Significance
Large carnivore population dynamics operate at large spatial scales (across populations), yet most studies investigating their mortality are conducted at local scales within populations. We estimated survival and quantified mortality risk from humans for mountain lions across the state of California to evaluate these processes at the scale appropriate for conservation. Our data indicate that human-caused mortality is additive to natural mortality as it was not compensated for by reductions in natural mortality and instead was associated with reduced population-level survival. Despite protection from hunting, mortality risk for mountain lions in California is strongly influenced by the presence of rural development and by variation in the mindset of humans as captured by their voting patterns on statewide environmental ballot initiatives.
Keywords: additive mortality, compensatory mortality, human–wildlife conflict, Puma concolor, survival
Abstract
Mitigating human-caused mortality for large carnivores is a pressing global challenge for wildlife conservation. However, mortality is almost exclusively studied at local (within-population) scales creating a mismatch between our understanding of risk and the spatial extent most relevant to conservation and management of wide-ranging species. Here, we quantified mortality for 590 radio-collared mountain lions statewide across their distribution in California to identify drivers of human-caused mortality and investigate whether human-caused mortality is additive or compensatory. Human-caused mortality, primarily from conflict management and vehicles, exceeded natural mortality despite mountain lions being protected from hunting. Our data indicate that human-caused mortality is additive to natural mortality as population-level survival decreased as a function of increasing human-caused mortality and natural mortality did not decrease with increased human-caused mortality. Mortality risk increased for mountain lions closer to rural development and decreased in areas with higher proportions of citizens voting to support environmental initiatives. Thus, the presence of human infrastructure and variation in the mindset of humans sharing landscapes with mountain lions appear to be primary drivers of risk. We show that human-caused mortality can reduce population-level survival of large carnivores across large spatial scales, even when they are protected from hunting.
Understanding and mitigating human-caused mortality for large carnivores is a pressing global challenge for wildlife ecology and conservation (1, 2). High rates of human-caused mortality can limit or threaten populations of large carnivores because of the strong influence of adult survival on population growth and potential disruption to interpopulation connectivity (3, 4). Declining large carnivore populations can have ecosystem-level consequences given the important roles played by top predators in ecological communities (5). Thus, effective conservation in human-dominated landscapes requires an understanding of how mortality risk varies spatially across the large geographic regions occupied by large carnivores and the degree to which human-caused mortality impacts their overall survival (6, 7). Unfortunately, mortality is mostly studied at relatively small spatial scales, within single populations or management units (e.g., refs. 4 and 7–12), creating a mismatch between our understanding of mortality risk and the spatial extent relevant to conservation of viable populations of large carnivores.
A fundamental question in wildlife population ecology is whether anthropogenic mortality adds to natural mortality and therefore reduces overall survival (13). The compensatory mortality hypothesis suggests that anthropogenic mortality is compensated for by density-dependent reductions in natural mortality under the premise that there is a “doomed surplus” of individuals that can be killed without decreasing population-level survival (14). Compensatory mortality is an implicit assumption of many natural resource agencies that often must operate without sufficient information about cause-specific mortality and other dynamics of the populations they manage (4, 15, 16). Empirical evidence suggests that anthropogenic mortality of large carnivores can be compensatory, partially compensatory, or additive to natural mortality (e.g., refs. 17–19). The degree to which compensation occurs appears to vary relative to life history characteristics, population density, and factors influencing natural mortality (e.g., habitat quality; 19–21). However, these conclusions are complicated by limitations of most previous tests of the compensatory mortality hypothesis. First, most studies are conducted at relatively small spatial scales by comparing dynamics of two populations or within single management units (e.g., refs. 4, 6, and 18). Given the vast spaces used by large carnivores, rigorous tests of the compensatory mortality hypothesis should involve metapopulations occupying larger geographic extents (6). Second, most studies have ignored the considerable sampling variation in estimates of survival and cause-specific mortality in wild populations (but see ref. 6). Thus, although critical for sustainable management of large carnivores, unambiguous tests of the compensatory mortality hypothesis remain elusive.
Spatial variation in human activity and infrastructure influences the probability of human-caused mortality of large carnivores, highlighting the importance of considering risk across gradients of human disturbance (2, 22, 23). However, there is a more cryptic source of spatial variation in risk associated with differences in attitudes of humans that interact with large carnivores in local areas (24). Indeed, human tolerance is often the key factor influencing persistence of conflict-prone large carnivores (25). Unfortunately, reliable indices of tolerance are rarely included in models evaluating spatial mortality risk for large carnivores, limiting our ability to predict and effectively mitigate conflict and associated mortality.
Mountain lions (Puma concolor) in California provide an important case study with which to understand the influence of human-caused mortality on large carnivores across a wide diversity of landscape types and human viewpoints. California has perhaps the greatest diversity of ecoregions and the steepest gradient of human density of any US state (26). California is also the only western state that has permanently banned hunting of mountain lions, following a 1990 ballot initiative that passed by popular vote. Despite their protected status, mountain lions are regularly killed legally in California, mainly in response to livestock depredation (27). Thus, their protection and conflict with humans in California epitomize the polarizing nature of large carnivores in shared landscapes. Recently (2017 to 2020), threats to connectivity and viability of mountain lion populations along the central coast and in southern California (28–30) led to stricter regulations for lethal removal following depredation and the designation of mountain lions in these regions as a candidate species for listing as “threatened” under the California Endangered Species Act. In contrast, large tracts of contiguous habitat in undeveloped public lands in the Sierra Nevada mountains and northern California appear to be occupied by abundant, genetically diverse populations of mountain lions (30). A comprehensive understanding of human-caused mortality at the statewide scale, relative to the wide variation in socioecological context of landscapes occupied by mountain lions in California would inform the ongoing listing consideration and provide insight into large carnivore population dynamics.
We studied survival and spatially varying mortality risk across the distribution of the species in California by radio tracking 590 mountain lions to address several hypotheses (Fig. 1 and SI Appendix, Table S1). First, we hypothesized that despite their protected status in California, human-caused mortality would exceed natural mortality. Specifically, we predicted that management killing, vehicle collisions, and other anthropogenic mortality combined would exceed death from natural causes (P1). Second, we tested the compensatory mortality hypothesis by predicting that overall survival would not decline with higher rates of human-caused mortality (P2) and that natural mortality would decline with greater human-caused mortality across California (P3). Third, we hypothesized that humans drive spatial variation in mortality risk for this protected large carnivore, although in unintuitive ways. Here, we predicted that mortality risk would be highest in areas of intermediate human presence at broad and fine scales (P4). Conflict over livestock is often higher in rural areas occupied by humans compared to both urban centers and more remote areas (23, 27). However, variation in human tolerance may influence whether livestock depredation results in carnivore mortality (25). Thus, we also predicted that spatial variation in human viewpoints would influence mortality risk more strongly than the presence of livestock itself (P5), highlighting the complexity in mitigating human–mountain lion conflict. Our work advances mechanistic understanding of human-caused mortality risk for large carnivores occupying steep gradients in human density and perspective.
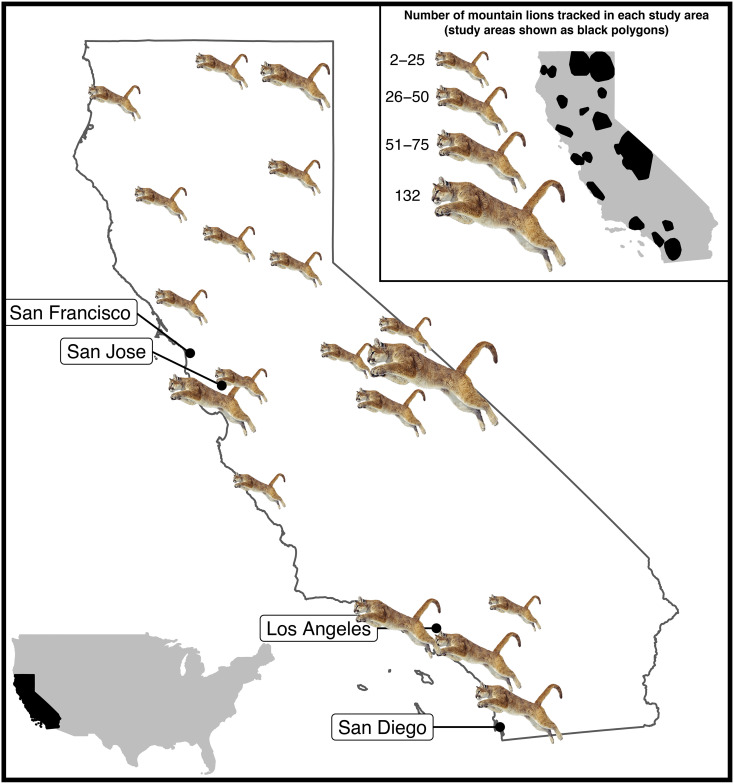
Fig. 1.
Study areas where we monitored survival and mortality of 590 radio-collared mountain lions across California, 1974 to 2020. Mountain lions are sized proportionally to the number of animals tracked.
Results
Survival and Cause-Specific Mortality.
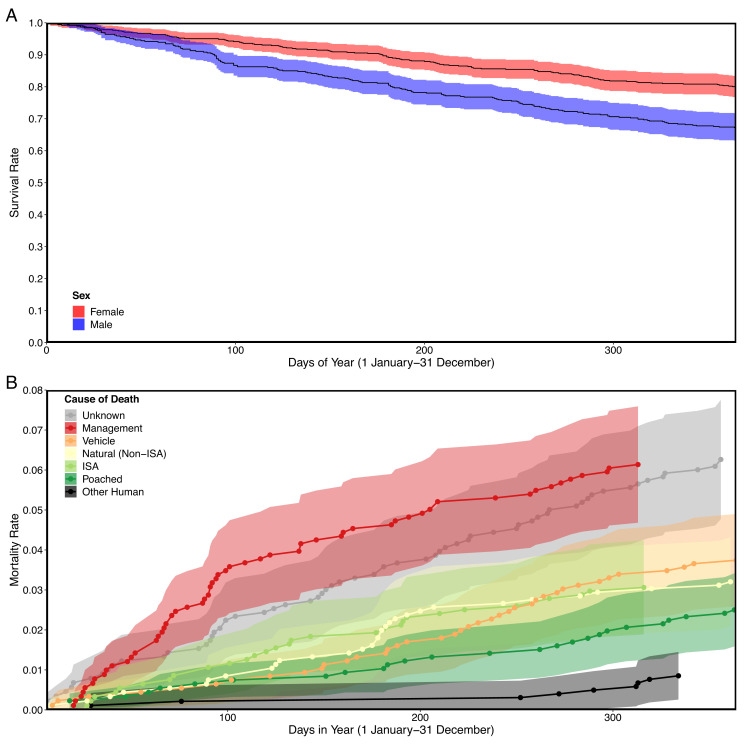
We tracked 590 radio-collared mountain lions across California and documented 263 mortalities during the monitoring period from 1974 to 2020. Most (76%) of our data were collected since 2000. Estimated annual survival of radio-collared mountain lions was 0.67 for males [95% CI (0.63, 0.72), n = 297 males, and n = 151 deaths] and 0.80 for females [95% CI (0.77, 0.83), n = 293 females, and n = 112 deaths; Fig. 2A]. We determined cause of death for 199 of 263 mortalities (76%). The annual rate of human-caused mortality [0.13, 95% CI (0.11, 0.15), and n = 135 deaths] was greater than the annual rate of natural mortality [0.06, 95% CI (0.05, 0.08), and n = 64 deaths; supporting P1] or unknown mortality [0.06, 95% CI (0.05, 0.08), and n = 64]. The leading specific cause of known mortality was management killing [0.06, 95% CI (0.05, 0.08), and n = 61], followed by vehicles [0.04, 95% CI (0.03, 0.05), and n = 39], nonstrife natural mortality [0.03, 95% CI (0.02, 0.04), and n = 33], intraspecific strife [0.03, 95% CI (0.02, 0.04), and n = 31], poaching [0.03, 95% CI (0.02, 0.03), and n = 26], and other human causes [0.01, 95% CI (0.00, 0.01), and n = 9; Fig. 2B]. Numbers of animals dying from each cause are summarized by sex and age class in SI Appendix, Table S2.
Fig. 2.
Annual survival (for males and females; A) and cause-specific mortality (B) curves for 590 mountain lions tracked across California, 1974 to 2020. These analyses were done with the full dataset of GPS- and VHF-collared animals. Circles along trend lines in cause-specific mortality curves represent the timing of specific mortality events.
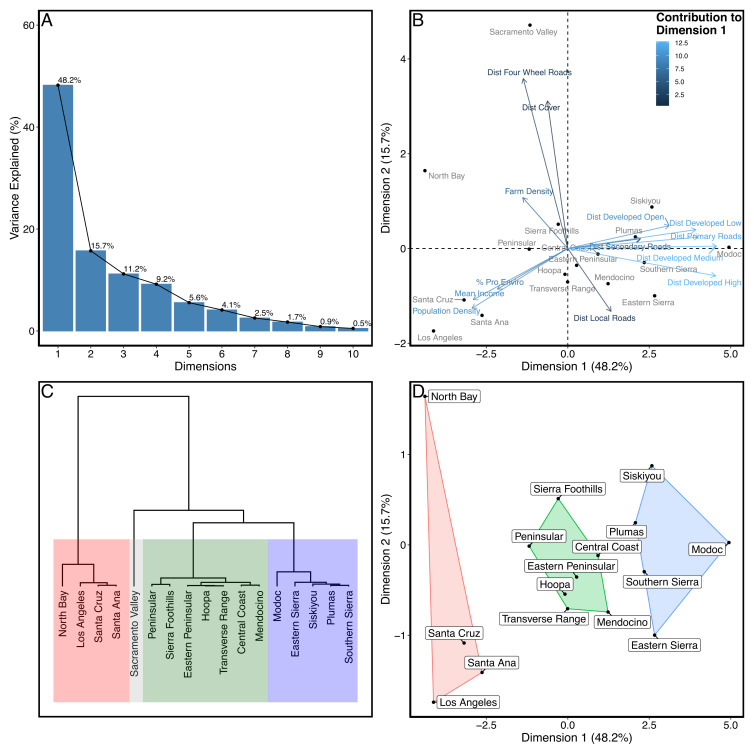
The first two dimensions of a principal components analysis (PCA) explained 64% of the variation in landscape features and attributes of human populations in areas occupied by global positioning system (GPS)-collared mountain lions across the state (Fig. 3). Hierarchical clustering suggested four clusters of study areas with similar characteristics (Fig. 3 and SI Appendix, Table S1). However, one cluster contained only a single study area (Sacramento Valley) where we only tracked two animals, so we excluded that cluster and used the other three clusters to compare survival and mortality across the state. Annual survival of all mountain lions and adult females (which have the greatest influence on population growth) was lowest in the study areas with intermediate human presence (Cluster 2; Table 1). Although CIs overlapped with rates from other clusters, the rates of human-caused and natural mortality were both greatest in the study areas with intermediate human presence (Table 1). Interestingly, survival was similar in the most remote study areas and those with the greatest human presence and infrastructure, within and adjacent to the Los Angeles, San Jose, and San Francisco Bay areas (Cluster 1; Table 1). The point estimates for overall and adult female survival were highest in the study areas with the greatest human presence (Table 1).
Fig. 3.
Principal components analysis (PCA) plots for landscape features and characteristics of human populations associated with mountain lion (n = 389) GPS telemetry locations across California. Shown are the percentage of variance explained by each dimension (A), contribution to variation in landscape and human populations of first dimensions of PCA (B), a dendrogram identifying 4 clusters of study areas with similar characteristics (C), and a plot showing the 3 main clusters of study areas identified with the PCA (D).
Table 1.
Survival and mortality rates within 3 clusters of study areas where we tracked mountain lions with GPS telemetry (n = 387) in California grouped by similar landscape and human characteristics with a principal components analysis
| Sample size | Overall survival | Adult female survival | Cause-specific mortality rates | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cluster | Animals | Deaths | ŝall | 95% CI | ŝAF | 95% CI | Human | 95% CI | Natural | 95% CI |
| 1* | 165 | 70 | 0.75 | (0.70, 0.80) | 0.80 | (0.73, 0.88) | 0.14 | (0.11, 0.18) | 0.04 | (0.02, 0.07) |
| 2† | 98 | 39 | 0.59 | (0.50, 0.70) | 0.71 | (0.58, 0.86) | 0.23 | (0.16, 0.31) | 0.11 | (0.06, 0.17) |
| 3‡ | 124 | 33 | 0.72 | (0.64, 0.81) | 0.79 | (0.70, 0.91) | 0.16 | (0.11, 0.22) | 0.09 | (0.05, 0.14) |
Shown are sample sizes, annual overall survival rates, adult female survival rates, human-caused mortality rates, and natural mortality rates with 95% CIs. We excluded animals tracked by VHF telemetry due to sparse location data.
*Greatest human presence.
†Intermediate human presence.
‡Lowest human presence.
Additive vs. Compensatory Mortality.
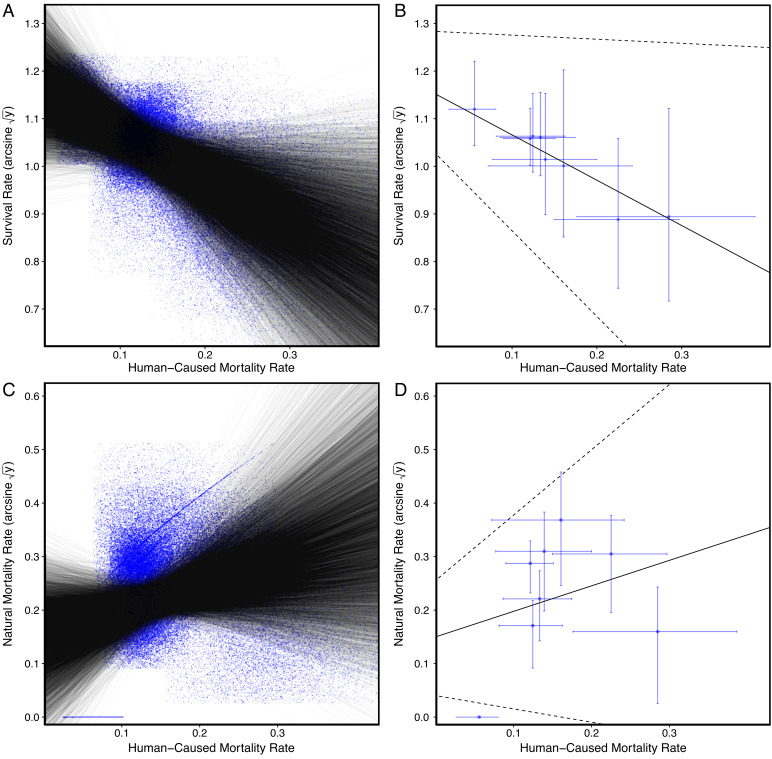
Overall survival decreased as a function of increasing human-caused mortality in populations across California [failing to support P2; β = −0.95, 90% highest posterior density interval (HPD; −0.09, −1.80), and n = 8; Fig. 4]. Natural mortality did not decline and instead showed a positive, nonsignificant trend in relation to increasing human-caused mortality [failing to support P3; β = 0.48, 90% HPD (−0.25, 1.22), and n = 8; Fig. 4]. When we attributed the unknown mortality to varying proportions of human-caused and natural mortality (ranging from 100% natural to 100% human caused) to explore the uncertainty associated with unknown mortality, natural mortality still did not significantly decline or increase as a function of human-caused mortality (SI Appendix, Appendix S1 and Table S3). Thus, our analyses provided no support for the compensatory mortality hypothesis and suggested that human-caused mortality was additive. We restricted these analyses to data from 8 study areas with data from ≥29 animals (431 total animals and 231 mortalities) to ensure the survival and mortality rates were representative of these areas. However, we also relaxed these restrictions in exploratory analyses with data from 13 study areas where we tracked ≥ 10 animals, and the results were consistent (SI Appendix, Appendix S2).
Fig. 4.
Plots showing relationships of overall survival and human-caused mortality (A and B) and natural mortality and human-caused mortality (C and D) across 8 study areas (n = 431 mountain lions) to test the compensatory mortality hypothesis. Shown are data points and regression lines (A and C) generated by drawing 10,000 samples from beta distributions to account for uncertainty in our estimates and regression plots used to estimate mean β and 90% highest posterior density intervals for these relationships (B and D).
Spatial Mortality Risk.
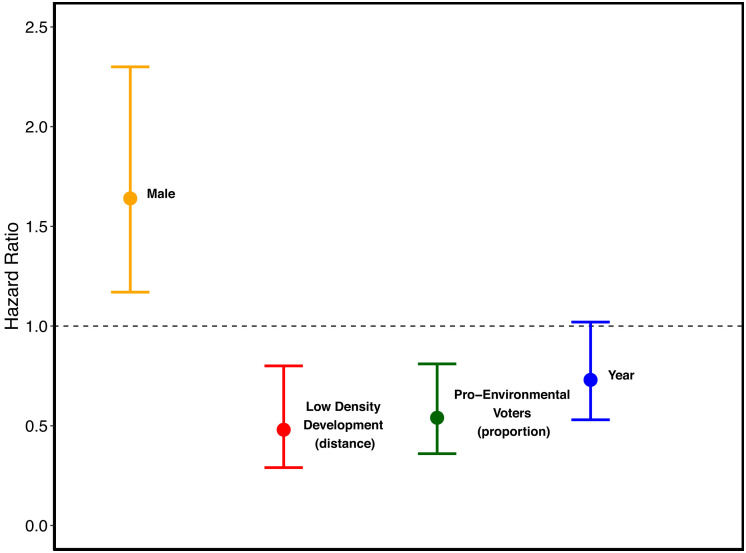
The most strongly supported model explaining mortality risk across California from 2001 to 2020 retained sex, distance to low-density development, proportion of voters supporting environmental ballot propositions, and a continuous variable for year (GPS-collared animals only, n = 389 animals and n = 142 mortalities; Table 2). We only included GPS-collared animals in the spatial mortality risk models because location data needed to link mortality data to spatial covariates across California were insufficient for animals tracked with very high–frequency (VHF) telemetry (Materials and Methods). There was greater mortality risk for males relative to females [hazard ratio = 1.64 and 95% CI (1.17, 2.30)]. Mortality risk was lower for mountain lions farther from low-density development [supporting P4; hazard ratio = 0.48 and 95% CI (0.29, 0.80); Fig. 5]. Competing risk models showed that risk specifically from human-caused mortality was lower with increasing distance to low-density development [hazard ratio = 0.30 and 95% CI (0.14, 0.65)], whereas other causes of mortality were not significantly influenced by distance to low-density development. Mortality risk was reduced for mountain lions in areas with a higher proportion of voters supporting environmentally focused ballot propositions [supporting P5; hazard ratio = 0.54 and 95% CI (0.36, 0.81); Fig. 5]. Although density of goat and sheep farms was retained in one plausible competing model (ΔAICc <2), this variable did not significantly influence mortality risk [supporting P5; hazard ratio = 1.03 and 95% CI (0.70, 1.51)]. Mortality risk specifically from intentional killing (management and poaching) was lower for mountain lions in areas with higher proportions of voters supporting environmental ballot propositions [hazard ratio = 0.53 and 95% CI (0.29, 0.99)]. Other (unintentional) forms of human-caused mortality (e.g., vehicle collisions), nonstrife-related natural mortality, and unknown mortality were not significantly influenced by the proportion of voters supporting environmental causes. Interestingly, risk of intraspecific strife was also lower for mountain lions in areas with higher proportions of voters supporting environmental causes [hazard ratio = 0.33 and 95% CI (0.14, 0.78)]. There was a trend toward reduced mortality risk across years from 2001 to 2020 that approached significance, but the CI for the hazard ratio overlapped one [hazard ratio = 0.73 and 95% CI (0.53, 1.02)]. Risk of management killing decreased across years from 2001 to 2020 [hazard ratio = 0.93 and 95% CI (0.87, 0.99)], whereas risk of natural [hazard ratio = 0.98 and 95% CI (0.92, 1.10)] and other human-caused [hazard ratio = 1.01 and 95% CI (0.96, 1.07)] mortality did not vary significantly across years. Other variables retained in plausible competing models did not significantly influence mortality risk, including distance to cover [hazard ratio = 1.07 and 95% CI (0.97, 1.17)], distance to secondary roads [hazard ratio = 0.85 and 95% CI (0.57, 1.26)], age class [hazard ratio = 0.85 and 95% CI (0.58, 1.24), reference = subadult], and distance to local roads [hazard ratio = 0.93 and 95% CI (0.55, 1.58); Table 2].
Table 2.
Model fit for strongly supported (ΔAICc < 2) mixed effects Cox proportional hazards models and the null model used to investigate spatial mortality risk for GPS-collared mountain lions (n = 389) across the state of California
| Model | k | AICc | ΔAICc |
|---|---|---|---|
| Male* + developed low† + voted proenvironment‡ + year§ | 4 | 1,675.02 | 0 |
| Male + developed low + voted proenvironment + year + cover¶ | 5 | 1,675.91 | 0.89 |
| Male + developed low + voted proenvironment + year + adult# | 5 | 1,676.41 | 1.39 |
| Male + developed low + voted proenvironment + year + secondary roads|| | 5 | 1,676.62 | 1.60 |
| Male + developed low + voted proenvironment + year + farm density** | 5 | 1,676.80 | 1.78 |
| Male + developed low + voted proenvironment | 3 | 1,676.83 | 1.81 |
| Male + developed low + voted proenvironment + year + local roads†† | 5 | 1,676.92 | 1.90 |
| Null model | 0 | 1,697.08 | 20.05 |
Shown are number of predictor variables (k), Akaike’s information criteria corrected for small samples (AICc), and the change in model fit relative to best model (ΔAICc).
*Dummy-coded variable for sex (0 = female and 1 = male).
†Distance to low-density development.
‡Proportion of voters supporting environmental ballot propositions.
§Continuous variable of year (2001 to 2020).
¶Distance to nearest forest or shrub patch.
#Dummy-coded variable for age class (0 = subadult and 1 = adult).
||Distance to secondary roads.
**Number of goat and sheep farms per square kilometer.
††Distance to smaller roads, residential streets.
Fig. 5.
Hazard ratio plot from the most strongly supported mixed-effects Cox proportional hazards model investigating factors influencing spatially varying mortality risk of GPS-collared mountain lions (n = 389) across the state of California. Hazard ratios above 1 indicate greater mortality risk, whereas those below 1 indicate reduced risk. Variables retained in the model included male (female is reference), distance to low-density development, proportion of voters supporting proenvironmental legislation, and the year (continuous, 2001 to 2020).
Discussion
Despite the importance of survival and human-caused mortality for understanding large carnivore population dynamics, these processes are mostly studied at spatial scales below what is most relevant to the demography and management of wide-ranging species (6, 31). Across the occupied range of mountain lions in California, encompassing an anthropogenic gradient from large tracts of undeveloped public land to major metropolitan areas, variation in mortality risk was driven by the presence of humans and potentially by variation in their tolerance. Although designated as a “specially protected mammal” in California, the most common known sources of mortality were intentional killing following livestock depredation and unintentional killing by vehicular collisions (Fig. 2A). However, mountain lion mortality did not increase linearly with human presence. Statewide, mountain lion mortality was highest in areas of intermediate human presence (Table 1). At finer scales, risk increased when mountain lions were closer to low-density development and relative to different human viewpoints (Fig. 5).
We found no support for the compensatory mortality hypothesis consistent with previous research with mountain lions (4, 6, 8, 18). Our analysis addressed limitations inherent in many previous tests of compensatory mortality as we conducted our analysis at a large scale (statewide), accounted for uncertainty in survival and cause-specific mortality estimates (6), and explored potential uncertainty associated with mortality events for which we could not determine cause of death (SI Appendix, Table S3). In terms of the point estimates, survival was lowest in the cluster of study areas with the greatest human-caused mortality, which also had the highest rate of natural mortality (Table 1), further suggesting that human-caused mortality is not compensated for by reductions in natural mortality. Adult female survival, the demographic parameter that has the strongest influence on population growth of mountain lions (e.g., refs. 28 and 32), tracked overall survival and was lowest in the cluster with the highest human-caused mortality (Table 1). Previous research suggests that mountain lion populations often decline with adult female survival lower than 0.78 (reviewed by ref. 8) but can also decline at higher rates of adult female survival (33). Adult female survival was below this threshold in the cluster of intermediate human presence (0.71) and slightly above it for the other two clusters (0.79 and 0.80; Table 1). It is important to recognize that our current results are limited to survival and mortality. Thus, integrating these survival rates into population models for different regions of California will be needed to fully evaluate relationships between human-caused mortality, survival rates, and population growth.
Human-caused mortality of mountain lions is generally not compensated for by changes in natural mortality, reproduction, or recruitment (4, 8, 18). Rather, immigration from connected populations with higher survival is required to maintain stable or positive population growth (4, 8, 18). Genetic analyses suggest that connectivity for mountain lions is compromised along the central coast and in southern California, given the strong genetic structure between regions and low genetic diversity of some populations (30, 34). Thus, high rates of human-caused mortality (e.g., ref. 9) and demographic stochasticity (e.g., ref. 28) could result in local population declines in some areas (e.g., southern California) if immigration is not sufficient due to lack of connectivity or high rates of mortality in potential source populations (35). Risk of mortality from management killing declined for mountain lions from 2001 to 2020, which drove a trend in reduced overall mortality risk during this period that approached significance. There was no change in natural mortality concurrent with reduced mortality from management, perhaps providing additional evidence that human-caused mortality is not compensated for by changes in natural mortality.
Our third hypothesis, that humans drive mortality risk for mountain lions, was supported as the strongest predictors of mountain lion mortality risk across the state were distance to low-density development and the views of humans as captured by their voting record on environmental issues. Recent work in the Santa Cruz Mountains found that risk of mountain lions being killed following depredation did not scale directly with development as risk was highest in areas of intermediate housing density (23). Our competing risk analysis showed that risk of both intentional human-caused mortality and vehicle mortality increased closer to low-density development. Our measure of human attitudes was a better predictor of both overall mortality risk and risk specifically from intentional human-caused mortality than the density of goat and sheep farms, suggesting that variation in human viewpoints influences risk more strongly than the actual sources of conflict on the landscape. We acknowledge that environmental voting records are not a direct reflection of the complex viewpoints of humans with respect to tolerance of mountain lions. However, the strength of this predictor suggests that human mindset could be an important driver of risk. In California, landowners experiencing depredation of livestock or pets do not always request a lethal permit from the CDFW, and not all permits result in mortality (27). Risk of mortality from management killing declined from 2001 to 2020, which might reflect increased tolerance of mountain lions in California during this period. Although challenging, understanding human dimensions underlying decisions made by individual landowners following depredation of domestic animals could provide valuable insight. Collaboration between ecologists and social scientists could facilitate integration of more direct data about tolerance into future mortality risk models for large carnivores.
Our competing risk analysis showed that mortality risk from intraspecific strife was also reduced in areas with higher proportions of citizens voting to support environmental ballot propositions. Why would strife, a seemingly natural form of mortality, be greater in areas where conflict with humans is common? Previous work has shown that mortality from hunting and management removal of mountain lions can cause increased immigration of dispersing male subadults (4, 36). Intraspecific aggression in mountain lions most often occurs in territorial disputes involving dispersing males (10). Thus, increased intraspecific mortality in areas of high human-caused mortality in California could be the result of changes in age and social structure precipitated by an influx of dispersing young males into areas following the removal of resident adult males by humans. Previous work has also documented correlations between the removal rate of mountain lions and other large carnivores and the rate of livestock depredation the following year, which might also be due to increased immigration of younger, conflict-prone animals following mortality (27, 37–39). Future research should investigate mechanistic relationships between human-caused mortality, changes in age structure, intraspecific aggression, and livestock depredation to elucidate consequences of killing mountain lions for both population dynamics and livestock depredation.
Recent policy changes (2017 to 2020; the “three-strikes” policy) mandate that at least two attempts at nonlethal management must be attempted prior to issuing permits to kill mountain lions in populations of conservation concern in the central coast and southern California. This policy appears to be a prudent first step in reducing human-caused mortality given concerns about connectivity and viability in these regions. Although the policy was specific to certain regions and only in place for the last 4 y of our study, this change in management could have contributed to the reduced mortality risk from management killing over time we documented. Additionally, education and improvements to animal husbandry practices might help to reduce loss of livestock and address the source of conflict (27). Highway crossing structures in combination with exclusionary fencing around roadways in areas occupied by mountain lions can be effective at reducing vehicular mortality and promoting connectivity for large carnivores (e.g., ref. 40). In 2022, ground was broken on two, high-profile wildlife crossing structures in central and southern California with the intention of improving connectivity for mountain lions (41). Given the publicity and locations of these structures, close to major urban centers of Los Angeles and San Jose, they may provide examples leading to construction of additional wildlife crossings that would help mitigate mortality and restore connectivity in California and beyond.
Human-caused mortality and conflict are ubiquitous where large carnivores share landscapes with humans (42). However, large carnivore conservation is possible when human tolerance results in sustainable levels of mortality (43, 44). California provides an important case study showing that providing legal protection from hunting is not always sufficient to prevent intentional and unintentional human-caused mortality from impacting population-level survival rates of large carnivores. Reduced survival from human-caused mortality can be problematic for small, isolated populations given that human-caused mortality can negatively influence local extinction probability (28, 42). Additionally, high rates of human-caused mortality can negatively impact metapopulation dynamics by reducing the rate, distance, and success of dispersal for mountain lions and other large carnivores (35, 45). Thus, future work should investigate mountain lion dispersal throughout California with movement data to identify behavioral mechanisms underlying observed genetic structure between populations (30, 34). Modeling population growth at regional and statewide scales is also needed to further evaluate the implications of human-caused mortality. To date, most population modeling with California mountain lions has focused on the small, isolated populations in highly developed areas in southern California (28, 29, 46). Modeling the dynamics of a broader diversity of mountain lion populations across the state would be valuable to understand the functioning of larger populations and evaluate their potential to contribute to source–sink dynamics. Understanding mortality and population dynamics of large carnivores at scales appropriate to inform conservation requires inference transcending local study areas. Large, multiorganizational collaborative networks are required to address fundamental questions about demography, behavior, and genetics of large carnivores at the broad scales occupied by metapopulations.
Materials and Methods
Study System and Field Methods.
We captured and tracked 590 mountain lions in 24 study areas across their occupied range in the state of California from 1974 to 2020 (Fig. 1 and SI Appendix, Table S1). Most of our data were collected after the statewide hunting ban in June 1990 (87%), including 76% since 2000. Our study areas reflected the considerable diversity in landscapes occupied by mountain lions in the state ranging from remote wilderness areas (e.g., Sierra Nevada mountains) to major metropolitan areas (e.g., city of Los Angeles; Fig. 1). We captured adult and subadult mountain lions using cage traps, cable restraints, or trained hounds. Capture and handling were done in accordance with multiple Institutional Animal Care and Use Committee protocols (Humboldt State University 98/99.W.22B; University of California, Berkeley R129-0394; University of California, Santa Cruz: Wilmc1912, Wilmc1509, and Wilmc1811; University of California, Davis 15341, 16886, 17233, and 22408; and National Park Service PWR_SAMO_Riley_Mt.Lion_2014.A3) and with approval from the CDFW under scientific collecting permits (SC-011968, SC-00703, SC-7303, SC-002730, SC-009875, SC-013416, and SC-0005636). We deployed mortality-sensitive very high–frequency (VHF, n = 199) or global positioning system (GPS, n = 391) collars on mountain lions and monitored their movements and survival with ground and aerial tracking (VHF) or by remote download of GPS data. VHF data are appropriate for estimating survival and cause-specific mortality rates, but we excluded data from animals tracked exclusively with VHF telemetry from spatial analyses requiring abundant, highly accurate location data (i.e., hierarchical clustering analysis and spatial mortality risk modeling, see below). We estimated age of captured animals using size and characteristics of teeth or because we knew their age from tagging them at natal dens (11). We monitored survival at least approximately once per week (VHF) or once per day (GPS) and usually more frequently. We investigated mortality sites for evidence of cause of death and submitted carcasses for necropsy by veterinarians if we found sufficient remains, and cause of death was not apparent in the field.
Survival and Cause-Specific Mortality Estimation.
We estimated annual survival rates for independent-aged mountain lions using the nonparametric Kaplan–Meier product limit estimator and an annual recurrent timescale (47). We considered nonbreeding animals that were independent from their mothers to be subadults and breeding animals to be adults (10). We classified animals by estimated age classes (subadults: 12 to 24 mo for females and 12 to 36 mo for males, and adults: >24 mo for females and >36 mo for males) and adjusted age classes where possible using field observations regarding independence and breeding status. We entered animals into the survival model in a staggered manner on the day that they were fit with a GPS or VHF transmitter (48). Animals exited upon death (coded 1) or were right censored if the monitoring period ended prior to death (coded 0). Monitoring periods ended prior to death due to collar failure, timed release of collars, emigration from the study area (VHF only), or at the end of the study period (31 December 2020). We censored all living animals with an active collar on the last day of each year (31 December) and reentered them on the first day of the following year (1 January). Thus, we clustered data from multiple years for the same individuals and estimated robust SEs. Prior to our main analyses, we evaluated potential differences in survival before and after the 1990 legislation banning mountain lion hunting in study areas where we had data both before and after 1990 and did not detect significant differences (SI Appendix, Appendix S3). Thus, we used all VHF and GPS telemetry data from 1976 to 2020 for our statewide survival and cause-specific mortality rates.
To evaluate the relative importance of different, exclusive causes of mortality, we estimated annual cause-specific mortality rates using the nonparametric cumulative incidence function estimator (49). We estimated annual rates for two categories of natural mortality resulting from intraspecific strife and nonstrife natural causes (e.g., starvation and disease). We estimated annual rates for four categories of human-caused mortality: management, vehicles, poaching, and other human causes. Management mortality included killing animals following livestock depredation (75%), killing of federally endangered Sierra Nevada bighorn sheep (Ovis canadensis sierrae, 23%; 50), or because they were perceived as a threat to human safety (2%). Depredation of domestic animals by mountain lions in California primarily involves smaller livestock (e.g., goats and sheep), but also lower proportions of larger livestock (e.g., cattle and horses) and pets (27). During our study, when the CDFW received reports of domestic animal depredation suspected to be caused by mountain lions, they coordinated a site visit to evaluate evidence. If a mountain lion was determined to be responsible and the landowner requested a permit, the CDFW issued a permit allowing the mountain lion to be legally killed (additional details available in ref. 27). Most mountain lions killed under these permits were shot at the original depredation site. Poaching was intentional illegal killing. Other human causes were all other forms of (unintentional) anthropogenic mortality including death from rodenticide poisoning or wildfires ignited by humans. Sample sizes for specific causes of death within each mortality class are provided in SI Appendix, Table S4. The unknown mortality events in our data occurred because a) carcasses were too decomposed or scavenged to yield informative evidence or b) field and laboratory necropsies were inconclusive even with a relatively intact carcass. We also combined all natural causes and all human causes to estimate overall annual human and natural mortality rates and compared these rates to test the prediction that human-caused mortality exceeded natural mortality (P1).
Hierarchical Cluster Analysis (HCA).
We identified groups of similar study areas using a hierarchical cluster analysis (HCA). Specifically, we estimated a number of landscape features and characteristics of the human populations (SI Appendix, Table S5) associated with location data of mountain lions in the 17 study areas within which we had GPS telemetry (SI Appendix, Table S1). We limited this analysis to animals with functioning GPS collars (n = 389) because we were evaluating the location of mountain lions relative to multiple spatial covariates, which required abundant, highly accurate location data that were not available for animals tracked by VHF telemetry. Thus, we excluded animals with VHF collars and two GPS-collared animals whose units failed immediately and recorded no locations. We then used a principal components analysis (PCA) to reduce the dimensions of the data into a set of principal components containing the most important information. Next, we performed agglomerative clustering using Ward’s minimum variance criterion on the first two principal components to estimate a dendrogram depicting clusters of study areas with similar characteristics (51). We selected the number of clusters that minimized within-cluster variance while maximizing variance across clusters (51). We implemented the HCA and PCA in the package ‘FactoMineR’ in R version 4.1.2 (R Development Core Team, 2022). We estimated survival and cause-specific mortality data within each cluster to make comparisons across clusters of areas occupied by mountain lions with similarities in human populations and landscape conditions.
Additive vs. Compensatory Mortality.
We tested predictions of the compensatory mortality hypothesis by modeling annual rates of overall survival, human-caused mortality, and natural mortality from 8 study areas across California within which we tracked at least 29 independent-aged mountain lions to estimate survival and mortality rates (n = 431 animals, range = 29 to 132 animals per study area; SI Appendix, Table S1). We limited this analysis to the study areas with ≥29 animals in an effort to ensure the survival and mortality estimates were representative of those areas. However, we also tested the same predictions with additional study areas (n = 13 with ≥10 animals) to evaluate whether the results were robust to sample size and additional spatial variation (SI Appendix, Appendix S2). We fit a linear regression model with survival rate of each population as the response variable and human-caused mortality rate as the predictor variable to test our prediction that overall survival did not decline as a function of human-caused mortality (P2). Next, we fit a model where natural mortality rate was the response variable and human-caused mortality rate was the predictor variable to test the prediction that natural mortality would decline with increased human-caused mortality (P3). If natural mortality did not decline, we concluded that human-caused mortality was additive, whereas if natural mortality actually increased as a function of human-caused mortality, this suggests overadditive human-caused mortality (6). We transformed the proportional response variables for both models using the arcsine square root transformation to meet the assumptions of regression. We accounted for the uncertainty in our estimates by drawing 10,000 samples from the 95th percentile of beta distributions derived from the uncertainty surrounding our estimates of survival and mortality rates for each study area. We fit the linear regressions described above with each sample draw and estimated mean beta coefficients across all runs along with 90% highest posterior density (HPD) intervals to test our two predictions. Given that cause of death was not known for all mortality events, we also investigated the influence of this uncertainty on the relationship between human-caused and natural mortality by attributing the unknown mortality to varying proportions of human- and natural-caused mortality (including extreme scenarios attributing 100% of unknown mortality to human causes and 100% to natural causes) and rerunning the model with natural mortality as a function of human-caused mortality to ensure this prediction test (P3) was robust to our failure to identify cause of death in all cases (SI Appendix, Appendix S1 and Table S3).
Mortality Risk Modeling.
We investigated intrinsic and extrinsic factors influencing mortality risk of mountain lions with semiparametric Cox proportional hazards regression models with mixed effects (shared frailty models; 52). We investigated the potential influence of sex (females = 0 and males = 1) and age class (subadults = 0 and adults = 1) with discrete, dummy-coded predictor variables. We also considered the potential influence of continuous, spatially varying environmental variables by linking the GPS location data for each individual to landscape features or characteristics of local human populations. Similar to the HCA, we restricted our spatial mortality risk models to animals tracked with GPS telemetry (n = 389) because these analyses required abundant, highly accurate location data. In these models, we considered the distance of mountain lions to developed open space (mostly lawn grasses with 0 to 20% impervious surfaces) and low-intensity development (20 to 49% impervious surfaces) from the National Land Cover Database [NLCD; 30-m resolution]), which we combined into a single class of development that we refer to as low-density development. We also estimated the distance of mountain lions to secondary (secondary highways or major connector roads) and local (residential streets and all other paved roads smaller than secondary roads) roads from the US Geological Survey National Transportation Dataset distance to buildings (from Microsoft building footprint dataset; 30-m resolution), density of goat and sheep farms (farms/km2; from the US Department of Agriculture Census), and distance to cover (any pixel classified as forest or shrub habitat from the NLCD). Additional details of spatial variables are in SI Appendix, Table S5. We initially planned to consider the influence of primary roads and higher density development, but these features were rare or nonexistent in many areas occupied by mountain lions across California, so they were excluded from the analysis. We predicted that differences in human viewpoints about the environment across California influenced their tolerance of large carnivores. In the absence of direct data on human views of large carnivores, we created a variable reflecting broader support for the environment to serve as a proxy by accessing publicly available records on voting records by citizens of California for five environmentally focused statewide ballot propositions from 2010 to 2018 (details in SI Appendix, Table S5). From these records, we used voting data at the county level to generate proportions of voters that supported proenvironmental propositions that we linked with locations of mountain lions as they moved around the landscape.
For both landscape features and voting records, we intersected all mountain lion telemetry locations with spatially and temporally varying data for each covariate and then averaged the values for each week of monitoring. We estimated landscape features based on the NLCD with layers from the closest year to the telemetry data (updated every 2 to 3 y from 2001 to 2019). We averaged voting records across the results of the environmental propositions (SI Appendix, Table S5) such that these values only changed spatially when mountain lions moved between counties. Thus, in our spatial mortality risk model, we included a separate row for each individual during each week of monitoring and updated the spatial covariate data according to their position in space and time. We also fit a continuous variable of year to investigate and account for temporal trends in mortality risk across the years for which we had data (2001 to 2020). We included frailty terms (random effects assuming a Gaussian distribution) for individual mountain lions nested within each study area to account for the lack of independence between the multiple data rows for each individual and study area (52). We predicted that mortality risk would increase when mountain lions were closer to low-density development (P4), buildings, and roads and farther from vegetative cover. We also predicted that mortality risk would be more strongly influenced by human attitudes (captured through voting record) regarding the environment than the density of livestock operations in local areas (P5).
We used competing risks models to verify that the anthropogenic variables we considered influenced risk of the specific causes of death consistent with our predictions and to explore temporal trends of specific causes of death (22, 53). Here, we created multiple records for each individual (one record for each cause of death) with an associated stratum variable indicating the specific cause. Then, we fit models with this stratum variable in the model statement to allow for separate hazard functions for each cause of death. We included interactions between dummy-coded cause of death variables and a predictor variable of interest (distance to low-density development, proportion of voters supporting proenvironmental propositions, and year in separate competing risk models). In the presence of the interactions, the main effect of the variable of interest provided a beta coefficient for the influence of that variable on the cause of death withheld as the reference category (11). Thus, we were able to quantify the degree to which risk from specific causes increased or decreased with our predictors.
We centered and scaled all continuous variables included in our models by subtracting the mean and dividing by two SDs. We did not include correlated variables (r > 0.5, i.e., distance to buildings and distance to low-density development) in the same models within our model set. We compared relative fit of mortality risk models with all combinations of variables that were not strongly correlated using Akaike’s information criterion corrected for small samples (AICc; with n = number of events; 54). We considered models to be strongly supported if the difference in AICc from the top model (ΔAICc) was less than 2 (54). We verified the proportional hazards assumption of Cox models by examining the distribution of Schoenfeld residuals with a chi-square test using the cox.zph function in the “survival” package (52). We examined parameter estimates for strongly supported models (ΔAICc < 2) and present exponentiated beta coefficients (hazard ratios), SEs, and 95% CIs. We considered variables with 95% CIs that did not overlap 1 to have significantly increased or decreased mortality risk. We conducted all analyses using the “survival”, “coxme”, “MASS”, and “MuMIn” packages in R version 4.1.2.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
We thank M. Gabriel, P. Johnston, D. Krucki, and P. Mahoney. This work was funded and supported by the School of Natural Resources, University of Nebraska-Lincoln (J.F.B. and K.D.D.); California Department of Fish and Wildlife (B.C., D.J.G., T.R.S., H.U.W., and J.A.D.); Wildlife Restoration Fund to the California Department of Fish and Wildlife; US Forest Service; US Department of Agriculture—Wildlife Services; California Department of Parks and Recreation; Hoopa Tribe; and Department of Defense (J.A.D.); The California State Department of Parks and Recreation, the California Department of Fish and Wildlife, The Nature Conservancy, the McBeth Foundation, the Anza-Borrego Foundation, The Nature Reserve of Orange County, the NSF, the Foothill/Eastern Transportation Corridor Agency, San Diego County Association of Governments Environmental Mitigation Program, the San Diego Foundation, Felidae Conservation Fund, the Mountain Lion Foundation, the Santa Rosa Plateau Foundation, the Institute for Wildlife Studies, and private donors (W.M.B. and T.W.V.); the National Park Service, California State Parks, Santa Monica Mountains Fund, Santa Monica Mountains Conservancy/Mountains Recreation and Conservation Authority, and Calabasas Landfill (S.P.D.R. and J.A.S.); Audubon Canyon Ranch and its donors (QEM); NSF grants 0963022 and 1255913 and by the support of the Gordon and Betty Moore Foundation and the Peninsula Open Space Trust (C.C.W.). We also thank the many field technicians, graduate students, volunteers, and other organizations that bolstered data collection efforts.
Author contributions
J.F.B., K.D.D., and J.A.D. designed research; J.F.B., K.D.D., P.B., W.M.B., B.C., D.J.G., D.K.G., J.M.H., Q.E.M., A.C.N., S.P.D.R., J.A.S., T.R.S., T.W.V., G.M.W., C.C.W., H.U.W., and J.A.D. performed research; J.F.B. and K.D.D. analyzed data; K.D.D., P.B., W.M.B., B.C., D.J.G., D.K.G., J.M.H., Q.E.M., A.C.N., S.P.D.R., J.A.S., T.R.S., T.W.V., G.M.W., C.C.W., H.U.W., and J.A.D. contributed to revising paper; and J.F.B. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Data, Materials, and Software Availability
All data needed to reproduce the analyses and results in this paper can be found at the following link: https://github.com/jfbenson22/BensonCalLionMortality (55).
Supporting Information
References
- 1.Darimont C. T., Fox C. H., Bryan H. M., Reimchen T. T., The unique ecology of human predators. Science 349, 858–860 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Lamb C. T., et al. , The ecology of human–carnivore coexistence. Proc. Natl. Acad. Sci. U.S.A. 117, 17876–17883 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weaver J. L., Paquet P. C., Ruggiero L. F., Resilience and conservation of large carnivores in the Rocky Mountains. Conserv. Biol. 10, 964–976 (1996). [Google Scholar]
- 4.Cooley H. S., Wielgus R. B., Koehler G. M., Robinson H. S., Maletzke B. T., Does hunting regulate cougar populations? A test of the compensatory mortality hypothesis. Ecology 90, 2913–2921 (2009). [DOI] [PubMed] [Google Scholar]
- 5.Estes J. A., et al. , Trophic downgrading of planet Earth. Science 333, 301–306 (2011). [DOI] [PubMed] [Google Scholar]
- 6.Wolfe L. M., et al. , Is anthropogenic cougar mortality compensated by changes in natural mortality in Utah? Insight from long-term studies. Biol. Conserv. 182, 187–196 (2015). [Google Scholar]
- 7.Loveridge A. J., Valeix M., Elliot N. B., Macdonald D. W., The landscape of anthropogenic mortality: How African lions respond to spatial variation in risk. J. Appl. Ecol. 54, 815–825 (2017). [Google Scholar]
- 8.Logan K., Runge M., Effects of hunting on a puma population in Colorado. Wildl. Monog. 209, 1–35 (2021). [Google Scholar]
- 9.Vickers T. W., et al. , Survival and mortality of pumas (Puma concolor) in a fragmented, urbanizing landscape. PloS one 10, e0131490 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Logan K. A., Sweanor L. L., Desert Puma: Evolutionary Ecology and Conservation of an Enduring Carnivore (Island Press, Washington, D.C., 2001). [Google Scholar]
- 11.Benson J. F., Sikich J. A., Riley S.P.D., Survival and competing mortality risks of mountain lions in a major metropolitan area. Biol. Conserv. 241, 108294 (2020). [Google Scholar]
- 12.Nisi A., Benson J. F., King R., Wilmers C. C., Habitat fragmentation reduces survival and drives source-sink dynamics for a large carnivore. Ecol. Appl. (2023), in press. [DOI] [PubMed] [Google Scholar]
- 13.Burnham K. P., Anderson D. R., Tests of compensatory vs. additive hypotheses of mortality in mallards. Ecology 65, 105–112 (1984). [Google Scholar]
- 14.Boyce M. S., Sinclair A. R. E., White G. C., Seasonal compensation of predation and harvesting. Oikos 87, 419–426 (1999). [Google Scholar]
- 15.Pöysä H., Ecological basis of sustainable harvesting: Is the prevailing paradigm of compensatory mortality still valid? Oikos 104, 612–615 (2004). [Google Scholar]
- 16.Creel S., Rotella J. J., Meta-analysis of relationships between human offtake, total mortality and population dynamics of gray wolves (Canis lupus). PLoS one 5, e12918 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuller T. K., Mech L. D., Cochrane J. F., “Wolf population dynamics” in Wolves: Behavior, Ecology, and Conservation, Mech L., Boitani L., Eds. (University of Chicago Press, 2003), pp. 161–191. [Google Scholar]
- 18.Robinson H. S., et al. , A test of the compensatory mortality hypothesis in mountain lions: A management experiment in west-central Montana. J. Wildl. Manage. 78, 791–807 (2014). [Google Scholar]
- 19.Stenglein J. L., Wydeven A. P., Van Deelen T. R., Compensatory mortality in a recovering top carnivore: Wolves in Wisconsin, USA (1979–2013). Oecologia 187, 99–111 (2018). [DOI] [PubMed] [Google Scholar]
- 20.Murray D. L., et al. , Death from anthropogenic causes is partially compensatory in recovering wolf populations. Biol. Conserv. 143, 2514–2524 (2010). [Google Scholar]
- 21.Creel S., et al. , Questionable policy for large carnivore hunting. Science 350, 1473–1475 (2015). [DOI] [PubMed] [Google Scholar]
- 22.Benson J. F., Patterson B. R., P. J. Mahoney, A protected area influences genotype-specific survival and the structure of a Canis hybrid zone. Ecology 95, 254–264 (2014). [DOI] [PubMed] [Google Scholar]
- 23.Nisi A., Benson J. F., Wilmers C. C., Puma responses to unreliable human cues suggest an ecological trap in a fragmented landscape. Oikos 2022, e09051 (2022). [Google Scholar]
- 24.Bruskotter J. T., Wilson R. S., Determining where the wild things will be: Using psychological theory to find tolerance for large carnivores. Conserv. Lett. 7, 158–165 (2014). [Google Scholar]
- 25.Treves A., Bruskotter J., Tolerance for predatory wildlife. Science 344, 476–477 (2014). [DOI] [PubMed] [Google Scholar]
- 26.Lenihan J. M., Drapek R., Bachelet D., Neilson R. P., Climate change effects on vegetation distribution, carbon, and fire in California. Ecol. Appl. 13, 1667–1681 (2003). [Google Scholar]
- 27.Dellinger J. A., Macon D. K., Rudd J. L., Clifford D. L., Torres S. G., Temporal trends and drivers of mountain lion depredation in California, USA. Hum-Wildl. Interact. 15, 21 (2021). [Google Scholar]
- 28.Benson J. F., et al. , Interactions between demography, genetics, and landscape connectivity increase extinction probability for a small population of large carnivores in a major metropolitan area. Proc. Roy. Soc. Lond. B. Biol. Sci. 283, 20160957 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benson J. F., et al. , Extinction vortex dynamics of top predators isolated by urbanization. Ecol. Appl. 29, e01868 (2019). [DOI] [PubMed] [Google Scholar]
- 30.Gustafson K. D., et al. , Genetic source–sink dynamics among naturally structured and anthropogenically fragmented puma populations. Conserv. Genet. 20, 215–227 (2019). [Google Scholar]
- 31.Liberg O., et al. , Shoot, shovel and shut up: Cryptic poaching slows restoration of a large carnivore in Europe. Proc. Roy. Soc. B. Biol. Sci. 279, 910–915 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lambert C. M., et al. , Cougar population dynamics and viability in the Pacific Northwest. J. Wildl. Manage. 70, 246–254 (2006). [Google Scholar]
- 33.Ruth T. K., et al. , Cougar survival and source-sink structure on Greater Yellowstone’s Northern Range. J. Wildl. Manage. 75, 1381–1398 (2011). [Google Scholar]
- 34.Gustafson K. D., et al. , Multi-population puma connectivity could restore genomic diversity to at-risk coastal populations in California. Evol. Appl. 15, 286–299 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Newby J. R., et al. , Human-caused mortality influences spatial population dynamics: Pumas in landscapes with varying mortality risks. Biol. Conserv. 159, 230–239 (2013). [Google Scholar]
- 36.Robinson H. S., Wielgus R. B., Cooley H. S., Cooley S. W., Sink populations in carnivore management: Cougar demography and immigration in a hunted population. Ecol. Appl. 18, 1028–1037 (2008). [DOI] [PubMed] [Google Scholar]
- 37.Peebles K. A., Wielgus R. B., Maletzke B. T., Swanson M. E., Effects of remedial sport hunting on cougar complaints and livestock depredations. PLoS one 8, e79713 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wielgus R. B., Peebles K. A., Effects of wolf mortality on livestock depredations. PloS One 9, e113505 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Teichman K. J., Cristescu B., Darimont C. T., Hunting as a management tool? Cougar-human conflict is positively related to trophy hunting. BMC Ecol. 16, 1–8 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gilhooly P. S., Nielsen S. E., Whittington J., St Clair C. C., Wildlife mortality on roads and railways following highway mitigation. Ecosphere 10, e02597 (2019). [Google Scholar]
- 41.Pittman C., World’s largest wildlife bridge could save mountain lions. Sci. Amer. 325, 72–79 (2021). [Google Scholar]
- 42.Ripple W. J., et al. , Status and ecological effects of the world’s largest carnivores. Science 343, 1241484 (2014). [DOI] [PubMed] [Google Scholar]
- 43.Balme G. A., Slotow R., Hunter L. T., Impact of conservation interventions on the dynamics and persistence of a persecuted leopard (Panthera pardus) population. Biol. Conserv. 142, 2681–2690 (2009). [Google Scholar]
- 44.Chapron G., et al. , Recovery of large carnivores in Europe’s modern human-dominated landscapes. Science 346, 1517–1519 (2014). [DOI] [PubMed] [Google Scholar]
- 45.Morales-González A., Fernández-Gil A., Quevedo M., Revilla E., Patterns and determinants of dispersal in grey wolves (Canis lupus). Biol. Rev. 97, 466–480 (2022). [DOI] [PubMed] [Google Scholar]
- 46.Beier P., Determining minimum habitat areas and habitat corridors for cougars. Conserv. Biol. 7, 94–108 (1993). [Google Scholar]
- 47.Fieberg J., DelGiudice G. D., What time is it? Choice of time origin and scale in extended proportional hazards models. Ecology 90, 1687–1697 (2009). [DOI] [PubMed] [Google Scholar]
- 48.Pollock K. H., Winterstein S. R., Bunck C. M., Curtis P. D., Survival analysis in telemetry studies: The staggered entry design. J. Wildl. Manage. 53, 7–15 (1989). [Google Scholar]
- 49.Heisey D. M., Patterson B. R., A review of methods to estimate cause-specific mortality in presence of competing risks. J. Wildl. Manage. 70, 1544–1555 (2006). [Google Scholar]
- 50.Gammons D. J., et al. , Predation impedes recovery of Sierra Nevada bighorn sheep. Calif. Fish Wildl. Special CESA issue, 444–470 (2021). [Google Scholar]
- 51.Husson F., Josse J., Pages J., Principal component methods-hierarchical clustering-partitional clustering: Why would we need to choose for visualizing data. Appl. Math. Depart. 1–17 (2010). [Google Scholar]
- 52.Therneau T. M., Grambsch P. M., Modeling Survival Data: Extending the Cox Model (Springer Science & Business Media, 2000). [Google Scholar]
- 53.Lunn M., McNeil D., Applying Cox regression to competing risks. Biometrics 51, 524–532 (1995). [PubMed] [Google Scholar]
- 54.Burnham K. P., Anderson D. R., Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach (Springer-Verlag, New York, 2002). [Google Scholar]
- 55.Benson J. F., et al. , Data for “Benson et al. 2023. The ecology of human-caused mortality for a protected large carnivore. PNAS.” https://github.com/jfbenson22/BensonCalLionMortality. Accessed 27 February 2023. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
All data needed to reproduce the analyses and results in this paper can be found at the following link: https://github.com/jfbenson22/BensonCalLionMortality (55).