Abstract
The transcriptional factor liver receptor homolog 1 (LRH1) regulates pancreatic development, and may participate in pancreatic oncogenesis through activation of growth factor signaling transduction cascades. We measured transcriptional activity of β-catenin in response to LRH1 stimulation by a Top-flash reporter assay. The pancreatic cancer (PC) phenotype was then characterized by cell migration, wound healing, invasion, and sphere formation in vitro, as well as tumor formation and distant metastatic spread in vivo. We compared results between vector control and LRH1-overexpressing stable PC cell lines. In addition, tumor burden, angiogenesis, histologic characteristics, and hepatic spread were assessed in orthotopic and experimental liver metastatic murine models. Expression of downstream LRH1 related genes was evaluated by Western blot and immunohistochemistry in PC cell lines and human tumor specimens. Specific inhibition of LRH1 expression and function was accomplished by shRNAs ‘‘knockdown’’ experiments. It was found that LRH1 enhanced transcriptional activity of β-catenin and the expression of downstream target genes (c-Myc, MMP2/9), as well as promoted migration, wound healing, invasion, and sphere formation of PC cell lines. Specific inhibition of LRH1 by shRNAs reduced cell migration, invasion, sphere formation and expression of c-Myc and MMP2/9 target genes. Mice injected with LRH1 overexpressing stable PC cells developed tumors with increased size and exhibited striking hepatic metastatic spread. More important, LRH1 was overexpressed in PC tumors compared to adjacent normal pancreas. Our findings demonstrate that LRH1 overexpression is associated with increased PC growth and metastatic spread, indicating that LRH1-targeted therapy could inhibit tumor progression.
Keywords: Liver receptor homolog 1, c-Myc, MMP2, MMP9, Pancreatic cancer
Introduction
Pancreatic cancer (PC) is the eighth most common cause of cancer-related deaths worldwide with an extremely poor prognosis [22]. To improve clinical outcome, progress requires development of diagnostic methods and identification of new therapeutic targets. Signaling pathways involved in the development of the embryonic pancreas have been implicated as critical elements in the malignant transformation process of the adult pancreas [18,24]. The liver receptor homolog 1 (LRH1) (also known as nuclear receptor subfamily 5 group A member 2 NR5A2) regulates pancreatic embryogenesis [6] and may participate in pancreatic tumorigenesis. LRH1 appears to be involved in liver and pancreas differentiation during early embryonic development, and modulates cholesterol/bile-acid homeostasis and steroidogenesis in adulthood [6,12].
Previous studies have found that LRH1 overexpression may be involved in oncogenesis of breast [25], gastric [27], colon [21], and pancreatic tumors [2]. LRH1 promotes cell proliferation by enhancing ERα transcription of growth-related target genes mediated by estrogen in breast cancer 1 [5]. LRH1 promotes breast cancer motility and invasion by remodeling of the actin cytoskeleton and E-cadherin cleavage [4]. LRH1 also promotes intestinal tumor formation via regulating cell cycle and inflammatory pathways [21]. Moreover, LRH1 genetic variants have been associated with individual susceptibility to PC [20]. Interestingly, LRH1 is highly expressed in some PC cell lines [2]. We have found that LRH1 upregulates cyclin D1/E1 and promotes cell proliferation and tumor growth in PC [14]. However, it is unclear how LRH1 contributes to PC oncogenesis and tumor progression. Thus, using cell-based assays, xenografts in nude mice, tumor and tissue specimens derived from PC patients, we investigated the mechanism(s) by which LRH1 promotes PC growth, progression and metastatic spread through activation of β-catenin mediated signal transduction pathways.
Materials and methods
Cell culture
The human PC cell lines AsPC-1, Capan-1, and MIA PaCa2, human embryonic kidney (HEK) 293 (ATCC) have been authenticated by short tandem repeat profiling to reduce the frequency of cell misidentification [17]. Cells were cultured at 37 °C in a humidified atmosphere containing 5% CO2 in corresponding medium supplemented with 10% fetal bovine serum (FBS) and antibiotics (penicillin and streptomycin). Cells were passaged when they reached 80% confluence.
Topflash assay
Capan-1, AsPC-1, MIA PaCa2 and HEK 293 cells were seeded in 24 well plates at 50–60% confluence. After 24 h, cells were transfected with 100 ng of the Topflash reporter and 10 ng of pRL-SV40 Vector plasmids with/without 10 ng β-catenin and 60 ng LRH1 expression plasmids per well in the serum free medium. Total amount of plasmids was balanced with an empty pc-DNA3 plasmid. After 4 h of transfection, serum free medium was replaced with normal medium with/without DMSO or β-catenin inhibitor Quercetin (Sigma–Aldrich) at 160 μM, and incubated for another 20 h. Then the samples were harvested to measure luciferase reporter gene expression (Promega Dual luciferase kit). The luciferase activity was normalized to Renilla luciferase activity from co-transfected internal control plasmid pRL-SV40.
Lentiviral infected stable cell lines with overexpression or knockdown of LRH1
We established two stable Capan-1 and AsPC-1 cell lines with constitutive expression of LRH1, and three stable MIA PaCa2 cell lines with constitutive knock-down of LRH1 by specific shRNAs using the lentiviral expression system (GeneCopoeia, #EX-Z2607-Lv105 for overexpressing and #HSH006437 for knockdown). Three shRNAs sequences used to target LRH1 were as follows: CACGGACTTACACCT-ATTG (nucleotides 252–270), GTTGTCCTTACTGTCGTTT (nucleotides 478–496), and TTGCCTCCTACAGACTATG (nucleotides 756–774). After 48 h of transfection with packaging plasmids and pLentiviral plasmids of target genes in 293 T cells, the supernatants containing lentiviral particles were filtered by 0.45 μm nitrocellulose membrane and mixed with polybrene at 8 μg/ml. Twenty-four hours after lentiviral infection, cells were incubated with the corresponding media for 24 h, then selected with 8 μg/mL (for Capan-1), 3 μg/mL (for AsPC-1) or 4 μg/mL (for MIA PaCa2) puromycin overtime to eliminate un-infected cells. The LRH1 protein level in stable PC cell lines was confirmed by Western blot. The selected cell lines were routinely cultured in corresponding puromycin media until two days before the experiments, then the cells were cultured in media without puromycin.
Western blot analysis
Cell lysates were treated with ConA-sepharose beads overnight followed by centrifugation to remove cadherin-bound β-catenin. Total Cell lysates and non-membrane bound cell lysates were separated by SDS PAGE and transferred to nitrocellulose membranes. Western blot analysis was performed using primary antibodies against LRH1 (Abcam, ab18293), c-Myc, β-catenin and MMP2 (Cell Signaling Technology, #5605, #9562, and #3132, respectively), and MMP9 (Santa Cruz, sc-21733). Protein bands were visualized by IRDye® 680RD Infrared Dye and IRDye® 800CW Infrared Dye and exposed on Odyssey image system (LI-COR).
Migration
Cell migration was assessed using a modified 24-well Boyden chamber. The top chamber (Transwell) with 8.0-mm pores on the filter membrane (BD Biosciences) was inserted into a 24-well plate (bottom chamber). 10% FBS containing medium was placed in the bottom chambers as a chemoattractant. Vectors-, LRH1- and LRH1 shRNA-stable PC cells (AsPC-1, Capan-1 and MIA PaCa2) (5 × 104 cells per well) were seeded onto the top chambers in 400 μl serum-free corresponding medium. After incubation for 24 h at 37 °C, migrated cells on the bottom surface of the filters were fixed and stained with crystal violet. The cells on the top surface of the membrane were mechanically removed, and the cells that had migrated to the bottom surface of the membrane were observed. The average number of migrated cells from 5 to 7 randomly chosen fields on the bottom surface of the membrane was counted. All data were obtained from at least three independent experiments.
Wound healing assay
Vectors- and LRH1-stable PC cells (AsPC-1 and Capan-1) were seeded in a 6-well plate and cultured to reach 80% confluence. Initially, cells were starved for 24 h in media containing 1% fetal calf serum (FCS). Afterwards a scratch was made using a 200 μl pipette tip. The wounded monolayer was washed with PBS, incubated with 1% FCS, and photographed using microscope. Cells were incubated for an additional 24 h after which the photographs were taken for the wounded area.
Invasion
Vectors-, LRH1- and LRH1 shRNA-stable PC cells (AsPC-1, Capan-1 and MIA PaCa2) growing in log phase were incubated in serum-free medium for 24 h. Matrigel invasion chambers (BD BioCoat Matrigel Invasion 24-well Chamber, 8 μm pores, BD Biosciences) were rehydrated for 2 h at 37 °C with serum-free medium. Immediately prior to the addition of dissociated cells to the upper chamber (2.5 × 104 cells/500μL), 10% FBS media were added to the lower chamber. After 36 h, Matrigel and non-migrating cells were removed from the top chamber with a Q-tip. Invading cells on the bottom of the membrane were fixed in ethanol and stained with crystal violet. After drying overnight, stained cells were counted under a microscope.
Sphere formation
45 μL of Matrigel was spread evenly to each well of an 8-well chamber slide (BD Falcon) and the slides were placed in a cell culture incubator for 30 min. Single-cell suspensions derived from Vectors-, LRH1- and LRH1 shRNA-stable PC cells (AsPC-1, Capan-1 and MIA PaCa2) were suspended in corresponding medium containing 2% Matrigel at a concentration of 2000 cells per 400 μL. Cells were allowed to grow in the incubator for 6 days. Fresh media containing 2% Matrigel were changed every 2 days. The spheres formed after 5 days were evaluated for their sizes by light microscopy [11].
Orthotopic tumor model
Athymic BALB/c male nude mice (Charles River laboratory) were housed in laminar flow cabinets under specific pathogen-free conditions and used at 5–6 weeks of age. All animal protocols were approved by the Institutional Animal Care and Use Committee of Rhode Island Hospital. Vector- and LRH1-stable Capan-1 cells in exponential growth phase were harvested, washed, and resuspended in Ca2+/Mg2+-free HBSS to the desired cell concentration. Cell viability was determined by trypan blue exclusion and single-cell suspensions of >90% viability was used. For intrapancreatic injections, the mice were anesthetized and a 0.5–1-cm incision was made in the left subcostal region. Vector- and LRH1-expressing tumor cells (3 × 106) in a volume of 50 μL were injected into the body of the pancreas of the nude mice (n = 5) [16,29]. The peritoneum and skin were closed with a 4.0 surgical suture. The animals were euthanized with an overdose of CO2 exposure when their tumor sizes reached 2 cm in diameter or the animals developed ascites during the observation period, and the time of euthanasia was recorded as mortality. All surviving mice were euthanized after 3 weeks and evaluated macroscopically for the presence of orthotopic tumors and the presence of metastases in the abdominal cavity. Primary tumors were removed, weighed and fixed with 10% neutral buffered formalin. Histologic characteristics were obtained from tumor tissue sections stained with H&E, and the microscopic images were analyzed with NIH ImageJ to define the area of the tumor involvement from the surrounding non-tumor area. We employed Immunohistochemical staining (IHC) of the cell proliferation marker Ki67 (Cell Signaling Technology, #9449) on 4-μm sections of orthotopic tumors. The Ki67 index (percentage of Ki67-positive PC cell nuclei) was determined by counting positive and negative staining nuclei.
Hepatic metastatic tumor model [15]
For the experimental liver metastasis model, Vector (control) and Capan-1 cells stable expressing LRH1 (5 × 106 in 0.5 mL serum free media) were injected directly into the spleen of nude mice. Several minutes after the injection, the PC cells travel into the portal vein through the splenic vein, and then the spleen was removed; tumor cells will subsequently migrate through the portal vein into the parenchyma of the liver to form multiple metastatic foci. The mice were sacrificed 12 days after splenic injection, and the livers were harvested, washed in water, weighed and fixed with 10% neutral buffered formalin. Sections of the livers were stained with hematoxylin and eosin (H&E) to assess invasion and tumor burden and to monitor the presence and type of micrometastases.
Immunohistochemistry (IHC)
Tissue specimens from 18 individuals with stage I/II pancreatic ductal adenocarcinoma were studied. The study was approved by the Institutional Review Board of Lifespan Rhode Island and The Miriam Hospital. Immunohistochemical staining was conducted on 4-μm formalin-fixed paraffin-embedded (FFPE) unstained sections using antibodies to LRH1 (Abcam, ab18293), c-Myc (Cell Signaling Technology, #5605) and β-catenin (Abcam, ab32572). The sections were deparaffinized in xylene and rehydrated in a descending ethanol gradient. Antigen retrieval was performed using Citric Acid Based Antigen Unmasking Solution (Vector Laboratories, Burlingame, CA) in a microwave pressure cooker (Nordic Ware, Minneapolis, MN) for 6 min at full power, followed by a 30 min cool down process. Endogenous peroxidase activity was quenched by a 30-min treatment with 3% hydrogen peroxide in methanol. The remaining steps of the staining procedure, including blocking, secondary antibody incubation, and ABC reagent incubation, were performed using the VECTASTAIN Elite ABC Kit (Vector Laboratories, Burlingame, CA) according to the manufacturer’s protocol. Primary antibodies were diluted in TBS/0.1% Tween-20 with 5% normal goat serum and were incubated at 4 °C overnight. Color development was done using DAB Tablet (Wako Chemicals, Richmond, VA) as a substrate per manufacturer’s instruction. Finally, the sections were counterstained by hematoxylin, dehydrated in an ascending ethanol gradient, and mounted with VECTASHIELD Mounting Medium (Vector Laboratories, Burlingame, CA).
Statistical analysis
All obtained data were calculated and expressed as mean ± SD. Statistical analyses were performed with the two-tailed Student’s or paired t-test using SPSS software. A p < 0.05 was considered statistically significant.
Results
LRH1 synergistically enhanced transcriptional activity of β-catenin
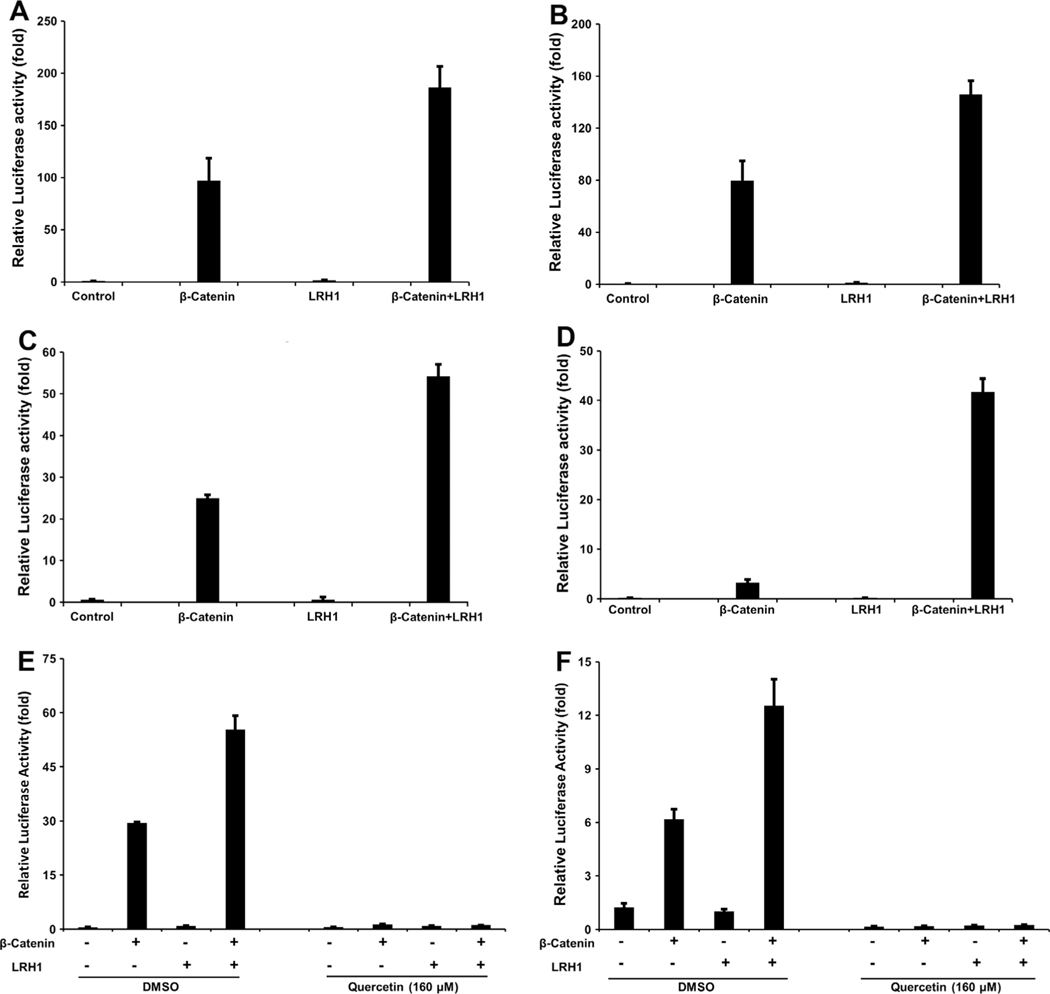
LRH1–β-catenin interaction results in enhanced activation of the β-catenin signaling pathway. Synergistic effects of LRH1 on transcriptional activity of β-catenin were observed in HEK 293, Capan-1, AsPC-1, and MIA PaCa-2 cells (Fig. 1A–D).
Fig. 1.
Synergistic effect of LRH1 on transcriptional activity of β-catenin was reversed by quercetin. LRH1 potentiates β-catenin/Tcf4-mediated transactivation in PC cell lines (A) Capan-1, (B) AsPC-1, (C) MIA PaCa-2, as well as (D) HEK 293 cells. Reversal of LRH1-induced effect was obtained by quercetin. The above LRH1–β-catenin/Tcf4 synergy was reversed by quercetin in (E) Capan-1 and (F) AsPC-1 cells.
Reversal of LRH1-induced effects by β-catenin inhibition
Quercetin significantly inhibited the transcriptional activity of the β-catenin/Tcf4 complex in Capan-1 and AsPC-1 PC cell lines (Fig. 1E and F). The synergism was reversed by β-catenin inhibition, indicating the importance of a functional action between the LRH1 and β-catenin proteins.
LRH1 upregulates downstream target genes c-Myc, and MMP2/9
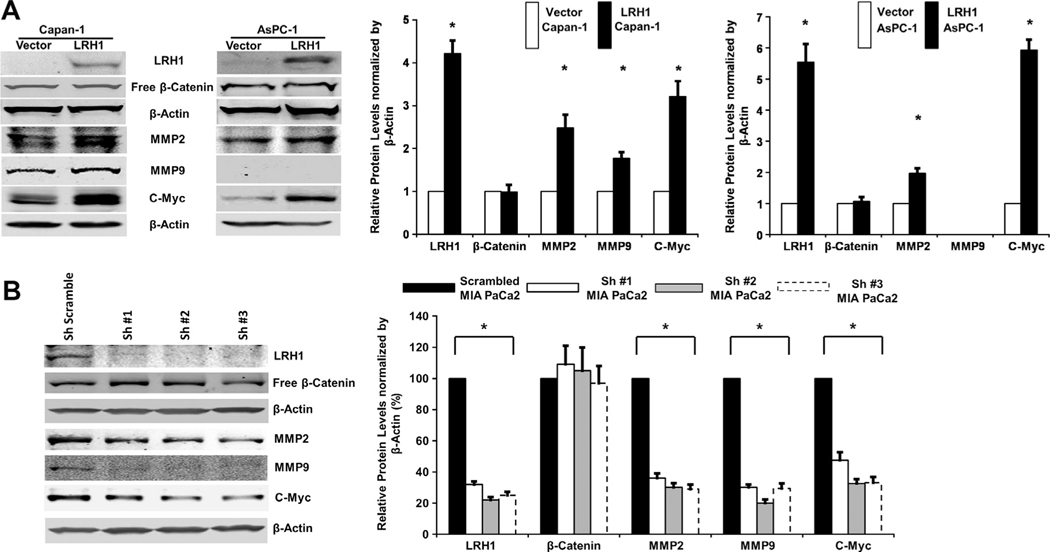
We examined the potential mechanisms of LRH1 involvement in PC by measuring activation of its downstream target genes. Western blot analysis revealed that c-Myc, MMP2 and MMP9 (in Capan-1) were upregulated by LRH1 overexpression in stable PC cells (Fig. 2A).
Fig. 2.
LRH1 upregulated downstream target genes in PC. (A) Expression levels of c-Myc, MMP2 and MMP9 (in Capan-1) were significantly enhanced in LRH1 overexpressed stable Capan-1 and AsPC-1 cells than vector control and (B) LRH1 expression in stable MIA PaCa2 cells with constitutive expression of specific shRNAs was significantly reduced, and the expression of c-Myc, MMP2 and MMP9 was downregulated in response to LRH1 knockdown.
Knockdown of LRH1 by specific shRNAs
Quantitative PCR analysis showed nearly complete inhibition of LRH1 gene transcription after shRNAs transfection, whereas the transcription of LRH1 was not affected in MIA PaCa2 cells transfected with scrambled shRNA (data not shown). Western blot analysis revealed that expression of LRH1 protein in MIA PaCa2 cells transfected with receptor specific shRNAs was significantly reduced; whereas transfection with scrambled shRNA had no effect on LRH1 expression (Fig. 2B). Accordingly, the downstream expression of c-Myc, MMP2 and MMP9 was significantly reduced in response to LRH1 knockdown by shRNAs (Fig. 2B).
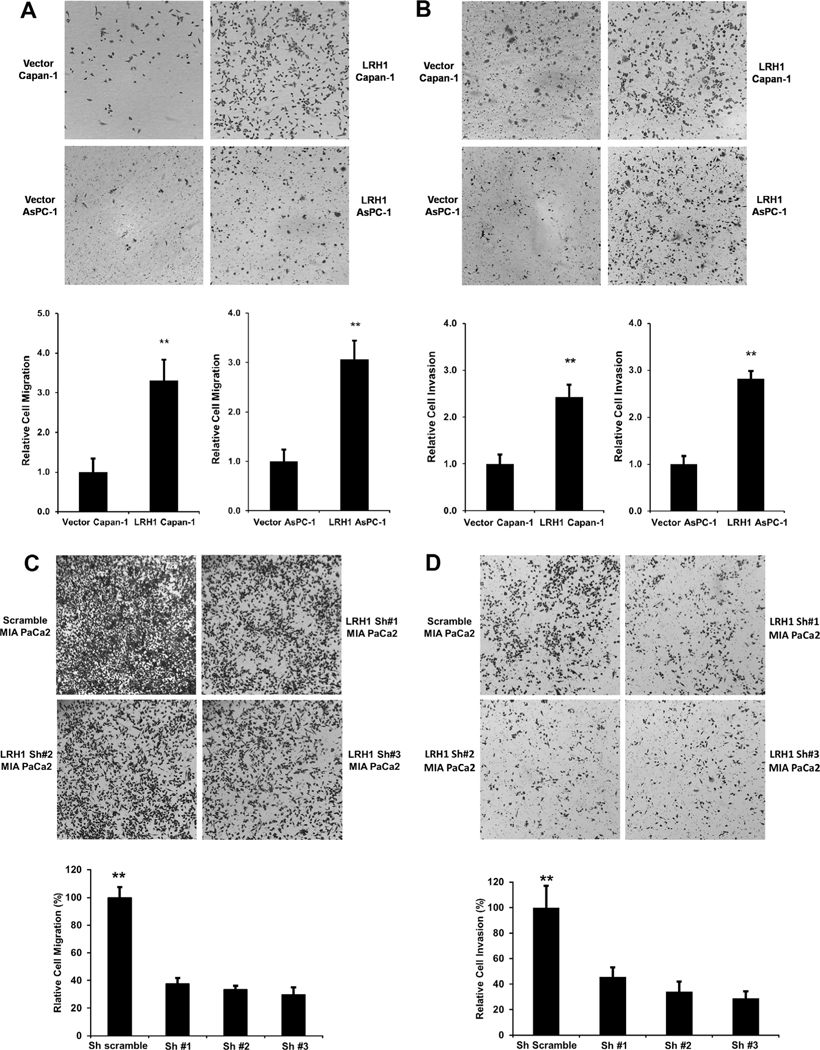
LRH1 enhances PC cell migration and invasion
A transwell cell migration assay demonstrated that LRH1 overexpression resulted in increased cell migration (Fig. 3A) and invasion (Fig. 3B) in Capan-1 and AsPC-1 cells. Similar to the results found with the Western blot assay demonstrating down-regulation of c-Myc, MMP2 and MMP9, significant inhibition of cell migration (Fig. 3C) and invasion (Fig. 3D) were observed in LRH1-shRNAs transfected MIA PaCa2 cells.
Fig. 3.
LRH1 promoted PC cell migration and invasion. (A) Pro-migration of LRH1 on Capan-1 and AsPC-1 cells. LRH1 promoted Capan-1 and AsPC-1 cell Migration using BD Bioscience Chamber. Compared to vectors, LRH1 expressing stable Capan-1 and AsPC-1 cells showed enhanced cell migration at 24 h in Boyden chamber assay. A substantial increase in migration was present in LRH1 stable transfected Capan-1 and AsPC-1 cells compared to vector control (**p < 0.01). (B) LRH1 overexpression promoted Capan-1 and AsPC-1 cell invasion. Compared to vector control, LRH1 stable overexpressing Capan-1 and AsPC-1 cells conferred enhanced cell invasion at 24 h. Enhanced invasion was present in LRH1 stable transfected Capan-1 and AsPC-1 cells compared to vector control (**p < 0.01). Significant attenuation of cell migration (C) and invasion (D) was observed at 36 h in three stable MIA PaCa2 cell lines expressing LRH1-shRNAs (**p < 0.01).
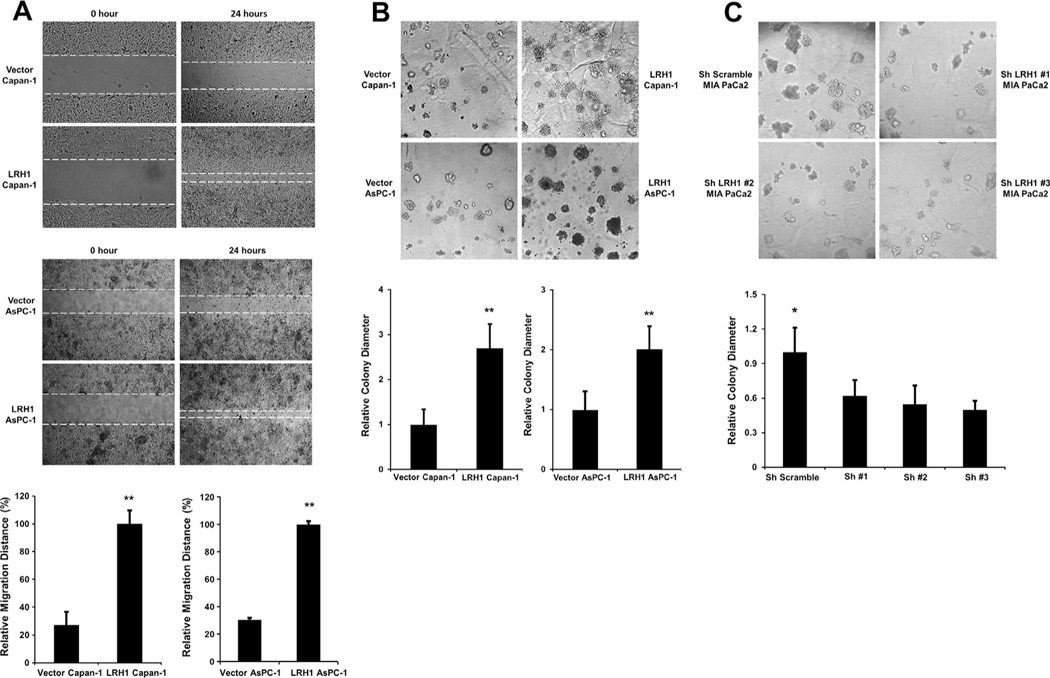
LRH1 promotes wound healing and sphere formation
LRH1 overexpression significantly enhanced wound healing (Fig. 4A) in Capan-1 and AsPC-1 cells. LRH1 upregulation also promoted sphere formation, which was characterized by increased number and size of spheres of stable Capan-1 and AsPC-1 cells compared to vector-transfected control (Fig. 4B). Significant inhibition of sphere formation (Fig. 4C) was observed in MIA PaCa2 cells transfected with specific shRNAs directed against LRH1.
Fig. 4.
LRH1 promoted wound healing and sphere formation. (A) LRH1 accelerated wound healing of PC cell lines Capan-1 and AsPC-1. Compared to vector control, LRH1 stable expressing Capan-1 and AsPC-1 cells exhibited accelerated wound healing at 24 h. An increase in relative migration distance was present in LRH1 stable cells compared to vector control (**p < 0.01), (B) LRH1 stable overexpression promoted sphere formation in Capan-1 and AsPC-1 cells. Morphologic changes were observed in LRH1 stable overexpressing Capan-1 and AsPC-1 cells. Compared to vector control, LRH1 stable overexpressing Capan-1 and AsPC-1 cells produced spheres with increased size (**p < 0.01) and (C) Significant reduction in the size of spheres was detected in three MIA PaCa2 cells expressing specific LRH1-shRNAs (**p < 0.05).
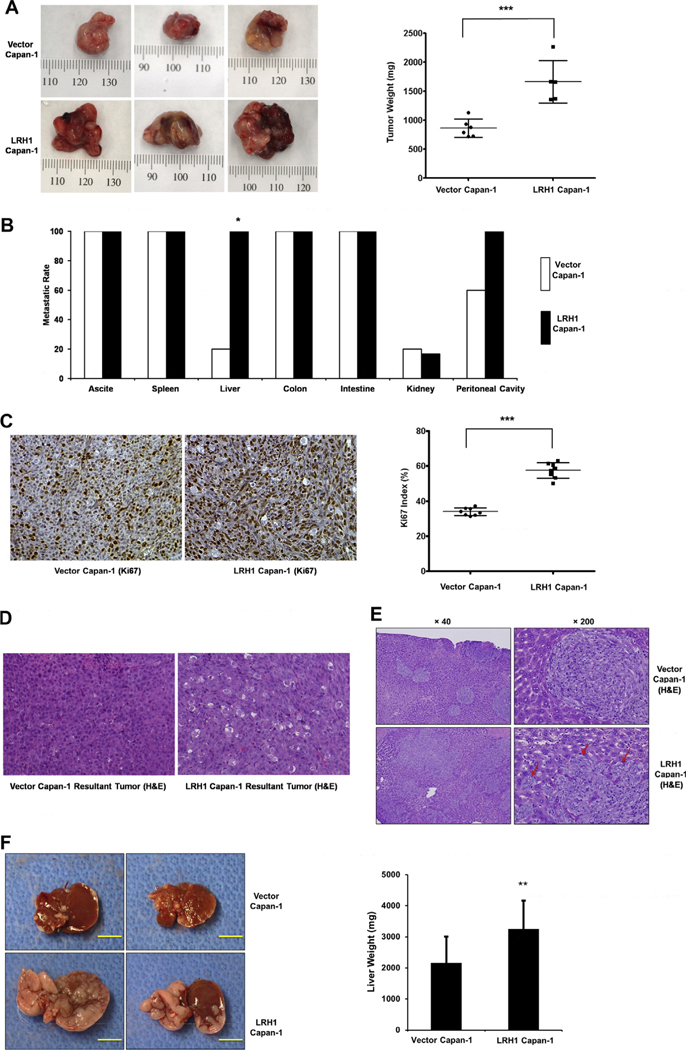
LRH1 overexpression promotes PC growth and progression in the murine orthotopic xenograft model
Capan-1–LRH1 cells produced tumors with higher weight compared with the Capan-1–Vector control cells (1665 ± 164 mg vs. 864 ± 64 mg, p < 0.001, Fig. 5A). Furthermore, mice injected with Capan-1–LRH1 cells showed peritoneal dissemination (100%) and liver metastasis (100%). In contrast, 60% of Capan-1–Vector cell injected mice showed peritoneal dissemination and only 20% developed liver metastasis (Fig. 5B). As shown in Fig. 5C, tumors from Capan-1–LRH1-cell injected mice revealed an increased cell proliferation index as indicated by the staining of Ki67, compared with that of the Capan-1–Vector mice (Ki67 index of 33.9% vs. 57.5%, p < 0.001). Furthermore, the majority of the tumors from the Capan-1–LRH1 group were poorly differentiated, and the area of tumor involvement varied between 70% and 95%, whereas most of the tumors from the Capan-1–Vector group were moderately differentiated, and the area of tumor spread was 40–60% (Fig. 5D). The expression of LRH1 in orthotopic tumors from the Capan1–LRH1 group was confirmed by real time RT-PCR, and the expression of LRH1 was increased by 60% in the Capan-1–LRH1 overexpressing cell tumor group compared with that in the Capan-1–Vector group at 3 weeks after cell injection (data not shown). Therefore, LRH1 significantly contributes to PC growth and progression in vivo.
Fig. 5.
LRH1 promoted PC tumor growth and distant metastasis in nude mice. (A) Orthotopic PC tumor growth. Representative established orthotopic tumors produced by inoculation of 3 × 106 Capan-1 cells transfected with LRH1 or vector control cells into the body of the pancreas. Primary tumors were removed and three representative pictures from each group are shown. Tumor weights were analyzed at 3 weeks after inoculation of Capan-1 cells stably transfected with LRH1 or vector (n = 5). Compared to vector control, LRH1 expressing stable Capan-1 cells produced much larger tumors (paired t test, 2-tailed, **p < 0.01). Vertical bars, standard error, (B) metastatic rate of orthotopic tumors. Liver metastatic rate of Capan-1–LRH1 and Capan-1–Vector mice was 100% vs. 20%, respectively (*p < 0.05), (C) IHC for Ki67 and the calculation of the proliferation Index as measured by positive Ki67 staining of tumor nuclei (***p < 0.0001), (D) H&E staining of two orthotopic tumors, (E) PC liver metastatic model produced by injection of Capan-1 cells transfected with vector or LRH1 into the spleen (upper and bottom, respectively). H&E staining of representative sections of the metastatic liver foci derived from vector cells and LRH1 expressing cells (upper and bottom, respectively). Magnification was 40 × (left) and 200 × (right), respectively. Black arrows indicate the tip of infiltrated tumor derived from the LRH1 expressing cells and (F) compared to vector control cells, LRH1 expressing stable Capan-1 cells aggressively invade through the portal vein into the liver parenchyma. The yellow bar is 1 cm in length. LRH1 expressing stable Capan-1 cells produced much larger tumors in the liver compared to vector-transfected control cells (paired t test, 2-tailed, *p < 0.01).
LRH1 overexpression promotes PC liver metastasis
In vitro observations suggest that LRH1 expression may be important for PC cell migration and invasion. Therefore, we explored its potential oncogenic role using in vivo models (Fig. 5). We measured the resulting tumor growth and development of hepatic metastasis in the immune deficient mice after injection of parental and LRH1-transfected PC cells. LRH1 expressing cells also displayed enhanced ability to form liver metastasis in vivo (Fig. 5E and F). Histologic observations of the liver revealed that the metastatic foci derived from the vector-transfected cells were confirmed to the lumen of the portal vein with great expansion of this venous system due to intravascular proliferation. In contrast, the metastatic foci derived from the LRH1 transfected cells were strikingly different in appearance since there was a direct and aggressive invasion through the endothelial cells of the portal vein into the hepatic parenchyma and sinusoidal space which greatly expanded the metastatic foci (Fig. 5E). Necropsies performed in animals 2 weeks following injection of LRH1 expressing Capan-1 cells into the spleen compared with mice injected with vector-transfected control cells revealed a higher liver weight due to increased tumor burden (3248 ± 919 mg vs. 2166 ± 840 mg, p < 0.05, Fig. 5F). These findings suggest that LRH1 overexpression generates a highly malignant phenotype and may act as a driving factor in producing a robust proliferative, invasive and metastatic phenotype of PC growth, progression and metastasis.
LRH1 is overexpressed in human PC
The specificity of LRH1 antibody was confirmed using positive (liver), negative (heart muscle) and non-relevant antibody IgG controls. IHC experiments revealed significant differences in the levels of LRH1 between the adjacent normal and the malignant portion of the pancreas. In normal exocrine pancreas, no expression of the LRH1 protein was present. 15/18 (83.3%) of PC patients presented increased LRH1 expression with heightened levels detected in the nuclei as well as the cytoplasm of tumor cells (Fig. 6A). Negative, weak-moderate, moderate, and strong immunoreactivity was observed in 16.67%, 49.10%, 22.91% and 11.33% of the tumors, respectively.
Fig. 6.
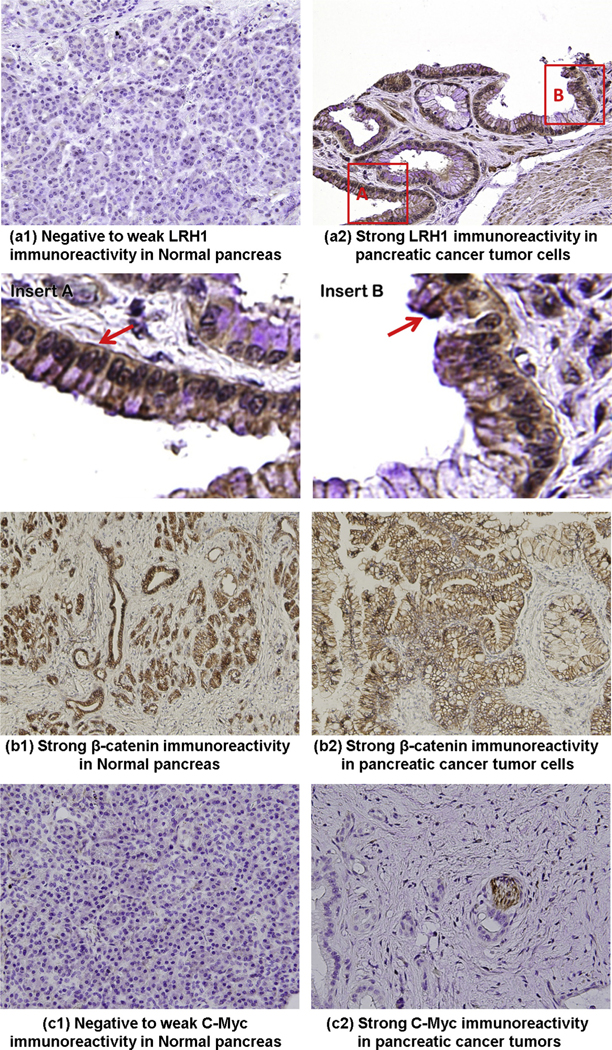
Expression of LRH1 and related genes in PC. LRH1 was overexpressed in tumor tissues (a2) compared with adjacent normal pancreas (a1) of patients with PC. Negative staining was observed for all components of normal exocrine pancreas. In tumor cells, an elevated level of LRH1 was detected either in the nuclei (insert A) or in the cytoplasm (insert B) or both. The original magnification (40×) is specified. The expression of β-catenin (b1–b2) was comparable between the tumorous and adjacent normal pancreas. Weak-negative staining for c-Myc (c1) was observed for all components of normal exocrine pancreas. In tumor cells, an elevated level of c-Myc (c2) was detected. The original magnification (40×) is specified.
Expression of β-catenin and c-Myc
To test the hypothesis that LRH1 may upregulate and activate downstream target genes in PC patients, we examined the expression of LRH1-related genes in pathologic specimens. The expression of β-catenin was comparable between the tumorous and normal pancreas. Consistent with the in vitro findings, c-Myc was overexpressed in tumorous tissues by IHC compared to adjacent normal pancreas in PC patients (Fig. 6B).
Discussion
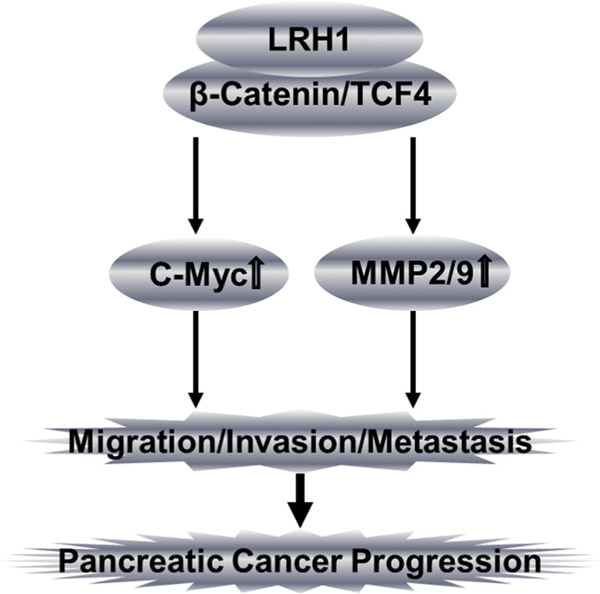
We have observed that LRH1 overexpression enhanced transcriptional activity of β-catenin and expression of its downstream target genes leading to a phenotype characterized by enhanced cell migration, wound healing, invasion and sphere formation in PC cell lines, as well as promoting tumor formation and liver metastasis in nude mice. LRH1 expression correlated with more aggressive tumor characteristics in vivo using two types of murine models. More important, LRH1-enhanced cell migration, invasion and sphere formation, as well as expression of downstream targets were significantly attenuated by knockdown of the receptor using specific shRNAs. These observations suggest that LRH1 plays a role in PC growth, progression and metastasis (Fig. 7).
Fig. 7.
Diagram illustrating the proposed oncogenic role of LRH1 in PC. We hypothesize that LRH1 promotes pancreatic oncogenesis (cell migration, invasion, tumor formation, angiogenesis and liver metastasis) by upregulating c-Myc and MMP2/9.
LRH1 is overexpressed in some PC cell lines and most human tumors of PC patients. Similarly, expression and subcellular localization of LRH1 are also altered in adenocarcinomas of the colon compared with normal human colon [21]. We have found that LRH1 promotes PC cell proliferation by inducing cyclin D1/E1, which drives cell cycle transition from G1 to S phase [14]. LRH1 enhances the transcriptional activity of β-catenin and upregulates downstream targets (c-Myc, MMP2/9) in PC cells. This effect was reversed by the β-catenin inhibitor quercetin, suggesting that LRH1-induced phenotype is β-catenin dependent. c-Myc is frequently overexpressed in gastrointestinal tumors [2,3,21] and promotes tumor formation in vivo [28]. Our study extends and confirms the synergy between LRH1 and β-catenin/Tcf4 signaling in PC, which further emphasizes its contribution to pancreatic tumorigenesis.
The c-Myc is a potent oncogene that promotes tumorigenesis in a variety of tissues and its overexpression predicts poor clinical outcomes [19]. It can amplify the output of the existing gene expression program to reduce rate-limiting constraints for tumor cell growth and proliferation [13]. Importantly, direct programming initiated by oncogenic Ras and Myc has been implicated in the spontaneous PC KrasG12D mouse model [10]. Constant activation of KrasG12D confers phenotypic plasticity that is susceptible to malignant transformation and acquisition of stem cell characteristics [10]. Moreover, c-Myc controls the generation of self-renewing metastatic cancer cells [10]. These findings raise the hypothesis that LRH1-induced c-Myc upregulation in conjunction with Kras activation may enable non-stem cancer cells with high metastatic capacity to dedifferentiate or reprogram to acquire stem cell properties in PC [10].
LRH1 spurs PC cell migration and invasion through upregulating MMP2/9. In breast cancer, LRH1 stimulates migration and invasion through remodeling actin, upregulating MMP9, and posttranslational inactivation of E-cadherin, indicating that LRH1 promotes epithelial-mesenchymal transition or transformation (EMT) process [4]. Cell migration is central to the EMT process and requisite for cancer progression and metastasis [4]. The initiating step in tumor metastasis is the dislodgement of cells from the primary sites and subsequent invasion into adjacent tissues followed by metastasis [4]. LRH1 overexpression substantially increased cell invasion. Given the role of LRH1 in embryonic stem cells [8] and the implication that LRH1 may be involved in the EMT, further investigation of enhanced LRH1 activity in pancreatic cancer stem cells (PCSCs) is warranted.
Sphere formation assay using matrigel matrix involves three dimensional culture systems of cancer stem cells (CSCs) isolated from solid tumors, tumor derived cell lines and genetically modified cells [23]. Based on its physiological composition (laminin, collagen IV, heparin sulfate proteoglycans, entactin, nidogen and growth factors) and functionality, matrigel matrix effectively mimics the physical interplay between CSCs and the extracellular matrix in the tumor microenvironment in vivo, which is critical to demonstrate the role of the microenvironment in maintaining the CSC niche [9]. We found that LRH1 upregulates CSCs markers like CD44 and EpCAM (data not shown) as well as promotes sphere formation, indicating the involvement of LRH1 in pancreatic cancer stem cells (PCSCs) initiation.
Notably, since the only difference between the two Capan-1 cell lines is the overexpression of LRH1, the liver metastatic model was most illustrative since LRH1 promoted vascular invasion through the portal veins into the liver parenchyma and sinusoidal space. Thus, LRH1 can apparently activate genes necessary for a more malignant phenotype which is characterized by tumor cell infiltration, invasion and metastatic spread in the liver.
We found that LRH1 overexpression promotes tumor formation and distant metastasis in nude mice. Interestingly, recent studies indicate that loss of Lrh1/Nr5a2 may contribute to pancreatic oncogenesis. In KrasG12V;Ptf1a-Cre;Nr5a2+/− mice, Nr5a2+/− may sensitize to and/or cooperate with inflammation in KrasG12V-driven pancreatic tumorigenicity [7]. In PdxCrelate;KrasG12D;Nr5a2c/c mice, Nr5a2 was proposed to maintain acinar cell differentiation and constrain oncogenic Kras-mediated pancreatic neoplastic initiation [26]. However, the mechanisms underlying LRH1/Nr5a2 dysfunction, inflammation and PC development has yet to be clarified. Dysfunction of LRH1/Nr5a2 at early stage may promote tumor development by impairing acinar cell differentiation, and its overexpression at later stage may obtain a growth advantage and thus accelerate malignant transformation.[26] Further studies to explore the effect of LRH1/Nr5a2 overexpression coactivation with Kras-mutant during PC initiation and progression in transgenic models will be needed to demonstrate the complicated role of Nr5a2 and the potential cellular origin of PC.
Structure-based discovery of LRH1 antagonists has identified ligands that inhibit LRH1 transcriptional activity and diminish expression of the receptor’s target genes [1]. However, in our model, we did not observe any inhibitory effect of compounds Cpd3 and Cpd3d2 in PC cell lines (data not shown) as previously reported [1]. To explore whether inhibition of LRH1 can block PC progression, several potential LRH1 antagonists are under investigation to demonstrate the pathological role of LRH1 in PC oncogenesis and metastasis.
In summary, our study revealed that overexpressed LRH1 promotes cell migration, wound healing, invasion and sphere formation in vitro, as well as tumor formation, and liver metastasis in vivo. Our observations demonstrate that the LRH1 receptor plays a critical role in progression and metastasis of PC (Fig. 7). These findings also suggest that among the regulatory molecules that have been identified as important factors in pancreatic tumorigenesis, LRH1 appears to be a key driver of PC malignant phenotypes. Collectively our findings indicate the importance of LRH1 in the progression of PC and implicate LRH1 as a potential target for drug development.
Acknowledgements
Human tissue samples were provided by Dr. Murray Resnick at Department of Pathology and Laboratory Medicine, Lifespan/Rhode Island and Miriam Hospital, Warren Alpert Medical School, Brown University.
Grant Support:
National Institute for General Medical Sciences (NIGMS), National Institutes of Health (NIH), United States, RI-INBRE Faculty Development Research Project Grant (8P20GM103430-12, 5P20GM103430-13), 2012 URI Division of Research & Economic Development and URI Council for Research Proposal Development Grants, 2011 AACR-FNAB Fellows Grant for Translational Pancreatic Cancer Research (11-30-14-DONG).
Footnotes
Conflict of interest
There is no conflict of interest among authors.
References
- [1].Benod C, Carlsson J, Uthayaruban R, Hwang P, Irwin JJ, Doak AK, Shoichet BK, Sablin EP, Fletterick RJ, Structure-based discovery of antagonists of nuclear receptor LRH-1, J. Biol. Chem 288 (2013) 19830–19844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Benod C, Vinogradova MV, Jouravel N, Kim GE, Fletterick RJ, Sablin EP, Nuclear receptor liver receptor homologue 1 (LRH-1) regulates pancreatic cancer cell growth and proliferation, Proc. National Acad. Sci. United States America 108 (2011) 16927–16931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Botrugno OA, Fayard E, Annicotte JS, Haby C, Brennan T, Wendling O, Tanaka T, Kodama T, Thomas W, Auwerx J, Schoonjans K, Synergy between LRH-1 and beta-catenin induces G1 cyclin-mediated cell proliferation, Mol. Cell 15 (2004) 499–509. [DOI] [PubMed] [Google Scholar]
- [4].Chand AL, Herridge KA, Thompson EW, Clyne CD, The orphan nuclear receptor LRH-1 promotes breast cancer motility and invasion, Endocrine-related Cancer 17 (2010) 965–975. [DOI] [PubMed] [Google Scholar]
- [5].Chand AL, Wijayakumara DD, Knower KC, Herridge KA, Howard TL, Lazarus KA, Clyne CD, The orphan nuclear receptor LRH-1 and ERalpha activate GREB1 expression to induce breast cancer cell proliferation, PloS 7 (2012) e31593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fayard E, Auwerx J, Schoonjans K, LRH-1: an orphan nuclear receptor involved in development, metabolism and steroidogenesis, Trends Cell. Biol 14 (2004) 250–260. [DOI] [PubMed] [Google Scholar]
- [7].Flandez M, Cendrowski J, Canamero M, Salas A, Del Pozo N, Schoonjans K, Real FX, Nr5a2 heterozygosity sensitises to, and cooperates with, inflammation in KRasG12V-driven pancreatic tumourigenesis, Gut 63 (2013) 647–655. [DOI] [PubMed] [Google Scholar]
- [8].Heng JC, Feng B, Han J, Jiang J, Kraus P, Ng JH, Orlov YL, Huss M, Yang L, Lufkin T, Lim B, Ng HH, The nuclear receptor Nr5a2 can replace Oct4 in the reprogramming of murine somatic cells to pluripotent cells, Cell Stem Cell 6 (2010) 167–174. [DOI] [PubMed] [Google Scholar]
- [9].Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C, Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer, Cell Stem Cell 1 (2007) 313–323. [DOI] [PubMed] [Google Scholar]
- [10].Ischenko I, Zhi J, Moll UM, Nemajerova A, Petrenko O, Direct reprogramming by oncogenic Ras and Myc, Proc. National Acad. Sci. United States of America 110 (2013) 3937–3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kanwar SS, Yu Y, Nautiyal J, Patel BB, Majumdar AP, The Wnt/beta-catenin pathway regulates growth and maintenance of colonospheres, Mol. Cancer 9 (2010) 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lee YK, Moore DD, Liver receptor homolog-1, an emerging metabolic modulator, Front Biosci. 13 (2008) 5950–5958. [DOI] [PubMed] [Google Scholar]
- [13].Lin CY, Loven J, Rahl PB, Paranal RM, Burge CB, Bradner JE, Lee TI, Young RA, Transcriptional amplification in tumor cells with elevated c-Myc, Cell 151 (2012) 56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lin Q, Aihara A, Chung W, Li Y, Huang Z, Chen X, Weng S, Carlson RI, Wands JR, Dong X, LRH1 as a driving factor in pancreatic cancer growth, Cancer Lett. 345 (2014) 85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mohanty S, Xu L, Experimental metastasis assay, J. Vis. Exp 42 (2010) pii: 1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ni X, Yang J, Li M, Imaging-guided curative surgical resection of pancreatic cancer in a xenograft mouse model, Cancer Lett. 324 (2012) 179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Nims RW, Sykes G, Cottrill K, Ikonomi P, Elmore E, Short tandem repeat profiling: part of an overall strategy for reducing the frequency of cell misidentification, In vitro cellular & developmental biology, Animal 46 (2010) 811–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Pasca di Magliano M, Biankin AV, Heiser PW, Cano DA, Gutierrez PJ, Deramaudt T, Segara D, Dawson AC, Kench JG, Henshall SM, Sutherland RL, Dlugosz A, Rustgi AK, Hebrok M, Common activation of canonical Wnt signaling in pancreatic adenocarcinoma, PloS 2 (2007) e1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Pelengaris S, Khan M, Evan G, C-MYC: more than just a matter of life and death, Nat. Rev. Cancer 2 (2002) 764–776. [DOI] [PubMed] [Google Scholar]
- [20].Petersen GM, Amundadottir L, Fuchs CS, Kraft P, Stolzenberg-Solomon RZ, Jacobs KB, Arslan AA, Bueno-de-Mesquita HB, Gallinger S, Gross M, Helzlsouer K, Holly EA, Jacobs EJ, Klein AP, LaCroix A, Li D, Mandelson MT, Olson SH, Risch HA, Zheng W, Albanes D, Bamlet WR, Berg CD, Boutron-Ruault MC, Buring JE, Bracci PM, Canzian F, Clipp S, Cotterchio M, de Andrade M, Duell EJ, Gaziano JM, Giovannucci EL, Goggins M, Hallmans G, Hankinson SE, Hassan M, Howard B, Hunter DJ, Hutchinson A, Jenab M, Kaaks R, Kooperberg C, Krogh V, Kurtz RC, Lynch SM, McWilliams RR, Mendelsohn JB, Michaud DS, Parikh H, Patel AV, Peeters PH, Rajkovic A, Riboli E, Rodriguez L, Seminara D, Shu XO, Thomas G, Tjonneland A, Tobias GS, Trichopoulos D, Van Den Eeden SK, Virtamo J, Wactawski-Wende J, Wang Z, Wolpin BM, Yu H, Yu K, Zeleniuch-Jacquotte A, Fraumeni JF Jr., Hoover RN, Hartge P, Chanock SJ, A genome-wide association study identifies pancreatic cancer susceptibility loci on chromosomes 13q22.1, 1q32.1 and 5p15.33, Nat. Genet 42 (2010) 224–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Schoonjans K, Dubuquoy L, Mebis J, Fayard E, Wendling O, Haby C, Geboes K, Auwerx J, Liver receptor homolog 1 contributes to intestinal tumor formation through effects on cell cycle and inflammation, Proc. National Acad. Sci. United States of America 102 (2005) 2058–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Siegel R, Naishadham D, Jemal A, Cancer statistics, 2012, CA: Cancer J. Clin 62 (2012) 10–29. [DOI] [PubMed] [Google Scholar]
- [23].Tang DG, Understanding cancer stem cell heterogeneity and plasticity, Cell Res. 22 (2012) 457–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Thayer SP, di Magliano MP, Heiser PW, Nielsen CM, Roberts DJ, Lauwers GY, Qi YP, Gysin S, Fernandez-del Castillo C, Yajnik V, Antoniu B, McMahon M, Warshaw AL, Hebrok M, Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis, Nature 425 (2003) 851–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Thiruchelvam PT, Lai CF, Hua H, Thomas RS, Hurtado A, Hudson W, Bayly AR, Kyle FJ, Periyasamy M, Photiou A, Spivey AC, Ortlund EA, Whitby RJ, Carroll JS, Coombes RC, Buluwela L, Ali S, The liver receptor homolog-1 regulates estrogen receptor expression in breast cancer cells, Breast Cancer Res. Treat 127 (2011) 385–396. [DOI] [PubMed] [Google Scholar]
- [26].von Figura G, Morris J.P.t., Wright CV, Hebrok M, Nr5a2 maintains acinar cell differentiation and constrains oncogenic Kras-mediated pancreatic neoplastic initiation, Gut 63 (2013) 656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wang SL, Zheng DZ, Lan FH, Deng XJ, Zeng J, Li CJ, Wang R, Zhu ZY, Increased expression of hLRH-1 in human gastric cancer and its implication in tumorigenesis, Mol. Cell. Biochem 308 (2008) 93–100. [DOI] [PubMed] [Google Scholar]
- [28].Yu Q, Geng Y, Sicinski P, Specific protection against breast cancers by cyclin D1 ablation, Nature 411 (2001) 1017–1021. [DOI] [PubMed] [Google Scholar]
- [29].Zhang Y, Yang J, Cui X, Chen Y, Zhu VF, Hagan JP, Wang H, Yu X, Hodges SE, Fang J, Chiao PJ, Logsdon CD, Fisher WE, Brunicardi FC, Chen C, Yao Q, Fernandez-Zapico ME, Li M, A novel epigenetic CREB-miR-373 axis mediates ZIP4-induced pancreatic cancer growth, EMBO Mol. Med 5 (2013) 1322–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]