Abstract
Background
Corneal inflammation after alkali burns often results in vision loss due to neovascularization and corneal opacification. Mesenchymal stem cells (MSCs) and their secreted factors (secretome) have been studied for their anti-inflammatory and antiangiogenic properties with encouraging results. However, topical instillation of MSCs or their secretome are often accompanied by issues related to delivery or rapid washout. Polyethylene glycol (PEG) and collagen are well-known biomaterials used extensively in scaffolds for tissue engineering. To effectively suppress alkaline burn-induced corneal injury, we proposed encapsulating MSCs within collagen gels crosslinked with multifunctional PEG succinimidyl esters as a means to deliver the secretome of immobilized MSCs.
Methods
Human MSCs were added to a neutralized collagen solution and mixed with a solution of four-arm PEG-N-hydroxysuccinimide (NHS). An ex vivo organ culture was conducted using rabbit corneas injured by alkali burn. MSC were encapsulated within PEG-collagen hydrogels and injected onto the wounded cornea immediately following alkali burn and washing. Photographs of the ocular surface were taken over a period of 7 days after the alkali burn and processed for immunohistochemical evaluation. Samples were split into three groups: injury without treatment, MSCs alone, and MSCs encapsulated within PEG-collagen hydrogels.
Results
All corneas in ex vivo organ culture lost their transparency immediately after alkali burn and only the groups treated with MSC and MSC encapsulated within PEG-collagen hydrogels recovered some transparency after 7 days. Immunohistochemistry revealed the increased expression of vimentin in the anterior corneal stroma of the no treatment group (indicative of fibrotic healing), while less stromal vimentin was detected in the group containing MSCs encapsulated within the PEG-collagen hydrogels.
Discussion
PEG-collagen hydrogels enable the encapsulation of viable MSCs capable of releasing secreted factors onto the ocular surface. Encapsulating MSCs within PEG-collagen hydrogels may be a promising method for delivering their therapeutic benefits in cases of ocular inflammatory diseases, such as alkali-burn injuries.
Keywords: collagen, cornea, encapsulation, mesenchymal stem cells, regeneration, wound healing
1. Introduction
The severity of chemical injuries to the external eye can vary from mild irritation to complete destruction of the ocular surface, which lead to corneal opacification, persistent ulceration, angiogenesis, and vision loss[1]. The most severe form of chemical injury arises from alkaline solutions, resulting in the saponification of fatty acids in cell membranes and cellular disruption[2]. With damage to the epithelium, the alkaline agent penetrates the corneal stroma where it destroys proteoglycans and collagen fibers[2]. Injury management usually focuses on decreasing inflammation, preventing angiogenesis, and promoting re-epithelialization of the cornea[2–4]. Intense polymorphonucleocyte (PMN) infiltration of the corneal stroma can dissolve stromal collagen in the acute phase of alkali burns [5].
Corticosteroids are excellent inhibitors of PMNs and prevent further damage to stromal melting; however, they are often associated with epithelial wound healing retardation, increased intraocular pressure (IOP), and development of cataract or secondary infection.[6–8] Although a number of treatment modalities have been tested, such as antibiotics, ascorbic acid, collagenase inhibitors, and surgery, structural and functional restoration of the cornea from alkali burn injuries remains a challenge[2,9].
Mesenchymal stem cells (MSC) are multipotent stromal cells derived from the mesoderm that can differentiate into a variety of cell types[10]. MSC and their secreted factors (secretome) have been studied for their multifunctional properties- from tissue repair/regeneration to immunomodulatory/anti-inflammatory functions[11–13]. MSC have been shown to be a promising treatment modality for corneal chemical burns[4,14,15]. However, topical instillation of MSC or their secretome does not always yield good treatment results, due to dilution into tears or rapid washout through the naso-lacrimal pump[16,17]. Thus, in order to achieve clinical application, the development of biomaterial matrices to deliver and retain MSCs onto the ocular surface remains crucial. Conventional two-dimensional systems for stem cell culture have demonstrated a non-physiologic environment that could affect cell signaling[18]. Recently, stem cell cellular and acellular niches have been emphasized regarding cell function[19]. Cross-linked collagen has not only been used to prepare scaffolds for tissue repair and engineering by increasing stability and control degradation, but also have been shown to be as a three-dimensional (3D) MSC niche that mimics the in vivo environment[18,20–22].
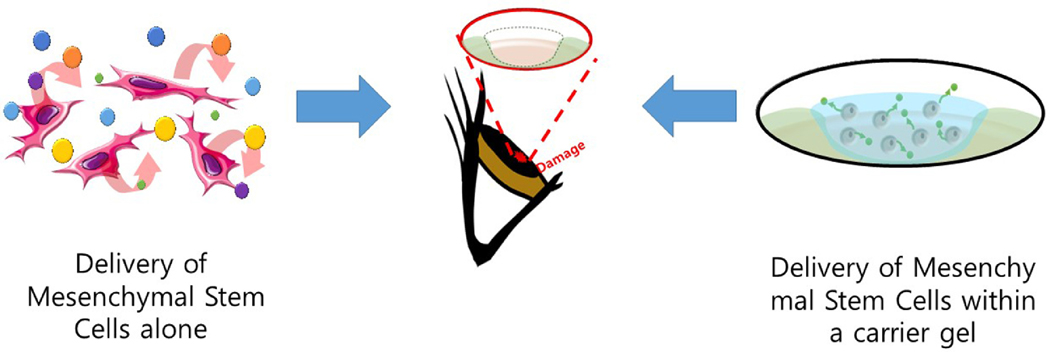
We have been using an organ-cultured rabbit cornea model to investigate a series of modalities that take advantage of the therapeutic benefits of MSC and its secretome, subsequently applying our findings to mitigate the deleterious sequelae of alkaline burns on the ocular surface[23]. We chose to encapsulate bone marrow-derived human MSCs within collagen gels cross-linked by multi-arm polyethylene glycol (PEG)-N-hydroxysuccinimide MSC (Figure 1) to deliver these cells and their beneficial factors to the corneal surface.
Figure 1.
Schematic diagram showing the two main approaches to deliver the benefits of bone marrow-derived human MSC. MSC were either administered in cell culture media topically or encapsulated within PEG-collagen hydrogels
2. Materials and methods
Materials
Unless otherwise noted, all chemicals and solvents were of analytical grade and used as advised by the manufacturer. 4 and 8-arm PEG succinimidyl glutarate (PEG) was purchased from JenKem Technology (Texas, USA). Collagenase, dimethyl sulfoxide (DMSO), fluorescamine, sodium hydroxide solution (1.0 N), bovine serum albumin (BSA), fibronectin, cholera subunit A, insulin, Triton-X, and Cell Counting kit-8 were purchased from Sigma-Aldrich (St. Louis, MO, USA). Phosphate-buffered saline (PBS) pH 7.4, 10X PBS, Slide-A-Lyzer dialysis kit (3.5 kDa MWCO), collagen I bovine protein solution (5 mg/mL), collagenase, Dulbecco’s modified eagle medium nutrient mixture F-12 (DMEM/F-12) with 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), insulin-transferrinselenium (ITS), Dulbecco’s phosphate-buffered saline (DPBS), antibiotic-antimycotic, Live/Dead viability/cytotoxicity staining kit, and paraformaldehyde (PFA) were purchased from Thermo Fisher Scientific (Massachusetts, USA).
Collagen crosslinking by 4-arm and 8 PEG-NHS
The pH of type I bovine collagen was neutralized using a solution of 1 M sodium hydroxide solution, deionized (DI) water and 10× PBS in a 3:57:20 ratio, as previously described[24]. Briefly, the 5 mg mL−1 collagen solution was mixed with the neutralization solution in a 3:2 ratio so that the final concentration of collagen was 3 mg mL−1. Collagen consists of two alpha-1 chains and one alpha-2 chain, each of which contains approximately 1000 amino acids. Since conjugation of the click chemistry functional groups occurs with the primary amine of lysine by NHS ester reaction, the number of lysine residues is important to determine the concentration of each chemical. Lysine constitutes 2.5–3.0% of collagen, amounting to a total of 75 – 100 lysine residues in the triple helix. Three mg of collagen corresponds to 7.5 × 10−9 mol and 7.5 × 10−7 mol of primary amine. The 4-arm PEG-NHS has four NHS groups per molecule; the molar equivalent amount of NHS groups is 1.875 mg per three milligram of primary amine group in collagen. Thus, 18.75 µL of 100 mg.mL−1 of 4-arm PEG-NHS provided the equivalent amount of NHS groups. Four-arm PEG-NHS was dissolved in PBS at 100 mg.mL−1 and the solution was subsequently mixed with the neutralized collagen solution at the desired concentration (0.2, 0.4, 0.8, and 1.6% w/v) for 30 s at room temperature. Subsequently, the collagen gels were incubated for 10–15 min at 37 °C for completion of gelation.
Human mesenchymal stem cell culture and cell encapsulation in PEG-collagen hydrogels
As previously described, MSC (PT-2501) were purchased from Lonza and the cells expressed CD29, CD44, CD105, CD166, CD90 and CD73, but not CD14, CD34, CD45, or CD19[23]. MSC were cultured in culture medium (10% v/v FBS and 1% v/v antibiotic/antimycotic solution in DMEM) and incubated at 37 °C in 5% CO2. For MSC encapsulation, MSC were added to the neutralized collagen solution in the fourth passage, resulting in a final concentration of 1.0 × 105 cells.mL−1 in the PEG-collagen hydrogel followed by the addition of 4-arm PEG-NHS solution.
Cell encapsulation within hydrogels on porcine corneas
To evaluate PEG- collagen hydrogel on the cornea, we formed the PEG hydrogel using collagen conjugated to FITC. MSC were incubated with cell tracker for 30 minutes. After incubation, we encapsulated the cells in the PEG- collagen hydrogel following the same procedure described above and the final PEG concentration was 4 mg/mL. As a negative control we applied only collagen conjugated to FITC. The corneas were washed three times with PBS and immersed in PFA 4% for 1 hour, sucrose 10 and 20% for 2 hours. Next, the corneas were put in tissue freezing medium and sectioned for histological evaluations.
To avoid collagen autofluorescence masking the results, corneas were incubated with Alexa Fluor® 647 IgG Fraction Monoclonal Mouse Anti-Fluorescein (FITC) in blocking solution (1:100), and left for 2 hours at room temperature. The sections were washed three times with PBS and a solution of DAPI in PBS (1:1000) was added for 5 minutes. After washing three times with PBS, the sections were mounted and observed under confocal microscopy. The encapsulated cells were observed by exciting the cell tracker dye under confocal microscopy (ZEISS LSM 880, Carl Zeiss Ag).
Human mesenchymal stem cell biocompatibility and morphology
In evaluating whether PEG-collagen hydrogels provided a suitable substrate for their growth, we examined cell viability, cellular morphological changes, and phenotypes of MSC through in vitro cell culture experiments. The sustained viability of MSC using Live/Dead staining revealed that the gels with different concentrations were the cytocompatible. PEG-collagen hydrogels were formed in a 48-well plate as previously mentioned and incubated with supplemented KSFM overnight. Afterwards, 2 × 105 MSC cells were encapsulated and cultured within the hydrogels for two days. Live/dead solution was added, and the cells were evaluated under an inverted microscope. At 2, 4 and 8 days, we collected the supernatant, and the remaining hydrogel was degraded by the addition of collagenase 2.5 mg/mL for 10 hours. After this time, the suspension was centrifuged at 350 RCF and the degraded hydrogel was collected for protein analysis (Luminex).
The conditioned media from encapsulated MSCs was collected and centrifuged at 500 × g for 10 min. This assay was performed by the Human Immune Monitoring Center at Stanford University. Human 73-plex eBiosciences with modifications were used, closely following the manufacturer’s recommendations. Beads were added to a 96-well plate and washed in a Biotek ELx405 washer. Samples were added to the plate containing the mixed antibody-linked beads and incubated at room temperature for an hour, followed by overnight incubation at 4°C with consistent shaking. Here, cold (4°C) and room temperature incubation plates were executed on an orbital shaker at 500–600rpm. Following overnight incubation, plates were washed in a Biotek ELx405 washer; then, the biotinylated detection antibody was added at room temperature with consistent shaking for 75 minutes. The same protocol of washing as described above was performed before adding streptavidin-PE. The same washing protocol was used after incubation at room temperature for 30 minutes, and the reading buffer was added to the wells. Each sample was measured in duplicates. Plates were read using a Luminex 200 or a FM3D FlexMap instrument with a lower bound of 50 beads per sample per cytokine. Custom Assay Chex control beads that were added were purchased from Radix Biosolutions located in Georgetown, Texas, and all well supernatants were analyzed by Luminex multiplex assay at the Human Immune Monitoring Center at Stanford University. Using the cell number for each condition, the raw MFI was normalized and compared.
To evaluate the morphology of MSCs encapsulated within the PEG-collagen hydrogels, samples were fixed with 4% paraformaldehyde and permeabilized with 0.1% Triton-X at each time point. The cells were stained with Alexa Fluor 647 Phalloidin (Thermo Fisher Scientific; A12379) and incubated for 20 min. After washing three times with DPBS, 5 mg/mL of DAPI solution in DPBS was added, followed by a 5 min incubation. The glass plates were mounted and the cell morphology was observed using fluorescence confocal microscopy. Lamellipodia were visually quantified as thin cytoplasmic sheets that extended at the front of the cells, whereas filopodia were visually quantified as finger-like projections at the edges of the cells.
Rabbit corneal organ culture
Rabbit eyes were obtained from Vision Tech (Sunnyvale, TX, USA) within 2 h after death, transported to the laboratory at 4°C and used immediately. Fresh eyeballs were disinfected with 10% povidone-iodine solution twice and washed in PBS. The ocular surface of the eyeballs was air-dried for 1 min. Meanwhile, Whatman filter paper was punched with a 5 mm diameter biopsy punch (Miltex, Integra LifeSciences, Plainsboro, NJ) and the circular cut filter papers were completely soaked in 1 M sodium hydroxide solution for 10 s. The sodium hydroxide-absorbed filter paper was placed on the cornea center for 1 min and the eyes were rinsed with flowing water for 5 min immediately after the removal of the filter paper.
For the air/liquid interface organ culture system, we followed the method described in a previous study [23, 25]. After the injury, corneoscleral preparations of the wounded corneas were made from the globes with a 1 mm scleral rim, grasping only scleral rims to avoid touching the limbus. Excised corneas were immediately transferred onto individual agar plugs to maintain normal culture and nutritional support. Agar plugs were made from a 1:1 mixture of serum-free medium containing double-strength antibiotic/antimycotic and 2% agar in distilled water. The agar plugs were made using polydimethylsiloxane (PDMS) molds. The base and curing agents of Sylgard 184 (Dow Corning, Midland, MI, USA) were mixed at a 10:1 weight ratio and the 10 mL round bottom tubes were posted to the PDMS precursor mixture. The tubes were removed after PDMS curing and the PDMS mold was autoclaved before use. Wounded corneas on the agar plugs were placed in a 12-well plate with 1 mL of complete serum-free culture medium, which was sufficient in bringing the medium to the level of the limbal area. DMEM/F-12 containing 120 μg/mL penicillin G, 200 μg/mL streptomycin sulfate and ITS premix was used as the culture medium. Samples were incubated at 37 °C in a 5% CO2 atmosphere and the cornea apical surface was irrigated to remove debris and maintain moisture; the culture medium was changed daily.
For the MSC group, MSC (1.0 × 105 cells) were seeded onto the wounded corneal tissue directly. For the MSC encapsulated in the PEG-collagen hydrogel group, MSC (1.0 × 105 cells) were added to the neutralized collagen and 4-arm PEG-NHS solution was added at a final volume of 1 mL to match the MSC numbers in both groups. The 4-arm PEG (0.8%) hydrogel was topically applied onto the corneal wounded surface to form an in situ hydrogel on the corneal surface. Photographs of fluorescein-stained corneas were taken with a smartphone ophthalmic imaging adapter (Paxos Scope, by Digisight Technologies, now Verana Health, San Francisco, CA, USA) equipped with a 15× magnifying lens and a blue LED in the same manner as previously reported.
Immunofluorescence staining
Upon conclusion of the ex vivo organ culture experiment, corneas were cut in half, fixed in 4% PFA for 15 min at 4 °C, and processed with graded sucrose solutions, 15% sucrose for 2 h. Afterwards, they were left in a 30% sucrose solution overnight at 4 °C. Subsequently, corneas were embedded in optimal critical temperature compound and 18micron sections were cut with a cryostat (Leica CM3050 S Research Cryostat). The eyes were sectioned and permeabilized for 30 min with 5% normal goat serum and 0.5% Triton-X. Next, anti-Vimentin antibody (Abcam: ab137321, 1:100) was added to the corneal sections and incubated overnight in the same permeabilization solution. After three PBS washes, secondary antibodies (1:1000) and Alexa Fluor 546-coupled goat anti-rabbit IgG were added for 2 h. Finally, after washing with PBS, DAPI was added for 5 min in PBS solution (1:1000). The corneas were mounted and observed using confocal microscopy (ZEISS LSM 880, Carl Zeiss Ag). Negative controls were not incubated with primary antibodies. Images were taken with a confocal microscope at 10X magnification. Additionally, Alexa Fluor 647 was conjugated to the PEG-collagen to visualize the collagen gels formed on the corneal wounds by fluorescence microscopy following the manufacturer’s protocol. Briefly, Alexa Fluor 647 reactive dye was mixed with collagen, incubated for 2 h at 4 °C, and dialyzed via a Slide-A-Lyzer dialysis kit overnight at 4 °C in PBS. Finally, the 4-arm PEG-NHS dissolved in PBS was added to the neutralized collagen.
Statistical Analysis
All data are expressed as mean ± standard deviation (SD). Each experiment was performed at least three times, unless otherwise indicated. Statistical evaluation was performed using one-way ANOVA, Kruskal-Wallis test and uncorrected Dunn’s test for multiple comparisons. Results with p < 0.05 were considered statistically significant. Statistical analysis was performed using the statistical software GraphPad Prism 7.
3. Results
Adhesion of PEG-collagen hydrogel on corneal stroma and gel biocompatibility
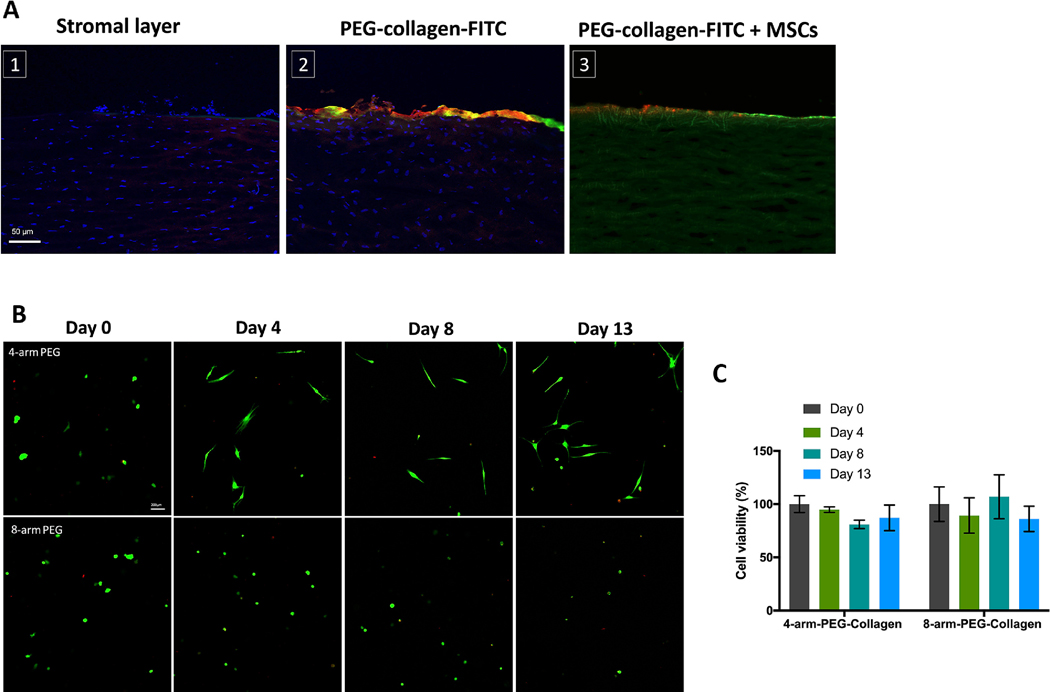
Our goal was for the PEG-collagen hydrogel to adhere to corneal stroma while also providing a favorable physiological environment for MSC. We applied NHS ester chemistry to crosslink low concentrations of exogenously applied collagen type I using multi-arm PEG and expected the NHS group to also react with the primary amine groups on the collagen of porcine corneal stroma. First, we evaluated whether the PEG-collagen hydrogel could bind to an ex vivo corneal stromal layer after removing the epithelial layer. To visualize the gel, collagen was conjugated with FITC before crosslinking with PEG. After washing 3 times in PBS, we observed that the gel could form and be retained on the stromal surface (Figure 2A). To confirm our results, we incubated the corneas with anti-FITC antibody 647. Figure 2A shows the 488 and 647 emission confirming that the green fluorescence stems from the gel and not from the autofluorescence of corneal collagen. Next, we aimed to evaluate the presence of MSCs delivered by the gel onto the cornea. Figure 2A shows how encapsulated MSC marked with DiI cell tracker are observed on the ex vivo cornea stroma after application of the PEG-collagen hydrogel.
Figure 2.
(a) Presence of PEG-collagen hydrogel on the stromal layer of ex vivo porcine cornea. Cornea autofluorescence on the Bowman’s membrane can be observed. PEG-collagen-FTIC (green) and anti-FITC antibody (red) confirmed that the gel adheres to the stromal layer of the cornea. MSC are observed on the stromal layer after application of the gel. (b) MSC viability using the LIVE/DEAD assay after encapsulated in 4 and 8 arm PEG-collagen gel at 0.4% w/v at 13 days in culture. Live cells were stained green, while dead cells were stained red. (c) Quantification of live/dead assay shows that cell viability remained around 100 % at 13 days in culture.
To analyze the gels’ biocompatibility, MSC were encapsulated in the gels and a LIVE/DEAD cell assay was performed (Figure 2B). Cell viability was compared to cells encapsulated 30 minutes after the gelation. PEG-collagen gels demonstrated great biocompatibility after 13 days of encapsulation. Cells encapsulated in 4 arm PEG-collagen spread during the evaluated time frame, but the cells encapsulated in 8 arm-PEG-collagen gel did not spread very well. Cell viabilities for 4 arm-PEG collagen and 8 arm-PEG collagen hydrogels were both high, nearly 100% for 13 days (Figure 2C). No difference in cell viability was observed between the 4 arm PEG and 8 arm PEG-collagen hydrogels.
MSC morphology encapsulated on PEG-collagen hydrogels at varying PEG concentration.
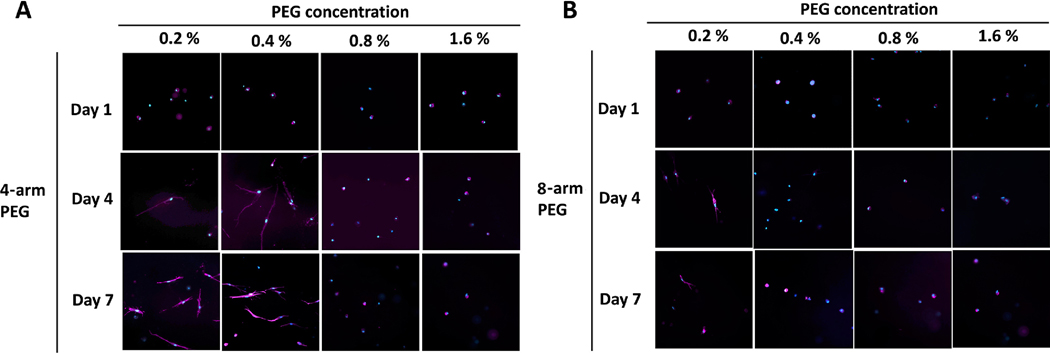
Next, we evaluated MSC behavior as a function of time, PEG arm number, and concentration. Figure 3 shows F-actin staining of MSC encapsulated within PEG-collagen hydrogels. At day 1, the cells maintained a round shape in the case of both 4 arm and 8 arm-PEG-collagen at all concentrations. At day 4, cells encapsulated in 4 arm-PEG-collagen at 0.2 % and 0.4 % w/v and 8 arm-PEG-collagen at 0.2 % showed spread morphology compared to the other conditions. No changes on cell morphology were observed at day 7 for all the evaluated groups. These results suggest that the functionality and concentration of the crosslinker can modulate cell spreading. A lower degree of cell spreading occurred with higher PEG concentration. In addition, we found the cells to be less spread in 8 arm PEG-collagen at the same concentration as for 4 arm-PEG-collagen hydrogel, underscoring that the number of PEG arms plays an additional role in encapsulated cell behavior.
Figure 3.
MSC morphology encapsulated in (a) 4 arm and (b) 8 arm PEG-collagen hydrogels on days 1, 4, and 7. Immunofluorescence staining was performed with filamentous actin (F-actin, stained in magenta) using Alexa Fluor Phalloidin 647 and a nuclear counterstain with DAPI (in blue).
MSC secretome production comparison between encapsulation PEG-collagen hydrogel vs cells on tissue culture plate.
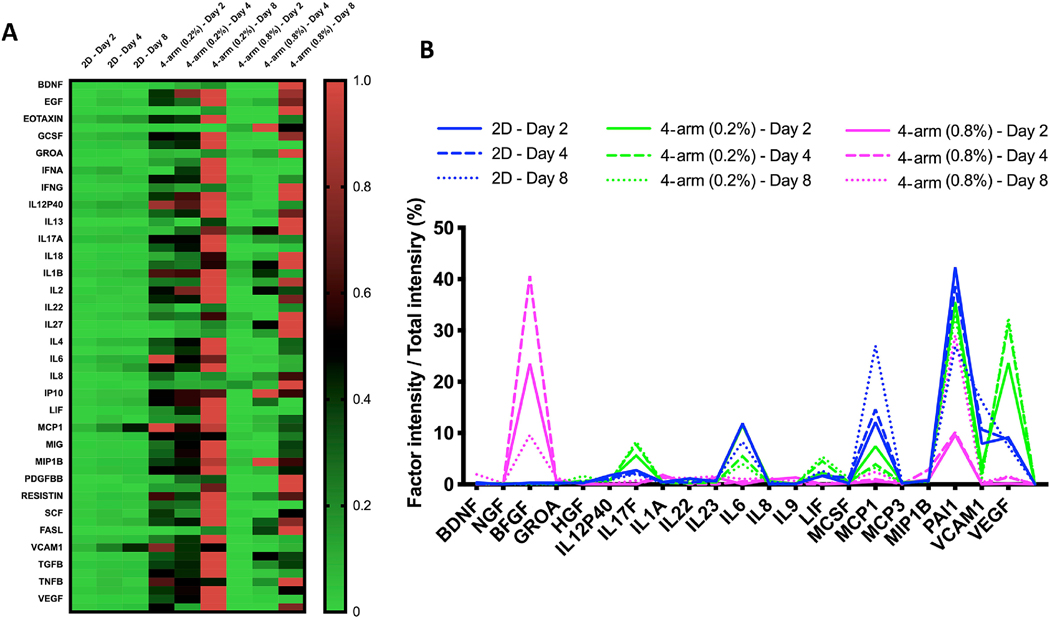
The production of growth factors and cytokines for encapsulated MSC in 4 arm PEG-collagen hydrogels as a function of PEG concentration was evaluated after 2, 4 and 8 days. We chose to work with 4 arm PEG-collagen at two different concentrations: 0.2 % and 0.8% w/v. We observed that cells encapsulated in 4 arm PEG 0.2 % spread more compared to the cells encapsulated in 0.8% PEG that were round during the evaluated time frame. We thus questioned whether cell morphology impacted secretome production. In addition, we compared secretome production of encapsulated cells to cells plated on tissue culture plate (TCP). Figure 4 reveals how the cells plated in the 2D substrate produced relatively lower amounts of protein (growth factors, chemokine and cytokines) at all evaluated times as opposed to encapsulated cells. Cells encapsulated in 0.2% 4 arm-PEG-collagen hydrogels secreted higher protein concentrations at all the times compared to the 2D condition and 0.8 % 4 arm-PEG-collagen hydrogels. Nevertheless, cells encapsulated in 0.8 % 4 arm PEGcollagen secreted more proteins compared to those in 2D condition. The amount of protein secreted increased with time for all the groups. Such results imply how the behavior and environment of cells hold important modulatory roles for the production of MSC secreted factors. We then analyzed which proteins were highly secreted by the cells under the different conditions. Figure 4b and Table 1 shows that a distinct secreted profile was observed for MSC encapsulated in the gels and plated on the TCP. Growth factors were secreted to a greater extent by cells encapsulated in the gels compared to the 2D conditions. VEGF production was increased by cells encapsulated in 4 arm PEG-collagen hydrogels with 0.2 % PEG content compared to cells encapsulated in 4 arm PEG-collagen hydrogels with 0.8 % PEG content. On the other hand, FGF-B was increased in cells encapsulated in 4 arm PEGcollagen with 0.8 % PEG compared to 4 arm PEG-collagen hydrogels with 0.2% PEG. Cells plated on the TCP plate exhibited an increase in pro-inflammatory proteins compared to those encapsulated within the gels.
Figure 4.
(a) Heat map showing secretome production by the MSC encapsulated in 4 armPEG-collagen hydrogels at 0.2 % and 0.8 % w/v PEG to collagen. (b) Qualitative secretome profile showing the most abundant proteins secreted by the cells in different conditions.
Table 1.
Top 5 factors secreted by MSC under different culture conditions.
| 2D | 4arm (0.2 %) | 4arm (0.8 %) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| day 2 | day 4 | day 8 | day 2 | day 4 | day 8 | day 2 | day 4 | day 8 | |
| 1 | PAI1 | PAI1 | MCP1 | PAI1 | PAI1 | PAI1 | FGFB | FGFB | PAI1 |
| 2 | MCP1 | MCP1 | PAI1 | VEGF | VEGF | VEGF | PAI1 | PAI1 | FGFB |
| 3 | IL6 | IL6 | VCAM1 | IL6 | IL17F | IL17F | IL1A | MIP1B | MCP1 |
| 4 | VEGF | VCAM1 | IL6 | MCP1 | IL6 | LIF | IL23 | VEGF | BDNF |
| 5 | VCAM1 | VEGF | VEGF | IL17F | LIF | IL6 | IL9 | IL1A | IL23 |
Corneal alkaline burn organ culture model
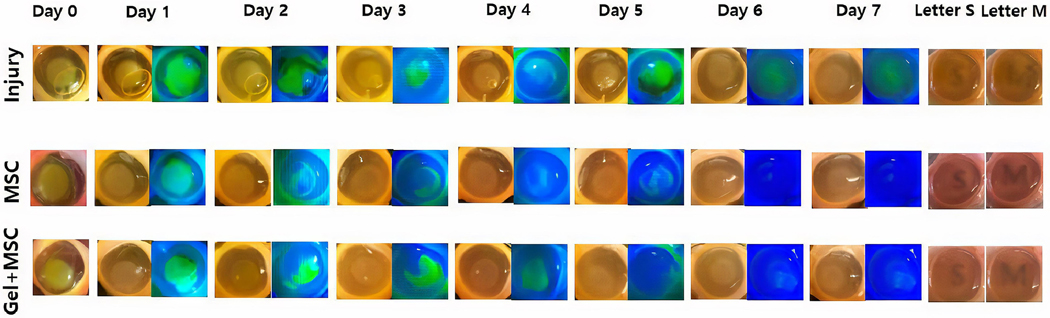
Given that MSC encapsulated in PEG-collagen hydrogels secrete an increased amount of proteins compared to cells under 2D conditions, we studied how such a finding impacts healing after alkali burn in a rabbit corneal organ culture model. Topically applied MSCs and MSC encapsulated in PEG-collagen hydrogel were applied onto the injured corneal surfaces, which were then monitored for epithelial wound healing and stromal scarring. In an organ culture setup, an air-liquid interface is created on the corneal surface, where nutrients make it to the ocular surface either through diffusion from an underlying agar plug on the posterior side of the cornea, or through capillary wicking from a moat of culture media in which the corneal-scleral rim was partially submersed along its periphery, as previously described. When MSCs are applied topically, the cells are likely to settle onto the culture well surfaces in contact with the culture media. Thus, any secreted factors from the MSCs would actively condition the media and nourish the cornea through a capillary wicking effect. The MSCs encapsulated in the PEG-collagen hydrogel were applied topically and underwent in situ gelation on the corneal surface. Thus, factors secreted from MSCs within the gel should directly affect wound healing. There were 3 rabbit corneas per treatment group. Photographs of the ocular surface taken over a period of 7 days after the alkali burn demonstrated that corneal damage occurred in a time-dependent manner. Daily photographs were taken under white light and blue LED light with fluorescein dye applied to the cornea to observe wound size. Corneas treated with MSC alone or MSC encapsulated in PEG-collagen hydrogels had similar rates of wound closure, and by day 6, all corneal wounds had healed in both the groups (Figure 5). In the injury-only group, corneal wounds were not completely healed until the end of the experiment (Day 7).
Figure 5.
Representative rabbit cornea organ cultures in the three treatment groups following alkali burn until day 7. Corneas treated with MSC healed faster and were more transparent than those of the control group after one week. Corneas treated with MSC encapsulated within the PEG-collagen hydrogel exhibited the highest degree of transparency. Fluorescein dye was used to track the closure of epithelial wounds.
The cornea became opaque almost immediately after the alkali burn. At the conclusion of the experiment, we quantified corneal transparency by placing printed letters on a sheet of paper placed below the corneal tissue culture dish. Representative images of MSC and MSC encapsulated in the PEG-collagen hydrogel group highlighted how stromal haze formation was relatively lower in treated groups than of non-treated group. Corneal opacity remained in all 3 groups, but it was most significant in the injury-only (no treatment) group and least significant in the group treated with MSC encapsulated within the PEG-collagen hydrogel. The MSCs-only and the MSCs encapsulated within PEG-collagen hydrogel both facilitated similar rates of corneal epithelial wound healing. Corneal haze was graded manually by authors KN, HL, and YS, and were as follows for the injury, MSC, and MSC encapsulated in PEG-collagen hydrogel groups (0 as totally opaque and 5 as totally transparent): 1.2 ± 0.2, 3.0 ± 0.6, and 3.6 ± 0.5, respectively.
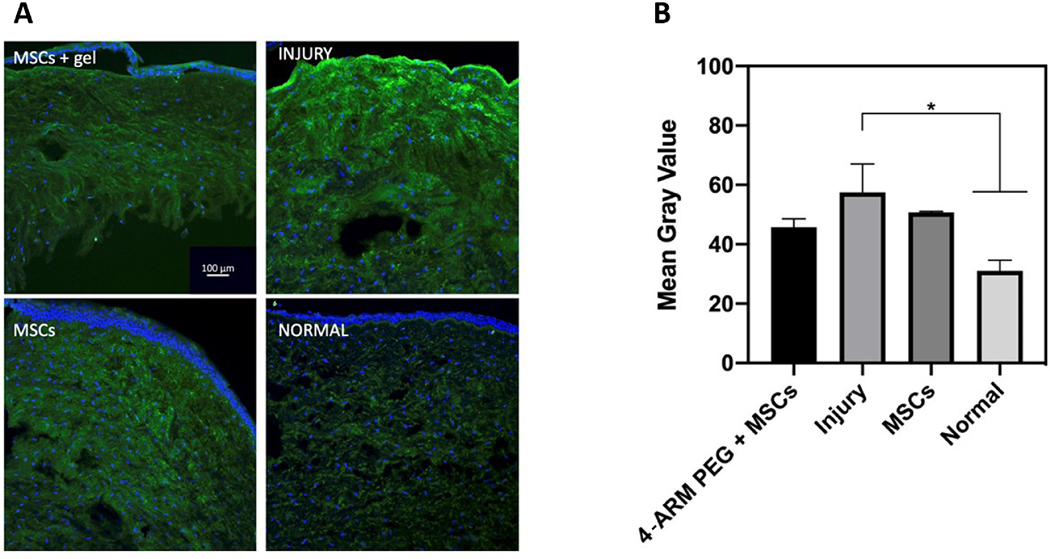
Immunofluorescence confocal imaging showed anterior stromal vimentin (green) was highest in the untreated corneas and lowest in those treated with MSC encapsulated within 4-arm PEG-collagen gels (Figure 6a). There was basal expression of vimentin in keratocytes in unwounded normal cornea as well. After quantifying the expression of vimentin, there was a difference between the MSCs-only group and the gel-encapsulated group, but the difference was not statistically significant (Figure 6b).
Figure 6.
(a) Fluorescent confocal microscopy images of alkali-burned rabbit corneas in the normal, injury-only, MSC, and MSC encapsulated in PEG-collagen gel groups upon completion of the organ culture experiment. Tissue was stained for vimentin (green) and DAPI (blue). (b) Quantification of vimentin expression in the anterior stroma of normal, injury-only, MSC, and MSC encapsulated in PEG-collagen gel groups.
4. Discussion
In this study, we successfully encapsulated MSC within a PEG-collagen hydrogel as an injectable biomaterial for corneal alkali burn treatment. We optimized the concentration of PEG-collagen hydrogel as a vehicle for cell delivery on the ocular surface. Based on our results, in situ forming PEG-collagen hydrogels exhibited good adherence to the corneal surface and satisfactory MSC survival after encapsulation. Data from an alkali burn organ culture model demonstrated PEG-collagen hydrogels were suitable cell vehicles for the delivery of MSCs to promote epithelial healing and reduce corneal haze.
MSCs have gathered significant interest due to their immunomodulatory capacity, which allows for their use in allogeneic settings [11,25]. A large body of evidence has shown that MSCs secrete a variety of growth factors, chemokines and cytokines[26–29]. Their secretome is known to have benefits in the absence of the cells themselves, and thus cell-free secretome therapy has garnered interest as a therapeutic option. However, the properties of the secretome vary widely depending on the host age and niche[25,26]. It is unclear whether the secretome of MSC grown in vitro can display the tissue-repairing and immunomodulatory effects[25]. Previous studies strongly support the safety of MSC use in cell-based therapies by demonstrating that no tumors have originated from systemic infusions of MSCs [30,31]. Autologous and allogeneic MSCs have been used in a number of clinical trials for a wide range of clinical conditions. A promising way to deliver MSC onto the corneal surface involves the use of scaffolds to adhere them to target surfaces while also mimicking their three dimensional physiological environment. Previously, we showed that MSCs cultured on electrospun fibers composed of gelatin (to mimic the collagenous matrix of the cornea) and polycaprolactone (PCL) enhanced healing in corneal fibroblast cells in vitro, as well as in a rabbit corneal alkali burn organ culture model [23].
In the present study, we selected in situ forming PEG-collagen hydrogels as delivery vehicles for MSCs. Encapsulation of cells in hydrogels has some advantages over seeding onto a prefabricated porous scaffold[22]. This strategy allows for cells suspended in a liquid precursor solution to be delivered in vivo directly to the site of interest with a simple injection. If hydrogels encapsulated with MSCs adhere to the ocular surface, they can be directly applied to a corneal burn site without the need for sutures or glue. Encapsulating cells in biodegradable hydrogels presents a number of advantages, including ease of handling, and a highly hydrated tissue-like environment for cell and tissue growth[32,33]. Consequently, collagen has been commonly used to create scaffolds for many other clinical applications[34,35]. Collagen— a major component of the extracellular matrix (ECM)— is one of the most well-established natural polymers for biomedical applications [32]. Despite its demonstrated potential, its mechanical properties remain difficult to control which can pose a challenge[36]. Meanwhile, synthetic polymer-based materials such as PEG have been developed as scaffolds. One of PEG’s most prominent advantages is its tunability—the ability to control its physical and chemical properties particularly in the setting of crosslinked networks, including mechanical strength and degradation kinetics[33]. PEG is widely used for its ability to be easily modified and create variable structures with different reactive groups[37–39]. In addition, PEG-based materials are bioinert and are used in FDA-approved ocular sealants [40]. Combining both natural and synthetic polymers into hydrogels has gained widespread attention for tissue engineering and drug delivery owing to their high water content, tissue-like elasticity, and biocompatibility[41,42].
In the current study, we observed that cytocompatibility for PEG collagen hydrogels were high with nearly 100% viability for 2 weeks. We measured cell proliferation in the hydrogel after 2,4 and 8 days (Supplementary Figure 2). We observed that cell proliferation decreased over time in the hydrogel probably due to hydrogel degradation. Although we understand the importance for these cells to maintain their properties and stemness, here we focused on evaluating secretome release and hope to pursue this line of inquiry in a future study. We tried to simplify our study by evaluating encapsulation parameters, including PEG concentration. We will in the future evaluate how cell differentiation and spread is impacted by gel mechanical properties. In the future, we aim to assess the impact of gel mechanical properties on cell differentiation and spreading.
We found that single encapsulated MSCs showed dendritic morphology by 8 days within the PEG-collagen hydrogel. MSCs undergo spontaneous differentiation in response to certain physical cues in the environment, such as matrix stiffness and mechanical forces[43]. The actin cytoskeleton in MSC is composed of a large number of thin, parallel microfilament bundles that extend across the entire cytoplasm, and it is still unclear whether the cytoskeletal changes of MSC affect their fate [44]. We found that the cells encapsulated within PEGcollagen hydrogels were viable and that their morphology changed in response to their surrounding environment. We used 4-arm PEG (0.2%) in organ culture, because we surmised that MSCs in the lower PEG concentration and with the lower PEG number may spread more freely and favor stem-like cellular phenotype in the softer gel, and consequently affect the secretory properties of MSC. We found that the cells could migrate from the hydrogel to the plate as a consequence hydrogel degradation (Supplementary Figure 1). However, our experimental design offered no space for the cells to migrate vertically within the hydrogel covering the entire well spanning approximately 300 to 400 micrometers.
In a previous study, we reported on the rheological characterization of these PEG-collagen hydrogels in vitro as well as regeneration of mechanically injured epithelium over the in situ forming PEG-collagen hydrogel in vivo [45]. Here, we conducted an ex vivo rabbit organ culture experiment to analyze the therapeutic effects of MSCs encapsulated in PEGhydrogels after alkali burn injury to the cornea. Corneal organ culture creates a biomimetic air–liquid interface along with nutrient flow that emulates the tear film (cell culture media encircling a cornea-scleral rim) and the aqueous humor (agar plug posterior to the cornea)[46]. Epithelial wound closure was measured with blue light and fluorescein dye, which stains epithelial defects. Scarring and corneal haze were also evaluated through the rabbit corneal organ culture experiments. MSCs alone and MSC-encapsulated within PEG-collagen gels promoted faster epithelial wound healing rates and reduced corneal opacification compared to injured corneas without treatment. Topically instilled MSCs tended to slide off of the cornea and grow in the culture medium in which the corneoscleral rim is soaked and secretome-enriched media can reach the cornea via capillary action and diffusion through the underlying agar matrix. PEG-collagen gels, on the other hand, are successfully maintained on the curved surface of the cornea along with the encapsulated cells, without flowing off into the underlying media. Based on the epithelial wound healing data of the group consisting of PEG-collagen gels with encapsulated MSCs, it is possible that the hydrogels may prevent epithelial wound closure initially. However, epithelial healing was achieved by day 5, and corneal transparency was improved compared to the corneas treated with MSCs only and to untreated corneas at day 7.
In our proteomic analysis of MSC secreted factors, protein content was normalized to the number of cells and subsequently compared to their 2D conditions for the collected secretome. The normalization allowed us to indirectly investigate and characterize the released secretome of encapsulated MSC, based on the PEG concentration and encapsulation time. We observed that the secretome released by these cells increased at lower PEG concentrations and over time. The observed increase of VEGF in the MSC secretome in the context of the known anti-angiogeneic properties of MSCs requires further explanation. The MSC secretome has a multitude of factors, the makeup of which can be modulated toward pro-angiogenic and pro-inflammatory verses and anti-angiogenic and anti-inflammatory depending on culture conditions [47]. Thus, the same secretome has factors which may have opposite effects, but with certain factors being at a higher effective concentration. Several studies have reported that MSCs promote vasculogenesis [48], whereas other groups showed they inhibit angiogenesis [49]. Previous studies have reported that an exogenous VEGF isoform (VEGF-B) promoted corneal nerve fiber regeneration along with restoration of sensation and healing of the epithelium [50]. In our group’s recent publication, we showed the MSC secretome alone with and without a viscoelastic gel carrier could prevent corneal neovascularization in after alkali burn injury in rodents in vivo [51]. In this context, we hypothesized that the MSC secretome produced by MSCs encapsulated within collagen-PEG gels may also facilitate corneal epithelial wound healing and similarly prevent neovascularization although further in vivo testing is required to confirm this. In addition to the therapeutic potential of the MSC secretome, exosomes have been well-studied for their capacity to accelerate corneal epithelial wound healing as well [52].
Immunofluorescence imaging of corneal cryosections revealed differences in the expression of vimentin between the three groups. The injury-only group exhibited increased vimentin expression in the stroma, as did the group treated with MSCs alone, albeit to a lesser degree. The gel-encapsulated MSC group had the lowest degree of stromal vimentin expression, which is suggestive of the beneficial effects that this delivery modality may have on MSC activity and, in turn, long-term stromal scarring. Previous study has reported that vimentin, a major class of intermediate filament proteins, plays an essential role in the early and intermediate stages of the formation of myofibroblasts during corneal wound healing [53]. Charurasia et al showed that there was an increase in stromal cells expressing vimentin at higher levels at one week after photorefractive keratectomy in rabbits, followed by alpha-SMA by 2 weeks and desmin by 4 weeks [53]. As we tested eyes 1 week after corneal burns, we selected vimentin as our marker of fibrotic wound healing. This result correlates with our macroscopic observations of corneal haze in organ culture over 8 days. The highest levels of vimentin expression and corneal haze were seen in the injury-only group, followed by the MSC-only group. The group treated with MSC encapsulated within PEG-collagen hydrogels presented the lowest vimentin expression and corneal haze levels.
There are a number of limitations inherent to this study. First limitation was the fact organ culture, due to the lack of vessels and the lymphatic system, fails to model the host immune response after the alkali burn of the cornea. However, Zhao et al. showed that the response of in vitro organ culture models to severe alkali burn is similar to that observed by other groups in clinical and in vivo studies[46]. Overt changes, as well as ultrastructure of corneal surface photographed over a period of 7 days after the alkali burn, can be see on ex vivo corneas in a time-dependent manner. Although animal models are generally used for the study of pathologic changes after alkali burn, there has been a demand for the reduction of animal use in research over the last few years. In addition, there are some benefits to using in vitro and ex vivo models, such as easier manipulation and monitoring, and controlled applications of chemical agents and treatment modalities.
In conclusion, we found that PEG-collagen hydrogels are suitable encapsulated vehicles for MSCs, and that MSCs can be reliably delivered to the corneal surface using this platform. MSCs in PEG-collagen hydrogels accelerated epithelial wound closure and reduced corneal scar formation to a significant extent compared to untreated corneal controls. Furthermore, immunohistochemical differences were observed in the gel-encapsulated MSC treatment group, including decreased α-SMA expression and reduced corneal haze. Future work is merited to evaluate and validate these effects in in vivo models, as well as to reduce the batch-to-batch variability and avoid the risk of host immune rejection through the use of human-derived collagen rather than animal-derived collagen. Our data, together with previous reports demonstrating the mechanical and physical properties of PEG-collagen hydrogels, underscores that in situ-forming PEG-collagen hydrogels may have potential as a delivery modality for MSCs in a clinical setting.
Supplementary Material
Supplemental Figure 1. Hydrogel degradation.
Supplemental Figure 2. Cell proliferation fold change (normalized by day 2).
Funding
This work was supported by the National Institutes of Health (National Eye Institute K08EY028176 and a Departmental P30-EY026877 core grant), the Stanford SPARK Translational Research Grant (D.M.), a core grant and Career Development Award from Research to Prevent Blindness (RPB), the Matilda Ziegler Foundation, the VA Rehabilitation Research and Development Small Projects in Rehabilitation Effectiveness (SPiRE) program (I21 RX003179), and the Byers Eye Institute at Stanford. This study was also supported by a grant from the National Research Foundation of Korea (NRF) (2019R1F1A1061421) and by a grant of the Catholic University Yeouido St.Mary’s Hospital made in the program year of 2020.
Abbreviations
- MSCs
Mesenchymal stem cells
- PEG
Polyethylene glycol
- NHS
N-hydroxysuccinimide
- PMN
polymorphonucleocyte
- IOP
increased intraocular pressure
Footnotes
Declaration of Competing Interest
Authors DM, HJ, and GMFC have a patent application on the gel technology described in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Bunker DJ, George RJ, Kleinschmidt A, Kumar RJ, Maitz P. Alkali-related ocular burns: a case series and review. J Burn Care Res 2014;35(3):261–8. [DOI] [PubMed] [Google Scholar]
- [2].Wagoner MD. Chemical injuries of the eye: current concepts in pathophysiology and therapy. Surv Ophthalmol 1997;41(4):275–313. [DOI] [PubMed] [Google Scholar]
- [3].Bakunowicz-Łazarczyk A, Urban B. Assessment of therapeutic options for reducing alkali burn-induced corneal neovascularization and inflammation. Adv Med Sci 2016;61(1):101–12. [DOI] [PubMed] [Google Scholar]
- [4].Sharma N, Kaur M, Agarwal T, Sangwan VS, Vajpayee RB. Treatment of acute ocular chemical burns. Surv Ophthalmol 2018;63(2):214–35. [DOI] [PubMed] [Google Scholar]
- [5].Soiberman U, Kambhampati SP, Wu T, Mishra MK, Oh Y, Sharma R, et al. Subconjunctival injectable dendrimer-dexamethasone gel for the treatment of corneal inflammation. Biomaterials 2017;125:38–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Petroutsos G, Guimaraes R, Giraud JP, Pouliquen Y. Corticosteroids and corneal epithelial wound healing. Br J Ophthalmol 1982;66(11):705–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Pleyer U, Ursell PG, Rama P. Intraocular pressure effects of common topical steroids for post-cataract inflammation: are they all the same? Ophthalmol Ther 2013;2(2):55–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Skalka HW, Prchal JT. Effect of corticosteroids on cataract formation. Arch Ophthalmol 1980;98(10):1773–7. [DOI] [PubMed] [Google Scholar]
- [9].Ghosh S, Salvador-Culla B, Kotagiri A, Pushpoth S, Tey A, Johnson ZK, et al. Acute Chemical Eye Injury and Limbal Stem Cell Deficiency-A Prospective Study in the United Kingdom. Cornea 2019;38(1):8–12. [DOI] [PubMed] [Google Scholar]
- [10].Dulak J, Szade K, Szade A, Nowak W, Józkowicz A. Adult stem cells: hopes and hypes of regenerative medicine. Acta Biochim Pol 2015;62(3):329–37. [DOI] [PubMed] [Google Scholar]
- [11].Gao F, Chiu SM, Motan DA, Zhang Z, Chen L, Ji HL, et al. Mesenchymal stem cells and immunomodulation: current status and future prospects. Cell Death Dis 2016;7(1):e2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Maumus M, Jorgensen C, Noël D. Mesenchymal stem cells in regenerative medicine applied to rheumatic diseases: role of secretome and exosomes. Biochimie 2013;95(12):2229–34. [DOI] [PubMed] [Google Scholar]
- [13].Regulski MJ. Mesenchymal Stem Cells: “Guardians of Inflammation”. Wounds 2017;29(1):20–7. [PubMed] [Google Scholar]
- [14].Lee DE, Ayoub N, Agrawal DK. Mesenchymal stem cells and cutaneous wound healing: novel methods to increase cell delivery and therapeutic efficacy. Stem Cell Res Ther 2016;7:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Navas A, Magaña-Guerrero FS, Domínguez-López A, Chávez-García C, Partido G, Graue-Hernández EO, et al. Anti-Inflammatory and Anti-Fibrotic Effects of Human Amniotic Membrane Mesenchymal Stem Cells and Their Potential in Corneal Repair. Stem Cells Transl Med 2018;7(12):906–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hosoya K, Lee VH, Kim KJ. Roles of the conjunctiva in ocular drug delivery: a review of conjunctival transport mechanisms and their regulation. Eur J Pharm Biopharm 2005;60(2):227–40. [DOI] [PubMed] [Google Scholar]
- [17].Wu Y, Liu Y, Li X, Kebebe D, Zhang B, Ren J, et al. Research progress of in-situ gelling ophthalmic drug delivery system. Asian J Pharm Sci 2019;14(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Jang M, Lee ST, Kim JW, Yang JH, Yoon JK, Park JC, et al. A feeder-free, defined three-dimensional polyethylene glycol-based extracellular matrix niche for culture of human embryonic stem cells. Biomaterials 2013;34(14):3571–80. [DOI] [PubMed] [Google Scholar]
- [19].Thomson H. Bioprocessing of embryonic stem cells for drug discovery. Trends Biotechnol 2007;25(5):224–30. [DOI] [PubMed] [Google Scholar]
- [20].Koivusalo L, Karvinen J, Sorsa E, Jönkkäri I, Väliaho J, Kallio P, et al. Hydrazone crosslinked hyaluronan-based hydrogels for therapeutic delivery of adipose stem cells to treat corneal defects. Mater Sci Eng C Mater Biol Appl 2018;85:68–78. [DOI] [PubMed] [Google Scholar]
- [21].Koivusalo L, Kauppila M, Samanta S, Parihar VS, Ilmarinen T, Miettinen S, et al. Tissue adhesive hyaluronic acid hydrogels for sutureless stem cell delivery and regeneration of corneal epithelium and stroma. Biomaterials 2019;225:119516. [DOI] [PubMed] [Google Scholar]
- [22].Nicodemus GD, Bryant SJ. Cell encapsulation in biodegradable hydrogels for tissue engineering applications. Tissue Eng Part B Rev 2008;14(2):149–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Carter K, Lee HJ, Na KS, Fernandes-Cunha GM, Blanco IJ, Djalilian A, et al. Characterizing the impact of 2D and 3D culture conditions on the therapeutic effects of human mesenchymal stem cell secretome on corneal wound healing in vitro and ex vivo. Acta Biomater 2019;99:247–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lee HJ, Fernandes-Cunha GM, Na KS, Hull SM, Myung D. Bio-Orthogonally Crosslinked, In Situ Forming Corneal Stromal Tissue Substitute. Adv Healthc Mater 2018;7(19):e1800560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].L PK, Kandoi S, Misra R, S V, K R, Verma RS. The mesenchymal stem cell secretome: A new paradigm towards cell-free therapeutic mode in regenerative medicine. Cytokine Growth Factor Rev 2019;46:1–9. [DOI] [PubMed] [Google Scholar]
- [26].Gaceb A, Barbariga M, Özen I, Paul G. The pericyte secretome: Potential impact on regeneration. Biochimie 2018;155:16–25. [DOI] [PubMed] [Google Scholar]
- [27].Mardpour S, Hamidieh AA, Taleahmad S, Sharifzad F, Taghikhani A, Baharvand H. Interaction between mesenchymal stromal cell-derived extracellular vesicles and immune cells by distinct protein content. J Cell Physiol 2019;234(6):8249–58. [DOI] [PubMed] [Google Scholar]
- [28].Shi Y, Wang Y, Li Q, Liu K, Hou J, Shao C, et al. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat Rev Nephrol 2018;14(8):493–507. [DOI] [PubMed] [Google Scholar]
- [29].Wang M, Yuan Q, Xie L. Mesenchymal Stem Cell-Based Immunomodulation: Properties and Clinical Application. Stem Cells Int 2018;2018:3057624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Galipeau J, Sensébé L. Mesenchymal Stromal Cells: Clinical Challenges and Therapeutic Opportunities. Cell Stem Cell 2018;22(6):824–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Squillaro T, Peluso G, Galderisi U. Clinical Trials With Mesenchymal Stem Cells: An Update. Cell Transplant 2016;25(5):829–48. [DOI] [PubMed] [Google Scholar]
- [32].Lee KY, Mooney DJ. Hydrogels for tissue engineering. Chem Rev 2001;101(7):1869–79. [DOI] [PubMed] [Google Scholar]
- [33].Sargeant TD, Desai AP, Banerjee S, Agawu A, Stopek JB. An in situ forming PEGcollagen hydrogel for tissue regeneration. Acta Biomater 2012;8(1):124–32. [DOI] [PubMed] [Google Scholar]
- [34].Irawan V, Sung TC, Higuchi A, Ikoma T. Collagen Scaffolds in Cartilage Tissue Engineering and Relevant Approaches for Future Development. Tissue Eng Regen Med. 2018;15(6):673–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Stoppel WL, Ghezzi CE, McNamara SL, Black LD 3rd, Kaplan DL. Clinical applications of naturally derived biopolymer-based scaffolds for regenerative medicine. Ann Biomed Eng. 2015;43(3):657–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Schmidt CE, Baier JM. Acellular vascular tissues: natural biomaterials for tissue repair and tissue engineering. Biomaterials 2000;21(22):2215–31. [DOI] [PubMed] [Google Scholar]
- [37].Deshmukh M, Singh Y, Gunaseelan S, Gao D, Stein S, Sinko PJ. Biodegradable poly(ethylene glycol) hydrogels based on a self-elimination degradation mechanism. Biomaterials 2010;31(26):6675–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lutolf MP, Lauer-Fields JL, Schmoekel HG, Metters AT, Weber FE, Fields GB, et al. Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: engineering cell-invasion characteristics. Proc Natl Acad Sci U S A 2003;100(9):5413–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Tessmar JK, Göpferich AM. Customized PEG-derived copolymers for tissue-engineering applications. Macromol Biosci 2007;7(1):23–39. [DOI] [PubMed] [Google Scholar]
- [40].Nallasamy N, Grove KE, Legault GL, Daluvoy MB, Kim T. Hydrogel ocular sealant for clear corneal incisions in cataract surgery. J Cataract Refract Surg 2017;43(8):1010–4. [DOI] [PubMed] [Google Scholar]
- [41].Lee HJ, Lee JS, Chansakul T, Yu C, Elisseeff JH, Yu SM. Collagen mimetic peptide-conjugated photopolymerizable PEG hydrogel. Biomaterials 2006;27(30):5268–76. [DOI] [PubMed] [Google Scholar]
- [42].Vanderhooft JL, Mann BK, Prestwich GD. Synthesis and characterization of novel thiol-reactive poly(ethylene glycol) cross-linkers for extracellular-matrix-mimetic biomaterials. Biomacromolecules 2007;8(9):2883–9. [DOI] [PubMed] [Google Scholar]
- [43].Steward AJ, Kelly DJ. Mechanical regulation of mesenchymal stem cell differentiation. J Anat 2015;227(6):717–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Cesarz Z, Tamama K. Spheroid Culture of Mesenchymal Stem Cells. Stem Cells Int 2016;2016:9176357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Fernandes-Cunha GM, Chen K. In situ-forming collagen hydrogel crosslinked via multi-functional PEG as a matrix therapy for corneal defects. Sci Rep, 2020;10(1):16671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Zhao B, Ma A, Martin FL, Fullwood NJ. An investigation into corneal alkali burns using an organ culture model. Cornea 2009;28(5):541–6. [DOI] [PubMed] [Google Scholar]
- [47].Kasper G, Dankert N, Tuischer J, Hoeft M, Gaber T, Glaeser JD et al. Mesenchymal stem cells regulate angiogenesis according to their mechanical environment. Stem Cells. 2007;25(4):903–10. [DOI] [PubMed] [Google Scholar]
- [48].Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007t;25:2648–59. [DOI] [PubMed] [Google Scholar]
- [49].Ho IA, Toh HC, Ng WH, Teo YL, Guo CM, Hui KM et al. Human bone marrowderived mesenchymal stem cells suppress human glioma growth through inhibition of angiogenesis. Stem Cells. 2013;31(1):146–55. [DOI] [PubMed] [Google Scholar]
- [50].Di G, Zhao X, Qi X, Zhang S, Feng L, Shi W et al. VEGF-B promotes recovery of corneal innervations and trophic functions in diabetic mice. Sci Rep. 2017;7:40582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Fernandes-Cunha GM, Na KS, Putra I, Lee HJ, Hull S, Cheng YC et al. Corneal Wound Healing Effects of Mesenchymal Stem Cell Secretome Delivered Within a Viscoelastic Gel Carrier. Stem Cells Transl Med. 2019;8(5):478–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Samaeekia R, Rabiee B, Putra I, Shen X, Park YJ, Hematti P. Effect of Human Corneal Mesenchymal Stromal Cell-derived Exosomes on Corneal Epithelial Wound Healing. Invest Ophthalmol Vis Sci. 2018;59(12):5194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Chaurasia SS, Kaur H, de Medeiros FW, Smith SD, Wilson SE. Dynamics of the expression of intermediate filaments vimentin and desmin during myofibroblast differentiation after corneal injury. Exp. Eye Res. 2009;89(2):133–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Hydrogel degradation.
Supplemental Figure 2. Cell proliferation fold change (normalized by day 2).