Abstract
Bacterial imbalances are observed in intestinal diseases and fecal microbiota transplantation (FMT) has been used to restore the intestinal microbiota of horses. However, there is evidence that the current methods proposed for FMT in horses have limited efficacy. The objective of this study was to concentrate the bacteria present in the donor stool by centrifugation, and to test the effect in horses with antibiotic-induced dysbiosis. One healthy 11-year-old horse was selected as a fecal donor and 9 horses were given trimethoprim sulfadiazine (TMS) for 5 days to induce dysbiosis. Horses received either a concentrated FMT (cFMT, n = 3), fresh unconcentrated FMT (fFMT, n = 3), or 10% glycerol solution (vehicle, VEH, n = 3) by nasogastric tube for 3 days. Fecal samples were collected on Days 0, 4, 9, 11, and 21 for microbiota analysis (Illumina sequencing). The TMS significantly changed the bacterial composition of horses’ feces (D0 versus D4). The composition of the cFMT and fFMT recipient horses was significantly different after transplantation compared to after antibiotic-induced dysbiosis (D4 versus D11), whereas the microbiota of the vehicle recipients was not, indicating that both protocols induced transient changes. However, preparation of FMT solutions markedly changed the original composition present in the donor’s feces, with significant enrichment of Escherichia genus in the cFMT. Individual susceptibility to restoration of the microbiota was observed in horses, similar to what is known for other species. Our results suggest that concentrating bacteria should not be recommended in preparation of FMT solutions and that further research is required to improve current methods recommended to perform FMT in horses.
Résumé
Des déséquilibres bactériens sont observés dans les maladies intestinales et la transplantation de microbiote fécal (FMT) a été utilisée pour la restaurer le microbiote intestinal des chevaux. Cependant, que les méthodes actuelles proposées pour FMT chez les chevaux ont une efficacité limitée. L’objectif de cette étude était de concentrer les bactéries présentes dans les selles du donneur par centrifugation, et de tester leur effet chez des chevaux atteints de dysbiose induite par les antibiotiques. Un cheval sain de 11 ans a été sélectionné comme donneur fécal et 9 chevaux ont reçu du triméthoprime sulfadiazine (TMS) pendant cinq jours pour induire une dysbiose. Les chevaux ont reçu soit une FMT concentrée (cFMT, n = 3), une FMT fraîche non concentrée (fFMT, n = 3) ou une solution de glycérol à 10 % (véhicule, VEH, n = 3) par sonde naso-gastrique pendant 3 jours. Des échantillons fécaux ont été prélevés aux jours 0, 4, 9, 11 et 21 pour analyse du microbiote (séquençage Illumina). Le TMS a significativement modifié la composition bactérienne des matières fécales des chevaux (D0 versus D4). La composition des chevaux receveurs cFMT et fFMT était significativement différente après la transplantation par rapport à la dysbiose induite par les antibiotiques (D4 versus D11), alors que le microbiote des receveurs de véhicules ne l’était pas, indiquant que les deux protocoles induisaient des changements transitoires. Cependant, la préparation des solutions FMT a considérablement modifié la composition originale présente dans les matières fécales du donneur, avec un enrichissement significatif du genre Escherichia dans le cFMT. Une susceptibilité individuelle à la restauration du microbiote a été observée chez les chevaux, à l’instar de ce qui est connu chez d’autres espèces. Nos résultats suggèrent que la concentration des bactéries ne devrait pas être recommandée dans la préparation des solutions FMT et que des recherches supplémentaires sont nécessaires pour améliorer les méthodes actuelles recommandées pour effectuer la FMT chez les chevaux.
(Traduit par les auteurs)
Introduction
Gut bacteria of the host, such as in nutrition (1), energy metabolism (2), immune development (3), and host defense against harmful pathogens (4). For example, the equine gut microbiota plays an important role in cellulose fermentation and short-chain fatty acid (SCFA) production to provide a horse with an important source of energy (5). The gut microbiota are composed of many microorganisms such as viruses, fungi, and archaea, with bacteria comprising the vast majority of them (6).
Although the use of the term dysbiosis remains a topic of discussion, it can be defined as significant changes of the normal composition of the microbiota. Many factors are associated with changes in the equine intestinal microbiota and dysbiosis which are present in cases of gastrointestinal (GI) diseases, such as colitis and colic (7,8) and with antimicrobial administration (9,10). Importantly, GI diseases are the leading causes of morbidity and mortality in horses (11,12), which can reach up to 40% (12). Therefore, advances in therapeutic approaches of equine colitis are warranted.
There are many methods of microbiota manipulation that might be used to restore a dysbiotic environment including probiotics, prebiotics, synbiotics, postbiotics, and fecal microbiota transplantation (FMT) (13,14). Of these methods, FMT is increasingly being used as therapy for GI diseases due to its success in treating human patients with recurrent Clostridioides difficile infection (15). Fecal microbiota transplantation involves administration of stool from a healthy donor to a patient with dysbiosis (16). Fecal microbiota transplantation has also seen success in several other species such as dogs (17), cattle (18,19), and pigs (20,21).
In the absence of well-controlled clinical studies demonstrating the efficacy of FMT in the treatment of equine dysbiosis, controversial results are reported in the literature. Although some reports claim that the procedure can restore the microbiota of patients with diarrhea (22,23), other studies reported no changes before and after FMT (24). Therefore, additional controlled studies of FMT treatment in horses are needed.
The use of FMT administered via colonoscopy is more efficient in treating colonic dysbiosis compared to the oral route in humans, but the long equine small colon precludes the solution infused by enema from reaching the large colon and cecum (25). Consequently, FMT needs to be administered via nasogastric tube, which decreases bacterial viability by exposure to gastric acidity, enzymatic activity in the small intestine, and fermentation in the cecum (26). Therefore, alternative protocols are required to enhance FMT capacity of distal gut colonization in horses.
The development of culture-independent DNA-sequencing technologies, such as next generation sequencing, has made it possible for in-depth characterization of the bacterial communities present in the intestinal microbiota (13). Trimethoprim sulfadiazine (TMS) has been shown to significantly alter equine intestinal microbiota (9,10,27), suggesting that oral administration of TMS could be used as a model for equine dysbiosis.
The objective of this study was to test a protocol using concentrated bacteria to improve FMT in horses. Thus, we tested the hypothesis that concentrating bacteria by centrifugation would more rapidly correct antibiotic-induced dysbiosis in horses compared to the current FMT recommendations.
Materials and methods
Ethics statement
Experimental procedures were performed in accordance with the Canadian Council for Animal Care guidelines and were approved by the Animal Care Committee of the Université de Montréal (#19Rech2025).
Animal selection
One healthy 11-year-old female Standardbred horse (teaching animal housed at the institution) weighing 490 kg was selected as a fecal donor (DON). The mare had no history of gastrointestinal disease and did not receive antimicrobials or other medications during the 3 mo prior to the study. The donor horse was fed hay with daily access to a paddock and access to a salt block with mineral supplements. Feces from the donor horse tested negative for the presence of Salmonella enterica (4 consecutive cultures), Clostridium perfringens and Clostridioides difficile (culture), and parasites eggs (egg counting using the quantitative Wisconsin technique) (25). The microbiological tests were conducted at the Centre de diagnostic vétérinaire de l’Université de Montréal.
Nine adult horses belonging to a research herd of asthmatic animals housed at a research facility located within 5 km from the donor horse were enrolled. All horses were in remission and had no history of gastrointestinal diseases or antimicrobial administration during the previous 3 mo. Three months before the study, horses received methylprednisolone, and 2 mo before the study, horses received a dewormer (ivermectin and praziquantel) and a Vetera Gold vaccine. The animals were housed on turnout with shelter, kept on grass pasture, fed silage, and had access to a salt block. Table S1 (available online from: www.canadianveterinarians.net) summarizes the studied population including previous treatments received.
Study design
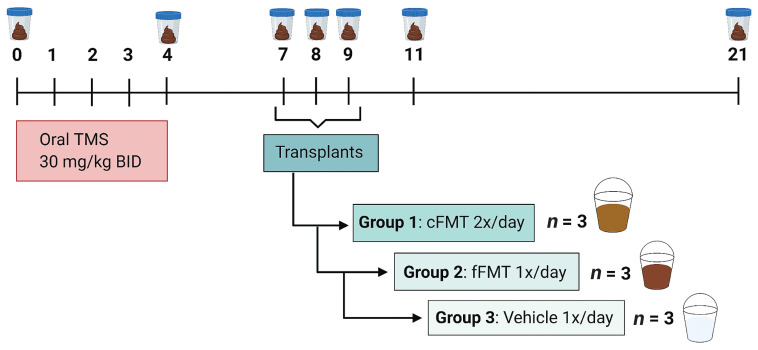
All 9 horses received TMS (30 mg/kg, PO, q12h) for 5 d (D0 to D4). Three days after the last dose (Day 7), the 9 horses were randomly assigned to each of 2 treatment groups or a vehicle group (controls). The first group received 3.2 L of concentrated FMT (cFMT), q12h by nasogastric tube for 3 consecutive days (D7 to D9). The solution of cFMT was prepared by thawing and mixing 8 plastic bags each containing 400 mL of cFMT (total volume 3.2 L). The second group received 3.2 L of fresh FMT (fFMT) as per current recommendations, q24h for 3 d (D7 to D9), prepared the same morning of administration. The vehicle group (VEH) received 3.2 L of 10% glycerol in 0.9% saline solution q24h for 3 d (D7 to D9). The same donor was used for all horses receiving cFMT and fFMT. All horses received 500 mL of 0.1 molar solution of sodium bicarbonate by nasogastric tube minutes before treatment administration to increase the pH of the stomach (28). Figure 1 shows a detailed experimental timeline. Horses were monitored daily (physical examination, fecal consistency) from Days 0 to 9, and again on Days 11 and 21. The experiment was carried out concomitantly in all 9 horses.
Figure 1.
Experimental timeline. Trimethoprim sulfadiazine (TMS) administration for 5 consecutive days (Days 0 to 4). Transplants were performed by nasogastric tube for 3 consecutive days (Days 7, 8, 9). Group 1 (3 horses) received 3.2 L of concentrated FMT (cFMT) q12h for 3 d. Group 2 (3 horses) received 3.2 L fresh unconcentrated fFMT (fFMT) q24h for 3 d. Group 3 (3 horses), the vehicle recipients (VEH) received 3.2 L 10% glycerol in 0.9% saline solution q24h for 3 d. Fecal samples were collected on Days 0, 4, 7, 8, 9, 11, and 21.
Fecal samples were collected from the rectum before and after antibiotic administration (Days 0 and 4, respectively), before and during the transplants (Days 7 and 8), as well as after the transplants (Days 9, 11, and 21). A fecal sample from the rectum of the donor horse was collected during the cFMT fecal collection period. Samples of the fFMT and cFMT solutions were also collected on each of the 3 d of FMT administration. Fecal samples were refrigerated and stored at −80°C within 3 h of collection until DNA extraction.
Protocol for bacterial concentration
Feces from the donor horse were collected using a fecal collector (Equisan Marketing Pty, Southbank VIC, Australia) that was attached to the horse and maintained overnight to obtain approximately 10 kg of feces per day, over a 23-day period. The concentrated solution (cFMT) was made by adding 2 L of distilled water to 1 kg of feces, mixing thoroughly to break the fecal balls, and then filtered with a cheesecloth to remove large particles. The filtered feces were then added to 500 mL centrifuge bottles and centrifuged at 24 470 × g for 30 min. The supernatant was discarded, and the pellet was resuspended in 400 mL of 10% glycerol in 0.9% saline solution. The cFMT was transferred into plastic bags and stored at −80°C until use.
Protocol for fresh FMT preparation
The fresh FMT (fFMT) was made from feces collected using the fecal collector on the donor horse the evening before each treatment day (Days 6, 7, and 8). On the day of the transplants, 3.2 L of water were added to 1.6 kg of feces, mixed thoroughly, and the mixture strained through a cheesecloth to remove large particles (25). This procedure was repeated until enough fFMT was made to treat the 3 horses (3.2 L × 3 horses = 9.6 L).
Bacterial quantification
Samples of the cFMT and fFMT solutions that were given to the respective recipient horses were collected on each day of transplantation (D7, D8, D9) for bacterial quantification using fluorescence-activated cell sorter (FACS) analysis. Briefly, samples were centrifuged at 10 000 × g for 2 min to pellet the cells and resuspended in 0.85% NaCl. The previous steps were repeated twice for a final resuspension in 0.85% NaCl. Samples were diluted 10-fold, and then 10 μL were added to 977 μL 0.85% NaCl along with 10 μL of the microsphere standard, as per the manufacturer’s recommendations (LIVE/DEAD BacLight Bacterial Viability and Counting Kit; Molecular Probes, Thermo-Fisher Scientific, Mississauga, Ontario). Data acquisition was performed on an LSR Fortessa X-20 (BD Biosciences Canada, Mississauga, Ontario) using FACSDiva software v9.0 (BD Biosciences Canada). The data were further analyzed using FlowJo software v10.7.0 (FlowJo, LLC). The number of bacteria per milliliter was calculated using the following formula as per manufacturer’s recommendations (LIVE/DEAD BacLight Bacterial Viability and Counting Kit, Molecular Probes, Thermo-Fisher):
Microbiota analysis
Total DNA was extracted using a commercial kit (DNeasy PowerSoil Kit, QIAGEN, Toronto, Ontario) following the manufacturer’s instructions. Polymerase chain reaction amplification of the V4 region of the 16S rRNA gene was performed using the primers 515F (GTGCCAGCMGCCGCGGTAA) and 806R (GGACTACHVGGGTWTCTAAT) as previously recommended (29). Sequencing was performed using an Illumina MiSeq platform for 250 cycles from each end at the Génome Québec Innovation Centre.
Sequencing and statistical analysis
Bioinformatic analysis was performed using the software mothur (30) following the Standard Operating Procedure previously described (31). Sequencing reads were aligned with the SILVA reference, clustered at 97% similarity and taxonomic classification was obtained using the Ribosomal Databank Project (RDP). Sequences classified as the same genus (94% similarity) were clustered together for further analyses (Phylotypes).
The Chao richness estimator, Simpson’s index, and Shannon index were calculated for characterization of richness (number of different genera present in a community) and diversity (number of genera present and their relative abundances). Those indices were compared between donor solution and recipients, and between recipients at the different time points using a paired Student’s t-test and 1-way repeated measures analysis of variance (ANOVA) using the software GraphPad Prism v9.0.0. Beta diversity (comparison of taxonomic composition between each sample) was characterized by the Jaccard index and the Yue and Clayton index to evaluate community composition (membership) and structure, respectively. It is important to note that membership analysis considers only the presence or absence of bacterial taxa, whereas the structure also considers how often that bacteria appeared in the analysis (relative abundance). A 2-dimensional Principal Coordinate Analysis (PCoA) plot was generated to visualize the similarity between samples. Analysis of molecular variance (AMOVA) was used to determine significance of clustering between recipients in different treatment groups at different time points.
The most abundant bacteria (> 1%) were visualized by generating bar charts representing the relative abundance of the main phyla and genera in each horse. The linear discriminant analysis effect size (LEfSe), which uses a non-parametric factorial Kruskal-Wallis with a subsequent unpaired Wilcoxon test, was used to detect significant differences in relative abundances with respect to each group of interest (recipients before and after antibiotic administration, and after treatment), followed by a linear discriminant analysis (LDA) to estimate the effect size of each differentially abundant group (32). A P-value of less than 0.05 and LDA higher than 3 were used to determine significance.
Data from bacterial quantification using FACS analysis were expressed as mean ± SD and analyzed for significance using Student’s unpaired t-test (GraphPad Prism v9.0.0).
Results
Horses
The recipient horses presented no evident changes in behavior, appetite, temperature, respiratory and cardiac frequency, GI motility, and stool consistency.
Bacterial quantification
The number of bacteria per milliliter was significantly higher (P < 0.001) in the cFMT solution (3.96 × 109 ± 4.5 × 108) compared to the fFMT solution (1.36 × 109 ± 9.8 × 107), indicating that the protocol successfully concentrated the bacteria.
Microbiota analysis
A total of 11 084 884 reads were obtained from 78 samples 6 543 737 of which passed all quality filters and were used in the analysis. To normalize the number of reads across all samples and decrease bias of non-uniform sizes, a subsample of 12 144 reads per sample was used for analysis.
Alpha diversity
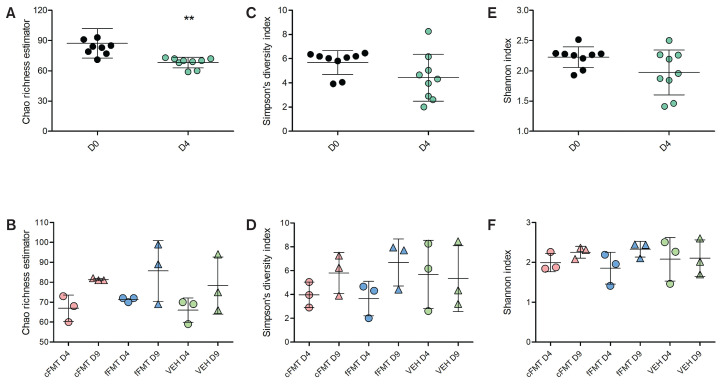
As expected, a significant decrease in richness (Chao richness estimator) was observed after antibiotic administration (P < 0.01, paired Student’s t-test of D0 fecal samples versus D4 fecal samples, Figure 2 A). No significant difference in richness was observed after microbiota transplant (D9) in either cFMT, fFMT, or control groups compared to D4 addressed by the ANOVA analysis (Figure 2 B). However, as richness was consistently numerically increased in all horses with cFMT, a post-hoc t-test comparison was performed and demonstrated significance (P = 0.03). Diversity (Simpson’s and Shannon indices) was not significantly different after antibiotic administration, or when comparing samples after antibiotic administration to those after the microbiota transplant (Figure 2 C–F).
Figure 2.
Alpha diversity indices. Chao richness estimator of all horses before (D0) and after (D4) antibiotic administration (A) and before (D7) and after (D9) transplantation (B). Simpson’s index of all horses before (D0) and after (D4) antibiotic administration (C) and before (D7) and after (D9) transplantation (D). Shannon index of all horses before (D0) and after (D4) antibiotic administration (E) and before (D7) and after (D9) transplantation (F). cFMT represents horses receiving the concentrated FMT, whereas fFMT represents the horses receiving the fresh FMT. VEH represents the horses receiving 10% glycerol in 0.9% saline solution. Statistical analysis was performed using paired Student’s t-tests (A, C, E) and 1-way ANOVA (B, D, F). Bars represent mean and SD.
** P ≤ 0.01.
Beta diversity
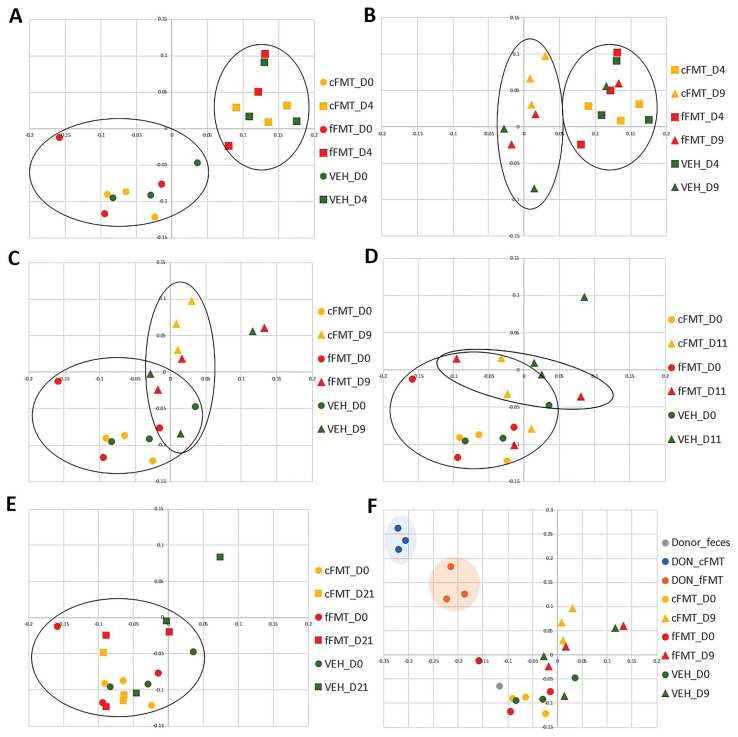
A complete list of P-values for beta-diversity comparisons obtained from the AMOVA test is provided in Table I. A significant difference was observed in community composition after antibiotic administration compared to baseline (P < 0.001, D0 versus D4, Figure 3 A), confirming the capacity of TMS to induce changes in the distal gut microbiota. Composition of cFMT and fFMT recipients after transplantation was significantly different than after antibiotic administration (D4 versus D9), whereas vehicle recipients were not (P = 0.004 for cFMT; P = 0.04 for fFMT; P = 0.26 for VEH; Figure 3 B). When compared to baseline values, the composition of cFMT and fFMT recipients after transplantation (D0 versus D9) was significantly different, but so was the composition of vehicle recipients (P = 0.004 for cFMT; P = 0.004 for fFMT; P = 0.02 for VEH; Figure 3 C).
Table I.
P-values obtained from the AMOVA test for structure (Yue & Clayton index) and composition (Jaccard index) comparing groups at different sampling times. Bolded data represent significant P-values < 0.05.
| Group comparisons | Structure | Composition |
|---|---|---|
| D0 versus D4 | 0.11 | < 0.001 |
| cFMT_D4 versus cFMT_D9 | 0.44 | 0.004 |
| fFMT_D4 versus fFMT_D9 | 0.22 | 0.04 |
| VEH_D4 versus VEH_D9 | 0.11 | 0.26 |
| cFMT_D0 versus cFMT_D9 | 0.38 | 0.004 |
| fFMT_D0 versus fFMT_D9 | 0.27 | 0.004 |
| VEH_D0 versus VEH_D9 | 0.06 | 0.02 |
| cFMT_D9 versus VEH_D9 | 0.03 | 0.37 |
| fFMT_D9 versus VEH_D9 | 0.02 | 0.89 |
| cFMT_D9 versus fFMT_D9 | 0.71 | 0.16 |
| D4 versus cFMT_D11 | 0.13 | 0.008 |
| D4 versus fFMT_D11 | 0.01 | < 0.001 |
| D0 versus cFMT_D11 | 0.68 | 0.008 |
| D0 versus fFMT_D11 | 0.08 | 0.13 |
| cFMT_D11 versus fFMT_D11 | 0.17 | 0.7 |
| cFMT_D0 versus DON_cFMT | 0.003 | 0.004 |
| fFMT_D0 versus DON_fFMT | 0.005 | 0.003 |
| cFMT_D9 versus DON_cFMT | 0.09 | 0.09 |
| fFMT_D9 versus DON_fFMT | 0.11 | 0.11 |
| DON_cFMT versus DON_fFMT | 0.1 | 0.1 |
Figure 3.
Principal coordinate analysis (PCoA) of bacterial communities’ composition present in the feces of healthy donor horse, FMT recipients, and vehicle recipients. Bidimensional representation of the principal coordinate analysis of bacterial communities’ composition addressed by the Classic Jaccard analysis. A — Membership before antibiotic administration (D0) and after antibiotic administration (D4) of recipients receiving the concentrated FMT (cFMT), recipients receiving the fresh FMT (fFMT) and recipients receiving the vehicle (VEH). B — Membership after antibiotic administration (D4) and after transplantation (D9). C — Membership before antibiotic administration (D0) and after transplantation (D9). D — Membership before antibiotic administration (D0) and 6 d after transplantation (D15). E — Membership before antibiotic administration (D0) and 12 d after transplantation (D21). F — Membership of the donor feces, the donor’s fecal suspensions (DON_cFMT, DON_fFMT), and of the recipients before antibiotic administration (D0), and after transplantation (D9). Circles were used to highlight the major clustering.
The community composition from the cFMT and fFMT donor’s fecal suspensions were significantly different from the cFMT and fFMT recipients’ baseline values (D0 versus DON), respectively (P = 0.004 for cFMT; P = 0.003 for fFMT; Figure 3 F). Both fecal suspensions were also different from the donor fecal microbiota (fresh sample, pre-processing), indicating that the preparation of FMT alters the microbiota present in the feces of the healthy donor. Indeed, the fecal sample obtained from the donor clustered with the baseline composition of all recipients (grey circle in Figure 3 F).
No significant difference was observed in community structure after antibiotic administration compared with baseline values of all recipients (P = 0.11, all recipients on D0 versus D4, Figure S1 A; all supplementary figures are available online from: www.canadianveterinarians.net), indicating that TMS affects the rare populations of a community, but not the most abundant. The structure was not significantly different when comparing values after transplantation to values after antibiotic administration (D4 versus D9) (P = 0.44 for cFMT; P = 0.22 for fFMT; P = 0.11 for VEH; Figure S1 B), indicating no impact of treatment. Similarly, no significant difference was observed when comparing the structure of baseline values to after transplantation (D0 versus D9) (P = 0.38 for cFMT; P = 0.27 for fFMT; P = 0.06 for VEH; Figure S1 C). The fecal microbiota structure of the donor clustered together with the baseline structure of all recipients (Figure S1 F). The structures from the cFMT and fFMT of the donor’s fecal suspensions were significantly different from the cFMT and fFMT recipients’ baseline structure (D0 versus DON) (P = 0.003 for cFMT; P = 0.005 for fFMT; Figure S1 F), as well as from the donor’s fecal microbiota (Figure S1 F).
Relative abundances
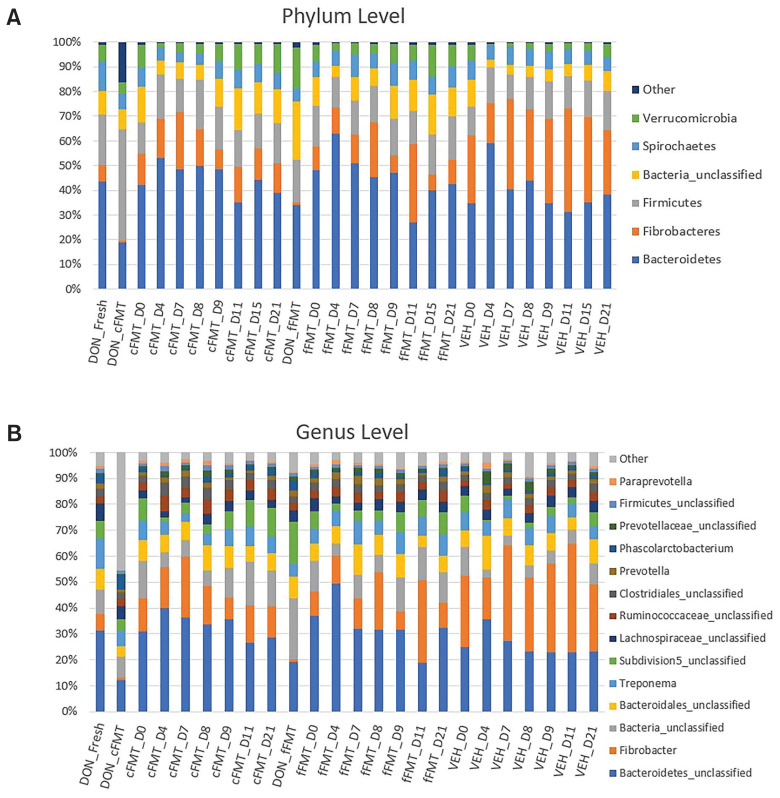
The relative abundances at the phylum and genus levels found in each group at the various sampling times are shown in Figure 4. Bacteroidetes was the most abundant phylum among recipient horses (45%), followed by Fibrobacteres (19%), Firmicutes (15%), unclassified bacteria (9%), Spirochaetes (7%), and Verrucomicrobia (5%) (Figure 4 A). The most abundant taxa classified at lower taxonomic levels included unclassified Bacteroidetes, Fibrobacter, unclassified bacteria, unclassified Bacteroidales, Treponema, unclassified subdivision 5 (Verrucomicrobia), unclassified Lachnospiraceae, unclassified Ruminococcaceae, unclassified Clostridiales, Prevotella, Phascolarctobacterium, unclassified Prevotellaceae, and unclassified Firmicutes (Figure 4 B).
Figure 4.
Relative abundance of predominant bacteria at the phylum (A) and genus (B) levels. Recipients before and after antibiotic administration, after transplantation, and vehicle recipients are represented. Fecal samples collected directly from the donor (DON_Fresh) and from the transplanted solutions (DON_cFMT, DON_fFMT) are represented as well. Only the 6 most common phyla and 14 most common genera are represented.
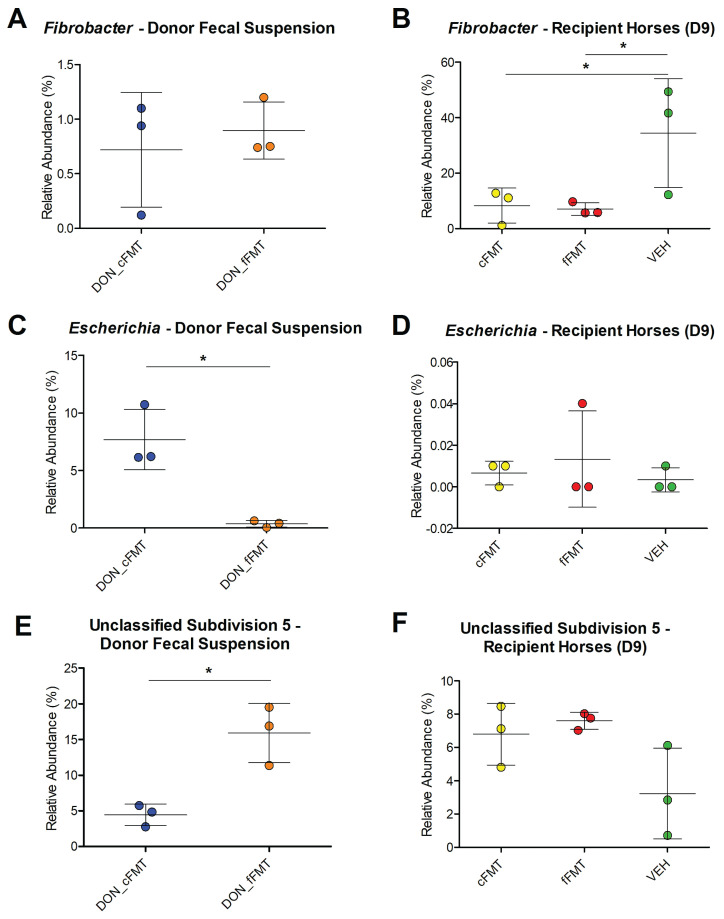
The relative abundance of Fibrobacter was similar between cFMT and fFMT donor fecal suspensions (P = 0.63, Figure 5 A), but significantly lower in the cFMT and fFMT groups at D9 compared to VEH (P < 0.05, Figure 5 B). The relative abundance of the Escherichia genus was significantly increased in the cFMT donor fecal suspension (P = 0.009, Figure 5 C); but this was not represented in the cFMT group on D9 compared to fFMT and VEH (P = 0.69, Figure 5 D). A significant greater relative abundance of the unclassified subdivision 5 genus was observed in the fFMT donor fecal suspension (P = 0.01, Figure 5 E); however, no significant difference was observed in horses treated with that solution (P = 0.07, Figure 5 F).
Figure 5.
Relative abundances of genera in donor fecal suspensions and treatment groups. Relative abundances in the cFMT donor fecal suspension (DON_cFMT) and fFMT donor fecal suspension (DON_fFMT) of Fibrobacter (A), Escherichia (C) and unclassified subdivision 5 (E) on D7, D8, and D9. Relative abundances in the cFMT recipients (cFMT), fFMT recipients (fFMT), and vehicle recipients (VEH) of Fibrobacter (B), Escherichia (D), and unclassified subdivision 5 (F) on D9. Note that the scale of relative abundances (y-axis) is different between each graph.
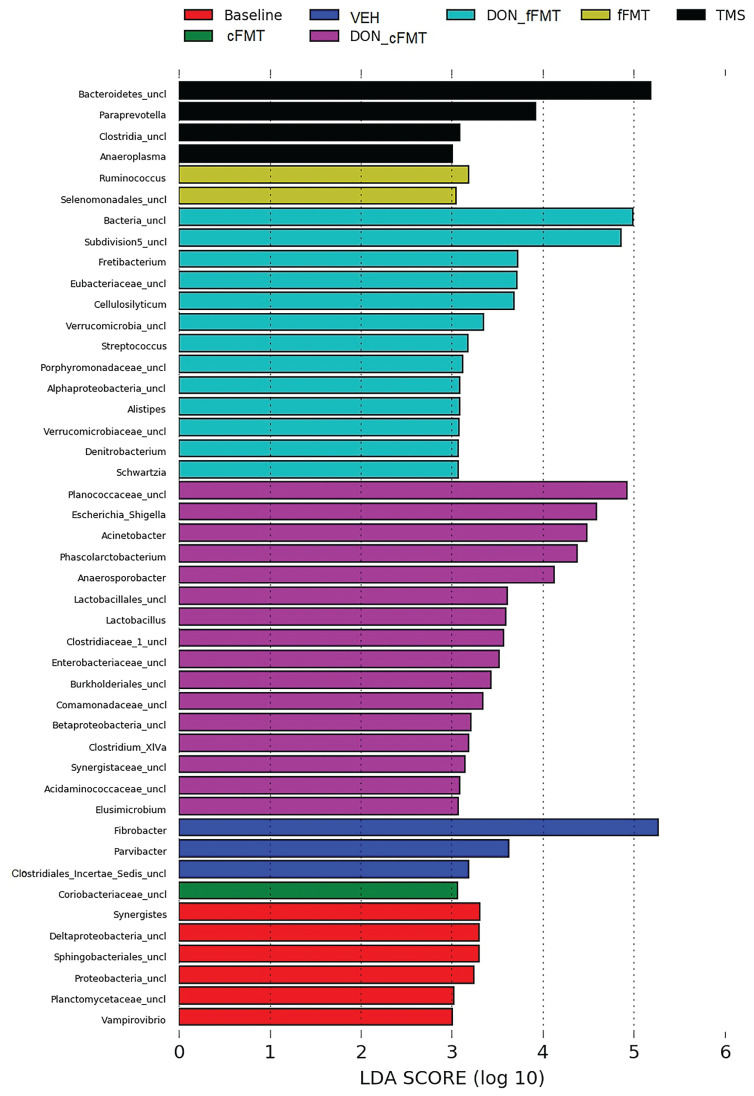
Overrepresentation in taxa between donor fecal suspensions, and between recipients before and after antibiotic administration, and before and after transplantation seen using LEfSe analysis is represented in Figure 6.
Figure 6.
Linear Discriminant Analysis Effect Size (LEfSe) of treatment groups and time points. LEfSe analysis showing taxa that were significantly overrepresented in cFMT donor fecal suspensions (DON_cFMT), fFMT donor fecal suspensions (DON_fFMT), in the recipients before antibiotic administration at D0 (Baseline), after antibiotic administration at D4 (TMS), in the cFMT recipient group at D9 (cFMT), fFMT recipient group at D9 (fFMT), and the vehicle recipients at D9 (VEH).
Discussion
This study tested the effect of a concentrated bacterial suspension on the fecal microbiota after antibiotic induced dysbiosis in horses. Administration of concentrated (cFMT) and fresh unconcentrated (fFMT) solutions was associated with changes in microbiota composition after 3 d of treatment (D4 versus D9), although this was not observed in the control group. Interestingly, it can be observed from the PCoA results that although cFMT solution had a homogeneous effect on animals receiving the solution, there was great individual variation in horses receiving fFMT and in controls (VEH). It is noteworthy that the changes induced by administration of cFMT did not resemble the composition of the normal microbiota of horses seen at baseline (D0). Therefore, it is unclear whether those changes are in fact beneficial for horses recovering from a dysbiotic state. Data from this study suggest that FMT has the potential to change, but not to restore the microbiota composition of horses after treatment with antimicrobials.
Conversely, neither antibiotic treatment nor FMT affected the community structure, which considers the relative abundance of each taxon, suggesting that the main groups of bacteria were not affected by treatment. It is possible that further studies should focus on diseases models (i.e., colitis patients) rather than antibiotic-induced dysbiosis.
Current recommendations would be to perform FMT in horses once a day and that is what the few studies published in the literature have used (22–24,27). In this study, we used a concentrated solution containing 3× more bacteria, which was administered q12h (thereby increasing the dose to 6× what is normally used) in an attempt to increase the number of bacteria donated to the horses thus increasing the chances of successful manipulation of the microbiota. Despite no side effects being observed in the 3 horses receiving the cFMT, the protocol cannot be recommended at this time considering lack of information regarding the benefits of the induced changes, as well as the increased abundance of Escherichia in the cFMT solution. This might be especially important when treating sick animals.
As expected, the donor’s fecal microbiota before processing for FMT preparation clustered with the fecal microbiota of all recipients at baseline (D0), indicating that their microbiota was similar before treatment even though the donor and recipients were housed at different facilities. However, the microbiota of both cFMT and fFMT donor fecal suspensions substantially differed from the donor feces, suggesting a potential marked impact of handling feces. Interestingly, although the cFMT donor fecal suspension had a high relative abundance of Proteobacteria (15.7%) mainly caused by increased Escherichia genus, the feces of the cFMT recipient horses had low abundances of this phylum (< 1%) on all days. Proteobacteria are part of the gut microbiota of healthy horses (33,34), but it has also been associated with dysbiosis (35,36). Studies have reported that handling feces at room temperature in ambient air greatly decreases the abundance of anaerobic bacteria and increases the abundance of opportunistic facultative aerobic bacteria such as E. coli (37) and Proteobacteria (38). In this study, the cFMT donor fecal suspension was exposed to ambient air for 3 to 5 h longer than the fFMT donor fecal suspension with the addition of the centrifugation step. Furthermore, cFMT donor fecal suspensions underwent freeze-thawing, which was also shown to affect bacterial viability and composition (40). Although this study did not measure bacterial viability of the fFMT and cFMT suspensions prior to treatment, studies have shown a negative impact of oxygen exposure and freeze-thawing cycles (26,37).
In addition, abundance of Subdivision 5, which are also part of a healthy equine gut (39,40), was higher in the fFMT donor fecal suspension compared to the cFMT donor fecal suspension. These results suggest that the relative abundance of Subdivision 5 might be negatively affected by longer exposure to oxygen or freezing cycles. Therefore, it might be important to minimize exposure time to oxygen to prevent the overgrowth of potential pathogenic bacteria, but further studies investigating the best conditions for FMT preparation in horses are required.
The use of FMT to treat GI issues in horses is widely adopted. The current FMT protocol proposed to correct dysbiosis in horses (25) using fresh feces failed to induce microbiota changes in 6 horses with diarrhea (24). Another study in which FMT was administered to geriatric horses with diarrhea reported a significant increase in alpha-diversity in 3 out of 5 horses; however, no control group was included in this study (22). Improvements in fecal scores and alpha-diversity was reported in 12 horses with colitis treated with FMT, compared to 10 control horses treated at another hospital (23). Therefore, larger controlled clinical trials demonstrating clinical and microbiological benefits of FMT in horses remain to be performed.
This study observed the most abundant phyla to be Bacteroidetes, followed by Fibrobacteres and Firmicutes. Compared with other studies, horses with colitis have a high relative abundance of Bacteroidetes (40%), Firmicutes (30.3%), and Proteobacteria (18.7%), whereas healthy horses have a high relative abundance of Firmicutes (68.1%), Bacteroidetes (14.2%), and Proteobacteria (10.2%) (35). Differences can be due to the variation in methodologies used. One study using the same methods on the same horses had similar results to the study herein (39).
Furthermore, the Fibrobacteres phylum, and correspondingly, the Fibrobacter genus, were observed in significantly relatively high levels in vehicle recipients, compared to horses receiving cFMT or fFMT. Fibrobacter was also increased in healthy horses compared to horses with metabolic syndrome (41) and asthma (39) and was part of the core gut microbiota of the horse (42), with abundances increasing with age in foals (43).
The composition of one vehicle recipient was different from all other recipients, suggesting that the horse remained in a dysbiotic state up to 17 d after antibiotic administration. This result had been previously observed in horses and humans in whom the gut microbiota of individuals can take days or months to recover after antibiotic administration and return to the original composition (9,44,45).
As frequently observed in other studies investigating the microbiota of horses (22,42,46), the main limitation of this study was the small sample size. However, this study brings new information to guide future research on microbiota manipulation in horses. Furthermore, a great degree of interindividual variability is present in response to treatments aimed at manipulating the gut microbiota, such as FMT (47), probiotics (48), prebiotics (49), and dietary interventions (50), highlighting the importance of larger studies and the inclusion of control animals. Lastly, this study was performed in healthy horses with antibiotic-induced dysbiosis, which might substantially differ from inflammation-driven dysbiosis, such as in cases of naturally occurring colitis.
In conclusion, FMT has the potential to transiently change, but not to restore the microbiota composition of horses after antibiotic-induced dysbiosis. The composition of the transplanted solution greatly differed from the microbiota in healthy horses, possibly caused by oxygen exposure and extended preparation time at room temperature. Thus, the concentration protocol evaluated in this study cannot be recommended to treat clinical patients.
Acknowledgment
We acknowledge Dr. Jean-Pierre Lavoie for his horses and Khristine Picotte for her help during the experimental process.
Footnotes
All supplementary figures are available online from: www.canadianveterinarians.net
References
- 1.Moron R, Galvez J, Colmenero M, Anderson P, Cabeza J, Rodriguez-Cabezas ME. The Importance of the microbiome in critically ill patients: Role of nutrition. Nutrients. 2019;11:3002. doi: 10.3390/nu11123002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud D-J, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54:2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gensollen T, Iyer SS, Kasper DL, Blumberg RS. How colonization by microbiota in early life shapes the immune system. Science. 2016;352:539–544. doi: 10.1126/science.aad9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baumler AJ, Sperandio V. Interactions between the microbiota and pathogenic bacteria in the gut. Nature. 2016;535:85–93. doi: 10.1038/nature18849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glinsky MJ, Smith RM, Spires HR, Davis CL. Measurement of volatile fatty acid production rates in the cecum of the pony. J Anim Sci. 1976;42:1465–1470. doi: 10.2527/jas1976.4261465x. [DOI] [PubMed] [Google Scholar]
- 6.Rinninella E, Raoul P, Cintoni M, et al. What is the Healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms. 2019;7:14. doi: 10.3390/microorganisms7010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abutarbush SM, Carmalt JL, Shoemaker RW. Causes of gastrointestinal colic in horses in western Canada: 604 cases (1992 to 2002) Can Vet J. 2005;46:800–805. [PMC free article] [PubMed] [Google Scholar]
- 8.Stewart HL, Pitta D, Indugu N, et al. Changes in the faecal bacterial microbiota during hospitalisation of horses with colic and the effect of different causes of colic. Equine Vet J. 2021;53:1119–1131. doi: 10.1111/evj.13389. [DOI] [PubMed] [Google Scholar]
- 9.Costa MC, Stämpfli HR, Arroyo LG, Allen-Vercoe E, Gomes RG, Weese JS. Changes in the equine fecal microbiota associated with the use of systemic antimicrobial drugs. BMC Vet Res. 2015;11:19. doi: 10.1186/s12917-015-0335-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Pietro R, Arroyo LG, Leclere M, Costa MC. Species-level gut microbiota analysis after antibiotic-induced dysbiosis in horses. Animals (Basel) 2021;11:2859. doi: 10.3390/ani11102859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al Jassim RAM, Andrews FM. The bacterial community of the horse gastrointestinal tract and its relation to fermentative acidosis, laminitis, colic, and stomach ulcers. Veterinary Clinics of North America: Equine practice. 2009;25:199–215. doi: 10.1016/j.cveq.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 12.International Equine Colitis Research G. Science-in-brief: Report on the Havemeyer Foundation workshop on acute colitis of the adult horse. Equine Vet J. 2020;52:163–164. doi: 10.1111/evj.13223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Costa MC, Weese JS. Understanding the intestinal microbiome in health and disease. Veterinary clinics of North America: Equine practice. 2018;34:1–12. doi: 10.1016/j.cveq.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Halkjær SI, Christensen AH, Lo BZS, et al. Faecal microbiota transplantation alters gut microbiota in patients with irritable bowel syndrome: Results from a randomised, double-blind placebo-controlled study. Gut. 2018;67:2107–2115. doi: 10.1136/gutjnl-2018-316434. [DOI] [PubMed] [Google Scholar]
- 15.Quraishi MN, Widlak M, Bhala N, et al. Systematic review with meta-analysis: The efficacy of faecal microbiota transplantation for the treatment of recurrent and refractory Clostridium difficile infection. Aliment Pharmacol Ther. 2017;46:479–493. doi: 10.1111/apt.14201. [DOI] [PubMed] [Google Scholar]
- 16.Zhang F, Luo W, Shi Y, Fan Z, Ji G. Should we standardize the 1,700-year-old fecal microbiota transplantation? Am J Gastroenterol. 2012;107:1755. doi: 10.1038/ajg.2012.251. author reply p. 1755–1756. [DOI] [PubMed] [Google Scholar]
- 17.Pereira GQ, Gomes LA, Santos IS, Alfieri AF, Weese JS, Costa MC. Fecal microbiota transplantation in puppies with canine parvovirus infection. J Vet Intern Med. 2018;32:707–711. doi: 10.1111/jvim.15072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ribeiro GO, Oss DB, He Z, et al. Repeated inoculation of cattle rumen with bison rumen contents alters the rumen microbiome and improves nitrogen digestibility in cattle. Sci Rep. 2017;7:1276. doi: 10.1038/s41598-017-01269-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rager KD, George LW, House JK, DePeters EJ. Evaluation of rumen transfaunation after surgical correction of left-sided displacement of the abomasum in cows. J Am Vet Med Assoc. 2004;225:915–920. doi: 10.2460/javma.2004.225.915. [DOI] [PubMed] [Google Scholar]
- 20.Niederwerder MC, Constance LA, Rowland RRR, et al. Fecal microbiota transplantation is associated with reduced morbidity and mortality in porcine circovirus associated disease. Front Microbiol. 2018;9:1631. doi: 10.3389/fmicb.2018.01631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCormack UM, Curião T, Wilkinson T, et al. Fecal microbiota transplantation in gestating sows and neonatal offspring alters lifetime intestinal microbiota and growth in offspring. mSystems. 2018;3:e00134–17. doi: 10.1128/mSystems.00134-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKinney CA, Oliveira BCM, Bedenice D, et al. The fecal microbiota of healthy donor horses and geriatric recipients undergoing fecal microbial transplantation for the treatment of diarrhea. PloS One. 2020;15:e0230148. doi: 10.1371/journal.pone.0230148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McKinney CA, Bedenice D, Pacheco AP, et al. Assessment of clinical and microbiota responses to fecal microbial transplantation in adult horses with diarrhea. PloS One. 2021;16:e0244381. doi: 10.1371/journal.pone.0244381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Costa M, Di Pietro R, Bessegatto JA, et al. Evaluation of changes in microbiota after fecal microbiota transplantation in 6 diarrheic horses. Can Vet J. 2021;62:1123–1130. [PMC free article] [PubMed] [Google Scholar]
- 25.Mullen KR, Yasuda K, Divers TJ, Weese JS. Equine faecal microbiota transplant: Current knowledge, proposed guidelines and future directions. Equine Vet Educ. 2018;30:151–160. doi: 10.1111/eve.12559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kopper JJ, Alexander TL, Kogan CJ, Berreta AR, Burbick CR. In vitro evaluation of the effect of storage at −20°C and proximal gastrointestinal conditions on viability of equine fecal microbiota transplant. J Equine Vet Sci. 2021;98:103360. doi: 10.1016/j.jevs.2020.103360. [DOI] [PubMed] [Google Scholar]
- 27.Kinoshita Y, Niwa H, Uchida-Fujii E, Nukada T, Ueno T. Simultaneous daily fecal microbiota transplantation fails to prevent metronidazole-induced dysbiosis of equine gut microbiota. J Equine Vet Sci. 2022;114:104004. doi: 10.1016/j.jevs.2022.104004. [DOI] [PubMed] [Google Scholar]
- 28.Greenhaff PL, Snow DH, Harris RC, Roberts CA. Bicarbonate loading in the thoroughbred: Dose, method of administration and acid-base changes. Equine Vet J Suppl. 1990;9:83–85. doi: 10.1111/j.2042-3306.1990.tb04741.x. [DOI] [PubMed] [Google Scholar]
- 29.Walters W, Hyde ER, Berg-Lyons D, et al. Improved bacterial 16S rRNA gene (V4 and V4–5) and fungal internal transcribed spacer marker gene primers for microbial community surveys. mSystems. 2015;1:e0009–15. doi: 10.1128/mSystems.00009-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schloss PD, Westcott SL, Ryabin T, et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009;75:7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kozich JJ, Westcott SL, Baxter NT, Highlander SK, Schloss PD. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq illumina sequencing platform. Appl Environ Microbiol. 2013;79:5112–5120. doi: 10.1128/AEM.01043-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Segata N, Izard J, Waldron L, et al. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12:R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dougal K, de la Fuente G, Harris PA, Girdwood SE, Pinloche E, Newbold CJ. Identification of a core bacterial community within the large intestine of the horse. PloS One. 2013;8:e77660-e. doi: 10.1371/journal.pone.0077660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moreau MM, Eades SC, Reinemeyer CR, Fugaro MN, Onishi JC. Illumina sequencing of the V4 hypervariable region 16S rRNA gene reveals extensive changes in bacterial communities in the cecum following carbohydrate oral infusion and development of early-stage acute laminitis in the horse. Vet Microbiol. 2014;168:436–441. doi: 10.1016/j.vetmic.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 35.Costa MC, Arroyo LG, Allen-Vercoe E, et al. Comparison of the fecal microbiota of healthy horses and horses with colitis by high throughput sequencing of the V3–V5 region of the 16S rRNA gene. PLoS One. 2012;7:e41484. doi: 10.1371/journal.pone.0041484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weese JS, Holcombe SJ, Embertson RM, et al. Changes in the faecal microbiota of mares precede the development of post partum colic. Equine Vet J. 2015;47:641–649. doi: 10.1111/evj.12361. [DOI] [PubMed] [Google Scholar]
- 37.Papanicolas LE, Choo JM, Wang Y, et al. Bacterial viability in faecal transplants: Which bacteria survive? EBioMedicine. 2019;41:509–516. doi: 10.1016/j.ebiom.2019.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin de Bustamante M, Plummer C, MacNicol J, Gomez D. Impact of ambient temperature sample storage on the equine fecal microbiota. Animals (Basel) 2021;11:819. doi: 10.3390/ani11030819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leclere M, Costa MC. Fecal microbiota in horses with asthma. J Vet Intern Med. 2020;34:996–1006. doi: 10.1111/jvim.15748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Costa MC, Silva G, Ramos RV, et al. Characterization and comparison of the bacterial microbiota in different gastrointestinal tract compartments in horses. Vet J. 2015;205:74–80. doi: 10.1016/j.tvjl.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 41.Elzinga SE. Comparison of the fecal microbiota in horses with equine metabolic syndrome and metabolically normal controls fed a similar all-forage diet. J Equine Vet Sci. 2016;44:9–16. [Google Scholar]
- 42.O” Donnell MM, Harris HMB, Jeffery IB, et al. The core faecal bacterial microbiome of Irish Thoroughbred racehorses. Lett Appl Microbiol. 2013;57:492–501. doi: 10.1111/lam.12137. [DOI] [PubMed] [Google Scholar]
- 43.Faubladier C, Julliand V, Danel J, Philippeau C. Bacterial carbohydrate-degrading capacity in foal faeces: Changes from birth to pre-weaning and the impact of maternal supplementation with fermented feed products. Br J Nutr. 2013;110:1040–1052. doi: 10.1017/S0007114512006162. [DOI] [PubMed] [Google Scholar]
- 44.Buffie CG, Jarchum I, Equinda M, et al. Profound alterations of intestinal microbiota following a single dose of clindamycin results in sustained susceptibility to Clostridium difficile-induced colitis. Infect Immun. 2012;80:62–73. doi: 10.1128/IAI.05496-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jernberg C, Löfmark S, Edlund C, Jansson JK. Long-term ecological impacts of antibiotic administration on the human intestinal microbiota. ISME J. 2007;1:56–66. doi: 10.1038/ismej.2007.3. [DOI] [PubMed] [Google Scholar]
- 46.Schoster A, Mosing M, Jalali M, Staempfli HR, Weese JS. Effects of transport, fasting and anaesthesia on the faecal microbiota of healthy adult horses. Equine Vet J. 2016;48:595–602. doi: 10.1111/evj.12479. [DOI] [PubMed] [Google Scholar]
- 47.Vaughn BP, Vatanen T, Allegretti JR, et al. Increased intestinal microbial diversity following fecal microbiota transplant for active Crohn’s disease. Inflammatory bowel diseases. 2016;22:2182–2190. doi: 10.1097/MIB.0000000000000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferrario C, Taverniti V, Milani C, et al. Modulation of fecal Clostridiales bacteria and butyrate by probiotic intervention with Lactobacillus paracasei DG varies among healthy adults. J Nutr. 2014;144:1787–1796. doi: 10.3945/jn.114.197723. [DOI] [PubMed] [Google Scholar]
- 49.Walker AW, Ince J, Duncan SH, et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. 2011;5:220–230. doi: 10.1038/ismej.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Salonen A, Lahti L, Salojärvi J, et al. Impact of diet and individual variation on intestinal microbiota composition and fermentation products in obese men. ISME J. 2014;8:2218–2230. doi: 10.1038/ismej.2014.63. [DOI] [PMC free article] [PubMed] [Google Scholar]