Abstract
Tidal volumes have tremendously decreased over the last decades from <15 ml kg−1 to ∼6 ml kg−1 actual body weight. Guidelines, widely agreed and used, exist for patients with acute lung injury or acute respiratory distress syndrome (ARDS). However, it is questionable if data created in patients with acute lung injury or ARDS from ventilation on intensive care units can be transferred to healthy patients undergoing surgery. Consensus criteria regarding this topic are still missing because only a few randomised controlled trials have been performed to date, focussing on the use of the best intra-operative tidal volume. The same problem has been observed regarding the application of positive end-expiratory pressure (PEEP) and intra-operative lung recruitment.
This article provides an overview of the current literature addressing the size of tidal volume, the use of PEEP and the application of the open-lung concept in patients without acute lung injury or ARDS. Pathophysiological aspects of mechanical ventilation are elucidated.
Keywords: tidal volume, ventilation strategy, open lung concept, positive end-expiratory pressure, one-lung ventilation
Tidal volume in patients with acute lung injury/acute respiratory distress syndrome
Early interest in low-tidal volume (VT) ventilation in acute lung injury (ALI)/acute respiratory distress syndrome (ARDS) was prompted by animal studies, showing that ventilation with higher V T as well as high peak pressure results in pulmonary changes which mimic ALI. Ventilation-induced ALI is defined as injury with diffuse alveolar damage with pulmonary oedema, recruitment of inflammatory cells and production of pro-inflammatory mediators (Fig. 1, Fig. 2 ). Results from randomised controlled trials, evaluating the possible benefit of low-V T ventilation in patients with ARDS compared with traditional V T ventilation, were divergent, showing a positive or no effect on mortality.1, 2, 3, 4 From 1996 to 1999, the National Heart, Lung, and Blood Institute ARDS Network enrolled 861 patients at 10 institutions in a randomised controlled trial, known as the Respiratory Management in Acute Lung Injury/ARDS (ARMA) trial.5 Low versus high V T and plateau pressure of <30 cmH2O were compared with liberal ventilation strategies. Hospital mortality rate was significantly reduced in the low-V T group compare to the control group (31% vs. 39.8%, p = 0.007). Additionally, these patients had a greater number of ventilator-free days (12 ± 11 vs. 10 ± 11 days, p = 0.007) as well as a increased number of days free of non-pulmonary organ failure (15 ± 11 vs. 12 ± 11 days, p = 0.006).
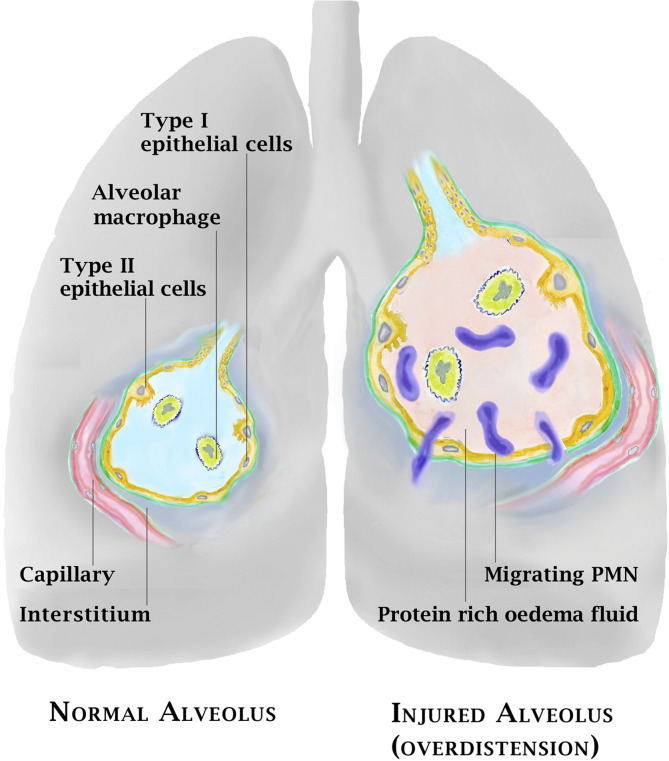
Fig. 1.
Left side: Normal alveolus. Right side: Alveolar overdistention induces endothelial and epithelial cell injury with alveolar formation of oedema.
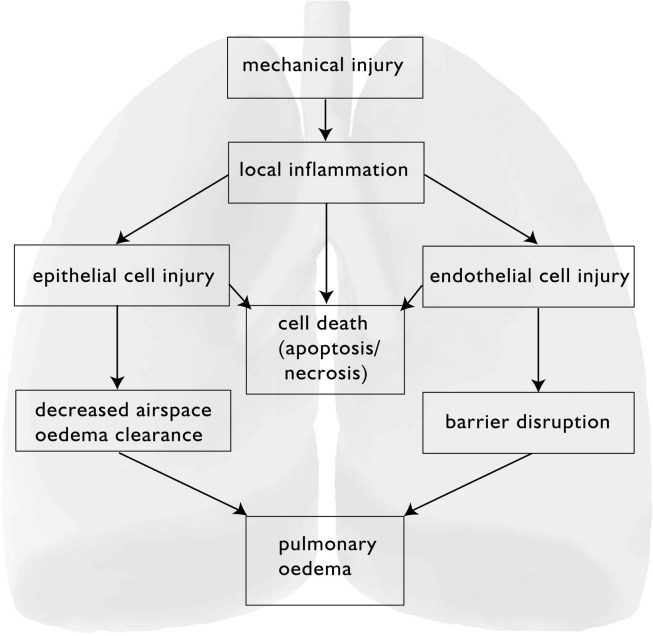
Fig. 2.
Mechanism of ventilation-induced lung injury.
Tidal volumes in healthy lungs
With the knowledge of the benefit of low-V T ventilation for patients with ALI/ARDS, the crucial question arose whether this ventilation strategy might also be applied to healthy patients. There are several reasons why this lung-protective procedure should also be considered in healthy patients, even in the absence of clinical trial results. A first argument is the fact that patients under mechanical ventilation may develop lung injury during surgery. An example is surgery for empyaema. These patients might suddenly experience a septic state with pulmonary involvement and would, therefore – in the presence of ALI – benefit from a protective ventilation strategy. Secondly, patients with lung injury might not meet the criteria for ALI anymore, but would still benefit from a ventilation with lower V T. A third group is represented by very ill patients, who are always at increased risk to develop ALI, such as transfusion-related lung injury, ventilator-associated pneumonia and silent aspiration. Although it seems likely that these patients would benefit from a lung-protective procedure, there is currently no evidence available to support these assumptions.
Retrospective and observational studies
Numerous reports can be found about the topic of protective ventilation procedures for the lung, although the number is not as high as compared with studies focussing on patients suffering from ALI/ARDS. While most of these studies have been performed as prospective randomised controlled trials, several retrospective or observational studies on V T exist.6, 7, 8, 9, 10, 11 Van der Werff retrospectively studied 197 patients and determined the incidence of post-pneumonectomy pulmonary oedema.6 The incidence of pre-manifest post-pneumonectomy pulmonary oedema was 12.2% as opposed to an incidence of manifest oedema of 2.5%. Higher mechanical ventilation pressures during surgery were considered as a significant risk factor (relative risk: 4.3; 95% confidence interval (CI): 1.3–14.4, corrected for age and gender). Licker et al. performed a database study of patients operated for lung cancer between 1990 and 1997 in order to identify predictive risk factors of operative death.7 A trend towards improved outcome was observed during the second period of the observation time, from 1994 to 1997, which could reflect a better understanding of the pathophysiological changes associated with thoracotomy under an improved ventilation strategy. Esteban et al. were the first to analyse a large number of patients, (n = 15 757), who had been prospectively enrolled in a multi-centric international study on mechanical ventilation in intensive care units (ICUs).8 The analysis aimed at the identification of factors influencing survival. The study showed that plateau pressures of >35 cmH2O were associated with increased mortality. Similarly, Gajic and his colleagues retrospectively analysed ventilated patients.10 In this group of 3261 patients, they reported an odds ratio (OD) of 2.6 for V T of >700 ml (p < 0.001) amongst other factors such as high peak-airway pressure. Additionally, Gajic et al. reported on the development of ALI/ARDS upon mechanical ventilation in patients with healthy lungs.9 Within 5 days of mechanical ventilation, 24% of the patients developed ALI/ARDS. The main risk factors associated with the development of lung injury, as assessed by a multivariate analysis, were the use of large V T, transfusion of blood products, acidosis and a history of restrictive lung disease. Interestingly, female patients were ventilated with larger V T (per predicted body weight) and tended to develop lung injury more frequently. Fernandez et al. collected intra-operative data regarding V T in 170 patients undergoing pneumonectomy.11 Of these, 18% experienced postoperative respiratory failure, and half of these patients even met criteria for ALI/ARDS. Risk factors for postoperative respiratory failure were intra-operative large V T in addition to increased application of intra-operative fluids the affected patients had been exposed to. All these retrospective and/or observational studies provide interesting insight with the caveat that they were not prospectively randomised trials.
Randomised controlled studies
Several studies were prospectively performed, testing the hypothesis that mechanical ventilation with large V T and/or high inspiratory pressure could induce injury in healthy lungs and, as a consequence could be deleterious. Most of these studies were performed during surgery with or without a short postoperative ventilation period in the ICU. Therefore, the observational time was, in general, rather short. Additionally, levels of plasma or pulmonary inflammatory mediators were endpoints of these trials, and no outcome markers were assessed. Lee et al. postoperatively included 103 patients in ICUs and randomised them to a ventilation regime with a V T of either 6 or 12 ml kg−1 of actual body weight.12 They found that the incidence of pulmonary infection tended to be lower with a smaller V T, while the duration of intubation seemed to be shorter. Results of these trials were very promising. Wrigge et al. conducted a randomised controlled trial in 39 patients during elective surgery with all patients having anaesthesiologists physical status I and II13 and measured plasma levels of various cytokines as indicators of inflammatory processes. V T of 6 ml kg−1 with 10 cmH2O positive end-expiratory pressure (PEEP) and zero PEEP (ZEEP) were tested versus 15 ml kg−1 with ZEEP. There was no significant difference in plasma levels of tumour necrosis factor alpha (TNF-α), interleukin (IL)-1 receptor antagonist, IL-6 and IL-10 after 1 h of mechanical ventilation. Similar results were found in 44 patients undergoing elective coronary artery-bypass grafting surgery with three ventilatory strategies: 6 ml kg−1 with 5 cmH2O PEEP, 10 ml kg−1 V T with 5 cmH2O PEEP and 10 ml kg−1 with ZEEP.14 No difference in plasma levels of TNF-α and IL-6 were detected. Wrigge et al. also performed a study including 64 patients with major thoracic (n = 34) and abdominal (n = 30) surgery.15 V T of 6, 12 and 15 ml kg−1 were chosen with 10 cmH2O for the V T of 6 ml kg−1 and no PEEP for the larger V T. Determination of tracheal aspirate and plasma TNF-α, IL-1, IL-6, IL-8, IL-12 as well as IL-10 after 3 h of ventilation showed, again, no intergroup differences. A first clinical trial with differences in TNF-α concentrations in bronchoalveolar lavage fluid (BALF) was detected in patients undergoing cardiopulmonary bypass surgery with V T of 6 versus 12 ml kg−1 predicted body weight for 6h.16 The concentration of TNF-α was higher in the group of patients with larger V T, while values of IL-6 and IL-8 did not differ between the groups. Zupancich et al. evaluated 40 patients with elective coronary artery-bypass-grafting surgery, comparing V T of 10–12 ml kg−1 with 2–3 cmH2O PEEP versus 8 ml kg−1 with 10 cmH2O PEEP.17 Larger V T were correlated with an increase in BALF and plasma levels of IL-6 and IL-8. A clinical trial with the focus on coagulopathy was performed with 6 ml kg−1 predicted body-weight V T compared with 10 cmH2O PEEP and 12 ml kg−1 with ZEEP.18 Ventilation with lower V T prevented pulmonary coagulopathy as compared with ventilation with larger V T, finding a less impressive increase in soluble thrombomodulin in BALF as well as lower levels of bronchoalveolar activated protein C after 5 h of ventilation. Bronchoalveolar fibrinolytic activity did not differ by either ventilation strategy. A recent study was performed in 40 patients undergoing an elective surgical procedure, randomising for 12 ml kg−1 V T and ZEEP or 6 ml kg−1 and 10 cmH2O PEEP.19 Pulmonary myeloperoxidase release was increased in the patients managed with the higher V T. BALF and plasma levels of TNF-α, IL-α, IL-1β, IL-6, macrophage inflammatory protein-1α and -1β were not affected by the type of mechanical ventilation.
Rationale of using low tidal volume in healthy lungs
Many of the above-mentioned clinical trials did not observe a statistical difference in the primary end points, such as inflammatory mediators in BALF or plasma, compared with controls. It is important to note, regarding the interpretation of the clinical trials, that the studies were not powered to draw clinically relevant conclusions on outcome measurements. They were performed with a relative small number of patients in different fields of surgery. The value of inflammatory mediators as surrogate markers of a clinical outcome is unproven. Additionally, a variety of cofactors, such as positioning or the extent of surgical trauma, were not assessed, which beside the V T might be crucial for the development of pulmonary injury. In the past, ventilation strategies were recommended from various experts on this topic.*20, *21, *22, *23 Based on the paucity of clinical trials, which have been performed up to date, the following recommendations can be made:
Practice points.
-
-
For a patient with a healthy lung, a tidal volume <10 ml kg−1 of predicted body weight should be used, while in a patient with an injured lung a tidal volume <6 ml kg−1 is preferable.
-
-
A tidal volume <10 ml kg−1 of predicted body weight can be applied to patients without risk factors for the development of perioperative ALI/ARDS.
PEEP and alveolar recruitment in patients with ALI/ARDS
PEEP is an essential component of mechanical ventilation. Several randomised trials have evaluated the efficacy of high levels of PEEP in the treatment of ARDS. Amato et al. compared a significantly higher PEEP in their intervention group compared with the control group (n = 53; 13.2 ± 0.4 vs. 9.3 ± 0.5 cmH2O; p < 0.01).1 In addition, Villar and colleagues investigated, in a similar trial (n = 103), higher PEEP values (14.1 ± 2.8 vs. 9 ± 2.7 cmH2O; p < 0.001).24 Both studies showed a significantly lower ICU mortality rate in the patients with higher PEEP. Contrary to these data, Ranieri et al., with a similar study design (n = 44), were not able to lower mortality rate with a high-PEEP ventilation strategy.25 The end points of this trial, however, aimed at levels of inflammatory mediators rather than at assessment of mortality. In order to determine a specific benefit of high-PEEP ventilation in ALI/ARDS patients, a large randomised controlled trial (Assessment of Low tidal Volume and Elevated End-Expiratory Pressure to Obviate Lung Injury (ALVEOLO)) was designed by Brower et al. (n = 549).26 Patients were randomised to ventilation with high or low PEEP (14.7 ± 3.5 vs. 8.9 ± 3.5 cmH2O). Oxygenation was clearly increased in the intervention group with higher PEEP. However, ICU-mortality rate was similar in the two groups.
Gattinoni et al. showed that patients with early ARDS have multiple areas of atelectasis, most commonly in the dependent lung regions.27 This consequently leads to a reduced volume of the aerated lung. The importance of early lung recruitment and stabilisation is a crucial aspect of ventilatory physiology. The ‘open lung’ concept was first described by Lachmann in the injured lung with impaired surfactant system, requiring higher airway pressures to stabilise the alveoli.28 The open lung represents a lung with little or no atelectasis and an optimal gas exchange. In the last few years, this concept has led to the open-lung procedure, in which the lung is opened and kept open to minimise cyclic forces of alveolar opening and closing. The goal of this technique is to minimise cycle alveolar collapse and reopening. Recruitment is performed during 5–15 s by pressure-controlled ventilation with a peak pressure of 40–60 cmH2O and a ratio of duration of inspiration to expiration of 1:1 or 1:2. The peak inspiratory pressure is adjusted to the lowest pressure, which keeps the lung open, ideally 15–30 cmH2O with a PEEP of 10–20 cmH2O to avoid alveolar collapse.
PEEP and alveolar recruitment in healthy lungs
What about patients with a healthy lung? Even if ventilation is performed in a healthy lung over a short time, this might represent a crucial factor for a potential development of ALI/ARDS. The open-lung concept clearly aims at the injured lung, which is characterised by an impaired surfactant system with increased surface tension requiring higher airway pressures to stabilise the alveoli. However, atelectasis is also observed in healthy lungs during mechanical ventilation. Mead et al. demonstrated that, already in 1970, in atelectatic areas, shear forces act on the fragile alveolar membrane due to the pulmonary interdependence of the alveoli.29 This leads to enormous shear forces between atelectatic and normal lung areas of up to 140 cmH2O with transpulmonary pressures of 30 cmH2O. These shear forces may be an important reason for epithelial disruption and the loss of the alveolar epithelium’s barrier function. Epithelial disruption leads to high-permeability oedema with dilution of the surfactant and/or an inactivation of the surfactant by plasma components.30 This surfactant impairment causes an aggravation of atelectasis. An important aspect, herein, is the fact that formation of atelectasis already starts at induction of anaesthesia and, consequently, countermeasures should start early.*31, 32
To avoid atelectasis, the strategy of ‘open lung’ might be useful even in non-injured lungs. No studies investigating open-lung protective ventilation strategies regarding outcome have yet been performed during surgery. However, it was recently suggested that the open-lung strategy may decrease pulmonary inflammatory processes, triggered by cardiopulmonary bypass.33 Reis Miranda and colleagues performed a prospective single-centre randomised controlled study with 62 patients undergoing elective coronary artery-bypass graft and/or valve surgery with cardiopulmonary bypass. Patients were randomly assigned to three groups with conventional mechanical ventilation, late or early open-lung ventilation. Cardiopulmonary bypass caused a significant increase of IL-6, -8 and -10 in all groups. IL-8 decreased significantly more rapidly in both open-lung concept groups, IL-10 only in the early open-lung group; TNF-α, interferon gamma (IFN-γ) and IL-6, however, did not differ significantly between groups. Another focus in a study from Reis Miranda’s group was the functional residual capacity after extubation.34 The study showed that early application of the open-lung concept resulted in a significantly higher functional residual capacity and fewer episodes of hypoxaemia than with conventional mechanical ventilation.
What are the potential contraindications for an open-lung strategy? Recruitment manoeuvres with high inspiratory pressures have the potential risk for barotraumas. A recent study showed a correlation of high inspiratory pressures and elevated PEEP levels with an increased rate of pneumothorax.35, 36 However, both retrospective studies were performed in patients suffering from ARDS. Weg et al. found no significant correlation between high ventilatory pressures and the development of pneumothorax in a large prospective study with 725 ARDS patients.37 Another concern of the open-lung management might be the impairment of the circulatory system. Increased intra-thoracic pressures are associated with a decrease in cardiac output.38 Dyhr et al., however, reported that the recruitment manoeuvre with two 20-s inflations to 45 cmH2O in combination with PEEP of 14 ± 3 cmH2O can be performed safely in ventilated patients, even after coronary artery-bypass surgery.39 Cardiac index did not decrease after alveolar recruitment and application of PEEP. Similar results were found in other studies. Reis Miranda et al. investigated the effect of the open-lung concept with recruitment manoeuvres, followed by low-V T ventilation with elevated PEEP, on right ventricular outflow impedance during inspiration and expiration in 28 patients after cardiac surgery using trans-oesophageal echocardiography.40 Right ventricular outflow impedance during expiration was not changed by open-lung strategy; during inspiration, right ventricular outflow impedance was decreased.
Rationale of using PEEP in healthy lungs
Prevention or attenuation of atelectasis formation should be performed by the following practice points:
Practice points.
-
-
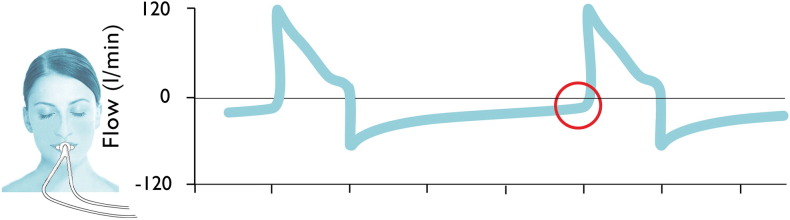
PEEP should be used in selected patients (e.g., patients undergoing general anaesthesia in lateral position, video laparoscopic surgery) at level not below 5 cmH2O. Caution must be taken in chronic obstructive pulmonary disease (COPD) patients as well as in patients whose expiratory flow does not reach baseline zero (Fig. 3).
-
-
In selected patients, such as severely obese patients, PEEP should be used already at induction of anaesthesia.
-
-
‘Best’ PEEP should be evaluated during anaesthesia according to clinical parameters, such as plateau pressure, alveolar dead space or end tidal CO2, arterial oxygenation and haemodynamics.
Rationale of using open-lung manoeuvre in healthy lungs
The open-lung concept of mechanical ventilation with recruitment and stabilisation of the lung is, according to the literature, a useful ventilatory strategy in patients with ALI/ARDS. For patients with ventilatory support only during surgery, a paucity of clinical trials exists, although the literature is growing. However, the number of elective surgery in patients with – in part – significant pulmonary co-morbidity is constantly increasing. Therefore, to avoid further damage (i.e., second hit), it seems plausible to apply open-lung techniques even during elective surgery. This, on the other hand, has to be adapted individually as a variety of surgical factors (prone position, Trendelenburg position, pneumo-peritoneum, etc.) additionally influence ventilation physiology. Mechanical ventilation during video laparoscopic surgery and in morbidly obese patients is widely treated in another article.
Practice points.
-
-
It is suggested that patients with impaired arterial oxygenation or affected by high chest-wall impedance (e.g., morbidly obese patients or patients with high abdominal pressure) might benefit from ventilation according to the open-lung concept (recruitment manoeuvre over 5–10 s under pressure-controlled ventilation with a peak pressure of ∼40 cmH2O, inspiration-to-expiration ratio 1:1, PEEP>5 cmH2O, followed by adjustment of set pressures.
-
-
Gentle recruitment technique is required.
Fig. 3.
Flow diagram of a ventilated patient with suspected compromised exspiration (flow at the end of the expiration does not return to zero baseline).
One-lung ventilation
Patients with ventilation for lung resection have an increased risk to develop ALI. ALI after lung resection is relatively infrequent, occurring in 2.5% of all lung resections combined, with a peak incidence of 7.9% after pneumonectomy. However, it is associated with a high morbidity and mortality rate of ∼40%.41 Causative factors of lung injury besides inflammatory processes induced by the surgical procedure are ventilatory trauma, transfusion, hypoxia–re-oxgenation upon one-lung ventilation (OLV), etc.42, *43 A prospective trial underlined the hypothesis that alveolar hypoxia, followed by re-oxygenation and re-expansion, respectively, triggers the release of inflammatory mediators.44 In a retrospective analysis of risk factors for ALI after lung resections, increased duration of OLV represents a crucial risk factor for the development of ALI.43
Ventilatory strategy
As a consequence, the ventilatory management for patients undergoing thoracic surgery is very demanding. A special challenge, thereby, is the procedure of OLV regarding ventilatory considerations as well as the device, which should be used for lung isolation.
VT
For many years, hypoxaemia was considered as the most important problem during OLV. Therefore, guidelines were based on the results of clinical trials focussing on an improved oxygenation, independent of the V T. Katz et al. showed, in the early 1980s, that large V T produced the highest partial pressure of oxygen (pO2) values during OLV.45 As a consequence, the common recommendation were the use of a V T of 8–12 ml kg−1 for two-, as well one-, lung-ventilation procedures. With increasing amount of study results, it became clear that a protective ventilatory strategy, as mentioned earlier, is also crucial in situations of OLV to prevent ALI. One of the most important and most widely cited animal studies came from Gama de Abreu, who investigated, in detail, V T reduction for OLV in isolated rabbit lungs.46 This group showed that OLV with high VT and ZEEP is injurious in the isolated rabbit lung model. Wrigge and colleagues investigated cytokine levels in patients undergoing OLV.15 However, with high- or low-V T ventilatory strategies, no difference in pulmonary or systemic levels of measured inflammatory markers was found. Schilling et al. assessed the increased production of pulmonary inflammatory mediators during OLV upon ventilation of the depended lung with V T of 10 ml kg−1 compared to 5 ml kg−1.47 A study with 52 randomised patients undergoing oesophagectomy, from Michelet et al., with OLV and 9 ml kg−1 ZEEP versus 5 ml kg−1 PEEP of 5 cmH2O revealed evidence for a lower cytokine levels in the protective ventilation group.48 Again, these studies did not focus on the outcome of the patients.
The fraction of inspired oxygen (FiO2)
Hypoxaemia is a constant threat during OLV. Traditionally, 100% oxygen was used to prevent hypoxaemia during OLV, to improve peripheral oxygenation, increase blood flow to the ventilated, non-deflated lung, decrease nausea as well as improve wound healing.49, 50, 51 Despite the advantages the application of 100% oxygen offers, it is correlated with certain risks for the patient. High FiO2 can cause absorption atelectasis and, therefore, might indirectly trigger ALI. Additionally, hyperoxia may cause airway hyper-responsiveness – although only demonstrated in an animal model – and may promote the formation of radical oxygen species, which could jeopardise the lungs, inducing inflammatory responses.52, 53 Diseased lungs may be more susceptible to injury than healthy lungs.54
PEEP
Application of PEEP for OLV faces some additional aspects as for two-lung ventilation. The patient is in a lateral decubitus/decubital position with the dependent lung being compressed by additional gravitation, being implied with a potential for elevated airway pressure and atelectasis. Fujiwara et al. performed a study, examining patients undergoing OLV with different PEEP values.55 The dependent lung was ventilated for 20 min with 10 ml kg−1 VT with ZEEP in a stepwise fashion, followed by ventilation with 4 cmH2O PEEP. Application of PEEP significantly increased oxygenation and decreased the shunt fraction. Similarly, Senturk et al. demonstrated that PEEP improved oxygenation during OLV.56 Response to PEEP during OLV seems to be individual and, therefore, has to be evaluated during ventilation.57 Slinger et al., titrating external PEEP according to the lung–chest compliance of each patient, found that with the application of 5 cmH2O PEEP oxygenation improved in 14%; however, it had no effect in 65% and even decreased oxygenation in 21% of the patients. An important aspect seems to be the interaction between V T and PEEP to minimise alveolar damage.58 All these studies are very interesting, but with the exception of Michelet et al., they did not define ALI as an end point.
Alveolar recruitment
Lung recruitment during OLV seems to be efficient as shown in the study of Cinnella et al.59 In this study, lung-recruiting manoeuvres improved oxygenation, although transient haemodynamic derangement occurred during the manoeuvre. Tusman et al. studied the effects of alveolar recruitment manoeuvres in 12 patients undergoing thoracic surgery in the dependent lung during OLV.60 Patients undergoing this strategy showed improved oxygenation as well as increased ventilation efficiency. However, re-expansion of the lung may be harmful, as evaluated in an animal model.61
Ventilation
Normally, a volume-controlled ventilation strategy (volume-controlled ventilation (VCV)) is used in the operating room during surgery. For OLV, the question arises whether a pressure-controlled management (pressure-controlled ventilation (PCV)) would be better, offering a more homogeneous distribution of ventilation and improving ventilation–perfusion mismatch. This research question was evaluated in 48 patients undergoing thoracotomy.62 In the first group of patients (n = 24), OLV was initiated with VCV, followed by a switch to the other mode of PCV. Ventilation modes were performed in the opposite order in the second group (n = 24). Peak airway and plateau pressure were significantly higher in the VCV, oxygen tension smaller and intra-pulmonary shunt more pronounced. Other studies have confirmed these findings of improved oxygenation, reporting significant lower peak- and plateau-pressure values when using PCV.56 Despite the application of 4 cmH2O PEEP in the study of Senturk et al., airway pressures in the PCV–PEEP mode were still lower compared with VCV with ZEEP. In a more recent study, however, no significant difference between VCV and PCV was found.63 Fifty-eight patients undergoing thoracic surgery were ventilated as follows: in the VCP group, a V T of 9 ml kg−1 with ZEEP was chosen to reach the same inspiratory airway pressure as in the PCV group. No significant difference in oxygenation was observed between both ventilation strategies, while peak airway pressures were higher during OLV with VCV. Based on the literature, no clear arguments exist for a PCV.
The following recommendations are based on several reviews.64, 65, 66
Practice points.
-
-
A reduction of the tidal volume to a maximum of 6 ml kg−1 predicted body weight and a limitation of the plateau airway pressure to <20 cmH2O is recommended.
-
-
Inspiratory oxygen concentration should be minimised as much as possible.
-
-
The use of 5–10 cmH2O PEEP should prevent atelectasis.
-
-
Although the issue between volume- and pressure-controlled ventilation is not yet clearly defined, PCV should be preferably used.
-
-
Recruitment manoeuvre is recommended with caution according to the above-mentioned settings by taking into account all the possible negative effects of heart–lung interactions.
-
-
Gentle recruitment technique is required.
Devices for lung isolation
Double-lumen endotracheal tubes (DLTs) and bronchial blockers seem to be clinically equivalent regarding performance in providing lung collapse for patients with normal airways.67 Each device offers its advantages depending on the patient and the type of surgery. Intubation manoeuvre with DLT is more demanding because of the larger size and the special shape of the tube. In patients requiring a rapid sequence induction, a DLT is more difficult to insert than a single-lumen tube, followed by induction of a bronchus blocker.68 The use of a wire-guided endobronchial blocker via the nasal route also offers safe way for patients with restricted mouth opening for oesophagectomy.69 Comparing effectiveness of lung isolation, lung collapse was achieved within 17 min with a DLT, compared with 19–26 min for the bronchial blocker.70
Recommendations
Based on the recommendations of an article by Campos, absolute lung separation can be better achieved with a DLT, while the use of a bronchus blocker is recommended in situations with difficult airways.67 Both devices are tools for only well-trained thoracic anaesthesiologists.
Research agenda.
Many clinical trials have been performed in patients with ALI/ARDS, elucidating application of low and high tidal volume and/or PEEP, and the use of the open-lung concept. Only a paucity of studies is available in patients with healthy lungs. As a consequence, an immense need emerges to design prospective randomised clinical trials.
-
-
Clinical trials are warranted regarding surrogate endpoints, such as morbidity and mortality, for ventilatory strategies.
-
-
Differential aspects of ventilatory procedures and their impact on outcome remain to be determined.
References
- 1.Amato M.B., Barbas C.S., Medeiros D.M., et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. The New England Journal of Medicine. 1998;338:347–354. doi: 10.1056/NEJM199802053380602. [DOI] [PubMed] [Google Scholar]
- 2.Stewart T.E., Meade M.O., Cook D.J., et al. Evaluation of a ventilation strategy to prevent barotrauma in patients at high risk for acute respiratory distress syndrome. Pressure- and Volume-Limited Ventilation Strategy Group. The New England Journal of Medicine. 1998;338:355–361. doi: 10.1056/NEJM199802053380603. [DOI] [PubMed] [Google Scholar]
- 3.Brochard L., Roudot-Thoraval F., Roupie E., et al. Tidal volume reduction for prevention of ventilator-induced lung injury in acute respiratory distress syndrome. The Multicenter Trail Group on Tidal Volume reduction in ARDS. American Journal of Respiratory and Critical Care Medicine. 1998;158:1831–1838. doi: 10.1164/ajrccm.158.6.9801044. [DOI] [PubMed] [Google Scholar]
- 4.Brower R.G., Shanholtz C.B., Fessler H.E., et al. Prospective, randomized, controlled clinical trial comparing traditional versus reduced tidal volume ventilation in acute respiratory distress syndrome patients. Critical Care Medicine. 1999;27:1492–1498. doi: 10.1097/00003246-199908000-00015. [DOI] [PubMed] [Google Scholar]
- 5.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. The New England Journal of Medicine. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 6.van der Werff Y.D., van der Houwen H.K., Heijmans P.J., et al. Postpneumonectomy pulmonary edema. A retrospective analysis of incidence and possible risk factors. Chest. 1997;111:1278–1284. doi: 10.1378/chest.111.5.1278. [DOI] [PubMed] [Google Scholar]
- 7.Licker M., de Perrot M., Hohn L., et al. Perioperative mortality and major cardio-pulmonary complications after lung surgery for non-small cell carcinoma. European Journal of Cardio-thoracic Surgery. 1999;15:314–319. doi: 10.1016/s1010-7940(99)00006-8. [DOI] [PubMed] [Google Scholar]
- 8.Esteban A., Anzueto A., Frutos F., et al. Characteristics and outcomes in adult patients receiving mechanical ventilation: a 28-day international study. The Journal of the American Medical Association. 2002;287:345–355. doi: 10.1001/jama.287.3.345. [DOI] [PubMed] [Google Scholar]
- 9.Gajic O., Dara S.I., Mendez J.L., et al. Ventilator-associated lung injury in patients without acute lung injury at the onset of mechanical ventilation. Critical Care Medicine. 2004;32:1817–1824. doi: 10.1097/01.ccm.0000133019.52531.30. [DOI] [PubMed] [Google Scholar]
- 10.Gajic O., Frutos-Vivar F., Esteban A., et al. Ventilator settings as a risk factor for acute respiratory distress syndrome in mechanically ventilated patients. Intensive Care Medicine. 2005;31:922–926. doi: 10.1007/s00134-005-2625-1. [DOI] [PubMed] [Google Scholar]
- 11.Fernandez-Perez E.R., Keegan M.T., Brown D.R., et al. Intraoperative tidal volume as a risk factor for respiratory failure after pneumonectomy. Anesthesiology. 2006;105:14–18. doi: 10.1097/00000542-200607000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Lee P.C., Helsmoortel C.M., Cohn S.M., et al. Are low tidal volumes safe? Chest. 1990;97:430–434. doi: 10.1378/chest.97.2.430. [DOI] [PubMed] [Google Scholar]
- 13.Wrigge H., Zinserling J., Stuber F., et al. Effects of mechanical ventilation on release of cytokines into systemic circulation in patients with normal pulmonary function. Anesthesiology. 2000;93:1413–1417. doi: 10.1097/00000542-200012000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Koner O., Celebi S., Balci H., et al. Effects of protective and conventional mechanical ventilation on pulmonary function and systemic cytokine release after cardiopulmonary bypass. Intensive Care Medicine. 2004;30:620–626. doi: 10.1007/s00134-003-2104-5. [DOI] [PubMed] [Google Scholar]
- 15.Wrigge H., Uhlig U., Zinserling J., et al. The effects of different ventilatory settings on pulmonary and systemic inflammatory responses during major surgery. Anesthesia and Analgesia. 2004;98:775–781. doi: 10.1213/01.ane.0000100663.11852.bf. table of contents. [DOI] [PubMed] [Google Scholar]
- 16.Wrigge H., Uhlig U., Baumgarten G., et al. Mechanical ventilation strategies and inflammatory responses to cardiac surgery: a prospective randomized clinical trial. Intensive Care Medicine. 2005;31:1379–1387. doi: 10.1007/s00134-005-2767-1. [DOI] [PubMed] [Google Scholar]
- 17.Zupancich E., Paparella D., Turani F., et al. Mechanical ventilation affects inflammatory mediators in patients undergoing cardiopulmonary bypass for cardiac surgery: a randomized clinical trial. The Journal of Thoracic and Cardiovascular Surgery. 2005;130:378–383. doi: 10.1016/j.jtcvs.2004.11.061. [DOI] [PubMed] [Google Scholar]
- 18.Choi G., Wolthuis E.K., Bresser P., et al. Mechanical ventilation with lower tidal volumes and positive end-expiratory pressure prevents alveolar coagulation in patients without lung injury. Anesthesiology. 2006;105:689–695. doi: 10.1097/00000542-200610000-00013. [DOI] [PubMed] [Google Scholar]
- 19.Wolthuis E.K., Choi G., Dessing M.C., et al. Mechanical ventilation with lower tidal volumes and positive end-expiratory pressure prevents pulmonary inflammation in patients without preexisting lung injury. Anesthesiology. 2008;108:46–54. doi: 10.1097/01.anes.0000296068.80921.10. [DOI] [PubMed] [Google Scholar]
- 20.Putensen C., Wrigge H. Tidal volumes in patients with normal lungs: one for all or the less, the better? Anesthesiology. 2007;106:1085–1087. doi: 10.1097/01.anes.0000265424.07486.8c. [DOI] [PubMed] [Google Scholar]
- 21.Schultz M.J., Haitsma J.J., Slutsky A.S., et al. What tidal volumes should be used in patients without acute lung injury? Anesthesiology. 2007;106:1226–1231. doi: 10.1097/01.anes.0000267607.25011.e8. [DOI] [PubMed] [Google Scholar]
- 22.Licker M., Diaper J., Ellenberger C. Perioperative protective ventilatory strategies in patients without acute lung injuries. Anesthesiology. 2008;108:335–336. doi: 10.1097/01.anes.0000300053.91799.27. author reply 336–7. [DOI] [PubMed] [Google Scholar]
- 23.Schultz M.J. Lung-protective mechanical ventilation with lower tidal volumes in patients not suffering from acute lung injury: a review of clinical studies. Medical Science Monitor. 2008;14:RA22–RA26. [PubMed] [Google Scholar]
- 24.Villar J., Kacmarek R.M., Perez-Mendez L., et al. A high positive end-expiratory pressure, low tidal volume ventilatory strategy improves outcome in persistent acute respiratory distress syndrome: a randomized, controlled trial. Critical Care Medicine. 2006;34:1311–1318. doi: 10.1097/01.CCM.0000215598.84885.01. [DOI] [PubMed] [Google Scholar]
- 25.Ranieri V.M., Suter P.M., Tortorella C., et al. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. The Journal of the American Medical Association. 1999;282:54–61. doi: 10.1001/jama.282.1.54. [DOI] [PubMed] [Google Scholar]
- 26.Brower R.G., Lanken P.N., MacIntyre N., et al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. The New England Journal of Medicine. 2004;351:327–336. doi: 10.1056/NEJMoa032193. [DOI] [PubMed] [Google Scholar]
- 27.Gattinoni L., Presenti A., Torresin A., et al. Adult respiratory distress syndrome profiles by computed tomography. Journal of Thoracic Imaging. 1986;1:25–30. doi: 10.1097/00005382-198607000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Lachmann B. Open up the lung and keep the lung open. Intensive Care Medicine. 1992;18:319–321. doi: 10.1007/BF01694358. [DOI] [PubMed] [Google Scholar]
- 29.Mead J., Takishima T., Leith D. Stress distribution in lungs: a model of pulmonary elasticity. Journal of Applied Physiology. 1970;28:596–608. doi: 10.1152/jappl.1970.28.5.596. [DOI] [PubMed] [Google Scholar]
- 30.Seeger W., Gunther A., Walmrath H.D., et al. Alveolar surfactant and adult respiratory distress syndrome. Pathogenetic role and therapeutic prospects. The Clinical Investigator. 1993;71:177–190. doi: 10.1007/BF00180100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rusca M., Proietti S., Schnyder P., et al. Prevention of atelectasis formation during induction of general anesthesia. Anesthesia and Analgesia. 2003;97:1835–1839. doi: 10.1213/01.ANE.0000087042.02266.F6. [DOI] [PubMed] [Google Scholar]
- 32.von Ungern-Sternberg B.S., Regli A., Schibler A., et al. The impact of positive end-expiratory pressure on functional residual capacity and ventilation homogeneity impairment in anesthetized children exposed to high levels of inspired oxygen. Anesthesia and Analgesia. 2007;104:1364–1368. doi: 10.1213/01.ane.0000261503.29619.9c. table of contents. [DOI] [PubMed] [Google Scholar]
- 33.Reis Miranda D., Gommers D., Struijs A., et al. Ventilation according to the open lung concept attenuates pulmonary inflammatory response in cardiac surgery. European Journal of Cardio-thoracic Surgery. 2005;28:889–895. doi: 10.1016/j.ejcts.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 34.Reis Miranda D., Struijs A., Koetsier P., et al. Open lung ventilation improves functional residual capacity after extubation in cardiac surgery. Critical Care Medicine. 2005;33:2253–2258. doi: 10.1097/01.ccm.0000181674.71237.3b. [DOI] [PubMed] [Google Scholar]
- 35.Boussarsar M., Thierry G., Jaber S., et al. Relationship between ventilatory settings and barotrauma in the acute respiratory distress syndrome. Intensive Care Medicine. 2002;28:406–413. doi: 10.1007/s00134-001-1178-1. [DOI] [PubMed] [Google Scholar]
- 36.Eisner M.D., Thompson B.T., Schoenfeld D., et al. Airway pressures and early barotrauma in patients with acute lung injury and acute respiratory distress syndrome. American Journal of Respiratory and Critical Care Medicine. 2002;165:978–982. doi: 10.1164/ajrccm.165.7.2109059. [DOI] [PubMed] [Google Scholar]
- 37.Weg J.G., Anzueto A., Balk R.A., et al. The relation of pneumothorax and other air leaks to mortality in the acute respiratory distress syndrome. The New England Journal of Medicine. 1998;338:341–346. doi: 10.1056/NEJM199802053380601. [DOI] [PubMed] [Google Scholar]
- 38.Cournand A., Motley H.L., et al. Physiological studies of the effects of intermittent positive pressure breathing on cardiac output in man. The American Journal of Physiology. 1948;152:162–174. doi: 10.1152/ajplegacy.1947.152.1.162. [DOI] [PubMed] [Google Scholar]
- 39.Dyhr T., Laursen N., Larsson A. Effects of lung recruitment maneuver and positive end-expiratory pressure on lung volume, respiratory mechanics and alveolar gas mixing in patients ventilated after cardiac surgery. Acta Anaesthesiologica Scandinavica. 2002;46:717–725. doi: 10.1034/j.1399-6576.2002.460615.x. [DOI] [PubMed] [Google Scholar]
- 40.Reis Miranda D., Klompe L., Mekel J., et al. Open lung ventilation does not increase right ventricular outflow impedance: an echo-Doppler study. Critical Care Medicine. 2006;34:2555–2560. doi: 10.1097/01.CCM.0000239118.05093.EE. [DOI] [PubMed] [Google Scholar]
- 41.Dulu A., Pastores S.M., Park B., et al. Prevalence and mortality of acute lung injury and ARDS after lung resection. Chest. 2006;130:73–78. doi: 10.1378/chest.130.1.73. [DOI] [PubMed] [Google Scholar]
- 42.Jordan S., Mitchell J.A., Quinlan G.J., et al. The pathogenesis of lung injury following pulmonary resection. The European Respiratory Journal. 2000;15:790–799. doi: 10.1034/j.1399-3003.2000.15d26.x. [DOI] [PubMed] [Google Scholar]
- 43.Licker M., de Perrot M., Spiliopoulos A., et al. Risk factors for acute lung injury after thoracic surgery for lung cancer. Anesthesia and Analgesia. 2003;97:1558–1565. doi: 10.1213/01.ANE.0000087799.85495.8A. [DOI] [PubMed] [Google Scholar]
- 44.De Conno E., Steurer M.P., Wittlinger M., et al. Anesthetic-induced improvement of the inflammatory response to one-lung ventilation. Anesthesiology. 2009;110:1316–1326. doi: 10.1097/ALN.0b013e3181a10731. [DOI] [PubMed] [Google Scholar]
- 45.Katz J.A., Laverne R.G., Fairley H.B., et al. Pulmonary oxygen exchange during endobronchial anesthesia: effect of tidal volume and PEEP. Anesthesiology. 1982;56:164–171. doi: 10.1097/00000542-198203000-00002. [DOI] [PubMed] [Google Scholar]
- 46.Gama de Abreu M., Heintz M., Heller A., et al. One-lung ventilation with high tidal volumes and zero positive end-expiratory pressure is injurious in the isolated rabbit lung model. Anesthesia and Analgesia. 2003;96:220–228. doi: 10.1097/00000539-200301000-00045. table of contents. [DOI] [PubMed] [Google Scholar]
- 47.Schilling T., Kozian A., Huth C., et al. The pulmonary immune effects of mechanical ventilation in patients undergoing thoracic surgery. Anesthesia and Analgesia. 2005;101:957–965. doi: 10.1213/01.ane.0000172112.02902.77. table of contents. [DOI] [PubMed] [Google Scholar]
- 48.Michelet P., D’Journo X.B., Roch A., et al. Protective ventilation influences systemic inflammation after esophagectomy: a randomized controlled study. Anesthesiology. 2006;105:911–919. doi: 10.1097/00000542-200611000-00011. [DOI] [PubMed] [Google Scholar]
- 49.Kabon B., Kurz A. Optimal perioperative oxygen administration. Current Opinion in Anaesthesiology. 2006;19:11–18. doi: 10.1097/01.aco.0000192775.24774.15. [DOI] [PubMed] [Google Scholar]
- 50.Belda F.J., Aguilera L., Garcia de la Asuncion J., et al. Supplemental perioperative oxygen and the risk of surgical wound infection: a randomized controlled trial. The Journal of the American Medical Association. 2005;294:2035–2042. doi: 10.1001/jama.294.16.2035. [DOI] [PubMed] [Google Scholar]
- 51.Puckridge P.J., Saleem H.A., Vasudevan T.M., et al. Perioperative high-dose oxygen therapy in vascular surgery. ANZ Journal of Surgery. 2007;77:433–436. doi: 10.1111/j.1445-2197.2007.04089.x. [DOI] [PubMed] [Google Scholar]
- 52.Szarek J.L., Ramsay H.L., Andringa A., et al. Time course of airway hyperresponsiveness and remodeling induced by hyperoxia in rats. The American Journal of Physiology. 1995;269:L227–L233. doi: 10.1152/ajplung.1995.269.2.L227. [DOI] [PubMed] [Google Scholar]
- 53.Carvalho C.R., de Paula Pinto Schettino G., Maranhao B., et al. Hyperoxia and lung disease. Current Opinion in Pulmonary Medicine. 1998;4:300–304. doi: 10.1097/00063198-199809000-00010. [DOI] [PubMed] [Google Scholar]
- 54.Witschi H.R., Haschek W.M., Klein-Szanto A.J., et al. Potentiation of diffuse lung damage by oxygen: determining variables. The American Review of Respiratory Disease. 1981;123:98–103. doi: 10.1164/arrd.1981.123.1.98. [DOI] [PubMed] [Google Scholar]
- 55.Fujiwara M., Abe K., Mashimo T. The effect of positive end-expiratory pressure and continuous positive airway pressure on the oxygenation and shunt fraction during one-lung ventilation with propofol anesthesia. Journal of Clinical Anesthesia. 2001;13:473–477. doi: 10.1016/s0952-8180(01)00310-5. [DOI] [PubMed] [Google Scholar]
- 56.Senturk N.M., Dilek A., Camci E., et al. Effects of positive end-expiratory pressure on ventilatory and oxygenation parameters during pressure-controlled one-lung ventilation. Journal of Cardiothoracic and Vascular Anesthesia. 2005;19:71–75. doi: 10.1053/j.jvca.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 57.Slinger P.D., Kruger M., McRae K., et al. Relation of the static compliance curve and positive end-expiratory pressure to oxygenation during one-lung ventilation. Anesthesiology. 2001;95:1096–1102. doi: 10.1097/00000542-200111000-00012. [DOI] [PubMed] [Google Scholar]
- 58.Michelet P. Protective ventilation during one-lung ventilation. Anesthesiology. 2007;107:176–177. doi: 10.1097/01.anes.0000268569.01129.d6. [DOI] [PubMed] [Google Scholar]
- 59.Cinnella G., Grasso S., Natale C., et al. Physiological effects of a lung-recruiting strategy applied during one-lung ventilation. Acta Anaesthesiologica Scandinavica. 2008;52:766–775. doi: 10.1111/j.1399-6576.2008.01652.x. [DOI] [PubMed] [Google Scholar]
- 60.Tusman G., Bohm S.H., Suarez-Sipmann F., et al. Alveolar recruitment improves ventilatory efficiency of the lungs during anesthesia. Canadian Journal of Anaesthesia. 2004;51:723–727. doi: 10.1007/BF03018433. [DOI] [PubMed] [Google Scholar]
- 61.Sivrikoz M.C., Tuncozgur B., Cekmen M., et al. The role of tissue reperfusion in the reexpansion injury of the lungs. European Journal of Cardio-thoracic Surgery. 2002;22:721–727. doi: 10.1016/s1010-7940(02)00447-5. [DOI] [PubMed] [Google Scholar]
- 62.Tugrul M., Camci E., Karadeniz H., et al. Comparison of volume controlled with pressure controlled ventilation during one-lung anaesthesia. British Journal of Anaesthesia. 1997;79:306–310. doi: 10.1093/bja/79.3.306. [DOI] [PubMed] [Google Scholar]
- 63.Unzueta M.C., Casas J.I., Moral M.V. Pressure-controlled versus volume-controlled ventilation during one-lung ventilation for thoracic surgery. Anesthesia and Analgesia. 2007;104:1029–1033. doi: 10.1213/01.ane.0000260313.63893.2f. tables of contents. [DOI] [PubMed] [Google Scholar]
- 64.Lohser J. Evidence-based management of one-lung ventilation. Anesthesiology Clinics. 2008;26:241–272. doi: 10.1016/j.anclin.2008.01.011. v. [DOI] [PubMed] [Google Scholar]
- 65.Lytle F.T., Brown D.R. Appropriate ventilatory settings for thoracic surgery: intraoperative and postoperative. Seminars in Cardiothoracic and Vascular Anesthesia. 2008;12:97–108. doi: 10.1177/1089253208319869. [DOI] [PubMed] [Google Scholar]
- 66.Grichnik K.P., Shaw A. Update on one-lung ventilation: the use of continuous positive airway pressure ventilation and positive end-expiratory pressure ventilation–clinical application. Current Opinion in Anaesthesiology. 2009;22:23–30. doi: 10.1097/ACO.0b013e32831d7b41. [DOI] [PubMed] [Google Scholar]
- 67.Campos J.H. Which device should be considered the best for lung isolation: double-lumen endotracheal tube versus bronchial blockers. Current Opinion in Anaesthesiology. 2007;20:27–31. doi: 10.1097/ACO.0b013e3280111e2a. [DOI] [PubMed] [Google Scholar]
- 68.Vanner R. Arndt Endobronchial Blocker during oesophagectomy. Anaesthesia. 2005;60:295–296. doi: 10.1111/j.1365-2044.2005.04134.x. [DOI] [PubMed] [Google Scholar]
- 69.Angie Ho C.Y., Chen C.Y., Yang M.W., et al. Use of the Arndt wire-guided endobronchial blocker via nasal for one-lung ventilation in patient with anticipated restricted mouth opening for esophagectomy. European Journal of Cardio-thoracic Surgery. 2005;28:174–175. doi: 10.1016/j.ejcts.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 70.Campos J.H., Kernstine K.H. A comparison of a left-sided Broncho-Cath with the torque control blocker univent and the wire-guided blocker. Anesthesia and Analgesia. 2003;96:283–289. doi: 10.1097/00000539-200301000-00056. table of contents. [DOI] [PubMed] [Google Scholar]