Abstract
Introduction:
Whether atrial cardiopathy is associated with stroke prognosis remains unclear. We evaluated the association between atrial cardiopathy markers and outcomes in patients with ischemic stroke using a nationwide prospective registry.
Patients and methods:
Based on the Third China National Stroke Registry, we evaluated different atrial cardiopathy markers including increased P-wave terminal force in V1 (PTFV1), advanced interatrial block (aIAB), prolonged P-wave duration, prolonged P-wave dispersion, paroxysmal supraventricular tachycardia, premature atrial contractions, prolonged PR interval, and severe left atrial enlargement in ischemic stroke patients. The outcomes were death and ischemic stroke recurrence at 1 year. The association between atrial cardiopathy markers and outcomes was analyzed using Cox regression models.
Results:
At 1-year follow-up, 486 (3.4%) patients had died and 1317 (9.3%) patients had experienced ischemic stroke recurrence. After adjustment for clinical risk factors including atrial fibrillation, PTFV1 > 5000 μV·ms (adjusted hazard ratio [HR] 1.70, 95% confidence interval [CI]: 1.18–2.45, p = 0.004) and aIAB (adjusted HR 1.47, 95% CI: 1.14–1.91, p = 0.003) were significantly associated with mortality. PTFV1 > 5000 μV·ms was significantly associated with ischemic stroke recurrence (adjusted HR 1.54, 95% CI: 1.22–1.96, p = 0.0004). This association was observed although we excluded patients diagnosed with atrial fibrillation.
Discussion and Conclusion:
Atrial cardiopathy markers, especially PTFV1 and aIAB, are significantly associated with a higher risk of poor prognosis in patients with ischemic stroke.
Keywords: Atrial cardiopathy, prognosis, stroke
Introduction
Stroke is a leading cause of death and disability worldwide. 1 The death rate for cerebrovascular disease in China was 149.49 per 100,000 in 2018. 2 Atrial cardiopathy refers to atrial structural and pathophysiologic changes that can occur before atrial fibrillation (AF). 3 A series of previous studies have found that atrial cardiopathy markers such as increased PTFV1, 4 aIAB, 5 prolonged P-wave duration, 4 prolonged P-wave dispersion, 6 paroxysmal supraventricular tachycardia, 7 premature atrial contractions, 8 prolonged PR interval, 9 and increased left atrial (LA) size 8 are associated with ischemic stroke in patients with or without AF. However, the relationship between atrial cardiopathy markers and stroke prognosis has not been thoroughly verified in large cohorts. Therefore, this study was conducted in a nationwide registry to explore the predictive value of atrial cardiopathy markers for prognosis in patients with ischemic stroke.
Methods
Study design
The Third China National Stroke Registry (CNSR-III) is a nationwide prospective registry for patients with ischemic stroke or transient ischemic attack (TIA) between August 2015 and March 2018. The study recruited consecutive 15,166 patients visiting 201 participating hospitals in 22 provinces and 4 municipalities in China. The patients were >18-year-old and encountered ischemic stroke or TIA within 7 days after onset of symptoms. CNSR-III was approved by the Ethics Committee of Beijing Tiantan Hospital (institutional review board approval number: KY2015-001-01) and all participating hospitals. Informed consent was obtained from all patients or their legally authorized representatives. The details of CNSR-III have been published previously. 10
Atrial cardiopathy markers
A standard 12-lead ECG was recorded for each patient at a speed of 25 mm/s and a calibration of 10 mm/mV at the time of admission. All ECGs were digitalized. ECG image was amplified by up to five times its original size. P-wave indices were measured using digital calipers by a well-trained cardiologist who was blinded to the clinical data of patients. All measurements were repeated three times, and the average values were obtained. P-wave duration was measured from P-wave onset to return to baseline. For biphasic P wave, P-wave duration encompassed both positive and negative deflections from baseline. P-wave duration was measured in all 12 leads to acquire the maximum and minimum durations. P-wave dispersion was defined as the difference between the maximum and minimum P-wave durations. aIAB was identified as the presence of prolonged P-wave duration and biphasic (positive negative) morphology in leads II, III, and aVF or biphasic (positive negative) morphology in leads III and aVF and notched P (positive positive) in lead II. 5 PTFV1 was defined as the duration (ms) of the downward deflection (terminal portion) of P wave in lead V1 multiplied by the absolute value of its amplitude (μV). PR interval was defined as the interval from the onset of P wave to the end of PR segment (junction with the QRS complex). Previous studies have shown excellent intrarater correlations for manual measurements of P-wave morphology. 11 A second well-trained cardiologist independently performed blinded measurements of a random sample of 200 ECGs to assess the interrater reliability of P-wave indices measurements. The interrater intraclass correlation coefficient (ICC) was 0.67 (95% CI 0.59–0.74) for maximum P-wave duration, 0.73 (95% CI 0.65–0.79) for P-wave dispersion, and 0.78 (95% CI 0.67–0.85) for PTFV1.
Atrial cardiopathy markers were defined according to the previous studies. Prolonged P-wave duration was determined as being present if the maximum P-wave duration was >120 ms. 4 Prolonged P-wave dispersion was defined as >40 ms. 12 Increased PTFV1 was defined as >5000 μV·ms. 13 Prolonged PR interval was defined as >200 ms. 14 LA enlargement was measured as anterior-posterior linear LA diameter (mm) on a baseline 2D echocardiogram (UCG), with severe enlargement defined using sex-specific cut-offs (⩾47 mm for women and ⩾52 mm for men). 15 Atrial ectopy and paroxysmal supraventricular tachycardia were measured on the baseline 24-h Holter. Excessive atrial ectopy was defined as a frequency of atrial premature beats per hour of >30. 8
Outcomes and follow-up
In this study, the outcomes included all-cause mortality and ischemic stroke recurrence at 1 year. Patients were followed up at 3 months, 6 months, and 1 year after onset. Patients were interviewed face-to-face at 3 months and contacted over the telephone by trained research coordinators at 6 months and 1 year. Confirmation of cerebrovascular events were sought from the treating hospital, and suspected recurrent cerebrovascular events without hospitalization were judged by independent endpoint judgment committee. Each case fatality was either confirmed on a death certificate from the attended hospital or the local citizen registry. Details of the determination of outcome events have been published in previous study. 10
Statistical analysis
Continuous variables were presented as means with standard deviations or medians with interquartile ranges. Statistical comparisons were performed with one-way analysis of variance or the Kruskal–Wallis test. Categorical variables were presented as frequencies with percentages and were compared by χ 2 test. The risks of outcomes were evaluated by Kaplan–Meier survival analysis and the significance of differences was evaluated using the log-rank test. Multivariable Cox regressions were used to determine significant predictors of outcomes. In multivariable Cox regressions, we excluded the patients without complete marker data, respectively. The receiver-operator curve (ROC) with area under curve was calculated to evaluate improvement in risk classification by atrial cardiopathy markers over iScore and Essen Stroke Risk Score. In the sensitivity analysis, we excluded patients diagnosed with AF. We also tested the association between atrial cardiopathy markers and vascular mortality at 1 year as a sensitivity analysis. All tests were two-sided. A p < 0.05 was considered statistically significant. All analyses were conducted with SAS software, version 9.4 (SAS Institute, Inc, Cary, NC).
Results
Baseline characteristics
Between August 2015 and March 2018, 15,166 patients with acute ischemic stroke or TIA were recruited for the CNSR-III study. After excluding patients with TIA, 14,146 ischemic stroke patients (mean age, 62.3 years; median National Institutes of Health Stroke Scale (NIHSS) score, three [interquartile range, 2–6]) were finally included in this analysis. Of these patients, 68.7% were men. Among 14,146 ischemic stroke patients, besides patients with a medical history of AF, some patients were newly detected with AF during hospitalization. After excluding patients both with known and newly detected AF, 13,015 patients were included. At 1-year follow-up, 486 (3.4%) patients had died, and 1317 (9.3%) patients had experienced ischemic stroke recurrence. The baseline characteristics of patients are summarized in Table 1 and Supplemental Table 1. All atrial cardiopathy markers, except prolonged P-wave dispersion (p = 0.06), were more frequent in patients who had succumbed during the 1-year period. Patients with ischemic stroke recurrence had a significantly higher frequency of PTFV1 > 5000 μV·ms, aIAB, prolonged PR interval, and severe LA enlargement than patients without. Among 14,146 ischemic stroke patients, the proportion of patients who underwent ECG, UCG, and 24-h Holter was 94.1%, 94.3%, and 82.3%, respectively. The median timing of ECG, UCG, and Holter with respect to the index stroke was 1, 3, and 4.8 days, respectively. The baseline characteristics of patients with and without cardiac examination variables were presented in Supplemental Table 2–4, respectively. Baseline characteristics between two groups were largely comparable.
Table 1.
Characteristics of patients with or without death and ischemic stroke recurrence.
| Total (n = 14,146) | Patients who did not survive (n = 486) | Patients who survived (n = 13,660) | p-Value | Patients with ischemic stroke recurrence (n = 1317) | Patients without ischemic stroke recurrence (n = 12,829) | p-Value | |
|---|---|---|---|---|---|---|---|
| Age, mean (SD), y | 62.3 (11.3) | 70.6 (11.1) | 62.0 (11.2) | <0.0001 | 63.8 (11.0) | 62.1 (11.3) | <0.0001 |
| Sex, n (%), male | 9720 (68.7) | 308 (63.4) | 9412 (68.9) | <0.01 | 864 (65.6) | 8856 (69.0) | 0.01 |
| Medical history, n (%) | |||||||
| Hypertension | 8887 (62.8) | 314 (64.6) | 8573 (62.8) | 0.41 | 860 (65.3) | 8027 (62.6) | 0.05 |
| Diabetes mellitus | 3310 (23.4) | 138 (28.4) | 3172 (23.2) | 0.008 | 370 (28.1) | 2940 (22.9) | <0.0001 |
| Dyslipidemia | 1075 (7.6) | 25 (5.1) | 1050 (7.7) | 0.04 | 96 (7.3) | 979 (7.6) | 0.65 |
| Current tobacco smoker | 4503 (31.8) | 111 (22.8) | 4392 (32.1) | <0.0001 | 384 (29.2) | 4119 (32.1) | 0.03 |
| Coronary artery disease | 1486 (10.5) | 94 (19.3) | 1392 (10.2) | <0.0001 | 190 (14.4) | 1296 (10.1) | <0.0001 |
| Atrial fibrillation | 986 (7.0) | 110 (22.6) | 876 (6.4) | <0.0001 | 124 (9.4) | 862 (6.7) | 0.0003 |
| Heart failure | 92 (0.6) | 12 (2.5) | 80 (0.6) | <0.0001 | 17 (1.3) | 75 (0.6) | 0.002 |
| Myocardial infarction | 336 (2.4) | 24 (5.1) | 312 (2.3) | 0.0001 | 44 (3.4) | 292 (2.3) | 0.01 |
| Peripheral vascular disease | 107 (0.8) | 9 (1.8) | 98 (0.7) | 0.005 | 12 (0.9) | 95 (0.7) | 0.50 |
| Stroke | 3134 (22.1) | 164 (33.7) | 2970 (21.7) | <0.0001 | 414 (31.4) | 2720 (21.2) | <0.0001 |
| TIA | 306 (2.2) | 9 (1.8) | 297 (2.2) | 0.63 | 39 (3.0) | 267 (2.1) | 0.04 |
| Body-mass index, mean (SD) | 24.7 (3.3) | 23.8 (3.5) | 24.7 (3.3) | <0.0001 | 24.7 (3.4) | 24.7 (3.3) | 0.37 |
| NIHSS at admission, median (IQR) | 3 (2–6) | 6 (3–12) | 3 (2–6) | <0.0001 | 4 (2–7) | 3 (2–6) | <0.0001 |
| Medication at discharge, n (%) | |||||||
| Antiplatelet | 12,814 (90.6) | 347 (71.4) | 12,467 (91.3) | <0.0001 | 1132 (86.0) | 11,682 (91.1) | <0.0001 |
| Anticoagulants | 428 (3.0) | 24 (4.9) | 404 (3.0) | 0.01 | 43 (3.3) | 385 (3.0) | 0.59 |
| Antihypertensive agent | 6981 (49.3) | 206 (42.4) | 6775 (49.6) | 0.002 | 645 (49.0) | 6336 (49.4) | 0.77 |
| Hypoglycemic agent | 3369 (23.8) | 101 (20.8) | 3268 (23.9) | 0.11 | 352 (26.7) | 3017 (23.5) | 0.009 |
| Lipid-lowering agent | 12,947 (91.5) | 361 (74.3) | 12,586 (92.1) | <0.0001 | 1143 (86.8) | 11,804 (92.0) | <0.0001 |
| Atrial cardiopathy markers, n (%) | |||||||
| PTFV1 > 5000 μV·ms | 572 (5.1) | 36 (11.7) | 536 (4.9) | <0.0001 | 79 (7.9) | 493 (4.8) | <0.0001 |
| aIAB | 2033 (17.9) | 88 (28.7) | 1945 (17.6) | <0.0001 | 207 (20.2) | 1826 (17.7) | 0.04 |
| Prolonged P-wave duration | 3921 (35.0) | 129 (42.3) | 3792 (34.8) | 0.007 | 372 (36.8) | 3549 (34.8) | 0.21 |
| Prolonged P-wave dispersion | 6029 (53.8) | 180 (59.0) | 5849 (53.7) | 0.06 | 568 (56.2) | 5461 (53.6) | 0.11 |
| Prolonged PR interval | 929 (7.8) | 41 (12.9) | 888 (7.7) | 0.0007 | 104 (9.7) | 825 (7.7) | 0.02 |
| Supraventricular tachycardia | 3350 (33.9) | 110 (40.1) | 3240 (33.8) | 0.03 | 310 (35.1) | 3040 (33.8) | 0.44 |
| Excessive atrial ectopy | 3929 (46.7) | 131 (62.4) | 3798 (46.3) | <0.0001 | 350 (47.7) | 3579 (46.6) | 0.56 |
| Severe LA enlargement | 216 (1.7) | 21 (5.5) | 195 (1.6) | <0.0001 | 29 (2.6) | 187 (1.6) | 0.02 |
aIAB: advanced interatrial block; IQR: interquartile range; LA: left atrial; NIHSS: National Institutes of Health Stroke Scale; PTFV1: P-wave terminal force in V1; SD: standard deviation; TIA: transient ischemic attack.
Associations of atrial cardiopathy markers with mortality
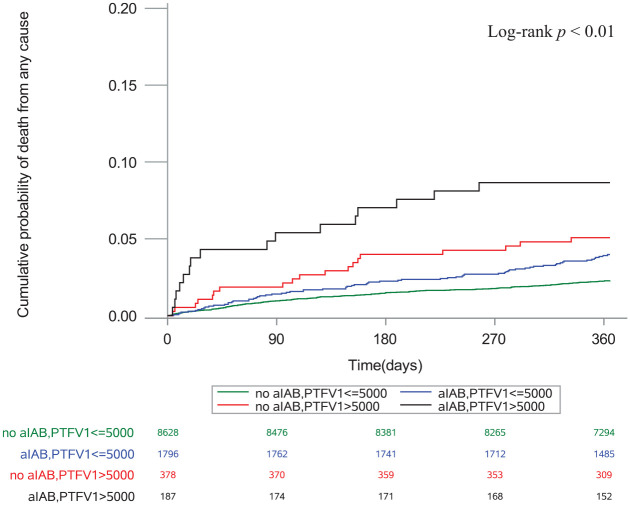
In multivariable models, PTFV1 > 5000 μV·ms (adjusted HR 1.70, 95% CI: 1.18–2.45, p = 0.004) and aIAB (adjusted HR 1.47, 95% CI: 1.14–1.91, p = 0.003) were significantly associated with mortality (Table 2). The association was true after we excluded patients diagnosed with AF; PTFV1 > 5000 μV·ms (adjusted HR 1.77, 95% CI: 1.21–2.59, p = 0.003) and aIAB (adjusted HR 1.43, 95% CI: 1.09–1.88, p = 0.009) still exhibited significant associations with mortality (Table 2). Kaplan–Meier survival curves demonstrated that the cumulative incidence of mortality was highest in patients with both markers (log-rank test, p < 0.01; Figure 1).
Table 2.
Risk of death for atrial cardiopathy markers.
| Markers | Total patients | AF patients excluded | |||
|---|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | ||
| PTFV1 > 5000 μV·ms | Model 1 | 2.17 (1.53–3.08) | <0.0001 | 2.28 (1.58–3.30) | <0.0001 |
| Model 2 | 1.70 (1.18–2.45) | 0.004 | 1.77 (1.21–2.59) | 0.003 | |
| aIAB | Model 1 | 1.57 (1.23–2.02) | 0.0004 | 1.54 (1.18–2.00) | 0.001 |
| Model 2 | 1.47 (1.14–1.91) | 0.003 | 1.43 (1.09–1.88) | 0.009 | |
| Prolonged P-wave duration | Model 1 | 1.22 (0.97–1.53) | 0.09 | 1.21 (0.96–1.54) | 0.11 |
| Model 2 | 1.17 (0.93–1.48) | 0.18 | 1.16 (0.91–1.48) | 0.23 | |
| Prolonged P-wave dispersion | Model 1 | 1.17 (0.93–1.47) | 0.19 | 1.22 (0.96–1.55) | 0.10 |
| Model 2 | 1.16 (0.92–1.47) | 0.21 | 1.21 (0.95–1.54) | 0.12 | |
| Prolonged PR interval | Model 1 | 1.32 (0.94–1.84) | 0.11 | 1.29 (0.91–1.84) | 0.16 |
| Model 2 | 1.26 (0.89–1.78) | 0.19 | 1.18 (0.82–1.71) | 0.37 | |
| Supraventricular tachycardia | Model 1 | 0.81 (0.63–1.04) | 0.10 | 1.02 (0.76–1.37) | 0.88 |
| Model 2 | 0.95 (0.73–1.24) | 0.73 | 0.95 (0.70–1.29) | 0.76 | |
| Excessive atrial ectopy | Model 1 | 1.09 (0.81–1.48) | 0.57 | 1.09 (0.79–1.49) | 0.61 |
| Model 2 | 1.03 (0.75–1.41) | 0.84 | 1.07 (0.77–1.48) | 0.69 | |
| Severe LA enlargement | Model 1 | 2.55 (1.63–3.98) | <0.0001 | 2.30 (0.85–6.18) | 0.10 |
| Model 2 | 1.41 (0.87–2.29) | 0.16 | 1.72 (0.63–4.68) | 0.29 | |
Model 1: Model 1 was adjusted for age, sex.
Model 2: Model 2 was adjusted for age, sex, NIHSS, atrial fibrillation, hypertension, diabetes mellitus, dyslipidemia, current tobacco smoker, heart failure, coronary artery disease, peripheral artery disease, myocardial infarction, prior history of stroke or TIA, medication at discharge (antiplatelet, anticoagulants, antihypertensive agent, hypoglycemic agent, lipid-lowering agent).
AF: atrial fibrillation; aIAB: advanced interatrial block; CI: confidence interval; HR: hazard ratio; LA: left atrial; PTFV1: P-wave terminal force in V1.
Figure 1.
Cumulative probability of death stratified by aIAB and PTFV1.
aIAB: advanced interatrial block; PTFV1: P-wave terminal force in V1.
The multivariable model showed a trend that PTFV1 > 5000 μV·ms increased the risk of vascular mortality in ischemic stroke patients (adjusted HR 1.71, 95% CI: 0.95–3.09, p = 0.07), although there is no statistical significance. After we excluded patients diagnosed with AF. PTFV1 > 5000 μV·ms was the only marker associated with vascular mortality (adjusted HR 2.03, 95% CI: 1.13–3.66, p = 0.02; Supplemental Table 5, Supplemental Figure 1).
Associations of atrial cardiopathy markers with ischemic stroke recurrence
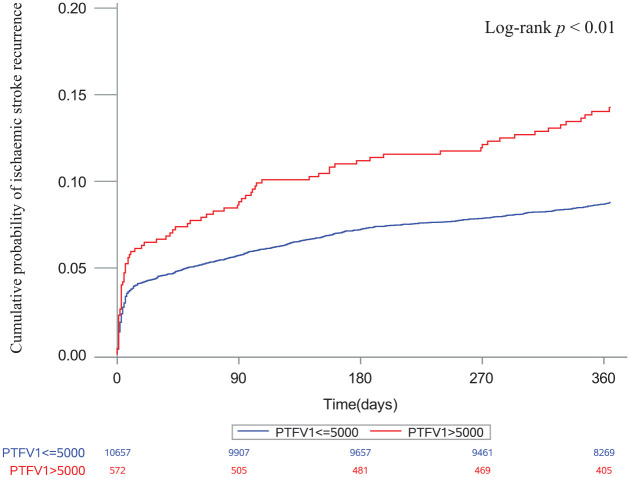
In the multivariable models, PTFV1 > 5000 μV·ms was the only marker associated with ischemic stroke recurrence (adjusted HR 1.54, 95% CI: 1.22–1.96, p = 0.0004; Table 3). After excluding patients diagnosed with AF, PTFV1 > 5000 μV·ms still exhibited a significant association with ischemic stroke recurrence (adjusted HR 1.62, 95% CI: 1.27–2.07, p = 0.0001; Table 3). The Kaplan–Meier survival curves depicted that the cumulative incidence of ischemic stroke recurrence was significantly higher in patients with increased PTFV1 than in those without increased PTFV1 (log-rank test, p < 0.01; Figure 2).
Table 3.
Risk of ischemic stroke recurrence for atrial cardiopathy markers.
| Markers | Total patients | AF patients excluded | |||
|---|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | ||
| PTFV1 > 5000 μV·ms | Model 1 | 1.64 (1.30–2.07) | <0.0001 | 1.71 (1.35–2.18) | <0.0001 |
| Model 2 | 1.54 (1.22–1.96) | 0.0004 | 1.62 (1.27–2.07) | 0.0001 | |
| aIAB | Model 1 | 1.13 (0.97–1.32) | 0.12 | 1.14 (0.97–1.33) | 0.11 |
| Model 2 | 1.08 (0.92–1.26) | 0.33 | 1.09 (0.93–1.28) | 0.27 | |
| Prolonged P-wave duration | Model 1 | 1.06 (0.93–1.21) | 0.36 | 1.04 (0.92–1.19) | 0.51 |
| Model 2 | 1.02 (0.90–1.16) | 0.76 | 1.01 (0.88–1.15) | 0.90 | |
| Prolonged P-wave dispersion | Model 1 | 1.09 (0.97–1.24) | 0.15 | 1.08 (0.95–1.23) | 0.21 |
| Model 2 | 1.09 (0.96–1.23) | 0.19 | 1.08 (0.95–1.23) | 0.23 | |
| Prolonged PR interval | Model 1 | 1.23 (1.01–1.51) | 0.04 | 1.24 (1.01–1.53) | 0.04 |
| Model 2 | 1.16 (0.94–1.44) | 0.16 | 1.16 (0.94–1.45) | 0.17 | |
| Supraventricular tachycardia | Model 1 | 0.97 (0.84–1.12) | 0.67 | 1.00 (0.85–1.16) | 0.96 |
| Model 2 | 1.01 (0.87–1.18) | 0.84 | 1.00 (0.85–1.17) | 0.97 | |
| Excessive atrial ectopy | Model 1 | 0.95 (0.81–1.12) | 0.56 | 0.96 (0.82–1.13) | 0.63 |
| Model 2 | 0.98 (0.83–1.15) | 0.81 | 1.00 (0.85–1.18) | 0.98 | |
| Severe LA enlargement | Model 1 | 1.42 (0.98–2.06) | 0.06 | 1.28 (0.57–2.86) | 0.54 |
| Model 2 | 1.27 (0.85–1.90) | 0.24 | 1.21 (0.54–2.72) | 0.64 | |
Model 1: Model 1 was adjusted for age, sex.
Model 2: Model 2 was adjusted for age, sex, NIHSS, atrial fibrillation, hypertension, diabetes mellitus, dyslipidemia, current tobacco smoker, heart failure, coronary artery disease, peripheral artery disease, myocardial infarction, prior history of stroke or TIA, medication at discharge (antiplatelet, anticoagulants, antihypertensive agent, hypoglycemic agent, lipid-lowering agent).
AF: atrial fibrillation; aIAB: advanced interatrial block; CI: confidence interval; HR: hazard ratio; LA: left atrial; PTFV1: P-wave terminal force in V1.
Figure 2.
Cumulative probability of ischemic stroke recurrence stratified by PTFV1.
PTFV1: P-wave terminal force in V1.
Improvement in prediction model for mortality and ischemic stroke recurrence by addition of atrial cardiopathy markers
We established the performance of iScore for predicting mortality and Essen Stroke Risk Score for predicting ischemic stroke recurrence at 1 year. Adding PTFV1 and aIAB slightly increased the area under the ROC for iScore (0.013, p = 0.02) (Supplemental Table 6). Besides, adding PTFV1 slightly increased the area under the ROC for Essen Stroke Risk Score (0.008, p = 0.04) (Supplemental Table 6).
Discussion
Fibrotic atrial remodeling and enlargement are crucial components of the prothrombotic and proarrhythmic substrates in the development of LA thromboembolism. The measurement of P-wave indices generates the basis for the ECG-based detection of the substrate. PTFV1 and aIAB are both markers of atrial fibrosis and LA abnormalities.3,16 In this study, we found that PTFV1 was a predictor of death and ischemic stroke recurrence in patients with ischemic stroke. In addition, aIAB was associated with a higher risk of death after ischemic stroke.
In several different longitudinal cohorts, PTFV1 has been related to incident stroke in populations free of stroke or AF at baseline, and the associations remain constant even after adjusting for incident AF.4,11,17 –19 The relationship of increased PTFV1 with a new stroke occurring in patients without AF further supports the hypothesis that the increased risk of stroke may be mediated via atrial cardiopathy, independent of the effects of AF. 3 PTFV1 may reflect atrial changes, such as LA hypertrophy and inter-atrial conduction defects, 20 and its increase has been associated with impaired LA function.21,22 Furthermore, PTFV1 has been associated with low LA appendage ejection velocity on transoesophageal echocardiography. 23 The Cardiovascular Health Study reported PTFV1 to be associated with prevalent magnetic resonance imaging (MRI)-defined infarcts, especially nonlacunar infarcts. 24 The results suggest that PTFV1 may reflect a thromboembolic mechanism that induces ischemic stroke.
Recent work has confirmed an association between abnormal PTFV1 and left ventricular fibrosis on cardiac MRI. 21 In addition, PTFV1 may be an early manifestation of left ventricular diastolic dysfunction 25 which is an independent predictor of mortality. 26 In the general population, PTFV1 is also associated with sudden cardiac death, cardiovascular death, 27 and all-cause death 28 independent of clinical cardiovascular risk factors.
Patients with aIAB present low atrial mobility and reduced strain as assessed by speckle-tracking echocardiography. 29 Furthermore, aIAB has been reported to be associated with larger LA volumes, lower LA emptying fraction, and increased fibrosis on MRI. 30 There is growing evidence that aIAB is a powerful marker of increased risk of a poor prognosis in different clinical groups, such as the general population, 5 and patients with heart failure, 31 acute myocardial infarction, 32 stress cardiomyopathy, 33 and ischemic stroke. 34 The results indicate that electrocardiographic abnormalities as markers of changes in the cardiac structure and function are effective predictors of poor prognosis.
This study has several limitations. First, as we excluded some patients without complete markers, potential biases may exist in our study. However, the characteristics of patients with and without complete cardiac examination variables were well balanced. Second, All-cause mortality was lower in our study because patients in CNSR-III were younger and had relatively mild stroke severity. This could have the tendency to underestimate the magnitude of associations between atrial cardiopathy markers and mortality. Finally, this study relied on manual measurements rather than automated measurements. However, blinded measurements were performed by two independent investigators to confirm the objectivity of the results.
Conclusions
In conclusion, this study revealed the effect of PTFV1 and aIAB on the prognosis of ischemic stroke. Clinicians should apply these easily accessible markers to help the risk classification and more well-designed clinical studies are needed to explore optimal strategies for stroke patients with these atrial cardiopathy markers.
Supplemental Material
Supplemental material, sj-docx-1-eso-10.1177_23969873221126000 for Prognostic significance of atrial cardiopathy in patients with acute ischemic stroke by Yueyang Wu, Xiaomeng Yang, Jing Jing, Xia Meng, Zixiao Li, Yuesong Pan, Yong Jiang, Hongyi Yan, Xinying Huang, Liping Liu, Xingquan Zhao, Yilong Wang, Hao Li and Yongjun Wang in European Stroke Journal
Acknowledgments
The authors thank the staff and participants of the CNSR-III study for their contribution.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Capital’s Funds for Health Improvement and Research (grant number: 2020-1-2041), Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (grant number: 2019-I2M-5-029), and National Natural Science Foundation of China (grant number: 81870905, U20A20358, 82001237).
Ethical approval: CNSR-III was approved by the Ethics Committee of Beijing Tiantan Hospital (institutional review board approval number: KY2015-001-01) and all participating hospitals.
Informed consent: Informed consent was obtained from all patients or their legally authorized representatives.
Guarantor: YW
Contributorship: All authors were involved in the design and planning of the work. YP, YJ, HY, and XH performed data analysis. YW and XY wrote the first draft of the manuscript. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
ORCID iDs: Yueyang Wu  https://orcid.org/0000-0001-8100-0392
https://orcid.org/0000-0001-8100-0392
Jing Jing  https://orcid.org/0000-0001-9822-5758
https://orcid.org/0000-0001-9822-5758
Supplemental material: Supplemental material for this article is available online.
References
- 1. Feigin VL, Norrving B, Mensah GA. Global Burden of stroke. Circ Res 2017; 120: 439–448. [DOI] [PubMed] [Google Scholar]
- 2. Wang YJ, Li ZX, Gu HQ, et al. China Stroke Statistics 2019: A report from the National Center for Healthcare Quality Management in neurological diseases, China National Clinical Research Center for Neurological Diseases, the Chinese Stroke Association, National Center for Chronic and Non-communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention and Institute for Global Neuroscience and Stroke Collaborations. Stroke Vasc Neurol 2020; 5: 211–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kamel H, Okin PM, Longstreth WT, Jr., et al. Atrial cardiopathy: a broadened concept of left atrial thromboembolism beyond atrial fibrillation. Future Cardiol 2015; 11: 323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. He J, Tse G, Korantzopoulos P, et al. P-wave indices and risk of ischemic stroke: A systematic review and meta-analysis. Stroke 2017; 48: 2066–2072. [DOI] [PubMed] [Google Scholar]
- 5. O’Neal WT, Kamel H, Zhang ZM, et al. Advanced interatrial block and ischemic stroke: the atherosclerosis risk in communities study. Neurology 2016; 87: 352–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dogan U, Dogan EA, Tekinalp M, et al. P-wave dispersion for predicting paroxysmal atrial fibrillation in acute ischemic stroke. Int J Med Sci 2012; 9: 108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kamel H, Elkind MS, Bhave PD, et al. Paroxysmal supraventricular tachycardia and the risk of ischemic stroke. Stroke 2013; 44: 1550–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Edwards JD, Healey JS, Fang J, et al. Atrial cardiopathy in the absence of atrial fibrillation increases risk of ischemic stroke, incident atrial fibrillation, and mortality and improves stroke risk prediction. J Am Heart Assoc 2020; 9: e013227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thijs VN, Brachmann J, Morillo CA, et al. Predictors for atrial fibrillation detection after cryptogenic stroke: results from CRYSTAL AF. Neurology 2016; 86: 261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang Y, Jing J, Meng X, et al. The third China National Stroke Registry (CNSR-III) for patients with acute ischaemic stroke or transient ischaemic attack: design, rationale and baseline patient characteristics. Stroke Vasc Neurol 2019; 4: 158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kamel H, O’Neal WT, Okin PM, et al. Electrocardiographic left atrial abnormality and stroke subtype in the atherosclerosis risk in communities study. Ann Neurol 2015; 78: 670–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Acampa M, Lazzerini PE, Guideri F, et al. Inflammation and atrial electrical remodelling in patients with embolic strokes of undetermined source. Heart Lung Circ 2019; 28: 917–922. [DOI] [PubMed] [Google Scholar]
- 13. Jalini S, Rajalingam R, Nisenbaum R, et al. Atrial cardiopathy in patients with embolic strokes of unknown source and other stroke etiologies. Neurology 2019; 92: e288–e294. [DOI] [PubMed] [Google Scholar]
- 14. Cheng S, Keyes MJ, Larson MG, et al. Long-term outcomes in individuals with prolonged PR interval or first-degree atrioventricular block. JAMA 2009; 301: 2571–2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification. Eur J Echocardiogr 2006; 7: 79–108. [DOI] [PubMed] [Google Scholar]
- 16. Bayés de, Luna A, Platonov P, Cosio FG, et al. Interatrial blocks. A separate entity from left atrial enlargement: a consensus report. J Electrocardiol 2012; 45: 445–451. [DOI] [PubMed] [Google Scholar]
- 17. Kamel H, Soliman EZ, Heckbert SR, et al. P-wave morphology and the risk of incident ischemic stroke in the multi-ethnic study of Atherosclerosis. Stroke 2014; 45: 2786–2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kamel H, Hunter M, Moon YP, et al. Electrocardiographic left atrial abnormality and risk of stroke: Northern Manhattan Study. Stroke 2015; 46: 3208–3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kamel H, Bartz TM, Elkind MSV, et al. Atrial cardiopathy and the risk of ischemic stroke in the CHS (Cardiovascular Health Study). Stroke 2018; 49: 980–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alpert MA, Munuswamy K. Electrocardiographic diagnosis of left atrial enlargement. Arch Intern Med 1989; 149: 1161–1165. [PubMed] [Google Scholar]
- 21. Tiffany Win T, Ambale Venkatesh B, Volpe GJ, et al. Associations of electrocardiographic P-wave characteristics with left atrial function, and diffuse left ventricular fibrosis defined by cardiac magnetic resonance: the PRIMERI Study. Heart Rhythm 2015; 12: 155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lebek S, Wester M, Pec J, et al. Abnormal P-wave terminal force in lead V1 is a marker for atrial electrical dysfunction but not structural remodelling. ESC Heart Fail 2021; 8: 4055–4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McConkey N, Malamas P, Norby FL, et al. Abnormal P-wave terminal force in lead V1 is associated with low left atrial appendage ejection velocity. J Electrocardiol 2021; 67: 142–147. [DOI] [PubMed] [Google Scholar]
- 24. Kamel H, Bartz TM, Longstreth WT, Jr, et al. Association between left atrial abnormality on ECG and vascular brain injury on MRI in the Cardiovascular Health Study. Stroke 2015; 46: 711–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tanoue MT, Kjeldsen SE, Devereux RB, et al. Relationship between abnormal P-wave terminal force in lead V1 and left ventricular diastolic dysfunction in hypertensive patients: the LIFE study. Blood Press 2017; 26: 94–101. [DOI] [PubMed] [Google Scholar]
- 26. Halley CM, Houghtaling PL, Khalil MK, et al. Mortality rate in patients with diastolic dysfunction and normal systolic function. Arch Intern Med 2011; 171: 1082–1087. [DOI] [PubMed] [Google Scholar]
- 27. Maheshwari A, Norby FL, Soliman EZ, et al. Association of P-wave abnormalities with sudden cardiac and cardiovascular death: the ARIC Study. Circ Arrhythm Electrophysiol 2021; 14: e009314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Eranti A, Aro AL, Kerola T, et al. Prevalence and prognostic significance of abnormal P terminal force in lead V1 of the ECG in the general population. Circ Arrhythm Electrophysiol 2014; 7: 1116–1121. [DOI] [PubMed] [Google Scholar]
- 29. Lacalzada-Almeida J, Izquierdo-Gómez MM, García-Niebla J, et al. Advanced interatrial block is a surrogate for left atrial strain reduction which predicts atrial fibrillation and stroke. Ann Noninvasive Electrocardiol 2019; 24: e12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ciuffo L, Bruña V, Martínez-Sellés M, et al. Association between interatrial block, left atrial fibrosis, and mechanical dyssynchrony: electrocardiography-magnetic resonance imaging correlation. J Cardiovasc Electrophysiol 2020; 31: 1719–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Escobar-Robledo LA, Bayés-de-Luna A, Lupón J, et al. Advanced interatrial block predicts new-onset atrial fibrillation and ischemic stroke in patients with heart failure: the “Bayes’ Syndrome-HF” study. Int J Cardiol 2018; 271: 174–180. [DOI] [PubMed] [Google Scholar]
- 32. Bruña V, Velásquez-Rodríguez J, Valero-Masa MJ, et al. Prognostic of Interatrial Block after an acute ST-Segment elevation myocardial infarction. Cardiology 2019; 142: 109–115. [DOI] [PubMed] [Google Scholar]
- 33. Martín-Demiguel I, Núñez-Gil IJ, Pérez-Castellanos A, et al. Prevalence and significance of interatrial block in Takotsubo syndrome (from the RETAKO Registry). Am J Cardiol 2019; 123: 2039–2043. [DOI] [PubMed] [Google Scholar]
- 34. Baturova MA, Lindgren A, Shubik YV, et al. Interatrial block in prediction of all-cause mortality after first-ever ischemic stroke. BMC Cardiovasc Disord 2019; 19: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-eso-10.1177_23969873221126000 for Prognostic significance of atrial cardiopathy in patients with acute ischemic stroke by Yueyang Wu, Xiaomeng Yang, Jing Jing, Xia Meng, Zixiao Li, Yuesong Pan, Yong Jiang, Hongyi Yan, Xinying Huang, Liping Liu, Xingquan Zhao, Yilong Wang, Hao Li and Yongjun Wang in European Stroke Journal