Abstract
Background
The treatment of drug-eluting stent (DES) in-stent restenosis (ISR) is challenging as it has a high risk of recurrence.
Aims
The aim of this analysis was to develop and validate a model to predict the risk of repeat percutaneous coronary intervention (PCI) for recurrent DES-ISR.
Methods
A retrospective, observational analysis was performed including consecutive patients treated with PCI for DES-ISR at two centres in Germany. Included patients were randomly divided into training and validation cohorts. Two regression analyses identified factors associated with repeat PCI for recurrent DES-ISR up to 1 year. The discriminative ability of the resultant model was then compared to a benchmark ISR classification model using bootstrap resampling. A classification and regression tree analysis and a numerical scoring system (the ISAR score) were used to predict the risk of repeat PCI for recurrent DES-ISR based on the identified predictors.
Results
We included 1,986 patients in the current analysis, divided randomly into training (1,471 patients, 1,778 lesions) and validation (515 patients, 614 lesions) cohorts. Four factor variables (a non-focal ISR pattern, a time interval to ISR of <6 months, ISR of the left circumflex artery and ISR in a calcified vessel) were associated with repeat PCI for recurrent DES-ISR at 1-year follow-up. On bootstrap resampling analysis, the C-statistic for the model including these four variables was 0.60 (95% confidence interval [CI]: 0.57-0.63), whereas the C-statistic for the benchmark ISR classification model was 0.54 (95% CI: 0.52-0.57), a difference that was statistically significant (delta C-statistic 0.062; 95% CI: 0.035-0.094; p<0.001). The cumulative incidence of repeat PCI for recurrent DES-ISR was over three times higher in DES-ISR lesions with an ISAR score of ≥3 in comparison to lesions with an ISAR score of 0.
Conclusions
This study developed and validated a risk prediction model for repeat PCI for recurrent DES-ISR at 1-year follow-up. This model served to generate the ISAR score, a standardised tool that can be used to predict the 1-year risk of repeat PCI for recurrent DES-ISR.
Introduction
In-stent restenosis (ISR) is the most common cause of stent failure in the drug-eluting stent (DES) era1,2,3. A commonly used ISR classification system, based on angiographic patterns of ISR, was developed in the bare metal stent (BMS) era and has been shown to provide prognostic information with respect to the risk of recurrent BMS-ISR requiring repeat percutaneous coronary intervention (PCI)4. Given that there are recognised differences between DES-ISR and BMS-ISR, the value of this classification system in the setting of DES-ISR is less certain5.
DES-ISR is challenging to treat, with a high risk of recurrence requiring repeat PCI after the initial treatment3. However, there are limited studies investigating the factors that are predictive of repeat PCI for recurrent DES-ISR in adequately sized patient populations. In fact, many of the previous studies on this topic reported on relatively small patient populations, which may have limited their ability to identify relevant predictors of repeat PCI for recurrent DES-ISR6,7,8,9,10,11,12,13,14.
The development of statistical models with the ability to predict repeat PCI for recurrent DES-ISR may be useful, both in order to risk stratify patients as well as to guide clinical follow-up after DES-ISR PCI. In this study, we report on the development and validation of a model to predict repeat PCI for recurrent DES-ISR.
Methods
Patient selection
Consecutive patients undergoing PCI for DES-ISR from September 2005 to December 2013 in two centres in Germany were included in the current analysis. Clinical, procedural and angiographic characteristics were analysed for consecutive patients undergoing PCI for DES-ISR. Relevant data were collected and entered into a computer database by specialised personnel of the clinical data management centre. Digitally recorded baseline, post-procedural, and follow-up coronary angiograms were sent to a centralised imaging core laboratory (ISAResearch Center, Munich, Germany). The Mehran classification was used to classify the angiographic pattern of ISR4. This system classifies ISR into groups based on three characteristics: ISR length (≤10 mm: focal, >10 mm: diffuse), ISR location (within or beyond stent margins) and occlusion (yes or no). Application of this classification system results in four groups: type I: focal; type II: diffuse, within stent; type III: diffuse, within and beyond stent; and type IV: occlusive. The presence or absence of coronary artery calcification was adjudicated by the angiographic core laboratory based on the system described by Mintz et al, with moderate or severe calcification classified as coronary artery calcification for the purposes of this analysis15. Full details of this system are provided in the Supplementary Appendix 1.
Study aims and endpoints
The primary aim of this study was to develop and validate a risk model to predict repeat PCI for recurrent DES-ISR at 1-year follow-up. Accordingly, the main endpoint of interest for the current analysis was repeat PCI for recurrent DES-ISR, defined as any repeat percutaneous intervention of the initially treated target ISR lesion. Additional data were collected on other endpoints of interest, including all-cause mortality, myocardial infarction (MI), definite stent thrombosis (ST) and coronary artery bypass grafting (CABG) after PCI for DES-ISR. Myocardial infarction was defined as per the universal definition of myocardial infarction on the basis of clinical symptoms, electrocardiographic changes, and increases in cardiac biomarkers16,17,18. Stent thrombosis was defined as per the Academic Research Consortium (ARC) criteria19.
Statistical analysis
For the purposes of this analysis, the patients were randomly divided into training and validation populations in a 3:1 ratio. Continuous variables are expressed as means±standard deviations and were compared using the Student’s t-test or the non-parametric Wilcoxon rank-sum test. Categorical variables are reported as frequencies and percentages and were compared using the Chi-square test or Fisher’s exact test. Data distribution was tested for normality by using the Kolmogorov-Smirnov test for goodness of fit. Adverse event rates were calculated with the use of the Kaplan-Meier method (for all-cause death) or cumulative incidence after accounting for the competing risk of death (for all events other than all-cause death) and compared using a Cox proportional hazards model. The cumulative incidences of repeat PCI for recurrent DES-ISR and definite ST were calculated at the lesion level, and the incidences of the remaining endpoints were calculated at the patient level.
Firstly, the least absolute shrinkage and selection operator (LASSO) method was used to select clinical, angiographic and procedural variables for the logistic regression analysis. Missing data were imputed using the multiple imputation by chained equations (R package mice) method. The use of LASSO regression was deemed appropriate in order to improve the prediction accuracy and interpretability of the regression model and to prevent overfitting19. The complete list of variables considered for selection by the LASSO regression model are reported in the Supplementary Appendix 2.
Secondly, a logistic regression analysis was performed using the variables selected by LASSO to examine factors associated with repeat PCI for recurrent DES-ISR at 1-year follow-up20. Regression coefficients were corrected for intracluster correlation in patients with multiple ISR lesions (R package bootcov)21. An exploratory analysis was also performed to predict repeat PCI for recurrent DES-ISR at longer-term follow-up (namely, from 1 to 5 years after the first reintervention for DES-ISR). The overall performance of the risk prediction model was assessed using the C-statistic. The training cohort was used to create the model with all LASSO variables, whilst the validation cohort was used to qualify the performance of the model. Using 400 cycles of bootstrap resampling, we performed an internal validation of the model (including only the significant variables) and repeated this analysis for a model based on the Mehran classification, after dichotomisation of the original four Mehran classification ISR categories into focal or non-focal (this latter group included diffuse intrastent, diffuse proliferative and total occlusion ISR lesions)4. This analysis allowed empirical bootstrap distributions of sample means and bootstrap confidence intervals of the C-statistics and integrated discrimination improvements (IDI) to be calculated. The C-statistic is a measure of the predictive accuracy of a model, and the IDI is a measure to quantify risk discrimination improvement22,23. We calculated the delta C-statistic and delta IDI to determine whether any differences in the predictive accuracy and the risk discrimination improvement between the two models were statistically significant.
A classification and regression tree (CART) analysis was performed with regression trees constructed using only the independent predictors of repeat PCI for recurrent DES-ISR. In addition, a numerical scoring system based on the four significant predictors served to help determine the risk of repeat PCI for recurrent DES-ISR at 1-year follow-up according to the number of predictor variables present. The risk of repeat PCI for recurrent DES-ISR was calculated as a cumulative incidence after accounting for the competing risk of death and compared using a Cox proportional hazards model with correction for intracluster correlation for lesions with increasing numbers of predictor variables compared to those without any predictor variables. These results are presented as hazard ratios (HR) and 95% confidence intervals (CI). Rates of repeat PCI for recurrent DES-ISR for patients as per the identified predictor variables were also calculated as cumulative incidences after accounting for the competing risk of death and compared using a Cox proportional hazards model with correction for intracluster correlation during two time periods (namely from 0 to 1 year and from 1 to 5 years after PCI). The results are presented as cumulative incidences, HRs and 95% CIs for both time periods. All analyses were in accordance with the TRIPOD statement (Supplementary Table 1)24. Statistical significance was defined at a p-value of <0.05. Statistical analysis was performed using the R 3.6.0 Statistical Package (The R Foundation for Statistical Computing). All of the authors had access to the data used in this study and take full responsibility for its integrity and the data analysis.
Results
We included 1,986 patients treated with PCI for DES-ISR. These patients were randomly divided into training (1,471 patients with 1,778 lesions) and validation cohorts (515 patients with 614 lesions). Overall, the median duration of follow-up (25th, 75th percentiles) after treatment with PCI for DES-ISR was 7.4 (4.2, 10.4) years.
Baseline clinical features for the overall population, as well as in the training and the validation populations, are shown in Table 1. Of note, most patients undergoing PCI for DES-ISR were male, and approximately one-quarter of patients presented with acute coronary syndrome (ACS). Lesion-level angiographic features in the overall population, as well as in the training and the validation populations, are shown in Table 2. The majority of the ISR lesions after DES implantation were focal as per the Mehran classification, and the initial repeat PCI type was comparable in the training and validation populations. The indication for repeat PCI for recurrent DES-ISR from 0-1 years and from 1-5 years is demonstrated in Supplementary Table 2.
Table 1. Baseline characteristics.
| Characteristic | All patients (N=1,986) | Training population (N=1,471) | Validation population (N=515) | p-value | |
|---|---|---|---|---|---|
| Age, median [IQR], years | 69.8 [61.6-76.2] | 69.2 [60.8-76.1] | 70.9 [63.5-76.7] | 0.003 | |
| Sex, female – n (%) | 398 (20.0) | 295 (20.1) | 103 (20.0) | >0.999 | |
| BMI, median [IQR], kg/m2 | 27.2 [24.7-30.0] | 27.2 [24.7-30.1] | 27.2 [24.7-29.7] | 0.453 | |
| First re-PCI interval, median [IQR], days | 247 [197-840] | 254 [197-886] | 230 [196-744] | 0.093 | |
| Restenosis interval – n (%) | <6 months | 326 (16.4) | 237 (16.1) | 89 (17.3) | 0.276 |
| 6-12 months | 833 (41.9) | 603 (41.0) | 230 (44.7) | ||
| >12-24 months | 247 (12.4) | 186 (12.6) | 61 (11.8) | ||
| >24 months | 580 (29.2) | 445 (30.3) | 135 (26.2) | ||
| Short restenosis interval – n (%) | 326 (16.4) | 237 (16.1) | 89 (17.3) | 0.584 | |
| Diabetes – n (%) | 748/1,980 (37.8) | 586/1,468 (39.9) | 162/512 (31.6) | 0.001 | |
| Insulin-treated – n (%) | 293/1,980 (14.8) | 229/1,468 (15.6) | 64/512 (12.5) | 0.103 | |
| Arterial hypertension – n (%) | 1,886/1,982 (95.2) | 1,406/1,468 (95.8) | 480/512 (93.4) | 0.040 | |
| Hypercholesterolaemia – n (%) | 1,505/1,971 (76.4) | 1,115/1,461 (76.3) | 390/510 (76.5) | 0.992 | |
| Smoker – n (%) | 302/1,981 (15.2) | 231/1,468 (15.7) | 71/513 (13.8) | 0.339 | |
| ACS – n (%) | 506/1,982 (25.5) | 369/1,467 (25.2) | 137 (26.6) | 0.555 | |
| NYHA classification – n (%) | I | 732 (36.9) | 553 (37.6) | 179 (34.8) | 0.159 |
| II | 964 (48.5) | 709 (48.2) | 255 (49.5) | ||
| III | 241 (12.1) | 179 (12.2) | 62 (12.0) | ||
| IV | 49 (2.5) | 30 (2.0) | 19 (3.7) | ||
| Coronary artery disease – n (%) | Single-vessel | 151 (7.6) | 113 (7.7) | 38 (7.4) | 0.929 |
| Two-vessel | 359 (18.1) | 268 (18.2) | 91 (17.7) | ||
| Three-vessel | 1,476 (74.3) | 1,090 (74.1) | 386 (75.0) | ||
| Multivessel disease – n (%) | 1,835 (92.4) | 1,358 (92.3) | 477 (92.6) | 0.899 | |
| Prior myocardial infarction – n (%) | 830/1,979 (41.9) | 608/1,466 (41.5) | 222/513 (43.3) | 0.509 | |
| Prior CABG – n (%) | 275/1,984 (13.9) | 192/1,469 (13.1) | 83 (16.1) | 0.099 | |
| Atrial fibrillation – n (%) | 97 (4.9) | 76 (5.2) | 21 (4.1) | 0.385 | |
| LVEF – n (%) | <35% | 64 (3.2) | 51 (3.5) | 13 (2.5) | 0.030 |
| 35-50% | 576 (29.0) | 425 (28.9) | 151 (29.4) | ||
| >50-55% | 297 (15.0) | 201 (13.7) | 96 (18.7) | ||
| >55% | 1,048 (52.8) | 794 (54.0) | 254 (49.4) | ||
| Missing continuous data: Training Population: body mass index, 2 patients, Validation Population: body mass index, 1 patient. The remaining continuous data were complete. ACS: acute coronary syndrome; BMI: body mass index; CABG: coronary artery bypass grafting; IQR: interquartile range; LVEF: left ventricular ejection fraction; MI: myocardial infarction; NYHA: New York Heart Association; PCI: percutaneous coronary intervention | |||||
Table 2. Angiographic characteristics.
| Characteristic | All lesions (N=2,392) | Training population lesions (N=1,778) | Validation population lesions (N=614) | p-value | |
|---|---|---|---|---|---|
| Restenosis morphology – n (%) | I (focal) | 1,563 (65.3) | 1,163 (65.4) | 400 (65.1) | 0.092 |
| II (diffuse intrastent) | 581 (24.3) | 420 (23.6) | 161 (26.2) | ||
| III (diffuse proliferative) | 57 (2.4) | 40 (2.3) | 17 (2.8) | ||
| IV (total occlusion) | 191 (8.0) | 155 (8.7) | 36 (5.9) | ||
| Vessel – n (%) | LCA | 97 (4.1) | 64 (3.6) | 33 (5.4) | 0.067 |
| LAD | 870 (36.4) | 645 (36.3) | 225 (36.6) | ||
| LCx | 700 (29.3) | 540 (30.4) | 160 (26.1) | ||
| RCA | 725 (30.3) | 529 (29.8) | 196 (31.9) | ||
| Initially implanted DES type | BP-BES | 166 (6.9%) | 119 (6.7%) | 47 (7.7%) | 0.51 |
| BP-SES | 543 (22.7%) | 402 (22.6%) | 141 (23.0%) | ||
| PF-SES | 465 (19.4%) | 355 (20.0%) | 110 (17.9%) | ||
| PP-EES | 885 (37.0%) | 664 (37.3%) | 221 (36.0%) | ||
| PP-ZES | 333 (13.9%) | 238 (13.4%) | 95 (15.5%) | ||
| Initial repeat PCI type | DES | 1,178 (49.2%) | 862 (48.5%) | 316 (51.5%) | 0.219 |
| PTCA (DCB or POBA) | 1,214 (50.8%) | 916 (51.5%) | 298 (48.5%) | ||
| DCB | 635 (26.5%) | 479 (26.9%) | 156 (25.4%) | – | |
| POBA | 579 (24.2%) | 437 (24.6%) | 142 (23.1%) | – | |
| Scoring balloon | 160 (6.7%) | 122 (6.9%) | 38 (6.2%) | 0.630 | |
| Rotational atherectomy | 2 (0.1%) | 2 (0.1%) | 0 (0.0%) | 0.628 | |
| IVUS | 4 (0.2%) | 2 (0.1%) | 2 (0.3%) | 0.577 | |
| OCT | 59 (2.5%) | 44 (2.5%) | 15 (2.4%) | >0.999 | |
| Calcification – n (%) | 680 (28.5) | 512 (28.9) | 168 (27.4) | 0.506 | |
| Ostial lesion – n (%) | 173 (7.3) | 129 (7.3) | 44 (7.2) | >0.999 | |
| Bifurcation – n (%) | 818 (34.3) | 608 (34.3) | 210 (34.2) | >0.999 | |
| CTO – n (%) | 169 (7.1) | 135 (7.6) | 34 (5.5) | 0.101 | |
| Restenosis ≥90% – n (%) | 343/2,387 (14.4) | 270/1,773 (15.2) | 73 (11.9) | 0.049 | |
| Device diameter, median [IQR] mm | 3.00 [2.50-3.50] | 3.00 [2.50-3.50] | 3.00 [2.75-3.50] | 0.054 | |
| BES: biolimus-eluting stent; BP: biodegradable polymer; CTO: chronic total occlusion; DCB: drug-coated balloon; DES: drug-eluting stent; EES: everolimus-eluting stent; IQR: interquartile range; IVUS: intravascular ultrasound; LAD: left anterior descending; LCA: left coronary artery; LCx: left circumflex coronary artery; OCT: optical coherence tomography; PF: polymer-free; POBA: plain old balloon angioplasty; PP: permanent polymer; PTCA: percutaneous transluminal coronary angioplasty; RCA: right coronary artery; SES: sirolimus-eluting stent; ZES: zotarolimus-eluting stent | |||||
Cumulative incidences
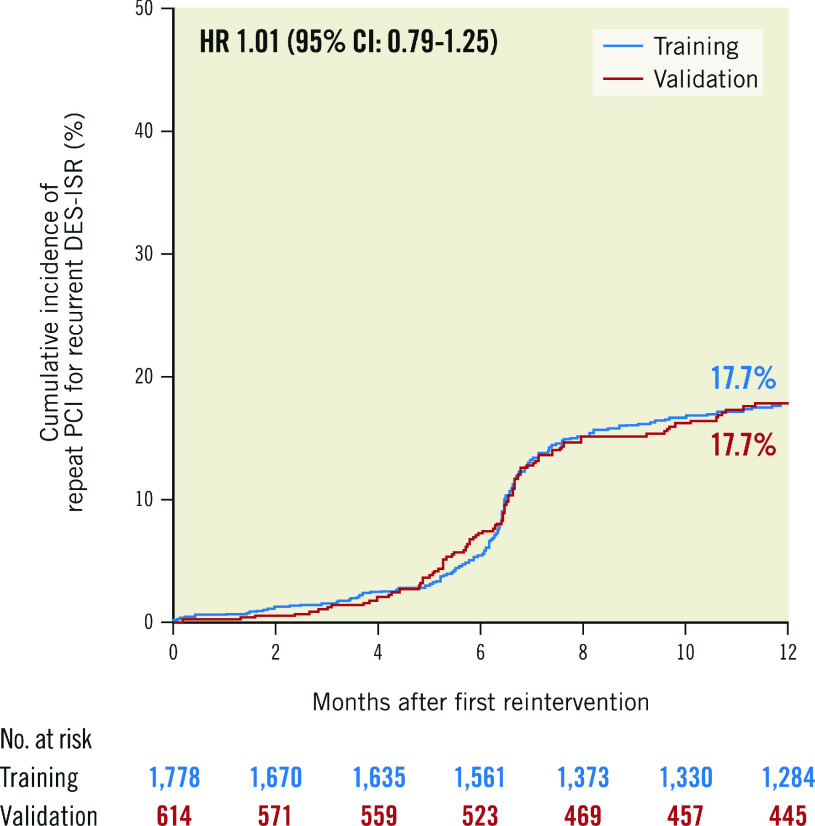
Clinical outcomes at 1-year follow-up for the overall, training and validation populations are shown in Table 3. The cumulative incidence of repeat PCI for recurrent DES-ISR at 1-year follow-up in the training and the validation populations is shown in Figure 1.
Table 3. Clinical outcomes through to 1 year in the entire population, training population and validation population.
| Lesion-level outcomes | All lesions (N=2,392) | Training (N=1,778) | Validation (N=614) | Hazard ratio (95% CI) |
|---|---|---|---|---|
| Repeat PCI for recurrent DES-ISR | 402 (17.7) | 299 (17.7) | 103 (17.7) | 1.01 (0.79-1.25) |
| Definite stent thrombosis | 3 (0.1) | 2 (0.1) | 1 (0.2) | 1.47 (0.00-9734.2) |
| Patient-level outcomes | All patients (N=1,986) | Training (N=1,471) | Validation (N=515) | Hazard ratio (95% CI) |
| All-cause mortality | 90 (4.7) | 63 (4.5) | 27 (5.5) | 1.23 (0.79-1.93) |
| Myocardial infarction | 31 (1.6) | 24 (1.7) | 7 (1.4) | 0.83 (0.36-1.94) |
| Coronary artery bypass grafting | 18 (1.0) | 15 (1.1) | 3 (0.6) | 0.57 (0.17-1.98) |
| Data are number of events with Kaplan-Meier estimates for all-cause mortality (%) or cumulative incidence (%) after accounting for competing risk of death for all other events. The hazard ratio and 95% confidence interval (95% CI) reported is for the comparison between the training and validation populations. DES: drug-eluting stent; ISR: in-stent restenosis; PCI: percutaneous coronary intervention | ||||
Figure 1. Repeat PCI for recurrent DES-ISR at 1 year in the training and validation populations.
CI: confidence interval; DES: drug-eluting stent; HR: hazard ratio; ISR: in-stent restenosis; PCI: percutaneous coronary intervention
Predictors of repeat PCI for recurrent DES-ISR from 0 to 1 year
The LASSO method selected the following variables for the logistic regression model from 0 to 1 year after PCI for DES-ISR: ISR type, age, hypercholesterolaemia, smoking, ACS, multivessel disease, left circumflex (LCx) coronary artery ISR, vessel calcification, ostial ISR lesion, bifurcational ISR, ISR severity (>90%), maximum device (stent/balloon) diameter and ISR interval. Of note, the variables ISR type and ISR interval were dichotomised (into non-focal/focal and <6 months/≥6 months, respectively) based on the analysis of the crude frequencies of repeat PCI at 1 year for DES-ISR in the training population as per groups identified by ISR type and ISR interval (Supplementary Table 3).
The logistic regression model including all the above-mentioned variables identified four independent predictors of repeat PCI for recurrent DES-ISR from 0 to 1 year: a non-focal ISR pattern, a time interval to restenosis of <6 months, ISR in the LCx coronary artery, and vessel calcification (Table 4). The C-statistic for the regression model including all variables identified by the LASSO analysis was 0.62 in the training population and 0.64 in the validation population.
Table 4. Results of logistic regression analysis for repeat PCI for recurrent DES-ISR from 0 to 1 year after after the first reintervention for DES-ISR.
| Variable | Regression coefficient | p-value |
|---|---|---|
| Non-focal ISR at index PCI | 0.346 | 0.029 |
| Age | −0.007 | 0.297 |
| Hypercholesterolaemia | 0.161 | 0.310 |
| Smoking | 0.197 | 0.328 |
| Acute coronary syndrome | 0.054 | 0.736 |
| Multivessel disease | 0.259 | 0.401 |
| Left circumflex coronary artery | 0.288 | 0.048 |
| Vessel calcification | 0.359 | 0.020 |
| Ostial lesion | 0.378 | 0.169 |
| Bifurcation lesion | 0.143 | 0.333 |
| ISR severity >90% | 0.178 | 0.383 |
| Device diameter | −0.174 | 0.220 |
| Restenosis interval <6 months | 0.506 | 0.008 |
| Significant correlates are in bold. DES: drug-eluting stent; ISR: in-stent restenosis; PCI: percutaneous coronary intervention | ||
Bootstrap analysis
Internal validation was performed using bootstrapping with 400 resamples for the four-variable model in the training and validation populations and to compare the performance of the four-variable and the Mehran classification models. Of note, the model based on the Mehran classification included only the non-focal ISR morphology predictor variable.
The C-statistic for the four-variable model was 0.61 (95% CI: 0.57-0.64) in the training population and 0.61 (95% CI: 0.55-0.67) in the validation population. The delta C-statistic was −0.003 (95% CI: −0.070 to 0.074; p=0.91) indicating that there was no difference in the discriminative performance of the four-variable model in the training and validation populations.
The C-statistic for the four-variable model developed to predict the likelihood of repeat PCI for recurrent DES-ISR at 1 year was 0.60 (95% CI: 0.57-0.63) and the C-statistic for the model based on the Mehran classification was 0.54 (95% CI: 0.52-0.57). The IDI for the four-variable model was 0.021 (95% CI: 0.010-0.033) and the IDI for the model based on the Mehran classification was 0.005 (95% CI: 0.001-0.011). Both the delta C-statistic (0.062, 95% CI: 0.035-0.094) and delta IDI (0.016, 95% CI: 0.007-0.029) between the four-variable model and the model based on the Mehran classification were statistically significant (p<0.001).
CART model and predictors of repeat PCI for recurrent DES-ISR from 0 to 1 year
The CART model (Figure 2) demonstrates the variables with an impact on the likelihood of repeat PCI for recurrent DES-ISR at 1-year follow-up. The likelihood of repeat PCI for recurrent DES-ISR ranged from 12.4% to 30.9% based on the presence or absence of the predictors identified in the final regression model. We also analysed the risk of repeat PCI for recurrent DES-ISR according to whether the DES-ISR lesions presented with one of the four significant predictors derived from the final regression model (Table 5, Figure 3).
Figure 2. Classification and regression tree analysis. Classification and regression tree (CART) analysis demonstrating the variables which influence the likelihood of repeat PCI for recurrent DES-ISR at 1-year follow-up.
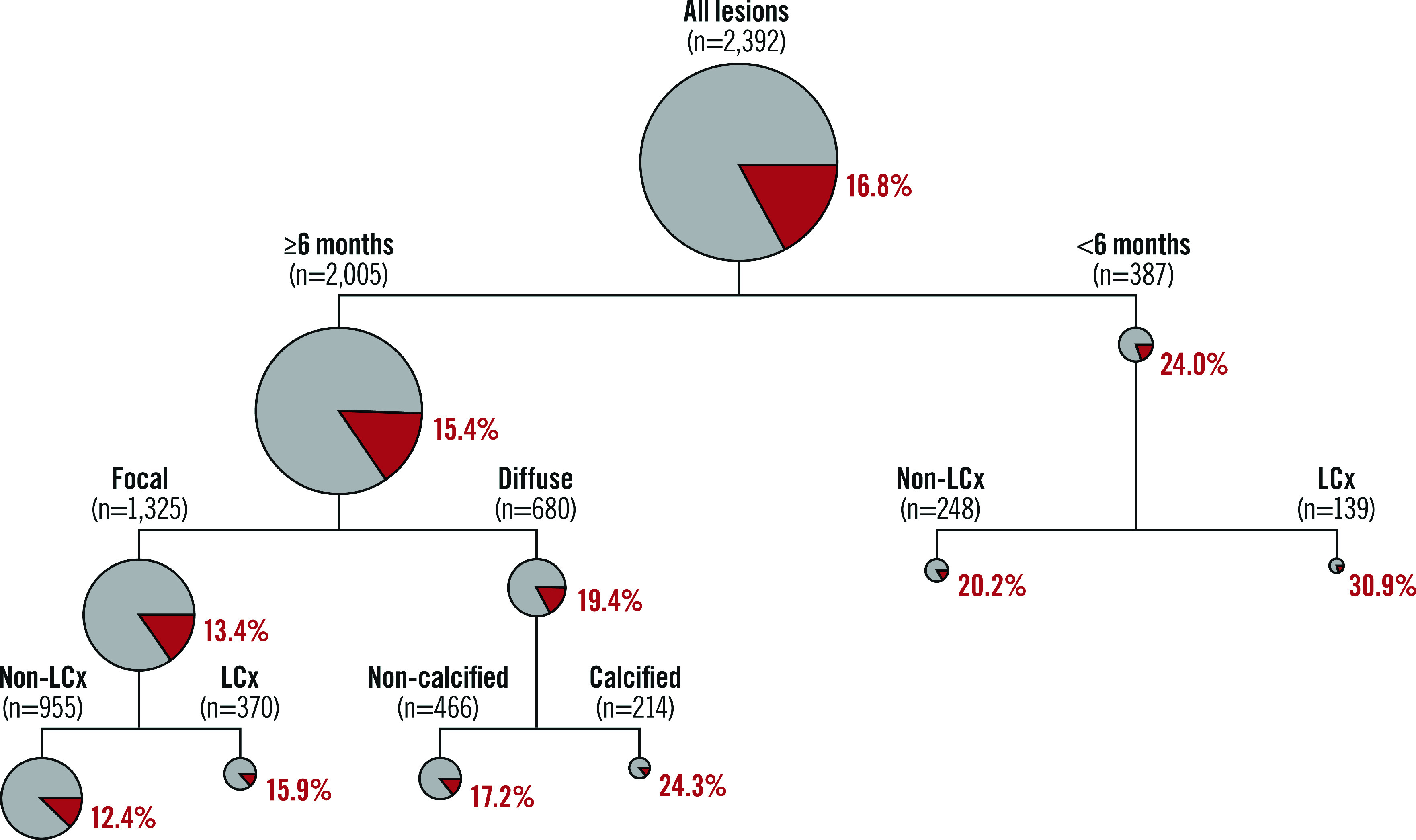
The size of the circles are proportional to the size of the subgroup. The red segments of the circles indicate the percentage of DES-ISR lesions undergoing repeat PCI at 1-year follow-up. DES: drug-eluting stent; LCx: left circumflex coronary artery; ISR: in-stent restenosis; PCI: percutaneous coronary intervention
Table 5. Cumulative incidence of repeat PCI for recurrent DES-ISR from 0 to 1 year for lesions with and without the four predictor variables identified in the logistic regression model.
| Predictor | Predictor present (n/N, KM%) | Predictor not present (n/N, KM%) | Hazard ratio (95% CI) |
|---|---|---|---|
| Restenosis interval <6 months | 93/387 (24.4%) | 309/2,005 (16.4%) | 1.62 (1.22-2.10) |
| Non-focal in-stent restenosis morphology | 173/829 (22.4%) | 229/1,563 (15.3%) | 1.56 (1.28-1.85) |
| Vessel calcification | 134/680 (20.9%) | 268/1,707 (16.5%) | 1.35 (1.09-1.63) |
| Left circumflex artery | 147/700 (21.9%) | 255/1,692 (16.0%) | 1.44 (1.19-1.80) |
| Data are number of events with cumulative incidence (%) after accounting for competing risk of death. CI: confidence interval; KM: Kaplan-Meier | |||
Figure 3. Cumulative incidence of repeat PCI for recurrent DES-ISR at 1-year follow-up for lesions with and without the four predictor variables identified in the logistic regression model.
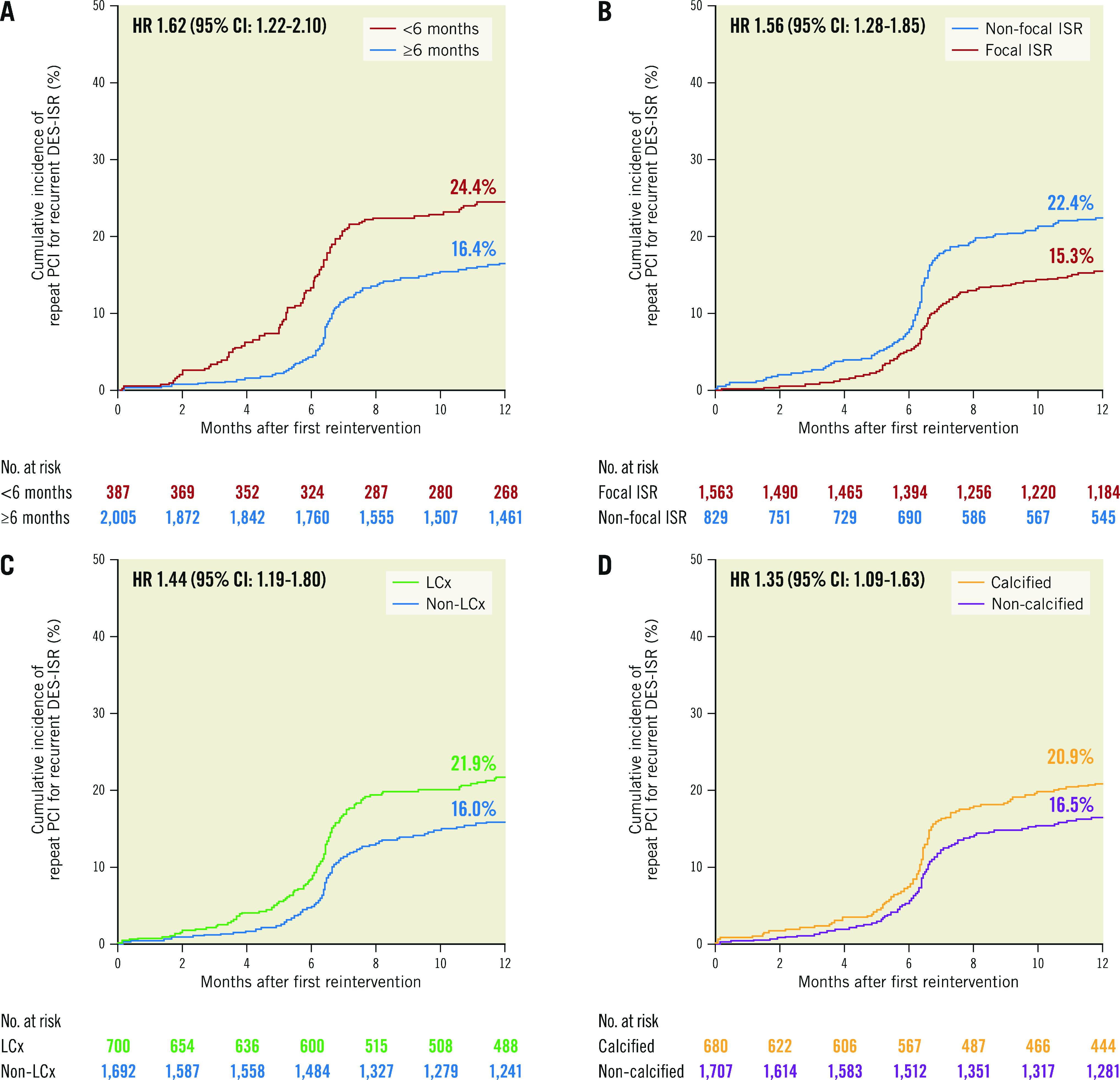
A) Restenosis interval <6 months versus restenosis interval ≥6 months. B) Restenosis morphology: non-focal versus restenosis morphology: focal. C) Artery involved: left circumflex artery versus non-left circumflex artery. D) Vessel calcification: calcified vessel versus non-calcified vessel. CI: confidence interval; DES: drug-eluting stent; HR: hazard ratio; ISR: in-stent restenosis; LCx: left circumflex coronary artery; PCI: percutaneous coronary intervention
The ISAR score
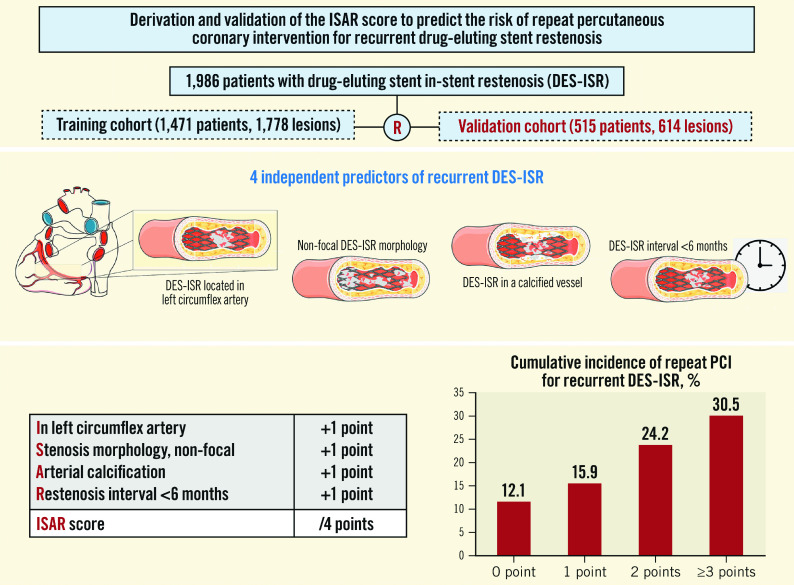
From the final regression model, including the significant predictors of repeat PCI for recurrent DES-ISR at 1 year, we developed a four-item score, namely the ISAR score, to estimate the 1-year incidence of repeat PCI for recurrent DES-ISR. The score is obtained by assigning 1 point to each DES-ISR lesion based on the following criteria: ISR location In the LCx coronary artery; non-focal Stenosis morphology; presence of Arterial calcification of the target vessel; Restenosis interval <6 months (ISAR). As such, the ISAR score for a DES-ISR lesion undergoing PCI can range from 0 to 4 points. In the current study population, the 1-year incidences of repeat PCI for recurrent DES-ISR in lesions with ISAR scores of 0, 1, 2 and ≥3 points were 12.1%, 15.9%, 24.2% and 30.5%, respectively (p for trend <0.001). Compared to lesions with an ISAR score of 0, the incidence of repeat PCI for recurrent DES-ISR was increased in lesions with an ISAR score of 1 (HR 1.37, 95% CI: 1.04-1.79), 2 (HR 2.27, 95% CI: 1.69-3.07) and ≥3 (HR 3.11, 95% CI: 2.15-4.81). The cumulative incidences of repeat PCI for recurrent DES-ISR at 1-year as per the ISAR score are demonstrated in the Central illustration. Of note, the ISAR score was significantly correlated with the 1-year cumulative incidence of repeat PCI for recurrent DES-ISR in both the patient populations treated with stent- (p for trend=0.0016) and balloon-based (p for trend <0.001) modalities. There was no interaction between the ISAR score and ACS presentation with regard to repeat PCI for recurrent DES-ISR. The ISAR score was significantly correlated with the 1-year cumulative incidence of repeat PCI for recurrent DES-ISR in both the patient populations presenting with ACS (p for trend <0.001) and chronic coronary syndrome (CCS) (p for trend <0.001). The ISAR score was also significantly correlated with the incidence of repeat PCI for recurrent DES-ISR at 5-year follow-up (p for trend <0.001).
Central illustration. The ISAR score to estimate the risk of repeat PCI for recurrent DES-ISR at 1-year follow-up.
The ISAR score can range from 0-4 based on the presence or absence of the four identified predictors of repeat PCI for recurrent DES-ISR. Patients receive +1 point for each of the following criteria they meet: DES-ISR In the LCx coronary artery, non-focal Stenosis morphology, Arterial calcification and Restenosis interval of <6 months (ISAR). The cumulative incidence of repeat PCI for recurrent DES-ISR increases with an increasing ISAR score, from 12.1% for patients with an ISAR score of 0 to 30.5% for patients with an ISAR score of ≥3. DES: drug-eluting stent; LCx: left circumflex coronary artery; ISR: in-stent restenosis; PCI: percutaneous coronary intervention; R: Patients were randomly divided into training (1,471 patients with 1,778 lesions) and validation (515 patients with 614 lesions) cohorts
Predictors of repeat PCI for recurrent DES-ISR from 1 to 5 years
From 1 to 5 years, repeat PCI for recurrent DES-ISR occurred in 209 of 1,729 lesions.
An exploratory logistic regression model, searching for predictors of repeat PCI for recurrent DES-ISR from 1 to 5 years, did not identify any independent significant predictors of repeat PCI for recurrent DES-ISR from 1 to 5 years (Supplementary Table 4). In addition, a landmark analysis (from 0 to 1 year and from 1 to 5 years) explored a possible time dependence in the incidence of repeat PCI for recurrent DES-ISR according to whether the DES-ISR lesions presented with one of the four significant predictors derived from the final multivariable model at 1-year follow-up (Supplementary Figure 1).
Discussion
We studied nearly 2,000 patients treated with PCI for DES-ISR in order to develop and validate a risk prediction model for repeat PCI due to recurrent DES-ISR. As far as we are aware, the current study is the largest analysis of the predictors of repeat PCI for recurrent DES-ISR. The main findings of the present analysis are as follows:
A non-focal DES-ISR pattern, a DES-ISR interval of <6 months, DES-ISR in the LCx coronary artery and DES-ISR in a calcified coronary vessel are independent predictors of repeat PCI for recurrent DES-ISR at 1-year follow-up.
These four variables were used to develop and validate a risk prediction model for repeat PCI due to recurrent DES-ISR. Although the discriminative power of this risk prediction model was modest, it was stronger than a model based on a previous ISR classification system introduced in the BMS era.
The four-variable model was then used to create the four-item ISAR score, a scoring system which allows for practical estimation of the risk of repeat PCI for recurrent DES-ISR.
The present data demonstrate that patients with DES-ISR treated with PCI have a relatively high rate of recurrence, with repeat PCI for recurrent DES-ISR occurring in over one in six treated DES-ISR lesions at 1-year follow-up. This analysis focused on a follow-up duration of 1 year for two reasons. Firstly, a large proportion of trials dealing with a similar research topic were designed to capture major clinical endpoints up to 1-year follow-up2,25,26,27. Secondly, the 1-year follow-up limit allowed us to compare the developed risk prediction model with a model based on the Mehran classification, which originally reported on clinical outcomes up to the same timepoint4.
A model to predict repeat PCI for recurrent DES-ISR could potentially be used to identify patients at the highest risk of ISR recurrence and could help to stratify patients for the purposes of clinical follow-up. Overall, the model developed in our analysis had a relatively modest discriminative power for the prediction of repeat PCI for recurrent DES-ISR at 1-year follow-up. However, the discriminative power of this model was greater than that of a model based on the Mehran classification system, which was developed in the BMS era4.
Our analysis confirms previous observations that the typical DES-ISR pattern is focal in morphology28. A study which included a relatively small number of patients with a median follow-up of about 1 year reported that the ISR morphology (defined in that study as focal versus diffuse) retained prognostic importance in the early DES era with regard to both angiographic restenosis and repeat PCI for recurrent DES-ISR13. While that study excluded patients with occlusive restenosis, another smaller study subsequently reported that occlusive DES-ISR was also associated with an increased risk of both angiographic restenosis and recurrent target lesion revascularisation (TLR)6. A larger study, including 392 patients with a median follow-up of nearly 3 years, confirmed the importance of the initial DES-ISR morphology on longer-term outcomes after PCI for DES-ISR14. Our analysis, which includes a population that is between 6 to 10 times larger than in previous studies and with approximately 2,500 DES-ISR lesions, demonstrates that in addition to the DES-ISR morphology, the restenosis interval, the involved vessel and the presence of arterial calcification are predictors of repeat PCI for recurrent DES-ISR at 1-year follow-up.
Some studies have previously suggested that these factors may be associated with an increased risk of TLR in the setting of DES-ISR. We previously reported that ISR occurring <12 months after DES implantation is associated with an increased risk of adverse events in DES-ISR treated with drug-coated balloon (DCB) angioplasty29. While the development of early DES-ISR may suggest a more aggressive ISR phenotype, definitive data in this regard are limited. It has been reported that early DES-ISR is mainly caused by neointimal hyperplasia, whereas DES-ISR that occurs later tends to be caused by neoatherosclerosis, and this may also be relevant in this regard30. It has been previously suggested that ostial ISR lesions in the LCx coronary artery have an increased TLR rate in comparison to non-LCx lesions31. Similar findings were reported in another study assessing patients with ostial ISR treated with DCB therapy, with an increased risk of TLR in patients treated for LCx DES-ISR32. Both vessel and lesion calcification have also been previously associated with an increased risk of DES-ISR33,34. In addition, another small study including 276 patients noted that patients undergoing repeat PCI of recurrent DES-ISR commonly had moderate to severe target lesion calcification35.
While several risk prediction models have been proposed for the prediction of developing ISR after PCI with DES implantation, our current analysis is the first risk prediction model that has been developed and validated in a large number of patients for repeat PCI for recurrent DES-ISR6,7,8,9. The overall modest discriminative power of our model may reflect the heterogeneous nature of DES-ISR. In our study, this was particularly evident from 1 to 5 years after PCI for DES-ISR, a time period in which we were unable to identify any significant predictor of repeat PCI for recurrent DES-ISR. The risk of recurrent DES-ISR PCI may be increased because of multiple factors, relating not only to the patient, lesion and initial procedure but also to the initial treatment of the DES-ISR10,11,12. This complexity may become even more apparent at longer-term follow-up, and this may have contributed to the inability of our model to identify predictors of repeat PCI for recurrent DES-ISR beyond 1 year.
Overall, these results highlight that it remains challenging to accurately predict the likelihood of a patient requiring repeat PCI for recurrent DES-ISR. In this context, the ISAR score may be a prediction tool of certain relevance. An ISAR score of 0 was associated with a 12.1% risk of repeat PCI for recurrent DES-ISR at 1-year follow-up, while an ISAR score of ≥3 was associated with a risk of 30.5%. From a practical perspective, an advantage of the ISAR score is that it can be quickly calculated in the cardiac catheterisation laboratory using readily available angiographic and clinical information. Vessel calcification, DES-ISR morphology and ISR vessel location can all be assessed angiographically, while the restenosis interval can be identified from the clinical history, clinical referral report or previous angiogram report in the majority of cases. The ISAR score may be useful not only to guide clinical follow-up but also when counselling patients regarding their risk of requiring repeat PCI for recurrent DES-ISR within the first year. However, these data also demonstrate that even the lowest risk DES-ISR patients (with an ISAR score of 0) are still at a relatively high risk of repeat PCI for recurrent DES-ISR at 1-year follow-up, highlighting the challenge that remains in treating DES-ISR in real-world practice.
Limitations
This study has several limitations which warrant consideration. This is a retrospective analysis of patient data from a registry database and so carries the inherent limitations associated with this form of analysis. Although we used randomly assigned training and validation populations, an external prospective validation of the ISAR score is still missing. Therefore, while the model was internally validated, it lacks external validation. Most patients presented with stable coronary artery disease at the time of PCI for DES-ISR, with only 25% of the entire cohort presenting with ACS. In this regard, the predictive performance of the ISAR score in a population different from that investigated here remains to be addressed, although there was no interaction between the ISAR score and clinical presentation with regard to repeat PCI for recurrent DES-ISR. This point also holds true regarding the DES platforms studied, given that the latest-generation DES were not included in the present analysis. The presence of coronary artery calcification, one of the significant predictors of repeat PCI for recurrent DES-ISR at 1 year, was evaluated only at coronary angiography, which may have led to underreporting or to underestimation of calcification. Similarly, given the low use of intravascular imaging in this analysis, it was not possible to assess imaging parameters for inclusion into our model. Indeed, despite encouragement from consensus documents36, the use of intravascular imaging in clinical practice in Europe and the US remains relatively low37,38. We had no information regarding antithrombotic medications and other secondary prevention measures at the time of PCI for DES-ISR. In this regard, the possible impact of common cardioactive drugs and risk reduction pathways on a subsequent repeat PCI for recurrent DES-ISR cannot be determined in this context and should be the subject of future investigations. This analysis does not have detailed procedural data demonstrating the mechanism of the initial DES-ISR or regarding lesion preparation techniques used prior to the index PCI. The identification of recurrent ISR after a first reintervention for ISR may be dependent on the nature of follow-up. A final important potential limitation of the ISAR score is that in some cases it may not be possible to determine the restenosis interval at the time of the repeat PCI procedure.
Conclusions
Restenosis morphology and interval, vessel calcification and involvement of the LCx coronary artery are independent predictors of repeat PCI for recurrent DES-ISR at 1-year follow-up. A four-variable model for risk prediction of repeat PCI for recurrent DES-ISR at 1-year follow-up demonstrated a modest discriminative performance overall, although superior to that of the current benchmark for ISR classification. Based on the four-variable prediction model, we developed the ISAR score, a four-item scoring system that can be used to estimate the risk of repeat PCI for recurrent DES-ISR at 1-year follow-up.
Impact on daily practice
At 1-year follow-up after the first DES-ISR intervention, four factor variables (a non-focal ISR pattern, a time interval to ISR of <6 months, ISR of the left circumflex artery and ISR in a calcified vessel) are associated with an increased risk of repeat PCI for recurrent DES-ISR. These four factors were used to generate the ISAR score, a scoring system which was significantly correlated with the 1-year cumulative incidence of repeat PCI for recurrent DES-ISR. The 1-year risk of repeat PCI for recurrent DES-ISR can be predicted using the ISAR score, a standardised tool that can be quickly calculated in the cardiac catheterisation laboratory using readily available angiographic and clinical information.
Supplementary data
Methods.
List of variables considered by the LASSO model.
TRIPOD checklist: prediction model development and validation.
Indications for repeat PCI for recurrent DES-ISR at 1- and 5-year follow-up.
Repeat PCI for recurrent DES-ISR from 0 to 1 year and from 1 to 5 years as per ISR interval and morphology.
Results of logistic regression analysis for repeat PCI for recurrent DES-ISR from 1 to 5 years after first reintervention for DES-ISR.
Landmark analysis demonstrating the cumulative incidence of repeat PCI for recurrent DES-ISR from 0-1 years and from 1-5 years for lesions with and without the four predictor variables identified in the logistic regression model.
Acknowledgments
Conflict of interest statement
J. Wiebe reports lecture fees from AstraZeneca and lecture fees and an institutional grant from Abbott Vascular. The other authors have no conflicts of interest to declare.
Abbreviations
- ACS
acute coronary syndrome
- BMS
bare metal stent
- CABG
coronary artery bypass grafting
- CART
classification and regression tree
- CI
confidence interval
- DCB
drug-coated balloon
- DES
drug-eluting stent
- HR
hazard ratio
- IDI
integrated discrimination improvement
- ISR
in-stent restenosis
- LASSO
least absolute shrinkage and selection operator
- LCx
left circumflex artery
- MI
myocardial infarction
- PCI
percutaneous coronary intervention
- ST
stent thrombosis
- TLR
target lesion revascularisation
Contributor Information
JJ Coughlan, Klinik für Herz- und Kreislauferkrankungen, Deutsches Herzzentrum München, Technische Universität München, Munich, Germany.
Alp Aytekin, Klinik für Herz- und Kreislauferkrankungen, Deutsches Herzzentrum München, Technische Universität München, Munich, Germany.
Shqipdona Lahu, Klinik für Herz- und Kreislauferkrankungen, Deutsches Herzzentrum München, Technische Universität München, Munich, Germany.
Maria Scalamogna, Klinik für Herz- und Kreislauferkrankungen, Deutsches Herzzentrum München, Technische Universität München, Munich, Germany; Division of Cardiology, Department of Advanced Biomedical Sciences, University of Naples Federico II, Naples, Italy.
Jens Wiebe, Klinik für Herz- und Kreislauferkrankungen, Deutsches Herzzentrum München, Technische Universität München, Munich, Germany.
Susanne Pinieck, Klinik für Herz- und Kreislauferkrankungen, Deutsches Herzzentrum München, Technische Universität München, Munich, Germany.
Sebastian Kufner, Klinik für Herz- und Kreislauferkrankungen, Deutsches Herzzentrum München, Technische Universität München, Munich, Germany.
Erion Xhepa, Klinik für Herz- und Kreislauferkrankungen, Deutsches Herzzentrum München, Technische Universität München, Munich, Germany.
Michael Joner, Klinik für Herz- und Kreislauferkrankungen, Deutsches Herzzentrum München, Technische Universität München, Munich, Germany; DZHK (German Center for Cardiovascular Research), Partner site Munich Heart Alliance, Munich, Germany.
Constantin Kuna, Klinik für Herz- und Kreislauferkrankungen, Deutsches Herzzentrum München, Technische Universität München, Munich, Germany.
Felix Voll, Klinik für Herz- und Kreislauferkrankungen, Deutsches Herzzentrum München, Technische Universität München, Munich, Germany.
Karl Ludwig Laugwitz, DZHK (German Center for Cardiovascular Research), Partner site Munich Heart Alliance, Munich, Germany; Medizinische Klinik und Poliklinik, Klinikum rechts der Isar, Technische Universität München, Munich, Germany.
Heribert Schunkert, Klinik für Herz- und Kreislauferkrankungen, Deutsches Herzzentrum München, Technische Universität München, Munich, Germany; DZHK (German Center for Cardiovascular Research), Partner site Munich Heart Alliance, Munich, Germany.
Adnan Kastrati, Klinik für Herz- und Kreislauferkrankungen, Deutsches Herzzentrum München, Technische Universität München, Munich, Germany; DZHK (German Center for Cardiovascular Research), Partner site Munich Heart Alliance, Munich, Germany.
Salvatore Cassese, Klinik für Herz- und Kreislauferkrankungen, Deutsches Herzzentrum München, Technische Universität München, Munich, Germany.
References
- Cassese S, Byrne RA, Tada T, Pinieck S, Joner M, Ibrahim T, King LA, Fusaro M, Laugwitz KL, Kastrati A. Incidence and predictors of restenosis after coronary stenting in 10 004 patients with surveillance angiography. Heart. 2014;100:153–9. doi: 10.1136/heartjnl-2013-304933. [DOI] [PubMed] [Google Scholar]
- Kastrati A, Mehilli J, von Beckerath, Dibra A, Hausleiter J, Pache J, Schühlen H, Schmitt C, Dirschinger J, Schömig A ISAR-DESIRE Study Investigators. Sirolimus-eluting stent or paclitaxel-eluting stent vs balloon angioplasty for prevention of recurrences in patients with coronary in-stent restenosis: a randomized controlled trial. JAMA. 2005;293:165–71. doi: 10.1001/jama.293.2.165. [DOI] [PubMed] [Google Scholar]
- Giacoppo D, Alfonso F, Xu B, Claessen B, Adriaenssens T, Jensen C, Pérez-Vizcayno MJ, Kang DY, Degenhardt R, Pleva L, Baan J, Cuesta J, Park DW, Kukla P, Jiménez-Quevedo P, Unverdorben M, Gao R, Naber CK, Park SJ, Henriques JPS, Kastrati A, Byrne RA. Drug-Coated Balloon Angioplasty Versus Drug-Eluting Stent Implantation in Patients With Coronary Stent Restenosis. J Am Coll Cardiol. 2020;75:2664–78. doi: 10.1016/j.jacc.2020.04.006. [DOI] [PubMed] [Google Scholar]
- Mehran R, Dangas G, Abizaid AS, Mintz GS, Lansky AJ, Satler LF, Pichard AD, Kent KM, Stone GW, Leon MB. Angiographic patterns of in-stent restenosis: classification and implications for long-term outcome. Circulation. 1999;100:1872–8. doi: 10.1161/01.cir.100.18.1872. [DOI] [PubMed] [Google Scholar]
- Byrne RA, Joner M, Tada T, Kastrati A. Restenosis in bare metal and drug-eluting stents: distinct mechanistic insights from histopathology and optical intravascular imaging. Minerva Cardioangiol. 2012;60:473–89. [PubMed] [Google Scholar]
- Takasawa Y, Iijima R, Shiba M, Nakamura M, Sugi K. Predictor of subsequent target lesion revascularization in patients with drug-eluting stent restenosis undergoing percutaneous coronary intervention. J Cardiol. 2010;55:391–6. doi: 10.1016/j.jjcc.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Ozawa M, Okamura A, Date M, Higuchi Y, Nagai H, Shibuya M, Ryusuke K, Inoue K, Koyama Y, Iwakura K, Fujii K. Third in-stent restenosis in sirolimus eluting stents: predictors of the next restenosis. Catheter Cardiovasc Interv. 2012;79:91–6. doi: 10.1002/ccd.22916. [DOI] [PubMed] [Google Scholar]
- Nojima Y, Yasuoka Y, Kume K, Adachi H, Hattori S, Matsutera R, Kohama Y, Sasaki T. Switching types of drug-eluting stents does not prevent repeated in-stent restenosis in patients with coronary drug-eluting stent restenosis. Coron Artery Dis. 2014;25:638–44. doi: 10.1097/MCA.0000000000000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto H, Ruparelia N, Latib A, Miyazaki T, Sato K, Mangieri A, Contri R, Stella S, Figini F, Chieffo A, Carlino M, Montorfano M, Colombo A. Drug-Coated Balloons Versus Second-Generation Drug-Eluting Stents for the Management of Recurrent Multimetal-Layered In-Stent Restenosis. JACC Cardiovasc Interv. 2015;8:1586–94. doi: 10.1016/j.jcin.2015.04.032. [DOI] [PubMed] [Google Scholar]
- Alfonso F, García J, Pérez-Vizcayno MJ, Hernando L, Hernandez R, Escaned J, Jiménez-Quevedo P, Bañuelos C, Macaya C. New stent implantation for recurrences after stenting for in-stent restenosis: implications of a third metal layer in human coronary arteries. J Am Coll Cardiol. 2009;54:1036–8. doi: 10.1016/j.jacc.2009.04.082. [DOI] [PubMed] [Google Scholar]
- Kubo S, Kadota K, Otsuru S, Hasegawa D, Shigemoto Y, Habara S, Tada T, Tanaka H, Fuku Y, Katoh H, Goto T, Mitsudo K. Optimal treatment of recurrent restenosis lesions after drug-eluting stent implantation for in-stent restenosis lesions. EuroIntervention. 2013;9:788–96. doi: 10.4244/EIJV9I7A131. [DOI] [PubMed] [Google Scholar]
- Clever YP, Cremers B, von Scheidt, Bohm M, Speck U, Scheller B. Compassionate use of a paclitaxel coated balloon in patients with refractory recurrent coronary in-stent restenosis. Clin Res Cardiol. 2014;103:21–7. doi: 10.1007/s00392-013-0617-7. [DOI] [PubMed] [Google Scholar]
- Cosgrave J, Melzi G, Biondi-Zoccai GG, Airoldi F, Chieffo A, Sangiorgi GM, Montorfano M, Michev I, Carlino M, Bonizzoni E, Colombo A. Drug-eluting stent restenosis the pattern predicts the outcome. J Am Coll Cardiol. 2006;47:2399–404. doi: 10.1016/j.jacc.2006.02.046. [DOI] [PubMed] [Google Scholar]
- Latib A, Mussardo M, Ielasi A, Tarsia G, Godino C, Al-Lamee R, Chieffo A, Airoldi F, Carlino M, Montorfano M, Colombo A. Long-term outcomes after the percutaneous treatment of drug-eluting stent restenosis. JACC Cardiovasc Interv. 2011;4:155–64. doi: 10.1016/j.jcin.2010.09.027. [DOI] [PubMed] [Google Scholar]
- Mintz GS, Popma JJ, Pichard AD, Kent KM, Satler LF, Chuang YC, Ditrano CJ, Leon MB. Patterns of calcification in coronary artery disease. A statistical analysis of intravascular ultrasound and coronary angiography in 1155 lesions. Circulation. 1995;91:1959–65. doi: 10.1161/01.cir.91.7.1959. [DOI] [PubMed] [Google Scholar]
- Thygesen K, Alpert JS, White HD Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction, Jaffe AS, Apple FS, Galvani M, Katus HA, Newby LK, Ravkilde J, Chaitman B, Clemmensen PM, Dellborg M, Hod H, Porela P, Underwood R, Bax JJ, Beller GA, Bonow R, Van der Wall EE, Bassand JP, Wijns W, Ferguson TB, Steg PG, Uretsky BF, Williams DO, Armstrong PW, Antman EM, Fox KA, Hamm CW, Ohman EM, Simoons ML, Poole-Wilson PA, Gurfinkel EP, Lopez-Sendon JL, Pais P, Mendis S, Zhu JR, Wallentin LC, Fernández-Avilés F, Fox KM, Parkhomenko AN, Priori SG, Tendera M, Voipio-Pulkki LM, Vahanian A, Camm AJ, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Morais J, Brener S, Harrington R, Morrow D, Lim M, Martinez-Rios MA, Steinhubl S, Levine GN, Gibler WB, Goff D, Tubaro M, Dudek D, Al-Attar N. Universal definition of myocardial infarction. Circulation. 2007;116:2634–53. [Google Scholar]
- Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es, Steg PG, Morel MA, Mauri L, Vranckx P, McFadden E, Lansky A, Hamon M, Krucoff MW, Serruys PW Academic Research Consortium. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344–51. doi: 10.1161/CIRCULATIONAHA.106.685313. [DOI] [PubMed] [Google Scholar]
- Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined--a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol. 2000;36:959–69. doi: 10.1016/s0735-1097(00)00804-4. [DOI] [PubMed] [Google Scholar]
- Kastrati A, Dibra A, Mehilli J, Mayer S, Pinieck S, Pache J, Dirschinger J, Schömig A. Predictive factors of restenosis after coronary implantation of sirolimus- or paclitaxel-eluting stents. Circulation. 2006;113:2293–300. doi: 10.1161/CIRCULATIONAHA.105.601823. [DOI] [PubMed] [Google Scholar]
- Tibshirani R. The lasso method for variable selection in the Cox model. Stat Med. 1997;16:385–95. doi: 10.1002/(sici)1097-0258(19970228)16:4<385::aid-sim380>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Harrell F. Predicting Outcomes: Applied Survival Analysis and Logistic Regression. Charlottesville,VA: University of Virginia Press; 1997. pp. 23–4. [Google Scholar]
- Pencina MJ, D’Agostino Sr, D’Agostino JR, Vasan RS. Comments on ‘Integrated discrimination and net reclassification improvements—Practical advice’. Statistics in Medicine. 2007;27:207–12. [Google Scholar]
- Pencina MJ, D'Agostino RB, Pencina KM, Janssens AC, Greenland P. Interpreting incremental value of markers added to risk prediction models. Am J Epidemiol. 2012;176:473–81. doi: 10.1093/aje/kws207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moons KG, Altman DG, Reitsma JB, Ioannidis JP, Macaskill P, Steyerberg EW, Vickers AJ, Ransohoff DF, Collins GS. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med. 2015;162:W1–73. doi: 10.7326/M14-0698. [DOI] [PubMed] [Google Scholar]
- Mehilli J, Byrne RA, Tiroch K, Pinieck S, Schulz S, Kufner S, Massberg S, Laugwitz KL, Schömig A, Kastrati A ISAR-DESIRE 2 Investigators. Randomized trial of paclitaxel- versus sirolimus-eluting stents for treatment of coronary restenosis in sirolimus-eluting stents: the ISAR-DESIRE 2 (Intracoronary Stenting and Angiographic Results: Drug Eluting Stents for In-Stent Restenosis 2) study. J Am Coll Cardiol. 2010;55:2710–6. doi: 10.1016/j.jacc.2010.02.009. [DOI] [PubMed] [Google Scholar]
- Alfonso F, Zueco J, Cequier A, Mantilla R, Bethencourt A, López-Minguez JR, Angel J, Augé JM, Gómez-Recio M, Morís C, Seabra-Gomes R, Perez-Vizcayno MJ, Macaya C Restenosis Intra-stent: Balloon Angioplasty Versus Elective Stenting (RIBS) Investigators. A randomized comparison of repeat stenting with balloon angioplasty in patients with in-stent restenosis. J Am Coll Cardiol. 2003;42:796–805. doi: 10.1016/s0735-1097(03)00852-0. [DOI] [PubMed] [Google Scholar]
- Alfonso F, Pérez-Vizcayno MJ, Hernandez R, Bethencourt A, Martí V, López-Mínguez JR, Angel J, Mantilla R, Morís C, Cequier A, Sabaté M, Escaned J, Moreno R, Bañuelos C, Suárez A, Macaya C RIBS-II Investigators. A randomized comparison of sirolimus-eluting stent with balloon angioplasty in patients with in-stent restenosis: results of the Restenosis Intrastent: Balloon Angioplasty Versus Elective Sirolimus-Eluting Stenting (RIBS-II) trial. J Am Coll Cardiol. 2006;47:2152–60. doi: 10.1016/j.jacc.2005.10.078. [DOI] [PubMed] [Google Scholar]
- Solinas E, Dangas G, Kirtane AJ, Lansky AJ, Franklin-Bond T, Boland P, Syros G, Kim YH, Gupta A, Mintz G, Fahy M, Collins M, Kodali S, Stone GW, Moses JW, Leon MB, Mehran R. Angiographic patterns of drug-eluting stent restenosis and one-year outcomes after treatment with repeated percutaneous coronary intervention. Am J Cardiol. 2008;102:311–5. doi: 10.1016/j.amjcard.2008.03.060. [DOI] [PubMed] [Google Scholar]
- Koch T, Cassese S, Xhepa E, Mayer K, Tölg R, Hoppmann P, Laugwitz KL, Byrne RA, Kastrati A, Kufner S. Efficacy of drug-coated balloon angioplasty in early versus late occurring drug-eluting stent restenosis: A pooled analysis from the randomized ISAR DESIRE 3 and DESIRE 4 trials. Catheter Cardiovasc Interv. 2020;96:1008–15. doi: 10.1002/ccd.28638. [DOI] [PubMed] [Google Scholar]
- Jinnouchi H, Kuramitsu S, Shinozaki T, Tomoi Y, Hiromasa T, Kobayashi Y, Domei T, Soga Y, Hyodo M, Shirai S, Ando K. Difference of Tissue Characteristics Between Early and Late Restenosis After Second-Generation Drug-Eluting Stents Implantation - An Optical Coherence Tomography Study. Circ J. 2017;81:450–7. doi: 10.1253/circj.CJ-16-1069. [DOI] [PubMed] [Google Scholar]
- Chezar-Azerrad C, Musallam A, Shea C, Zhang C, Torguson R, Yerasi C, Case BC, Forrestal BJ, Khalid N, Khan JM, Shlofmitz E, Chen Y, Satler LF, Bernardo NL, Ben-Dor I, Rogers T, Hashim H, Mintz GS, Waksman R. One-Year Outcomes After Treatment of Ostial In-Stent Restenosis in Left Circumflex Versus Left Anterior Descending or Right Coronary Artery. Am J Cardiol. 2021;151:45–50. doi: 10.1016/j.amjcard.2021.03.045. [DOI] [PubMed] [Google Scholar]
- Lee WC, Fang HY, Chung WJ, Hsueh SK, Chen CJ, Yang CH, Yip HK, Hang CL, Wu CJ, Fang CY. One-year outcomes following drug-eluting balloon use for coronary ostial restenosis. Int J Cardiol Heart Vasc. 2015;10:25–8. doi: 10.1016/j.ijcha.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Yasuda S, Kataoka Y, Morii I, Kawamura A, Miyazaki S. Significant association of coronary artery calcification in stent delivery route with restenosis after sirolimus-eluting stent implantation. Circ J. 2009;73:1856–63. doi: 10.1253/circj.cj-09-0080. [DOI] [PubMed] [Google Scholar]
- Kawaguchi R, Tsurugaya H, Hoshizaki H, Toyama T, Oshima S, Taniguchi K. Impact of lesion calcification on clinical and angiographic outcome after sirolimus-eluting stent implantation in real-world patients. Cardiovasc Revasc Med. 2008;9:2–8. doi: 10.1016/j.carrev.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Theodoropoulos K, Mennuni MG, Dangas GD, Meelu OA, Bansilal S, Baber U, Sartori S, Kovacic JC, Moreno PR, Sharma SK, Mehran R, Kini AS. Resistant in-stent restenosis in the drug eluting stent era. Catheter Cardiovasc Interv. 2016;88:777–85. doi: 10.1002/ccd.26559. [DOI] [PubMed] [Google Scholar]
- Stefanini GG, Alfonso F, Barbato E, Byrne RA, Capodanno D, Colleran R, Escaned J, Giacoppo D, Kunadian V, Lansky A, Mehilli J, Neumann FJ, Regazzoli D, Sanz-Sanchez J, Wijns W, Baumbach A. Management of myocardial revascularisation failure: an expert consensus document of the EAPCI. EuroIntervention. 2020;16:e875–90. doi: 10.4244/EIJ-D-20-00487. [DOI] [PubMed] [Google Scholar]
- Elgendy IY, Ha LD, Elbadawi A, Ogunbayo GO, Olorunfemi O, Mahmoud AN, Mojadidi MK, Abuzaid A, Anderson RD, Bavry AA. Temporal Trends in Inpatient Use of Intravascular Imaging Among Patients Undergoing Percutaneous Coronary Intervention in the United States. JACC Cardiovasc Interv. 2018;11:913–5. doi: 10.1016/j.jcin.2018.01.254. [DOI] [PubMed] [Google Scholar]
- Koskinas KC, Nakamura M, Räber L, Colleran R, Kadota K, Capodanno D, Wijns W, Akasaka T, Valgimigli M, Guagliumi G, Windecker S, Byrne RA. Current use of intracoronary imaging in interventional practice - Results of a European Association of Percutaneous Cardiovascular Interventions (EAPCI) and Japanese Association of Cardiovascular Interventions and Therapeutics (CVIT) Clinical Practice Survey. EuroIntervention. 2018;14:e475–84. doi: 10.4244/EIJY18M03_01. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Methods.
List of variables considered by the LASSO model.
TRIPOD checklist: prediction model development and validation.
Indications for repeat PCI for recurrent DES-ISR at 1- and 5-year follow-up.
Repeat PCI for recurrent DES-ISR from 0 to 1 year and from 1 to 5 years as per ISR interval and morphology.
Results of logistic regression analysis for repeat PCI for recurrent DES-ISR from 1 to 5 years after first reintervention for DES-ISR.
Landmark analysis demonstrating the cumulative incidence of repeat PCI for recurrent DES-ISR from 0-1 years and from 1-5 years for lesions with and without the four predictor variables identified in the logistic regression model.