Abstract
Objective
Pacing in a univentricular circulation has been associated with worsened outcomes. We investigated the long‐term outcomes of pacing in children with a univentricular circulation compared to a complex biventricular circulation. We also identified predictors of adverse outcomes.
Methods
A retrospective study of all children with major congenital heart disease who underwent pacemaker implantation under the age of 18 years between November 1994 and October 2017.
Results
Eighty‐nine patients were included; 19 with a univentricular and 70 with a complex biventricular circulation. A total of 96% of pacemaker systems were epicardial. Median follow up was 8.3 years. The incidence of adverse outcome was similar between the two groups. Five (5.6%) patients died and two (2.2%) underwent heart transplantation. Most adverse events occurred within the first 8 years after pacemaker implantation. Univariate analysis identified five predictors of adverse outcomes in the patients in the biventricular but none in the univentricular group. The predictors of adverse outcome in the biventricular circulation were a right morphologic ventricle as the systemic ventricle, age at first congenital heart disease (CHD) operation, number of CHD operations, and female gender. The nonapical lead position was associated with a much higher risk of an adverse outcome.
Conclusions
Children with a pacemaker and a complex biventricular circulation have similar survival to the ones with a pacemaker and a univentricular circulation. The only modifiable predictor was the epicardial lead position on the paced ventricle, emphasizing the importance of apical placement of the ventricular lead.
Keywords: biventricular, children, congenital heart disease, pacemaker, univentricular
1. INTRODUCTION
There has been a lot of interest in the outcomes of pacing in univentricular congenital heart disease (CHD). A recent large registry study 1 , 2 and a large cross‐sectional study 3 demonstrated worsened outcomes (decreased functional status and decreased ventricular systolic function) and survival associated with pacing in these patients. Bulic and colleagues similarly found an association between chronic ventricular pacing in children with a single ventricle circulation and a higher risk of moderate to severe ventricular dysfunction and death or transplantation. 4
We sought to investigate how the outcomes of pacing in children with a single ventricle circulation compare to the outcomes of pacing in complex biventricular CHD. In addition, we aimed to identify predictors of adverse outcomes for the two groups.
2. METHODS
2.1. Study population
The study cohort was identified by a retrospective review of medical records and the pacemaker database at the Royal Children's Hospital Melbourne. It included all pediatric patients with major CHD who underwent pacemaker implantation under the age of 18 years between November 1994 and October 2017. Patients who had the initial or subsequent device implantation or revision outside the study institution were included, as long as they received subsequent cardiac and pacemaker follow up at the study institution. We also included patients who underwent congenital cardiac surgery and device implantations or revisions at our institution but were subsequently discharged to the external cardiology units. For those patients, the respective units and cardiologists were contacted and up to date data were collected as per the study protocol. We excluded patients with a structurally normal heart, noncomplex CHD that required a single surgical repair and those patients who received multisite ventricular pacing as the initial implant. We excluded three patients due to insufficient follow‐up data after their care was transferred to another center. The start of the follow‐up period was from the first pacemaker implantation procedure. The end of the study period for each subject was August 2019 or any of the following events before that date: death, transplantation, or upgrade to multisite pacing before August 2019. We collected data on patient characteristics such as age, gender, follow‐up duration, type of CHD, genetic abnormalities, congenital and pacemaker‐related surgery, pacing indication, complications, device interrogation, clinical status, ventricular function as assessed by echocardiographic indices, and adverse events.
We defined adverse events as death, transplantation, ventricular assist device implantation, echocardiographic evidence of ventricular dysfunction, upgrade to multisite pacing for ventricular dysfunction or deterioration of New York Heart Association (NYHA) class. The echocardiographic evidence of ventricular dysfunction was based on qualitative assessment of the systemic ventricular function, which included calculation of the ejection fraction by M‐mode for the biventricular circulation group. The echocardiograms were reported by a senior cardiac physiologist and a consultant cardiologist. In order to avoid duplication errors, we defined deterioration of the NYHA class as a significant change in the exercise capacity or the development of heart failure symptoms without echocardiographic evidence of ventricular dysfunction.
The primary outcome was to compare the incidence of adverse events between the paced patients with a complex biventricular circulation and those with a univentricular circulation. The secondary outcome was to identify predictors of adverse events.
Data were entered into a Research Electronic Data Capture (REDCap) database, hosted by Murdoch Children's Research Institute (MCRI). The study protocol was approved by The Royal Children's Hospital Melbourne Human Research Ethics Committee and received governance authorization at the Melbourne Children's Campus prior to study commencement. Patients were not involved in the design or conduct of this study.
2.2. Statistical analysis
Analysis was performed in the R software (version 4.0.1, R Foundation). Patient characteristics are presented for the whole sample and by type of circulation (univentricular vs biventricular). Continuous data are summarized as the mean and standard deviation or median and interquartile range, depending on normality of distribution. Calculation of proportions was based on nonmissing data. For comparisons of continuous variables, the t‐test was used if the distribution was approximately symmetrical; otherwise the Wilcoxon rank‐sum test was used. Fisher's exact test was used for comparisons of categorical variables. The Kaplan–Meier method was used to analyze the time‐to‐failure event endpoint which was defined as the time from pacemaker implantation to the adverse outcome, with 95% confidence intervals (CIs) being given when providing point estimates. Cox regression models and likelihood ratio tests were used to assess the effect of patient characteristics on the time‐to‐failure endpoint. The proportional hazards assumption was assessed based on the method of Harrell and Lee and via diagnostic plots. p ˂ .05 were considered statistically significant.
The survival curves were created using the GraphPad Prism 9.0.0 (GraphPad Software).
3. RESULTS
3.1. Patients and pacemaker characteristics
A total of 89 patients were included in this study, of which 19 (21%) were children with a single‐ventricle circulation and 70 (79%) were children postcomplex biventricular repair. All children had undergone permanent pacemaker implantation. The median follow‐up duration was 8.3 years. Demographic and clinical characteristics are summarized in Table 1. The type of CHD is described in Table 2. The pacemaker characteristics are described in Table 3.
TABLE 1.
Demographic and clinical characteristics.
| Total (n = 89) | Univentricular (n = 19) | Biventricular (n = 70) | p value | |
|---|---|---|---|---|
| Gender | ||||
| Male | 52 (58%) | 10 (53%) | 42 (60%) | .607 |
| Female | 37 (42%) | 9 (47%) | 28 (40%) | |
| Systemic ventricle | ||||
| Right | 32 (36%) | 11 (58%) | 21 (30%) | .032 |
| Left | 57 (64%) | 8 (42%) | 49 (70%) | |
| Genetic condition | ||||
| No | 79 (89%) | 18 (95%) | 61 (87%) | .683 |
| Yes | 10 (11%) | 1 (5%) | 9 (13%) | |
| Heterotaxy | ||||
| No | 76 (85%) | 14 (74%) | 62 (89%) | .140 |
| Yes | 13 (15%) | 5 (26%) | 8 (11%) | |
| Years of follow up | ||||
| Mean | 9.20 | 8.73 | 9.33 | .681 |
| Median [IQR] | 8.3 [4.69–13.76] | 7.19 [4.7–14.3] | 8.38 [4.8–13.49] | .681 |
| Age at pacing (years) | ||||
| Median [IQR] | 1.81 [0.46–5.83] | 5.21 [1.47–6.59] | 1.14 [0.4–4.05] | .062 |
| Weight at pacing (kg) | ||||
| Median [IQR] | 10 [5.8–19.6] | 15.5 [8.04–19.3] | 9 [5.5–19.8] | .281 |
| Age at first CHD‐op | ||||
| Median [IQR] | 0.37 [0.04–0.92] | 0.12 [0.01–0.36] | 0.48 [0.11–1.12] | .0032 |
| Pacing indication | ||||
| Postop CAV block | 56 (63%) | 10 (53%) | 46 (66%) | .046 |
| Congenital CAV block | 5 (6%) | 1 (5%) | 4 (6%) | |
| Sinus node dysfunction | 12 (13%) | 7 (37%) | 5 (7%) | |
| Second degree AV block | 12 (13%) | 1 (5%) | 11 (16%) | |
| Paroxysmal CAV block | 2 (2%) | 0 (0%) | 2 (3%) | |
| Atrial tachy | 2 (2%) | 0 (0%) | 2 (3%) | |
Note: Comparison of the demographic and clinical characteristics of the patients between the univentricular and biventricular circulation groups.
Bold indicates p value <.05, demonstrating statistical significance.
TABLE 2.
Type of congenital heart disease.
| Number of patients | Type of circulation | |
|---|---|---|
| Congenitally corrected transposition of the great arteries | 12 | Biventricular |
| Tetralogy of Fallot | 10 | Biventricular |
| Atrioventricular septal defect | 9 | Biventricular |
| Transposition of the great arteries | 9 | Biventricular |
| Pulmonary atresia | 4 | Biventricular |
| Double‐outlet right ventricle | 3 | Biventricular |
| Shone's complex | 2 | Biventricular |
| Severe aortic stenosis | 2 | Biventricular |
| Mixed aortic valve disease | 2 | Biventricular |
| Aortic stenosis, ventricular septal defect, and hypoplastic arch | 2 | Biventricular |
| Interrupted aortic arch | 2 | Biventricular |
| Multiple ventricular septal defects, aortic coarctation | 2 | Biventricular |
| Partial anomalous pulmonary venous drainage, and left atrial isomerism | 2 | Biventricular |
| Ventricular septal defect, subaortic stenosis, and subpulmonary stenosis | 1 | Biventricular |
| Ventricular septal defect, subaortic stenosis, and hypoplastic arch | 1 | Biventricular |
| Subaortic stenosis, hypoplastic aortic arch | 1 | Biventricular |
| Atrioventricular septal defect/tetralogy of Fallot | 1 | Biventricular |
| Atrioventricular septal defect/‐Double‐outlet right ventricle | 1 | Biventricular |
| Cleft mitral valve, ventricular septal defect | 1 | Biventricular |
| Ebstein's anomaly | 1 | Biventricular |
| Atrioventricular discordance, ventricular septal defect | 1 | Biventricular |
| Loeys–Dietz syndrome, Aortic root dilatation | 1 | Biventricular |
| Unbalanced atrioventricular septal defect | 5 | Univentricular |
| Hypoplastic left heart syndrome | 3 | Univentricular |
| Hypoplastic left ventricle with a ventricular septal defect | 2 | Univentricular |
| Double‐inlet left ventricle | 2 | Univentricular |
| Tricuspid atresia | 2 | Univentricular |
| Mitral atresia | 1 | Univentricular |
| Congenitally corrected transposition of the great arteries | 1 | Univentricular |
| Atrioventricular discordance, double‐outlet right ventricle | 1 | Univentricular |
| Double‐outlet right ventricle | 1 | Univentricular |
| Crisscross heart with atrioventricular discordance | 1 | Univentricular |
Note: List of congenital heart disease diagnoses for the two groups.
TABLE 3.
Pacemaker characteristics.
| Total (n = 89) | Univentricular (n = 19) | Biventricular (n = 70) | p value | |
|---|---|---|---|---|
| Pacemaker lead position | ||||
| Epicardial | 85 (96%) | 19 (100%) | 66 (94%) | .574 |
| Transvenous | 4 (4%) | 0 (0%) | 4 (6%) | |
| Pacemaker system | ||||
| Atrial | 6 (7%) | 4 (21%) | 2 (3%) | .0014 |
| Ventricular | 18 (20%) | 0 (0%) | 18 (26%) | |
| Dual chamber | 65 (73%) | 15 (79%) | 50 (71%) | |
| Ventricular pacing | ||||
| <50% | 27 (30%) | 9 (47%) | 18 (26%) | .092 |
| >50% | 62 (70%) | 10 (53%) | 52 (74%) | |
| Ventricular lead position | ||||
| Apex | 44 (54%) | 8 (53%) | 36 (54%) | >.99 |
| Nonapical | 38 (46%) | 7 (47%) | 31 (46%) | |
| Ventricle paced | ||||
| Subpulmonary | 32 (39%) | 0 (0%) | 32 (47%) | .0003 |
| Systemic | 51 (61%) | 15 (100%) | 36 (53%) | |
| Paced QRS width (ms) | ||||
| <140 | 15 (24%) | 1 (10%) | 14 (27%) | .427 |
| ≥140 | 47 (76%) | 9 (90%) | 38 (73%) | |
| Complications postimplantation | ||||
| No | 79 (89%) | 17 (89%) | 62 (89%) | >.99 |
| Yes | 10 (11%) | 2 (11%) | 8 (11%) | |
| Reoperation within a month | ||||
| No | 82 (92%) | 18 (95%) | 64 (91%) | >.99 |
| Yes | 7 (8%) | 1 (5%) | 6 (9%) | |
| Long‐term pacing complications | ||||
| No | 82 (92%) | 19 (100%) | 63 (90%) | |
| Yes | 7 (8%) | 0 (0%) | 7 (10%) | |
| Pacemaker replacement | ||||
| No | 28 (31%) | 8 (42%) | 20 (29%) | |
| Yes | 61 (69%) | 11 (58%) | 50 (71%) | |
| Number of replacements | ||||
| Median [IQR] | 1 [0–2] | 1 [0–1.5] | 1 [0–2] | |
Note: Comparison of the pacemaker characteristics between the two groups.
Bold indicates p value <.05, demonstrating statistical significance.
Most (96%) pacemaker systems were epicardial. We routinely place bipolar Medtronic leads (4965 and 4968 CapSure Epi). The position of the epicardial ventricular lead was derived from the operation note and was confirmed with the chest X‐ray for each implant and each lead repositioning. The most recent lead position was included in the final analysis. The ventricular pacing percentage was assessed based on the pacemaker interrogation within 2 years of last follow up. We used the cutoff of 50% to divide the patients into low (<50%) and high (≥50%) ventricular pacing in line with previous studies that assessed the effect of ventricular pacing on ventricular function and heart failure. 1 , 5 , 6 Sixty‐two out of 89 patients received a high percentage ventricular pacing (>50%). For each patient, the most recent 15‐lead ECG at the time of last follow up was reviewed and the QRS duration was recorded. For those patients whose ECG at the time of last follow up was not available for review, the most recent available ECG was reviewed.
The complications post implantation (11%) included lead malfunction or dislodgement requiring revision, pneumothorax, postoperative pneumonia or fever, wound dehiscence, and a retained pleural drain. Seven patients had to be reoperated within a month of pacemaker implantation (8%), with one of them requiring an atrial lead revision for under sensing. Late pacemaker complications (8%) included ventricular lead fracture (in three patients) leading to symptomatic bradycardia, migration of the generator requiring surgery, pacemaker infection, and superior vena cava thrombosis.
3.2. Adverse outcomes
Table 4 summarizes the type and incidence of adverse outcomes in the two groups. A total of 22 patients (25%) suffered an adverse outcome. The incidence of adverse outcome was similar between the two groups (univentricular 31.6%, biventricular 22.9%; p = .484). Death and transplantation were the most serious adverse outcomes. Five (5.6%) patients died and two (2.2%) underwent heart transplantation.
TABLE 4.
Adverse outcomes.
| Total (n = 89) | Univentricular (n = 19) | Biventricular (n = 70) | |
|---|---|---|---|
| Worsening ventricular function | 5 (6%) | 0 (0%) | 5 (7%) |
| Upgrade to multisite pacing | 7 (8%) | 0 (0%) | 7 (12%) |
| Death | 5 (6%) | 2 (11%) | 3 (4%) |
| Worsening NYHA class | 3 (3%) | 3 (16%) | 0 (0%) |
| Heart transplant | 2 (2%) | 1 (5%) | 1 (1%) |
| Total | 22 (25%) | 6 (32%) | 16 (23%) |
Note: The type and incidence of adverse outcomes in the two groups.
The Kaplan–Meier survival curve demonstrates similar freedom from adverse outcomes for both groups (Figure 1; 95% CIs represented by the dotted lines). Most adverse events occur within the first 8 years after pacemaker implantation.
FIGURE 1.
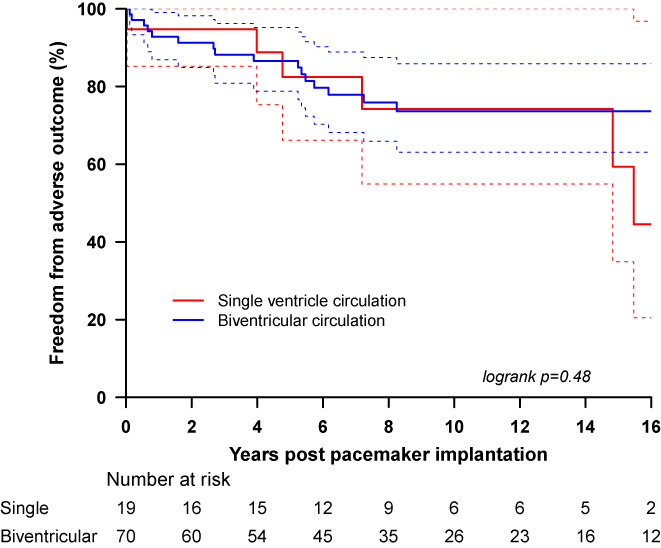
Sixteen‐year postpacemaker implantation adverse event‐free survival for the univentricular and the biventricular groups.
3.3. Predictors of adverse outcomes
Univariate analysis identified five predictors of adverse outcomes in the patients with a complex biventricular circulation. No predictors of adverse outcome were identified in the single ventricle circulation. The results of the univariate analysis are described in Table 5.
TABLE 5.
Univariate analysis.
| Variables | Total (n = 89) | Univentricular (n = 19) | Biventricular (n = 70) | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | p‐value | HR (95% CI) | p‐value | HR (95% CI) | p‐value | |
| Circulation | ||||||
| Univentricular | 1 | .484 | ||||
| Biventricular | 0.72 (0.28–1.83) | |||||
| Gender | ||||||
| Male | 1 | .018 | 1 | .464 | 1 | .036 |
| Female | 2.74 (1.15–6.55) | 1.87 (0.34–10.35) | 2.82 (1.02–7.76) | |||
| Systemic ventricle | ||||||
| Morphologic right | 1 | .004 | 1 | .165 | 1 | .018 |
| Morphologic left | 0.30 (0.13–0.71) | 0.24 (0.03–2.10) | 0.32 (0.12–0.87) | |||
| Genetic condition | ||||||
| No | 1 | .289 | 1 | .341 | ||
| Yes | 0.35 (0.05–2.64) | 0.39 (0.05–2.94) | ||||
| Heterotaxy | ||||||
| No | 1 | .788 | 1 | .866 | 1 | .913 |
| Yes | 1.16 (0.39–3.43) | 1.16 (0.21–6.39) | 1.09 (0.25–4.78) | |||
| Age at pacing (years) | ||||||
| Per unit decrease | 0.92 (0.83–1.01) | .088 | 0.95 (0.75–1.19) | .651 | 0.92 (0.82–1.02) | .122 |
| Weight at initial pacing | ||||||
| Per unit decrease | 0.99 (0.96–1.02) | .587 | 0.97 (0.90–1.04) | .398 | 0.99 (0.96–1.03) | .723 |
| Age at first CHD operation | ||||||
| Per unit decrease | 0.76 (0.66–0.88) | <.001 | 0.78 (0.06–9.53) | .844 | 0.74 (0.64–0.86) | <.001 |
| Number of CHD operations | ||||||
| Per unit increase | 1.50 (1.15–1.97) | .003 | 1.87 (0.73–4.79) | .196 | 1.46 (1.08–1.97) | .015 |
| Number of pacemaker operations | ||||||
| Per unit increase | 0.66 (0.41–1.05) | .078 | 0.52 (0.21–1.30) | .161 | 0.70 (0.41–1.20) | .193 |
| Pacing indication | ||||||
| Postop complete AV block | 1 | .723 | 1 | .193 | 1 | .897 |
| Congenital complete AV block | 0.72 (0.09–5.60) | 1.15 (0.15–9.10) | ||||
| Sinus node dysfunction | 2.13 (0.68–6.67) | 1.09 (0.14–8.63) | ||||
| Second degree AV block | 1.48 (0.42–5.28) | 1.71 (0.46–6.33) | ||||
| Intermittent complete AV block | 1.74 (0.22–13.39) | 2.07 (0.26–16.36) | ||||
| Atrial tachyarrhythmia | 2.65 (0.34–20.65) | 2.63 (0.33–20.90) | ||||
| Pacing lead position | ||||||
| Epicardial | 1 | .939 | 1 | 1 | 1 | .906 |
| Transvenous | 0.92 (0.12–6.93) | 1.13 (0.15–8.57) | ||||
| Pacing system | ||||||
| Atrial | 1 | .285 | 1 | .453 | 1 | .194 |
| Ventricular | 0.64 (0.13–3.14) | 0.65 (0.08–5.32) | ||||
| Dual chamber | 0.38 (0.08–1.69) | 0.42 (0.04–4.26) | 0.29 (0.04–2.35) | |||
| Percentage of ventricular pacing | ||||||
| <50 | 1 | .839 | 1 | .766 | 1 | .533 |
| >50 | 1.10 (0.43–2.82) | 0.78 (0.16–3.90) | 1.49 (0.42–5.22) | |||
| Ventricle paced | ||||||
| Subpulmonary | 1 | .426 | 1 | .198 | ||
| Systemic | 0.70 (0.29–1.69) | 0.50 (0.17–1.47) | ||||
| V lead position in the >50% VP | ||||||
| Apical | 1 | .039 | 1 | .647 | 1 | .034 |
| Nonapical | 2.95 (1.01–8.63) | 1.76 (0.15–20.11) | 3.39 (1.02–11.26) | |||
| Paced QRS width (ms) | ||||||
| <140 | 1 | .296 | 1 | .689 | ||
| ≥140 | 0.57 (0.19–1.66) | 0.79 (0.24–2.56) | ||||
| Complications postimplantation | ||||||
| No | 1 | .238 | 1 | .41 | ||
| Yes | 0.32 (0.04–2.37) | 0.44 (0.06–3.31) | ||||
| Reoperation within a month | ||||||
| No | 1 | .513 | 1 | .663 | ||
| Yes | 0.52 (0.07–3.86) | 0.64 (0.08–4.85) | ||||
Note: Univariate analysis for identification of predictors of adverse outcome in the two groups.
Bold indicates p value <.05, demonstrating statistical significance.
The predictors of adverse outcome in the paced children with a complex biventricular circulation were the right morphologic ventricle as the systemic ventricle (p = .018), young age at first CHD operation (p < .001), number of CHD operations (the higher the number of operations, the higher the risk of an adverse outcome) (p = .015), and female gender (p = .036). Lastly, of the patients who received a high percentage ventricular pacing, the nonapical lead position was associated with a higher risk of an adverse outcome. The estimated hazard ratio for an adverse outcome was 3.39 (95% CI 1.02–11.26, p = .034).
We did not demonstrate a difference in survival between the high percentage ventricular pacing group and the low percentage group when all the patients were considered. Both the high and low ventricular percentage pacing groups have a similar incidence of adverse outcomes (high ventricular percentage pacing group 25.8%; low ventricular percentage pacing group 22.2%, p = .839). The Kaplan–Meier survival curve demonstrates similar freedom from adverse outcomes for both groups (Figure 2). Comparison of the high and low percentage ventricular pacing in children with a univentricular repair revealed similar incidence of adverse events (30% vs 33.3%; p = .77). For the children with a complex biventricular circulation, analysis revealed a higher incidence of adverse events in the high ventricular percentage pacing group (25% vs 16.7%), but the difference did not reach statistical significance (p = .53). However, when focusing on the high percentage ventricular pacing patients for both the univentricular and the complex biventricular groups, there was an obvious difference in survival with worse outcomes in the patients with a nonapical ventricular lead position (HR 2.95 [1.01–8.63], p = .039). This was demonstrated both by the univariate analysis (Table 5) and the Kaplan–Meier survival curve (Figure 3). This difference did not reach statistical significance when examining exclusively the univentricular group of patients, but for the biventricular group, there a threefold increase in the risk of adverse outcomes was demonstrated (HR 3.39 [1.02–11.26], p‐value .034).
FIGURE 2.
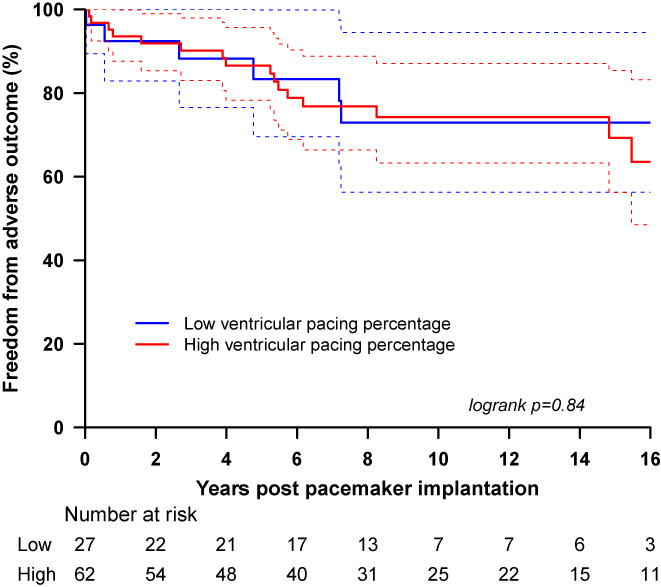
Sixteen‐year postpacemaker implantation adverse event‐free survival for all the patients (with a univentricular or a biventricular circulation) according to the requirement of high percentage ventricular pacing (≥50%) or low percentage ventricular pacing (<50%).
FIGURE 3.
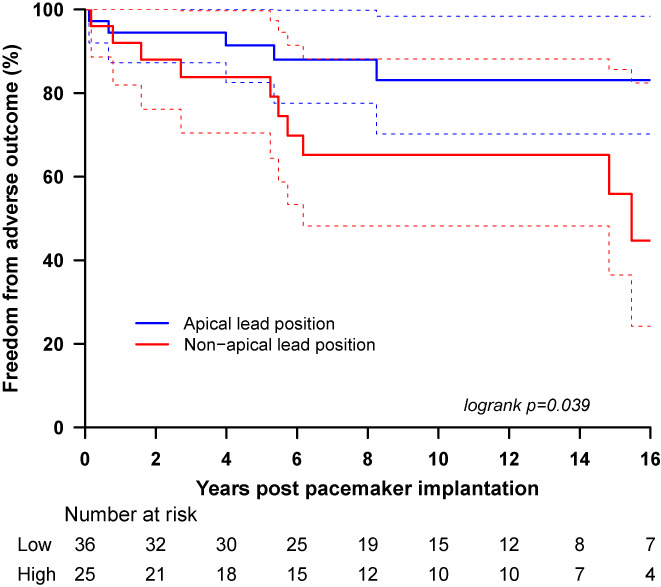
Sixteen‐year postpacemaker implantation adverse event‐free survival for all the patients requiring high percentage ventricular pacing divided in two groups; the group with an apical lead position (epicardial) and the group with any position outside the ventricular apex (epicardial).
4. DISCUSSION
Chronic pacing and high ventricular pacing percentage (greater than 50%) has been identified as a risk factor for adverse outcomes (death or transplantation) in children with a univentricular circulation. 4 The majority of those patients had received their first pacemaker after the Fontan completion, as in our population. The Pediatric Heart Network's Fontan Cross‐Sectional Study similarly reported that paced Fontan patients had a worse functional status and decreased ventricular systolic function compared to those without a pacemaker. 3 However, most of their patients were not ventricular paced. These findings raised the question of whether the requirement for a pacemaker is a surrogate marker for the severity of disease or whether pacing in itself affects the ventricular function and clinical outcomes. Poh and colleagues 1 addressed this question by propensity‐matching Fontan patients with existing risk factors of late mortality to assess the impact of pacing without the confounding factors of disease severity. This large study from the Australia and New Zealand Fontan Registry showed that chronic ventricular pacing in a Fontan circulation resulted in a significant risk of death and transplantation. In summary, it has been shown that in patients with a univentricular circulation, the need for a pacemaker is a risk factor for a less favorable outcome, 1 , 2 , 3 , 4 though the mechanism or cause is unclear. To further elucidate the relationship between outcome and pacemaker implantation, we looked into comparing the outcomes of pacing in children with complex CHD with a biventricular circulation to the children with a univentricular circulation.
In our experience, children with a complex biventricular circulation who require long‐term pacing are at higher risk of adverse outcomes too. Despite the interest in outcomes of pacing in patients with a single ventricle circulation, there is not much data available on the outcomes of pacing in the biventricular patients. A large study by Midha and colleagues 7 showed that patients with severe structural heart disease (which included single and biventricular circulations) have a very high risk of late device‐related complications. An older pediatric study by Smerup and colleagues 8 found an association between lower age at implantation of the first pacemaker system and the presence of epicardial leads as predictors of mortality and failure of the first pacemaker systems. Our study is the first one to compare the clinical outcomes of pacing in children with complex CHD with a biventricular circulation to the children with univentricular circulation. We included exclusively patients who had their pacemaker implanted before the age of 18. In our biventricular group, we included children with major CHD. These patients face similar challenges 9 as the patients with a single ventricle. They too have underlying CHD that predisposes them to ventricular dysfunction (i.e., patients with congenitally corrected transposition of the great arteries, patients with chronically volume or pressure‐loaded ventricles); they require multiple sternotomies leading to extensive adhesions and often ventriculotomies causing ventricular scarring.
Therefore, it was not surprising that the incidence of adverse outcomes was very high (24.7% in total) and similar in both groups (univentricular 31.6%, biventricular 22.9%) A higher percentage of patients suffered death or underwent heart transplantation in the univentricular group (15.8%) compared to the biventricular group (5.7%), but a meaningful comparison was not possible due to the small size of the univentricular group compared to the biventricular one. The survival curves demonstrate that the majority of the adverse outcomes occur within the first 8 years of pacemaker implantation for both groups, indicating that they already occur in childhood.
A total of 96% of the pacemaker systems in our study were epicardial. It is established practice to avoid endocardial pacing leads in the univentricular circulation to decrease the risk of pulmonary and systemic thrombosis and embolism. 10 In our center, we routinely opt for epicardial pacemaker systems for the patients with a biventricular circulation too, as evident by the low incidence of endocardial leads (6%). Epicardial placement of pacing leads is the preferred approach by many even for the children with a biventricular circulation, for weights less than 15 kg. This is mainly due to concerns regarding stretching of the lead with growth, venous stenosis or occlusion and risks of future lead extraction. 11 Epicardial leads have the additional advantage of more options with regard to lead positioning on the paced ventricle. Optimal lead positioning in chronic pacing is crucial, even for children with structurally normal hearts. As Janoušek and colleagues demonstrated in a multicenter pediatric study, the left ventricular apical and left ventricular lateral wall pacing are associated with the best preservation of ventricular contraction. 12 Their findings were further supported by a previous large retrospective pediatric multicenter survey 13 and previous studies that showed preservation of left ventricular function with left ventricular apical or left ventricular lateral wall pacing. 14 , 15 , 16 , 17 , 18 , 19
A major finding of our study was that the nonapical ventricular lead position was associated with a threefold increase in the risk of adverse outcomes for the children with a complex biventricular circulation. This was not surprising given the findings of other studies on optimal lead positioning, 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 but we believe our study to be the first to demonstrate the importance of this for children with a biventricular CHD. The ventricular lead position was the only modifiable predictor of adverse outcome and although an apical position may not always be achievable due to extensive adhesions and the presence of ventricular scar, this finding stresses the importance of aiming for the apex at implantation of epicardial leads.
Another important finding of the study was that the right morphologic ventricle as the systemic ventricle was a predictor of adverse outcomes in a biventricular circulation. It is well established that children with congenitally corrected transposition of the great arteries are at high risk of adverse outcomes, even after the anatomic repair. 21 , 22 A number of studies have attempted to investigate whether biventricular pacing is beneficial in these patients. The guidelines are cautious with biventricular pacing recommendations as these studies are small and in addition, there is a lack of long‐term outcomes, 23 though the outcomes of our study shows the importance of considering an alternative approach.
Other predictors of adverse outcomes were the age and the number of CHD operations. This indicates that the younger the patient at the time of the first CHD operation (not pacemaker insertion) and the higher number of CHD operations (excluding pacemaker‐related procedures) increase the risk. Lastly, female gender was identified as a risk factor in our cohort.
Interestingly, the percentage of ventricular pacing itself as a single factor did not predict outcomes for either the single or biventricular patients in our study. This may be a reflection of the dominance of other confounding factors that increase the risk of adverse events in children with complex CHD. The patients in both the univentricular and biventricular groups had major CHD with complex surgical history; the average number of CHD operations in the univentricular group was five while in the biventricular group was two but with some patients undergoing up to eight separate CHD operations. It is also possible that with good selection of an epicardial pacing site, the effect of ventricular pacing on adverse outcomes might be significantly reduced.
No predictors of adverse outcome were identified for the single ventricle patients. It is possible that the small cohort of patients with follow up longer than 8 years may have limited our ability to reach statistical significance and therefore identify predictors.
Lastly, we had a high incidence of complications (11%) postpacemaker implantation. This is in accordance with the reported incidence of complications in CHD in other studies. Similar to our findings, Midha and colleagues reported a 10‐year cumulative risk of device complications of 12.5%. 7
5. LIMITATIONS
This was a retrospective study performed in a single tertiary center. As with most exclusively pediatric studies, it was limited by the small number of patients, particularly for the univentricular circulation group. Therefore, a large multicenter study would be required to identify predictors of adverse outcomes in this group.
6. CONCLUSIONS
Children with a pacemaker and a complex biventricular circulation have similar adverse outcomes and survival as the children with a pacemaker and a univentricular circulation. This study identified specific predictors of adverse outcomes in the biventricular group like age at first surgical intervention and a right systemic ventricle. Onset of these complications was predominantly within the first 8 years of follow up. The only easily modifiable predictor was the epicardial lead position on the paced ventricle, stressing the importance of good apical lead placement.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts to disclose.
Spentzou G, Taylor L, Zhang Y, D’Udekem Y, Zannino D, Davis A, et al. Long‐term outcomes of pacemaker implantation in children with univentricular versus complex biventricular surgical repair. J Arrhythmia. 2023;39:207–216. 10.1002/joa3.12832
REFERENCES
- 1. Poh CL, Celermajer DS, Grigg LE, Kalman JM, McGuire MA, Gentles TL, et al. Pacemakers are associated with a higher risk of late death and transplantation in the Fontan population. Int J Cardiol. 2019;282:33–7. [DOI] [PubMed] [Google Scholar]
- 2. Poh CL, d'Udekem Y. Life after surviving Fontan surgery: a meta‐analysis of the incidence and predictors of late death. Heart Lung Circ. 2018;27:552–9. [DOI] [PubMed] [Google Scholar]
- 3. Williams RV, Travison T, Kaltman JR, Cecchin F, Colan SD, Idriss SF, et al. Comparison of Fontan survivors with and without pacemakers: a report from the Pediatric Heart Network Fontan cross‐sectional study. Congenit Heart Dis. 2013;8:32–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bulic A, Zimmerman FJ, Ceresnak SR, Shetty I, Motonaga KS, Freter A, et al. Ventricular pacing in single ventricles—a bad combination. Hear Rhythm. 2020;14:853–7. [DOI] [PubMed] [Google Scholar]
- 5. Barsheshet A, Moss AJ, McNitt S, Jons C, Glikson M, Klein HU, et al. Long‐term implications of cumulative right ventricular pacing among patients with an implantable cardioverter‐defibrillator. Heart Rhythm. 2011;8(2):212–8. [DOI] [PubMed] [Google Scholar]
- 6. Steinberg JS, Fischer A, Wang P, Schuger C, Daubert J, McNitt S, et al. The clinical implications of cumulative right ventricular pacing in the multicenter automatic defibrillator trial II. J Cardiovasc Electrophysiol. 2005;16(4):359–65. [DOI] [PubMed] [Google Scholar]
- 7. Midha D, Chen Z, Jones DG, Williams HJ, Lascelles K, Jarman J, et al. Pacing in congenital heart disease—a four‐decade experience in a single tertiary centre. Int J Cardiol. 2017;241:177–81. [DOI] [PubMed] [Google Scholar]
- 8. Smerup M, Hjertholm T, Johnsen SP, Pedersen AK, Hansen PS, Mortensen PT, et al. Pacemaker implantation after congenital heart surgery: risk and prognosis in a population‐based follow‐up study. Eur J Cardiothorac Surg. 2005. Jul;28(1):61–8. [DOI] [PubMed] [Google Scholar]
- 9. O'Leary ET, Gauvreau K, Alexander ME, Banka P, Bezzerides VJ, Fynn‐Thompson F, et al. Dual‐site ventricular pacing in patients with Fontan physiology and heart block: does it mitigate the detrimental effects of single‐site ventricular pacing? JACC Clin Electrophysiol. 2018;4(10):1289–97. [DOI] [PubMed] [Google Scholar]
- 10. Cohen MI, Vetter VL, Wernovsky G, Bush DM, Gaynor JW, Iyer VR, et al. Epicardial pacemaker implantation and follow‐up in patients with a single ventricle after the Fontan operation. J Thorac Cardiovasc Surg. 2001;121(4):804–11. [DOI] [PubMed] [Google Scholar]
- 11. McLeod KA. Cardiac pacing in infants and children. Heart. 2010;96(18):1502–8. [DOI] [PubMed] [Google Scholar]
- 12. Janoušek J, van Geldorp IE, Krupičková S, Rosenthal E, Nugent K, Tomaske M, et al. Permanent cardiac pacing in children: choosing the optimal pacing site: a multicenter study. Circulation. 2013;127(5):613–23. [DOI] [PubMed] [Google Scholar]
- 13. van Geldorp IE, Delhaas T, Gebauer RA, Frias P, Tomaske M, Friedberg MK, et al. Impact of the permanent ventricular pacing site on left ventricular function in children: a retrospective multicentre survey. Heart. 2011;97(24):2051–5. [DOI] [PubMed] [Google Scholar]
- 14. Vanagt WY, Verbeek XA, Delhaas T, Mertens L, Daenen WJ, Prinzen FW. The left ventricular apex is the optimal site for pediatric pacing: correlation with animal experience. Pace. 2004;27:837–43. [DOI] [PubMed] [Google Scholar]
- 15. Vanagt WY, Verbeek XA, Delhaas T, Gewillig M, Mertens L, Wouters P, et al. Acute hemodynamic benefit of left ventricular apex pacing in children. Ann Thorac Surg. 2005;79:932–6. [DOI] [PubMed] [Google Scholar]
- 16. van Geldorp IE, Vanagt WY, Bauersfeld U, Tomaske M, Prinzen FW, Delhaas T. Chronic left ventricular pacing preserves left ventricular function in children. Pediatr Cardiol. 2009;30:125–32. [DOI] [PubMed] [Google Scholar]
- 17. Tomaske M, Breithardt OA, Bauersfeld U. Preserved cardiac synchrony and function with single‐site left ventricular epicardial pacing during mid‐term follow‐up in paediatric patients. Europace. 2009;11:1168–76. [DOI] [PubMed] [Google Scholar]
- 18. Gebauer RA, Tomek V, Kubus P, Rázek V, Matejka T, Salameh A, et al. Differential effects of the site of permanent epicardial pacing on left ventricular synchrony and function in the young: implications for lead placement. Europace. 2009;11:1654–9. [DOI] [PubMed] [Google Scholar]
- 19. Silvetti MS, Muzi G, Unolt M, D'Anna C, Saputo FA, Di Mambro C, et al. Left ventricular (LV) pacing in newborns and infants: Echo assessment of LV systolic function and synchrony at 5‐year follow‐up. Pacing and Clinical Electrophysiology. 2020;43(6):535–41. [DOI] [PubMed] [Google Scholar]
- 20. Kodama Y, Kuraoka A, Ishikawa Y, Nakamura M, Ushinohama H, Sagawa K, et al. Outcome of patients with functional single ventricular heart after pacemaker implantation: what makes it poor, and what can we do? Heart Rhythm. 2019;16(12):1870–4. [DOI] [PubMed] [Google Scholar]
- 21. Lenoir M, Bouhout I, Gaudin R, Raisky O, Vouhé P. Outcomes of the anatomical repair in patients with congenitally corrected transposition of the great arteries: lessons learned in a high‐volume Centre. Eur J Cardiothorac Surg. 2018;54(3):532–8. [DOI] [PubMed] [Google Scholar]
- 22. Brizard CP, Lee A, Zannino D, Davis AM, Fricke TA. Long‐term results of anatomic correction for congenitally corrected transposition of the great arteries: a 19‐year experience. J Thorac Cardiovasc Surg. 2017;154:256–65. [DOI] [PubMed] [Google Scholar]
- 23. Kharbanda RK, Moore JP, Taverne YJHJ, Bramer WM, Bogers AJJC, de Groot NMS. Cardiac resynchronization therapy for the failing systemic right ventricle: a systematic review. Int J Cardiol. 2020;318:74–81. [DOI] [PubMed] [Google Scholar]


