Abstract
Introduction:
Endovascular thrombectomy (EVT) increases the chance of good functional outcome after ischemic stroke caused by a large vessel occlusion, but the risk of death in the first 90 days is still considerable. We assessed the causes, timing and risk factors of death after EVT to aid future studies aiming to reduce mortality.
Patients and methods:
We used data from the MR CLEAN Registry, a prospective, multicenter, observational cohort study of patients treated with EVT in the Netherlands between March 2014, and November 2017. We assessed causes and timing of death and risk factors for death in the first 90 days after treatment. Causes and timing of death were determined by reviewing serious adverse event forms, discharge letters, or other written clinical information. Risk factors for death were determined with multivariable logistic regression.
Results:
Of 3180 patients treated with EVT, 863 (27.1%) died in the first 90 days. The most common causes of death were pneumonia (215 patients, 26.2%), intracranial hemorrhage (142 patients, 17.3%), withdrawal of life-sustaining treatment because of the initial stroke (110 patients, 13.4%) and space-occupying edema (101 patients, 12.3%). In total, 448 patients (52% of all deaths) died in the first week, with intracranial hemorrhage as most frequent cause. The strongest risk factors for death were hyperglycemia and functional dependency before the stroke and severe neurological deficit at 24–48 h after treatment.
Discussion and conclusion:
When EVT fails to decrease the initial neurological deficit, strategies to prevent complications like pneumonia and intracranial hemorrhage after EVT could improve survival, as these are often the cause of death.
Keywords: Stroke, pneumonia, mortality, ischemic stroke, intracerebral hemorrhage, endovascular treatment, thrombectomy, death, space-occupying edema, malignant infarction
Introduction
Endovascular treatment (EVT) strongly reduces the risk of poor functional outcome in patients with ischemic stroke caused by large-vessel occlusion in the anterior circulation, but the benefit of this treatment in terms of survival is less convincing. In only one of five landmark randomized trials of EVT versus no EVT a statistically significant reduction of death was found, 1 and in a meta-analysis of 11 randomized trials of EVT plus best medical treatment versus best medical treatment alone EVT reduced the risk of death at 90 days by 4%–15%. 2
The limited effect of EVT on survival is supported by death rates in large multi-center observational studies of EVT for ischemic stroke, which ranged from 24% to 29%3–5 in Europe and 19% to 22%6,7 in the US. One study from Japan, including 1121 patients from 46 centers, reported a rate of 9.8%. 8
The timing and causes of death after EVT in routine clinical practice have remained largely unknown. Registry-based studies focusing on hemorrhagic complications,9–12 space-occupying edema formation13–15 or infectious complications as pneumonia16,17 have suggested that these are frequent causes of death after EVT, but the relative frequencies of the complications and the timing of their occurrence are unknown. A better understanding of the causes of death after EVT may inform future studies aiming to improve outcomes and increase the chance of survival. We therefore assessed the timing, causes, risk factors and predictors of death in the first 90 days after EVT in routine clinical practice.
Methods
Study protocol and population
We used data from the Multicenter Randomized Controlled Trial of Endovascular Treatment for Acute Ischemic Stroke (MR CLEAN) Registry – a prospective, multicenter, observational study of patients with acute ischemic stroke treated with EVT in routine clinical practice in the Netherlands in 17 intervention centers. 3 The current study is based on data from patients included in parts 1 and 2 of the Registry between March 16, 2014 and November 1, 2017. Inclusion criteria for this analysis were: age ⩾18 years; clinical diagnosis of acute ischemic stroke with a proximal arterial occlusion in the anterior circulation (internal carotid artery, middle cerebral artery [M1 and M2], or anterior cerebral artery [A1 and A2]), demonstrated with computed tomography angiography, magnetic resonance angiography or digital subtraction angiography; and EVT, defined as groin puncture, initiated within 6.5 h of symptom onset or last seen well. According to the intention-to-treat-principle, all patients in whom a groin puncture was performed were analyzed. We adhered to the RECORD reporting guideline. 18 The associated checklist can be found in the Supplemental Table 1. The protocol of the MR CLEAN Registry was reviewed by the Erasmus University Medical Center Ethics Committee. The requirement for written informed consent was waived, but patients or their representatives were provided with information on the study orally and in writing and were given the opportunity to refuse participation.
Data collection
Local study investigators collected clinical and demographic data from electronic patient records and assessed the score on the modified Rankin Scale (mRS) at 90 days (±14 days).
Baseline imaging characteristics, including occlusion site, ASPECTS and collateral status by the method of Tan et al., 19 intervention results using the extended treatment in cerebral infarction score (eTICI) 20 and follow-up imaging including assessment of hemorrhagic transformation with the Heidelberg Bleeding Classification, 21 and space-occupying edema were assessed by the MR CLEAN Registry imaging core laboratory, blinded to clinical findings.
For each deceased patient the cause and timing of death were either determined by serious adverse event (SAE) forms that were reviewed by the serious adverse event committee of the Registry or discharge letters when no SAE that resulted in death was recorded. All discharge letters were reviewed independently by two investigators (WS and LP). Disagreements were solved in a consensus meeting. We assessed whether death occurred during admission (in-hospital death) or after discharge, and on days 0–7 or days 8–90. In case of missing data, additional information was obtained by contacting the physicians who treated the patient at the time of death, when patients died at home or in a nursing home this was the general practitioner or nursing home physician.
Outcome and definitions of causes of mortality
Predefined expected causes of death were classified as (1) pneumonia when there were signs of infection combined with respiratory symptoms or relevant abnormalities on chest radiograph leading the adverse event committee or treating physician to diagnose a pneumonia leading to death; (2) intracranial hemorrhage in case of evidence of either Heidelberg Bleeding Classification class I, II or III on imaging and neurological deterioration (National Institutes of Health Stroke Scale (NIHSS) increase ⩾4) eventually leading to death according to the adverse event committee or treating physician; (3) space-occupying edema when there was neurological deterioration with clinical and imaging signs of space-occupying edema leading to death; (4) cardiac death in case of heart failure, cardiac asthma, or myocardial infarction with respiratory insufficiency or cardiac arrest leading to death; (5) withdrawal of life-sustaining treatment in case of withdrawal or withholding life-sustaining treatments because of a lack of improvement of initial stroke symptoms, without any other apparent cause of death; (6) recurrent ischemic stroke in case of a new ischemic stroke in the same or different territory resulting in neurological deterioration and withdrawal of life-sustaining treatment; (7) stroke progression if there was neurological deterioration (NIHSS increase ⩾4) without clinical signs of a space-occupying infarction, imaging signs of midline shift or edema and without any other apparent cause leading to withdrawal of life-sustaining treatment.
Statistical analysis
Baseline characteristics, distribution of causes of death, and timing and location of death are described using descriptive statistics. To investigate a possible time trend, we compared causes of death between part 1 and part 2 of the registry. Comparisons between patients who died and those who survived were made using χ 2 or Student’s t test where appropriate. For the multivariable regression analyses, multiple imputation was performed for predictor values with missing data. Variables with a p value less than 0.15 in the univariable model were considered to be possible independent predictors and subsequently included in a multivariable logistic regression analysis to identify predictors of death. A sensitivity analysis was performed without imputing data. Statistical significance was defined as a p value <0.05. All analyses were performed using R studio version 1.3.1056. (Rstudio PBC).
Results
A total of 3180 patients with a median age of 72 years (IQR 61–81) were included in this analysis (Supplemental Figure 1) and details of missing variables are listed in Supplemental Table 3. Spontaneous reperfusion after groin puncture was observed in 284 (8.9%) patients and in 187 (5.9%) the occlusion could not be reached. Of all included patients, 863 (27.1%) died in the first 90 days. Median time to death was 7 days (IQR: 3–19 days). In total, 629 deaths (73.1%) occurred during the admission for acute stroke.
The cause of death was known in 821 (95.1%) patients. Pneumonia was reported as the cause of death in 215 patients (26.2%); intracranial hemorrhage in 142 (17.3%); withdrawal of life-sustaining treatment in 110 (13.4%); space-occupying edema in 101 (12.3%); and a different complication in 253 (30.8%; Table 1). No difference in causes of death between the two time periods in the Registry was observed (Supplemental Table 4).
Table 1.
Determined causes of death.
| Cause* | All patients n = 821 | Death <7 days n = 448 | Death at 8–90 days n = 373 |
|---|---|---|---|
| Pneumonia | 215 (26.2) | 102 (22.8) | 113 (30.3) |
| Intracranial hemorrhage † | 142 (17.3) | 114 (25.4) | 28 (7.5) |
| Withdrawal of life-sustaining treatment | 110 (13.4) | 41 (9.2) | 69 (18.5) |
| Space-occupying infarction | 101 (12.3) | 98 (21.9) | 3 (0.8) |
| Stroke progression | 56 (6.8) | 33 (7.4) | 23 (6.2) |
| Cardiac death | 45 (5.5) | 24 (5.4) | 21 (5.6) |
| Recurrent ischemic stroke | 29 (3.5) | 9 (2.0) | 20 (5.4) |
| Withholding artificial food | 18 (2.2) | 1 (0.2) | 17 (4.6) |
| Infection, other | 16 (1.9) | 2 (0.4) | 14 (3.8) |
| Cancer | 15 (1.8) | 2 (0.4) | 13 (3.5) |
| Neurological deterioration e.c.i. ‡ | 15 (1.8) | 6 (1.3) | 9 (2.4) |
| Sudden death | 14 (1.7) | 3 (0.7) | 11 (2.9) |
| Extracranial hemorrhage | 11 (1.3) | 3 (0.7) | 8 (2.1) |
| Respiratory insufficiency, other | 11 (1.3) | 6 (1.3) | 5 (1.3) |
| Urinary tract infection | 10 (1.2) | 1 (0.2) | 9 (2.4) |
| Renal failure | 4 (0.5) | 0 (0.0) | 4 (1.1) |
| Pulmonary embolism | 3 (0.4) | 1 (0.2) | 2 (0.5) |
| Multi-organ failure | 2 (0.2) | 0 (0.0) | 2 (0.5) |
| Endocarditis | 1 (0.1) | 1 (0.2) | 0 (0.0) |
| Acute abdomen | 1 (0.1) | 1 (0.2) | 0 (0.0) |
| Euthanasia | 1 (0.1) | 0 (0.0) | 1 (0.3) |
| Status epilepticus | 1 (0.1) | 0 (0.0) | 1 (0.3) |
c.i.: e causa ignota; COPD: chronic obstructive pulmonary disease.
All numbers are n (%) unless stated otherwise.
Any type of symptomatic (NIHSS increase ⩾4) intracranial hemorrhage (Heidelberg Bleeding Classification I, II, or III).
Patients who died after new focal deficits or coma without an apparent cause in whom no imaging was performed.
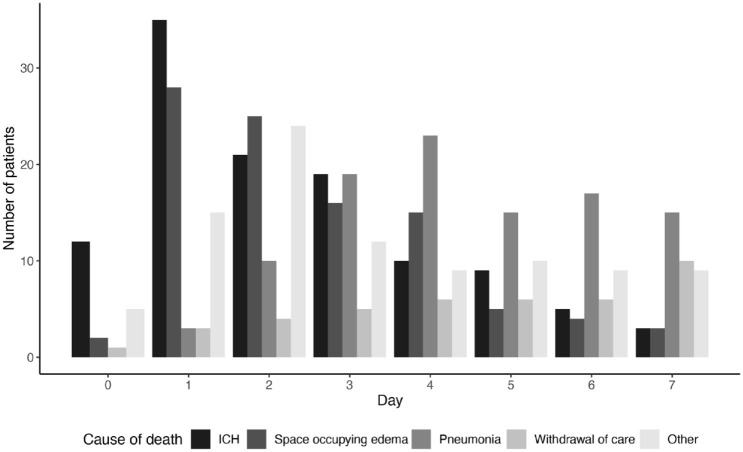
Any type of symptomatic intracranial hemorrhage and space-occupying infarction were the main causes of death in the first 3 days following EVT, followed by pneumonia later in the first week (Figure 1).
Figure 1.
Most frequent causes of death in the first 7 days.
ICH: intracranial hemorrhage.
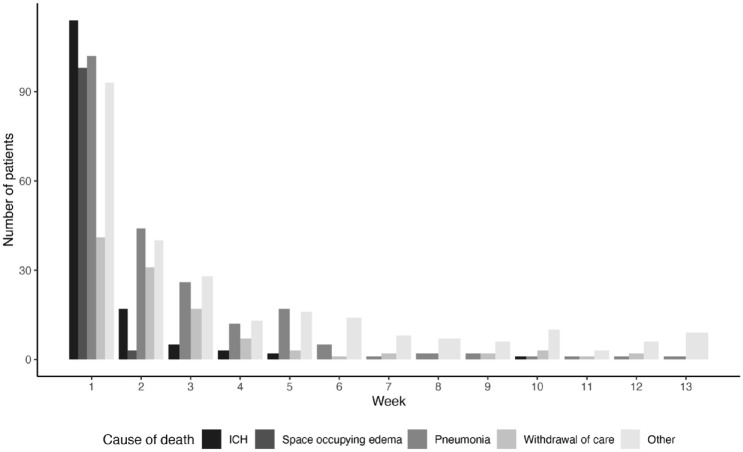
Intracranial hemorrhage was the most frequent cause of death in the first 7 days, pneumonia between day 8 and 90, during the admission for stroke and after discharge (Table 1 and Figure 2).
Figure 2.
Most frequent causes of death within 90 days.
ICH: intracranial hemorrhage.
Of all 215 patients who died of pneumonia, treatment with antibiotics was initiated in 166 (77.2%). In the remaining patients life-sustaining treatments were stopped after suspicion of pneumonia. If these patients were categorized as withdrawal of life-sustaining treatment (as it can be hypothesized that no treatment was initiated because of the initial stroke severity), pneumonia remained the most frequent cause of death (20.2%).
Patients who died differed from patients who survived in almost every demographic, clinical, and imaging characteristic (Table 2).
Table 2.
Baseline characteristics of patients who died or survived.
| Characteristics* | All patients n = 3180 | Alive n = 2317 | Deceased n = 863 | p-Value |
|---|---|---|---|---|
| Demographics | ||||
| Age, years (median; IQR) | 72.0 (61.2–80.5) | 69.2 (58.2–77.8) | 79.0 (70.6–86.0) | p < 0.001 |
| Sex (female) | 1524 (48.0) | 1073 (46.4) | 451 (52.3) | p = 0.003 |
| In-hospital stroke | 324 (10.2) | 209 (9.0) | 115 (13.3) | p = 0.002 |
| Clinical characteristics | ||||
| NIHSS (median; IQR) | 16.0 (11.0–19.0) | 15.0 (10.0–19.0) | 18.0 (14.0–22.0) | p < 0.001 |
| Left hemisphere | 1684 (53.1) | 1180 (51.1) | 504 (58.4) | p = 0.001 |
| Systolic BP (mean; SD) | 149.9 (24.8) | 148.1 (24.0) | 154.5 (26.3) | p < 0.001 |
| Functional dependent † | 360 (11.6) | 163 (7.2) | 197 (23.8) | p < 0.001 |
| Treatment with IVT | 2424 (76.4) | 1826 (79.1) | 598 (69.4) | p < 0.001 |
| Laboratory characteristics | ||||
| INR (median: IQR) | 1.0 (1.0–1.0) | 1.0 (1.0–1.1) | 1.1 (1.0–1.2) | p < 0.001 |
| CRP (median; IQR) | 4.0 (2.0–10.0) | 4.0 (1.9–8.0) | 6.0 (2.5–17.0) | p < 0.001 |
| Glucose (median; IQR) | 6.8 (5.9–8.1) | 6.6 (5.9–7.8) | 7.4 (6.2–9.1) | p < 0.001 |
| Imaging characteristics | ||||
| Occlusion site | p < 0.001 | |||
| ICA | 155 (5.1) | 115 (5.2) | 40 (4.9) | |
| ICA-T | 640 (21.1) | 397 (17.9) | 242 (29.7) | |
| M1 | 1760 (57.9) | 1340 (60.3) | 420 (51.3) | |
| M2 | 461 (15.2) | 355 (16.0) | 106 (13.0) | |
| Other | 24 (0.8) | 15 (0.7) | 9 (1.1) | |
| ASPECTS | p < 0.001 | |||
| 0–4 | 144 (4.7) | 87 (3.9) | 57 (6.9) | |
| 5–7 | 628 (20.5) | 446 (19.9) | 182 (22.1) | |
| 8–10 | 2298 (74.9) | 1712 (76.3) | 586 (71.0) | |
| Collateral score ‡ | p < 0.001 | |||
| 0 | 186 (6.3) | 90 (4.1) | 96 (12.0) | |
| 1 | 1068 (35.9) | 715 (32.9) | 353 (44.0) | |
| 2 | 1153 (38.8) | 891 (41.1) | 262 (32.6) | |
| 3 | 566 (19.0) | 474 (21.8) | 92 (11.5) | |
| Comorbidities | ||||
| Hypertension | 1629 (52.4) | 1096 (48.4) | 533 (63.2) | p < 0.001 |
| Atrial fibrillation | 755 (24.1) | 468 (20.5) | 287 (33.8) | p < 0.001 |
| Diabetes | 510 (16.2) | 293 (12.8) | 217 (25.4) | p < 0.001 |
| Hypercholesterolemia | 935 (30.8) | 654 (29.5) | 281 (34.5) | p = 0.008 |
| Previous ischemic stroke | 529 (16.8) | 333 (14.5) | 196 (23.0) | p < 0.001 |
| Previous myocardial infarction | 440 (14.1) | 289 (12.7) | 151 (18.0) | p < 0.001 |
| Peripheral arterial disease | 292 (9.4) | 188 (8.3) | 104 (12.4) | p < 0.001 |
| Medication | ||||
| Antiplatelet therapy | 980 (31.3) | 653 (28.5) | 327 (38.7) | p < 0.001 |
| DOAC | 104 (3.3) | 68 (3.0) | 36 (4.2) | p = 0.079 |
| Coumarins | 409 (13.0) | 249 (10.8) | 160 (18.8) | p < 0.001 |
| Heparin | 98 (3.1) | 61 (2.7) | 37 (4.4) | p = 0.014 |
| Statins | 1096 (35.3) | 764 (33.7) | 332 (39.7) | p = 0.002 |
| Anti-hypertensive agents | 1684 (54.1) | 1130 (49.7) | 554 (65.9) | p < 0.001 |
IQR: interquartile range; NIHSS: national institute of health stroke scale; BP: blood pressure; SD: standard deviation; IVT: intravenous thrombolysis; INR: international normalized ratio; CRP: c-reactive protein; ICA: internal carotid artery; ICA-T: tandem occlusion of the ICA and proximal middle cerebral artery; M1/M2: first and second segment of the middle cerebral artery; ASPECTS: Alberta stroke program early ct score; DOAC: direct oral anti-coagulant.
All numbers are n (%) unless stated otherwise.
mRS of 3 or higher.
0 = absent collaterals; 1 = less than 50% filling of occluded area with collaterals; 2 = more than 50 but less than 100% filling; 3 = 100% filling.
Patients who died were older, more likely to be functionally dependent at baseline, had more cardiovascular risk factors and comorbidities, had worse collaterals, a lower ASPECTS score on baseline imaging, and less frequently excellent reperfusion, defined as eTICI 2C/3. In patients who died after assessment of the NIHSS score at 24–48 h, this score had not changed as compared with the score on admission (median 0.0 points (IQR −3.0 to 4.0)) whereas this had decreased by 6.0 points (IQR 1.0–10.0) in patients who survived up to 90 days (p < 0.001) (Tables 2 and 3).
Table 3.
Treatment characteristics and outcome of patients who died or survived.
| Treatment characteristics* | All patients n = 3180 | Alive n = 2317 | Deceased n = 863 | p-Value |
|---|---|---|---|---|
| Time parameters | ||||
| Onset to groin (mean; SD) † | 203.4 (73.1) | 198 (72.7) | 216.2 (72.8) | p < 0.001 |
| Duration of procedure (mean; SD) † | 63.7 (34.3) | 60.6 (33.4) | 72.1 (35.2) | p < 0.001 |
| Onset to end of procedure (mean; SD) † | 258.1 (80.4) | 250.5 (80.0) | 279.3 (78.1) | p < 0.001 |
| Admission at off-hours ‡ | 2025 (63.8) | 1459 (63.1) | 566 (65.6) | p = 0.196 |
| Procedural characteristics | ||||
| General anesthesia | 758 (25.4) | 532 (24.5) | 226 (27.8) | p = 0.069 |
| Treatment outcome | ||||
| Reperfusion (TICI 2B-3) | 1910 (61.8) | 1502 (66.8) | 408 (48.5) | p < 0.001 |
| Excellent reperfusion (TICI 2C-3) | 1216 (39.3) | 970 (43.1) | 246 (29.2) | p < 0.001 |
| Decrease of NIHSS (median; IQR) § | 4.0 (0.0–9.0) | 6.0 (1.0–10.0) | 0.0 (−4.0–3.0) | p < 0.001 |
SD: standard deviation; TICI: thrombolysis in cerebral infarction scale; NIHSS: national institute of health stroke scale.
All numbers are n (%) unless stated otherwise
In minutes
Monday to Friday 17:00–08:00, weekends and national holidays.
NIHSS measured 24–48 h post EVT.
Probability of survival was smaller in patients who deteriorated or failed to improve after EVT. (Supplemental Figure 2).
Independent predictors for death within 90 days were higher age; stroke onset while a patient was hospitalized; functional dependency before the stroke; hyperglycemia and a history of diabetes mellitus; previous use of anti-platelet therapy; absent or poor collaterals; procedure under general anesthesia and NIHSS 24–48 h after treatment. The NIHSS at baseline was not an independent predictor of death, in contrast to the NIHSS after treatment. An occlusion of the first segment of the middle cerebral artery was associated with a lower risk of death (Table 4).
Table 4.
Independent predictors of death within 90 days after EVT.
| Predictors* | Odds ratio (95%CI) |
|---|---|
| Age | 1.07 (1.06–1.09) |
| In hospital stroke | 1.81 (1.17–2.80) |
| Functional dependency † | 2.28 (1.66–3.11) |
| Glucose at baseline | 1.09 (1.04–1.15) |
| M1 occlusion | 0.29 (0.09–0.89) |
| Absent collaterals | 2.12 (1.28–3.52) |
| Collateral filling <50% of occluded area | 1.49 (1.05–2.14) |
| History of diabetes | 1.52 (1.11–2.08) |
| Previous use of APT | 1.47 (1.11–1.95) |
| General anesthesia | 1.34 (1.03–1.74) |
| NIHSS at 24–48 hours | 1.19 (1.17–1.21) |
CRP: c-reactive protein; APT: anti platelet therapy; NIHSS: national institute of health stroke scale.
All numbers are odds ratio’s with corresponding 95% confidence interval.
mRS of 3 or higher.
A sensitivity analysis without imputed data had similar results (Supplemental Table 2 and 3).
Discussion
During a follow-up period of 90 days, about one quarter of patients with ischemic stroke caused by a large vessel occlusion in the anterior circulation died despite EVT, and half of these patients died within the first week. In the first days the most common causes of death were any type of symptomatic intracranial hemorrhage and space-occupying edema, followed by a shift toward pneumonia, which was the most common cause of death overall.
Independent predictors of death within 90 days were age, stroke in already hospitalized patients, functional dependency at baseline, a history of diabetes, hyperglycemia, the use of general anesthesia, absent or poor collaterals, previous anti-platelet therapy and a higher NIHSS score at 24–48 h after treatment.
The cause of death of patients in studies on EVT is often not reported. In a long-term follow up study of patients in the REVASCAT trial the most frequent cause of death was related to the initial stroke. 22 This was either malignant edema, hemorrhagic transformation or withdrawal of life-sustaining treatment because of poor prognosis. A small observational study reported that 80% of deaths after EVT was related to the initial stroke, but this was not further specified. 23 Studies on causes of death in all patients with ischemic stroke, regardless of treatment type, are more prevalent.24–26 Frequent causes were the initial stroke, pneumonia, and other cardiovascular disease.
Predictors of death after EVT have been studied more frequently. Common predictors are a higher age, pre-stroke functional dependency and diabetes at baseline, severe stroke caused by a proximal occlusion or indicated by a high NIHSS, and lack of reperfusion.12,27–30 The occurrence of complications after treatment, such as infections and intracerebral hemorrhage has also been reported as a predictor of death.12,31
It is well established that EVT increases the chance of good functional outcome in patients with ischemic stroke. Unfortunately, it does not prevent a considerable proportion of patients from dying and most of these patients die from post-stroke complications such as intracranial hemorrhage, pneumonia or malignant edema. In addition to improving techniques and broadening indications of EVT, prevention and treatment of these complications holds potential to improve survival in patients with ischemic stroke. Surgical decompression reduces the risk of death in patients with space-occupying hemispheric infarction if performed within 48 h of stroke onset but it is uncertain whether this is also of benefit if performed later. 32 , 33 Lowering blood pressure or plasma glucose did not result in better outcomes.34,35 A pharmacological strategy to reduce the rates of pneumonia and fever is under investigation in the ongoing PRECIOUS trial (ISRCTN82217627). 36
Our study has limitations. First, determination of the cause of death was done retrospectively in an observational registry, and apart from patients who died from a reported serious adverse event, no standardized manner of reporting causes of death was used. In none of the patients we investigated, autopsy was performed and while still alive patients did not provide consent to investigate death certificates for this study. In patients who died without a reported serious adverse event, we relied on information we obtained from discharge letters and contact with treating physicians, therefore causes of patients who died later in the 90-day window are more uncertain.
Second, in 1.8%of patients who deteriorated no imaging was performed. In the present study, we have classified the cause of death in these patients as “neurological deterioration without known cause.” These patients could have died of a different cause that was not diagnosed by the clinicians.
Third, in this dataset there were missing data on baseline variables as laboratory markers and vital signs. In previous studies some of these variables were predictors for mortality.37,38 We imputed these data for the regression analysis, and did a sensitivity analysis by excluding patients with missing data, which gave comparable results.
Conclusions
Greater post-procedural stroke severity, higher age, hyperglycemia before treatment, and pre-stroke functional dependency are associated with a greater risk of death in the first 90 days after EVT. The most frequently reported causes of death were pneumonia and other post-stroke complications. About half of the deaths occurred in the first week. When EVT fails to decrease the neurological deficit, strategies to prevent complications after EVT could improve survival.
Supplemental Material
Supplemental material, sj-pdf-1-eso-10.1177_23969873221143210 for Timing and causes of death after endovascular thrombectomy in patients with acute ischemic stroke by Wouter M Sluis, Wouter H Hinsenveld, Robert-Jan B Goldhoorn, Lianne H Potters, Agnetha AE Bruggeman, Anouk van der Hoorn, Joseph CJ Bot, Robert J van Oostenbrugge, Hester F Lingsma, Jeannette Hofmeijer, Wim H van Zwam, Charles BLM Majoie and H Bart van der Worp in European Stroke Journal
Acknowledgments
We would like to thank all the MR CLEAN Registry investigators; a complete list can be found in the Supplemental Table 4.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: WMS is supported by the European Union’s Horizon 2020 research and innovation program (634809). CBLMM received funds from TWIN Foundation (related to this project, paid to institution), CVON/Dutch Heart Foundation, Stryker, European Commission, Healthcare Evaluation Netherlands (unrelated to this project; paid to institution) and is shareholder of Nicolab. HBvdW has received speaker’s fees from Bayer and Boehringer Ingelheim; served as a consultant to Bayer, Boehringer Ingelheim, and LivaNova; and reports grants from Stryker.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The MR CLEAN Registry was partly funded by the Toegepast Wetenschappelijk Instituut voor Neuromodulatie Foundation, Erasmus MC Medical Center, Maastricht University Medical Center, and Academic Medical Center Amsterdam.
Ethical approval and informed consent: The protocol of the MR CLEAN Registry was reviewed by the Erasmus University Medical Center Ethics Committee. The requirement for written informed consent was waived, but patients or their representatives were provided with information on the study orally and in writing and were given the opportunity to refuse participation.
Guarantor: HBvdW
Contributorship: WMS contributed to study concept, statistical analysis, interpretation of results and drafting of the manuscript. WH, RBG, LHP, AAB, AvdH, JCJB, RJvO, HFL, JH, WHvZ, CBLMM contributed to critical revision of the manuscript and HBvdW contributed to study concept, interpretation of results and critical revision of the manuscript.
ORCID iDs: Wouter M Sluis  https://orcid.org/0000-0003-3057-2010
https://orcid.org/0000-0003-3057-2010
Wim H van Zwam  https://orcid.org/0000-0003-1631-7056
https://orcid.org/0000-0003-1631-7056
H Bart van der Worp  https://orcid.org/0000-0001-9891-2136
https://orcid.org/0000-0001-9891-2136
Supplemental material: Supplemental material for this article is available online.
References
- 1. Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015; 372: 1019–1030. [DOI] [PubMed] [Google Scholar]
- 2. Katsanos AH, Malhotra K, Goyal N, et al. Mortality risk in acute ischemic stroke patients with large vessel occlusion treated with mechanical thrombectomy. J Am Heart Assoc 2019; 8: e014425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jansen IGH, Mulder MJHL, Goldhoorn RB. Endovascular treatment for acute ischaemic stroke in routine clinical practice: prospective, observational cohort study (MR CLEAN Registry). BMJ 2018; 360: k949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Compagne KCJ, Kappelhof M, Hinsenveld WH, et al. Improvements in endovascular treatment for acute ischemic stroke: a longitudinal study in the MR CLEAN Registry. Stroke 2022; 53: 1863–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stösser S, Bode FJ, Dorn F, et al. Workflow Times and outcome of endovascular therapy in stroke patients with initial MRI or CT. Cerebrovasc Dis 2022; 51: 45–51. [DOI] [PubMed] [Google Scholar]
- 6. Zaidat OO, Castonguay AC, Nogueira RG, et al. TREVO stent-retriever mechanical thrombectomy for acute ischemic stroke secondary to large vessel occlusion registry. J Neurointerv Surg 2018; 10: 516–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Almallouhi E, Al Kasab S, Hubbard Z, et al. Outcomes of mechanical thrombectomy for patients with stroke presenting with Low Alberta Stroke Program early computed tomography score in the early and extended window. JAMA Netw Open 2021; 4: e2137708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yoshimura S, Sakai N, Uchida K, et al. Endovascular therapy in ischemic stroke with acute large-vessel occlusion: recovery by endovascular salvage for cerebral ultra-acute embolism Japan Registry 2. J Am Heart Assoc 2018; 7: e008796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van Kranendonk KR, Treurniet KM, Boers AMM, et al. Hemorrhagic transformation is associated with poor functional outcome in patients with acute ischemic stroke due to a large vessel occlusion. J Neurointerv Surg 2019; 11: 464–468. [DOI] [PubMed] [Google Scholar]
- 10. Hao Y, Yang D, Wang H, et al. Predictors for symptomatic intracranial hemorrhage after endovascular treatment of acute ischemic stroke. Stroke 2017; 48: 1203–1209. [DOI] [PubMed] [Google Scholar]
- 11. Kinjo N, Yoshimura S, Uchida K, et al. Incidence and prognostic impact of intracranial hemorrhage after endovascular treatment for acute large vessel occlusion. Cerebrovasc Dis 2020; 49: 540–549. [DOI] [PubMed] [Google Scholar]
- 12. Linfante I, Walker GR, Castonguay AC, et al. Predictors of mortality in acute ischemic stroke intervention: analysis of the North American Solitaire Acute Stroke Registry. Stroke 2015; 46: 2305–2308. [DOI] [PubMed] [Google Scholar]
- 13. Bernsen MLE, Kauw F, Martens JM, et al. Malignant infarction after endovascular treatment: incidence and prediction. Int J Stroke 2022; 17: 198–206. [DOI] [PubMed] [Google Scholar]
- 14. Peng G, Huang C, Chen W, et al. Risk factors for decompressive craniectomy after endovascular treatment in acute ischemic stroke. Neurosurg Rev 2020; 43: 1357–1364. [DOI] [PubMed] [Google Scholar]
- 15. Alzayiani M, Schmidt T, Veldeman M, et al. Risk profile of decompressive hemicraniectomy for malignant stroke after revascularization treatment. J Neurol Sci 2021; 420: 117275. [DOI] [PubMed] [Google Scholar]
- 16. van de, Graaf RA, Samuels N, Chalos V, et al. Predictors of poor outcome despite successful endovascular treatment for ischemic stroke: results from the MR CLEAN Registry. J Neurointerv Surg 2022; 14: 660–665. [DOI] [PubMed] [Google Scholar]
- 17. Zhu Y, Gao J, Lv Q, et al. Risk factors and outcomes of stroke-associated pneumonia in patients with stroke and acute large artery occlusion treated with endovascular thrombectomy. J Stroke Cerebrovasc Dis 2020; 29: 105223. [DOI] [PubMed] [Google Scholar]
- 18. Benchimol EI, Smeeth L, Guttmann A, et al. The REporting of studies conducted using observational routinely-collected health data (RECORD) statement. PLoS Med 2015; 12: e1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tan JC, Dillon WP, Liu S, et al. Systematic comparison of perfusion-CT and CT-angiography in acute stroke patients. Ann Neurol 2007; 61: 533–543. [DOI] [PubMed] [Google Scholar]
- 20. Liebeskind DS, Bracard S, Guillemin F, et al. eTICI reperfusion: defining success in endovascular stroke therapy. J Neurointerv Surg 2019; 11: 433–438. [DOI] [PubMed] [Google Scholar]
- 21. von Kummer R, Broderick JP, Campbell BC, et al. The Heidelberg bleeding classification: classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke 2015; 46: 2981–2986. [DOI] [PubMed] [Google Scholar]
- 22. Dávalos A, Cobo E, Molina CA, et al. Safety and efficacy of thrombectomy in acute ischaemic stroke (REVASCAT): 1-year follow-up of a randomised open-label trial. Lancet Neurol 2017; 16: 369–376. [DOI] [PubMed] [Google Scholar]
- 23. Zhao W, Shang S, Li C, et al. Long-term outcomes of acute ischemic stroke patients treated with endovascular thrombectomy: a real-world experience. J Neurol Sci 2018; 390: 77–83. [DOI] [PubMed] [Google Scholar]
- 24. Hartmann A, Rundek T, Mast H, et al. Mortality and causes of death after first ischemic stroke: the Northern Manhattan Stroke Study. Neurology 2001; 57: 2000–2005. [DOI] [PubMed] [Google Scholar]
- 25. Vernino S, Brown RD, Jr., Sejvar JJ, et al. Cause-specific mortality after first cerebral infarction: a population-based study. Stroke 2003; 34: 1828–1832. [DOI] [PubMed] [Google Scholar]
- 26. Kimura K, Minematsu K, Kazui S, et al. Mortality and cause of death after hospital discharge in 10,981 patients with ischemic stroke and transient ischemic attack. Cerebrovasc Dis 2005; 19: 171–178. [DOI] [PubMed] [Google Scholar]
- 27. Gattringer T, Posekany A, Niederkorn K, et al. Predicting early mortality of acute ischemic stroke. Stroke 2019; 50: 349–356. [DOI] [PubMed] [Google Scholar]
- 28. Nogueira RG, Liebeskind DS, Sung G, et al. Predictors of good clinical outcomes, mortality, and successful revascularization in patients with acute ischemic stroke undergoing thrombectomy: pooled analysis of the mechanical Embolus removal in cerebral ischemia (MERCI) and multi MERCI Trials. Stroke 2009; 40: 3777–3783. [DOI] [PubMed] [Google Scholar]
- 29. Oliveira ADP, Andrade-Valença LPA, Valença MM. Factors associated with In-hospital mortality in very elderly patients with ischemic stroke: a Cohort Study. J Stroke Cerebrovasc Dis 2019; 28: 104281. [DOI] [PubMed] [Google Scholar]
- 30. Li H, Ye SS, Wu YL, et al. Predicting mortality in acute ischaemic stroke treated with mechanical thrombectomy: analysis of a multicentre prospective registry. BMJ Open 2021; 11: e043415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Westendorp WF, Nederkoorn PJ, Vermeij JD, et al. Post-stroke infection: a systematic review and meta-analysis. BMC Neurol 2011; 11: 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Reinink H, Jüttler E, Hacke W, et al. Surgical decompression for space-occupying hemispheric infarction: a systematic review and individual patient meta-analysis of randomized clinical trials. JAMA Neurol 2021; 78: 208–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. van der Worp HB, Hofmeijer J, Jüttler E, et al. European Stroke Organisation (ESO) guidelines on the management of space-occupying brain infarction. Eur Stroke J 2021; 6: XC–CX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sandset EC, Anderson CS, Bath PM, et al. European Stroke Organisation (ESO) guidelines on blood pressure management in acute ischaemic stroke and intracerebral haemorrhage. Eur Stroke J 2021; 6: XLVIII–LXXXIX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Johnston KC, Bruno A, Pauls Q, et al. Intensive vs standard treatment of hyperglycemia and functional outcome in patients with acute ischemic stroke: the SHINE Randomized Clinical Trial. JAMA 2019; 322: 326–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Reinink H, de Jonge JC, Bath PM, et al. PRECIOUS: PREvention of complications to improve OUtcome in elderly patients with acute Stroke. Rationale and design of a randomised, open, phase III, clinical trial with blinded outcome assessment. Eur Stroke J 2018; 3: 291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Greer DM, Funk SE, Reaven NL, et al. Impact of fever on outcome in patients with stroke and neurologic injury: a comprehensive meta-analysis. Stroke 2008; 39: 3029–3035. [DOI] [PubMed] [Google Scholar]
- 38. Rowat AM, Dennis MS, Wardlaw JM. Hypoxaemia in acute stroke is frequent and worsens outcome. Cerebrovasc Dis 2006; 21: 166–172. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-eso-10.1177_23969873221143210 for Timing and causes of death after endovascular thrombectomy in patients with acute ischemic stroke by Wouter M Sluis, Wouter H Hinsenveld, Robert-Jan B Goldhoorn, Lianne H Potters, Agnetha AE Bruggeman, Anouk van der Hoorn, Joseph CJ Bot, Robert J van Oostenbrugge, Hester F Lingsma, Jeannette Hofmeijer, Wim H van Zwam, Charles BLM Majoie and H Bart van der Worp in European Stroke Journal