Abstract
Introduction
In 2019, a new virus from the coronavirus family called SARS-CoV-2, infected populations throughout the world. Coronavirus disease 2019 (COVID-19), an illness induced by this virus, attacks vital organs in the body, such as the respiratory system and the gastrointestinal tract. Recent studies have confirmed changes in the gut microbiome caused by the COVID-19 disease. We examined the alteration of the gut microbiome in COVID-19 patients compared to healthy individuals.
Materials and methods
in this study, the 16s metagenomics dataset, publicly available in the Sequence Read Archive (SRA) database, was used for analysis (accession number PRJNA636824). The analysis processes were performed using the CLC Microbial Genomics Module 20.1.1 (Qiagen). At first, the sequence reads of samples were trimmed and classified into operational taxonomic units (OTUs) with 97% similarity and then assigned to the Greengenes reference database (v138). Differential abundance analysis was used to determine statistically significant differences in OTUs between COVID-19 and healthy groups. Next, biodiversity analyses including the alpha diversity (intragroup diversity) and beta diversity (intergroup diversity) using defined indexes were estimated. Then, the co-occurrence network at the species level was constructed using the Pearson correlation coefficient calculation between pairs of OTUs in R software and visualized using Cytoscape software. Ultimately, the hub OTUs at the species level were identified using the cytoHubba plugin of Cytoscape based on Maximal Clique Centrality (MCC) algorithm.
Results
The results of the metagenomic analysis revealed that the intestinal microbiome in healthy individuals has a higher biodiversity compared to COVID-19 patients. Indeed, healthy people also have a higher percentage of beneficial bacteria such as bifidobacteria adolescentis compared to COVID-19 patients; in contrast, COVID-19 patients have higher levels of opportunistic and pathogenic bacteria such as Streptococcus anginosus than healthy people. Also, by constructing a co-occurrence network at the species level, Bifidobacterium longum in the healthy group and Veillonella parvulain the COVID-19 group were found as hub species.
Conclusion
The results of this study shed light on the relationship between the gut microbiome and COVID-19. These results could be helpful for understanding the pathogenesis, clinical features, and treatment of COVID-9.
Keywords: Microbiome, Gut, COVID-19, Healthy individuals, Biodiversity, Co-occurrence network
1. Introduction
In 2019, a new virus spread all around the world. Coronavirus disease (COVID-19) is a contagious disease of the respiratory system caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1]. This virus can lead to different clinical symptoms in patients. The severity of disease complications is associated with having some underlying diseases such as diabetes, severe obesity, hypertension, and cardiovascular disease that may worsen illness symptoms [[2], [3], [4], [5], [6], [7], a, b]. In addition to the respiratory system, the disease has adverse effects on the nervous [8], cardiovascular [9], and gastrointestinal systems [10]. Gastrointestinal symptoms include various complications such as diarrhea, anorexia, and nausea that are observed in some patients [11]. The virus invades the cell through the angiotensin-converting enzyme 2 (ACE2) receptor [12,13], which is expressed at high levels in the gastrointestinal and respiratory tracts [14]. Studies have indicated the presence of the SARS-CoV-2 in fecal samples [15]. The intestinal microbiome is a critical component of the gastrointestinal tract that plays an essential role in human health and disease [16]. Evidence has implicated respiratory viruses in altering the gut microbiome [17], which in turn can cause an imbalance in the immune system and induce secondary bacterial pneumonia [18]. Recent studies have also shown that COVID-19 alters the intestinal microbiome [19]. According to the results, healthy individuals have a higher gut microbial diversity than COVID-19 patients. Indeed, such patients have higher bacterial pathogens and lower beneficial bacteria [20,21].
The aim of this study is to explain the relationship between the gut microbiome and COVID-19 disease in terms of taxonomy and microbial ecology. Accordingly, we investigated alterations of the gut microbiome in COVID-19 patients relative to healthy individuals using a new approach.
2. Material and methods
2.1. Data collection and preprocessing
Following the coronavirus outbreak, Gu et al. (2019) evaluated gut microbiome alterations in COVID-19 and H1N1 patients as compared to healthy people [20]. Stool samples of healthy individuals and patients were analyzed by 16S rRNA sequencing. We utilized the metagenomic dataset reported in Gu et al. [20]. This dataset is deposited in the Sequence Read Archive (SRA) under the accession number PRJNA636824. This dataset includes 30 COVID-19 patients, 30 healthy controls (HC), and 24 H1N1 patient samples. We excluded samples of H1N1 patients from this study. Also, samples of healthy people and COVID-19 patients were imported into this study for analysis. Bioinformatics analysis was performed using the CLC Microbial Genomics Module 20.1.1 (Qiagen). Information on the dataset is included in Table 1 .
Table 1.
Information of dataset.
| NGS platform | ILLUMINA |
|---|---|
| Library layout | paired end |
| Total number of sequences | 6,751,658 sequences |
| Total number of nucleotides | 2,032,249,058 nucleotides |
2.2. OTUs clustering ad taxonomic assignment
In this phase, the reads of samples were trimmed and classified into operational taxonomic units (OTUs) with 97% similarity and then assigned to the Greengenes reference database (v138) based on the default settings of the software. The OTUs clustering workflow includes five tools. “Optional Merge Paired Reads” is the first tool that merges two sets of sequences. The merged reads were trimmed with the “Trim sequence” tool. Alternatively, sequences were measured by the “Fixed Length Trimming” tool. Then, the sequences were filtered through the “Filer Samples Based on the Number of Reads” tool that generated output containing high-quality sequences. Ultimately, the sequences were clustered into operational taxonomic units (OTUs) using the “OTU clustering” tool. The relative abundance of OTUs was visualized using a bar chart at the phylum, class, family, and genus levels.
2.3. Differential abundance analysis
In this step, for evaluating differential abundance analysis, OTUs with low abundance were removed with a combined abundance of less than ten, and then statistically significant differences in OTUs between COVID-19 patients versus the healthy groups were determined.
2.4. Diversity analyses
In order to estimate the alpha diversity (intragroup diversity) and beta diversity (intergroup diversity), OTUs were aligned with the “MUSCLE” tool. This tool produces a phylogenetic tree based on the maximum likelihood phylogeny approach, which is used to estimate alpha and beta diversity. Alpha and beta diversity are two indicators in microbial ecology that are commonly estimated in various studies. Alpha diversity represents diversity within the groups, and beta diversity indicates diversity between groups. For alpha diversity, the Total number, Shannon entropy, Choa 1, Simpson's index, and phylogenetic diversity Indices were estimated. The Kruskal-Wallis nonparametric test was used to determine statistical significance differences within groups for alpha diversity. The statistically significant level was consideredP< 0.05. Bray-Curtis, Jaccard, Unweighted Unifrac, and Weighted Unifrac Indices were calculated and visualized using the principal coordinate analysis (PCoA) for beta diversity. Indeed, the Permutational Multivariate Analysis of Variance (PERMANOVA) was employed to determine the statistical significance differences between groups. These tests were carried out with the CLC Microbial Genomics Module.
2.5. Co-occurrence network and selection of hub OTUs at the species level
To construct the bacterial co-occurrence network, the Pearson correlation coefficient between pairs of OTUs was calculated in R software using the Hmisc package [22]. Correlation coefficients with an r > 0.3 or r < 0.3 and p-value<0.05 were selected as significant relationships for network drawing and were visualized using Cytoscape software [23]. The co-occurrence network was constructed at the species level in COVID-19 and HC groups. Afterward, the hub OTUs at the species level were identified using the cytoHubba plugin of Cytoscape based on Maximal Clique Centrality (MCC) algorithm [24].
3. Results
3.1. Comparing the microbial community in COVID-19 patients and healthy individuals
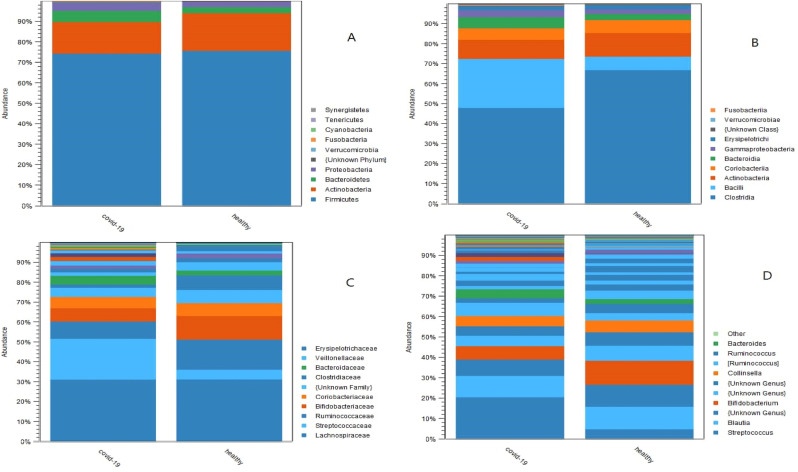
Based on taxonomic analysis (Fig. 1 ), Actinobacteria and Firmicutes were the two predominant phyla that comprise about 90% of the relative abundance distribution in both groups. At the class level, Clostridia (67%), Actinobacteria (12%), and Bacilli (6.7%) were the three dominant classes in healthy individuals. Whereas, in COVID-19 patients, Clostridia (48%), Actinobacteria (25%), and Bacilli (9.6%) were the three prevalent classes. At the family level, Lachnospiraceae (31%), Ruminococcaceae (15%), and Bifidobacteriaceae (12%) were the three dominant families in healthy individuals. Whereas, Lachnospiraceae (31%), Streptococcaceae (21%), and Ruminococcaceae (8.6%) were the three dominant families in COVID-19 patients.
Fig. 1.
Results of taxonomic analysis, the relative abundance of bacterial populations among COVID-19 patients and healthy individuals were visualized by using stacked bar charts; respectively, phylum level (A), class level (B), family level (C), genus level (D).
3.2. Differential abundance analysis between COVID-19 patients and healthy individuals
Based on the differential abundance analysis results (Table 2 ), respectively, Rothia mucilaginosa (species), Blautia (genus), Streptococcus (genus), and Streptococcus anginosus (species) were the four taxonomic levels with the highest fold change in COVID-19 group. On the other hand, SMB53 (genus), Bifidobacterium adolescentis (species), Dorea (genus), Clostridiaceae (family), and Faecalibacterium prausnitzii (species) were the most prevalent taxonomic levels with the highest fold change in healthy individuals.
Table 2.
Results of differential abundance analysis.
| OTUs names (specific number) | Log₂ fold change: log(FC) in COVID-19 vs healthy | Taxonomy |
|---|---|---|
| Positive abundances | ||
| s__mucilaginosa, (1017181) | 9.82563 | Rothia mucilaginosa |
| g__Blautia,(174009) | 6.41008 | Blautia |
| g__Streptococcus, (2024840) | 5.50363 | Streptococcus |
| s__anginosus, (1888677) | 4.14302 | Streptococcus anginosus |
| Negative abundances | ||
| s__prausnitzii, (189937) | −3.105632 | Faecalibacterium prausnitzii |
| f__Clostridiaceae, (180516) | −3.695406 | Clostridiaceae |
| g__Dorea, (189559) | −3.86359 | Dorea |
| s__adolescentis, (235262) | −7.172547 | Bifidobacterium adolescentis |
| g__SMB53, (555945) | −9.061805 | SMB53 |
Positive abundances indicate that OTUs has the highest log(FC) in COVID-19; Negative abundances indicate that OTUs has the highest log(FC) in healthy individuals; f, family level; g, genus level; s, species level.
3.3. Diversity analyses
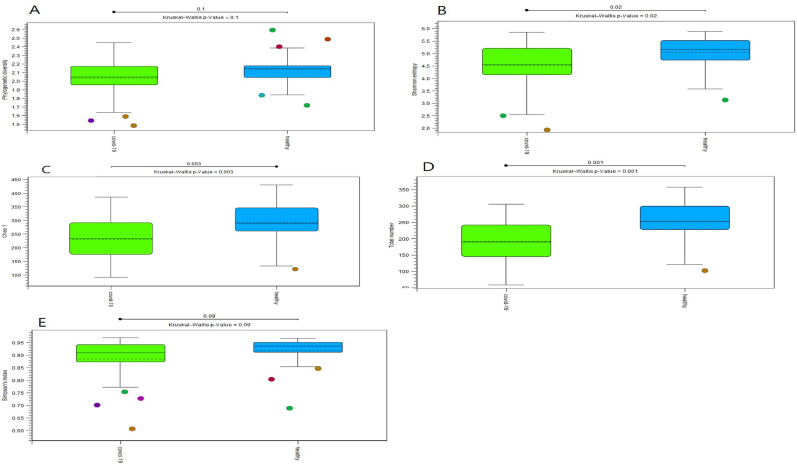
According to the alpha diversity results (Fig. 2 ), the Shannon entropy (P = 0.02), Total number (P = 0.001), and Chao1 (P = 0.003) indices were significantly higher in healthy people compared to COVID-19 patients. The phylogenetic diversity and Simpson's indices were not statistically significant between the groups. The results revealed that the richness and evenness of healthy individuals' gut microbiomes are higher than those of COVID-19 patients.
Fig. 2.
Alpha diversity results, results were visualized with Box and whisker plot; respectively, Phylogenetic diversity (A), Shannon entropy (B), Chao1 (C), Total number (D), and Simpson's index (E).
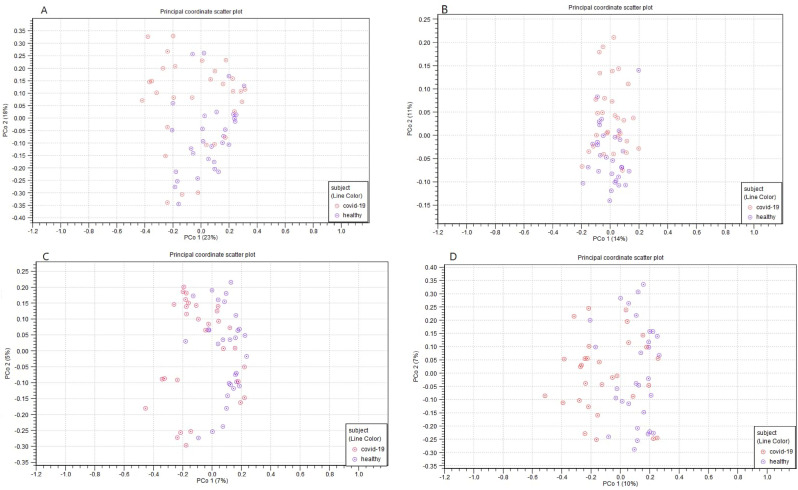
The beta diversity results (Fig. 3 ) revealed that COVID-19 patients and healthy individuals have completely distinct gut microbiome compositions. According to the PERMANOVA analysis, the Unweighted UniFrac (P = 0.00003), Weighted UniFrac (P = 0.00028), Bray-Curtis (P = 0.00001), and Jaccard (P = 0.00001) indices were found to be statistically significant in COVID-19 patients versus healthy individuals.
Fig. 3.
Beta diversity results; results were visualized by using a principal coordinate aalysis (PCoA); respectively, Weighted UniFrac (A), Unweighted UniFrac (B), Jaccard (C), and Bray-Curtis (D).
3.4. Co-occurrence network and selection of hub OTUs at the species level
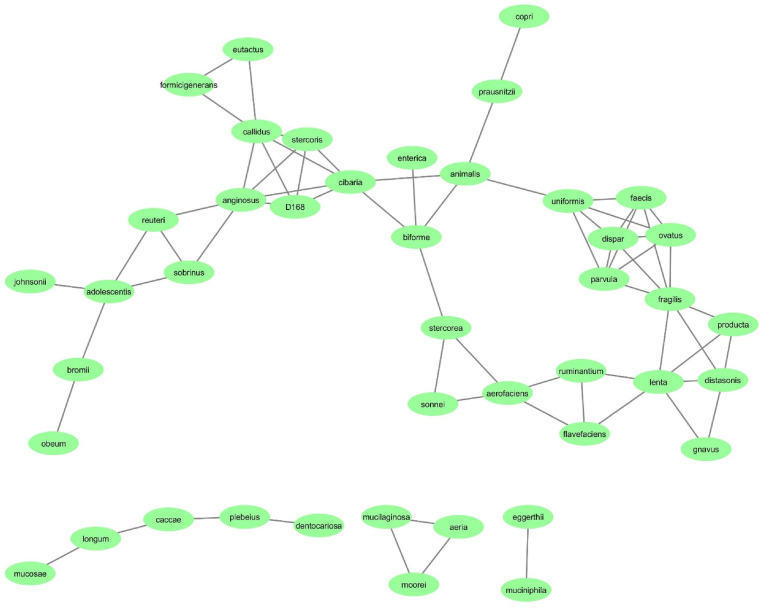
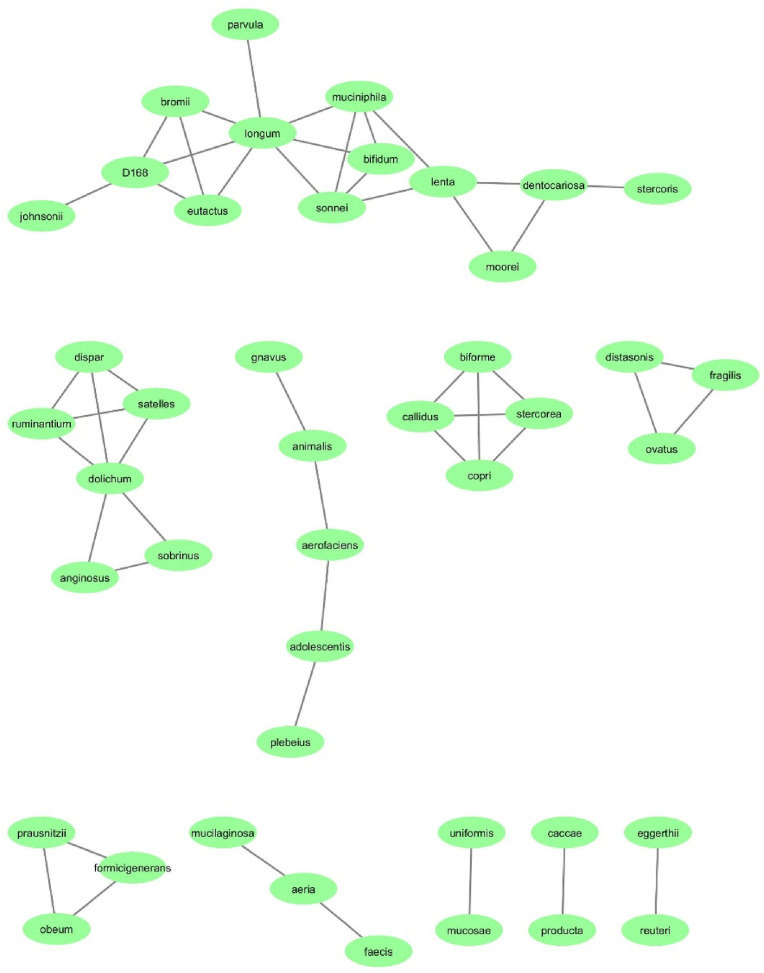
The co-occurrence network in COVID-19 and healthy groups was constructed at the species level. The co-occurrence network of the COVID-19 group had 43 nodes and 67 edges (Fig. 5 ); whereas, the co-occurrence network of the HC group had 43 nodes and 50 edges (Fig. 4). According to the results, 67 connections were found in the COVID-19 group; All connections are positive. The strongest correlations were between the species Rothia mucilaginosa and Rothia aeria (r = 0.996), Bacteroides eggerthii and Akkermansia muciniphila (r = 0.995) and Bulleidia moorei and Rothia mucilaginosa (r = 0.989). Also, in the HC group, 50 connections were found that all connections are positive. The strongest correlations were between the species Akkermansia muciniphila and Shigella sonnei (r = 0.996), Eubacterium biforme and Ruminococcus callidus (r = 0.995) and Eubacterium biforme and Prevotella copri (r = 0.995). Furthermore, the top 10 hub OTUs at the species level were determined based on MCC algorithms. According to the result, Bifidobacterium longum is the hub species in HC group with the highest MCC score. On the other hand, Veillonella parvula is the hub species in COVID-19 group with the highest MCC score. The top 10 hub OTUs in COVID-19 and HC groups are inserted in Supplementary file 1.
Fig. 5.
The bacterial co-occurrence network of the COVID-19 group at the species level; the green rectangles indicted bacterial species and black lines indicate interactions between them. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 4.
The bacterial co-occurrence network of the HC group at the species level; the green rectangles indicted bacterial species and black lines indicate interactions between them. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
In this study, the intestinal microbiome of healthy individuals and COVID-19 patients are evaluated ecologically and taxonomically. According to the results, the microbiome of COVID-19 patients has less biodiversity than healthy people, and the microbiome of healthy people is much richer than that of COVID-19 patients. The results also revealed that opportunistic bacteria and pathogens are prevalent in the microbiome of COVID-19 patients. On the other hand, beneficial bacteria are dominant in the microbiome of healthy people, which demonstrates the dynamism and healthy status of their intestines.
In the current study, R. mucilaginosa was found that have the highest log(FC) in the microbiome of the COVID-19 group. R. mucilaginosa is one of the oral and respiratory tract flora. This Gram-positive coccus is occasionally observed in the gastrointestinal system [25], which can appear as a pathogen that causes several infections, such as bacteremia, meningitis, pneumonia, and other manifestations, notably under immunocompromised conditions [26]. Wu et al. showed that R. mucilaginosa was enriched in COVID-19 patients' feces that seemed associated with growth potential of pathogenic bacteria or the extra-intestinal microbe's migration into the gut [27]. Furthermore, previous research confirmed that COVID-19 suffered patients, especially in severe cases, with cardiovascular disorder tended to have a greater prevalence of the genus Rothia related with SARS-CoV-2, and can be used as a marker to detect the increased risk in people with covid-19 [28]. Also, S. anginosus is another Gram-positive opportunistic bacterium that can lead to various diseases. Complications of infection with this bacterium include pneumonia and lung abscess under certain circumstances [29]. S. anginosus and Olsenella normally form a biofilm in oropharynx to increase bacterial thriving and adherence [30]. De Pascale et al. reported that in the patients of COVID-19 group, S. anginosus was significantly enriched in the lung microbiota compared to the non–COVID-19 group [31]. Indeed, two important bacteria that display a highist log (FC) in healthy people are B. adolescentis and F. prausnitzii. One of the prevalent and dominant bacteria in the intestinal flora of healthy people is F. prausnitzii that is regarded as an indicator of gastrointestinal health [32]. Based on the evidence, an imbalance in the composition of this bacterium is correlated with several diseases [33]. He et al. found that there was a negative correlation between the abundance of F. prausnitzii and severity of COVID-19 [34]. Additionally, Zuo et al. reported that F. prausnitzii was one of the most important bacterial species which showed an inverse association with severity of COVID-19 [35]. Another beneficial bacterium is B. adolescentis, which is commonly found in the intestines of healthy people [35]. Several benefits have been reported for this bacterium, one of which is antiviral properties against certain viruses [[36], [37], [38]]. The presence of B. adolescentis was related with the higher effectivness of neutralising antibodies to CoronaVac suggesting that the B. adolescentis may use as an adjuvant to overcoming waning immunity of inactivated vaccine [39].
Additionally, our result showed that Bifidobacterium longum and Veillonella parvula are the hub species with the highest MCC score in HC and COVID-19 groups, respectively. V. parvula is an anaerobic opportunistic coccus that leading to serious infection, in particular in individuals with immunological defects [40]. Several studies presented that V. parvula is a marker for COVID-19 that can stimulate pro-inflammatory cytokines production such as TNF-a, and might induce responses of pro-oxidative and inflammatory which result in various respiratory infections outcomes [41].
Furthermore, B. longum is an obligatory anaerobic bacterium that inhabits in the intestine of human predominantly from premature infants to elderly individuals [42]. Multiple conventional probiotics such as B. longum potentially enhanced the antibodies level in viral infections [43]. Li et al. reported that Bifidobacterium sp. significantly declined in the fecal samples of COVID-19 patients. They confirmed that immune responses of covid-19 patient to SARS-CoV-2 was improved by increasing the level of this bacteria and their metabolite inosine [44].
This study has illustrated the relationship between the intestinal microbiome and COVID-19. Consequently, considering the critical role of the intestinal microbiome in the human immune system and health, studies and clinical trials using beneficial probiotics such asBifidobacterium and Lactobacillus to enrich and reinforce the composition of the intestinal microbiome can be evaluated for the prevention and treatment of COVID-19 patients. It should be noted that not all probiotics have the same function. Bifidobacteria and Lactobacilli can be used as two types of non-pathogenic and beneficial probiotics. However, the arbitrary use of probiotics and other fermented products containing probiotics is not recommended. The effects of probiotics combating COVID-19 should be tested in randomized clinical trials (RCTs) to evaluate their impacts on the modulation and balance of intestinal microbial composition. It is likely that probiotic therapy may be used as a new therapeutic method to harmonize intestinal microbiota so as to prevent or treat COVID-19.
5. Conclusion
In this study, the alterations of the gut microbiome in COVID-19 patients compared to HC were evaluated using the metagenomic approach, the results of which revealed lower microbial biodiversity in the intestine of COVID-19 patients, when compared to healthy individuals. In fact, beneficial bacteria such as bifidobacteria were found to be more prevalent in healthy individuals than in COVID-19 patients, who were reported to have higher levels of opportunistic and pathogenic bacteria. Therefore, the restoration of dysbiotic gut microbiota with the use of probiotics for the prevention or treatment of COVID-19 could be examined as a therapeutic strategy.
Data availability statement
In this study, an available public dataset was analyzed. This dataset is located at the following address:
Funding
Not applicable for this study.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We would like to express our gratitude to the National Institute of Genetic Engineering and Biotechnology (Tehran, Iran) for supporting us during the course of this research.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.imu.2023.101239.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Hu B., Guo H., Zhou P., Shi Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2020;19:1–14. doi: 10.1038/s41579-020-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mantovani A., Byrne C.D., Zheng M.H., Targher G. Diabetes as a risk factor for greater COVID-19 severity and in-hospital death: a meta-analysis of observational studies. Nutr Metabol Cardiovasc Dis. 2020;30:1236–1248. doi: 10.1016/j.numecd.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gao F., Zheng K.I., Wang X.B., Sun Q.F., Pan K.H., Wang T.Y., Chen Y.P., Targher G., Byrne C.D., George J., Zheng M.H. Obesity is a risk factor for greater COVID-19 severity. Diabetes Care. 2020;43:e72–e74. doi: 10.2337/dc20-0682. [DOI] [PubMed] [Google Scholar]
- 4.Parveen R., Sehar N., Bajpai R., Agarwal N.B. Association of diabetes and hypertension with disease severity in covid-19 patients: a systematic literature review and exploratory meta-analysis. Diabetes Res Clin Pract. 2020;166 doi: 10.1016/j.diabres.2020.108295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aggarwal G., Cheruiyot I., Aggarwal S., Wong J., Lippi G., Lavie C.J., Henry B.M., Sanchis-Gomar F. Association of cardiovascular disease with coronavirus disease 2019 (COVID-19) severity: a meta-analysis. Curr Probl Cardiol. 2020;45 doi: 10.1016/j.cpcardiol.2020.100617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun P., Qie S., Liu Z., Ren J., Li K., Xi J. Clinical characteristics of hospitalized patients with SARS‐CoV‐2 infection: a single arm meta‐analysis. J Med Virol. 2020 Jun;92(6):612–617. doi: 10.1002/jmv.25735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.a Mubarak M., Nasri H. COVID-19 nephropathy; an emerging condition caused by novel coronavirus infection. Journal of Nephropathology. 2020 Jul 1;(3):9. [Google Scholar]; b Wu Y., Xu X., Chen Z., Duan J., Hashimoto K., Yang L., Liu C., Yang C. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. 2020;87:18–22. doi: 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu P.P., Blet A., Smyth D., Li H. The science underlying COVID-19: implications for the cardiovascular system. Circulation. 2020;142:68–78. doi: 10.1161/CIRCULATIONAHA.120.047549. [DOI] [PubMed] [Google Scholar]
- 9.Zhou Z., Zhao N., Shu Y., Han S., Chen B., Shu X. Effect of gastrointestinal symptoms in patients with COVID-19. Gastroenterology. 2020;158:2294–2297. doi: 10.1053/j.gastro.2020.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ai J.W., Zi H., Wang Y., Huang Q., Wang N., Li L.Y., Pei B., Ji J., Zeng X.T. Clinical characteristics of COVID-19 patients with gastrointestinal symptoms: an analysis of seven patients in China. Front Med. 2020;7:308. doi: 10.3389/fmed.2020.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jing Y., Run-Qian L., Hao-Ran W., Hao-Ran C., Ya-Bin L., Yang G., Fei C. Potential influence of COVID-19/ACE2 on the female reproductive system. Mol Hum Reprod. 2020;26:367–373. doi: 10.1093/molehr/gaaa030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu H., Zhong L., Deng J., Peng J., Dan H., Zeng X., Li T., Chen Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12:1–5. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valizadeh R., Baradaran A., Mirzazadeh A., Bhaskar L.V. Coronavirus-nephropathy; renal involvement in COVID-19. J Ren Inj Prev. 2020 Mar 9;9(2):e18. [Google Scholar]
- 14.Zhang J., Wang S., Xue Y. Fecal specimen diagnosis 2019 novel coronavirus–infected pneumonia. J Med Virol. 2020;92:680–682. doi: 10.1002/jmv.25742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kinross J.M., Darzi A.W., Nicholson J.K. Gut microbiome-host interactions in health and disease. Genome Med. 2011;3:1–12. doi: 10.1186/gm228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Groves H.T., Higham S.L., Moffatt M.F., Cox M.J., Tregoning J.S. Respiratory viral infection alters the gut microbiota by inducing inappetence. mBio. 2020;11 doi: 10.1128/mBio.03236-19. e03236-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanada S., Pirzadeh M., Carver K.Y., Deng J.C. Respiratory viral infection-induced microbiome alterations and secondary bacterial pneumonia. Front Immunol. 2018;9:2640. doi: 10.3389/fimmu.2018.02640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zuo T., Zhang F., Lui G.C., Yeoh Y.K., Li A.Y., Zhan H., Wan Y., Chung A.C., Cheung C.P., Chen N., Lai C.K. Alterations in gut microbiota of patients with COVID-19 during time of hospitalization. Gastroenterology. 2020;159:944–955. doi: 10.1053/j.gastro.2020.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu R., Lu R., Zhang T., Wu Q., Cai W., Han X., Wan Z., Jin X., Zhang Z., Zhang C. Temporal association between human upper respiratory and gut bacterial microbiomes during the course of COVID-19 in adults. Commun Biol. 2021;4:1–11. doi: 10.1038/s42003-021-01796-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gu S., Chen Y., Wu Z., Chen Y., Gao H., Lv L., Guo F., Zhang X., Luo R., Huang C., Lu H. Alterations of the gut microbiota in patients with coronavirus disease 2019 or H1N1 influenza. Clin Infect Dis. 2020;71:2669–2678. doi: 10.1093/cid/ciaa709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alharbi K.S., Singh Y., Rawat S., Afzal O., Altamimi A.S., Kazmi I., Al-Abbasi F.A., Alzarea S.I., Singh S.K., Bhatt S., Chellappan D.K. Gut microbiota disruption in COVID-19 or post-COVID illness association with severity biomarkers: a possible role of pre/pro-biotics in manipulating microflora. Chem Biol Interact. 2022 May 1;358 doi: 10.1016/j.cbi.2022.109898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harrell F.E., Jr., Harrell M.F., Jr. Package ‘hmisc’. CRAN2018. 2019 Jan 25;2019:235–236. [Google Scholar]
- 23.Smoot M.E., Ono K., Ruscheinski J., Wang P.L., Ideker T. Cytoscape 2.8: new features for data integration and network visualization. Bioinformatics. 2011 Feb 1;27(3):431–432. doi: 10.1093/bioinformatics/btq675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chin C.H., Chen S.H., Wu H.H., Ho C.W., Ko M.T., Lin C.Y. cytoHubba: identifying hub objects and sub-networks from complex interactome. BMC Syst Biol. 2014 Dec;8(4):1–7. doi: 10.1186/1752-0509-8-S4-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maraki S., Papadakis I.S. Rothia mucilaginosa pneumonia: a literature review. Infectious Diseases. 2015;47:125–129. doi: 10.3109/00365548.2014.980843. [DOI] [PubMed] [Google Scholar]
- 26.Ramanan P., Barreto J.N., Osmon D.R., Tosh P.K. Rothia bacteremia: a 10-year experience at mayo clinic, rochester, Minnesota. J Clin Microbiol. 2014;52:3184. doi: 10.1128/JCM.01270-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu Y., Cheng X., Jiang G., Tang H., Ming S., Tang L., Lu J., Guo C., Shan H., Huang X. Altered oral and gut microbiota and its association with SARS-CoV-2 viral load in COVID-19 patients during hospitalization. npj Biofilms and Microbiomes. 2021;7(1):61. doi: 10.1038/s41522-021-00232-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marotz C., Belda-Ferre P., Ali F., Das P., Huang S., Cantrell K., Jiang L., Martino C., Diner R.E., Rahman G., McDonald D. SARS-CoV-2 detection status associates with bacterial community composition in patients and the hospital environment. Microbiome. 2021 Dec;9(1):1–5. doi: 10.1186/s40168-021-01083-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang S., Li M., Fu T., Shan F., Jiang L., Shao Z. Clinical characteristics of infections caused by Streptococcus Anginosus Group. Sci Rep. 2020;10:1–6. doi: 10.1038/s41598-020-65977-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Su T.Y., Lee M.H., Huang C.T., Liu T.P., Lu J.J. The clinical impact of patients with bloodstream infection with different groups of Viridans group streptococci by using matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) Medicine. 2018 Dec;97(50) doi: 10.1097/MD.0000000000013607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Pascale G., De Maio F., Carelli S., De Angelis G., Cacaci M., Montini L., Bello G., Cutuli S.L., Pintaudi G., Tanzarella E.S., Xhemalaj R. Staphylococcus aureus ventilator-associated pneumonia in patients with COVID-19: clinical features and potential inference with lung dysbiosis. Crit Care. 2021 Dec;25(1):1–2. doi: 10.1186/s13054-021-03623-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sokol H., Thomas M., Wells J.M., Langella Faecalibacterium prausnitzii and human intestinal health. Curr Opin Microbiol. 2013;16:255–261. doi: 10.1016/j.mib.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 33.Martín R., Bermúdez-Humarán L.G., Langella P. Searching for the bacterial effector: the example of the multi-skilled commensal bacterium Faecalibacterium prausnitzii. Front Microbiol. 2018;9:346. doi: 10.3389/fmicb.2018.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He X., Zhao S., Li Y. Faecalibacterium prausnitzii: a next-generation probiotic in gut disease improvement. Can J Infect Dis Med Microbiol. 2021 Mar 5;2021 1-0. [Google Scholar]
- 35.Zuo T., Zhang F., Lui G.C., Yeoh Y.K., Li A.Y., Zhan H., Wan Y., Chung A.C., Cheung C.P., Chen N., Lai C.K. Alterations in gut microbiota of patients with COVID-19 during time of hospitalization. Gastroenterology. 2020 Sep 1;159(3):944–955. doi: 10.1053/j.gastro.2020.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duranti S., Ruiz L., Lugli G.A., Tames H., Milani C., Mancabelli L., Mancino W., Longhi G., Carnevali L., Sgoifo A., Margolles A. Bifidobacterium adolescentis as a key member of the human gut microbiota in the production of GABA. Sci Rep. 2020;10:1–13. doi: 10.1038/s41598-020-70986-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cha M.K., Lee D.K., An H.M., Lee S.W., Shin S.H., Kwon J.H., Kim K.J., Ha N.J. Antiviral activity of Bifidobacterium adolescentis SPM1005-A on human papillomavirus type 16. BMC Med. 2012;10:1–6. doi: 10.1186/1741-7015-10-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim M.J., Lee D.K., Park J.E., Park I.H., Seo J.G., Ha N.J. Antiviral activity of Bifidobacterium adolescentis SPM1605 against coxsackievirus B3. Biotechnol Biotechnol Equip. 2014;28:681–688. doi: 10.1080/13102818.2014.945237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ng S.C., Peng Y., Zhang L., Mok C.K., Zhao S., Li A., Ching J.Y., Liu Y., Yan S., Chan D.L., Zhu J. Gut microbiota composition is associated with SARS-CoV-2 vaccine immunogenicity and adverse events. Gut. 2022 Jun 1;71(6):1106–1116. doi: 10.1136/gutjnl-2021-326563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strach M., Siedlar M., Kowalczyk D., Zembala M., Grodzicki T. Sepsis caused by Veillonella parvula infection in a 17-year-old patient with X-linked agammaglobulinemia (Bruton's disease) J Clin Microbiol. 2006 Jul;44(7):2655–2656. doi: 10.1128/JCM.00467-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Devi P., Maurya R., Mehta P., Shamim U., Yadav A., Chattopadhyay P., Kanakan A., Khare K., Vasudevan J.S., Sahni S., Mishra P. Increased abundance of Achromobacter xylosoxidans and Bacillus cereus in upper airway transcriptionally active microbiome of COVID-19 mortality patients indicates role of co-infections in disease severity and outcome. Microbiol Spectr. 2022 Jun 29;10(3):e02311–e02321. doi: 10.1128/spectrum.02311-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kageyama Y., Nishizaki Y., Aida K., Yayama K., Ebisui T., Akiyama T., Nakamura T. Lactobacillus plantarum induces innate cytokine responses that potentially provide a protective benefit against COVID-19: a single-arm, double-blind, prospective trial combined with an in vitro cytokine response assay. Exp Ther Med. 2022 Jan 1;23(1):1–3. doi: 10.3892/etm.2021.10942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mirashrafi S., Moravejolahkami A.R., Zehi Z.B., Kermani M.A., Bahreini-Esfahani N., Haratian M., Dashti M.G., Pourhossein M. The efficacy of probiotics on virus titres and antibody production in virus diseases: a systematic review on recent evidence for COVID-19 treatment. Clinical nutrition ESPEN. 2021 Dec 1;46:1–8. doi: 10.1016/j.clnesp.2021.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li S., Yang S., Zhou Y., Disoma C., Dong Z., Du A., Zhang Y., Chen Y., Huang W., Chen J., Song D. Microbiome profiling using shotgun metagenomic sequencing identified unique microorganisms in COVID-19 patients with altered gut microbiota. Front Microbiol. 2021 Oct 11;12 doi: 10.3389/fmicb.2021.712081. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
In this study, an available public dataset was analyzed. This dataset is located at the following address: