Abstract
The diagnosis and treatment of rectal cancer have evolved dramatically over the past several decades. At the same time, its incidence has increased in younger populations. This review will inform the reader of advances in both diagnosis and treatment. These advances have led to the watch-and-wait approach, otherwise known as nonsurgical management. This review briefly outlines changes in medical and surgical treatment, advances in MRI technology and interpretation, and landmark studies or trials that have led to this exciting juncture. Herein, the authors delve into current state-of-the-art methods to assess response to treatment with MRI and endoscopy. Currently, these methods for avoiding surgery can be used to detect a complete clinical response in as many as 50% of patients with rectal cancer. Finally, the limitations of imaging and endoscopy and future challenges will be discussed.
© RSNA, 2023
Summary
MRI plays a critical role in assessing clinical complete response for patient selection and monitoring with the watch-and-wait strategy, otherwise known as nonsurgical management, in rectal cancer management.
Essentials
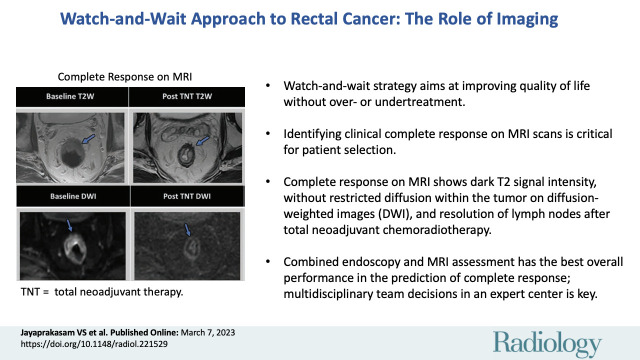
■ Watch-and-wait (W&W) strategy, otherwise known as nonsurgical care, is an emerging and attractive option in the care of patients with rectal cancer, aimed at improving quality of life without over- or undertreatment.
■ Accurate assessment of clinical complete response (cCR) on MRI scans is critical for optimal patient selection and monitoring under W&W.
■ The cCR on MRI scans is signified by presence of dark T2-weighted MRI signal intensity, without any intermediate signal intensity or restricted diffusion within the tumor bed on diffusion-weighted images, and resolution of lymph nodes on MRI scans after neoadjuvant chemoradiotherapy.
■ Combined endoscopy and MRI assessment has the best overall performance in the prediction of cCR.
■ One of the key challenges in the implementation of W&W is the accurate radiologic and clinical assessment of cCR, which, as such, should be based on a multidisciplinary team decision in an expert center.
Introduction
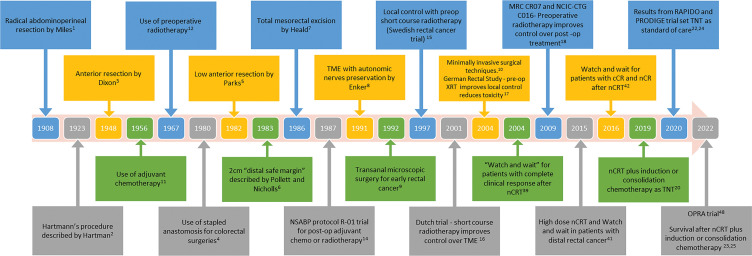
The evolving landscape of rectal cancer management is fascinating yet challenging. Management of rectal cancer has rapidly evolved since the days of radical abdominoperineal resection, first described by William Ernest Miles in 1908 (1,2). Attempts to perform less radical, more sphincter-saving procedures and to improve local recurrence rates led to the development of anterior resections for upper and middle rectal cancers in the 1940s, low anterior resection for low-lying rectal cancers in the 1970s, and total mesorectal excision in the 1980s (3–10). Thereafter, large randomized controlled trials conducted in the early 2000s, notably the German Rectal Trial and the Swedish Rectal Trial, demonstrated the superiority of neoadjuvant chemoradiotherapy (nCRT) over postoperative chemoradiotherapy and established a combined multimodality sequence of treatments as the standard of care worldwide (11–18) (Fig 1). This regimen was associated with 15%–20% of patients achieving a pathologic complete response. In addition, local recurrence rates plummeted from about 40% to 7%; however, the rate of distant metastases remained high at about 20%–30% (19).
Figure 1:
Timeline of development of various surgical techniques and some of the important trials and studies influencing the management of rectal cancer in the past century. cCR = clinical complete response, LARC = locally advanced rectal cancer, nCR = near complete response, nCRT = neoadjuvant chemoradiotherapy, OPRA = Organ Preservation of Rectal Adenocarcinoma, TME = total mesorectal excision, TNT = total neoadjuvant therapy, XRT = radiation therapy.
Given the persistently high rates of distant metastases, variations in treatment modality sequencing have been investigated. Prospective trials (eg, RAPIDO, PRODIGE-23, CAO/ARO/AIO-12, STELLAR) have ushered in the current era of total neoadjuvant therapy followed by total mesorectal excision as the standard of care for locally advanced rectal cancer (20–24). Total neoadjuvant therapy shifts postoperative (adjuvant) chemotherapy to before surgery. This shift is designed to more immediately address potential distant micrometastases; it can be given either before (induction) or after (consolidation) chemoradiotherapy. Total neoadjuvant therapy has been noted to improve local response and the likelihood of pathologic complete response (23–26). Strategies to further achieve higher pathologic complete response rates have been accomplished using longer intervals between nCRT and surgery (supported by the TIMING trial) and longer intervals between the end of nCRT and imaging—in recognition of the delayed effects of radiation therapy (27,28).
Up to one-third of patients still develop disease-related treatment failure (eg, distant metastases, treatment-related death, or local-regional failure) (24). Treatment-related morbidity due to surgery and radiation therapy includes bowel, bladder, and sexual dysfunction, and risk of permanent stoma, affecting quality of life (29,30). Thus, treatment de-intensification strategies aiming to exclude one modality have been attempted in clinical trials (eg, chemotherapy with only selective radiation therapy followed by surgery [PROSPECT], nCRT and surgery without adjuvant chemotherapy [Spanish GCR-3], and chemotherapy and nCRT without surgery [Organ Preservation of Rectal Adenocarcinoma, or OPRA]) (31–33). The increasing awareness that patients who undergo surgery may have no tumor in the specimen (ie, a pathologic complete response) has led to possible organ-preserving strategies. Organ preservation after a clinical complete response (cCR) to neoadjuvant therapy seeks to avoid unnecessary surgery that would remove a tumor-free rectum. This approach is referred to as watch and wait (W&W), wait and see, or nonsurgical management.
High-spatial-resolution pelvic MRI is essential to rectal cancer management. It is critical for anatomic delineation of the rectal wall and mesorectal fascia and has become the standard of care for preoperative assessment of prognostic factors in locally advanced rectal cancer, such as bowel wall invasion, extramural spread, extramural vascular invasion, and lymph node and peritoneal involvement. Its routine use has taken a firm hold in the work-up of patients with locally advanced rectal cancer based on results from the MERCURY study (34–36). Technologic advances in the past 15 years, including higher magnetic field strength, improved surface coils, and functional sequences, such as diffusion-weighted imaging (DWI) and dynamic contrast-enhanced sequences, have further improved MRI in rectal cancer assessment (37,38).
Improvement of survival outcomes with an emphasis on better quality of life and avoiding over- or undertreatment informs the current goals of rectal cancer management. This review will provide insight into the pivotal role of MRI in the increasingly practiced W&W approach to treatment. It will also explore the current state-of-the-art methods for staging and response assessment to ensure the success of the W&W approach in standard clinical practice.
Clinical Aspects: A Brief Recap of Key Studies and Trials
The foundation for W&W was laid by Habr-Gama and colleagues in 2004 when they compared surgical with nonsurgical treatment in patients with distal rectal cancer after nCRT (39). In this seminal study, patients assigned to W&W who had cCR after nCRT were compared with patients who had pathologic complete response after surgery. The 5-year overall survival rate was better in the observation group (100% vs 88%, P = .01), but there was no evidence of a difference in the disease-free survival (DFS) rate (92% vs 83%, P = .09).
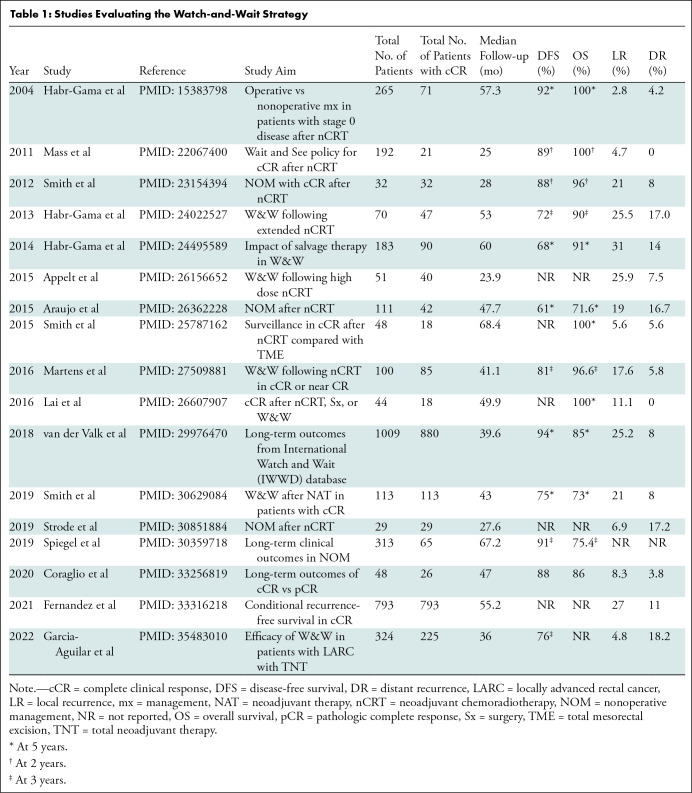
In a follow-up study spanning 20 years, Habr-Gama et al reported that most local regrowth was endoluminal, detected with endoscopy, and occurred within the first 12 months of follow-up, with local or pelvic recurrence in 31% of patients (40). Most local regrowth was treated with salvage surgery, leading to overall recurrence-free survival in 94% of patients, sphincter preservation in 85%, and organ preservation in 78%. The study highlighted two important findings. First, most early regrowth is local, amenable to R0 salvage surgery, and probably due to failure to identify residual disease clinically or radiologically. Second, there was a substantial proportion of ypT3-4 disease (35%) in the resected specimens, suggesting residual and deeper foci of viable cancer. Both findings highlight the importance of appropriate clinical and radiologic assessment of cCR in selecting patients for W&W. In the years since the seminal work of Habr-Gama et al, several studies have been published assessing the survival outcomes and benefits of W&W (41–44) (Table 1).
Table 1:
Studies Evaluating the Watch-and-Wait Strategy
To understand the risks and benefits of organ preservation strategies and to systematically collect retrospective and prospective data across centers worldwide, an International Watch and Wait Database was initiated in 2014 by the European Registration of Cancer Care and the Champalimaud Foundation (45). Results from this web-based database showed a 5-year overall survival of 85% and a 5-year disease-specific survival of 94% (46). The largest North American cohort of patients in the W&W protocol who developed a cCR after nCRT (n = 113) showed a high rate of rectal preservation (82%) and effective surgical salvage after regrowth (91%) (47). Of note, patients with local regrowth showed higher rates of distant metastasis compared with patients with sustained cCR (36% vs 1%). Similar findings related to regrowth were noted in the International Watch and Wait Database (18% vs 5%) (46).
Recently, results were published from the Organ Preservation of Rectal Adenocarcinoma trial, the first prospective trial integrating W&W and total neoadjuvant therapy (48). In this randomized phase II trial with a median follow-up of 3 years, 152 patients underwent induction chemotherapy followed by long-course chemoradiation, and 155 underwent consolidation chemotherapy after long-course chemoradiation. A three-tiered response schema using physical examination, endoscopy, and MRI was used to determine whether patients underwent total mesorectal excision or W&W (49). Three-year DFS was 76% in both groups. Although the trial did not meet its primary end point of a 10% improvement in DFS compared with historical control subjects (also 76%), it revealed that the 3-year total mesorectal excision–free survival rate (surrogate for organ preservation rate) was 41% in the induction group and 53% in the consolidation group. There were no differences in local recurrence-free survival, distant metastasis–free survival, or overall survival. Patients who underwent total mesorectal excision after restaging and patients who underwent total mesorectal excision after local regrowth had similar DFS rates.
Overall, these data favor safe integration of W&W in a total neoadjuvant therapy strategy wherein half of patients with locally advanced rectal cancer can avoid surgery, and it highlights the important role that MRI plays in decision making.
Imaging Assessment of Response and cCR
MRI
The success of W&W depends on accurate restaging and identification of cCR, as well as appropriate patient selection and monitoring. MRI is the imaging modality of choice for treatment response assessment, surveillance, and detection of local regrowth in patients with rectal cancer. The MERCURY study showed that the MRI-based tumor regression grade and circumferential resection margin assessment on post-nCRT MRI scans provided information regarding DFS, overall survival, and risk for local recurrence (50). There is growing evidence that functional MRI sequences such as DWI allow for qualitative and tumor microenvironment-based quantitative assessment of the posttreatment tumor bed, but further large-scale prospective studies are required (51). To obtain optimal diagnostic-quality images and provide accurate and standardized response evaluation, MRI should be performed and reported according to recommended parameters. The 2016 European Society of Gastrointestinal and Abdominal Radiology and the similar 2017 Society of Abdominal Radiology rectal cancer disease–focused panel consensus recommendations and guidelines are considered the standard of care. These societies recommend guidelines on the acquisition, interpretation, and reporting of MRI scans for baseline clinical staging and posttreatment response evaluation of rectal cancer (52,53). The U.S. National Accreditation Program in Rectal Cancer has adopted these standards as well as the synoptic report from the Society of Abdominal Radiology (54).
MRI protocol.—The principles of MRI scanning in the response assessment setting are similar to those in the staging setting but with greater emphasis on DWI sequences. It is recommended that MRI be performed with an external phased-array surface coil (preferably with between eight and 32 elements) with a minimum magnetic field strength of 1.5 T. There is no recommended preference between 1.5 and 3.0 T. However, significant signal intensity differences have been reported between pre- and post-DWI scans and apparent diffusion coefficient–calculated images between responders and nonresponders, with possibly better visual assessment of treatment response at 3.0 T compared with 1.5 T (55). This must be balanced with the potential for more artifacts on 3.0-T DWI scans. In Europe, where users have more experience, 1.5 T is slightly preferred. An endorectal coil is not recommended. Rectal filling is optional. Spasmolytics and a rectal microenema can improve image quality of DWI scans, especially for high-lying tumors, in the post-total neoadjuvant therapy setting and with 3.0-T scanners (56,57). A study comparing b values of 800 and 1500 sec/mm2 indicated a preference and suggested greater diagnostic accuracy for cCR using a b value of 1500 sec/mm2 (58). High-spatial-resolution two-dimensional T2-weighted axial and coronal oblique sequences perpendicular and parallel to the tumor axis with a section thickness of 3 mm or less are essential for accurate response assessment within the primary tumor and for determining the presence or regression of extramural vascular invasion, lymph nodes, or tumor deposits within the mesorectal fascia, as well as for determining the circumferential resection margin. A nonenhanced T1-weighted sequence is recommended by the Society of Abdominal Radiology (52). Additionally, intravenous contrast material is not routinely recommended. MRI protocols for all vendors can be found online at the Society of Abdominal Radiology website (59).
Reporting proforma.—Structured reporting of restaging rectal MRI (available from the Society of Abdominal Radiology website) is essential for the accurate and consistent analysis of primary tumor response and the evaluation of prognostic features and posttreatment changes compared with the baseline MRI findings (60,61). When reporting restaging MRI, the radiologist should be aware of the patient’s prior treatment (eg, induction chemotherapy, nCRT, total neoadjuvant therapy, or transanal excision). Restaging MRI, reported in comparison with baseline MRI, must contain information regarding changes in tumor morphology (eg, tumor length, wall thickness, relationship to the anal sphincter complex, mesorectal fascia, and peritoneum). Differences in T2-weighted signal characteristics of the tumor and the presence or absence of mucin should also be reported. MRI-based features of tumor regression after nCRT are better at predicting treatment response than the posttreatment T category (62,63). Describing changes within the mesorectal and pelvic side wall lymph nodes, including node borders and signal intensity features (known as the Dutch Criteria), in addition to site, size, and location, is helpful for a more reliable reassessment of lymph node involvement (35,64).
T2-weighted MRI sequences and MRI-based tumor regression grade.—Multiplanar high-spatial-resolution two-dimensional T2-weighted MRI sequences are the mainstay for rectal cancer restaging. MRI assessment of treatment response after nCRT or total neoadjuvant therapy is usually performed within 6–8 weeks of completion of therapy. However, longer intervals, such as 8–10 weeks and even 10–12 weeks, are increasingly common in recognition of the delayed effects of radiation (65). At baseline, the untreated rectal adenocarcinoma typically appears as an intermediate T2-weighted signal intensity lesion when compared with the muscularis propria. Mucinous tumors, comprising 10%–15% of all rectal adenocarcinomas, are associated with worse prognosis and have high T2-weighted signal intensity areas (66). On the post-nCRT or total neoadjuvant therapy MRI scan, progressive fibrosis in the primary tumor leads to darkening of T2 signal intensity and a reduction in size.
Assessment of MRI-based T category restaging is extremely limited, and radiologists should not assign this. A meta-analysis demonstrated that the ability of posttreatment MRI to depict residual tumor had a sensitivity of 50% and a specificity of 91% (67). Interestingly, among the included studies that used DWI MRI, the sensitivity improved to 84%, with little reduction in the specificity, which was 85%. Most inaccuracies in restaging T category are due to overstaging, particularly of those small residual superficial T0–T2 lesions with associated fibrosis or peritumoral desmoplastic reactions (68). The Response Evaluation Criteria in Solid Tumors, version 1.1, does not apply to luminal enteric tumors due to differences in degree of luminal distention, circumferential growth pattern, and luminal contents interfering with assessment.
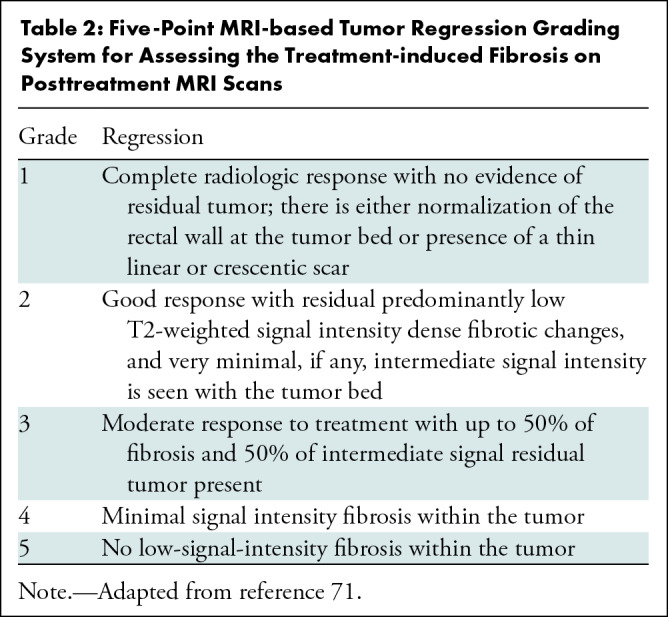
While there are many methods proposed to quantitate response, such as volume and length or maximal thickness reduction, these are not widely applied or validated. A qualitative description of primary tumor fibrosis may be descriptive or semiquantitative to approximate the scale used by pathologists. The MRI-based tumor regression grading system, adapted from the pathologic tumor regression grading system, is used to assess the degree of treatment-induced fibrosis on posttreatment MRI scans (69–71) (Table 2). Patients with MRI-based tumor regression grades of 1–3 are considered good responders with favorable pathologic findings, better overall survival, and better DFS than poor responders with MRI-based tumor regression grades of 4–5 (71,72). More recently, the creators of this system have pointed out some important shortcomings that may explain its lack of widespread use. For example, MRI-based tumor regression grades often do not correctly predict pathologic tumor regression grading (range, 28%–34%), with equal under- and overestimations (73). Sensitivity for the prediction of pathologic complete response is limited at 61%, with a specificity of 89% (74). Interobserver agreement ranges from 60% to 67%, with modest κ interreader agreement values of 0.25 to 0.36 (75). Use of MRI-based tumor regression grades as imaging markers to validate MRI-directed patient care based on imaging response to nCRT is currently being tested in the Magnetic Resonance Tumour Regression Grade as Biomarker for Stratified Management of Rectal Cancer Patients (TRIGGER) trial (76).
Table 2:
Five-Point MRI-based Tumor Regression Grading System for Assessing the Treatment-induced Fibrosis on Posttreatment MRI Scans
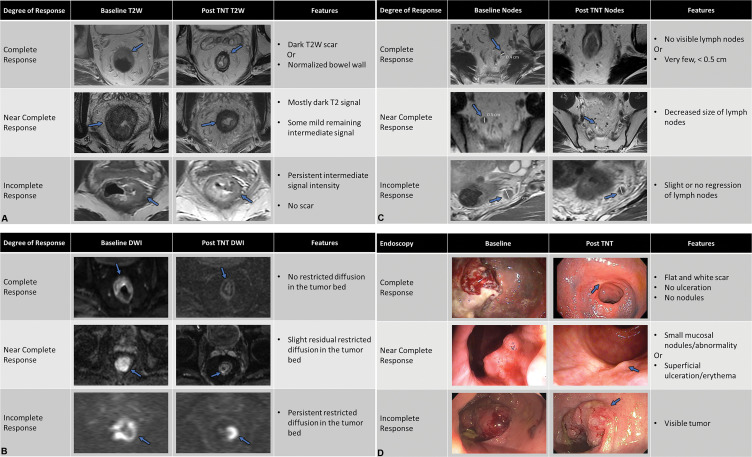
As such, the focus in day-to-day practice is to simplify the qualitative assessment of the degree of T2 darkening and scar formation. Use of a three-tiered system for response assessment showed no loss of accuracy compared with a five-tier system and correlated well with survival outcomes (77). The modified response assessment used in the recent Organ Preservation of Rectal Adenocarcinoma trial is similar to these three-point scales and is used as a measure of MRI-based response assessment within the primary tumor and lymph nodes on T2-weighted and DWI scans, in conjunction with endoscopic findings (Fig 2) (48). Within the primary tumor, complete response refers to either normal-appearing bowel wall without any fibrosis in the tumor bed or presence of only dark T2 signal intensity without any evidence of intermediate T2 signal intensity. Near-complete response refers to mostly dark T2 signal intensity scar with some remaining intermediate signal intensity within the tumor bed. A persistent intermediate signal intensity and the absence of a T2 scar is deemed an incomplete response. This qualitative grading is admittedly subjective and requires experience and validation. Also, it does not apply well to mucinous tumors.
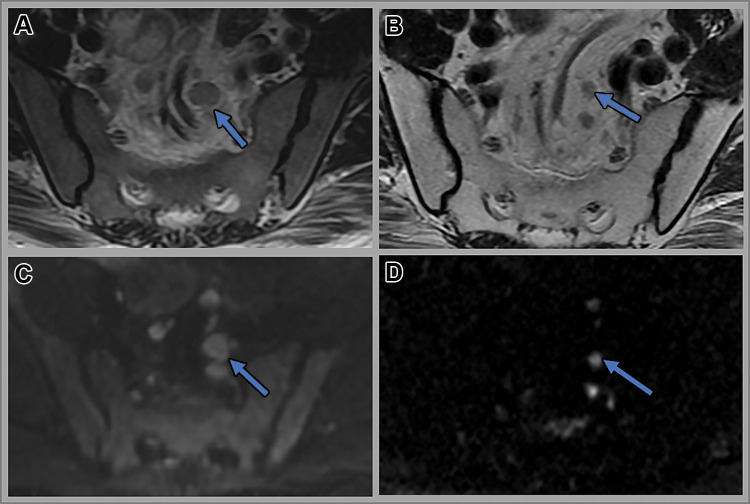
Figure 2:
(A) Complete, near complete, and incomplete response within the primary tumor (arrows) on axial T2-weighted (T2W) MRI scans at restaging performed after completion of total neoadjuvant therapy. (B) Complete, near complete, and incomplete response (arrows) within the primary tumor on axial diffusion-weighted images at restaging performed after completion of total neoadjuvant therapy (TNT). (C) Complete, near complete, and incomplete response (arrows) within the lymph nodes on axial T2-weighted MRI scans at restaging performed after completion of total neoadjuvant therapy (TNT). (D) Endoscopy images depicting complete, near complete, and incomplete response (arrows) within the primary tumor obtained after completion of TNT.
With respect to residual tumor outside the bowel wall, margin assessment is critical for successful curative resection. High-spatial-resolution T2-weighted sequences predict the involvement of the mesorectal fascia, referred to as the circumferential resection margin, when the distance between the lateral-most edge of the tumor is 1 mm or less from the mesorectal fascia (Fig 3) (78). It is important to understand that the circumferential resection margin is determined by the surgeon at surgery. The two terms are equated because the ideal circumferential resection margin is equivalent to the mesorectal fascia, but such surgery is challenging. Thus, it is better to refer to the tumor distance to the mesorectal fascia in radiologic reports. On posttreatment studies, nearly 36% of patients are overstaged for tumor invasion of the mesorectal fascia, probably due to the desmoplastic changes seen in more than 50% of patients (Figs 4, 5). Although MRI has a relatively high sensitivity and negative predictive value (both 100%) in the prediction of mesorectal fascia invasion, it has moderate specificity (range, 32%–59%), positive predictive value (range, 57%–68%), and interreader agreement (κ = 0.38) (79). As expected, the accuracy of MRI to assess circumferential resection margin response is relatively low for patients with a higher pretreatment T category than for those with early pretreatment T category disease (79,80).
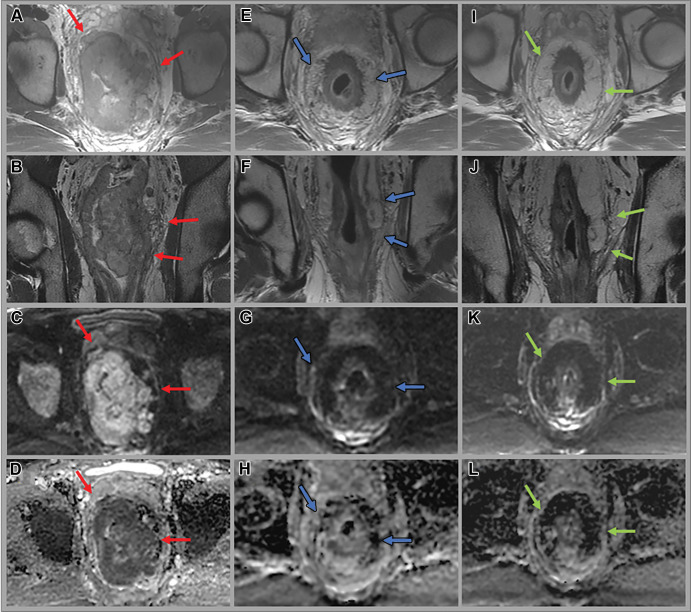
Figure 3:
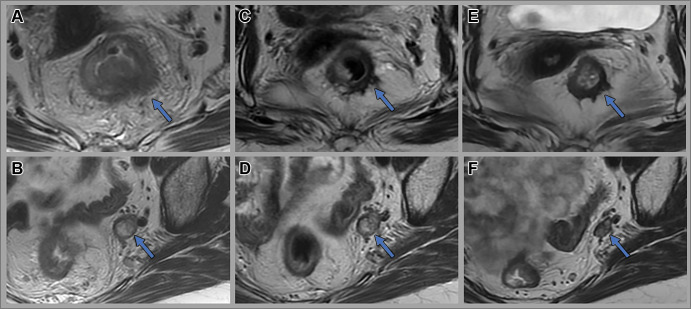
Images in a 53-year-old man with bulky middle to upper rectal adenocarcinoma involving the mesorectal fascia. (A, E, I) Axial and (B, F, J) coronal oblique T2-weighted MRI scans, (C, G, K) axial diffusion-weighted images (b value = 800 sec/mm2), and (D, H, L) apparent diffusion coefficient maps through the mid rectum at baseline (A–D), 12 weeks after total neoadjuvant therapy (E–H), and 14 months surveillance after total neoadjuvant therapy while the patient was on a watch-andwait (W&W) strategy (I–L). Baseline images show the primary rectal tumor with multifocal involvement of the mesorectal fascia (red arrows). Post-total neoadjuvant therapy images at 12 weeks show some T2-weighted mixed dark and intermediate signal intensity within the tumor and desmoplastic reactions extending up to the mesorectal fascia (blue arrows). Endoscopy images show intense inflammation (images not shown). Surveillance images at 14 months while the patient was on the W&W strategy show darker T2-weighted dark signal intensity in the scar, no tumor regrowth, clear mesorectal fascia (green arrows), and continued absence of restricted diffusion.
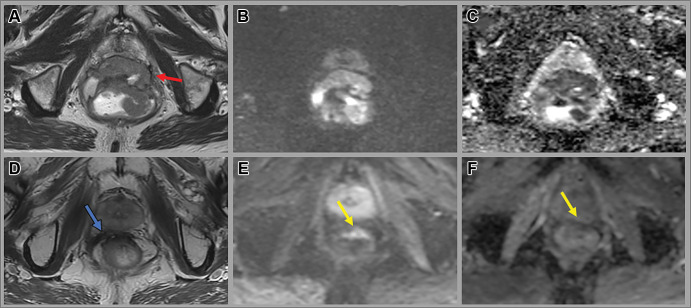
Figure 4:
Images in a 65-year-old man with a low rectal tumor with anterior perforation extending to the anterior mesorectal fascia (red arrow). (A, D) Axial T2-weighted MRI scans, (B, E) axial diffusion-weighted images (b value, 800 sec/mm2), and (C, F) apparent diffusion coefficient maps at baseline (A–C) and 12 weeks after completion of total neoadjuvant therapy (D–F). Post-total neoadjuvant therapy images show decreased size of the tumor with new scar (blue arrow) and some residual restricted diffusion (yellow arrow), consistent with near-complete response. The patient opted for nonsurgical management and remains free of tumor regrowth at 3.5 years of surveillance.
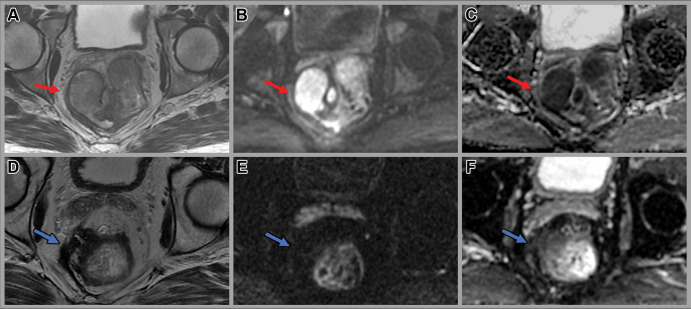
Figure 5:
Images in a 58-year-old man with rectal adenocarcinoma. Baseline (A) axial T2-weighted MRI scan, (B) axial diffusion-weighted image (b value, 800 sec/mm2), and (C) apparent diffusion coefficient map show a large rectal mass with an extraluminal component on the right side contacting the mesorectal fascia (red arrow) with restricted diffusion. Axial (D) T2-weighted MRI scan, (E) axial diffusion-weighted image (b value, 800 sec/mm2), and (F) apparent diffusion coefficient image at 4 weeks after completion of total neoadjuvant therapy show a scar in the tumor bed extending to the mesorectal fascia and minimal residual restricted diffusion (blue arrow), consistent with near-complete response. The patient underwent low anterior resection. Final histology revealed a few foci of residual cancer and no involvement of the mesorectal fascia.
Assessment of residual tumor spread to vessels and nodes is equally important for safe resection margins. Extramural vascular invasion is seen as serpiginous tumor signal intensity and nodular expansion of the mesorectal vessels, which may or may not be contiguous with the primary tumor mass. Assessment of posttreatment extramural vascular invasion has emerged as an important risk factor associated with reduced overall survival (hazard ratio, 2.3) and reduced DFS (hazard ratio, 5.0) (72) (Fig 6). The significant reduction in DFS in patients with positive posttreatment extramural vascular invasion is independent of yT and yN stage (81). Regression of extramural vascular invasion after nCRT is associated with improved survival outcomes and longer DFS compared with those with persistent extramural vascular invasion (82).
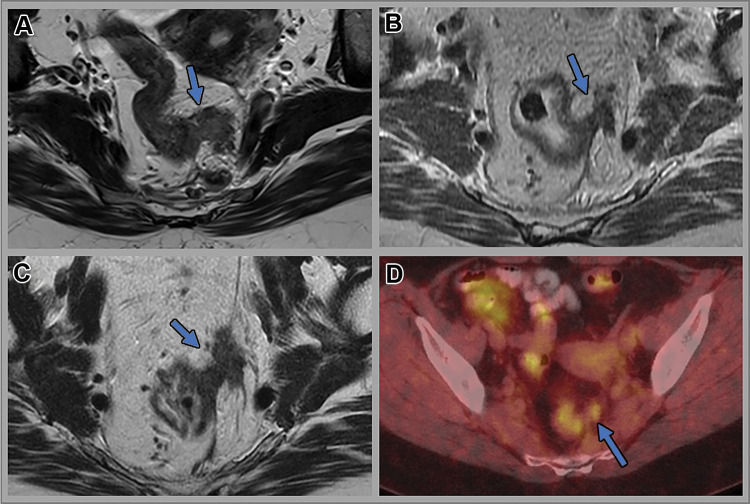
Figure 6:
Images in a 53-year-old woman with locally advanced upper rectal tumor. Axial T2-weighted MRI scans (A) at baseline, (B) 12 weeks after total neoadjuvant therapy, and (C) 12 months after total neoadjuvant therapy (surveillance). (D) Subsequent fused axial fluorine 18 (18F) fluorodeoxyglucose (FDG) PET/CT image. In A, intermediate T2-weighted signal intensity left lateral extramural vascular invasion (arrow) is seen. In B, partial regression of extramural vascular invasion (arrow) is shown. No residual tumor was seen at endoscopy. In C, tumor regrowth within the extramural vascular invasion site (arrow) is visible. Moderately intense 18F-FDG uptake (maximum standard uptake value, 4.1) is seen within the extramural vascular invasion (arrow) on D.
Lymph nodes within the mesorectum are considered suspicious at baseline if they measure at least 0.9 cm in the short-axis dimension, have two or more suspicious morphologic features (eg, round shape, irregular border, heterogenous signal intensity) when they are 0.5–0.8 cm, or have all three suspicious morphologic features when they are smaller than 0.5 cm (24). Assessment of nodal involvement using size criteria on MRI scans obtained after treatment is actually less limited than at baseline for the following reasons: (a) many irradiated nodes disappear (30% fewer are harvested at surgery after radiation), leading to fewer interpretation errors; (b) 80% of remaining nodes are sterilized; (c) one has the ability to compare pre- and posttreatment size and appearance; and (d) remaining enlarged nodes are more likely to be malignant (83). Use of a 0.5-cm cutoff, while not ideal, has been promulgated, in part based on studies and in part based on a pragmatic choice after an accumulation of combined experience (Fig 7). For example, in a node-for-node validation study between MRI and pathology findings, only 20 of 178 nodes (11%) 0.5 cm or smaller were malignant (84). In another node-for-node validation study that examined posttreatment decreases in size of nodes (in parallel with a good responding tumor), nodes 0.5 cm or smaller were benign 86% of the time (85). Of note, in tumors that did not respond well, the 0.5-cm cutoff size had a 33% false-negative rate instead of 14%, confirming the need to assess the primary tumor, as it usually parallels the response in the nodes (Fig 8). Nonetheless, no one size cutoff is perfect; each represents a trade-off between sensitivity and specificity.
Figure 7:
Images in a 58-year-old man with rectal adenocarcinoma. (A, C) Axial T2-weighted MRI scans and (B, D) axial diffusion-weighted (b value, 800 sec/mm2) images at baseline (A, B) and 4 weeks after completion of total neoadjuvant therapy (C, D). Baseline images shows several superior rectal lymph nodes with intermediate signal intensity measuring up to 1.0 cm in short-axis dimension (arrow in A). Post-total neoadjuvant therapy image shows decreased size of the lymph nodes with uniform signal intensity measuring up to 0.5 cm in the short-axis dimension (arrow in B). Nodes are well seen on diffusion-weighted images, which helps in detection of the nodes (arrows in B and D). The patient underwent low anterior resection. Final histologic examination showed no evidence of metastasis in the lymph nodes.
Figure 8:
Images in a 56-year-old woman with locally advanced rectal cancer with enlarged 1.3-cm left internal iliac lymph nodes containing heterogenous signal intensity. Axial T2-weighted MR images at (A, B) baseline, (C, D) 8 weeks after total neoadjuvant therapy, and (E, F) 3.5 years after total neoadjuvant therapy (surveillance). Post-total neoadjuvant therapy images show near-complete response within the primary tumor (arrow in C) and only slight regression of the pelvic node measuring 1.3 cm (arrow in D). Endoscopic biopsy of the tumor bed at this assessment timepoint was negative for malignancy. The patient opted for nonsurgical management and remains free of tumor regrowth at 3.5-year follow-up (arrow in E). The left internal iliac lymph node shows regression with decreased size and mixed T2-weighted signal changes measuring 0.8 cm (arrow in F).
The Lateral Lymph Node Consortium has shown different size criteria for nodes in the pelvic sidewall (compared with the mesorectum discussed previously), an area not routinely dissected in the West due to high morbidity regarding bladder and sexual function. In Japan, this procedure has been more routine (86). Ogura et al reported that extramesorectal lymph nodes (specifically internal iliac and obturator lymph nodes) measuring less than 0.7 cm in short-axis diameter at baseline MRI have a higher chance of complete regression than lymph nodes measuring 0.7 cm or more (87). Furthermore, a reduction from 0.7 cm or larger to 0.4 cm or smaller (internal iliac nodes) and to 0.6 cm or smaller (obturator nodes) results in a lower risk of lateral lymph node recurrence at 3 years (87). As with mesorectal nodes, no cutoff size is absolute; however, the consortium reported that posttreatment lymph node size was a better predictor of lateral local recurrence pretreatment lymph node size. For the internal iliac nodes that remain larger than 0.4 cm and the obturator nodes that remain larger than 0.6 cm after all treatment, lateral pelvic lymph node dissection is strongly advised. The external iliac lymph nodes, considered as nonregional lymph nodes according to the American Joint Committee on Cancer, are associated with a twofold increase in distant recurrence but do not result in increased local recurrence rates (87).
DWI sequence.—DWI, a commonly used functional MRI sequence accomplished by the addition of motion-probing magnetic gradients, is now included in the routine posttreatment rectal MRI protocol and is specifically recommended in the assessment of residual tumor and tumor regrowth (53). Both the European Society of Gastrointestinal and Abdominal Radiology (ESGAR) and the Society of Abdominal Radiology recommend its use at restaging MRI, with guidelines of the latter suggesting its use at baseline as well as to help in the detection of small tumors. While the optimal value and number of magnetic gradient pulses (b values) to apply are still under investigation, it is common to use DWI with b values of 0 and 800 sec/mm2 or greater to assess tumor in the rectum.
Qualitative visual assessment of DWI scans, the apparent diffusion coefficient map, and T2-weighted images constitute the most common and accepted methods to predict and monitor treatment response. In a pooled analysis of individual patient data from 14 publications, the qualitative analysis of DWI scans had better accuracy in predicting pathologic complete response than quantitative analysis (87% vs 74%–78%) (88). The absence of residual high signal intensity on DWI scans together with complete normalization of rectal wall or scar in tumor bed on T2-weighted images signifies complete response, although uniform linear signal intensity in the wall above the tumor is also acceptable. Near-complete response is suggested by marked regression of the DWI signal intensity on images with b values of 800–1000 sec/mm2. Persistence of areas of high signal intensity without much regression represents incomplete response. Combined T2-weighted and DWI sequences improved the diagnostic performance of MRI in the assessment of complete response, with an accuracy of almost 79% (89). It is challenging to perform DWI well and obtain high-quality images. Air in the rectum causes magnetic susceptibility artifact, especially at higher field strengths, such as 3.0 T. For this reason, the use of a rectal microenema (5 mL of fluid) was found to improve image quality and reduce the severity of gas-induced artifact over DWI performed without the microenema (56,57).
Nonetheless, there are many DWI naysayers in the radiologic community. They suggest that it is too hard to achieve reliable sequences with DWI, that DWI is too time consuming, and especially, that T2-weighted sequences are adequate. As mentioned previously, the literature contradicts this stance. A recent meta-analysis indicated a pooled sensitivity and specificity, respectively, of 49% and 86% for T2-weighted sequences alone, 85% and 80% for DWI alone, and 62% and 89% for studies combining both sequences in the assessment of pathologic complete response. This confirms a very real need to use both T2-weighted and DWI sequences complimentarily to best assess complete response (90). Small studies have provided preliminary evidence that the assessment of DWI sequences may assist in posttreatment extramural vascular invasion detection (91) and ypN0 status assessment (92). Also, the addition of DWI sequences to assess tumor regression grades at MRI improves interobserver agreement (72). Lee et al proposed that in patients with locally advanced rectal cancer, a modified MRI-based tumor regression grading system incorporating DWI improved accuracy and interreader agreement and was independently associated with 3-year DFS rate (93). A similar study from a national clinical trial (ClinicalTrials.gov/, NCT02921256) analyzed three expert radiologists’ readings of tumor regression grades at MRI and DWI. Using a similar modification of the MRI-based tumor regression grade score, essentially fortified by DWI, they found that the addition of DWI to tumor regression grades at MRI showed improved specificity and sensitivity over MRI-based tumor regression grades alone for the diagnosis of pathologic complete response (P = .02) (94). Importantly, in a meta-analysis, studies with experienced MRI observers showed better results (higher sensitivity [P = .01]) compared with studies with less experienced MRI observers for tumor staging (67). Not surprisingly, there is a steep learning curve and many pitfalls. The reader is referred to a helpful expert tutorial that shows the most common interpretation pitfalls in the post-nCRT setting, which includes low signal intensity on the apparent diffusion coefficient map due to fibrosis (T2 dark-through), susceptibility artifacts, and T2 shine-through effects (95). Quantitative use of DWI using the apparent diffusion coefficient is still not validated and remains in the research realm along with many other types of quantitative assessments in MRI for rectal cancer response assessment. These are beyond the scope of this review, but the reader is referred to the excellent review by Joye et al (88).
Other Modalities and Techniques
Fluorine 18 fluorodeoxyglucose (FDG) is currently the most used radiopharmaceutical for oncologic molecular imaging. A systematic review assessing the early response of rectal cancer during neoadjuvant therapy showed that FDG PET/CT had good early predictive value (sensitivity, 79%; specificity, 78%) and higher diagnostic accuracy when there was a minimum percentage decrease of 42% in standard uptake value compared with baseline studies (96). In a prospective study of 68 patients, FDG PET/CT showed an overall accuracy of 92% in the identification of patients with cCR to nCRT, with higher accuracy (96%) when PET/CT was combined with a clinical examination (97). At baseline, PET/CT has lower sensitivity but higher specificity in the detection of regional lymph nodes when compared with MRI (98). Persistence of FDG uptake within the inguinal lymph nodes in patients with distal rectal cancer 12 weeks after the completion of nCRT suggests worse prognosis (99). Initial experiences with combined whole-body PET/MRI have shown a slightly better accuracy for ypT and ypN staging than with MRI alone (100). In an exploratory pilot study, increasing the PET acquisition time from 3 to 15 minutes in the pelvis during PET/MRI improved the detection of FDG-avid lymph nodes, but histopathologic validation was lacking (101). The major drawback for FDG PET/CT or PET/MRI in the clinical setting is the higher background FDG uptake in the rectum, which could be a combination of physiologic uptake and inflammatory changes after radiation therapy. The reduced sensitivity of the regional lymph nodes is in part due to the small size of these nodes and the reduced spatial resolution close to the usually intense primary tumor. For these reasons, FDG PET/CT is currently not recommended in the routine management of locally advanced rectal cancer. Tracers other than FDG have been used in a few studies to assess various metabolic parameters as imaging markers for response to neoadjuvant treatment, although these studies are few in number with small study samples. Negative post-nCRT fluoride 18 fluorothymidine PET/CT findings revealed more histopathologic responders than did FDG PET/CT findings (102). In a small pilot study using copper 60 diacetyl-bis (N4-methylthiosemicarbazone) PET, a marker for hypoxia, response to neoadjuvant treatment, overall survival, and progression-free survival were significantly worse for hypoxic tumors than for nonhypoxic tumors (103).
In recent years, an increasing number of research studies have focused on radiomics and deep learning techniques in various oncologic settings. In rectal cancer, radiomic features have been analyzed for various clinical outcomes (eg, T and N staging, response to treatment, and survival prediction). In a recent study, an area under the receiver operating characteristic curve (AUC) of 0.93 (95% CI: 0.87, 0.96; diagnosis of pathologic complete response) was demonstrated using a radiomic model created from posttreatment MRI in patients with locally advanced rectal cancer, outperforming qualitative assessment of T2-weighted imaging and DWI (P < .001) (104). Another study developed and validated a radiomics nomogram with a logistic regression classifier differentiating good responders from poor responders to nCRT in patients with locally advanced rectal cancer, with an AUC of 0.90 in the validation set (P = .02) (105). A radiomics nomogram incorporating radiomics score, histologic grade, and T staging demonstrated a better diagnostic performance than clinical and quantitative models in predicting extramural vascular invasion (106). Finally, a meta-analysis showed that radiomics and deep learning models had a per-patient AUC of 0.81 and 0.92, respectively, in the detection of lymph node metastases compared with the radiologist (AUC = 0.69) (107). Radiomics and machine learning algorithms are currently not implemented in rectal cancer MRI readings. Large-scale prospective validation studies are required to address such challenges as combining MRI studies from many different MRI vendors and sequence types. Recently, circulating tumor DNA combined with MRI has been shown to predict nCRT response and help select patients for the W&W strategy (108).
Challenges
Despite the improvements in techniques and in the understanding of rectal cancer pathophysiology, there remain challenges in imaging assessment of patients with rectal cancer. Strict adherence to the imaging protocol at both baseline and posttreatment timepoints is essential to achieve optimal diagnostic quality images. Assessment can be limited by poor acquisition techniques (eg, inadequate scanner magnet strength, failure to use appropriate surface coils, lack of patient preparation, or motion artifacts). Lack of appropriately positioned high-spatial-resolution small field-of-view sequences through parallel and perpendicular planes of the tumor can lead to inaccurate T staging. Posttreatment submucosal edema or postradiation peri- and mesorectal stranding and fibrosis can mimic tumor. DWI sequences may be affected by geometric distortion, susceptibility artifact from intraluminal gas, and T2 shine-through effects from mural or luminal fluid content. Careful scrutiny of the apparent diffusion coefficient map is also required. Mucinous degeneration of a solid tumor or lymph node in the posttreatment setting is generally considered a sign of treatment response. However, differentiation of cellular mucin from acellular mucin is limited on MRI scans and can lead to challenges during response assessment (109,110). A final and substantial challenge is radiologist experience with W&W imaging surveillance. The volume of studies performed at most centers is quite low, and there is a learning curve in interpretation of MRI results.
Endoscopic or Digital Rectal Examination Assessment of cCR
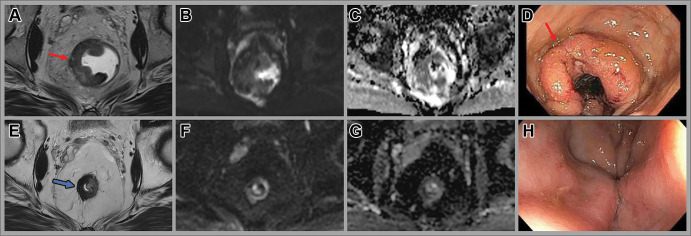
The landmark study by Maas et al was the first to show that combining endoscopy with MRI allowed for the best overall performance in the assessment of complete tumor response (89). In their 2015 study, the AUC for a combined T2-weighted and DWI sequence was 79% compared with a combined digital rectal endoscopy and endoscopy AUC of 88%. Notably, the combination of all three methods to detect pathologic complete response led to the highest sensitivity and specificity (71% and 97%, respectively), with a posttest probability of 98%. Similar results were seen using clinical and multisequence MRI reading strategies (111). Interestingly, when all three modalities indicated residual tumor, 15% of patients still experienced complete response (89). Patients with complete or near-complete response are offered rectal organ preservation with close follow-up, which includes flexible sigmoidoscopy, digital rectal examination, and rectal MRI (Fig 9). Near-complete responders are reassessed in an additional 4–8 weeks to allow more time for progression to a cCR.
Figure 9:
Images in a 46-year-old man with locally advanced rectal cancer. Axial (A) T2-weighted MRI scan, (B) axial diffusion-weighted image, and (C) apparent diffusion coefficient map at baseline show a tumor with intermediate T2-weighted signal intensity (arrow in A), concordant with (D) the endoscopic findings of a fungating and ulcerating rectal mass (arrow). Note the rectal gel in the lumen masking the high signal intensity on B. (E) Posttotal neoadjuvant therapy image obtained 8 weeks after completion of therapy shows T2-weighted dark signal intensity in the tumor bed (arrow) and (F, G) minimal restricted diffusion. (H) No residual tumor was seen at endoscopy, consistent with complete response.
The correlation between endoscopic findings and individual tumor response has not been deeply studied. Felder et al found a strong association with specific endoscopic criteria used in the Memorial Sloan-Kettering Cancer Center Rectal Cancer Regression Schema and tumor grade response (112). These criteria allow for objectivity in assessment and include flat white scar, telangiectasias, absence of ulceration and nodularity, small mucosal nodules or minor mucosal abnormality, superficial ulceration, mild persisting erythema, and visible tumor. The mean diagnostic accuracy for surgeons assigning a cCR after evaluating pre- and posttreatment endoscopy photographs was 89%. The study also showed that surgeons more accurately assigned cCR than nCR, suggesting that the criteria used to assign tumor response may underestimate it. This supports the importance of other modalities to assess clinical response to allow near-complete responders the potential for rectal organ preservation.
Monitoring Tumor Response over Time
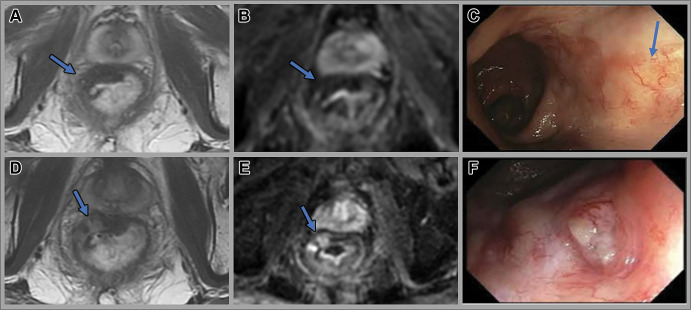
International consensus recommendations from a multidisciplinary and interprofessional team identified several key outcome measures for successful implementation of W&W, including determination of cCR, time point of tumor response assessment, and follow-up methods (113). The optimal response assessment timepoint to determine cCR is 12 weeks from the start of treatment in patients undergoing standard short-course radiation therapy or nCRT for early-stage disease, 14 weeks from start of treatment in patients undergoing nCRT followed by brachytherapy, and 24 weeks after start of treatment after total neoadjuvant therapy. In patients with near-complete response at initial assessment, a repeat assessment 4–10 weeks later is recommended. Local regrowth is seen in approximately 25% of patients, with the majority occurring within the 1st year; regrowths are mainly endoluminal and treated with salvage surgery (Fig 10) (46). After completion of treatment, consensus recommends the patient be assessed every 3–4 months with digital rectal examination, endoscopy, and pelvic MRI for the first 2 years and then every 6 months for 3–5 years after treatment. CT of the chest and abdomen should be performed every 6 months for the first 2 years and then annually for 3–5 years.
Figure 10:
Images in a 68-year-old man with locally advanced rectal cancer. Axial (A) T2-weighted MRI scan and (B) diffusion-weighted image obtained 12 weeks after total neoadjuvant therapy show a T2-weighted dark scar (arrow). (C) Endoscopy 8 months after total neoadjuvant therapy shows radiation-related telangiectatic changes (arrow), with no evidence of tumor. (D) Surveillance scans obtained 12 months after total neoadjuvant therapy show higher intermediate signal intensity tumor regrowth (arrow in D), with restricted diffusion (arrow in E). (F) Endoscopy at 12-month follow-up shows a 0.5-cm ulcerated nodule in the tumor bed, consistent with tumor regrowth.
Summary and Current Status
The watch-and-wait (W&W) approach in rectal cancer treatment represents a safe strategy for selected patients who want to preserve their rectum and undergo surveillance. Not all patients will be eligible, and more data are needed to ensure long-term safety. Prime among the outstanding questions is the potential for worse long-term outcomes due to distant metastases by potentially leaving small amounts of undetectable residual tumor behind in the rectum. Longer follow-up of the Organ Preservation of Rectal Adenocarcinoma trial is required. Many patients have become aware of the W&W approach and often ask if this is available at diagnosis or during treatment. For now, the care of these patients is best approached in a multidisciplinary setting at expert centers where many imaging, clinical, and sociologic factors can be weighed carefully by an experienced multidisciplinary team. While quality control measures for the management of rectal cancer are lacking, it is critical that they be promoted across centers, and efforts to do so have been supported by the National Accreditation Program in Rectal Cancer (114). It is quite clear from the authors’ experience that current obstacles to progress include the following: (a) widespread inexperience with MRI interpretation; (b) variability in surgeon and radiologist interpretation of endoscopy and MRI, respectively; (c) lack of agreement on a standard follow-up approach and schedule; and (d) the intrinsic limitations of advanced technology to detect minimal residual disease in a scar. However, the advances that have been made have ushered in an exciting era in cancer treatment wherein many more patients may be able to attain a higher quality of life despite aggressive multimodality treatment and even avoid surgery altogether, as has become the case in anal cancer. We anticipate a growing number of resources for both radiologists and surgeons to learn about and attain expertise in the care of patients undergoing W&W. We also anticipate investigation into the use of other noninvasive approaches during surveillance, such as circulating tumor DNA, that have the potential to further improve the efficacy and safety of this approach.
Supported in part by a National Institutes of Health and National Cancer Institute Cancer Center Support Grant (P30 CA008748).
Disclosures of conflicts of interest: V.S.J. No relevant relationships. J.A. No relevant relationships. D.M.O. No relevant relationships. M.J.G. No relevant relationships. J.J.S. Consultant and speaker for Johnson & Johnson; travel support from Intuitive Surgical; clinical advisor for Guardant Health and Foundation Medicine. I.P. No relevant relationships.
Abbreviations:
- AUC
- area under the receiver operating characteristic curve
- cCR
- clinical complete response
- DFS
- disease-free survival
- DWI
- diffusion-weighted imaging
- FDG
- fluorodeoxyglucose
- nCRT
- neoadjuvant chemoradiotherapy
- W&W
- watch and wait
References
- 1. Miles WE . A method of performing abdomino-perineal excision for carcinoma of the rectum and of the terminal portion of the pelvic colon (1908) . CA Cancer J Clin 1971. ; 21 ( 6 ): 361 – 364 . [DOI] [PubMed] [Google Scholar]
- 2. Galler AS , Petrelli NJ , Shakamuri SP . Rectal cancer surgery: a brief history . Surg Oncol 2011. ; 20 ( 4 ): 223 – 230 . [DOI] [PubMed] [Google Scholar]
- 3. Dixon CF . Anterior Resection for Malignant Lesions of the Upper Part of the Rectum and Lower Part of the Sigmoid . Ann Surg 1948. ; 128 ( 3 ): 425 – 442 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Heald RJ . Towards fewer colostomies--the impact of circular stapling devices on the surgery of rectal cancer in a district hospital . Br J Surg 1980. ; 67 ( 3 ): 198 – 200 . [DOI] [PubMed] [Google Scholar]
- 5. Parks AG , Percy JP . Resection and sutured colo-anal anastomosis for rectal carcinoma . Br J Surg 1982. ; 69 ( 6 ): 301 – 304 . [DOI] [PubMed] [Google Scholar]
- 6. Pollett WG , Nicholls RJ . The relationship between the extent of distal clearance and survival and local recurrence rates after curative anterior resection for carcinoma of the rectum . Ann Surg 1983. ; 198 ( 2 ): 159 – 163 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Heald RJ , Ryall RD . Recurrence and survival after total mesorectal excision for rectal cancer . Lancet 1986. ; 1 ( 8496 ): 1479 – 1482 . [DOI] [PubMed] [Google Scholar]
- 8. Enker WE . Mesorectal excision (TME) in the operative treatment of rectal cancer . Int J Surg Investig 1999. ; 1 ( 3 ): 253 – 255 . [PubMed] [Google Scholar]
- 9. Buess G , Mentges B , Manncke K , Starlinger M , Becker HD . Technique and results of transanal endoscopic microsurgery in early rectal cancer . Am J Surg 1992. ; 163 ( 1 ): 63 – 69 ; discussion 69–70. [DOI] [PubMed] [Google Scholar]
- 10. Anthuber M , Fuerst A , Elser F , Berger R , Jauch KW . Outcome of laparoscopic surgery for rectal cancer in 101 patients . Dis Colon Rectum 2003. ; 46 ( 8 ): 1047 – 1053 . [DOI] [PubMed] [Google Scholar]
- 11. Heidelberger C , Chaudhuri NK , Danneberg P , et al . Fluorinated pyrimidines, a new class of tumour-inhibitory compounds . Nature 1957. ; 179 ( 4561 ): 663 – 666 . [DOI] [PubMed] [Google Scholar]
- 12. Tepper M , Vidone RA , Hayes MA , Lindenmuth WW , Kligerman MM . Preoperative irradiation in rectal cancer: initial comparison of clinical tolerance, surgical and pathologic findings . Am J Roentgenol Radium Ther Nucl Med 1968. ; 102 ( 3 ): 587 – 595 . [DOI] [PubMed] [Google Scholar]
- 13. Morson BC , Bussey HJ . Surgical pathology of rectal cancer in relation to adjuvant radiotherapy . Br J Radiol 1967. ; 40 ( 471 ): 161 – 165 . [DOI] [PubMed] [Google Scholar]
- 14. Fisher B , Wolmark N , Rockette H , et al . Postoperative adjuvant chemotherapy or radiation therapy for rectal cancer: results from NSABP protocol R-01 . J Natl Cancer Inst 1988. ; 80 ( 1 ): 21 – 29 . [DOI] [PubMed] [Google Scholar]
- 15. Cedermark B , Dahlberg M , Glimelius B , et al . Improved survival with preoperative radiotherapy in resectable rectal cancer . N Engl J Med 1997. ; 336 ( 14 ): 980 – 987 . [DOI] [PubMed] [Google Scholar]
- 16. Kapiteijn E , Marijnen CA , Nagtegaal ID , et al. ; Dutch Colorectal Cancer Group . Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer . N Engl J Med 2001. ; 345 ( 9 ): 638 – 646 . [DOI] [PubMed] [Google Scholar]
- 17. Sauer R , Becker H , Hohenberger W , et al. ; German Rectal Cancer Study Group . Preoperative versus postoperative chemoradiotherapy for rectal cancer . N Engl J Med 2004. ; 351 ( 17 ): 1731 – 1740 . [DOI] [PubMed] [Google Scholar]
- 18. Sebag-Montefiore D , Stephens RJ , Steele R , et al . Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial . Lancet 2009. ; 373 ( 9666 ): 811 – 820 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sauer R , Liersch T , Merkel S , et al . Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years . J Clin Oncol 2012. ; 30 ( 16 ): 1926 – 1933 . [DOI] [PubMed] [Google Scholar]
- 20. Fokas E , Allgäuer M , Polat B , et al. ; German Rectal Cancer Study Group . Randomized Phase II Trial of Chemoradiotherapy Plus Induction or Consolidation Chemotherapy as Total Neoadjuvant Therapy for Locally Advanced Rectal Cancer: CAO/ARO/AIO-12 . J Clin Oncol 2019. ; 37 ( 34 ): 3212 – 3222 . [DOI] [PubMed] [Google Scholar]
- 21. Jin J , Tang Y , Hu C , et al . Multicenter, Randomized, Phase III Trial of Short-Term Radiotherapy Plus Chemotherapy Versus Long-Term Chemoradiotherapy in Locally Advanced Rectal Cancer (STELLAR) . J Clin Oncol 2022. ; 40 ( 15 ): 1681 – 1692 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Conroy T , Bosset JF , Etienne PL , et al. ; Unicancer Gastrointestinal Group and Partenariat de Recherche en Oncologie Digestive (PRODIGE) Group . Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): a multicentre, randomised, open-label, phase 3 trial . Lancet Oncol 2021. ; 22 ( 5 ): 702 – 715 . [DOI] [PubMed] [Google Scholar]
- 23. Fokas E , Schlenska-Lange A , Polat B , et al. ; German Rectal Cancer Study Group . Chemoradiotherapy Plus Induction or Consolidation Chemotherapy as Total Neoadjuvant Therapy for Patients With Locally Advanced Rectal Cancer: Long-term Results of the CAO/ARO/AIO-12 Randomized Clinical Trial . JAMA Oncol 2022. ; 8 ( 1 ): e215445 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bahadoer RR , Dijkstra EA , van Etten B , et al . RAPIDO collaborative investigators. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial . Lancet Oncol 2021. ; 22 ( 1 ): 29 – 42 . [DOI] [PubMed] [Google Scholar]
- 25. Kim JK , Marco MR , Roxburgh CSD , et al . Survival After Induction Chemotherapy and Chemoradiation Versus Chemoradiation and Adjuvant Chemotherapy for Locally Advanced Rectal Cancer . Oncologist 2022. ; 27 ( 5 ): 380 – 388 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cercek A , Roxburgh CSD , Strombom P , et al . Adoption of Total Neoadjuvant Therapy for Locally Advanced Rectal Cancer . JAMA Oncol 2018. ; 4 ( 6 ): e180071 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Marco MR , Zhou L , Patil S , et al . Timing of Rectal Cancer Response to Chemoradiation Consortium. Consolidation mFOLFOX6 Chemotherapy After Chemoradiotherapy Improves Survival in Patients With Locally Advanced Rectal Cancer: Final Results of a Multicenter Phase II Trial . Dis Colon Rectum 2018. ; 61 ( 10 ): 1146 – 1155 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Garcia-Aguilar J , Chow OS , Smith DD , et al . Timing of Rectal Cancer Response to Chemoradiation Consortium. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trial . Lancet Oncol 2015. ; 16 ( 8 ): 957 – 966 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pucciarelli S , Del Bianco P , Efficace F , et al . Patient-reported outcomes after neoadjuvant chemoradiotherapy for rectal cancer: a multicenter prospective observational study . Ann Surg 2011. ; 253 ( 1 ): 71 – 77 . [DOI] [PubMed] [Google Scholar]
- 30. Chen TY , Wiltink LM , Nout RA , et al . Bowel function 14 years after preoperative short-course radiotherapy and total mesorectal excision for rectal cancer: report of a multicenter randomized trial . Clin Colorectal Cancer 2015. ; 14 ( 2 ): 106 – 114 . [DOI] [PubMed] [Google Scholar]
- 31. Schrag D , Weiser M , Saltz L , et al . Challenges and solutions in the design and execution of the PROSPECT Phase II/III neoadjuvant rectal cancer trial (NCCTG N1048/Alliance) . Clin Trials 2019. ; 16 ( 2 ): 165 – 175 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fernandez-Martos C , Garcia-Albeniz X , Pericay C , et al . Chemoradiation, surgery and adjuvant chemotherapy versus induction chemotherapy followed by chemoradiation and surgery: long-term results of the Spanish GCR-3 phase II randomized trial† . Ann Oncol 2015. ; 26 ( 8 ): 1722 – 1728 . [DOI] [PubMed] [Google Scholar]
- 33. Garcia-Aguilar J , Patil S , Kim JK , et al . Preliminary results of the organ preservation of rectal adenocarcinoma (OPRA) trial . J Clin Oncol 2020. ; 38 ( 15_suppl ): 4008 . [Google Scholar]
- 34. Brown G , Radcliffe AG , Newcombe RG , Dallimore NS , Bourne MW , Williams GT . Preoperative assessment of prognostic factors in rectal cancer using high-resolution magnetic resonance imaging . Br J Surg 2003. ; 90 ( 3 ): 355 – 364 . [DOI] [PubMed] [Google Scholar]
- 35. Brown G , Richards CJ , Bourne MW , et al . Morphologic predictors of lymph node status in rectal cancer with use of high-spatial-resolution MR imaging with histopathologic comparison . Radiology 2003. ; 227 ( 2 ): 371 – 377 . [DOI] [PubMed] [Google Scholar]
- 36. Beets-Tan RG , Beets GL , Vliegen RF , et al . Accuracy of magnetic resonance imaging in prediction of tumour-free resection margin in rectal cancer surgery . Lancet 2001. ; 357 ( 9255 ): 497 – 504 . [DOI] [PubMed] [Google Scholar]
- 37. Attenberger UI , Pilz LR , Morelli JN , et al . Multi-parametric MRI of rectal cancer - do quantitative functional MR measurements correlate with radiologic and pathologic tumor stages? Eur J Radiol 2014. ; 83 ( 7 ): 1036 – 1043 . [DOI] [PubMed] [Google Scholar]
- 38. Park MJ , Kim SH , Lee SJ , Jang KM , Rhim H . Locally advanced rectal cancer: added value of diffusion-weighted MR imaging for predicting tumor clearance of the mesorectal fascia after neoadjuvant chemotherapy and radiation therapy . Radiology 2011. ; 260 ( 3 ): 771 – 780 . [DOI] [PubMed] [Google Scholar]
- 39. Habr-Gama A , Perez RO , Nadalin W , et al . Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results . Ann Surg 2004. ; 240 ( 4 ): 711 – 717 ; discussion 717–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Habr-Gama A , Gama-Rodrigues J , São Julião GP , et al . Local recurrence after complete clinical response and watch and wait in rectal cancer after neoadjuvant chemoradiation: impact of salvage therapy on local disease control . Int J Radiat Oncol Biol Phys 2014. ; 88 ( 4 ): 822 – 828 . [DOI] [PubMed] [Google Scholar]
- 41. Appelt AL , Pløen J , Harling H , et al . High-dose chemoradiotherapy and watchful waiting for distal rectal cancer: a prospective observational study . Lancet Oncol 2015. ; 16 ( 8 ): 919 – 927 . [DOI] [PubMed] [Google Scholar]
- 42. Martens MH , Maas M , Heijnen LA , et al . Long-term Outcome of an Organ Preservation Program After Neoadjuvant Treatment for Rectal Cancer . J Natl Cancer Inst 2016. ; 108 ( 12 ): djw171 . [DOI] [PubMed] [Google Scholar]
- 43. Araujo RO , Valadão M , Borges D , et al . Nonoperative management of rectal cancer after chemoradiation opposed to resection after complete clinical response. A comparative study . Eur J Surg Oncol 2015. ; 41 ( 11 ): 1456 – 1463 . [DOI] [PubMed] [Google Scholar]
- 44. Lai CL , Lai MJ , Wu CC , Jao SW , Hsiao CW . Rectal cancer with complete clinical response after neoadjuvant chemoradiotherapy, surgery, or “watch and wait” . Int J Colorectal Dis 2016. ; 31 ( 2 ): 413 – 419 . [DOI] [PubMed] [Google Scholar]
- 45. Beets GL , Figueiredo NL , Habr-Gama A , van de Velde CJ . A new paradigm for rectal cancer: Organ preservation: Introducing the International Watch & Wait Database (IWWD) . Eur J Surg Oncol 2015. ; 41 ( 12 ): 1562 – 1564 . [DOI] [PubMed] [Google Scholar]
- 46. van der Valk MJM , Hilling DE , Bastiaannet E , et al. ; IWWD Consortium . Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): an international multicentre registry study . Lancet 2018. ; 391 ( 10139 ): 2537 – 2545 . [DOI] [PubMed] [Google Scholar]
- 47. Smith JJ , Strombom P , Chow OS , et al . Assessment of a Watch-and-Wait Strategy for Rectal Cancer in Patients With a Complete Response After Neoadjuvant Therapy . JAMA Oncol 2019. ; 5 ( 4 ): e185896 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Garcia-Aguilar J , Patil S , Gollub MJ , et al . Organ Preservation in Patients With Rectal Adenocarcinoma Treated With Total Neoadjuvant Therapy . J Clin Oncol 2022. ; 40 ( 23 ): 2546 – 2556 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Smith JJ , Chow OS , Gollub MJ , et al. ; Rectal Cancer Consortium . Organ Preservation in Rectal Adenocarcinoma: a phase II randomized controlled trial evaluating 3-year disease-free survival in patients with locally advanced rectal cancer treated with chemoradiation plus induction or consolidation chemotherapy, and total mesorectal excision or nonoperative management . BMC Cancer 2015. ; 15 ( 1 ): 767 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Patel UB , Blomqvist LK , Taylor F , et al . MRI after treatment of locally advanced rectal cancer: how to report tumor response--the MERCURY experience . AJR Am J Roentgenol 2012. ; 199 ( 4 ): W486 – W495 . [DOI] [PubMed] [Google Scholar]
- 51. Padhani AR , Miles KA . Multiparametric imaging of tumor response to therapy . Radiology 2010. ; 256 ( 2 ): 348 – 364 . [DOI] [PubMed] [Google Scholar]
- 52. Gollub MJ , Arya S , Beets-Tan RG , et al . Use of magnetic resonance imaging in rectal cancer patients: Society of Abdominal Radiology (SAR) rectal cancer disease-focused panel (DFP) recommendations 2017 . Abdom Radiol (NY) 2018. ; 43 ( 11 ): 2893 – 2902 . [DOI] [PubMed] [Google Scholar]
- 53. Beets-Tan RGH , Lambregts DMJ , Maas M , et al . Magnetic resonance imaging for clinical management of rectal cancer: Updated recommendations from the 2016 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting . Eur Radiol 2018. ; 28 ( 4 ): 1465 – 1475 . [Published correction appears in Eur Radiol 2018;28(6):2711.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. American College of Surgeons . Optimal Resources for Rectal Cancer Care: 2020 Standards . Chicago, IL: : American College of Surgeons; . https://www.facs.org/media/nj2i4frt/optimal_resources_for_rectal_cancer_care_2020_standards.pdf. Published 2020. Accessed May 20, 2022. [Google Scholar]
- 55. Caruso D , Zerunian M , De Santis D , et al . Magnetic Resonance of Rectal Cancer Response to Therapy: An Image Quality Comparison between 3.0 and 1.5 Tesla . BioMed Res Int 2020. ; 2020 : 9842732 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jayaprakasam VS , Javed-Tayyab S , Gangai N , et al . Does microenema administration improve the quality of DWI sequences in rectal MRI? Abdom Radiol (NY) 2021. ; 46 ( 3 ): 858 – 866 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. van Griethuysen JJM , Bus EM , Hauptmann M , et al . Gas-induced susceptibility artefacts on diffusion-weighted MRI of the rectum at 1.5 T - Effect of applying a micro-enema to improve image quality . Eur J Radiol 2018. ; 99 : 131 – 137 . [DOI] [PubMed] [Google Scholar]
- 58. Bates DDB , Golia Pernicka JS , Fuqua JL 3rd , et al . Diagnostic accuracy of b800 and b1500 DWI-MRI of the pelvis to detect residual rectal adenocarcinoma: a multi-reader study . Abdom Radiol (NY) 2020. ; 45 ( 2 ): 293 – 300 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Society of Abdominal Radiology Rectal and Anal Cancer Disease-Focused Panel . MR imaging protocol for rectal cancer . https://abdominalradiology.org/wp-content/uploads/2021/03/MR-Protocols.pdf. Accessed May 16, 2022. [DOI] [PMC free article] [PubMed]
- 60. MRI pelvis Rectal Cancer RESTAGING (12/2020) . Society of Abdominal Radiology Rectal and Anal Cancer Disease-Focused Panel . https://abdominalradiology.org/wp-content/uploads/2021/03/Updated-MRI-pelvis-Rectal-Cancer-RESTAGING.pdf. Accessed October 25, 2022.
- 61. Taylor F , Mangat N , Swift IR , Brown G . Proforma-based reporting in rectal cancer . Cancer Imaging 2010. ; 10 Spec no A ( 1A ): S142 – S150 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Siddiqui MR , Bhoday J , Battersby NJ , et al . Defining response to radiotherapy in rectal cancer using magnetic resonance imaging and histopathological scales . World J Gastroenterol 2016. ; 22 ( 37 ): 8414 – 8434 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Patel UB , Taylor F , Blomqvist L , et al . Magnetic resonance imaging-detected tumor response for locally advanced rectal cancer predicts survival outcomes: MERCURY experience . J Clin Oncol 2011. ; 29 ( 28 ): 3753 – 3760 . [DOI] [PubMed] [Google Scholar]
- 64. Kim JH , Beets GL , Kim MJ , Kessels AG , Beets-Tan RG . High-resolution MR imaging for nodal staging in rectal cancer: are there any criteria in addition to the size? Eur J Radiol 2004. ; 52 ( 1 ): 78 – 83 . [DOI] [PubMed] [Google Scholar]
- 65. Aker M , Boone D , Chandramohan A , Sizer B , Motson R , Arulampalam T . Diagnostic accuracy of MRI in assessing tumor regression and identifying complete response in patients with locally advanced rectal cancer after neoadjuvant treatment . Abdom Radiol (NY) 2018. ; 43 ( 12 ): 3213 – 3219 . [DOI] [PubMed] [Google Scholar]
- 66. Zhu L , Ling C , Xu T , et al . Clinicopathological Features and Survival of Signet-Ring Cell Carcinoma and Mucinous Adenocarcinoma of Right Colon, Left Colon, and Rectum . Pathol Oncol Res 2021. ; 27 : 1609800 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. van der Paardt MP , Zagers MB , Beets-Tan RG , Stoker J , Bipat S . Patients who undergo preoperative chemoradiotherapy for locally advanced rectal cancer restaged by using diagnostic MR imaging: a systematic review and meta-analysis . Radiology 2013. ; 269 ( 1 ): 101 – 112 . [DOI] [PubMed] [Google Scholar]
- 68. Kuo LJ , Chern MC , Tsou MH , et al . Interpretation of magnetic resonance imaging for locally advanced rectal carcinoma after preoperative chemoradiation therapy . Dis Colon Rectum 2005. ; 48 ( 1 ): 23 – 28 . [DOI] [PubMed] [Google Scholar]
- 69. Dworak O , Keilholz L , Hoffmann A . Pathological features of rectal cancer after preoperative radiochemotherapy . Int J Colorectal Dis 1997. ; 12 ( 1 ): 19 – 23 . [DOI] [PubMed] [Google Scholar]
- 70. Mandard AM , Dalibard F , Mandard JC , et al . Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations . Cancer 1994. ; 73 ( 11 ): 2680 – 2686 . [DOI] [PubMed] [Google Scholar]
- 71. Patel UB , Brown G , Rutten H , et al . Comparison of magnetic resonance imaging and histopathological response to chemoradiotherapy in locally advanced rectal cancer . Ann Surg Oncol 2012. ; 19 ( 9 ): 2842 – 2852 . [DOI] [PubMed] [Google Scholar]
- 72. Yoen H , Park HE , Kim SH , et al . Prognostic Value of Tumor Regression Grade on MR in Rectal Cancer: A Large-Scale, Single-Center Experience . Korean J Radiol 2020. ; 21 ( 9 ): 1065 – 1076 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sclafani F , Brown G , Cunningham D , et al . Comparison between MRI and pathology in the assessment of tumour regression grade in rectal cancer . Br J Cancer 2017. ; 117 ( 10 ): 1478 – 1485 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Nahas SC , Nahas CSR , Cama GM , et al . Diagnostic performance of magnetic resonance to assess treatment response after neoadjuvant therapy in patients with locally advanced rectal cancer . Abdom Radiol (NY) 2019. ; 44 ( 11 ): 3632 – 3640 . [DOI] [PubMed] [Google Scholar]
- 75. van den Broek JJ , van der Wolf FS , Lahaye MJ , et al . Accuracy of MRI in Restaging Locally Advanced Rectal Cancer After Preoperative Chemoradiation . Dis Colon Rectum 2017. ; 60 ( 3 ): 274 – 283 . [DOI] [PubMed] [Google Scholar]
- 76. Battersby NJ , Dattani M , Rao S , et al . A rectal cancer feasibility study with an embedded phase III trial design assessing magnetic resonance tumour regression grade (mrTRG) as a novel biomarker to stratify management by good and poor response to chemoradiotherapy (TRIGGER): study protocol for a randomised controlled trial . Trials 2017. ; 18 ( 1 ): 394 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Trakarnsanga A , Gönen M , Shia J , et al . Comparison of tumor regression grade systems for locally advanced rectal cancer after multimodality treatment . J Natl Cancer Inst 2014. ; 106 ( 10 ): dju248 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Taylor FG , Quirke P , Heald RJ , et al . Magnetic Resonance Imaging in Rectal Cancer European Equivalence Study Study Group. Preoperative magnetic resonance imaging assessment of circumferential resection margin predicts disease-free survival and local recurrence: 5-year follow-up results of the MERCURY study . J Clin Oncol 2014. ; 32 ( 1 ): 34 – 43 . [DOI] [PubMed] [Google Scholar]
- 79. Vliegen RF , Beets GL , Lammering G , et al . Mesorectal fascia invasion after neoadjuvant chemotherapy and radiation therapy for locally advanced rectal cancer: accuracy of MR imaging for prediction . Radiology 2008. ; 246 ( 2 ): 454 – 462 . [DOI] [PubMed] [Google Scholar]
- 80. Kim SH , Lee JM , Park HS , Eun HW , Han JK , Choi BI . Accuracy of MRI for predicting the circumferential resection margin, mesorectal fascia invasion, and tumor response to neoadjuvant chemoradiotherapy for locally advanced rectal cancer . J Magn Reson Imaging 2009. ; 29 ( 5 ): 1093 – 1101 . [DOI] [PubMed] [Google Scholar]
- 81. Chand M , Evans J , Swift RI , et al . The prognostic significance of postchemoradiotherapy high-resolution MRI and histopathology detected extramural venous invasion in rectal cancer . Ann Surg 2015. ; 261 ( 3 ): 473 – 479 . [DOI] [PubMed] [Google Scholar]
- 82. Prampolini F , Taschini S , Pecchi A , et al . Magnetic resonance imaging performed before and after preoperative chemoradiotherapy in rectal cancer: predictive factors of recurrence and prognostic significance of MR-detected extramural venous invasion . Abdom Radiol (NY) 2020. ; 45 ( 10 ): 2941 – 2949 . [DOI] [PubMed] [Google Scholar]
- 83. Heijnen LA , Maas M , Beets-Tan RG , et al . Nodal staging in rectal cancer: why is restaging after chemoradiation more accurate than primary nodal staging? Int J Colorectal Dis 2016. ; 31 ( 6 ): 1157 – 1162 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Lahaye MJ , Beets GL , Engelen SM , et al . Locally advanced rectal cancer: MR imaging for restaging after neoadjuvant radiation therapy with concomitant chemotherapy. Part II. What are the criteria to predict involved lymph nodes? Radiology 2009. ; 252 ( 1 ): 81 – 91 . [DOI] [PubMed] [Google Scholar]
- 85. Sassen S , de Booij M , Sosef M , et al . Locally advanced rectal cancer: is diffusion weighted MRI helpful for the identification of complete responders (ypT0N0) after neoadjuvant chemoradiation therapy? Eur Radiol 2013. ; 23 ( 12 ): 3440 – 3449 . [DOI] [PubMed] [Google Scholar]
- 86. Fujita S , Mizusawa J , Kanemitsu Y , et al . Colorectal Cancer Study Group of Japan Clinical Oncology Group. Mesorectal Excision With or Without Lateral Lymph Node Dissection for Clinical Stage II/III Lower Rectal Cancer (JCOG0212): A Multicenter, Randomized Controlled, Noninferiority Trial . Ann Surg 2017. ; 266 ( 2 ): 201 – 207 . [DOI] [PubMed] [Google Scholar]
- 87. Ogura A , Konishi T , Beets GL , et al. ; Lateral Node Study Consortium . Lateral Nodal Features on Restaging Magnetic Resonance Imaging Associated With Lateral Local Recurrence in Low Rectal Cancer After Neoadjuvant Chemoradiotherapy or Radiotherapy . JAMA Surg 2019. ; 154 ( 9 ): e192172 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Joye I , Deroose CM , Vandecaveye V , Haustermans K . The role of diffusion-weighted MRI and (18)F-FDG PET/CT in the prediction of pathologic complete response after radiochemotherapy for rectal cancer: a systematic review . Radiother Oncol 2014. ; 113 ( 2 ): 158 – 165 . [DOI] [PubMed] [Google Scholar]
- 89. Maas M , Lambregts DM , Nelemans PJ , et al . Assessment of Clinical Complete Response After Chemoradiation for Rectal Cancer with Digital Rectal Examination, Endoscopy, and MRI: Selection for Organ-Saving Treatment . Ann Surg Oncol 2015. ; 22 ( 12 ): 3873 – 3880 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Park SH , Cho SH , Choi SH , et al . Korean Society of Abdominal Radiology Study Group for Rectal Cancer. MRI Assessment of Complete Response to Preoperative Chemoradiation Therapy for Rectal Cancer: 2020 Guide for Practice from the Korean Society of Abdominal Radiology . Korean J Radiol 2020. ; 21 ( 7 ): 812 – 828 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Fornell-Perez R , Vivas-Escalona V , Aranda-Sanchez J , et al . Primary and post-chemoradiotherapy MRI detection of extramural venous invasion in rectal cancer: the role of diffusion-weighted imaging . Radiol Med (Torino) 2020. ; 125 ( 6 ): 522 – 530 . [DOI] [PubMed] [Google Scholar]
- 92. van Heeswijk MM , Lambregts DM , Palm WM , et al . DWI for Assessment of Rectal Cancer Nodes After Chemoradiotherapy: Is the Absence of Nodes at DWI Proof of a Negative Nodal Status? AJR Am J Roentgenol 2017. ; 208 ( 3 ): W79 – W84 . [DOI] [PubMed] [Google Scholar]
- 93. Lee MA , Cho SH , Seo AN , et al . Modified 3-Point MRI-Based Tumor Regression Grade Incorporating DWI for Locally Advanced Rectal Cancer . AJR Am J Roentgenol 2017. ; 209 ( 6 ): 1247 – 1255 . [DOI] [PubMed] [Google Scholar]
- 94. Hall WA , Li J , You YN , et al . Prospective Validation of the Magnetic Resonance Tumor Regression Grade (MR-TRG) and Correlation With Pathologic Endpoints Score in NRG Oncology GI002 . Int J Radiat Oncol Biol Phys 2021. ; 111 ( 3, Supplement ): S37 . [Google Scholar]
- 95. Lambregts DMJ , van Heeswijk MM , Delli Pizzi A , et al . Diffusion-weighted MRI to assess response to chemoradiotherapy in rectal cancer: main interpretation pitfalls and their use for teaching . Eur Radiol 2017. ; 27 ( 10 ): 4445 – 4454 . [DOI] [PubMed] [Google Scholar]
- 96. Maffione AM , Chondrogiannis S , Capirci C , et al . Early prediction of response by 18F-FDG PET/CT during preoperative therapy in locally advanced rectal cancer: a systematic review . Eur J Surg Oncol 2014. ; 40 ( 10 ): 1186 – 1194 . [DOI] [PubMed] [Google Scholar]
- 97. López-López V , Abrisqueta Carrión J , Luján J , et al . Assessing tumor response to neoadjuvant chemoradiation in rectal cancer with rectoscopy and 18F-FDG PET/CT: results from a prospective series . Rev Esp Enferm Dig 2021. ; 113 ( 5 ): 307 – 312 . [DOI] [PubMed] [Google Scholar]
- 98. Cerny M , Dunet V , Prior JO , et al . Initial Staging of Locally Advanced Rectal Cancer and Regional Lymph Nodes: Comparison of Diffusion-Weighted MRI With 18F-FDG-PET/CT . Clin Nucl Med 2016. ; 41 ( 4 ): 289 – 295 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Perez RO , Habr-Gama A , São Julião GP , et al . Clinical relevance of positron emission tomography/computed tomography-positive inguinal nodes in rectal cancer after neoadjuvant chemoradiation . Colorectal Dis 2013. ; 15 ( 6 ): 674 – 682 . [DOI] [PubMed] [Google Scholar]
- 100. Crimì F , Spolverato G , Lacognata C , et al . 18F-FDG PET/MRI for Rectal Cancer TNM Restaging After Preoperative Chemoradiotherapy: Initial Experience . Dis Colon Rectum 2020. ; 63 ( 3 ): 310 – 318 . [DOI] [PubMed] [Google Scholar]
- 101. Bailey JJ , Jordan EJ , Burke C , et al . Does Extended PET Acquisition in PET/MRI Rectal Cancer Staging Improve Results? AJR Am J Roentgenol 2018. ; 211 ( 4 ): 896 – 900 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Rendl G , Rettenbacher L , Holzmannhofer J , et al . Assessment of response to neoadjuvant radiochemotherapy with F-18 FLT and F-18 FDG PET/CT in patients with rectal cancer . Ann Nucl Med 2015. ; 29 ( 3 ): 284 – 294 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Dietz DW , Dehdashti F , Grigsby PW , et al . Tumor hypoxia detected by positron emission tomography with 60Cu-ATSM as a predictor of response and survival in patients undergoing Neoadjuvant chemoradiotherapy for rectal carcinoma: a pilot study . Dis Colon Rectum 2008. ; 51 ( 11 ): 1641 – 1648 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Horvat N , Veeraraghavan H , Khan M , et al . MR Imaging of Rectal Cancer: Radiomics Analysis to Assess Treatment Response after Neoadjuvant Therapy . Radiology 2018. ; 287 ( 3 ): 833 – 843 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Wang J , Liu X , Hu B , Gao Y , Chen J , Li J . Development and validation of an MRI-based radiomic nomogram to distinguish between good and poor responders in patients with locally advanced rectal cancer undergoing neoadjuvant chemoradiotherapy . Abdom Radiol (NY) 2021. ; 46 ( 5 ): 1805 – 1815 . [DOI] [PubMed] [Google Scholar]
- 106. Yu X , Song W , Guo D , et al . Preoperative Prediction of Extramural Venous Invasion in Rectal Cancer: Comparison of the Diagnostic Efficacy of Radiomics Models and Quantitative Dynamic Contrast-Enhanced Magnetic Resonance Imaging . Front Oncol 2020. ; 10 : 459 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Bedrikovetski S , Dudi-Venkata NN , Kroon HM , et al . Artificial intelligence for pre-operative lymph node staging in colorectal cancer: a systematic review and meta-analysis . BMC Cancer 2021. ; 21 ( 1 ): 1058 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Wang Y , Yang L , Bao H , et al . Utility of ctDNA in predicting response to neoadjuvant chemoradiotherapy and prognosis assessment in locally advanced rectal cancer: A prospective cohort study . PLoS Med 2021. ; 18 ( 8 ): e1003741 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Horvat N , Hope TA , Pickhardt PJ , Petkovska I . Mucinous rectal cancer: concepts and imaging challenges . Abdom Radiol (NY) 2019. ; 44 ( 11 ): 3569 – 3580 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Wnorowski AM , Menias CO , Pickhardt PJ , Kim DH , Hara AK , Lubner MG . Mucin-Containing Rectal Carcinomas: Overview of Unique Clinical and Imaging Features . AJR Am J Roentgenol 2019. ; 213 ( 1 ): 26 – 34 . [DOI] [PubMed] [Google Scholar]
- 111. Gollub MJ , Blazic I , Felder S , et al . Value of adding dynamic contrast-enhanced MRI visual assessment to conventional MRI and clinical assessment in the diagnosis of complete tumour response to chemoradiotherapy for rectal cancer . Eur Radiol 2019. ; 29 ( 3 ): 1104 – 1113 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Felder SI , Patil S , Kennedy E , Garcia-Aguilar J . Endoscopic Feature and Response Reproducibility in Tumor Assessment after Neoadjuvant Therapy for Rectal Adenocarcinoma . Ann Surg Oncol 2021. ; 28 ( 9 ): 5205 – 5223 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Fokas E , Appelt A , Glynne-Jones R , et al . International consensus recommendations on key outcome measures for organ preservation after (chemo)radiotherapy in patients with rectal cancer . Nat Rev Clin Oncol 2021. ; 18 ( 12 ): 805 – 816 . [DOI] [PubMed] [Google Scholar]
- 114. Wexner SD , White CM . Improving Rectal Cancer Outcomes with the National Accreditation Program for Rectal Cancer . Clin Colon Rectal Surg 2020. ; 33 ( 5 ): 318 – 324 . [DOI] [PMC free article] [PubMed] [Google Scholar]