Abstract
Background
The proper imaging modality for use in the selection of patients for endovascular thrombectomy (EVT) presenting in the late window remains controversial, despite current guidelines advocating the use of advanced imaging in this population. We sought to understand if clinicians with different specialty training differ in their approach to patient selection for EVT in the late time window.
Methods
We conducted an international survey of stroke and neurointerventional clinicians between January and May 2022 with questions focusing on imaging and treatment decisions of large vessel occlusion (LVO) patients presenting in the late window. Interventional neurologists, interventional neuroradiologists, and endovascular neurosurgeons were defined as interventionists whereas all other specialties were defined as non-interventionists. The non-interventionist group was defined by all other specialties of the respondents: stroke neurologist, neuroradiologist, emergency medicine physician, trainee (fellows and residents) and others.
Results
Of 3000 invited to participate, 1506 (1027 non-interventionists, 478 interventionists, 1 declined to specify) physicians completed the study. Interventionist respondents were more likely to proceed directly to EVT (39.5% vs. 19.5%; p < 0.0001) compared to non-interventionist respondents in patients with favorable ASPECTS (Alberta Stroke Program Early CT Score). Despite no difference in access to advanced imaging, interventionists were more likely to prefer CT/CTA alone (34.8% vs. 21.0%) and less likely to prefer CT/CTA/CTP (39.1% vs. 52.4%) for patient selection (p < 0.0001). When faced with uncertainty, non-interventionists were more likely to follow clinical guidelines (45.1% vs. 30.2%) while interventionists were more likely to follow their assessment of evidence (38.7% vs. 27.0%) (p < 0.0001).
Conclusion
Interventionists were less likely to use advanced imaging techniques in selecting LVO patients presenting in the late window and more likely to base their decisions on their assessment of evidence rather than published guidelines. These results reflect gaps between interventionists and non-interventionists reliance on clinical guidelines, the limits of available evidence, and clinician belief in the utility of advanced imaging.
Supplementary Information
The online version of this article (10.1007/s00062-023-01284-0) contains supplementary material, which is available to authorized users.
Keywords: ASPECTS, Endovascular thrombectomy, Mechanical thrombectomy, Late window, Large vessel occlusion
Introduction
Endovascular thrombectomy (EVT) benefits certain patients with large vessel occlusion (LVO) acute ischemic stroke presenting in the late window, as first demonstrated by the DEFUSE 3 and DAWN randomized trials [1, 2] and later with the large core thrombectomy trials [3–5]. Patient selection in DEFUSE 3 and DAWN trials was highly restrictive and based on advanced imaging techniques, CT perfusion (CTP) and MRI, to assess for potentially salvageable brain tissue within penumbra regions. In spite of methodological flaws that limit trial generalizability, the selection criteria of these trials have been incorporated into current major stroke guidelines and recommend the use of advanced imaging in the selection of patients for EVT presenting in the late window [6–8]. Moreover, ongoing thrombectomy device trials mandate advanced neuroimaging for patients presenting in the 6–24h window (e.g. ENVI trial, NCT05107206), in accordance with the FDA’s adherence to clinical guidelines. Recent guidelines have not been as restrictive, reflecting the medical uncertainty on this issue [9].
Advanced imaging requires substantially increased cost and resources compared to standard imaging (CT and CT angiography [CTA]) which may present a barrier to the use of these techniques and, in turn, the use of EVT in the late window [10–12]. The potential delay in care from the use of advanced imaging may diminish the benefits of EVT, and might exclude beneficiaries from thrombectomy on the basis of rigid parameters. Additionally, quantitative perfusion thresholds that were used as selection criteria had not been properly justified before the conduct of the trials, and there are reasons to suspect they were too restrictive, as shown by the exceptionally good results of thrombectomy in late comers [1, 2]. Recent research such as the CT for Late Endovascular Reperfusion (CLEAR) study has cast doubt on the requirement of advanced imaging for patient selection [13–15]. In CLEAR, there was no difference in outcomes between patients selected for EVT using CT/CTA alone versus patients selected using MRI or CTP, and the median door-to-puncture time was 20 min shorter for patients selected by CT/CTA compared to advanced imaging [13]. Moreover, the rate of reperfusion without functional independence (or previously termed futile reperfusion) was not worse in those who were selected by non-contrast CT (NCCT) vs. advanced imaging [10]. In the early window, large strides have been made to decrease the door-to-puncture time [16–20] including standardized stroke protocols and direct-to-thrombectomy workflow pathways [21, 22]. Interventionists, as practitioners of EVT in the angiography suite, are often at the forefront of patient workflow with vascular neurologists, neuroradiologists, and emergency room physicians, and have first-hand experience with the delays encountered in arterial puncture affecting optimal delivery of care. Additionally, a previous survey of specialist perceptions of EVT in basilar artery occlusion revealed differences in opinion as to the benefit of EVT [23] and patient selection between interventionists and non-interventionists [24].
In the context of new evidence about patient selection in the late window, we sought to understand if clinicians with different specialty training differ in their approach to patient selection for EVT in the late time window.
Methods
Approval was obtained via Boston Medical Center Institutional Review Board (IRB H‑37519). Data are available upon request to the corresponding author.
Design
The online survey consisted of demographic questions followed by 11 questions examining views on the imaging selection of patients for EVT in the late window [25]. Senior authors developed the questions, and the survey had an estimated completion time of less than 5 min. Participants were exposed to simulated case scenarios including direct questions about opinions toward the availability of imaging and management decisions. Responses to the online survey were collected using the Research Electronic Database Capture (REDCap) system, a web-based application hosted by the Boston University Clinical and Translational Science Institute 1UL1TR001430.1. A complete copy of the survey is available in the Supplement.
Distribution
The survey link was distributed through national and international groups including: Argentina Neurology Society, Brazil Stroke Society, German Stroke Trial Network, Italian Stroke Society, Colombia Association of Neurology, Norway Stroke Organization, the Japanese Society for Neuroendovascular Therapy, Dutch Neurovascular Society, British and Irish Association of Stroke Physicians, Welsh Association of Stroke Physicians, Stroke Clinical Trials Network in Ireland, Madrid Association of Neurology, Indonesian Neurointerventionalists, the Lithuanian Stroke Association, Society of Vascular and Interventional Neurology (SVIN), Global SVIN COVID-19 stroke registry, Whatsapp or Telegram group for three Neurointerventional groups, WeChat Stroke Network in China, MT2020, and Women in Neurointervention.
Statistical Analysis
Statistical analysis was conducted using JMP 15 software (SAS Institute, Cary, NC, USA). Responses without an email address provided, responses only to demographic questions, and duplicate or blank responses were excluded from the analysis. Country income was stratified according to the World Bank classification. The interventionist group was defined by the following specialties: interventional neurologist, interventional neuroradiologist, and endovascular neurosurgeon. The non-interventionist group was defined by all other specialties of the respondents: stroke neurologist, neuroradiologist, emergency medicine physician, trainee (fellows and residents) and others (Table 1).
Table 1.
Characteristics of respondents
| Overall | Interventionist | Non-interventionist | p | |
|---|---|---|---|---|
| Age (years) | ||||
| ≤ 50 | 1256 (83.46) | 419 (87.66) | 836 (81.48) | 0.0027 |
| > 50 | 249 (16.54) | 59 (12.34) | 190 (18.52) | – |
| Interventionist | 478 (31.76) | 478 (31.76) | – | – |
| Interventional neurologist | 215 (14.29) | 215 (14.29) | – | – |
| Interventional neuroradiologist | 189 (12.56) | 189 (12.56) | – | – |
| Endovascular neurosurgeon | 74 (4.92) | 74 (4.92) | – | – |
| Non-interventionist | 1027 (68.24) | – | 1027 (68.24) | – |
| Stroke neurologist | 747 (49.64) | – | 747 (49.64) | – |
| – At PSC or non-stroke center | 141 (9.37) | – | 141 (9.37) | – |
| – At CSC | 606 (40.27) | – | 606 (40.27) | – |
| Neuroradiologist | 27 (1.79) | – | 27 (1.79) | – |
| Emergency medicine | 10 (0.66) | – | 10 (0.66) | – |
| Trainee | ||||
| – Fellow | 104 (6.91) | – | 104 (6.91) | – |
| – Resident | 97 (6.45) | – | 97 (6.45) | – |
| Other | 42 (2.79) | – | 42 (2.79) | – |
| Practice Setting | ||||
| Comprehensive Stroke Center | 1348 (89.63) | 461 (96.44) | 886 (86.44) | < 0.0001 |
| Primary Stroke Center | 106 (7.05) | 7 (1.46) | 99 (9.66) | – |
| Non-stroke center | 50 (3.32) | 10 (2.09) | 40 (3.90) | – |
| Country Income | ||||
| High | 1093 (74.91) | 303 (65.87) | 790 (79.16) | < 0.0001 |
| Low or middle | 366 (25.09) | 157 (34.13) | 208 (20.84) | – |
| Continent | ||||
| Africa | 9 (0.62) | 1 (0.22) | 8 (0.80) | < 0.0001 |
| Asia | 351 (24.06) | 170 (36.96) | 181 (18.14) | – |
| Europe | 25 (1.71) | 104 (22.61) | 450 (45.09) | – |
| North America | 554 (37.97) | 156 (33.91) | 279 (27.96) | – |
| Oceania | 436 (29.88) | 5 (1.09) | 20 (2.00) | – |
| South America | 84 (5.76) | 24 (5.22) | 60 (6.01) | – |
All numbers represent: column n (%)
Where appropriate, intergroup differences were assessed with the χ2-test or Fisher’s exact test. Missing values were not imputed. Statistical significance was set at α = 0.05.
Results
Approximately 3000 participants were invited to participate, yielding 1696 total responses. Responses without email identification, those responding only to demographic questions, duplicate responses, and blank responses were excluded. This yielded 1506 responses for analysis, of which 1027 (68.2%) were from non-interventionists, 478 (31.8%) from interventionists, and 1 was without a specialty provided.
Demographics
Overall, 83.5% were younger than 50 years old, 75.0% were practicing in a high-income country, 89.6% were practicing at a comprehensive stroke center, and 68.2% were non-interventionists. Characteristics of the cohort are provided in Table 1.
Interventionist respondents were more likely to be ≤ 50 years old (87.7% vs. 81.5%; p = 0.0027), more likely to practice at a comprehensive stroke center (96.4% vs. 86.4%; p < 0.0001), and more likely to practice in a low-income or middle-income country (34.1% vs. 20.8%; p < 0.0001) compared to their non-interventionist counterparts. Interventionists were more likely to be from Asian countries (37.0% vs. 18.1%) while non-interventionists were more likely to be from European countries (45.1% vs. 22.6%) (p < 0.0001). Characteristics of both groups are reported in Table 1.
Patient Selection for Reperfusion Therapy
Participants exposed to a case scenario of a wake-up stroke, 9 hours from last seen well with CT ASPECTS of 9 and a left middle cerebral artery M1 occlusion, were asked about their preferred next step in management. When provided options to proceed with additional imaging or endovascular intervention, the most common recommendation among interventionists and non-interventionists was to request a CTP or MRI prior to EVT (45.6% and 39.7%, respectively). Interventionists were more likely to select a direct-to-EVT approach (39.5% vs. 19.5%) and less likely to recommend CTP or MRI prior to combined intravenous thrombolysis (IVT) and EVT (11.0% vs. 32.2%), as compared to non-interventionists (p < 0.0001, Table 2, Q0).
Table 2.
Survey responses: interventionist vs. non-interventionist
| Interventionist | Non-interventionist | p | |
|---|---|---|---|
| Q0: 78-year-old woman from assisted living facility (due to mild cognitive impairment, MoCA 20) presents as wake-up stroke, 9 h from last seen well. CT ASPECTS 9. CTA shows left M1 occlusion. How would you manage the patient? | |||
| Complete CTP or MRI prior to EVT | 206 (45.57) | 385 (39.69) | < 0.0001 |
| Complete CTP or MRI prior to combined IVT and EVT | 50 (11.04) | 312 (32.16) | – |
| Direct to EVT | 179 (39.51) | 189 (19.48) | – |
| IVT alone as wake-up stroke | 2 (0.44) | 17 (1.75) | – |
| Medical management | 11 (2.43) | 40 (4.12) | – |
| Refer to EVT center | 5 (1.10) | 27 (2.78) | – |
| Q1: Would you agree to base reperfusion therapies for patients presenting in the late time window (6–24 h) on CT + CTA as opposed to advanced brain imaging (CTP/MRI) done in the DAWN/DEFUSE3 studies? | |||
| I agree with CT/CTA/CTP for patient selection in the late window | 184 (39.07) | 521 (52.41) | < 0.0001 |
| I agree with just CT/CTA modalities for patient selection in late window | 164 (34.82) | 209 (21.03) | – |
| I agree with MRI/MRP for patient selection in the late window | 59 (12.53) | 77 (7.75) | – |
| Given the uncertainty about the best strategy, I make individual decisions | 64 (13.59) | 187 (18.81) | – |
| Q2: When making treatment decisions under uncertainty, which option below are you most comfortable with? | |||
| Following standard of care as established in my region or country | 29 (6.26) | 103 (10.49) | < 0.0001 |
| Following recommendations from current clinical guidelines | 140 (30.24) | 443 (45.11) | – |
| Following your standard clinical practice based on your expertise and evidence | 179 (38.66) | 265 (26.99) | – |
| I do not apply a consistent strategy for every therapeutic decision | 115 (24.84) | 171 (17.41) | – |
| Q3. Is advanced imaging (CTP/MRI) available 24/7 at your institution? | |||
| No, it is not available | 21 (4.56) | 70 (7.14) | 0.2254 |
| No, it is only available on weekdays | 17 (3.69) | 46 (4.69) | – |
| Only available as a special request | 18 (3.90) | 49 (4.99) | – |
| Yes, we use it routinely | 339 (73.54) | 680 (69.32) | – |
| Yes, but it is not always immediately available | 66 (14.32) | 136 (13.86) | – |
| Q4. Do you routinely use advanced imaging (CTP/MRI) at your center for thrombectomy decision making in patients presenting with LVO in the 6–24h time window? | |||
| Advanced imaging (CTP/MRI) is not available | 14 (3.02) | 49 (5.02) | 0.3472 |
| No, we use it in some cases | 121 (26.13) | 239 (24.49) | – |
| Treatment decisions are based on CT/CTA at my institution | 34 (7.34) | 67 (6.86) | – |
| Yes, we use it in every case | 294 (63.50) | 621 (63.63) | – |
| Q5. If you do not have advanced imaging readily available, and a patient presents to you with LVO in the 6–24 h window based on CT/CTA imaging, how do you treat this patient? | |||
| Medical management | 12 (2.69) | 93 (10.01) | < 0.0001 |
| Enroll in RCT | 14 (3.14) | 23 (2.48) | – |
| Refer to EVT based upon CT alone | 373 (83.63) | 591 (63.62) | – |
| Refer to CSC | 19 (4.26) | 147 (15.82) | – |
| Wait for advanced imaging | 28 (6.28) | 75 (8.07) | – |
| Q6. If you use advanced imaging (CTP/MRI) for selecting patients in the 6–24 h window, compared to CT imaging, what additional time does it usually take in your center to obtain these images to decide a patient’s candidacy for thrombectomy? | |||
| 5 min | 101 (24.51) | 206 (24.38) | 0.8431 |
| 10 min | 135 (32.77) | 292 (34.56) | – |
| 20 min | 93 (22.57) | 161 (19.05) | – |
| 30 min | 49 (11.89) | 106 (12.54) | – |
| 45 min | 16 (3.88) | 37 (4.38) | – |
| 60 min | 13 (3.16) | 29 (3.43) | – |
| 90 min | 2 (0.49) | 9 (1.07) | – |
| 120 min | 3 (0.73) | 5 (0.59) | – |
| Q7. If you use advanced imaging (CTP/MRI) for selection of patients in the 6–24 h window, compared to CT imaging, what additional time delay do you believe is acceptable to obtain these images to decide a patient’s candidacy for thrombectomy? | |||
| 0 min | 12 (2.90) | 23 (2.72) | 0.6089 |
| 5 min | 79 (19.08) | 141 (16.69) | – |
| 10 min | 147 (35.51) | 295 (34.91) | – |
| 20 min | 80 (19.32) | 201 (23.79) | – |
| 30 min | 65 (15.70) | 139 (16.45) | – |
| 45 min | 8 (1.93) | 14 (1.66) | – |
| 60 min | 15 (3.62) | 24 (2.84) | – |
| 90 min | 3 (0.72) | 3 (0.36) | – |
| 120 min | 5 (1.21) | 5 (0.59) | – |
| Q8. The RESCUE Japan study showed benefit for endovascular therapy compared to medical management in the treatment of patients in Japan with large core infarct (ASPECTS 3–5), up to 24 h from symptom onset. Most patients in this study (86%) were selected by MRI. A 70-year-old patient presents 7 h from symptom onset, NIHSS 17, left M1 occlusion, CT ASPECTS 4. How would you next manage this patient? | |||
| CTP, then triage | 129 (31.31) | 324 (37.72) | 0.0692 |
| Direct to angio for thrombectomy | 61 (14.81) | 87 (10.13) | – |
| I would randomize into an ongoing large core infarct trial | 103 (25.00) | 204 (23.75) | – |
| Medical management | 58 (14.08) | 122 (14.20) | – |
| MRI, then triage | 61 (14.81) | 122 (14.20) | – |
Another case scenario included a patient with a stroke, 7 hours from symptom onset with National Institutes of Health Stroke Scale (NIHSS) of 17, CT ASPECTS of 4 and a left M1 occlusion. The most common recommendation among interventionists and non-interventionists was to perform CTP and then triage the patient for further treatment (31.3% and 37.7%, respectively). Interventionists were more likely to proceed directly to thrombectomy than non-interventionists, but this difference was not significant (14.8% vs. 10.1%; p = 0.0692) (Table 2, Q8).
We also investigated another paradigm of care of uncertainty based on the availability of practice guidelines and standards of care. Non-interventionists were more likely to follow clinical guidelines (45.1% vs. 30.2%) while interventionists were more likely to follow standard clinical practice based on their assessment of evidence (38.7% vs. 27.0%) (p < 0.0001). No consistent strategy for selection was employed by 24.8% of interventionists and 17.4% of non-interventionists (Table 2, Q2).
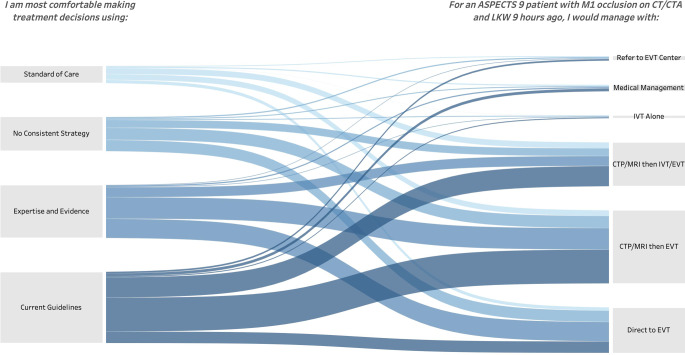
Respondents who indicated that they would base treatment on their assessment of evidence were more likely to recommend a direct to EVT approach for Q0 compared to those who indicated they would base treatment on clinical guidelines (36.56% vs. 16.16%; p < 0.0001); however, the method of decision making reported by the respondent did not directly map to the response to a real-world clinical scenario. At least one respondent in each decision-making group of Q2 selected each management option of Q0 (Fig. 1). Among the respondents who indicated they would follow practice guidelines, only 57.58% of interventionists and 44.58% of non-interventionists followed the AHA guidelines by recommending to proceed with CTP or MRI followed by EVT for Q0 (p < 0.0001).
Fig. 1.
Sankey flow diagram of clinician decision making
Advanced Imaging Availability and Timing
Advanced imaging was available and used routinely by 73.5% of interventionists and 69.3% of non-interventionists. There were 14.3% of interventionists and 13.9% of non-interventionists who responded that advanced imaging was possible irrespective of date and time but was not always immediately available (p = 0.23) (Table 2, Q3).
When advanced imaging is not available, interventionists were more likely to use CT alone for patient selection (83.6% vs. 63.6%) and less likely to select medical management only (2.7% vs. 10.0%) compared to non-interventionists (p < 0.0001). There was no difference between interventionists and non-interventionists in the length of delay they believed acceptable to obtain advanced imaging or in the delay it currently takes their center to obtain advanced imaging. The majority of both groups believed a delay of 20 min or less was acceptable to obtain advanced imaging. The majority of both groups reported it currently takes their center 20 min or less to obtain advanced imaging.
When asked their preferred imaging method for selection of reperfusion therapy in patients presenting in the late window, interventionists were more likely than non-interventionists to prefer CT/CTA alone (34.8% vs. 21.0%) and less likely to prefer CT/CTA/CTP (39.1% vs. 52.4%) (p < 0.0001) (Table 2, Q1); however, when asked about their practice for these patients, most interventionists and non-interventionists responded that they used advanced imaging in every case (63.5% vs. 63.6%; p = 0.35). Furthermore, only 7.3% of interventionists and 6.9% of non-interventionists would select patients for reperfusion therapy in the late window using CT/CTA alone (Table 2, Q4).
Discussion
Endovascular reperfusion has been proven as an effective therapy in the late window, but decisions around patient selection are still under debate [13]. We investigated how interventionist and non-interventionist colleagues make such decisions. We found that interventionists are less likely to prefer advanced imaging techniques in selecting patients for LVO presenting in the late window, but a similar majority of each group reported using advanced imaging in every case in their own practice. Advanced imaging is routinely available to most respondents, irrespective of specialty, and a delay of 20 min was deemed acceptable by the respondents. Interventionists were more likely to base their decisions on their expertise and assessment of evidence (e.g. observational studies or RCTs) rather than published guidelines.
Advanced imaging is recommended for patient selection for EVT in the late window by major society guidelines including the European Stroke Organization and the American Heart Association (AHA) [6, 7]. The CLEAR and other studies challenge these stringent parameters and indicate good outcomes can be achieved using CT without advanced imaging [13, 26, 27]. Compared to CT/CTA, advanced imaging represents increased costs, staffing, radiation dose, and image acquisition time. Nonetheless, advanced imaging was available 24/7 to more than 80% of respondents and used for every patient by 63%. Approximately half (52.7%) of interventionists preferred advanced imaging while one third (34.8%) preferred the use of CT/CTA, and the remaining interventionists made individualized decisions. Only one third of interventionists based their treatment decisions on clinical guidelines. This result indicates a strong adherence to stroke guidelines, despite many respondents indicating that their preferred patient selection method did not include advanced imaging and the minority of respondents stating that their treatment decisions are based upon published guidelines alone.
In the case of M1 occlusion wake-up stroke presenting 9 hours after last known well (LKW) with ASPECTS 9, interventionists were more likely to proceed directly to EVT than their non-interventionist colleagues. In a third of non-interventionist respondents, this divergence in management may be related to further selection of their patient for IVT prior to EVT in the wake of the extended window intravenous thrombolysis trial data [28]. While the bridging IVT/EVT trials for patients with LVO failed to demonstrate non-inferiority of EVT alone to IVT/EVT bridge in the early window (IVT < 4.5 h), it is unknown whether bridging thrombolysis should be performed or skipped for patients with LVO in the extended window [29, 30].
With regards to patients presenting with large core infarct, in the case of a M1 occlusion stroke presenting 7 hours after LKW with ASPECTS 4, there was no difference in responses between the specialty categories. There was a pattern toward interventionists choosing to proceed directly to EVT compared to non-interventionists. These results suggest hesitance among respondents to proceed to EVT in patients with low ASPECTS scores. The recent RESCUE-Japan LIMIT trial found a benefit over medical management for EVT in patients with a MRI-ASPECTS or CT-ASPECTS of 3–5 presenting up to 24h [3]. These results were summarized for survey respondents prior to the question involving a patient with an ASPECTS of 4. Approximately one quarter of both interventionists and non-interventionists stated they would randomize such a patient to an ongoing large infarct clinical trial [31–33], perhaps reflecting uncertainty whether these results were generalizable to their practice.
The results of the RESCUE-Japan LIMIT trial and the CLEAR study have not been incorporated into most guidelines. The responses seen in this survey may reflect strong adherence to current guidelines, despite respondents’ beliefs that their decisions are based upon clinical evidence or individualized decisions. Nonetheless, the results of this survey reflect gaps, especially among interventionists, between clinical guidelines, clinician beliefs and perceptions, and clinicians’ current practice. Given a rapidly evolving field, closing these gaps may necessitate more frequent guideline updates and a focus on conducting broadly inclusive trials [34].
As with any survey, the results of this study reflect opinions of providers and cannot be used to infer best practices in the treatment of patients. First, the behavior of clinicians willing to respond to a survey about practice patterns may be more aligned to guidelines than clinicians unwilling to respond, creating a response bias. Second, while this survey captured many responses from across the globe, clinicians from Africa, South America, Central America, and Oceania were underrepresented. Given that these regions are home to many low-income and middle-income nations where advanced imaging may present an increased burden [35] due to its high cost, the perceptions of these clinicians can inform the divergent preferences between non-interventionists and interventionists in the acute management of patients with large vessel occlusion stroke presenting in the extended time window. Third, there was no external validation. As a result, participants may have overestimated or underestimated the use of advanced imaging at their center and the time of the delay associated with advanced imaging. Finally, the results of the survey were presented prior to the presentation of the MR CLEAN LATE results in October 2022, demonstrating in patients with LVO presenting in the 6–24 h window, better outcomes were achieved in those selected by CT and CTA collaterals compared to medical management [36, 37]. The survey was also conducted prior to publication of the ANGEL-ASPECT and SELECT2 randomized trials demonstrating benefit of EVT in patients with large ischemic core infarct [4, 5].
Conclusion
In the evolving field of reperfusion therapies for acute ischemic stroke, interventionists were less likely to use advanced imaging techniques in the selection of patients for LVO presenting in the late window and more likely to base their decisions on their own expertise and assessment of evidence rather than published guidelines. These results reflect gaps between clinical guidelines, clinician beliefs and perceptions, and the current practice of interventionists. Closing these gaps may require a shift in age-old paradigms, and the acceptance of a growing body of evidence [38] supporting pragmatic criteria with lower cost and accessible technology (i.e., NCCT) in the selection of patients for EVT in the late window.
Supplementary Information
Checklist for survey reporting. Survey questions
Conflict of interest
S. Nagel: consultancy for Brainomix, speaker with Boehringer Ingelheim, Pfizer. T.S. Field: research grants from Bayer, consultancy for HLS Therapeutics, Roche. V. Puetz: lecturer Daiichi Sankyo. U. Fischer: research grants from Medtronic, Stryker, Rapid Medical, Penumbra, Phenox, Swiss Science Foundation, Swiss Heart Foundation; consultant Medtronic, Stryker, CSL Behring; advisory board for Alexion/Portola, Boehringer Ingelheim. H. Yamagami: research grants Bristol Myers Squibb, lecturer Bayer, Daiichi-Sankyo, Stryker, Bristol-Myers Squib; advisory Daiichi-Sankyo. S. Sacco: research grants Novartis and Uriach; advisor/speaker Abbott, Allergan-Abbvie, AstraZeneca, Lilly, Lundbeck, Novartis, Novo Nordisk, Pfizer, Teva. R. Mikulik: project IRENE COST Action-Implementation Research Network in Stroke Care Quality, INTER-EXCELLENCE INTER-COST program of Ministry of Education, Youth and Sports of Czech Republic. J.E. Siegler: speaker with AstraZeneca. R. Masiliūnas: IRENE COST Action-Implementation Research Network in Stroke Care Quality. J.P. Marto: consulting Amicus Therapeutics, Boehringer Ingelheim; Speaker Boehringer Ingelheim. J.D. Diestro: Honoraria from Medtronic, travel grant from Microvention. G. Thomalla: consultant Acandis, Alexion, Amarin, Bayer, Bristol Myers Squibb/Pfizer, Boehringer Ingelheim, Portola, Stryker. M. Parsons: research Siemens, Canon, Apollo Medical Imaging. R.G. Nogueira: consulting Anaconda, Biogen, Cerenovus, Genentech, Hybernia, Imperative Care, Medtronic, Phenox, Philips, Prolong Pharmaceuticals, Stryker Neurovascular, Shanghai Wallaby, Synchron; advisory Astrocyte, Brainomix, Cerebrotech, Ceretrieve, Corindus Vascular Robotics, Vesalio, Viz-AI, RapidPulse, Perfuze; investments in Viz-AI, Perfuze, Cerebrotech, Reist/Q’Apel Medical, Truvic, Viseon. G. Saposnik: research grants, consulting from Roche; Editor-in-chief, World Stroke Academy. T.N. Nguyen: research support Medtronic, SVIN. P. Klein, X. Huo, Y. Chen, M. Abdalkader, Z. Qiu, J. Raymond, L. Liu, J.E. Siegler, D. Strbian, S. Yaghi, M.M. Qureshi, J. Demeestere, A. Berberich, P. Michel, J. Kaesmacher, F. Alemseged, G. Tsivgoulis, W.J. Schonewille, W. Hu, X. Liu, C. Li, X. Ji, B. Drumm, S. Banerjee, E.C. Sandset, E.S. Kristoffersen, P. Slade, M. Romoli, F. Diana, K. Krishnan, P. Dhillon, E. Kasper, H. Dasenbrock, M.D. Ton, A.A. Arsovska, A.A. Dmytriw, R.W. Regenhardt, G.S. Silva, T. Siepmann, D. Sun, H. Sang, P. Yang, M.H. Mohammaden, F. Li, H.E. Masoud, A. Ma, Raynald, A. Ganesh, J. Liu, L. Meyer, D.W.J. Dippel, A.I. Qureshi, M. Goyal, A.J. Yoo, B. Lapergue, O.O. Zaidat, H.-S. Chen, B.C.V. Campbell, T.G. Jovin, and Z. Miao declare that they have no competing interests.
Footnotes
The authors Thanh N. Nguyen and Gustavo Saposnik contributed equally to the manuscript.
References
- 1.Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, Yavagal DR, Ribo M, Cognard C, Hanel RA, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378:11–21. doi: 10.1056/NEJMoa1706442. [DOI] [PubMed] [Google Scholar]
- 2.Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, McTaggart RA, Torbey MT, Kim-Tenser M, Leslie-Mazwi T, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378:708–718. doi: 10.1056/NEJMoa1713973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoshimura S, Sakai N, Yamagami H, Uchida K, Beppu M, Toyoda K, Matsumaru Y, Matsumoto Y, Kimura K, Takeuchi M, et al. Endovascular therapy for acute stroke with a large ischemic region. N Engl J Med. 2022;386:1303–1313. doi: 10.1056/NEJMoa2118191. [DOI] [PubMed] [Google Scholar]
- 4.Huo X, Ma G, Tong X, Zhang X, Pan Y, Nguyen TN, Yuan G, Han H, Chen W, Wei M, et al. Trial of endovascular therapy for acute ischemic stroke with large infarct. N Engl J Med. 2023 doi: 10.1056/NEJMoa2213379. [DOI] [PubMed] [Google Scholar]
- 5.Sarraj A, Hassan AE, Abraham MG, Ortega-Gutierrez S, Kasner SE, Hussain MS, Chen M, Blackburn S, Sitton CW, Churilov L, et al. Trial of endovascular thrombectomy for large ischemic strokes. n Engl J Med. 2023 doi: 10.1056/NEJMoa2214403. [DOI] [PubMed] [Google Scholar]
- 6.Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, et al. Guidelines for the early management of patients with acute Ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. 2019;50:e344–e418. doi: 10.1161/STR.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 7.Turc G, Bhogal P, Fischer U, Khatri P, Lobotesis K, Mazighi M, Schellinger PD, Toni D, de Vries J, White P, et al. European stroke organisation (ESO)—European society for minimally invasive neurological therapy (ESMINT) guidelines on mechanical thrombectomy in acute ischaemic strokeendorsed by stroke alliance for europe (SAFE) Eur Stroke J. 2019;4:6–12. doi: 10.1177/2396987319832140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eskey CJ, Meyers PM, Nguyen TN, Ansari SA, Jayaraman M, McDougall CG, DeMarco JK, Gray WA, Hess DC, Higashida RT, et al. Indications for the performance of intracranial endovascular neurointerventional procedures: a scientific statement from the American heart association. Circulation. 2018;137:e661–e689. doi: 10.1161/CIR.0000000000000567. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen TN, Castonguay AC, Siegler JE, Nagel S, Lansberg MG, de Havenon A, Sheth SA, Abdalkader M, Tsai J, Albers GW, et al. Mechanical thrombectomy in the late presentation of anterior circulation large vessel occlusion stroke: guideline recommendations from the society of vascular and Interventional neurology guidelines and practice standards (GAPS) committee. Stroke: Vasc Interv Neurol. 2023 doi: 10.1161/SVIN.122.000512. [DOI] [Google Scholar]
- 10.Roushdy T, Aref H, Kesraoui S, Temgoua M, Nono KP, Gebrewold MA, Peter W, Gopaul U, Belahsen MF, Ben-Adji D, et al. Stroke services in Africa: what is there and what is needed. Int J Stroke. 2022;17(9):972–982. doi: 10.1177/17474930211066416. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen TN, Raymond J, Nogueira RG, Fischer U, Siegler JE. The problem of restrictive thrombectomy trial eligibility criteria. Stroke. 2022;53:2988–2990. doi: 10.1161/STROKEAHA.122.040006. [DOI] [PubMed] [Google Scholar]
- 12.Abdalkader M, Siegler JE, Lee JS, Yaghi S, Qiu Z, Huo X, Miao Z, Campbell BCV, Nguyen TN. Neuroimaging of acute ischemic stroke: multimodal imaging approach for acute endovascular therapy. J Stroke. 2023;25:55–71. doi: 10.5853/jos.2022.03286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguyen TN, Abdalkader M, Nagel S, Qureshi MM, Ribo M, Caparros F, Haussen DC, Mohammaden MH, Sheth SA, Ortega-Gutierrez S, et al. Noncontrast computed tomography vs computed tomography perfusion or magnetic resonance imaging selection in late presentation of stroke with large-vessel occlusion. JAMA Neurol. 2022;79:22–31. doi: 10.1001/jamaneurol.2021.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seker F, Qureshi MM, Möhlenbruch MA, Nogueira RG, Abdalkader M, Ribo M, Caparros F, Haussen DC, Mohammaden MH, Sheth SA, et al. Reperfusion without functional independence in late presentation of stroke with large vessel occlusion. Stroke. 2022;53:3594–3604. doi: 10.1161/STROKEAHA.122.039476. [DOI] [PubMed] [Google Scholar]
- 15.Sheth KN, Terry JB, Nogueira RG, Horev A, Nguyen TN, Fong AK, Gandhi D, Prabhakharan S, et al. Advanced modality imaging evaluation in acute ischemic stroke may lead to delayed endovascular reperfusion therapy without improvement in clinical outcomes. J NeuroIntervent Surg. 2013;5:62–65. doi: 10.1136/neurintsurg-2012-010512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun Chung-Huan J, Ribo M, Goyal M, Yoo AJ, Jovin T, Cronin CA, Zaidat O, Nogueira R, Nguyen TN, Hussain S, et al. Door-to-puncture: a practical metric for capturing and enhancing system processes associated with endovascular stroke care, preliminary results from the rapid reperfusion registry. J Am Heart Assoc. 2014;3:e000859. doi: 10.1161/JAHA.114.000859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siegler JE, Ortega-Gutierrez S, Hester T, Haussen DC, Nogueira RG, Liebeskind DS, Zaidat OO, Vora N, Desai S, Jadhav AP, et al. Interaction of ethnicity and arrival method on thrombectomy delay: the society of vascular and interventional neurology collaboration. Stroke Vasc Interv Neurol. 2022;2:e000217. [Google Scholar]
- 18.Raymaekers V, Demeestere J, Bellante F, De Blauwe S, De Raedt S, Dusart A, Jodaitis L, Lemmens R, Loos C, Noémie L, et al. The impact of COVID-19 on acute stroke care in Belgium. Acta Neurol Belg. 2021;121:1251–1258. doi: 10.1007/s13760-021-01726-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siegler JE, Zha AM, Czap AL, Ortega-Gutierrez S, Farooqui M, Liebeskind DS, Desai SM, Hassan AE, Starosciak AK, Linfante I, et al. Influence of the COVID-19 pandemic on treatment times for acute Ischemic stroke: the society of vascular and Interventional neurology multicenter collaboration. Stroke. 2021;52:40–47. doi: 10.1161/STROKEAHA.120.032789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fischer U, Branca M, Bonati LH, Carrera E, Vargas MI, Platon A, Kulcsar Z, Wegener S, Luft A, Seiffge DJ, et al. Magnetic resonance imaging or computed tomography for suspected acute stroke: association of admission image modality with acute recanalization therapies, workflow metrics, and outcomes. Ann Neurol. 2022;92:184–194. doi: 10.1002/ana.26413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohammaden MH, Doheim MF, Elfil M, Al-Bayati AR, Pinheiro A, Nguyen TN, Bhatt NR, Haussen DC, Nogueira RG. Direct to angiosuite versus conventional imaging in suspected large vessel occlusion: a systemic review and meta-analysis. Stroke. 2022;53:2478–2487. doi: 10.1161/STROKEAHA.121.038221. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen TN, Abdalkader M, Jovin TG, Nogueira RG, Jadhav AP, Haussen DC, Hassan AE, Novakovic R, Sheth SA, Ortega-Gutierrez S, et al. Mechanical thrombectomy in the era of the COVID-19 pandemic: emergency preparedness for neuroscience teams: a guidance statement from the society of vascular and interventional neurology. Stroke. 2020;51:1896–1901. doi: 10.1161/STROKEAHA.120.030100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drumm B, Banerjee S, Qureshi MM, Schonewille WJ, Klein P, Huo X, Chen Y, Strbian D, Fischer U, Puetz V, et al. Current opinions on optimal management of basilar artery occlusion: after the BEST of BASICS survey. Stroke Vasc Interv Neurol. 2022;2:e000538. [Google Scholar]
- 24.Edwards C, Drumm B, Siegler JE, Schonewille WJ, Klein P, Huo X, Chen Y, Abdalkader M, Qureshi MM, Strbian D, et al. Basilar artery occlusion management: specialist perspectives from an international survey. J Neuroimaging. 2023 doi: 10.1111/jon.13084. [DOI] [PubMed] [Google Scholar]
- 25.Nguyen TN, Klein P, Berberich A, Nagel S, Abdalkader M, Herning A, Chen Y, Huo X, Miao Z, Sheth SA, et al. Late window imaging selection for endovascular therapy of large vessel occlusion stroke: an international survey. Stroke Vasc Interv Neurol. 2023 doi: 10.1161/SVIN.122.000595. [DOI] [Google Scholar]
- 26.Nagel S, Herweh C, Pfaff JAR, Schieber S, Schönenberger S, Möhlenbruch MA, Bendszus M, Ringleb PA. Simplified selection criteria for patients with longer or unknown time to treatment predict good outcome after mechanical thrombectomy. J Neurointerv Surg. 2019;11:559–562. doi: 10.1136/neurintsurg-2018-014347. [DOI] [PubMed] [Google Scholar]
- 27.Jovin TG, Nogueira RG, Lansberg MG, Demchuk AM, Martins SO, Mocco J, Ribo M, Jadhav AP, Ortega-Gutierrez S, Hill MD, et al. Thrombectomy for anterior circulation stroke beyond 6 h from time last known well (AURORA): a systematic review and individual patient data meta-analysis. Lancet. 2022;399:249–258. doi: 10.1016/S0140-6736(21)01341-6. [DOI] [PubMed] [Google Scholar]
- 28.Thomalla G, Simonsen CZ, Boutitie F, Andersen G, Berthezene Y, Cheng B, Cheripelli B, Cho T-H, Fazekas F, Fiehler J, et al. MRI-guided thrombolysis for stroke with unknown time of onset. N Engl J Med. 2018;379:611–622. doi: 10.1056/NEJMoa1804355. [DOI] [PubMed] [Google Scholar]
- 29.Masoud H, Havenon A, Castonguay AC, Asif KS, Nguyen TN, Mehta B, Abdalkader M, Ortega-Gutierrez S, Leslie-Mazwi TM, Mansour OY, et al. Brief practice update on intravenous thrombolysis before thrombectomy in patients with large vessel occlusion acute ischemic stroke. Stroke Vasc Interv Neurol. 2021;2022(2):e000276. doi: 10.1161/SVIN.121.000276. [DOI] [Google Scholar]
- 30.Fischer U, Kaesmacher J, Strbian D, Eker O, Cognard C, Plattner PS, Bütikofer L, Mordasini P, Deppeler S, Pereira VM, et al. Thrombectomy alone versus intravenous alteplase plus thrombectomy in patients with stroke: an open-label, blinded-outcome, randomised non-inferiority trial. Lancet. 2022;400:104–115. doi: 10.1016/S0140-6736(22)00537-2. [DOI] [PubMed] [Google Scholar]
- 31.Huo X, Ma G, Zhang X, Pan Y, Tong X, Sun D, Liu L, Wang Y, Liebeskind DS, Wang Y, et al. Endovascular therapy in acute anterior circulation large vessel occlusive patients with a large infarct core (ANGEL-ASPECT): protocol of a multicentre randomised trial. Stroke Vasc Neurol. 2022 doi: 10.1136/svn-2022-001865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campbell BCV, Nguyen TN. Advances in stroke: treatments-interventional. Stroke. 2022;53:264–267. doi: 10.1161/STROKEAHA.121.037039. [DOI] [PubMed] [Google Scholar]
- 33.Sarraj A, Hassan AE, Abraham M, Ribo M, Blackburn S, Chen M, Hussain MS, Pereira VM, Ortega-Gutierrez S, Sitton C, et al. A randomized controlled trial to optimize patient’s selection for endovascular treatment in acute ischemic stroke (SELECT2): study protocol. Int J Stroke. 2022;17:689–693. doi: 10.1177/17474930211035032. [DOI] [PubMed] [Google Scholar]
- 34.Castonguay AC, de Havenon A, Leslie-Mazwi TM, Kenmuir C, Sheth SA, Asif KS, Nguyen TN, Janardhan V, Mansour OY, Novakovic R, et al. Society of vascular and Interventional neurology standards and parameters for guideline development and publication. Stroke Vasc Interv Neurol. 2021;1:e000112. doi: 10.1161/SVIN.121.000112. [DOI] [Google Scholar]
- 35.Mai DT, Dao XC, Luong NK, Nguyen TK, Nguyen HT, Nguyen TN. Current state of stroke care in Vietnam. Stroke Vasc Interv Neurol. 2022;2:e000331. [Google Scholar]
- 36.Olthuis SGH, Pirson FAV, Pinckaers FME, Hinsenveld WH, Nieboer D, Ceulemans A, Knapen RRM, Quirien Robbe MM, Berkhemer OA, van Walderveen MAA, et al. Endovascular treatment versus no endovascular treatment after 6–24 h in patients with ischaemic stroke and collateral flow on CT angiography (MR CLEAN-LATE) in the Netherlands: a multicentre, open-label, blinded-endpoint, randomised, controlled, phase 3 trial. Lancet. 2023. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(23)00575-5/fulltext [DOI] [PubMed]
- 37.Campbell BCV, Hill MD, Nguyen TN, Broderick JP. Acute and interventional treatments. Stroke. 2023;54:591–594. doi: 10.1161/STROKEAHA.122.041254. [DOI] [PubMed] [Google Scholar]
- 38.Siegler JE, Qureshi MM, Nogueira RG, Tanaka K, Nagel S, Michel P, Vigilante N, Ribo M, Yamagami H, Yoshimura S, et al. Endovascular vs medical management for late anterior large vessel occlusion with prestroke disability: analysis of CLEAR and RESCUE-Japan. Neurology. 2022 doi: 10.1212/WNL.0000000000201543. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Checklist for survey reporting. Survey questions