Abstract
Anemia is associated with increased risk of Acute Kidney Injury (AKI), stroke and mortality in perioperative patients. We sought to understand the mechanism(s) by assessing the integrative physiological responses to anemia (kidney, brain), the degrees of anemia-induced tissue hypoxia, and associated biomarkers and physiological parameters. Experimental measurements demonstrate a linear relationship between blood Oxygen Content (CaO2) and renal microvascular PO2 (y = 0.30x + 6.9, r2 = 0.75), demonstrating that renal hypoxia is proportional to the degree of anemia. This defines the kidney as a potential oxygen sensor during anemia. Further evidence of renal oxygen sensing is demonstrated by proportional increase in serum Erythropoietin (EPO) during anemia (y = 93.806*10−0.02, r2 = 0.82). This data implicates systemic EPO levels as a biomarker of anemia-induced renal tissue hypoxia. By contrast, cerebral Oxygen Delivery (DO2) is defended by a profound proportional increase in Cerebral Blood Flow (CBF), minimizing tissue hypoxia in the brain, until more severe levels of anemia occur. We hypothesize that the kidney experiences profound early anemia-induced tissue hypoxia which contributes to adaptive mechanisms to preserve cerebral perfusion. At severe levels of anemia, renal hypoxia intensifies, and cerebral hypoxia occurs, possibly contributing to the mechanism(s) of AKI and stroke when adaptive mechanisms to preserve organ perfusion are overwhelmed. Clinical methods to detect renal tissue hypoxia (an early warning signal) and cerebral hypoxia (a later consequence of severe anemia) may inform clinical practice and support the assessment of clinical biomarkers (i.e., EPO) and physiological parameters (i.e., urinary PO2) of anemia-induced tissue hypoxia. This information may direct targeted treatment strategies to prevent adverse outcomes associated with anemia.
KEYWORDS: Anemia, Brain, Kidney, Hypoxia, Erythropoietin, Perioperative period
Introduction: evidence that anemia poses a risk to patients
Anemia is prevalent worldwide, and negatively influences diverse patient populations including neonates,1,2 children,3 young adults,4 pregnant women5,6 and the elderly.7,8 The risk posed by anemia has been assessed in terms of its global impact9 and its impact on clinical outcomes in patients undergoing surgical procedures.10,11 In perioperative patients, acute and chronic anemia are associated with increased brain, heart and kidney injury, and increased mortality by currently undefined mechanisms.10,12 Assessment of patients with severe acute surgical-induced anemia, particularly those who refuse treatment with blood transfusion, strongly suggest that mortality is proportional to the severity of anemia.13, 14, 15, 16, 17, 18 Thus, assessing the impact of anemia on oxygen delivery to tissues and the physiological adaptation to anemia may provide a clinically relevant understanding of potential mechanisms of anemia-induced organ injury, and additional underlying mechanisms responsible for anemia-induced morbidity and mortality. We propose that careful review of experimental and clinical studies may help to direct the development of novel treatment pathways to improve outcomes for anemic patients.
Anemia is associated with perioperative morbidity and mortality
As previously reviewed,10,12 preoperative anemia is prevalent and has been associated with severe adverse outcomes, including Acute Kidney Injury (AKI), myocardial injury, stroke and mortality. In a large systematic review and meta-analysis, in which 39.1% (371,594 of 949,445) of patients were anemic, the odds ratios for increased mortality in anemic patients undergoing non-cardiac (OR = 2.87 [2.10, 3.93]) (p < 0.001) and cardiac surgery (OR = 2.98 [2.02, 4.38]) (p < 0.001) indicate that anemia poses a significant risk to perioperative patients.19 In addition to increasing the utilization of acute treatments, including RBC transfusions (OR = 5.04 (4.12, 6.17]) (p < 0.001), anemia was associated with increased risk of Acute Kidney Injury (AKI) (OR = 3.75 (2.95, 4.76]) (p < 0.001) and stroke (OR = 1.28 (1.06, 1.55]) (p = 0.009).19 Given the association between anemia, Acute Kidney Injury (AKI) and stroke, we focused our review and analysis on the potential mechanism of injury for these vital organs.
Evidence of acute anemia-induced kidney injury
Based on analysis of retrospective data, anemia is a predictor of acute kidney injury following both non-cardiac20 and cardiac surgery.19,21, 22, 23 The incidence and severity are more prominent for patients undergoing cardiac surgery, in which the severity of intraoperative anemia is proportional to the degree of AKI.24 Experimental studies suggest that the mechanism of anemia-induced AKI may be related to the degree of anemia-induced renal tissue hypoxia.25,26 The importance of renal tissue hypoxia during heart surgery and Cardiopulmonary Bypass (CPB) has been emphasized by studies which demonstrate an association between low urinary PO2, which reflects renal medullary PO2, and AKI post-cardiac surgery.27,28 The link between anemia and renal hypoxia is strengthened by an experimental study demonstrating that the combination of anemia and CPB resulted in the lowest levels of renal medullary tissue PO2.29 Furthermore, in a small prospective study, acute hemodilutional anemia was associated with increased serum Erythropoietin (EPO) levels (hypoxic response), and acute decline in renal function.30 Further clinical studies utilizing these available techniques are needed to determine if there is a direct link between anemia, renal hypoxia, and AKI, and if treatments (including treatment of preoperative anemia and targeted RBC transfusion) can positively influence outcomes.
Evidence of anemia-induced cerebral injury (stroke)
Case series in severely anemic patients secondary to acute blood loss demonstrated evidence of “watershed” cerebral infarction associated with inadequate cerebral tissue perfusion.31 Assessment of patients with sickle cell anemia32 demonstrate evidence of cerebral ischemia and stroke at Hemoglobin (Hb) thresholds below 70 g.L−1. In these young patients, a baseline Hb below 70 g.L−1 was associated with an increased hazard ratio for silent ischemic infarcts (HR = 2.79 [1.43, 6.17]) (p = 0.004).32 For patients undergoing non-cardiac surgery, coordinated experimental33, 34, 35, 36 and clinical studies37,38 suggest that the impact of anemia, in combination with β-blockade, accentuates cerebral tissue hypoxia and stroke incidence.35, 36, 37 Anemic patients undergoing cardiac surgery and CPB have an increased risk of stroke.19,21, 22, 23 An experimental study suggests that the mechanism includes global reduction in brain Oxygen Delivery (DO2).39 In clinical studies, the severity of acute intraoperative anemia secondary to blood loss and hemodilution during CPB was associated with increased stroke incidence,24 supporting a possible causal relationship between anemia-induced cerebral hypoxia and stroke.39,40 Understanding the mechanism(s) associated with anemia-induced cerebral hypoxia is a central goal of experimental studies, which define the hemoglobin threshold for cerebral hypoxia associated with acute anemia.41
Summary of the cardiovascular adaptation and renal and cerebral blood flow responses to anemia
In 1807, Halle published one of the earliest descriptions of the physiological responses of severe acute anemia: “We think it proper to give this disease the name of Anemia (deficiency of blood)” in which “the heart…beat(s) very strongly against the ribs”.42 This clinical observation strongly links the impact of anemia on cardiovascular responses, including Cardiac Output (CO) and cerebral blood flow.43,44 Experimental and clinical studies demonstrate that CO and CBF increase in proportion to the degree of anemia in animal25,41,43, 44, 45, 46 and human studies.47,48 These well characterized cardiovascular responses to anemia tightly defend brain perfusion. However, despite these cardiovascular adaptations to maintain cerebral perfusion, at more sever levels of anemia (Hb ∼50 g.L−1) healthy volunteers demonstrate evidence of cognitive dysfunction and reduced neuronal transmission, suggesting that the compensation to severe anemia is incomplete.49,50
In order to explore the potential mechanisms, translational studies in mammals have assessed the impact of acute anemia on cardiovascular responses and characterize different patterns of kidney and brain perfusion.25,41,43, 44, 45, 46 These studies assessed the adaptive responses to anemia, and the point at which these mechanisms are overwhelmed.25,26,34,41,51, 52, 53, 54 Measurement of organ specific hypoxic gene expression has been utilized to demonstrate specific levels of anemia-induced in the kidney and brain. At comparable Hb levels, more severe levels of tissue hypoxia are observed in the kidney, relative to the brain.41
In these studies, we have characterized that stabilization of the hypoxic transcription factor Hypoxia Inducible Factor-α (HIF-α) occurs in both kidney and brain during anemia.41,54 At the cellular level, HIF-α is a hypoxic transcription factor that activates a number of hypoxia responsive genes including Erythropoietin (EPO). In addition, we have demonstrated that the HIF-α response requires upregulation of another hypoxia regulated gene, neuronal Nitric Oxide Synthase (nNOS).41,51,54 When nNOS is genetically deleted, the HIFα response is greatly attenuated leading to impaired cardiovascular responses to anemia and reduced survival in acutely anemic rodents.54 These data are consistent with the hypotheses that: 1) Acute anemia leads to organ specific levels of tissue hypoxia, which is most severe in the kidney;25,26 2) Renal tissue hypoxia may activate local hypoxic responses (EPO production/secretion in the kidney) and adaptive cardiovascular responses (increased cardiac output, cerebral blood flow) to maintain brain perfusion;30,41,54 and 3) These cellular and cardiovascular responses can be measured in anemic patients as potential biomarkers of anemic tissue hypoxia.
At severe levels of anemia, renal hypoxia may become a maladaptive mechanism associated with AKI.25, 26, 27,30 Similarly, during mild to moderate anemia, adaptive changes in CO and Cerebral Blood Flow (CBF) may maintain cerebral DO2 at a level that maintains cerebral function. However, at more severe levels of anemia, cerebral perfusion may become inadequate, resulting in cellular evidence of tissue hypoxia, and possible stroke.35,36,41,51,54 In order to better understand the adaptive integrative physiological responses to acute anemia, and potential biomarkers of anemia-induced hypoxia, we undertook a re-analysis of the primary data from a number of previously published studies.25,36,40,41,52, 53, 54 A summary of these integrated physiological responses to anemia, and potential means of their clinical assessment are outlined in Figure 1.
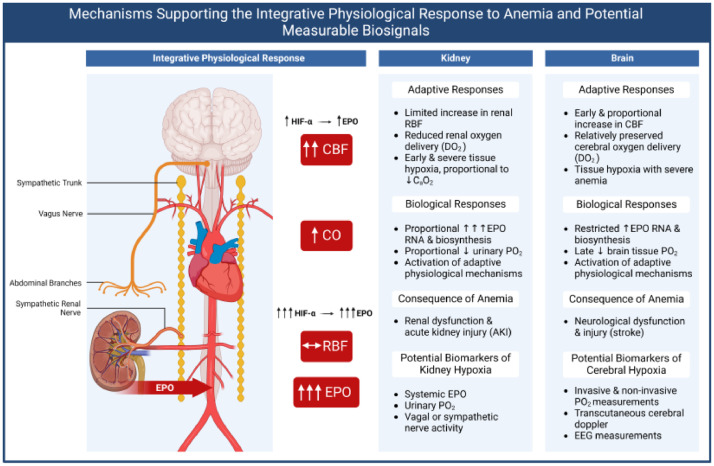
Figure 1.
Summary of integrative physiological responses to anemia and potential means of clinical assessment. Under anemic conditions, renal blood flow is maintained but associated oxygen delivery is decreased. This allows the kidney to sense decreases in blood oxygen content. Renal Erythropoietin (EPO) production is greatly stimulated at all levels of anemia in response to stabilization of Hypoxia-Inducible Factor-alpha (HIF-α). The lack of increased Renal Blood Flow (RBF) makes the kidney susceptible to hypoxia and Acute Kidney Injury (AKI). By contrast, the brain is protected to an extent during anemia via an increase in cerebral blood flow, allowing maintenance of oxygen delivery in mild to moderate anemia. Brain tissue hypoxia in response to severe anemia is associated with increased HIFα and EPO expression and may contribute to neurological injury and stroke. Biomarkers to identify renal and brain hypoxia are listed. This figure was created in BioRender.com. CBF, Cerebral Blood Flow; CO, Cardiac Output; DO2, Oxygen Delivery; CaO2, Arterial Oxygen Content.
Strategy for assessment of the integrative physiological responses to acute anemia in animal models
In order to assess the impact of changes in blood Oxygen Content (CaO2) on physiological parameters, including brain and kidney microvascular PO2, cardiac output, organ blood flow, and Oxygen Delivery (DO2), we searched for experimental studies using the following search strategy based on outcomes from several previously published studies:25,36,40,41,52, 53, 54 “tissue oxygen or PO2” and “kidney or brain” and “anemia or hemodilution”. We captured 897 studies on our initial search and selected studies that: 1) Provided data for calculation of blood Oxygen Content (CaO2) and that also included 2) Assessed physiological responses at more than one level of anemia and/or 3) That provided measurements for both kidney and brain tissue hypoxia (PO2). Five of 897 searched studies met the inclusion criteria.25,26,36,41,54 (Table 1). Of the 892 excluded studies, 4 additional experimental studies provided data which support our proposed hypothesis but were excluded due to inadequate data to directly correlate CaO2 with measured parameters including kidney and brain microvascular PO2.44,53,55,56
Table 1.
Inclusion criteria for references that provided adequate data for analysis.
| Inclusion Criteria (A plus B and/or C) | ||||
|---|---|---|---|---|
| Reference | Experimental model | A | B | C |
| Individual data for CaO2 available | Assessed > One level of anemia | Measure PO2 outcomes in both kidney and brain | ||
| Abrahamson JR. Am J Physiol. 202025 | Rat hemodilutional anemia | Yes | Yes | No |
| Chin K. Can J Anesth. 202126 | Rat hemodilutional anemia | Yes | Yes | No |
| Ragoonanan T. Anesthesiology 200936 | Rat hemodilutional anemia | Yes | No | Yes |
| Tsui AKY. Proc Nat Acad Sci. 201154 | Mouse hemodilutional anemia | Yes | Yes | Yes |
| Tsui AKY. Am J Physiol. 201441 | Mouse hemodilutional anemia | Yes | Yes | Yes |
We re-evaluated data from the five selected studies and performed new analysis utilizing blood CaO2 as the independent variable. All data are reported as individual data points, scatter plots or box plots, and Analysis of Variance (ANOVA) was performed to compare changes in measured parameters vs. changes in CaO2. Appropriate statistical analysis and regressions were performed utilizing SigmaPlot 14.0 with statistical significance being assessed at and alpha value of p < 0.05.
Evidence that the kidney is a biosensor of blood oxygen content (CaO2)
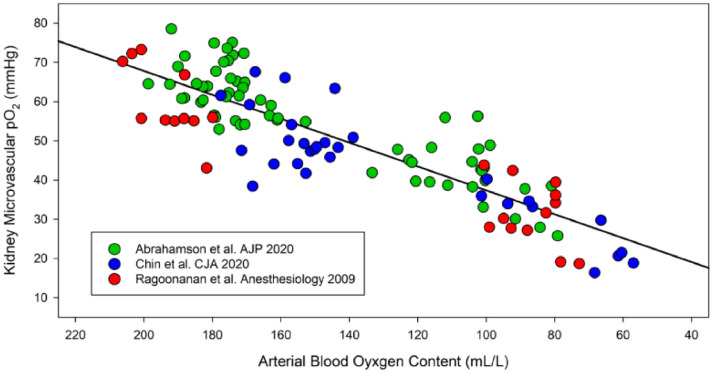
Measurements of microvascular kidney tissue PO2 (PkO2) were made using an intravascular oxygen sensitive phosphorescent dye (Oxyphor G4).25,26,36 From the available arterial blood gas and co-oximetry measurements, the arterial Oxygen Content (CaO2) was calculated from the hemoglobin concentration, pO2 and SO2 (CaO2 = 1.34*Hb*SO2+0.0031*pO2). We plotted PkO2 vs. CaO2 and generated a regression line and r2 value (Fig. 2). The results demonstrate that there was a direct correlation between changes in CaO2 and microvascular renal PO2, providing a means for the kidney to translate changes in CaO2 to local tissue PO2. Sensing and responding to local changes in PO2 developed teleologically as a survival mechanism for early aerobic life,57 and continues to be critical for complex organism (mammalian) survival.58 There is also evidence of a proportional increase in sympathetic nerve activity with progressive anemia, which supports the hypothesis that “sensed” tissue hypoxia is translated into an autonomic signal during acute anemia.59 Evidence that the kidney is important in both sensing and transmitting an afferent “warning” signal to the central nervous system is supported by preliminary data demonstrating that removal of the kidneys (bilateral nephrectomy) is associated with profound brain hypoxia in control and anemic animals.60 Thus, oxygen sensing by the kidney and other cells appears to be a critical starting point which initiates adaptive responses to anemia. Measurements of biomarkers of renal tissue hypoxia may help us to utilize this warning signal to optimally treat acutely anemic patients.27,30
Figure 2.
Scatterplot of the relationship between kidney microvascular PO2 versus arterial blood oxygen content in anesthetized Sprague-Dawley rats exposed to acute hemodilutional anemia. A significant correlation (y = 0.30x + 6.9, r2 = 0.75) is observed between microvascular kidney tissue PO2 and arterial oxygen content. As arterial oxygen content decreases, microvascular kidney tissue PO2 decreases proportionally in a linear manner. This data demonstrates the ability of the kidney to translate CaO2 into a local regional microvascular PO2 based on organ blood flow and tissue metabolic requirements. Data from Abrahamson et al., AJP 202025 (n = 8); Chin et al., CJA 2021 (n = 5);26 and Ragoonanan et al., Anesthesiology 200936 (n = 5).
Adaptive cardiovascular responses to severe anemia result in differential degrees of tissue hypoxia in the kidney and brain
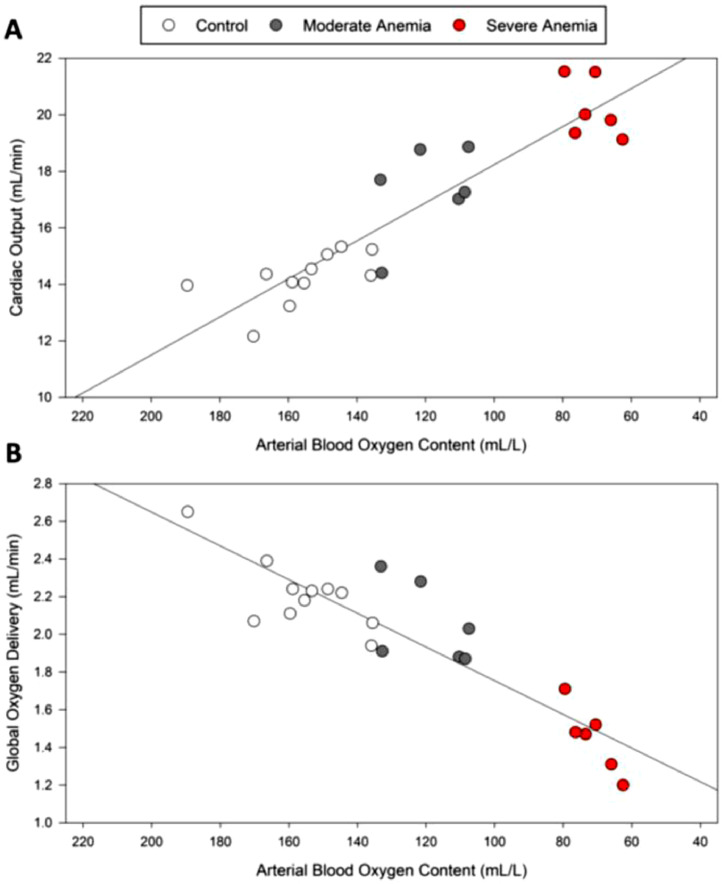
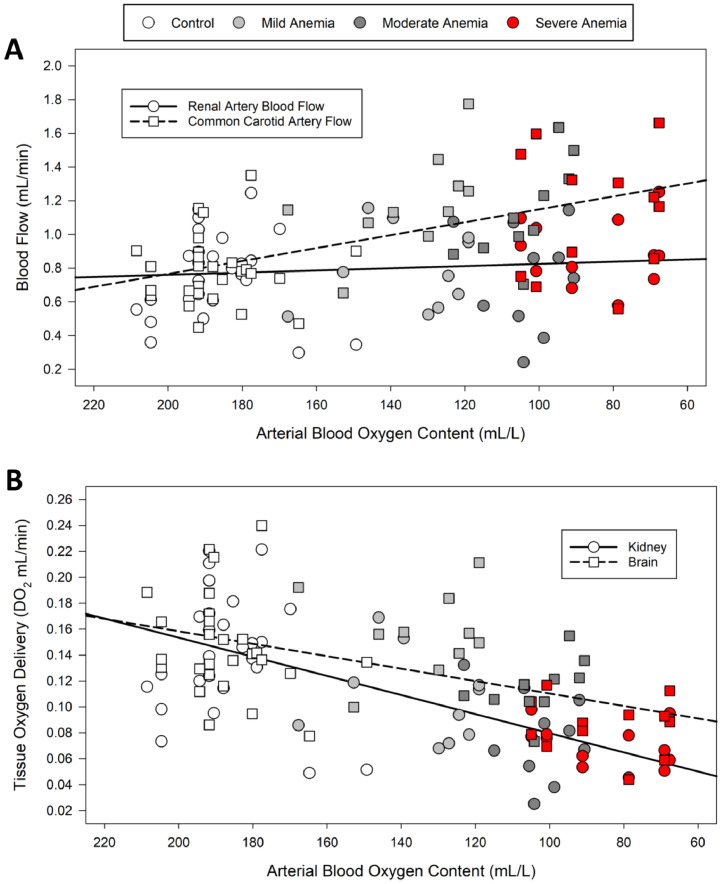
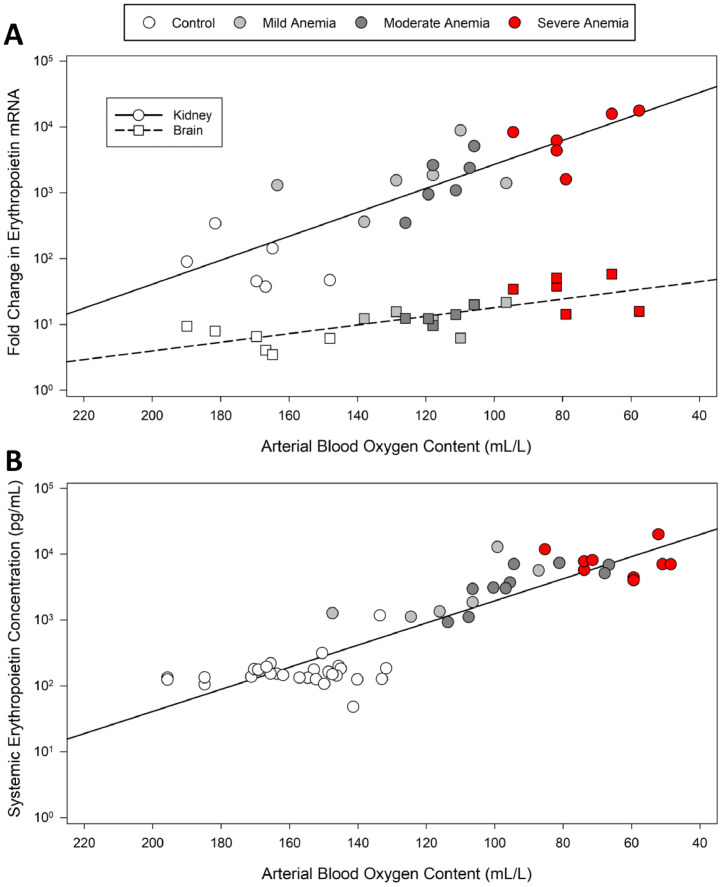
Our dataset reflects the most fundamental finding during acute anemia: that there is an immediate and proportional increase in CO observed with acute anemia (Fig. 3, upper panel). Despite this clear increase in CO, global DO2 decreases (Fig. 3, lower panel), particularly at more severe levels of anemia. This finding has been reported in mammals and humans,41,48 however, the subsequent impact on end organ perfusion, tissue hypoxia, and hypoxic gene expression has been lacking. We assessed the impact of different levels of acute anemia on cerebral and renal blood flow and DO2 in response to mild, moderate, and severe anemia (Hb ∼ 90, 70 and 50 g.L−1, respectively)41 (Fig. 4). Comparison of the renal and brain perfusion demonstrate that renal blood flow is not increased during anemia and therefore, renal DO2 decreases progressively (Fig. 4). This leads to a dramatic increase in hypoxic gene expression including EPO whose RNA expression increased 10,000-fold (Fig. 5, upper panel). By contrast, the brain is relatively protected from tissue hypoxia by a progressive increase in CBF, resulting in a less profound decrease in DO2 and relatively preserved brain PO2 as indicated by a relatively small increase in hypoxia EPO RNA expression (10-fold) (Figs. 4 and 5).
Figure 3.
Cardiac output and global oxygen delivery in rats at varying levels of anemia. (A) A strong inverse relationship (y = -0.07x + 24.9, r2 = 0.83) is observed between cardiac output and arterial blood oxygen content. Decreasing arterial oxygen content and increasing severity of anemia results in a proportional increase in cardiac output. (B) A strong positive relationship (y = 0.01x + 0.86, r2 = 0.82) is observed between global oxygen delivery and arterial oxygen content. Reduced arterial oxygen content results in decreased global oxygen delivery, especially under severe anemic conditions. Data from Tsui et al. 2014.41
Figure 4.
Blood flow and tissue oxygen delivery to the brain and kidney of rats at varying levels of anemia. (A) Renal artery blood flow does not change significantly regardless of anemic status (y = -0.0006x + 0.89, r2 = 0.14). Common carotid artery flow increases proportionally as arterial blood oxygen content decreases and anemic status increases in severity (y = -0.004x + 1.53, r2 = 0.29) (n = 5). (B) Both kidney and brain DO2 decrease proportionally as arterial blood oxygen content decreases and anemic status increases in severity. Brain DO2 (y = 0.0005x + 0.06, r2 = 0.28) decreases less profoundly as compared to kidney DO2 at lower arterial blood oxygen content (y = 0.0007x + 0.006, r2 = 0.48) (n = 15). Data from Tsui et al. 2014.41
Figure 5.
Organ-specific Erythropoietin (EPO) mRNA and systemic EPO versus arterial blood oxygen content in anemic rats. (A) EPO mRNA expression increases exponentially in both kidney tissue and brain tissue as arterial oxygen content decreases (n = 6). Kidney EPO mRNA expression (y = 178590*10−0.018, r2 = 0.71) increases more dramatically than brain EPO mRNA expression (y = 81.72*10−0.007, r2 = 0.57) in severely anemic conditions. (B) Systemic EPO protein concentration increases exponentially (y = 93806*10−0.02, r2 = 0.82) as arterial oxygen content decreases and the severity of anemic status increases (n = 10). Data from Tsui et al. 2011 and 2014.41,54
A key systemic response to anemia induced renal tissue hypoxia is the secretion of EPO into the blood, the magnitude of which is proportional to the degree of acute anemia (Fig. 5, lower panel). While knowledge of this response is decades old,61 the clinical utilization of this robust bio signal of tissue hypoxia has been lacking. Our animal studies demonstrate that EPO is amongst the most sensitive HIF responsive molecule in the kidney and brain.25,41,53,54 Furthermore, in a small prospective study in humans undergoing heart surgery, we have identified that postoperative serum EPO levels correlated to reduce Hb on CPB and systemic lactate levels.30 In addition, elevated postoperative EPO levels were associated with a trend toward increased creatinine and mild AKI.30 These data raise the possibility that systemic EPO may be a potential biomarker of anemia induced tissue hypoxia and that a threshold for renal injury may be identified.
Summary of adaptive physiological responses to acute anemia
The key findings of this analysis are as follows: 1) The kidney is capable of detecting changes in blood oxygen content during acute anemia and converting these changes into a local microvascular and tissue PO2 response providing a mechanism for local hypoxia sensing and increased expression and secretion of EPO during acute anemia;25,26 2) Removal of the kidney can severely impair brain perfusion during anemia;60 3) The kidney can respond to anemia-induced tissue hypoxia by increasing EPO production in proportion to the degree of anemia,41 and this response may serve autocrine and paracrine as well as endocrine functions; 4) Adaptive cardiovascular responses to acute anemia may protect vital organs, including the brain, from hypoxic injury;62 5) Inhibiting these cardiovascular responses resulted in elevated levels of brain tissue hypoxia, suggesting that they are critical for maintaining cerebral oxygen homeostasis during acute anemia;34, 35, 36 6) Differences in renal and cerebral hypoxia are reflected by the magnitude of the local EPO mRNA responses, which are directly reflected in EPO transcription and secretion.41,45
Human studies reflect the physiological adaptations defined by animal models
The constellations of adaptive responses to acute anemia in animal models is reflected by a number of published clinical studies, suggesting that they represent highly conserved mechanism of mammalian adaptation to acute blood loss and anemia.40,45 In an elegant series of studies in human volunteers, Weiskopf and colleagues, have clearly defined the human cardiovascular responses to acute anemia. They have demonstrated that: 1) The increased cardiac output (heart rate and stroke volume) is directly proportional to the degree of acute anemia;48 2) The heart rate response to acute anemia follows a tight linear relationship, suggesting that a sensitive mechanism for anemia detection and proportional responses to acute reduction in CaO2 occur;63 3) Despite a robust cardiovascular response to acute anemia, evidence of cognitive impairment suggest an inadequacy of cerebral perfusion, and impaired oxygen homeostasis, at severe levels of anemia;49,50 and 4) Despite these adaptive changes there is a relationship between severe anemia, cardiac injury and mortality. The inability for complete compensation for acute reductions in blood CaO2 and subsequent tissue DO2 are reflected by studies which demonstrate an incidence of vital organ injury (kidney and brain) that is proportional to the degree of anemia following surgery.22, 23, 24 Finally, treatment of anemia with iron, EPO, or blood transfusion improves clinical outcomes and survival in critically ill patients,64, 65, 66, 67, 68 providing proof of concept that treatment of anemia may change the injurious mechanism responsible for organ injury and mortality associated with anemia.67
Can measurement of kidney and urinary PO2 predict hypoxic kidney injury?
Acute kidney injury is prevalent in anemic patients undergoing non-cardiac and cardiac surgery with an estimated 3‒4-fold increased risk of AKI in anemic patients (OR = 3.75, 95% CI 2.95–4.76) (p < 0.001).19 Reduced urinary PO2 is a surrogate of renal medullary hypoxia, and has been associated with an increased risk for AKI.27,28,69 Measured of urinary PO2 has been performed during cardiac surgery with the aid of modified urinary catheters placed in the bladder, which contain a real-time PO2 probe. Two different studies have identified that low levels of urinary PO2, predominantly at the time of CPB, are associated with impaired renal function.27,28 In one study, a urinary PO2 of ≤ 15 mmHg for greater than 4.8 minutes per hour, increased the odds ratio for AKI by about 5 fold (OR = 4.9, 95% CI 1.6–14.4) (p = 0.004).27 In another study, the unadjusted risk of AKI increased by 50% if the mean urinary PO2 was below 25 mmHg (RR = 1.51 [1.08–2.10]) (p = 0.015).28 A multivariate analysis identified mean urinary PO2 to be an independent predictor of AKI (RR = 0.82, 95% CI 0.71–0.950, p = 0.009) for every 10 mmHg decrease in urinary PO2.28 Despite experimental evidence that low Hb on CPB worsens renal medullary PO2,29 low Hb or Hct was not a risk factor for AKI in these studies.27,28 Utilizing methods to measure urinary PO2 may also help to elucidate the impact of fluid management for patients in the ICU and operating room. For example, the negative impact of utilizing intravascular starch for fluid resuscitation on AKI and mortality may be reflected by changes in renal PO2.70,71 Experimental evidence suggests that the mechanism of starch induced renal dysfunction may lower kidney PO2.25 Further clinical studies would be required to test this hypothesis and assess whether or not assessing the impact of anemia on renal hypoxia, and its treatment, can improve renal outcomes in anemic patients undergoing surgery and critical care.
Can cerebral monitoring predicate the risk of stroke and cerebral dysfunction in anemia surgical patients?
The risk of stroke associated with anemia (OR = 1.28, 95% CI 1.06–1.55) (p = 0.009)19 may be of a lower magnitude than that of AKI, in part due to the strong adaptive increase in CBF to maintain cerebral DO2. However, the profound negative impact of stroke in perioperative patients,38 particularly in patients undergoing cardiac surgery,24 warrants continued re-assessment. Novel monitoring approaches to minimizing stroke risk include: 1) Near Infrared Spectroscopy (NIRS);72,73 2) Direct cerebral oximetry;74 3) Transcranial Doppler;75,76 and 4) Processed EEG measurements.77 In cardiac surgery, NIRS methodology is routinely utilized to assess levels of cerebral perfusion in many centers performing cardiac surgery.72 These methods effectively detect severe reduction in cerebral perfusion associated with interruption of CPB bypass circuits and during prolonged circulatory arrest.78 Utilization of NIRS has been proposed for standard monitoring of cerebral perfusion during CPB. Established protocols for optimizing cerebral perfusion have been performed in a few small RCTs. While the clinical demonstration of benefits remains uncertain, the demonstration of feasibility supports the ongoing development of this approach.72,73 More recently, decline in cerebral oxygen saturation has been associated with increased incidence of delirium and stroke in patients undergoing percutaneous valve replacement, supporting the value of monitoring and treating cerebral oxygen desaturation.79
Few clinical settings warrant the placement of direct cerebral oximetry probes in the brain. However, invasive Clarke-type electrodes have been used to assess cerebral perfusion in patients with severe closed head injury and following craniotomy.74 The hopeful results of the BOOST 2 trial suggest that active treatment of low brain oxygen tension can significantly improve patient outcomes.74 These data may be confirmed in the ongoing BOOST 3 trial, and may provide evidence that measuring and treating low brain PO2 may improve outcomes in other type of patients, including those undergoing heart surgeries who have a higher risk of stroke associated with acute anemia.24
The well-characterized proportional increase in CBF, associated with anemia, has been assessed by transcranial Doppler in several clinical settings. In the case of anemia, dilation of resistance arterioles results in an increased flow velocity associated with physiological mechanisms to optimize cerebral DO2. This signal has been used to assess the degree of fetal anemia in utero76,80 as well as the risk of stroke in young adults with sickle cell anemia.32,75 The approach in both cases is to use the increased blood flow velocity signal as a biomarker of adaptive changes in blood flow during anemia, the magnitude of which reflects the degree of anemia. Correlation of the CBF with severity of anemia is used to determine a threshold after which potential cerebral injury and stroke may occur. Once this predefined threshold is reached, the clinical decision to transfuse RBCs has been shown to reduce stroke associated with severe anemia.32,75 As the physiological mechanism (increased CBF) is shared between differing types of anemia, this approach may be used in surgical patients facing severe acute blood loss. Finally, new advances in assessing changes in evoked EEG patterns may provide means of assessing outcomes including level of sedation, postoperative cognitive decline, and stroke.77
Systemic biomarkers of tissue hypoxia may predict risk of organ injury
Traditional biomarker of kidney injury, including creatinine, Neutrophil Gelatinase-Associated Lipocalin (NGAL), and cystatin C may predict renal injury following CPB.69 More recently, there was evidence that the hypoxia-induced molecule EPO may also provide an indicator of AKI.30 EPO is an erythrocyte-stimulating hormone best characterized by its molecular response to acute anemia.81 Data supports this physiological response but, with more severe anemia, a strong serum erythropoietic response is associated with hypoxic kidney injury,30 suggesting that at extremes of anemic stress, the adaptive physiological responses may not be adequate to preserve end-organ function.30
While systemic EPO levels are thought to be primarily of renal origin, new evidence has emerged that non-renal sources of EPO may contribute to both local tissue levels and systemic EPO levels.82 As such, EPO may provide a cytoprotective role via autocrine, paracrine, and endocrine mechanisms. Studies have explored the pleiotropic effects of EPO and provided evidence for potential neuroprotective effects.83 EPO receptors found on brain tissue modulate anti-apoptotic, antioxidant and other neuroprotective functions particularly in the context of ischemia-reperfusion injury.83
Clinical trials have demonstrated a number of interesting findings: critically ill patients with anemia, both acute and chronic, do exhibit a relative increase in serum EPO levels compared to non-anemic patients.84 In a series of clinical trials, treatment of anemic, critically ill patients with EPO resulted in improved outcomes, including improved survival.65,66 However, a meta-analysis by Mesgarpour et al. (34 randomized controlled trials and 14 observational studies including 944,856 patients from clinical studies conducted up to 2012) demonstrated no benefit and a possible increased risk of thrombotic events, tempering the use of EPO in this patient population.85 By contrast, other analyses in patients undergoing elective cardiac and non-cardiac surgery demonstrated efficacy of EPO in avoiding RBC transfusion without any adverse thrombotic events.86,87 Finally, recent work during the Coronavirus-2019 (COVID-19) pandemic has shown the potential for EPO therapy in patients with COVID-19 ARDS.88 Thus, systemic EPO levels may serve as a biomarker of anemia-induced tissue hypoxia and organ injury.30 In addition, exogenous EPO may be effective in treating anemia and subsequent anemia-related organ injury and mortality.
Evidence that targeted treatments of anemia improve outcomes
While the summary assessment of several meta-analysis and systematic reviews suggests that, overall, restrictive transfusion strategies are non-inferior to more liberal strategies,89,90 a number of patient-specific factors must be considered. In the FOCUS trail, in which the restrictive group was randomized to a Hb threshold of 80 g.L−1, about 10% of this group were transfused more liberally due to cardiovascular symptoms and signs of hypoperfusion, including hypotension and tachycardia, indicating that a restrictive transfusion threshold may not be tolerated by all patients.91 Evidence supports that patients with cardiovascular disease may not benefit from a more restrictive transfusion strategy.92,93 A sub-analysis of the TRICS 3 study demonstrated that when stratified by age, younger patients had a more favorable composite outcome with more liberal transfusion.94,95 Finally, the positive outcomes from liberal transfusion, including improved survival in surgical patients undergoing extensive abdominal surgery,64 support the continuation trials to assess evidence of end-organ hypo-perfusion and injury in specific patients during acute anemia. These positive outcomes also support the concept that appropriate targeting of RBC transfusion may improve patient outcome and survival.
Can iron therapy improve outcomes for anemic patients?
While few studies have assessed the impact of intravenous iron therapy on both hemoglobin levels and patient outcomes, two recent studies warrant mention. The PREVENTT trial assessed the impact of preoperative iron infusion on anemic patients undergoing abdominal procedures. While no difference in mortality, or major morbidity, were observed, the authors did report that iron increased perioperative Hb and significantly reduced hospital readmission in the iron intervention group.68 Another small but important clinical trial demonstrated that treatment of severely iron-deficient patients in the ICU, with biomarker evidence of tissue hypoxia (reduced hepcidin), resulted in improved survival in these patients.67 These two studies demonstrate the potential for treatment of anemia to improve important clinical outcomes in perioperative patients. Completion of larger RCTS will be needed to provide evidence which support, or refute, that targeted therapies to treat anemia may benefit patients in terms of improved event free survival.
Limitations
There are limitations to our review. It reanalyzes previous experimental data and thus carries the limitations of the previous work which may limit the generalizability of the reported outcomes. As no long-term chronic assessments of the impact of anemia were analyzed, we lack further research into the chronic effects of anemia. In addition, as the analysis was limited by measurements that focused on the brain and kidney, the effect of anemia on other organs was not investigated.
Conclusions
Comprehensive analysis of experimental studies characterizes the integrated physiological responses to acute anemia and demonstrates differential responses in the kidney and brain. During acute anemia, kidney perfusion is relatively restricted, and Oxygen Delivery (DO2) is attenuated, thereby contributing to the kidneys' ability to detect acute changes in blood oxygen content, enabling the kidney to function as an oxygen sensor during acute anemia. At more severe levels of anemia, this mechanism may place the kidney at risk of hypoxic injury. Conversely, cardiovascular adaptation to acute anemia, including a proportional increase in cardiac output and cerebral blood flow, ensures preservation of cerebral DO2 and brain tissue PO2. These mechanisms defend against hypoxic cerebral injury, until severe levels of anemia occur. Assessing functional biomarkers and physiological parameters of acute anemia-induced tissue hypoxia (i.e., urinary hypoxia, serum EPO, CBF, cerebral oximetry) may help to detect anemia-induced tissue hypoxia and direct the development of effective treatment strategies, including treatment of anemia and targeted RBC transfusion30,40 to optimize the management of anemic patients and improve clinical outcomes.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
Dr. GMT Hare received CAS – CARF support from the RA Gordon Research Award. Drs. Baker, Hare and Mazer receive support from the University of Toronto, Department of Anesthesia Merit Award Program.
References
- 1.Goobie SM, DiNardo JA, Faraoni D. Relationship between transfusion volume and outcomes in children undergoing noncardiac surgery. Transfusion. 2016;56:2487–2494. doi: 10.1111/trf.13732. [DOI] [PubMed] [Google Scholar]
- 2.Patel RM. Short- and Long-Term Outcomes for Extremely Preterm Infants. Am J Perinatol. 2016;33:318–328. doi: 10.1055/s-0035-1571202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clarke SE, Jukes MC, Njagi JK, et al. Effect of intermittent preventive treatment of malaria on health and education in schoolchildren: a cluster-randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:127–138. doi: 10.1016/S0140-6736(08)61034-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kyu HH, Pinho C, Wagner JA, et al. Global and National Burden of Diseases and Injuries Among Children and Adolescents Between 1990 and 2013: Findings From the Global Burden of Disease 2013 Study. JAMA Pediatr. 2016;170:267–287. doi: 10.1001/jamapediatrics.2015.4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daru J, Zamora J, Fernández-Félix BM, et al. Risk of maternal mortality in women with severe anaemia during pregnancy and post partum: a multilevel analysis. Lancet Glob Health. 2018;6:e548–e554. doi: 10.1016/S2214-109X(18)30078-0. [DOI] [PubMed] [Google Scholar]
- 6.Tort J, Rozenberg P, Traoré M, Fournier P, Dumont A. Factors associated with postpartum hemorrhage maternal death in referral hospitals in Senegal and Mali: a cross-sectional epidemiological survey. BMC Pregnancy Childbirth. 2015;15:235. doi: 10.1186/s12884-015-0669-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zakai NA, Katz R, Hirsch C, et al. A prospective study of anemia status, hemoglobin concentration, and mortality in an elderly cohort: the Cardiovascular Health Study. Arch Intern Med. 2005;165:2214–2220. doi: 10.1001/archinte.165.19.2214. [DOI] [PubMed] [Google Scholar]
- 8.Penninx BW, Pahor M, Woodman RC, Guralnik JM. Anemia in old age is associated with increased mortality and hospitalization. J Gerontol A Biol Sci Med Sci. 2006;61:474–479. doi: 10.1093/gerona/61.5.474. [DOI] [PubMed] [Google Scholar]
- 9.Kassebaum NJ, Jasrasaria R, Naghavi M, et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood. 2014;123:615–624. doi: 10.1182/blood-2013-06-508325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hare GMT, Mazer CD. Anemia: perioperative risk and treatment opportunity. Anesthesiology. 2021;135:520–530. doi: 10.1097/ALN.0000000000003870. [DOI] [PubMed] [Google Scholar]
- 11.Warner MA, Shore-Lesserson L, Shander A, Patel SY, Perelman SI, Guinn NR. Perioperative anemia: prevention, diagnosis, and management throughout the spectrum of perioperative care. Anesth Analg. 2020;130:1364–1380. doi: 10.1213/ANE.0000000000004727. [DOI] [PubMed] [Google Scholar]
- 12.Shander A, Javidroozi M, Ozawa S, Hare GM. What is really dangerous: anaemia or transfusion? Br J Anaesth. 2011;107(1):i41–i59. doi: 10.1093/bja/aer350. Suppl. [DOI] [PubMed] [Google Scholar]
- 13.Carson JL, Duff A, Poses RM, et al. Effect of anaemia and cardiovascular disease on surgical mortality and morbidity. Lancet. 1996;348:1055–1060. doi: 10.1016/S0140-6736(96)04330-9. [DOI] [PubMed] [Google Scholar]
- 14.Carson JL, Poses RM, Spence RK, Bonavita G. Severity of anaemia and operative mortality and morbidity. Lancet. 1988;1:727–729. doi: 10.1016/s0140-6736(88)91536-x. [DOI] [PubMed] [Google Scholar]
- 15.Guinn NR, Cooter ML, Villalpando C, Weiskopf RB. Severe anemia associated with increased risk of death and myocardial ischemia in patients declining blood transfusion. Transfusion. 2018;58:2290–2296. doi: 10.1111/trf.14768. [DOI] [PubMed] [Google Scholar]
- 16.Guinn NR, Cooter ML, Weiskopf RB. Lower hemoglobin concentration decreases time to death in severely anemic patients for whom blood transfusion is not an option. J Trauma Acute Care Surg. 2020;88:803–808. doi: 10.1097/TA.0000000000002632. [DOI] [PubMed] [Google Scholar]
- 17.Shander A, Javidroozi M, Naqvi S, et al. An update on mortality and morbidity in patients with very low postoperative hemoglobin levels who decline blood transfusion (CME) Transfusion. 2014;54:2688–2695. doi: 10.1111/trf.12565. [DOI] [PubMed] [Google Scholar]
- 18.Weiskopf RB, Glassberg E, Guinn NR, James MFM, Ness PM, Pusateri AE. The need for an artificial oxygen carrier for disasters and pandemics, including COVID-19. Transfusion. 2020;60:3039–3045. doi: 10.1111/trf.16122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fowler AJ, Ahmad T, Phull MK, Allard S, Gillies MA, Pearse RM. Meta-analysis of the association between preoperative anaemia and mortality after surgery. Br J Surg. 2015;102:1314–1324. doi: 10.1002/bjs.9861. [DOI] [PubMed] [Google Scholar]
- 20.Musallam KM, Tamim HM, Richards T, et al. Preoperative anaemia and postoperative outcomes in non-cardiac surgery: a retrospective cohort study. Lancet. 2011;378:1396–1407. doi: 10.1016/S0140-6736(11)61381-0. [DOI] [PubMed] [Google Scholar]
- 21.Padmanabhan H, Siau K, Curtis J, et al. Preoperative anemia and outcomes in cardiovascular surgery: systematic review and meta-analysis. Ann Thorac Surg. 2019;108:1840–1848. doi: 10.1016/j.athoracsur.2019.04.108. [DOI] [PubMed] [Google Scholar]
- 22.Karkouti K, Wijeysundera DN, Beattie WS. Risk associated with preoperative anemia in cardiac surgery: a multicenter cohort study. Circulation. 2008;117:478–484. doi: 10.1161/CIRCULATIONAHA.107.718353. [DOI] [PubMed] [Google Scholar]
- 23.Kulier A, Levin J, Moser R, et al. Impact of preoperative anemia on outcome in patients undergoing coronary artery bypass graft surgery. Circulation. 2007;116:471–479. doi: 10.1161/CIRCULATIONAHA.106.653501. [DOI] [PubMed] [Google Scholar]
- 24.Karkouti K, Djaiani G, Borger MA, et al. Low hematocrit during cardiopulmonary bypass is associated with increased risk of perioperative stroke in cardiac surgery. Ann Thorac Surg. 2005;80:1381–1387. doi: 10.1016/j.athoracsur.2005.03.137. [DOI] [PubMed] [Google Scholar]
- 25.Abrahamson JR, Read A, Chin K, et al. Renal tissue PO2 sensing during acute hemodilution is dependent on the diluent. Am J Physiol Regul Integr Comp Physiol. 2020;318:R799–R812. doi: 10.1152/ajpregu.00323.2019. [DOI] [PubMed] [Google Scholar]
- 26.Chin K, Cazorla-Bak MP, Liu E, et al. Renal microvascular oxygen tension during hyperoxia and acute hemodilution assessed by phosphorescence quenching and excitation with blue and red light. Can J Anaesth. 2021;68:214–225. doi: 10.1007/s12630-020-01848-5. [DOI] [PubMed] [Google Scholar]
- 27.Zhu MZL, Martin A, Cochrane AD, et al. Urinary hypoxia: an intraoperative marker of risk of cardiac surgery-associated acute kidney injury. Nephrol Dial Transplant. 2018;33(12):2191–2201. doi: 10.1093/ndt/gfy047. [DOI] [PubMed] [Google Scholar]
- 28.Silverton NA, Lofgren LR, Hall IE, et al. Noninvasive Urine Oxygen Monitoring and the Risk of Acute Kidney Injury in Cardiac Surgery. Anesthesiology. 2021;135:406–418. doi: 10.1097/ALN.0000000000003663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Darby PJ, Kim N, Hare GM, et al. Anemia increases the risk of renal cortical and medullary hypoxia during cardiopulmonary bypass. Perfusion. 2013;28:504–511. doi: 10.1177/0267659113490219. [DOI] [PubMed] [Google Scholar]
- 30.Hare GMT, Han K, Leshchyshyn Y, et al. Potential biomarkers of tissue hypoxia during acute hemodilutional anemia in cardiac surgery: A prospective study to assess tissue hypoxia as a mechanism of organ injury. Can J Anaesth. 2018;65:901–913. doi: 10.1007/s12630-018-1140-0. [DOI] [PubMed] [Google Scholar]
- 31.Tsai CF, Yip PK, Chen CC, Yeh SJ, Chung ST, Jeng JS. Cerebral infarction in acute anemia. J Neurol. 2010;257:2044–2051. doi: 10.1007/s00415-010-5657-6. [DOI] [PubMed] [Google Scholar]
- 32.Bernaudin F, Verlhac S, Arnaud C, et al. Chronic and acute anemia and extracranial internal carotid stenosis are risk factors for silent cerebral infarcts in sickle cell anemia. Blood. 2015;125:1653–1661. doi: 10.1182/blood-2014-09-599852. [DOI] [PubMed] [Google Scholar]
- 33.El Beheiry MH, Heximer SP, Voigtlaender-Bolz J, et al. Metoprolol impairs resistance artery function in mice. J Appl Physiol. 2011;111:1125–1133. doi: 10.1152/japplphysiol.01340.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hare GM, Worrall JM, Baker AJ, Liu E, Sikich N, Mazer CD. Beta2 adrenergic antagonist inhibits cerebral cortical oxygen delivery after severe haemodilution in rats. Br J Anaesth. 2006;97:617–623. doi: 10.1093/bja/ael238. [DOI] [PubMed] [Google Scholar]
- 35.Hu T, Beattie WS, Mazer CD, et al. Treatment with a highly selective β1 antagonist causes dose-dependent impairment of cerebral perfusion after hemodilution in rats. Anesth Analg. 2013;116:649–662. doi: 10.1213/ANE.0b013e318280e26d. [DOI] [PubMed] [Google Scholar]
- 36.Ragoonanan TE, Beattie WS, Mazer CD, et al. Metoprolol reduces cerebral tissue oxygen tension after acute hemodilution in rats. Anesthesiology. 2009;111:988–1000. doi: 10.1097/ALN.0b013e3181b87f0e. [DOI] [PubMed] [Google Scholar]
- 37.Ashes C, Judelman S, Wijeysundera DN, et al. Selective β1-antagonism with bisoprolol is associated with fewer postoperative strokes than atenolol or metoprolol: a single-center cohort study of 44,092 consecutive patients. Anesthesiology. 2013;119:777–787. doi: 10.1097/ALN.0b013e3182a17f12. [DOI] [PubMed] [Google Scholar]
- 38.Vlisides P, Mashour GA. Perioperative stroke. Can J Anaesth. 2016;63:193–204. doi: 10.1007/s12630-015-0494-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Homi HM, Yang H, Pearlstein RD, Grocott HP. Hemodilution during cardiopulmonary bypass increases cerebral infarct volume after middle cerebral artery occlusion in rats. Anesth Analg. 2004;99:974–981. doi: 10.1213/01.ANE.0000131504.90754.D0. [DOI] [PubMed] [Google Scholar]
- 40.Hare GM, Tsui AK, Ozawa S, Shander A. Anaemia: can we define haemoglobin thresholds for impaired oxygen homeostasis and suggest new strategies for treatment? Best Pract Res Clin Anaesthesiol. 2013;27:85–98. doi: 10.1016/j.bpa.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 41.Tsui AKY, Marsden PA, David Mazer C, et al. Differential HIF and NOS responses to acute anemia: defining organ-specific hemoglobin thresholds for tissue hypoxia. Am J Physiol Regul Integr Comp Physiol. 2014;307:R13–R25. doi: 10.1152/ajpregu.00411.2013. [DOI] [PubMed] [Google Scholar]
- 42.Halle M. Concise Observations on Anæmia. Edinb Med Surg J. 1807;3:170–180. [PMC free article] [PubMed] [Google Scholar]
- 43.Murray JF, Rapaport E. Coronary blood flow and myocardial metabolism in acute experimental anaemia. Cardiovasc Res. 1972;6:360–367. doi: 10.1093/cvr/6.4.360. [DOI] [PubMed] [Google Scholar]
- 44.van Bommel J, Trouwborst A, Schwarte L, Siegemund M, Ince C. Henny Ch P. Intestinal and cerebral oxygenation during severe isovolemic hemodilution and subsequent hyperoxic ventilation in a pig model. Anesthesiology. 2002;97:660–670. doi: 10.1097/00000542-200209000-00021. [DOI] [PubMed] [Google Scholar]
- 45.Cain SM, Chapler CK. Circulatory adjustments to anemic hypoxia. Adv Exp Med Biol. 1988;227:103–115. doi: 10.1007/978-1-4684-5481-9_9. [DOI] [PubMed] [Google Scholar]
- 46.Chapler CK, Cain SM. The physiologic reserve in oxygen carrying capacity: studies in experimental hemodilution. Can J Physiol Pharmacol. 1986;64:7–12. doi: 10.1139/y86-002. [DOI] [PubMed] [Google Scholar]
- 47.Brannon ES, Merrill AJ, Warren JV. Stead EA. The Cardiac Output in Patients with Chronic Anemia as Measured by the Technique of Right Atrial Catheterization. J Clin Invest. 1945;24:332–336. doi: 10.1172/JCI101610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weiskopf RB, Viele MK, Feiner J, et al. Human cardiovascular and metabolic response to acute, severe isovolemic anemia. JAMA. 1998;279:217–221. doi: 10.1001/jama.279.3.217. [DOI] [PubMed] [Google Scholar]
- 49.Weiskopf RB, Kramer JH, Viele M, et al. Acute severe isovolemic anemia impairs cognitive function and memory in humans. Anesthesiology. 2000;92:1646–1652. doi: 10.1097/00000542-200006000-00023. [DOI] [PubMed] [Google Scholar]
- 50.Weiskopf RB, Toy P, Hopf HW, et al. Acute isovolemic anemia impairs central processing as determined by P300 latency. Clin Neurophysiol. 2005;116:1028–1032. doi: 10.1016/j.clinph.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 51.Hare GMT, Mazer CD, Mak W, et al. Hemodilutional anemia is associated with increased cerebral neuronal nitric oxide synthase gene expression. J Appl Physiol. 2003;94:2058–2067. doi: 10.1152/japplphysiol.00931.2002. [DOI] [PubMed] [Google Scholar]
- 52.McLaren AT, Marsden PA, Mazer CD, et al. Increased expression of HIF-1α, nNOS, and VEGF in the cerebral cortex of anemic rats. Am J Physiol Regul Integr Comp Physiol. 2007;292:403–414. doi: 10.1152/ajpregu.00403.2006. [DOI] [PubMed] [Google Scholar]
- 53.Mistry N, Mazer CD, Sled JG, et al. Red blood cell antibody-induced anemia causes differential degrees of tissue hypoxia in kidney and brain. Am J Physiol Regul Integr Comp Physiol. 2018;314:R611–R622. doi: 10.1152/ajpregu.00182.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsui AKY, Marsden PA, Mazer CD, et al. Priming of hypoxia-inducible factor by neuronal nitric oxide synthase is essential for adaptive responses to severe anemia. Pro Natl Acad Sci USA. 2011;108:17544–17549. doi: 10.1073/pnas.1114026108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Johannes T, Mik EG, Ince C. Dual-wavelength phosphorimetry for determination of cortical and subcortical microvascular oxygenation in rat kidney. J Appl Physiol. 2006;100:1301–1310. doi: 10.1152/japplphysiol.01315.2005. [DOI] [PubMed] [Google Scholar]
- 56.van Bommel J, Siegemund M, Henny Ch.P, Ince C. Heart, kidney, and intestine have different tolerances for anemia. Transl Res. 2008;151:110–117. doi: 10.1016/j.trsl.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 57.Loenarz C, Coleman ML, Boleininger A, et al. The hypoxia-inducible transcription factor pathway regulates oxygen sensing in the simplest animal, Trichoplax adhaerens. EMBO Rep. 2011;12:63–70. doi: 10.1038/embor.2010.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rytkönen KT, Williams TA, Renshaw GM, Primmer CR, Nikinmaa M. Molecular evolution of the metazoan PHD-HIF oxygen-sensing system. Mol Biol Evol. 2011;28:1913–1926. doi: 10.1093/molbev/msr012. [DOI] [PubMed] [Google Scholar]
- 59.Hatcher JD, Chiu LK, Jennings DB. Anemia as a stimulus to aortic and carotid chemoreceptors in the cat. J Appl Physiol Respir Environ Exerc Physiol. 1978;44:696–702. doi: 10.1152/jappl.1978.44.5.696. [DOI] [PubMed] [Google Scholar]
- 60.Chin K, Steinberg BE, Goldenberg NM, Baker AJ, Mazer CD, Hare GMT. Bilateral Nephrectomy Impairs Cerebral Oxygen Delivery After Acute Hemodilution Anemia in Rats. FASEB J. 2022;36(S1) [Google Scholar]
- 61.Tan CC, Eckardt KU, Firth JD, Ratcliffe PJ. Feedback modulation of renal and hepatic erythropoietin mRNA in response to graded anemia and hypoxia. Am J Physiol. 1992;263:F474–F481. doi: 10.1152/ajprenal.1992.263.3.F474. [DOI] [PubMed] [Google Scholar]
- 62.Hare GMT, Mazer CD, Hutchison JS, et al. Severe hemodilutional anemia increases cerebral tissue injury following acute neurotrauma. J Appl Physiol. 2007;103:1021–1029. doi: 10.1152/japplphysiol.01315.2006. [DOI] [PubMed] [Google Scholar]
- 63.Feiner JR, Finlay-Morreale HE, Toy P, et al. High oxygen partial pressure decreases anemia-induced heart rate increase equivalent to transfusion. Anesthesiology. 2011;115:492–498. doi: 10.1097/ALN.0b013e31822a22be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bergamin FS, Almeida JP, Landoni G, et al. Liberal versus restrictive transfusion strategy in critically Ill oncologic patients: the transfusion requirements in critically Ill oncologic patients randomized controlled trial. Crit Care Med. 2017;45:766–773. doi: 10.1097/CCM.0000000000002283. [DOI] [PubMed] [Google Scholar]
- 65.Corwin HL, Gettinger A, Fabian TC, et al. Efficacy and safety of epoetin alfa in critically ill patients. N Engl J Med. 2007;357:965–976. doi: 10.1056/NEJMoa071533. [DOI] [PubMed] [Google Scholar]
- 66.Corwin HL, Gettinger A, Pearl RG, et al. Efficacy of recombinant human erythropoietin in critically ill patients: a randomized controlled trial. JAMA. 2002;288:2827–2835. doi: 10.1001/jama.288.22.2827. [DOI] [PubMed] [Google Scholar]
- 67.Lasocki S, Asfar P, Jaber S, et al. Impact of treating iron deficiency, diagnosed according to hepcidin quantification, on outcomes after a prolonged ICU stay compared to standard care: a multicenter, randomized, single-blinded trial. Crit Care. 2021;25:62. doi: 10.1186/s13054-020-03430-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Richards T, Baikady RR, Clevenger B, et al. Preoperative intravenous iron to treat anaemia before major abdominal surgery (PREVENTT): a randomised, double-blind, controlled trial. Lancet. 2020;396:1353–1361. doi: 10.1016/S0140-6736(20)31539-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Noe KM, Ngo JP, Martin A, et al. Intra-operative and early post-operative prediction of cardiac surgery-associated acute kidney injury: Urinary oxygen tension compared with plasma and urinary biomarkers. Clin Exp Pharmacol Physiol. 2022;49:228–241. doi: 10.1111/1440-1681.13603. [DOI] [PubMed] [Google Scholar]
- 70.Futier E, Garot M, Godet T, et al. Effect of Hydroxyethyl Starch vs Saline for Volume Replacement Therapy on Death or Postoperative Complications Among High-Risk Patients Undergoing Major Abdominal Surgery: The FLASH Randomized Clinical Trial. JAMA. 2020;323:225–236. doi: 10.1001/jama.2019.20833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zarychanski R, Abou-Setta AM, Turgeon AF, et al. Association of Hydroxyethyl Starch Administration With Mortality and Acute Kidney Injury in Critically Ill Patients Requiring Volume Resuscitation: A Systematic Review and Meta-analysis. JAMA. 2013;309:678–688. doi: 10.1001/jama.2013.430. [DOI] [PubMed] [Google Scholar]
- 72.Deschamps A, Hall R, Grocott H, et al. Cerebral Oximetry Monitoring to Maintain Normal Cerebral Oxygen Saturation during High-risk Cardiac Surgery: A Randomized Controlled Feasibility Trial. Anesthesiology. 2016;124:826–836. doi: 10.1097/ALN.0000000000001029. [DOI] [PubMed] [Google Scholar]
- 73.Murkin JM, Adams SJ, Novick RJ, et al. Monitoring brain oxygen saturation during coronary bypass surgery: a randomized, prospective study. Anesth Analg. 2007;104:51–58. doi: 10.1213/01.ane.0000246814.29362.f4. [DOI] [PubMed] [Google Scholar]
- 74.Okonkwo DO, Shutter LA, Moore C, et al. Brain oxygen optimization in severe traumatic brain injury phase-II: A Phase II Randomized Trial. Crit Care Med. 2017;45:1907–1914. doi: 10.1097/CCM.0000000000002619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Adams RJ, McKie VC, Hsu L, et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med. 1998;339:5–11. doi: 10.1056/NEJM199807023390102. [DOI] [PubMed] [Google Scholar]
- 76.Dodd JM, Andersen C, Dickinson JE, et al. Fetal middle cerebral artery Doppler to time intrauterine transfusion in red-cell alloimmunization: a randomized trial. Ultrasound Obstet Gynecol. 2018;51:306–312. doi: 10.1002/uog.18807. [DOI] [PubMed] [Google Scholar]
- 77.Baron Shahaf D, Hare GMT, Shahaf G. The effects of anesthetics on the cortex-lessons from event-related potentials. Front Syst Neurosci. 2020;14(2) doi: 10.3389/fnsys.2020.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zavriyev AI, Kaya K, Farzam P, et al. The role of diffuse correlation spectroscopy and frequency-domain near-infrared spectroscopy in monitoring cerebral hemodynamics during hypothermic circulatory arrests. JTCVS Tech. 2021;7:161–177. doi: 10.1016/j.xjtc.2021.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Seppelt PC, Mas-Peiro S, De Rosa R, et al. Dynamics of cerebral oxygenation during rapid ventricular pacing and its impact on outcome in transfemoral transcatheter aortic valve implantation. Catheter Cardiovasc Interv. 2021;97:E146–e153. doi: 10.1002/ccd.28975. [DOI] [PubMed] [Google Scholar]
- 80.Leon RL, Ortigoza EB, Ali N, Angelis D, Wolovits JS, Chalak LF. Cerebral blood flow monitoring in high-risk fetal and neonatal populations. Front Pediatr. 2021;9 doi: 10.3389/fped.2021.748345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Koury ST, Koury MJ, Bondurant MC, Caro J, Graber SE. Quantitation of erythropoietin-producing cells in kidneys of mice by in situ hybridization: correlation with hematocrit, renal erythropoietin mRNA, and serum erythropoietin concentration. Blood. 1989;74:645–651. [PubMed] [Google Scholar]
- 82.Weidemann A, Kerdiles YM, Knaup KX, et al. The glial cell response is an essential component of hypoxia-induced erythropoiesis in mice. J Clin Invest. 2009;119:3373–3383. doi: 10.1172/JCI39378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hernández CC, Burgos CF, Gajardo AH, et al. Neuroprotective effects of erythropoietin on neurodegenerative and ischemic brain diseases: the role of erythropoietin receptor. Neural Regen Res. 2017;12:1381–1389. doi: 10.4103/1673-5374.215240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rogiers P, Zhang H, Leeman M, et al. Erythropoietin response is blunted in critically ill patients. Intensive Care Med. 1997;23:159–162. doi: 10.1007/s001340050310. [DOI] [PubMed] [Google Scholar]
- 85.Mesgarpour B, Heidinger BH, Schwameis M, et al. Safety of off-label erythropoiesis stimulating agents in critically ill patients: a meta-analysis. Intensive Care Med. 2013;39:1896–1908. doi: 10.1007/s00134-013-3030-9. [DOI] [PubMed] [Google Scholar]
- 86.Kei T, Mistry N, Curley G, et al. Efficacy and safety of erythropoietin and iron therapy to reduce red blood cell transfusion in surgical patients: a systematic review and meta-analysis. Can J Anaesth. 2019;66:716–731. doi: 10.1007/s12630-019-01351-6. [DOI] [PubMed] [Google Scholar]
- 87.Cho BC, Serini J, Zorrilla-Vaca A, et al. Impact of preoperative erythropoietin on allogeneic blood transfusions in surgical patients: results from a systematic review and meta-analysis. Anesth Analg. 2019;128:981–992. doi: 10.1213/ANE.0000000000004005. [DOI] [PubMed] [Google Scholar]
- 88.Ehrenreich H, Weissenborn K, Begemann M, Busch M, Vieta E, Miskowiak KW. Erythropoietin as candidate for supportive treatment of severe COVID-19. Mol Med. 2020;26:58. doi: 10.1186/s10020-020-00186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Carson JL, Stanworth SJ, Dennis JA, et al. Transfusion thresholds for guiding red blood cell transfusion. Cochrane Database Syst Rev. 2021;12 doi: 10.1002/14651858.CD002042.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shehata N, Mistry N, da Costa BR, et al. Restrictive compared with liberal red cell transfusion strategies in cardiac surgery: a meta-analysis. Eur Heart J. 2019;40:1081–1088. doi: 10.1093/eurheartj/ehy435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Carson JL, Terrin ML, Noveck H, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365:2453–2462. doi: 10.1056/NEJMoa1012452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Carson JL, Brooks MM, Abbott JD, et al. Liberal versus restrictive transfusion thresholds for patients with symptomatic coronary artery disease. Am Heart J. 2013;165:964–971. doi: 10.1016/j.ahj.2013.03.001. e961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gonzalez-Juanatey JR, Lemesle G, Puymirat E, et al. One-year major cardiovascular events after restrictive versus liberal blood transfusion strategy in patients with acute myocardial infarction and anemia: The REALITY randomized trial. Circulation. 2022;145:486–488. doi: 10.1161/CIRCULATIONAHA.121.057909. [DOI] [PubMed] [Google Scholar]
- 94.Hare GMT, Cazorla-Bak MP, Ku SFM, et al. When to transfuse your acute care patient? A narrative review of the risk of anemia and red blood cell transfusion based on clinical trial outcomes. Can J Anaesth. 2020;67:1576–1594. doi: 10.1007/s12630-020-01763-9. [DOI] [PubMed] [Google Scholar]
- 95.Mazer CD, Whitlock RP, Fergusson DA, et al. Six-month outcomes after restrictive or liberal transfusion for cardiac surgery. N Engl J Med. 2018;379:1224–1233. doi: 10.1056/NEJMoa1808561. [DOI] [PubMed] [Google Scholar]