Abstract
Introduction:
Poor adoption of stroke guidelines is a problem internationally. The Quality in Acute Stroke Care (QASC) trial demonstrated significant reduction in death and disability with facilitated implementation of nurse-initiated
Methods:
This was a multi-country, multi-centre, pre-test/post-test study (2017–2021) comparing post implementation data with historically collected pre-implementation data. Hospital clinical champions, supported by the Angels Initiative conducted multidisciplinary workshops discussing pre-implementation medical record audit results, barriers and facilitators to FeSS Protocol implementation, developed action plans and provided education, with ongoing support co-ordinated remotely from Australia. Prospective audits were conducted 3-month after FeSS Protocol introduction. Pre-to-post analysis and country income classification comparisons were adjusted for clustering by hospital and country controlling for age/sex/stroke severity.
Results:
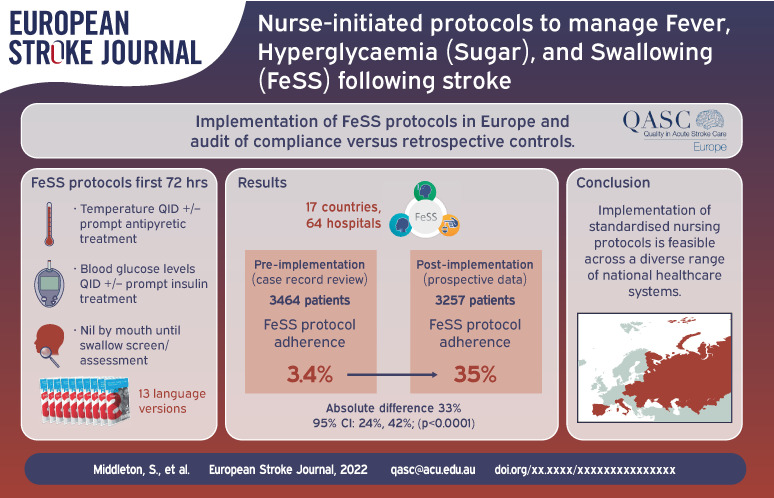
Data from 64 hospitals in 17 countries (3464 patients pre-implementation and 3257 patients post-implementation) showed improvement pre-to-post implementation in measurement recording of all three FeSS components, all p < 0.0001: fever elements (pre: 17%, post: 51%; absolute difference 33%, 95% CI 30%, 37%); hyperglycaemia elements (pre: 18%, post: 52%; absolute difference 34%; 95% CI 31%, 36%); swallowing elements (pre: 39%, post: 67%; absolute difference 29%, 95% CI 26%, 31%) and thus in overall FeSS Protocol adherence (pre: 3.4%, post: 35%; absolute difference 33%, 95% CI 24%, 42%). In exploratory analysis of FeSS adherence by countries’ economic status, high-income versus middle-income countries improved to a comparable extent.
Discussion and conclusion:
Our collaboration resulted in successful rapid implementation and scale-up of FeSS Protocols into countries with vastly different healthcare systems.
Keywords: Stroke, implementation, nurses, QASC, FeSS, fever, hyperglycaemia, swallow, dysphagia, translation
Graphical abstract.
Introduction
Despite evidence that early intervention for stroke results in better patient outcomes, and European guideline recommendations, evidence is not always effectively implemented. 1 Thrombolysis rates vary widely by country from <1% to 20.6% 1 and only 30% of European stroke patients receive stroke unit care.2,3 These evidence-practice gaps are particularly notable in Eastern European and in Lower-Middle Income Countries where many hospitals have limited access to hyper-acute stroke therapies.4,5
Nurses have a major role in accelerating the delivery of acute stroke care within hospitals including the management of physiological variables known to influence stroke outcome, 6 such as elevated body temperature, hyperglycaemia and swallowing dysfunction. 6 Our landmark Australian trial demonstrated that patients cared for in stroke units where staff had received facilitated implementation of nurse-initiated protocols to manage Fever, hyperglycaemia (Sugar) and Swallowing (FeSS) had a significant 16% absolute reduction in death and dependency 90-day following stroke, 7 with a sustained 20% relative improvement in survival 4 years post stroke. 8 Two independent economic evaluations have estimated significant savings in healthcare costs ($65 M) and societal costs ($252 M) with the use of FeSS protocols in stroke units.9,10 Subsequent successful state-wide scale-up of the FeSS Protocols demonstrated improvements in adherence. 11
There are few rigorous international studies that have evaluated systematic implementation and scale-up of a proven stroke intervention.12–15 The series of BRIDGE cluster randomised trials were predicated on rigorous evidence from earlier randomised controlled trials.12,13,15 These trials used multifaceted quality improvement interventions to promote the use of evidence-based therapies and were evaluated using performance measures based on the American Heart Association/American Stroke Association’s ‘Get With the Guidelines’ (GWTG) quality improvement programme. 14 The translational capacity of the intervention was tested through each upscale phase that included different medical conditions (acute coronary syndrome (BRIDGE-ACS, Brazil), 12 acute ischaemic stroke (Golden-BRIDGE, China), 15 transient ischaemic attack (BRIDGE-Stroke, Brazil, Peru, Argentina) 13 and in different countries that included both lower and middle-income cohorts. The significant increase in the use of evidence-based therapies reported in the BRIDGE-ACS and Golden-BRIDGE trials were not as impressive in the BRIDGE-Stroke trial possibly due to the inclusion of patients with transient ischaemic attacks in the latter study sample. However, scaling up complex, multidisciplinary interventions can pose considerable challenges, 16 particularly in the context of different health systems and resources (e.g. Lower-Middle Income Countries); economic, political and organisational barriers; and variations in stroke care practices. 17
Several opportunities for scale-up and spread of the FeSS Protocols into routine acute stroke care across Europe were identified. They were, the focus on research priorities for implementation stroke research in the 2018–2030 Stroke Action Plan for Europe (SAP-E), 18 and the urgent global initiative to improve access to stroke units and the specialist stroke nursing care provided in these units. A partnership was formed between QASC Trial researchers, the European Stroke Organisation (ESO), the European Acute Networks Striving for Excellence in Stroke (Angels) Initiative 19 and the Registry of Stroke Care Quality (RES-Q) to target management of these three complications following stroke.19,20 The Angels Initiative is a not-for-profit organisation that operates to optimise the quality of treatment in all existing stroke centres.
We aimed to examine the real-world effectiveness of supported implementation of the FeSS Protocols by: (i) examining changes in monitoring and treatment for fever, hyperglycaemia and swallowing pre-to-post-FeSS Protocol implementation; (ii) determining whether pre-to-post-implementation changes in monitoring and treatment differed between high-income countries (HIC) and countries with lower incomes (lower-middle income and upper-middle income 21 – hereafter jointly classified as middle income countries (MIC)) and (iii) comparing post-implementation changes between HIC and MIC.
Methods
Study design
This pre-test/post-test study involved collection of data from medical records for consecutive patients with stroke admitted to participating hospitals. Our study is reported using the SQUIRE reporting guidelines. 22
The study was overseen by the QASC Europe Steering Committee with a part-time study liaison officer based in the Netherlands. The Nursing Research Institute based in Australia led the independent evaluation, and guided and supported the Angels consultants, country nurse coordinators and hospital clinical champions in the implementation processes at a local level.
Ethics approval
Ethics approval was obtained from Australian Catholic University (2017-11H). The project protocol as approved by Australian Catholic University HREC is available as Supplemental Appendix A. As the study was observational and all data were de-identified, individual patient consent was not required as no identifying patient information was provided by hospitals. Some hospitals in Italy, Portugal and Spain required ethical clearance (Supplemental Appendix B).
Participants
Hospitals with stroke units/stroke services already participating in the European Angels Initiative programme were eligible to participate. Eligible patients were those with a discharge diagnosis of ischaemic stroke, intracerebral haemorrhage or stroke of undetermined origin, presenting within 48 h of symptom onset. Patients identified for palliative care only on admission were excluded.
Procedures
FeSS Protocols and resources were translated into 12 languages: Bulgarian, Czech, French, Hungarian, Italian, Polish, Portuguese, Romanian, Russian, Slovakian, Spanish and Ukrainian.
Implementation strategy
The FeSS Protocols and implementation strategy used in the QASC trial7,8 and the QASC Implementation Project 9 were adapted for international use and included: audit and feedback, clinical champions, barrier and enabler assessments, educational workshops and reminders (Box 1).23,24
Box 1.
QASC Europe intervention component.
|
Fever, hyperglycaemia (sugar) swallow (FeSS) protocols
Fever ■ Temperature readings monitored and recorded four times per day for the first 72 h ■ If temperature ⩾37.5°C treat within 1 h with paracetamol or other antipyretic Sugar (hyperglyceamia) ■ Formal venous glucose on admission to Emergency Department or stroke service ■ Finger-prick blood glucose level readings monitored and recorded four times per day for the first 48 h ■ If glucose >10 mmol/L (>180 mg/dL) treat within 1 h with insulin Swallowing ■ Swallow screen or swallow assessment within 24 h of admission and prior to being given oral food, fluids or medications ■ Referral to speech and language therapist/pathologist for full assessment for those who fail the swallow screen Implementation strategy Multidisciplinary workshops Workshop 1 – (one at each hospital) ■ Presentation of pre-implementation audit results ■ Identification of barriers and enablers to FeSS Protocol use ■ Development of local Action Plan Workshop 2 – (one at each hospital) ■ Revisit Action Plan ■ Ascertain actions already taken ■ Explore any further local barriers Education ■ PowerPoint provided by Nursing Research Institute research team ■ Sessions for all clinicians run by local clinical champions Use of clinical opinion leaders ■ National clinical opinion leaders available for support (reactive) ■ Clinical site champions at each hospital Site Support ■ Local: clinical champions at each hospital ■ External: Angels consultants visited sites to discuss progress. They were supported by Project European Liaison Officer who did not visit sites but briefed the Angels consultants on processes and provided on-going reactive support to Angel consultants. ■ Country champions Audit and Feedback ■ Pre-implementation audit results provided to each hospital 1 week following completion of data entry Reminders ■ Milestones poster provided to hospitals for display outlining steps in the implementation process ■ Proactive face-to-face,* contact from Angels Consultant following completion of pre-implementation audit data collection and reactive contact when required ■ Emails ○ Proactive email from Nursing Research Institute research team to clinical champions following each milestone ○ Reactive emails from Nursing Research Institute research team to Angels Consultants ○ Reactive emails from Nursing Research Institute team to Country Champions ○ Reactive emails from Country champions to local clinical champions when required or requested |
Pre COVID-19, face-to-face; during COVID-19 pandemic, by telephone.
Intervention
Hospital clinicians undertook a pre-implementation audit of fever, hyperglycaemia and swallow management during the first 72 hours after stroke using standardised indicators. Hospital clinical champions were provided with a self-directed PowerPoint® training module on how to complete the audit as well as the data collection tool and data dictionary translation in their native language. Local hospital clinical champions then conducted two multidisciplinary workshop and education sessions with the support of stroke physicians, and speech and language therapists. Angels Consultants were briefed on FeSS Protocols and implementation strategies by our European liaison officer, and then assisted the clinical champions with workshops. Pre-implementation (baseline) audit data were presented at the first workshop by hospital clinical champions. Teams then identified potential barriers and enablers to FeSS Protocol implementation, developing local solutions recorded on a hospital-specific Action Plan which was revisited at the second workshop. Facilitation of the intervention was co-ordinated remotely from Australia (Supplemental Appendix C). Following workshops and education sessions, hospital clinical champions notified the researchers of the date of commencement of FeSS Protocol use.
Data collection
The hospital clinicians from each participating hospital completed an Organisational Survey on workplace characteristics reporting: presence of a stroke unit (derived from whether they reported co-located beds, interprofessional stroke team, interprofessional team meetings and staff education),23,25 stroke service certification from ESO, bed capacity, types of stroke services available and employment of any stroke expert nurses (stroke specialist nurse, stroke nurse practitioner, clinical nurse educator, clinical nurse consultant, advanced practice nurse).
Hospital clinicians retrospectively audited between 40 and 100 consecutive eligible stroke admissions (number determined by each hospital and depended on hospital stroke presentation volume) commencing from time of study enrolment until their chosen number of records was reached, to provide pre-implementation data. Pre-implementation audits were conducted between March 2018 and June 2020.
The post-implementation audits was conducted 3 months after FeSS Protocol commencement at each hospital, which allowed a ‘bedding down’ period for the FeSS Protocols to become normal practice.7,8 Hospital clinicians undertook medical record audits for a similar number of prospectively identified consecutive stroke admissions (post-implementation data), conducted between January 2019 and February 2021. (As with the pre-implementation audit, the number of records audited were determined by the hospital and all other audit methods were identical.)
Data were entered by hospital clinical champions into the on-line Global Registry of Stroke Care Quality (RES-Q) 26 database based in Brno, Czech Republic and operating in 75 countries. Data collected were: patient demographic characteristics (age, sex), stroke type, stroke severity (National Institutes of Health Stroke Scale (NIHSS)), 27 premorbid risk factors, functional status (modified Rankin Scale (mRS)), 28 treatment with thrombolysis, monitoring and treatment according to the FeSS Protocols, discharge mRS and discharge destination. A sample of five records for each hospital was randomly selected by the statistician and independently audited by a second local auditor to assess inter-rater reliability. An a priori process evaluation of implementation barriers and facilitators will be reported elsewhere.
Outcome measures
The primary outcome was a post-hoc binary measure of adherence with all monitoring and treatment elements of the FeSS Protocols as recorded in the medical records. While we do not have a formal published protocol, the protocol submitted to the ACU ethics committee included all the outcomes related to adherence to the individual elements of the protocols and a summary measure for each of the fever, hyperglycaemia and swallowing protocols. A priori, we did not specify primary or secondary outcomes. Prior to analysis we decided that a composite outcome measure would aid interpretation. The a priori secondary outcomes were adherence to each of the combined monitoring and treatment elements for: (i) fever, (ii) hyperglycaemia and (iii) swallowing. The tertiary outcomes were adherence to the individual elements of the FeSS Protocols; Due to increasing interest of a clinical impact of the FeSS Protocols, we decided prior to analysis to include the following treatment outcomes (receipt of thrombolysis; stroke unit care) and discharge clinical outcomes (discharge destination; ability to walk on discharge; duration of hospital stay; discharge mRS) (Box 2). We also generated a FeSS adherence score as the proportion of six FeSS Protocol elements (as listed in Box 1, excluding formal venous glucose on admission) correctly implemented for each individual.
Box 2.
QASC Europe outcome measures.
|
• Primary outcome (Post-hoc)*
■ Adherence to all monitoring and treatment elements of the FeSS Protocols • Secondary outcomes ■ Adherence to all monitoring and treatment elements of the Fever Protocol ■ Adherence to all monitoring and treatment elements of the Hyperglycaemia (Sugar) Protocol ■ Adherence to all monitoring and treatment elements of the Swallow Protocol • Tertiary outcomes ■ Fever Protocol ○ Temperature monitored at least four times per day on day of admission ○ Temperature monitored at least four times per day on day 2 of admission ○ Temperature monitored at least four times per day on day 3 of admission ○ Paracetamol (or other anti-pyretic) given for first temperature ⩾37.5°C ○ Paracetamol (or other anti-pyretic) given with 1 h from first temperature ⩾37.5°C ■ Hyperglycaemia (Sugar) Protocol ○ Venous blood glucose level sample collected and sent to laboratory ○ Blood Glucose Levels (BGL) monitored at least four times per day on day of admission ○ BGLs monitored at least four times per day on day 2 of admission ○ BGLs monitored at least four times per day on day 3 of admission (if BGLs unstable) ○ Insulin given for first BGL ⩾10 mmol/L ○ Insulin given within 1 h from first BGL ⩾10 mmol/L ■ Swallow Protocol ○ Formal swallow screen performed ○ Failed screen and subsequently had swallow assessment ○ Swallow screen performed within 24 h ○ Swallow assessment recorded ○ Swallow screen OR assessment recorded ○ Swallow screen or assessment performed before being given oral medications ○ Swallow screen or assessment performed before being given oral food or fluids ■ Treatment Outcomes (Post-hoc)* ○ Receipt of thrombolysis ○ Stroke unit care ■ Discharge Outcomes (Post-hoc)* ○ Discharge destination ○ Ability to walk on discharge ○ Duration of hospital stay ○ Discharge modified Rankin Score |
Post-hoc outcomes were determined prior to analysis.
Statistical analysis
Patient demographic and clinical characteristics of pre and post-implementation patients were compared using the chi-squared test or Fisher’s exact test.
Hospitals that provided data for both pre and post implementation were included in the main analyses. The number and proportion of patients with each outcome pre and post implementation was reported. Pre-to-post implementation change in outcomes were assessed separately for each outcome using mixed effects logistic regression which included variables for implementation status (pre or post), age group, sex and stroke severity (NIHSS) and adjusted for correlation of outcomes within hospital and country. Although inclusion of the covariates in the model was not mentioned in the ethics protocol, their use is considered conventional. Adjusted differences in proportions from pre-to-post-implementation are reported with 95% Confidence Interval (CIs).
Post-hoc, to determine if the pre-to-post changes in primary or secondary outcomes differed between HIC and MIC 21 we repeated the above analyses including a term for country income classification (HIC vs MIC) and an interaction between time (pre/post) and country income classification. A statistically significant estimate for this term indicated that the changes pre-to-post differed by country income classification. The differences in proportions with the outcome from pre-to-post implementation are reported separately for country income classification, with 95% CIs.
Post-implementation primary and secondary outcomes were compared between HIC and MIC using mixed effects logistic regression models with post-implementation data only, and including country income classification (HIC/MIC), age, sex and stroke severity (NIHSS), and adjusted for correlation of outcomes within hospital and country; differences in proportions are reported with 95% CIs. As an additional comparison of HIC and MIC post-implementation practices, we graphed the mean FeSS adherence score, with 95% CI, by country income status and hospital.
Observations with missing values for covariates were excluded from the regression models (complete case analyses) but are included in descriptive tables where appropriate. Due to the number of missing values for NIHSS we undertook sensitivity analyses including a category for missing NIHSS.
Cohen’s Kappa coefficient was calculated to measure inter-rater agreement at the patient level for the primary outcome of overall FeSS adherence.
Role of the funding source: The funder and industry collaborators had no role in the study design, data collection, data analysis, data interpretation or report writing.
Results
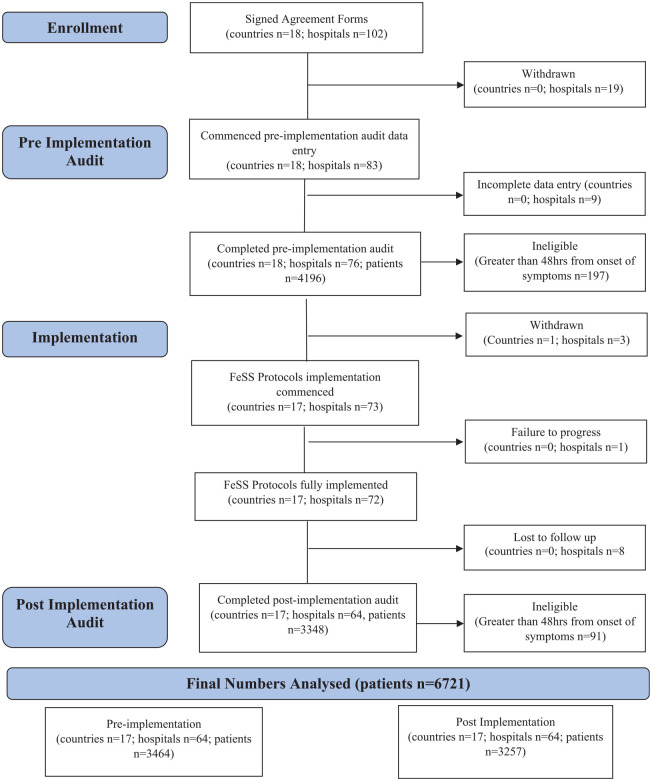
Of the 102 hospitals from 18 countries (eight HIC and 10 MIC) who volunteered to participate; 19 hospitals withdrew without reason before entering any data.
Pre-implementation data entry was commenced by 83 hospitals with 76 hospitals completing (n = 4196 patients); 197 patients were ineligible due to arriving at the hospital 48 h after symptom onset (total n = 3999 patients from 18 countries). FeSS Protocol implementation was commenced in 73 hospitals in 17 countries (one country (France) withdrew all three hospitals as they no longer had an Angels consultant), one hospital failed to complete implementation due to COVID-19. Eight hospitals failed to complete post-implementation data entry due to COVID-19, leaving 64 hospitals from 17 countries (as for pre-implementation excluding France) providing pre-implementation data for 3464 and post-implementation data for 3348 patients; 91 patients were ineligible due to arriving at hospitals 48 h after symptoms onset (total n = 3257) (Figure 1).
Figure 1.
QASC Europe project flow diagram.
Hospital characteristics
Of the 56 hospitals from 16 countries providing organisational data, 84% (n = 37) were public hospitals, 61% (n = 34) had a dedicated stroke unit, 25 23% (n = 13) had a stroke service certification by the ESO, 29 70% (n = 39) were a Comprehensive Stroke Centre and 80% (n = 45) reported employing a Stroke Expert Nurse (Table 1); there were a median of 496 strokes admitted per year.
Table 1.
QASC Europe hospital characteristics (n = 56 hospital, n = 16 countries a ).
| n (%) | |
|---|---|
| Hospital type | |
| Public | 37 (84) |
| Private | 7 (16) |
| (Missing) | 12 (27) |
| Stroke unit b | 34 (61) |
| Co-located beds | 55 (98) |
| Interprofessional stroke team | 51 (91) |
| Interprofessional team meetings | 39 (70) |
| Staff education | 48 (86) |
| ESO stroke service certification | 13 (23) |
| Stroke admission per year | |
| <250 | 5 (9.1) |
| 250–499 | 22 (40) |
| 500–749 | 11 (20) |
| 750–999 | 7 (13) |
| >1000 | 10 (18) |
| (Missing) | 1 (1.8) |
| Stroke service b | |
| Comprehensive stroke centre | 39 (70) |
| Primary stroke centre | 17 (30) |
| General hospital | 0 (0) |
| Any stroke expert nurse c | 45 (80) |
| Stroke specialist nurse | 33 (59) |
| Stroke nurse practitioner | 23 (41) |
| Clinical nurse educator | 15 (27) |
| Clinical nurse consultant | 10 (18) |
| Advanced practice nurse | 23 (41) |
Numbers may not add to total sample size due to missing data.
Armenia, Belarus, Bulgaria, Czech Republic, France, Georgia, Italy, Kazakhstan, Lithuania, Moldova, Poland, Portugal, Romania, Russia, Slovakia, Spain and Ukraine.
Stroke Foundation Framework (Stroke Foundation. National Acute Stroke Services Framework 2019. Melbourne, 2019).
These positions are filled by a Registered Nurse with extensive experience and additional training or education who work in an advanced practice stroke specific role.
Patient demographics and characteristics
Patient characteristics were generally similar for pre- and post-implementation groups. However, there were significant differences in pre- and post-implementation distribution for full range pre-morbid mRS however there was no difference in the dichotomised pre-morbid mRS (Table 2).
Table 2.
QASC Europe: patient demographic, clinical characteristics.
| Pre N = 3464 | Post N = 3257 | p | |
|---|---|---|---|
| n (%) | n (%) | ||
| Participating sites | 64 | 64 | |
| Gender | |||
| Female | 1717 (51) | 1617 (50) | 0.3062 |
| Male | 1653 (49) | 1639 (50) | |
| (Missing) | 94 (2.7) | 1 (0.03) | – |
| Age group (years) | |||
| <65 | 1041 (30) | 910 (28) | 0.1096 |
| 65–<75 | 931 (27) | 954 (29) | |
| 75–<85 | 970 (28) | 915 (28) | |
| 85+ | 505 (15) | 477 (15) | |
| (Missing) | 17 (0.49) | 1 (0.03) | – |
| Premorbid risk factors | |||
| Previous stroke | 483 (14) | 416 (13) | 0.1696 |
| Diabetes | 616 (18) | 629 (19) | 0.1138 |
| None | 2172 (63) | 2021 (62) | 0.5992 |
| Premorbid modified Rankin Score | |||
| 0: No symptoms at all | 1883 (59) | 1757 (60) | 0.0357 |
| 1: No significant disability despite symptoms | 465 (14) | 401 (14) | |
| 2: Slight disability | 307 (9.6) | 283 (9.6) | |
| 3: Moderate disability | 300 (9.3) | 232 (7.9) | |
| 4: Moderately severe disability | 137 (4.3) | 170 (5.8) | |
| 5: Severe disability | 119 (3.7) | 106 (3.6) | |
| mRS ⩽ 2 | 2348 (73) | 2158 (73) | 0.9850 |
| (Missing) | 253 (7.3) | 308 (9.5) | – |
| Stroke type | |||
| Ischaemic stroke | 3057 (88) | 2879 (88) | 0.8825 |
| Intracerebral haemorrhage | 368 (11) | 338 (10) | |
| Undetermined/(missing) | 39 (1.1) | 40 (1.2) | |
| National Institutes Health Stroke Scale | |||
| 0–7 (mild stroke) | 1546 (53) | 1419 (52) | 0.0691 |
| 8–16 (moderate stroke) | 882 (30) | 799 (29) | |
| 17+ (severe stroke) | 482 (17) | 517 (19) | |
| (Missing) | 554 (16) | 522 (16) | – |
| Able to walk unassisted on admission | |||
| Yes | 1425 (45) | 1409 (46) | 0.7850 |
| No | 1723 (55) | 1678 (54) | |
| (Missing) | 316 (9.1) | 170 (5.2) | – |
| Time from onset of symptoms to Emergency Department (min) | |||
| N: Median (Q1, Q3) | 2528: 186 (87, 438) | 2287: 187 (90, 470) | 0.3305 |
| Time from onset of symptoms to stroke unit (min) | |||
| N: Median (Q1, Q3) | 2113: 370 (180, 870) | 2144: 390 (190, 904) | 0.1687 |
p-values from chi squared test for categorical data, Wilcoxon rank-sum test for continuous data. Hypothesis tests omit missing data.
Bold indicates significant values
FeSS management and treatment: Overall adherence (primary and secondary outcomes)
Statistically significantly larger proportions of patients adhered with all management and treatment elements of the FeSS Protocols from pre-to-post implementation (Pre: 3.4%, Post: 35%; Absolute difference 33%, 95% CI 24%, 42%; p < 0.0001). Similarly, significantly greater proportion of patients from pre-to-post implementation had care adhering to all elements of: the fever elements combined (Pre: 17%, Post: 51%; Absolute difference 33%, 95% CI 30%, 37%; p < 0.0001); the hyperglycaemia elements combined (Pre: 18%, Post: 52%; Absolute difference 34%; 95% CI 31%, 36%; p < 0.0001); and the swallowing elements combined (Pre: 39%, Post: 67%; Absolute difference 29%, 95% CI 26%, 31%; p < 0.0001) (Table 3).
Table 3.
QASC Europe FeSS management results.
| Pre N = 3464 | Post N = 3257 | Differences in proportion (95% CI) a | p * | |
|---|---|---|---|---|
| n (%) | n (%) | |||
| Participating sites | 64 | 64 | – | – |
| Patient records entered | 3464 (100) | 3257 (100) | – | – |
| Overall adherence to the FeSS protocol c | 119 (3.4) | 1128 (35) | 33% (24%, 42%) | <0.0001 |
| Temperature monitored at least four times per day | ||||
| Day of admission d | 1493 (43) | 2565 (79) | 35% (32%, 38%) | <0.0001 |
| Day 2 of admission d | 1267 (37) | 2267 (70) | 33% (31%, 36%) | <0.0001 |
| Day 3 of admission d | 943 (27) | 1936 (59) | 31% (28%, 33%) | <0.0001 |
| Temperature >37.5°C recorded within 72 h of admission | 514 (15) | 580 (18) | 2.6% (0.72%, 4.4%) | 0.0058 |
| Paracetamol (or other anti-pyretic) given for first temperature ⩾37.5°C | 328 (64) | 487 (84) | 19% (12%, 26%) | <0.0001 |
| Paracetamol (or other anti-pyretic) given with 1 h from first temperature ⩾37.5°C d | 291 (57) | 458 (79) | 20% (13%, 27%) | <0.0001 |
| Monitored and treated according to the Fever Protocol d | 607 (17) | 1675 (51) | 33% (30%, 37%) | <0.0001 |
| Venous blood glucose level sample collected and sent to laboratory | 3143 (91) | 3172 (97) | 6.9% (4.9%, 8.9%) | <0.0001 |
| Blood glucose level (BGL) monitored at least four times per day | ||||
| Day of admission e | 1312 (40) | 2438 (75) | 36% (33%, 38%) | <0.0001 |
| Day 2 of admission e | 1122 (32) | 2224 (68) | 35% (33%, 38%) | <0.0001 |
| Day 3 of admission b | 736 (21) | 1691 (52) | 30% (27%, 33%) | <0.0001 |
| BGL ⩾ 10 mmol/L within 48 h of admission | 645 (19) | 693 (21) | 4% (1.8%, 6.1%) | 0.0002 |
| Insulin given for first BGL ⩾ 10 mmol/L | 417 (65) | 543 (78) | 15% (9.8%, 21%) | <0.0001 |
| Insulin given within 1 h from first BGL ⩾ 10 mmol/L e | 377 (58) | 519 (75) | 17% (12%, 23%) | <0.0001 |
| Monitored and treated according to the Hyperglycaemic (Sugar) Protocol e | 608 (18) | 1702 (52) | 34% (31%, 36%) | <0.0001 |
| Formal swallow screen performed | 2172 (63) | 2817 (86) | 22% (15%, 28%) | <0.0001 |
| Failed swallow screen | 475 (22) | 717 (25) | 1.7% (−0.51%, 3.9%) | 0.1325 |
| Failed screen and subsequently had swallow assessment f | 299 (63) | 472 (66) | 6.9% (2.2%, 12%) | 0.0036 |
| Swallow screen performed within 24 h f | 1729 (50) | 2584 (79) | 30% (26%, 34%) | <0.0001 |
| Swallow assessment recorded | 1499 (43) | 1887 (58) | 15% (13%, 17%) | <0.0001 |
| Swallow screen OR assessment recorded? | 2339 (67) | 2865 (88) | 19% (12%, 26%) | <0.0001 |
| Swallow screen or assessment performed before being given oral medications f | 1958 (56) | 2667 (82) | 23% (19%, 28%) | <0.0001 |
| Swallow screen or assessment performed before being given oral food or fluids f | 1974 (57) | 2688 (82) | 24% (19%, 29%) | <0.0001 |
| Monitored and treated according to the Swallow Protocol f | 1360 (39) | 2178 (67) | 29% (26%, 31%) | <0.0001 |
Paracetamol, insulin administration and subsequent swallow assessment outcomes include only patients with a fever (Model N = 411 Pre: N = 477 Post), hyperglycaemic event (Model N = 512 Pre: N = 594 Post) or failed swallow screen (Model N = 404: N = 571 Post) within relevant time period respectively. Monitoring and treatment practices were based on the documentation of activities in the medical records and therefore absent values were recorded as not carried out rather than missing.
Estimated marginal mean difference in proportion from mixed effects model calculated using the R margins package, standard errors for confidence interval obtained using delta method.
Only monitored if BGL unstable in first 48 h.
Must meet (d–f) to be deemed as having been monitored and treated according to the complete FeSS Protocol. This was a post-hoc, but pre-analysis outcome, comprised of a priori individual FeSS processes of care measures.
Must meet all elements to be deemed as having been monitored and treated according to the Fever Protocol.
Must meet all elements to be deemed as having been monitored and treated according to the Hyperglycaemic (Sugar) Protocol.
Must meet all elements to be deemed as having been monitored and treated according to the Swallow Protocol.
Mixed effects logistic regression controlling for age, sex, NIHSS and correlation within site and country. Model omits patients with missing covariate data (Model N = 2814 Pre: N = 2734 Post).
Bold indicates significant values
FeSS adherence: Individual elements (tertiary outcomes)
We also found that larger proportions of patients had improved monitoring and treatment for individual elements of the FeSS Protocols post-implementation, compared to pre-implementation (Table 3).
Treatment and discharge outcomes (tertiary outcomes)
Significantly more patients were treated in a stroke unit (p < 0.001) and were able to walk unassisted on discharge (p = 0.0105) post-implementation. There were no differences from pre- to post-implementation in receipt of intravenous thrombolysis, discharge destination, duration of hospital stay or disability using a dichotomised mRS (mRS < 2 vs mRS ⩾ 2). Our tertiary outcome analysis showed a shift towards worse outcome on the discharge mRS (Table 4).
Table 4.
QASC Europe: patient treatment and discharge outcomes.
| Pre N = 3464 | Post N = 3257 | Difference in proportion (95% CI) b | p a | |
|---|---|---|---|---|
| n (%) | n (%) | |||
| Treatment | ||||
| Patient received intravenous thrombolysis | 813 (23) | 738 (23) | −1.2% (−3.4%, 0.99%) | 0.2770 |
| Patient treated in a stroke unit at any time during their stay | 2904 (84) | 2974 (91) | 4.1% (2%, 6.2%) | <0.0001 |
| Discharge outcomes | ||||
| Discharge destination | ||||
| Home | 1965 (61) | 1799 (61) | n/a | 0.6774 |
| Rehabilitation facility | 402 (13) | 363 (12) | ||
| Long-term care/nursing home | 198 (6.2) | 208 (7.1) | ||
| Other hospital | 323 (10) | 293 (9.9) | ||
| Deceased | 294 (9.2) | 280 (9.5) | ||
| (Missing) | 253 (7.3) | 308 (9.5) | – | – |
| Discharge modified Rankin Score | ||||
| 0: No symptoms at all | 489 (15) | 390 (13) | n/a | 0.0328 |
| 1: No significant disability despite symptoms | 572 (18) | 505 (17) | ||
| 2: Slight disability | 413 (13) | 413 (14) | ||
| 3: Moderate disability | 472 (15) | 402 (14) | ||
| 4: Moderately severe disability | 521 (16) | 522 (18) | ||
| 5: Severe disability | 450 (14) | 437 (15) | ||
| 6: Deceased | 294 (8.5) | 280 (9.5) | ||
| mRS ⩾ 2 | 2150 (67) | 2054 (70) | 1.6% (−0.65%, 3.9%) | 0.1614 |
| (Missing) | 243 (7.0) | 308 (9.5) | – | – |
| Able to walk unassisted on discharge | ||||
| Yes | 1791 (53) | 1799 (56) | 2.9% (0.68%, 5.2%) | 0.0105 |
| No | 1562 (47) | 1410 (44) | ||
| (Missing) | 111 (3.2) | 48 (1.5) | – | – |
| Duration of hospital stay (days) | ||||
| Median (Q1, Q3) | 8 (5, 12) | 8 (5, 12) | −0.20 (−0.53, 0.14) | 0.1257 |
n/a: due to the multiple nominal distribution of this variable it was not appropriate to calculate pre-post difference in proportions.
Mixed effects logistic regression controlling for age, sex, NIHSS and correlation within site and country.
Estimated marginal mean difference in proportion from mixed effects model, standard errors for confidence interval obtained using delta method.
Bold indicates significant values
A sensitivity analysis including pre-implementation data from hospitals who subsequently withdrew or were lost to follow-up showed consistent results (Supplemental Appendix D) as did analyses including a missing data category for NIHSS. There were no differences for FeSS adherence outcomes between those hospitals that completed the project and those that didn’t. Hospitals that did not complete the project had a higher proportion of patients with moderate to severe premorbid disability at pre-intervention. (Supplemental Appendix E and F).
FeSS monitoring and treatment practices between HIC and MIC
Comparison of pre-post changes for HIC and MIC (exploratory analysis)
Pre-implementation data were received for 2397 patients and 2199 patients post-implementation from 44 hospitals in seven HICs (Lithuania, Czech Republic, Italy, Poland, Portugal, Slovakia, Spain); and 1067 patients pre-implementation and 1058 patients post-implementation from 20 hospitals in 10 MICs (Armenia, Belarus, Bulgaria, Romania, Russia, Georgia, Kazakhstan, Moldova, Ukraine, Uzbekistan). 21 Patients from MIC were younger, more likely to have had a previous stroke, a more severe stroke and a higher pre-morbid mRS. They were less likely to be able to walk unassisted on admission, have diabetes and took longer to arrive at the ED but were faster to be admitted to a stroke unit relative to patients from HIC (Supplemental Appendix G).
Pre-post implementation changes in the overall FeSS Protocol compliance were significantly greater for HIC compared with MIC. However, the relationship was reversed for compliance with each of the combined fever, hyperglycaemia and swallow elements (Table 5).
Table 5.
QASC Europe FeSS management per country income status.
| High income countries | Middle income countries | p * | |||||
|---|---|---|---|---|---|---|---|
| Pre N = 2397, n (%) | Post N = 2199, n (%) | Pre/post changes (95% CI) a | Pre N = 1067, n (%) | Post N = 1058, n (%) | Pre/post changes (95% CI) a | ||
| Participating sites | 44 | 44 | 20 | 20 | |||
| Monitored and treated according to the combined FeSS Protocol b | 87 (3.6) | 773 (35) | 33% (21%, 46%) | 32 (3.0) | 355 (34) | 31% (19%, 43%) | 0.0365 |
| Monitored and treated according to the Fever Protocol c | 468 (20) | 1115 (51) | 31% (28%, 35%) | 139 (13) | 560 (53) | 39% (31%, 47%) | 0.0001 |
| Monitored and treated according to the Hyperglycaemic Protocol d | 525 (22) | 1178 (54) | 31% (28%, 33%) | 83 (7.8) | 524 (50) | 37% (28%, 46%) | <0.0001 |
| Monitored and treated according to the Swallow Protocol e | 835 (35) | 1404 (64) | 27% (25%, 30%) | 525 (49) | 774 (73) | 29% (21%, 37%) | 0.0045 |
Paracetamol, insulin administration and subsequent swallow assessment outcomes include only patients with a fever (Model N = 411 Pre: N = 477 Post), hyperglycaemic event (Model N = 512 Pre: N = 594 Post) or failed swallow screen (Model N = 404: N = 571 Post) within relevant time period respectively. The CIs for difference in proportions overlap due to methods of estimating marginal mean. Odds ratio confidence intervals do not overlap where p < 0.05 (data not shown).
Estimated marginal mean difference in proportion from mixed effects model, standard errors for confidence interval obtained using delta method.
Must meet d, e and f to be deemed as having been monitored and treated according to the combined FeSS Protocol.
Must meet all elements to be deemed as having been monitored and treated according to the Fever Protocol.
Must meet all elements to be deemed as having been monitored and treated according to the Hyperglycaemic (Sugar) Protocol.
Must meet all elements to be deemed as having been monitored and treated according to the Swallow Protocol. *Mixed effects logistic regression controlling for age, sex, NIHSS and correlation within site and country. Model omits patients with missing covariate data (Model N = 2814 Pre: N = 2734 Post).
Changes in treatment and discharge outcomes between HIC and MIC were similar, with greater improvements in proportion of patients treated in a stroke unit and able to walk unassisted on discharge in MIC (Supplemental Appendix H).
Post-implementation changes between HIC and MIC
Post-implementation primary and secondary outcomes were similar for HIC and MIC (Supplemental Appendix 1).
Mean FeSS adherence score varied greatly between hospitals, ranging from 38% to 100% with 11% hospitals recording less than 50% mean overall FeSS Protocol adherence and 14% recording over 95% adherence (Supplemental Appendix J). There was no discernible difference in distribution of mean overall FeSS Protocol adherence with country income class.
Data quality
Inter-rater reliability data were received and successfully matched for 265 post-implementation patients from 53 hospitals. Post intervention overall FeSS Protocol adherence had a Kappa value of 0.86 (95% CI 0.77, 0.92) indicating moderate to strong inter-rater reliability. 30
Discussion
The QASC Europe Study was a pan-European study to support clinicians to implement the evidence-based FeSS Protocols. We achieved significant improvements in overall FeSS Protocol adherence, as well as individual fever, hyperglycaemia and swallowing elements of the FeSS Protocols. Given published behaviour change improvements of between 4% and 12%, 24 the improvements in clinical practice observed in this study of up to 30% and across multiple elements of the Protocols, is laudable. Our data support that the improvement in FeSS Protocol adherence is likely due to our implementation strategy and not external factors because other metrics of stroke care quality such as thrombolysis remained unchanged.
While evidence from several studies show the FeSS Protocols reduce death and dependency at 90-days 7 and up to 4 years post-stroke, 8 in this non-clinical trial study, worse outcomes were shown for discharge mRS. This maybe be because FeSS Protocols do not improve discharge mRS and/or that our measuring of the recording of information does not accord with actual care provided. Although differences in study design and definition of measures mean that we cannot directly compare our results to the process measures for the original QASC Trial results, 31 pre-post differences seen in QASC Europe were generally greater than those seen between intervention and control hospitals in the original QASC Trial.
We note a discrepancy between the number of hospitals who reported presence of a stroke unit and the number of patients who received stroke unit care. This could possibly be due to inconsistencies around the definition of a stroke unit, or new stroke units being set up during the study.
Pre-implementation, monitoring and treatment was low for the combined fever (16%), and combined hyperglycaemia (16%) protocols and somewhat higher but still sub-optimal for combined swallowing elements (41%). Such low baseline level of FeSS can explain the large size of the pre-to-post improvements. Also, while pre-to-post improvements were substantial these were received by only half to two thirds of patients indicating the need for further concentrated efforts to improve compliance.
Significant pre-to-post improvements were made in both HIC and MIC. Improvements in overall FeSS Protocol adherence were significantly higher in HIC when compared with MIC. This relationship was reversed for the each of the combined fever, hyperglycaemia and swallow elements; while potentially counter intuitive, this reversal was due to a lower adjusted baseline adherence in MIC. However, these were exploratory tests and we do not consider that they show any meaningful difference between MIC and HIC in terms of FeSS adherence; rather, the important message is that the countries’ income status did not influence the improvements in monitoring and treatment (Table 5).
Post-implementation, adherence with individual elements of the FeSS Protocols were broadly similar between MIC and HIC noting that while combined swallowing management and treatment practices were higher for MIC (73%) than HIC (64%) this difference was not statistically significant (Supplemental Appendix H). However, the clinical importance of these improvements in MIC is noteworthy in that many hospitals in MIC have no access to stroke unit care nor reperfusion therapies with corresponding limited resources for patient care, 5 hence, use of the FeSS Protocols has the potential to significantly improve stroke patient outcomes. These results also help contradict any assumption that lack of resources is a barrier to achieving improvements in quality stroke care delivery in MIC.4,5
Our study had multiple strengths. Firstly, post-implementation patients were prospectively identified consecutive stroke admissions. Secondly, our inter-rater reliability checks were rigorous, with reliability cases randomly selected by the study statistician (BM) and not the hospital clinicians and showed moderate to strong agreement. Other strengths included stakeholder engagement with ESO, use of an established stroke audit platform and importantly, the involvement of the Angels Consultants who were already active in these hospitals. External facilitation previously has been shown to improve stroke care processes. 32 Building on this evidence, our study also provides evidence of successful remote offshore facilitation (from Australia) of a complex intervention delivered simultaneously to multiple international stakeholders (in Europe). In addition, with implementation scale-up there is always the risk of ‘voltage drop’ 33 where the intervention effect is minimised or lost – this was not observed in the QASC Europe Study.
An additional strength of our study is the examination of the performance for all three physiological measures which has not been previously undertaken in Europe. In the BRIDGE stroke trials,12,15,34 whilst dysphagia management was evaluated, acute management of fever and assessment of blood glucose levels was not, despite many international clinical practice guidelines specific to these.35–38 Our large-scale, multi-country, multi-centre implementation study with an embedded a priori process evaluation, demonstrated successful large-scale implementation of the evidence-based FeSS Protocols with significant improvements in FeSS management. Our study had a focused multi-country approach to fever, hyperglycaemia and swallowing management following stroke, which prior to this study was not part of routine data collection within Europe. Routine collection of these variables is now embedded in the RES-Q platform for future evaluation. Further, there have been no international stroke implementation studies describing remote facilitation and action planning methods as used in our study. Prior efforts to improve guideline adherence has concentrated primarily on audit and feedback mechanisms.
Our study had some limitations. As the primary outcome measure was not included in the original protocol submitted to ACU ethics committee, this needs to be interpreted accordingly, however, composite outcomes are more difficult to achieve, and this post-hoc analysis was done for ease of study results interpretation. We had a volunteer rather than random sample of hospitals and the implementation of the FeSS Protocols was not randomly assigned. However, the project reflected real-time, real-world clinical practice with minimal patient exclusions and this enabled us to include a wide range of European countries. Further, 90-day patient outcome data were not collected as this was a focused implementation and scale up study and not a randomised trial with clinical outcomes. The study also used retrospective pre-implementation historical data from the medical records but data from prospectively recruited patients for the post-implementation data. This difference may have resulted in recording bias. Data also were self-reported by hospital clinicians, acknowledging potential for response bias. However, the retrospective nature of clinical audit and the use of self-reported data in not unconventional. The study was severely impacted by the COVID-19 pandemic with many stroke units across Europe co-opted, or stroke-specialist staff redeployed39,40 producing an extended period for post-implementation audit commencement/completion. This may have resulted in a longer time to ‘bed down’ the intervention potentially increasing the impact of the intervention. Conversely, international stroke data showed that due to COVID-19, many patients with stroke did not receive access to time critical stroke care, rehabilitation and prevention therapies 40 ; stroke unit care; antihypertensives, antithrombotics on discharge; nor access to rehabilitation. 41 That our participating hospitals did manage to initiate the FeSS Protocols during this disruptive time is testament to the study’s ability to drive behaviour change and establish a common purpose to manage these nursing aspects of stroke care more effectively. Twelve hospitals which provided pre-implementation data did not provide any post-implementation data; this may also have been impacted by the COVID-19 pandemic. There were also a small amount of missing data for some patient demographic characteristics (⩽3%) and a larger amount for some clinical characteristics (pre-morbid mRS (8.3%); NIHSS (16%)). However, sensitivity analyses including a missing value category for NIHSS in regression models produced similar results to the analyses excluded missing values, thus missing data were unlikely to have an impact on results and conclusions.
The potential of this study to leave a positive legacy is substantial. This was the first time many of our collaborating nurses had participated in such a study, particularly notable for countries where nursing autonomy is not high and physicians usually determine care protocols. 42 Nurses can apply their experience and learnings to lead other similar evidence-implementation studies in the future, providing an opportunity to work to the top of their scope of practice whilst also building capacity in research skill development. One country already has adopted the FeSS Protocols into their National Stroke Guidelines. 38 Of interest, since completion of the seminal trial 10 years ago, FeSS recommendations have appeared in guidelines in the United Kingdom, 43 United States of America 35 and Europe. 44
In line with research and development priorities outlined in the 2018–2030 European Stroke Action Plan 18 to reduce the burden of stroke across Europe, our study fostered international collaboration among researchers, hospital executives, governments, professional societies and industry in HICs and MICs. This level of collaboration is often difficult to achieve, and demonstrates how benefits to patients outweigh geopolitical and sociocultural challenges and complexities and demands of international health research collaborations. 45
Finally, the findings from the QASC Europe Study will be used to further scale-up the proven FeSS intervention internationally (QASC Global) including free access to the FeSS data collection tool via RES-Q (contact authors). This could be of particular benefit in low-resourced hospitals in LMICs with limited access to hyper-acute stroke therapies, education and stroke specialists.
Conclusion
This pan-European, multi-site implementation study with a nurse-initiated intervention, demonstrated on a large scale what can be achieved when healthcare and an industry-initiated healthcare initiative combine dedicated resources with collaborative, multidisciplinary relationships and commit to a shared vision of improving stroke outcomes. Our study was conducted with the International Year of the Nurse (2020) and in direct alignment with the World Health Organisation’s ‘Nursing Now’ 46 campaign goal to raise the status and profile of nurses.. This international knowledge implementation and scale-up study targeted one of the largest occupational groups in the global health workforce to implement the evidence-based nurse-initiated FeSS Protocols. Undertaken in countries with vast differences in access to the latest stroke therapies, resources and healthcare systems confirms how extensive the global reach can be to improve care when nurses are empowered to lead.
Supplemental Material
Supplemental material, sj-docx-11-eso-10.1177_23969873221126027 for Translation of nurse-initiated protocols to manage fever, hyperglycaemia and swallowing following stroke across Europe (QASC Europe): A pre-test/post-test implementation study by Sandy Middleton, Simeon Dale, Benjamin McElduff, Kelly Coughlan, Elizabeth McInnes, Robert Mikulik, Thomas Fischer, Jan Van der Merwe, Dominique Cadilhac, Catherine D’Este, Christopher Levi, Jeremy M Grimshaw, Andreea Grecu, Clare Quinn, Ngai Wah Cheung, Tereza Koláčná, Sabina Medukhanova, Estela Sanjuan Menendez, Susana Salselas, Gert Messchendorp, Anne-Kathrin Cassier-Woidasky, Marcelina Skrzypek-Czerko, Merce Slavat-Plana, Urso Antonella and Waltraud Pfeilschifter in European Stroke Journal
Supplemental material, sj-docx-2-eso-10.1177_23969873221126027 for Translation of nurse-initiated protocols to manage fever, hyperglycaemia and swallowing following stroke across Europe (QASC Europe): A pre-test/post-test implementation study by Sandy Middleton, Simeon Dale, Benjamin McElduff, Kelly Coughlan, Elizabeth McInnes, Robert Mikulik, Thomas Fischer, Jan Van der Merwe, Dominique Cadilhac, Catherine D’Este, Christopher Levi, Jeremy M Grimshaw, Andreea Grecu, Clare Quinn, Ngai Wah Cheung, Tereza Koláčná, Sabina Medukhanova, Estela Sanjuan Menendez, Susana Salselas, Gert Messchendorp, Anne-Kathrin Cassier-Woidasky, Marcelina Skrzypek-Czerko, Merce Slavat-Plana, Urso Antonella and Waltraud Pfeilschifter in European Stroke Journal
Supplemental material, sj-docx-3-eso-10.1177_23969873221126027 for Translation of nurse-initiated protocols to manage fever, hyperglycaemia and swallowing following stroke across Europe (QASC Europe): A pre-test/post-test implementation study by Sandy Middleton, Simeon Dale, Benjamin McElduff, Kelly Coughlan, Elizabeth McInnes, Robert Mikulik, Thomas Fischer, Jan Van der Merwe, Dominique Cadilhac, Catherine D’Este, Christopher Levi, Jeremy M Grimshaw, Andreea Grecu, Clare Quinn, Ngai Wah Cheung, Tereza Koláčná, Sabina Medukhanova, Estela Sanjuan Menendez, Susana Salselas, Gert Messchendorp, Anne-Kathrin Cassier-Woidasky, Marcelina Skrzypek-Czerko, Merce Slavat-Plana, Urso Antonella and Waltraud Pfeilschifter in European Stroke Journal
Supplemental material, sj-docx-4-eso-10.1177_23969873221126027 for Translation of nurse-initiated protocols to manage fever, hyperglycaemia and swallowing following stroke across Europe (QASC Europe): A pre-test/post-test implementation study by Sandy Middleton, Simeon Dale, Benjamin McElduff, Kelly Coughlan, Elizabeth McInnes, Robert Mikulik, Thomas Fischer, Jan Van der Merwe, Dominique Cadilhac, Catherine D’Este, Christopher Levi, Jeremy M Grimshaw, Andreea Grecu, Clare Quinn, Ngai Wah Cheung, Tereza Koláčná, Sabina Medukhanova, Estela Sanjuan Menendez, Susana Salselas, Gert Messchendorp, Anne-Kathrin Cassier-Woidasky, Marcelina Skrzypek-Czerko, Merce Slavat-Plana, Urso Antonella and Waltraud Pfeilschifter in European Stroke Journal
Supplemental material, sj-docx-5-eso-10.1177_23969873221126027 for Translation of nurse-initiated protocols to manage fever, hyperglycaemia and swallowing following stroke across Europe (QASC Europe): A pre-test/post-test implementation study by Sandy Middleton, Simeon Dale, Benjamin McElduff, Kelly Coughlan, Elizabeth McInnes, Robert Mikulik, Thomas Fischer, Jan Van der Merwe, Dominique Cadilhac, Catherine D’Este, Christopher Levi, Jeremy M Grimshaw, Andreea Grecu, Clare Quinn, Ngai Wah Cheung, Tereza Koláčná, Sabina Medukhanova, Estela Sanjuan Menendez, Susana Salselas, Gert Messchendorp, Anne-Kathrin Cassier-Woidasky, Marcelina Skrzypek-Czerko, Merce Slavat-Plana, Urso Antonella and Waltraud Pfeilschifter in European Stroke Journal
Supplemental material, sj-docx-6-eso-10.1177_23969873221126027 for Translation of nurse-initiated protocols to manage fever, hyperglycaemia and swallowing following stroke across Europe (QASC Europe): A pre-test/post-test implementation study by Sandy Middleton, Simeon Dale, Benjamin McElduff, Kelly Coughlan, Elizabeth McInnes, Robert Mikulik, Thomas Fischer, Jan Van der Merwe, Dominique Cadilhac, Catherine D’Este, Christopher Levi, Jeremy M Grimshaw, Andreea Grecu, Clare Quinn, Ngai Wah Cheung, Tereza Koláčná, Sabina Medukhanova, Estela Sanjuan Menendez, Susana Salselas, Gert Messchendorp, Anne-Kathrin Cassier-Woidasky, Marcelina Skrzypek-Czerko, Merce Slavat-Plana, Urso Antonella and Waltraud Pfeilschifter in European Stroke Journal
Supplemental material, sj-docx-7-eso-10.1177_23969873221126027 for Translation of nurse-initiated protocols to manage fever, hyperglycaemia and swallowing following stroke across Europe (QASC Europe): A pre-test/post-test implementation study by Sandy Middleton, Simeon Dale, Benjamin McElduff, Kelly Coughlan, Elizabeth McInnes, Robert Mikulik, Thomas Fischer, Jan Van der Merwe, Dominique Cadilhac, Catherine D’Este, Christopher Levi, Jeremy M Grimshaw, Andreea Grecu, Clare Quinn, Ngai Wah Cheung, Tereza Koláčná, Sabina Medukhanova, Estela Sanjuan Menendez, Susana Salselas, Gert Messchendorp, Anne-Kathrin Cassier-Woidasky, Marcelina Skrzypek-Czerko, Merce Slavat-Plana, Urso Antonella and Waltraud Pfeilschifter in European Stroke Journal
Supplemental material, sj-docx-8-eso-10.1177_23969873221126027 for Translation of nurse-initiated protocols to manage fever, hyperglycaemia and swallowing following stroke across Europe (QASC Europe): A pre-test/post-test implementation study by Sandy Middleton, Simeon Dale, Benjamin McElduff, Kelly Coughlan, Elizabeth McInnes, Robert Mikulik, Thomas Fischer, Jan Van der Merwe, Dominique Cadilhac, Catherine D’Este, Christopher Levi, Jeremy M Grimshaw, Andreea Grecu, Clare Quinn, Ngai Wah Cheung, Tereza Koláčná, Sabina Medukhanova, Estela Sanjuan Menendez, Susana Salselas, Gert Messchendorp, Anne-Kathrin Cassier-Woidasky, Marcelina Skrzypek-Czerko, Merce Slavat-Plana, Urso Antonella and Waltraud Pfeilschifter in European Stroke Journal
Supplemental material, sj-docx-9-eso-10.1177_23969873221126027 for Translation of nurse-initiated protocols to manage fever, hyperglycaemia and swallowing following stroke across Europe (QASC Europe): A pre-test/post-test implementation study by Sandy Middleton, Simeon Dale, Benjamin McElduff, Kelly Coughlan, Elizabeth McInnes, Robert Mikulik, Thomas Fischer, Jan Van der Merwe, Dominique Cadilhac, Catherine D’Este, Christopher Levi, Jeremy M Grimshaw, Andreea Grecu, Clare Quinn, Ngai Wah Cheung, Tereza Koláčná, Sabina Medukhanova, Estela Sanjuan Menendez, Susana Salselas, Gert Messchendorp, Anne-Kathrin Cassier-Woidasky, Marcelina Skrzypek-Czerko, Merce Slavat-Plana, Urso Antonella and Waltraud Pfeilschifter in European Stroke Journal
Supplemental material, sj-pdf-1-eso-10.1177_23969873221126027 for Translation of nurse-initiated protocols to manage fever, hyperglycaemia and swallowing following stroke across Europe (QASC Europe): A pre-test/post-test implementation study by Sandy Middleton, Simeon Dale, Benjamin McElduff, Kelly Coughlan, Elizabeth McInnes, Robert Mikulik, Thomas Fischer, Jan Van der Merwe, Dominique Cadilhac, Catherine D’Este, Christopher Levi, Jeremy M Grimshaw, Andreea Grecu, Clare Quinn, Ngai Wah Cheung, Tereza Koláčná, Sabina Medukhanova, Estela Sanjuan Menendez, Susana Salselas, Gert Messchendorp, Anne-Kathrin Cassier-Woidasky, Marcelina Skrzypek-Czerko, Merce Slavat-Plana, Urso Antonella and Waltraud Pfeilschifter in European Stroke Journal
Supplemental material, sj-png-10-eso-10.1177_23969873221126027 for Translation of nurse-initiated protocols to manage fever, hyperglycaemia and swallowing following stroke across Europe (QASC Europe): A pre-test/post-test implementation study by Sandy Middleton, Simeon Dale, Benjamin McElduff, Kelly Coughlan, Elizabeth McInnes, Robert Mikulik, Thomas Fischer, Jan Van der Merwe, Dominique Cadilhac, Catherine D’Este, Christopher Levi, Jeremy M Grimshaw, Andreea Grecu, Clare Quinn, Ngai Wah Cheung, Tereza Koláčná, Sabina Medukhanova, Estela Sanjuan Menendez, Susana Salselas, Gert Messchendorp, Anne-Kathrin Cassier-Woidasky, Marcelina Skrzypek-Czerko, Merce Slavat-Plana, Urso Antonella and Waltraud Pfeilschifter in European Stroke Journal
Acknowledgments
We thank all clinical champions, nurses, stroke unit directors and speech and language therapist from participating hospitals and all the Angels consultants past and present who were involved in the project (Supplemental Appendix K). All members of the Steering and management committee.^,# All members of the RES-Q team,* European Stroke Organisation Presidents present and past,^^ QASC Europe Study team## and the Nursing Research Institute team.**
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: SD, SM and RM received speakers’ fees, conference registration and travel expenses from the Angels Initiative. Other authors have no competing interests.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by the European Stroke Organisation. The funder and industry collaborators had no role in the study design, data collection, data analysis, data interpretation or report writing.
Ethical approval: Ethics approval was obtained from Australian Catholic University (2017-11H). Some hospitals in Italy, Portugal and Spain required governance clearance (Supplemental Appendix B).
Informed consent: As all data were de-identified, individual patient consent was not required as no identifying patient information was provided by hospitals.
Guarantor: ACU? Steering Committee?
Contributorship: SM, SD, EMc, DC, CDE, CL JG, AG, CQ, WC, WP conceptualised the research. SM and SD secured funding. SM provided oversight and leadership of the project. SM, SD, LMc, DC, JG, CQ, WC, WP, KC, BMc, CDE developed and designed the method. SM, SD, KC, RM, TK, SM, ESM, SS, GM MSC, MSP, AU oversaw the research conduct. SD, KC, SM were responsible for project administration. BMc, CDE, AG were responsible for data curation. SM, SD, CDE, BMc verified the underlying data. BMc, CDE conducted the analyses. SM, SD, KC, TF JVDM, RM, TK, SM, ESM, SS, MSC, MSP, AU were responsible for resource development. SM, SD, RM, AG, BM designed the RES-Q data collection form. SM, SD, KC, BMc were responsible for the visualisation of the project. SM, KC, SD, BMc, CDE, WP, CL, JG, DC, EM wrote the initial draft of the manuscript. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
Data sharing: Deidentified individual participant data will be made available on request to the corresponding author (sandy.middleton@acu.edu.au), subject to ethical approval.
^Quality in Acute Stroke Care (QASC) Europe Steering Committee: Professor Waltraud Pfeilschifter (Chair, Goethe University Frankfurt, Germany)
Professor Dominique Cadilhac (Monash University, Australia)
Dr Luca Casertano (Manager of the Hospital Network Planning, Lazio region, Italy)
Professor Anne-Kathrin Cassier-Woidasky (University of Applied Sciences Saarland, Germany)
Professor N Wah Cheung (Sydney University, Australia)
Dr Igor Crivorucica (Institute of Emergency Medicine, Moldova)
Ms Simeon Dale (Australian Catholic University, Australia)
Professor Cate D’Este (Sax Institute, Sydney; Australian National University, Australia)
Dr Oganes Ezoyen (Erebouni Medical Centre, Armenia)
Dr. Joerg Glahn (Department of Neurology Johannes Wesling Klinikum, Germany)
Ms Andreea Grecu (RES-Q, St. Anne’s University Hospital, Brno, Czech Republic)
Professor Jeremy M. Grimshaw (Ottawa University, Canada)
Ms Olga Jarmak (Kharkiv Railway Clinical Hospital, Ukraine)
Professor Chris Levi (University of New South Wales, Australia)
Ms Tereza Loucna (Faculty Hospital Motol, Prague, Czech Republic)
Professor Elizabeth McInnes (Australian Catholic University, Australia)
Ms Sabina Medukhanova (National Center for Neurosurgery, Kazakhstan)
Mr Gert Messchendorp (University Medical Center Groningen, Netherlands)
Professor Sandy Middleton (Australian Catholic University, Australia)
Ms Clare Quinn (Prince of Wales Hospital, Australia)
Ms Marieke Rijksen (SPM Consult, Netherlands)
Ms Mercè Salvat Plana (Catalan Stroke Programme, Spain)
Ms Susana Salselas (Stroke Unit, UH Macedo Cavaleiros, Portugal)
Dr Estela Sanjuan Menendez (Vall d’Hebron University Hospital, Spain)
Dr Marcelina Skrzypek-Czerko (Medical University of Gdańsk, Poland)
Ms Antonella Urso (Hospital Network Area-Regional Health Department, Regione Lazio, Italy)
#QASC Europe Study Implementation Committee: Professor Sandy Middleton (Australian Catholic University)
Ms Simeon Dale (Australian Catholic University)
Professor Elizabeth McInnes (Australian Catholic University)
Professor Dominique Cadilhac (Monash University, Australia)
Ms Marieke Rijksen (Australian Catholic University)
Mr Thomas Fischer (Head of the Global Angel Initiative, Germany)
Mr Jan Van der Merwe (Head of the Angels Initiative Europe, Germany)
Ms Rita Rodrigues (Angels Initiative Europe, Germany)
Mr Robert Havalda (Angels Initiative Europe, Czech Republic)
*Global Registry of Stroke Care Quality (RES-Q): Professor Robert Mikulik (RES-Q Founder, Data Custodian, Czech Republic)
Ms Andreea Grecu (RES-Q Platform Architect, Czech Republic)
Mr Miroslav Varecha (RES-Q Data Scientist, Czech Republic)
Ms Nina V. Chufarova (RES-Q coordinator, Czech Republic)
Mr Steven Simsic (RES-Q Senior Developer, Czech Republic)
Ms Rupal Sedani (RES-Q Manager, Czech Republic)
Ms Veronika Svobodova (Strategy Officer, Czech Republic)
Mr Vaclav Pasacek (RES-Q Senior Developer, Czech Republic)
^^European Stroke Organisation: Prof. Martin Dichgans (President, European Stroke Organisation, Ludwig-Maximilians-Universität, LMU, Munich, Germany)
Dr Bart van der Worp (Immediate Past-President, European Stroke Organisation; University Medical Centre, Utrecht, Netherlands)
Professor Valeria Caso (Past-President, European Stroke Organisation; University of Perugia, Italy)
##Quality in Acute Stroke Care (QASC) Europe Study Team: Professor Sandy Middleton (Principal Investigator, Australian Catholic University, Australia)
Ms Simeon Dale (Study Manager, Australian Catholic University, Australia)
Ms Kelly Coughlan (Study Officer Australian Catholic University, Australia)
Professor Cate D’Este (Senior Biostatistician, Sax institute, Australia; Australian National University, Australia)
Mr Benjamin McElduff (Biostatistician, Australian Catholic University, Australia)
Professor Elizabeth McInnes (Australian Catholic University, Australia)
Ms Marieke Rijksen (European Liaison Officer, Netherlands)
**Nursing Research Institute Team: Professor Sandy Middleton (Principal Investigator, Australian Catholic University, Australia)
Ms Simeon Dale (Study Manager, Australian Catholic University, Australia)
Ms Kelly Coughlan (Study Officer Australian Catholic University, Australia)
Mr Benjamin McElduff (Biostatistician, Australian Catholic University, Australia)
Professor Elizabeth McInnes (Australian Catholic University, Australia)
Dr Cintia Mayel Martinez-Garduno (Research Officer, Australian Catholic University, Australia)
Dr Oyebola Fasugba (Research Fellow, Australian Catholic University, Australia)
Ms Joylynn Israel (Research Assistant, Australian Catholic University, Australia)
Ms Patty Zenenos, (Executive Officer, Australian Catholic University, Australia)
Ms Carmel Parker (Executive Assistant, Australian Catholic University, Australia)
Ms Tara Doyle (Executive Assistant, Australian Catholic University, Australia)
ORCID iDs: S Middleton  https://orcid.org/0000-0002-7201-4394
https://orcid.org/0000-0002-7201-4394
DA Cadilhac  https://orcid.org/0000-0001-8162-682X
https://orcid.org/0000-0001-8162-682X
A-K Cassier-Woidasky  https://orcid.org/0000-0002-0282-2185
https://orcid.org/0000-0002-0282-2185
Supplemental material: Supplemental material for this article is available online.
References
- 1. Aguiar de Sousa D, von Martial R, Abilleira S, et al. Access to and delivery of acute ischaemic stroke treatments: a survey of national scientific societies and stroke experts in 44 European countries. Eur Stroke J 2019; 4: 13–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stroke Unit Trialists Collaboration. Organised inpatient (stroke unit) care for stroke. Cochrane Database Syst Rev 2013; 9: 1–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wardlaw JM, Murray V, Berge E, et al. Recombinant tissue plasminogen activator for acute ischaemic stroke: an updated systematic review and meta-analysis. Lancet 2012; 379: 2364–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stroke Alliance for Europe (SAFE). The burden of stroke in Europe. Report. London, 2017. [Google Scholar]
- 5. Owolabi MO, Thrift AG, Martins S, et al. The state of stroke services across the globe: report of World Stroke Organization-World Health Organization Surveys. Int J Stroke 2021; 16: 889–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Green TL, McNair ND, Hinkle JL, et al. Care of the patient with acute ischemic stroke (posthyperacute and prehospital discharge): update to 2009 comprehensive nursing care scientific statement: a scientific statement from the American Heart Association. Stroke 2021; 52: e179–e197. [DOI] [PubMed] [Google Scholar]
- 7. Middleton S, McElduff P, Ward J, et al. Implementation of evidence-based treatment protocols to manage fever, hyperglycaemia, and swallowing dysfunction in acute stroke (QASC): a cluster randomised controlled trial. Lancet 2011; 378: 1699–1706. [DOI] [PubMed] [Google Scholar]
- 8. Middleton S, Coughlan K, Mnatzaganian G, et al. Mortality reduction for fever, hyperglycemia, and swallowing nurse-initiated stroke intervention: QASC trial (quality in acute stroke care) follow-up. Stroke 2017; 48: 1331–1336. [DOI] [PubMed] [Google Scholar]
- 9. Australian Commission on Safety and Quality in Health Care. Economic evaluation of investigator-initiated clinical trials conducted by networks, https://www.safetyandquality.gov.au/publications/economic-evaluation-of-investigator-initiated-clinical-trials-conducted-by-networks/ (2017, accessed June 2020).
- 10. Marquina C, Ademi Z, Zomer E, et al. Cost burden and cost-effective analysis of the nationwide implementation of the quality in acute stroke care protocol in Australia. J Stroke Cerebrovasc Dis 2021; 30: 105931. [DOI] [PubMed] [Google Scholar]
- 11. Middleton S, Lydtin A, Comerford D, et al. From QASC to QASCIP: successful Australian translational scale-up and spread of a proven intervention in acute stroke using a prospective pre-test/post-test study design. BMJ Open 2016; 6: e011568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Berwanger O, Guimarães HP, Laranjeira LN, et al. Effect of a multifaceted intervention on use of evidence-based therapies in patients with acute coronary syndromes in Brazil: the BRIDGE-ACS randomized trial. JAMA 2012; 307: 2041–2049. [DOI] [PubMed] [Google Scholar]
- 13. Machline-Carrion MJ, Santucci EV, Damiani LP, et al. Effect of a quality improvement intervention on adherence to therapies for patients with acute ischemic stroke and transient ischemic attack: a cluster randomized clinical trial. JAMA Neurol 2019; 76: 932–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ormseth CH, Sheth KN, Saver JL, et al. The American Heart Association’s get with the guidelines (GWTG)-stroke development and impact on stroke care. Stroke Vasc Neurol 2017; 2: 94–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang Y, Li Z, Zhao X, et al. Effect of a multifaceted quality improvement intervention on hospital personnel adherence to performance measures in patients with acute ischemic stroke in China: a randomized clinical trial. JAMA 2018; 320: 245–254. [DOI] [PubMed] [Google Scholar]
- 16. Zomahoun HTV, Ben Charif A, Freitas A, et al. The pitfalls of scaling up evidence-based interventions in health. Glob Health Action 2019; 12: 1670449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mikulik R, Caso V, Wahlgren N. Past & future of stroke care in Europe. Oruen–CNS J 2017; 2: 19–26. [Google Scholar]
- 18. Norrving B, Barrick J, Davalos A, et al. Action plan for stroke in Europe 2018–2030. Eur Stroke J 2018; 3: 309–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mikulik R, Ylikotila P, Roine RO, et al. Leaving a legacy of stroke in Europe: a community of dedicated professionals is changing the face of stroke in Europe. CNS J 2017; 3: 8–18. [Google Scholar]
- 20. Middleton S, Pfeilschifter W. International translation of fever, sugar, swallow protocols: the quality in acute Stroke Care Europe Project. Int J Stroke 2020; 15: 591–594. [DOI] [PubMed] [Google Scholar]
- 21. The World Bank. World Bank country and lending groups, https://datahelpdesk.worldbank.org/knowledgebase/articles/906519 (2021, accessed September 2021).
- 22. Ogrinc G, Davies L, Goodman D, et al.SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. J Contin Educ Nurs 2015; 46: 501–507. [DOI] [PubMed] [Google Scholar]
- 23. Miech EJ, Rattray NA, Flanagan ME, et al. Inside help: an integrative review of champions in healthcare-related implementation. SAGE Open Med 2018; 6: 2050312118773261–2050312118773311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Grimshaw JM, Eccles MP, Lavis JN, et al. Knowledge translation of research findings. Implement Sci 2012; 7: 1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stroke Foundation. National acute stroke services framework 2019. Melbourne: Stroke Foundation, 2019. [Google Scholar]
- 26. International Clinical Research Center. Registry of stroke care quality, https://qualityregistry.eu/ (2021, accessed January 2021).
- 27. Brott T, Adams HP, Jr, Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke 1989; 20: 864–870. [DOI] [PubMed] [Google Scholar]
- 28. de Haan R, Limburg M, Bossuyt P, et al. The clinical meaning of Rankin ‘handicap’ grades after stroke. Stroke 1995; 26: 2027–2030. [DOI] [PubMed] [Google Scholar]
- 29. Waje-Andreassen U, Nabavi DG, Engelter ST, et al. European Stroke Organisation certification of stroke units and stroke centres. Eur Stroke J 2018; 3: 220–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977; 33: 159–174. [PubMed] [Google Scholar]
- 31. Drury P, Levi C, D'Este C, et al. Quality in acute stroke care (QASC): process evaluation of an intervention to improve the management of fever, hyperglycemia, and swallowing dysfunction following acute stroke. Int J Stroke 2014; 9: 766–776. [DOI] [PubMed] [Google Scholar]
- 32. Cadilhac DA, Grimley R, Kilkenny MF, et al. Multicenter, prospective, controlled, before-and-after, quality improvement study (Stroke123) of acute stroke care. Stroke 2019; 50: 1525–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chambers DA, Glasgow RE, Stange KC. The dynamic sustainability framework: addressing the paradox of sustainment amid ongoing change. Implement Sci 2013; 8: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Machline-Carrion MJ, Santucci EV, Damiani LP, et al. An international cluster-randomized quality improvement trial to increase the adherence to evidence-based therapies for acute ischemic stroke and transient ischemic attack patients: rationale and design of the Bridge Stroke Trial. Am Heart J 2019; 207: 49–57. [DOI] [PubMed] [Google Scholar]
- 35. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2019; 50: e344–e418. [DOI] [PubMed] [Google Scholar]
- 36. Fuentes B, Ntaios G, Putaala J, et al. European Stroke Organisation (ESO) guidelines on glycaemia management in acute stroke. Eur Stroke J 2018; 3: 5–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Boulanger J, Lindsay M, Gubitz G, et al. Canadian stroke best practice recommendations for acute stroke management: prehospital, emergency department, and acute inpatient stroke care, 6th edition, update 2018. Int J Stroke 2018; 13: 949–984. [DOI] [PubMed] [Google Scholar]
- 38. Romanian Government. Acțiunea prioritară pentru Tratamentul intervențional Al pacienților Cu avc Acut, https://www.ms.ro/wp-content/uploads/2018/12/Protocol-pentru-tratamentul-intervent%CC%A6ional-al-pacient%CC%A6ilor-cu-accident-vascular-cerebral-acut-.pdf (2018, accessed May 2020). [Google Scholar]
- 39. Brainin M. Stroke care and the COVID19 pandemic words from our president, https://www.world-stroke.org/news-and-blog/news/stroke-care-and-the-covid19-pandemic (2020, accessed 2 June 2021).
- 40. Markus HS, Brainin M. COVID-19 and stroke – a global World Stroke Organization perspective. Int J Stroke 2020; 15: 361–364. [DOI] [PubMed] [Google Scholar]
- 41. Cadilhac DA, Kim J, Tod EK, et al. COVID-19 pandemic impact on care for stroke in Australia: emerging Evidence from the Australian Stroke Clinical Registry. Front Neurol 2021; 12: 621495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Papathanassoglou ED, Karanikola MN, Kalafati M, et al. Professional autonomy, collaboration with physicians, and moral distress among European Intensive Care Nurses. Am J Crit Care 2012; 21: e41–e52. [DOI] [PubMed] [Google Scholar]
- 43. Intercollegiate Stroke Working Party. National clinical guideline for stroke, 2016. [Google Scholar]
- 44. Ntaios G, Dziedzic T, Michel P, et al. European Stroke Organisation (ESO) guidelines for the management of temperature in patients with acute ischemic stroke. Int J Stroke 2015; 10: 941–949. [DOI] [PubMed] [Google Scholar]
- 45. Green LA, Fryer GE, Froom P, et al. Opportunities, challenges, and lessons of international research in practice-based research networks: the case of an international study of acute otitis media. Ann Fam Med 2004; 2: 429–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Holloway ATA, Stilwell B, Finch H, et al. Agents of change: the story of the nursing now campaign, https://www.nursingnow.org/ (2021, accessed June 2021).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-11-eso-10.1177_23969873221126027 for Translation of nurse-initiated protocols to manage fever, hyperglycaemia and swallowing following stroke across Europe (QASC Europe): A pre-test/post-test implementation study by Sandy Middleton, Simeon Dale, Benjamin McElduff, Kelly Coughlan, Elizabeth McInnes, Robert Mikulik, Thomas Fischer, Jan Van der Merwe, Dominique Cadilhac, Catherine D’Este, Christopher Levi, Jeremy M Grimshaw, Andreea Grecu, Clare Quinn, Ngai Wah Cheung, Tereza Koláčná, Sabina Medukhanova, Estela Sanjuan Menendez, Susana Salselas, Gert Messchendorp, Anne-Kathrin Cassier-Woidasky, Marcelina Skrzypek-Czerko, Merce Slavat-Plana, Urso Antonella and Waltraud Pfeilschifter in European Stroke Journal
Supplemental material, sj-docx-2-eso-10.1177_23969873221126027 for Translation of nurse-initiated protocols to manage fever, hyperglycaemia and swallowing following stroke across Europe (QASC Europe): A pre-test/post-test implementation study by Sandy Middleton, Simeon Dale, Benjamin McElduff, Kelly Coughlan, Elizabeth McInnes, Robert Mikulik, Thomas Fischer, Jan Van der Merwe, Dominique Cadilhac, Catherine D’Este, Christopher Levi, Jeremy M Grimshaw, Andreea Grecu, Clare Quinn, Ngai Wah Cheung, Tereza Koláčná, Sabina Medukhanova, Estela Sanjuan Menendez, Susana Salselas, Gert Messchendorp, Anne-Kathrin Cassier-Woidasky, Marcelina Skrzypek-Czerko, Merce Slavat-Plana, Urso Antonella and Waltraud Pfeilschifter in European Stroke Journal
Supplemental material, sj-docx-3-eso-10.1177_23969873221126027 for Translation of nurse-initiated protocols to manage fever, hyperglycaemia and swallowing following stroke across Europe (QASC Europe): A pre-test/post-test implementation study by Sandy Middleton, Simeon Dale, Benjamin McElduff, Kelly Coughlan, Elizabeth McInnes, Robert Mikulik, Thomas Fischer, Jan Van der Merwe, Dominique Cadilhac, Catherine D’Este, Christopher Levi, Jeremy M Grimshaw, Andreea Grecu, Clare Quinn, Ngai Wah Cheung, Tereza Koláčná, Sabina Medukhanova, Estela Sanjuan Menendez, Susana Salselas, Gert Messchendorp, Anne-Kathrin Cassier-Woidasky, Marcelina Skrzypek-Czerko, Merce Slavat-Plana, Urso Antonella and Waltraud Pfeilschifter in European Stroke Journal
Supplemental material, sj-docx-4-eso-10.1177_23969873221126027 for Translation of nurse-initiated protocols to manage fever, hyperglycaemia and swallowing following stroke across Europe (QASC Europe): A pre-test/post-test implementation study by Sandy Middleton, Simeon Dale, Benjamin McElduff, Kelly Coughlan, Elizabeth McInnes, Robert Mikulik, Thomas Fischer, Jan Van der Merwe, Dominique Cadilhac, Catherine D’Este, Christopher Levi, Jeremy M Grimshaw, Andreea Grecu, Clare Quinn, Ngai Wah Cheung, Tereza Koláčná, Sabina Medukhanova, Estela Sanjuan Menendez, Susana Salselas, Gert Messchendorp, Anne-Kathrin Cassier-Woidasky, Marcelina Skrzypek-Czerko, Merce Slavat-Plana, Urso Antonella and Waltraud Pfeilschifter in European Stroke Journal
Supplemental material, sj-docx-5-eso-10.1177_23969873221126027 for Translation of nurse-initiated protocols to manage fever, hyperglycaemia and swallowing following stroke across Europe (QASC Europe): A pre-test/post-test implementation study by Sandy Middleton, Simeon Dale, Benjamin McElduff, Kelly Coughlan, Elizabeth McInnes, Robert Mikulik, Thomas Fischer, Jan Van der Merwe, Dominique Cadilhac, Catherine D’Este, Christopher Levi, Jeremy M Grimshaw, Andreea Grecu, Clare Quinn, Ngai Wah Cheung, Tereza Koláčná, Sabina Medukhanova, Estela Sanjuan Menendez, Susana Salselas, Gert Messchendorp, Anne-Kathrin Cassier-Woidasky, Marcelina Skrzypek-Czerko, Merce Slavat-Plana, Urso Antonella and Waltraud Pfeilschifter in European Stroke Journal
Supplemental material, sj-docx-6-eso-10.1177_23969873221126027 for Translation of nurse-initiated protocols to manage fever, hyperglycaemia and swallowing following stroke across Europe (QASC Europe): A pre-test/post-test implementation study by Sandy Middleton, Simeon Dale, Benjamin McElduff, Kelly Coughlan, Elizabeth McInnes, Robert Mikulik, Thomas Fischer, Jan Van der Merwe, Dominique Cadilhac, Catherine D’Este, Christopher Levi, Jeremy M Grimshaw, Andreea Grecu, Clare Quinn, Ngai Wah Cheung, Tereza Koláčná, Sabina Medukhanova, Estela Sanjuan Menendez, Susana Salselas, Gert Messchendorp, Anne-Kathrin Cassier-Woidasky, Marcelina Skrzypek-Czerko, Merce Slavat-Plana, Urso Antonella and Waltraud Pfeilschifter in European Stroke Journal
Supplemental material, sj-docx-7-eso-10.1177_23969873221126027 for Translation of nurse-initiated protocols to manage fever, hyperglycaemia and swallowing following stroke across Europe (QASC Europe): A pre-test/post-test implementation study by Sandy Middleton, Simeon Dale, Benjamin McElduff, Kelly Coughlan, Elizabeth McInnes, Robert Mikulik, Thomas Fischer, Jan Van der Merwe, Dominique Cadilhac, Catherine D’Este, Christopher Levi, Jeremy M Grimshaw, Andreea Grecu, Clare Quinn, Ngai Wah Cheung, Tereza Koláčná, Sabina Medukhanova, Estela Sanjuan Menendez, Susana Salselas, Gert Messchendorp, Anne-Kathrin Cassier-Woidasky, Marcelina Skrzypek-Czerko, Merce Slavat-Plana, Urso Antonella and Waltraud Pfeilschifter in European Stroke Journal
Supplemental material, sj-docx-8-eso-10.1177_23969873221126027 for Translation of nurse-initiated protocols to manage fever, hyperglycaemia and swallowing following stroke across Europe (QASC Europe): A pre-test/post-test implementation study by Sandy Middleton, Simeon Dale, Benjamin McElduff, Kelly Coughlan, Elizabeth McInnes, Robert Mikulik, Thomas Fischer, Jan Van der Merwe, Dominique Cadilhac, Catherine D’Este, Christopher Levi, Jeremy M Grimshaw, Andreea Grecu, Clare Quinn, Ngai Wah Cheung, Tereza Koláčná, Sabina Medukhanova, Estela Sanjuan Menendez, Susana Salselas, Gert Messchendorp, Anne-Kathrin Cassier-Woidasky, Marcelina Skrzypek-Czerko, Merce Slavat-Plana, Urso Antonella and Waltraud Pfeilschifter in European Stroke Journal
Supplemental material, sj-docx-9-eso-10.1177_23969873221126027 for Translation of nurse-initiated protocols to manage fever, hyperglycaemia and swallowing following stroke across Europe (QASC Europe): A pre-test/post-test implementation study by Sandy Middleton, Simeon Dale, Benjamin McElduff, Kelly Coughlan, Elizabeth McInnes, Robert Mikulik, Thomas Fischer, Jan Van der Merwe, Dominique Cadilhac, Catherine D’Este, Christopher Levi, Jeremy M Grimshaw, Andreea Grecu, Clare Quinn, Ngai Wah Cheung, Tereza Koláčná, Sabina Medukhanova, Estela Sanjuan Menendez, Susana Salselas, Gert Messchendorp, Anne-Kathrin Cassier-Woidasky, Marcelina Skrzypek-Czerko, Merce Slavat-Plana, Urso Antonella and Waltraud Pfeilschifter in European Stroke Journal
Supplemental material, sj-pdf-1-eso-10.1177_23969873221126027 for Translation of nurse-initiated protocols to manage fever, hyperglycaemia and swallowing following stroke across Europe (QASC Europe): A pre-test/post-test implementation study by Sandy Middleton, Simeon Dale, Benjamin McElduff, Kelly Coughlan, Elizabeth McInnes, Robert Mikulik, Thomas Fischer, Jan Van der Merwe, Dominique Cadilhac, Catherine D’Este, Christopher Levi, Jeremy M Grimshaw, Andreea Grecu, Clare Quinn, Ngai Wah Cheung, Tereza Koláčná, Sabina Medukhanova, Estela Sanjuan Menendez, Susana Salselas, Gert Messchendorp, Anne-Kathrin Cassier-Woidasky, Marcelina Skrzypek-Czerko, Merce Slavat-Plana, Urso Antonella and Waltraud Pfeilschifter in European Stroke Journal
Supplemental material, sj-png-10-eso-10.1177_23969873221126027 for Translation of nurse-initiated protocols to manage fever, hyperglycaemia and swallowing following stroke across Europe (QASC Europe): A pre-test/post-test implementation study by Sandy Middleton, Simeon Dale, Benjamin McElduff, Kelly Coughlan, Elizabeth McInnes, Robert Mikulik, Thomas Fischer, Jan Van der Merwe, Dominique Cadilhac, Catherine D’Este, Christopher Levi, Jeremy M Grimshaw, Andreea Grecu, Clare Quinn, Ngai Wah Cheung, Tereza Koláčná, Sabina Medukhanova, Estela Sanjuan Menendez, Susana Salselas, Gert Messchendorp, Anne-Kathrin Cassier-Woidasky, Marcelina Skrzypek-Czerko, Merce Slavat-Plana, Urso Antonella and Waltraud Pfeilschifter in European Stroke Journal