Abstract
Background
Among patients with heart failure (HF), patient-reported health status provides information beyond standard clinician assessment. Although HF management guidelines recommend collecting patient-reported health status as part of routine care, there is minimal data on the impact of this intervention.
Study Design
The Patient-Reported Outcomes in Heart Failure Clinic (PRO-HF) trial is a pragmatic, randomized, implementation-effectiveness trial testing the hypothesis that routine health status assessment via the Kansas City Cardiomyopathy Questionnaire-12 (KCCQ-12) leads to an improvement in patient-reported health status among patients treated in a tertiary health system HF clinic. PRO-HF has completed randomization of 1,248 participants to routine KCCQ-12 assessment or usual care. Patients randomized to the KCCQ-12 arm complete KCCQ-12 assessments before each HF clinic visit with the results shared with their treating clinician. Clinicians received education regarding the interpretation and potential utility of the KCCQ-12. The primary endpoint is the change in KCCQ-12 over 1 year. Secondary outcomes are HF therapy patterns and healthcare utilization, including clinic visits, testing, hospitalizations, and emergency department (ED) visits. As a sub-study, PRO-HF also evaluated the impact of routine KCCQ-12 assessment on patient experience and the accuracy of clinician-assessed health status. In addition, clinicians completed semi-structured interviews to capture their perceptions on the trial’s implementation of routine KCCQ-12 assessment in clinical practice.
Conclusions
PRO-HF is a pragmatic, randomized trial based in a real-world HF clinic to determine the feasibility of routinely assessing patient-reported health status and the impact of this intervention on health status, care delivery, patient experience, and the accuracy of clinician health status assessment.
Keywords: heart failure, quality of care, quality of life, health services research
Introduction
Heart failure (HF) can dramatically impact patient quality of life.1 Clinician assessment of HF symptom burden is frequently discordant with health status reported by patients.2–5 Patient-reported outcome (PRO) measures can more accurately detect clinically meaningful changes in HF health status as compared to routine clinical assessment using New York Heart Association (NYHA) classification. As such, there has been increasing interest in expanding PROs from their traditional role in clinical trials to routine clinical practice.6–9 The 2022 American College of Cardiology/American Heart Association Heart Failure Clinical Practice Guidelines included a 2A recommendation to incorporate patient-reported health status into routine HF care.10 In addition, the Centers for Medicare & Medicaid Services now include the collection of HF patient-reported health status as a quality measure.11
Despite the significant evidence supporting the validity and potential utility of PROs in HF care, the feasibility and impact of routine PRO assessment among HF patients is unknown. There are no prior studies evaluating the effect of collecting PROs on the quality of HF care. In addition, gaps remain in our understanding of how clinician engagement leads to successful PRO implementation and integration. This is critical as patients are less willing to complete PROs if they are not actively incorporated into clinical care.12
Understanding the impact of PRO assessment on HF patient outcomes is necessary to determine the value of routinely collecting this data and to inform the extent to which health systems, clinical societies, and government agencies should invest in promoting widespread adoption of PROs in HF care. In addition, identifying factors that lead to successful PRO implementation will guide future interventions. We sought to determine the impact of routine collection of PROs from adult outpatients treated in HF clinic on patient-reported health status, therapy patterns, care utilization, patient experience, and the accuracy of clinician assessment of health status. We also sought to evaluate clinician perceptions regarding PRO implementation in a HF clinic.
The PRO-HF Trial
The Patient-Reported Outcome Measurement in Heart Failure Clinic (PRO-HF) trial (NCT04164004) is a pragmatic, single-center, randomized controlled trial to evaluate the routine assessment of patient-reported health status among adults treated in HF clinic utilizing the Kansas City Cardiomyopathy Questionnaire-12 (KCCQ-12), a validated measure of patient-reported HF health status (Figure 1).3 The study protocol is available on ClinicalTrials.gov.13 The study is approved by the Stanford Institutional Review Board (Protocol # 58104) and is consistent with the Declaration of Helsinki. Trial enrollment started on August 30, 2021, and was completed on June 30, 2022. The study is funded by the National Heart, Lung, and Blood Institute (1K23HL151672–01). An independent safety monitor is notified of any safety concerns and adverse events related to KCCQ-12 assessment. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper, and its final contents.
Figure 1.
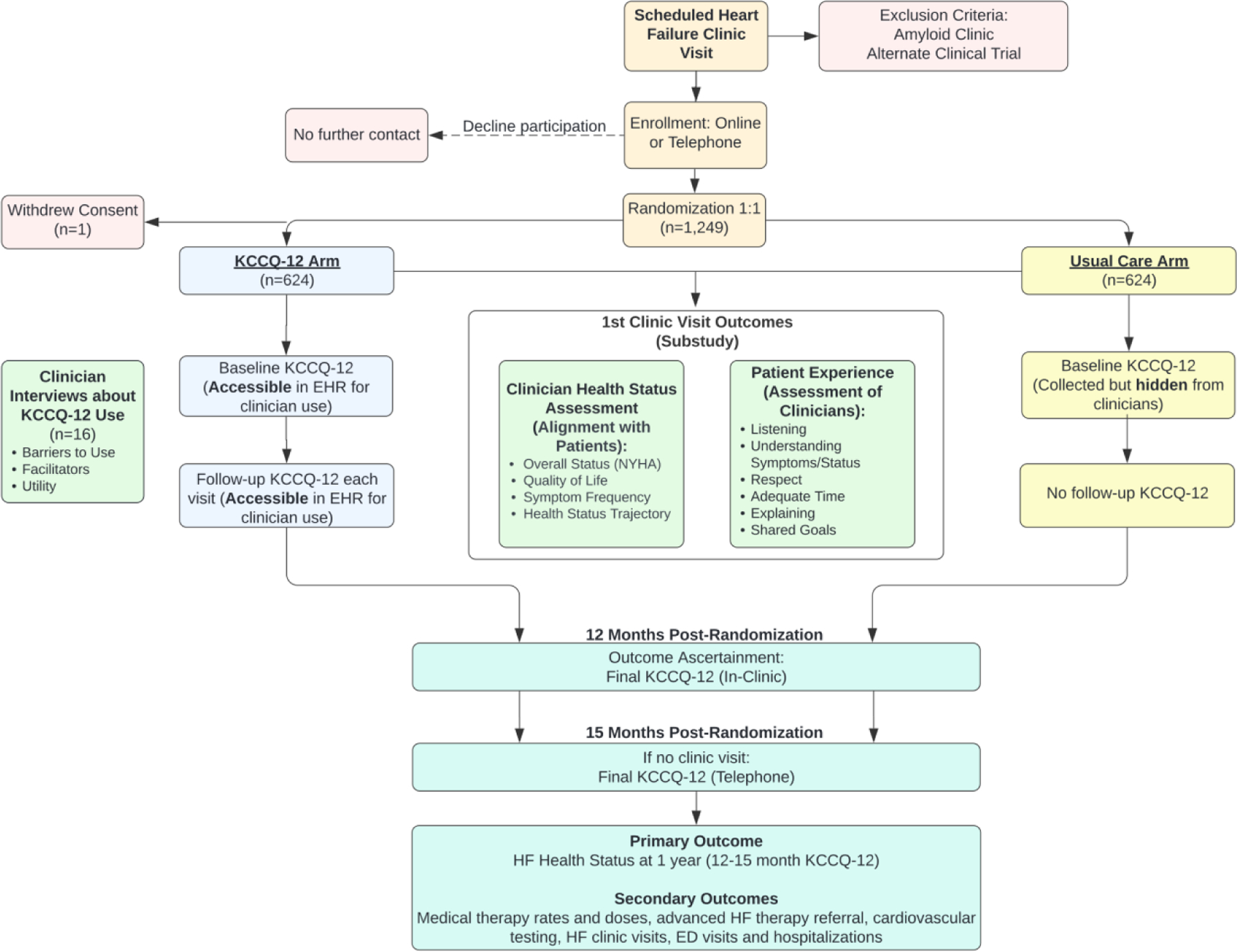
Study Flow Diagram
This figure displays the overall study design. The diagram lists the outcomes ascertained in the PRO-HF trial, including the sub-study that evaluates clinician health status assessment and patient experience following the first visit post-randomization, the semi-structured clinician interviews, and the final study outcomes evaluated 12–15 months after randomization.
Study Population
The trial recruited patients with scheduled visits (in-person and telemedicine) in the Stanford Health Care (SHC) adult heart failure clinic. Patients were included regardless of the presence or absence of a HF diagnosis. Patients were excluded at the discretion of their treating cardiologist if they were enrolled in an alternate clinical trial with a KCCQ-based outcome or if they were being seen in the amyloid clinic since these patients were eligible for other actively recruiting trials that included the KCCQ among the outcomes.
Enrollment and Randomization
Eligible patients were identified and contacted via automated emails after linking the Epic (Epic Systems, Verona, Wisconsin, USA) electronic health record (EHR) to the REDCap electronic data capture tool (Figure 2).14 We sent secure emails to potential trial participants 7–10 days before their upcoming clinic visit. The email included a link to an online consent form with information on the trial and contact information for further inquiries. Patients could either consent to enroll in the trial or decline, in which case they received no further contact from the study team. Patients who did not respond to the email were contacted by text message and then via phone call from a study team member 3–5 days before their clinic visit.
Figure 2.
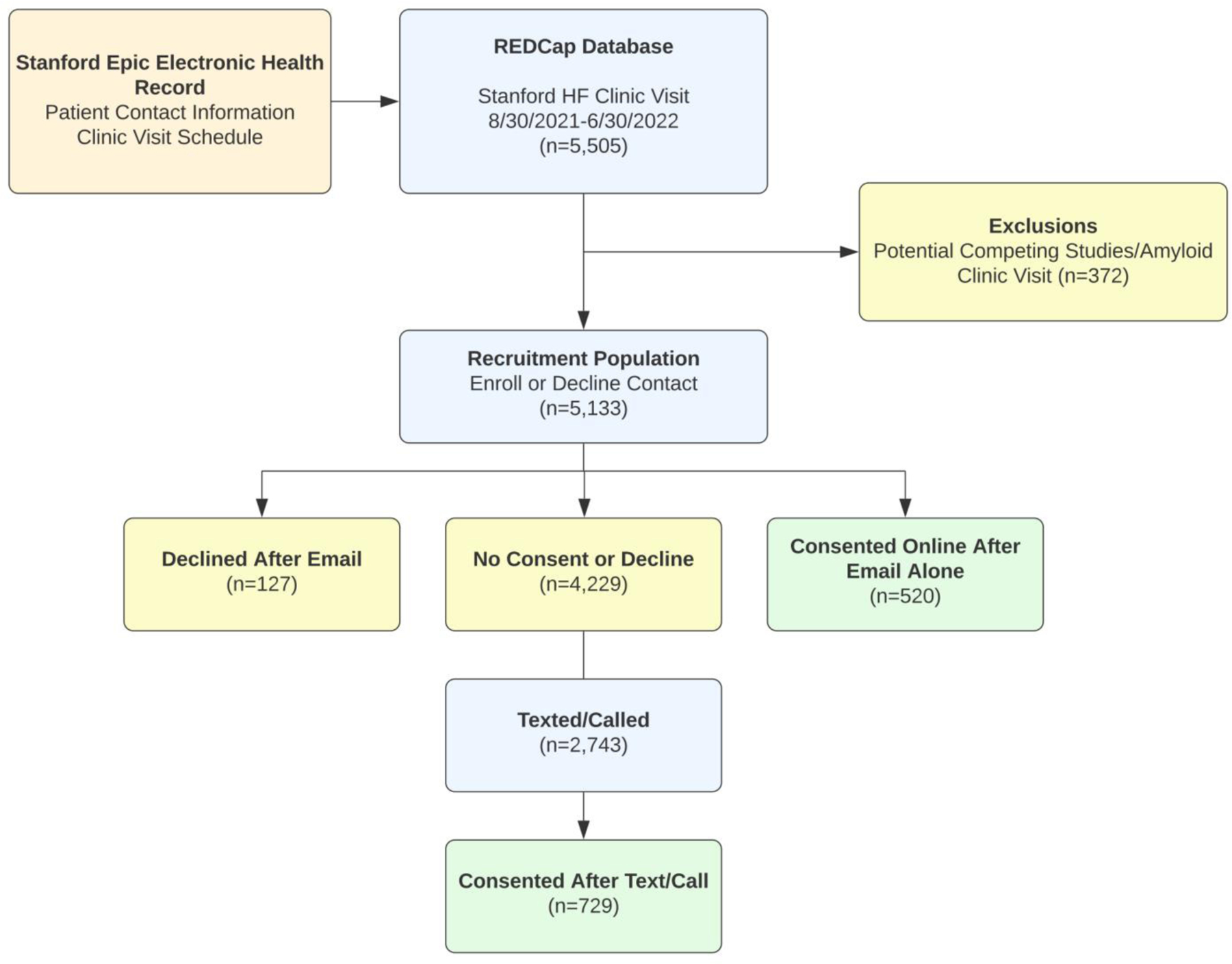
Enrollment Process
This figure displays the enrollment process. This includes both automated emails and text messages to the recruitment population and telephone calls from the study team.
Of 5,133 eligible patients, 1,362 (24.7%) declined enrollment and 1,249 patients (24.3%) consented to the trial. There were 520 patients who consented using the online link via email or automated text message. Among the 2,743 patients contacted manually by research staff via telephone or text, 729 (26.6%) were enrolled. One patient withdrew consent. Table 1 displays the characteristics of the enrolled patients.
Table 1.
Patient Characteristics1
| Total N=1,248 | |
|---|---|
| Demographics | |
| Age, years | 63.9 (51.8–72.8) |
| Female Sex | 485 (38.9%) |
| Race | |
| American Indian or Alaska Native | 7 (0.6%) |
| Asian | 143 (11.5%) |
| Black | 57 (4.6%) |
| Pacific Islander | 15 (1.2%) |
| Unknown | 216 (17.3%) |
| White | 810 (64.9%) |
| Ethnicity | |
| Hispanic/Latino | 101 (8.1%) |
| Non-Hispanic | 1,077 (86.3%) |
| Unknown | 70 (5.6%) |
|
| |
| Baseline HF Characteristics 2 | |
| Heart Failure or Cardiomyopathy Diagnosis | 1,089 (87.3%) |
| Prior HF Clinic Encounter | 1,046 (83.8%) |
| Left ventricular ejection fraction, % | 52 (39–60) |
| Left ventricular ejection fraction ≤40% | 348 (27.9%) |
| Left ventricular ejection fraction 41–50% | 230 (18.4%) |
| Left ventricular ejection fraction >50% | 667 (53.4%) |
| Missing left ventricular ejection fraction | 3 (0.2%) |
| KCCQ-12 Overall Summary Score | 82 (58–95) |
| KCCQ-12 Physical Limitation Score | 83 (58–100) |
| KCCQ-12 Symptom Frequency Score | 88 (67–100) |
| KCCQ-12 Quality of Life Score | 75 (50–100) |
| KCCQ-12 Social Limitations Score | 83 (58–100) |
|
| |
| Comorbidities, % 2 | |
| Atrial Fibrillation/Atrial Flutter | 437 (35.0%) |
| Coronary Artery Disease | 500 (40.1%) |
| Cancer | 169 (13.5%) |
| Chronic Kidney Disease | 276 (22.1%) |
| Chronic Obstructive Pulmonary Disease | 176 (14.1%) |
| Depression | 159 (12.7%) |
| Diabetes Mellitus | 235 (18.8%) |
| Hypertension | 658 (52.7%) |
| Peripheral Vascular Disease | 481 (38.5%) |
|
| |
| Vitals and Labs 3 | |
| Systolic Blood Pressure, mmHg | 120 (109–132) |
| Diastolic Blood Pressure, mmHg | 69 (61–77) |
| Heart Rate, bpm | 72.0 (64.0–82.0) |
| Body Mass Index, kg/m2 | 27 (24–31) |
| Creatinine, mmol/L | 1.0 (0.8–1.2) |
| Potassium, mEq/dL | 4.4 (4.1–4.7) |
| Sodium, mmol/L | 139 (137–140) |
|
| |
| Baseline Medication Therapies, % | |
| ACEI/ARB/ARNI | 499 (40.0%) |
| Beta-blocker | 823 (65.9%) |
| Loop Diuretics | 352 (28.2%) |
| MRA | 472 (37.8%) |
| SGLT2I | 115 (9.2%) |
Abbreviations: ACEI: angiotensin-converting-enzyme inhibitor; ARB: angiotensin receptor blocker; ARNI: angiotensin receptor-neprilysin inhibitor; MRA: mineralocorticoid receptor antagonist; SGLT2I: sodium/glucose cotransporter-2 inhibitor.
Continuous variables displayed as median (interquartile range); binary variables displayed as outcome (percentage).
Comorbidities based on electronic health record diagnoses within 2 years preceding index visit. Heart failure diagnosis based on electronic health record diagnosis and abstraction of first clinic visit post-randomization.
Vitals and laboratory values based on most recent values within 2 years preceding or including the index visit.
Consented patients were randomized using a secure online randomization module through REDCap. Randomization was stratified by the primary treating clinician at the first post-randomization clinic visit with block sizes of 2 and 4. Randomization occurred immediately on the online platform after a patient consented to participate in the trial.
Enrolled patients were treated by 17 clinicians in this HF clinic, who were consented as study participants (13 advanced HF physicians and 4 advanced practice providers).
Intervention
The Kansas City Cardiomyopathy Questionnaire summarizes the impact of HF on patients’ symptoms, function, and quality of life.15 It is the most widely used PRO measure in HF care. The KCCQ-12 is a shorter version of 12 questions developed for use in routine clinical care.16 The KCCQ-12 has four domain scores: physical limitations, symptom frequency, quality of life, and social limitations. The overall summary score (KCCQ-OSS) is the average of these four scores. Each score ranges from 0–100.
Patients were randomized to the KCCQ-12 or usual care arms. Participants randomized to the KCCQ-12 arm are asked to complete the KCCQ-12 prior to their initial clinic visit following trial enrollment and prior to each subsequent visit. These patients complete the KCCQ-12 via the EHR patient portal application as part of the pre-visit check-in process 3 days before their visit. Patients who do not complete the KCCQ-12 by the day preceding their clinic visit receive a reminder notification through the patient portal to complete the questionnaire. Patients who do not complete the KCCQ-12 pre-visit can also complete it during in-person visit check-in.
For patients in the KCCQ-12 arm, the KCCQ-12 results were provided for treating clinicians as physical printouts at the time of the first clinic visit. The KCCQ-12 results are also available in the EHR (Figure 3). The EHR includes graphical displays that overlay labs and vitals with the KCCQ-12 results and allow the results to be incorporated into clinical notes. Because patients in the KCCQ-12 arm complete the assessment with each visit, clinicians can track the change in KCCQ-OSS over time.
Figure 3.
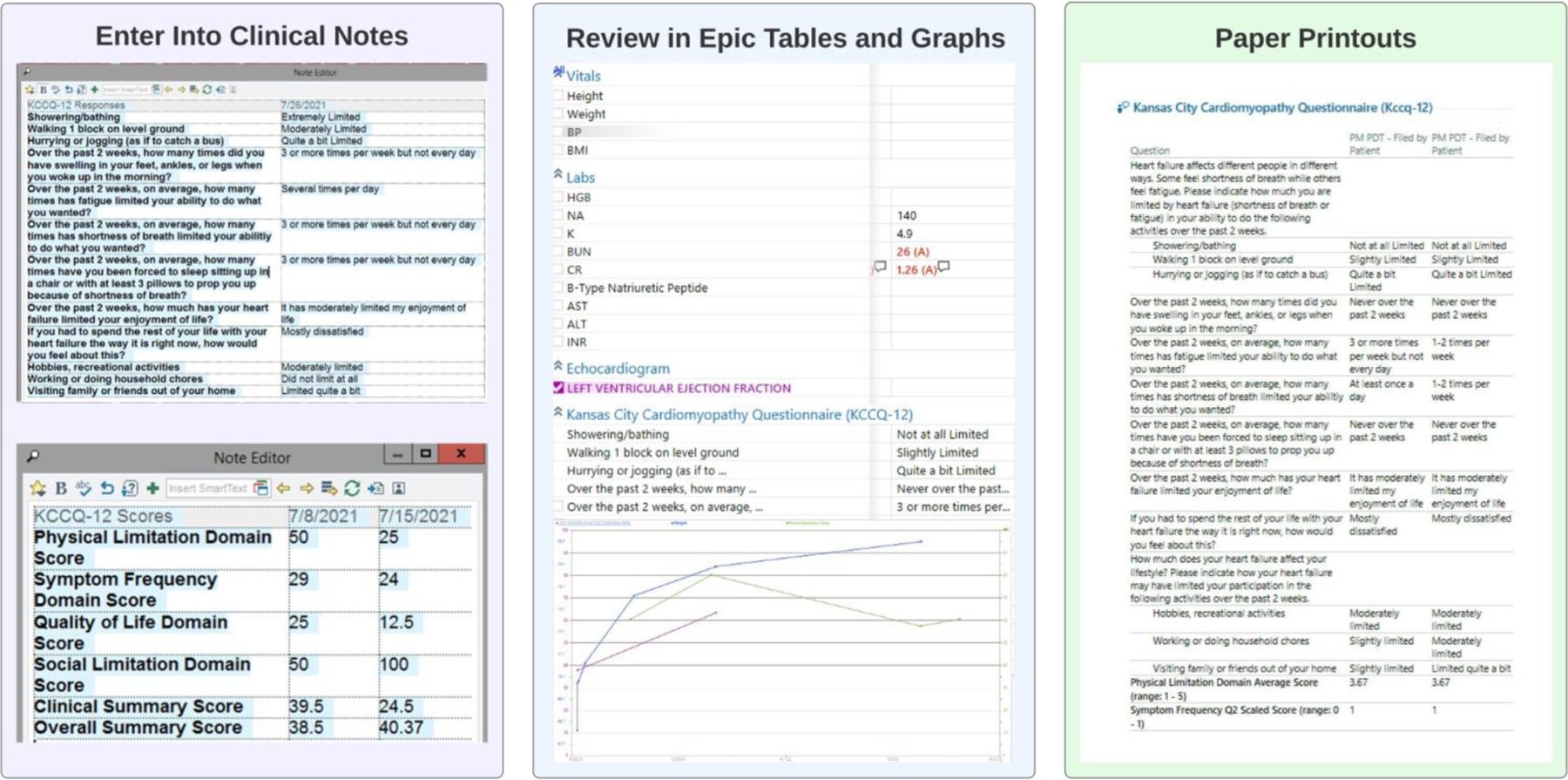
KCCQ-12 Visualization for Clinicians
This figure shows how clinicians can visualize the KCCQ-12 results for patients in the KCCQ-12 arm. First, they can enter the KCCQ-12 results (current and historical) into their clinic note using automated smartphrases. Second, they can review tables in the EHR showing results over time along with other clinical data, including vitals, laboratory values, and left ventricular ejection fraction. Finally, clinicians were provided a paper printout of the results during the initial enrollment period.
Patients in the usual care arm underwent baseline KCCQ-12 assessment prior to their first clinic visit following enrollment. They received the KCCQ-12 via an online form. Those enrolled by telephone had the opportunity to complete the KCCQ-12 during the call. KCCQ-12 results for these patients are not provided to their treating clinician or made available in the EHR. These patients do not undergo any further KCCQ-12 assessment until the end of the trial.
In both arms, participants with missing KCCQ-12 results the day before their initial visit received a phone call from the study team to assist with questionnaire completion. While completion rates of the KCCQ-12 in real-world implementations will likely be lower, we took this approach to ensure KCCQ-12 data will be available to better test the efficacy of KCCQ-12 assessment. Clinicians in both arms were allowed to make all diagnostic and treatment decisions at their discretion.
Blinding for clinicians was not possible since the intervention being tested was to provide clinicians with the KCCQ-12 results. Patients are also not blinded; however, patients in both arms are asked to complete the KCCQ-12 at trial enrollment and conclusion.
Provider Engagement
For patient-reported health status measures to be beneficial in clinical care, clinicians need to be able to interpret and apply these measures. All HF clinicians and clinic staff received training and implementation support. The study team leveraged multiple strategies to disseminate education regarding the KCCQ-12. The PRO-HF team presented data on the importance and interpretation of the KCCQ-12 at multiple educational seminars and meetings, including local cardiology grand rounds, a HF lecture series, faculty meetings, and clinic staff meetings. The trial PI (AS) met individually with each clinician before commencement of the trial to answer questions regarding the KCCQ-12, demonstrate how to access the results in the EHR, and distribute educational materials. In addition, the PI reached out to clinicians on the first day they saw an enrolled participant and again during the first 3 months of the study. The study team posted KCCQ-12 infographics throughout the clinic as a reference (Figure 4). Finally, the PRO-HF team worked closely with the clinic staff to provide resources and develop protocols on how to integrate the KCCQ-12 into clinic workflows.
Figure 4.
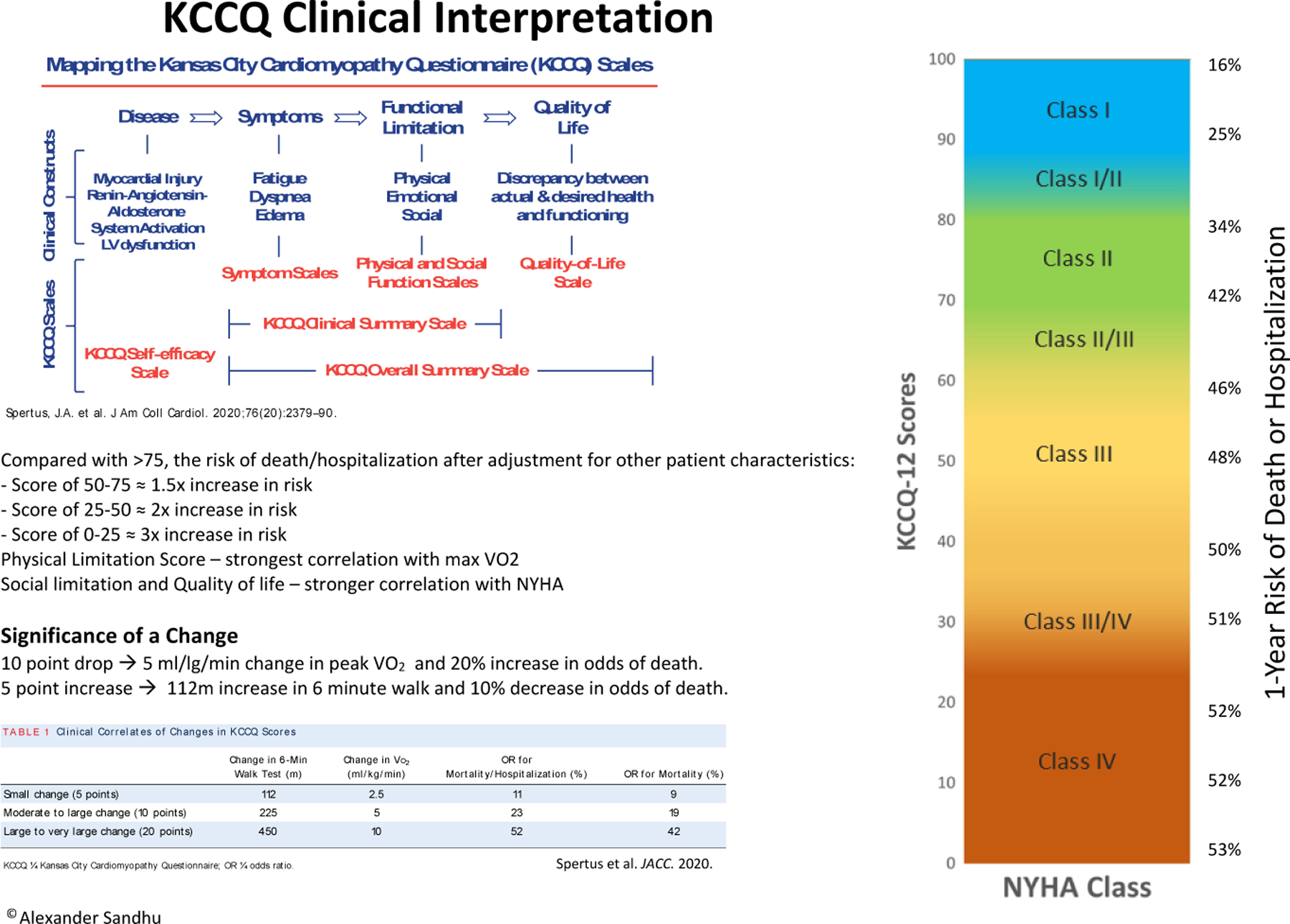
KCCQ-12 Interpretation Flyer
This figure displays a flyer that was posted in workrooms in the Stanford HF clinic as a reminder to clinicians and clinic staff regarding the interpretation and utility of the KCCQ-12.
Outcomes
The primary outcome of the PRO-HF trial is the change in KCCQ-OSS after 1 year of follow-up. This primary outcome was selected because it was hypothesized that the greatest effect of routinely collecting patient-reported health status and sharing this information with clinicians would be to improve patient health status itself, which is most accurately measured through the KCCQ-12. In addition, due to the single-center design and short duration of this trial, it was not powered to detect changes in other outcomes, such as mortality or HF admissions.
In both treatment arms, patients will be prompted to complete the KCCQ-12 for any clinic visit at least 1-year post-randomization via the EHR patient portal. Patients who do not complete the KCCQ-12 within 15 months post-randomization will be contacted to complete the KCCQ-12.
Secondary outcomes of the PRO-HF trial include HF therapy rates, testing rates, and healthcare resource utilization. Therapy rates will include the use of guideline-recommended medication therapies based on left ventricular ejection fraction at enrollment, cardiac procedures (e.g., cardiac defibrillator implantation, cardiac resynchronization therapy, or valvular intervention), advanced HF therapy evaluation, and referral to other specialties (e.g., palliative care and psychiatry). Testing rates will include cardiovascular imaging, rhythm monitoring, right heart catheterization, and invasive coronary angiography. Resource utilization will include outpatient visit frequency, emergency department visits, and hospitalizations for HF in addition to all-cause hospitalizations. Each of these outcomes will be derived from the EHR with telephone ascertainment as needed for missing data. Patients will also be surveyed at the end of the trial regarding hospitalizations and ED visits outside of the SHC system.
Sub-Study of Patient Experience and Accuracy of Clinician Health Status Assessment
PRO-HF includes a sub-study evaluating patient experience and the accuracy of clinician health status assessment among a subset of trial participants using patient and clinician surveys. We planned to enroll half of trial participants based on the month of their first post-randomization clinic visit. We excluded patients enrolled in the first month (August 31, 2021, to September 30, 2021), so clinicians could gain experience incorporating the KCCQ-12 into their workflows prior to assessment. We initially planned to enroll half of patients in alternating months in the sub-study. During the second month of enrollment (first month of the sub-study), we revised the protocol to include all subsequent patients in the sub-study. This revision was based on increased research team capacity.
We assessed patient experience using a 10-question survey (Supplement Figure 1) that was emailed to participants after their first clinic visit post-randomization. Patients who did not respond to the survey via email were contacted by text message and phone call. The survey included a Likert scale with 5 levels of agreement (ranging from “strongly agree” to “strongly disagree”) for statements related to their interaction with their clinician. Specifically, the survey evaluated their perception of their clinician’s understanding of their health status and quality of communication and their overall agreement regarding their treatment plan. The survey questions were based on existing ambulatory patient experience surveys with a focus on perceptions that may be influenced by patient-reported health status assessment.17 We also asked patients about the factors that have the greatest impact on their quality of life.
To evaluate the accuracy of clinician health status assessment, we provided an 8-question paper survey to the treating clinicians following the initial clinic visit (Supplement Figure 2). Clinicians who did not complete the paper survey were sent the survey via email. The survey solicited clinician perceptions of their patients’ NYHA class, quality of life, symptom frequency for each of the 4 HF symptoms assessed by the KCCQ-12, health status trajectory, and the primary condition influencing their quality of life. We categorized each response as “concordant,” “discordant,” or “intermediate” based on comparison with patient reporting. Supplement Table 1 details these assignments. While determining concordance between NYHA class and KCCQ-OSS has inherent challenges given the imprecision of NYHA classification, we used previously established estimates.16 For example, we considered NYHA class I concordant with a KCCQ-OSS of ≥80, discordant with a KCCQ-OSS of <70, and intermediate for KCCQ-OSS between 70 and <80.
Semi-Structured Clinician Interviews on KCCQ-12 Implementation
We conducted semi-structured interviews with HF clinicians to better understand their perspectives regarding KCCQ-12 implementation. These interviews focused on clinician perceptions on the value of routinely assessing the KCCQ-12 as part of clinical care, how they utilize KCCQ-12 data, and barriers they experienced in implementing the KCCQ-12 into their practice.
At SHC, 13 HF clinicians agreed to be interviewed. We also recruited 3 clinicians from 3 sites outside SHC that use the KCCQ-12 in clinical practice to assess other potential approaches to KCCQ-12 implementation and determine the generalizability of conclusions drawn. We conducted 30-minute interviews with each clinician between April 2022 and May 2022 after clinicians in the PRO-HF trial had been using the KCCQ-12 for over 7 months. Interviews were conducted by a trained qualitative researcher (AA). The interview protocol was designed by the study team using previously described focus group questions, the Consolidated Framework for Implementation Research 2.0, and design thinking approaches.18,19
Statistical Analysis
The primary analysis compares the KCCQ-OSS at 1 year across study arms with adjustment for baseline KCCQ-OSS using a mixed-effects linear regression model. We will account for within-clinician clustering by including random intercepts for the treating clinicians. The baseline KCCQ-OSS will be modeled as a restricted cubic spline with 4 knots. The primary analysis will be limited to patients with follow-up scores. As a secondary analysis, we will include adjustment for other pre-specified baseline characteristics (age and left ventricular ejection fraction) that have been shown to be associated with health status.20–22 As secondary analyses, we will also compare the change in domain scores (physical limitation, symptom frequency, quality of life, and social limitation) across arms. Subgroup analyses will be performed evaluating for heterogeneity by age, sex, left ventricular ejection fraction, Charlson comorbidity index, baseline KCCQ-OSS/domain score, and baseline intensity of medical therapy.
The sample size is based on the primary outcome. Prior work suggests that a 5–6-point change in the KCCQ-OSS represents a small change, and 10–11-point change represents a moderate change for an individual patient.23,24 A smaller mean change across a population may be clinically meaningful.3 With 1,200 patients, with 20% loss to follow-up, we estimate a 97% power to detect a mean difference of 6, and 73% power for a mean difference of 4 in the KCCQ-OSS. The statistical power should be amplified with baseline covariate adjustment. We assume baseline KCCQ-OSS would explain approximately 35% of the variance in the final KCCQ-OSS based on available data from prior clinical trials using the KCCQ-12.25–28 With baseline adjustment, we estimate approximately 55% power to detect a mean difference of 3 in the KCCQ-OSS and 81% power for a mean difference of 4 via simulation modeling.
Secondary outcomes will be evaluated using mixed-effects multivariable regression with a random intercept for the treating clinicians. The models will include adjustment for prespecified patient characteristics: age, left ventricular ejection fraction, baseline therapy use, and Charlson Comorbidity Index. A standard regression model will be selected based on the distribution of the outcome (e.g., logistic regression for binary outcomes). Each model will be adjusted for the baseline frequency (e.g., hospitalizations or telephone encounters in the prior year) or baseline treatment (e.g., baseline therapies and doses) to improve model precision. This trial evaluates whether the KCCQ-12 impacts treatment decisions; the actual effect on treatment may vary by baseline KCCQ-OSS. Therefore, for each treatment or testing outcome, we will evaluate whether there is an interaction between baseline KCCQ-OSS and the treatment arm by stratifying analyses and modeling the KCCQ-OSS as a continuous variable interacting with the treatment arm. Secondary analyses will not be adjusted for multiplicity and will therefore be considered hypothesis generating.
The sub-study surveys include ordinal responses based on the degree of agreement for the patient experience survey or classifications of concordant, intermediate, and discordant for the clinician survey assessing patient health status. We will compare responses across arms via mixed effects logistic regression models with random intercepts for the treating clinicians. We will perform subgroup analyses for the characteristics described above. We will conduct a sensitivity analysis with adjustment for the prespecified variables described above. Finally, to understand variation in the effect of routine KCCQ-12 assessment on the accuracy of clinician health status assessment across clinicians, we will compare concordance categories across arms within each clinician’s practice.
Missing Data
Given the intervention design, there may be systematic differences in missing data between the study arms. For the primary outcome, we will include multiple sensitivity analyses for missing data. First, a KCCQ-OSS score of 0 will be used for all patients who die during follow-up. Second, sensitivity analyses via two approaches will account for living patients with missing data. First, we will predict follow-up KCCQ-OSS via multiple imputation using baseline KCCQ-OSS and baseline health characteristics. The second approach will assume patients with missing data have worse outcomes by using the minimum observed KCCQ-OSS in the trial. For secondary outcomes and the patient experience and clinician assessment sub-study, we will repeat the analyses with imputation for missing data using both multiple imputation and imputation assuming missing values are systematically better or systematically worse than expected.
Discussion
The PRO-HF trial will be the first randomized study to evaluate the effect of integrating patient-reported outcomes into routine cardiovascular care. The trial aims to determine the effect of systematic collection of the KCCQ-12, the most widely used and validated PRO in HF, on changes in patient-reported health status, therapy patterns, and care utilization. This trial will also evaluate the impact of routine KCCQ-12 assessment on patient experience and the accuracy of clinician assessment of health status. Finally, the PRO-HF trial includes an examination of its own implementation of the KCCQ-12 through clinician interviews.
Understanding the impact of PRO integration into routine cardiovascular care is critical. Although PRO assessment is low-cost and low-risk for a given patient, systematic collection of PRO data and integration into standard workflows requires clinician buy-in and the redirection of limited resources. The PRO-HF trial will help to determine the value of these investments. If the routine use of PROs improves HF care delivery, it will justify significant efforts to institute PRO measurement across all HF clinics and expand PRO use to other domains of cardiology.
The PRO-HF trial uses a novel, pragmatic approach to investigate the impact of KCCQ-12 assessment in a real-world clinic setting rather than under idealized conditions with a narrow patient population. Clinicians are not required to review KCCQ-12 data and are not asked to respond to results using a specific protocol. In addition, patients were only required to have a HF clinic visit and not a HF diagnosis for enrollment. This approach was selected because it more closely approximates the conditions under which PROs will likely be implemented at the clinic level and will thus increase the relevance of trial results. A consequence of this strategy, however, is that enrolled patients were healthier than the population typically enrolled in HF trials (as seen in Table 1). The PRO-HF trial also leverages the health system’s information technology infrastructure for a highly efficient design. Relying on largely automated recruitment via EHR data and the REDCap database, we were able to quickly enroll, consent, and randomize over 1,200 patients with minimal cost. Similar strategies can be used to evaluate other low-risk interventions to improve quality of care, delivering on the promise of learning health systems.29
This trial is also novel because it combines effectiveness and implementation evaluations. We aim to both assess the impact of routine KCCQ-12 collection on health status and to understand how the KCCQ-12 is used by clinicians in this trial. The effect of KCCQ-12 assessment is highly dependent on the clinician: how they interpret it, how they discuss it with patients, and how they incorporate it into their decision-making. Including a qualitative implementation assessment will thus support a more nuanced interpretation of the trial results by highlighting whether and how clinicians incorporated the KCCQ-12 into their clinical practice. Identifying key barriers and facilitators to the routine use of PROs in HF care will also support iterative improvement of KCCQ-12 implementation at our center and in other settings, including future clinical trials.
There are several reasons to believe that PRO integration will improve the quality of HF care, as it has for other conditions.30 Providing clinicians with more granular and accurate data on patient health status, including changes over time, may decrease clinician and patient inertia to optimize guideline-directed medical therapies. The KCCQ-12 may also facilitate identification of patients with low or worsening health status who may benefit from procedural interventions, palliative care, or earlier referral for advanced therapies. We hypothesize that these care delivery changes, driven by a better understanding of patient health status, will ultimately lead to improvements in patient health status itself, as measured by the change in KCCQ-OSS over 1 year, the trial’s primary outcome. One challenge with this outcome is that more frequent surveying and discussion of KCCQ-12 results may influence KCCQ-12 responses independent from a true change in health status. The trial’s secondary outcomes, which capture clinical processes, such as HF medication rates, and the accuracy of clinician health status assessment, will help to validate the primary outcome by suggesting potential mechanisms of effect. Ultimately, we hope the PRO-HF trial will provide critical preliminary data to inform the design of a larger, multicenter trial evaluating the impact of routine PRO assessment on downstream clinical outcomes, such as mortality and hospital admission.
There are important limitations to the PRO-HF trial that should be considered when evaluating the results. The PRO-HF trial only includes patients from 17 clinicians (13 advanced HF physicians and 4 advanced practice providers) from 1 specialized HF clinic. The effect and the implementation of routine KCCQ-12 assessment will likely vary across clinicians and settings. For example, implementation in a general cardiology clinic with less HF specialization may require additional education regarding KCCQ-12 interpretation, but it may also result in greater benefit from the addition of structured symptom assessment. This increases the importance of the PRO-HF trial’s implementation evaluation to clarify the role of clinician and site-specific factors that influence the intervention and its effect.
The lack of patient and clinician blinding may impact patient and clinician behavior and survey responses. Patients may change their reporting during clinic visits because their clinician did not receive their KCCQ-12 results. For those in the usual care arm, their patient experience surveys may also be impacted by the lack of clinician acknowledgement of their KCCQ-12 responses. Blinding is challenging in pragmatic health system implementation studies, especially when evaluating interventions like PRO assessment that aim to modify the interaction between patients and clinicians.
Finally, the PRO-HF trial used patient-level randomization rather than randomizing entire clinician practices due to the modest number of HF clinicians in this trial. Subsequently, clinicians only have KCCQ-12 data for a subset of patients. The resulting inconsistent use of the KCCQ-12 for patient care may make it difficult for clinicians to incorporate the data into routine practice. The 1-year follow-up period may also be too brief for clinicians to learn how to fully integrate PROs into their care decisions. Patient-level randomization in a nonblinded trial may also introduce contamination between arms. Clinicians may learn from their experience using the KCCQ-12 with patients in the intervention arm and apply those lessons to patients in the usual care arm. Future multicenter studies could use cluster randomization of entire clinician practices with longer follow-up periods to ensure clinicians are able to fully integrate routine PRO assessment into their clinical workflows and minimize contamination concerns.
The routine use of PROs in HF treatment has the potential to place the patient’s voice at the center of HF care, leading to improvements in patient health status. The PRO-HF trial is a pragmatic, hybrid implementation-effectiveness study incorporating a quantitative randomized controlled trial of the impact of routine KCCQ-12 assessment on care delivery as well as a qualitative study of clinician perspectives on the integration of the KCCQ-12 into clinical practice. The results will provide insights on how to optimize PRO implementation in HF care and inform the future widespread adoption of these measures.
Supplementary Material
Acknowledgments
This work was funded by the National Heart, Lung, and Blood Institute (1K23HL151672–01) and Stanford University institutional funding.
Footnotes
Declaration of Conflicting Interests/Disclosures:
The other authors have no conflicts of interest to disclose.
Trial Registration: https://clinicaltrials.gov/ct2/show/NCT041640
REFERENCES
- 1.Juenger J, Schellberg D, Kraemer S, et al. Health related quality of life in patients with congestive heart failure: comparison with other chronic diseases and relation to functional variables. Heart 2002;87(3):235–241. doi: 10.1136/heart.87.3.235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kelkar AA, Spertus J, Pang P, Pierson RF, Cody RJ, Pina IL, Hernandez A and Butler J. Utility of Patient-Reported Outcome Instruments in Heart Failure. JACC Heart Fail 2016;4:165–75. [DOI] [PubMed] [Google Scholar]
- 3.Spertus JA, Jones PG, Sandhu AT and Arnold SV. Interpreting the Kansas City Cardiomyopathy Questionnaire in Clinical Trials and Clinical Care: JACC State-of-the-Art Review. J Am Coll Cardiol 2020;76:2379–2390. [DOI] [PubMed] [Google Scholar]
- 4.Greene SJ, Butler J, Spertus JA, Hellkamp AS, Vaduganathan M, DeVore AD, Albert NM, Duffy CI, Patterson JH, Thomas L, Williams FB, Hernandez AF and Fonarow GC. Comparison of New York Heart Association Class and Patient-Reported Outcomes for Heart Failure With Reduced Ejection Fraction. JAMA Cardiol 2021;6:522–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tran AT, Chan PS, Jones PG and Spertus JA. Comparison of Patient Self-reported Health Status With Clinician-Assigned New York Heart Association Classification. JAMA Netw Open 2020;3:e2014319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anker SD, Agewall S, Borggrefe M, Calvert M, Jaime Caro J, Cowie MR, Ford I, Paty JA, Riley JP, Swedberg K, Tavazzi L, Wiklund I and Kirchhof P. The importance of patient-reported outcomes: a call for their comprehensive integration in cardiovascular clinical trials. Eur Heart J 2014;35:2001–9. [DOI] [PubMed] [Google Scholar]
- 7.Spertus JA, Birmingham MC, Nassif M, Damaraju CV, Abbate A, Butler J, Lanfear DE, Lingvay I, Kosiborod MN and Januzzi JL. The SGLT2 inhibitor canagliflozin in heart failure: the CHIEF-HF remote, patient-centered randomized trial. Nat Med 2022;28:809–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Food and Drug Administration. Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. Guidance for Industry 2009. [DOI] [PMC free article] [PubMed]
- 9.Heidenreich PA. The Growing Case for Routine Collection of Patient-Reported Outcomes. JAMA Cardiol 2021;6:497–498. [DOI] [PubMed] [Google Scholar]
- 10.Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, Deswal A, Drazner MH, Dunlay SM, Evers LR, Fang JC, Fedson SE, Fonarow GC, Hayek SS, Hernandez AF, Khazanie P, Kittleson MM, Lee CS, Link MS, Milano CA, Nnacheta LC, Sandhu AT, Stevenson LW, Vardeny O, Vest AR and Yancy CW. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022;145:e895–e1032. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Medicare & Medicaid Services. Functional Status Assessments for Congestive Heart Failure Accessed 29 September 2022. https://ecqi.healthit.gov/sites/default/files/ecqm/measures/CMS90v9.html.
- 12.Stover AM, Haverman L, van Oers HA, et al. Using an implementation science approach to implement and evaluate patient-reported outcome measures (PROM) initiatives in routine care settings. Qual Life Res 2021;30(11):3015–3033. doi: 10.1007/s11136-020-02564-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patient Reported Outcome Measurement in Heart Failure Clinic (PRO-HF) Accessed 1 September, 2022. https://clinicaltrials.gov/ct2/show/NCT04164004.
- 14.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N and Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol 2000. Apr;35(5):1245–55. [DOI] [PubMed] [Google Scholar]
- 16.Spertus JA and Jones PG. Development and Validation of a Short Version of the Kansas City Cardiomyopathy Questionnaire. Circ Cardiovasc Qual Outcomes 2015;8:469–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.CAHPS Clinician and Group Survey. Accessed March 15, 2021. https://www.ahrq.gov/cahps/surveys-guidance/cg/index.html.
- 18.Wohlfahrt P, Zickmund SL, Slager S, et al. Provider Perspectives on the Feasibility and Utility of Routine Patient‐Reported Outcomes Assessment in Heart Failure: A Qualitative Analysis. JAHA 2020;9(2). doi: 10.1161/JAHA.119.013047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Damschroder Laura. Introducing CFIR 2.0 Updates. Presented at: Academy Health Dissemination and Implementation Conference, Washington DC, December 2021. [Google Scholar]
- 20.Heidenreich PA, Spertus JA, Jones PG, et al. Health status identifies heart failure outpatients at risk for hospitalization or death. J Am Coll Cardiol 2006;47(4):752–756 [DOI] [PubMed] [Google Scholar]
- 21.Joseph SM, Novak E, Arnold SV, et al. Comparable performance of the Kansas City Cardiomyopathy Questionnaire in patients with heart failure with preserved and reduced ejection fraction. Circ Heart Fail 2013;6(6):1139–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pokharel Y, Khariton Y, Tang 21 Y, et al. Association of Serial Kansas City Cardiomyopathy Questionnaire Assessments With Death and Hospitalization in Patients With Heart Failure With Preserved and Reduced Ejection Fraction: A Secondary Analysis of 2 Randomized Clinical Trials. JAMA Cardiol 2017;2(12):1315–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hawwa N, Vest AR, Kumar R, et al. Comparison Between the Kansas City Cardiomyopathy Questionnaire and New York Heart Association in Assessing Functional Capacity and Clinical Outcomes. Journal of Cardiac Failure 2017;23(4):280–285. doi: 10.1016/j.cardfail.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Butler J, Shahzeb Khan M, Lindenfeld J, Abraham WT, Savarese G, Salsali A, Zeller C, Peil B, Filippatos G, Ponikowski P, Anker SD. Minimally Clinically Important Difference in Health Status Scores in Patients With HFrEF vs HFpEF. JACC Heart Fail 2022. Sep;10(9):651–661. [DOI] [PubMed] [Google Scholar]
- 25.Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O’Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM; TOPCAT Investigators. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014. Apr 10;370(15):1383–92. [DOI] [PubMed] [Google Scholar]
- 26.O’Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, Rendall DS, Miller NH, Fleg JL, Schulman KA, McKelvie RS, Zannad F, Piña IL; HF-ACTION Investigators. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009. Apr 8;301(14):1439–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Velazquez EJ, Lee KL, Jones RH, Al-Khalidi HR, Hill JA, Panza JA, Michler RE, Bonow RO, Doenst T, Petrie MC, Oh JK, She L, Moore VL, Desvigne-Nickens P, Sopko G, Rouleau JL; STICHES Investigators. Coronary-Artery Bypass Surgery in Patients with Ischemic Cardiomyopathy. N Engl J Med 2016. Apr 21;374(16):1511–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Felker GM, Anstrom KJ, Adams KF, Ezekowitz JA, Fiuzat M, Houston-Miller N, Januzzi JL Jr, Mark DB, Piña IL, Passmore G, Whellan DJ, Yang H, Cooper LS, Leifer ES, Desvigne-Nickens P, O’Connor CM. Effect of Natriuretic Peptide-Guided Therapy on Hospitalization or Cardiovascular Mortality in High-Risk Patients With Heart Failure and Reduced Ejection Fraction: A Randomized Clinical Trial. JAMA 2017. Aug 22;318(8):713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horwitz LI, Kuznetsova M, Jones SA. Creating a Learning Health System through Rapid-Cycle, Randomized Testing. N Engl J Med 2019. Sep 19;381(12):1175–1179. doi: 10.1056/NEJMsb1900856. [DOI] [PubMed] [Google Scholar]
- 30.Basch E, Deal AM, Dueck AC, Scher HI, Kris MG, Hudis C, Schrag D. Overall Survival Results of a Trial Assessing Patient-Reported Outcomes for Symptom Monitoring During Routine Cancer Treatment. JAMA 2017. Jul 11;318(2):197–198. doi: 10.1001/jama.2017.7156. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


