Significance
A rare neurodegenerative disorder endemic to Guam features several types of protein lesions scattered throughout the nervous system and manifests clinical signs of amyotrophic lateral sclerosis, parkinsonism, and dementia. Despite many genetic and environmental studies, the disease etiology remains a mystery. Histological and biochemical investigations have well characterized the insoluble protein aggregates, but no study has determined if self-propagating tau, α-synuclein, or β-amyloid (Aβ) protein conformers exist in the diseased brains of Guam. Using a panel of cellular bioassays, the authors demonstrate that donor brain extracts contain tau and Aβ prions with a molecular phenotype distinct from the prions found in Alzheimer’s disease. The importance of these findings may extend well beyond mechanistic implications for Guam neurodegenerative disease.
Keywords: prions, Aβ, tau, neurodegeneration, Guam
Abstract
The amyotrophic lateral sclerosis–parkinsonism dementia complex (ALS-PDC) of Guam is an endemic neurodegenerative disease that features widespread tau tangles, occasional α-synuclein Lewy bodies, and sparse β-amyloid (Aβ) plaques distributed in the central nervous system. Extensive studies of genetic or environmental factors have failed to identify a cause of ALS-PDC. Building on prior work describing the detection of tau and Aβ prions in Alzheimer’s disease (AD) and Down syndrome brains, we investigated ALS-PDC brain samples for the presence of prions. We obtained postmortem frozen brain tissue from 26 donors from Guam with ALS-PDC or no neurological impairment and 71 non-Guamanian donors with AD or no neurological impairment. We employed cellular bioassays to detect the prion conformers of tau, α-synuclein, and Aβ proteins in brain extracts. In ALS-PDC brain samples, we detected high titers of tau and Aβ prions, but we did not detect α-synuclein prions in either cohort. The specific activity of tau and Aβ prions was increased in Guam ALS-PDC compared with sporadic AD. Applying partial least squares regression to all biochemical and prion infectivity measurements, we demonstrated that the ALS-PDC cohort has a unique molecular signature distinguishable from AD. Our findings argue that Guam ALS-PDC is a distinct double-prion disorder featuring both tau and Aβ prions.
In 1944, after United States (US) troops recaptured Guam from Japanese forces, the US Navy asked Thomas Rivers, president of the Rockefeller Institute, to assemble a group of medical scientists to work at a small hospital on the island. Among those enlisted was Harry Zimmerman, a neuropathologist. In May 1945, following observations on the health of the 28,000 native inhabitants living on the island known as the Chamorro people, Zimmerman wrote to his Navy superiors in Washington, D.C.: “During the past few months there were admitted to the medical wards of the Civilian Hospital [on Guam] 7 or 8 patients with a full-blown clinical picture of amyotrophic lateral sclerosis. This was quite surprising in view of the infrequency with which this neurologic disorder is encountered in the United States. Two of the patients died during the month of May and the diagnosis was confirmed at autopsy” (1). Zimmerman expressed that this unusual finding might be of scientific interest and suggested that the pathogenesis of the disease be studied following the end of the war.
In the 1950s, investigators found that amyotrophic lateral sclerosis (ALS) among the Chamorros of Guam was clinically indistinguishable from ALS seen elsewhere (2–4). Door-to-door surveys for neurologic diseases among the Chamorro people revealed an additional disorder (or possibly a spectrum of disease) consisting of parkinsonism associated with severe progressive dementia (referred to as parkinsonism–dementia complex of Guam, or PDC) (5). In the 1960s and 1970s, epidemiologists found that ALS among the Chamorros was more than 100 times greater than seen elsewhere in the world and was almost 1,000 times greater in selected villages (6). PDC, on the other hand, was a unique disorder found only on Guam. Importantly, neuropathology studies revealed that both forms of the disorder showed widespread, severe neurofibrillary tangle (NFT) formation in the absence of β-amyloid (Aβ) plaques (7–9). Although amyotrophic lateral sclerosis–parkinsonism dementia complex (ALS-PDC) was initially considered to be a hereditary disorder within an island-bound geographic isolate, subsequent studies indicated that migrants from the Philippines could also develop the condition after living on Guam for more than two decades (10).
The remarkably high incidence of neurodegenerative phenotypes developed by the Chamorro people on Guam in the 1950s stimulated numerous hypotheses on the cause of this disorder, but none have proved fruitful. Clinically, this neurodegenerative disease (ND) resembled ALS with profound weakness as well as dementia and signs of Parkinson’s disease (PD). Over the past 80 y, ALS-PDC has slowly disappeared on Guam (11), and there are virtually no new cases encountered on the island today. The dramatic change in incidence has reinforced the suggestion that some environmental factor(s) on Guam had initiated or caused the disease, but exposure to this factor must have been eliminated or at least mitigated, eventually leading to its disappearance among the at-risk population. The implications of this dramatic shift in incidence of a prion disorder are likely to be profound.
Despite the variation in clinical manifestations, the one constant has been the widespread presence of NFTs that stain for the tau protein in the brain (12, 13). NFTs include both 3R and 4R tau isoforms with phosphorylated epitopes, similar to Alzheimer’s disease (AD) (14). However, unlike AD, tau NFTs are distributed in both neuronal and glial cells in the gray and white matter (15–17). Deposits of another protein, α-synuclein, are often restricted to the amygdala and cerebellum and only found in a fraction of autopsied brains from ALS-PDC patients (18–20). Lastly, despite the rarity of extracellular Aβ plaques (21–23), there is histological evidence of intracellular Aβ inclusions in ALS-PDC (24–27). Because much of the characterization of Guam ALS-PDC has been determined from histology of fixed tissue sections, there remain open questions about the precise biochemical and biophysical properties of the putative proteins involved in its neuropathogenesis. Moreover, given the spectrum of clinical signs in ALS-PDC and the lack of microscopic protein deposits, it is unclear if α-synuclein and Aβ are involved in neuropathogenesis. Thus, quantitative methods in fresh-frozen tissues are needed to better understand the molecular nature of ALS-PDC and how it differs from PD, AD, and the spectrum of primary tauopathies.
Over the past 20 y, there has been cumulative evidence arguing that other than prion protein (PrP), tau, α-synuclein, and Aβ proteins also adopt pathogenic, self-propagating conformations characteristic of prions (28). Prions induce the misfolding of additional copies of the naive protein in a self-perpetuating process that spreads within and between cells (infectivity at the cellular level). Indeed, readily transmissible PrP prions cause the rare kuru and iatrogenic Creutzfeldt–Jakob disease (29), but there is no definitive evidence to suggest that AD or PD are communicable disorders. While experimental transmission of human brain-derived tau, α-synuclein, and Aβ proteins was first demonstrated in susceptible mice (30–33), these inoculation-based bioassays required incubation periods of several months to a year. To our knowledge, there has been only one study to transmit brain extract from Guam ALS-PDC donors into primates; after incubation periods up to ~4 y, the primates developed only modest neurological signs, and no microscopic protein deposits were noted in the brains postmortem (34–36). Interestingly, upon the second and third passage of brain extract into primates, neurologic symptoms were observed again but lesions were not. It is possible that either the silver-staining techniques of that era did not detect immature protein deposits that can now be detected with antibodies or that the incubation periods were too short to manifest robust histopathology. Nevertheless, we postulated that prion conformers of tau or α-synuclein might have been present in those tissues but were undetectable using the available methods.
Based on the recent development of a rapid human-cell bioassay engineered for the measurement of tau prions (37), we built parallel cell lines to measure α-synuclein, Aβ, and tau prions derived from several NDs on the same platform (38–42). Here, we applied a similar approach to measure prions in autopsied, frozen brain samples from people of Guam who manifested ALS-PDC syndrome or died from other illnesses without any neurological disorder. We found that all of the neocortical brain samples from 20 donors with ALS-PDC contained tau prions, but none of the samples had detectable α-synuclein prions. Unexpectedly, we found that all of the ALS-PDC samples contained Aβ prions. Similar to earlier studies that demonstrated that AD (40) and Down syndrome are double-prion disorders (42), our findings establish ALS-PDC as the third example of a double-prion disorder. To determine if the biochemical profile of tau and Aβ prions in ALS-PDC could be differentiated from AD, we performed a battery of quantitative enzyme-linked immunosorbent assays (ELISAs) to measure distinct proteoforms of tau and Aβ. While the etiology remains a mystery, we hypothesized that such detailed investigations in brain samples from Guam ALS-PDC might foster a fresh perspective in dissecting the molecular pathogenesis of more prevalent NDs caused by prions.
Results
Cellular Bioassays Detect Tau and Aβ Prions in Guam ALS-PDC Brain Samples.
Following earlier studies (37–40), we employed a panel of engineered cell lines to measure the prion infectivity of α-synuclein, tau, and Aβ proteins present in extracts from frozen human brain samples. In brief, we used transfected human embryonic kidney–293T (HEK293T) cells stably expressing the yellow fluorescent protein (YFP) reporter fused to the following proteins: 1) α-synuclein, 2) 3R tau (repeat domain), 3) 4R tau (repeat domain), 4) Aβ40, and 5) Aβ42 (Materials and Methods). These cell lines express the fusion protein in the cytoplasm, which serves as a soluble substrate for prion templating after exposure to a brain extract containing proteins in a prion conformation. Prion infectivity is determined from the number of cells (percent positive cells) showing yellow fluorescent puncta due to the accumulation of aggregated YFP fusion proteins in the cytoplasm. In prior work, we defined the specificity of each cell line (38–40), which exhibit homotypic propagation for a specific prion type, and found that immunodepletion of prions in brain extracts eliminates cell infectivity (43).
Thus, our panel of cellular assays enabled the parallel detection of α-synuclein, tau, and Aβ prions in a collection of human postmortem brain samples including 20 donors from Guam with ALS-PDC (median age at death = 75 y; SI Appendix, Table S1), 6 donors from Guam with no neurological impairment (median age at death = 75 y; SI Appendix, Table S1), 14 donors from the United States with no neurological impairment (median age at death = 48 y; SI Appendix, Table S1), and 57 donors from the United States with sporadic AD (median age at death = 77 y; SI Appendix, Table S2). For each brain sample, we used phosphotungstic acid (PTA) to selectively precipitate the prions from brain homogenates. To further liberate prions from membrane lipids and proteins, we performed a limited digestion with proteinase K (PK) prior to the PTA precipitation. Next, we applied the PTA and PK+PTA extracts to each cell line to measure in parallel the infectivity of α-synuclein, tau, and Aβ prions (Figs. 1 and 2 and SI Appendix, Fig. S1).
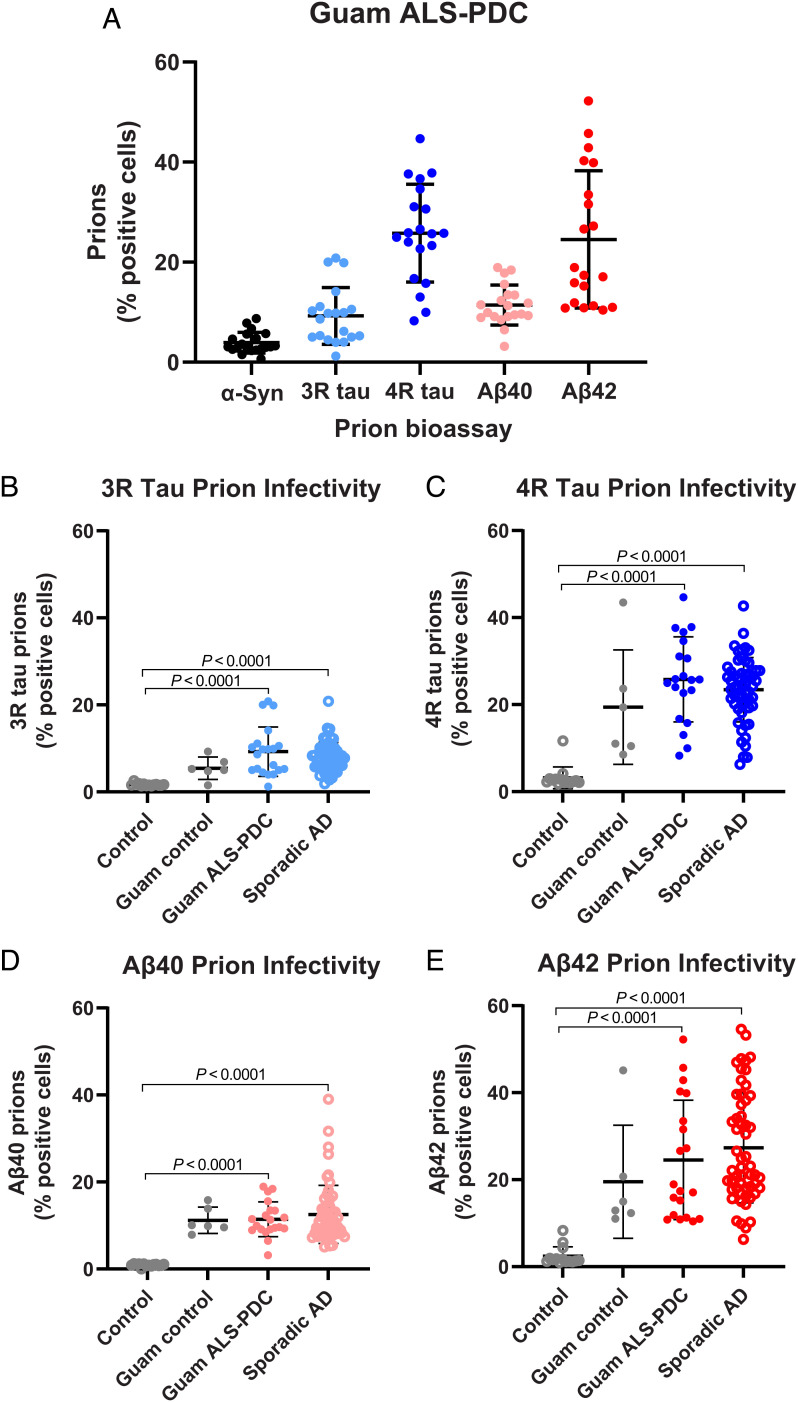
Fig. 1.
The measurement of α-synuclein, tau, and Aβ prions in Guam ALS-PDC and sporadic AD brain extracts using a cellular bioassay panel. (A) Prion infectivity measurements in Guam ALS-PDC brain samples (PK+PTA extracts) using transfected HEK293T cells stably expressing the α-syn(A53T)-YFP (black), 3R tau(K19VM)-YFP (light blue), 4R tau(K18LM)-YFP (blue), YFP-Aβ40 (pink), or YFP-Aβ42 (red) fusion proteins (Materials and Methods). Comparison of prion infectivity in Guam ALS-PDC (filled circles) or sporadic AD (open circles) patient brain extracts in (B) 3R tau(K19VM)-YFP cells (light blue), (C) 4R tau(K18LM)-YFP cells (blue), (D) YFP-Aβ40 cells (pink), or (E) YFP-Aβ42 cells (red). Data shown are individual donors plotted with mean ± SD as determined from four images per well in four wells per donor sample.
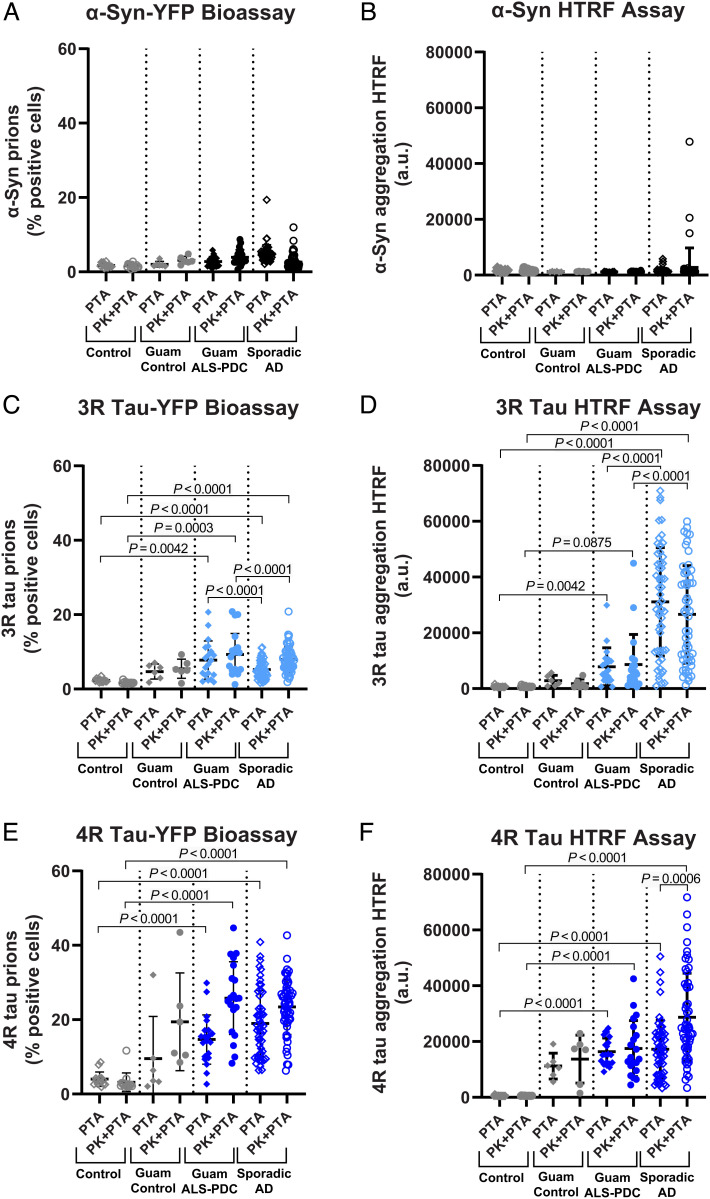
Fig. 2.
Comparison of YFP-tagged and untagged cellular bioassays for α-synuclein and tau prion infectivity. Prion bioassays using (A) HEK293T cells expressing YFP-tagged α-synuclein or (B) HEK293T cells expressing untagged α-synuclein (measured by HTRF) show α-synuclein prion infectivity in PTA (diamond symbols) and PK+PTA extracts (circle symbols) from Guam ALS-PDC (filled symbols) and sporadic AD (open symbols). Prion bioassays using (C) HEK293T cells expressing YFP-tagged repeat domain of 3R tau or (D) HEK293T cells expressing untagged full-length 0N3R tau (measured by HTRF) show 3R tau prion infectivity in PTA (diamond symbols) and PK+PTA extracts (circle symbols) from Guam ALS-PDC (filled symbols) and sporadic AD (open symbols). Prion bioassays using (E) HEK293T cells expressing YFP-tagged repeat domain of 4R tau or (F) HEK293T cells expressing untagged full-length 0N4R tau (measured by HTRF) show 4R tau prion infectivity in PTA (diamond symbols) and PK+PTA extracts (circle symbols) from Guam ALS-PDC (filled symbols) and sporadic AD (open symbols). Data shown are individual donors plotted with mean ± SD as determined from four images per well in four wells per donor sample.
Despite the signs of parkinsonism in patients with ALS-PDC, we did not observe any statistically significant change in α-synuclein prion infectivity (Fig. 1A). In contrast, consistent with widespread tau NFT pathology, we measured appreciable 3R tau and robust 4R tau prion infectivity in Guam cases (Fig. 1A). Unexpectedly, we detected Aβ40 and Aβ42 prions in most of the Guam ALS-PDC extracts (Fig. 1A), which was surprising given the scarcity of amyloid deposits observed histologically (21–23). Even more striking, we found appreciable tau and Aβ prion infectivity in Guam donors without neurological impairment (“Guam controls”). This suggests the accrual of ALS-PDC prions in these asymptomatic individuals who died from other illnesses but whose autopsied brains contained insufficient neuropathology to cause a clinical phenotype (44, 45). Because tau and Aβ prions appear to predominate in the Guam ALS-PDC cases, we compared these measurements with those in brain extracts from sporadic AD, a double-prion disorder featuring tau and Aβ prions (40). Overall, prion infectivity measurements were indistinguishable between both patient cohorts—no statistically significant differences were observed in the 3R tau, 4R tau, Aβ40, and Aβ42 cellular assays (Fig. 1 B–E). Notably, in both Guam ALS-PDC and sporadic AD, the Aβ42 prion infectivity became much more robust after the limited digestion step with PK (PK+PTA) (SI Appendix, Fig. S1), suggesting this step in the extraction protocol is critical for the enhanced detection of Aβ prions.
While deposition of tau NFTs in Guam ALS-PDC differs from sporadic AD, these cohorts do share some histological and biochemical similarities; thus, it remained unclear if the tau prions were distinct in each patient cohort. We speculated that using the 3R or 4R repeat domain of tau in our cellular assays may not be sufficient to efficiently template all the possible structural variations of the tau prion conformation. To further probe if differences exist in tau prion infectivity between cohorts, we engineered transfected HEK293T cell lines expressing the human full-length 0N3R tau or 0N4R tau isoform without the YFP reporter tag. In addition, we built an analogous cell line expressing human α-synuclein lacking the YFP fusion. We reasoned that the bulky YFP tag may hinder prion templating and thus decrease sensitivity to more subtle differences in prion abundance or conformation. Following exposure to brain extracts (PTA or PK+PTA), the untagged cells were lysed, and prion infectivity was measured using a commercial antibody-based Förster resonance energy transfer assay. This assay measures the abundance of tau or α-synuclein aggregation in a bulk volume of cell lysate using antibodies and homogeneous time-resolved fluorescence (HTRF) readout (Materials and Methods). Using both the untagged α-synuclein cells and YFP-tagged α-synuclein cells, we confirmed that the neocortical brain samples from Guam ALS-PDC and sporadic AD donors are largely devoid of α-synuclein prion infectivity (Fig. 2 A and B).
Next, using the untagged 0N3R tau cells, we observed robust 3R tau prion infectivity in sporadic AD brain extracts, but only modest 3R prion levels in Guam ALS-PDC. These data starkly contrast with the results obtained using the YFP-tagged 3R tau (repeat domain) cell assay, which does not differentiate between cohorts (Fig. 2 C and D). However, the statistical trends of 4R tau prion infectivity data observed in both the untagged 0N4R cells and the YFP-tagged 4R (repeat domain) cells appear to be in better agreement, showing relatively robust 4R tau prion infectivity in both cohorts (Fig. 2 E and F). Interestingly, the Guam controls exhibited no 3R tau prions and showed a more modest 4R tau prion load. We concluded that the HTRF-based bioassay in untagged cells is more sensitive and has a larger dynamic range compared with the YFP fusion–based bioassay, thus providing further differentiation between ALS-PDC and asymptomatic individuals from Guam. Taken together, these data suggest that Guam ALS-PDC brain extracts primarily contain 4R tau prions, while sporadic AD extracts have more equal proportions of 3R and 4R tau prions, consistent with prior immunoassays of 3R and 4R tau isoforms in brain tissues.
Biochemical Profile of Tau and Aβ Species Differentiates Guam ALS-PDC Brain Samples.
To determine if the comparable titers of tau and Aβ prions in brain samples from both cohorts can be attributed to similar biochemical forms of tau and Aβ, we used ELISA to measure the abundance of these proteins in different brain fractions. Soluble and insoluble Aβ42 was markedly lower in Guam ALS-PDC compared with sporadic AD (SI Appendix, Fig. S2 A and B); moreover, the level of insoluble Aβ42 in Guam controls was negligible. While soluble Aβ40 was not statistically different between groups, we observed reduced insoluble Aβ40 (SI Appendix, Fig. S2 C and D), thus matching the low amount of insoluble Aβ42 in Guam ALS-PDC and controls. These biochemical data are consistent with reports of few to no amyloid plaques observed by silver stain and immunohistochemistry (21–24, 34). Notably, we calculated a lower ratio of Aβ42 to Aβ40 in the soluble and insoluble brain fractions for Guam ALS-PDC compared with sporadic AD (SI Appendix, Fig. S2 E and F).
While prior studies report qualitative similarities in the histochemical and biochemical forms of tau in Guam ALS-PDC brain samples compared with sporadic AD (14, 15, 21), we performed ELISA to quantify the abundance of total tau and several ND-associated phosphorylated tau (p-tau) epitopes in soluble and insoluble brain fractions. We measured no statistically significant difference in soluble total tau (SI Appendix, Fig. S3A), suggesting that MAPT expression is unaffected in the Guam ALS-PDC cases. Likewise, we measured no statistically significant difference in soluble p-tau S199 and T231 epitopes; however, soluble p-tau S396 was markedly lower in Guam ALS-PDC samples and negligible in the Guam controls (SI Appendix, Fig. S3 B–D). Notably, we found a relatively low abundance of insoluble total tau and p-tau in Guam ALS-PDC samples (SI Appendix, Fig. S3 E–H), which was unexpected because histological reports indicate comparable tau NFT burden in end-stage ALS-PDC when compared with AD neuropathology (12, 14, 21). Similarly, the Guam control samples exhibited a minimal abundance of insoluble total tau and p-tau. To further examine if any one phospho-tau epitope differentiates ALS-PDC from AD, we normalized the insoluble p-tau levels to insoluble total tau for each donor sample but found no statistically significant differences between disease cohorts (data not shown). These data suggest that, despite overall less insoluble tau in ALS-PDC, it is hyperphosphorylated to a similar extent as it is in sporadic AD (13).
Aβ42 Prion Infectivity is More Potent in Guam ALS-PDC Compared with Sporadic AD.
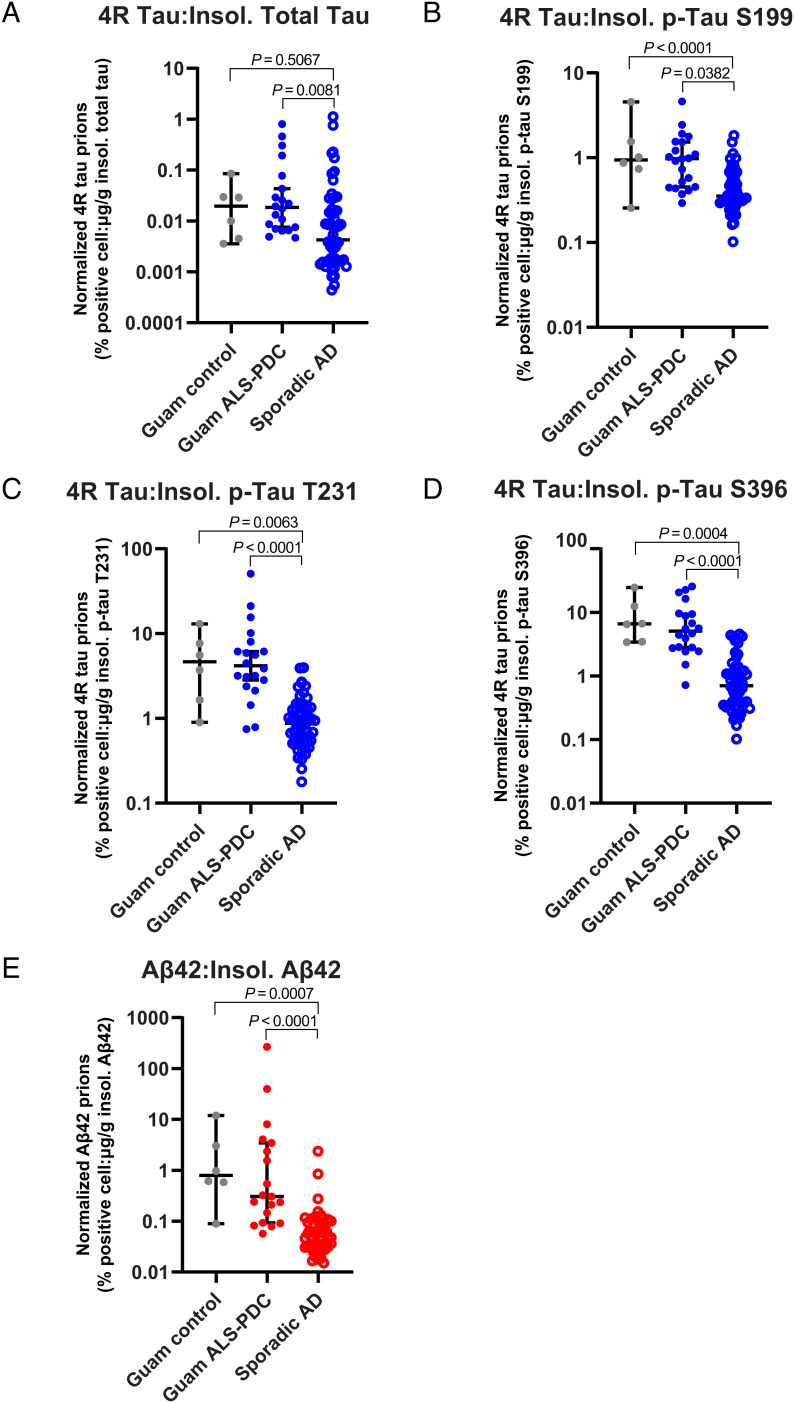
Because tau and Aβ prion infectivity is comparable between the patient cohorts despite clear differences in the abundance of tau and Aβ proteins, we reasoned that Guam ALS-PDC samples may contain more potent prions when adjusted to their respective protein concentration in the brain. To estimate the “specific activity” of tau and Aβ prions, we normalized the prion infectivity (percent positive cells) measured in the 4R tau and Aβ42 cell bioassays to the brain concentration (ELISA) of insoluble tau (total tau or p-tau) or Aβ42, respectively (Fig. 3). The specific activity of 4R tau normalized to insoluble total tau abundance (Fig. 3A) was five times greater in Guam ALS-PDC compared with sporadic AD (P = 0.0081). Further, we found that the specific activity for 4R tau prions normalized to insoluble p-tau S199 (P = 0.0382), p-tau T231 (P < 0.0001), and p-tau S396 (P < 0.0001) abundance was three to nine times greater in Guam ALS-PDC compared with sporadic AD cases (Fig. 3 B–D). We also observed a five times greater increase (P < 0.0001) in the specific activity for Aβ42 prions present in Guam ALS-PDC brain samples (Fig. 3E), suggesting a more potent species of Aβ prion. Strikingly, we observed similar significant statistical trends for prion-specific activities in the Guam control samples compared with AD samples (Fig. 3).
Fig. 3.
Specific activity of Aβ and tau prions in PK+PTA brain extracts. (A–E) Prion activity in brain tissue (PK+PTA extract) was normalized to the adjusted value of total tau, Aβ40, or Aβ42 in the insoluble brain fraction as measured by ELISA. Shown are normalized data for each donor in the Guam ALS-PDC (filled circles) and sporadic AD (open circles) cohorts. (A) 4R tau prion activity normalized by concentration of insoluble total tau, (B–D) 4R tau prion activity normalized by concentration of insoluble S199 p-tau, T231 p-tau, or S396 p-tau, respectively, and (E) Aβ42 prion activity normalized by concentration of insoluble Aβ42. Data shown are individual donors plotted with median ± 95% CI on a log-10 scale. See SI Appendix, Figs. S2 and S3 for ELISA data used for normalization.
Guam ALS-PDC is a Double-Prion Disorder with a Distinct Molecular Profile.
To simultaneously consider all of the biochemical data and the prion infectivity measurements, we constructed a heat map to visualize the molecular profile of tau and Aβ species we measured in the Guam ALS-PDC and sporadic AD samples (Fig. 4). We normalized the raw values obtained for each set of data from a given cell bioassay or ELISA measurement, which revealed a distinctive global pattern for prion infectivity and biochemical proteoforms in the Guam ALS-PDC cohort. Next, we performed a partial least squares (PLS) regression analysis on the normalized dataset for sporadic AD and Guam ALS-PDC samples, which distinguished each disease cohort as a unique cluster (Fig. 5); the data did not segregate further between female and male donors. Interestingly, the Guam control cases clustered with the Guam ALS-PDC cases, suggesting that these donor samples derived from asymptomatic individuals who might have developed signs of ALS-PDC later in life. Consistent with this notion, earlier histological studies reported evidence that such asymptomatic cases were likely in the process of developing the disease but had yet to achieve a sufficient burden of pathology to manifest symptoms (44, 45). To exclude the contribution of host genetic factors in differentiating these cohorts, we performed whole-genome sequencing and risk variant analysis on the Guam ALS-PDC and control cases. We found no evidence for increased genetic risk of ND phenotypes (SI Appendix, Table S3), which is consistent with a prior genetic study (46). Altogether, these data indicate that the biochemical milieu comprising the various prion species is unique to Guam ALS-PDC, differentiating this neurological disorder from sporadic AD.
Fig. 4.
Molecular profile of Aβ and tau prions differentiates Guam ALS-PDC from sporadic AD. Heatmap showing normalized data from cell-based prion infectivity and ELISA measurements of protein abundance in every brain sample. Data in each column represent a set of values normalized on a 0 to 100 scale. Two donor samples (2344.11 and 2837) were excluded because the prion infectivity (PTA) data were not determined due to instrument malfunction.
Fig. 5.
ALS-PDC has a distinguishable molecular signature. Partial least squares (PLS) regression analysis was performed on the normalized dataset for Guam controls (yellow circles), Guam ALS-PDC (red circles), and sporadic AD (green circles). Seventy-five percent of the variance can be explained by the first two PLS components. The centroid (mean value) of all the individual data points is represented by a diamond symbol in the Guam controls cluster (yellow), the Guam ALS-PDC cluster (red), and the sporadic AD cluster (green).
Discussion
Growing evidence from animal models and postmortem human tissue studies bolsters the argument that tau and Aβ adopt pathological conformations and spread through the brain by a prion mechanism to cause AD and related disorders, such as frontotemporal lobar degeneration with tau inclusions (FTLD-tau) or cerebral amyloid angiopathy (CAA). Our recent study provided unequivocal evidence for the presence of both tau and Aβ prions in human sporadic and familial AD brain tissue (40), thus laying a foundation to study AD as a double-prion disorder. Building on this concept, the results of our present study argue that the ALS-PDC syndrome of Guam is also a double-prion disorder (Fig. 1).
In the case of Guam ALS-PDC, the finding of Aβ prions in donor brain tissue is truly surprising. Most histological reports describe the lack of amyloid plaque pathology; if plaques are noted, they are found in older ALS-PDC donors and the amyloid burden is consistent with normal aging (21–24, 34), suggesting Aβ plaques are not involved in pathogenesis. Interestingly, in our prior work, we did not detect any Aβ prion infectivity in brain tissues from aged cognitively normal subjects or donors with FTLD-tau that had the sparse amyloid plaque burden consistent with normal aging (40). This finding argues that the Aβ prions found in Guam ALS-PDC are unique, and their infectivity is less likely to be dependent on scarce plaque pathology. Our finding highlights an important concept: the prion forms of a protein can be present in the absence of overt histopathological deposits (47–50).
Aside from amyloid plaques, the presence of intraneuronal Aβ accumulation is another histological feature in Guam ALS-PDC cases, which is also found in cases without any amyloid plaques and may be comingled with tau NFTs (24–27). These findings are reminiscent of the early-stage intraneuronal Aβ pathology observed in AD and Down syndrome (51–54). However, the continued presence of sparse intraneuronal Aβ, rare extracellular plaque deposition, and robust Aβ prion infectivity in the end-stage ALS-PDC brain calls into question whether these findings are merely signs of a late-blooming Aβ pathology. Interestingly, we observed that the ratio of Aβ42 to Aβ40 is markedly lower in ALS-PDC brain samples compared with AD (SI Appendix, Fig. S2), but the Aβ42 prion infectivity rivals that of AD. This may be one clue that the Aβ prion strain in ALS-PDC is distinct because of its composition (32, 55). Moreover, considering its relatively high specific activity in ALS-PDC (Fig. 3), a unique prion strain of Aβ may explain the predilection for intraneuronal accumulation. Consistent with this hypothesis, data in mouse models demonstrate that intracellular membrane–associated Aβ is a very potent species in Aβ propagation (56, 57). Ongoing research should elucidate the biochemical and biophysical properties of Aβ found in different subcellular fractions (58) of the ALS-PDC brain to better understand its role in disease.
In contrast to the observed discordance between Aβ prion infectivity and plaque load, our results show that the high 4R tau prion infectivity in ALS-PDC donor samples is consistent with the widespread tau NFT histopathology observed in previous reports (12, 14, 21). While a Braak stage score was unavailable, we showed that the bulk accumulation of insoluble tau was much lower in Guam ALS-PDC samples compared with AD samples (SI Appendix, Fig. S3), and yet the specific activity of 4R tau prions in ALS-PDC was markedly higher than AD tau prions (Fig. 3). Importantly, using the untagged 0N3R tau cell assay paradigm, we saw only a modest amount of 3R tau prions compared with AD (Fig. 2), suggesting tau prions in ALS-PDC more closely resemble 4R tauopathies such as progressive supranuclear palsy and corticobasal degeneration. The biophysical and biochemical basis for this remains unclear, but it is possible that ALS-PDC tau prions are a distinct conformational strain. This premise is supported by data showing that tau deposits in ALS-PDC appear in neurons and glial cells in CNS gray and white matter, which is similar to FTLD-tau but unlike AD in which tau NFTs primarily feature in gray matter neurons (15–17, 59). Indeed, a recent report argues that after adjusting for tau protein accumulation, tau prion infectivity is more robust in glial globular tauopathy compared with AD (60), suggesting that glial cells alone can produce a potent tau prion species. Future research on ALS-PDC and related disorders should focus on further defining tau prion infectivity by anatomical location and cellular source.
Despite histological studies reporting α-synuclein Lewy bodies in a subset of ALS-PDC donors, we did not observe any appreciable α-synuclein prion infectivity (Fig. 2). One explanation for this negative result is that we used neocortical brain samples; most α-synuclein deposits are observed in the amygdala and cerebellum (18–20). Our cellular assays are sensitive and specific for α-synuclein prions such as those found in multiple system atrophy and dementia with Lewy bodies (33, 38, 41). Conversely, we found no appreciable amount of α-synuclein prions in nearly all 55 cases of sporadic AD (Fig. 2) and ~100 cases of AD, CAA, and FTLD-tau in our prior study (40). Future research on ALS-PDC should investigate subcortical brain regions to explore the possibility of α-synuclein prion infectivity. Beyond tau, Aβ, and α-synuclein pathology, histological studies report that insoluble cellular inclusions of TAR DNA-binding protein 43 (TDP-43) are found in a fraction of Guam ALS-PDC patients. As with α-synuclein, the deposition patterns of these cellular inclusions appear restricted to subcortical brain regions and the spinal cord (61–63). While analogous cellular assays to measure TDP-43 prions are lacking, we are actively building comparable cell lines to expand the panel of prion bioassays to study ALS-PDC and related disorders such as FTLD with TDP-43 inclusions. Taken together, future research may highlight that ALS-PDC features more than two prions.
Perhaps our most fascinating finding is that the levels and specific activities of tau and Aβ prions in the Guam control brain extracts are equivalent to those in the ALS-PDC cases. Remarkably, even the PLS analysis shows the Guam control cases clustering with the ALS-PDC cases. The most parsimonious interpretation is that the Guam control cases represent asymptomatic people who would eventually develop ALS-PDC but died from nonneurological illness. Earlier histological investigations of large cohorts of subclinical cases reported the presence of protein deposits albeit fewer compared with symptomatic cases (34, 44, 45). That all of these people would eventually develop ALS-PDC would be consistent with an extremely high prevalence in the population and would be expected from a slowly developing disorder that is endemic, like ALS-PDC among the Chamorros. However, an alternative interpretation would be that there is an extremely high rate of permanent asymptomatic carriage of the disorder, and of the prions that cause it, in a subset of Chamorros. While it seems unlikely that robust levels of prions would not have neurological consequences, there are examples of some individuals who accumulate abundant AD plaques and tangles (amounts associated with neurological deficits) but remain cognitively normal until death (64). Interestingly, Aβ from “AD-resilient” cases can still transmit pathology to mice (65), suggesting that this condition does not generate inert prion conformers. Whether the asymptomatic Guam control cases represent individuals with unknown genetic or functional resilience, as hypothesized in the AD-resilient cases, remains to be elucidated. Future investigations should conduct a systematic evaluation of monogenic and polygenic ND risk in a large cohort stratified by specific activity of prions. Another consideration is that we only looked at one neocortical region; if we examined several other regions to generate a composite score of prion infectivity across the brain, we would gain more clarity on this unexpected result. Ongoing work in this vein is warranted and will have implications for understanding the relationship of prions to the prodromal stages of other NDs or to individuals resistant to them.
The importance of our findings may extend well beyond mechanistic implications for ALS-PDC in Guam. A similar clinical syndrome with a near identical neuropathological phenotype including tau, α-synuclein, Aβ, and TDP-43 lesions in the brain and spinal cord is described in the Kii Peninsula of Japan (66–69). There is a strong possibility that investigating postmortem tissues from the Kii cohort using our prion bioassays will reveal that Kii ALS-PDC is also a multiprion disorder. Furthermore, an endemic ALS-PDC clinical phenotype has been described in other geographical foci such as the southern coastal area of Papua New Guinea (70, 71) and Marie-Galante, an island of the Guadeloupe archipelago in the Caribbean (72). No autopsy reports are known to be published yet, but given the similar neurological syndromes, these cases should be investigated for the presence of different prions using our cellular bioassay approach.
In summary, our study demonstrates that both tau and Aβ prions accumulate in the brains of people who carry the clinical diagnosis of the ALS-PDC syndrome of Guam. Unexpectedly, we found that ALS-PDC is another double-prion disorder that resembles both AD and Down syndrome in some respects. Classical neuropathology was inadequate in elucidating the etiology of ALS-PDC. It remains to be established how many more double-prion diseases will be identified in future investigations.
Materials and Methods
Study Design.
This case–control study used deidentified human biospecimens from deceased individuals and is exempt from approval by an institutional review board (IRB; i.e., this study is not considered human subject research) in accordance with the University of California, San Francisco (UCSF) IRB policy. Samples were procured for this study retrospectively based on availability for distribution and known case criteria. As such, we have followed the Strengthening the Reporting of Observational Studies in Epidemiology reporting guidelines in this article.
Human Brain Sample Procurement and Processing.
All tissue donors provided written or verbal consent to donate autopsied brains for use in biomedical research in accordance with the standards of each institution. Fresh-frozen autopsied brain tissue was procured from the brain biorepositories at UCSF and the Guam Brain Specimen repository of the Micronesian Health Study, directed by Dr. Daniel Perl and funded by the National Institute on Aging for over 20 y. It is currently housed at the Uniformed Services University of the Health Sciences in Bethesda, MD. SI Appendix, Tables S1 and S2 include available demographic and clinicopathological information of patient donors. Frozen brain tissues were thawed and weighed to determine the mass in grams. Tissue was mechanically homogenized in nine volumes of cold Dulbecco's phosphate-buffered saline (DPBS) containing Halt Protease Inhibitor Cocktail (1×, Thermo Fisher Scientific), using a handheld probe-tip homogenizer (OMNI International). The homogenate was clarified by centrifugation at 5,000 × g for 5 min at 4 °C, and supernatants were collected and stored at −80 °C.
Whole Genome Sequencing and Neurodegenerative Disease Risk Variant Interpretation.
Distinct samples of frozen brain tissues were used as input for genomic DNA isolation using the DNeasy Blood & Tissue Kit (Qiagen). PCR-free DNA sequencing library preparation was conducted using 1 μg gDNA using the TruSeq® DNA Library Preparation Kit (Illumina) and unique dual indexes (Integrated DNA Technologies) on an automated liquid handling platform (Hamilton). Generated sequencing libraries were assessed for size distribution and absence of free adapters and dimers on a 5300 Fragment Analyzer System (Agilent) and assessed for yield using quantitative PCR on a LightCycler® 480 Instrument II (Roche). Pooled libraries were sequenced on a NovaSeq™ 6000 System (Illumina) using paired-end 150-bp read length on an S4 flow cell. Raw data demultiplexing, alignment, variant calling, and annotation were conducted using the HAS2.2 workflow (Illumina). A clinical molecular geneticist conducted variant interpretation after variant filter and prioritization for a 111 gene panel for screening variants associated with neurological diseases (73).
Phosphotungstic Acid Precipitation and Proteinase K Digestion of Aβ, Tau, and α-Synuclein Prions in Frozen Brain Samples.
Phosphotungstic acid (PTA) precipitation of human postmortem brain samples was performed as described (40, 74). Briefly, 10% brain homogenate was incubated in 2% sarkosyl and 0.5% benzonase (Sigma) at 37 °C with constant agitation (900 rpm) in an orbital shaker for 2 h. For sample preparation including limited proteolysis, proteinase K (Thermo Fisher Scientific) was added to the samples and incubated for 1 h at 37 °C with constant agitation in an orbital shaker. After incubation for 1 h, phenylmethylsulfonyl fluoride (PMSF) (Thermo Fisher Scientific) was added to stop the PK reaction. PTA was dissolved in double-distilled H2O, and the pH was adjusted to 7.0. PTA was added to the solution to a final concentration of 2%, which was then incubated overnight under the same conditions. The sample was centrifuged at 16,000 × g for 1 h and 15 min at room temperature, and the supernatant was removed. The resulting pellet was resuspended in DPBS using 10% of the initial starting volume and stored at −80 °C.
To establish bioassays for measuring Aβ, tau, or α-synuclein prion activity, we prepared a dilution series (e.g., 0.01, 0.03, and 0.1×) of all PTA-precipitated brain samples to perform the initial experiments. Once we established the dilution factor that best suited most of the samples, we performed subsequent experiments with only one or two dilution factors to conserve sample stocks. Using this approach, we ensured that our aggregation-inducing infectivity measurements were well within the dynamic range of the bioassay. Immunoprecipitation methods notably yield comparable results to PTA precipitated prion infectivity in the cellular bioassay (38) but are much less cost-efficient.
YFP-Tagged HEK293T Cell Bioassay for Measuring Prion Infectivity.
Previously, we developed monoclonal HEK293T cell lines expressing constructs encoding human WT Aβ42 or Aβ40 fused with yellow fluorescent protein (YFP) at the N terminus (40). Cell lines expressed either human tau containing the repeat domain of 4R tau with the mutations P301L and V337M or human tau containing the repeat domain of 3R tau with the mutations L266V and V337M. Both constructs were fused with YFP at the C terminus and generated as described (39). The cell line expressing human full-length α-synuclein (1 to 140 aa) with the A53T mutation fused with YFP at the C terminus was generated as described (38). To perform the bioassay, 3,000 cells per well (containing 0.1 μg/mL Hoechst 33342) were plated at 70 μL/well onto 384-well plates (Greiner) and incubated for 2 h before treatment with samples. Based on prior work (40), samples [(0.015×) 20% final volume] were incubated with Lipofectamine 2000 (1.5% final volume; Thermo Fisher Scientific) and OptiMEM (78.5% final volume; Thermo Fisher Scientific) for 2 h. Following incubation, samples were plated onto 384-well plates in four replicate wells (10 μL/well). Plates were incubated and imaged for 4'6-diamidino-2-phenylindole (DAPI) and fluorescein (FITC) channels every 24 h (five images/well) for 3 d using the GE Healthcare IN Cell Analyzer 6000 followed by image analysis using IN Cell Developer software and custom protocols containing algorithms to detect intracellular aggregates in live cells.
Formic Acid Extraction of Insoluble Proteins in Brain Tissue for Enzyme-Linked Immunosorbent Assay.
Fifty μL formic acid were added to 25 μL 10% brain homogenate and placed in an ultracentrifuge tube. The samples were vortexed, sonicated for 20 min at 37 °C in a water-bath sonicator, then centrifuged at 100,000 × g for 1 h. We removed 50 μL of the supernatant and neutralized it with 950 μL neutralization buffer in a low-binding tube. The neutralization buffer consisted of 1 M Tris Base and 500 mM dibasic sodium phosphate with no pH adjustment. If a very small pellet or layer of lipids formed at the top of the supernatant, we aspirated the sample from the middle of the supernatant to maximize the protein in the extract. Samples were aliquoted into low-binding tubes and flash frozen in liquid nitrogen. The following ELISA kits from Thermo Fisher Scientific were used according to the manufacturer’s protocol: amyloid precursor protein (KHB0051), Aβ40 (KHB3481), Aβ42 (KHB3441), total tau (KHB0041), p-tau S396 (KHB7031), p-tau S199 (KHB7041), and p-tau T231 (KHB8051). Each sample was analyzed in duplicate. We adjusted the raw ELISA values to total brain protein (grams) in the clarified 10% brain homogenate as determined by bicinchoninic acid (BCA) assay (Pierce/Thermo Fisher Scientific).
Quantification of Total Protein in Brain Homogenate.
Total protein content in the PBS- (clarified 10% brain homogenate) and detergent-soluble fractions was quantified using the Pierce BCA Protein Assay Kit (Thermo Fisher Scientific) following the manufacturer’s protocol.
Untagged HEK293T Cells Expressing Full-Length Tau and α-Synuclein.
The HEK293T cellular bioassays expressing untagged proteins were developed as previously described (37–39). Cell lines expressed human tau containing the full-length 0N4R tau with the mutations P301L and V337M or human tau containing the full-length 0N3R tau with the mutations L266V and V337M. A cell line expressing human full-length α-synuclein (1 to 140 aa) with the A53T mutation was developed using the same approach. It should be noted that we also created untagged Aβ40 and Aβ42 cell lines but could not implement a similar infectivity readout because the untagged Aβ spontaneously aggregated in the cytoplasm, which would confound data interpretation.
Homogeneous Time-Resolved Fluorescence Assay for Tau and α-Synuclein Prions.
HTRF analysis was performed on cell lysate using Tau and Alpha-Synuclein Aggregation Kits (Cisbio) with 384-well shallow microplates (Perkin Elmer). Briefly, antibodies were diluted to a concentration of 50× with detection buffer and premixed 1:1 right before plating. Samples (10 μL) were added to each well followed by 10 μL premixed antibodies. Plates were sealed and incubated overnight at room temperature. Plates were read at 665 nm and 620 nm using the PHERAstar FSX Microplate Reader followed by analysis using MARS Data Analysis Software.
Statistical Analysis.
Statistical analyses were performed with GraphPad Prism version 9. Data are shown as mean ± SD. Comparisons between multiple groups were performed using one-way ANOVA (Brown-Forsythe and Welch tests; general linear model) with post hoc Dunnett’s T3 multiple comparison test. For the prion-specific activity calculations, we plotted the data on a Log10 scale and used one-way ANOVA (Kruskal–Wallis test; general linear model) with post hoc Dunn’s multiple comparison test. A value of P < 0.05 (two-sided) was considered significant. Partial least squares (PLS) regression was carried out using MATLAB R2019b (MathWorks) with normalized cell-based prion infectivity and ELISA-based protein abundance data (75). “Sporadic AD” was set as the predictor variable and “Guam ALS-PDC” and “Guam control” as the response variables. From the plot of percent of variance explained in the response variable as a function of the number of components, the number of components was chosen. The scores from the first and second PLS components were plotted and labeled accordingly.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
We thank Charles Rice, Jonathan Woodson, Walter Tinling, and Kevin Kelly for their support and encouragement in many different phases of this study. This work was supported by grants from the NIH (AG002132 and AG031220), as well as by the Dana Foundation, the Glenn Foundation, the Henry M. Jackson Foundation, the Rainwater Charitable Foundation, and the Sherman Fairchild Foundation. We thank Amanda L. Woerman (UCSF) for her contribution to the initial study. We thank Ann A. Lazar (Biostatistics, UCSF) for her assistance with statistical analyses. We thank Joaquin Villar (Center for Military Precision Health, Uniformed Services University of the Health Sciences) for his assistance with genome sequencing analysis and gene variant interpretation. We thank Douglas Galasko (University of California San Diego) for providing the clinical reports associated with the Guamanian ALS-PDC and control samples. Human brain tissue was received from the UCSF Neurodegenerative Disease Brain Bank, which is supported by the NIH (AG023501 and AG19724 to W.W.S.), the Tau Consortium, and the Consortium for Frontotemporal Dementia Research. The work was also supported by the NIH (P01AG14382), the Department of Defense/Uniformed Services University Brain Tissue Repository and Neuropathology Core [Defense Health Agency (DHA) Grant #HU0001-17-2-0029], and the UCSF/Uniformed Services University of the Health Sciences Partnership to Develop Tau Prion Therapeutics for Chronic Traumatic Encephalopathy—Brain Injury and Disease Prevention, Treatment & Research (DHA Grant #HU0001-19-2-000). The opinions expressed here are those of the authors and are not necessarily representative of those of the Uniformed Services University, the United States Department of Defense or the United States Army, Navy, Air Force, or any other Federal agency.
Author contributions
C.C. and S.B.P. designed research; C.C., J.I.A., C.L.D., M.M.G.G., and B.M.R. performed research; W.W.S. and D.P.P. contributed new reagents/analytic tools; C.C., C.L.D., D.P.P., and S.B.P. analyzed data; and C.C. and S.B.P. wrote the paper.
Competing interests
The authors have organizational affiliations to disclose: S.B.P. is the acting president of Prio-Pharma. The authors have stock ownership to disclose: J.I.A. and S.B.P. are shareholders in Prio-Pharma. The authors have patent filings to disclose: Ayers, Paras, and Prusiner, U.S. Application Serial No. 17/943,988 “Assays for Classifying Alpha-Synuclein Prion Diseases.” Paras, Merz, et al., U.S. Application Serial No. 63/247,230 “Drugs for Neurodegenerative Diseases.”
Footnotes
Reviewers: W.C.M., University of California San Diego; and G.C.T., Colorado State University.
Contributor Information
Carlo Condello, Email: carlo.condello@ucsf.edu.
Stanley B. Prusiner, Email: stanley.prusiner@ucsf.edu.
Data, Materials, and Software Availability
All study data are included in the article and/or SI Appendix.
Supporting Information
References
- 1.Zimmerman H. M., Memorandum to U.S.N. medical research unit No. 2 (1945).
- 2.Arnold A., Edgren D. C., Palladino V. S., Amyotrophic lateral sclerosis; fifty cases observed on Guam. J. Nerv. Ment. Dis. 117, 135–139 (1953). [PubMed] [Google Scholar]
- 3.Mulder D. W., Kurland L. T., Iriarte L. L., Neurologic diseases on the island of Guam. U.S. Armed Forces. Med. J. 5, 1724–1739 (1954). [PubMed] [Google Scholar]
- 4.Kurland L. T., et al. , Amyotrophic lateral sclerosis in the Mariana Islands. AMA Arch. Neurol. Psychiatry 75, 435–441 (1956). [DOI] [PubMed] [Google Scholar]
- 5.Hirano A., Kurland L. T., Krooth R. S., Lessell S., Parkinsonism-dementia complex, an endemic disease on the island of Guam. I. Clinical features. Brain 84, 642–661 (1961). [DOI] [PubMed] [Google Scholar]
- 6.Reed D. M., Brody J. A., Amyotrophic lateral sclerosis and parkinsonism-dementia on Guam, 1945–1972. I. Descriptive epidemiology. Am. J. Epidemiol. 101, 287–301 (1975). [DOI] [PubMed] [Google Scholar]
- 7.Hirano A., Malamud N., Kurland L. T., Parkinsonism-dementia complex, an endemic disease on the island of Guam. II. Pathological features. Brain 84, 662–679 (1961). [DOI] [PubMed] [Google Scholar]
- 8.Malamud N., Hirano A., Kurland L. T., Pathoanatomic changes in amyotrophic lateral sclerosis on Guam. Special reference to the occurrence of neurofibrillary changes. Arch. Neurol. 5, 401–415 (1961). [DOI] [PubMed] [Google Scholar]
- 9.Hirano A., Arumugasamy N., Zimmerman H. M., Amyotrophic lateral sclerosis. A comparison of Guam and classical cases. Arch. Neurol. 16, 357–363 (1967). [DOI] [PubMed] [Google Scholar]
- 10.Garruto R. M., Gajdusek D. C., Chen K. M., Amyotrophic lateral sclerosis and parkinsonism-dementia among Filipino migrants to Guam. Ann. Neurol. 10, 341–350 (1981). [DOI] [PubMed] [Google Scholar]
- 11.Plato C. C., et al. , Amyotrophic lateral sclerosis and parkinsonism-dementia complex of Guam: Changing incidence rates during the past 60 years. Am. J. Epidemiol. 157, 149–157 (2003). [DOI] [PubMed] [Google Scholar]
- 12.Hof P. R., et al. , Amyotrophic lateral sclerosis/parkinsonism-dementia complex of Guam: Quantitative neuropathology, immunohistochemical analysis of neuronal vulnerability, and comparison with related neurodegenerative disorders. Acta Neuropathol. 88, 397–404 (1994). [DOI] [PubMed] [Google Scholar]
- 13.Buée-Scherrer V., et al. , Neurofibrillary degeneration in amyotrophic lateral sclerosis/parkinsonism-dementia complex of Guam. Immunochemical characterization of tau proteins. Am J Pathol. 146, 924–932 (1995). [PMC free article] [PubMed] [Google Scholar]
- 14.Mawal-Dewan M., et al. , Identification of phosphorylation sites in PHF-TAU from patients with Guam amyotrophic lateral sclerosis/parkinsonism-dementia complex. J. Neuropathol. Exp. Neurol. 55, 1051–1059 (1996). [PubMed] [Google Scholar]
- 15.Oyanagi K., et al. , Distinct pathological features of the gallyas- and tau-positive glia in the parkinsonism-dementia complex and amyotrophic lateral sclerosis of Guam. J. Neuropathol. Exp. Neurol. 56, 308–316 (1997). [DOI] [PubMed] [Google Scholar]
- 16.Yamazaki M., et al. , Tau-positive fine granules in the cerebral white matter: A novel finding among the tauopathies exclusive to parkinsonism-dementia complex of Guam. J. Neuropathol. Exp. Neurol. 64, 839–846 (2005). [DOI] [PubMed] [Google Scholar]
- 17.Winton M. J., et al. , Characterization of tau pathologies in gray and white matter of Guam parkinsonism-dementia complex. Acta Neuropathol. 111, 401–412 (2006). [DOI] [PubMed] [Google Scholar]
- 18.Yamazaki M., et al. , α-Synuclein inclusions in amygdala in the brains of patients with the parkinsonism-dementia complex of Guam. J. Neuropathol. Exp. Neurol. 59, 585–591 (2000). [DOI] [PubMed] [Google Scholar]
- 19.Forman M. S., et al. , Tau and α-synuclein pathology in amygdala of Parkinsonism-dementia complex patients of Guam. Am. J. Pathol. 160, 1725–1731 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sebeo J., Hof P. R., Perl D. P., Occurrence of α-synuclein pathology in the cerebellum of Guamanian patients with parkinsonism-dementia complex. Acta Neuropathol. 107, 497–503 (2004). [DOI] [PubMed] [Google Scholar]
- 21.Hof P. R., Perl D. P., Loerzel A. J., Morrison J. H., Neurofibrillary tangle distribution in the cerebral cortex of parkinsonism-dementia cases from Guam: Differences with Alzheimer’s disease. Brain Res. 564, 306–313 (1991). [DOI] [PubMed] [Google Scholar]
- 22.Schwab C., Steele J. C., Akiyama H., McGeer P. L., Distinct distribution of apolipoprotein E and β-amyloid immunoreactivity in the hippocampus of Parkinson dementia complex of Guam. Acta Neuropathol. 92, 378–385 (1996). [DOI] [PubMed] [Google Scholar]
- 23.Schmidt M. L., et al. , Amyloid plaques in Guam amyotrophic lateral sclerosis/parkinsonism-dementia complex contain species of Aβ similar to those found in the amyloid plaques of Alzheimer’s disease and pathological aging. Acta Neuropathol. 95, 117–122 (1998). [DOI] [PubMed] [Google Scholar]
- 24.Gentleman S. M., et al. , β(A4)-amyloid protein and parkinsonian dementia complex of Guam. Lancet 337, 55–56 (1991). [DOI] [PubMed] [Google Scholar]
- 25.Ito H., Hirano H., Yen S. H., Kato S., Demonstration of β amyloid protein-containing neurofibrillary tangles in parkinsonism-dementia complex on Guam. Neuropathol. Appl. Neurobiol. 17, 365–373 (1991). [DOI] [PubMed] [Google Scholar]
- 26.Guiroy D. C., et al. , Neurofibrillary tangles of Guamanian amyotrophic lateral sclerosis, parkinsonism-dementia and neurologically normal Guamanians contain a 4- to 4.5-kilodalton protein which is immunoreactive to anti-amyloid β/A4-protein antibodies. Acta Neuropathol. 86, 265–274 (1993). [DOI] [PubMed] [Google Scholar]
- 27.Schwab C., Steele J. C., Akiyama H., McGeer E. G., McGeer P. L., Relationship of amyloid β/A4 protein to the neurofibrillary tangles in Guamanian parkinsonism-dementia. Acta Neuropathol. 90, 287–298 (1995). [DOI] [PubMed] [Google Scholar]
- 28.Prusiner S. B., Cell biology. A unifying role for prions in neurodegenerative diseases. Science 336, 1511–1513 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prusiner S. B., Some speculations about prions, amyloid, and Alzheimer’s disease. N. Engl. J. Med. 310, 661–663 (1984). [DOI] [PubMed] [Google Scholar]
- 30.Meyer-Luehmann M., et al. , Exogenous induction of cerebral beta-amyloidogenesis is governed by agent and host. Science 313, 1781–1784 (2006). [DOI] [PubMed] [Google Scholar]
- 31.Clavaguera F., et al. , Transmission and spreading of tauopathy in transgenic mouse brain. Nat. Cell Biol. 11, 909–913 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watts J. C., et al. , Serial propagation of distinct strains of Aβ prions from Alzheimer’s disease patients. Proc. Natl. Acad. Sci. U.S.A. 111, 10323–10328 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prusiner S. B., et al. , Evidence for α-synuclein prions causing multiple system atrophy in humans with parkinsonism. Proc. Natl. Acad. Sci. U.S.A. 112, E5308–E5317 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hirano A., Malamud N., Elizan T. S., Kurland L. T., Amyotrophic lateral sclerosis and Parkinsonism-dementia complex on Guam. Further pathologic studies. Arch. Neurol. 15, 35–51 (1966). [DOI] [PubMed] [Google Scholar]
- 35.Gibbs C. J. Jr., Gajdusek D. C., Amyotrophic lateral sclerosis, Parkinson's disease, and the amyotrophic lateral sclerosis-Parkinsonism-dementia complex on Guam: A review and summary of attempts to demonstrate infection as the aetiology. J. Clin. Pathol. Suppl. (R. Coll. Pathol.) 6, 132–140 (1972). [PMC free article] [PubMed] [Google Scholar]
- 36.Gibbs C. J. Jr., Gajdusek D. C., An update on long-term in vivo and in vitro studies designed to identify a virus as the cause of amyotrophic lateral sclerosis, parkinsonism dementia, and Parkinson’s disease. Adv. Neurol. 36, 343–353 (1982). [PubMed] [Google Scholar]
- 37.Sanders D. W., et al. , Distinct tau prion strains propagate in cells and mice and define different tauopathies. Neuron 82, 1271–1288 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woerman A. L., et al. , Propagation of prions causing synucleinopathies in cultured cells. Proc. Natl. Acad. Sci. U.S.A. 112, E4949–E4958 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woerman A. L., et al. , Tau prions from Alzheimer’s disease and chronic traumatic encephalopathy patients propagate in cultured cells. Proc. Natl. Acad. Sci. U.S.A. 113, E8187–E8196 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aoyagi A., et al. , Aβ and tau prion-like activities decline with longevity in the Alzheimer’s disease human brain. Sci. Transl. Med. 11, eaat8462 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ayers J. I., et al. , Different α-synuclein prion strains cause dementia with Lewy bodies and multiple system atrophy. Proc. Natl. Acad. Sci. U.S.A. 119, e2113489119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Condello C., et al. , Aβ and tau prions feature in the neuropathogenesis of Down syndrome. Proc. Natl. Acad. Sci. U.S.A. 119, e2212954119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnson N. R., et al. , Evidence for sortilin modulating regional accumulation of human tau prions in transgenic mice. Proc. Natl. Acad. Sci. U.S.A. 114, E11029–E11036 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anderson F. H., Richardson E. P. Jr., Okazaki H., Brody J. A., Neurofibrillary degeneration on Guam: frequency in Chamorros and non Chamorros with no known neurological disease. Brain 102, 65–77 (1979). [DOI] [PubMed] [Google Scholar]
- 45.Perl D. P., Hof P. R., Purohit D. P., Loerzel A. J., Kakulas B. A., Hippocampal and entorhinal cortex neurofibrillary tangle formation in Guamanian Chamorros free of overt neurologic dysfunction. J. Neuropathol. Exp. Neurol. 62, 381–388 (2003). [DOI] [PubMed] [Google Scholar]
- 46.Morris H. R., et al. , Genome-wide analysis of the parkinsonism-dementia complex of Guam. Arch. Neurol. 61, 1889–1897 (2004). [DOI] [PubMed] [Google Scholar]
- 47.Furman J. L., et al. , Widespread tau seeding activity at early Braak stages. Acta Neuropathol. 133, 91–100 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Woerman A. L., et al. , Kinetics of human mutant tau prion formation in the brains of two transgenic mouse lines. JAMA Neurol. 74, 1464–1472 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ye L., et al. , Aβ seeding potency peaks in the early stages of cerebral β-amyloidosis. EMBO Rep. 18, 1536–1544 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Woerman A. L., et al. , Kinetics of α-synuclein prions preceding neuropathological inclusions in multiple system atrophy. PLOS Pathog. 16, e1008222 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schwab C., McGeer P. L., Aβ42-carboxy-terminal-like immunoreactivity is associated with intracellular neurofibrillary tangles and pick bodies. Exp. Neurol. 161, 527–534 (2000). [DOI] [PubMed] [Google Scholar]
- 52.Mori C., et al. , Intraneuronal Aβ42 accumulation in Down syndrome brain. Amyloid 9, 88–102 (2002). [PubMed] [Google Scholar]
- 53.Cataldo A. M., et al. , Aβ localization in abnormal endosomes: Association with earliest Aβ elevations in AD and Down syndrome. Neurobiol. Aging. 25, 1263–1272 (2004). [DOI] [PubMed] [Google Scholar]
- 54.Baker-Nigh A., et al. , Neuronal amyloid-β accumulation within cholinergic basal forebrain in ageing and Alzheimer’s disease. Brain 138, 1722–1737 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Condello C., et al. , Structural heterogeneity and intersubject variability of Aβ in familial and sporadic Alzheimer’s disease. Proc. Natl. Acad. Sci. U.S.A. 115, E782–E791 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nagarathinam A., et al. , Membrane-anchored Aβ accelerates amyloid formation and exacerbates amyloid-associated toxicity in mice. J. Neurosci. 33, 19284–19294 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marzesco A.-M., et al. , Highly potent intracellular membrane-associated Aβ seeds. Sci. Rep. 6, 28125 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Steinerman J. R., et al. , Distinct pools of beta-amyloid in Alzheimer disease-affected brain: a clinicopathologic study. Arch. Neurol. 65, 906–912 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kovacs G. G., Robinson J. L., Perl D. P., Lee V. M., Trojanowski J. Q., Thorn-shaped astrocytes in the depth of cortical sulci in Western Pacific ALS/Parkinsonism-Dementia complex. Acta Neuropathol. 140, 591–593 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chung D. C., et al. , Tau exhibits unique seeding properties in globular glial tauopathy. Acta Neuropathol. Commun. 7, 36 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hasegawa M., et al. , TDP-43 is deposited in the Guam parkinsonism–dementia complex brains. Brain 130, 1386–1394 (2007). [DOI] [PubMed] [Google Scholar]
- 62.Geser F., et al. , Pathological TDP-43 in parkinsonism-dementia complex and amyotrophic lateral sclerosis of Guam. Acta Neuropathol. 115, 133–145 (2008). [DOI] [PubMed] [Google Scholar]
- 63.Miklossy J., et al. , Enduring involvement of tau, β-amyloid, α-synuclein, ubiquitin and TDP-43 pathology in the amyotrophic lateral sclerosis/parkinsonism–dementia complex of Guam (ALS/PDC). Acta Neuropathol. 116, 625–637 (2008). [DOI] [PubMed] [Google Scholar]
- 64.Gómez-Isla T., Frosch M. P., Lesions without symptoms: Understanding resilience to Alzheimer disease neuropathological changes. Nat. Rev. Neurol. 18, 323–332 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Duran-Aniotz C., Morales R., Moreno-Gonzalez I., Hu P. P., Soto C., Brains from non-Alzheimer’s individuals containing amyloid deposits accelerate Aβ deposition in vivo. Acta Neuropathol. Commun. 1, 76 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wakayama I., Kihira T., Yoshida S., Garruto R. M., Rare neuropil threads in amyotrophic lateral sclerosis and parkinsonism-dementia on Guam and in the Kii Peninsula of Japan. Dementia 4, 75–80 (1993). [DOI] [PubMed] [Google Scholar]
- 67.Itoh N., et al. , Biochemical and ultrastructural study of neurofibrillary tangles in amyotrophic lateral sclerosis/parkinsonism-dementia complex in the Kii peninsula of Japan. J. Neuropathol. Exp. Neurol. 62, 791–798 (2003). [DOI] [PubMed] [Google Scholar]
- 68.Kuzuhara S., Kokubo Y., Atypical parkinsonism of Japan: Amyotrophic lateral sclerosis-parkinsonism-dementia complex of the Kii peninsula of Japan (Muro disease): An update. Mov. Disord. 20, S108–S113 (2005). [DOI] [PubMed] [Google Scholar]
- 69.Mimuro M., Yoshida M., Kuzuhara S., Kokubo Y., Amyotrophic lateral sclerosis and parkinsonism-dementia complex of the Hohara focus of the Kii Peninsula: A multiple proteinopathy? Neuropathology 38, 98–107 (2018). [DOI] [PubMed] [Google Scholar]
- 70.Gajdusek D. C., Salazar A. M., Amyotrophic lateral sclerosis and parkinsonian syndromes in high incidence among the Auyu and Jakai people of West New Guinea. Neurology 32, 107–126 (1982). [DOI] [PubMed] [Google Scholar]
- 71.Okumiya K., et al. , Amyotrophic lateral sclerosis and parkinsonism in Papua, Indonesia: 2001–2012 survey results. BMJ Open 4, e004353 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lannuzel A., et al. , Clinical varieties and epidemiological aspects of amyotrophic lateral sclerosis in the Caribbean island of Guadeloupe: A new focus of ALS associated with Parkinsonism. Amyotroph. Lateral Scler. Frontotemporal Degener. 16, 216–223 (2015). [DOI] [PubMed] [Google Scholar]
- 73.Blauwendraat C., et al. , NeuroChip, an updated version of the NeuroX genotyping platform to rapidly screen for variants associated with neurological diseases. Neurobiol. Aging. 57, 247.e9–247.e13 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Levine D. J., et al. , Mechanism of scrapie prion precipitation with phosphotungstate anions. ACS Chem. Biol. 10, 1269–1277 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Abdi H., Partial least squares regression and projection on latent structure regression (PLS Regression). Wiley Interdiscip. Rev. Comput. Stat. 2, 97–106 (2010). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
All study data are included in the article and/or SI Appendix.