Abstract
Background:
Autologous matrix-induced chondrogenesis (AMIC) for the treatment of osteochondral lesions of the talus (OLT) results in favorable clinical outcomes, yet high reoperation rates. The aim of this study was to report and analyze typical complications and their risk factors after AMIC for OLT.
Methods:
A total of 127 consecutive patients with 130 AMIC procedures for OLT were retrospectively assessed. All AMIC procedures were performed in an open fashion with 106 (81.5%) cases requiring a malleolar osteotomy (OT) to access the OLT. Seventy-one patients (54.6%) underwent subsequent surgery. These cases were evaluated at a mean follow-up of 3.1 years (±2.5) for complications reviewing postoperative imaging and intraoperative findings during revision surgery. Six patients (8.5%) were lost to follow-up. Regression model analysis was conducted to identify factors that were associated with AMIC-related complications.
Results:
Among the 65 (50%) patients who required revision surgery, 18 patients (28%) demonstrated AMIC-related complications with deep fissuring (83%) and thinning (17%) of the AMIC graft. Conversely, 47 patients (72%) underwent subsequent surgery due to AMIC-unrelated reasons including isolated removal of symptomatic hardware (n = 17) and surgery addressing concomitant pathologies with (n = 25) and without hardware removal (n = 5). Previous prior cartilage repair surgery was significantly associated with AMIC graft-associated complications in patients undergoing revision surgery (P = .0023). Among age, body mass index, defect size, smoking, and bone grafting, smoking was the only factor showing statistical significance with an odds ratio of 3.7 (95% CI 1.24, 10.9; P = .019) to undergo revision surgery due to graft-related complications, when adjusted for previous cartilage repair surgery.
Conclusion:
The majority of revision surgeries after AMIC for OLT are unrelated to the performed AMIC graft but frequently address symptomatic hardware and concomitant pathologies. Both smoking and previous cartilage repair surgery seem to significantly increase the risk of undergoing revision surgery due to AMIC-related complications.
Level of evidence:
Level IV, case series.
Keywords: cartilage repair, bone marrow stimulation, autologous matrix-induced chondrogenesis, AMIC, talus, ankle
Introduction
Osteochondral lesions of the talus (OLTs) may be caused by trauma, ankle instability, or osteochondritis dissecans (OD).12,43,44 Although nonoperative management of these lesions can result in favorable outcomes in selected patients,42 operative treatment often becomes necessary because of the poor regeneration potential of human hyaline cartilage.37 In addition to cartilage surgery, associated pathoanatomic morphologies are known causes for cartilage lesions and thus have to be addressed during treatment.1 To address the OLT, various treatment options exist including simple debridement with or without microfracturing,13 osteochondral autograft transfer,19 osteochondral allograft transplantation,31 autologous chondrocyte implantation (ACI),3 and autologous matrix-induced chondrogenesis (AMIC).7
The latter combines bone marrow stimulation of microfracturing and the augmentation of a collagen type I/III bilayer membrane to contain the subchondral bleeding and provide a matrix for repair tissue maturation.18 This technique has several benefits over other cartilage restoration methods as it is a single-step procedure without the risk of donor site morbidity, which makes it advantageous to ACI or osteochondral allograft transplantation, showing improved outcomes compared to microfracture, especially in larger lesions.17,25,28 Reported mid- to long-term results after AMIC for OLT are favorable, yet with reoperation rates ranging from 5% to 58%, with the majority undergoing symptomatic hardware removal after malleolar osteotomy.2,4,10,16,24,27,41 However, a systematic analyzation of complications after AMIC for the treatment of OLT, particularly distinguishing between AMIC graft-related and unrelated complications, is still missing.
The purpose of this study, therefore, was to report and analyze typical complications and their risk factors after AMIC for OLT. It was hypothesized that patient- and lesion-specific factors lead to an increased rate of complications and subsequent surgery.
Materials and Methods
Ethical approval was granted by the local research ethics committee and all patients included in this study gave their informed consent.
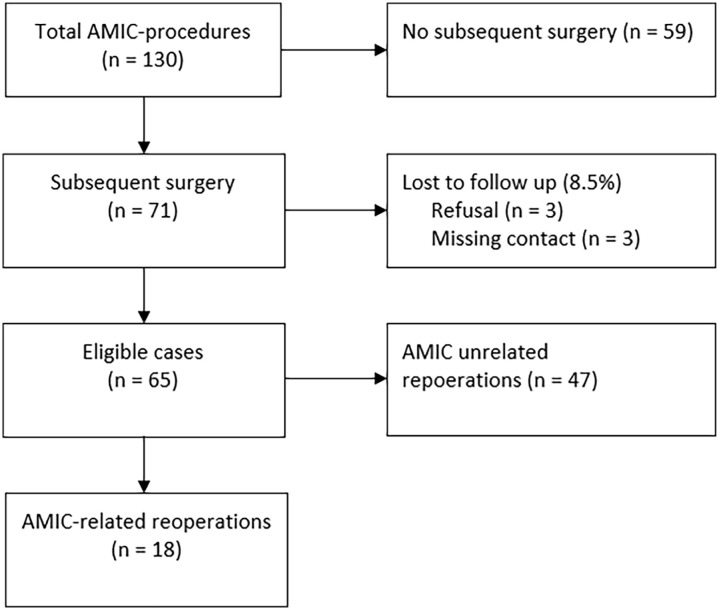
Data are regularly collected and stored for patients undergoing elective surgery at our institution. A total of 127 patients (130 ankles) who underwent cartilage repair with AMIC for isolated OLT between 2008 and 2018 were identified. The treatment with AMIC was indicated in patients with symptomatic focal full-thickness chondral or osteochondral defects of the talus and failed conservative management, which was initiated for a minimum of 3-6 months (Figure 1). Contraindications comprised inflammatory arthritis, advanced osteoarthritis, and infection. A total of 71 patients (54%) undergoing subsequent surgery were identified. Three of them refused to participate in the study and 3 could not be contacted and, hence, were excluded from the study (loss to follow-up, 8.5%). The remaining 65 patients were included in the study and evaluated for patient factors, lesion morphology and location, details of complication, follow-up imaging, and intraoperative findings during revision and treatment (Figure 2). Radiographic and macroscopic graft morphology during revision surgery was analyzed and evaluated.
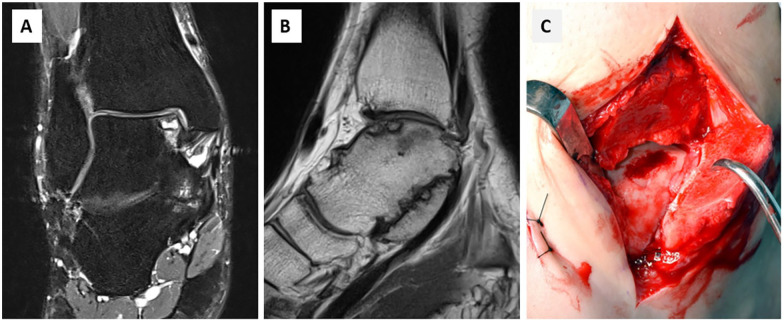
Figure 1.
(A, B) Preoperative coronal and sagittal T2- and T1-weighted magnetic resonance image of the ankle of a 38-year-old female patient showing a large OLT on the medial talar dome. (C) Intraoperative view after medial malleolar osteotomy with a defect with intact border of cartilage suitable for autologous matrix-induced chondrogenesis (AMIC) technique.
Figure 2.
Flowchart of study participants.
Clinical and Radiographic Assessment
Clinical notes, operative reports, and available radiographic imaging were reviewed to determine patient’s age at the time of surgery, sex, body mass index (BMI), smoking status, defect size, lesion location, autologous bone grafting, complications, and reoperations. Radiographic analysis was based on magnetic resonance imaging or Arthro-CT for preoperative defect size, and the diameter of the lesion was measured at the largest point in the coronal and sagittal planes. Magnetic resonance imaging included T1-weighted sequence and fat-suppressed T2-weighted images. The lesion area was calculated by use of the elliptical area formula (sagittal length × coronal length × 0.79) as described by Choi et al.9
Surgical Technique
All AMIC procedures were performed in an open fashion. A malleolar osteotomy was necessary in 106 ankles (81.5%) through a medial (n = 94), lateral (n = 12), or bilateral (n = 1) approach. In the remaining ankles an anteromedial (n = 13) or anterolateral (n = 10) approach without the need for osteotomy was used.
After identifying the OLT, the defect was carefully debrided followed by creating a defect with vertical walls. Loose and fragmented necrotic/cystic bone was excised until vital and stabile bone tissue was visible. Then, microfracturing of the bone was achieved by drilling with an awl or K-wire (1.2-mm-diameter; DePuy Synthes, Oberdorf, Switzerland). In case of a large bony defect, the joint surface of the talus was reconstructed with autologous spongiosa graft from the ipsilateral distal tibia (n = 74), proximal tibia ( = 4), calcaneus (n = 4), or distal fibula (n = 1). Allograft (Tutoplast; Novomedics GmbH, Zurich, Switzerland) was used in 3 cases. Thereafter, the bilayer type I/III collagen matrix (Chondro-Gide; Geistlich Pharma AG, Wolhusen, Switzerland) was cut to fit the defect and laid on the lesion. After complete coverage of the defect, surgical fibrin glue was applied to secure the membrane to the adjacent cartilage (Tissucol Duo S; Baxter International Inc, Deerfield, IL) (Figure 3). To ensure stable membrane fixation, the ankle was brought through full range of motion. Concomitant corrective surgery for deformity or instability was performed as indicated. The malleolar osteotomy was fixed with two 3.5-cm titanium screws (medial) or compression plating (lateral).
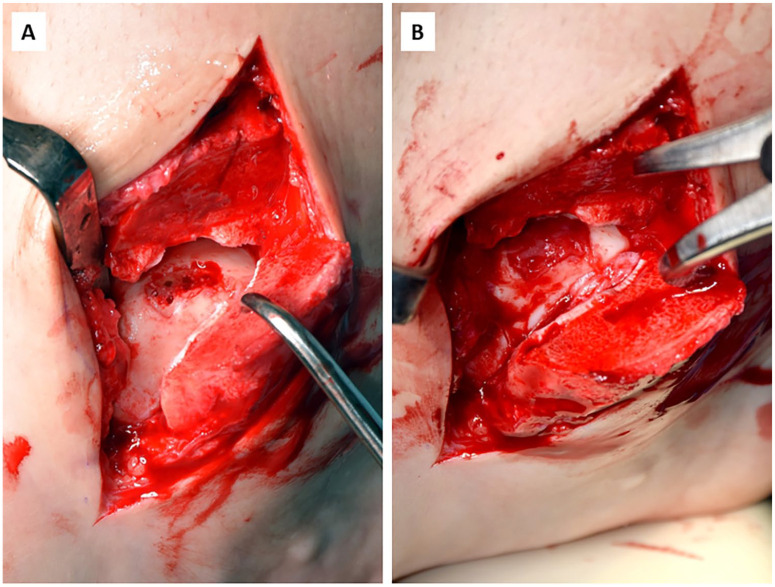
Figure 3.
(A) Osteochondral defect of the talus after debridement of loose cartilage fragments and microdrilling creating a well-bordered defect area with vital subchondral bone. (B) Complete cartilage repair after fixation of the bilayer type I/III collagen matrix with fibrin glue.
Postoperative Rehabilitation
Postoperative passive range of motion exercises were initiated after the first change of dressing. Nonweightbearing for 6 weeks was prescribed, followed by progression to weightbearing after a clinical and radiologic follow-up in the outpatient clinic 6 weeks after surgery. Low-impact sports were allowed 12 weeks and contact sports 6 month postoperatively.
Definition of AMIC-Related Complication Undergoing Revision
Patients who presented with persistent ankle pain localized over the cartilage repair without any other plausible cause of symptoms in combination with radiologic or intraoperative signs of graft anomalies or insufficiency.
Statistical Analysis
Sociodemographic and clinical characteristics of patients were determined using descriptive statistics. Data are presented as mean ± SD. Chi-square test and bi- and multivariate regression analyses were performed to assess associations between patient- and lesion-specific factors and revision surgery due to AMIC-related complications. Presented odds ratios (ORs) are accompanied by 95% CIs. All statistical analyses were performed in SPSS for Mac (version 23.0; SPSS Inc, Chicago, IL). Significance was set at P <.05.
Results
The 127 patients (130 ankles) underwent surgery at a mean age at the time of surgery of 35.0 ± 2.1 years and a BMI averaging 27.0 ± 3.3. Of these, 19 cases underwent concomitant ligament reconstruction (14.6%), 11 cases had osseous corrective procedures (8.5%), 5 patients received soft tissue surgery (3.9%), and 9 cases had a combination of these procedures (6.9%) because of ankle instability, foot malalignment, tendon or ligament injuries, ganglions, or other concomitant pathologies (Table 1). Although 41 ankles (31.5%) had prior surgery, of which 27 (65.9%) had cartilage surgery (1 debridement, 10 microdrilling, 3 retrograde drilling, 4 microfracturing, 4 spongiosa autografts, 1 iliac bone graft, 1 AMIC, and 3 OLT refixation), the remaining 89 ankles (68.5%) received primary talar AMIC.
Table 1.
Patient and lesion characteristics.
| Variable | AMIC (n=130) |
|---|---|
| Female sex, n (%) | 54 (41.5) |
| Smoker, n (%) | 45 (34.6) |
| Side, right/left, n | 75/55 |
| Lesion size, mm2, mean ± SD | 83 ± 58 |
| OLT/OCD, n | 115/15 |
| Lesion location, n (%) | |
| Medial | 107 (82.3) |
| Lateral | 22 (16.9) |
| Bilateral | 1 (0.8) |
| Bone grafting, n (%) | 86 (66.2) |
| Concomitant surgery, n (%) | |
| Ankle stabilization | 27 (20.7) |
| Corrective osteotomies | 12 (9.2) |
| Soft tissue and tendon procedures | 6 (4.6) |
| Previous surgery, n (%) | 41 (31.5) |
| Cartilage surgery | 27 (20.7) |
Abbreviations: AMIC, autologous matrix-induced chondrogenesis; OCD, osteochondrosis dissecans; OLT, osteochondral lesion of the talus; R/L, right/left ankle.
Reoperations and Complications
The reviewed cohort consisted of 65 (50%) patients undergoing subsequent surgery after a mean of 22.9 ± 28.5 months.
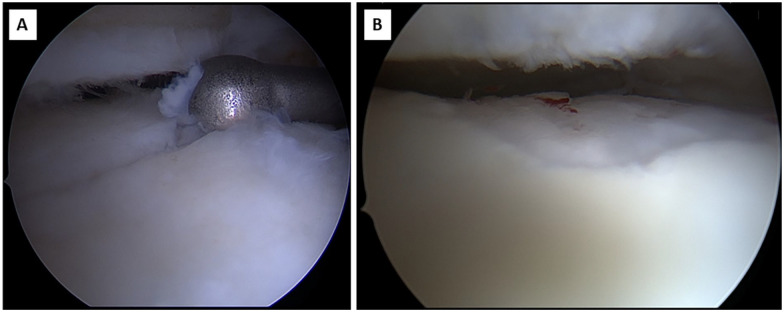
Revision surgery for AMIC-related complications was indicated in 18 cases (27.7%) at a mean follow-up of 3.7 ± 2.8 years. Macroscopic evaluation during revision surgery demonstrated deep fissuring of the fibrocartilage (83%) and thinning (17%) of the AMIC graft (Figure 4). Revision surgery included predominantly re-AMIC or arthroscopic microfracturing (56%), screw removal and steroid infiltration for symptom relief (17%), debridement (17%), total ankle replacement (6%), and arthrodesis (6%). Corrective osteotomy was performed in 1 patient with persistent hindfoot varus malalignment despite lateral sliding calcaneus osteotomy, 1 for an acquired planovalgus, which was not evident during the initial treatment, and 1 with only minor hindfoot varus that was not corrected during the initial surgery (Table 2).
Figure 4.
Typical morphology of AMIC graft complication with (A) deep fissuring and (B) thinning.
Table 2.
Detailed Graft-Associated Complications.
| Patient | Age (y) | BMI | Smoking | Defect Location | Lesion Size (mm2) | Isolated AMIC | Malleolar OT and/or Additional Procedures | Spongiosa Autografting | Graft Complication |
Reoperations |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 37 | 40.9 | No | Medial | 44 | Yes | Malleolar OT | Yes | Fissuring | Screw removal, infiltration |
| 2 | 47 | 25.4 | Yes | Medial | 152 | Yes | Malleolar OT | Yes | Fissuring | TAR |
| 3 | 32 | 24.8 | Yes | Medial | 175 | Yes | Malleolar OT | Yes | Fissuring | Debridement |
| 4 | 25 | 24.1 | Yes | Medial | 237 | Yes | Malleolar OT | Yes | Fissuring | Re-AMIC |
| 5 | 34 | 31.6 | Yes | Medial | 142 | Yes | Malleolar OT | Yes | Fissuring | Arthrodesis |
| 6 | 19 | 18.8 | Yes | Medial | 87 | Yes | Malleolar OT | No | Fissuring | Screw removal, infiltration |
| 7 | 29 | 22.7 | Yes | Medial | 51 | Yes | Malleolar OT | Yes | Thinning | Corrective supramalleolar OT, arthroscopy, MF |
| 8 | 36 | 29.8 | Yes | Medial | 64 | Yes | Malleolar OT | Yes | Fissuring | Arthroscopy, MF |
| 9 | 15 | 22.4 | Yes | Medial | 152 | No | Malleolar OT, lateral ankle ligament reconstruction | Yes | Fissuring | Screw removal, infiltration |
| 10 | 20 | 21.4 | No | Medial | 79 | Yes | Malleolar OT | Yes | Fissuring | Arthroscopy, MF |
| 11 | 21 | 36.3 | No | Medial | 119 | Yes | No | Yes | Fissuring | Re-AMIC |
| 12 | 28 | 25.9 | No | Lateral | 22 | Yes | No | No | Thinning | Re-AMIC, corrective OT |
| 13 | 35 | 24.2 | No | Lateral | 40 | No | Lateral ankle ligament reconstruction | No | Fissuring | Arthroscopy, MF |
| 14 | 54 | 30.8 | Yes | Medial | 14 | No | Malleolar OT, corrective calcaneus osteotomy | Yes | Thinning | Dedridement |
| 15 | 29 | 22.8 | Yes | Medial | 269 | Yes | Malleolar OT | Yes | Fissuring | Re-AMIC |
| 16 | 43 | 30.4 | No | Medial | 35 | No | Lateral ankle ligament reconstruction | No | Fissuring | Re-AMIC, corrective OT |
| 17 | 40 | 24.1 | Yes | Medial | 19 | No | Lateral ankle ligament reconstruction | Yes | Fissuring | Debridement |
| 18 | 29 | 31.6 | Yes | Medial | 148 | No | Lateral ankle ligament reconstruction | Yes | Fissuring | Arthroscopy, MF |
Abbreviations: AMIC, autologous matrix-induced chondrogenesis; BMI, body mass index; MF, microfracturing; OLT, osteochondral lesion of the talus; OT, osteotomy; TAR, total ankle replacement.
Graft-unrelated revision was indicated in 47 (72%) cases undergoing reoperation due to symptomatic hardware removal (n = 30; 46%), hardware removal alone (n=17; 26%) or with concomitant procedures unrelated to the AMIC graft (n = 25; 38%), and other procedures unrelated to the AMIC graft (n = 5; 8%).
Failure Analysis
Of the 18 AMIC graft–associated complications, 16 cases (88.9%) presented with a medial OLT and in 14 cases (77.8%) a spongiosa autograft augmentation was performed because of the subchondral defect. Five cases (27.3%) underwent concomitant lateral ankle ligament reconstruction due to instability, and 1 patient (5.6%) underwent concomitant medializing calcaneus osteotomy. The mean defect size was 102 ± 76 mm2. Eight cases (44.4%) had a history of a prior cartilage repair surgery (5 microdrilling, 2 autografts, and 1 AMIC).
Previous prior cartilage repair surgery was significantly associated with AMIC graft–associated complications in patients undergoing revision surgery (8/18 vs 5/47; P = .0023).
Among age, BMI, defect size, smoking, and bone grafting, smoking was the only factor showing statistical significance with an OR of 4.4 (95% CI 1.527, 12.679; P = .006) to undergo revision surgery due to graft-related complications after AMIC for OLT (Table 3). This OR slightly decreased to 3.7 (95% CI 1.24, 10.9; P = .019) when adjusted for prior cartilage repair surgery.
Table 3.
Bivariate Correlation Analysis for Reoperations due to AMIC-Related Complications.
| Factors | Pearson Correlation | Significance (2-Tailed)a |
|---|---|---|
| BMI | 0.007 | .941 |
| Smoking | 0.255 | .003 |
| Age | -0.095 | .282 |
| Defect Size | -0.008 | .925 |
| Bone grafting | 0.098 | .265 |
Abbreviations: AMIC, autologous matrix-induced chondrogenesis; BMI, body mass index.
Bold indicates significance at a level of P < .05.
In a subgroup analysis of the 86 patients who underwent isolated AMIC without any concomitant procedure, 12 showed AMIC graft–related complications (Table 4). Bivariate correlation analysis indicated a significant correlation of smoking (r = 0.257; P = .017) and previous cartilage repair (r = 0.368; P < .001) with a graft-related complication after AMIC. In the multivariate regression model, previous cartilage repair was the only variable showing a significant association with AMIC graft-related complications (OR 6.7, 95% CI 1.444, 31.168; P = .015).
Table 4.
Patient and Lesion Characteristics for Isolated AMIC.
| Variable | AMIC (n=86) |
|---|---|
| Female sex, n (%) | 32 (37.2) |
| Age, y, mean ± SD | 34.8 ± 13.8 |
| BMI, mean ± SD | 26.9 ± 5.0 |
| Smoker, n (%) | 31 (36) |
| Lesion size, mm2, mean ± SD | 89 ± 61.9 |
| Bone grafting, n (%) | 64 (74.4) |
| Previous cartilage surgery, n (%) | 14 (16.3) |
| Graft-related complication, n (%) | 12 (14%) |
Abbreviations: AMIC, autologous matrix-induced chondrogenesis; BMI, body mass index.
Discussion
The main finding of this study is that revision surgeries after AMIC for OLT are predominantly caused by graft-unrelated complications, namely, symptomatic hardware removal. In the current cohort, only 14% of patients underwent revision surgery and had an evident AMIC graft-related complication. Interestingly, failure analysis revealed that smoking significantly increases the risk for AMIC-related complications requiring revision surgery adding further evidence to the existing literature on the harming effect of smoking on cartilage repair.
The AMIC technique appears effective, improving the symptoms and function of OLT and resulting in favorable mid- to long-term outcomes.21,26 However, revision surgery is frequent, with the majority of cases requiring hardware removal as patients often report pain over the screwheads after osteosynthesis of the malleolar osteotomy.41 The current study confirms these findings with a revision rate due to symptomatic hardware in 46% of all revisions. However, the current study focuses on graft-related complications, which are only 28% of all revisions. Especially in patients undergoing revision cartilage repair with AMIC, graft-associated complications occurred significantly more often.
Because of the complications of an osteotomy, it should be avoided if possible. However, malleolar osteotomy is often required in combination with the AMIC cartilage repair to obtain good exposition and achieve optimal defect coverage. Alternatively, the all-arthroscopic AMIC technique (AT-AMIC) can be performed without the need for malleolar osteotomy, yet this is an challenging procedure with a long learning curve.36 In a follow-up study of 20 patients observed for 24 months, Usuelli et al35 reported that AT-AMIC is safe and effective in treatment of OLTs, with 1 patient suffering from a hypertrophic reaction with impingement requiring repeat arthroscopy. However, the study excluded patients with prior ankle surgery and/or associated procedures. Another study by Baumfield et al5 showed similar results of 17 cases with no complications and an average AOFAS Score of 89.5 points at the last follow-up after a mean time of 10.8 months. This study also included posterior lesions, thus showing the possibility to treat defects even in usually difficult to access locations of the talus. However, the selection criteria are not reported, and therefore the application of the treatment to every case remains questionable. Furthermore, both studies reported only short- to midterm results and could therefore underestimate the complication rate after cartilage treatment. Nevertheless, in light of the findings of the current study with high reoperations rates due to symptomatic hardware after AMIC, the apparent advantage of this arthroscopic or mini-open technique has to be emphasized, but further research is warranted to assess and compare clinical results after these AMIC techniques.
The type of complication after cartilage repair depends on the technique and is unique to each specific treatment used. Microfracturing induces regeneration of the chondral layer with the production of fibrocartilaginous tissue by stimulating the subchondral bone marrow.34 The repair tissue is biomechanically inferior to native cartilage and results in graft insufficiency in long-term results.13,23 Especially in larger cartilage defects, AMIC has shown superior outcomes compared with microfracture.39 Although the mean defect size in the current study is smaller than reported in other studies investigating AMIC for talar defects,22 there is no established minimum cutoff defect size for AMIC in the talus available. Therefore, smaller defects also were treated with AMIC as an advancement of microfracturing to improve the biomechanical properties of the repair tissue.7,25 In fact, favorable outcomes have been shown after microfracturing as well as AMIC for smaller cartilage lesions of the talus.6 Furthermore, the current study reports the lesion size based on preoperative imaging, which has been shown to often underestimate articular cartilage defect size8; thus, it is likely that the actual defect size might have been slightly larger than reported.
The detailed evaluation of the current study underlines the favorable outcomes after AMIC for OLT with a low rate of graft failures at a mean follow-up of 3 years. However, typical complications resemble those seen after microfracturing, with biomechanical insufficiency showing deep fissuring and thinning of the graft. In contrast to bone marrow stimulation techniques, cell-based techniques like ACI aim to restore the native cartilage by implanting autologous hyaline cartilage tissue into the defect after culturing chondrocytes ex vivo.30,32 Typical complications after ACI, as reported by Niemeyer et al,29 differ from the current study. They describe typical graft-associated complications in 15% of cases after ACI for the knee with symptomatic hypertrophy of the transplant, disturbed fusion of the graft with the adjacent native cartilage, delamination, and graft failure. Another technique used for the treatment of OLT is the restoration of the defect using osteochondral allograft transplantation. This can result in good outcomes with restoration of the OLT with viable cartilage tissue. However, the reported complication rate is 43%, with typical complications comprising surface incongruity of transplants, uncovered area between plugs, and donor site morbidity.14
The current study also analyzed the effect of patient- and lesion-specific factors influencing graft-related complications. In the studied cohort, when adjusted for previous cartilage repair surgery, smokers were still almost 4 times as likely to undergo revision surgery due to graft-related complications after AMIC for OLT compared to nonsmokers.
Although not fully understood, smoking has multiple negative effects on postoperative regeneration such as changes in blood supply and healing potential of soft tissue.15,20,33 This finding supports the growing evidence of the negative effect of smoking after cartilage repair.15,20,33,38
The following limitations have to be acknowledged. First, this is a retrospective study with its associated biases, including infrequently documented patient-reported outcome measurements, which did not allow for a comprehensive clinical outcome analysis. Second, revision surgery was only performed in symptomatic patients and therefore intraoperative assessment of the repair tissue quality was not available in all patients. Third, because of the limited clinical value of routine imaging after cartilage repair,11,40 this study did not assess or report postoperative MR or arthro-CT imaging outcomes of asymptomatic patients. Lastly, the effect of coexisting pathologies and concomitant procedures makes it sometimes challenging to differentiate between true graft-related and nonrelated complications in these cases.
Despite these limitations, the herein presented study is an important contribution to the current literature of cartilage repair in the ankle by systematically assessing complications after AMIC for OLT in a large cohort of patients treated at a single institution.
Conclusion
The majority of revision surgeries after AMIC for OLT are unrelated to the performed AMIC graft but frequently address symptomatic hardware and concomitant pathologies. Both smoking and previous cartilage repair surgery seem to significantly increase the risk of undergoing revision surgery due to AMIC-related complications.
Footnotes
Ethical Approval: Ethical approval was granted by the local research ethics committee, and all patients included in this study gave their informed consent.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. ICMJE forms for all authors are available online.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Manuel Waltenspül, MD,  https://orcid.org/0000-0002-8192-0233
https://orcid.org/0000-0002-8192-0233
References
- 1. Ackermann J, Casari FA, Germann C, Weigelt L, Wirth SH, Viehöfer AF. Autologous matrix-induced chondrogenesis with lateral ligament stabilization for osteochondral lesions of the talus in patients with ankle instability. Orthop J Sports Med. 2021;9(5):23259671211007439. doi: 10.1177/23259671211007439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Albano D, Martinelli N, Bianchi A, Messina C, Malerba F, Sconfienza LM. Clinical and imaging outcome of osteochondral lesions of the talus treated using autologous matrix-induced chondrogenesis technique with a biomimetic scaffold. BMC Musculoskelet Disord. 2017;18:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aurich M, Bedi HS, Smith PJ, et al. Arthroscopic treatment of osteochondral lesions of the ankle with matrix-associated chondrocyte implantation: early clinical and magnetic resonance imaging results. Am J Sports Med. 2011;39(2):311-319. doi: 10.1177/0363546510381575 [DOI] [PubMed] [Google Scholar]
- 4. Ayyaswamy B, Salim M, Sidaginamale R, Elsayed M, Karpe P, Limaye R. Early to medium term outcomes of osteochondral lesions of the talus treated by autologous matrix induced chondrogenesis (AMIC). Foot Ankle Surg. 2021;27(2):207-212. [DOI] [PubMed] [Google Scholar]
- 5. Baumfeld T, Baumfeld D, Prado M, Nery C. All-arthroscopic AMIC® (AT-AMIC) for the treatment of talar osteochondral defects: a short follow-up case series. Foot. 2018;37:23-27. doi: 10.1016/j.foot.2018.07.006 [DOI] [PubMed] [Google Scholar]
- 6. Becher C, Malahias MA, Ali MM, Maffulli N, Thermann H. Arthroscopic microfracture vs.Arthroscopic autologous matrix-induced chondrogenesis for the treatment of articular cartilage defects of the talus. Knee Surg Sports Traumatol Arthrosc. 2019;27(9):2731-2736. doi: 10.1007/s00167-018-5278-7 [DOI] [PubMed] [Google Scholar]
- 7. Behrens P. Matrixgekoppelte Mikrofrakturierung [Matrix-coupled microfracture]. Arthroskopie. 2005;18(3):193-197. doi: 10.1007/s00142-005-0316-0 [DOI] [Google Scholar]
- 8. Campbell AB, Knopp MV, Kolovich GP, et al. Preoperative MRI underestimates articular cartilage defect size compared with findings at arthroscopic knee surgery. Am J Sports Med. 2013;41(3):590-595. doi: 10.1177/0363546512472044 [DOI] [PubMed] [Google Scholar]
- 9. Choi WJ, Park KK, Kim BS, Lee JW. Osteochondral lesion of the talus: is there a critical defect size for poor outcome? Am J Sports Med. 2009;37(10):1974-1980. doi: 10.1177/0363546509335765 [DOI] [PubMed] [Google Scholar]
- 10. D’Ambrosi R, Usuelli FG. All-arthroscopic technique with autologous bone graft for talar osteochondral defects. Minerva Ortop Traumatol. 2019;70(2):95-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Windt TS, Welsch GH, Brittberg M, et al. Is magnetic resonance imaging reliable in predicting clinical outcome after articular cartilage repair of the knee? A systematic review and meta-analysis. Am J Sports Med. 2013;41(7):1695-1702. doi: 10.1177/0363546512473258 [DOI] [PubMed] [Google Scholar]
- 12. DiGiovanni BF, Fraga CJ, Cohen BE, Shereff MJ. Associated injuries found in chronic lateral ankle instability. Foot Ankle Int. 2000;21(10):809-815. doi: 10.1177/107110070002101003 [DOI] [PubMed] [Google Scholar]
- 13. Ferkel RD, Zanotti RM, Komenda GA, et al. Arthroscopic treatment of chronic osteochondral lesions of the talus: long-term results. Am J Sports Med. 2008;36(9):1750-1762. doi: 10.1177/0363546508316773 [DOI] [PubMed] [Google Scholar]
- 14. Ferreira C, Vuurberg G, Oliveira JM, et al. Good clinical outcome after osteochondral autologous transplantation surgery for osteochondral lesions of the talus but at the cost of a high rate of complications: a systematic review. J ISAKOS. 2016;1(4):184-191. doi: 10.1136/jisakos-2015-000020 [DOI] [Google Scholar]
- 15. Frey C, Halikus NM, Vu-Rose T, Ebramzadeh E. A review of ankle arthrodesis: predisposing factors to nonunion. Foot Ankle Int. 1994;15(11):581-584. doi: 10.1177/107110079401501102 [DOI] [PubMed] [Google Scholar]
- 16. Galla M, Duensing I, Kahn TL, Barg A. Open reconstruction with autologous spongiosa grafts and matrix-induced chondrogenesis for osteochondral lesions of the talus can be performed without medial malleolar osteotomy. Knee Surg Sports Traumatol Arthrosc. 2019;27:2789-2795. [DOI] [PubMed] [Google Scholar]
- 17. Giannini S, Battaglia M, Buda R, Cavallo M, Ruffilli A, Vannini F. Surgical treatment of osteochondral lesions of the talus by open-field autologous chondrocyte implantation: a 10-year follow-up clinical and magnetic resonance imaging T2-mapping evaluation. Am J Sports Med. 2009;37(1 suppl):112-118. [DOI] [PubMed] [Google Scholar]
- 18. Gille J, Meisner U, Ehlers EM, Müller A, Russlies M, Behrens P. Migration pattern, morphology and viability of cells suspended in or sealed with fibrin glue: a histomorphologic study. Tissue Cell. 2005;37(5):339-348. doi: 10.1016/j.tice.2005.05.004 [DOI] [PubMed] [Google Scholar]
- 19. Hangody L, Kish G, Módis L, et al. Mosaicplasty for the treatment of osteochondritis dissecans of the talus: two to seven year results in 36 patients. Foot Ankle Int. 2001;22(7):552-558. doi: 10.1177/107110070102200704 [DOI] [PubMed] [Google Scholar]
- 20. Jaiswal PK, Macmull S, Bentley G, Carrington RW, Skinner JA, Briggs TW. Does smoking influence outcome after autologous chondrocyte implantation?: a case-controlled study. J Bone Joint Surg Br. 2009;91(12):1575-1578. doi: 10.1302/0301-620x.91b12.22879 [DOI] [PubMed] [Google Scholar]
- 21. Jantzen C, Ebskov LB, Johansen JK. AMIC procedure for treatment of osteochondral lesions of talus—a systematic review of the current literature. J Foot Ankle Surg. 2022;61(4):888-895. doi: 10.1053/j.jfas.2021.12.017 [DOI] [PubMed] [Google Scholar]
- 22. Malahias MA, Kostretzis L, Megaloikonomos PD, et al. Autologous matrix-induced chondrogenesis for the treatment of osteochondral lesions of the talus: a systematic review. Orthop Rev (Pavia). 2020;12(4):8872. doi: 10.4081/or.2020.8872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Matsunaga D, Akizuki S, Takizawa T, Yamazaki I, Kuraishi J. Repair of articular cartilage and clinical outcome after osteotomy with microfracture or abrasion arthroplasty for medial gonarthrosis. Knee. 2007;14(6):465-471. doi: 10.1016/j.knee.2007.06.008 [DOI] [PubMed] [Google Scholar]
- 24. Meisterhans M, Valderrabano V, Wiewiorski M. Medial oblique malleolar osteotomy for approach of medial osteochondral lesion of the talus. Arch Orthop Trauma Surg. Published online September 5, 2022. doi: 10.1007/s00402-022-04598-9 [DOI] [PubMed] [Google Scholar]
- 25. Migliorini F, Eschweiler J, Maffulli N, et al. Autologous matrix induced chondrogenesis (AMIC) compared to microfractures for chondral defects of the talar shoulder: a five-year follow-up prospective cohort study. Life. 2021;11(3):244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Migliorini F, Maffulli N, Schenker H, et al. Surgical management of focal chondral defects of the talus: a bayesian network meta-analysis. Am J Sports Med. 2022;50(10):2853-2859. doi: 10.1177/03635465211029642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Murphy EP, Fenelon C, Egan C, Kearns SR. Matrix-associated stem cell transplantation is successful in treating talar osteochondral lesions. Knee Surg Sports Traumatol Arthrosc. 2019;27:2737-2743. [DOI] [PubMed] [Google Scholar]
- 28. Nguyen A, Ramasamy A, Walsh M, McMenemy L, Calder JD. Autologous osteochondral transplantation for large osteochondral lesions of the talus is a viable option in an athletic population. Am J Sports Med. 2019;47(14):3429-3435. [DOI] [PubMed] [Google Scholar]
- 29. Niemeyer P, Pestka JM, Kreuz PC, et al. Characteristic complications after autologous chondrocyte implantation for cartilage defects of the knee joint. Am J Sports Med. 2008;36(11):2091-2099. doi: 10.1177/0363546508322131 [DOI] [PubMed] [Google Scholar]
- 30. Nixon AJ, Rickey E, Butler TJ, Scimeca MS, Moran N, Matthews GL. A chondrocyte infiltrated collagen type I/III membrane (MACI® implant) improves cartilage healing in the equine patellofemoral joint model. Osteoarthritis Cartilage. 2015;23(4):648-660. doi: 10.1016/j.joca.2014.12.021 [DOI] [PubMed] [Google Scholar]
- 31. Raikin SM. Fresh osteochondral allografts for large-volume cystic osteochondral defects of the talus. J Bone Joint Surg Am. 2009;91(12):2818-2826. doi: 10.2106/jbjs.I.00398 [DOI] [PubMed] [Google Scholar]
- 32. Roberts S, McCall IW, Darby AJ, et al. Autologous chondrocyte implantation for cartilage repair: monitoring its success by magnetic resonance imaging and histology. Arthritis Res Ther. 2003;5(1):R60-73. doi: 10.1186/ar613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sørensen LT, Jørgensen S, Petersen L, et al. Acute effects of nicotine and smoking on blood flow, tissue oxygen, and aerobe metabolism of the skin and subcutis. J Surg Res. 2008;152:224-230. doi: 10.1016/j.jss.2008.02.066 [DOI] [PubMed] [Google Scholar]
- 34. Steadman JR, Rodkey WG, Rodrigo JJ. Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop Relat Res. 2001;391:S362-S369. [DOI] [PubMed] [Google Scholar]
- 35. Usuelli FG, D’Ambrosi R, Maccario C, Boga M, de Girolamo L. All-arthroscopic AMIC® (AT-AMIC®) technique with autologous bone graft for talar osteochondral defects: clinical and radiological results. Knee Surg Sports Traumatol Arthrosc. 2018;26(3):875-881. doi: 10.1007/s00167-016-4318-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Usuelli FG, de Girolamo L, Grassi M, D’Ambrosi R, Montrasio UA, Boga M. All-arthroscopic autologous matrix-induced chondrogenesis for the treatment of osteochondral lesions of the talus. Arthrosc Tech. 2015;4(3):e255-e259. doi: 10.1016/j.eats.2015.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Verhagen RA, Struijs PA, Bossuyt PM, van Dijk CN. Systematic review of treatment strategies for osteochondral defects of the talar dome. Foot Ankle Clin. 2003;8(2):233-242, viii-ix. doi: 10.1016/s1083-7515(02)00064-5 [DOI] [PubMed] [Google Scholar]
- 38. Viehöfer AF, Casari F, Waibel FWA, et al. Smoking is associated with anterior ankle impingement after isolated autologous matrix-induced chondrogenesis for osteochondral lesions of the talus. Cartilage. 2021;13(1 suppl):1366s-1372s. doi: 10.1177/1947603520959405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Volz M, Schaumburger J, Frick H, Grifka J, Anders S. A randomized controlled trial demonstrating sustained benefit of autologous matrix-induced chondrogenesis over microfracture at five years. Int Orthop. 2017;41(4):797-804. doi: 10.1007/s00264-016-3391-0 [DOI] [PubMed] [Google Scholar]
- 40. Waltenspül M, Zindel C, Altorfer FCS, Wirth S, Ackermann J. Correlation of Postoperative Imaging With MRI and Clinical Outcome After Cartilage Repair of the Ankle: A Systematic Review and Meta-analysis. Foot Ankle Orthop. 2022. Apr 29;7(2):24730114221092021. doi: 10.1177/24730114221092021. PMID: 35520475; PMCID: PMC9067057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Weigelt L, Hartmann R, Pfirrmann C, Espinosa N, Wirth SH. Autologous matrix-induced chondrogenesis for osteochondral lesions of the talus: a clinical and radiological 2- to 8-year follow-up study. Am J Sports Med. 2019;47(7):1679-1686. doi: 10.1177/0363546519841574 [DOI] [PubMed] [Google Scholar]
- 42. Weigelt L, Laux CJ, Urbanschitz L, et al. Long-term prognosis after successful nonoperative treatment of osteochondral lesions of the talus: an observational 14-year follow-up study. Orthop J Sports Med. 2020;8(6):2325967120924183. doi: 10.1177/2325967120924183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wiewiorski M, Barg A, Valderrabano V. Chondral and osteochondral reconstruction of local ankle degeneration. Foot Ankle Clin. 2013;18(3):543-554. doi: 10.1016/j.fcl.2013.06.009 [DOI] [PubMed] [Google Scholar]
- 44. Zanon G, Di Vico G, Marullo M. Osteochondritis dissecans of the talus. Joints. 2014;2(3):115-123. doi: 10.11138/jts/2014.2.3.115 [DOI] [PMC free article] [PubMed] [Google Scholar]