Abstract
Bone marrow mesenchymal stem cells (BMMSCs) transplantation methods are promising candidates for osteoarthritis (OA) treatment. However, inflammatory factors (such as TNF‐α) that occur at cell transplantation sites are critical factors that impair the effectiveness of the treatment. Previous studies have shown that aspirin (AS) had a regulatory role in stem cell differentiation. However, little is known about the role of AS on the chondrogenesis of BMMSCs. The purpose of this study is to explore the protective role of AS against the negative effects of TNF‐α on BMMSC chondrogenesis. In this study, we investigated the effects of AS and TNF‐α on BMMSCs chondrogenesis by performing the Alcian Blue staining, safranin O‐fast green staining, haematoxylin and eosin staining, and immunohistochemical staining, as well as real‐time RT‐PCR and western blot assays. Our results demonstrated that TNF‐α inhibited chondrogenic differentiation of BMMSCs by disrupting the balance of cartilage metabolism and promoting oxidative stress in BMMSCs, while AS treatment attenuated these effects. Furthermore, a detailed molecular mechanistic analysis indicated that Yes‐associated protein (YAP) played a critical regulatory role in this process. In addition, AS treatment mitigated the progression of cartilage degeneration in a mouse destabilization of the medial meniscus (DMM) model. AS alleviated the inhibitory effect of TNF‐α on chondrogenesis of BMMSCs by stabilizing YAP, which may provide new therapeutic strategies for OA treatment.
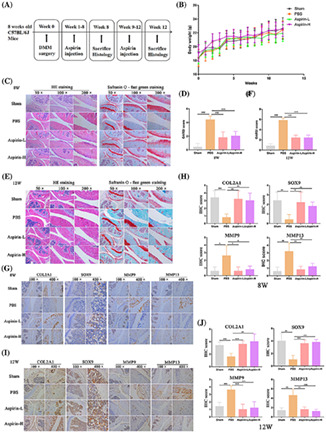
Aspirin mitigates the progression of cartilage degeneration in a mouse DMM model. (A) A schematic of the in vivo experiment. After DMM surgery, mice were injected with aspirin or PBS for 12 weeks (n = 10 per group). (B) The body weights of mice from different treatment groups were measured after 12 weeks. (C) Representative H&E and safranin O‐fast green staining images of the left knee joint of mice after exposure to different treatments for 8 weeks (n = 5 per group). (D) The severity of the OA‐like phenotype 8 weeks after DMM surgery in (C) was analysed by the Osteoarthritis Research Society International (OARSI) score system. (E) Representative H&E and safranin O‐fast green staining images of the left knee joint of mice after exposure to different treatments for 12 weeks (n = 5 per group). (F) The severity of the OA‐like phenotype 12 weeks after DMM surgery in (E) was analysed by the Osteoarthritis Research Society International (OARSI) score system. (G) IHC staining of chondrogenic markers (COL2A1 and SOX9) and catabolic markers (MMP9 and MMP13) in the left knee joint of mice after different treatments for 8 weeks. (H) IHC staining scores of (G). (I) IHC staining of chondrogenic markers (COL2A1 and SOX9) and catabolic markers (MMP9 and MMP13) in the left knee joint of mice after different treatments for 12 weeks. (J) IHC staining scores of (I). *p < 0.05, **p < 0.01, ***p < 0.001. Scale bars: 0.5 mm (50× figures), 250 μm (100× figures), 100 μm (200× figures), 50 μm (400× figures).
1. INTRODUCTION
Osteoarthritis (OA), a chronic disease characterized by degenerative changes in articular cartilage accompanied by subchondral bone sclerosis and synovitis, is the cause of both socioeconomic and personal health burdens. 1 , 2 With an aging global population and increasing obesity, the incidence of OA is increasing year by year. 3 At present, OA is generally treated with conservative drugs in the early stage of the disease, and surgical treatment such as joint replacement in the later stages. 4 However, these treatment strategies are limited by factors such as poor efficacy of drugs, 5 large surgical trauma, and high cost. Therefore, the development of alternative therapeutic strategies is critical. In recent years, the use of bone marrow mesenchymal stem cells (BMMSCs) that differentiate into cartilage has shown multiple advantages over traditional therapies in the treatment of OA. However, although this treatment strategy has the potential for clinical application, 6 , 7 , 8 its use is limited by the low differentiation rate of BMMSCs due to the presence of inflammatory factors, such as tumour necrosis factor‐alpha (TNF‐α). 9 , 10 Thus, the development of strategies to improve the differentiation of BMMSCs into chondrocytes in an inflammatory environment is an important issue that requires further attention.
Aspirin (AS), also known as acetylsalicylic acid, is a commonly used nonsteroidal anti‐inflammatory drug, which plays an important role in many physiological processes in the human body, such as anti‐inflammation and analgesia, and has been implicated in anti‐tumour responses and prevention of thrombosis. 11 , 12 , 13 , 14 Previous studies have shown that AS can regulate the physiological functions of mesenchymal stem cells (MSCs) in multiple ways, including inhibition of stem cell proliferation, promotion of stem cell apoptosis, promotion of myocardial differentiation and osteogenesis, and inhibition of adipogenic differentiation. 15 , 16 , 17 , 18 , 19 However, whether AS can reverse the inhibitory effect of TNF‐α on chondrogenic differentiation of BMMSCs remains unknown. Therefore, the purpose of this study was to determine whether AS can reverse the TNF‐α‐induced chondrogenic damage of BMMSCs, in order to provide an experimental basis for the application of AS in the prevention and treatment of OA in the future.
2. MATERIALS AND METHODS
2.1. Isolation and culture of human BMMSCs
The BMMSCs used in this study were isolated and extracted from healthy volunteers as previously described. 20 , 21 , 22 Isolated BMMSCs were cultured in low glycemic DMEM mediums containing 10% foetal bovine serum (FBS), 100 U/ml penicillin, and 100 mg/ml streptomycin. The culture medium was changed every 3 days.
2.2. Chondrogenic differentiation of BMMSCs
The high‐density hanging drop method was used to culture cartilage pellets as described previously. 20 Pellets were cultured in the Mesenchymal Stem Cell Chondrogenic Differentiation Medium (#7551, ScienCell, USA) with or without TNF‐α (10 ng/ml) and AS (100 μM) for 7, 14, and 21 days. Cartilage pellets were collected for follow‐up experiments.
2.3. Antibodies and reagents
Antibodies against COL2A1, ACAN, SOX9, ADAMTS4, MMP9, MMP13, NOX1, NOX2, SOD1, SOD2, P‐YAP, and YAP were purchased from Abcam (Cambridge, UK). Antibodies against GAPDH were purchased from Cell Signaling Technology (Boston, USA). Goat anti‐rabbit IgG H&L (HRP) and Goat anti‐mouse IgG H&L (HRP) were purchased from Cell Signaling Technology (Boston, USA). TNF‐α was purchased from NovoProtein (China) and AS was purchased from MedChemExpress (New Jersey, USA).
2.4. Alcian Blue staining
Cartilage pellets were fixed in 4% paraformaldehyde (Phygene, Fujian, China) for 6 h, then embedded in paraffin. Paraffin‐embedded samples were sliced to a thickness of 3–5 μm and placed on glass slides. The slides were deparaffinized before samples were incubated in Alcian Blue solution (pH 2.5; Servicebio, Wuhan, China) at room temperature for 30 min. The slides were dehydrated using increasing concentrations of ethanol three times (5 min each time), cleared with xylene for 5 min, then sealed with neutral resin. Finally, samples were visualized and images captured using an Olympus BX63 microscope (Olympus, Japan).
2.5. Safranin O‐fast green staining
Cartilage pellets and mouse knee joint tissues were fixed in 4% paraformaldehyde for 6 h, then embedded in paraffin. Paraffin‐embedded samples were sliced to a thickness of 3–5 μm and placed on glass slides. The slides were deparaffinized before samples were incubated in Fast Green Solution (Servicebio, Wuhan, China) at room temperature for 3–5 min. Excess stain was removed by washing with water, then samples were immersed in hydrochloric acid in alcohol for 3–5 s, and incubated with Safranin O Solution (Servicebio, Wuhan, China) for 10–15 s. The slides were dehydrated using increasing concentrations of ethanol three times (5 min each time), cleared with xylene for 5 min, then sealed with neutral resin. Finally, samples were visualized and images captured using an Olympus BX63 microscope (Olympus, Japan).
2.6. Real‐time RT‐PCR assay
Total RNA samples were extracted from cultured BMMSCs using the Total RNA Extraction Kit (#15596018; GIBCO, USA), as per the manufacturer's instructions. Using the NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, USA) detected the RNA concentrations. The cDNAs were subsequently synthesized from 1 μg total RNA using the Evo M‐MLVRT Kit (#AG11706; Accurate Biotechnology, Hunan, China). Then, a qRT‐PCR assay was performed using the SYBR Green Pro Taq HS Premix II Kit (#AG11702; Accurate Biotechnology, Hunan, China), as per the manufacturer's instructions. The mRNA expression levels were calculated using the standard 2−ΔΔCt method based on at least three biological replicates. The primer sequences of genes involved in this manuscript were shown in Table 1.
TABLE 1.
Sequences of primers used for quantitative PCR
| Gene | Primer sequence (5′‐3′) |
|---|---|
| GAPDH |
Forward: GGAGCGAGATCCCTCCAAAAT; Reverse: GGCTGTTGTCATACTTCTCATGG |
| COL2A1 |
Forward: GCTGCGGATGCTCTCAATCT; Reverse: TGGACGATCAGGCGAAACC |
| ACAN |
Forward: ACTCTGGGTTTTCGTGACTCT; Reverse: ACACTCAGCGAGTTGTCATGG |
| SOX9 |
Forward: AGCGAACGCACATCAAGAC; Reverse: CTGTAGGCGATCTGTTGGGG |
| ADAMTS4 |
Forward: GAGGAGGAGATCGTGTTTCCA; Reverse: CCAGCTCTAGTAGCAGCGTC |
| MMP9 |
Forward: TGTACCGCTATGGTTACACTCG; Reverse: GGCAGGGACAGTTGCTTCT |
| MMP13 |
Forward: ACTGAGAGGCTCCGAGAAATG; Reverse: GAACCCCGCATCTTGGCTT |
| NOX1 |
Forward: GCACACCTGTTTAACTTTGACTG; Reverse: GGACTGGATGGGATTTAGCCA |
| NOX2 |
Forward: AACGAATTGTACGTGGGCAGA; Reverse: GAGGGTTTCCAGCAAACTGAG |
| SOD1 |
Forward: GGTGGGCCAAAGGATGAAGAG; Reverse: CCACAAGCCAAACGACTTCC |
| SOD2 |
Forward: GCTCCGGTTTTGGGGTATCTG; Reverse: GCGTTGATGTGAGGTTCCAG |
| CTGF |
Forward: CAGCATGGACGTTCGTCTG; Reverse: AACCACGGTTTGGTCCTTGG |
| CYR61 |
Forward: CTCGCCTTAGTCGTCACCC; Reverse: CGCCGAAGTTGCATTCCAG |
| YAP |
Forward: TAGCCCTGCGTAGCCAGTTA; Reverse: TCATGCTTAGTCCACTGTCTGT |
Abbreviations: ACAN, aggrecan; ADAMTS4, ADAM metallopeptidase with thrombospondin type 1 motif 4; COL2A1, collagen type II alpha 1 chain; CTGF, connective tissue growth factor; CYR61, cysteine‐rich angiogenic inducer 61; GAPDH, glyceraldehyde‐3‐phosphate dehydrogenase; MMP9, matrix metallopeptidase 9; MMP13, matrix metallopeptidase 13; NOX1, NADPH oxidase 1; NOX2, NADPH oxidase 2; SOD1, superoxide dismutase 1; SOD2, superoxide dismutase 2; SOX9, SRY‐box transcription factor 9; YAP, yes‐associated protein.
2.7. Western blot assay
Total protein samples were extracted from cultured BMMSCs using RIPA lysis solution (#P0013C, Beyotime, USA) containing proteinase and phosphatase inhibitors (1:100). Protein concentrations were determined using a BCA Protein Concentration Determination Kit (#P0012, Beyotime, USA), and protein samples were separated using a PAGE Gel Fast Preparation Kit (#PG112, EpiZyme, China) and transferred onto a polyvinylidene fluoride (PVDF) membrane. Membranes were blocked with 5% lipid‐free milk solution at room temperature for 60 min, then incubated with anti‐COL2A1 (1:1000), anti‐ACAN (1:1000), anti‐SOX9 (1:1000), anti‐ADAMTS4 (1:1000), anti‐MMP9 (1:1000), anti‐MMP13 (1:1000), anti‐NOX1(1:1000), anti‐NOX2 (1:1000), anti‐SOD1 (1:1000), anti‐SOD2 (1:1000), anti‐phospho‐YAP (1:1000), anti‐YAP (1:1000), and anti‐GAPDH (1:2000) antibodies overnight at 4°C. Next, membranes were incubated with secondary antibodies (1:2000) at room temperature for 60 min. Protein bands were visualized using an HRP Chemiluminescent Western Blot Detection Kit (Merck Millipore, USA).
2.8. Plasmids and lentivirus construction
Plasmids were obtained from Addgene (Cambridge, MA, USA), and transfection was performed using Lipofectamine 3000 reagent, according to the manufacturer's instructions. The human Yes‐associated protein (YAP) coding sequence was cloned into the lentiviral transfer plasmid pSin‐puro to construct plasmid pSin‐YAP‐FLAG. A control vector plasmid was also constructed. To silence YAP expression, the shRNA sequence was cloned into the lentiviral transfer plasmid pSin‐puro to construct pSin‐YAP‐shRNA. Control scramble‐shRNA was also constructed. Lentiviral infection was carried out as described in the previously published article. 23
2.9. Hematoxylin and eosin (H&E) staining
Mouse knee joints and organ tissues (heart, liver, spleen, and kidney) of mice were fixed in 4% paraformaldehyde for 6 h and subsequently embedded in paraffin. Four‐micrometre thick sections were stained with hematoxylin (#G1005, Servicebio, Wuhan, China) for 8–10 min and with eosin (#G1005, Servicebio, Wuhan, China) for 1–2 min. Finally, samples were visualized and images captured using an Olympus BX63 microscope (Olympus, Japan).
2.10. Immunohistochemical (IHC) staining
Paraffin sections of the cartilage pellets and mouse knee joints were prepared for IHC experiments using the Histostain‐Plus Kit (ZSGB‐BIO, China). The antibodies included anti‐COL2A1 (1:200), anti‐SOX9 (1:500), anti‐MMP9 (1:200) and anti‐MMP13 (1:1000) antibodies. Detection was performed using a DAB Horseradish Peroxidase Color Development Kit (ZSGB‐BIO, China), and staining intensity was scored according to the previously published article. 24
2.11. Animal experiments
A total of 40 specific‐pathogen‐free (SPF) female mice aged 6–8 weeks were purchased from the East Campus Animal Center of Sun Yat‐sen University (Guangzhou, China). The mice were housed in a 12‐h light/12‐h dark condition. Mice were randomly assigned to the following four groups (n = 10 per group): (i) Sham group, (ii) destabilization of the medial meniscus (DMM) model group (PBS group), (iii) DMM model + low dose AS group (1 mg/kg/d, 3 days per week, solvent: PBS), (iv) DMM model + high dose AS group (10 mg/kg/d, 3 days per week, solvent: PBS). An OA mice model was established using DMM surgery to the left knee joint of the mice, AS was administered by intraperitoneal injection after DMM surgery. The body weights of the mice were recorded weekly for 12 weeks. After 8 or 12 weeks of AS treatment, the left knee joint and organ tissues of the mice were removed, and H&E, Safranin O‐fast green, and IHC staining were performed on the samples. This study was approved by the Experimental Animal Ethics Committee of Sun Yat‐sen University (Animal approval certificate information ID: SYSU‐IACUC‐2021‐000788).
2.12. Statistical analysis
All quantitative data have been analysed using SPSS 23.0 software. The differences between the 2 and >2 groups were determined by the Student's t‐test and analysis of variance methods, respectively. p < 0.05 is statistically significant. Important notations are as follows: “*” represents p < 0.05, “**” represents p < 0.01, “***” represents p < 0.001.
3. RESULTS
3.1. AS alleviates TNF‐α‐induced inhibition of BMMSC cartilage pellet growth
After 7, 14, and 21 days of BMMSC chondrogenic differentiation, we used a stereomicroscope to observe the cartilage pellets cultured from BMMSCs under different treatment conditions. We found that TNF‐α inhibited the growth of cartilage pellets on days 7, 14, and 21, whereas AS treatment alleviated this inhibitory effect (Figure 1A). The quantitative data showing the average diameter of the cartilage pellets for each treatment group are shown in Figure 1B. In conclusion, AS alleviated the inhibitory effect of TNF‐α on the differentiation of cartilage pellets from BMMSCs.
FIGURE 1.
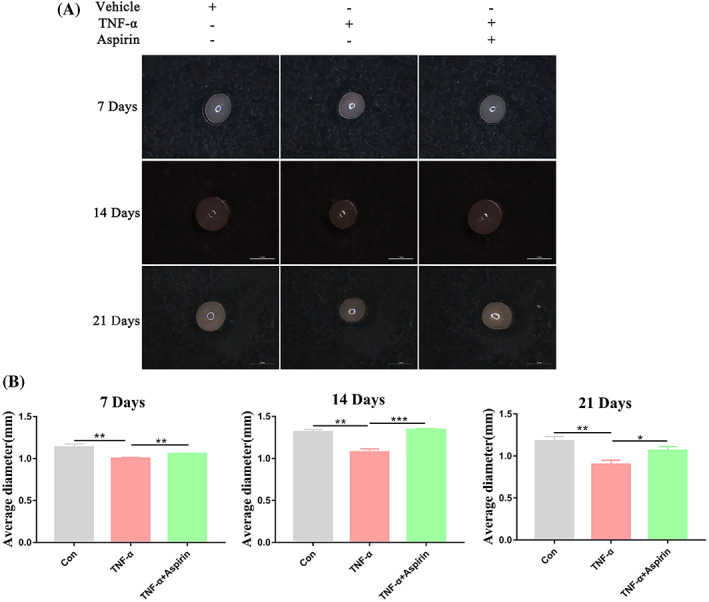
Impact of TNF‐α and aspirin on cartilage pellet development during chondrogenesis of BMMSCs. (A) Representative microscopic images showing cartilage pellets cultured under different treatment conditions for 7, 14, and 21 days. (B) Quantitative data showing the mean diameter of cartilage pellets of different treatment groups. Data in (B) are given as the mean ± SD of three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001. Scale bars: 1 mm
3.2. AS reduces the inhibitory effect of TNF‐α on the synthesis and accumulation of cartilage matrix during BMMSC chondrogenic differentiation
Next, we used Alcian Blue and safranin O‐fast green staining to detect the cartilage matrix synthesis and accumulation during BMMSCs chondrogenic differentiation in the different treatment groups. We found that TNF‐α and AS treatment had no significant effect on cartilage matrix synthesis after 7 days of chondrogenic differentiation (Figure 2A,B). However, treatment with TNF‐α significantly reduced cartilage matrix synthesis on days 14 and 21, while AS treatment reversed this inhibitory effect (Figure 2A,B). These findings suggested that AS reduced the inhibitory effect of TNF‐α on cartilage matrix synthesis and accumulation during BMMSC chondrogenic differentiation.
FIGURE 2.
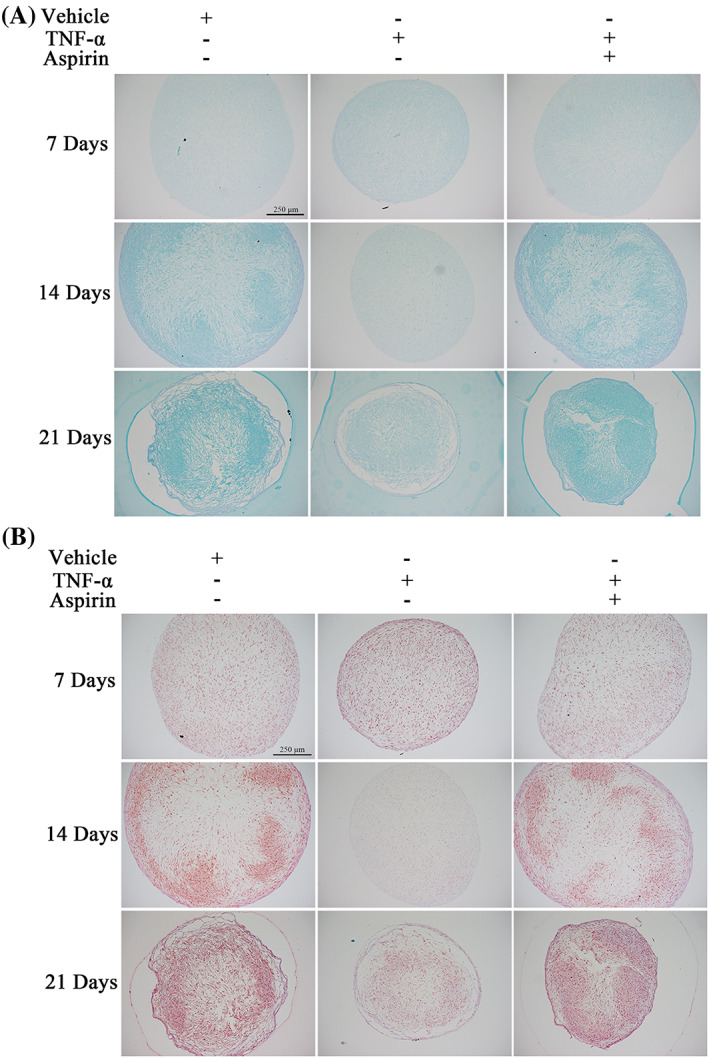
Impact of TNF‐α and aspirin on matrix accumulation during chondrogenesis of BMMSCs. (A) Representative microscopic images showing Alcian Blue staining of cartilage pellets cultured under different treatment conditions for 7, 14, and 21 days. (B) Representative microscopic images showing safranin O‐fast green staining of cartilage pellets cultured under different treatment conditions for 7, 14, and 21 days. Scale bars: 250 μm
3.3. AS reduces the inhibitory effect of TNF‐α on the expression of BMMSC chondrogenic differentiation markers
We used RT‐PCR and western blot assays to detect the expression of chondrogenic differentiation markers (COL2A1, ACAN, SOX9) in the cartilage pellets of the different treatment groups. We found that TNF‐α significantly reduced mRNA and protein expression of these markers on 7, 14, and 21 days, while AS treatment reversed these inhibitory effects of TNF‐α (Figure 3A–F). Furthermore, immunohistochemistry (IHC) staining of COL2A1 in the different treatment groups was consistent with these findings (Figure 3G,H). These results indicated that AS reduced the inhibitory effect of TNF‐α on the expression of BMMSC chondrogenic differentiation markers.
FIGURE 3.
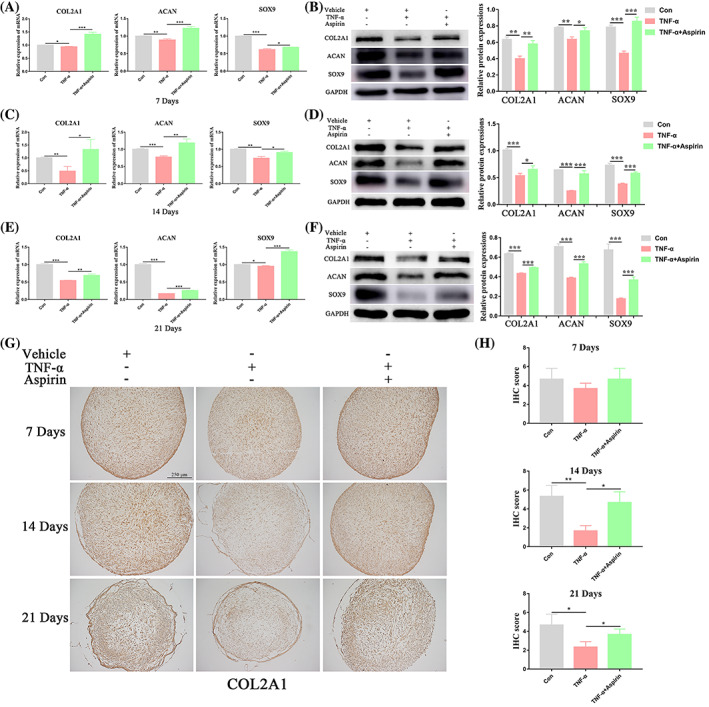
Aspirin reverses TNF‐α‐inhibited chondrogenic markers expression during chondrogenesis of BMMSCs. (A, B) Relative mRNA and protein expression levels of chondrogenic markers (COL2A1, ACAN, and SOX9) were measured in BMMSCs treated with or without TNF‐α and aspirin for 7 days by qRT‐PCR and western blot analyses, respectively. (C, D) Relative mRNA and protein expression levels of chondrogenic markers (COL2A1, ACAN, and SOX9) were measured in BMMSCs treated with or without TNF‐α and aspirin for 14 days by qRT‐PCR and western blot analyses, respectively. (E, F) Relative mRNA and protein expression levels of chondrogenic markers (COL2A1, ACAN, and SOX9) were measured in BMMSCs treated with or without TNF‐α and aspirin for 21 days by qRT‐PCR and western blot analyses, respectively. (G) IHC staining of COL2A1 in cartilage pellets cultured under different treatment conditions for 7, 14, and 21 days. (H) Quantification of the COL2A1 IHC staining data. Data in (A), (C), (E), and (H) are given as the mean ± SD of three independent experiments. ACAN, aggrecan; COL2A1, collagen type II alpha 1 chain; GAPDH, glyceraldehyde‐3‐phosphate dehydrogenase; SOX9, SRY‐box transcription factor 9. *p < 0.05, **p < 0.01, ***p < 0.001. Scale bars: 250 μm
3.4. AS alleviates the stimulatory effect of TNF‐α on the expression of catabolic markers of BMMSC chondrogenic differentiation
In this part of the study, we detected mRNA and protein expression of catabolic markers (ADAMTS4, MMP9, MMP13) in the cartilage pellets of different treatment groups. We found that TNF‐α significantly promoted the mRNA and protein expression of these markers on 7, 14, and 21 days. While treatment with AS reduced this stimulatory effect (Figure 4A–F), demonstrating that AS reduced the stimulatory effects of TNF‐α on the expression of catabolic markers of BMMSC chondrogenic differentiation.
FIGURE 4.
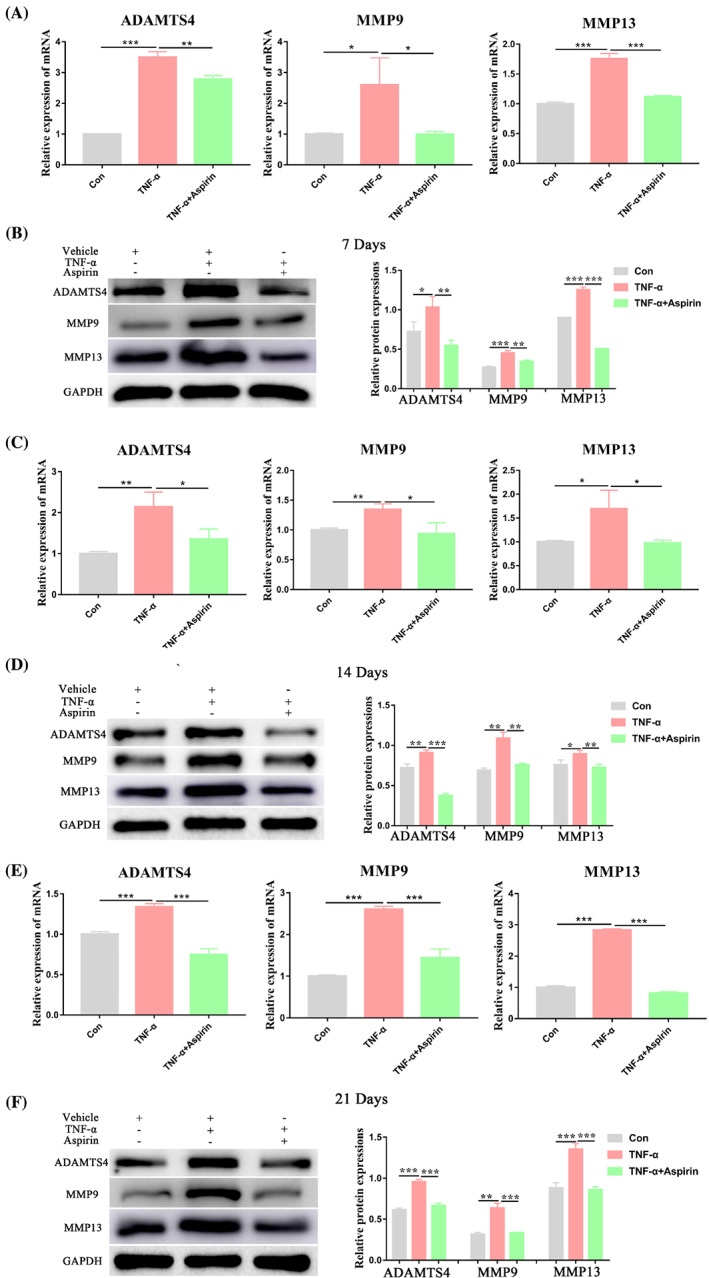
Aspirin reversed TNF‐α‐promoted catabolic markers expression during chondrogenesis of BMMSCs. (A, B) Relative mRNA and protein expression levels of catabolic markers (ADAMTS4, MMP9, and MMP13) were measured in BMMSCs treated with or without TNF‐α and aspirin for 7 days by qRT‐PCR and western blot analyses, respectively. (C, D) Relative mRNA and protein expression levels of catabolic markers (ADAMTS4, MMP9, and MMP13) were measured in BMMSCs treated with or without TNF‐α and aspirin for 14 days by qRT‐PCR and western blot analyses, respectively. (E, F) Relative mRNA and protein expression levels of catabolic markers (ADAMTS4, MMP9, and MMP13) were measured in BMMSCs treated with or without TNF‐α and aspirin for 21 days by qRT‐PCR and western blot analyses, respectively. Data in A, C, and E are given as the mean ± SD of three independent experiments. ADAMTS4, ADAM metallopeptidase with thrombospondin type 1 motif 4; GAPDH, glyceraldehyde‐3‐phosphate dehydrogenase; MMP9, matrix metallopeptidase 9; MMP13, matrix metallopeptidase 13. *p < 0.05, **p < 0.01, ***p < 0.001
3.5. AS alleviates the stimulatory effects of TNF‐α on oxidative stress levels during chondrogenic differentiation of BMMSCs
Because TNF‐α is an inflammatory factor that may affect oxidative stress levels in stem cells, we next detected the mRNA and protein expression levels of oxidative stress markers (NOX1, NOX2, SOD1, and SOD2) in the different treatment groups. We found that TNF‐α significantly increased the expression of the oxidase markers (NOX1, NOX2), while inhibiting the expression of the antioxidant enzyme markers (SOD1, SOD2) on 7, 14, and 21 days. Treatment with AS reversed these effects (Figure 5A–F). Together, these data showed that AS alleviated the stimulatory effects of TNF‐α on oxidative stress levels during chondrogenic differentiation of BMMSCs.
FIGURE 5.
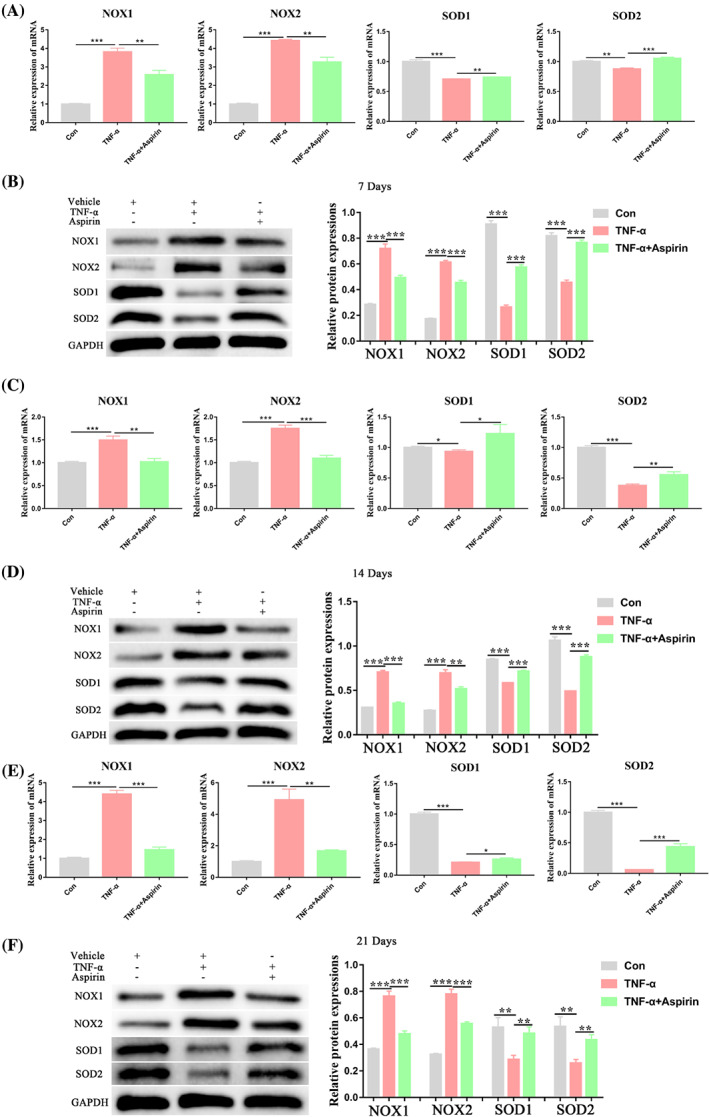
Impact of TNF‐α and aspirin on oxidative stress markers expression during chondrogenesis of BMMSCs. (A, B) Relative mRNA and protein expression levels of oxidative stress markers (NOX1, NOX2, SOD1, and SOD2) were measured in BMMSCs treated with or without TNF‐α and aspirin for 7 days by qRT‐PCR and western blot analyses, respectively. (C, D) Relative mRNA and protein expression levels of oxidative stress markers (NOX1, NOX2, SOD1, and SOD2) were measured in BMMSCs treated with or without TNF‐α and aspirin for 14 days by qRT‐PCR and western blot analyses, respectively. (E, F) Relative mRNA and protein expression levels of oxidative stress markers (NOX1, NOX2, SOD1, and SOD2) were measured in BMMSCs treated with or without TNF‐α and aspirin for 21 days by qRT‐PCR and western blot analyses, respectively. Data in (A), (C), and (E) are given as the mean ± SD of three independent experiments. GAPDH, glyceraldehyde‐3‐phosphate dehydrogenase; NOX1, NADPH oxidase 1; NOX2, NADPH oxidase 2; SOD1, superoxide dismutase 1; SOD2, superoxide dismutase 2. *p < 0.05, **p < 0.01, ***p < 0.001
3.6. AS reduces the inhibitory effect of TNF‐α on the chondrogenic differentiation of BMMSCs by stabilizing YAP
Because the Hippo pathway plays an important regulatory role in the physiological processes of stem cells, we speculated that YAP, a key component of the Hippo pathway, might be involved in mediating the inhibitory effects of TNF‐α during chondrogenic differentiation of BMMSCs. To test our hypothesis, we first detected mRNA expression of YAP and the typical YAP‐targeted genes connective tissue growth factor (CTGF) and cysteine‐rich angiogenic inducer 61 (CYR61) after TNF‐α treatment. We found that TNF‐α reduced mRNA expression of YAP, CTGF, and CYR61 (Figure 6A). Next, we examined p‐YAP and YAP protein expression and found that treatment with TNF‐α did not affect the p‐YAP/YAP ratio (Figure 6B).
FIGURE 6.
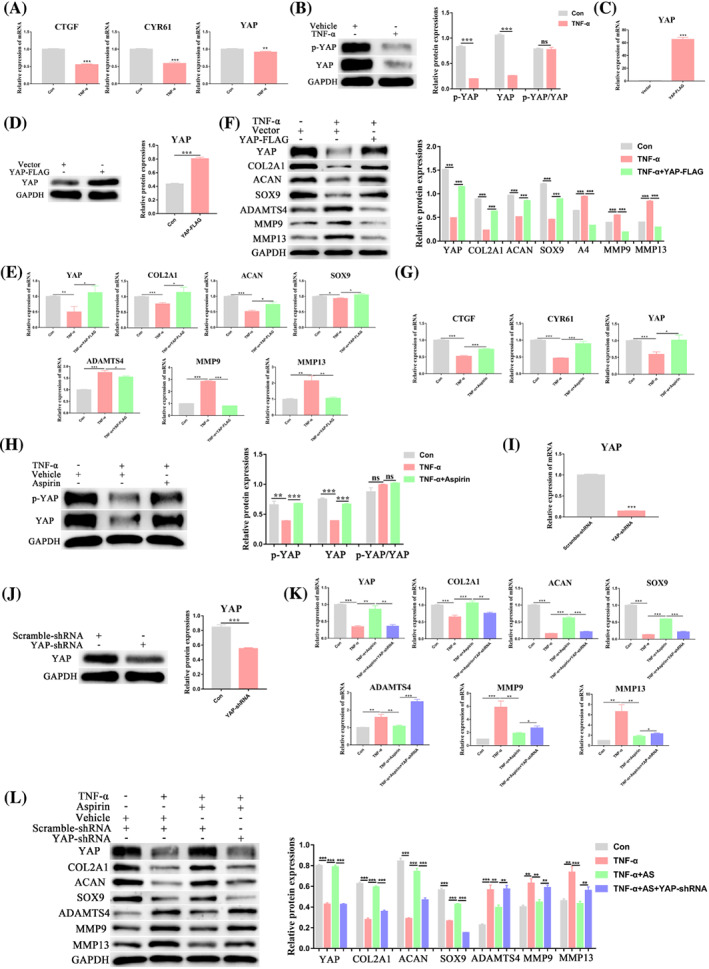
TNF‐α suppresses chondrogenesis of BMMSCs by downregulating YAP, while aspirin reverses these TNF‐α‐induced effects through stabilization of YAP. (A) The mRNA levels of YAP and key Hippo pathway target genes, including CTGF and CYR61, were measured by qPCR. (B) The p‐YAP and YAP protein expression levels were examined using western blot assays. (C, D) Stable overexpression of YAP‐FLAG or vector in BMMSCs was conducted. qPCR (C) and western blot (D) assays were performed to ensure overexpression efficiency. Chondrogenesis was then induced in BMMSCs for 14 days with or without TNFα, and expressions of YAP, COL2A1, ACAN, SOX9, ADAMTS4, MMP9, and MMP13 were detected by qPCR (E) and western blot (F). (G, H) Expressions of CTGF, CYR61 and YAP in different groups were examined by qPCR (G). The p‐YAP and YAP protein expression levels were assessed by western blot (H). (I, J) YAP expression was silenced in BMMSCs by transduction with lentivirus encoding YAP‐shRNA. Scramble‐shRNA was used as the control. qPCR (I) and western blot (J) were conducted to determine the silencing efficiency. Then, chondrogenesis differentiation was induced in BMMSCs for 14 days with or without vehicle, 10 ng/ml TNFα, and 100 μmol/L aspirin, and the expressions of YAP, COL2A1, ACAN, SOX9, ADAMTS4, MMP9, and MMP13 were detected using qPCR (K) and western blot (L) analyses. Values in A, C, E, G, I, and K are the mean ± SD of three independent experiments. ACAN, aggrecan; ADAMTS4, ADAM metallopeptidase with thrombospondin type 1 motif 4; COL2A1, collagen type II alpha 1 chain; CTGF, connective tissue growth factor; CYR61, cysteine‐rich angiogenic inducer 61; GAPDH, glyceraldehyde‐3‐phosphate dehydrogenase; MMP9, matrix metallopeptidase 9; MMP13, matrix metallopeptidase 13; SOX9, SRY‐box transcription factor 9; YAP, yes‐associated protein. *p < 0.05, **p < 0.01, ***p < 0.001
Through YAP overexpression studies (Figure 6C,D), we found that overexpression of YAP reversed the inhibitory effect of TNF‐α on the chondrogenic synthesis markers (COL2A1, ACAN, SOX9), the antioxidant enzyme markers (SOD1, SOD2) and its stimulatory effect on the chondrogenic catabolic markers (ADAMTS4, MMP9, MMP13) and the oxidase markers (NOX1, NOX2) (Figure 6E,F, Figure S1A). These findings suggested that the inhibitory effect of TNF‐α on the chondrogenic differentiation of BMMSCs was mediated through YAP.
Subsequently, we found that AS treatment reversed the inhibitory effects of TNF‐α on YAP, CTGF, and CYR61 mRNA expression (Figure 6G), but did not affect the p‐YAP/YAP ratio (Figure 6H). Using RNA interference to inhibit YAP expression (Figure 6I,J), we found that inhibition of YAP reversed the stimulatory effects of AS on the expression of chondrogenic differentiation synthesis markers (COL2A1, ACAN, SOX9), the antioxidant enzyme markers (SOD1, SOD2), as well as its inhibitory effects on the expression of catabolic markers (ADAMTS4, MMP9, MMP13) and the oxidase markers (NOX1, NOX2) (Figure 6K,L, Figure S1B). These results suggested that the protective effects of AS on BMMSC chondrogenic differentiation were mediated by YAP.
Taken together, our findings demonstrated that AS alleviated the inhibitory effect of TNF‐α on the chondrogenic differentiation of BMMSCs by stabilizing YAP expression.
3.7. AS mitigates the progression of cartilage degeneration in a DMM mouse model
Next, we constructed an in vivo OA model by performing DMM surgery on the left knee of mice. Low (1 mg/kg/d, 3 days per week) and high (10 mg/kg/d, 3 days per week) doses of AS were administered intraperitoneally for 8 or 12 weeks post‐surgery to determine the effect of AS treatment on the progression of cartilage degeneration (Figure 7A). We found that treatment with both high and low concentrations of AS did not affect mouse body weight compared to the Sham and PBS groups (Figure 7B). Next, we performed H&E staining on heart, liver, spleen, and kidney samples collected 8 and 12 weeks after AS treatment and found that AS treatment was not toxic to these organs (Figure S2).
FIGURE 7.
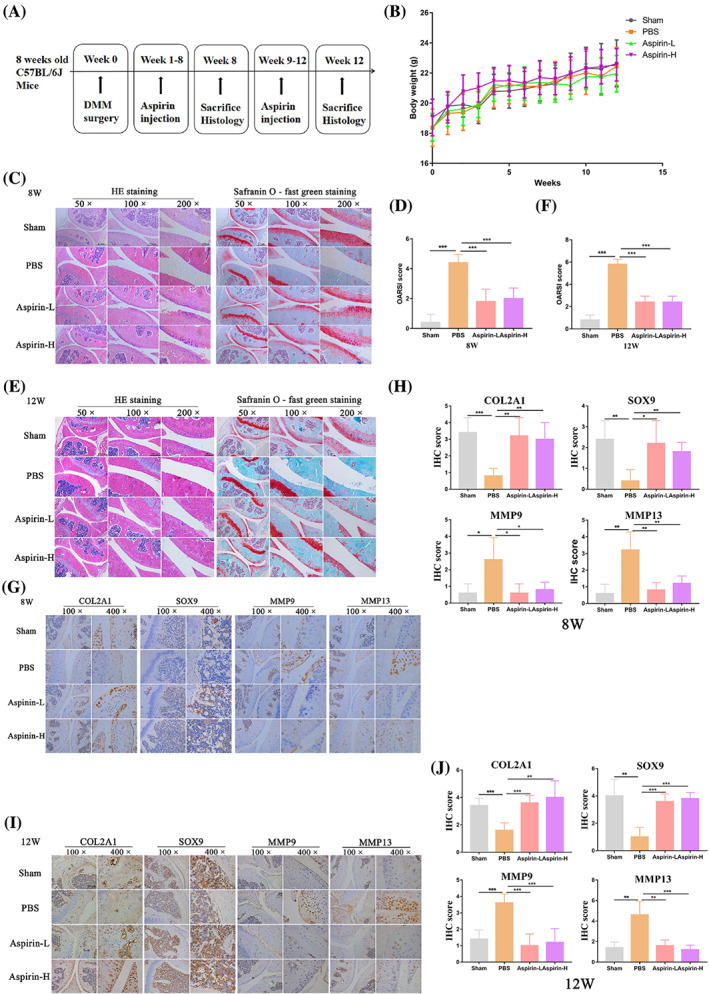
Aspirin mitigates the progression of cartilage degeneration in a mouse DMM model. A schematic of the in vivo experiment. After DMM surgery, mice were injected with aspirin or PBS for 12 weeks (n = 10 per group). (B) The body weights of mice from different treatment groups were measured after 12 weeks. (C) Representative H&E and safranin O‐fast green staining images of the left knee joint of mice after exposure to different treatments for 8 weeks (n = 5 per group). (D) The severity of the OA‐like phenotype 8 weeks after DMM surgery in (C) was analysed by the Osteoarthritis Research Society International (OARSI) score system. (E) Representative H&E and safranin O‐fast green staining images of the left knee joint of mice after exposure to different treatments for 12 weeks (n = 5 per group). (F) The severity of the OA‐like phenotype 12 weeks after DMM surgery in (E) was analysed by the Osteoarthritis Research Society International (OARSI) score system. (G) IHC staining of chondrogenic markers (COL2A1 and SOX9) and catabolic markers (MMP9 and MMP13) in the left knee joint of mice after different treatments for 8 weeks. (H) IHC staining scores of (G). (I) IHC staining of chondrogenic markers (COL2A1 and SOX9) and catabolic markers (MMP9 and MMP13) in the left knee joint of mice after different treatments for 12 weeks. (J) IHC staining scores of (I). COL2A1, collagen type II alpha 1 chain; MMP9, matrix metallopeptidase 9; MMP13, matrix metallopeptidase 13; SOX9, SRY‐box transcription factor 9. *p < 0.05, **p < 0.01, ***p < 0.001. Scale bars: 0.5 mm (50× figures), 250 μm (100× figures), 100 μm (200× figures), 50 μm (400× figures)
Eight and 12 weeks after surgical treatment of DMM, the mouse knee joints showed signs of OA, including cartilage wear and articular surface irregularities. Treatment with AS delayed the progression of knee joint cartilage degeneration in the DMM mouse model as measured by H&E staining and safranin O‐fast green staining (Figure 7C–F). IHC staining of cartilage synthesis markers (COL2A1, SOX9) and catabolic markers (MMP9, MMP13) in the knee joint tissues revealed that AS treatment led to the promotion of the expression of cartilage synthesis markers and suppression of the expression of catabolic markers in the DMM mouse model (Figure 7G,I). The quantitative data for the IHC staining of each treatment group are shown in Figure 7H,J. In conclusion, AS mitigated the progression of cartilage degeneration in the DMM mouse model by promoting cartilage synthesis and inhibiting cartilage catabolism.
4. DISCUSSION
The present study explored the effects of AS and TNF‐α on the chondrogenic differentiation of BMMSCs. We found that TNF‐α inhibited BMMSC chondrogenic differentiation by disrupting the balance of cartilage metabolism and promoting oxidative stress in BMMSCs, while AS treatment attenuated these effects. Mechanistically, YAP played an important regulatory role in this process. We further demonstrated in our in vivo DMM model of OA that AS treatment mitigated the progression of cartilage degeneration. Taken together, our data showed that AS alleviated the inhibitory effect of TNF‐α on BMMSC chondrogenic differentiation by stabilizing the expression of YAP. To the best of our knowledge, this is the first study to investigate the effects of AS on the chondrogenic differentiation of BMMSCs. Our study aimed to provide an experimental basis for the application of AS in the prevention and treatment of OA in the future.
The inflammatory factor TNF‐α has a negative impact on the effectiveness of stem cell therapy. Here, we found that TNF‐α inhibited chondrogenic differentiation of BMMSCs by disrupting the balance of cartilage metabolism, promoting the oxidative stress level of BMMSCs, and decreasing YAP expression. These findings are consistent with previous studies, which reported that TNF‐α can impair the physiological processes in stem cells through multiple pathways. For example, Wang et al. showed that TNF‐α impaired the stemness maintenance of BMMSCs by inhibiting the expression of YAP, 9 while Ding et al. found that TNF‐α inhibited chondrogenic differentiation of BMMSCs, thereby promoting OA progression. 10 TNF‐α has also been found to inhibit osteogenic differentiation of BMMSCs by promoting the production of reactive oxygen species (ROS) or degradation of SMAD1 protein. 21 , 23 Taken together, these studies highlighted the importance of developing therapeutic strategies to reverse the damaging effects of TNF‐α on stem cells.
AS plays an important role in regulating stem cell function. In the current study, we found that AS blocked the inhibitory effect of TNF‐α on chondrogenic differentiation of BMMSCs by stabilizing the expression of YAP, promoting cartilage metabolic homeostasis, and suppressing oxidative stress in BMMSCs. Previous studies have also demonstrated that AS can affect not only the proliferative activity of BMMSCs but also their ability to differentiate into multiple cell types. For example, Wen et al. showed that different concentrations of AS inhibited the growth of BMMSCs, as well as enhanced cardiomyocyte differentiation. 25 In addition, AS was found to inhibit osteogenic differentiation and promote adipogenic differentiation of BMMSCs. 26 , 27 AS was also shown to promote cranial regeneration, as well as reduce bone loss in ovariectomized rats, and could therefore have clinical potential in the treatment of osteoporosis. 28 , 29 , 30 Finally, AS was found to improve the immunomodulatory properties of BMMSCs through the 15d‐PGJ2/PPARγ/TGF‐β1 signalling axis. 31 These studies indicate that AS has the potential to improve the efficacy of stem cell therapy.
AS also plays a critical role in OA prevention and treatment. Our study showed that AS delayed the progression of cartilage degeneration in a DMM mouse model by promoting cartilage synthesis and inhibiting cartilage catabolism. Our findings are consistent with data from a cohort study, which showed that the use of low‐dose AS was associated with reduced medial tibial cartilage loss over 2 years in patients with knee OA, suggesting that low‐dose AS may be used to slow the progression of knee OA. 16 Meesawatsom et al. demonstrated that AS‐triggered resolvin D1 had an inhibitory effect on spinal cord damage in a rat model of chronic OA pain, and may therefore be beneficial in the treatment of inflammatory pain. 18 AS may also have clinical potential in the treatment of secondary nociceptive hypersensitivity in OA. 19 These studies suggested that AS may be used for OA prevention and treatment in the future.
YAP, a key molecule of the Hippo pathway, plays an important regulatory role in the physiological processes of stem cells. Here, we found that TNF‐α decreased YAP expression resulting in inhibition of BMMSCs chondrogenic differentiation, while AS treatment upregulated YAP expression, thereby delaying the inhibitory effects of TNF‐α. These findings suggest that YAP has a critical regulatory role in the chondrogenic differentiation of BMMSCs. Similarly, Wang et al. found that YAP played a regulatory role in the process of melatonin maintaining the stemness of BMMSCs 25 ; Wang et al. demonstrated that BMMSC‐derived extracellular vesicles induced cartilage reconstruction in temporomandibular joint OA through the autotaxin‐YAP signalling axis, 6 while Mao et al. found that BMMSC‐derived exosomes regulated the GPRC5A‐YAP signalling axis to improve sulfur mustard‐induced acute lung injury. 32 Liu et al. found that the AMOT130/YAP signalling axis is an important pathway mediating micro/nano‐topography (MNT) to promote osteogenic differentiation of BMMSCs. 33 In addition, YAP has also been shown to play an important regulatory role in reducing apoptosis and regulating osteogenic and adipogenic differentiation of BMMSCs. 34 , 35 These studies, together with our findings, suggested that YAP played an important regulatory role in the physiological function of stem cells.
YAP plays a significant regulatory role in stem cell chondrogenic differentiation. Yamashita et al. 36 found that YAP knockdown could promote the chondrogenic differentiation of human‐induced pluripotent stem cells; Li et al. 37 discovered that microtubule stabilization could inhibit YAP expression and promote the chondrogenic differentiation of synovial MSCs. Furthermore, Nie et al. 38 found that dasatinib promoted the chondrogenic differentiation of mesenchymal stem cells by increasing the phosphorylation level of YAP. In addition to regulating the chondrogenic differentiation of stem cells, YAP has been shown to play a key role in the chondrogenesis of C3H10T1/2 and ATDC5 cells. 39 , 40 , 41 Taken together, YAP plays an essential regulatory role in the process of chondrogenesis in various cell types.
YAP is also important in the regulation of oxidative stress. In this study, we discovered that YAP overexpression could partially reverse the oxidative stress‐promoting effect of TNF‐α. Silencing YAP, on the other hand, could partially reverse the down‐regulatory effect of AS on oxidative stress, indicating that YAP plays a negative regulatory role in the oxidative stress process. This is consistent with the findings of the majority of previously published literature. White et al. 42 discovered that YAP maintains redox balance and prevents oxidative stress‐induced cell death by reducing mitochondrial respiration capacity, whereas excessive consumption of YAP can increase mitochondrial respiration and ROS accumulation, leading to oxidative stress‐induced cell death. Melatonin, according to Sun et al., 43 can alleviate doxorubicin‐induced mitochondrial oxidative stress injury and ferroptosis in cardiomyocytes by up‐regulating YAP expression. Cucci et al. 44 found that by inhibiting YAP expression, Ailanthone increased oxidative stress in anti‐CDDP ovarian and bladder cancer cells. Furthermore, Huang et al. 45 discovered that FoxO4 might exacerbate the apoptosis and oxidative stress of H9C2 cells induced by hypoxia/reoxygenation by blocking the Hippo/YAP pathway.
However, some studies have demonstrated that YAP can promote oxidative stress. Yu et al. 46 discovered that overexpression of (Pro)renin receptor can aggravate oxidative stress and myocardial fibrosis in diabetic cardiomyopathy by increasing the expression of YAP and that YAP blockade can reverse these pathological changes. Moreover, Bai et al. 47 established a mouse model of unilateral ureteral obstruction (UUO) and discovered that ruxolitinib treatment attenuated UUO‐induced inflammation, oxidative stress, and apoptosis by inhibiting the activation of the Akt/mTOR/Yap pathway. These studies, together with our findings, indicated that YAP played a crucial regulatory role in oxidative stress.
In this study, we chose 1 mg/kg/d and 10 mg/kg/d as the doses of AS for treatment, based on the usual doses of AS utilized in prior studies. 48 , 49 , 50 , 51 According to the findings of this study, both doses of AS alleviated cartilage degeneration in the DMM mouse model, indicating that AS has an anti‐OA effect in the DMM mouse model. According to the calculation methods in Suman et al., 52 1 mg/kg/d and 10 mg/kg/d administered to mice in our study are equivalent to 22.2 mg/d and 222 mg/d in humans, which are within the range of medication doses and are safe to administer to patients in clinical practice. Nonetheless, no studies have been conducted to evaluate whether AS at these doses can be utilized clinically for the prevention and treatment of OA, and additional clinical researches are necessary to better validate the effect of AS on OA.
Our study had several limitations. First, the animal experiments in this study only evaluated the histology of articular cartilage and the expression of cartilage metabolic markers in mice, with no assessment of mouse mobility. Second, although our data suggested that AS reversed the inhibitory effects of TNF‐α on BMMSCs chondrogenic differentiation through the stabilization of YAP, the downstream mechanisms of YAP were not explored in depth. In addition, because this study was a preliminary investigative study at the cellular level and in an animal model of OA, clinical trials are still needed to further verify the preventive and therapeutic effects of AS on OA.
In conclusion, this study demonstrated that TNF‐α inhibited chondrogenic differentiation of BMMSCs by disrupting the balance of cartilage metabolism and promoting oxidative stress in BMMSCs in vitro, while AS treatment attenuated these effects. Furthermore, a detailed molecular mechanistic analysis indicated that YAP played a critical regulatory role in this process. In addition, AS treatment mitigated the progression of cartilage degeneration in a mouse DMM model. In conclusion, AS alleviated the inhibitory effects of TNF‐α on BMMSC chondrogenic differentiation by stabilizing YAP.
AUTHOR CONTRIBUTIONS
Ziji Zhang, Puyi Sheng, and Yan Kang designed the experiments. Xudong Wang, Hongyi Liao, Yong Liu, Yunze Kang, Qingqiang Tu, and Zhiwen Li conducted the experiments. Xudong Wang and Hongyi Liao acquired the data. Xudong Wang, Hongyi Liao, Ziji Zhang, Puyi Sheng, and Yan Kang analysed the data. Xudong Wang, Hongyi Liao, Ziji Zhang, Puyi Sheng, and Yan Kang wrote the manuscript. All authors read and approved the final manuscript.
CONFLICT OF INTEREST
All authors declare no conflict of interest.
Supporting information
Figure S1. TNF‐α promotes oxidative stress of BMMSCs by downregulating YAP, while aspirin reverses these TNF‐α‐induced effects through stabilization of YAP. (A) After overexpression of YAP, expressions of YAP, NOX1, NOX2, SOD1, and SOD2 were detected by western blot. (B) After silencing the expression of YAP, the expressions of YAP, NOX1, NOX2, SOD1, and SOD2 were detected using western blot analyses. YAP, yes‐associated protein; NOX1, NADPH oxidase 1; NOX2, NADPH oxidase 2; SOD1, superoxide dismutase 1; SOD2, superoxide dismutase 2. *p < 0.05, **p < 0.01, ***p < 0.001.
Figure S2. The pathology images of heart, liver, spleen, and kidney in different groups mice. (A) Pathology images of heart, liver, spleen, and kidney in mice with different treatments after 8 weeks (n = 5 per group). (B) Pathology images of heart, liver, spleen, and kidney in mice with different treatments after 12 weeks (n = 5 per group). Scale bars: 250 μm (100× figures), 50 μm (400× figures).
Wang X, Liao H, Liu Y, et al. Aspirin reverses inflammatory suppression of chondrogenesis by stabilizing YAP. Cell Prolif. 2023;56(4):e13380. doi: 10.1111/cpr.13380
Xudong Wang and Hongyi Liao contributed equally to this work.
Funding information National Natural Science Foundation of China, Grant/Award Number: 81874014; Natural Science Foundation of Guangdong Province, China, Grant/Award Number: 2022A1515012279
Contributor Information
Yan Kang, Email: kangyan2@mail.sysu.edu.cn.
Puyi Sheng, Email: shengpy@mail.sysu.edu.cn.
Ziji Zhang, Email: zhangziji@mail.sysu.edu.cn.
DATA AVAILABILITY STATEMENT
The datasets analysed during the current research are available from the corresponding author on reasonable request.
REFERENCES
- 1. Prietoalhambra D, Judge A, Kassim Javaid M, Cooper C, Diez‐Perez A, Arden NK. Incidence and risk factors for clinically diagnosed knee, hip and hand osteoarthritis: influences of age, gender and osteoarthritis affecting other joints. Ann Rheum Dis. 2014;73:1659‐1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hunter DJ, Schofield D, Callander E. The individual and socioeconomic impact of osteoarthritis. Nat Rev Rheumatol. 2014;10:437‐441. [DOI] [PubMed] [Google Scholar]
- 3. Safiri S, Kolahi AA, Smith E, et al. Global, regional and national burden of osteoarthritis 1990–2017: a systematic analysis of the Global Burden of Disease Study 2017. Ann Rheum Dis. 2020;79:819‐828. [DOI] [PubMed] [Google Scholar]
- 4. Sofat N, Watt FE, Lyn TA. Development of medical therapeutics in osteoarthritis: time for action to improve patient care. Rheumatology (Oxford). 2021;60:3487‐3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Toyoda E, Maehara M, Watanabe M, Sato M. Candidates for intra‐articular administration therapeutics and therapies of osteoarthritis. Int J Mol Sci. 2021;22:3594. doi: 10.1002/art.34453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang Y, Zhao M, Li W, et al. BMSC‐derived small extracellular vesicles induce cartilage reconstruction of temporomandibular joint osteoarthritis via autotaxin‐YAP signaling axis. Front Cell Dev Biol. 2021;9:656153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu Y, Peng L, Li L, et al. 3D‐bioprinted BMSC‐laden biomimetic multiphasic scaffolds for efficient repair of osteochondral defects in an osteoarthritic rat model. Biomaterials. 2021;279:121216. [DOI] [PubMed] [Google Scholar]
- 8. Kim SH, Djaja YP, Park YB, Park JG, Ko YB, Ha CW. Intra‐articular injection of culture‐expanded mesenchymal stem cells without adjuvant surgery in knee osteoarthritis: a systematic review and meta‐analysis. Am J Sports Med. 2020;48:2839‐2849. [DOI] [PubMed] [Google Scholar]
- 9. Wang X, Liang T, Qiu J, et al. Melatonin reverses the loss of stemness induced by TNF‐α in human bone marrow mesenchymal stem cells through upregulation of YAP expression. Stem Cells Int. 2019;2019:6568394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ding N, Zeng MY, Song WJ, Xiao CX, Li EM, Wei B. SP600125 restored tumor necrosis factor‐α‐induced impaired chondrogenesis in bone mesenchymal stem cells and its anti‐osteoarthritis effect in mice. Stem Cells Dev. 2021;30:1028‐1036. doi: 10.1089/scd.2021.0146 [DOI] [PubMed] [Google Scholar]
- 11. Arango‐Varela SS, Luzardo‐Ocampo I, Maldonado‐Celis ME, Campos‐Vega R. Andean berry (Vaccinium meridionale Swartz) juice in combination with Aspirin modulated anti‐inflammatory markers on LPS‐stimulated RAW 264.7 macrophages. Food Res Int. 2020;137:109541. [DOI] [PubMed] [Google Scholar]
- 12. Khan SA, Chatterjee SS, Kumar V. Low dose aspirin like analgesic and anti‐inflammatory activities of mono‐hydroxybenzoic acids in stressed rodents. Life Sci. 2016;148:53‐62. [DOI] [PubMed] [Google Scholar]
- 13. Schade DS, Burchiel S, Eaton RP. A pathophysiologic primary prevention review of Aspirin administration to prevent cardiovascular thrombosis. Endocr Pract. 2020;26:787‐793. [DOI] [PubMed] [Google Scholar]
- 14. Hua H, Zhang H, Kong Q, Wang J, Jiang Y. Complex roles of the old drug aspirin in cancer chemoprevention and therapy. Med Res Rev. 2019;39:114‐145. [DOI] [PubMed] [Google Scholar]
- 15. Fan W, Li J, Chen J, et al. Aspirin inhibits the proliferation of synovium‐derived mesenchymal stem cells by arresting the cell cycle in the G0/G1 phase. Am J Transl Res. 2017;9:5056‐5062. [PMC free article] [PubMed] [Google Scholar]
- 16. Hao W, Shi S, Zhou S, Wang X, Nie S. Aspirin inhibits growth and enhances cardiomyocyte differentiation of bone marrow mesenchymal stem cells. Eur J Pharmacol. 2018;827:198‐207. [DOI] [PubMed] [Google Scholar]
- 17. Deng L, Hu S, Baydoun AR, Chen J, Chen X, Cong X. Aspirin induces apoptosis in mesenchymal stem cells requiring Wnt/beta‐catenin pathway. Cell Prolif. 2009;42:721‐730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang Y, Xiong Y, Chen X, Chen C, Zhu Z, Li L. Therapeutic effect of bone marrow mesenchymal stem cells pretreated with acetylsalicylic acid on experimental periodontitis in rats. Int Immunopharmacol. 2018;54:320‐328. [DOI] [PubMed] [Google Scholar]
- 19. Zhan Y, He Z, Liu X, et al. Aspirin‐induced attenuation of adipogenic differentiation of bone marrow mesenchymal stem cells is accompanied by the disturbed epigenetic modification. Int J Biochem Cell Biol. 2018;98:29‐42. [DOI] [PubMed] [Google Scholar]
- 20. Gao B, Gao W, Wu Z, et al. Melatonin rescued interleukin 1beta‐impaired chondrogenesis of human mesenchymal stem cells. Stem Cell Res Ther. 2018;9:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Qiu X, Wang X, Qiu J, et al. Melatonin rescued reactive oxygen species‐impaired osteogenesis of human bone marrow mesenchymal stem cells in the presence of tumor necrosis factor‐alpha. Stem Cells Int. 2019;2019:6403967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang X, Chen T, Deng Z, et al. Melatonin promotes bone marrow mesenchymal stem cell osteogenic differentiation and prevents osteoporosis development through modulating circ_0003865 that sponges miR‐3653‐3p. Stem Cell Res Ther. 2021;12:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lian C, Wu Z, Gao B, et al. Melatonin reversed tumor necrosis factor‐alpha‐inhibited osteogenesis of human mesenchymal stem cells by stabilizing SMAD1 protein. J Pineal Res. 2016;61:317‐327. [DOI] [PubMed] [Google Scholar]
- 24. Lian C, Wang X, Qiu X, et al. Collagen type II suppresses articular chondrocyte hypertrophy and osteoarthritis progression by promoting integrin β1‐SMAD1 interaction. Bone Res. 2019;7:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wluka AE, Ding C, Wang Y, Jones G, Urquhart DM, Cicuttini FM. Aspirin is associated with reduced cartilage loss in knee osteoarthritis: data from a cohort study. Maturitas. 2015;81:394‐397. [DOI] [PubMed] [Google Scholar]
- 26. Meesawatsom P, Burston J, Hathway G, Bennett A, Chapman V. Inhibitory effects of aspirin‐triggered resolvin D1 on spinal nociceptive processing in rat pain models. J Neuroinflammation. 2016;13:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Niibori M, Kudo Y, Hayakawa T, et al. Mechanism of aspirin‐induced inhibition on the secondary hyperalgesia in osteoarthritis model rats. Heliyon. 2020;6:e03963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cao Y, Xiong J, Mei S, et al. Aspirin promotes bone marrow mesenchymal stem cell‐based calvarial bone regeneration in mini swine. Stem Cell Res Ther. 2015;6:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu H, Li W, Liu Y, Zhang X, Zhou Y. Co‐administration of aspirin and allogeneic adipose‐derived stromal cells attenuates bone loss in ovariectomized rats through the anti‐inflammatory and chemotactic abilities of aspirin. Stem Cell Res Ther. 2015;6:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lin S, Lee WYW, Huang M, et al. Aspirin prevents bone loss with little mechanical improvement in high‐fat‐fed ovariectomized rats. Eur J Pharmacol. 2016;791:331‐338. [DOI] [PubMed] [Google Scholar]
- 31. Tang J, Xiong J, Wu T, et al. Aspirin treatment improved mesenchymal stem cell immunomodulatory properties via the 15d‐PGJ2/PPARγ/TGF‐β1 pathway. Stem Cells Dev. 2014;23:2093‐2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mao G, Gong CC, Wang Z, et al. BMSC‐derived exosomes ameliorate sulfur mustard‐induced acute lung injury by regulating the GPRC5A‐YAP axis. Acta Pharmacol Sin. 2021;42:2082‐2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu X, Hou W, He L, et al. AMOT130/YAP pathway in topography‐induced BMSC osteoblastic differentiation. Colloids Surf B Biointerfaces. 2019;182:110332. [DOI] [PubMed] [Google Scholar]
- 34. Wang Z, Cui M, Qu Y, et al. Hypoxia protects rat bone marrow mesenchymal stem cells against compression‐induced apoptosis in the degenerative disc microenvironment through activation of the HIF‐1α/YAP signaling pathway. Stem Cells Dev. 2020;29:1309‐1319. [DOI] [PubMed] [Google Scholar]
- 35. Pan H, Xie Y, Zhang Z, et al. YAP‐mediated mechanotransduction regulates osteogenic and adipogenic differentiation of BMSCs on hierarchical structure. Colloids Surf B Biointerfaces. 2017;152:344‐353. [DOI] [PubMed] [Google Scholar]
- 36. Yamashita A, Yoshitomi H, Kihara S, Toguchida J, Tsumaki N. Culture substrate‐associated YAP inactivation underlies chondrogenic differentiation of human induced pluripotent stem cells. Stem Cells Transl Med. 2021;10:115‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li J, Sun Z, Lv Z, et al. Microtubule stabilization enhances the chondrogenesis of synovial mesenchymal stem cells. Front Cell Dev Biol. 2021;9:748804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nie P, Li Y, Suo H, Jiang N, Yu D, Fang B. Dasatinib promotes chondrogenic differentiation of human mesenchymal stem cells via the Src/hippo‐YAP signaling pathway. ACS Biomater Sci Eng. 2019;5:5255‐5265. [DOI] [PubMed] [Google Scholar]
- 39. Thorup A‐S, Strachan D, Caxaria S, et al. ROR2 blockade as a therapy for osteoarthritis. Sci Transl Med. 2020;12:eaax3063. doi: 10.1126/scitranslmed.aax3063 [DOI] [PubMed] [Google Scholar]
- 40. Li M, Ning J, Wang J, Yan Q, Zhao K, Jia X. SETD7 regulates chondrocyte differentiation and glycolysis via the Hippo signaling pathway and HIF‐1α. Int J Mol Med. 2021;48:210. doi: 10.3892/ijmm.2021.5043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chen P, Yang B, Wu Y, Wang J. YAP1 regulates chondrogenic differentiation of ATDC5 promoted by temporary TNF‐α stimulation through AMPK signaling pathway. Mol Cell Biochem. 2020;474:209‐218. [DOI] [PubMed] [Google Scholar]
- 42. White SM, Avantaggiati ML, Nemazanyy I, et al. YAP/TAZ inhibition induces metabolic and signaling rewiring resulting in targetable vulnerabilities in NF2‐deficient tumor cells. Dev Cell. 2019;49:425‐443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sun X, Sun P, Zhen D, et al. Melatonin alleviates doxorubicin‐induced mitochondrial oxidative damage and ferroptosis in cardiomyocytes by regulating YAP expression. Toxicol Appl Pharmacol. 2022;437:115902. [DOI] [PubMed] [Google Scholar]
- 44. Cucci MA, Grattarola M, Dianzani C, et al. Ailanthone increases oxidative stress in CDDP‐resistant ovarian and bladder cancer cells by inhibiting of Nrf2 and YAP expression through a post‐translational mechanism. Free Radic Biol Med. 2020;150:125‐135. [DOI] [PubMed] [Google Scholar]
- 45. Huang J, Liu Y, Wang M, Wang R, Ling H, Yang Y. FoxO4 negatively modulates USP10 transcription to aggravate the apoptosis and oxidative stress of hypoxia/reoxygenation‐induced cardiomyocytes by regulating the Hippo/YAP pathway. J Bioenerg Biomembr. 2021;53:541‐551. [DOI] [PubMed] [Google Scholar]
- 46. Yu S, Dong X, Yang M, et al. (Pro)renin receptor involves in myocardial fibrosis and oxidative stress in diabetic cardiomyopathy via the PRR‐YAP pathway. Sci Rep. 2021;11:3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bai Y, Wang W, Yin P, et al. Ruxolitinib alleviates renal interstitial fibrosis in UUO mice. Int J Biol Sci. 2020;16:194‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Li Y, Cao J, Hao Z, et al. Aspirin ameliorates the cognition impairment in mice following benzo[a]pyrene treatment via down‐regulating BDNF IV methylation. Neurotoxicology. 2022;89:20‐30. [DOI] [PubMed] [Google Scholar]
- 49. Zhu T, Chen X, Qiu H, et al. Aspirin alleviates particulate matter induced asymptomatic Orchitis of mice via suppression of cGAS‐STING signaling. Front Immunol. 2021;12:734546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mahlangu TJ, Dludla PV, Mxinwa V, et al. Elevated T‐helper 2 cytokine levels in high fat diet‐fed C57BL/6 mice are attenuated by short‐term 6‐week treatment with a combination of low‐dose aspirin and metformin. Cytokine. 2020;128:154999. [DOI] [PubMed] [Google Scholar]
- 51. Arango‐Varela SS, Luzardo‐Ocampo i, Maldonado‐Celis ME. Andean berry (Vaccinium meridionale Swartz) juice, in combination with Aspirin, displayed antiproliferative and pro‐apoptotic mechanisms in vitro while exhibiting protective effects against AOM‐induced colorectal cancer in vivo. Food Res Int. 2022;157:111244. [DOI] [PubMed] [Google Scholar]
- 52. Suman S, Kumar S, Moon BH, et al. Effects of dietary aspirin on high‐LET radiation‐induced prostaglandin E2 levels and gastrointestinal tumorigenesis in Apc mice. Life Sci Space Res (Amst). 2021;31:85‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. TNF‐α promotes oxidative stress of BMMSCs by downregulating YAP, while aspirin reverses these TNF‐α‐induced effects through stabilization of YAP. (A) After overexpression of YAP, expressions of YAP, NOX1, NOX2, SOD1, and SOD2 were detected by western blot. (B) After silencing the expression of YAP, the expressions of YAP, NOX1, NOX2, SOD1, and SOD2 were detected using western blot analyses. YAP, yes‐associated protein; NOX1, NADPH oxidase 1; NOX2, NADPH oxidase 2; SOD1, superoxide dismutase 1; SOD2, superoxide dismutase 2. *p < 0.05, **p < 0.01, ***p < 0.001.
Figure S2. The pathology images of heart, liver, spleen, and kidney in different groups mice. (A) Pathology images of heart, liver, spleen, and kidney in mice with different treatments after 8 weeks (n = 5 per group). (B) Pathology images of heart, liver, spleen, and kidney in mice with different treatments after 12 weeks (n = 5 per group). Scale bars: 250 μm (100× figures), 50 μm (400× figures).
Data Availability Statement
The datasets analysed during the current research are available from the corresponding author on reasonable request.


