Abstract
Cardiovascular disease (CVD) continues to be the leading cause of death in adults, highlighting the need to develop novel strategies to mitigate cardiovascular risk. The advancing obesity epidemic is now threatening the gains in CVD risk reduction brought about by contemporary pharmaceutical and surgical interventions. There are sex differences in the development and outcomes of CVD; premenopausal women have significantly lower CVD risk than men of the same age, but women lose this advantage as they transition to menopause, an observation suggesting potential role of sex hormones in determining CVD risk. Clear differences in obesity and regional fat distribution among men and women also exist. While men have relatively high fat in the abdominal area, women tend to distribute a larger proportion of their fat in the lower body. Considering that regional body fat distribution is an important CVD risk factor, differences in how men and women store their body fat may partly contribute to sex-based alterations in CVD risk as well. This article presents findings related to the role of obesity and sex hormones in determining CVD risk. Evidence for the role of sex hormones in determining body composition in men and women is also presented. Lastly, the clinical potential for using sex hormones to alter body composition and reduce CVD risk is outlined.
Introduction
Cardiovascular disease (CVD) including coronary heart disease (CHD), myocardial infarction (MI), and heart failure (HF) continues to be the leading cause of death in the United States (260). Alarmingly, declines in CVD mortality has diminished over the past decade and the CVD prevention goals set up by the US Department of Health and Human Services, such as the Million Heart Initiatives, are unlikely to be met (368, 531, 593). The stalling of declines in CVD mortality in recent years may be partly ascribed to the continued obesity epidemic and the aging population (287). Evidence for the role of obesity in CVD mortality is apparent from the upward trends in deaths attributed to CVD causes in middle-aged Americans (35–65 years) in geographic locations overlapping with high prevalence of obesity (368, 593). Importantly, obesity continues to be widespread in the US adult population with 36.0% men and 40.4% women reported to have body mass index (BMI) of >30 kg/m2 (603). Being overweight and obese are also associated with an earlier development of CVD. Notably, the CVD risk attributed to being obese is equivalent to that of traditional risk factors such as hypertension, dyslipidemia, and diabetes in women and men (422).
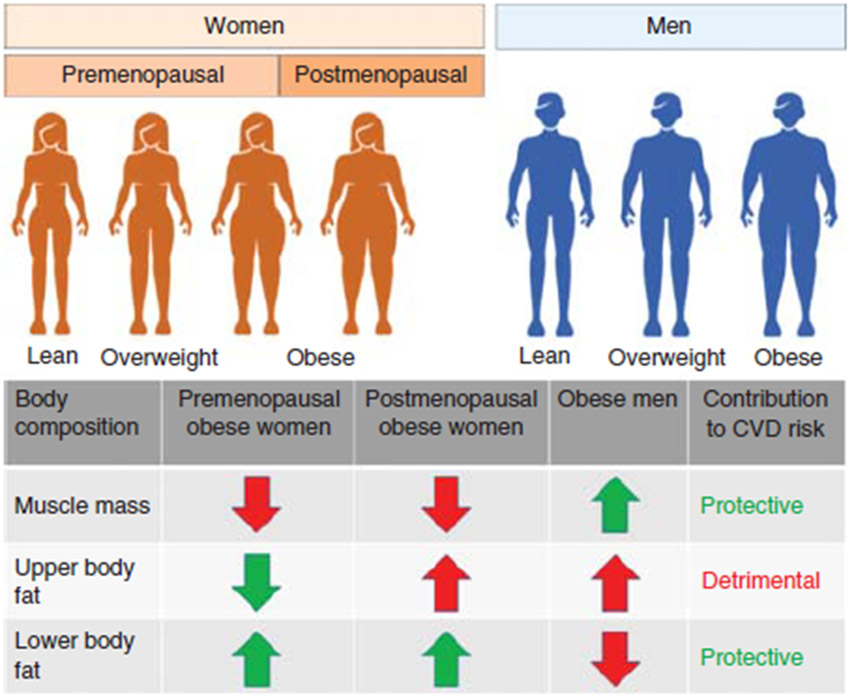
Another important aspect of CVD mortality is related to age and sex. For example, mortality statistics are largely driven by deaths in adults aged ≥65 years where CVD events account for 25.3% of all deaths (260). In younger adults, heart disease accounts for 10.3% and 20.9% of deaths in the age group between 25 and 44 years, as well as 45 and 64 years, respectively. Importantly, death from CVD is similar between men and women in the United States (260). In 2016, approximately 1 in 4 deaths (24.2%) in men and 1 in 5 deaths (22.0%) in women were attributable to CVD. Strikingly, in earlier decades of life, CVD mortality in women is much lower than in similarly aged men suggesting important sex differences in the development and progression of the disease (59). Also, men are more likely than women to have traditional CVD risk factors, namely, hypertension, dyslipidemia, and smoking (208). Therefore, it is not surprising that the common CVDs including HF, atrial fibrillation, and ischemic heart disease are also sex specific (52, 422). Of note, sex-specific differences have also been well documented for obesity, body composition, and regional fat deposition (422). Some of these differences are highlighted in Figure 1.
Figure 1.
Schematic highlighting the differential body composition and fat distribution in men and women along with its contribution towards CVD risk. Changes in body fat distribution in postmenopausal women are emphasized. Arrows indicate prevalence of body composition variables in obese women and men. Downward arrows indicate low prevalence and upward arrows indicate high prevalence. Green-colored text and arrows indicate cardioprotective effects and red-colored text and arrows indicate detrimental cardiovascular effects.
Together, the above trends highlight the need for continued efforts to reduce CVD burden in the US adult population and a heightened focus on sex-specific primary and secondary prevention strategies. In this article, we aim to discuss findings related to obesity and sex as CVD risk factors along with an in-depth presentation of the literature related to the influence of sex hormones on CVD risk and body fat distribution. To conclude, we will discuss the potential of sex hormones as an intervention for CVD prevention. The article does not examine the molecular mechanisms through which the hormones may contribute to the CVD risk and mortality (Table 1).
Table 1.
List of Abbreviations
| ADT | androgen deprivation therapy |
| AI | aromatase inhibitors |
| ARIC | Atherosclerosis Risk in Communities |
| BMI | body mass index |
| BPA | bisphenol A |
| CEE | conjugated equine estrogen |
| CIMT | carotid intima-media thickness |
| CRP | C-reactive protein |
| CV | cardiovascular |
| CVD | cardiovascular disease |
| CAD | coronary artery disease |
| CHD | coronary heart disease |
| DHEA | dehydroepiandrosterone |
| DHEAS | dehydroepiandrosterone and its sulfate |
| E2 | estradiol |
| GnRH | gonadotrophin-releasing hormone |
| HF | heart failure |
| HDL-C | high-density lipoprotein cholesterol |
| LDL-C | low-density lipoprotein cholesterol |
| MI | myocardial infarction |
| MHT | menopausal hormone therapy |
| MPA | medroxyprogesterone acetate |
| NEN-EN | norethisterone enanthate |
| NHANES | National Health and Nutrition Examination Surveys |
| POC | progestin-only contraceptive |
| RCT | randomized clinical trial |
| SERMs | selective estrogen receptor modulators |
| SWAN | Study of Women’s Health Across the Nation |
| T | testosterone |
| T2D | type 2 diabetes |
| TRT | testosterone replacement therapy |
Obesity as CVD Risk Factor
Despite unwavering public health focus, the prevalence of obesity has continued to rise over the past 20 years. From 1999 through 2018, age-standardized estimates from the National Health and Nutrition Examination Surveys (NHANES) showed that obesity prevalence in the US adult population, defined as a BMI equal to or greater than 30 kg/m2, increased from 30.5% to 42.4%. At the same time, severe obesity (BMI ≥40 kg/m2) nearly doubled, from 4.7% to 9.2% (245). Obesity rates are largely similar between sexes and across age groups, rendering it a ubiquitous condition. However, severe obesity is more prevalent in women (11.5%) than men (6.9%). These growing national trends reflect worldwide statistics pointing towards a soaring burden of obesity. The Global Burden of Disease study evaluated 35 years of data collected from over 68 million individuals across 195 countries to analyze temporal gradients in obesity status and obesity-related disease. Since 1980, the proportion of individuals with BMI >30 kg/m2 has increased in near every country and doubled in more than 70 countries (214). As a result, in 2015, there were over 600 million obese adults globally. Even more sobering are the projections that anticipate further increases in obesity prevalence in the next decades (197, 298).
These estimates become especially alarming when considering the health hazards of excess adiposity. Obesity is a well-recognized leading determinant of morbidity and mortality, with 4 million deaths globally attributable to high BMI, of which nearly 70% are attributable to CVD (214). Importantly, the growing obesity trend is thought to contribute to the decelerated rates of improved cardiovascular (CV) mortality seen in recent years (403). However, the association between BMI and mortality is typically described as curvilinear rather than monotonic, with lower survival rates seen in individuals at both extremes of the BMI continuum (62, 119, 474, 479, 487). The Prospective Studies Collaboration pooled data from nearly 900,000 adults worldwide and showed that, in subjects with BMI >25 kg/m2, risk of all-cause and CVD mortality increased by 29% and 41% for each 5-unit increase in BMI, respectively (479). Optimal survival was noted in those with BMI between 22.5 and 25 kg/m2, while a negative relation with mortality was manifest in those with lower BMI. These association patterns were consistent in men and women and across age strata. Accordingly, multiple meta-analyses provide summary evidence consistent with a U- or J-shape association between BMI and mortality (27, 108, 200).
With regard to CVD, elevated BMI independently predicts development of atrial fibrillation (609, 635), HF (26), CHD (77, 379, 422), and stroke (379). During a 5.7 year follow-up of a population-based cohort aged 50 to 64 years (207), adjusted hazard ratios for atrial fibrillation or flutter were 1.75 to 1.39 times greater in overweight men and women, respectively, compared to normal weight. Among adults with obesity, the risk of developing atrial fibrillation or flutter was twofold greater than those with normal weight. Obesity also predisposes to postoperative new-onset atrial fibrillation (87, 656). In a large community sample, the probability of incident HF increased by 5% in men and 7% in women per each 1-unit increase in BMI, and adults with obesity had a double risk of developing HF compared to those with normal weight (328). Considering the HF categories, elevated BMI appears to especially predispose to the subtype with preserved ejection fraction (268, 455). With regard to CHD, obesity accelerates progression of subclinical atherosclerosis as determined by coronary artery calcification (111), and increments in BMI over time are associated with increases in CHD risk score (630). Relative to normal weight, hazard ratios for MI were 1.38 and 2.04 in adults with overweight and obesity, respectively, in the Copenhagen General Population Study (579). Greater risk of ischemic stroke was associated with BMI ≥30 kg/m2 at study entry and weight changes over time in 15,792 subjects from the Atherosclerosis Risk in Communities (ARIC) Study (557).
Overall, compelling data from several cohorts including the Framingham Heart Study (278), the Nurses’ Health Study (395, 396), and Buffalo Health Study (175) have identified obesity as a predictor of composite CVD endpoints. A follow-up examination of the Framingham Heart Study on individuals aged 35 to 75 years found that, compared to normal weight, the age-adjusted risk for future CVD events (angina, MI, CHD, and stroke) was 1.21 to 1.20 times greater in overweight men and women and 1.46 to 1.64 times greater in obese men and women, respectively (631). A similar graded increase in the relative risk of incident CVD, starting from a BMI ≥25 kg/m2, was noted in a 10-year follow-up of the Nurses’ Health Study and the Health Professional Follow up Study (195), which also reported comparable estimates for new-onset diabetes, hypertension, and hypercholesterolemia. In this regard, individual BMI data for 221,934 people from 58 cohorts pooled by the Emerging Risk Factors Collaboration (184) showed that BMI predicted future CVD in models adjusted for age, sex, and smoking status, with stronger hazard ratios for CHD than for stroke. However, further correction for established prognostic markers such as total cholesterol, high-density lipoprotein cholesterol (HDL-C), systolic blood pressure, and diabetes largely attenuated these associations, in line with previous studies (395). Nevertheless, these observations highlight the pathogenic role of other obesity-related complications in the premature CVD mortality connected with obesity.
In addition to direct biological actions inducing structural and functional alterations in the CV system, excess adiposity predisposes to a multitude of CVD risk factors, including dyslipidemia, hypertension, and diabetes, to name a few. The prevalence of these metabolic syndrome components progressively increases with higher BMI, accentuating the related health burden. Dyslipidemia is a hallmark of excess body fat, resulting from multiple mechanisms including increased free fatty acid fluxes to the liver, upregulation of hepatic very-low-density lipoprotein synthesis, defective free fatty acid uptake, and reduced lipolysis of circulating triglycerides (340). The lipid profile in obese patients typically shows high levels of circulating triglycerides and free fatty acid, lower levels of HDL-C, and increased small dense low-density lipoprotein cholesterol (LDL-C). Apolipoprotein B is also often elevated. Serum total cholesterol levels progressively increase with increasing BMI in both men and women, while HDL-C displays a reverse profile (90). Consistently, while dyslipidemia is present in 28.6% of normal-weight individuals, its prevalence increases to 49.7% among those with obesity (514). A prominent CVD risk factor in the general population, type 2 diabetes (T2D) affects 18.5% of adults with obesity versus 5.4% with normal weight, and prevalence peaks at 23.2% in those with severe obesity (514). The risk of developing T2D is three- and sevenfold higher in individuals with overweight and obesity, respectively, relative to those with normal weight (2). Obesity-related diabetes is primarily caused by insulin resistance, in turn, largely mediated by increased plasma free fatty acid and aggravated by systemic inflammation and oxidative stress. Insulin deficiency due to pancreatic beta-cells dysfunction also ensues as a result of hepatic and local triglycerides accumulation and contributes to diabetes onset (596). More than one-third of patients with obesity suffer from hypertension, compared to one-fifth of those with normal weight (514). Pooled relative risk of incident hypertension is 1.49 for each 5-unit increment in BMI (302), and up to 60% to 80% of new cases of hypertension can be ascribed to obesity (213). Similar to other obesity-related complications, blood pressure elevation due to excess adiposity is elicited by the interplay of numerous mechanisms, including sympathetic hyperactivation, impaired baroreflex, endothelial dysfunction, increased renal sodium reabsorption, renin-angiotensin system stimulation, and insulin resistance (520).
Even though obesity is a well-recognized CVD risk factor, certain nuances in the relationship between obesity and CVD need to be considered. These are highlighted in the sections below.
BMI as a Surrogate Marker for Obesity and CVD Risk
Although collective evidence strongly supports a detrimental impact of excess adiposity on CVD outcomes, it is important to point out that large heterogeneity exists. In this regard, a critical aspect of the assessment of the health implications of body fatness pertains to the definition of obesity. In the vast majority of the literature, especially epidemiological studies, adiposity status is typically defined according to BMI categories. This approach, based on height and weight measures, has gained popularity due to being inexpensive, easily obtainable, and yielding immediate results. Nevertheless, this anthropometric measure suffers from considerable limitations, being merely a proxy for fatness. Compared to classifications based on body fat percentages, the BMI has elevated (90%) specificity for the detection of obesity, but only modest (50%) sensitivity (448), thus failing to correctly identify approximately one in two individuals with obesity. It follows that the true prevalence of obesity is likely largely underestimated, and its burden even more dramatic. Furthermore, it has also been suggested that obesity in midlife women may be more appropriately defined by BMI ≥25 kg/m2 rather than the generally accepted definition of ≥30 kg/m2 (38, 73). In spite of the close relationship between BMI and body fat percentage and mass (40), there is ample variability in body fat content at any given BMI, particularly in the normal weight and overweight BMI ranges (293). Sex and racial/ethnic differences exist, with fat percentage being about 10% greater in women compared to men at a given BMI (293), and Black women exhibiting lower body fat than white women at a similar BMI (190). These observations underscore how the BMI is an imperfect anthropometric index, as it does not discriminate between fat mass and fat-free mass, or takes into account body shapes.
Body Fat Distribution in CV Health
In addition to the differential prognostic significance of body composition, evidence has been gathered highlighting the role of fat localization, rather than general body fatness, for risk stratification. As mentioned above, another limitation of using BMI, as well as of direct indices of total body fat, is the lack of information on regional fat partitioning. The expression “android” or “upper body” obesity refers to the body shape more frequently noted in men, in whom fat is mainly stored in the abdominal (trunk) region, while “gynoid” or “lower body” obesity indicates accumulation of fat in the lower body (gluteo-femoral region) and is more common in women (322). These body shapes are associated with distinct risk trajectories based on the location of excess body fat. It is now well recognized that fat accumulated in the android region is strongly linked to adverse CV outcomes, while fat stored in the gynoid region appears to be protective (465, 590, 594, 626). As documented by a cross-sectional analysis of the Dallas Heart Study, lower body fat quantified from dual-energy x-ray absorptiometry (a gold standard to determine body composition and regional fat distribution), negatively correlated with the HOMA index, C-reactive protein (CRP), triglyceride/HDL-C ratio, and systolic blood pressure after adjusting for total body fat (594). Conversely, abdominal (trunk) fat was positively associated with these cardiometabolic risk indicators, and the magnitude of these associations was greater compared to those with total body fat percentage. Opposite relations of android and gynoid fat with arterial stiffness have also been observed (193). In Chinese individuals aged 50 to 70 years, higher trunk fat mass was positively associated with higher levels of plasminogen activator inhibitor 1, CRP, interleukin-6, retinol-binding protein 4, and lower levels of adiponectin. Conversely, higher leg fat mass correlated with higher adiponectin and lower plasminogen activator inhibitor-1 and retinol-binding protein 4 (638). Additionally, odds ratio of metabolic syndrome for those in the highest tertile of leg fat mass compared to the lowest tertile were 0.33 for men and 0.43 for women, further corroborating the concept that lower body adiposity conveys metabolic benefits. The gluteo-femoral compartment is indeed regarded as a “metabolic sink”, allowing storage of excess fatty acids thus protecting from ectopic fat deposition and related adverse consequences. This is mediated by increased adipose tissue lipoprotein lipase activity and decreased hormone-sensitive lipase which favor storage of fatty acids and inhibit lipolysis, respectively. Furthermore, the secretive profile of lower-body adipose tissue is more benign compared to upper body fat, producing greater quantities of adiponectin and lower proinflammatory cytokines (393).
In lieu of gold standard imaging modalities, anthropometric measures of central fatness such as waist circumference, waist-to-hip ratio, or waist-to-height ratio are more typically used in epidemiological studies. Despite being closely correlated with BMI, these measures are superior as indices of total body fat content and abdominal adiposity (102, 500, 594), and convey better predictive value. Anthropometric measurements of central fatness discriminate cardiometabolic abnormalities better than BMI in both men and women (359). In a nationally representative sample of 12,606 adults, waist circumference was a stronger predictor of hypertension, diabetes, and low HDL-C than BMI and total body fat in both men and women (540). Greater prognostic power of abdominal adiposity indices is evident also with regard to incident fatal and nonfatal CVD events. A large case-control study on incident coronary events found that the association between waist-to-hip ratio and MI was 3 times more robust than that with BMI (655).
The abdominal fat compartment is further partitioned in subcutaneous and intra-abdominal (visceral) fat depots, with the former being additionally dissected into deep and superficial adipose tissue. There is a gradient in the abdominal adipose tissue characteristics and metabolism from the outermost to the innermost depots that is reflected in their prognostic implications. Compared to the superficial compartment, deep subcutaneous fat is more closely associated with measures of hepatic and peripheral insulin sensitivity, triglycerides, HDL-C, and blood pressure (327, 398, 417). mRNA expression of inflammatory, lipogenic, and lipolytic genes is higher in the deep subcutaneous depot, together with higher saturated fatty acids content (398). Notably, the abdominal superficial adipose tissue follows a similar pattern compared to leg fat (327), suggesting a more benign profile exhibited by this depot. On the other hand, visceral adiposity is more pathogenic than the combined subcutaneous compartments. Using data from the Multi-Ethnic Study of Atherosclerosis cohort, studies have shown that individuals with higher visceral fat, as quantified from abdominal computed tomography, have lower HDL-C, and higher triglycerides, HbA1C, and blood pressure, and are more likely to have diabetes than those with lower visceral fat (420). Visceral fat is also a stronger predictor of incident metabolic syndrome, CVD, and mortality than subcutaneous fat (89, 344, 420, 523). The greater hazard posed by visceral adiposity is thought to be due to its peculiar structural and functional attributes. While subcutaneous fat secretes more leptin and adiponectin, visceral adipose tissue produces more inflammatory and proatherogenic adipokines such as interleukin-6, plasminogen activator inhibitor 1, vascular endothelial growth factor, and monocyte chemoattractant protein-1, along with higher number of resident macrophages. Visceral fat is also more lipolytically active, innervated, and vascularized than subcutaneous fat, and renin-angiotensin system components are overexpressed in visceral fat cells (285). Excess visceral adiposity may also contribute indirectly to raise CV risk, being an indicator of adipose tissue dysregulation that results in ectopic fat accumulation. According to the adipose tissue expandability hypothesis, subcutaneous adipose tissue has a limited capacity to expand to accommodate excess energy in the form of triglycerides. Once subcutaneous adipose tissue expansion reaches a threshold, excess energy begin to be stored in ectopic sites and adipose tissue dysfunction ensues, with profound health consequences. Ectopic fat deposition within and around tissues and organs such as the heart, liver, and pancreas may cause dysfunction due to mechanical compression or by exposing the organ to substances secreted by the ectopic adipose tissue cells in a paracrine way. Hepatic fat accumulation favors development of systemic insulin resistance and T2D (468), while epicardial adipose tissue is associated with coronary atherosclerosis and CVD risk (543).
Measures of abdominal adiposity are not only associated with CVD risk factors and outcomes independently of total adiposity or BMI, but they confer incremental predictive values. Thus, supplementing conventional BMI-based characterization with waist circumference or waist-to-hip ratio yields better assessment of risk. Central obesity defined by waist circumference is associated with increased prevalence of cardiometabolic risk markers across BMI-defined normal weight, overweight, and class 1 obese (BMI of 30–34.9 kg/m2) subjects (468). In the European Prospective Investigation into Cancer and Nutrition (EPIC) study, adjusting for BMI in models testing the relation between waist circumference or waist-to-hip ratio and mortality significantly improved risk prediction (474): at a given BMI, a 5 cm increase in WC portended a 1.17-times and 1.13-times increased risk of death in men and women, respectively. A recently published dose-response meta-analysis that combined data from over 2.5 million participants of 72 cohorts confirmed peculiar patterns of associations with mortality across central adiposity metrics (303). A J-shaped relation emerged for waist circumference and waist-to-hip ratio, while a positive linear relation was observed for waist-to-hip ratio. However, aggregate data showed that the slope of these relations increased markedly after taking into account BMI, further supporting the notion that both indicators of general and central fatness should be used for risk stratification. Combination of standard BMI classification with measures of body composition and fat partitioning yields various obesity phenotypes, with related different health status and prognosis.
As mentioned above, there is large variability in body fat percentage at any given BMI. It follows that even within the normal weight BMI category, individuals may have high adiposity and be obese based on their body fat content. Using the NHANES datasets, Romero-Corral et al. (496) categorized 6171 normal BMI (18.5–24.9 kg/m2) individuals into sex-specific tertiles according to their body fat percentage and found that prevalence of metabolic syndrome and its components increased with higher body fat. Compared to their respective lower tertiles of body fat, men with normal BMI but body fat >23% (higher tertile) were more likely to have dyslipidemia and hypertension, while normal weight women with body fat >33% were more likely to have dyslipidemia and CVD. Other investigations have corroborated these findings, reporting greater risk of cardiometabolic abnormalities in subjects with normal BMI but elevated body fat relative to their low body fat counterparts (305, 341, 524). Normal weight obesity also predicts CVD mortality in older adults (50). When localization of excess adiposity is taken into account, mortality risks associated with this obesity phenotype appear to be exacerbated. Individuals with normal BMI but high waist-to-hip ratio are at greater risk of total and CVD death than both those with normal BMI and normal waist-to-hip ratio and those who are overweight or obese based only on BMI, across all ages (508). A prospective evaluation of the Women’s Health Initiative (WHI) cohort corroborated these results, with normal weight women with central obesity having 25% greater risk of CVD mortality than those without central obesity, and similar to overweight and obese women (564). These data collectively underscore the health hazards posed by normal weight obesity (202), which are under-recognized.
Metabolically Healthy Obesity and CV Health
The notion of metabolically healthy obesity, which integrates fatness information with clinical and laboratory parameters, has been gaining attention in recent times. This phenotype refers to individuals who are obese based on BMI, waist circumference, or body fat content but do not manifest the expected pattern of complications such as insulin resistance, hyperlipidemia, or arterial hypertension. Due to the heterogeneous definitions used in studies to identify metabolically healthy obesity, estimates of its prevalence diverge considerably, ranging between 6% and 75% (490). Earlier studies noted that subjects with obesity lacking cardiometabolic abnormalities also exhibited a favorable risk profile relative to those with metabolically abnormal obesity, and comparable to normal weight populations (476). Stefan et al. (553) showed that subjects with obesity who are insulin sensitive had lower intrahepatic fat accumulation and skeletal muscle fat infiltration, and lower carotid intima-media thickness (CIMT) than those with obesity and insulin resistance, in spite of similar total body fat. Additionally, insulin sensitivity and CIMT were similar between insulin-sensitive obese and normal weight subjects. Metabolically healthy obese individuals also display better cardiorespiratory fitness (450), higher adiponectin (7), lower CRP (320), and interleukin-6 (527). However, the clinical significance and prognostic value of this obesity phenotype remain controversial, potentially in part because of the lack of a standardized definition. Longitudinal studies have largely shown that metabolically healthy obesity may not be advantageous in the long term, failing to protect against CV events and mortality. Even in the absence of concurrent metabolic derangements, subjects with obesity are at similar (421) or higher risk of incident CVD compared to their normal weight counterparts (99). Collective evidence indicates that those with metabolically healthy obesity have 52% higher risk of CVD and 23% higher risk of total mortality compared to metabolically healthy normal weight individuals (650), thus rejecting the concept that it is a benign condition. Furthermore, data suggest that nearly half of metabolically healthy obese individuals (48%) transition to a metabolically abnormal phenotype (421), suggesting that this population should be regarded as being at a preclinical stage of metabolic dysfunction.
Muscle Mass in CV Health
While the health impact of body fat is complex, as described above, a large body of evidence consistently supports protective effects of lean (muscle) mass. Muscle mass is inversely related to T2D and HbA1C levels (250). While the prevalence of hypertension decreases with increasing skeletal muscle, a positive relation is seen with body fat percentage (251). Lean mass is also negatively associated with arterial stiffness (193, 443). In terms of mortality, loss of muscle mass, indicative of sarcopenia, is a robust predictor of mortality (19). The opposite implications of high-fat mass and low fat-free mass (body weight minus fat mass) for mortality risk are also thought to contribute to the curvilinear pattern of lower life expectancy typically noted at both ends of the BMI continuum. In a large Danish cohort of 50 to 64 years old men and women (69), in whom body composition was assessed via bioelectrical impedance, a J-shaped association was found between fat mass and risk of death, while a reverse J-shaped association was evident for fat-free mass. Thus, the U-shaped relation between BMI and mortality likely reflects the combination of such opposite patterns. Comparable trajectories were reported by a study using dual-energy X-ray absorptiometry to determine body composition, with total fat mass predicting increased risk of death and lean mass predicting better survival (582).
Sarcopenic Obesity and CV Health
Another obesity phenotype is sarcopenic obesity. This condition is characterized by loss of skeletal muscle mass and strength accompanied by gain in fat mass. Because the BMI may remain stable, sarcopenic obesity remains often masked when only using this anthropometric measure. Sarcopenic obesity is thought to be present in 5% to 10% of older adults (360), although prevalence estimates vary greatly (49), likely as a function of the different operational definitions. Currently, there is indeed no consensus on this classification, as multiple indices and cutoffs of both sarcopenia and obesity are applied (641). With regard to the former, muscle strength, muscle quality and quantity, and physical performance can be used to indicate sarcopenia. A number of studies have suggested that sarcopenic obesity may confer disadvantages compared to obesity alone. In comparison to subjects with nonsarcopenic obesity, those with sarcopenic obesity have been found to have higher circulating triglycerides, insulin, lower HDL-C (121), higher blood pressure (461), and altered glucose metabolism (545). However, others have disputed these findings, failing to find greater degrees of cardiometabolic dysfunction in sarcopenic obesity versus nonsarcopenic obesity, especially in women (176, 411). Mixed observations have been reported also with regard to future CVD risk in individuals with sarcopenic obesity (25, 191). However, in pooled estimates, sarcopenic obesity was associated with 21% increased risk of mortality in older adults (657). Notably, the association persisted irrespective of the indices used to define sarcopenia or obesity.
Obesity Paradox in CVD
The discrete predictive values of body composition and fat distribution may be implicated in the so-called “obesity paradox.” This expression has been coined to describe the paradoxically better prognosis associated with elevated BMI in patients with established CVD and challenges the concept of obesity as a predictor of adverse CV sequelae. An obesity paradox has been observed in relation to outcomes of multiple manifestations of CVD, including acute coronary syndromes (18, 172, 611), stable CHD (161, 356), atrial fibrillation (29, 509), HF (236, 357), and stroke (15, 595). A large Swedish registry of patients with acute coronary syndromes showed a curvilinear relation between BMI and mortality over a 3-year period, with the lowest risk of death in overweight patients and decreased risk in obese patients up to a BMI of 35 kg/m2 (18). Combining three cohorts of patients following coronary artery bypass grafting, Schwann et al. (518) showed that a BMI of 29 kg/m2 was associated with best survival, and overweight and class 1 obese patients had better CV and non-CV free survival over a 15-year follow-up. However, there was a temporal gradient showing that overall superior life expectancy was evident in the early (0–1 year) and intermediate (1–8 years) postoperative time, but the advantage dissipated over the late period (8–15 years). Pooled estimates support a time trend, with overweight and obesity conferring survival advantages in the short- and long-term period (≥6 months), but with benefits curbing after 5 years (615). In patients with chronic HF, the obesity paradox is evident in both preserved and reduced ejection fraction forms, with lower mortality risk in those with BMI of 25 to 34.9 kg/m2 (452). Overall, the nadir mortality is seen within the overweight and moderately obese range, while data are more conflicting with regard to severe and morbid obesity (172, 219, 236, 329). It has been argued whether the obesity paradox represents a true phenomenon or is rather a manifestation of residual confounding, unmeasured risk factors, unintentional weight loss, or selection bias (194). CVD patients with obesity are younger, more likely to exhibit multimorbidity and to receive CVD pharmacotherapy (172, 452). Superior control of comorbidities and risk factors may improve neurohormonal status thus enhancing survival. Other mechanisms include increased calorie reserve, lower circulating levels of natriuretic peptides, and greater cardiorespiratory fitness (107, 355). Additionally, aspects related to adiposity assessment are likely to play a role. Indeed, when other adiposity measures are used, or when fat distribution is considered, no obesity paradox is noted. Abdominal obesity, determined by waist circumference, is positively associated with mortality (144). Patients with HF and abdominal obesity had 1.52 greater risk of all-cause mortality and 1.50 times greater risk of CVD mortality than those without in multivariable analysis (588). Conversely, when using BMI, the expected obesity paradox with improved life expectancy in the patient groups with overweight and obesity emerged. Furthermore, increased lean mass has been linked to improved survival in stable CHD (356) and adjustment for lean mass nullified the association between high-fat mass and lower mortality in HF patients (162).
Brown Adipose Tissue and CV Health
To evaluate the effects of excess fat mass, an important parameter to consider is the type of fat. Most fat in adult humans consists of white fat depots which store excess energy. However, metabolically active brown fat depots are known to exist as well and are being targeted to improve metabolic profile (126, 147, 604, 652). A recent study examined the relationship between brown fat and CV health using 18F-flurodeoxyglucose PET/CT scans from 52,487 cancer patients (53). This study showed that the presence of brown fat depots was negatively associated with BMI and age. Also, compared to men (4.9%), the presence of brown fat was noted to be more in women (13.8%). The higher prevalence of brown fat in women has been previously documented as well (147). Importantly, Becher et al. show that individuals with brown fat have lower prevalence of CV risk factors and were associated with decreased odds for T2D, dyslipidemia, hypertension, atrial fibrillation, coronary artery disease (CAD), and HF. Moreover, the beneficial effects of presence of brown fat were more pronounced in overweight and obese individuals. Another study has shown that polymorphism in key proteins associated with brown fat metabolism such as uncoupling proteins are associated with development of obesity, dyslipidemia, T2D, and CVD (475). Evidence from clinical studies suggests that increased brown fat may improve CV health via increasing energy expenditure, enhancing glucose and free-fatty acid disposal, and improving lipid utilization (45, 118, 252, 652, 653) These findings are concordant with a recent study showing a positive relationship between high brown fat activity and future improvement in CV risk parameters (483). However, factors contributing to inter-individual variability in brown fat depots are not completely understood and represent an active area of research. Overall, studies suggest even though brown fat may improve CV risk, it is unlikely to play a role in weight control (399).
Lipodystrophy and CV Health
While excess body fat is detrimental, reduced body fat also has adverse effects on CV health. Body fat may be diminished in conditions of lipodystrophy resulting in increases in traditional CV risk factors including hypertriglyceridemia, low HDL-C, insulin resistance, and diabetes (91, 281). Lipodystrophy is also often accompanied with body fat redistribution resulting in lipoatrophy in lower body fat and face, and lipohypertrophy of fat depots located in abdomen, breasts, and the supraclavicular region (281). While metabolic and hepatic effects of lipodystrophy are well studied, there is a paucity of data related to its CV effects. Based on limited data, lipodystrophy patients often have hypertrophic cardiomyopathy, CHD, diabetes, and hypertension (91, 281, 366, 380, 510). The frequent cardiomyopathies contribute to sudden cardiac arrest and a shortened lifespan as well. Importantly, sex differences exist, and compared to men, diabetes, dyslipidemia, and atherosclerotic disease burden including CHD and stroke have been reported to be prominent in women with familial partial lipodystrophy (212). Importantly, mechanisms adversely affecting CV health in lipodystrophy are not completely understood but alterations in lipid metabolism, lipotoxicity, insulin resistance, and leptin deficiency are considered important contributors (281).
Sex as a CVD Risk Factor
Along with age, hypertension, smoking, dyslipidemia, and diabetes, sex is recognized as major risk factor for developing CVD. Sex-based differences in development and progression of CVD are well established (215) and clinical calculators routinely used to estimate CVD risks such as Atherosclerotic CVD Risk Estimator (369) and Framingham Risk Calculator (148) use sex-specific algorithms to predict 10-year risk for individual CVD events to inform potential preventive measures. For exactly similar age and CV health profile—total cholesterol, HDL-C, blood pressure, smoking status, and his-tory of diabetes, young and middle-aged men (20–59 years) have approximately twice as high probability to develop CVD in the next 10 years than women.
Sexual dimorphism is also evident in prevalence of certain CVD risk factors including dyslipidemia (110), hypertension (603), diabetes (603), and certain CVDs such as HF and atrial fibrillation (391). For example, the prevalence of unhealthy total cholesterol (≥200 mg/dL) in women is 40.4% compared to 35.4% in men (603). On the other hand, more men are likely to have low HDL-C (<40 mg/dL) than women (28.5%in men vs. 8.9% in women) (110). Prevalence data for sex differences in other risk factors are presented in Table 2. Importantly, these sex differences in risk factors translate to differences in development of different CVDs as well. An increased age-adjusted prevalence of all heart diseases (11.8% vs. 9.5%, men vs. women, respectively), CAD (7.2%vs. 4.2%), and hypertension (26.0% vs. 23.1%) is observed in men (603). For HF, it is well documented that women constitute the majority of patients with preserved ejection fraction while HF with reduced ejection fraction is more common in men (267, 268).
Table 2.
Sex Differences in Prevalence of CV Risk Factors
| Risk factor | Prevalence in adult men (%) | Prevalence in adult women (%) |
|---|---|---|
| High LDL-C (>240 mg/dL) | 10.7 | 12.4 |
| Low HDL-C (<40 mg/dL) | 28.5 | 8.9 |
| Hypertensiona | 49.0 | 42.8 |
| Diabetes | ||
| Diagnosed | 10.9 | 8.9 |
| Undiagnosedb | 4.6 | 2.8 |
| Prediabetesc | 44.0 | 31.3 |
All data are age-adjusted (603).
Using 2017 definition—SBP ≥ 130 mmHg or DBP ≥ 80 mmHg or self-reported antihypertensive medication use or having been informed by a physician or other health professional that one has high blood pressure on at least two occasions.
Undiagnosed diabetes is defined as presence of fasting blood glucose (FBG) ≥ 126 mg/dL but who did not report being told by a healthcare provider that they had diabetes.
Prediabetes is defined as fasting blood glucose of 100 to <126 mg/dL. Based on, with permission, Virani SS, et al., 2020 (603)
The sex differences in CVD are specifically prominent in certain age groups (603). The prevalence of CVD in women aged 20 to 39 years is much lower than similarly aged men (17.2% vs. 29.8% in women vs. men, respectively). In the age group of 40 to 59 years, the prevalence of CVD increases to 56.9% in men and 51.6% in women but still remains lower in women. In contrast, however, the CV protective effects of female sex are diminished after menopause, and women over the age of 60 years have CVD prevalence and CVD mortality which is comparable to men (78.2% vs. 77.2%; women vs. men respectively) (517, 603). The marked change in CVD risk profile in women during aging occurs in parallel to menopause transition suggesting that the sex differences in cardiometabolic risk profile maybe partly attributed to gonadal hormones such as estrogens (mainly estradiol, E2) and testosterone (T).
Contradictory to cardioprotective effects seen in healthy women, established CVD risk factors such as diabetes, and smoking, confer higher CVD risk and consequent CVD mortality in women than in men (282, 283, 469, 614). Compared to men, women with type 1 diabetes have 154%higher incidence of CHD and 86% higher mortality related to CV causes (282). In a meta-analysis including more than 5 million participants from 86 prospective cohort studies, Wang et al. (614) showed a 13% greater risk for all-cause mortality and a 30% greater risk for CVD mortality in women with diabetes compared with men. The increase in CVD mortality in women was driven by increased relative risk for CHD. Notably, these sex differences were not evident for cancer-related mortality in this study, suggesting that the existence of sex-specific all-cause mortality differences may likely stem from CV causes. Likewise, cigarette smoking confers 25% higher CVD risk in women than in men (283) and hypertension increases the risk for HF 3 times in women compared to 2 times in men (363).
These sex differences extend to CVD prevention as well. As an example, in spite of overall lower prevalence of hypertension in women, it is projected that elimination of hypertension could reduce CVD mortality by 30.4% among men and 38.0% among women (464). Furthermore, elimination of hypertension would be most effective in women aged 65 to 79 years where it may have the potential to reduce CVD mortality by 48.8%. This discordance in prevalence of hypertension and CVD mortality reduction may be explained by higher blood pressure-related mortality in women (52.1%) than in men (47.9%) (603).
There are several factors which may contribute to sexual dimorphism in CVD. These factors may include differential regulation of traditional CVD risk factors including lipid metabolism, insulin sensitivity, and hypertension in men and women. Compared to men, women especially at younger ages tend to have a more favorable lipid profile and remain insulin-sensitive even with higher adiposity (418). In addition, the underlying pathophysiological responses are also different in men and women which contribute the sexual cardiometabolic dimorphism. For example, fibrosis is an important pathological mechanism which differs in men and women. Myocardial fibrosis affects men to a greater degree than women partially due to greater induction of the renin-angiotensin system in men than in women (319). During stress, men activate more pro-fibrotic genes and generate more collagen and fibrous tissue than women (470). How-ever, among these factors, sex hormones play a prominent role through the interplay with other possibly sex-specific processes such as lipid metabolism, insulin sensitivity, and adiposity (388, 389, 467). Of note, cellular receptors through which sex hormones such as T and E2 may directly regulate metabolism and pathological processes are expressed throughout the CV system. To gain further understanding, we provide below an in-depth review of findings related to sex hormones and CVD risk in men and women.
Sex Hormones and CV Health in Men
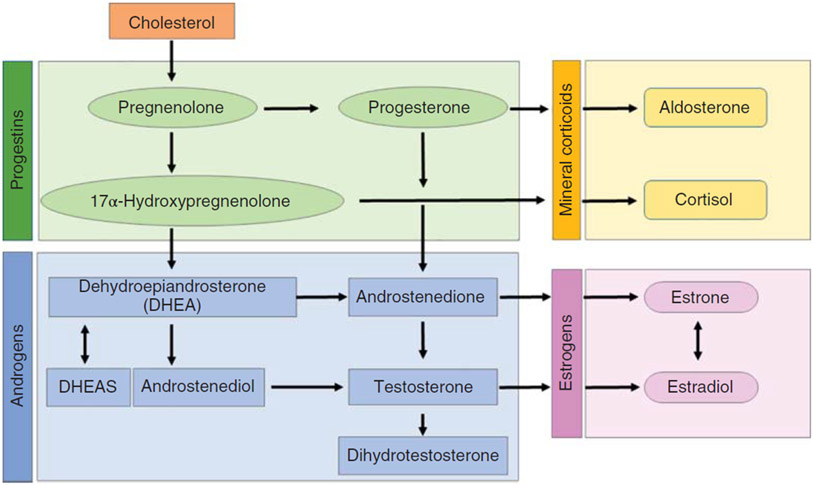
In adult men, T is the predominant sex hormone, which is enzymatically converted to other sex hormones relevant to CV system including E2, progesterone, and dehydroepiandrosterone (DHEA) (338, 371). The conversion of T to other sex hormones occurs in testis and other tissues of importance to CV physiology including adipose tissue, skeletal muscle, heart, blood vessels, kidney, brain, and bone where these hormones can directly mediate cellular effects via specific androgen and E2 receptors (133, 599). A simplified schematic of the process of steroidogenesis is presented in Figure 2.
Figure 2.
Simplified schematic highlighting pathways involved in synthesis of sex hormones. Cholesterol is a precursor for progestins which are converted to androgens and mineral corticoids through enzymatic processes in specific cell types. Estrogen production relies on aromatization of androgens.
Relationship between T and CVD in men
An understanding of the role of T in CVD in men can be gained through several sources including observational and interventional studies in different populations. These studies are presented below.
Evidence from observational studies
Circulating T concentrations are known to decrease with age and certain metabolic conditions established to contribute to cardiometabolic pathophysiologies such as obesity, T2D, and metabolic syndrome (255, 339). Therefore, it is not surprising that several cross-sectional epidemiological studies show that low T is associated with increased risk for CVD (137, 254, 353) and consequent CVD mortality as well (20, 140). Conversely, high T levels are associated with reduced CVD risk (332, 446). In a meta-analysis including 37 prospective observational studies examining the relationship between endogenous T levels and CVD risk in more than 43,000 men with a mean age of 63.5 years and an approximately 6.4 years of follow-up, Corona et al. (137) showed that low endogenous T was associated with 54% higher risk of CVD mortality and 17% higher risk for incident CVD. However, the higher risks for CVD morbidity and mortality were evident only after adjustment for age, diabetes, and smoking were considered and the strength of these associations varied with method used to measure T. Importantly, this meta-analysis acknowledged several limitations including publication biases related to studies reporting CVD mortality, lack of precise definition related to CVD events, and missing data which confounded the findings (137). Nevertheless, findings from this latest meta-analysis confirm reports from previous pooled analysis which showed low T to have a tendency to increase CVD mortality and increased risk for CVD events in men (20, 140).
Similar to CV mortality and CV events, low T is also associated with higher prevalence of CV risk factors. In a post hoc analysis of 2118 participants from the AIM-HIGH trial, compared to men with high T, the study reported a clustering of CVD risk factors including diabetes, hyper-tension, high triglycerides, and obesity in men with low T (76). Similarly, in a recent prospective study of 612 men with stable CAD and a median follow-up of 5 years, high serum free T levels were shown to be associated with less CVD mortality independent of traditional risk factors including age, diabetes, cholesterol, hypertension, smoking, and family history of CAD (484). However, these findings are in contrast with another meta-analysis (503), which included nested case-control studies along with prospective studies of population-based cohorts and showed no association between endogenous T and CV disease in men younger than 70 years and a weak protective effect of T in older men aged ≥70 years. Few studies also noted no association of T levels with CVD events and mortality (22, 129, 253, 546).
An important confounding in our understanding of the physiological effects of T relates to total circulating versus bioavailable T. Most studies rely on the measurement of total T which includes both free molecules and those which are bound to other molecules including albumin and sex-hormone binding globulin. The distinction between free and bound form of T is important as when bound with sex-hormone bind-ing globulin, the circulating T is unable to activate cellular signaling. Therefore, in certain conditions, high circulating T may not translate into high bioavailable T. Nevertheless, in conditions of low circulating T, bioavailable T levels will also be low and unlikely to change the relationship between T and CVD. Indeed, in the Osteoporotic Fractures in Men study low total circulating T levels continued to associate with high CV risk even after adjustment for sex-hormone binding globulin levels (446). This study included 2416 men aged 69 to 81 years with a median 5-year follow-up for CVD events and showed that men with T levels higher than 550 ng/dL had an approximately 30% lower risk for CVD events and death.
The overall consensus from these observational studies agrees to the presence of an inverse relationship between T and CVD risk in men.
Evidence from observational T replacement studies
Glimpses into the causal role of T in development and progression of CVD can be derived from findings from studies of testosterone replacement therapy (TRT) in hypogonadal men with decreased T levels. Considering the inverse relationship between T and CVD, a TRT-associated increase in circulating T would be expected to decrease CV risk. However, most observational studies comparing CVD events between men using versus not using TRT do not report differences in CVD events or CVD mortality and only few studies report decrease in CVD risk in T users (34, 171, 237, 505, 529).
Overall, the findings from T replacement in patients with CVD are controversial. In four clinical trials including HF patients, T therapy was associated with improvement in exercise capacity without incident CVD events (581). However, in a subsequent clinical trial, 12-month TRT in hypogonadal men with chronic HF with reduced ejection fraction was not associated with any improvements in clinical symptoms or functional capacity (436). In contrast, a retrospective study including participants with coronary angiography showed that TRT was associated with 29%increased risk for all-cause mortality, MI, and ischemic stroke (601). However, this study generated scientific controversy due to their analytical approach and several professional societies called for a retraction (428). Similarly, in a case-control study, Etminan et al. (188) showed that TRT was associated with a 41% increased risk for MI and a retrospective cohort study including individuals with prior history of heart disease also showed increased risk for nonfatal MI among T users (199). Importantly, the findings from these observational studies should be cautiously interpreted as they have marked methodological heterogeneity and overall evidence has been rated as very low quality (10, 428). Nevertheless, evidence from observational studies of anabolic androgen substance abuse also supports androgen excesses to increased CVD risk (31, 205, 441). In a study of 86 experienced male weight lifters with ≥2 years of cumulative use of anabolic androgen and 54 nonusing controls, a decrease in left ventricular systolic and diastolic function, as well as increased prevalence of advanced coronary artery plaque volume, was observed among androgen abusers (31). The detrimental effects of anabolic androgens on CV system are also corroborated by histopathological findings from deaths attributed to anabolic androgen which showed cardiac hypertrophy and fibrosis, thrombotic events, and cardiac necrosis (205).
Evidence from randomized clinical trials (RCTs)
Similar to the observational T replacement studies, there are several inconsistencies in findings from randomized clinical trials (RCT)s of TRT as well (10, 78, 100, 135, 138, 171, 192, 238, 428, 581, 642). The US Food and Drug Administration has approved T products for replacement therapy in men with classical hypogonadism but not age-related hypogonadism. However, T is frequently over-prescribed in middle-aged and elderly men without discernable T deficiencies and the effects of such therapy on CVD are not completely understood (438). This prompted the American College of Physicians to undertake a meta-analysis of TRT-associated harms and benefits and they determined that the evidence related to CVD risks and events were insufficient (171). Among the clinical trials examining the effects of T therapy in hypogonadal men, only 14 clinical trials including 2415 participants reported CVD events as adverse events. Also, participants with high CVD risk including recent history of MI or stroke, and advanced HF were not included in these clinical trials thereby limiting our understanding of the beneficial or harmful effects of TRT on CVD. In spite of these limitations, this meta-analysis revealed that, com-pared to placebo, TRT did not alter the risk for CVD events (171, 481). These findings are concordant with a previous meta-analysis including 20 RCTs which demonstrated that TRT did not change the risk for MI, stroke, or mortality (10). However, these findings are discordant from another meta-analysis which only included placebo-controlled RCTs of TRT lasting more than 12 weeks (conducted prior to 2012) which demonstrated that TRT was associated with 54% higher risk of CVD events (642). Interestingly, this meta-analysis showed that the results were biased by the source of funding with increases in CVD events apparent only in studies not funded by the pharmaceutical industry.
An important aspect to consider when examining effects of TRT on CVD may be related to the route of administration. In a meta-analysis of RCTs, Borst et al. (78) observed no significant of effect of TRT on CVD risk when all routes of administration were grouped together. However, when the studies were analyzed separately based on administration routes, increased CVD risk was apparent when T was administrated orally. Interestingly, the oral formulation of T has been shown to increase blood pressure and is contraindicated in men with age-related hypogonadism (171).
Among the RCTs conducted so far, the TOM trial (T in Older men with Mobility Limitations) may be considered landmark for its association of TRT with increased incidence of adverse CV events (46). Of note, the TOM trial was not included in the recent meta-analysis conducted on behalf of the American College of Physicians to reduce risk of bias based on attrition rate (171). This placebo-controlled RCT included community-dwelling men aged 65 years or older with hypogonadism and mobility limitations and was designed primarily to compare the effects of transdermal T gel versus placebo on muscle strength. While the study showed improvement in muscular strength in the T-treated participants, it required an early termination due to higher occurrence of CVD events in the participants assigned to TRT (46). However, it is noteworthy that the adverse events were only evaluated as a part of safety monitoring and were not a part of the planned primary or secondary endpoints. Importantly, in a subsequent RCT of TRT in older men, the rate for adverse CV events did not differ between in the placebo and T treated group (537). The difference in these two RCTs may be related to degree of therapy-induced increases in circulating T levels. The latter study aimed to achieve increases in T only to the levels compatible with mid-normal range observed in younger men aged 19 to 40 years while the increase in T in the former study were much higher (135, 199, 601, 642). Indeed, most adverse CV events were noted in men in with highest quartile of T during the interventional period (46).
Considering the inconsistent findings of the effects of TRT on CVD, a double-blind placebo-controlled 12-month T supplementation trial was undertaken to examine the effects of T supplementation on CV risk factors. This study enrolled 788 men with ≥65 years of age and T levels less than 275 ng/dL (419). The main outcome measures of this study included traditional cardiometabolic risk factors such as lipid profile, glucose metabolism, fibrinolysis, inflammation, and myocardial damage. However, individuals with high risk for CVD including prior history of MI, hypertension, elevated creatinine, and diabetes were excluded from participation. The study showed that compared to placebo, TRT decreased total cholesterol, HDL-C, and LDL-C. A slight reduction in fasting insulin and HOMA-IR were also seen. However, TRT was not associated with changes in triglycerides, d-dimer, CRP, interleukin-6, troponin, glucose, or HbA1C. In other words, T supplementation was not associated with overall improvement in CV risk profile.
The evidence for the effect of TRT on insulin sensitivity is conflicting with some studies showing improvement (168, 318) and others reporting no benefit (220, 233, 277). Similarly, with regard to the effects of TRT on glycemic control, conflicting findings have also been reported. Meta-analyses of heterogeneous studies that included open-label and cross-over studies of varying durations reported that TRT led to a small reduction in HbA1C (98, 136, 365). In a recent 2-year clinical trial including 1007 men of 50 to 74 years with central obesity and impaired glucose homeostasis, TRT was associated with decreased prevalence of T2D beyond the effects of lifestyle interventions (633). Compared to participants who received placebo, the risk for T2D in the TRT group was reduced by 40%. However, increased incidence of hematocrit greater than 54% was noted in the TRT group (22% vs. 1%, TRT vs. placebo, respectively), suggesting that TRT would potentially have safety concerns including increased CVD risk. With regard to CVD risk with TRT, meta-analyses have also yielded conflicted findings of increased risk (642) or no overall increased risk (9, 10, 100, 138, 183, 192, 238, 458). These meta-analyses were, how-ever, limited by inclusion of low-to-medium quality trials, variable treatment durations, heterogeneous patient characteristics, varied definitions, and low numbers of adverse CV events, thus lacking adequate statistical power. Considering differential effects of TRT on various CV risk factors, its clinical importance with regard to overall health and CVD risk reduction remains undiscernible.
Evidence from androgen deprivation therapy in cancer patients
Sex-hormone targeted therapy is frequently used for treatment of cancer patients sensitive to these hormones. In patients with metastatic prostate cancer, androgen deprivation therapy (ADT) is the main therapeutic approach (362). T reduction in these patients is mainly achieved via use of gonadotropin-releasing hormone (GnRH) agonists, orchiectomy, GnRH antagonists, estrogen analogs (diethylstilbestrol), antiandrogens, and androgen receptor antagonists. While ADT is effective in management of prostate cancer, the adverse CVD effects of ADT therapy are not completely understood. A meta-analysis including 8 observational studies showed that compared to men not treated with ADT, use of GnRH agonist increases the risk for nonfatal CVD by 38% (80). Similar increases in risk were observed for use of GnRH agonist and increases in ischemic heart disease, MI and stroke as well. Orchiectomy and antiandrogen therapy also showed increased risk of nonfatal CVD events by 44% and 21%, respectively. In contrast, the findings from RCTs do not consistently demonstrate that ADT is associated with increased CVD mortality. In a meta-analysis of 8 RCTs including 4141 patients, Nguyen et al. (439) showed that the CVD death was not increased in patients receiving ADT. In another study including a long-term follow-up of RCTs of with and without ADT, survival benefits from CV causes for not using ADT were noted in participants who had presence of moderate or severe comorbidities (151). Compared to patients receiving ADT, CVD mortality was similar without ADT use when patients had none or minimal comorbidities. In spite of all the nuances and controversies around clinical recommendations related to use of ADT in prostate cancer patients (68, 362, 504), the increased CVD risk in men undergoing ADT suggests that T might have cardioprotective effects (275, 362).
Summary
Overall evidence from observational studies and RCTs do not support the causal role between low T and CVD risk. It is likely that low T may not contribute to CVD but rather be a biomarker of poor health and lifestyle associated with CVD. However, an important aspect related to the age range of participants included in the studies remains lacking. Representation from hypogonadal men aged 18 to 45 years is deficient (171). The discrepancy related to age range may be important as the CV risk directly related to low T may be more apparent in younger men in absence of other prevalent confounding comorbidities such as diabetes and hypertension. The higher prevalence of cardiometabolic comorbidities in older population may lead to a lesser contribution for low T with consequent smaller improvements with T therapy. In the context of the inconclusive evidence, the US Food and Drug Administration and professional medical societies have called for a rigorous clinical trial to overcome the shortcomings from prior studies including short follow-up duration, exclusion of participants with high CVD risk, and lack of CVD endpoints as primary outcomes. Consequently, the TRT for assessment of long-term vascular events and efficacy response in hypogonadal men trial (TRAVERSE trial, NCT03518034) has begun in 2018 with expected completion date in June 2022 to include 6000 participants aged 45 to 80 years with preexisting CVD or significant CVD risk factors and examine participants for 5 years for CV safety (210). The primary endpoint for this study is a composite of major adverse cardiac events comprising of nonfatal MI, nonfatal stroke, or death due to CVD. The secondary out-comes include cardiac revascularization procedures, cardiac percutaneous interventions, and coronary artery bypass graft.
Relationship between estrogen and CVD in men
In men, estrogens are formed by the aromatization of andro-gens and have several important roles in male physiology (133). Importantly, in the last few decades, there has been a paradigm shift in our understanding of E2 in men and its role in development and progression of CVD is being increasingly recognized (133, 312). The circulating levels of E2 in an individual are largely determined by levels of T, aromatase activity, and rates of estrogen breakdown and clearance (374, 534). Therefore, evidence for the role of E2 in CVD in men can be largely gathered from (i) observational cross-sectional and longitudinal studies; (ii) case reports of estrogen deficiencies resulting from mutation in aromatase and estrogen receptors; and (iii) longitudinal studies of aromatase inhibition or estrogen treatment. Lastly, estrogen activity in men may also be modulated by estrogen-mimicking compounds associated with environmental and dietary factors. The findings from these sources are presented below.
Evidence from observational studies
Compared to T, studies investigating E2 in men are very few which has led to inconsistent findings. In a meta-analysis including 14 prospective population-based cohorts and nested case-control studies, Vandenplas et al. (592) reported no association between E2 and incident CVD. These findings are also concordant with findings from subsequent studies which show no significant relationship between E2 and CVD or CVD mortality (114, 253, 271, 272, 446, 646, 647). In contrast, in a previous meta-analysis of cross-sectional and prospective studies, elevated circulating E2 was reported in men with CVD (140). This analysis showed that after adjustment for age and BMI, each 1 pmol/L increment of E2 increased CVD risk by 1.5%. However, unlike T, baseline E2 did not predict future CVD development (140). Indeed, earlier studies examining the effects of conjugated E2 on CVD in the Coronary Drug Projects were shown to associate high 5.0 mg/day of estrogen therapy with increased incidence of MI, pulmonary embolism, and thrombophlebitis. However, these adverse events were not observed at lower E2 dose of 2.5 mg/day. In contrast, other studies have shown that low E2 levels are associated with higher risk for CVD and mortality (22, 580). In a study including 1114 men with a median follow-up of 16 years, men with low E2 were shown to be associated with a significantly higher risk of CVD mortality during the baseline to 9-year follow-up but not during the 9 to 18 year follow-up period (410). Likewise, compared to healthy controls, low E2 levels were observed in men with progressive CAD (36). These inconsistencies in findings may be partly related to limited availability of the data and also a result of complex relationship between T and E2. Since T is substrate for E2; T deficiency is likely to cause lowering of E2 as well. Therefore, low T and low E2 may be expected in individuals with high CVD risk. On the other hand, elevated E2 can decrease T expression via inhibitory effects on release of gonadotropin hormone through the hypothalamic-pituitary axis. Therefore, it is likely that high E2 may cause T deficiency and be asso-ciated with high CVD risk. It is also possible that the relationship between E2 and CVD risk is U shaped rather than linear. Indeed, in men with chronic systolic HF, both low and high levels of E2, compared to middle quintile, have been shown to be associated with increased CVD mortality (295).
Evidence from rare genetic estrogen deficiencies
An important source of evidence for the physiological role of E2 in men comes from the rare, documented cases of aromatase and estrogen-receptor deficiencies. In all cases of E2 deficiencies arising from loss-of-function mutation, adults have sought medical attention due to bone pain or continuous increases in height, emphasizing the importance of E2 in bone metabolism, namely bone turnover, and mineralization (51, 70, 82, 106, 116, 263, 350, 386, 387, 414, 429). The metabolic effects of E2 deficiency are varied with dyslipidemia being a fairly common feature (51, 70, 106, 116, 262, 263, 350, 387, 429, 493). E2 deficiency is also frequently characterized by alterations in glucose metabolism with increased fasting insulin and glucose along with impaired insulin sensitivity being reported in several individuals (70, 116, 350, 387, 429, 536). Hypertension has also been reported in some individuals (310). Importantly, the detrimental effects on glucose homeostasis and lipid metabolism are reversed during E2 replacement therapy in men with dysfunction aromatase protein (263). The variations in metabolic manifestation of the mutations may be related to the degree of functional loss as well as exposure to dietary and environmental E2 which may obscure the full manifestation of endogenous E2 deficiency. Nevertheless, these frequently documented metabolic effects of E2 deficiency are also likely to increase CVD risk. Indeed, flow-mediated brachial artery vasodilation (an early marker for CVD) was shown to be absent in a man reported to have estrogen receptor-1 deficiency along with a subsequent documentation for accelerated coronary arteriosclerosis in the same individual (501, 561, 562). Likewise, a polymorphism in aromatase gene has also been shown to be associated with CHD and hypertension in men (56, 124). On the other hand, mutations in aromatase with increase in function have also been identified which shows that having T in normal range along with elevated E2 does not have any metabolic effects (93, 125, 163, 209, 229, 402). Collectively, data from these case reports suggest that E2 may have cardioprotective role in men.
Evidence from E2 supplementation or inhibition therapy
E2 is targeted for management of certain conditions in men. Supplemental E2 and synthetic E2 analogs may be therapeutically used to induce androgen deficiency in prostate cancer patients. On the other hand, inhibition of E2 is targeted via use of aromatase inhibitors (AIs) and selective estrogen receptor modulators (SERMs) in patients with hormone-sensitive breast cancer. AIs are also used for the management of gynecomastia and T deficiency in some men. Synthetic estrogens including diethylstilbestrol were successfully used to treat prostate cancer in the 1960s and 1970s but were later found to be associated with increased risk for MI and its use was significantly curtailed (32). However, compared to other ADT strategies, E2 supplementation at optimal dose and route is not associated with further elevation of CV risk (488). This suggests that increases in E2 in itself may not be detrimental to CV health. In fact, transdermal E2 therapy has been shown to improve lipid profile without changes in inflammatory profile in men with prostate cancer in small RCTs (480). However, these findings related to E2 in lipid measures were not corroborated in other studies (95, 333, 377, 494, 539). Nonetheless, AI and SERM use has not been shown to be associated with any change in CVD risk markers nor any major CV adverse events (28, 378, 623).
Evidence from exposure to environmental estrogen analogs
In addition to endogenous E2, there are several environmental pollutants such as bisphenol A (BPA), and dichloro-diphenyl-trichloroethane, and dietary compounds (phytoestrogens) such as isoflavones derived from soybeans and other legumes, and resveratrol, found in grapes and wine with E2 mimicking ability which can contribute to estrogenic effects in men (174, 227). Indeed, a number of cross-sectional epidemiological studies have implicated excessive exposure to environmental E2 signaling modulators with obesity, diabetes, and CVD which led the US Endocrine Society to issue a scientific statement on the impact of endocrine-disrupting chemicals on health (120, 227, 249). In a prospective cohort study from the European Investigators of the Cancer-Norfolk UK including 1619 participants and mean follow-up of 10.7 years, Melzer et al. (408) showed that each standard deviation increase of urinary BPA (4.56 ng/mL) was associated with 13% increased incidence for CAD after adjustments for age, sex, and urinary creatinine. Similarly, data from two nested case-control studies (ESTHER and SURDIAGENE cohorts) have shown that exposure to BPA as determined by urine analysis, is associated with 97% higher incidence of MI (274). In a representative US population derived from NHANES, compared to individuals with lowest levels of urinary BPA, high levels of urinary BPA were shown to be associated with 73% increased prevalence of MI and 61%increased prevalence of stroke (97). Interestingly, a cross-over RCT showed that BPA exposure from canned drinks acutely caused increase in blood pressure by approximately 4.5 mmHg (30). Importantly, in this study, the associations between BPA and CVD were more evident in men.
Evidence from epidemiological studies and clinical trials suggests that the phytoestrogens may affect CV health, but well-controlled studies are lacking (174). In an RCT examining the effects of soy isoflavones in 200 men with hypogonadism and diabetes, 3 months of soy protein intake with 66 mg of isoflavones was associated with improvement in glycemic control and vascular endothelial function along with reduction in triglycerides, CRP, and diastolic blood pressure (513). In another RCT including healthy men and postmenopausal women, soy protein intake with 118 mg of isoflavones for 3 months was shown to associate with greater reduction in blood pressure and improvements in lipid profile (573). However, a decline in vascular endothelial function was observed only in men. Studies examining the role of resveratrol on CVD are more complex as it has been shown to have both E2 agonist as well as antagonist properties based on cell type (375). Nevertheless, these studies corroborate the potential cardioprotective effects of dietary E2 supplementation especially in T-deficient men and warrant further investigation.
Lastly, it should be noted that these E2 mimicking com-pounds do not entirely represent the actions of endogenous E2 mainly due to differential selectivity to E2 receptors along with their ability to activate receptors for other metabolically important hormones such as thyroid receptors (591). These caveats must be kept in mind when drawing conclusions about the role of E2 in CV physiology from these studies of environmental and dietary compounds. Nevertheless, these studies highlight that exogenous environmental and dietary factors may confound our understanding to the role of E2 and estrogen-like compounds in development and progression of CV diseases.
Summary
Together, the studies examining the role of E2 in development and progression of CVD in men suggest possible cardioprotective effects, but further clinical studies are needed to delineate the underlying mechanisms with a goal to develop interventions aimed at activation of estrogenic pathways which may be targeted to reduce CV risk in men.
Relationship between progesterone and CVD in men
Progesterone is one of the earlier products of steroidogenesis which may act as a precursor for T as well as cortisol and aldosterone (570). In men, progesterone is synthesized in the testicles and adrenal glands and remains largely stable with age (57, 444). Progesterone is well established in reproductive fields but is also recognized for its anxiolytic, antidepressant, anesthetic, anticonvulsant, and analgesic effects making it a potential tool for the management of other clinical conditions (392, 473). While the role of progesterone in CVD in women has received a lot of attention, its role in men remains scarce leading to inconsistent findings (444).
Evidence from observational studies
Some cross-sectional and longitudinal studies have shown high circulating progesterone to be associated with increased CVD risk (442, 451, 662). In a case-control study including 411 participants, compared to healthy participants with no family history of premature CAD, high progesterone levels were found to be associated with family history of premature CAD. Importantly, these associations were independent of traditional risk factors for CAD (451). In this study, progesterone was also shown to be weakly correlated with factors associated with insulin resistance, including waist-to-hip ratio, fasting glucose, and HbA1C. In another study, Zitzmann et al. (662) reported a positive association between inflammatory markers such as interleukin-6, CRP, VCAM, e-selectins, and leptin with progesterone in 67 healthy men aged 20 to 50 years. The role of progesterone in progression of CVD was also corroborated in a prospective study including 230 men at baseline and 132 men at 8-year follow-up which showed that progesterone was elevated in men with chronic HF and in participants who died during the follow-up period. Importantly, men who developed chronic HF also demonstrated increases in progesterone (442). In contrast, in a cross-sectional study including 181 elderly men aged 71.8 ± 7.1 years, an inverse relationship between CIMT and circulating progesterone was observed independent of several traditional CV risk factors including age, triglycerides, total cholesterol, LDL-C, CRP, systolic blood pressure, waist-to-hip ratio, and BMI (381). Individuals with low progesterone were also shown to have elevated risk for higher CIMT. In another study of 95 men with idiopathic pulmonary arterial hypertension and median follow-up of 65 months, low levels of progesterone were associated with increased risk of pulmonary arterial hypertension but not mortality (640).
Evidence from progesterone supplementation studies
Progesterone therapy has been examined in the settings of contraceptive use and is also prescribed to increase body weight in elderly men to overcome cachexia. In men, efficacious hormonal contraception requires administration of both T and progesterone. RCTs of T and progesterone for male contraception revealed inconsistent effects on CV risk factors (55, 315, 661). While decreases in HDL-C have been consistently reported, clinical trials examining different progesterone analogs for contraception have shown inconsistent effects on other measures of lipid profile and glucose metabolism (541, 661). Megestrol, a synthetic progesterone analog, has been shown to decrease total cholesterol and HDL-C in elderly men treated for cachexia (349). However, in middle-aged men, in addition to reproductive effects on sperm counts, studies show that a single injection of 200 mg norethisterone enanthate (NEN-EN, synthetic progesterone analog) was associated with depletion of T and E2, no changes in lipid profile and glucose tolerance, and marked shift in hemostatic parameters (315, 663). In contrast, detrimental effects of progesterone analogs on lipid profile were apparent when administered in combination with T (316, 317). A deterioration of insulin sensitivity as determined by QUICKI index was also seen (664). It is noteworthy that synthetic progesterone analogs do not exactly mimic the actions of endogenous progesterone and their downstream consequences may be different (23). Importantly, synthetic progestin analogs can bind to progesterone receptors as well as to androgen receptors leading to ambiguous findings for the physiological role of progesterone.
Summary
Together, the current studies suggest that progesterone may have some detrimental as well as protective CV effects which largely work in conjunction with other sex hormones including T and E2. However, well-controlled prospective studies are needed to elucidate the relationships along with interventional studies to determine causality.
Relationship between DHEA and CVD in men
DHEA is produced by the adrenals and is a precursor of bioactive androgens and E2 (177, 343, 522). Dehydroepiandrosterone and its sulfate (DHEAS) are abundantly present in circulation and are known to decline with age (449). DHEA and DHEAS are implicated in a broad range of metabolic dis-orders including osteoporosis, cancer, and mental disorders.
Evidence from observational studies
Earlier studies from the 1960s and 1980s laid foundation for potential cardioprotective effects of DHEA. Several cross-sectional and case-control studies showed that low levels of DHEA accompanied CV risk factors such as hypertension, dyslipidemia along with CVD (323, 401, 485). In a prospective 12-year study of 242 men aged 50 to 79 years, Barrett-Connor et al. (44) showed that DHEAS levels decreased with age and were lower in men with a history of heart disease. Importantly, in men with low DHEAS levels (below 140 μg/dL) in whom there was no history of heart disease, the risk for CVD mortality was 3.3 times higher during the follow-up period. Similar high risk was also observed for death from ischemic heart disease. While the inverse relationship between DHEA/DHEAS and CVD has been reported by several studies, there are some inconsistent findings as well. DHEAS has been shown to be low in patients with chronic HF and associated with decreased all-cause mortality, but this association was abolished when adjusted for age and HF severity (234). In another study of 1251 men participating in the Framingham Heart Study, DHEAS was not associated with increased risk for atrial fibrillation (390). Similarly, DHEAS but not DHEA has been shown to be predictive of adverse adrenal functional outcomes in ischemic stroke patients but not mortality (75). In a prospective study including 46 men with CAD and 124 healthy men and a mean follow-up of 9.5 years, no difference in DHEAS was found between healthy men and patients with CAD. Furthermore, among healthy men, DHEAS was not predictive of CAD risk (132). Therefore, it was not surprising that a meta-analysis of 11 cross-sectional studies in men (conducted up to 2011) showed that DHEA is not associated with CVD or mortality (140).
In contrast, a longitudinal study including 4255 Vietnam-era US army veterans with 15 years of follow-up, high DHEA was associated with low all-cause mortality and CVD mortality in models adjusted for age and other covariates including smoking, comorbidities, household income, ethnicity, and marital status (471). Of note, this study was not included in the above meta-analysis. In a more recent meta-analysis including 18 prospective studies of 92,489 patients with CVD conducted up to 2016 and a follow-up period ranging from 1 to 19 years, Wu et al. (639) showed that low DHEAS were associated with increased risk for all-cause mortality, CVD mortality, and nonfatal CVD events. Importantly, these associations remained significant when analyzed only for men (639). A subsequent meta-analysis in 6744 elderly individuals (aged 65 years or more) also confirmed the increased risk associated with low DHEAS for all-cause mortality and CVD mortality (364). The follow-up duration for the studies included in this meta-analysis ranged from 2.7 to 8.0 years and the increased risk of all-cause mortality and CVD mortality was only apparent in men (364).
In a recent report of 4107 men participating in the ARIC study, Zhao et al. (658) showed that each standard deviation decrease in DHEAS was accompanied by 7% increase in HF risk in men during the median 19.2-year follow-up period. In a separate analysis of 3650 healthy men participating in the ARIC study, low baseline DHEAS was shown to be associated with 30% increased hospitalization for HF but not CHD (306). The low DHEAS were also associated with higher age, BMI, triglycerides, and comorbidities such as hypertension, and dyslipidemia. Furthermore, the study showed that the greater decreases in DHEAS during the follow-up period were predictive of higher risk for HF hospitalization. Importantly, these associations between DHEAS and HF were not significant when adjusted for age and other HF-associated covariates such as proBNP. The relationship between low DHEA and DHEAS with higher risk for T2D has also been reported in a study of 2171 middle-aged and elderly men during a median 10.9-year follow-up period (85). In another study, a positive relationship between DHEAS and ambulatory diastolic blood pressure but not systolic blood pressure was noted only in 229 normotensive men (308).
Notwithstanding some inconsistencies, most observational studies suggest that low DHEA/DHEAS levels may be associated with increased risk for at least certain CVDs in men. The discrepancies in findings from different studies may likely stem from age of the participants as well as different pathological mechanisms involved during the development and progression of various CVDs.
Evidence from DHEA supplementation studies
Over the years, the cross-sectional and longitudinal studies have encouraged the use of DHEA as dietary supplement to improve health. However, the benefits of DHEA supplementation remain inconclusive. In a meta-analysis of all RCTs conducted up to 2013 which included 1353 elderly men with a median follow-up of 36 weeks, DHEA supplementation led to 1.24 nMol/L increases in T and 24.76 pMol/L increase in E2. But these alterations in sex hormones did not translate to any changes in glycemia, insulin, or total cholesterol (139). Similar lack of protective effects of DHEA supplementation on lipid profile was also noted in a more recent meta-analysis of RCTs in men and women. Qin et al. (482) showed that compared to control group, DHEA intake was associated with a mean 3.13 mg/dL decrease in HDL-C while other parameters including total cholesterol, LDL-C, and triglycerides remained unchanged. However, the decreases in HDL-C were lacking in men and the overall decreases were largely driven by women. Notably, the meta-analysis also highlighted the heterogeneity in the clinical trials including differences in inclusion criteria, DHEA dosage, and length of treatment duration. In contrast to the previous meta-analysis, Wang et al. (613) showed that DHEA consumption was associated with a mean 2.2 mg/dL decrease in fasting glucose with no effects on insulin and HOMA-IR. However, the decreases in glucose were not apparent in men.
Summary
Overall, the findings from the RCT suggested a lack of direct causal effects of DHEA on CVD and did not support the use of DHEA supplementation to improve cardiometabolic health. It is likely the low DHEA is a mere marker for poor CV health and may be used to identify CVD risk in men.
Sex Hormones and CV Health in Women
While E2 is predominant sex hormone in women, other sex hormones including T, progesterone, and DHEA have been shown to be associated with CV risk as well. The prevalence of CVD increases with age in both men and women. While women have a lower prevalence of CVD until midlife, the prevalence rates are similar between men and women in the decades just after menopause (58, 498, 659). As a result, it has been suggested that menopause represents an ideal time to monitor women’s CV and metabolic risk and a critical window for implementing early intervention strategies to reduce CVD risk in women as they age (130, 178). The lower prevalence of CVD in premenopausal women has been attributed to estrogens—particularly estradiol (E2)—which declines substantially with the menopause transition. The impact of E2, as well as other hormones like T, progesterone, and DHEA, on vascular aging may explain the age-related increases in CVD risk in midlife women.
Relationship between T and CVD in women
In healthy premenopausal women, adrenal contribution to endogenous T is relatively constant throughout the menstrual cycle (varying between 40% and 66%) (3). Furthermore, the ovarian contribution of endogenous T in healthy women reaches maximum levels during midcycle (60%), and the early follicular and late luteal phases of the menstrual cycle both contributing to approximately one-third of total endogenous T levels (3). As women get older, ovarian function declines, menstrual cycles become more irregular and cease, and T levels decline. T also declines with oophorectomy, hypopituitarism, adrenal insufficiency, chemotherapy and radiation therapy, oral contraception, glucocorticoid therapy, and oral E2 therapy. Although CVD risk increases in women as they age which coincides with declining T levels, this relationship is complex.
Evidence from observational studies
Traditionally, the effect of hyperandrogenemia on CV risk was mostly studied in women with polycystic ovarian syn-drome (PCOS). Women with PCOS have the hallmark trait of high levels of circulating free T, which is shown to be associated with endothelial dysfunction (460) and arterial disease (156). Premenopausal women with hyperandrogenemia also have a higher prevalence of CVD risk factors including central obesity, insulin resistance, dyslipidemia, and chronic inflammation (627). Studies investigating the association between endogenous T levels and CVD risk in non-PCOS women often examine older women and demonstrate more inconsistent findings. In these studies, both high and low levels of endogenous T have been associated with CVD, while other studies report no such association. One study that demonstrates the complexity of such associations includes older women with both lower and higher levels of T having increased risk of CHD compared to women with normal T (354).
Associations between lower T and higher CVD risk and mortality have also been reported. In a large, population-based cohort of women over 40 years of age, lower levels of T were marginally associated with risk of acute MI (149). Similarly, lower T was also associated with increased CAD, as well as increased CVD, and all-cause mortality in postmenopausal women with and without diabetes (313, 617). A large cohort of 2914 women in Germany between the ages 18 and 75 years revealed that lower baseline T levels were associated with increased all-cause mortality and incident CVD events that were independent of traditional CV risk factors (532). Studies demonstrating an association between higher T and CVD risk are also available. For example, in a Danish prospective cohort study, women with very high T levels had a 68%higher risk of ischemic heart disease and a 36% higher risk of death compared to women with lower T levels (60). Another large cohort of postmenopausal women participating in the Multi-Ethnic Study of Atherosclerosis study revealed that higher T-to-E2 ratio, as well as higher T levels alone, were associated with higher risk for incident CVD, CHD, and HF (659). Other studies show no relationship with CVD risk and total T, free T, or low or high total T (273, 515). Potential mechanisms that may explain this increased risk include the link between T and vasoconstriction of blood vessels and increased platelet aggregation (8). T is also associated with increased accumulation of visceral (abdominal) fat, lipids, as well as increased blood pressure, CRP, and insulin resistance (145, 225, 406, 565, 612, 617).
Evidence from T supplementation studies
Even though T is frequently prescribed (off-label) for hypoactive sexual desire disorder, (155, 625), its effects on CVD have not been thoroughly explored. One study has demonstrated that 6 weeks of parenteral T improved endothelial function in postmenopausal women who were chronically taking E2 therapy (637). Conversely, in premenopausal women, microvascular vasodilation in response to administration of GnRH antagonist with and without T add-on does not appear to be different (621). In another RCT, administration of 300 μg transdermal T in women with chronic HF was associated with improvement in 6-min walk test and insulin sensitivity (286).
Summary
Although data from observational studies suggest that T might have CV protective effects in women, there is inadequate research on therapeutic application of exogenous T for reduction of CV risk (155).
Relationship between E2 and CVD in women
The hallmark decline in estrogen with aging (particularly E2), with its protective effects against CVD in women is thought to be the primary reason for these increases in CVD risk in women.
Evidence from observational studies
The incidence of CHD increases after menopause, as well as after bilateral oophorectomy in the absence of E2 supplementation (127, 178). Early menopause is also associated with shorter CHD-free survival (619), while older age at menopause onset is associated with reduced CVD mortality (157). Nevertheless, studies of age of natural menopause and incident morbidity and mortality have yielded inconsistent findings (178).
The cardioprotective effects of E2 are thought to be partly related to its positive effects on vasculature. Specifically, E2 contributes to reduction in blood pressure via promoting vasodilation through increasing endothelial nitric oxide synthesis and inhibiting the renin-angiotensin system (415). E2 is also thought to regulate specific inflammatory markers and cytokines (37). The loss of antioxidant and anti-inflammatory effects observed with E2 decline is linked with low-grade inflammatory condition and development of CVD (4, 618). Flow-mediated vascular dilation, an established early marker for CVD risk, has been reported to decline in women as they near menopause onset (425) but improves with E2 treatment (426, 427, 526, 606). Similarly, other markers of CV risk including pulse wave velocity and arterial compliance also decline with age in women (548, 608). Furthermore, data from the Study of Women’s Health Across the Nation (SWAN) revealed associations between low E2 and increased CIMT, a strong predictor of subclinical CVD, in perimenopausal women (179). Interestingly, improvements in endothelial function in postmenopausal women during endurance training appear to be effective in only those women treated with E2, suggesting an essential role of E2 in vascular adaptations to endurance training in women (427). Furthermore, certain metabolites are converted into cardio-protective molecules only in presence of E2. For instance, arachidonic acids are converted in epoxyeicosanoids, which have antiarrhythmic properties and are partly responsible for lower prevalence of atrial fibrillation only in women (431, 622).
Evidence from E2 supplementation studies
The observational and mechanistic studies suggest favorable effects of E2 on lipid profile, and CVD risk reduction (43, 96, 230, 409, 492, 549, 576). This led to off-label use of Premarin (conjugated equine estrogens, CEE) and Prempro (CEE plus medroxyprogesterone) which skyrocketed in the 1990s (342). Although these hormonal therapies were approved for relief of menopausal symptoms and prevention of osteoporosis, they were largely prescribed for preventing heart disease and other chronic diseases with the hope of slowing or reversing the manifestations of aging among postmenopausal women. Following publication of the principal results of the WHI trials in 2002 and 2004 which suggested more harm than benefit from treatment with CEE in combination with progestin (499) or CEE alone (17), the use of menopausal hormone therapy (MHT) for prevention of chronic diseases decreased dramatically and remained at a low level (544).
The WHI hormone trials comprised of two large RCTs—one comparing CEE 0.625 mg/day plus medroxyprogesterone acetate (MPA) 2.5 mg/day vs. placebo, and the other comparing CEE-only vs. placebo. Because of the established risk of unopposed E2 for endometrial cancer, menopausal women with an intact uterus were enrolled in the CEE + MPA trial (N = 16,608) and those who had hysterectomy were included in the CEE-only trial (N = 10,739). In both trials, the primary efficacy outcome was CHD, a composite of nonfatal MI and CHD death. Based on observational data of the effect of E2 on the risk of breast cancer (555), invasive breast cancer was designated as the primary adverse outcome in both trials. The sample sizes were based on estimated event rates, and benefits and risks. Both trials were stopped earlier than the planned 8.5-year treatment period. The CEE + MPA (estrogen plus progestin) intervention was stopped as recommended by the study’s Data Safety Monitoring Board after a median 5.6-year treatment duration on the basis of increased risk of breast cancer in the active drug therapy arm (499), and the CEE (estrogen) monotherapy intervention was discontinued by a decision made by the National Institutes of Health after a median 7.2-year treatment period because of an increased risk of stroke in the CEE arm (17). The study participants in both trials were followed for several years after discontinuation of the interventions. Over a cumulative 18-year follow-up, there were 1088 and 6401 deaths during the intervention and postintervention phases, respectively, for a total of 7489 deaths in the CEE-only and CEE + MPA trials (394). In the pooled cohort, all-cause mortality was 27.1% in the hormone therapy group vs. 27.6% in the placebo group. Mortality rates related to CVD and cancer were also not different between hormone therapy and placebo groups. The results were not different between the CEE-only and CEE + MPA trials.
In the intervention phase of the CEE + MPA trial (499), several CV outcomes suggested harm. Increased risks for CHD, stroke, deep vein thrombosis, pulmonary embolism, and total CVD were observed in the CEE + MPA group vs. placebo. Interestingly, during the intervention phase of the CEE-only trial (17), no increased risk for CHD was observed with CEE vs. placebo although there were significantly increased risks for stroke, deep vein thrombosis, and total CVD. In both trials, CHD risk increased in the first year of intervention, albeit more significantly with CEE + MPA. Whereas CHD risk persisted with the combination therapy, the risk diminished with CEE-only with no overall increased risk after 6.8-year treatment. Stroke risk was significantly elevated with both CEE + MPA (41% increase; 29 vs. 21 per 10,000 person-years) and CEE-only (39% increase; 44 vs. 32 per 10,000 person-years). However, the increased CVD risks observed during treatment with CEE + MPI and CEE-only were no longer present during long-term follow-up after treatment discontinuation (258, 348). The primary publications of both WHI trials concluded that CEE alone or in combination with MPA should not be recommended for prevention of chronic disease in postmenopausal women.
The results of the WHI trials further confirmed the findings of the Heart and Estrogen/progestin Replacement Study, a placebo-controlled trial of CEE + MPA in 2763 women with preexisting CHD, which reported an increased risk of CHD events in the first year of treatment and no overall reduction in CHD events during an average treatment period of 4.1 years (280). Concordant with these findings, a recent meta-analysis (334) of 26 RCTs found that MHT was associated with a 14% increased risk of stroke, 70% increased risk for venous thromboembolism and 26% increased risk for pulmonary embolism, but no significant increase in the risk of MI or overall CHD.
While the MHT may not benefit all postmenopausal women, there are some caveats. Women who are 10 years or more beyond menopause onset are likely to not benefit from the cardioprotective effects of estrogen due, in part, to its coagulative effects (370). It is also recommended that hormone therapy be primarily estrogen-based and that progesterone use, to protect against endometrial hyperplasia, should be minimized as they may attenuate the coronary benefits of E2 (370). MHT has also been shown to lower LDL and total cholesterol, as well as increase HDL cholesterol (384). These improvements in cholesterol, as well as lowering of triglyceride levels, are diminished with use of orally administered E2 (but not transdermal) (224). MHT is also associated with increased nitric oxide production and decreased vascular inflammation (489). The “timing hypothesis” proposes that hormone therapy started in the perimenopausal, or early postmenopausal period is cardioprotective, while hormone therapy late after menopause increases CVD risk (221, 472). Indeed, data from the Early versus Late Estrogen Trial demonstrated that while women who were 10 years past menopause experience no coronary benefit from hormone therapy, women who were less than 6 years past menopause has less progression of subclinical atherosclerosis (269). Furthermore, clinical trials of secondary prevention of CHD and stroke also show no benefit with hormone therapy in women with average ages of 67 to 72 years, which is the age beyond 10 years of average menopause onset (280, 605). The WHI trials further demonstrate that hormone therapy appears to protect again CV diseases in recently menopausal women (550), and in those at greater CV risk. The literature supporting a critical role for the time of initiation of MHT use relative to menopause, with initiation at <60 years of age or within 10 years of menopause appearing to be associated with reduced CVD risk, strongly calls for further research assessing MHT use, including potential contrasts by form, route, and duration of administration, on cardiometabolic effects in women traversing menopause, a large proportion of whom experience menopausal symptoms before even reaching menopause.
Evidence from E2 agonists or inhibition therapy
Apart from changes in estrogen resulting from natural menopause, E2 levels are clinically modulated via use of AI and SERMS in hormone receptor-positive breast cancer patients. Contrasting effects of Ais and SERMS (tamoxifen) have been observed in these patients. AI use is associated with increase in incidence of hyperlipidemia and hyper-tension in some studies and not in others (228, 372, 616). Most studies have reported an improvement in lipid profile among tamoxifen users (14, 201). Compared to tamoxifen, use of AI was associated with increased risk for CVD by 30% (86, 123). Ais seem to accelerate CVD progression which is lacking in women receiving SERMs (14, 521). Cardioprotective effects of tamoxifen have also been shown for CVD (86, 123).
Summary
Together, evidence from cross-sectional studies and therapeutic trials suggests that E2 has cardio-protective effects. However, the use of E2 to improve cardiometabolic profile requires consideration of many other factors of which time from menopause is the most important consideration.
Relationship between progesterone and CVD in women
In women, progesterone plays a critical role during pregnancy and is responsible for many physiological adaptations required for a successful gestation (570). However, the impact of progesterone on CV health is not well understood.
Evidence from observational studies
Most studies examining the relationship between sex steroids and CVD have not measured progesterone. In a study of post-menopausal women, high serum progesterone was shown to be associated with increased mortality, and chronic HF (442). Considering the wide variation in circulating progesterone observed during the menstrual cycle, epidemiological studies have not evaluated the relationship between progesterone and CVD risk in premenopausal women. However, clues into the effects of progesterone on CVD risk can be derived from studies examining the effects of progestin-only contraceptive (POC) use. In a pooled analysis of 19 observational studies, compared to nonusers, POC users did not exhibit any changes in the risk for venous thromboembolism, MI, and stroke (223). This meta-analysis included data from 11,930 women using POCs. However, closer examination of the data revealed that injectable POC was associated while oral POCs were not associated with increased risk for venous thromboembolism risk. Furthermore, POC use was not associated with changes in blood pressure or diabetes risk. Based on these studies, the US medical guidelines for contraceptive use advocate POCs for women at high risk for CVD but contraindicate the use of injectable POCs in women with a history of MI and stroke (574).
Along with estrogen, women experience declines in endogenous progesterone levels with age. It is well known that estrogen and progesterone treatments are highly effective at treating vasomotor symptoms, including hot flashes and night sweats. The use of progesterone was initially limited by poor gastrointestinal absorption and only the development of an oral micronized form made it possible to achieve adequate and consistent physiologic blood levels. Now, progesterone is US Food and Drug Administration approved in oral micronized form, as well as in the form of a vaginal gel or as an over-the-counter cream. Progesterone is used in combination with estrogens to treat vasomotor symptoms in women during menopause transition. Use of progesterone alone for treatment of menopause symptoms has also been suggested (477).
Evidence from progesterone supplementation studies
Unlike estrogen, there is less known about the effects of endogenous progesterone and synthetic progestins on CVD risk. Additionally, the androgenic nature of progestogens suggests that different progestogen agents may have different effects on CVD risk. An in-depth meta-analysis of 248 prospective studies published from 1974 to 2000 summarized serum lipid levels that encompassed 42 different hormone therapy regimens (224). Unfortunately, separating the effects of progestins from estrogen-alone or combined therapies on CV risk was difficult. While progestogens did not appear to alter the estrogen effects on LDL-C and total cholesterol, estrogen-induced increases in HDL and triglycerides were diminished according to the type of progestogen (224). Specifically, they were opposed in the order of progestogen (from least to greatest effect): dydrogesterone and medrogestone, progesterone, cyproterone acetate, MPA, transdermal norethindrone acetate, norgestrel, and oral norethindrone acetate. One of the major trials examining the effect of hormone therapy with progestogens was the Postmenopausal Estrogen/Progestogen Intervention Study. Specifically, the study was a prospective, 3-year, randomized trial in 875 postmenopausal women between 45 and 65 years of age that compared placebo, unopposed CEE, CEE plus continuous MPA, CEE plus cyclical MPA, and CEE plus cyclical micronized progesterone. This study found that postmenopausal women treated with estrogen and micronized progesterone had significantly higher HDL cholesterol levels than women treated with estrogen and MPA (576). Importantly, HDL-C is more closely related to CVD than LDL-C, making these findings highly relevant (294). More recent studies in premenopausal women also indicate that administration of either MPA or micronized progesterone alone or combined with E2 abolished the beneficial effect of E2 on endothelial function (407, 416). In contrast, others have reported that micronized progesterone did not negate the effects of E2 on vasodilation in postmenopausal women (216).
Summary
Overall, the relationship between progesterone and CVD risk remains unclear. Studies exclusively examining the effects of progesterone on CVD are lacking.
Relationship between DHEA and CVD in women
Fewer studies have examined the relationship between DHEA and CVD risk in women, and most are associated with inconclusive findings.
Evidence from observational studies
In The Rancho Bernardo Study, investigators found that, unlike in men, DHEAS levels in postmenopausal women did not predict CV mortality (42). Another analysis from the ARIC cohort further supports the lack of association between CVD risk and DHEAS levels (226). Concordant with these findings, in a recent meta-analysis which included all longitudinal and observational studies up to 2019, the association of low DHEAS with all-cause mortality and CV mortality was shown to be only limited to men (364).
In contrast, a recent analysis of the ARIC cohort showed that 1 standard deviation decrease in DHEAS was associated with 1.17 higher odds of HF (658). Low DHEAS (<27.4 ug/dL) was also associated with increased HF hospitalization in women and increased CVD death (306). However, no association with CAD was noted. Low DHEAS have been also found to be associated with higher CVD and all-cause mortality in postmenopausal women with coronary risk factors (530). Low DHEAS has also been shown to be associated with higher risk for venous thromboembolism in women using MHT (495). Severe disease and worse outcomes in postmenopausal women with pulmonary arterial hypertension were also seen in presence of low DHEAS (35). Similarly, among patients with CVD, low DHEAS was associated with 67% higher all-cause mortality, and 52%higher nonfatal CVD events but not CVD mortality (639). Meanwhile, other studies have reported increased rates of CVD and all-cause mortality in women with higher DHEAS levels (309, 586). In a nested case-control study within the Nurses’ Health Study cohort, the risk for MI was shown to increase with increases in DHEA and DHEAS (453). In another study, Jimenez et al. (307) examined the relationship between DHEAS and CVD risk among Latinos participating in the Boston Puerto Rican Health study and observed higher DHEAS in women with hypertension and in women with a history of CVD. Adding to these inconsistencies, a U-shaped relationship between DHEAS and 5-year mortality has also been shown (104, 447). Interestingly, women with higher DHEAs levels tended to have higher cancer mortality and those with lower DHEAS tended to have a greater CVD mortality (104).
Evidence from DHEA supplementation studies
DHEA is a precursor to estrogen. Therefore, DHEA supplementation has the potential to improve hormonal profile in postmenopausal women (154). While exogenous DHEA treatment is shown to improve hormonal profile (345, 346, 457), no meaningful or consistent improvements in lipid pro-file or glucose metabolism have been observed in RCTs (154, 457). The discordant effects observed in the different DHEA supplementation studies may be partly due to differences in route of administration—oral versus transdermal, strength of the supplement (25–1600 mg/day), and length of treatment (4–52 weeks). However, most studies show that DHEA use is associated with decrease in HDL-C and total cholesterol which was more prominent in women (154, 482). Similar inconsistent findings were reported for insulin sensitivity as well (47, 48, 112, 351, 423, 430, 435, 456). Importantly, none of the RCTs of DHEA in women had sufficient size to provide data pertaining to CVD events (154).
Summary
Taken together, the relationship between DHEA, DHEAS, and CVD observed in men has not been consistently replicated among women (577). As with men, DHEA supplementation does not have any favorable effect on CVD risk profile but its effect on major adverse CV events remains largely unknown.
Sex hormones and CVD Risk in Transgender Individuals
The contributory role of gonadal hormones in CV health is highlighted by findings from transgender patients who undergo hormonal therapy to develop and maintain the physical characteristic consistent with their gender identity. Current evidence suggests that gender affirmative hormonal therapy increases the risk for CV disease including venous thromboembolism, MI, and stroke which is prominent in transwomen (131, 560). Further, while overall CV mortality is not increased in transgender population (74), an increased mortality from CV causes is noted in transwomen (24, 181). Hormonal therapy in transwomen affects coagulation factors such that it leads to a pro-thrombotic state and an increase in risk for venous thromboembolism. In a 4-year prospective study, transwomen were shown to have an elevated risk for venous thromboembolism than nontransmen and nontranswomen matched for BMI, smoking history, hypertension, and cholesterol values (217). This study also showed that compared to nontranswomen, transwomen were at a higher risk for ischemic stroke and MI. However, the risk for ischemic stroke and MI did not increase in comparison to nontransmen. Similar findings have been reported in other study cohorts (624). For transmen, the evidence is insufficient to allow conclusion regarding venous thromboembolism, stroke, and MI (131, 217).
In terms of CV risk factors, a meta-analysis of 29 studies evaluating the effect of hormonal therapy in transgender individuals showed elevated triglycerides and LDL-C along with decreased HDL-C in transmen (397). In transwomen, higher levels of triglycerides were observed with no other changes in lipid parameters. Most studies examining the effects of hormonal therapy on blood pressure in transgender individuals show no effect while some studies have shown that T treatment in transgender men is associated with a small increase in systolic and diastolic blood pressure values (292, 560). In contrast, hormone treatment in transwomen is associated with decreases in blood pressure. While T2D is a major CV risk factor, the effect of hormonal treatment in transgender individuals is not clear. A meta-analysis of limited studies examining the effects of hormonal therapy on insulin resistance in transgender individuals showed that T therapy in transmen does not impact insulin resistance while E2 therapy in transwomen may worsen insulin resistance (542). Nonetheless, compared to nontransgender individuals, T2D is more frequent in transwomen and transmen (624).
Overall, while few prospective studies of transgender CV outcomes exist, current evidence suggests that the use of gender affirmative hormonal therapy does not significantly alter the CV risk in transgender individuals. Therefore, clinical recommendations for CV prevention in transgender individuals differ to general population guidelines to include routine assessment of traditional risk factors including lipid profile, blood pressure, and diabetes (292, 497, 560).
Sex Hormones, Obesity, and Body Composition
Sex differences in body composition are present during the first several months after birth with boys having lower body fat and higher fat-free mass than girls (153). These sex differences may be due to the anabolic effects of T production in men (153). The sexual dimorphism in body composition increases markedly during puberty with growth of skeletal muscle in men exceeding that in women; these effects lead to a corresponding decrease in percent fat in men relative to women (525, 533). By early adulthood “Reference” Man (weight 70 kg; height 170 cm) and Woman (60 kg; 160 cm) have 19% and 28% of their body weight as fat, respectively (288). Corresponding estimates of total body skeletal muscle mass are 40% and 29% (288). Across the general population, adipose tissue mass increases, and skeletal muscle mass decreases with greater age, starting at about the fourth decade, independent of sex, race, weight, and height (265). These effects of senescence appear greater in men than in women (265). Activity levels independently account for some of the between-individual variation in muscularity (264).
There also is a sexual dimorphism in adipose tissue distribution. Men have relatively more visceral adipose tissue than women after controlling for body size and total adiposity (454, 525). By contrast, women have more subcutaneous adipose tissue than men, notably in the hips and thighs. The android body shape in men commutes greater CVD risk than the gynoid shape characteristic of women (454). Visceral adipose tissue increases with age, notably in the postmenopausal period in women. This pattern of adipose tissue distribution lends to more CVD risk in men and postmenopausal women.
As adults gain weight above an “ideal” baseline during periods of positive energy balance, the deposited “obesity tissue” includes not only adipose tissue but also skeletal muscle, liver, kidney, heart, and other “lean” organs and tissues (284). In other words, fat starts to accumulate in cell types other than adipocytes leading to lipotoxicity. In men, 48% of obesity tissue is adipose tissue compared to the 67% present in women. Obesity tissue is 29% skeletal muscle in men compared to half that, or 14%, in women. The deposition of fat in nonadipose tissue alters the metabolism contributing to increases in CVD risk factors such as insulin resistance and dyslipidemia.
In summary, there is a pronounced sexual dimorphism in body composition that appears early in life and remains present across the lifespan. Hormonal mechanisms are the primary drivers of these sex differences in body size and shape, although diet and activity patterns are contributing factors. Importantly, while obesity is more prevalent in women, men are more likely to experience obesity-related disorders (324).
Sex Hormones and Body Composition in Men
As stated above, the body composition in men is defined by the circulating levels of sex hormones. While all sex hormones may have effects on muscle mass, adiposity, and regional fat distribution, most studies have focused on the effects of T and estrogen in men.
Relationship between T and body composition in men
Evidence from observational studies
Obese men mostly display low levels of circulating T, especially in presence of other comorbidities such as T2D and metabolic syndrome (232). The decreases in T among obese men were first reported in the 1970s (13, 222). Since then, not only several cross-sectional studies have consistently reported low levels of T in obese men of all ages but have also shown that low T predicts abdominal adiposity as determined by waist circumference and waist-to-hip ratio (12, 211, 331, 484, 567, 665). In fact, prospective studies have shown that obesity is associated with rapid age-related decline in T and low T predicts future weight gain and central obesity (101, 165, 331, 585, 587). The European Male aging study which included 3369 men with ages between 40 and 79 years showed that obese men had 30% lower T and 18%lower free-T compared to lean men (569). The Boston area community health survey included 1822 men and showed that central obesity was the most important contributor to symptomatic low T (246). Symptoms of low T were defined as low libido, erectile dysfunction, osteoporosis or two or more of sleep disturbances, depressed mood, lethargy, or diminished physical performance. Concordant with the association of abdominal adiposity with CVD risk, low T has been shown to be associated with prevalent metabolic syndrome (88). However, association of T with metabolic syndrome varies according to BMI, and the magnitude of associations was shown to be largest in non overweight men. This meta-analysis also showed that the associations of T were strong for abdominal obesity, hyperglycemia, and hypertriglyceridemia (88). Further, weight loss is associated with increases in T. A meta-analysis of 22 studies showed that weight loss achieved with diet or bariatric surgery leads to rise in total and free T levels, and the extent of the rise is dependent on the degree of weight loss (141). These findings were also established in a more recent meta-analysis of studies including severely obese men undergoing weight loss after bariatric surgery (186, 361).
Evidence from T supplementation studies
The causal relationship between obesity and low T is exemplified in hypogonadal men who are mostly obese but experience weight loss upon T therapy (434, 610). Obesity and increased waist circumference are highly prevalent in hypogonadal men with T deficiency (109, 538, 587). On the other hand, hypogonadism is a common finding in men with obesity (167, 169, 270, 563). A recent meta-analysis examining the prevalence of endocrine disorders in obese men reported the prevalence of hypogonadism to be 42.8% when deter-mined by measuring total T (589). Numerous studies have also reported that TRT was associated with significant weight loss among men with hypogonadism and obesity (203, 204, 244, 314, 506, 507, 583, 644, 584). However, most of such reports were based on observational data gathered via one or two registries at various intervals of time. Nevertheless, these reports led to claims that T therapy could revert obesity.
In a study of 682,915 participants from the Veterans Affair system, among the patients without diagnosed hypogonadism, obesity was one of the strongest predictor for T prescription (301). Notably, some RCTs have reported no change in weight (11, 142, 290, 311, 507, 538, 554) or slight weight gain (79, 206) with TRT. However, the verdict is not completely out as there is conflicting emerging evidence as well. In a 1-year open-label clinical trial during which 88 participants with late-onset hypogonadism received intramuscular T, decreases in BMI along with lowering of total cholesterol, and glycated hemoglobin were observed (103).
Unlike the effects of T therapy on CVD, CVD mortality, or the total body weight, the effects of T therapy on body composition have been consistently shown. T is a key determinant of body composition and is closely associated with lean mass (67, 597). Hypogonadal men exhibit increased fat mass, abdominal obesity, and reduced lean mass (331, 568, 597, 654). Conversely, higher T levels are associated with less loss of muscle mass in older men (358). Numerous studies reported that T treatment of hypogonadal men led to decreased fat mass and increased lean mass (6, 11, 54, 64, 66, 79, 83, 185, 311, 330, 538, 547, 566, 634). TRT has also been shown to restore loss of lean mass associated with weight loss induced by a very-low-calorie diet among men with obesity (437). Of note, TRT reduces subcutaneous fat, but effects on visceral adipose tissue are not consistent (206, 220, 437). In a 26-week interventional trial in elderly men, T administration was associated with 2.9% increase in lean mass but no change in muscle strength (71). In a recent 2-year RCT, compared to placebo, T therapy was associated with reduction in total and abdominal fat mass along with increase in total muscle mass (633). This study also noted an increase in muscle strength for nondominant hand with T treatment. These favorable effects of T therapy on body composition are also evident in men without obesity. Compared to placebo, transdermal T therapy for 52 weeks increases total body fat-free mass along with preventing gain in visceral adipose tissue and loss of skeletal muscle in men aged ≥55 years without obesity (11). It is noteworthy that the total body weight and waist circumference did not change during the 52-week intervention in either the placebo or the T-treated groups. A meta-analysis of RCTs comparing T therapy on body composition and metabolic outcomes demonstrated that TRT is associated with significant reduction in fat mass and an increase in fat-free mass along with a reduction in fasting glucose and insulin resistance (134). These findings were also reiterated in a recent meta-analysis of TRT in patients with T2D or metabolic syndrome (365). Adding to the evidence of the role of T in determining body composition, ADT is associated with muscle loss and a gain of fat mass (84, 117, 362, 558).
Evidence from experimental studies
Several experimental studies in healthy men have been under-taken to examine the role of T deficiency in determining body composition. These studies show that short-term suppression of endogenous sex hormones is associated with decreases in fat-free mass and increases in fat mass. The decrease in fat-free mass that occurs with suppression of endogenous T production can be attenuated with T supplementation (65, 198, 311, 578). In these experiments, T increased fat-free mass in graded T dose-dependent manner (65, 198). Importantly, the increases in fat-free mass with T replacement were apparent even when T was administered in the presence of AI (198, 311). These studies support the causal role of T in altering body lean mass.
Summary
Together, evidence from observational, experimental, and interventional studies supports the role of T in determining body composition but not body weight. Specifically, T is involved in maintaining lean mass but not adiposity in men.
Relationship between estrogen and body composition in men
Evidence from observational studies
The associations between estrogen and body composition in men are complex and intricately linked to T. While obese men consistently have low T, estrogen levels in obese men have shown to be elevated, not changed, as well as decreased. Several studies have shown that men with obesity have higher E2 (248, 289, 337, 432, 516, 551, 559, 599). In these men, excess adiposity with increased aromatization may augment conversion of T to E2 with consequent elevated E2 in obesity (338, 383, 516). However, compared to adipose tissue of lean men, some studies have reported reduced aromatase activity in adipose tissue of men with obesity (218). Conversely, decreases in fat mass during weight loss in these obese men are associated with decreases in estrogen (141, 248, 502). Though some studies failed to show decreases in estrogen upon weight loss as well (141, 551, 559)—these may be related to the lack of sensitivity of E2 assays used in these studies (279). In a recent study of 224 healthy men, E2 was not found to change with increasing BMI (552). On the other hand, men with obesity and underlying hypogonadism are frequently found to have decreased E2 (166, 279). The low levels of E2 in these men may be attributed to T deficiency and during T therapy an increase in estrogen is frequently experienced along with T-dependent concomitant weight loss. The disparate relationship between obesity and estrogen in men may also stem from fat distribution. Compared to subcutaneous abdominal and femoral fat, visceral fat has low aromatase activity. Indeed, a study evaluating the associations between different fat depots showed that circulating E2 was closely associated with subcutaneous fat (599).
Evidence from experimental studies
In spite of the inconsistent findings related to E2 in obese men, experimental studies show that estrogen deficiency causes increases in fat mass in healthy men (115, 198, 311). In a study that included 400 healthy men, Finkelstein et al.(198) showed that drug-induced suppression of endogenous sex hormones (both T and E2 through goserelin acetate) led to increases in body fat. However, the increases in body fat were blunted when replacement T was provided at adequate concentrations. Importantly, replacement T was unable to prevent fat gain when administered in conjunction with AI, proving that estrogen deficiency contributes to increases in fat mass (198). The role of E2 in regulating fat mass was also apparent in another RCT that included 57 obese T deficient men (311). In this study, TRT led to significant reduction in adiposity which was lacking when T was administered in presence of AI. In another RCT, short-term estrogen withdrawal was also shown to be associated with fat mass accrual (115). Concordant with these experimental studies, aromatase-deficient and ESR1-deficient men are frequently overweight or obese with increased visceral fat noted in some individuals (51, 82, 105, 106, 116, 263, 350, 386, 387, 429). The role of E2 in determining adiposity is also demonstrated in men undergoing ADT for prostate cancer who are at a higher risk for development of obesity (173, 247, 325).
Summary
Overall, the clinical interventional studies support the role of E2 as a determinant of adiposity, more so than T. Notably, E2 deficiency appears to be a key facet of the metabolic risk conferred by T deficiency via increases in adiposity. Still, the routine measurement of E2 as part of the clinical assessment for obesity-associated hypogonadism and metabolic dysfunction is not recommended.
Relationship between progesterone and body composition in men
Evidence from observational studies
Few studies have explored the associations between progesterone, body weight, and body composition. Nevertheless, several cross-sectional studies consistently showed a negative association between progesterone/17-hydroxyprogesterone and body weight and other indices of adiposity (72, 150, 256, 289, 404, 412). In a small study including 38 men whose BMI ranged from lean to severe obesity, Blanchette et al.(72) showed a moderate negative correlation between progesterone and body weight, BMI, and waist circumference. In another study including 270 men from the HERITAGE Family Study, while an inverse association between circulating 17-hydroxy progesterone and fat mass, abdominal fat along with visceral fat was also observed, the strength of these associations was comparatively low (256). The relationship between progesterone and visceral fat was also confirmed in a recent study including 266 nondiabetic obese men aged 18 to 49 years which showed that progesterone was negatively associated with body weight, BMI, waist circumference, and visceral fat (404). In this study, a positive relationship between fat-free mass percentage and progesterone was also observed. Of note, the relationship between progesterone and BMI may be altered in individuals with high risk for CVD. In a study of 411 young healthy men with or without family history of CAD, circulating progesterone was positively associated with waist-to-hip ratio but not BMI (451).
Evidence from progesterone supplementation studies
Contradictory to the findings from cross-sectional observational studies, administration of progesterone analogs in men is associated with increased body weight mainly through increases in fat mass. Progesterone has been shown to stimulate appetite with consequent weight gain in men and women via direct actions on neurons (444). In studies of male contraception, use of NEN-EN or other synthetic progesterone has been shown to associate with weight gain (317, 661). In fact, megestrol acetate is clinically used to increase weight in elderly, AIDS, and cancer patients (349). However, megestrol acetate-induced weight gain is a result of increases in fat mass alone, and the therapy is frequently associated with decreases in muscle mass. The reduction in fat-free mass may be partly attributed to decreases in T. Therefore, resistance training along with T therapy may be used along with megestrol acetate to prevent muscle loss and promote weight gain as well (349). However, the decreases in lean mass from megestrol acetate therapy may not be universal. In a small RCT including men aged 50 to 83 years undergoing hemodialysis, 20 weeks of megestrol acetate use was associated with increases in body weight resulting from increases in body fat mass and fat-free mass (649).
Summary
Together, low physiologic levels of circulating progesterone may be a potential marker for high adiposity and fat distribution pattern which is detrimental to CV health. However, this relationship does not prove causality, and increasing progesterone via replacement therapy will likely cause weight gain.
Relationship between DHEA and body composition in men
Evidence from observational studies
Cross-sectional studies examining the associations between circulating DHEA with BMI have mostly shown a negative relationship (143, 196, 571) with an exception of a study by Vermeulen et al. (598) which included 250 men and showed a tendency for positive relationship. Notably, all studies show a negative relationship between DHEA and adiposity as deter-mined by actual or relative fat mass (143, 571). High DHEA has also been shown to associate with decreased abdominal adiposity as determined by waist-to-hip ratio (143, 196, 571). In contrast, the relationship of BMI with DHEAS is less consistent with some studies showing increased DHEAS and others showing decreases or no change in circulating DHEAS with increasing BMI (1, 143, 239, 240, 242, 243, 261, 306, 478, 486, 571, 598, 600). Similar discordant findings among different studies are also evident in the associations observed between DHEAS and body fat distribution (1, 143, 196, 239, 240, 242, 243, 261, 478, 486, 571). The inconsistencies observed may be partly attributed to the limited number of studies in which this relationship has been evaluated and the age of the participants included in these studies (572). Nonetheless, together these findings suggest that low DHEA may be associated with increased body weight, adiposity, and abdominal adipose tissue distribution. Notably, in an experimental study of overfeeding induced weight gain, low DHEA was associated with greater increases in body weight and fat mass (81). However, the relationship between body weight and DHEA may not be sustained during weight loss. During a Roux-en-Y gastric bypass surgery-induced weight loss in 64 obese patients, no changes in DHEA were observed (636). Similarly, diet-induced weight loss has been shown to not accompany any changes in circulating DHEA or DHEAS (257, 598). But some studies have demonstrated increase in DHEA with weight loss (259, 266, 463).
Evidence from DHEA supplementation studies
Clinical trials evaluating the effects of DHEA supplementation vary greatly in terms of participant inclusion criteria, dosage of DHEA, and duration of treatment. In spite of these variations, in a meta-analysis including 25 RCTs of DHEA supplementation on metabolic outcomes in elderly men, Corona et al. (139) showed that increases in DHEA were associated with reduction of fat mass and no change in fat-free mass. Importantly, even though a significant decrease in fat mass was noted, the magnitude of change was not associated with clear metabolic consequences and may be partly attributed to increases in T and E2 (139). Of note, even though the decreases in fat mass upon DHEA supplementation among men have been shown in other pooled analyses as well (297), not all studies have similar outcomes. In a study including elderly men (>70 years) with low scores on strength tests, oral supplementation of 50 mg/day DHEA for 36 weeks was associated with an increase in BMI with no significant changes in fat mass or lean mass (433). In another study of 4-month DHEA supplementation in 22 healthy men aged 50 to 69 years with endogenous DHEAS levels below 4.1 μM, no change in BMI, waist-to-hip ratio, or body composition was observed (21). Similarly, following a 12-month oral supple-mentation of DHEA in men aged 60 to 80 years, no differences in fat mass, fat-free mass, or muscle strength were observed (466). This finding was in contrast to a previous study which showed that 100 mg/day supplementation for 6 months was associated with an increase in knee muscle strength (651).
Summary
Together, the cross-sectional and interventional studies high-light the inconsistencies observed and suggest that DHEA is not a major determinant of body weight and adiposity. In other words, targeting DHEA for reducing body weight or body fat may not be a viable strategy for CVD prevention.
Sex Hormones and Body Composition in Women
Parallel to increases in CV risk, detrimental changes in body weight, body composition, and body fat distribution occur during the transition to menopause. This suggests that sex hormones may be important in regulating body weight—findings for T, E2, DHEA, and progesterone are detailed below.
Relationship between T and body composition in women
Evidence from observational studies
It is well established that as women age, the reduction in E2 levels leads to a progressive shift towards androgenic dominance (94, 352). There is observational evidence to suggest a positive association between T levels and body adiposity, waist circumference, insulin resistance, and T2D in women (92, 299, 445). In young premenopausal women, there appears to be a positive relationship between T levels and overall fat mass, but not abdominal adiposity (326). A similar positive relationship between T and total body fat has been observed in middle-aged women adjusted for menopause status (39). Supporting data from the SWAN Fat Patterning Study demonstrates that bioavailable T in middle-aged women is positively associated with visceral fat even after adjusting for insulin resistance (300). This positive association between T and body adiposity may turn into a negative association in the postmenopausal years (113).
Evidence from T supplementation studies
Studies examining the effects of T supplementation in women are rare. However, one study found that short-term T administration for 24 weeks in hysterectomized women with low Tlevels (<31 ng/dL) at baseline (both with and without oophorectomy) revealed that while injections of 3, 6.25, 12.5, or 25 mg of T enanthate was not associated with worsening CVD risk factors, there were dose-dependent gains in lean mass (276). On the other hand, women with PCOS typically have higher total T and free T levels, which are associated with higher body adiposity (491).
Summary
Collectively, definitive findings related to the role of T in determining body composition remain inconclusive since most of the observational data are associative in nature and experimental (mechanistic) evidence of T therapy in women remains sparse.
Relationship between estrogen and body composition in women
Evidence from observational studies
The effect of estrogen on female body composition is most conveniently examined in relation to the menopause transition, which coincides with dramatic decline in estro-gens (particularly E2). Some observational studies of the menopause transition report that changes in weight are independent of age (405, 462), while others do not (632). Specifically, data from the SWAN study and other smaller observational trials demonstrate that body weight was both an important predictor of sex steroid levels and the degree that sex steroids change across menopause (575, 628). Furthermore, women with obesity often maintain their E2 levels across the menopause transition (400, 575). Other data from SWAN reveal that chronological age is a significant contributor to the increase in weight and waist circumference, while menopausal status is not (556). These observational reports from SWAN support that weight status is more likely to predict changes in reproductive hormone status (particularly estrogen) across the menopause transition and not the other way around. Nonetheless, the dramatic declines in E2 across the menopause transition occur in parallel with significant increases in abdominal and visceral adiposity (235, 300, 376, 400). Indeed, data from SWAN indicates that fat mass doubles and fat-free mass start to decline in the 2 years prior to the final menstrual cycle when E2 levels are declining the fastest (231).
Evidence from hormone supplementation studies
Clinical interventions indicate that hormone therapy may counter the increases in weight and rising adiposity levels observed during menopause. In the Postmenopausal Estro-gen/Progestogen Intervention Study, the Danish Osteoporosis Prevention Study, as well as the Kronos Early Estrogen Prevention Study, women who received oral estrogen therapy gained less weight and body fat than women taking placebo (122, 187, 304). In contrast, administration of GnRH agonist in premenopausal women, which results in a dramatic loss of E2, leads to gains in abdominal fat (643). Importantly, estrogen add-back in these patients seems to eliminate these detrimental gains (512). These mechanistic studies suggest the protective effect of E2 against abdominal fat gains.
Summary
Conclusions as to the exact role of E2 on body composition differs depending on whether you look to observational studies or clinical hormone supplementation studies. How-ever, evidence suggests that E2 may contribute to abdominal adiposity.
Relationship between progesterone and body composition in women
Evidence from observational studies
Studies examining the relationship between progesterone and body composition in women are lacking. Consequently, the findings related to the association between progesterone and obesity are conflicting. In a study of 325 healthy women, Mezzullo et al. (413) reported a decrease in progesterone with increases in BMI. In another study of 304 women aged 34 ± 14 years who participated in the HERITAGE study, progesterone was not found to be associated with BMI or other measures of fat distribution including waist circumference and waist-to-hip ratio (256). These findings from the HERITAGE study were in contrast from those observed in men where a negative association was shown. In a recent study of 2213 postmenopausal women from rural China, a positive nonlinear relationship of progesterone with measures of central adiposity including waist circumference, waist-to-hip ratio, and waist-to-height ratio was observed but not for BMI (440). It is likely that the nonlinear relationship with increased progesterone observed for both low and high abdominal adiposity may obscure the findings in studies with small sample size. Nonetheless, weight loss following ketogenic diet in women with polycystic ovary syndrome has been shown to associate with increases in progesterone (459).
Evidence from hormone supplementation studies
Evidence for the role of progesterone in altering body weight and body composition can be gathered from clinical trials examining the effects of progesterone usage for contraception as well as a part of hormonal replacement therapy. In a 1-year open-label study of oral POC pill including 1006 women with BMI ranging from normal weight to obese, no significant changes in body weight were observed with increased body weight being reported in only 3.5% of the participants (335). In contrast, a retrospective chart review study of 240 women receiving depot-MPA found that women gained weight while they were on depot-MPA irrespective of their starting BMI (535). Similar effects of depot-MPA-associated increases in body weight and fat mass were observed in meta-analysis of 24 observational studies (170). Of note, among contraceptive methods in the United States, depot-MPA products have the highest discontinuation rate attributable to side effects such as weight gain. In another analysis of studies comparing the effects of POC with other methods or no contraception on body weight, depot-MPA use was associated with body weight gain, increased fat mass, and decreased lean mass (373). However, the authors noted that the increase in weight during the 1-year duration was modest and similar to that observed in women using no or other birth control methods. Lastly, in a 1-year RCT of MHT in postmenopausal women comparing combination of CEE with low-/high-dose of micronized progesterone, high-dose progesterone combination was associated with a small but significant increase in lean mass along with decreases in both upper and lower body fat depots (164). Remarkably, in the low-dose progesterone group, change to android fat distribution was noted.
Summary
While the role of progesterone in altering body weight and body composition in women is not clear, current evidence does not suggest that progesterone may have clinically significant role in determining adiposity or lean mass.
Relationship between DHEA and body composition in women
Evidence from observational studies
Cross-sectional studies examining the association between DHEA and/or DHEAS with measures of body weight and body composition have shown mixed results with several studies showing absence of any relationship (42, 146, 154, 306, 511). However, a negative relationship between DHEA and BMI has also been observed in pre- and postmenopausal women in three studies (41, 158, 160). Notably, associations between DHEAS and BMI are more inconsistent with some studies showing a positive (1, 160, 241, 629) relationship while others showing no (382, 486, 600) or negative relationship as well (41, 159, 189, 291).
Evidence from hormone supplementation studies
Clinical studies examining the effects of DHEA supplementation reveal no beneficial effect of DHEA on body composition. In a small trial of 6 postmenopausal women, 1600 mg/day of DHEA was orally administered for 28 days and did not appear to have any effect on body weight or percent body fat (430). Even a longer-term study of DHEA supplementation (50 mg daily) over 6 months in 17 older women revealed that percent body fat was unaltered (424).
Larger-scale trials do not provide a more optimistic view of the potential beneficial effects of DHEA supplementation on body composition. Specifically, a 1-year RCT revealed that DHEA had no effect on fat mass or fat-free mass in older women (n = 70) with low serum DHEA-S levels (296). Similarly, another 1-year trial of oral DHEA supplementation (50 mg/day) also did not show any improvement in body composition in older women (n = 115) (607). In support of these findings, a longer 2-year trial of elderly women (n = 57) with low levels of DHEA also did not show benefit from oral DHEA supplementation (50 mg/day) with regards to body composition, physical performance, insulin sensitivity, or quality of life (435). Despite individual differences in the three above mentioned trials of DHEA supplementation and their inability to find a positive effect of DHEA on body composition, these trial data were pooled to determine if there were sex-specific differences. Pooled findings revealed that DHEA therapy increased fat-free mass in women by 0.5 kg, although these trials did not control for exercise behavior which might explain some of the increases in fat-free mass in these women (297). One exercise trial with DHEA replacement therapy was conducted. However, this trial demonstrated that 10 months of DHEA replacement therapy (50 mg/day) alone does not significantly increase muscle mass or strength in elderly women (602). Instead, the addition of weightlifting exercise training to DHEA supplementation may be required to improve muscle mass and strength (602).
Summary
Overall, the current literature does not support DHEA supplementation as a measure to reduce obesity or improve body composition in women.
Sex Hormones and Body Composition in Transgender Individuals
Hormonal treatment in transgender individuals is associated with increase in body weight (336). The effects of sex hormones on body composition in transgender individuals are similar to those observed in non transgender individuals. T-based hormonal treatment in transmen is associated with increased muscle mass and decreased fat mass. However, there are reports that T therapy in transmen may increase visceral adiposity as well (182). In contrast, E2-based hormonal therapy in transwomen is associated with increases in body fat and decreases in muscle mass (336, 542).
Clinical Implication for CV Prevention
The sexual dimorphism in development and progression of CVD suggests a potential role of gonadal hormones. Conversely, considering the relationship between sex hormones and CVD risk factors including obesity, the use of sex hormones for CVD prevention may also be envisaged. Nonetheless, any such use would need to consider effects on overall health and not just be limited to CV health.
TRT in Men—Potential Benefits, Risks, and Recommendations
As described above, low T levels in men have been associated with unfavorable metabolic profile including increased adiposity, decreased muscle mass, insulin resistance and bone loss, and sexual symptoms including decreased libido and erectile dysfunction. Low T is also associated with nonspecific symptoms including fatigue, decreased concentration, low-grade depressed mood, and sleep disturbances. Together, low T is associated with increased CVD risk although no causality has been established (63, 180, 528). In 2015, concerned by a steep increase in T prescriptions (33) for treatment of nonspecific symptoms of age-related hypogonadism and also due to conflicting findings of CVD risk with T use, the US Food and Drug Administration restricted the use of TRT only in men with classic hypogonadism (primary hypogonadism or hypogonadotropic hypogonadism) and mandated that the drug labels state that the efficacy and safety of this therapy have not been established for age-related hypogonadism (438).
Specifically, the recent American College of Physicians clinical guideline recommends that TRT be not initiated for improvement of energy, vitality, physical function, or cognition in men with age-related low T (481). Table 3 summarizes the potential benefits and risks of TRT based on the currently available evidence (648). Concordant with these findings, the US Endocrine Society guideline also does not recommend T therapy in hypogonadal men with organic (advanced age) or functional hypogonadism (obesity). Importantly, it is recommended that the decision to prescribe T therapy in hypogonadal men (with classic pathology) should be based on the presence of preexisting prostate and CV conditions (648). Notably, the Endocrine Society guideline notes that low T is not considered to be an etiology factor for obesity as well (519). Lastly, the 2018 Endocrine Society clinical practice guideline recommends against using TRT for glycemic control (63).
Table 3.
Potential Benefits and Risks of Testosterone Replacement Therapy (TRT) in Men with Low T Levels
| Possible benefit or risk | |
|---|---|
| Sexual function | Small to moderate improvement in libido, sexual activity |
| Erectile function | Possible small improvement |
| Quality of life | Small improvement, driven mostly by improvement in sexual function |
| Physical function | No significant effect on self-reported or objectively assessed performance measures |
| Vitality or fatigue | No significant effect |
| Cognitive function | No significant effect |
| Mood | No significant effect |
| Body composition | Increases lean mass; no significant effect on physical function |
| Bone mineral density | No effect or small increase; no reduction in fracture risk |
| Glycemic control | Uncertain effect; not recommended to improve glycemic control among patients with diabetes mellitus |
| Lipid profile | No significant effect; may improve TG or worsen HDL-C slightly |
| CV events | Uncertain |
| Mortality | Uncertain |
| Prostate cancer | Uncertain risk; monitor PSA; contraindicated in men with suspected prostate cancer |
| Erythrocytosis | Increases hemoglobin and hematocrit; monitor CBC |
| Fertility | Decreases sperm count; not appropriate for men who desire fertility in the near future |
| LUTS | No worsening |
| Fluid retention | May cause fluid retention and edema |
| OSA | May worsen sleep apnea among patients with OSA |
MHT in Women—Potential Benefits, Risks, and Recommendations
MHT, also described as Hormone Replacement Therapy or simply Hormone Therapy refers to use of estrogen, given either alone or in combination with a progestogen for relief of menopausal symptoms such as hot flushes, night sweats, and sleep disturbance, and for prevention of bone loss in post-menopausal women. MHT, estrogen alone or in combination with progestin, is highly effective in easing menopausal vasomotor symptoms (385) and in preventing osteoporosis and related risk of fractures (620, 660). However, these benefits do not persist long after cessation of the hormone therapy (258, 321, 348, 645, 660).
Importantly, there are some concerns related to MHT and cancer. A large cohort study of 44,241 postmenopausal women found that estrogen-only therapy for 10 or more years was associated with increased risk of ovarian cancer especially with longer duration of MHT use (16, 61, 128, 152, 347, 367). However, due to paucity of data from RCTs and inconsistent findings from epidemiological studies, the causality between MHT and ovarian cancer remains unproven. Similar relationship between MHT and increased risk for breast cancer has been observed in some studies as well (499).
Table 4 summarizes the potential benefits and risk of MHT. Briefly, MHT has an acceptable benefit-to-risk profile when used in women who are within 10 years of the onset of menopause and are aged <60 years. It is preferable to use the lowest dose for the shortest duration needed. However, current recommendations from American College of Obstetricians and Gynecologists do not support the use of hormonal therapy for primary or secondary prevention of CHD (5). MHT is also not recommended for prevention of dementia or other chronic diseases. Some professional societies are supportive of MHT for prevention of osteoporosis in selected women if other therapies are not suitable.
Table 4.
Potential Benefits and Risks of Menstrual Hormone Therapy (MHT) in Postmenopausal Women
| Possible benefit or risk | |
|---|---|
| Menopausal symptoms | Most beneficial in alleviating hot flashes, night sweats, and related menopausal symptoms |
| Osteoporosis and fracture prevention | Effective, but benefit is lost with therapy cessation. Consider MHT in selected women if other effective treatments are not suitable |
| CHD prevention | Not recommended |
| Stroke | Increased risk |
| VTE | Increased risk |
| Urinary incontinence | Increased risk |
| Dementia | Increased risk |
| Cancer | Increased breast cancer risk with CEE+MPA. Decreased breast cancer risk with CEE-only. Decreased risk of colorectal cancer with CEE+MPA while on treatment only |
| Mortality | Neutral |
CHD, coronary heart disease; VTE, venous thromboembolism; CEE, conjugated equine estrogen; MPA, medroxyprogesterone acetate.
Conclusion
The cardioprotective benefits of T and E2 are recognized in men and women which partly contribute to sexual dimorphism in CVD. However, current studies do not support the use of hormone replacement for CVD prevention. Similarly, with regard to obesity, studies do not support the use of T, E2, or DHEA for weight loss or improvement in body composition. The metrics with the greatest potential for improvement in the United States are health behaviors including body weight reduction along with improved diet quality and physical activity. Towards the goal to improve public CV health, the American Heart Association has identified seven domains including four healthy behaviors—(i) increased physical activity, (ii) improved eating habits, (iii) smoking cessation, and (iv) weight reduction, and three health factors—including controlled (i) blood pressure, (ii) total cholesterol, and (iii) fasting plasma glucose (AHA life 7). Hormonal replacement therapy has not demonstrated the ability to improve any of the health factors or contribute to weight reduction.
Nevertheless, sexual dimorphism in CVD risk and CVD mortality highlights the need to develop sex-specific risk assessment and therapeutic interventions for CVD prevention. Strong consideration needs to be given to understand sex differences with a goal to integrate them into therapeutic preventive strategies. An important area is to increase the recruitment of women in CVD clinical trials to allow preventive strategies to be optimized and individualized based on sex rather than one size fits all. Awareness related to prognosis of CVD in women needs to be generated with special emphasis on women with high CVD risk such as diabetes and smoking. It would also be important to make preventive care available in younger women. Lastly, in recognition of the sexual differences, there is a need to generate sex-specific clinical guideline for CVD prevention especially for conditions such as HF where the underlying pathophysiology is distinctly different in men and women.
Didactic Synopsis.
Major teaching points
CVD is a leading cause of death in both men and women.
Obesity contributes to CVD pathophysiology and abdominal fat confers the highest risk.
While low testosterone in men is associated with increased CVD risk and obesity, current evidence does not support testosterone replacement to mitigate CVD risk or reduce body weight.
In women, estrogens have cardio-protective effects, but current evidence does not support the use of estrogen replacement to reduce CVD risk in postmenopausal women.
Estrogen deficiency contributes to increases in abdominal fat in both men and women.
Progesterone increases fat mass in both men and women, but its role in modifying CVD risk is unclear.
Low DHEA is associated with increased CVD risk, but DHEA supplementation does not alter CVD risk in men or women.
Related Articles.
Sex, Gender, and Sex Hormones in Pulmonary Hypertension and Right Ventricular Failure
Sex Hormones and Cognition: Neuroendocrine Influences on Memory and Learning
Mechanisms of Sex Disparities in Cardiovascular Function and Remodeling
Neural Control of the Circulation: How Sex and Age Differences Interact in Humans
Androgens and the Regulation of Adiposity and Body Fat Distribution in Humans
Sex Differences in the HPA Axis
References
- 1.Abbasi A, Duthie EH Jr, Sheldahl L, Wilson C, Sasse E, Rudman I, Mattson DE. Association of dehydroepiandrosterone sulfate, body composition, and physical fitness in independent community-dwelling older men and women. J Am Geriatr Soc 46: 263–273, 1998. [DOI] [PubMed] [Google Scholar]
- 2.Abdullah A, Peeters A, de Courten M, Stoelwinder J. The magnitude of association between overweight and obesity and the risk of diabetes: A meta-analysis of prospective cohort studies. Diabetes Res Clin Pract 89: 309–319, 2010. [DOI] [PubMed] [Google Scholar]
- 3.Abraham GE. Ovarian and adrenal contribution to peripheral andro-gens during the menstrual cycle. J Clin Endocrinol Metab 39: 340–346, 1974. [DOI] [PubMed] [Google Scholar]
- 4.Abu-Taha M, Rius C, Hermenegildo C, Noguera I, Cerda-Nicolas JM, Issekutz AC, Jose PJ, Cortijo J, Morcillo EJ, Sanz MJ. Menopause and ovariectomy cause a low grade of systemic inflammation that may be prevented by chronic treatment with low doses of estrogen or losartan. J Immunol 183: 1393–1402, 2009. [DOI] [PubMed] [Google Scholar]
- 5.ACOG. ACOG Committee Opinion No. 565: Hormone therapy and heart disease. Obstet Gynecol 121: 1407–1410, 2013. [DOI] [PubMed] [Google Scholar]
- 6.Agledahl I, Hansen JB, Svartberg J. Impact of testosterone treatment on postprandial triglyceride metabolism in elderly men with subnor-mal testosterone levels. Scand J Clin Lab Invest 68: 641–648, 2008. [DOI] [PubMed] [Google Scholar]
- 7.Aguilar-Salinas CA, García EG, Robles L, Riano D, Ruiz-Gomez DG, García-Ulloa AC, Melgarejo MA, Zamora M, Guillen-Pineda LE, Mehta R. High adiponectin concentrations are associated with the metabolically healthy obese phenotype. J Clin Endocrinol Metab 93: 4075–4079, 2008. [DOI] [PubMed] [Google Scholar]
- 8.Ajayi AA, Mathur R, Halushka PV. Testosterone increases human platelet thromboxane A2 receptor density and aggregation responses. Circulation 91: 2742–2747, 1995. [DOI] [PubMed] [Google Scholar]
- 9.Albert SG, Morley JE. Testosterone therapy, association with age, initiation and mode of therapy with cardiovascular events: A systematic review. Clin Endocrinol (Oxf) 85: 436–443, 2016. [DOI] [PubMed] [Google Scholar]
- 10.Alexander GC, Iyer G, Lucas E, Lin D, Singh S. Cardiovascular risks of exogenous testosterone use among men: A systematic review and meta-analysis. Am J Med 130: 293–305, 2017. [DOI] [PubMed] [Google Scholar]
- 11.Allan CA, Strauss BJ, Burger HG, Forbes EA, McLachlan RI. Testosterone therapy prevents gain in visceral adipose tissue and loss of skeletal muscle in nonobese aging men. J Clin Endocrinol Metab 93: 139–146, 2008. [DOI] [PubMed] [Google Scholar]
- 12.Allen NE, Appleby PN, Davey GK, Key TJ. Lifestyle and nutritional determinants of bioavailable androgens and related hormones in British men. Cancer Causes Control 13: 353–363, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Amatruda JM, Harman SM, Pourmotabbed G, Lockwood DH. Depressed plasma testosterone and fractional binding of testosterone in obese males. J Clin Endocrinol Metab 47: 268–271, 1978. [DOI] [PubMed] [Google Scholar]
- 14.Amir E, Seruga B, Niraula S, Carlsson L, Ocana A. Toxicity of adjuvant endocrine therapy in postmenopausal breast cancer patients: A systematic review and meta-analysis. J Natl Cancer Inst 103: 1299–1309, 2011. [DOI] [PubMed] [Google Scholar]
- 15.Andersen KK, Olsen TS. The obesity paradox in stroke: Lower mortality and lower risk of readmission for recurrent stroke in obese stroke patients. Int J Stroke 10: 99–104, 2015. [DOI] [PubMed] [Google Scholar]
- 16.Anderson GL, Judd HL, Kaunitz AM, Barad DH, Beresford SA, Pet-tinger M, Liu J, McNeeley SG, Lopez AM, Women’s Health Initiative Investigators. Effects of estrogen plus progestin on gynecologic cancers and associated diagnostic procedures: The Women’s Health Initiative randomized trial. JAMA 290: 1739–1748, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, Bonds D, Brunner R, Brzyski R, Caan B, Chlebowski R, Curb D, Gass M, Hays J, Heiss G, Hendrix S, Howard BV, Hsia J, Hubbell A, Jackson R, Johnson KC, Judd H, Kotchen JM, Kuller L, LaCroix AZ, Lane D, Langer RD, Lasser N, Lewis CE, Manson J, Margolis K, Ockene J, O’Sullivan MJ, Phillips L, Prentice RL, Ritenbaugh C, Robbins J, Rossouw JE, Sarto G, Stefanick ML, Van Horn L, Wactawski-Wende J, Wallace R, Wassertheil-Smoller S, Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: The Women’s Health Initiative randomized controlled trial. JAMA 291: 1701–1712, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Angerås O, Albertsson P, Karason K, Råmunddal T, Matejka G, James S, Lagerqvist B, Rosengren A, Omerovic E. Evidence for obesity para-dox in patients with acute coronary syndromes: A report from the Swedish Coronary Angiography and Angioplasty Registry. Eur Heart J 34: 345–353, 2013. [DOI] [PubMed] [Google Scholar]
- 19.Arango-Lopera V, Arroyo P, Gutiérrez-Robledo LM, Perez-Zepeda M, Cesari M. Mortality as an adverse outcome of sarcopenia. JNutr Health Aging 17: 259–262, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Araujo AB, Dixon JM, Suarez EA, Murad MH, Guey LT, Wittert GA. Clinical review: Endogenous testosterone and mortality in men: A systematic review and meta-analysis. J Clin Endocrinol Metab 96: 3007–3019, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arlt W, Callies F, Koehler I, van Vlijmen JC, Fassnacht M, Strasburger CJ, Seibel MJ, Huebler D, Ernst M, Oettel M, Reincke M, Schulte HM, Allolio B. Dehydroepiandrosterone supplementation in healthy men with an age-related decline of dehydroepiandrosterone secretion. J Clin Endocrinol Metab 86: 4686–4692, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Arnlov J, Pencina MJ, Amin S, Nam BH, Benjamin EJ, Murabito JM, Wang TJ, Knapp PE, D’Agostino RB Sr, Bhasin S, Vasan RS. Endogenous sex hormones and cardiovascular disease incidence in men. Ann Intern Med 145: 176–184, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Asi N, Mohammed K, Haydour Q, Gionfriddo MR, Vargas OL, Prokop LJ, Faubion SS, Murad MH. Progesterone vs. synthetic progestins and the risk of breast cancer: A systematic review and meta-analysis. Syst Rev 5: 121, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Asscheman H, Giltay EJ, Megens JA, de Ronde WP, van Trotsenburg MA, Gooren LJ. A long-term follow-up study of mortality in transsexuals receiving treatment with cross-sex hormones. Eur J Endocrinol 164: 635–642, 2011. [DOI] [PubMed] [Google Scholar]
- 25.Atkins JL, Whincup PH, Morris RW, Lennon LT, Papacosta O, Wannamethee SG. Sarcopenic obesity and risk of cardiovascular disease and mortality: A population-based cohort study of older men. JAm Geriatr Soc 62: 253–260, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aune D, Sen A, Norat T, Janszky I, Romundstad P, Tonstad S, Vatten LJ. Body mass index, abdominal fatness, and heart failure incidence and mortality: A systematic review and dose–response meta-analysis of prospective studies. Circulation 133: 639–649, 2016. [DOI] [PubMed] [Google Scholar]
- 27.Aune D, Sen A, Prasad M, Norat T, Janszky I, Tonstad S, Romund-stad P, Vatten LJ. BMI and all cause mortality: Systematic review and non-linear dose-response meta-analysis of 230 cohort studies with 3.74 million deaths among 30.3 million participants. BMJ 353: i2156, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Awouters M, Vanderschueren D, Antonio L. Aromatase inhibitors and selective estrogen receptor modulators: Unconventional therapies for functional hypogonadism? Andrology 8: 1590–1597, 2020. [DOI] [PubMed] [Google Scholar]
- 29.Badheka AO, Rathod A, Kizilbash MA, Garg N, Mohamad T, Afonso L, Jacob S. Influence of obesity on outcomes in atrial fibrillation: Yet another obesity paradox. Am J Med 123: 646–651, 2010. [DOI] [PubMed] [Google Scholar]
- 30.Bae S, Hong YC. Exposure to bisphenol A from drinking canned beverages increases blood pressure: Randomized crossover trial. Hyper-tension 65: 313–319, 2015. [DOI] [PubMed] [Google Scholar]
- 31.Baggish AL, Weiner RB, Kanayama G, Hudson JI, Lu MT, Hoffmann U, Pope HG Jr. Cardiovascular Toxicity of Illicit Anabolic-Androgenic Steroid Use. Circulation 135: 1991–2002, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bailar JC 3rd, Byar DP. Estrogen treatment for cancer of the prostate. Early results with 3 doses of diethylstilbestrol and placebo. Cancer 26: 257–261, 1970. [DOI] [PubMed] [Google Scholar]
- 33.Baillargeon J, Urban RJ, Ottenbacher KJ, Pierson KS, Goodwin JS. Trends in androgen prescribing in the United States, 2001 to 2011. JAMA Intern Med 173: 1465–1466, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baillargeon J, Urban RJ, Zhang W, Zaiden MF, Javed Z, Sheffield-Moore M, Kuo YF, Sharma G. Testosterone replacement therapy and hospitalization rates in men with COPD. Chron Respir Dis 16: 1479972318793004, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baird GL, Archer-Chicko C, Barr RG, Bluemke DA, Foderaro AE, Fritz JS, Hill NS, Kawut SM, Klinger JR, Lima JAC, Mullin CJ, Ouyang P, Palevsky HI, Palmisicano AJ, Pinder D, Preston IR, Roberts KE, Smith KA, Walsh T, Whittenhall M, Ventetuolo CE. Lower DHEA-S levels predict disease and worse outcomes in post-menopausal women with idiopathic, connective tissue disease- and congenital heart disease-associated pulmonary arterial hypertension. Eur Respir J 51: 1800467, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bajelan M, Etehad Roodi N, Hasanzadeh Daloee M, Farhangnia M, Samadi KA. The effect of low testosterone and estrogen levels on pro-gressive coronary artery disease in men. Rep Biochem Mol Biol 8:168–171, 2019. [PMC free article] [PubMed] [Google Scholar]
- 37.Baker L, Meldrum KK, Wang M, Sankula R, Vanam R, Raiesdana A, Tsai B, Hile K, Brown JW, Meldrum DR. The role of estrogen in cardiovascular disease. J Surg Res 115: 325–344, 2003. [DOI] [PubMed] [Google Scholar]
- 38.Banack HR, Wactawski-Wende J, Hovey KM, Stokes A. Is BMI a valid measure of obesity in postmenopausal women? Menopause 25: 307–313, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bann D, Wu FC, Keevil B, Lashen H, Adams J, Hardy R, Muniz G, Kuh D, Ben-Shlomo Y, Ong KK. Changes in testosterone related to body composition in late midlife: Findings from the 1946 British birth cohort study. Obesity (Silver Spring) 23: 1486–1492, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barreira TV, Harrington DM, Staiano AE, Heymsfield SB, Katzmarzyk PT. Body adiposity index, body mass index, and body fat in white and black adults. JAMA 306: 828–830, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barrett-Connor E, Ferrara A. Dehydroepiandrosterone, dehydroepiandrosterone sulfate, obesity, waist-hip ratio, and noninsulin-dependent diabetes in postmenopausal women: The Rancho Bernardo Study. J Clin Endocrinol Metab 81: 59–64, 1996. [DOI] [PubMed] [Google Scholar]
- 42.Barrett-Connor E, Goodman-Gruen D. Dehydroepiandrosterone sulfate does not predict cardiovascular death in postmenopausal women. The Rancho Bernardo Study. Circulation 91: 1757–1760, 1995. [DOI] [PubMed] [Google Scholar]
- 43.Barrett-Connor E, Grady D. Hormone replacement therapy, heart dis-ease, and other considerations. Annu Rev Public Health 19: 55–72, 1998. [DOI] [PubMed] [Google Scholar]
- 44.Barrett-Connor E, Khaw KT, Yen SS. A prospective study of dehydroepiandrosterone sulfate, mortality, and cardiovascular disease. N Engl J Med 315: 1519–1524, 1986. [DOI] [PubMed] [Google Scholar]
- 45.Bartelt A, Bruns OT, Reimer R, Hohenberg H, Ittrich H, Peldschus K, Kaul MG, Tromsdorf UI, Weller H, Waurisch C, Eychmuller A, Gordts PL, Rinninger F, Bruegelmann K, Freund B, Nielsen P, Merkel M, Heeren J. Brown adipose tissue activity controls triglyceride clearance. Nat Med 17: 200–205, 2011. [DOI] [PubMed] [Google Scholar]
- 46.Basaria S, Coviello AD, Travison TG, Storer TW, Farwell WR, Jette AM, Eder R, Tennstedt S, Ulloor J, Zhang A, Choong K, Lakshman KM, Mazer NA, Miciek R, Krasnoff J, Elmi A, Knapp PE, Brooks B, Appleman E, Aggarwal S, Bhasin G, Hede-Brierley L, Bhatia A, Collins L, LeBrasseur N, Fiore LD, Bhasin S. Adverse events asso-ciated with testosterone administration. N Engl J Med 363: 109–122, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Basu R, Dalla Man C, Campioni M, Basu A, Nair KS, Jensen MD, Khosla S, Klee G, Toffolo G, Cobelli C, Rizza RA. Two years of treatment with dehydroepiandrosterone does not improve insulin secretion, insulin action, or postprandial glucose turnover in elderly men or women. Diabetes 56: 753–766, 2007. [DOI] [PubMed] [Google Scholar]
- 48.Bates GW Jr, Egerman RS, Umstot ES, Buster JE, Casson PR. Dehydroepiandrosterone attenuates study-induced declines in insulin sensitivity in postmenopausal women. AnnNYAcadSci 774: 291–293, 1995. [DOI] [PubMed] [Google Scholar]
- 49.Batsis JA, Barre LK, Mackenzie TA, Pratt SI, Lopez-Jimenez F, Bartels SJ. Variation in the prevalence of sarcopenia and sarcopenic obesity in older adults associated with different research definitions: Dual-energy X-ray absorptiometry data from the National Health and Nutrition Examination Survey 1999–2004. J Am Geriatr Soc 61: 974–980, 2013. [DOI] [PubMed] [Google Scholar]
- 50.Batsis JA, Sahakyan KR, Rodriguez-Escudero JP, Bartels SJ, Somers VK, Lopez-Jimenez F. Normal weight obesity and mortality in United States subjects ≥ 60 years of age (from the Third National Health and Nutrition Examination Survey). Am J Cardiol 112: 1592–1598, 2013. [DOI] [PubMed] [Google Scholar]
- 51.Baykan EK, Erdogan M, Ozen S, Darcan S, Saygili LF. Aromatase deficiency, a rare syndrome: Case report. J Clin Res Pediatr Endocrinol 5: 129–132, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beale AL, Meyer P, Marwick TH, Lam CSP, Kaye DM. Sex differences in cardiovascular pathophysiology: Why women are overrepresented in heart failure with preserved ejection fraction. Circulation 138: 198–205, 2018. [DOI] [PubMed] [Google Scholar]
- 53.Becher T, Palanisamy S, Kramer DJ, Eljalby M, Marx SJ, Wibmer AG, Butler SD, Jiang CS, Vaughan R, Schoder H, Mark A, Cohen P. Brown adipose tissue is associated with cardiometabolic health. Nat Med 27:58–65, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Behre HM, Tammela TL, Arver S, Tolra JR, Bonifacio V, Lamche M, Kelly J, Hiemeyer F, European Testogel Study Team, Giltay EJ, Gooren LJ. A randomized, double-blind, placebo-controlled trial of testosterone gel on body composition and health-related quality-of-life in men with hypogonadal to low-normal levels of serum testosterone and symptoms of androgen deficiency over 6 months with 12 months open-label follow-up. Aging Male 15: 198–207, 2012. [DOI] [PubMed] [Google Scholar]
- 55.Behre HM, Zitzmann M, Anderson RA, Handelsman DJ, Lestari SW, McLachlan RI, Meriggiola MC, Misro MM, Noe G, Wu FC, Festin MP, Habib NA, Vogelsong KM, Callahan MM, Linton KA, Colvard DS. Efficacy and safety of an injectable combination hormonal contraceptive for men. J Clin Endocrinol Metab 101: 4779–4788, 2016. [DOI] [PubMed] [Google Scholar]
- 56.Beitelshees AL, Johnson JA, Hames ML, Gong Y, Cooper-Dehoff RM, Wu J, Cresci S, Ma CX, Pepine CJ, Province MA, Spertus JA, McLeod HL. Aromatase gene polymorphisms are associated with survival among patients with cardiovascular disease in a sex-specific manner. PLoS One 5: e15180, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Belanger A, Candas B, Dupont A, Cusan L, Diamond P, Gomez JL, Labrie F. Changes in serum concentrations of conjugated and uncon-jugated steroids in 40- to 80-year-old men. J Clin Endocrinol Metab 79: 1086–1090, 1994. [DOI] [PubMed] [Google Scholar]
- 58.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P, American Heart Association Statistics Committee, and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation 135: e146–e603, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O’Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, VanWagner LB, Wilkins JT, Wong SS, Virani SS, American Heart Association Council on Epidemiology and Prevention Statistics Committee, and Stroke Statistics Subcommittee. Heart Dis-ease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation 139: e56–e528, 2019. [DOI] [PubMed] [Google Scholar]
- 60.Benn M, Voss SS, Holmegard HN, Jensen GB, Tybjaerg-Hansen A, Nordestgaard BG. Extreme concentrations of endogenous sex hormones, ischemic heart disease, and death in women. Arterioscler Thromb Vasc Biol 35: 471–477, 2015. [DOI] [PubMed] [Google Scholar]
- 61.Beral V, Million Women Study Collaborators, Bull D, Green J, Reeves G. Ovarian cancer and hormone replacement therapy in the Million Women Study. Lancet 369: 1703–1710, 2007. [DOI] [PubMed] [Google Scholar]
- 62.Berrington de Gonzalez A, Hartge P, Cerhan JR, Flint AJ, Hannan L, RJ MI, Moore SC, Tobias GS, Anton-Culver H, Freeman LB. Body-mass index and mortality among 1.46 million white adults. N Engl J Med 363: 2211–2219, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bhasin S, Brito JP, Cunningham GR, Hayes FJ, Hodis HN, Matsumoto AM, Snyder PJ, Swerdloff RS, Wu FC, Yialamas MA. Testosterone therapy in men with hypogonadism: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 103: 1715–1744, 2018. [DOI] [PubMed] [Google Scholar]
- 64.Bhasin S, Parker RA, Sattler F, Haubrich R, Alston B, Umbleja T, Shikuma CM, Team ACTGPAS. Effects of testosterone supplementation on whole body and regional fat mass and distribution in human immunodeficiency virus-infected men with abdominal obesity. J Clin Endocrinol Metab 92: 1049–1057, 2007. [DOI] [PubMed] [Google Scholar]
- 65.Bhasin S, Travison TG, Storer TW, Lakshman K, Kaushik M, Mazer NA, Ngyuen AH, Davda MN, Jara H, Aakil A, Anderson S, Knapp PE, Hanka S, Mohammed N, Daou P, Miciek R, Ulloor J, Zhang A, Brooks B, Orwoll K, Hede-Brierley L, Eder R, Elmi A, Bhasin G, Collins L, Singh R, Basaria S. Effect of testosterone supplementation with and without a dual 5alpha-reductase inhibitor on fat-free mass in men with suppressed testosterone production: A randomized con-trolled trial. JAMA 307: 931–939, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bhasin S, Woodhouse L, Casaburi R, Singh AB, Mac RP, Lee M, Yarasheski KE, Sinha-Hikim I, Dzekov C, Dzekov J, Magliano L, Storer TW. Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J Clin Endocrinol Metab 90: 678–688, 2005. [DOI] [PubMed] [Google Scholar]
- 67.Bhasin S, Woodhouse L, Storer TW. Androgen effects on body composition. Growth Horm IGF Res 13 Suppl A: S63–S71, 2003. [DOI] [PubMed] [Google Scholar]
- 68.Bhatia N, Santos M, Jones LW, Beckman JA, Penson DF, Morgans AK, Moslehi J. Cardiovascular effects of androgen deprivation ther-apy for the treatment of prostate cancer: ABCDE steps to reduce car-diovascular disease in patients with prostate cancer. Circulation 133:537–541, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bigaard J, Frederiksen K, Tjønneland A, Thomsen BL, Overvad K, Heitmann BL, Sørensen TI. Body fat and fat-free mass and all-cause mortality. Obes Res 12: 1042–1049, 2004. [DOI] [PubMed] [Google Scholar]
- 70.Bilezikian JP, Morishima A, Bell J, Grumbach MM. Increased bone mass as a result of estrogen therapy in a man with aromatase deficiency. N Engl J Med 339: 599–603, 1998. [DOI] [PubMed] [Google Scholar]
- 71.Blackman MR, Sorkin JD, Munzer T, Bellantoni MF, Busby-Whitehead J, Stevens TE, Jayme J, O’Connor KG, Christmas C, Tobin JD, Stewart KJ, Cottrell E, St Clair C, Pabst KM, Harman SM. Growth hormone and sex steroid administration in healthy aged women and men: A randomized controlled trial. JAMA 288: 2282–2292, 2002. [DOI] [PubMed] [Google Scholar]
- 72.Blanchette S, Marceau P, Biron S, Brochu G, Tchernof A. Circulating progesterone and obesity in men. Horm Metab Res 38: 330–335, 2006. [DOI] [PubMed] [Google Scholar]
- 73.Blew RM, Sardinha LB, Milliken LA, Teixeira PJ, Going SB, Ferreira DL, Harris MM, Houtkooper LB, Lohman TG. Assessing the validity of body mass index standards in early postmenopausal women. Obes Res 10: 799–808, 2002. [DOI] [PubMed] [Google Scholar]
- 74.Blosnich JR, Brown GR, Wojcio S, Jones KT, Bossarte RM. Mortality among veterans with transgender-related diagnoses in the Veter-ans Health Administration, FY2000-2009. LGBT Health 1: 269–276, 2014. [DOI] [PubMed] [Google Scholar]
- 75.Blum CA, Mueller C, Schuetz P, Fluri F, Trummler M, Mueller B, Katan M, Christ-Crain M. Prognostic value of dehydroepiandrosterone-sulfate and other parameters of adrenal function in acute ischemic stroke. PLoS One 8: e63224, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Boden WE, Miller MG, McBride R, Harvey C, Snabes MC, Schmidt J, McGovern ME, Fleg JL, Desvigne-Nickens P, Anderson T, Kashyap M, Probstfield JL. Testosterone concentrations and risk of cardiovascular events in androgen-deficient men with atherosclerotic cardiovascular disease. Am Heart J 224: 65–76, 2020. [DOI] [PubMed] [Google Scholar]
- 77.Bogers RP, Bemelmans WJ, Hoogenveen RT, Boshuizen HC, Wood-ward M, Knekt P, Van Dam RM, Hu FB, Visscher TL, Menotti A. Association of overweight with increased risk of coronary heart disease partly independent of blood pressure and cholesterol levels: A meta-analysis of 21 cohort studies including more than 300 000 persons. Arch Intern Med 167: 1720–1728, 2007. [DOI] [PubMed] [Google Scholar]
- 78.Borst SE, Shuster JJ, Zou B, Ye F, Jia H, Wokhlu A, Yarrow JF. Cardiovascular risks and elevation of serum DHT vary by route of testosterone administration: A systematic review and meta-analysis. BMC Med 12: 211, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Borst SE, Yarrow JF, Conover CF, Nseyo U, Meuleman JR, Lipinska JA, Braith RW, Beck DT, Martin JS, Morrow M, Roessner S, Beggs LA, McCoy SC, Cannady DF 2nd, Shuster JJ. Musculoskeletal and prostate effects of combined testosterone and finasteride administration in older hypogonadal men: A randomized, controlled trial. Am J Physiol Endocrinol Metab 306: E433–E442, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bosco C, Bosnyak Z, Malmberg A, Adolfsson J, Keating NL, Van Hemelrijck M. Quantifying observational evidence for risk of fatal and nonfatal cardiovascular disease following androgen deprivation therapy for prostate cancer: A meta-analysis. Eur Urol 68: 386–396, 2015. [DOI] [PubMed] [Google Scholar]
- 81.Bouchard C, Tchernof A, Tremblay A. Predictors of body composition and body energy changes in response to chronic overfeeding. Int J Obes (Lond) 38: 236–242, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bouillon R, Bex M, Vanderschueren D, Boonen S. Estrogens are essential for male pubertal periosteal bone expansion. J Clin Endocrinol Metab 89: 6025–6029, 2004. [DOI] [PubMed] [Google Scholar]
- 83.Bouloux PM, Legros JJ, Elbers JM, Geurts TB, Kaspers MJ, Meehan AG, Meuleman EJ, Study I. Effects of oral testosterone undecanoate therapy on bone mineral density and body composition in 322 aging men with symptomatic testosterone deficiency: A 1-year, randomized, placebo-controlled, dose-ranging study. Aging Male 16: 38–47, 2013. [DOI] [PubMed] [Google Scholar]
- 84.Braga-Basaria M, Dobs AS, Muller DC, Carducci MA, John M, Egan J, Basaria S. Metabolic syndrome in men with prostate cancer under-going long-term androgen-deprivation therapy. J Clin Oncol 24: 3979–3983, 2006. [DOI] [PubMed] [Google Scholar]
- 85.Brahimaj A, Muka T, Kavousi M, Laven JS, Dehghan A, Franco OH. Serum dehydroepiandrosterone levels are associated with lower risk of type 2 diabetes: The Rotterdam Study. Diabetologia 60: 98–106, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Braithwaite RS, Chlebowski RT, Lau J, George S, Hess R, Col NF. Meta-analysis of vascular and neoplastic events associated with tamoxifen. J Gen Intern Med 18: 937–947, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bramer S, van Straten AH, Soliman Hamad MA, Berreklouw E, van den Broek KC, Maessen JG. Body mass index predicts new-onset atrial fibrillation after cardiac surgery. Eur J Cardiothorac Surg 40: 1185–1190, 2011. [DOI] [PubMed] [Google Scholar]
- 88.Brand JS, Rovers MM, Yeap BB, Schneider HJ, Tuomainen TP, Har-ing R, Corona G, Onat A, Maggio M, Bouchard C, Tong PC, Chen RY, Akishita M, Gietema JA, Gannage-Yared MH, Unden AL, Hautanen A, Goncharov NP, Kumanov P, Chubb SA, Almeida OP, Wittchen HU, Klotsche J, Wallaschofski H, Volzke H, Kauhanen J, Salonen JT, Ferrucci L, van der Schouw YT. Testosterone, sex hormone-binding globulin and the metabolic syndrome in men: An individual participant data meta-analysis of observational studies. PLoS One 9: e100409, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Britton KA, Massaro JM, Murabito JM, Kreger BE, Hoffmann U, Fox CS. Body fat distribution, incident cardiovascular disease, cancer, and all-cause mortality. J Am Coll Cardiol 62: 921–925, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brown CD, Higgins M, Donato KA, Rohde FC, Garrison R, Obarzanek E, Ernst ND, Horan M. Body mass index and the prevalence of hypertension and dyslipidemia. Obes Res 8: 605–619, 2000. [DOI] [PubMed] [Google Scholar]
- 91.Bruder-Nascimento T, Kress TC, Belin de Chantemele EJ. Recent advances in understanding lipodystrophy: A focus on lipodystrophy-associated cardiovascular disease and potential effects of leptin therapy on cardiovascular function. F1000Res 8: F1000 Faculty Rev–1756, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bruns CM, Kemnitz JW. Sex hormones, insulin sensitivity, and diabetes mellitus. ILAR J 45: 160–169, 2004. [DOI] [PubMed] [Google Scholar]
- 93.Bulun SE, Rosenthal IM, Brodie AM, Inkster SE, Zeller WP, DiGeorge AM, Frasier SD, Kilgore MW, Simpson ER. Use of tissue-specific promoters in the regulation of aromatase cytochrome P450 gene expression in human testicular and ovarian sex cord tumors, as well as in normal fetal and adult gonads. J Clin Endocrinol Metab 77: 1616–1621, 1993. [DOI] [PubMed] [Google Scholar]
- 94.Burger HG, Dudley EC, Cui J, Dennerstein L, Hopper JL. A prospective longitudinal study of serum testosterone, dehydroepiandrosterone sulfate, and sex hormone-binding globulin levels through the menopause transition. J Clin Endocrinol Metab 85: 2832–2838, 2000. [DOI] [PubMed] [Google Scholar]
- 95.Burnett-Bowie SA, McKay EA, Lee H, Leder BZ. Effects of aromatase inhibition on bone mineral density and bone turnover in older men with low testosterone levels. J Clin Endocrinol Metab 94: 4785–4792, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bush TL, Barrett-Connor E, Cowan LD, Criqui MH, Wallace RB, Suchindran CM, Tyroler HA, Rifkind BM. Cardiovascular mortality and noncontraceptive use of estrogen in women: Results from the Lipid Research Clinics Program Follow-up Study. Circulation 75: 1102–1109, 1987. [DOI] [PubMed] [Google Scholar]
- 97.Cai S, Rao X, Ye J, Ling Y, Mi S, Chen H, Fan C, Li Y. Relationship between urinary bisphenol a levels and cardiovascular diseases in the U.S. adult population, 2003-2014. Ecotoxicol Environ Saf 192: 110300, 2020. [DOI] [PubMed] [Google Scholar]
- 98.Cai X, Tian Y, Wu T, Cao CX, Li H, Wang KJ. Metabolic effects of testosterone replacement therapy on hypogonadal men with type 2 diabetes mellitus: A systematic review and meta-analysis of randomized controlled trials. Asian J Androl 16: 146–152, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Caleyachetty R, Thomas GN, Toulis KA, Mohammed N, Gokhale KM, Balachandran K, Nirantharakumar K. Metabolically healthy obese and incident cardiovascular disease events among 3.5 million men and women. J Am Coll Cardiol 70: 1429–1437, 2017. [DOI] [PubMed] [Google Scholar]
- 100.Calof OM, Singh AB, Lee ML, Kenny AM, Urban RJ, Tenover JL, Bhasin S. Adverse events associated with testosterone replacement in middle-aged and older men: A meta-analysis of randomized, placebo-controlled trials. J Gerontol A Biol Sci Med Sci 60: 1451–1457, 2005. [DOI] [PubMed] [Google Scholar]
- 101.Camacho EM, Huhtaniemi IT, O’Neill TW, Finn JD, Pye SR, Lee DM, Tajar A, Bartfai G, Boonen S, Casanueva FF, Forti G, Giwercman A, Han TS, Kula K, Keevil B, Lean ME, Pendleton N, Punab M, Vanderschueren D, Wu FC, EMAS Group. Age-associated changes in hypothalamic-pituitary-testicular function in middle-aged and older men are modified by weight change and lifestyle factors: Longitudinal results from the European Male Ageing Study. Eur J Endocrinol 168:445–455, 2013. [DOI] [PubMed] [Google Scholar]
- 102.Camhi SM, Bray GA, Bouchard C, Greenway FL, Johnson WD, New-ton RL, Ravussin E, Ryan DH, Smith SR, Katzmarzyk PT. The relationship of waist circumference and BMI to visceral, subcutaneous, and total body fat: Sex and race differences. Obesity (Silver Spring) 19: 402–408, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Canguven O, Talib RA, El Ansari W, Yassin DJ, Salman M, Al-Ansari A. Testosterone therapy has positive effects on anthropometric measures, metabolic syndrome components (obesity, lipid profile, Diabetes Mellitus control), blood indices, liver enzymes, and prostate health indicators in elderly hypogonadal men. Andrologia 49: e12768, 2017. [DOI] [PubMed] [Google Scholar]
- 104.Cappola AR, Xue QL, Walston JD, Leng SX, Ferrucci L, Guralnik J, Fried LP. DHEAS levels and mortality in disabled older women: The Women’s Health and Aging Study I. J Gerontol A Biol Sci Med Sci 61:957–962, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Carani C, Granata AR, Rochira V, Caffagni G, Aranda C, Antunez P, Maffei LE. Sex steroids and sexual desire in a man with a novel mutation of aromatase gene and hypogonadism. Psychoneuroen-docrinology 30: 413–417, 2005. [DOI] [PubMed] [Google Scholar]
- 106.Carani C, Qin K, Simoni M, Faustini-Fustini M, Serpente S, Boyd J, Korach KS, Simpson ER. Effect of testosterone and estradiol in a man with aromatase deficiency. N Engl J Med 337: 91–95, 1997. [DOI] [PubMed] [Google Scholar]
- 107.Carbone S, Canada JM, Billingsley HE, Siddiqui MS, Elagizi A, Lavie CJ. Obesity paradox in cardiovascular disease: Where do we stand? Vasc Health Risk Manag 15: 89, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Carmienke S, Freitag M, Pischon T, Schlattmann P, Fankhaenel T, Goebel H, Gensichen J. General and abdominal obesity parameters and their combination in relation to mortality: A systematic review and meta-regression analysis. Eur J Clin Nutr 67: 573–585, 2013. [DOI] [PubMed] [Google Scholar]
- 109.Carrageta DF, Oliveira PF, Alves MG, Monteiro MP. Obesity and male hypogonadism: Tales of a vicious cycle. Obes Rev 20: 1148–1158, 2019. [DOI] [PubMed] [Google Scholar]
- 110.Carroll MD, Fryar CD, Nguyen DT. High total and low high-density lipoprotein cholestrole in adults: United States, 2015-2016. NCHS Data Brief 290: 1–8, 2017. [PubMed] [Google Scholar]
- 111.Cassidy AE, Bielak LF, Zhou Y, Sheedy PF, Turner ST, Breen JF, Araoz PA, Kullo IJ, Lin X, Peyser PA. Progression of subclinical coronary atherosclerosis: Does obesity make a difference? Circulation 111: 1877–1882, 2005. [DOI] [PubMed] [Google Scholar]
- 112.Casson PR, Santoro N, Elkind-Hirsch K, Carson SA, Hornsby PJ, Abraham G, Buster JE. Postmenopausal dehydroepiandrosterone administration increases free insulin-like growth factor-I and decreases high-density lipoprotein: A six-month trial. Fertil Steril 70: 107–110, 1998. [DOI] [PubMed] [Google Scholar]
- 113.Casson PR, Toth MJ, Johnson JV, Stanczyk FZ, Casey CL, Dixon ME. Correlation of serum androgens with anthropometric and metabolic indices in healthy, nonobese postmenopausal women. J Clin Endocrinol Metab 95: 4276–4282, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chan YX, Knuiman MW, Hung J, Divitini ML, Beilby JP, Handelsman DJ, Beilin J, McQuillan B, Yeap BB. Neutral associations of testosterone, dihydrotestosterone and estradiol with fatal and non-fatal cardiovascular events, and mortality in men aged 17-97 years. Clin Endocrinol (Oxf) 85: 575–582, 2016. [DOI] [PubMed] [Google Scholar]
- 115.Chao J, Rubinow KB, Kratz M, Amory JK, Matsumoto AM, Page ST. Short-term estrogen withdrawal increases adiposity in healthy men. J Clin Endocrinol Metab 101: 3724–3731, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chen Z, Wang O, Nie M, Elison K, Zhou D, Li M, Jiang Y, Xia W, Meng X, Chen S, Xing X. Aromatase deficiency in a Chinese adult man caused by novel compound heterozygous CYP19A1 mutations: Effects of estrogen replacement therapy on the bone, lipid, liver and glucose metabolism. Mol Cell Endocrinol 399: 32–42, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cheung AS, Hoermann R, Dupuis P, Joon DL, Zajac JD, Grossmann M. Relationships between insulin resistance and frailty with body composition and testosterone in men undergoing androgen deprivation therapy for prostate cancer. Eur J Endocrinol 175: 229–237, 2016. [DOI] [PubMed] [Google Scholar]
- 118.Chondronikola M, Volpi E, Borsheim E, Porter C, Saraf MK, Annamalai P, Yfanti C, Chao T, Wong D, Shinoda K, Labbe SM, Hurren NM, Cesani F, Kajimura S, Sidossis LS. Brown adipose tissue activation is linked to distinct systemic effects on lipid metabolism in humans. Cell Metab 23: 1200–1206, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Christakoudi S, Tsilidis KK, Muller DC, Freisling H, Weiderpass E, Overvad K, Söderberg S, Häggström C, Pischon T, Dahm CC. A Body Shape Index (ABSI) achieves better mortality risk stratification than alternative indices of abdominal obesity: Results from a large Euro-pean cohort. Sci Rep-Uk 10: 1–15, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chrysant SG. Association of exposure to bisphenol A and incidence of cardiovascular disease and hypertension. J Clin Hypertens (Greenwich) 17: 737–739, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chung J-Y, Kang H-T, Lee D-C, Lee H-R, Lee Y-J. Body composition and its association with cardiometabolic risk factors in the elderly: A focus on sarcopenic obesity. Arch Gerontol Geriatr 56: 270–278, 2013. [DOI] [PubMed] [Google Scholar]
- 122.Cintron D, Beckman JP, Bailey KR, Lahr BD, Jayachandran M, Miller VM. Plasma orexin A levels in recently menopausal women during and 3 years following use of hormone therapy. Maturitas 99: 59–65, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Coates AS, Keshaviah A, Thurlimann B, Mouridsen H, Mauriac L, Forbes JF, Paridaens R, Castiglione-Gertsch M, Gelber RD, Colleoni M, Lang I, Del Mastro L, Smith I, Chirgwin J, Nogaret JM, Pienkowski T, Wardley A, Jakobsen EH, Price KN, Goldhirsch A. Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: Update of study BIG 1-98. J Clin Oncol 25: 486–492, 2007. [DOI] [PubMed] [Google Scholar]
- 124.Coban N, Onat A, Guclu-Geyik F, Can G, Erginel-Unaltuna N. Sex-and obesity-specific Association of Aromatase (CYP19A1) gene variant with Apolipoprotein B and hypertension. Arch Med Res 46:564–571, 2015. [DOI] [PubMed] [Google Scholar]
- 125.Coen P, Kulin H, Ballantine T, Zaino R, Frauenhoffer E, Boal D, Inkster S, Brodie A, Santen R. An aromatase-producing sex-cord tumor resulting in prepubertal gynecomastia. N Engl J Med 324: 317–322, 1991. [DOI] [PubMed] [Google Scholar]
- 126.Cohade C, Osman M, Pannu HK, Wahl RL. Uptake in supraclavicular area fat ("USA-Fat"): Description on 18F-FDG PET/CT. JNuclMed 44: 170–176, 2003. [PubMed] [Google Scholar]
- 127.Colditz GA, Willett WC, Stampfer MJ, Rosner B, Speizer FE, Hennekens CH. Menopause and the risk of coronary heart disease in women. N Engl J Med 316: 1105–1110, 1987. [DOI] [PubMed] [Google Scholar]
- 128.Collaborative Group On Epidemiological Studies Of Ovarian Cancer, Beral V, Gaitskell K, Hermon C, Moser K, Reeves G, Peto R. Menopausal hormone use and ovarian cancer risk: Individual participant meta-analysis of 52 epidemiological studies. Lancet 385: 1835–1842, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Collet TH, Ewing SK, Ensrud KE, Laughlin GA, Hoffman AR, Varosy PD, Stefanick ML, Stone KL, Orwoll E, Bauer DC. Endogenous testosterone levels and the risk of incident cardiovascular events in elderly men: The MrOS Prospective Study. J Endocr Soc 4: bvaa038, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Collins P, Webb CM, de Villiers TJ, Stevenson JC, Panay N, Baber RJ. Cardiovascular risk assessment in women - An update. Climacteric 19:329–336, 2016. [DOI] [PubMed] [Google Scholar]
- 131.Connors JM, Middeldorp S. Transgender patients and the role of the coagulation clinician. J Thromb Haemost 17: 1790–1797, 2019. [DOI] [PubMed] [Google Scholar]
- 132.Contoreggi CS, Blackman MR, Andres R, Muller DC, Lakatta EG, Fleg JL, Harman SM. Plasma levels of estradiol, testosterone, and DHEAS do not predict risk of coronary artery disease in men. J Androl 11: 460–470, 1990. [PubMed] [Google Scholar]
- 133.Cooke PS, Nanjappa MK, Ko C, Prins GS, Hess RA. Estrogens in male physiology. Physiol Rev 97: 995–1043, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Corona G, Giagulli VA, Maseroli E, Vignozzi L, Aversa A, Zitzmann M, Saad F, Mannucci E, Maggi M. THERAPY OF ENDOCRINE DISEASE: Testosterone supplementation and body composition: Results from a meta-analysis study. Eur J Endocrinol 174: R99–R116, 2016. [DOI] [PubMed] [Google Scholar]
- 135.Corona G, Maseroli E, Rastrelli G, Isidori AM, Sforza A, Mannucci E, Maggi M. Cardiovascular risk associated with testosterone-boosting medications: A systematic review and meta-analysis. Expert Opin Drug Saf 13: 1327–1351, 2014. [DOI] [PubMed] [Google Scholar]
- 136.Corona G, Monami M, Rastrelli G, Aversa A, Sforza A, Lenzi A, Forti G, Mannucci E, Maggi M. Type 2 diabetes mellitus and testosterone: A meta-analysis study. Int J Androl 34: 528–540, 2011. [DOI] [PubMed] [Google Scholar]
- 137.Corona G, Rastrelli G, Di Pasquale G, Sforza A, Mannucci E, Maggi M. Endogenous testosterone levels and cardiovascular risk: Meta-analysis of observational studies. JSexMed 15: 1260–1271, 2018. [DOI] [PubMed] [Google Scholar]
- 138.Corona G, Rastrelli G, Di Pasquale G, Sforza A, Mannucci E, Maggi M. Testosterone and cardiovascular risk: Meta-analysis of interventional studies. JSex Med 15: 820–838, 2018. [DOI] [PubMed] [Google Scholar]
- 139.Corona G, Rastrelli G, Giagulli VA, Sila A, Sforza A, Forti G, Mannucci E, Maggi M. Dehydroepiandrosterone supplementation in elderly men: A meta-analysis study of placebo-controlled trials. J Clin Endocrinol Metab 98: 3615–3626, 2013. [DOI] [PubMed] [Google Scholar]
- 140.Corona G, Rastrelli G, Monami M, Guay A, Buvat J, Sforza A, Forti G, Mannucci E, Maggi M. Hypogonadism as a risk factor for cardio-vascular mortality in men: A meta-analytic study. Eur J Endocrinol 165: 687–701, 2011. [DOI] [PubMed] [Google Scholar]
- 141.Corona G, Rastrelli G, Monami M, Saad F, Luconi M, Lucchese M, Facchiano E, Sforza A, Forti G, Mannucci E, Maggi M. Body weight loss reverts obesity-associated hypogonadotropic hypogonadism: A systematic review and meta-analysis. Eur J Endocrinol 168: 829–843, 2013. [DOI] [PubMed] [Google Scholar]
- 142.Corona G, Vignozzi L, Sforza A, Mannucci E, Maggi M. Obesity and late-onset hypogonadism. Mol Cell Endocrinol 418 Pt 2: 120–133, 2015. [DOI] [PubMed] [Google Scholar]
- 143.Couillard C, Gagnon J, Bergeron J, Leon AS, Rao DC, Skinner JS, Wilmore JH, Despres JP, Bouchard C. Contribution of body fatness and adipose tissue distribution to the age variation in plasma steroid hormone concentrations in men: The HERITAGE Family Study. J Clin Endocrinol Metab 85: 1026–1031, 2000. [DOI] [PubMed] [Google Scholar]
- 144.Coutinho T, Goel K, de Sá DC, Kragelund C, Kanaya AM, Zeller M, Park J-S, Kober L, Torp-Pedersen C, Cottin Y. Central obesity and survival in subjects with coronary artery disease: A systematic review of the literature and collaborative analysis with individual subject data. J Am Coll Cardiol 57: 1877–1886, 2011. [DOI] [PubMed] [Google Scholar]
- 145.Crandall CJ, Barrett-Connor E. Endogenous sex steroid levels and cardiovascular disease in relation to the menopause: A systematic review. Endocrinol Metab Clin North Am 42: 227–253, 2013. [DOI] [PubMed] [Google Scholar]
- 146.Crawford S, Santoro N, Laughlin GA, Sowers MF, McConnell D, Sutton-Tyrrell K, Weiss G, Vuga M, Randolph J, Lasley B. Circulating dehydroepiandrosterone sulfate concentrations during the menopausal transition. J Clin Endocrinol Metab 94: 2945–2951, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 360: 1509–1517, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.D’Agostino RB Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: The Framingham Heart Study. Circulation 117: 743–753, 2008. [DOI] [PubMed] [Google Scholar]
- 149.Daka B, Langer RD, Larsson CA, Rosen T, Jansson PA, Rastam L, Lindblad U. Low concentrations of serum testosterone predict acute myocardial infarction in men with type 2 diabetes mellitus. BMC Endocr Disord 15: 35, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Damgaard-Olesen A, Johannsen TH, Holmboe SA, Soeborg T, Petersen JH, Andersson A, Aadahl M, Linneberg A, Juul A. Reference ranges of 17-hydroxyprogesterone, DHEA, DHEAS, androstenedione, total and free testosterone determined by TurboFlow-LC-MS/MS and associations to health markers in 304 men. Clin Chim Acta 454:82–88, 2016. [DOI] [PubMed] [Google Scholar]
- 151.D’Amico AV, Chen MH, Renshaw A, Loffredo M, Kantoff PW. Long-term follow-up of a randomized trial of radiation with or without androgen deprivation therapy for localized prostate cancer. JAMA 314: 1291–1293, 2015. [DOI] [PubMed] [Google Scholar]
- 152.Danforth KN, Tworoger SS, Hecht JL, Rosner BA, Colditz GA, Hankinson SE. A prospective study of postmenopausal hormone use and ovarian cancer risk. Br J Cancer 96: 151–156, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Davis SM, Kaar JL, Ringham BM, Hockett CW, Glueck DH, Dabelea D. Sex differences in infant body composition emerge in the first 5 months of life. J Pediatr Endocrinol Metab 32: 1235–1239, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Davis SR, Panjari M, Stanczyk FZ. Clinical review: DHEA replacement for postmenopausal women. J Clin Endocrinol Metab 96: 1642–1653, 2011. [DOI] [PubMed] [Google Scholar]
- 155.Davis SR, Worsley R. Androgen treatment of postmenopausal women. J Steroid Biochem Mol Biol 142: 107–114, 2014. [DOI] [PubMed] [Google Scholar]
- 156.de Groot PC, Dekkers OM, Romijn JA, Dieben SW, Helmerhorst FM. PCOS, coronary heart disease, stroke and the influence of obesity: A systematic review and meta-analysis. Hum Reprod Update 17: 495–500, 2011. [DOI] [PubMed] [Google Scholar]
- 157.de Kleijn MJ, van der Schouw YT, Verbeek AL, Peeters PH, Banga JD, van der Graaf Y. Endogenous estrogen exposure and cardiovascular mortality risk in postmenopausal women. Am J Epidemiol 155: 339–345, 2002. [DOI] [PubMed] [Google Scholar]
- 158.De Pergola G, Giagulli VA, Garruti G, Cospite MR, Giorgino F, Cignarelli M, Giorgino R. Low dehydroepiandrosterone circulating levels in premenopausal obese women with very high body mass index. Metabolism 40: 187–190, 1991. [DOI] [PubMed] [Google Scholar]
- 159.De Pergola G, Triggiani V, Giorgino F, Cospite MR, Garruti G, Cignarelli M, Guastamacchia E, Giorgino R. The free testosterone to dehydroepiandrosterone sulphate molar ratio as a marker of visceral fat accumulation in premenopausal obese women. IntJObesRelat Metab Disord 18: 659–664, 1994. [PubMed] [Google Scholar]
- 160.De Pergola G, Zamboni M, Sciaraffia M, Turcato E, Pannacciulli N, Armellini F, Giorgino F, Perrini S, Bosello O, Giorgino R. Body fat accumulation is possibly responsible for lower dehydroepiandrosterone circulating levels in premenopausal obese women. Int J Obes Relat Metab Disord 20: 1105–1110, 1996. [PubMed] [Google Scholar]
- 161.De Schutter A, Kachur S, Lavie C, Boddepalli R, Patel D, Milani R. The impact of inflammation on the obesity paradox in coronary heart disease. Int J Obes (Lond) 40: 1730–1735, 2016. [DOI] [PubMed] [Google Scholar]
- 162.De Schutter A, Lavie CJ, Kachur S, Patel DA, Milani RV. Body composition and mortality in a large cohort with preserved ejection fraction: Untangling the obesity paradox. Mayo Clin Proc 89: 1072–1079, 2014. [DOI] [PubMed] [Google Scholar]
- 163.Demura M, Martin RM, Shozu M, Sebastian S, Takayama K, Hsu WT, Schultz RA, Neely K, Bryant M, Mendonca BB, Hanaki K, Kanzaki S, Rhoads DB, Misra M, Bulun SE. Regional rearrangements in chromosome 15q21 cause formation of cryptic promoters for the CYP19 (aromatase) gene. Hum Mol Genet 16: 2529–2541, 2007. [DOI] [PubMed] [Google Scholar]
- 164.Deng Y, Xue W, Wang Y, Zhu S, Ma X, Sun A. Effects of different menopausal hormone replacement regimens on body composition in Chinese women. Climacteric 21: 607–612, 2018. [DOI] [PubMed] [Google Scholar]
- 165.Derby CA, Zilber S, Brambilla D, Morales KH, McKinlay JB. Body mass index, waist circumference and waist to hip ratio and change in sex steroid hormones: The Massachusetts Male Ageing Study. Clin Endocrinol (Oxf) 65: 125–131, 2006. [DOI] [PubMed] [Google Scholar]
- 166.Dhindsa S, Furlanetto R, Vora M, Ghanim H, Chaudhuri A, Dandona P.Low estradiol concentrations in men with subnormal testosterone concentrations and type 2 diabetes. Diabetes Care 34: 1854–1859, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Dhindsa S, Ghanim H, Batra M, Dandona P. Hypogonadotropic hypogonadism in men with diabesity. Diabetes Care 41: 1516–1525, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Dhindsa S, Ghanim H, Batra M, Kuhadiya ND, Abuaysheh S, Sandhu S, Green K, Makdissi A, Hejna J, Chaudhuri A, Punyanitya M, Dandona P. Insulin resistance and inflammation in hypogonadotropic hypogonadism and their reduction after testosterone replacement in men with type 2 diabetes. Diabetes Care 39: 82–91, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Dhindsa S, Miller MG, McWhirter CL, Mager DE, Ghanim H, Chaudhuri A, Dandona P. Testosterone concentrations in diabetic and non-diabetic obese men. Diabetes Care 33: 1186–1192, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Dianat S, Fox E, Ahrens KA, Upadhyay UD, Zlidar VM, Gallo MF, Stidd RL, Moskosky S, Dehlendorf C. Side effects and health benefits of depot medroxyprogesterone acetate: A systematic review. Obstet Gynecol 133: 332–341, 2019. [DOI] [PubMed] [Google Scholar]
- 171.Diem SJ, Greer NL, MacDonald R, McKenzie LG, Dahm P, Ercan-Fang N, Estrada A, Hemmy LS, Rosebush CE, Fink HA, Wilt TJ. Efficacy and safety of testosterone treatment in men: An evidence report for a clinical practice guideline by the American College of Physicians. Ann Intern Med 172: 105–118, 2020. [DOI] [PubMed] [Google Scholar]
- 172.Diercks DB, Roe MT, Mulgund J, Pollack CV Jr, Kirk JD, Gibler WB, Ohman EM, Smith SC Jr, Boden WE, Peterson ED. The obesity paradox in non–ST-segment elevation acute coronary syndromes: Results from the Can Rapid risk stratification of Unstable angina patients Suppress Adverse outcomes with Early implementation of the American College of Cardiology/American Heart Association Guidelines Quality Improvement Initiative. Am Heart J 152: 140–148, 2006. [DOI] [PubMed] [Google Scholar]
- 173.Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: A systematic review and meta-analysis. JAMA 295: 1288–1299, 2006. [DOI] [PubMed] [Google Scholar]
- 174.Dominguez-Lopez I, Yago-Aragon M, Salas-Huetos A, Tresserra-Rimbau A, Hurtado-Barroso S. Effects of dietary phytoestrogens on hormones throughout a human lifespan: A review. Nutrients 12: 2456, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Dorn JM, Schisterman EF, Winkelstein W Jr, Trevisan M. Body mass index and mortality in a general population sample women of men and women: The Buffalo Health Study. Am J Epidemiol 146: 919–931, 1997. [DOI] [PubMed] [Google Scholar]
- 176.dos Santos EP, Gadelha AB, Safons MP, Nóbrega OT, Oliveira RJ, Lima RM. Sarcopenia and sarcopenic obesity classifications and cardiometabolic risks in older women. Arch Gerontol Geriatr 59: 56–61, 2014. [DOI] [PubMed] [Google Scholar]
- 177.Ebeling P, Koivisto VA. Physiological importance of dehydroepiandrosterone. Lancet 343: 1479–1481, 1994. [DOI] [PubMed] [Google Scholar]
- 178.El Khoudary SR, Aggarwal B, Beckie TM, Hodis HN, Johnson AE, Langer RD, Limacher MC, Manson JE, Stefanick ML, Allison MA, American Heart Association Prevention Science Committee of the Council on Epidemiology and Prevention, Council on Cardiovascular and Stroke Nursing. Menopause transition and cardiovascular disease risk: Implications for timing of early prevention: A scientific statement from the American Heart Association. Circulation 142: e506–e532, 2020. [DOI] [PubMed] [Google Scholar]
- 179.El Khoudary SR, Wildman RP, Matthews K, Thurston RC, Bromberger JT, Sutton-Tyrrell K. Endogenous sex hormones impact the progression of subclinical atherosclerosis in women during the menopausal transition. Atherosclerosis 225: 180–186, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Elagizi A, Kohler TS, Lavie CJ. Testosterone and cardiovascular health. Mayo Clin Proc 93: 83–100, 2018. [DOI] [PubMed] [Google Scholar]
- 181.Elamin MB, Garcia MZ, Murad MH, Erwin PJ, Montori VM. Effect of sex steroid use on cardiovascular risk in transsexual individuals: A systematic review and meta-analyses. Clin Endocrinol (Oxf) 72: 1–10, 2010. [DOI] [PubMed] [Google Scholar]
- 182.Elbers JM, Asscheman H, Seidell JC, Megens JA, Gooren LJ. Long-term testosterone administration increases visceral fat in female to male transsexuals. J Clin Endocrinol Metab 82: 2044–2047, 1997. [DOI] [PubMed] [Google Scholar]
- 183.Elliott J, Kelly SE, Millar AC, Peterson J, Chen L, Johnston A, Kotb A, Skidmore B, Bai Z, Mamdani M, Wells GA. Testosterone therapy in hypogonadal men: A systematic review and network meta-analysis. BMJ Open 7: e015284, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Emerging Risk Factors Collaboration. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: Collaborative analysis of 58 prospective studies. Lancet 377: 1085–1095, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Emmelot-Vonk MH, Verhaar HJ, Nakhai Pour HR, Aleman A, Lock TM, Bosch JL, Grobbee DE, van der Schouw YT. Effect of testosterone supplementation on functional mobility, cognition, and other parameters in older men: A randomized controlled trial. JAMA 299:39–52, 2008. [DOI] [PubMed] [Google Scholar]
- 186.Escobar-Morreale HF, Santacruz E, Luque-Ramirez M, Botella Carretero JI. Prevalence of ‘obesity-associated gonadal dysfunction’ in severely obese men and women and its resolution after bariatric surgery: A systematic review and meta-analysis. Hum Reprod Update 23: 390–408, 2017. [DOI] [PubMed] [Google Scholar]
- 187.Espeland MA, Stefanick ML, Kritz-Silverstein D, Fineberg SE, Waclawiw MA, James MK, Greendale GA. Effect of postmenopausal hormone therapy on body weight and waist and hip girths. Post-menopausal Estrogen-Progestin Interventions Study Investigators. J Clin Endocrinol Metab 82: 1549–1556, 1997. [DOI] [PubMed] [Google Scholar]
- 188.Etminan M, Skeldon SC, Goldenberg SL, Carleton B, Brophy JM. Testosterone therapy and risk of myocardial infarction: A pharmacoepidemiologic study. Pharmacotherapy 35: 72–78, 2015. [DOI] [PubMed] [Google Scholar]
- 189.Evans DJ, Hoffmann RG, Kalkhoff RK, Kissebah AH. Relationship of androgenic activity to body fat topography, fat cell morphology, and metabolic aberrations in premenopausal women. J Clin Endocrinol Metab 57: 304–310, 1983. [DOI] [PubMed] [Google Scholar]
- 190.Evans E, Rowe D, Racette S, Ross K, McAuley E. Is the current BMI obesity classification appropriate for black and white postmenopausal women? Int J Obes (Lond) 30: 837–843, 2006. [DOI] [PubMed] [Google Scholar]
- 191.Farmer RE, Mathur R, Schmidt AF, Bhaskaran K, Fatemifar G, East-wood SV, Finan C, Denaxas S, Smeeth L, Chaturvedi N. Associations between measures of sarcopenic obesity and risk of cardiovascular disease and mortality: A cohort study and Mendelian randomization analysis using the UK Biobank. J Am Heart Assoc 8: e011638, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Fernandez-Balsells MM, Murad MH, Lane M, Lampropulos JF, Albuquerque F, Mullan RJ, Agrwal N, Elamin MB, Gallegos-Orozco JF, Wang AT, Erwin PJ, Bhasin S, Montori VM. Clinical review 1: Adverse effects of testosterone therapy in adult men: A systematic review and meta-analysis. J Clin Endocrinol Metab 95: 2560–2575, 2010. [DOI] [PubMed] [Google Scholar]
- 193.Ferreira I, Snijder MB, Twisk JW, van Mechelen W, Kemper HC, Seidell JC, Stehouwer CD. Central fat mass versus peripheral fat and lean mass: Opposite (adverse versus favorable) associations with arterial stiffness? The Amsterdam Growth and Health Longitudinal Study. J Clin Endocrinol Metab 89: 2632–2639, 2004. [DOI] [PubMed] [Google Scholar]
- 194.Ferreira I, Stehouwer CD. Obesity paradox or inappropriate study designs? Time for life-course epidemiology. J Hypertens 30: 2271–2275, 2012. [DOI] [PubMed] [Google Scholar]
- 195.Field AE, Coakley EH, Must A, Spadano JL, Laird N, Dietz WH, Rimm E, Colditz GA. Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Arch Intern Med 161: 1581–1586, 2001. [DOI] [PubMed] [Google Scholar]
- 196.Field AE, Colditz GA, Willett WC, Longcope C, McKinlay JB. The relation of smoking, age, relative weight, and dietary intake to serum adrenal steroids, sex hormones, and sex hormone-binding globulin in middle-aged men. J Clin Endocrinol Metab 79: 1310–1316, 1994. [DOI] [PubMed] [Google Scholar]
- 197.Finkelstein EA, Khavjou OA, Thompson H, Trogdon JG, Pan L, Sherry B, Dietz W. Obesity and severe obesity forecasts through 2030. Am J Prev Med 42: 563–570, 2012. [DOI] [PubMed] [Google Scholar]
- 198.Finkelstein JS, Lee H, Burnett-Bowie SA, Pallais JC, Yu EW, Borges LF, Jones BF, Barry CV, Wulczyn KE, Thomas BJ, Leder BZ. Gonadal steroids and body composition, strength, and sexual function in men. N Engl J Med 369: 1011–1022, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Finkle WD, Greenland S, Ridgeway GK, Adams JL, Frasco MA, Cook MB, Fraumeni JF Jr, Hoover RN. Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. PLoS One 9: e85805, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: A systematic review and meta-analysis. JAMA 309: 71–82, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Foglietta J, Inno A, de Iuliis F, Sini V, Duranti S, Turazza M, Tarantini L, Gori S. Cardiotoxicity of aromatase inhibitors in breast cancer patients. Clin Breast Cancer 17: 11–17, 2017. [DOI] [PubMed] [Google Scholar]
- 202.Franco LP, Morais CC, Cominetti C. Normal-weight obesity syn-drome: Diagnosis, prevalence, and clinical implications. Nutr Rev 74:558–570, 2016. [DOI] [PubMed] [Google Scholar]
- 203.Francomano D, Bruzziches R, Barbaro G, Lenzi A, Aversa A. Effects of testosterone undecanoate replacement and withdrawal on cardio-metabolic, hormonal and body composition outcomes in severely obese hypogonadal men: A pilot study. J Endocrinol Invest 37: 401–411, 2014. [DOI] [PubMed] [Google Scholar]
- 204.Francomano D, Ilacqua A, Bruzziches R, Lenzi A, Aversa A. Effects of 5-year treatment with testosterone undecanoate on lower urinary tract symptoms in obese men with hypogonadism and metabolic syndrome. Urology 83: 167–173, 2014. [DOI] [PubMed] [Google Scholar]
- 205.Frati P, Busardo FP, Cipolloni L, Dominicis ED, Fineschi V. Anabolic Androgenic Steroid (AAS) related deaths: Autoptic, histopathological and toxicological findings. Curr Neuropharmacol 13: 146–159, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Frederiksen L, Hojlund K, Hougaard DM, Brixen K, Andersen M. Testosterone therapy increased muscle mass and lipid oxidation in aging men. Age (Dordr) 34: 145–156, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Frost L, Hune LJ, Vestergaard P. Overweight and obesity as risk factors for atrial fibrillation or flutter: The Danish Diet, Cancer, and Health Study. Am J Med 118: 489–495, 2005. [DOI] [PubMed] [Google Scholar]
- 208.Fryar CD, Chen TC, Li X. Prevalence of uncontrolled risk factors for cardiovascular disease: United States, 1999-2010. NCHSDataBrief 103: 1–8, 2012. [PubMed] [Google Scholar]
- 209.Fukami M, Shozu M, Soneda S, Kato F, Inagaki A, Takagi H, Hanaki K, Kanzaki S, Ohyama K, Sano T, Nishigaki T, Yokoya S, Binder G, Horikawa R, Ogata T. Aromatase excess syndrome: Identification of cryptic duplications and deletions leading to gain of function of CYP19A1 and assessment of phenotypic determinants. J Clin Endocrinol Metab 96: E1035–E1043, 2011. [DOI] [PubMed] [Google Scholar]
- 210.Gagliano-Juca T, Basaria S. Testosterone replacement therapy and cardiovascular risk. Nat Rev Cardiol 16: 555–574, 2019. [DOI] [PubMed] [Google Scholar]
- 211.Gapstur SM, Gann PH, Kopp P, Colangelo L, Longcope C, Liu K. Serum androgen concentrations in young men: A longitudinal analysis of associations with age, obesity, and race. The CARDIA male hormone study. Cancer Epidemiol Biomarkers Prev 11: 1041–1047, 2002. [PubMed] [Google Scholar]
- 212.Garg A. Gender differences in the prevalence of metabolic complications in familial partial lipodystrophy (Dunnigan variety). J Clin Endocrinol Metab 85: 1776–1782, 2000. [DOI] [PubMed] [Google Scholar]
- 213.Garrison RJ, Kannel WB, Stokes J III, Castelli WP. Incidence and pre-cursors of hypertension in young adults: The Framingham Offspring Study. Prev Med 16: 235–251, 1987. [DOI] [PubMed] [Google Scholar]
- 214.GBD 2015 Obesity Collaborators. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med 377: 13–27, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Gerdts E, Regitz-Zagrosek V. Sex differences in cardiometabolic dis-orders. Nat Med 25: 1657–1666, 2019. [DOI] [PubMed] [Google Scholar]
- 216.Gerhard M, Walsh BW, Tawakol A, Haley EA, Creager SJ, Seely EW, Ganz P, Creager MA. Estradiol therapy combined with progesterone and endothelium-dependent vasodilation in postmenopausal women. Circulation 98: 1158–1163, 1998. [DOI] [PubMed] [Google Scholar]
- 217.Getahun D, Nash R, Flanders WD, Baird TC, Becerra-Culqui TA, Cromwell L, Hunkeler E, Lash TL, Millman A, Quinn VP, Robin-son B, Roblin D, Silverberg MJ, Safer J, Slovis J, Tangpricha V, Goodman M. Cross-sex hormones and acute cardiovascular events in transgender persons: A cohort study. Ann Intern Med 169: 205–213, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Ghanim H, Dhindsa S, Abuaysheh S, Batra M, Kuhadiya ND, Makdissi A, Chaudhuri A, Dandona P. Diminished androgen and estrogen receptors and aromatase levels in hypogonadal diabetic men: Reversal with testosterone. Eur J Endocrinol 178: 277–283, 2018. [DOI] [PubMed] [Google Scholar]
- 219.Ghanta RK, LaPar DJ, Zhang Q, Devarkonda V, Isbell JM, Yarboro LT, Kern JA, Kron IL, Speir AM, Fonner CE. Obesity increases risk-adjusted morbidity, mortality, and cost following cardiac surgery. JAm Heart Assoc 6: e003831, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Gianatti EJ, Dupuis P, Hoermann R, Strauss BJ, Wentworth JM, Zajac JD, Grossmann M. Effect of testosterone treatment on glucose metabolism in men with type 2 diabetes: A randomized controlled trial. Diabetes Care 37: 2098–2107, 2014. [DOI] [PubMed] [Google Scholar]
- 221.Giordano S, Hage FG, Xing D, Chen YF, Allon S, Chen C, Oparil S. Estrogen and cardiovascular disease: Is timing everything? Am J Med Sci 350: 27–35, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Glass AR, Swerdloff RS, Bray GA, Dahms WT, Atkinson RL. Low serum testosterone and sex-hormone-binding-globulin in massively obese men. J Clin Endocrinol Metab 45: 1211–1219, 1977. [DOI] [PubMed] [Google Scholar]
- 223.Glisic M, Shahzad S, Tsoli S, Chadni M, Asllanaj E, Rojas LZ, Brown E, Chowdhury R, Muka T, Franco OH. Association between progestin-only contraceptive use and cardiometabolic outcomes: A systematic review and meta-analysis. Eur J Prev Cardiol 25: 1042–1052, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Godsland IF. Effects of postmenopausal hormone replacement therapy on lipid, lipoprotein, and apolipoprotein (a) concentrations: Analysis of studies published from 1974-2000. Fertil Steril 75: 898–915, 2001. [DOI] [PubMed] [Google Scholar]
- 225.Golden SH, Dobs AS, Vaidya D, Szklo M, Gapstur S, Kopp P, Liu K, Ouyang P. Endogenous sex hormones and glucose tolerance status in postmenopausal women. J Clin Endocrinol Metab 92: 1289–1295, 2007. [DOI] [PubMed] [Google Scholar]
- 226.Golden SH, Maguire A, Ding J, Crouse JR, Cauley JA, Zacur H, Szklo M. Endogenous postmenopausal hormones and carotid atherosclerosis: A case-control study of the Atherosclerosis Risk in Communities Cohort. Am J Epidemiol 155: 437–445, 2002. [DOI] [PubMed] [Google Scholar]
- 227.Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, Toppari J, Zoeller RT. EDC-2: The Endocrine Society’s second Scientific Statement on endocrine-disrupting chemicals. Endocr Rev 36: E1–E150, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Goss PE, Ingle JN, Martino S, Robert NJ, Muss HB, Piccart MJ, Castiglione M, Tu D, Shepherd LE, Pritchard KI, Livingston RB, David-son NE, Norton L, Perez EA, Abrams JS, Therasse P, Palmer MJ, Pater JL. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med 349: 1793–1802, 2003. [DOI] [PubMed] [Google Scholar]
- 229.Gourgari E, Saloustros E, Stratakis CA. Large-cell calcifying Sertoli cell tumors of the testes in pediatrics. Curr Opin Pediatr 24: 518–522, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Grady D, Rubin SM, Petitti DB, Fox CS, Black D, Ettinger B, Ernster VL, Cummings SR. Hormone therapy to prevent disease and prolong life in postmenopausal women. Ann Intern Med 117: 1016–1037, 1992. [DOI] [PubMed] [Google Scholar]
- 231.Greendale GA, Sternfeld B, Huang M, Han W, Karvonen-Gutierrez C, Ruppert K, Cauley JA, Finkelstein JS, Jiang SF, Karlamangla AS. Changes in body composition and weight during the menopause transition. JCI Insight 4: e124865, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Grossmann M. Hypogonadism and male obesity: Focus on unresolved questions. Clin Endocrinol (Oxf) 89: 11–21, 2018. [DOI] [PubMed] [Google Scholar]
- 233.Grossmann M, Hoermann R, Wittert G, Yeap BB. Effects of testosterone treatment on glucose metabolism and symptoms in men with type 2 diabetes and the metabolic syndrome: A systematic review and meta-analysis of randomized controlled clinical trials. Clin Endocrinol (Oxf) 83: 344–351, 2015. [DOI] [PubMed] [Google Scholar]
- 234.Guder G, Frantz S, Bauersachs J, Allolio B, Ertl G, Angermann CE, Stork S. Low circulating androgens and mortality risk in heart failure. Heart 96: 504–509, 2010. [DOI] [PubMed] [Google Scholar]
- 235.Guthrie JR, Dennerstein L, Taffe JR, Lehert P, Burger HG. The menopausal transition: A 9-year prospective population-based study. The Melbourne Women’s Midlife Health Project. Climacteric 7:375–389, 2004. [DOI] [PubMed] [Google Scholar]
- 236.Haass M, Kitzman DW, Anand IS, Miller A, Zile MR, Massie BM, Carson PE. Body mass index and adverse cardiovascular outcomes in heart failure patients with preserved ejection fraction: Results from the Irbesartan in Heart Failure with Preserved Ejection Fraction (I-PRESERVE) trial. Circ Heart Fail 4: 324–331, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Hackett G, Cole N, Mulay A, Strange RC, Ramachandran S. Long-term testosterone therapy in type 2 diabetes is associated with reduced mortality without improvement in conventional cardiovascular risk factors. BJU Int 123: 519–529, 2019. [DOI] [PubMed] [Google Scholar]
- 238.Haddad RM, Kennedy CC, Caples SM, Tracz MJ, Bolona ER, Sideras K, Uraga MV, Erwin PJ, Montori VM. Testosterone and cardiovascular risk in men: A systematic review and meta-analysis of randomized placebo-controlled trials. Mayo Clin Proc 82: 29–39, 2007. [DOI] [PubMed] [Google Scholar]
- 239.Haffner SM, Karhapaa P, Mykkanen L, Laakso M. Insulin resistance, body fat distribution, and sex hormones in men. Diabetes 43: 212–219, 1994. [DOI] [PubMed] [Google Scholar]
- 240.Haffner SM, Mykkanen L, Valdez RA, Katz MS. Relationship of sex hormones to lipids and lipoproteins in nondiabetic men. J Clin Endocrinol Metab 77: 1610–1615, 1993. [DOI] [PubMed] [Google Scholar]
- 241.Haffner SM, Newcomb PA, Marcus PM, Klein BE, Klein R. Relation of sex hormones and dehydroepiandrosterone sulfate (DHEA-SO4) to cardiovascular risk factors in postmenopausal women. Am J Epidemiol 142: 925–934, 1995. [DOI] [PubMed] [Google Scholar]
- 242.Haffner SM, Valdez RA, Mykkanen L, Stern MP, Katz MS. Decreased testosterone and dehydroepiandrosterone sulfate concentrations are associated with increased insulin and glucose concentrations in nondiabetic men. Metabolism 43: 599–603, 1994. [DOI] [PubMed] [Google Scholar]
- 243.Haffner SM, Valdez RA, Stern MP, Katz MS. Obesity, body fat distribution and sex hormones in men. Int J Obes Relat Metab Disord 17:643–649, 1993. [PubMed] [Google Scholar]
- 244.Haider A, Yassin A, Doros G, Saad F. Effects of long-term testosterone therapy on patients with "diabesity": Results of observational studies of pooled analyses in obese hypogonadal men with type 2 diabetes. Int J Endocrinol 2014: 683515, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief 360: 1–8, 2020. [PubMed] [Google Scholar]
- 246.Hall SA, Esche GR, Araujo AB, Travison TG, Clark RV, Williams RE, McKinlay JB. Correlates of low testosterone and symptomatic andro-gen deficiency in a population-based sample. J Clin Endocrinol Metab 93: 3870–3877, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Hamilton EJ, Gianatti E, Strauss BJ, Wentworth J, Lim-Joon D, Bolton D, Zajac JD, Grossmann M. Increase in visceral and subcutaneous abdominal fat in men with prostate cancer treated with androgen deprivation therapy. Clin Endocrinol (Oxf) 74: 377–383, 2011. [DOI] [PubMed] [Google Scholar]
- 248.Hammoud A, Gibson M, Hunt SC, Adams TD, Carrell DT, Kolotkin RL, Meikle AW. Effect of Roux-en-Y gastric bypass surgery on the sex steroids and quality of life in obese men. J Clin Endocrinol Metab 94: 1329–1332, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Han C, Hong YC. Bisphenol A, hypertension, and cardiovascular dis-eases: Epidemiological, laboratory, and clinical trial evidence. Curr Hypertens Rep 18: 11, 2016. [DOI] [PubMed] [Google Scholar]
- 250.Han T, Al-Gindan Y, Govan L, Hankey C, Lean M. Associations of BMI, waist circumference, body fat, and skeletal muscle with type 2 diabetes in adults. Acta diabetologica 56: 947–954, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Han TS, Al-Gindan YY, Govan L, Hankey CR, Lean ME. Associations of body fat and skeletal muscle with hypertension. J Clin Hypertens 21: 230–238, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Hanssen MJ, Hoeks J, Brans B, van der Lans AA, Schaart G, van den Driessche JJ, Jorgensen JA, Boekschoten MV, Hesselink MK, Havekes B, Kersten S, Mottaghy FM, van Marken Lichtenbelt WD, Schrauwen P. Short-term cold acclimation improves insulin sensitivity in patients with type 2 diabetes mellitus. Nat Med 21: 863–865, 2015. [DOI] [PubMed] [Google Scholar]
- 253.Haring R, Teng Z, Xanthakis V, Coviello A, Sullivan L, Bhasin S, Murabito JM, Wallaschofski H, Vasan RS. Association of sex steroids, gonadotrophins, and their trajectories with clinical cardiovascular disease and all-cause mortality in elderly men from the Framingham Heart Study. Clin Endocrinol (Oxf) 78: 629–634, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Haring R, Volzke H, Steveling A, Krebs A, Felix SB, Schofl C, Dorr M, Nauck M, Wallaschofski H. Low serum testosterone levels are associated with increased risk of mortality in a population-based cohort of men aged 20-79. Eur Heart J 31: 1494–1501, 2010. [DOI] [PubMed] [Google Scholar]
- 255.Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR, Balti-more Longitudinal Study of Aging. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab 86: 724–731, 2001. [DOI] [PubMed] [Google Scholar]
- 256.He Z, Rankinen T, Leon AS, Skinner JS, Tchernof A, Bouchard C. Plasma steroids, body composition, and fat distribution: Effects of age, sex, and exercise training. Int J Obes (Lond) 42: 1366–1377, 2018. [DOI] [PubMed] [Google Scholar]
- 257.Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, Rood J, Nguyen T, Martin CK, Volaufova J, Most MM, Greenway FL, Smith SR, Deutsch WA, Williamson DA, Ravussin E, Pennington CT. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: A randomized controlled trial. JAMA 295: 1539–1548, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Heiss G, Wallace R, Anderson GL, Aragaki A, Beresford SA, Brzyski R, Chlebowski RT, Gass M, LaCroix A, Manson JE, Prentice RL, Rossouw J, Stefanick ML, Investigators WHI. Health risks and benefits 3 years after stopping randomized treatment with estrogen and progestin. JAMA 299: 1036–1045, 2008. [DOI] [PubMed] [Google Scholar]
- 259.Hendrikx A, Heyns W, De Moor P. Influence of a low-calorie diet and fasting on the metabolism of dehydroepiandosterone sulfate in adult obese subjects. J Clin Endocrinol Metab 28: 1525–1533, 1968. [DOI] [PubMed] [Google Scholar]
- 260.Heron M. Deaths: Leading causes for 2016. Natl Vital Stat Rep 67:1–77, 2018. [PubMed] [Google Scholar]
- 261.Herranz L, Megia A, Grande C, Gonzalez-Gancedo P, Pallardo F. Dehydroepiandrosterone sulphate, body fat distribution and insulin in obese men. Int J Obes Relat Metab Disord 19: 57–60, 1995. [PubMed] [Google Scholar]
- 262.Herrmann BL, Janssen OE, Hahn S, Broecker-Preuss M, Mann K. Effects of estrogen replacement therapy on bone and glucose metabolism in a male with congenital aromatase deficiency. Horm Metab Res 37: 178–183, 2005. [DOI] [PubMed] [Google Scholar]
- 263.Herrmann BL, Saller B, Janssen OE, Gocke P, Bockisch A, Sperling H, Mann K, Broecker M. Impact of estrogen replacement therapy in a male with congenital aromatase deficiency caused by a novel mutation in the CYP19 gene. J Clin Endocrinol Metab 87: 5476–5484, 2002. [DOI] [PubMed] [Google Scholar]
- 264.Heymsfield SB, Scherzer R, Pietrobelli A, Lewis CE, Grunfeld C. Body mass index as a phenotypic expression of adiposity: Quantitative contribution of muscularity in a population-based sample. Int J Obes (Lond) 33: 1363–1373, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Heymsfield SB, Stanley A, Pietrobelli A, Heo M. Simple skeletal muscle mass estimation formulas: What we can learn from them. Front Endocrinol (Lausanne) 11: 31, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Hill PB, Wynder EL. Effect of a vegetarian diet and dexamethasone on plasma prolactin, testosterone and dehydroepiandrosterone in men and women. Cancer Lett 7: 273–282, 1979. [DOI] [PubMed] [Google Scholar]
- 267.Ho JE, Gona P, Pencina MJ, Tu JV, Austin PC, Vasan RS, Kannel WB, D’Agostino RB, Lee DS, Levy D. Discriminating clinical features of heart failure with preserved vs. reduced ejection fraction in the community. Eur Heart J 33: 1734–1741, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Ho JE, Lyass A, Lee DS, Vasan RS, Kannel WB, Larson MG, Levy D. Predictors of new-onset heart failure: Differences in preserved versus reduced ejection fraction. Circ Heart Fail 6: 279–286, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Hodis HN, Mack WJ, Henderson VW, Shoupe D, Budoff MJ, Hwang-Levine J, Li Y, Feng M, Dustin L, Kono N, Stanczyk FZ, Selzer RH, Azen SP, ELITE Research Group. Vascular effects of early versus late postmenopausal treatment with estradiol. N Engl J Med 374: 1221–1231, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Hofstra J, Loves S, van Wageningen B, Ruinemans-Koerts J, Jansen I, de Boer H. High prevalence of hypogonadotropic hypogonadism in men referred for obesity treatment. Neth J Med 66: 103–109, 2008. [PubMed] [Google Scholar]
- 271.Holmboe SA, Vradi E, Jensen TK, Linneberg A, Husemoen LL, Scheike T, Skakkebaek NE, Juul A, Andersson AM. The association of reproductive hormone levels and all-cause, cancer, and cardio-vascular disease mortality in men. J Clin Endocrinol Metab 100: 4472–4480, 2015. [DOI] [PubMed] [Google Scholar]
- 272.Holmegard HN, Nordestgaard BG, Jensen GB, Tybjaerg-Hansen A, Benn M. Sex hormones and ischemic stroke: A prospective cohort study and meta-analyses. J Clin Endocr Metab 101: 68–77, 2016. [DOI] [PubMed] [Google Scholar]
- 273.Holmegard HN, Nordestgaard BG, Jensen GB, Tybjaerg-Hansen A, Benn M. Sex hormones and ischemic stroke: A prospective cohort study and meta-analyses. J Clin Endocrinol Metab 101: 69–78, 2016. [DOI] [PubMed] [Google Scholar]
- 274.Hu C, Schottker B, Venisse N, Limousi F, Saulnier PJ, Albouy-Llaty M, Dupuis A, Brenner H, Migeot V, Hadjadj S. Bisphenol A, chlorinated derivatives of bisphenol A and occurrence of myocardial infarc-tion in patients with type 2 diabetes: Nested case-control studies in two European cohorts. Environ Sci Technol 53: 9876–9883, 2019. [DOI] [PubMed] [Google Scholar]
- 275.Hu JR, Duncan MS, Morgans AK, Brown JD, Meijers WC, Freiberg MS, Salem JE, Beckman JA, Moslehi JJ. Cardiovascular effects of androgen deprivation therapy in prostate cancer: Contemporary meta-analyses. Arterioscler Thromb Vasc Biol 40: e55–e64, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Huang G, Basaria S, Travison TG, Ho MH, Davda M, Mazer NA, Miciek R, Knapp PE, Zhang A, Collins L, Ursino M, Appleman E, Dzekov C, Stroh H, Ouellette M, Rundell T, Baby M, Bhatia NN, Khorram O, Friedman T, Storer TW, Bhasin S. Testosterone dose-response relationships in hysterectomized women with or without oophorectomy: Effects on sexual function, body composition, muscle performance and physical function in a randomized trial. Menopause 21: 612–623, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Huang G, Pencina KM, Li Z, Basaria S, Bhasin S, Travison TG, Storer TW, Harman SM, Tsitouras P. Long-term testosterone administration on insulin sensitivity in older men with low or low-normal testosterone levels. J Clin Endocrinol Metab 103: 1678–1685, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: A 26-year follow-up of participants in the Framingham Heart Study. Circulation 67: 968–977, 1983. [DOI] [PubMed] [Google Scholar]
- 279.Huhtaniemi IT, Tajar A, Lee DM, O’Neill TW, Finn JD, Bartfai G, Boonen S, Casanueva FF, Giwercman A, Han TS, Kula K, Labrie F, Lean ME, Pendleton N, Punab M, Silman AJ, Vanderschueren D, Forti G, Wu FC, EMAS Group. Comparison of serum testosterone and estradiol measurements in 3174 European men using platform immunoassay and mass spectrometry; relevance for the diagnostics in aging men. Eur J Endocrinol 166: 983–991, 2012. [DOI] [PubMed] [Google Scholar]
- 280.Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, Vittinghoff E. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA 280: 605–613, 1998. [DOI] [PubMed] [Google Scholar]
- 281.Hussain I, Patni N, Garg A. Lipodystrophies, dyslipidaemias and atherosclerotic cardiovascular disease. Pathology 51: 202–212, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Huxley RR, Peters SA, Mishra GD, Woodward M. Risk of all-cause mortality and vascular events in women versus men with type 1 diabetes: A systematic review and meta-analysis. Lancet Diabetes Endocrinol 3: 198–206, 2015. [DOI] [PubMed] [Google Scholar]
- 283.Huxley RR, Woodward M. Cigarette smoking as a risk factor for coronary heart disease in women compared with men: A systematic review and meta-analysis of prospective cohort studies. Lancet 378: 1297–1305, 2011. [DOI] [PubMed] [Google Scholar]
- 284.Hwaung P, Bosy-Westphal A, Muller MJ, Geisler C, Heo M, Thomas DM, Kennedy S, Heymsfield SB. Obesity tissue: Composition, energy expenditure, and energy content in adult humans. Obesity (Silver Spring) 27: 1472–1481, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Ibrahim MM. Subcutaneous and visceral adipose tissue: Structural and functional differences. Obesity Rev 11: 11–18, 2010. [DOI] [PubMed] [Google Scholar]
- 286.Iellamo F, Volterrani M, Caminiti G, Karam R, Massaro R, Fini M, Collins P, Rosano GM. Testosterone therapy in women with chronic heart failure: A pilot double-blind, randomized, placebo-controlled study. J Am Coll Cardiol 56: 1310–1316, 2010. [DOI] [PubMed] [Google Scholar]
- 287.Imes CC, Burke LE. The obesity epidemic: The United States as a cautionary tale for the rest of the World. Curr Epidemiol Rep 1: 82–88, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.International Commission on Radiological Protection. Task Group on Reference Man. Report of the Task Group on Reference Man: A Report. Oxford; New York: Pergamon Press, 1975, p. xix, 480 p. [Google Scholar]
- 289.Isidori AM, Caprio M, Strollo F, Moretti C, Frajese G, Isidori A, Fab-bri A. Leptin and androgens in male obesity: Evidence for leptin contribution to reduced androgen levels. J Clin Endocrinol Metab 84: 3673–3680, 1999. [DOI] [PubMed] [Google Scholar]
- 290.Isidori AM, Giannetta E, Greco EA, Gianfrilli D, Bonifacio V, Isidori A, Lenzi A, Fabbri A. Effects of testosterone on body composition, bone metabolism and serum lipid profile in middle-aged men: A meta-analysis. Clin Endocrinol (Oxf) 63: 280–293, 2005. [DOI] [PubMed] [Google Scholar]
- 291.Ivandic A, Prpic-Krizevac I, Sucic M, Juric M. Hyperinsulinemia and sex hormones in healthy premenopausal women: Relative contribution of obesity, obesity type, and duration of obesity. Metabolism 47: 13–19, 1998. [DOI] [PubMed] [Google Scholar]
- 292.Iwamoto SJ, Grimstad F, Irwig MS, Rothman MS. Routine screening for transgender and gender diverse adults taking gender-affirming hormone therapy: A narrative review. J Gen Intern Med 36: 1380–1389, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Jackson AS, Stanforth P, Gagnon J, Rankinen T, Leon AS, Rao D, Skinner J, Bouchard C, Wilmore J. The effect of sex, age and race on estimating percentage body fat from body mass index: The Heritage Family Study. Int J Obes (Lond) 26: 789–796, 2002. [DOI] [PubMed] [Google Scholar]
- 294.Jacobs DR Jr, Mebane IL, Bangdiwala SI, Criqui MH, Tyroler HA. High density lipoprotein cholesterol as a predictor of cardiovascular disease mortality in men and women: The follow-up study of the Lipid Research Clinics Prevalence Study. Am J Epidemiol 131: 32–47, 1990. [DOI] [PubMed] [Google Scholar]
- 295.Jankowska EA, Rozentryt P, Ponikowska B, Hartmann O, Kustrzycka-Kratochwil D, Reczuch K, Nowak J, Borodulin-Nadzieja L, Polonski L, Banasiak W, Poole-Wilson PA, Anker SD, Ponikowski P. Circulating estradiol and mortality in men with systolic chronic heart failure. JAMA 301: 1892–1901, 2009. [DOI] [PubMed] [Google Scholar]
- 296.Jankowski CM, Gozansky WS, Schwartz RS, Dahl DJ, Kittelson JM, Scott SM, Van Pelt RE, Kohrt WM. Effects of dehydroepiandrosterone replacement therapy on bone mineral density in older adults: A randomized, controlled trial. J Clin Endocrinol Metab 91: 2986–2993, 2006. [DOI] [PubMed] [Google Scholar]
- 297.Jankowski CM, Wolfe P, Schmiege SJ, Nair KS, Khosla S, Jensen M, von Muhlen D, Laughlin GA, Kritz-Silverstein D, Bergstrom J, Bettencourt R, Weiss EP, Villareal DT, Kohrt WM. Sex-specific effects of dehydroepiandrosterone (DHEA) on bone mineral density and body composition: A pooled analysis of four clinical trials. Clin Endocrinol (Oxf) 90: 293–300, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Janssen F, Bardoutsos A, Vidra N. Obesity prevalence in the long-term future in 18 European countries and in the USA. Obesity Facts 13: 514–527, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Janssen I, Powell LH, Crawford S, Lasley B, Sutton-Tyrrell K. Menopause and the metabolic syndrome: The Study of Women’s Health Across the Nation. Arch Intern Med 168: 1568–1575, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Janssen I, Powell LH, Kazlauskaite R, Dugan SA. Testosterone and visceral fat in midlife women: The Study of Women’s Health Across the Nation (SWAN) fat patterning study. Obesity (Silver Spring) 18: 604–610, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Jasuja GK, Rose AJ. Who gets testosterone? Patient characteristics associated with testosterone prescribing in the veteran affairs system: A cross-sectional study. J Gen Intern Med 32: 1075, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Jayedi A, Rashidy-Pour A, Khorshidi M, Shab-Bidar S. Body mass index, abdominal adiposity, weight gain and risk of developing hypertension: A systematic review and dose–response meta-analysis of more than 2.3 million participants. Obesity Rev 19: 654–667, 2018. [DOI] [PubMed] [Google Scholar]
- 303.Jayedi A, Soltani S, Zargar MS, Khan TA, Shab-Bidar S. Central fatness and risk of all cause mortality: Systematic review and dose-response meta-analysis of 72 prospective cohort studies. BMJ 370: m3324, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Jensen LB, Vestergaard P, Hermann AP, Gram J, Eiken P, Abrahamsen B, Brot C, Kolthoff N, Sorensen OH, Beck-Nielsen H, Nielsen SP, Charles P, Mosekilde L. Hormone replacement therapy dissociates fat mass and bone mass, and tends to reduce weight gain in early postmenopausal women: A randomized controlled 5-year clinical trial of the Danish Osteoporosis Prevention Study. J Bone Miner Res 18:333–342, 2003. [DOI] [PubMed] [Google Scholar]
- 305.Jia A, Xu S, Xing Y, Zhang W, Yu X, Zhao Y, Ming J, Ji Q. Prevalence and cardiometabolic risks of normal weight obesity in Chinese population: A nationwide study. Nutr Metab Cardiovasc Dis 28: 1045–1053, 2018. [DOI] [PubMed] [Google Scholar]
- 306.Jia X, Sun C, Tang O, Gorlov I, Nambi V, Virani SS, Villareal DT, Taffet GE, Yu B, Bressler J, Boerwinkle E, Windham BG, de Lemos JA, Matsushita K, Selvin E, Michos ED, Hoogeveen RC, Ballantyne CM. Plasma dehydroepiandrosterone sulfate and cardiovascular dis-ease risk in older men and women. J Clin Endocrinol Metab 105: e4304–e4327, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Jimenez MC, Tucker KL, Rodriguez F, Porneala BC, Meigs JB, Lopez L. Cardiovascular risk factors and dehydroepiandrosterone sulfate among Latinos in the Boston Puerto Rican Health Study. J Endocr Soc 3: 291–303, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Jimenez MC, Wang L, Buring JE, Manson JE, Forman JP, Sesso HD. Association between sex hormones and ambulatory blood pressure. J Hypertens 36: 2237–2244, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Johannes CB, Stellato RK, Feldman HA, Longcope C, McKinlay JB. Relation of dehydroepiandrosterone and dehydroepiandrosterone sulfate with cardiovascular disease risk factors in women: Longitudinal results from the Massachusetts Women’s Health Study. J Clin Epidemiol 52: 95–103, 1999. [DOI] [PubMed] [Google Scholar]
- 310.Jones ME, Boon WC, Proietto J, Simpson ER. Of mice and men: The evolving phenotype of aromatase deficiency. Trends Endocrinol Metab 17: 55–64, 2006. [DOI] [PubMed] [Google Scholar]
- 311.Juang PS, Peng S, Allehmazedeh K, Shah A, Coviello AD, Herbst KL. Testosterone with dutasteride, but not anastrazole, improves insulin sensitivity in young obese men: A randomized controlled trial. JSex Med 11: 563–573, 2014. [DOI] [PubMed] [Google Scholar]
- 312.Kacker R, Traish AM, Morgentaler A. Estrogens in men: Clinical implications for sexual function and the treatment of testosterone deficiency. JSex Med 9: 1681–1696, 2012. [DOI] [PubMed] [Google Scholar]
- 313.Kaczmarek A, Reczuch K, Majda J, Banasiak W, Ponikowski P. The association of lower testosterone level with coronary artery disease in postmenopausal women. Int J Cardiol 87: 53–57, 2003. [DOI] [PubMed] [Google Scholar]
- 314.Kalinchenko SY, Tishova YA, Mskhalaya GJ, Gooren LJ, Giltay EJ, Saad F. Effects of testosterone supplementation on markers of the metabolic syndrome and inflammation in hypogonadal men with the metabolic syndrome: The double-blinded placebo-controlled Moscow study. Clin Endocrinol (Oxf) 73: 602–612, 2010. [DOI] [PubMed] [Google Scholar]
- 315.Kamischke A, Diebacker J, Nieschlag E. Potential of norethisterone enanthate for male contraception: Pharmacokinetics and suppression of pituitary and gonadal function. Clin Endocrinol (Oxf) 53: 351–358, 2000. [DOI] [PubMed] [Google Scholar]
- 316.Kamischke A, Ploger D, Venherm S, von Eckardstein S, von Eckard-stein A, Nieschlag E. Intramuscular testosterone undecanoate with or without oral levonorgestrel: A randomized placebo-controlled feasibility study for male contraception. Clin Endocrinol (Oxf) 53: 43–52, 2000. [DOI] [PubMed] [Google Scholar]
- 317.Kamischke A, Venherm S, Ploger D, von Eckardstein S, Nieschlag E. Intramuscular testosterone undecanoate and norethisterone enanthate in a clinical trial for male contraception. J Clin Endocrinol Metab 86:303–309, 2001. [DOI] [PubMed] [Google Scholar]
- 318.Kapoor D, Goodwin E, Channer KS, Jones TH. Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity and hypercholesterolaemia in hypogonadal men with type 2 diabetes. Eur J Endocrinol 154: 899–906, 2006. [DOI] [PubMed] [Google Scholar]
- 319.Kararigas G, Bito V, Tinel H, Becher E, Baczko I, Knosalla C, Albrecht-Kupper B, Sipido KR, Regitz-Zagrosek V. Transcriptome characterization of estrogen-treated human myocardium identifies myosin regulatory light chain interacting protein as a sex-specific element influencing contractile function. J Am Coll Cardiol 59:410–417, 2012. [DOI] [PubMed] [Google Scholar]
- 320.Karelis AD, Faraj M, Bastard J-P, St-Pierre DH, Brochu M, Prud’homme D, Rabasa-Lhoret R. The metabolically healthy but obese individual presents a favorable inflammation profile. J Clin Endocrinol Metab 90: 4145–4150, 2005. [DOI] [PubMed] [Google Scholar]
- 321.Karim R, Dell RM, Greene DF, Mack WJ, Gallagher JC, Hodis HN. Hip fracture in postmenopausal women after cessation of hormone therapy: Results from a prospective study in a large health management organization. Menopause 18: 1172–1177, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Karpe F, Pinnick KE. Biology of upper-body and lower-body adipose tissue—link to whole-body phenotypes. Nat Rev Endocrinol 11: 90–100, 2015. [DOI] [PubMed] [Google Scholar]
- 323.Kask E. 17-Ketosteroids and arteriosclerosis. Angiology 10: 358–368, 1959. [DOI] [PubMed] [Google Scholar]
- 324.Kautzky-Willer A, Handisurya A. Metabolic diseases and associated complications: Sex and gender matter! Eur J Clin Invest 39: 631–648, 2009. [DOI] [PubMed] [Google Scholar]
- 325.Keating NL, O’Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol 24: 4448–4456, 2006. [DOI] [PubMed] [Google Scholar]
- 326.Keller JL, Casson PR, Toth MJ. Relationship of androgens to body composition, energy and substrate metabolism and aerobic capacity in healthy, young women. Steroids 76: 1247–1251, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Kelley DE, Thaete FL, Troost F, Huwe T, Goodpaster BH. Subdivisions of subcutaneous abdominal adipose tissue and insulin resistance. Am J Physiol Endocrinol Metab 278: E941–E948, 2000. [DOI] [PubMed] [Google Scholar]
- 328.Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, Kannel WB, Vasan RS. Obesity and the risk of heart failure. N Engl J Med 347: 305–313, 2002. [DOI] [PubMed] [Google Scholar]
- 329.Kenchaiah S, Pocock S, Wang D, Finn P, Zornoff L, Skali H, Pfeffer M, Yusuf S, Swedberg K, Michelson E. Body mass index and prognosis in patients with chronic heart failure: Insights from the Candesartan in Heart failure: Assessment of Reduction in Mortality and Morbidity (CHARM) Program. Circulation 116: 627–636, 2007. [DOI] [PubMed] [Google Scholar]
- 330.Kenny AM, Prestwood KM, Gruman CA, Marcello KM, Raisz LG. Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels. J Gerontol A Biol Sci Med Sci 56: M266–M272, 2001. [DOI] [PubMed] [Google Scholar]
- 331.Khaw KT, Barrett-Connor E. Lower endogenous androgens predict central adiposity in men. Ann Epidemiol 2: 675–682, 1992. [DOI] [PubMed] [Google Scholar]
- 332.Khaw KT, Dowsett M, Folkerd E, Bingham S, Wareham N, Luben R, Welch A, Day N. Endogenous testosterone and mortality due to all causes, cardiovascular disease, and cancer in men: European prospective investigation into cancer in Norfolk (EPIC-Norfolk) Prospective Population Study. Circulation 116: 2694–2701, 2007. [DOI] [PubMed] [Google Scholar]
- 333.Kim ED, McCullough A, Kaminetsky J. Oral enclomiphene citrate raises testosterone and preserves sperm counts in obese hypogonadal men, unlike topical testosterone: Restoration instead of replacement. BJU Int 117: 677–685, 2016. [DOI] [PubMed] [Google Scholar]
- 334.Kim JE, Chang JH, Jeong MJ, Choi J, Park J, Baek C, Shin A, Park SM, Kang D, Choi JY. A systematic review and meta-analysis of effects of menopausal hormone therapy on cardiovascular diseases. Sci Rep 10: 20631, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Kimble T, Burke AE, Barnhart KT, Archer DF, Colli E, Westhoff CL. A 1-year prospective, open-label, single-arm, multicenter, phase 3 trial of the contraceptive efficacy and safety of the oral progestin-only pill drospirenone 4 mg using a 24/4-day regimen. Contracept X 2: 100020, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Klaver M, Dekker M, de Mutsert R, Twisk JWR, den Heijer M. Cross-sex hormone therapy in transgender persons affects total body weight, body fat and lean body mass: A meta-analysis. Andrologia 49, 2017. DOI: 10.1111/and.12660. [DOI] [PubMed] [Google Scholar]
- 337.Kley HK, Deselaers T, Peerenboom H. Evidence for hypogonadism in massively obese males due to decreased free testosterone. Horm Metab Res 13: 639–641, 1981. [DOI] [PubMed] [Google Scholar]
- 338.Kley HK, Deselaers T, Peerenboom H, Kruskemper HL. Enhanced conversion of androstenedione to estrogens in obese males. J Clin Endocrinol Metab 51: 1128–1132, 1980. [DOI] [PubMed] [Google Scholar]
- 339.Kloner RA, Carson C 3rd, Dobs A, Kopecky S, Mohler ER 3rd. Testosterone and cardiovascular disease. J Am Coll Cardiol 67: 545–557, 2016. [DOI] [PubMed] [Google Scholar]
- 340.Klop B, Elte JWF, Cabezas MC. Dyslipidemia in obesity: Mechanisms and potential targets. Nutrients 5: 1218–1240, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Kosmala W, Jedrzejuk D, Derzhko R, Przewlocka-Kosmala M, Mysiak A, Bednarek-Tupikowska G. Left ventricular function impairment in patients with normal-weight obesity: Contribution of abdominal fat deposition, profibrotic state, reduced insulin sensitivity, and proinflammatory activation. Circ Cardiovas Imaging 5: 349–356, 2012. [DOI] [PubMed] [Google Scholar]
- 342.Kreling D, Mott D, Wiederholt J, Lundy J, Levitt L. Prescription Drug Trends: A Chartbook Update. Menlo Park, CA: Kaiser Family Foundation, 2001. [Google Scholar]
- 343.Kroboth PD, Salek FS, Pittenger AL, Fabian TJ, Frye RF. DHEA and DHEA-S: A review. J Clin Pharmacol 39: 327–348, 1999. [DOI] [PubMed] [Google Scholar]
- 344.Kuk JL, Katzmarzyk PT, Nichaman MZ, Church TS, Blair SN, Ross R.Visceral fat is an independent predictor of all-cause mortality in men. Obesity (Silver Spring) 14: 336–341, 2006. [DOI] [PubMed] [Google Scholar]
- 345.Labrie F, Belanger A, Belanger P, Berube R, Martel C, Cusan L, Gomez J, Candas B, Chaussade V, Castiel I, Deloche C, Leclaire J. Metabolism of DHEA in postmenopausal women following percutaneous administration. J Steroid Biochem Mol Biol 103: 178–188, 2007. [DOI] [PubMed] [Google Scholar]
- 346.Labrie F, Cusan L, Gomez JL, Martel C, Berube R, Belanger P, Chaussade V, Deloche C, Leclaire J. Changes in serum DHEA and eleven of its metabolites during 12-month percutaneous administration of DHEA. J Steroid Biochem Mol Biol 110: 1–9, 2008. [DOI] [PubMed] [Google Scholar]
- 347.Lacey JV Jr, Mink PJ, Lubin JH, Sherman ME, Troisi R, Hartge P, Schatzkin A, Schairer C. Menopausal hormone replacement therapy and risk of ovarian cancer. JAMA 288: 334–341, 2002. [DOI] [PubMed] [Google Scholar]
- 348.LaCroix AZ, Chlebowski RT, Manson JE, Aragaki AK, Johnson KC, Martin L, Margolis KL, Stefanick ML, Brzyski R, Curb JD, Howard BV, Lewis CE, Wactawski-Wende J, Investigators WHI. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy: A randomized controlled trial. JAMA 305: 1305–1314, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Lambert CP, Sullivan DH, Evans WJ. Megestrol acetate-induced weight gain does not negatively affect blood lipids in elderly men: Effects of resistance training and testosterone replacement. J Gerontol A Biol Sci Med Sci 58: 644–647, 2003. [DOI] [PubMed] [Google Scholar]
- 350.Lanfranco F, Zirilli L, Baldi M, Pignatti E, Corneli G, Ghigo E, Aimaretti G, Carani C, Rochira V. A novel mutation in the human aromatase gene: Insights on the relationship among serum estradiol, longitudinal growth and bone mineral density in an adult man under estrogen replacement treatment. Bone 43: 628–635, 2008. [DOI] [PubMed] [Google Scholar]
- 351.Lasco A, Frisina N, Morabito N, Gaudio A, Morini E, Trifiletti A, Basile G, Nicita-Mauro V, Cucinotta D. Metabolic effects of dehydroepiandrosterone replacement therapy in postmenopausal women. Eur J Endocrinol 145: 457–461, 2001. [DOI] [PubMed] [Google Scholar]
- 352.Lasley BL, Santoro N, Randolf JF, Gold EB, Crawford S, Weiss G, McConnell DS, Sowers MF. The relationship of circulating dehydroepiandrosterone, testosterone, and estradiol to stages of the menopausal transition and ethnicity. J Clin Endocrinol Metab 87: 3760–3767, 2002. [DOI] [PubMed] [Google Scholar]
- 353.Laughlin GA, Barrett-Connor E, Bergstrom J. Low serum testosterone and mortality in older men. J Clin Endocrinol Metab 93: 68–75, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Laughlin GA, Goodell V, Barrett-Connor E. Extremes of endogenous testosterone are associated with increased risk of incident coronary events in older women. J Clin Endocrinol Metab 95: 740–747, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Lavie CJ, Alpert MA, Arena R, Mehra MR, Milani RV, Ventura HO. Impact of obesity and the obesity paradox on prevalence and prognosis in heart failure. JACC Heart Fail 1: 93–102, 2013. [DOI] [PubMed] [Google Scholar]
- 356.Lavie CJ, De Schutter A, Patel DA, Romero-Corral A, Artham SM, Milani RV. Body composition and survival in stable coronary heart disease: Impact of lean mass index and body fat in the “obesity para-dox”. J Am Coll Cardiol 60: 1374–1380, 2012. [DOI] [PubMed] [Google Scholar]
- 357.Lavie CJ, Osman AF, Milani RV, Mehra MR. Body composition and prognosis in chronic systolic heart failure: The obesity paradox. Am J Cardiol 91: 891–894, 2003. [DOI] [PubMed] [Google Scholar]
- 358.LeBlanc ES, Wang PY, Lee CG, Barrett-Connor E, Cauley JA, Hoff-man AR, Laughlin GA, Marshall LM, Orwoll ES. Higher testosterone levels are associated with less loss of lean body mass in older men. J Clin Endocrinol Metab 96: 3855–3863, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Lee CMY, Huxley RR, Wildman RP, Woodward M. Indices of abdominal obesity are better discriminators of cardiovascular risk factors than BMI: A meta-analysis. J Clin Epidemiol 61: 646–653, 2008. [DOI] [PubMed] [Google Scholar]
- 360.Lee D-C, Shook RP, Drenowatz C, Blair SN. Physical activity and sarcopenic obesity: Definition, assessment, prevalence and mechanism. Future Sci OA 2: FSO127, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Lee Y, Dang JT, Switzer N, Yu J, Tian C, Birch DW, Karmali S. Impact of bariatric surgery on male sex hormones and sperm quality: A systematic review and meta-analysis. Obes Surg 29: 334–346, 2019. [DOI] [PubMed] [Google Scholar]
- 362.Levine GN, D’Amico AV, Berger P, Clark PE, Eckel RH, Keating NL, Milani RV, Sagalowsky AI, Smith MR, Zakai N, American Heart Association Council on Clinical Cardiology, Council on Epidemiology and Prevention, the American Cancer Society, the American Urological Association. Androgen-deprivation therapy in prostate cancer and cardiovascular risk: A science advisory from the American Heart Association, American Cancer Society, and American Urological Association: Endorsed by the American Society for Radiation Oncology. Circulation 121: 833–840, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK. The progression from hypertension to congestive heart failure. JAMA 275: 1557–1562, 1996. [PubMed] [Google Scholar]
- 364.Li R, E L, Zha N. Circulating dehydroepiandrosterone sulfate level and cardiovascular or all-cause mortality in the elderly population: A meta-analysis. Ann Palliat Med 9: 3537–3545, 2020. [DOI] [PubMed] [Google Scholar]
- 365.Li SY, Zhao YL, Yang YF, Wang X, Nie M, Wu XY, Mao JF. Metabolic effects of testosterone replacement therapy in patients with type 2 diabetes mellitus or metabolic syndrome: A meta-analysis. Int J Endocrinol 2020: 4732021, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Lima JG, Nobrega LHC, Lima NN, Dos Santos MCF, Silva PHD, Baracho MFP, Lima DN, de Melo Campos JTA, Ferreira LC, Freire Neto FP, Mendes-Aguiar CO, Jeronimo SMB. Causes of death in patients with Berardinelli-Seip congenital generalized lipodystrophy. PLoS One 13: e0199052, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Liu Y, Ma L, Yang X, Bie J, Li D, Sun C, Zhang J, Meng Y, Lin J. Menopausal hormone replacement therapy and the risk of ovarian cancer: A meta-analysis. Front Endocrinol (Lausanne) 10: 801, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Lloyd-Jones DM. Slowing progress in cardiovascular mortality rates: You reap what you sow. JAMA Cardiol 1: 599–600, 2016. [DOI] [PubMed] [Google Scholar]
- 369.Lloyd-Jones DM, Huffman MD, Karmali KN, Sanghavi DM, Wright JS, Pelser C, Gulati M, Masoudi FA, Goff DC Jr. Estimating longitudinal risks and benefits from cardiovascular preventive therapies among medicare patients: The Million Hearts Longitudinal ASCVD Risk Assessment Tool: A special report from the American Heart Association and American College of Cardiology. J Am Coll Cardiol 69: 1617–1636, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Lobo RA, Pickar JH, Stevenson JC, Mack WJ, Hodis HN. Back to the future: Hormone replacement therapy as part of a prevention strategy for women at the onset of menopause. Atherosclerosis 254: 282–290, 2016. [DOI] [PubMed] [Google Scholar]
- 371.Longcope C, Kato T, Horton R. Conversion of blood androgens to estrogens in normal adult men and women. J Clin Invest 48: 2191–2201, 1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Lonning PE, Geisler J, Krag LE, Erikstein B, Bremnes Y, Hagen AI, Schlichting E, Lien EA, Ofjord ES, Paolini J, Polli A, Massimini G. Effects of exemestane administered for 2 years versus placebo on bone mineral density, bone biomarkers, and plasma lipids in patients with surgically resected early breast cancer. J Clin Oncol 23: 5126–5137, 2005. [DOI] [PubMed] [Google Scholar]
- 373.Lopez LM, Ramesh S, Chen M, Edelman A, Otterness C, Trussell J, Helmerhorst FM. Progestin-only contraceptives: Effects on weight. Cochrane Database Syst Rev 2016: CD008815, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Lorentzon M, Eriksson AL, Mellstrom D, Ohlsson C. The COMT val158met polymorphism is associated with peak BMD in men. J Bone Miner Res 19: 2005–2011, 2004. [DOI] [PubMed] [Google Scholar]
- 375.Louis XL, Raj P, Chan L, Zieroth S, Netticadan T, Wigle JT. Are the cardioprotective effects of the phytoestrogen resveratrol sex-dependent? (1). Can J Physiol Pharmacol 97: 503–514, 2019. [DOI] [PubMed] [Google Scholar]
- 376.Lovejoy JC, Champagne CM, de Jonge L, Xie H, Smith SR. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes (Lond) 32: 949–958, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Loves S, de Jong J, van Sorge A, Telting D, Tack CJ, Hermus A, Westerterp K, de Boer H. Somatic and psychological effects of low-dose aromatase inhibition in men with obesity-related hypogonadotropic hypotestosteronemia. Eur J Endocrinol 169: 705–714, 2013. [DOI] [PubMed] [Google Scholar]
- 378.Loves S, Ruinemans-Koerts J, de Boer H. Letrozole once a week normalizes serum testosterone in obesity-related male hypogonadism. Eur J Endocrinol 158: 741–747, 2008. [DOI] [PubMed] [Google Scholar]
- 379.Lu Y, Hajifathalian K, Ezzati M, Woodward M, Rimm EB, Danaei G. Metabolic mediators of the effects of body-mass index, overweight, and obesity on coronary heart disease and stroke: A pooled analysis of 97 prospective cohorts with 1⋅ 8 million participants. Lancet (London, England) 383: 970–983, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Lupsa BC, Sachdev V, Lungu AO, Rosing DR, Gorden P. Cardiomyopathy in congenital and acquired generalized lipodystrophy: A clinical assessment. Medicine (Baltimore) 89: 245–250, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Ma Q, Sun X, Chen Y, Chen X, Zhi G, Tan G. Progesterone levels and carotid intima-media thickness: A negative association in older northern Chinese men. Tex Heart Inst J 36: 303–308, 2009. [PMC free article] [PubMed] [Google Scholar]
- 382.Maccario M, Mazza E, Ramunni J, Oleandri SE, Savio P, Grottoli S, Rossetto R, Procopio M, Gauna C, Ghigo E. Relationships between dehydroepiandrosterone-sulphate and anthropometric, metabolic and hormonal variables in a large cohort of obese women. Clin Endocrinol (Oxf) 50: 595–600, 1999. [DOI] [PubMed] [Google Scholar]
- 383.MacDonald AA, Herbison GP, Showell M, Farquhar CM. The impact of body mass index on semen parameters and reproductive hormones in human males: A systematic review with meta-analysis. Hum Reprod Update 16: 293–311, 2010. [DOI] [PubMed] [Google Scholar]
- 384.Maclaran K, Stevenson JC. Primary prevention of cardiovascular dis-ease with HRT. Womens Health (Lond) 8: 63–74, 2012. [DOI] [PubMed] [Google Scholar]
- 385.Maclennan AH, Broadbent JL, Lester S, Moore V. Oral oestrogen and combined oestrogen/progestogen therapy versus placebo for hot flushes. Cochrane Database Syst Rev 2004: CD002978, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Maffei L, Murata Y, Rochira V, Tubert G, Aranda C, Vazquez M, Clyne CD, Davis S, Simpson ER, Carani C. Dysmetabolic syndrome in a man with a novel mutation of the aromatase gene: Effects of testosterone, alendronate, and estradiol treatment. J Clin Endocrinol Metab 89: 61–70, 2004. [DOI] [PubMed] [Google Scholar]
- 387.Maffei L, Rochira V, Zirilli L, Antunez P, Aranda C, Fabre B, Simone ML, Pignatti E, Simpson ER, Houssami S, Clyne CD, Carani C. A novel compound heterozygous mutation of the aromatase gene in an adult man: Reinforced evidence on the relationship between congenital oestrogen deficiency, adiposity and the metabolic syndrome. Clin Endocrinol (Oxf) 67: 218–224, 2007. [DOI] [PubMed] [Google Scholar]
- 388.Magkos F, Mohammed BS, Mittendorfer B. Effect of obesity on the plasma lipoprotein subclass profile in normoglycemic and normolipiemic men and women. Int J Obes (Lond) 32: 1655–1664, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Magkos F, Wang X, Mittendorfer B. Metabolic actions of insulin in men and women. Nutrition 26: 686–693, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Magnani JW, Moser CB, Murabito JM, Sullivan LM, Wang N, Ellinor PT, Vasan RS, Benjamin EJ, Coviello AD. Association of sex hormones, aging, and atrial fibrillation in men: The Framingham Heart Study. Circ Arrhythm Electrophysiol 7: 307–312, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Magnussen C, Niiranen TJ, Ojeda FM, Gianfagna F, Blankenberg S, Njolstad I, Vartiainen E, Sans S, Pasterkamp G, Hughes M, Costanzo S, Donati MB, Jousilahti P, Linneberg A, Palosaari T, de Gaetano G, Bobak M, den Ruijter HM, Mathiesen E, Jorgensen T, Soderberg S, Kuulasmaa K, Zeller T, Iacoviello L, Salomaa V, Schnabel RB, BiomarCaRE Consortium. Sex differences and similarities in atrial fibrillation epidemiology, risk factors, and mortality in community cohorts: Results from the BiomarCaRE Consortium (Biomarker for Cardiovascular Risk Assessment in Europe). Circulation 136: 1588–1597, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science 232: 1004–1007, 1986. [DOI] [PubMed] [Google Scholar]
- 393.Manolopoulos K, Karpe F, Frayn K. Gluteofemoral body fat as a determinant of metabolic health. Int J Obes (Lond) 34: 949–959, 2010. [DOI] [PubMed] [Google Scholar]
- 394.Manson JE, Aragaki AK, Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Chlebowski RT, Howard BV, Thomson CA, Margolis KL, Lewis CE, Stefanick ML, Jackson RD, Johnson KC, Martin LW, Shumaker SA, Espeland MA, Wactawski-Wende J, Investigators WHI. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: The Women’s Health Initiative Randomized Trials. JAMA 318: 927–938, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Manson JE, Colditz GA, Stampfer MJ, Willett WC, Rosner B, Monson RR, Speizer FE, Hennekens CH. A prospective study of obesity and risk of coronary heart disease in women. NEnglJMed 322: 882–889, 1990. [DOI] [PubMed] [Google Scholar]
- 396.Manson JE, Willett WC, Stampfer MJ, Colditz GA, Hunter DJ, Hankinson SE, Hennekens CH, Speizer FE. Body weight and mortality among women. N Engl J Med 333: 677–685, 1995. [DOI] [PubMed] [Google Scholar]
- 397.Maraka S, Singh Ospina N, Rodriguez-Gutierrez R, Davidge-Pitts CJ, Nippoldt TB, Prokop LJ, Murad MH. Sex steroids and cardiovascular outcomes in transgender individuals: A systematic review and meta-analysis. J Clin Endocrinol Metab 102: 3914–3923, 2017. [DOI] [PubMed] [Google Scholar]
- 398.Marinou K, Hodson L, Vasan SK, Fielding BA, Banerjee R, Brismar K, Koutsilieris M, Clark A, Neville MJ, Karpe F. Structural and functional properties of deep abdominal subcutaneous adipose tissue explain its association with insulin resistance and cardiovascular risk in men. Diabetes Care 37: 821–829, 2014. [DOI] [PubMed] [Google Scholar]
- 399.Marlatt KL, Chen KY, Ravussin E. Is activation of human brown adi-pose tissue a viable target for weight management? Am J Physiol Regul Integr Comp Physiol 315: R479–R483, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Marlatt KL, Redman LM, Beyl RA, Smith SR, Champagne CM, Yi F, Lovejoy JC. Racial differences in body composition and cardiometabolic risk during the menopause transition: A prospective, observational cohort study. Am J Obstet Gynecol 222: 365 e361–365 e318, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Marmorston J, Lewis JJ, Bernstein JL, Sobel H, Kuzma O, Alexander R, Magidson O, Moore FJ. Excretion of urinary steroids by men and women with myocardial infarction. Geriatrics 12: 297–300, 1957. [PubMed] [Google Scholar]
- 402.Martin RM, Lin CJ, Nishi MY, Billerbeck AE, Latronico AC, Russell DW, Mendonca BB. Familial hyperestrogenism in both sexes: Clinical, hormonal, and molecular studies of two siblings. J Clin Endocrinol Metab 88: 3027–3034, 2003. [DOI] [PubMed] [Google Scholar]
- 403.Martinez R, Soliz P, Mujica OJ, Reveiz L, Campbell NR, Ordunez P. The slowdown in the reduction rate of premature mortality from cardiovascular diseases puts the Americas at risk of achieving SDG 3.4: A population trend analysis of 37 countries from 1990 to 2017. J Clin Hypertens 22: 1296–1309, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Martinez-Montoro JI, Molina-Vega M, Asenjo-Plaza M, Garcia-Ruiz MC, Varea-Marineto E, Plaza-Andrade I, Alvarez-Millan JJ, Cabezas-Sanchez P, Tinahones FJ, Fernandez-Garcia JC. Adiposity is associated with decreased serum 17-hydroxyprogesterone levels in non-diabetic obese men aged 18-49: A cross-sectional study. J Clin Med 9: 3873, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Matthews KA, Abrams B, Crawford S, Miles T, Neer R, Powell LH, Wesley D. Body mass index in mid-life women: Relative influence of menopause, hormone use, and ethnicity. Int J Obes Relat Metab Disord 25: 863–873, 2001. [DOI] [PubMed] [Google Scholar]
- 406.Maturana MA, Breda V, Lhullier F, Spritzer PM. Relationship between endogenous testosterone and cardiovascular risk in early postmenopausal women. Metabolism 57: 961–965, 2008. [DOI] [PubMed] [Google Scholar]
- 407.Meendering JR, Torgrimson BN, Miller NP, Kaplan PF, Minson CT. Estrogen, medroxyprogesterone acetate, endothelial function, and biomarkers of cardiovascular risk in young women. Am J Physiol Heart Circ Physiol 294: H1630–H1637, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Melzer D, Osborne NJ, Henley WE, Cipelli R, Young A, Money C, McCormack P, Luben R, Khaw KT, Wareham NJ, Galloway TS. Urinary bisphenol A concentration and risk of future coronary artery disease in apparently healthy men and women. Circulation 125: 1482–1490, 2012. [DOI] [PubMed] [Google Scholar]
- 409.Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med 340: 1801–1811, 1999. [DOI] [PubMed] [Google Scholar]
- 410.Menke A, Guallar E, Rohrmann S, Nelson WG, Rifai N, Kanarek N, Feinleib M, Michos ED, Dobs A, Platz EA. Sex steroid hormone concentrations and risk of death in US men. Am J Epidemiol 171: 583–592, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Messier V, Karelis AD, Lavoie M-E, Brochu M, Faraj M, Strychar I, Rabasa-Lhoret R. Metabolic profile and quality of life in class I sarcopenic overweight and obese postmenopausal women: A MONET study. Appl Physiol Nutr Metab 34: 18–24, 2009. [DOI] [PubMed] [Google Scholar]
- 412.Mezzullo M, Di Dalmazi G, Fazzini A, Baccini M, Repaci A, Gambineri A, Vicennati V, Pelusi C, Pagotto U, Fanelli F. Impact of age, body weight and metabolic risk factors on steroid reference intervals in men. Eur J Endocrinol 182: 459–471, 2020. [DOI] [PubMed] [Google Scholar]
- 413.Mezzullo M, Gambineri A, Di Dalmazi G, Fazzini A, Magagnoli M, Baccini M, Vicennati V, Pelusi C, Pagotto U, Fanelli F. Steroid reference intervals in women: Influence of menopause, age and metabolism. Eur J Endocrinol 184: 399–411, 2021. [DOI] [PubMed] [Google Scholar]
- 414.Miedlich SU, Karamooz N, Hammes SR. Aromatase deficiency in a male patient -Case report and review of the literature. Bone 93: 181–186, 2016. [DOI] [PubMed] [Google Scholar]
- 415.Miller VM, Duckles SP. Vascular actions of estrogens: Functional implications. Pharmacol Rev 60: 210–241, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Miner JA, Martini ER, Smith MM, Brunt VE, Kaplan PF, Halliwill JR, Minson CT. Short-term oral progesterone administration antagonizes the effect of transdermal estradiol on endothelium-dependent vasodilation in young healthy women. Am J Physiol Heart Circ Physiol 301: H1716–H1722, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Misra A, Garg A, Abate N, Peshock RM, Stray-Gundersen J, Grundy SM. Relationship of anterior and posterior subcutaneous abdominal fat to insulin sensitivity in nondiabetic men. Obes Res 5: 93–99, 1997. [DOI] [PubMed] [Google Scholar]
- 418.Mittendorfer B. Sexual dimorphism in human lipid metabolism. JNutr 135: 681–686, 2005. [DOI] [PubMed] [Google Scholar]
- 419.Mohler ER 3rd, Ellenberg SS, Lewis CE, Wenger NK, Budoff MJ, Lewis MR, Barrett-Connor E, Swerdloff RS, Stephens-Shields A, Bhasin S, Cauley JA, Crandall JP, Cunningham GR, Ensrud KE, Gill TM, Matsumoto AM, Molitch ME, Pahor M, Preston PE, Hou X, Cifelli D, Snyder PJ. The effect of testosterone on cardiovascular biomarkers in the testosterone trials. J Clin Endocrinol Metab 103:681–688, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Mongraw-Chaffin M, Allison MA, Burke GL, Criqui MH, Matsushita K, Ouyang P, Shah RV, Shay CM, Anderson CA. CT-derived body fat distribution and incident cardiovascular disease: The Multi-Ethnic Study of Atherosclerosis. J Clin Endocrinol Metab 102: 4173–4183, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Mongraw-Chaffin M, Foster MC, Anderson CA, Burke GL, Haq N, Kalyani RR, Ouyang P, Sibley CT, Tracy R, Woodward M. Metabolically healthy obesity, transition to metabolic syndrome, and cardiovascular risk. J Am Coll Cardiol 71: 1857–1865, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Mongraw-Chaffin ML, Peters SA, Huxley RR, Woodward M. The sex-specific association between BMI and coronary heart disease: A systematic review and meta-analysis of 95 cohorts with 1⋅ 2 million participants. Lancet Diabetes Endocrinol 3: 437–449, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Morales AJ, Haubrich RH, Hwang JY, Asakura H, Yen SS. The effect of six months treatment with a 100 mg daily dose of dehydroepiandrosterone (DHEA) on circulating sex steroids, body composition and muscle strength in age-advanced men and women. Clin Endocrinol (Oxf) 49: 421–432, 1998. [DOI] [PubMed] [Google Scholar]
- 424.Morales AJ, Nolan JJ, Nelson JC, Yen SS. Effects of replacement dose of dehydroepiandrosterone in men and women of advancing age. J Clin Endocrinol Metab 78: 1360–1367, 1994. [DOI] [PubMed] [Google Scholar]
- 425.Moreau KL, Hildreth KL, Meditz AL, Deane KD, Kohrt WM. Endothelial function is impaired across the stages of the menopause transition in healthy women. J Clin Endocrinol Metab 97: 4692–4700, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Moreau KL, Meditz A, Deane KD, Kohrt WM. Tetrahydrobiopterin improves endothelial function and decreases arterial stiffness in estrogen-deficient postmenopausal women. Am J Physiol Heart Circ Physiol 302: H1211–H1218, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Moreau KL, Stauffer BL, Kohrt WM, Seals DR. Essential role of estrogen for improvements in vascular endothelial function with endurance exercise in postmenopausal women. J Clin Endocrinol Metab 98: 4507–4515, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Morgentaler A, Miner MM, Caliber M, Guay AT, Khera M, Traish AM. Testosterone therapy and cardiovascular risk: Advances and controversies. Mayo Clin Proc 90: 224–251, 2015. [DOI] [PubMed] [Google Scholar]
- 429.Morishima A, Grumbach MM, Simpson ER, Fisher C, Qin K. Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. J Clin Endocrinol Metab 80: 3689–3698, 1995. [DOI] [PubMed] [Google Scholar]
- 430.Mortola JF, Yen SS. The effects of oral dehydroepiandrosterone on endocrine-metabolic parameters in postmenopausal women. J Clin Endocrinol Metab 71: 696–704, 1990. [DOI] [PubMed] [Google Scholar]
- 431.Muller DN, Schmidt C, Barbosa-Sicard E, Wellner M, Gross V, Hercule H, Markovic M, Honeck H, Luft FC, Schunck WH. Mouse Cyp4a isoforms: Enzymatic properties, gender- and strain-specific expression, and role in renal 20-hydroxyeicosatetraenoic acid formation. Biochem J 403: 109–118, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Muller M, den Tonkelaar I, Thijssen JH, Grobbee DE, van der Schouw YT. Endogenous sex hormones in men aged 40-80 years. Eur J Endocrinol 149: 583–589, 2003. [DOI] [PubMed] [Google Scholar]
- 433.Muller M, van den Beld AW, van der Schouw YT, Grobbee DE, Lamberts SW. Effects of dehydroepiandrosterone and atamestane supplementation on frailty in elderly men. J Clin Endocrinol Metab 91: 3988–3991, 2006. [DOI] [PubMed] [Google Scholar]
- 434.Naharci MI, Pinar M, Bolu E, Olgun A. Effect of testosterone on insulin sensitivity in men with idiopathic hypogonadotropic hypogonadism. Endocr Pract 13: 629–635, 2007. [DOI] [PubMed] [Google Scholar]
- 435.Nair KS, Rizza RA, O’Brien P, Dhatariya K, Short KR, Nehra A, Vit-tone JL, Klee GG, Basu A, Basu R, Cobelli C, Toffolo G, Dalla Man C, Tindall DJ, Melton LJ 3rd, Smith GE, Khosla S, Jensen MD. DHEA in elderly women and DHEA or testosterone in elderly men. N Engl J Med 355: 1647–1659, 2006. [DOI] [PubMed] [Google Scholar]
- 436.Navarro-Penalver M, Perez-Martinez MT, Gomez-Bueno M, Garcia-Pavia P, Lupon-Roses J, Roig-Minguell E, Comin-Colet J, Bayes-Genis A, Noguera JA, Pascual-Figal DA. Testosterone replacement therapy in deficient patients with chronic heart failure: A randomized double-blind controlled pilot study. J Cardiovasc Pharmacol Ther 23: 543–550, 2018. [DOI] [PubMed] [Google Scholar]
- 437.Ng Tang Fui M, Prendergast LA, Dupuis P, Raval M, Strauss BJ, Zajac JD, Grossmann M. Effects of testosterone treatment on body fat and lean mass in obese men on a hypocaloric diet: A randomised controlled trial. BMC Med 14: 153, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Nguyen CP, Hirsch MS, Moeny D, Kaul S, Mohamoud M, Joffe HV. Testosterone and "Age-Related Hypogonadism" – FDA concerns. N Engl J Med 373: 689–691, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Nguyen PL, Je Y, Schutz FA, Hoffman KE, Hu JC, Parekh A, Beckman JA, Choueiri TK. Association of androgen deprivation therapy with cardiovascular death in patients with prostate cancer: A meta-analysis of randomized trials. JAMA 306: 2359–2366, 2011. [DOI] [PubMed] [Google Scholar]
- 440.Nie L, Wei D, Liu P, Zhang L, Fan K, Song Y, Wang M, Wang L, Xu Q, Wang J, Liu X, Li L, Mao Z, Huang H, Wang C, Huo W. C-Reactive protein mediates the effect of serum progesterone on obesity for men and postmenopausal women in henan rural cohort study. J Inflamm Res 14: 633–644, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Nieschlag E, Vorona E. Doping with anabolic androgenic steroids (AAS): Adverse effects on non-reproductive organs and functions. Rev Endocr Metab Disord 16: 199–211, 2015. [DOI] [PubMed] [Google Scholar]
- 442.Nilsson SE, Fransson E, Brismar K. Relationship between serum progesterone concentrations and cardiovascular disease, diabetes, and mortality in elderly Swedish men and women: An 8-year prospective study. Gend Med 6: 433–443, 2009. [DOI] [PubMed] [Google Scholar]
- 443.Ochi M, Kohara K, Tabara Y, Kido T, Uetani E, Ochi N, Igase M, Miki T. Arterial stiffness is associated with low thigh muscle mass in middle-aged to elderly men. Atherosclerosis 212: 327–332, 2010. [DOI] [PubMed] [Google Scholar]
- 444.Oettel M, Mukhopadhyay AK. Progesterone: The forgotten hormone in men? Aging Male 7: 236–257, 2004. [DOI] [PubMed] [Google Scholar]
- 445.Oh JY, Barrett-Connor E, Wedick NM, Wingard DL, Rancho Bernardo S.Endogenous sex hormones and the development of type 2 diabetes in older men and women: The Rancho Bernardo study. Diabetes Care 25: 55–60, 2002. [DOI] [PubMed] [Google Scholar]
- 446.Ohlsson C, Barrett-Connor E, Bhasin S, Orwoll E, Labrie F, Karlsson MK, Ljunggren O, Vandenput L, Mellstrom D, Tivesten A. High serum testosterone is associated with reduced risk of cardiovascular events in elderly men. The MrOS (Osteoporotic Fractures in Men) study in Sweden. J Am Coll Cardiol 58: 1674–1681, 2011. [DOI] [PubMed] [Google Scholar]
- 447.Ohlsson C, Vandenput L, Tivesten A. DHEA and mortality: What is the nature of the association? J Steroid Biochem Mol Biol 145: 248–253, 2015. [DOI] [PubMed] [Google Scholar]
- 448.Okorodudu D, Jumean M, Montori VM, Romero-Corral A, Somers V, Erwin P, Lopez-Jimenez F. Diagnostic performance of body mass index to identify obesity as defined by body adiposity: A systematic review and meta-analysis. Int J Obes (Lond) 34: 791–799, 2010. [DOI] [PubMed] [Google Scholar]
- 449.Orentreich N, Brind JL, Rizer RL, Vogelman JH. Age changes and sex differences in serum dehydroepiandrosterone sulfate concentra-tions throughout adulthood. J Clin Endocrinol Metab 59: 551–555, 1984. [DOI] [PubMed] [Google Scholar]
- 450.Ortega FB, Lee D-C, Katzmarzyk PT, Ruiz JR, Sui X, Church TS, Blair SN. The intriguing metabolically healthy but obese phenotype: Cardiovascular prognosis and role of fitness. Eur Heart J 34: 389–397, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Osadnik T, Pawlas N, Osadnik K, Bujak K, Goral M, Lejawa M, Fron-czek M, Regula R, Czarnecka H, Gawlita M, Strzelczyk JK, Gonera M, Gierlotka M, Polonski L, Gasior M. High progesterone levels are associated with family history of premature coronary artery disease in young healthy adult men. PLoS One 14: e0215302, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Padwal R, McAlister FA, McMurray JJ, Cowie M, Rich M, Pocock S, Swedberg K, Maggioni A, Gamble G, Ariti C. The obesity paradox in heart failure patients with preserved versus reduced ejection fraction: A meta-analysis of individual patient data. Int J Obes (Lond) 38: 1110–1114, 2014. [DOI] [PubMed] [Google Scholar]
- 453.Page JH, Ma J, Rexrode KM, Rifai N, Manson JE, Hankinson SE. Plasma dehydroepiandrosterone and risk of myocardial infarction in women. Clin Chem 54: 1190–1196, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Palmer BF, Clegg DJ. The sexual dimorphism of obesity. Mol Cell Endocrinol 402: 113–119, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Pandey A, LaMonte M, Klein L, Ayers C, Psaty BM, Eaton CB, Allen NB, de Lemos JA, Carnethon M, Greenland P. Relationship between physical activity, body mass index, and risk of heart failure. JAmColl Cardiol 69: 1129–1142, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Panjari M, Bell RJ, Jane F, Adams J, Morrow C, Davis SR. The safety of 52 weeks of oral DHEA therapy for postmenopausal women. Maturitas 63: 240–245, 2009. [DOI] [PubMed] [Google Scholar]
- 457.Panjari M, Bell RJ, Jane F, Wolfe R, Adams J, Morrow C, Davis SR. A randomized trial of oral DHEA treatment for sexual function, well-being, and menopausal symptoms in postmenopausal women with low libido. JSex Med 6: 2579–2590, 2009. [DOI] [PubMed] [Google Scholar]
- 458.Pantalone KM, George J, Ji X, Kattan MW, Milinovich A, Bauman JM, Burguera B, Zimmerman RS, Misra-Hebert AD. Testosterone replacement therapy and the risk of adverse cardiovascular outcomes and mortality. Basic Clin Androl 29: 5, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Paoli A, Mancin L, Giacona MC, Bianco A, Caprio M. Effects of a ketogenic diet in overweight women with polycystic ovary syndrome. JTranslMed 18: 104, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Paradisi G, Steinberg HO, Hempfling A, Cronin J, Hook G, Shepard MK, Baron AD. Polycystic ovary syndrome is associated with endothelial dysfunction. Circulation 103: 1410–1415, 2001. [DOI] [PubMed] [Google Scholar]
- 461.Park SH, Park JH, Song PS, Kim DK, Kim KH, Seol SH, Kim HK, Jang HJ, Lee JG, Park HY. Sarcopenic obesity as an independent risk factor of hypertension. J Am Soc Hypertens 7: 420–425, 2013. [DOI] [PubMed] [Google Scholar]
- 462.Pasquali R, Casimirri F, Labate AM, Tortelli O, Pascal G, Anconetani B, Gatto MR, Flamia R, Capelli M, Barbara L. Body weight, fat distribution and the menopausal status in women. The VMH Collaborative Group. Int J Obes Relat Metab Disord 18: 614–621, 1994. [PubMed] [Google Scholar]
- 463.Pasquali R, Casimirri F, Melchionda N, Fabbri R, Capelli M, Plate L, Patrono D, Balestra V, Barbara L. Weight loss and sex steroid metabolism in massively obese man. J Endocrinol Invest 11: 205–210, 1988. [DOI] [PubMed] [Google Scholar]
- 464.Patel SA, Winkel M, Ali MK, Narayan KM, Mehta NK. Cardiovascular mortality associated with 5 leading risk factors: National and state preventable fractions estimated from survey data. Ann Intern Med 163:245–253, 2015. [DOI] [PubMed] [Google Scholar]
- 465.Peppa M, Koliaki C, Hadjidakis DI, Garoflos E, Papaefstathiou A, Katsilambros N, Raptis SA, Dimitriadis GD. Regional fat distribution and cardiometabolic risk in healthy postmenopausal women. Eur J Intern Med 24: 824–831, 2013. [DOI] [PubMed] [Google Scholar]
- 466.Percheron G, Hogrel JY, Denot-Ledunois S, Fayet G, Forette F, Baulieu EE, Fardeau M, Marini JF, Double-blind placebo-controlled trial. Effect of 1-year oral administration of dehydroepiandrosterone to 60- to 80-year-old individuals on muscle function and cross-sectional area: A double-blind placebo-controlled trial. Arch Intern Med 163: 720–727, 2003. [DOI] [PubMed] [Google Scholar]
- 467.Perez-Lopez FR, Larrad-Mur L, Kallen A, Chedraui P, Taylor HS. Gender differences in cardiovascular disease: Hormonal and biochemical influences. Reprod Sci 17: 511–531, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Perry RJ, Samuel VT, Petersen KF, Shulman GI. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature 510:84–91, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Peters SA, Huxley RR, Woodward M. Diabetes as risk factor for incident coronary heart disease in women compared with men: A systematic review and meta-analysis of 64 cohorts including 858,507 individuals and 28,203 coronary events. Diabetologia 57: 1542–1551, 2014. [DOI] [PubMed] [Google Scholar]
- 470.Petrov G, Dworatzek E, Schulze TM, Dandel M, Kararigas G, Mah-moodzadeh S, Knosalla C, Hetzer R, Regitz-Zagrosek V. Maladap-tive remodeling is associated with impaired survival in women but not in men after aortic valve replacement. JACC Cardiovasc Imaging 7: 1073–1080, 2014. [DOI] [PubMed] [Google Scholar]
- 471.Phillips AC, Carroll D, Gale CR, Lord JM, Arlt W, Batty GD. Cortisol, DHEA sulphate, their ratio, and all-cause and cause-specific mortality in the Vietnam Experience Study. Eur J Endocrinol 163: 285–292, 2010. [DOI] [PubMed] [Google Scholar]
- 472.Phillips LS, Langer RD. Postmenopausal hormone therapy: Critical reappraisal and a unified hypothesis. Fertil Steril 83: 558–566, 2005. [DOI] [PubMed] [Google Scholar]
- 473.Piette P. The history of natural progesterone, the never-ending story. Climacteric 21: 308–314, 2018. [DOI] [PubMed] [Google Scholar]
- 474.Pischon T, Boeing H, Hoffmann K, Bergmann M, Schulze MB, Over-vad K, Van der Schouw Y, Spencer E, Moons K, Tjønneland A. General and abdominal adiposity and risk of death in Europe. N Engl J Med 359: 2105–2120, 2008. [DOI] [PubMed] [Google Scholar]
- 475.Pravednikova AE, Shevchenko SY, Kerchev VV, Skhirtladze MR, Larina SN, Kachaev ZM, Egorov AD, Shidlovskii YV. Association of uncoupling protein (Ucp) gene polymorphisms with cardiometabolic diseases. Mol Med 26: 51, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Primeau V, Coderre L, Karelis A, Brochu M, Lavoie M, Messier V, Sladek R, Rabasa-Lhoret R. Characterizing the profile of obese patients who are metabolically healthy. Int J Obes (Lond) 35: 971–981, 2011. [DOI] [PubMed] [Google Scholar]
- 477.Prior JC. Progesterone for treatment of symptomatic menopausal women. Climacteric 21: 358–365, 2018. [DOI] [PubMed] [Google Scholar]
- 478.Pritchard J, Despres JP, Gagnon J, Tchernof A, Nadeau A, Tremblay A, Bouchard C. Plasma adrenal, gonadal, and conjugated steroids before and after long-term overfeeding in identical twins. J Clin Endocrinol Metab 83: 3277–3284, 1998. [DOI] [PubMed] [Google Scholar]
- 479.Prospective Studies Collaboration. Body-mass index and cause-specific mortality in 900 000 adults: Collaborative analyses of 57 prospective studies. Lancet 373: 1083–1096, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Purnell JQ, Bland LB, Garzotto M, Lemmon D, Wersinger EM, Ryan CW, Brunzell JD, Beer TM. Effects of transdermal estrogen on levels of lipids, lipase activity, and inflammatory markers in men with prostate cancer. J Lipid Res 47: 349–355, 2006. [DOI] [PubMed] [Google Scholar]
- 481.Qaseem A, Horwitch CA, Vijan S, Etxeandia-Ikobaltzeta I, Kansagara D, Clinical Guidelines Committee of the American College of Physicians. Testosterone treatment in adult men with age-related low testosterone: A clinical guideline from the American College of Physicians. Ann Intern Med 172: 126–133, 2020. [DOI] [PubMed] [Google Scholar]
- 482.Qin Y, Santos HO, Khani V, Tan SC, Zhi Y. Effects of dehydroepiandrosterone (DHEA) supplementation on the lipid profile: A systematic review and dose-response meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis 30: 1465–1475, 2020. [DOI] [PubMed] [Google Scholar]
- 483.Raiko J, Orava J, Savisto N, Virtanen KA. High brown fat activity correlates with cardiovascular risk factor levels cross-sectionally and subclinical atherosclerosis at 5-year follow-up. Arterioscler Thromb Vasc Biol 40: 1289–1295, 2020. [DOI] [PubMed] [Google Scholar]
- 484.Rallidis LS, Kotakos C, Tsalavoutas S, Katsimardos A, Drosatos A, Rallidi M, Moustaka E, Zolindaki M. Low serum free testosterone association with cardiovascular mortality in men with stable CAD. J Am Coll Cardiol 72: 2674–2675, 2018. [DOI] [PubMed] [Google Scholar]
- 485.Rao LG. Urinary steroid-excretion patterns after acute myocardial infarction. Lancet 2: 390–391, 1970. [DOI] [PubMed] [Google Scholar]
- 486.Ravaglia G, Forti P, Maioli F, Boschi F, Bernardi M, Pratelli L, Pizzo-ferrato A, Gasbarrini G. The relationship of dehydroepiandrosterone sulfate (DHEAS) to endocrine-metabolic parameters and functional status in the oldest-old. Results from an Italian study on healthy free-living over-ninety-year-olds. J Clin Endocrinol Metab 81: 1173–1178, 1996. [DOI] [PubMed] [Google Scholar]
- 487.Reis JP, Macera CA, Araneta MR, Lindsay SP, Marshall SJ, Wingard DL.Comparison of overall obesity and body fat distribution in predicting risk of mortality. Obesity (Silver Spring) 17: 1232–1239, 2009. [DOI] [PubMed] [Google Scholar]
- 488.Reis LO, Zani EL, Garcia-Perdomo HA. Estrogen therapy in patients with prostate cancer: A contemporary systematic review. Int Urol Nephrol 50: 993–1003, 2018. [DOI] [PubMed] [Google Scholar]
- 489.Reslan OM, Khalil RA. Vascular effects of estrogenic menopausal hormone therapy. Rev Recent Clin Trials 7: 47–70, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Rey-Lopez J, De Rezende L, Pastor-Valero M, Tess B. The prevalence of metabolically healthy obesity: A systematic review and critical evaluation of the definitions used. Obes Rev 15: 781–790, 2014. [DOI] [PubMed] [Google Scholar]
- 491.Richardson MR. Current perspectives in polycystic ovary syndrome. Am Fam Physician 68: 697–704, 2003. [PubMed] [Google Scholar]
- 492.Rijpkema AH, van der Sanden AA, Ruijs AH. Effects of post-menopausal oestrogen-progestogen replacement therapy on serum lipids and lipoproteins: A review. Maturitas 12: 259–285, 1990. [DOI] [PubMed] [Google Scholar]
- 493.Rochira V, Faustini-Fustini M, Balestrieri A, Carani C. Estrogen replacement therapy in a man with congenital aromatase deficiency: Effects of different doses of transdermal estradiol on bone mineral density and hormonal parameters. J Clin Endocrinol Metab 85: 1841–1845, 2000. [DOI] [PubMed] [Google Scholar]
- 494.Roelfsema F, Yang RJ, Veldhuis JD. Estradiol does not influence lipid measures and inflammatory markers in testosterone-clamped healthy men. J Endocr Soc 2: 882–892, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Roetker NS, MacLehose RF, Hoogeveen RC, Ballantyne CM, Basu S, Cushman M, Folsom AR. Prospective study of endogenous hormones and incidence of venous thromboembolism: The Atherosclerosis Risk in Communities Study. Thromb Haemost 118: 1940–1950, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Romero-Corral A, Somers VK, Sierra-Johnson J, Korenfeld Y, Boarin S, Korinek J, Jensen MD, Parati G, Lopez-Jimenez F. Normal weight obesity: A risk factor for cardiometabolic dysregulation and cardio-vascular mortality. Eur Heart J 31: 737–746, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.Rosendale N, Goldman S, Ortiz GM, Haber LA. Acute clinical care for transgender patients: A review. JAMA Intern Med 178: 1535–1543, 2018. [DOI] [PubMed] [Google Scholar]
- 498.Rossouw JE. Hormones, genetic factors, and gender differences in cardiovascular disease. Cardiovasc Res 53: 550–557, 2002. [DOI] [PubMed] [Google Scholar]
- 499.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J, Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results From the Women’s Health Initiative randomized controlled trial. JAMA 288: 321–333, 2002. [DOI] [PubMed] [Google Scholar]
- 500.Rost S, Freuer D, Peters A, Thorand B, Holle R, Linseisen J, Meisinger C. New indexes of body fat distribution and sex-specific risk of total and cause-specific mortality: A prospective cohort study. BMC Public Health 18: 427, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Rubanyi GM, Kauser K, Johns A. Role of estrogen receptors in the vascular system. Vascul Pharmacol 38: 81–88, 2002. [DOI] [PubMed] [Google Scholar]
- 502.Rubinow KB. Estrogens and body weight regulation in men. Adv Exp Med Biol 1043: 285–313, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Ruige JB, Mahmoud AM, De Bacquer D, Kaufman JM. Endogenous testosterone and cardiovascular disease in healthy men: A meta-analysis. Heart 97: 870–875, 2011. [DOI] [PubMed] [Google Scholar]
- 504.Ryan C, Morgans A. Addressing cardiovascular risk of prostate cancer hormonal therapy. JACC CardioOncol 2: 82–83, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Saad F, Doros G, Haider KS, Haider A. Hypogonadal men with moderate-to-severe lower urinary tract symptoms have a more severe cardiometabolic risk profile and benefit more from testosterone therapy than men with mild lower urinary tract symptoms. Investig Clin Urol 59: 399–409, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.Saad F, Haider A, Doros G, Traish A. Long-term treatment of hypogonadal men with testosterone produces substantial and sustained weight loss. Obesity (Silver Spring) 21: 1975–1981, 2013. [DOI] [PubMed] [Google Scholar]
- 507.Saad F, Yassin A, Doros G, Haider A. Effects of long-term treatment with testosterone on weight and waist size in 411 hypogonadal men with obesity classes I-III: Observational data from two registry studies. Int J Obes (Lond) 40: 162–170, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508.Sahakyan KR, Somers VK, Rodriguez-Escudero JP, Hodge DO, Carter RE, Sochor O, Coutinho T, Jensen MD, Roger VL, Singh P. Normal-weight central obesity: Implications for total and cardiovascular mortality. Ann Intern Med 163: 827–835, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Sandhu RK, Ezekowitz J, Andersson U, Alexander JH, Granger CB, Halvorsen S, Hanna M, Hijazi Z, Jansky P, Lopes RD. The ‘obesity paradox’in atrial fibrillation: Observations from the ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) trial. Eur Heart J 37: 2869–2878, 2016. [DOI] [PubMed] [Google Scholar]
- 510.Sanon VP, Handelsman Y, Pham SV, Chilton R. Cardiac manifestations of congenital generalized lipodystrophy. Clin Diabetes 34: 181–186, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Santoro N, Torrens J, Crawford S, Allsworth JE, Finkelstein JS, Gold EB, Korenman S, Lasley WL, Luborsky JL, McConnell D, Sowers MF, Weiss G. Correlates of circulating androgens in mid-life women: The study of women’s health across the nation. J Clin Endocrinol Metab 90: 4836–4845, 2005. [DOI] [PubMed] [Google Scholar]
- 512.Santosa S, Bonnes SL, Jensen MD. Acute female hypogonadism alters adipose tissue fatty acid storage factors and chylomicronemia. J Clin Endocrinol Metab 101: 2089–2098, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Sathyapalan T, Rigby AS, Bhasin S, Thatcher NJ, Kilpatrick ES, Atkin SL. Effect of soy in men with type 2 diabetes mellitus and subclinical hypogonadism: A randomized controlled study. J Clin Endocrinol Metab 102: 425–433, 2017. [DOI] [PubMed] [Google Scholar]
- 514.Saydah S, Bullard KM, Cheng Y, Ali MK, Gregg EW, Geiss L, Imperatore G. Trends in cardiovascular disease risk factors by obesity level in adults in the United States, NHANES 1999-2010. Obesity (Silver Spring) 22: 1888–1895, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515.Schaffrath G, Kische H, Gross S, Wallaschofski H, Volzke H, Dorr M, Nauck M, Keevil BG, Brabant G, Haring R. Association of sex hormones with incident 10-year cardiovascular disease and mortality in women. Maturitas 82: 424–430, 2015. [DOI] [PubMed] [Google Scholar]
- 516.Schneider G, Kirschner MA, Berkowitz R, Ertel NH. Increased estro-gen production in obese men. J Clin Endocrinol Metab 48: 633–638, 1979. [DOI] [PubMed] [Google Scholar]
- 517.Schnohr P, Jensen JS, Scharling H, Nordestgaard BG. Coronary heart disease risk factors ranked by importance for the individual and community. A 21 year follow-up of 12 000 men and women from The Copenhagen City Heart Study. Eur Heart J 23: 620–626, 2002. [DOI] [PubMed] [Google Scholar]
- 518.Schwann TA, Ramia PS, Engoren MC, Bonnell MR, Goodwin M, Monroe I, Habib RH. Evidence and temporality of the obesity paradox in coronary bypass surgery: An analysis of cause-specific mortality. Eur J Cardiothorac Surg 54: 896–903, 2018. [DOI] [PubMed] [Google Scholar]
- 519.Schwartz MW, Seeley RJ, Zeltser LM, Drewnowski A, Ravussin E, Redman LM, Leibel RL. Obesity pathogenesis: An Endocrine Society scientific statement. Endocr Rev 38: 267–296, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520.Seravalle G, Grassi G. Obesity and hypertension. Pharmacol Res 122:1–7, 2017. [DOI] [PubMed] [Google Scholar]
- 521.Seruga B, Zadnik V, Kuhar CG, Marinko T, Cufer T, Zakotnik B, Zorman D, Ocana A, Amir E. Association of aromatase inhibitors with coronary heart disease in women with early breast cancer. Cancer Invest 32: 99–104, 2014. [DOI] [PubMed] [Google Scholar]
- 522.Shackleton CH, Roitman E, Phillips A, Chang T. Androstanediol and 5-androstenediol profiling for detecting exogenously administered dihydrotestosterone, epitestosterone, and dehydroepiandrosterone: Potential use in gas chromatography isotope ratio mass spectrometry. Steroids 62: 665–673, 1997. [DOI] [PubMed] [Google Scholar]
- 523.Shah RV, Murthy VL, Abbasi SA, Blankstein R, Kwong RY, Goldfine AB, Jerosch-Herold M, Lima JA, Ding J, Allison MA. Visceral adiposity and the risk of metabolic syndrome across body mass index: The MESA Study. JACC Cardiovasc Imaging 7: 1221–1235, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Shea J, King M, Yi Y, Gulliver W, Sun G. Body fat percentage is associated with cardiometabolic dysregulation in BMI-defined normal weight subjects. Nutr Metab Cardiovasc Dis 22: 741–747, 2012. [DOI] [PubMed] [Google Scholar]
- 525.Shen W, Punyanitya M, Silva AM, Chen J, Gallagher D, Sardinha LB, Allison DB, Heymsfield SB. Sexual dimorphism of adipose tissue dis-tribution across the lifespan: A cross-sectional whole-body magnetic resonance imaging study. Nutr Metab (Lond) 6: 17, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526.Sherwood A, Bower JK, McFetridge-Durdle J, Blumenthal JA, Newby LK, Hinderliter AL. Age moderates the short-term effects of trans-dermal 17beta-estradiol on endothelium-dependent vascular function in postmenopausal women. Arterioscler Thromb Vasc Biol 27: 1782–1787, 2007. [DOI] [PubMed] [Google Scholar]
- 527.Shin M-J, Hyun Y, Kim O, Kim J, Jang Y, Lee J. Weight loss effect on inflammation and LDL oxidation in metabolically healthy but obese (MHO) individuals: Low inflammation and LDL oxidation in MHO women. Int J Obes (Lond) 30: 1529–1534, 2006. [DOI] [PubMed] [Google Scholar]
- 528.Shores MM, Matsumoto AM. Testosterone, aging and survival: Biomarker or deficiency. Curr Opin Endocrinol Diabetes Obes 21:209–216, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529.Shores MM, Smith NL, Forsberg CW, Anawalt BD, Matsumoto AM. Testosterone treatment and mortality in men with low testosterone lev-els. J Clin Endocrinol Metab 97: 2050–2058, 2012. [DOI] [PubMed] [Google Scholar]
- 530.Shufelt C, Bretsky P, Almeida CM, Johnson BD, Shaw LJ, Azziz R, Braunstein GD, Pepine CJ, Bittner V, Vido DA, Stanczyk FZ, Bairey Merz CN. DHEA-S levels and cardiovascular disease mortality in postmenopausal women: Results from the National Institutes of Health—National Heart, Lung, and Blood Institute (NHLBI)-sponsored Women’s Ischemia Syndrome Evaluation (WISE). J Clin Endocrinol Metab 95: 4985–4992, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 531.Sidney S, Quesenberry CP Jr, Jaffe MG, Sorel M, Nguyen-Huynh MN, Kushi LH, Go AS, Rana JS. Recent trends in cardiovascular mortality in the United States and public health goals. JAMA Cardiol 1: 594–599, 2016. [DOI] [PubMed] [Google Scholar]
- 532.Sievers C, Klotsche J, Pieper L, Schneider HJ, Marz W, Wittchen HU, Stalla GK, Mantzoros C. Low testosterone levels predict all-cause mortality and cardiovascular events in women: A prospective cohort study in German primary care patients. Eur J Endocrinol 163: 699–708, 2010. [DOI] [PubMed] [Google Scholar]
- 533.Silva AM, Shen W, Heo M, Gallagher D, Wang Z, Sardinha LB, Heymsfield SB. Ethnicity-related skeletal muscle differences across the lifespan. Am J Hum Biol 22: 76–82, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534.Simpson ER, Zhao Y, Agarwal VR, Michael MD, Bulun SE, Hinshel-wood MM, Graham-Lorence S, Sun T, Fisher CR, Qin K, Mendelson CR. Aromatase expression in health and disease. Recent Prog Horm Res 52: 185–213; discussion 213-184, 1997. [PubMed] [Google Scholar]
- 535.Sims J, Lutz E, Wallace K, Kassahun-Yimer W, Ngwudike C, Shwayder J. Depo-medroxyprogesterone acetate, weight gain and amenorrhea among obese adolescent and adult women. Eur J Contracept Reprod Health Care 25: 54–59, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536.Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B, Williams TC, Lubahn DB, Korach KS. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. NEnglJMed 331: 1056–1061, 1994. [DOI] [PubMed] [Google Scholar]
- 537.Snyder PJ, Ellenberg SS, Farrar JT. Testosterone treatment in older men. N Engl J Med 375: 90, 2016. [DOI] [PubMed] [Google Scholar]
- 538.Snyder PJ, Peachey H, Hannoush P, Berlin JA, Loh L, Lenrow DA, Holmes JH, Dlewati A, Santanna J, Rosen CJ, Strom BL. Effect of testosterone treatment on body composition and muscle strength in menover65yearsofage. J Clin Endocrinol Metab 84: 2647–2653, 1999. [DOI] [PubMed] [Google Scholar]
- 539.Soares AH, Horie NC, Chiang LAP, Caramelli B, Matheus MG, Campos AH, Marti LC, Rocha FA, Mancini MC, Costa EMF, Cercato C. Effects of clomiphene citrate on male obesity-associated hypogonadism: A randomized, double-blind, placebo-controlled study. Int J Obes (Lond) 42: 953–963, 2018. [DOI] [PubMed] [Google Scholar]
- 540.Song X, Jousilahti P, Stehouwer C, Söderberg S, Onat A, Laatikainen T, Yudkin J, Dankner R, Morris R, Tuomilehto J. Cardiovascular and all-cause mortality in relation to various anthropometric measures of obesity in Europeans. Nutr Metab Cardiovasc Dis 25: 295–304, 2015. [DOI] [PubMed] [Google Scholar]
- 541.Soufir JC, Meduri G, Ziyyat A. Spermatogenetic inhibition in men taking a combination of oral medroxyprogesterone acetate and per-cutaneous testosterone as a male contraceptive method. Hum Reprod 26: 1708–1714, 2011. [DOI] [PubMed] [Google Scholar]
- 542.Spanos C, Bretherton I, Zajac JD, Cheung AS. Effects of gender-affirming hormone therapy on insulin resistance and body composition in transgender individuals: A systematic review. World J Diabetes 11: 66–77, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 543.Spearman JV, Renker M, Schoepf UJ, Krazinski AW, Herbert TL, De Cecco CN, Nietert PJ, Meinel FG. Prognostic value of epicardial fat volume measurements by computed tomography: A systematic review of the literature. Eur Radiol 25: 3372–3381, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544.Sprague BL, Trentham-Dietz A, Cronin KA. A sustained decline in postmenopausal hormone use: Results from the National Health and Nutrition Examination Survey, 1999-2010. Obstet Gynecol 120: 595–603, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 545.Srikanthan P, Hevener AL, Karlamangla AS. Sarcopenia exacerbates obesity-associated insulin resistance and dysglycemia: Findings from the National Health and Nutrition Examination Survey III. PLoS One 5: e10805, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 546.Srinath R, Gottesman RF, Hill Golden S, Carson KA, Dobs A. Association between endogenous testosterone and cerebrovascular disease in the ARIC study (Atherosclerosis Risk in Communities). Stroke 47: 2682–2688, 2016. [DOI] [PubMed] [Google Scholar]
- 547.Srinivas-Shankar U, Roberts SA, Connolly MJ, O’Connell MD, Adams JE, Oldham JA, Wu FC. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: A randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab 95: 639–650, 2010. [DOI] [PubMed] [Google Scholar]
- 548.Staessen JA, van der Heijden-Spek JJ, Safar ME, Den Hond E, Gasowski J, Fagard RH, Wang JG, Boudier HA, Van Bortel LM. Menopause and the characteristics of the large arteries in a population study. J Hum Hypertens 15: 511–518, 2001. [DOI] [PubMed] [Google Scholar]
- 549.Stampfer MJ, Colditz GA. Estrogen replacement therapy and coronary heart disease: A quantitative assessment of the epidemiologic evidence. Prev Med 20: 47–63, 1991. [DOI] [PubMed] [Google Scholar]
- 550.Stampfer MJ, Willett WC, Colditz GA, Rosner B, Speizer FE, Hennekens CH. A prospective study of postmenopausal estrogen therapy and coronary heart disease. N Engl J Med 313: 1044–1049, 1985. [DOI] [PubMed] [Google Scholar]
- 551.Stanik S, Dornfeld LP, Maxwell MH, Viosca SP, Korenman SG. The effect of weight loss on reproductive hormones in obese men. J Clin Endocrinol Metab 53: 828–832, 1981. [DOI] [PubMed] [Google Scholar]
- 552.Starka L, Hill M, Pospisilova H, Duskova M. Estradiol, obesity and hypogonadism. Physiol Res 69: S273–S278, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553.Stefan N, Kantartzis K, Machann J, Schick F, Thamer C, Rittig K, Balletshofer B, Machicao F, Fritsche A, Häring H-U. Identification and characterization of metabolically benign obesity in humans. Arch Intern Med 168: 1609–1616, 2008. [DOI] [PubMed] [Google Scholar]
- 554.Steidle C, Schwartz S, Jacoby K, Sebree T, Smith T, Bachand R, North American AA2500 T Gel Study Group. AA2500 testosterone gel normalizes androgen levels in aging males with improvements in body composition and sexual function. J Clin Endocrinol Metab 88: 2673–2681, 2003. [DOI] [PubMed] [Google Scholar]
- 555.Steinberg KK, Thacker SB, Smith SJ, Stroup DF, Zack MM, Flanders WD, Berkelman RL. A meta-analysis of the effect of estrogen replacement therapy on the risk of breast cancer. JAMA 265: 1985–1990, 1991. [PubMed] [Google Scholar]
- 556.Sternfeld B, Wang H, Quesenberry CP Jr, Abrams B, Everson-Rose SA, Greendale GA, Matthews KA, Torrens JI, Sowers M. Physical activity and changes in weight and waist circumference in midlife women: Findings from the Study of Women’s Health Across the Nation. Am J Epidemiol 160: 912–922, 2004. [DOI] [PubMed] [Google Scholar]
- 557.Stevens J, Erber E, Truesdale KP, Wang C-H, Cai J. Long-and short-term weight change and incident coronary heart disease and ischemic stroke: The Atherosclerosis Risk in Communities Study. Am J Epidemiol 178: 239–248, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 558.Storer TW, Miciek R, Travison TG. Muscle function, physical performance and body composition changes in men with prostate cancer undergoing androgen deprivation therapy. Asian J Androl 14: 204–221, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 559.Strain GW, Zumoff B, Kream J, Strain JJ, Deucher R, Rosenfeld RS, Levin J, Fukushima DK. Mild Hypogonadotropic hypogonadism in obese men. Metabolism 31: 871–875, 1982. [DOI] [PubMed] [Google Scholar]
- 560.Streed CG Jr, Harfouch O, Marvel F, Blumenthal RS, Martin SS, Mukherjee M. Cardiovascular disease among transgender adults receiving hormone therapy: A narrative review. Ann Intern Med 167:256–267, 2017. [DOI] [PubMed] [Google Scholar]
- 561.Sudhir K, Chou TM, Chatterjee K, Smith EP, Williams TC, Kane JP, Malloy MJ, Korach KS, Rubanyi GM. Premature coronary artery dis-ease associated with a disruptive mutation in the estrogen receptor gene in a man. Circulation 96: 3774–3777, 1997. [DOI] [PubMed] [Google Scholar]
- 562.Sudhir K, Chou TM, Messina LM, Hutchison SJ, Korach KS, Chatterjee K, Rubanyi GM. Endothelial dysfunction in a man with disruptive mutation in oestrogen-receptor gene. Lancet 349: 1146–1147, 1997. [DOI] [PubMed] [Google Scholar]
- 563.Sultan S, Patel AG, El-Hassani S, Whitelaw B, Leca BM, Vincent RP, le Roux CW, Rubino F, Aywlin SJB, Dimitriadis GK. Male obesity associated gonadal dysfunction and the role of bariatric surgery. Front Endocrinol (Lausanne) 11: 408, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 564.Sun Y, Liu B, Snetselaar LG, Wallace RB, Caan BJ, Rohan TE, Neuhouser ML, Shadyab AH, Chlebowski RT, Manson JE. Association of normal-weight central obesity with all-cause and cause-specific mortality among postmenopausal women. JAMA Netw Open 2: e197337, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 565.Sutton-Tyrrell K, Wildman RP, Matthews KA, Chae C, Lasley BL, Brockwell S, Pasternak RC, Lloyd-Jones D, Sowers MF, Torrens JI, Investigators S. Sex-hormone-binding globulin and the free andro-gen index are related to cardiovascular risk factors in multiethnic premenopausal and perimenopausal women enrolled in the Study of Women Across the Nation (SWAN). Circulation 111: 1242–1249, 2005. [DOI] [PubMed] [Google Scholar]
- 566.Svartberg J, Agledahl I, Figenschau Y, Sildnes T, Waterloo K, Jorde R. Testosterone treatment in elderly men with subnormal testosterone levels improves body composition and BMD in the hip. Int J Impot Res 20: 378–387, 2008. [DOI] [PubMed] [Google Scholar]
- 567.Svartberg J, von Muhlen D, Sundsfjord J, Jorde R. Waist circumference and testosterone levels in community dwelling men. The Tromso study. Eur J Epidemiol 19: 657–663, 2004. [DOI] [PubMed] [Google Scholar]
- 568.Szulc P, Duboeuf F, Marchand F, Delmas PD. Hormonal and lifestyle determinants of appendicular skeletal muscle mass in men: The MINOS study. Am J Clin Nutr 80: 496–503, 2004. [DOI] [PubMed] [Google Scholar]
- 569.Tajar A, Forti G, O’Neill TW, Lee DM, Silman AJ, Finn JD, Bartfai G, Boonen S, Casanueva FF, Giwercman A, Han TS, Kula K, Labrie F, Lean ME, Pendleton N, Punab M, Vanderschueren D, Huhtaniemi IT, Wu FC, EMAS Group. Characteristics of secondary, primary, and compensated hypogonadism in aging men: Evidence from the Euro-pean Male Ageing Study. J Clin Endocrinol Metab 95: 1810–1818, 2010. [DOI] [PubMed] [Google Scholar]
- 570.Taraborrelli S. Physiology, production and action of progesterone. Acta Obstet Gynecol Scand 94 Suppl 161: 8–16, 2015. [DOI] [PubMed] [Google Scholar]
- 571.Tchernof A, Despres JP, Belanger A, Dupont A, Prud’homme D, Moorjani S, Lupien PJ, Labrie F. Reduced testosterone and adrenal C19 steroid levels in obese men. Metabolism 44: 513–519, 1995. [DOI] [PubMed] [Google Scholar]
- 572.Tchernof A, Labrie F. Dehydroepiandrosterone, obesity and cardio-vascular disease risk: A review of human studies. Eur J Endocrinol 151: 1–14, 2004. [DOI] [PubMed] [Google Scholar]
- 573.Teede HJ, Dalais FS, Kotsopoulos D, Liang YL, Davis S, McGrath BP. Dietary soy has both beneficial and potentially adverse cardiovascular effects: A placebo-controlled study in men and postmenopausal women. J Clin Endocrinol Metab 86: 3053–3060, 2001. [DOI] [PubMed] [Google Scholar]
- 574.Tepper NK, Curtis KM, Jatlaoui TC, Whiteman MK. Updated guidance for safe and effective use of contraception. J Womens Health (Larchmt) 25: 1097–1101, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 575.Tepper PG, Randolph JF Jr, McConnell DS, Crawford SL, El Khoudary SR, Joffe H, Gold EB, Zheng H, Bromberger JT, Sutton-Tyrrell K. Trajectory clustering of estradiol and follicle-stimulating hormone during the menopausal transition among women in the Study of Women’s Health across the Nation (SWAN). J Clin Endocrinol Metab 97: 2872–2880, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 576.The Writing Group for the PEPI Trial. Effects of estrogen or estro-gen/progestin regimens on heart disease risk factors in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. The Writing Group for the PEPI Trial. JAMA 273: 199–208, 1995. [PubMed] [Google Scholar]
- 577.Thijs L, Fagard R, Forette F, Nawrot T, Staessen JA. Are low dehydroepiandrosterone sulphate levels predictive for cardiovascular dis-eases? A review of prospective and retrospective studies. Acta Cardiol 58: 403–410, 2003. [DOI] [PubMed] [Google Scholar]
- 578.Thirumalai A, Rubinow KB, Cooper LA, Amory JK, Marck BT, Matsumoto AM, Page ST. Dose-response effects of sex hormone concentrations on body composition and adipokines in medically castrated healthy men administered graded doses of testosterone gel. Clin Endocrinol (Oxf) 87: 59–67, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 579.Thomsen M, Nordestgaard BG. Myocardial infarction and ischemic heart disease in overweight and obesity with and without metabolic syndrome. JAMA Intern Med 174: 15–22, 2014. [DOI] [PubMed] [Google Scholar]
- 580.Tivesten A, Vandenput L, Labrie F, Karlsson MK, Ljunggren O, Mellstrom D, Ohlsson C. Low serum testosterone and estradiol predict mortality in elderly men. J Clin Endocrinol Metab 94: 2482–2488, 2009. [DOI] [PubMed] [Google Scholar]
- 581.Toma M, McAlister FA, Coglianese EE, Vidi V, Vasaiwala S, Bakal JA, Armstrong PW, Ezekowitz JA. Testosterone supplementation in heart failure: A meta-analysis. Circ Heart Fail 5: 315–321, 2012. [DOI] [PubMed] [Google Scholar]
- 582.Toss F, Wiklund P, Nordström P, Nordström A. Body composition and mortality risk in later life. Age and ageing 41: 677–681, 2012. [DOI] [PubMed] [Google Scholar]
- 583.Traish AM, Haider A, Doros G, Saad F. Long-term testosterone therapy in hypogonadal men ameliorates elements of the metabolic syn-drome: An observational, long-term registry study. IntJClin Pract 68:314–329, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 584.Traish AM, Haider A, Haider KS, Doros G, Saad F. Long-term testosterone therapy improves cardiometabolic function and reduces risk of cardiovascular disease in men with hypogonadism: A real-life observational registry study setting comparing treated and untreated (control) groups. J Cardiovasc Pharmacol Ther 22: 414–433, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 585.Travison TG, Araujo AB, Kupelian V, O’Donnell AB, McKinlay JB. The relative contributions of aging, health, and lifestyle factors to serum testosterone decline in men. J Clin Endocrinol Metab 92:549–555, 2007. [DOI] [PubMed] [Google Scholar]
- 586.Trivedi DP, Khaw KT. Dehydroepiandrosterone sulfate and mortality in elderly men and women. J Clin Endocrinol Metab 86: 4171–4177, 2001. [DOI] [PubMed] [Google Scholar]
- 587.Tsai EC, Boyko EJ, Leonetti DL, Fujimoto WY. Low serum testosterone level as a predictor of increased visceral fat in Japanese-American men. Int J Obes Relat Metab Disord 24: 485–491, 2000. [DOI] [PubMed] [Google Scholar]
- 588.Tsujimoto T, Kajio H. Abdominal obesity is associated with an increased risk of all-cause mortality in patients with HFpEF. JAm Coll Cardiol 70: 2739–2749, 2017. [DOI] [PubMed] [Google Scholar]
- 589.van Hulsteijn LT, Pasquali R, Casanueva F, Haluzik M, Ledoux S, Monteiro MP, Salvador J, Santini F, Toplak H, Dekkers OM. Preva-lence of endocrine disorders in obese patients: Systematic review and meta-analysis. Eur J Endocrinol 182: 11–21, 2020. [DOI] [PubMed] [Google Scholar]
- 590.Van Pelt R, Evans E, Schechtman K, Ehsani A, Kohrt W. Contributions of total and regional fat mass to risk for cardiovascular disease in older women. Am J Physiol Endocrinol Metab 282: E1023–8, 2002. [DOI] [PubMed] [Google Scholar]
- 591.Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR Jr, Lee DH, Shioda T, Soto AM, vom Saal FS, Welshons WV, Zoeller RT, Myers JP. Hormones and endocrine-disrupting chemicals: Low-dose effects and nonmonotonic dose responses. Endocr Rev 33: 378–455, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 592.Vandenplas G, De Bacquer D, Calders P, Fiers T, Kaufman JM, Ouwens DM, Ruige JB. Endogenous oestradiol and cardiovascular disease in healthy men: A systematic review and meta-analysis of prospective studies. Heart 98: 1478–1482, 2012. [DOI] [PubMed] [Google Scholar]
- 593.Vaughan AS, Ritchey MD, Hannan J, Kramer MR, Casper M. Widespread recent increases in county-level heart disease mortality across age groups. Ann Epidemiol 27: 796–800, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 594.Vega GL, Adams-Huet B, Peshock R, Willett D, Shah B, Grundy SM. Influence of body fat content and distribution on variation in metabolic risk. J Clin Endocrinol Metab 91: 4459–4466, 2006. [DOI] [PubMed] [Google Scholar]
- 595.Vemmos K, Ntaios G, Spengos K, Savvari P, Vemmou A, Pappa T, Manios E, Georgiopoulos G, Alevizaki M. Association between obesity and mortality after acute first-ever stroke: The obesity–stroke para-dox. Stroke 42: 30–36, 2011. [DOI] [PubMed] [Google Scholar]
- 596.Verma S, Hussain ME. Obesity and diabetes: An update. Diabetes Metab Syndr Clin Res Rev 11: 73–79, 2017. [DOI] [PubMed] [Google Scholar]
- 597.Vermeulen A, Goemaere S, Kaufman JM. Testosterone, body composition and aging. J Endocrinol Invest 22: 110–116, 1999. [PubMed] [Google Scholar]
- 598.Vermeulen A, Kaufman JM, Giagulli VA. Influence of some biological indexes on sex hormone-binding globulin and androgen levels in aging or obese males. J Clin Endocrinol Metab 81: 1821–1826, 1996. [DOI] [PubMed] [Google Scholar]
- 599.Vermeulen A, Kaufman JM, Goemaere S, van Pottelberg I. Estradiol in elderly men. Aging Male 5: 98–102, 2002. [PubMed] [Google Scholar]
- 600.Vettor R, De Pergola G, Pagano C, Englaro P, Laudadio E, Giorgino F, Blum WF, Giorgino R, Federspil G. Gender differences in serum leptin in obese people: Relationships with testosterone, body fat distribution and insulin sensitivity. Eur J Clin Invest 27: 1016–1024, 1997. [DOI] [PubMed] [Google Scholar]
- 601.Vigen R, O’Donnell CI, Baron AE, Grunwald GK, Maddox TM, Bradley SM, Barqawi A, Woning G, Wierman ME, Plomondon ME, Rumsfeld JS, Ho PM. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA 310: 1829–1836, 2013. [DOI] [PubMed] [Google Scholar]
- 602.Villareal DT, Holloszy JO. DHEA enhances effects of weight training on muscle mass and strength in elderly women and men. Am J Physiol Endocrinol Metab 291: E1003–E1008, 2006. [DOI] [PubMed] [Google Scholar]
- 603.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, Djousse L, MSV E, Ferguson JF, Fornage M, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Shay CM, Spartano NL, Stokes A, Tirschwell DL, VanWagner LB, Tsao CW, American Heart Association Council on Epidemiology, Prevention Statistics Committee, Stroke Statistics Subcommittee. Heart disease and stroke statistics-2020 update: A report from the American Heart Association. Circulation 141: e139–e596, 2020. [DOI] [PubMed] [Google Scholar]
- 604.Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerback S, Nuutila P. Functional brown adipose tissue in healthy adults. NEnglJMed 360: 1518–1525, 2009. [DOI] [PubMed] [Google Scholar]
- 605.Viscoli CM, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horwitz RI. A clinical trial of estrogen-replacement therapy after ischemic stroke. N Engl J Med 345: 1243–1249, 2001. [DOI] [PubMed] [Google Scholar]
- 606.Vitale C, Mercuro G, Cerquetani E, Marazzi G, Patrizi R, Pelliccia F, Volterrani M, Fini M, Collins P, Rosano GM. Time since menopause influences the acute and chronic effect of estrogens on endothelial function. Arterioscler Thromb Vasc Biol 28: 348–352, 2008. [DOI] [PubMed] [Google Scholar]
- 607.von Muhlen D, Laughlin GA, Kritz-Silverstein D, Bergstrom J, Bettencourt R. Effect of dehydroepiandrosterone supplementation on bone mineral density, bone markers, and body composition in older adults: The DAWN trial. Osteoporos Int 19: 699–707, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 608.Waddell TK, Dart AM, Gatzka CD, Cameron JD, Kingwell BA. Women exhibit a greater age-related increase in proximal aortic stiffness than men. J Hypertens 19: 2205–2212, 2001. [DOI] [PubMed] [Google Scholar]
- 609.Wanahita N, Messerli FH, Bangalore S, Gami AS, Somers VK, Stein-berg JS. Atrial fibrillation and obesity—results of a meta-analysis. Am Heart J 155: 310–315, 2008. [DOI] [PubMed] [Google Scholar]
- 610.Wang C, Swerdloff RS, Iranmanesh A, Dobs A, Snyder PJ, Cunning-ham G, Matsumoto AM, Weber T, Berman N, Testosterone Gel Study G. Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal men. J Clin Endocrinol Metab 85: 2839–2853, 2000. [DOI] [PubMed] [Google Scholar]
- 611.Wang L, Liu W, He X, Chen Y, Lu J, Liu K, Cao K, Yin P. Association of overweight and obesity with patient mortality after acute myocardial infarction: A meta-analysis of prospective studies. Int J Obes (Lond) 40: 220–228, 2016. [DOI] [PubMed] [Google Scholar]
- 612.Wang L, Szklo M, Folsom AR, Cook NR, Gapstur SM, Ouyang P. Endogenous sex hormones, blood pressure change, and risk of hypertension in postmenopausal women: The Multi-Ethnic Study of Atherosclerosis. Atherosclerosis 224: 228–234, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 613.Wang X, Feng H, Fan D, Zou G, Han Y, Liu L. The influence of dehydroepiandrosterone (DHEA) on fasting plasma glucose, insulin levels and insulin resistance (HOMA-IR) index: A systematic review and dose response meta-analysis of randomized controlled trials. Complement Ther Med 55: 102583, 2020. [DOI] [PubMed] [Google Scholar]
- 614.Wang Y, O’Neil A, Jiao Y, Wang L, Huang J, Lan Y, Zhu Y, Yu C. Sex differences in the association between diabetes and risk of cardio-vascular disease, cancer, and all-cause and cause-specific mortality: A systematic review and meta-analysis of 5,162,654 participants. BMC Med 17: 136, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 615.Wang ZJ, Zhou YJ, Galper BZ, Gao F, Yeh RW, Mauri L. Association of body mass index with mortality and cardiovascular events for patients with coronary artery disease: A systematic review and meta-analysis. Heart 101: 1631–1638, 2015. [DOI] [PubMed] [Google Scholar]
- 616.Wasan KM, Goss PE, Pritchard PH, Shepherd L, Palmer MJ, Liu S, Tu D, Ingle JN, Heath M, Deangelis D, Perez EA. The influence of letrozole on serum lipid concentrations in postmenopausal women with primary breast cancer who have completed 5 years of adjuvant tamoxifen (NCIC CTG MA.17L). Ann Oncol 16: 707–715, 2005. [DOI] [PubMed] [Google Scholar]
- 617.Wehr E, Pilz S, Boehm BO, Grammer TB, Marz W, Obermayer-Pietsch B. Low free testosterone levels are associated with all-cause and cardiovascular mortality in postmenopausal diabetic women. Diabetes Care 34: 1771–1777, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 618.Weiner CP, Lizasoain I, Baylis SA, Knowles RG, Charles IG, Moncada S. Induction of calcium-dependent nitric oxide synthases by sex hormones. Proc Natl Acad Sci U S A 91: 5212–5216, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 619.Wellons M, Ouyang P, Schreiner PJ, Herrington DM, Vaidya D. Early menopause predicts future coronary heart disease and stroke: The Multi-Ethnic Study of Atherosclerosis. Menopause 19: 1081–1087, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 620.Wells G, Tugwell P, Shea B, Guyatt G, Peterson J, Zytaruk N, Robin-son V, Henry D, O’Connell D, Cranney A, Osteoporosis Methodology Group, The Osteoporosis Research Advisory Group. Meta-analyses of therapies for postmenopausal osteoporosis. V. Meta-analysis of the efficacy of hormone replacement therapy in treating and preventing osteoporosis in postmenopausal women. Endocr Rev 23: 529–539, 2002. [DOI] [PubMed] [Google Scholar]
- 621.Wenner MM, Taylor HS, Stachenfeld NS. Androgens influence microvascular dilation in PCOS through ET-A and ET-B receptors. Am J Physiol Endocrinol Metab 305: E818–E825, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 622.Westphal C, Spallek B, Konkel A, Marko L, Qadri F, DeGraff LM, Schubert C, Bradbury JA, Regitz-Zagrosek V, Falck JR, Zeldin DC, Muller DN, Schunck WH, Fischer R. CYP2J2 overexpression protects against arrhythmia susceptibility in cardiac hypertrophy. PLoS One 8: e73490, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 623.Wibowo E, Pollock PA, Hollis N, Wassersug RJ. Tamoxifen in men: A review of adverse events. Andrology 4: 776–788, 2016. [DOI] [PubMed] [Google Scholar]
- 624.Wierckx K, Elaut E, Declercq E, Heylens G, De Cuypere G, Taes Y, Kaufman JM, T’Sjoen G. Prevalence of cardiovascular disease and cancer during cross-sex hormone therapy in a large cohort of trans persons: A case-control study. Eur J Endocrinol 169: 471–478, 2013. [DOI] [PubMed] [Google Scholar]
- 625.Wierman ME, Arlt W, Basson R, Davis SR, Miller KK, Murad MH, Rosner W, Santoro N. Androgen therapy in women: A reappraisal: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 99: 3489–3510, 2014. [DOI] [PubMed] [Google Scholar]
- 626.Wiklund P, Toss F, Weinehall L, Hallmans G, Franks PW, Nordstrom A, Nordstrom P. Abdominal and gynoid fat mass are associated with cardiovascular risk factors in men and women. J Clin Endocrinol Metab 93: 4360–4366, 2008. [DOI] [PubMed] [Google Scholar]
- 627.Wild RA, Carmina E, Diamanti-Kandarakis E, Dokras A, Escobar-Morreale HF, Futterweit W, Lobo R, Norman RJ, Talbott E, Dumesic DA. Assessment of cardiovascular risk and prevention of cardio-vascular disease in women with the polycystic ovary syndrome: A consensus statement by the Androgen Excess and Polycystic Ovary Syndrome (AE-PCOS) Society. J Clin Endocrinol Metab 95: 2038–2049, 2010. [DOI] [PubMed] [Google Scholar]
- 628.Wildman RP, Tepper PG, Crawford S, Finkelstein JS, Sutton-Tyrrell K, Thurston RC, Santoro N, Sternfeld B, Greendale GA. Do changes in sex steroid hormones precede or follow increases in body weight during the menopause transition? Results from the Study of Women’s Health Across the Nation. J Clin Endocrinol Metab 97: E1695–E1704, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 629.Williams DP, Boyden TW, Pamenter RW, Lohman TG, Going SB. Relationship of body fat percentage and fat distribution with dehydroepiandrosterone sulfate in premenopausal females. J Clin Endocrinol Metab 77: 80–85, 1993. [DOI] [PubMed] [Google Scholar]
- 630.Wilsgaard T, Arnesen E. Body mass index and coronary heart disease risk score: The Tromsø study, 1979 to 2001. Ann Epidemiol 17: 100–105, 2007. [DOI] [PubMed] [Google Scholar]
- 631.Wilson PW, D’Agostino RB, Sullivan L, Parise H, Kannel WB. Overweight and obesity as determinants of cardiovascular risk: The Framingham experience. Arch Intern Med 162: 1867–1872, 2002. [DOI] [PubMed] [Google Scholar]
- 632.Wing RR, Matthews KA, Kuller LH, Meilahn EN, Plantinga PL. Weight gain at the time of menopause. Arch Intern Med 151: 97–102, 1991. [PubMed] [Google Scholar]
- 633.Wittert G, Bracken K, Robledo KP, Grossmann M, Yeap BB, Handelsman DJ, Stuckey B, Conway A, Inder W, McLachlan R, Allan C, Jesudason D, Fui MNT, Hague W, Jenkins A, Daniel M, Gebski V, Keech A. Testosterone treatment to prevent or revert type 2 diabetes in men enrolled in a lifestyle programme (T4DM): A randomised, double-blind, placebo-controlled, 2-year, phase 3b trial. Lancet Dia-betes Endocrinol 9: 32–45, 2021. [DOI] [PubMed] [Google Scholar]
- 634.Wittert GA, Chapman IM, Haren MT, Mackintosh S, Coates P, Morley JE. Oral testosterone supplementation increases muscle and decreases fat mass in healthy elderly males with low-normal gonadal status. J Gerontol A Biol Sci Med Sci 58: 618–625, 2003. [DOI] [PubMed] [Google Scholar]
- 635.Wong CX, Sullivan T, Sun MT, Mahajan R, Pathak RK, Middeldorp M, Twomey D, Ganesan AN, Rangnekar G, Roberts-Thomson KC. Obesity and the risk of incident, post-operative, and post-ablation atrial fibrillation: A meta-analysis of 626,603 individuals in 51 studies. JACC Clin Electrophysiol 1: 139–152, 2015. [DOI] [PubMed] [Google Scholar]
- 636.Woodard G, Ahmed S, Podelski V, Hernandez-Boussard T, Presti J Jr, Morton JM. Effect of Roux-en-Y gastric bypass on testosterone and prostate-specific antigen. Br J Surg 99: 693–698, 2012. [DOI] [PubMed] [Google Scholar]
- 637.Worboys S, Kotsopoulos D, Teede H, McGrath B, Davis SR. Evidence that parenteral testosterone therapy may improve endothelium-dependent and -independent vasodilation in postmenopausal women already receiving estrogen. J Clin Endocrinol Metab 86: 158–161, 2001. [DOI] [PubMed] [Google Scholar]
- 638.Wu H, Qi Q, Yu Z, Sun Q, Wang J, Franco OH, Sun L, Li H, Liu Y, Hu FB. Independent and opposite associations of trunk and leg fat depots with adipokines, inflammatory markers, and metabolic syndrome in middle-aged and older Chinese men and women. J Clin Endocrinol Metab 95: 4389–4398, 2010. [DOI] [PubMed] [Google Scholar]
- 639.Wu TT, Chen Y, Zhou Y, Adi D, Zheng YY, Liu F, Ma YT, Xie X. Prognostic value of dehydroepiandrosterone sulfate for patients with cardiovascular disease: A systematic review and meta-analysis. JAm Heart Assoc 6: e00489, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 640.Wu WH, Yuan P, Zhang SJ, Jiang X, Wu C, Li Y, Liu SF, Liu QQ, Li JH, Pudasaini B, Hu QH, Dupuis J, Jing ZC. Impact of pituitary-gonadal axis hormones on pulmonary arterial hypertension in men. Hypertension 72: 151–158, 2018. [DOI] [PubMed] [Google Scholar]
- 641.Xie W-Q, Xiao G-L, Fan Y-B, He M, Lv S, Li Y-S. Sarcopenic obesity: Research advances in pathogenesis and diagnostic criteria. Aging Clin Exp Res 33: 247–252, 2019. [DOI] [PubMed] [Google Scholar]
- 642.Xu L, Freeman G, Cowling BJ, Schooling CM. Testosterone therapy and cardiovascular events among men: A systematic review and meta-analysis of placebo-controlled randomized trials. BMC Med 11: 108, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 643.Yamasaki H, Douchi T, Yamamoto S, Oki T, Kuwahata R, Nagata Y. Body fat distribution and body composition during GnRH agonist therapy. Obstet Gynecol 97: 338–342, 2001. [DOI] [PubMed] [Google Scholar]
- 644.Yassin A, Doros G. Testosterone therapy in hypogonadal men results in sustained and clinically meaningful weight loss. Clin Obes 3: 73–83, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 645.Yates J, Barrett-Connor E, Barlas S, Chen YT, Miller PD, Siris ES. Rapid loss of hip fracture protection after estrogen cessation: Evidence from the National Osteoporosis Risk Assessment. Obstet Gynecol 103:440–446, 2004. [DOI] [PubMed] [Google Scholar]
- 646.Yeap BB, Alfonso H, Chubb SA, Handelsman DJ, Hankey GJ, Almeida OP, Golledge J, Norman PE, Flicker L. In older men an optimal plasma testosterone is associated with reduced all-cause mortality and higher dihydrotestosterone with reduced ischemic heart disease mortality, while estradiol levels do not predict mortality. J Clin Endocrinol Metab 99: E9–E18, 2014. [DOI] [PubMed] [Google Scholar]
- 647.Yeap BB, Alfonso H, Chubb SA, Hankey GJ, Handelsman DJ, Golledge J, Almeida OP, Flicker L, Norman PE. In older men, higher plasma testosterone or dihydrotestosterone is an independent predictor for reduced incidence of stroke but not myocardial infarction. J Clin Endocrinol Metab 99: 4565–4573, 2014. [DOI] [PubMed] [Google Scholar]
- 648.Yeap BB, Wu FCW. Clinical practice update on testosterone therapy for male hypogonadism: Contrasting perspectives to optimize care. Clin Endocrinol (Oxf) 90: 56–65, 2019. [DOI] [PubMed] [Google Scholar]
- 649.Yeh SS, Marandi M, Thode HC Jr, Levine DM, Parker T, Dixon T, Schuster MW. Report of a pilot, double-blind, placebo-controlled study of megestrol acetate in elderly dialysis patients with cachexia. J Ren Nutr 20: 52–62, 2010. [DOI] [PubMed] [Google Scholar]
- 650.Yeh T-L, Chen H-H, Tsai S-Y, Lin C-Y, Liu S-J, Chien K-L. The relationship between metabolically healthy obesity and the risk of cardiovascular disease: A systematic review and meta-analysis. J Clin Med 8: 1228, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 651.Yen SS, Morales AJ, Khorram O. Replacement of DHEA in aging men and women. Potential remedial effects . AnnNYAcadSci 774: 128–142, 1995. [DOI] [PubMed] [Google Scholar]
- 652.Yoneshiro T, Aita S, Matsushita M, Kameya T, Nakada K, Kawai Y, Saito M. Brown adipose tissue, whole-body energy expenditure, and thermogenesis in healthy adult men. Obesity (Silver Spring) 19: 13–16, 2011. [DOI] [PubMed] [Google Scholar]
- 653.Yoneshiro T, Aita S, Matsushita M, Kayahara T, Kameya T, Kawai Y, Iwanaga T, Saito M. Recruited brown adipose tissue as an antiobesity agent in humans. J Clin Invest 123: 3404–3408, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 654.Yuki A, Otsuka R, Kozakai R, Kitamura I, Okura T, Ando F, Shimokata H. Relationship between low free testosterone levels and loss of muscle mass. Sci Rep 3: 1818, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 655.Yusuf S, Hawken S, Ounpuu S, Bautista L, Franzosi MG, Commerford P, Lang CC, Rumboldt Z, Onen CL, Lisheng L. Obesity and the risk of myocardial infarction in 27 000 participants from 52 countries: A case-control study. Lancet 366: 1640–1649, 2005. [DOI] [PubMed] [Google Scholar]
- 656.Zacharias A, Schwann TA, Riordan CJ, Durham SJ, Shah AS, Habib RH. Obesity and risk of new-onset atrial fibrillation after cardiac surgery. Circulation 112: 3247–3255, 2005. [DOI] [PubMed] [Google Scholar]
- 657.Zhang X, Xie X, Dou Q, Liu C, Zhang W, Yang Y, Deng R, Cheng AS. Association of sarcopenic obesity with the risk of all-cause mortality among adults over a broad range of different settings: A updated meta-analysis. BMC Geriatr 19: 183, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 658.Zhao D, Guallar E, Ballantyne CM, Post WS, Ouyang P, Vaidya D, Jia X, Ying W, Subramanya V, Ndumele CE, Hoogeveen RC, Michos ED. Sex hormones and incident heart failure in men and postmenopausal women: The Atherosclerosis Risk in Communities Study. J Clin Endocrinol Metab 105: e3798–e3807, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 659.Zhao D, Guallar E, Ouyang P, Subramanya V, Vaidya D, Ndumele CE, Lima JA, Allison MA, Shah SJ, Bertoni AG, Budoff MJ, Post WS, Michos ED. Endogenous sex hormones and incident cardiovascular disease in post-menopausal women. J Am Coll Cardiol 71: 2555–2566, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 660.Zhu L, Jiang X, Sun Y, Shu W. Effect of hormone therapy on the risk of bone fractures: A systematic review and meta-analysis of randomized controlled trials. Menopause 23: 461–470, 2016. [DOI] [PubMed] [Google Scholar]
- 661.Zitzmann M. Would male hormonal contraceptives affect cardiovascular risk? Asian J Androl 20: 145–148, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 662.Zitzmann M, Erren M, Kamischke A, Simoni M, Nieschlag E. Endogenous progesterone and the exogenous progestin norethisterone enanthate are associated with a proinflammatory profile in healthy men. J Clin Endocrinol Metab 90: 6603–6608, 2005. [DOI] [PubMed] [Google Scholar]
- 663.Zitzmann M, Junker R, Kamischke A, Nieschlag E. Contraceptive steroids influence the hemostatic activation state in healthy men. J Androl 23: 503–511, 2002. [PubMed] [Google Scholar]
- 664.Zitzmann M, Rohayem J, Raidt J, Kliesch S, Kumar N, Sitruk-Ware R, Nieschlag E. Impact of various progestins with or without trans-dermal testosterone on gonadotropin levels for non-invasive hormonal male contraception: A randomized clinical trial. Andrology 5: 516–526, 2017. [DOI] [PubMed] [Google Scholar]
- 665.Zumoff B, Strain GW, Miller LK, Rosner W, Senie R, Seres DS, Rosenfeld RS. Plasma free and non-sex-hormone-binding-globulin-bound testosterone are decreased in obese men in proportion to their degree of obesity. J Clin Endocrinol Metab 71: 929–931, 1990. [DOI] [PubMed] [Google Scholar]