IAP and the kidneys, an inseparable couple
There has been increased awareness about elevated intra-abdominal pressure (IAP) and particularly intra-abdominal hypertension (IAH) and abdominal compartment syndrome (ACS), which can occur with markedly elevated IAP (> 20 mmHg) [1], and that is associated with significant morbidity and mortality [2]. Increased IAP impacts each organ system within and far outside the abdominal cavity. The kidneys have been considered the canary in the coal mine for IAH, with oliguria as the usual first sign of acute kidney injury (AKI) [3]. Mean perfusion pressure (MPP) is the difference between mean arterial pressure (MAP) and central venous pressure (CVP) and has been associated with the progression of organ system injury [4, 5]. A more specific marker for resistive abdominal forces may be abdominal perfusion pressure (APP), calculated as the difference between MAP and IAP [6]. And more specifically, the filtration gradient (FG), calculated as the difference between MAP and twice the IAP, has been suggested to assess glomerular filtration and correlated moderately with renal blood flow and microcirculatory perfusion, whereas APP did not [7]. Increased renal vascular resistance with elevated IAP might account for this [7]. This warrants appropriate IAP monitoring, primarily done using homemade or commercial pressure measurements via the bladder catheter in an intermittent fashion [8]. As conventional bladder pressure monitoring requires the transient obstruction of the catheter, continuous monitoring of IAP could not be performed in the past and required human intervention (e.g. via the use of a 3-way Foley catheter with continuous irrigation). In this issue of the journal, Khanna and colleagues, describe a new monitoring technique that additionally allowed for assessing both cumulative (pressure time burden) and continuous (assessment of the effect of treatment) aspects of IAP, which had never previously been done via the bladder [9].
What does the study tell us?
First of all, we would like to thank and congratulate the authors on this study, which, in our opinion, takes us one step further in the monitoring realm of acutely ill patients [9]. Using a novel technology that requires no further invasiveness than the insertion of a dedicated Foley catheter (the Accuryn Monitoring System, Potrero Medical, Hayward, CA, USA), the authors monitored IAP continuously for 48 h in a cohort of postoperative cardiac surgery patients.
Interestingly, the authors described the presence of significant IAP elevation (IAH > 12 mmHg for > 12 h) in 93% of the patients, making it essentially a feature of the post-cardiac surgery course, which previously remained unnoticed. More importantly, the authors also presented a graph illustrating the relation between elevated IAP and decreased urine output, a key parameter in detecting and defining AKI. It would be interesting to see if a pressure–time integral, or area-under-the-curve (AUC) concept applies to splanchnic organ dysfunction and whether continuous abdominal perfusion pressure measurement can play a major prognostic role.
Intra-abdominal pressure and the kidneys: the relationship works both ways!
The effect of pressure dysregulation on renal function is established, both from the venous backpressure—measured either by CVP [10], or Doppler indices [11, 12] and IAP [13, 14]. Both of these (CVP and IAP) reflect the importance of considering organ perfusion pressure (taking into account the back pressure at the venous side) instead of focusing only on inflow pressure. In a sense, while elevated IAP may be a warning signal for AKI, the reverse may also hold true, that decreasing urine output (oliguria) may act as the canary in the coal mine for IAH and anuria for impending ACS. The ability to monitor both closely and accurately would bring, in our opinion, a potentially important safeguard against both.
We suspect that this study should prompt a similar one in patients with advanced decompensated heart failure (ADHF), who have also been found to have elevated IAP frequently [15]. The concept of cardio-abdominal-renal syndrome (CARS) has been proposed, as congestive heart failure can result in an elevated IAP due to several potential mechanisms such as ascites, bowel edema, ileus, and abdominal wall anasarca (Fig. 1, Panel A) [16]. Splanchnic venous congestion is associated with organ dysfunction [11, 12], likely through decreased renal perfusion pressure, which can be further compromised by external capsular pressure from IAP [15]. This pathophysiology would make ADHF patients potentially an important group to benefit from continuous urine output and IAP monitoring, particularly since mortality is significantly higher in the subgroup with elevated IAP and those with worsening renal failure.
Fig. 1.
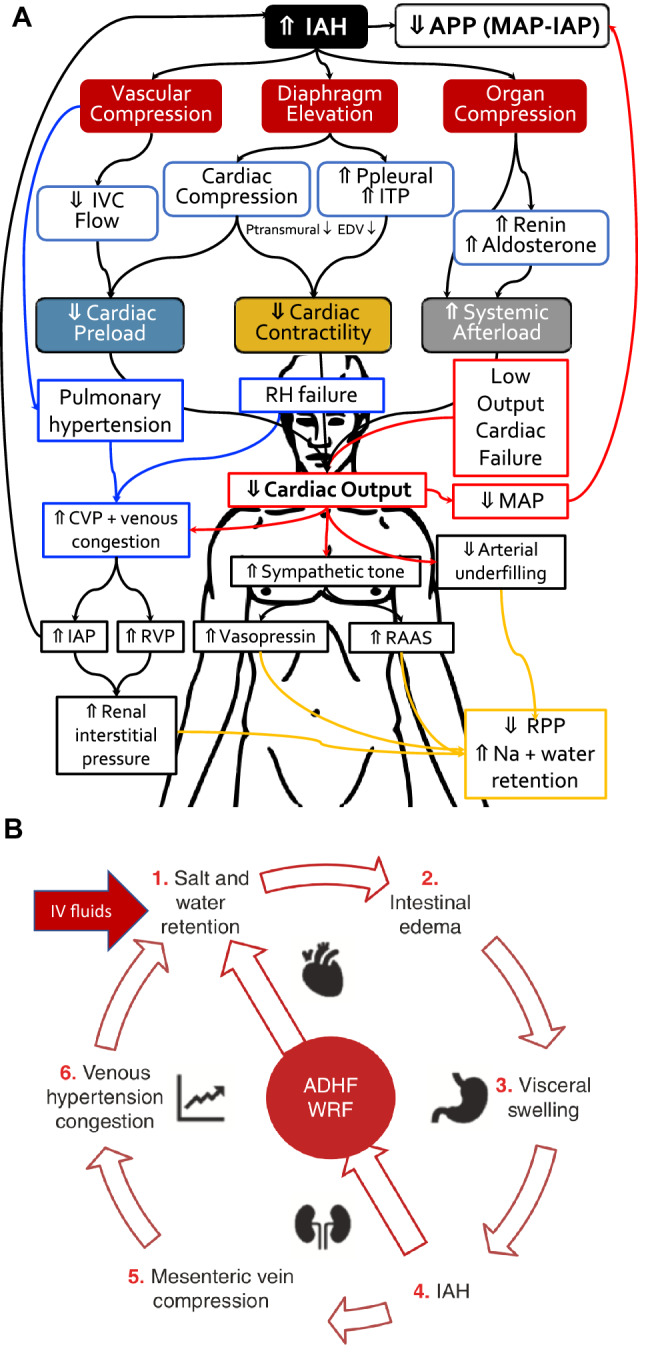
Panel A Pathophysiological effect of intra-abdominal hypertension and heart failure (RED arrows indicate forward failure) related venous congestion (BLUE arrows indicate backward failure) on organ function and net effects on salt and water homeostasis (ORANGE arrows).
Adapted from Minini et al. with permission [15]. APP abdominal perfusion pressure, CVP central venous pressure, IAP intra-abdominal pressure, ITP intrathoracic pressure, IVC inferior vena cava pressure MAP mean arterial pressure, RAAS renin angiotensin aldosterone system, RH right heart, RPP renal perfusion pressure. Panel B The pathophysiological vicious cycle of fluid overload leading to cardio-abdominal-renal syndrome (CARS). Adapted from Minini et al. with permission [15]. ADHF advanced decompensated heart failure, WRF worsening renal function
Intra-abdominal pressure increase during cardiac surgery
The present study is not the first to demonstrate elevated IAP in cardiac surgery patients. However, none of the previous work measured IAP continuously as Khanna and colleagues have done, thereby opening the possibility of uncovering an undermeasured and under detected physiological change. Most of the clinical studies showed increased IAP (measured intermittently) during the first 24–48 h after CABG completion [9, 17–30]. This IAP increase was associated with a decrease in APP and correlated with organ dysfunction [17, 18, 20]. Greater increases in IAP were observed in patients with higher BMI or more blood dilution following cardiopulmonary bypass, and the IAP increase was correlated with postoperative fluid balance and CVP [17, 22, 24, 26–28]. Additionally, patients with IAH received higher doses of vasoactive drugs [22]. In all studies the increase in IAP was temporary with IAP returning to the preoperative levels after 24 h in the majority of patients. However, the development of IAH (ranging from 30 to 50%) was associated with several postoperative complications, including AKI (Table 1).
Table 1.
The most important papers describing the effect of cardiac surgery on intra-abdominal pressure
| Author, year of publication [reference] | Type of surgery | Number of studied patients | Risk factors of IAH | IAP baseline (mmHg) | IAP after surgery (mmHg) | Adverse effect of elevated IAP and comments |
|---|---|---|---|---|---|---|
| Czajkowski [17] | CABG | 21 | Normovolemic hemodilution | 9 (7.5–0) |
BW < 75 kg: 12 (9.3–14) BW > 75 kg: 17 (17–18.5) |
Correlation between IAP and CVP. IAP higher in obesity. Median IAP returns to baseline (BL) of 9 mmHg after 18 h |
| Dabrowski [18] | CABG | 25 | Normovolemic hemodilution, cumulative fluid balance |
8.1 ± 1.8 9(8–9) |
12.2 ± 3.1 12 (10–16) |
IAP relates to disorders in venous outflow from the brain, increase in CVP. Median IAP returns to BL of 9 mmHg (8–10) after 18 h |
| Dabrowski [20] | CABG and aortic Valve surgery |
50 BMI < 25(18) BMI25-30 (23) BMI > 30 (9) |
Fluid balance, intra-operative blood dilution |
BMI < 25: 4.72 ± 1.19 BMI < 30: 6.69 ± 1.78 BMI > 30: 9.11 ± 0.99 |
11 ± 4.01 11.3 ± 3.4 12.9 ± 2.02 |
Decrease in abdominal perfusion pressure. Correlation IAP and BMI. Return to IAP 9 ± 2 mmHg, median of 8(6–11) after 18 h in all BMI groups |
| Dabrowski [36] | CABG | 45 | Not studied | 6.64 ± 1.87 | 11.17 ± 3.81 | Decrease in coronary perfusion pressure (CoPP). Correlation between IAP and CoPP and PCWP. Correlation CoPP and APP. IAP decreased to 9.08 ± 3.93 mmhg after 18 h |
| Dalfino et al. [22] | CABG and Off-pump cardiac by-pass surgery | 69 | Fluid balance |
8 (IAH in 32%) 6.5 (no IAH) |
14 9 |
IAP correlated with CVP, risk of AKI, prolonged mechanical ventilation, FB, higher doses of vasopressors. Higher IAP when on pump. Median IAP of 13 mmHg after 24 h (IAH) vs 7 (no IAH) |
| Iyer D. et al. [25] | CABG and Off-pump cardiac by-pass surgery | 108 | Fluid balance, duration of aorta cross-clamping | IAH in 46% (n = 50) | NA | Prolonged mechanical ventilation, higher doses of vasopressors, lower pH and PaO2/FiO2 ratio |
| Smit M. et al. [28] | CABG, CABG + Valve surgery, Thoracic aortic aneurysm | 186 | BMI | 9.1 ± 4.4 |
IAH in 26.9% (n = 50) ACS in 2.2% (n = 4) |
Correlation IAP with W:H ratio, waist circumference and BMI |
| Mazeffi et al. [24] | CABG, CABG + Valve surgery, | 50 | Not studied | Increased risk of AKI | ||
| Nazer et al. [27] | CABG | 50 (25 with BMI > 30) | BMI |
BMI > 30: 10.3 ± 3.3 BMI < 30: 8.4 ± 2.4 |
15.4 ± 1.6 10.6 ± 1.6 | Increased risk of AKI, liver dysfunction, prolonged postoperative mechanical ventilation |
| Kılıç et al. [21] | CABG, CABG + Valve surgery, | 100 | Age, hypertension, fluid balance, intra-operative blood dilution, duration of cardiopulmonary by-pass |
10.1 ± 2.4 (IAH in 49%) 8.1 ± 2.3 |
12.2 ± 0.7 (IAH) 9.5 ± 1.6 (no IAH) |
Increased incidence of atrial fibrillation, higher doses of vasopressors, higher lactate level, lower central venous saturation, AKI. Correlation with CVP. IAP after 24 h 14.7 ± 3.2 mmHg in IAH group |
| Ramser et al. [29] | CABG, CABG + Valve surgery, | 4128 | Risk factor for ACS: Perioperative ejection fraction, high Euroscore 2, duration of cardiopulmonary bypass | ACS in 1% (n = 42) | NA | In the 18 surviving patients, fascial closure was achieved in 72% after a median of 9 days. Outcome predictor in ACS: emergency, BMI, ASA, age |
| Richer-Séguin et al. [30] | CABG, CABG + Valve surgery, | 191 | BMI |
13 [9–15] (n = 191) 9 [7–10] (no IAH) 15 [13–17] (IAH in 55%, n = 105) |
13 [10–15] |
IAP independently associated with BMI, CVP and mean pulmonary artery pressure IAP measured 2 h after the admission to the postoperative cardiac intensive care unit was 8 [6–11]. |
| Khanna et al. (present study) [9] | Cardiac surgery | 137 | NA | 6.3 [4.0–8.1] | Within 6 h 10.2 [7.7–13.6] (ETT) and 17.2 [14.1–20.7] (postextubation) |
IAP first 24 h: 15.9 [13.6–18.7] IAP next 24–48 h 16.6 [14.5–19.1]. 93% (128/137) of patients spent at least 12 h in IAH grade I, 88% (113/128) of those patients in grade I also had grade II, 47% (53/113) of patients with grade II also had grade III, and 13% (7/53) of patients with grade III also had grade IV IAH |
Cardiopulmonary bypass can increase endothelial permeability leading to excessive fluid movement to the extravascular space, followed by tissue edema and increases in IAP [31, 32]. Inappropriate fluid administration perioperatively can lead to (intestinal) fluid accumulation, further contributing to IAH (Fig. 1, Panel B). The amount of extravascular water correlates significantly with the level of IAH [33]. Hypotonic priming, especially with cardiopulmonary bypass-related normovolemic hemodilution can exacerbate extravascular water build up [17]. Perioperative fluid administration should therefore be titrated with caution. Moreover, an increase in IAP above 15 mmHg impairs microcirculation including of the kidneys whereas IAP > 25 mmHg causes critical reduction of renal circulation, and these changes corresponded to a decrease in APP in experimental model of IAH [34]. An elevation of IAP to 15 mmHg for 120 min followed by IAP of 30 mmHg for 120 min caused a reduction in global perfusion, especially in the microcirculation of intestinal and ventricular mucosa, pancreas and the kidneys, and slightly increased cerebral perfusion which was associated with increase in intra-cranial pressure (ICP) [31]. Increased ICP with low cerebral perfusion can result from diminished venous return in IAH, which was observed in both cardiac surgery and critically ill patients [18, 35]. Interestingly, every disorder in cerebral circulation corresponded to increased risk of delirium and poor neurological outcome in cardiac surgery patients [19]. Elevated cerebral venous pressure led to cerebral damage as reflected by increased concentration of blood brain-injury biomarkers [36, 37]. Hence, disturbance in venous outflow following IAH after cardiopulmonary bypass can increase the risk of postoperative delirium and other neurological complications, potentially prolonging hospitalization duration after cardiac surgery.
The cephalic shift of the diaphragm in IAH impairs ventilation, both mechanical and spontaneous by reducing lung and chest wall compliance, lung volumes and increasing inspiratory resistance with high peak and plateau airway pressures [38]. An experimental study showed direct transmission of IAP to the thoracic cavity for approximately 50% [39]. The use of positive end-expiratory pressure (PEEP) counteracts the negative effect of IAH, therefore some PEEP is recommended [38, 40]. This was confirmed by Dalfino and colleagues who noted the prolonged duration of mechanical ventilation in patients with IAH [22]. Additionally, an increase in IAP can impair cardiac function leading to electrocardiographic abnormalities and increasing risk of cardiac arrhythmias [41]. Clinical observations showed an incidence of IAH in approximately 50% of patients undergoing elective cardiac surgery, and this increase was associated with a four-fold increase in postoperative atrial fibrillation [21]. Therefore, elevated IAP can be considered a risk factor that predisposes to postoperative complications after cardiac surgery.
IAH-induced cardiac dysfunction in cardiac surgery patients
Cardiac dysfunction caused by IAH has been well recognized (Fig. 1, Panel A). An experimental induction of IAH to 40 mmHg caused significant reduction of cardiac output and stroke volume and increase in vascular resistance [42]. Significant elevation of IAP also increases blood pressure in the pulmonary circulation and pulmonary capillary wedge pressure (PCWP) in a dose-related fashion [43]. Reduced cardiac output following IAH decreases microcirculatory perfusion in several organs, with the kidney, small bowel and colon mucosa being the most vulnerable [44]. The acute organ hypoperfusion together with massive inflammatory response increase the risk of organ insufficiency and a vicious cycle leading to ACS. Clinical observations showed that ACS developed in approximately 1% of patients after cardiopulmonary bypass, however it was associated with high mortality of 57% [29]. Importantly, the majority of patients with postoperative ACS were undergoing elective CABG surgery. This fact allows speculating that the stunned heart after the rapid changes in the cardiac perfusion following bypass together with cardiovascular depression following IAH can be a significant risk of ACS and poor outcome. Inappropriate fluid administration, especially fluid overload/accumulation and positive perioperative fluid balance was recognized as one of the most important risk factors for IAH and ACS, while restrictive fluid administration, avoidance of hypotonic crystalloids and use of hypertonic saline to control or slightly increase plasma osmolality was recommended to reduce the risk of IAH [45]. Avoiding IAH in the postoperative period hence eliminates a potential risk factor for cardiac dysfunction.
Limitations of the present study
Khanna et al. admit to several limitations in their study which is essentially pilot data for the nearly 10 times larger registry that is currently being created by the same group as part of an ongoing prospective study. This study does provide a lot of food for future thoughts. First, the sample size was relatively small and the study may have been underpowered to demonstrate causal relations. Second, since this study, a sub-study of an ongoing data registry had limited information on patient demographics, it was merely observational, and no interventions were prescribed upon increased IAP or presence of AKI. Third, the authors presented a graphical decrease in urinary output that was associated with elevated IAP. However, they did not analyze a potential relationship between IAP and length of postoperative mechanical ventilation, the incidence of delirium and the incidence of postoperative cardiac arrhythmias. Fourth, as IAP data is captured continuously it does not take into account potential confounders like patient position, sedation, pain, delirium, non-invasive ventilation, etc. Fifth, baseline IAP values could only be obtained after induction of anaesthesia (and muscle relaxation). Sixth, important data on fluid administration, fluid balance and concomitant medication (eg. diuretics), are missing. Seventh, it remains unclear why such relatively high IAP values were observed in this specific patient population. The median IAP values after 24 h remain elevated above 15 mmHg and are not in line with previous literature results, albeit performed with intermittent IAP. To play the devil’s advocate one could even argue on the importance of IAP if > 90% of patients exhibit IAH and do relatively well. Eight, unfortunately IAP monitoring stops at ICU discharge—but some patients still have high IAP > 20 mmHg—it would have been interesting to see what happened afterwards. Ninth, the authors provide no information on the relation between KDIGO or AKIN criteria with respect to duration of IAH (above 12 mmHg and other grades) and the duration of low urine output (below 0.5 or 0.3 ml/kg/min). In analogy to the cerebral compartment, the pressure–time burden of IAP is probably closely linked to AKI development. Finally, so far, no validation of continuous IAP has been done compared to the gold standard technique, e.g. intermittent bladder pressure measurement using the height of urine column, with patient supine, at end-expiration, without abdominal muscle contractions and zeroed at the level where the midaxillary line crosses the iliac crest. When examining an evidence based monitoring device we must ask ourselves four questions: (1) does the new device perform as well as the traditional gold standard; (2) does the new device offer us new information (new measured or derived parameters e.g. area under the curve, time above a certain threshold, pressure time burden, compliance, etc.; (3) can we guide/adapt our treatment based on this new information and finally (4) and if we do so, will this new parameter drive treatment effect and improve outcomes? The present paper is on the second point. The other questions need to be answered by future validation studies on continuous IAP in different patient populations, with normal and high BMI and with/without mechanical ventilation, following the WSACS recommendations and guidelines for research [46]. Finally, as we come to a point where the WSACS guidelines for IAH need to be updated [1], should we consider new paradigms of IAH grade based on continuous IAP thresholds (including also spontaneously breathing patients) different from the traditional sedated, mechanically ventilated patients, with intermittent IAP thresholds remains an important question. Should we titrate to a MAP or an APP to ensure that we provide an individualized precision medicine-based approach to organ protection is the next most important question that deserves an answer as well.
Take home messages
Khanna et al. deserve compliments for executing this study, and while the data presented are hypothesis generating, this will certainly open the doors for several follow-up and validation studies that will answer the questions regarding the prognostic power of pressure time burden, continuity, and accuracy of continuous IAP in a broader population of critically ill patients.
In summary, IAP and AKI go hand in hand and the novel continuous IAP monitoring tool presented herein opens a very interesting door into personalized physiological medicine for critically ill and ADHF patients, prompting both observational and interventional studies to determine how management could be altered with this available information.
Author contributions
WD, PR and MLNGM wrote the main manuscript text, WD prepared Table 1, and MLNGM prepared Fig. 1. All authors reviewed the manuscript.
Funding
The authors have not disclosed any funding, except for an unrestricted educational grant provided by Potrero Medical to cover the Open Access CC By Licence 4.0 fee.
Declarations
Conflict of interest
MLNGM is Professor of Critical Care Research at the 1st Department of Anaesthesiology and Intensive Therapy, Medical University of Lublin, Poland. He is co-founder, past-President and current Treasurer of WSACS (The Abdominal Compartment Society, http://www.wsacs.org). He is member of the medical advisory Board of Pulsion Medical Systems (part of Maquet-Getinge group), Serenno Medical, Potrero Medical, Sentinel Medical Technologies and Baxter. He consults for BBraun, Becton Dickinson, ConvaTec, Spiegelberg, and Holtech Medical, and received speaker's fees from PeerVoice. He holds stock options for Serenno and Potrero. He is co-founder and President of the International Fluid Academy (IFA). The IFA (http://www.fluidacademy.org) is integrated within the not-for-profit charitable organization iMERiT, International Medical Education and Research Initiative, under Belgian law. The other authors have no potential conflicts of interest in relation to the content of this manuscript.
Ethical statement
This paper is a brief report with mini-review of the existing literature on intra-abdominal pressure monitoring in cardiac surgery patients and a commentary based on the personal experience of the co-authors. Therefore an ethical statement is not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kirkpatrick AW, Roberts DJ, De Waele J, Jaeschke R, Malbrain ML, De Keulenaer B, Duchesne J, Bjorck M, Leppaniemi A, Ejike JC, et al. Intra-abdominal hypertension and the abdominal compartment syndrome: updated consensus definitions and clinical practice guidelines from the World Society of the abdominal compartment syndrome. Intensive Care Med. 2013;39(7):1190–1206. doi: 10.1007/s00134-013-2906-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malbrain ML, Chiumello D, Cesana BM, Reintam Blaser A, Starkopf J, Sugrue M, Pelosi P, Severgnini P, Hernandez G, Brienza N, et al. A systematic review and individual patient data meta-analysis on intra-abdominal hypertension in critically ill patients: the wake-up project world initiative on abdominal hypertension epidemiology a unifying project (WAKE-Up!) Minerva Anestesiol. 2014;80(3):293–306. [PubMed] [Google Scholar]
- 3.De laet I, Malbrain ML, Jadoul JL, Rogiers P, Sugrue M. Renal implications of increased intra-abdominal pressure: are the kidneys the canary for abdominal hypertension? Acta Clin Belg Suppl. 2007;62(1):119–130. doi: 10.1179/acb.2007.62.s1.015. [DOI] [PubMed] [Google Scholar]
- 4.Panwar R, Tarvade S, Lanyon N, Saxena M, Bush D, Hardie M, Attia J, Bellomo R, Van Haren F, Investigators RSS, et al. Relative hypotension and adverse kidney-related outcomes among critically Ill patients with shock. A multicenter prospective cohort study. Am J Respir Crit Care Med. 2020;202(10):1407–1418. doi: 10.1164/rccm.201912-2316OC. [DOI] [PubMed] [Google Scholar]
- 5.Ostermann M, Hall A, Crichton S. Low mean perfusion pressure is a risk factor for progression of acute kidney injury in critically ill patients—a retrospective analysis. BMC Nephrol. 2017;18(1):151. doi: 10.1186/s12882-017-0568-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheatham ML, White MW, Sagraves SG, Johnson JL, Block EF. Abdominal perfusion pressure: a superior parameter in the assessment of intra-abdominal hypertension. J Trauma. 2000;49(4):621–626. doi: 10.1097/00005373-200010000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Wauters J, Claus P, Brosens N, McLaughlin M, Malbrain M, Wilmer A. Pathophysiology of renal hemodynamics and renal cortical microcirculation in a porcine model of elevated intra-abdominal pressure. J Trauma. 2009;66(3):713–719. doi: 10.1097/TA.0b013e31817c5594. [DOI] [PubMed] [Google Scholar]
- 8.Malbrain ML. Different techniques to measure intra-abdominal pressure (IAP): time for a critical re-appraisal. Intensive Care Med. 2004;30(3):357–371. doi: 10.1007/s00134-003-2107-2. [DOI] [PubMed] [Google Scholar]
- 9.Khanna A, Minear S, Prabhakar A, Kurz A, Stanton K, Essakalli L, Blackwell BA, Sweatt N, Flores K, Harris L, et al. Intra-abdominal hypertension in cardiac surgery patients: A multicenter observational pilot study. J Clin Monit Comput. 2022 doi: 10.1007/s10877-022-00878-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mullens W, Abrahams Z, Francis GS, Sokos G, Taylor DO, Starling RC, Young JB, Tang WH. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol. 2009;53(7):589–596. doi: 10.1016/j.jacc.2008.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beaubien-Souligny W, Rola P, Haycock K, Bouchard J, Lamarche Y, Spiegel R, Denault AY. Quantifying systemic congestion with point-of-care ultrasound: development of the venous excess ultrasound grading system. Ultrasound J. 2020;12(1):16. doi: 10.1186/s13089-020-00163-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iida N, Seo Y, Sai S, Machino-Ohtsuka T, Yamamoto M, Ishizu T, Kawakami Y, Aonuma K. Clinical implications of intrarenal hemodynamic evaluation by Doppler ultrasonography in heart failure. JACC Heart Fail. 2016;4(8):674–682. doi: 10.1016/j.jchf.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 13.Mullens W, Abrahams Z, Francis GS, Taylor DO, Starling RC, Tang WH. Prompt reduction in intra-abdominal pressure following large-volume mechanical fluid removal improves renal insufficiency in refractory decompensated heart failure. J Cardiac Fail. 2008;14(6):508–514. doi: 10.1016/j.cardfail.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 14.Mullens W, Abrahams Z, Skouri HN, Francis GS, Taylor DO, Starling RC, Paganini E, Tang WH. Elevated intra-abdominal pressure in acute decompensated heart failure: a potential contributor to worsening renal function? J Am Coll Cardiol. 2008;51(3):300–306. doi: 10.1016/j.jacc.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 15.Minini A, Rola P, Malbrain MLNG. Kidney failure associated with polycompartment syndrome. In: Coccolini F, Malbrain ML, Kirkpatrick AW, Gamberini E, editors. Compartment syndrome hot topics in acute care surgery and trauma. Cham, Switzerland: Springer; 2021. [Google Scholar]
- 16.Verbrugge FH, Dupont M, Steels P, Grieten L, Malbrain M, Tang WH, Mullens W. Abdominal contributions to cardiorenal dysfunction in congestive heart failure. J Am Coll Cardiol. 2013;62(6):485–495. doi: 10.1016/j.jacc.2013.04.070. [DOI] [PubMed] [Google Scholar]
- 17.Czajkowski M, Dabrowski W. Changes in intra-abdominal pressure during CABG with normovolemic hemodilution. Med Sci Monit: Int Med J Exp Clin Res. 2006;12(11):CR487–492. [PubMed] [Google Scholar]
- 18.Dabrowski W. Changes in intra-abdominal pressure and central venous and brain venous blood pressure in patients during extracorporeal circulation. Med Sci Monit: Int Med J Exp Clin Res. 2007;13(12):548–554. [PubMed] [Google Scholar]
- 19.Xu B, Qiao Q, Chen M, Rastogi R, Luo D, Bi Q. Relationship between neurological complications, cerebrovascular and cerebral perfusion following off-pump coronary artery bypass grafting. Neurol Res. 2015;37(5):421–426. doi: 10.1179/1743132815Y.0000000030. [DOI] [PubMed] [Google Scholar]
- 20.Dabrowski W, Rzecki Z. Intra-abdominal and abdominal perfusion pressure in patients undergoing coronary artery bypass graft surgery. Acta Clin Belg. 2009;64(3):216–224. doi: 10.1179/acb.2009.038. [DOI] [PubMed] [Google Scholar]
- 21.Kilic B, Yapici N, Yapici F, Kavakli AS, Kudsioglu T, Kilic A, Aykac Z. Factors associated with increased intra-abdominal pressure in patients undergoing cardiac surgery. Turk Gogus Kalp Damar Cerrahisi Derg. 2020;28(1):134–142. doi: 10.5606/tgkdc.dergisi.2020.18662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dalfino L, Sicolo A, Paparella D, Mongelli M, Rubino G, Brienza N. Intra-abdominal hypertension in cardiac surgery. Interact Cardiovasc Thorac Surg. 2013;17(4):644–651. doi: 10.1093/icvts/ivt272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tyson N, Efthymiou C. Predictive risk factors for intra-abdominal hypertension after cardiac surgery. Interact Cardiovasc Thorac Surg. 2021;32(5):719–723. doi: 10.1093/icvts/ivaa336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mazzeffi MA, Stafford P, Wallace K, Bernstein W, Deshpande S, Odonkor P, Grewal A, Strauss E, Stubbs L, Gammie J, et al. Intra-abdominal hypertension and postoperative kidney dysfunction in cardiac surgery patients. J Cardiothorac Vasc Anesth. 2016;30(6):1571–1577. doi: 10.1053/j.jvca.2016.05.028. [DOI] [PubMed] [Google Scholar]
- 25.Iyer D, D'Amours S, Aneman A. Intra-abdominal hypertension in postoperative cardiac surgery patients. Crit Care Resusc. 2014;16(3):214–219. [PubMed] [Google Scholar]
- 26.Dabrowski W, Wacinski P, Visconti J. Abdominal perfusion pressure and coronary arterial perfusion pressure in patients undergoing coronary artery bypass graft surgery. Exp Clin Cardiol. 2009;14(3):e84–88. [PMC free article] [PubMed] [Google Scholar]
- 27.Nazer R, Albarrati A, Ullah A, Alamro S, Kashour T. Intra-abdominal hypertension in obese patients undergoing coronary surgery: a prospective observational study. Surgery. 2019;166(6):1128–1134. doi: 10.1016/j.surg.2019.05.038. [DOI] [PubMed] [Google Scholar]
- 28.Smit M, Werner MJM, Lansink-Hartgring AO, Dieperink W, Zijlstra JG, van Meurs M. How central obesity influences intra-abdominal pressure: a prospective, observational study in cardiothoracic surgical patients. Ann Intensive Care. 2016;6(1):99. doi: 10.1186/s13613-016-0195-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramser M, Glauser PM, Glass TR, Weixler B, Grapow MTR, Hoffmann H, Kirchhoff P. Abdominal decompression after cardiac surgery: outcome of 42 patients with abdominal compartment syndrome. World J Surg. 2021;45(4):1242–1251. doi: 10.1007/s00268-020-05917-0. [DOI] [PubMed] [Google Scholar]
- 30.Richer-Seguin E, Ayoub C, Lebon JS, Cogan J, Jarry S, Lamarche Y, Denault AY, Beaubien-Souligny W. Intra-abdominal pressure during and after cardiac surgery: a single-centre prospective cohort study. Can J Anaesth. 2022;69(2):234–242. doi: 10.1007/s12630-021-02141-9. [DOI] [PubMed] [Google Scholar]
- 31.Elvevoll B, Husby P, Ovrebo K, Haugen O. Acute elevation of intra-abdominal pressure contributes to extravascular shift of fluid and proteins in an experimental porcine model. BMC Res Notes. 2014;7:738. doi: 10.1186/1756-0500-7-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cordemans C, De laet I, Van Regenmortel N, Schoonheydt K, Dits H, Huber W, Malbrain MLNG. Fluid management in critically ill patients: the role of extravascular lung water, abdominal hypertension, capillary leak and fluid balance. Ann Intensive Care. 2012;2(1):1. doi: 10.1186/2110-5820-2-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dabrowski W, Kotlinska-Hasiec E, Jaroszynski A, Zadora P, Pilat J, Rzecki Z, Zaluska W, Schneditz D. Intra-abdominal pressure correlates with extracellular water content. PLoS ONE. 2015;10(4):e0122193. doi: 10.1371/journal.pone.0122193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sui F, Zheng Y, Li WX, Zhou JL. Renal circulation and microcirculation during intra-abdominal hypertension in a porcine model. Eur Rev Med Pharmacol Sci. 2016;20(3):452–461. [PubMed] [Google Scholar]
- 35.Kotlinska-Hasiec E, Dabrowski W, Rzecki Z, Rybojad B, Pilat J, De Keulenaer B, Lng Malbrain M. Association between intra-abdominal pressure and jugular bulb saturation in critically ill patients. Minerva Anestesiol. 2014;80(7):785–795. [PubMed] [Google Scholar]
- 36.Kotlinska-Hasiec E, Czajkowski M, Rzecki Z, Stadnik A, Olszewski K, Rybojad B, Dabrowski W. Disturbance in venous outflow from the cerebral circulation intensifies the release of blood-brain barrier injury biomarkers in patients undergoing cardiac surgery. J Cardiothorac Vasc Anesth. 2014;28(2):328–335. doi: 10.1053/j.jvca.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 37.Dabrowski W, Kotlinska E, Rzecki Z, Czajkowski M, Stadnik A, Olszewski K. Raised jugular venous pressure intensifies release of brain injury biomarkers in patients undergoing cardiac surgery. J Cardiothorac Vasc Anesth. 2012;26(6):999–1006. doi: 10.1053/j.jvca.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 38.Regli A, Pelosi P, Malbrain M. Ventilation in patients with intra-abdominal hypertension: what every critical care physician needs to know. Ann Intensive Care. 2019;9(1):52. doi: 10.1186/s13613-019-0522-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wauters J, Claus P, Brosens N, McLaughlin M, Hermans G, Malbrain M, Wilmer A. Relationship between abdominal pressure, pulmonary compliance, and cardiac preload in a porcine model. Crit Care Res Pract. 2012;2012:763181. doi: 10.1155/2012/763181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pelosi P, Vargas M. Mechanical ventilation and intra-abdominal hypertension: 'beyond good and evil'. Crit Care (London, England) 2012;16(6):187. doi: 10.1186/cc11874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dabrowski W, Jaroszynski A, Jaroszynska A, Rzecki Z, Schlegel TT, Malbrain ML. Intra-abdominal hypertension increases spatial QRS-T angle and elevates ST-segment J-point in healthy women undergoing laparoscopic surgery. J Electrocardiol. 2017;50(2):214–222. doi: 10.1016/j.jelectrocard.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 42.Ivankovich AD, Miletich DJ, Albrecht RF, Heyman HJ, Bonnet RF. Cardiovascular effects of intraperitoneal insufflation with carbon dioxide and nitrous oxide in the dog. Anesthesiology. 1975;42(3):281–287. doi: 10.1097/00000542-197503000-00008. [DOI] [PubMed] [Google Scholar]
- 43.Ridings PC, Bloomfield GL, Blocher CR, Sugerman HJ. Cardiopulmonary effects of raised intra-abdominal pressure before and after intravascular volume expansion. J Trauma. 1995;39(6):1071–1075. doi: 10.1097/00005373-199512000-00010. [DOI] [PubMed] [Google Scholar]
- 44.Olofsson PH, Berg S, Ahn HC, Brudin LH, Vikstrom T, Johansson KJ. Gastrointestinal microcirculation and cardiopulmonary function during experimentally increased intra-abdominal pressure. Crit Care Med. 2009;37(1):230–239. doi: 10.1097/CCM.0b013e318192ff51. [DOI] [PubMed] [Google Scholar]
- 45.Duchesne JC, Kaplan LJ, Balogh ZJ, Malbrain ML. Role of permissive hypotension, hypertonic resuscitation and the global increased permeability syndrome in patients with severe hemorrhage: adjuncts to damage control resuscitation to prevent intra-abdominal hypertension. Anaesthesiol Intensiv Ther. 2015;47(2):143–155. doi: 10.5603/AIT.a2014.0052. [DOI] [PubMed] [Google Scholar]
- 46.De Waele JJ, Cheatham ML, Malbrain ML, Kirkpatrick AW, Sugrue M, Balogh Z, Ivatury R, De Keulenaer B, Kimball EJ. Recommendations for research from the international conference of experts on intra-abdominal hypertension and abdominal compartment syndrome. Acta Clin Belg. 2009;64(3):203–209. doi: 10.1179/acb.2009.036. [DOI] [PubMed] [Google Scholar]


