures. Otherwise, a QC test of the laser system on the day of clinical use is appropriate [21]. Information on these tests and other CT-specific QC tests including those applicable for radiotherapy applications have been published [21], [22], [23], [24].
MR QC
QC for MR systems requires a different approach than for nuclear medicine imaging devices. This section summarizes MR-specific QC measures but also points to tasks that do not strictly belong to quality control in a conventional sense but nevertheless critically affect the system performance.
During the daily startup, the MR component automatically performs a wide range of tests and calibrations, and reports to the user should any problems arise. Basically, at typical MR sites, this automated startup test together with the acceptance test after installation [25] and the vendor maintenance (typically every three or six months) is the most important that an MR user needs to know about quality control. The reason for this rather simple approach is the fact that MR images show instantaneous severe artifacts or are not generated at all if the acquisition fails or is compromised. Therefore, the acquisition of patient images without artifacts during clinical use is in itself a QC-test. Thus, it is crucial that all staff directly involved in the actual MR image generation, acquisition, and reporting process is experienced and well trained to recognize any artifact in the MR images, even if it is only minor [28].
As MR images in their vast majority contain relative rather than absolute information (in contrast to PET or CT), they fall into a different regimen when it comes to QC. This fact can also be deduced from the literature [25], [26], [27], even though the sources differ in the procedures and their frequency [14]. The procedure “center resonance frequency” might serve as an example. Whereas one reference suggests a weekly frequency [26], others favor daily [27] or no QC [25]. In all cases, water (doped e.g. with CuSO4) filled phantom would be required. In practice, however, this test is usually performed using the patient as source during the initial scan preparation. On failure, the scan is aborted. Over the last decades, the “internal” QC of MR systems replaced dedicated tests outside the aforementioned vendor maintenance, and modern systems are characterized by high stability of the signals [29]. Strictly speaking, this holds true for typical hybrid PET-MR scenarios. However, for applications in radiation oncology with the requirement of high contrast and spatial accuracies, dedicated measures including dedicated phantoms can be required but are outside the scope of this document [30], [31], [32]. In general – as MRI moved into very specific areas such as orthopedic imaging - many of the sophisticated QC sequences are not always visible to the user anymore but require a service mode - and this also holds true for PET-MR systems.
In the context of an integrated PET-MR system, the most relevant procedure is the test for spatial alignment between the MR and PET component. Moreover, it should be noted that, contrary to a PET-CT system, the quarterly and yearly PET uniformity and image quality test are not sensitive to misalignment errors between the PET- and MR-component since these tests use a predefined attenuation template and are thus independent of the MR system. Alignment between the PET- and MR-component is typically performed using a spatial calibration phantom and automated alignment software during the installation. This calibration is repeated after every maintenance procedure when the PET insert is moved or removed or software updates are applied and, thus, are typically the domain of the field service engineer as part of planned maintenance or of any unplanned repair or maintenance intervention that might be required. However, this procedure should be supervised by a medical physicist with the appropriate certification to use either fillable or sold line sources or which are needed for this procedure.
Preventive actions
Preventive actions serve to prevent results of QC tests from drifting outside of the range of acceptable values.
Preventive maintenance
As part of these preventive actions, a maintenance contract foresees a regular intervention by the manufacturer to assess the operational status of the PET-CT or PET-MR system and, if needed, to perform corrective actions to avoid possible future technical problems. Specifically, for a PET-MR system, it is recommended to check during maintenance potential loosening of PET detector elements or other connections to the systems electronic circuit because of the vibrations induced by the pulsing of gradient coils. In addition, table vibration can increase as the patient table mechanics become worn out because of intensive use. Proper preventive actions during planned maintenance of the PET-MR system can avoid potential vibration-related image artifacts from occurring. After each maintenance, it is mandatory that the PET-CT or PET-MR system is again released for clinical use by a certified medical physicist. Although such regulations are not in place everywhere, this shall be facilitated by having a certified Medical Physicist countersigning the respective service report.
Next to the preventive maintenance by the manufacturer, it is recommended to perform additional preventive actions on a routine basis to primarily anticipate problems and avoid any surprises. These additional actions can be scheduled with a frequency which is recommended by the manufacturer. The proposed schemes are given in [5] for the major PET-CT and PET-MR vendors. Generally, one should adhere to the following scheme for the daily and less regular, quarterly preventive actions.
Daily actions
On a daily basis, most manufacturers advise a partial or full reboot of all computers and (sub)systems of the equipment before any scanning is initiated. This is to ensure all memory is cleared and connections between different subsystems are reinitialized. A partial restart of the system is also the first corrective action which is generally carried out ad hoc to solve technical problems which interfere with the routine clinical use of the PET-CT or PET-MR system.
After this process, it is recommended to check time settings to ensure that the clock of the PET system is synchronized with a reference clock and thus other critical measurement equipment such as radionuclide calibrators and well counters. Generally, a deviation of less than 1 minute is considered acceptable for 18F-labelled PET tracers.
Before starting daily routine operations, it is also recommended to do a walk around to visually check the system to exclude visible defects or mechanical damage and ensure that critical system components such as the gantry, patient table, and console are expected to operate properly. In addition, it is recommended to check whether all peripheral devices as required for the scheduled PET protocols such as patient monitoring equipment, injection pumps, or triggering devices for respiratory or cardiac gating, are available and functional.
Specifically for a PET-MR system, a daily check of the cooling system’s water temperature is – depending on the general reliability of the MR system – a proven approach of avoiding issues, as is the check for the level of the liquid helium. In addition, a daily check of the bed for small metal parts (such as pens, coins, paper clips, face masks) is recommended. These metal parts have the potential to deteriorate the magnetic field homogeneity and negatively affect image quality in general [33]. A visual check of coil connectors and sockets as well as checking the area below removable coils (e.g. spine coils) again for small metal parts is recommended on a daily or at least on a weekly basis. Meanwhile, the location of MR compatible devices in the scanner room, such as injection pumps or physiological monitors, should be verified to avoid any electrical interference with the MR system. If a visual system is needed for MRI, it is worthwhile to check the setup of the system, including the mirror on the head coil and software.
Quarterly actions
It is recommended to perform a full detector setup at least quarterly. This full detector setup which includes compensation for detector gain changes and changes in crystal energy profiles as well as the generation of new crystal region maps and a renewed time alignment of detector electronics. Part of these calibrations can be done on a more regular basis based on the recommendations by the manufacturer and the initiative of the medical physicist. We recommend performing these calibrations on a more regular basis shortly after the installation of the system and gradually reducing the frequency once the stability of the system has been established. A full or partial detector setup also constitutes the first corrective action in case the daily QC test fails. However, a partial or full detector setup should always be an informed decision in consultation with the supervising medical physicist to make sure it is the proper corrective action after a daily QC failure.
It is also recommended to check the calibration factor on a quarterly basis. This calibration factor relates the signal measured with the PET system to the radionuclide calibrator such that actual activity concentration values can be measured with PET. This calibration procedure generally uses a water filled phantom and a known amount of 18F-activity measured with the radionuclide calibrator to correlate the measured numerical value in each PET image voxel (counts per second or CPS) to a specific activity measured in physical units (activity per volume or KBq/ml). We recommend a PET system calibration check instead of recalibrating the PET system by default each quarter to avoid introducing variations in the PET system calibration because of a potential drift in the dose calibrator measurements. If a new calibration of the PET system is required, it is worthwhile to compare the new calibration factor with previous calibration data and to validate the new PET system calibration with an independent phantom filling and scanning procedure.
In addition, it is recommended to update the normalization correction map preferably once every quarter. The correction map normalizes the PET data to account for differences in coincidence pair sensitivity among the scanner's lines of response at least biannually. Normalization is generally performed using a dedicated phantom provided by the manufacturer and includes a geometrical correction which corrects for spatial non-uniformity in the data sampling at any given view angle caused by the curvature of the detector, gaps between detector units, and gamma-ray penetration into crystals. In addition, it corrects for individual detector element efficiencies to obtain a uniform detector crystal sensitivity.
General considerations
A schematic overview of the appropriate QC tests and preventive actions is given in Table 1., together with the corresponding frequency scheme. After significant software updates, where processing software for data correction and image reconstruction may be modified, it is recommended to perform all QC tests to validate the new software modules and establish a new baseline for the PET system performance when appropriate. In terms of daily testing, it is recommended to check other ancillary equipment which is critical to the scheduled PET procedures and not subject to regular QC tests before patient scanning is initiated. In addition, we would again like to emphasise the importance of regular calibrator and clock checks [7], [15].
Table 1.
Overview of recommended QC tests and preventive actions, together with the appropriate frequency scheme. Quarterly and yearly actions can be done on a more regular basis based on recommendations by the manufacturer and on initiative of the medical physicist, especially shortly after system installation.
| Frequency | Actions | |
|---|---|---|
| Daily* | General |
|
| PET |
|
|
| PET-CT |
|
|
| PET-MR |
|
|
| Quarterly | PET |
|
| Yearly | General |
|
| PET |
|
|
or day of clinical use.
not standard practice yet but recommended.
or after maintenance with corrective interventions involving a recalibration of table position or table motion.
In terms of who performs the QC procedures, the daily QC can generally be performed by any user as long as this person is adequately trained to perform these tests correctly. However, the quarterly or yearly PET QC tests should be performed by or at least under the supervision of a certified medical physicist. In terms of follow-up, it is recommended to monitor the daily QC of the detector response as well as the quarterly and yearly uniformity and calibration tests over time to identify any drift in the system performance and initiate preventive or corrective actions in time. For a PET-CT system, the analysis and trending of the CT Hounsfield Units can be performed in the same manner that the PET SUV for the phantom is assessed and trended, using the CT scan acquired of a PET phantom.
The same holds for the preventive actions where daily actions can be performed by any user who is familiar with the procedure. On the other hand, quarterly preventive actions should at least be supervised by a certified medical physicist.
In terms of preventive actions, specifically for PET-MR, it is essential to prevent any patient, or clinical staff from bringing electrically loose conducting materials into the scanner room, ideally by using a metal detector at the entrance as this can seriously impact personnel and patient safety or image quality.
Conclusion
A scheme of recommended QC tests is presented for both PET-CT and PET-MR systems to make sure the scanner is functioning according to predefined criteria, together with preventive actions to ensure QC testing remains within the limits for optimal clinical use. In addition, we recommend synchronizing the QC program of the PET-CT or PET-MR system with the QC programs of other devices such as radionuclide calibrators or gamma counters which are critical for specific quantitative PET procedures. For this purpose, the quarterly calibration and uniformity test of the PET system can be used to check the cross-calibration with radionuclide calibrators or well counter systems. We also stress the importance of the daily QC where the rationale is to objectively assess the readiness of the system as much as possible before clinical scanning is initiated to guarantee sufficient PET image quality and avoid unnecessary radiation exposure to patients.
As these guidelines are to be considered a snapshot of the current practice, we do not cover the new generation of PET-CT scanners with an extended, long, or large axial FOV for which dedicated QC procedures have been developed. However, future guidelines will embrace this new technology once a wider clinical use of PET-CT systems with a long, or large axial FOV has been established.
While these guidelines focus on the performance of PET-CT or PET-MR systems, we also want to emphasize that adequate patient preparation is essential. This doesn’t just mean injecting the correct activity of the intended tracer but also means adhering to the time interval between injection and imaging. In addition, this includes taking the time to comfort the patient during the uptake period (to avoid any unintended uptake induced by the patient’s stress, activity, or discomfort) and reassuring the patient just before initiating the scan procedure to minimize motion artifacts. All these factors are of key importance to ensure optimal diagnostic image quality of PET scans.
Liability statement
This guideline summarizes the views of the EANM Physics Committee. It reflects recommendations for which the EANM cannot be held responsible. The recommendations should be taken into context of good practice of nuclear medicine and do not substitute for national and international legal or regulatory provisions.
Authors' contributions
MK, IA, JD, SN and BS were major contributors in writing the manuscript. All other authors critically reviewed and approved the final manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The guidelines were brought to the attention of the relevant EANM Committees and the National Societies of Nuclear Medicine. The comments and suggestions from the British, Danish and French Society are highly appreciated and have been considered for this guideline.
References
- 1.International Electrotechnical Commission. IEC Standard 61223-1: Evaluation and routine testing in medical imaging departments - Part 1: General aspects; 1993.
- 2.Directorate-General for Energy (European Commission): RADIATION PROTECTION N° 162: Criteria for acceptability of medical radiological equipment used in diagnostic radiology, nuclear medicine and radiotherapy; 2013.
- 3.International Atomic Energy Agency. IAEA Health Human Series No. 1: Quality Assurance for PET and PET/CT Systems. Vienna, Austria; 2009.
- 4.Mawlawi OR, Jordan DW, Halama JR, Schmidtlein CR, Wooten WW. Report No. 126 - PET/CT Acceptance Testing and Quality Assurance: The Report of AAPM Task Group 126, 2019. [Online]. Available: https://www.aapm.org/pubs/reports/detail.asp?docid=193.
- 5.Lopez B.P., Jordan D.W., Kemp B.J., Kinahan P.E., Schmidtlein C.R., Mawlawi O.R. PET/CT acceptance testing and quality assurance: Executive summary of AAPM Task Group 126 Report. Med Phys. 2021 Feb;48(2):e31–e35. doi: 10.1002/mp.14656. [DOI] [PubMed] [Google Scholar]
- 6.Pike L, Julyan P, Marsden P, Waddington W. Quality Assurance of PET and PET/CT Systems. Institute of Physics and Engineering in Medicine (IPEM) Report 108, York, UK, 2013.
- 7.EANM Physics Committee, Busemann Sokole E, Płachcínska A, Britten A; EANM Working Group on Nuclear Medicine Instrumentation Quality Control, Lyra Georgosopoulou M, Tindale W, Klett R. Routine quality control recommendations for nuclear medicine instrumentation. Eur J Nucl Med Mol Imaging. 2010 Mar;37(3):662–71. [DOI] [PubMed]
- 8.Association N.E.M. Rosslyn; Virginia, USA: 2018. NEMA Standards Publication NU 2–2018: Performance Measurements of Positron Emission Tomographs. [Google Scholar]
- 9.International Electrotechnical Commission. IEC Standard: Radionuclide imaging devices – characteristics and test conditions – Part 1: Positron emission tomographs; 1998.
- 10.Kaalep A., Sera T., Rijnsdorp S., Yaqub M., Talsma A., Lodge M.A., et al. Feasibility of state of the art PET/CT systems performance harmonisation. Eur J Nucl Med Mol Imaging. 2018 Jul;45(8):1344–1361. doi: 10.1007/s00259-018-3977-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaalep A., Sera T., Oyen W., Krause B.J., Chiti A., Liu Y., et al. EANM/EARL FDG-PET/CT accreditation - summary results from the first 200 accredited imaging systems. Eur J Nucl Med Mol Imaging. 2018 Mar;45(3):412–422. doi: 10.1007/s00259-017-3853-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aide N., Lasnon C., Veit-Haibach P., Sera T., Sattler B., Boellaard R. EANM/EARL harmonization strategies in PET quantification: from daily practice to multicentre oncological studies. Eur J Nucl Med Mol Imaging. 2017 Aug;44(Suppl 1):17–31. doi: 10.1007/s00259-017-3740-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang S.C. Anatomy of SUV. Standardized uptake value. Nucl Med Biol. 2000 Oct;27(7):643–646. doi: 10.1016/s0969-8051(00)00155-4. [DOI] [PubMed] [Google Scholar]
- 14.Valladares A., Beyer T., Boellaard R., Chalampalakis Z., Comtat C., DalToso L., et al. Clinically Valuable Quality Control for PET/MRI Systems: Consensus Recommendation From the HYBRID Consortium. Fronti Phys. 2019:7. [Google Scholar]
- 15.Carey JE, Byrne P, DeWerd L, Lieto R, Petry N. Report no 181 - The Selection, Use, Calibration, and Quality Assurance of Radionuclide Calibrators Used in Nuclear Medicine: The Report of AAPM Task Group 181, 2012. [Online]. Available: https://www.aapm.org/pubs/reports/detail.asp?docid=137.
- 16.International Atomic Energy Agency. IAEA Technical Report Series: Quality Assurance for Radioactivity Measurement in Nuclear Medicine. Vienna, Austria, 2006.
- 17.Rezaei A., Schramm G., Van Laere K., Nuyts J. Estimation of Crystal Timing Properties and Efficiencies for the Improvement of (Joint) Maximum-Likelihood Reconstructions in TOF-PET. IEEE Trans Med Imaging. 2020 Apr;39(4):952–963. doi: 10.1109/TMI.2019.2938028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.AMIDE; 2012. [Online]. Available: http://amide.sourceforge.net/.
- 19.EARL Guidelines [Online]. Available: http://earl.eanm.org/cms/website.php?id=/en/projects/fdg_pet_ct_accreditation/guidelines.htm.
- 20.American College of Radiology (ACR) CT Quality Control (QC) Manual for the ACR CT Accreditation Program (CTAP), 2017. Available: https://www.acr.org/-/media/ACR/Files/Clinical-Resources/QC-Manuals/CT_QCManual.pdf.
- 21.Edvyean S, Gray J, Heggie J, Hiles P, Homolka P, Le Heron J, et al. Quality Assurance for Computed Tomography - Diagnostic and Therapy Applications. IAEA, 2012. [Online]. Available: http://www.iaea.org/Publications/index.html.
- 22.Samei E., Bakalyar D., Boedeker K.L., Brady S., Fan J., Leng S., et al. Performance evaluation of computed tomography systems: Summary of AAPM Task Group 233. Med Phys. 2019 Nov;46(11):e735–e756. doi: 10.1002/mp.13763. [DOI] [PubMed] [Google Scholar]
- 23.Patel I, Weston SJ, Palmer AL. Physics Aspects of Quality Control in Radiotherapy. Institute of Physics and Engineering in Medicine (IPEM) Report 81, York, UK, 2018.
- 24.Bissonnette J.P., Balter P.A., Dong L., Langen K.M., Lovelock D.M., Miften M., et al. Quality assurance for image-guided radiation therapy utilizing CT-based technologies: A report of the AAPM TG-179. Med Phys. 2012;39:1946–1963. doi: 10.1118/1.3690466. [DOI] [PubMed] [Google Scholar]
- 25.Jackson EF BM, Drost DJ, Och J, Sobol WT, Clarke GD. AAPM Report No. 100 acceptance testing and quality assurance procedures for magnetic resonance imaging facilities report of MR subcommittee task group subcommittee American Association of Physicists in Medicine, College Park (MD) 2010.
- 26.Manual of Procedures Part C: MRI Technical Procedures. American College of Radiology, Philadelphia (PA). 2013.
- 27.Commission IA. The IAC Standard and Guidelines for MRI Accreditation, Ellicot City (MD). 2017.
- 28.Sattler B., Jochimsen T., Barthel H., Sommerfeld K., Stumpp P., Hoffmann K.-T., et al. Physical and organizational provision for installation, regulatory requirements and implementation of a simultaneous hybrid PET/MR-imaging system in an integrated research and clinical setting. MAGMA. 2013;26(1):159–171. doi: 10.1007/s10334-012-0347-2. [DOI] [PubMed] [Google Scholar]
- 29.Firbank M.J., Harrison R.M., Williams E.D., Coulthard A. Quality assurance for MRI: practical experience. Br J Radiol. 2000;73:376–383. doi: 10.1259/bjr.73.868.10844863. [DOI] [PubMed] [Google Scholar]
- 30.Otazo R., Lambin P., Pignol J.P., Ladd M.E., Schlemmer H.P., Baumann M., et al. MRI-guided Radiation Therapy: An Emerging Paradigm in Adaptive Radiation Oncology. Radiology. 2021;298:248–260. doi: 10.1148/radiol.2020202747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adjeiwaah M., Garpebring A., Nyholm T. Sensitivity analysis of different quality assurance methods for magnetic resonance imaging in radiotherapy. Phys Imaging Radiat Oncol. 2020;13:21–27. doi: 10.1016/j.phro.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brynolfsson P., Axelsson J., Holmberg A., Jonsson J.H., Goldhaber D., Jian Y., et al. Technical Note: Adapting a GE SIGNA PET/MR scanner for radiotherapy. Med Phys. 2018 doi: 10.1002/mp.13032. [DOI] [PubMed] [Google Scholar]
- 33.Adjeiwaah M., Bylund M., Lundman J.A., Soderstrom K., Zackrisson B., Jonsson J.H., et al. Dosimetric Impact of MRI Distortions: A Study on Head and Neck Cancers. Int J Radiat Oncol Biol Phys. 2019;103:994–1003. doi: 10.1016/j.ijrobp.2018.11.037. [DOI] [PubMed] [Google Scholar]