Abstract
During decades-long infections in the cystic fibrosis (CF) airway, Pseudomonas aeruginosa undergoes selection. One bacterial genetic adaptation often observed in CF isolates is mucA mutations. MucA inhibits the sigma factor AlgU. Mutations in mucA lead to AlgU misregulation, resulting in a mucoid phenotype that is associated with poor CF disease outcomes. Due to its ability to be mutated, mucA is assumed to be dispensable for bacterial viability. Here we show that, paradoxically, a portion of mucA is essential in P. aeruginosa. We demonstrate that mucA is no longer required in a strain lacking algU, that mucA alleles encoding for proteins that do not bind to AlgU are insufficient for viability, and that mucA is no longer essential in mutant strains containing AlgU variants with reduced sigma factor activity. Furthermore, we found that overexpression of algU prevents cell growth in the absence of MucA, and that this phenotype can be rescued by the overproduction of RpoD, the housekeeping sigma factor. Together, these results suggest that in the absence of MucA, the inability to regulate AlgU activity results in the loss of bacterial viability. Finally, we speculate that the essentiality of anti-sigma factors that regulate envelope function may be a widespread phenomenon in bacteria.
Keywords: AlgU, cystic fibrosis, envelope stress, sigma factor competition
1 |. INTRODUCTION
The major cause of death in people with cystic fibrosis (CF), a human autosomal recessive genetic disease, is respiratory failure due to chronic lung infection. Pseudomonas aeruginosa is a prevalent CF respiratory pathogen (Cystic Fibrosis Foundation Patient Registry, 2019). The CF lung environment selects for mucoid P. aeruginosa mutants, which overproduce the exopolysaccharide alginate and are associated with poor disease prognosis (Douglas et al., 2009; Emerson et al., 2002; Farrell et al., 2009; Henry et al., 1992; Li et al., 2005; Nixon et al., 2001; Parad et al., 1999; Pedersen et al., 1992; Strateva et al., 2010). Conversion to the mucoid phenotype in clinical P. aeruginosa isolates, which is thought to be advantageous for chronic infection, is often caused by mucA mutations (Martin et al., 1993).
MucA is an anti-sigma factor to the alternative sigma factor AlgU (also known as AlgT, σE, or σ22), which responds to envelope stress (Damron & Goldberg, 2012; Govan & Deretic, 1996). MucA is a transmembrane protein that sequesters AlgU away from RNA polymerase (RNAP) via its N-terminus (Li et al., 2019; Schurr et al., 1996; Xie et al., 1996). The C-terminus of MucA is in the periplasm, where it is protected from proteolysis by MucB (Mathee et al., 1997; Schurr et al., 1996). The envelope stress response is controlled via a regulated intramembrane proteolysis cascade: MucB dissociates from MucA upon stress detection, allowing the proteases AlgW and MucP to cleave MucA from the inner membrane (Qiu et al., 2007; Wood & Ohman, 2009). In the cytoplasm, MucA is further degraded by ClpXP, releasing AlgU to interact with RNAP and activate the AlgU regulon (Qiu et al., 2008). AlgU regulates at least 350 genes, including those responsible for the production of itself, MucA, and the alginate biosynthetic enzymes (Firoved & Deretic, 2003; Schulz et al., 2015; Wood & Ohman, 2009). This system is homologous to the well-studied envelope stress response in Escherichia coli: MucA shares 28% identity (72% similarity) to the anti-sigma factor RseA. The cognate sigma factor of the E. coli RseA, called RpoE, is homologous to and functionally interchangeable with AlgU (Yu et al., 1995), with 66% identity (93% similarity).
For several reasons, mucA is assumed to be dispensable for P. aeruginosa viability. First, after being released from the cell membrane, MucA is presumed to be fully degraded in the cytoplasm by ClpXP (Qiu et al., 2008). Second, mucA mutations often arise in CF clinical isolates (Boucher et al., 1997; Candido Cacador et al., 2018; Ciofu et al., 2008; Martin et al., 1993; Pulcrano et al., 2012), and many laboratory mucA mutants exist in the literature (Gallagher et al., 2011; Liberati et al., 2006; Skurnik et al., 2013; Turner et al., 2015). Third, there are three published strains in which the entirety of mucA is removed from the genome (Intile et al., 2014; Jones et al., 2010; Pritchett et al., 2015). Paradoxically, three different whole-genome studies in two different strains of P. aeruginosa identified mucA as an essential gene (Lee et al., 2015; Liberati et al., 2006; Skurnik et al., 2013). Additionally, it has been anecdotally noted that mucA could not be deleted from the P. aeruginosa PAO1 genome (Panmanee et al., 2019). To investigate this paradox, we systematically attempted to delete mucA from P. aeruginosa using allelic exchange. Our results show that a portion of mucA was required for bacterial viability in multiple P. aeruginosa strains, that mucA was no longer essential in a strain lacking algU, and that mucA alleles that encode for proteins that do not interact with AlgU were insufficient to rescue viability or led to a growth defect. We found that algU mutations encoding for a less active sigma factor could relieve mucA essentiality and that AlgU overproduction in the absence of MucA was toxic. Interestingly, our works suggest that mucA essentiality can be suppressed by increasing the levels of the housekeeping sigma factor RpoD. Together, our results strongly suggest that the unregulated activity of AlgU itself, in the absence of MucA, leads to bacterial cell death.
2 |. RESULTS
2.1 |. mucA is essential for viability in a diverse set of P. aeruginosa isolates
To determine if mucA is essential, we attempted to delete the gene from various P. aeruginosa strains, using a modified allelic exchange protocol to turn it into a robust assay (Figure S1). An allele of mucA missing >95% of the coding region was introduced into P. aeruginosa. Using PCR-confirmed isolates containing both the endogenous and deletion alleles in the genome, we counter-selected for loss of one allele and then determined which mucA allele isolates resolved to via PCR. For non-essential genes, we should observe isolates that resolved to the endogenous or deletion allele. However, for essential genes, only cells that resolved to the endogenous allele should be isolated, as cells that resolved to the deletion allele should not survive in the absence of the gene. For statistical power, we screened ≥125 isolates for strains resolving only to the endogenous mucA. We performed this assay on a diverse set of wild-type P. aeruginosa strains, including four laboratory, five clinical, and two environmental isolates (Table 1). These strains are not only isolated from diverse locations, but they also vary in their colony morphology and their exopolysaccharide production profile (Colvin et al., 2012). For all strains tested, all observed isolates resolved to the endogenous mucA allele (p < .0001, Fisher’s exact test), strongly suggesting that mucA is essential in wild-type P. aeruginosa. Since all tested strains required mucA for viability, we continued our experiments using PAO1 as a representative strain.
TABLE 1.
mucA is essential in a variety of wild-type P. aeruginosa strains
| Strain background | Description† | Number of isolates resolved
to |
|
|---|---|---|---|
| WT | ∆mucA | ||
| PAO1 | Laboratory strain, Class II | 168 | 0* |
| PA14 | Laboratory strain, Class I | 147 | 0* |
| PA103 | Laboratory strain, unclassified | 361 | 0* |
| PAK | Laboratory strain, unclassified | 191 | 0* |
| CF127 | Mucoid CF isolate, unclassified | 136 | 0* |
| CF18 | Non-mucoid CF isolate, unclassified | 129 | 0* |
| CF27 | Rugose CF Isolate, Class IV | 152 | 0* |
| X13273 | Blood isolate, Class II | 142 | 0* |
| X24509 | UTI isolate, Class II | 221 | 0* |
| MSH10 | Water isolate, Class III | 146 | 0* |
| E2 | Tomato plant isolate, Class II | 141 | 0* |
*p < .0001, Fischer’s exact test.
†Class identification is based on the exopolysaccharide expression profile as described in (Colvin et al., 2012): Class I, Pel-dominant matrix; Class II, Psl-dominant matrix; Class III, exopolysaccharide redundant matrix users; and Class IV, matrix overproducers. CF, cystic fibrosis; UTI, urinary tract infection.
2.2 |. Depletion of MucA leads to cell death
To evaluate the effect of nutrient conditions on mucA essentiality, we tested the effect of depleting MucA on P. aeruginosa viability in different media. We engineered a strain lacking the native mucA and containing a chromosomally-integrated rhamnose-inducible copy (∆mucA attTn7::PrhaBAD-mucA). We determined the viability of this strain grown without rhamnose over time. Since cells would cease producing MucA in the absence of rhamnose, the MucA present in the cells at the start of the experiment would be depleted as the cells divided. In all four media we tested, if rhamnose was not removed, the cells increased in density by ~2-logs over time. In comparison, when rhamnose was removed, the cells lost viability over time in all four media (Figure 1), showing that mucA is essential in P. aeruginosa in various nutritional environments.
FIGURE 1.
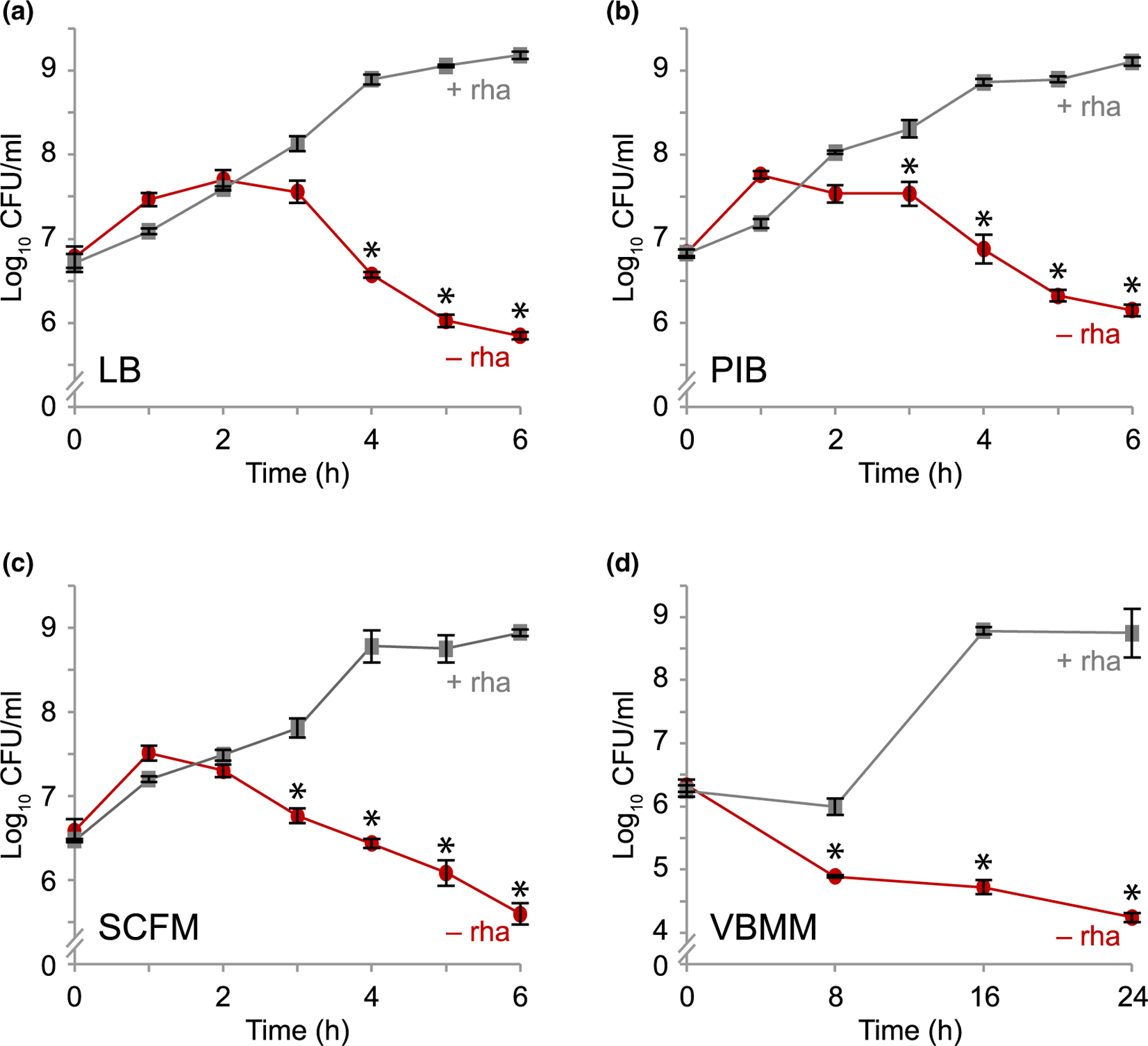
Depletion of MucA results in cell death. Viable colony counts of PAO1 ∆mucA attTn7::PrhaBAD-mucA over time in (a) LB, (b) PIB, (c) SCFM and (d) VBMM with (+ rha; gray squares) or without (− rha; red circles) 0.05% rhamnose. Viable colony counts after incubation in the indicated condition and time were determined by plating the cells on LB with 0.05% rhamnose to allow cells to recover and grow. Hash, broken y-axis; error bars, SEM (N = 3). Asterisk, statistically different from that at the same time point grown in the presence of rhamnose (p < .01, N = 3, mixed model ANOVA with post-hoc Bonferroni test)
2.3 |. Alginate biosynthesis is not solely responsible for mucA essentiality
Clinical isolates with mucA mutations are mucoid due to the overproduction of alginate (Martin et al., 1993). To determine if alginate overproduction is responsible for mucA essentiality, we attempted to delete mucA from a strain lacking algD, a key alginate biosynthesis gene (Deretic et al., 1987). We were unable to delete mucA in this background (Table S1).
Expression of the alginate biosynthesis genes is controlled by three AlgU-regulated transcription factors that are active in mucoid cells: AlgB, AlgR, and AmrZ (Martin et al., 1994; Wozniak & Ohman, 1994; Wozniak et al., 2003). Since these transcription factors regulate many genes in addition to those involved in alginate biosynthesis (Huang et al., 2019; Jones et al., 2014; Kong et al., 2015; Leech et al., 2008), we tested if overexpression of these three regulons underlies mucA essentiality. We could not delete mucA from strains lacking these transcription factors (Table S1). We conclude that eliminating alginate biosynthesis and the expression of ~50% AlgU regulon do not alleviate mucA essentiality.
2.4 |. The first 50 amino acids of MucA are necessary and sufficient for cell viability
While we were unable to delete mucA using an allele lacking >95% of the coding region, mucA is often mutated in clinical isolates and many mucA transposon mutants exist (Figure S2), suggesting that only a portion of mucA is essential. Based on the co-crystal structures of MucA with AlgU and MucB (Li et al., 2019), the first 78 residues of MucA interact with AlgU, and the last 48 residues of MucA interact with MucB. We attempted to delete the endogenous mucA from a series of strains containing an ectopic chromosomally integrated mucA, driven by its native promoter and encoding for truncation products (Figure 2a). We were unable to probe whether equivalent amounts of protein were produced across our strains, since our truncated versions of MucA are cleaved out of the membrane and degraded in the cytosol, as previously shown (Qiu et al., 2008). However, we expect that the ectopic alleles are expressed at similar levels prior to endogenous mucA deletion, as they are under the same promoter and at the same genomic site. These strains with two mucA alleles were non-mucoid, showing that the endogenous mucA allele, encoding full-length protein, is dominant over the ectopic alleles that encode for truncated MucA. We used a ∆algD background to make strains easier to manipulate, as deleting the endogenous mucA would result in alginate overproduction in some strains.
FIGURE 2.
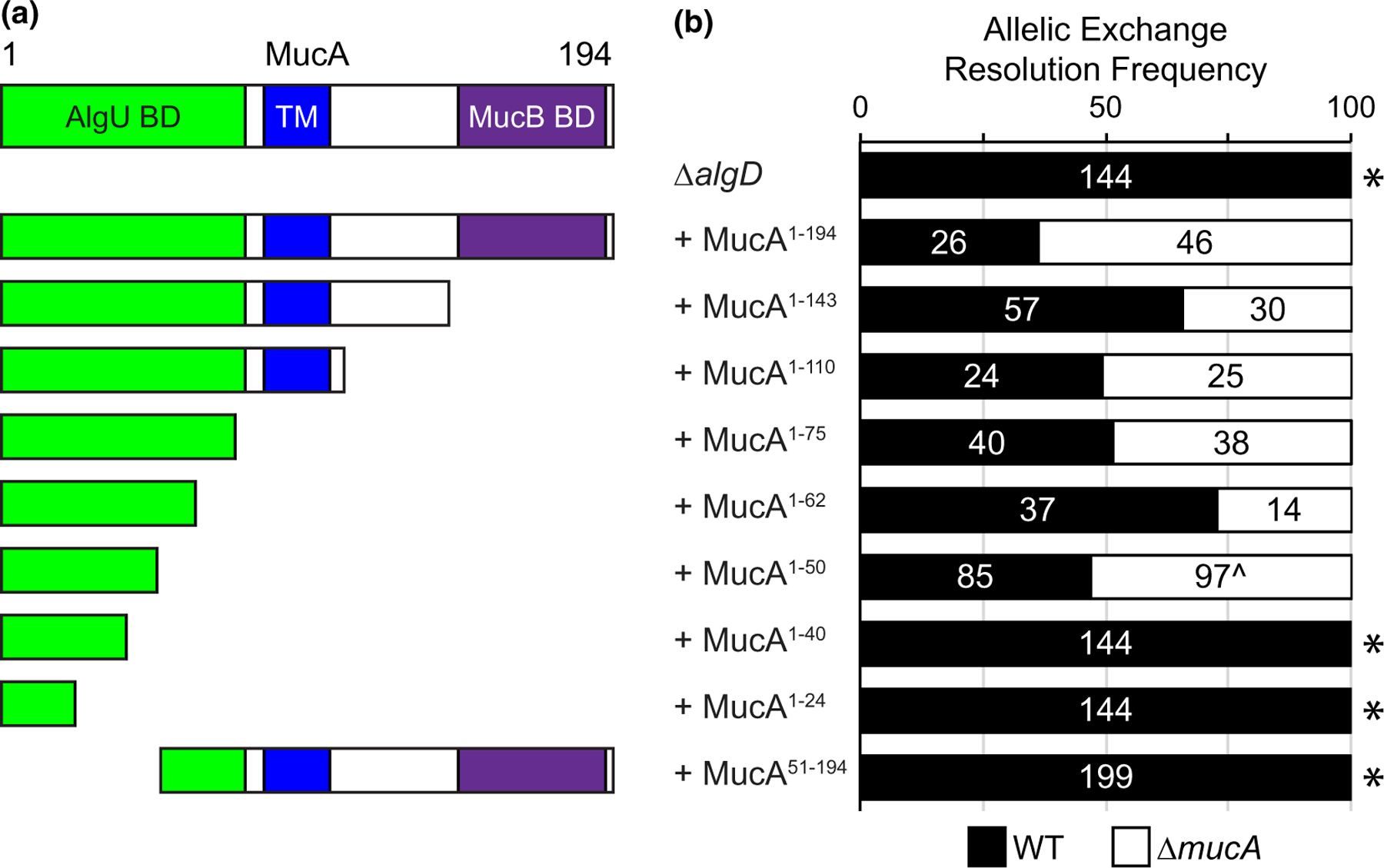
The first 50 amino acids of MucA are necessary and sufficient for viability. (a) Schematic of MucA encoded by the ectopic mucA alleles in PAO1 ∆algD strains tested for mucA essentiality in (b). MucA aa 1–143 lacks the MucB binding domain and is the product of the common mucA22 allele. MucA 1–110 lacks the entire periplasmic domain. MucA aa 1–75 contains most of the AlgU binding domain, while shorter truncations contain only parts of the AlgU binding domain (MucA aa 1–62, 1–50, 1–40, and 1–24). Green, AlgU binding domain (AlgU BD); blue, the transmembrane domain (TM); and purple, the MucB binding domain (MucB BD). (b) Frequency of observed isolates resolving to the endogenous mucA allele (WT, black) or the deletion allele (∆mucA, white) in the allelic exchange assay. Super-imposed on the bars are the number of isolates that were tested in each category. Asterisk, p < .0001; Fisher’s exact test. Caret, slower growing isolates
We then attempted to delete the endogenous mucA from these strains. Since the flanking regions of the two alleles differ, our deletion allele specifically targets the native allele. We were able to easily recover isolates that resolved to the mucA deletion allele from strains containing ectopic alleles encoding for the full-length MucA (aa 1–194), as well as MucA aa 1–143, aa 1–110, aa 1–75, and aa 1–62 (Figure 2b). Interestingly, while the endogenous mucA could be deleted from strains containing an ectopic mucA that encoded for aa 1–50 (Figure 2b), isolates resolving to the deletion allele grew up more slowly than those resolving to the native allele. This suggests that while this allele is sufficient for viability, it is not well tolerated. All tested isolates of strains carrying mucA alleles encoding shorter products (aa 1–40 and aa 1–24) resolved to the native allele. Furthermore, we were unable to delete the native mucA from a strain with an ectopic mucA that encodes for aa 51–194 (Figure 2b). These results suggest that the first 50 amino acids of MucA are necessary and sufficient for P. aeruginosa cell viability. Consistent with these results, reported mucA mutations (Boucher et al., 1997; Candido Cacador et al., 2018; Ciofu et al., 2008; Martin et al., 1993; Pulcrano et al., 2012; Turner et al., 2015) almost entirely fall outside the region of mucA encoding the first 50 residues (Figure S2).
2.5 |. The interaction of MucA with AlgU is required for cell survival
Since the only described function for MucA is to inhibit AlgU, we hypothesized that mucA essentiality is rooted in its regulation of AlgU. To test this, we attempted to delete mucA from a strain lacking algU (∆algU). We were able to do so, with 16 of the tested isolates resolving to the deletion allele out of the 48 colonies tested (see Figure 4a), showing that mucA essentiality is algU-dependent.
FIGURE 4.
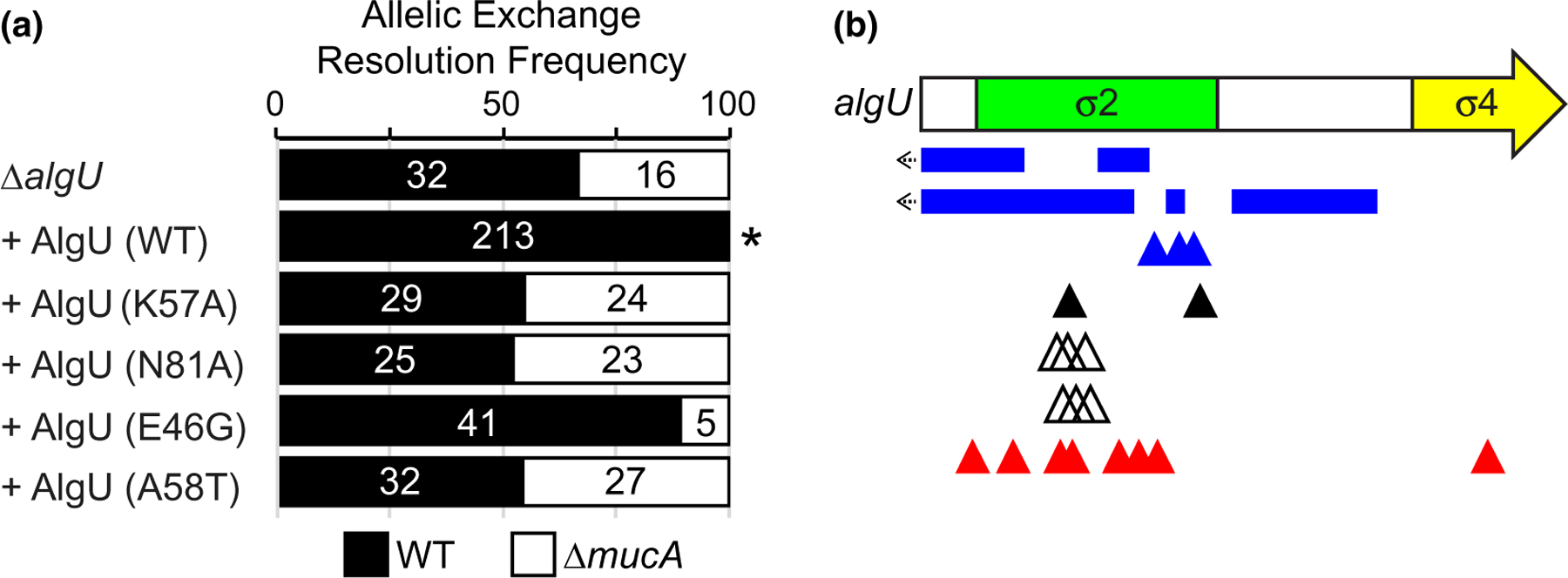
Mutations in AlgU can suppress mucA essentiality. (a) Frequency of observed isolates resolving to the endogenous mucA allele (WT, black) or the deletion allele (∆mucA, white) in the allelic exchange assay, using PAO1 ∆algU attTn7::PalgU-algU, where algU encodes for the indicated substitution. Super-imposed on the bars are the number of isolates that were tested in each category. Asterisk, p < .0001; Fisher’s exact test. (b) Schematic of mutations seen in revertants that could grow in the absence of mucA. Revertants were selected by growing PAO1 ∆mucA attTn7::PrhaBAD-mucA on media lacking rhamnose. Blue rectangles, multi-base pair deletions; left arrow, deletion extends into the promoter; blue triangles, single base pair deletions; black triangles, nonsense mutations; white triangles, duplications resulting in 3 or 4 amino acid insertions; red triangles, missense mutations. See Table S2 for a full description of the algU mutations
Because our data suggest that the AlgU-binding domain of MucA is required for viability (Figure 2b), we hypothesized that the physical interaction between MucA and AlgU is necessary for cell survival. In the co-crystal structure (Li et al., 2019), four residues in MucA make more than one hydrogen bond with AlgU: D15, E22, R42, and E72 (Figure 3a). We engineered alleles that encode for MucA D15A, E22A, R42A, or E72A to maximally affect the hydrogen bonding while limiting the effects on overall protein structure. We used MucA aa 1–75 as a base because the co-crystal structure includes the first 78 residues of MucA. As described above, an allele encoding MucA aa 1–75 was sufficient for viability (Figure 2b). We were unable to delete the native mucA from strains carrying alleles encoding the D15A and R42A substitutions (Figure 3b). While we were able to delete the native mucA from the strain containing MucA E22A, isolates that resolved to the deletion allele grew up slower than those that resolved to the wild-type allele, suggesting that the cells do not tolerate this allele well. As expected, due to being outside the required region of MucA (Figure 2b), we were able to delete the native mucA from a strain containing MucA E72A with 9 of 48 tested isolates resolving to the deletion allele.
FIGURE 3.
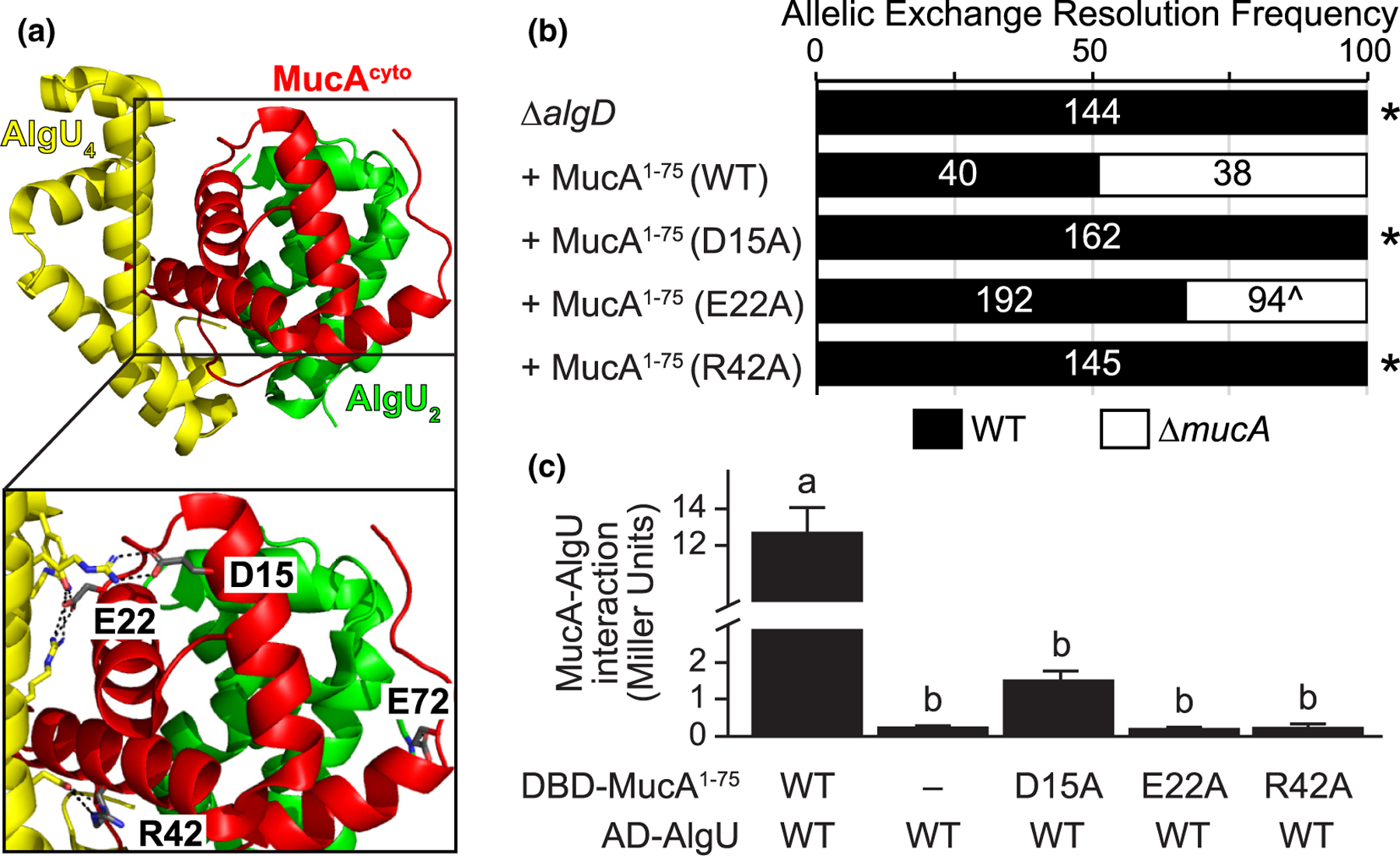
The physical interaction of MucA and AlgU is required for survival. (a) Four residues of MucA make greater than one predicted hydrogen bond with AlgU. The MucA-AlgU co-crystal structure (PDB 6IN7; Li et al., 2019) with the cytosolic domain of MucA (aa 1–78; red) and Regions 2 and 4 of AlgU (green, AlgU2; yellow, AlgU4) is shown. The residues of MucA that are predicted to make more than one hydrogen bond with AlgU (grey) are labeled in the inset. Black dotted lines, predicted hydrogen bonds; red atoms, oxygen; blue atoms, nitrogen. (b) Frequency of observed isolates resolving to the endogenous mucA allele (WT, black) or the deletion allele (∆mucA, white) in the allelic exchange assay, using PAO1 ∆algD attTn7::PalgU-mucA, where mucA encodes for the indicated substitution. Super-imposed on the bars are the number of isolates that were tested in each category. Asterisk, p < .0001; Fisher’s exact test. Caret, slower growing isolates. (c) Substitution of MucA residues at its interface with AlgU abolishes their binding via yeast two-hybrid. The first 75 residues of MucA were fused to the Gal4 DNA-binding domain (DBD-MucA1–75) and AlgU was fused to the Gal4 activation domain (AD-AlgU). Interaction of MucA and AlgU led to lacZ expression. Beta-galactosidase activity (in Miller units) was used as a proxy for the protein interaction strength. WT, wild-type protein sequence; −, no fusion protein included; hash, broken y-axis; error bars, SEM (N = 3); letters, statistical groupings (p < .01; biological triplicate with technical quadruplicates; ANOVA with post-hoc Tukey HSD)
To determine if the D15A, E22A, and R42A substitutions affect the ability of MucA to interact with AlgU, we used a yeast-two hybrid assay. Using beta-galactosidase activity as a proxy for their interaction, wild-type MucA aa 1–75 strongly interacted with AlgU (Figure 3c). This interaction was dependent on the presence of both MucA and AlgU, and the effect was not directional (Figure S3). In comparison, the D15A, E22A, and R42A MucA mutants failed to interact with AlgU (Figure 3c). We conclude that the interaction of MucA and AlgU is required for viability.
2.6 |. Mutations that reduce AlgU activity alleviate the requirement for MucA
If mucA essentiality is due to its inhibition of the AlgU regulon, strains containing a mutant AlgU with a lower affinity for DNA should alleviate mucA essentiality because the regulon expression is reduced in such mutants. We engineered ∆algU strains with an ectopic chromosomally-integrated algU allele encoding such DNA-binding mutants (K57A and N81A), based on homology to E. coli RpoE mutants that have decreased in vitro transcriptional activity (Campagne et al., 2014). As described above, we were able to delete mucA from a ∆algU strain (Figure 4a). This phenotype could be rescued via the ectopic addition of a wild-type algU allele, as we were no longer able to delete mucA from such a strain. In contrast, mucA could be deleted from strains carrying alleles encoding AlgU K57A or N81A, showing that these alleles failed to rescue the ∆algU phenotype (Figure 4a). To determine the effect of these substitutions on AlgU activity, we induced envelope stress using D-cycloserine (Wood et al., 2006) and measured AlgU activity using a plasmid-borne gfp reporter driven under the AlgU-regulated algD promoter (Damron et al., 2009). Similar to what is seen for RpoE (Campagne et al., 2014), these mutant strains had reduced AlgU activity upon induction of envelope stress (Figure 5a,b).
FIGURE 5.
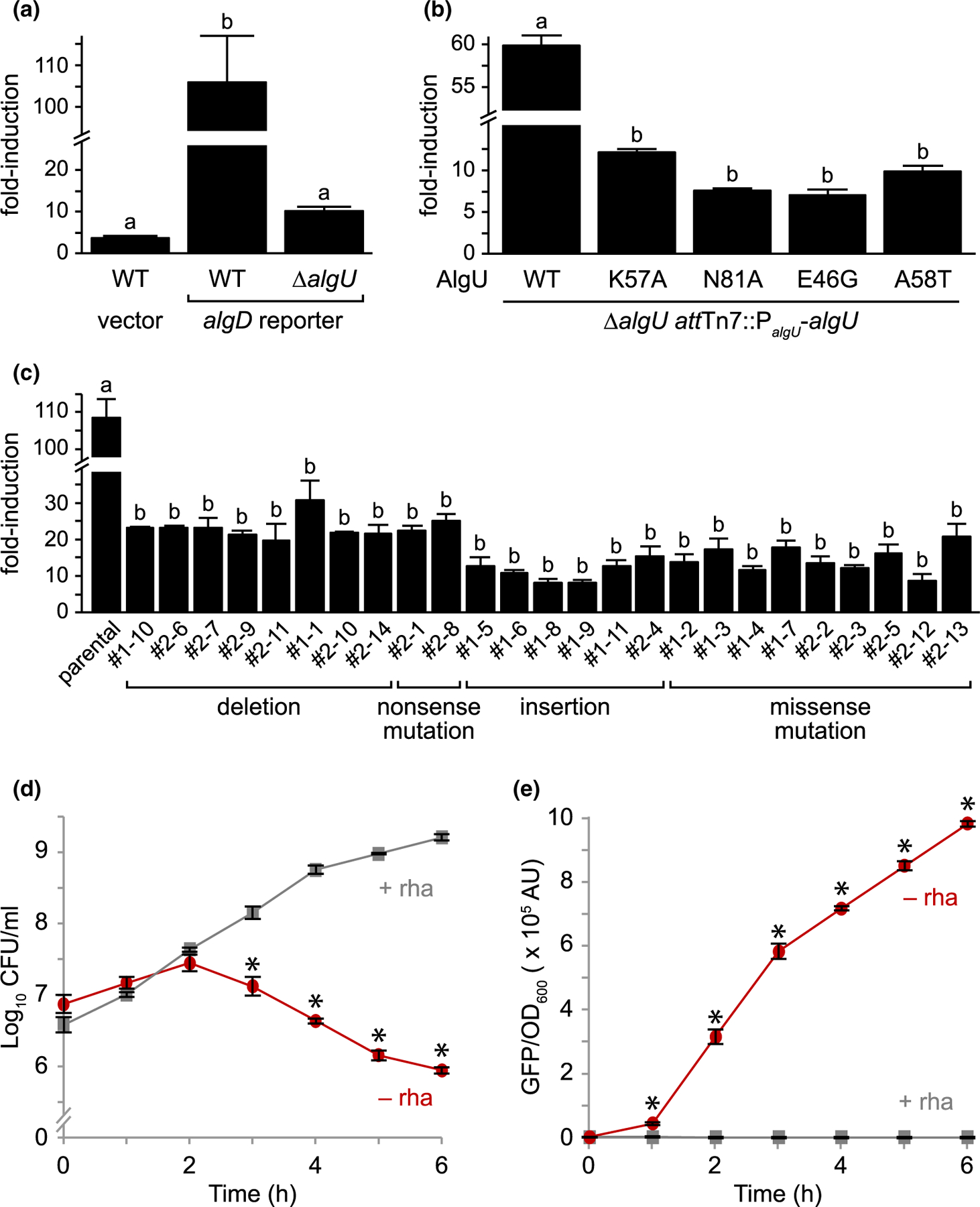
AlgU mutants that suppress mucA essentiality have decreased activity. (a) Fold-induction of GFP in PAO1 wild-type (WT) and ∆algU carrying a plasmid with either a promoter-less gfp (vector) or a gfp driven by the algD promoter (algD reporter), which is positively regulated by AlgU, after a 2h D-cycloserine treatment to activate envelope stress. GFP fluorescence was normalized to cell density and was then divided by the signal from untreated cells to determine the fold-induction. Hash, broken y-axis; error bars, SEM (N = 3); letters, statistical groupings (p < .01; biological triplicate with technical quadruplicates; ANOVA with post-hoc Tukey HSD). (b) Fold-induction of GFP in PAO1 ∆algU attTn7::PalgU-algU, where algU encodes for the indicated substitution. The experiment and statistics are as described in (a). WT, the wild-type AlgU sequence. (c) Fold-induction of GFP in the revertants isolated from PAO1 ∆mucA attTn7::PrhaBAD-mucA (parental) that can grow in the absence of MucA. The experiment and statistics are as described in (a), except all cells were grown in the presence of 0.05% rhamnose to allow for comparison to the parental strain, which grows only in the presence of rhamnose. The isolate identification numbers are shown and are grouped based on their algU mutation. (d) Viable colony counts of PAO1 ∆mucA attTn7::PrhaBAD-mucA carrying the algD reporter over time in LB with (+ rha; gray squares) or without (− rha; red circles) 0.05% rhamnose. Hash, broken y-axis; error bars, SEM (N = 3). Asterisk, statistically different from that at the same time point grown in the presence of rhamnose (p < .01, N = 3, mixed model ANOVA with post-hoc Bonferroni test). (e) Normalized GFP fluorescence of the samples in (d). GFP fluorescence was normalized to cell density. The statistical analysis is as described in (d)
Strains with the entirety of mucA deleted from P. aeruginosa PAO1, PAK, and PA103 exist (Intile et al., 2014; Jones et al., 2010; Pritchett et al., 2015). Since we were unable to delete mucA from these strain backgrounds (Table 1), we sequenced the genomes of these ∆mucA strains to determine if they contain suppressor mutations that allowed for their survival in the absence of mucA. In PAO1 ∆mucA (Pritchett et al., 2015), while the native mucA was deleted, the strain contained a second full-length copy of algU and a mucA allele that encoded for aa 1–155 elsewhere in the genome. As expected (Figure 2b), we were able to delete the native mucA allele from a PAO1 strain containing an ectopic allele that encoded for MucA aa 1–155 (with 5 of the 28 tested isolates resolving to the deletion allele), confirming that the ectopic mucA allele in the published PAO1 ∆mucA strain is sufficient for viability in the absence of the endogenous mucA. The PAK and PA103 ∆mucA strains (Intile et al., 2014; Jones et al., 2010) contain missense mutations in algU, which result in A58T and E46G, respectively. We replicated these mutations in PAO1 by inserting algU alleles encoding these substitutions in a ∆algU strain. We were able to delete mucA from these strains (Figure 4a). These results confirm that the algU alleles in the published PAK and PA103 ∆mucA strains suppress mucA essentiality. Using our reporter assay, we found that strains carrying these AlgU substitutions had reduced sigma factor activity (Figure 5b). We note that our reporter assay is not very sensitive to low levels of AlgU activity. Both PAK and PA103 ∆mucA strains are mucoid (Intile et al., 2014; Jones et al., 2010), suggesting that AlgU A58T and E46G are not completely inactive. Nevertheless, our results show that these AlgU mutants have significantly less transcriptional activity than the wild-type protein (Figure 5a,b).
To identify additional suppressors of mucA essentiality, we used the ∆mucA attTn7::PrhaBAD-mucA strain. This strain was not viable in the absence of rhamnose, but natural revertants arose at a frequency of less than 1 in 109 colony forming units. We sequenced the algU gene in 25 revertants, since AlgU mutants can suppress mucA essentiality (Figure 4a). All 25 isolates contained mutations in algU that are predicted to be hypomorphic (Figure 4b, Table S2). There were 10 revertants with deletions or nonsense mutations of algU, encoding either no product or a truncated product completely lacking Region 4 of the sigma factor. There were 6 revertants that contained multi-base pair duplications in algU that would lead to the insertion of 3 or 4 amino acids in Region 2 helix 3 of the sigma factor. There were 9 revertants containing missense mutations, 8 of which were unique, encoding the following substitutions: D18G, A21V, Y29C, A47T, D49G, Y59C, N81D, and R174G. Using a model of σE in complex with the RNAP core and the promoter element, we expect these insertions and substitutions to affect sigma factor folding, RNAP core interactions, or promoter interactions (Figure S4). Using our algD reporter assay in these natural revertants, we saw that all 25 revertants had much lower AlgU activity than the parental strain upon induction of envelope stress (Figure 5c), as predicted. Together, these data strongly suggest that algU mutations that encode for a protein with reduced transcriptional activity allow P. aeruginosa to survive in the absence of mucA.
If mucA essentiality is due to AlgU inhibition, AlgU activity should increase as MucA is depleted from cells, concomitant with the decrease in viability. To test this, the viability and fluorescence (as a proxy for AlgU activity) of a ∆mucA attTn7::PrhaBAD-mucA strain containing our algD reporter plasmid was measured over time in the absence of rhamnose. This strain had a similar viability to that seen in Figure 2 (Figure 5d), and as MucA was depleted, the AlgU activity increased (Figure 5e). These results show the cell death observed upon MucA depletion is correlated with a dramatic increase in AlgU activity.
2.7 |. Overexpression of algU in the absence of mucA leads to a growth defect
Our results suggest that mucA essentiality is due to unregulated AlgU activity. We reasoned that overexpression of algU should be toxic. However, algU can be overexpressed in wild-type P. aeruginosa that contain mucA (Qiu et al., 2008; Schulz et al., 2015). We, therefore, examined the effect of overproducing AlgU in wild type, ∆algU, and ∆algU ∆mucA strains, using a chromosomally integrated arabinose-inducible algU. Of note, the expression of algU is completely dependent on the inducer in the ∆algU and ∆algU ∆mucA strains, which lack positive feedback of AlgU on its own expression. Our results show that in the absence of an inducer when AlgU is not overexpressed, all three strains had similar growth rates (Figure 6a). In the presence of 1% arabinose (i.e., high expression of algU), all three strains had a growth defect with the strain lacking mucA failing to grow at all.
FIGURE 6.
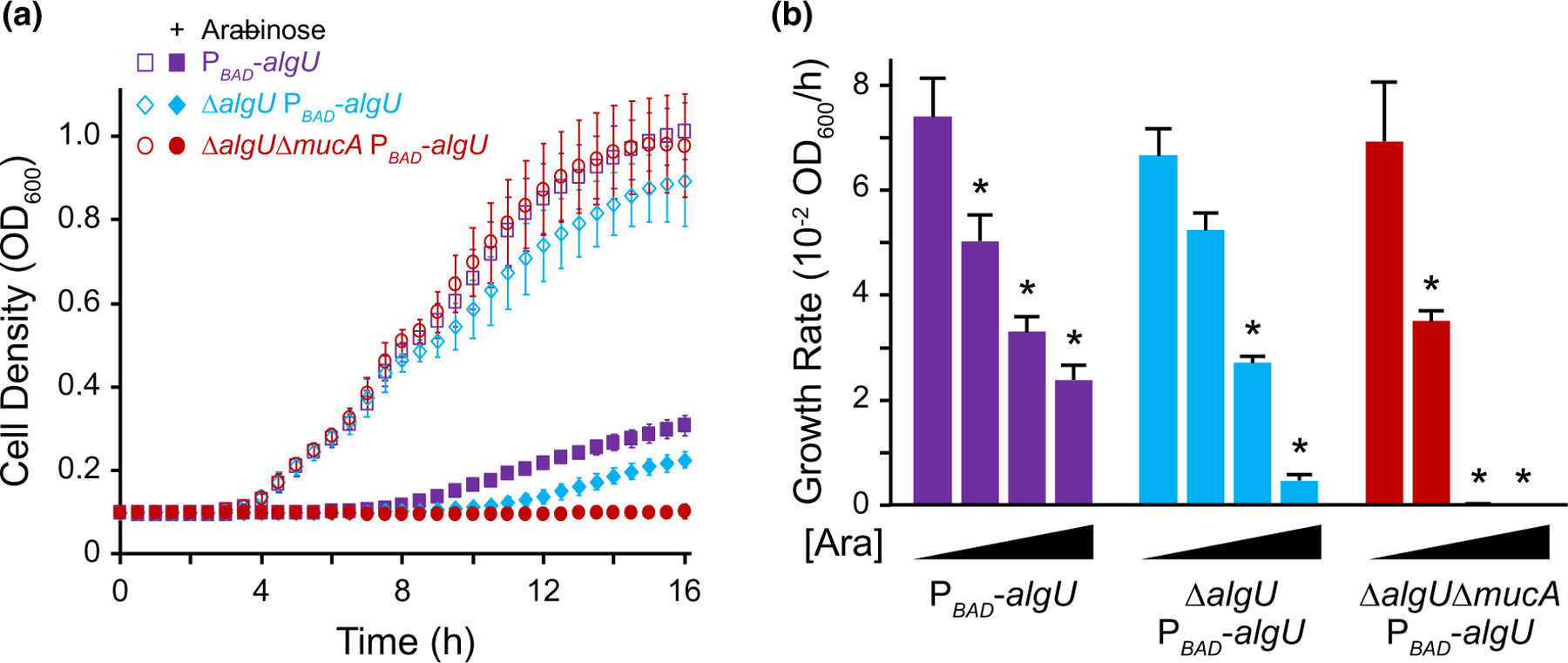
Overexpression of algU leads to a growth defect. (a) Growth curves of PAO1 strains containing an arabinose-inducible copy of algU in wild-type (purple squares), ∆algU (blue diamonds), and ∆algU ∆mucA (red circles) backgrounds in LB. Open symbols represent conditions in the absence of arabinose; closed symbols, with 1% arabinose. Error bars, SD (n = 18). (b) Growth rate of strains as described in (a), with increasing induction of algU. Strains were grown in LB with 0%, 0.1%, 0.25%, and 1% arabinose ([Ara]). Error bars, SEM (N = 3). Asterisk, statistically different from the same strain grown without arabinose (p < .01, N = 3, two-way ANOVA with post-hoc Bonferroni). See Table S3 for full statistical comparisons
To determine if the ∆algU ∆mucA attTn7::ParaBAD-algU strain can grow under lower levels of algU induction, we tested a range of inducer concentrations (Figure 6b, Table S3). We found that although there was a growth defect, ∆algU ∆mucA attTn7::ParaBAD-algU was able to grow in the presence of 0.1% arabinose. The drop in growth rate in comparison to the no arabinose condition was statistically larger for the ∆algU ∆mucA attTn7::ParaBAD-algU than for the other two strains (N = 3, p < .05, ANOVA with post hoc Tukey HSD), since this strain lacks the ability to produce any MucA to reduce AlgU activity. These results strongly suggest that in the absence of mucA, while a certain low level of AlgU activity is tolerated, high AlgU activity is fatal to the cell.
The above experiments were performed using only one medium. To determine if algU overexpression in the absence of mucA leads to a growth defect under other nutrient conditions, we determined the growth rate of the ∆algU ∆mucA attTn7::ParaBAD-algU strain in other media. This strain failed to grow in the presence of 1% arabinose for all four media we tested (Figure S5), showing that AlgU overproduction is toxic under various nutrient conditions.
2.8 |. Expression of rpoD can rescue the growth defect of algU overexpression
AlgU competes for RNAP binding with RpoD, the essential primary sigma factor (Yin et al., 2013). We, therefore, tested if the growth defect of high algU expression in the ∆algU ∆mucA background could be ameliorated by overexpressing rpoD under the same arabinose-inducible promoter. We tested growth in the presence of 2% arabinose, since these new strains contain two arabinose-inducible promoters, in contrast to the strains described in Figure 6. We confirmed that RpoD was overproduced in these new strains at similar levels upon arabinose induction (Figure S6). As expected (Figure 6), ∆algU ∆mucA attTn7::ParaBAD-algU failed to grow in the presence of 2% arabinose. The ∆algU ∆mucA attTn7::ParaBAD-algU attCTX::ParaBAD-rpoD strain, however, was able to grow with arabinose. Furthermore, the growth rate of this strain with arabinose was indistinguishable from that of ∆algU ∆mucA attCTX::ParaBAD-rpoD (Table S4).
There are two major explanations for how sigma factor competition may lead to toxicity during algU overexpression. First, the AlgU overproduction may reduce housekeeping gene expression due to the limited availability of RNAP to interact with RpoD. Second, algU overexpression results in high AlgU activity, which may lead to toxic expression of the AlgU regulon. We, therefore, constructed a strain containing an inducible algU encoding a K57A substitution in the ∆algU ∆mucA background. AlgU K57A exhibited reduced sigma factor activity (Figure 5a,b), and based on its location in the crystal structure (Campagne et al., 2014), we do not expect this substitution to affect RNAP affinity. In the presence of arabinose, the ∆algU ∆mucA attTn7::ParaBAD-algU K57A strain exhibited a growth rate greater than that of the strain overexpressing wild-type AlgU, but significantly less than that of the parental ∆algU ∆mucA strain (Figure 7, Table S4). RpoD overproduction in this background returned growth to a statistically similar level as that of a strain overexpressing RpoD alone (Table S4). Assuming that the K57A substitution does not affect AlgU affinity for RNAP, these results suggest that AlgU overexpression may lead to toxic expression of the AlgU regulon.
FIGURE 7.
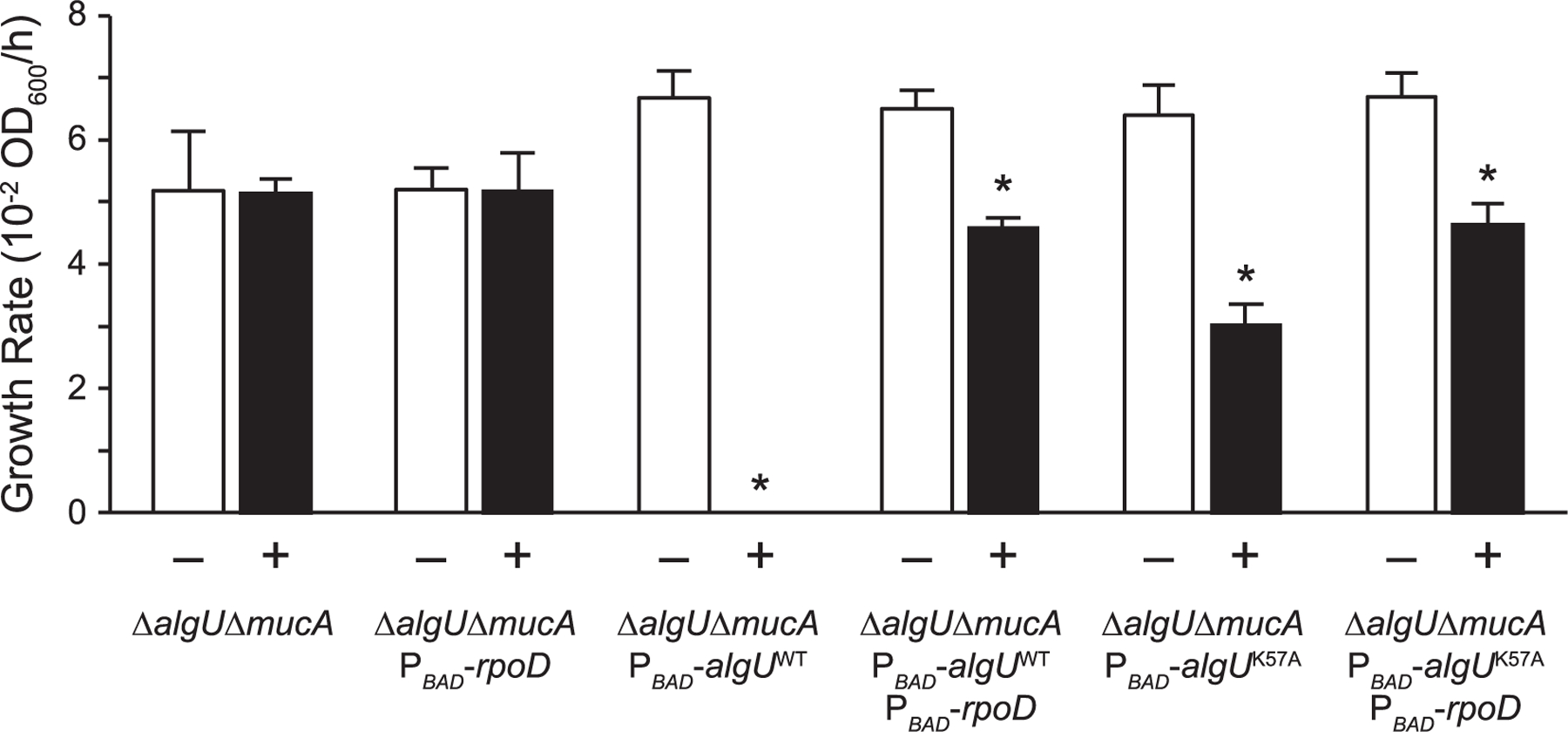
Growth defect caused by algU overexpression can be rescued via rpoD overexpression. Growth rate of indicated strains grown in LB with (+) or without (−) 2% arabinose. Error bars, SEM (N = 3). Asterisk, statistically different from the same strain grown without arabinose (p < .01, N = 3, two-way ANOVA with post-hoc Bonferroni). See Table S4 for full statistical comparisons
3 |. DISCUSSION
Contrary to its assumed dispensability, our work shows that mucA is essential in a variety of P. aeruginosa wild-type strains and under various nutrient conditions (Table 1, Figure 1). Our data strongly suggest that unchecked AlgU activity in the absence of MucA leads to cell death (Figure 8). Under non-stress conditions, MucA inhibits AlgU. Under envelope stress or in strains containing mucA mutations, the anti-sigma factor is cleaved. We propose that while this cleaved cytosolic form of MucA does not inhibit AlgU to the same extent as the full-length protein, it is still able to inhibit AlgU to some degree. Although AlgU is active under such conditions, because mucA is positively regulated by AlgU, this negative feedback keeps AlgU activity under control. In comparison, this ability to control the positive feedback of AlgU on its own expression and activity is lost in the absence of MucA, which we propose is the cause of cell death. Supporting this model are several lines of evidence. First, mucA essentiality is rooted in its interaction with algU (Figures 2 and 3), strongly suggesting that the ability of MucA to inhibit AlgU is required for viability. Second, AlgU mutants with decreased transcriptional activity can suppress mucA essentiality (Figures 4 and 5), supporting the idea that decreasing the positive feedback of AlgU on its own expression allows for survival in the absence of MucA. Lastly, algU overexpression led to a growth defect, which was lethal at high levels in the absence of mucA (Figure 6), suggesting that high AlgU activity leads to cell death when MucA is not present. Overall, our work strongly suggests that mucA essentiality is caused by unchecked AlgU activity, in agreement with previous studies suggesting that overproduction of AlgU is toxic (Cross et al., 2020; Hershberger et al., 1995; Schurr et al., 1994).
FIGURE 8.
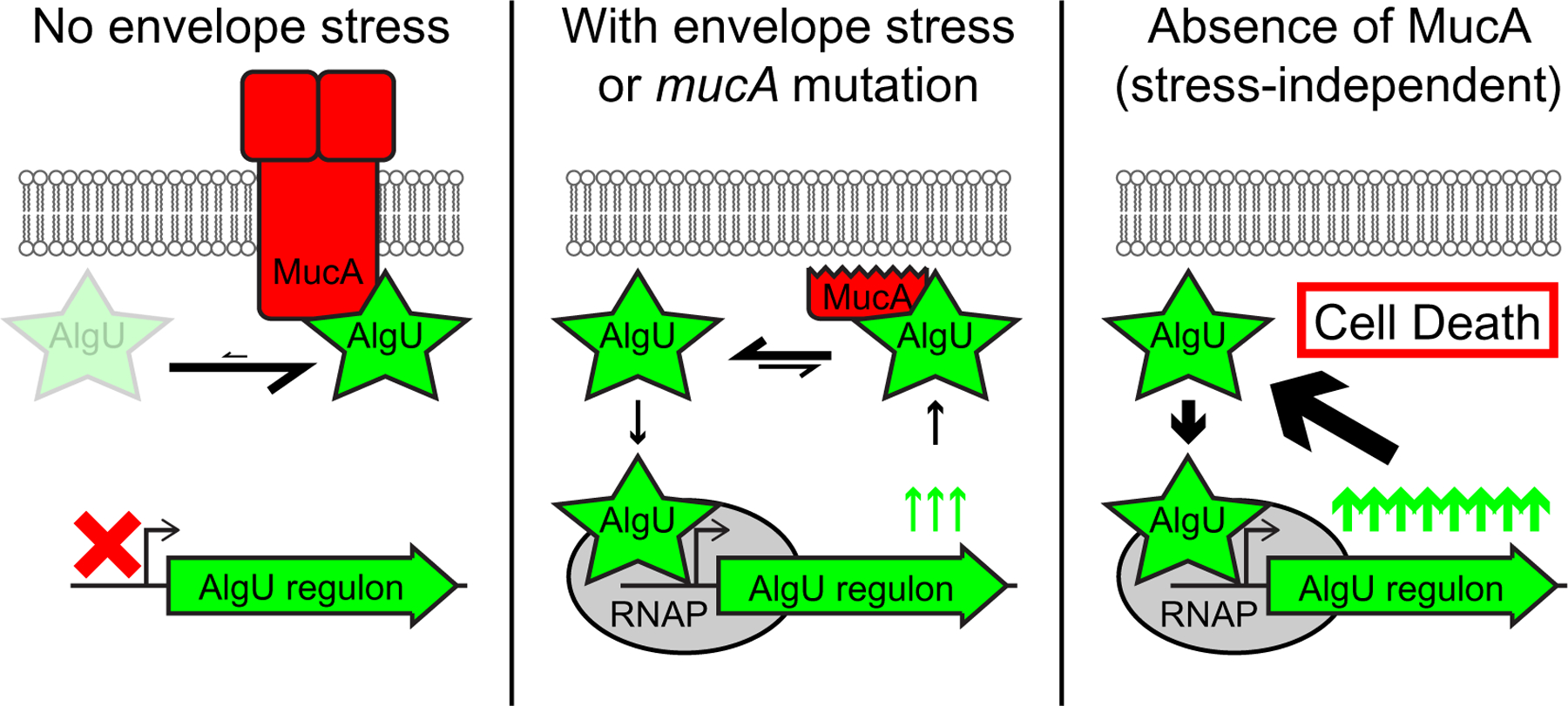
Unchecked AlgU activity in the absence of MucA leads to bacterial cell death. Under conditions of no envelope stress (left), the full-length MucA (red) binds strongly to AlgU (green star), leaving very little free AlgU to interact with the RNAP (grey oval). Therefore, the AlgU regulon (green arrow) is in the “off” state (red X). Under conditions of envelope stress or in strains containing mucA mutations that lead to a truncated product (middle), a cleaved cytosolic form of MucA is produced. This form can still interact with and inhibit AlgU. However, the strength of the interaction is weaker than with the full-length MucA, allowing for a pool of free AlgU that can then bind to and recruit RNAP to the promoters of its regulon. Under such conditions, the AlgU regulon, which includes algU and mucA, is activated and in the “on” state (green up arrows). However, because of the feedback on MucA, negative regulation of AlgU activity is still present. In the absence of mucA (right), there is no MucA to inhibit AlgU. All of the AlgU is free to interact with the core RNAP. This leads to high expression of the AlgU regulon and overproduction of AlgU itself. Under such strong positive feedback and in the absence of the negative regulator, the unchecked AlgU activity leads to cell death
AlgU overproduction may be toxic because of the reduced housekeeping gene expression (due to sigma factor competition with RpoD) and/or the increased expression of the AlgU regulon. Increasing RpoD levels alleviate the toxicity in both cases, by increasing the RNAP-RpoD complexes and reducing the RNAP-AlgU complexes. Our data do not definitively distinguish between these two mechanisms, which may not be mutually exclusive. Supporting a role for decreased housekeeping functions in AlgU toxicity, mucA was still essential in a strain lacking the three major AlgU-regulated transcription factors AlgB, AlgR, and AmrZ (Table S1), which regulate ~50% of the AlgU regulon (Huang et al., 2019; Jones et al., 2014; Leech et al., 2008; Schulz et al., 2015). However, we cannot exclude that a combination of genes in the other half of the AlgU regulon may be responsible for mucA essentiality. Supporting the hypothesis that increased AlgU regulon expression causes toxicity, mutations in algU, which is AlgU-regulated, suppress mucA essentiality (Figure 4). While this autoregulation of AlgU is important for mucA essentiality under physiological conditions, it does not lead to toxicity per se, since overexpressing algU in a strain lacking the positive feedback led to a severe growth defect (Figure 6). Under such conditions, the overproduction of AlgU K57A, which has reduced sigma factor activity (Figure 5b), caused a less severe growth defect than wild-type AlgU overproduction (Figure 7). Assuming that RNAP affinity is not affected by this AlgU substitution, this suggests that high AlgU activity and the ensuing regulon expression may be the mechanism underlying toxicity. Work in Bacillus subtilis has shown that the toxicity of unregulated SigM, an alternative sigma factor, is due specifically to membrane protein overproduction, which is alleviated by a gain-of-function mutation to the membrane insertase YidC1 (Zhao et al., 2019b). Since only ~80 of the ~200 AlgU-regulated predicted membrane proteins are regulated by AlgB, AlgR, and AmrZ (Huang et al., 2019; Jones et al., 2014; Leech et al., 2008; Schulz et al., 2015), it is possible that a gain-of-function YidC mutant may suppress mucA essentiality in P. aeruginosa. Such an experiment would help distinguish between the potential mechanisms underlying AlgU toxicity.
Anti-sigma factor essentiality is not unique to P. aeruginosa. In the defined transposon mutant library for Vibrio cholerae, an interruption in the mucA homolog rseA does not exist, suggesting that this anti-sigma factor may be essential (Cameron et al., 2008). Similarly, the Pseudomonas syringae mucA homolog is deemed essential based on transposon insertion analysis (Helmann et al., 2019). Furthermore, in Mycobacterium tuberculosis, the gene encoding the anti-sigma factor RslA is identified as essential (Griffin et al., 2011). Similar to our results (Figure 4), rslA can be deleted from M. tuberculosis lacking the gene encoding its cognate sigma factor SigL, which regulates genes involved in cell envelope processes (Dainese et al., 2006). Finally, there are two anti-sigma factors, YhdL and YxlC, in B. subtilis that are necessary for bacterial viability (Horsburgh & Moir, 1999; Koo et al., 2017; Mendez et al., 2012). The yhdL essentiality is notable as this anti-sigma factor negatively regulates SigM, an envelope stress response sigma factor (Horsburgh & Moir, 1999). Its essentiality could be suppressed by overproduction of SigA, the B. subtilis housekeeping sigma factor (Zhao et al., 2019a) and by a hyperactive form of the membrane insertase YidC1 (Zhao et al., 2019b). Interestingly, the YidC1 mutant ameliorated the toxicity of unregulated SigM by reducing the secretion stress associated with high membrane protein production (Zhao et al., 2019b). Taken together with our data, while not universal (as there are no essential anti-sigma factors in E. coli (Baba et al., 2006)), we speculate that essentiality of anti-sigma factors that regulate cell envelope function may be a widespread phenomenon in bacteria and that their essentiality is due to the increased production of membrane proteins when their sigma factors are not held in check.
In clinical CF isolates of P. aeruginosa, mucA mutations that lead to C-terminal truncations are common (Figure S2) and are expected to result in proteins that retain partial AlgU-inhibitory function. Interestingly, there is one P. aeruginosa CF isolate that is reported to have a mucA mutation that would result in a truncation within the first 50 aa of the protein (Boucher et al., 1997). Our sequencing data of the published ∆mucA strains (Intile et al., 2014; Jones et al., 2010; Pritchett et al., 2015) suggests that this isolate may contain a suppressor mutation that allows it to survive in the absence of a functional MucA. Nonetheless, in CF clinical isolates, mucA mutations that lead to C-terminal truncations are the norm, while null mutations in mucA with a presumed hypomorphic algU allele is rare (Figure S2). Furthermore, our results (Figure 4) agree with the literature showing that mucoid isolates with mucA mutations revert to a non-mucoid state via changes to algU (Ciofu et al., 2008; DeVries & Ohman, 1994; Sautter et al., 2012; Schurr et al., 1994). While these algU secondary site mutations are found in non-mucoid CF isolates with mucA mutations, such isolates are detected at a lower frequency (Ciofu et al., 2008). As suggested by Ciofu and associates, this may be due to the importance of AlgU for the survival of P. aeruginosa in the CF lung environment, suggesting that reducing AlgU activity may increase P. aeruginosa eradication from the CF airway. We propose that the MucA-AlgU interaction may serve as a good therapeutic target. Our results show that destabilizing the MucA-AlgU interaction results in bacterial cell death or mutations in algU that result in reduced sigma factor activity and likely reduced mucoid conversion, of which either outcome could be beneficial in the treatment of P. aeruginosa CF lung infections.
4 |. METHODS AND MATERIAL S
4.1 |. Bacterial strains and growth conditions
The bacterial strains, plasmids, and oligonucleotides used for this study are in Tables S5–S7. The construction of strains is described in Supplementary Information. Bacteria were grown at 37°C in LB with shaking or on semi-solid LB media, unless otherwise noted.
4.2 |. Allelic exchange assay
The protocol, depicted in Figure S1, was modified from (Hmelo et al., 2015). Briefly, a vector containing the mucA deletion allele flanked by approximately equal length homology regions is introduced via conjugation. Merodiploids were selected on semi-solid VBMM (Vogel & Bonner, 1956) with 60 mg/L gentamicin. Using OMS118 and OMS119, PCR was performed on at least six isolates to confirm that the presence of both the endogenous and deletion alleles of mucA. Confirmed merodiploids were then individually streaked on NSLB (10 g/L tryptone, 5 g/L yeast extract) with 10% sucrose semi-solid media for counterselection. PCR was performed on eight colonies per merodiploid, using OBT601 and OBT602 to determine the resolution to either the endogenous or deletion allele.
4.3 |. MucA depletion assay
Cells were grown in LB with 0.05% rhamnose at 37°C with shaking to an OD600 of 0.3. After washing, the culture was then divided in two, half resuspended with rhamnose and half without in its respective media base (LB, PIB, SCFM, or VBMM). Cells were incubated at 37°C with shaking. At the indicated time points, two aliquots were removed from each culture, serially diluted and plated in triplicate onto LB agar plates with 0.05% rhamnose for recovery. Colonies were counted and log10-transformed CFU/ml of the culture was calculated.
4.4 |. Yeast two-hybrid assay
The ProQuest Two-Hybrid System (Invitrogen) was used, per manufacturer’s instructions. Briefly, yeast containing the Gal4 activation domain-based prey and the Gal4 DNA-binding domain-based bait vectors were grown overnight in SD-Leu-Trp broth (Clontech). The OD600 value was recorded. ONPG (VWR) was added to lysed cells, and the mixture was incubated at 37°C until a light yellow color was achieved. The incubation time was recorded. The OD420 of the supernatants was determined using a Synergy Hybrid HTX Microplate Reader (Bio-Tek Instruments). Beta-galactosidase activity was determined using Miller units, based on the following equation: 1,000 × OD420/(time × culture volume × OD600).
4.5 |. AlgU activity assay
Strains of interest were transformed with a plasmid-borne algD reporter (pBT435) via electroporation (Choi et al., 2006). Strains were grown overnight in LSLB with 50 mg/L gentamicin and 0.05% rhamnose (where indicated) at 37°C with shaking. The overnight culture was diluted 1:100 and grown to an OD600 of 0.3. Cells were then treated with fresh 400 mg/L D-cycloserine for 2 hr at 37°C with shaking, as previously described (Wood et al., 2006). Cells were then pelleted and resuspended in PBS. GFP fluorescence and OD600 were determined using a Synergy Hybrid HTX Microplate Reader (Bio-Tek Instruments). To normalize the data, the GFP fluorescence was divided by the OD600 for each data point. To determine the fold induction, the ratios for the treated samples were divided by the average of that for the same strain not treated with D-cycloserine. The resulting ratios of the triplicate samples were averaged.
4.6 |. Natural revertant assay
P. aeruginosa ∆mucA attTn7::PrhaBAD-mucA was grown in LB with 0.05% rhamnose at 37°C with shaking to an OD600 of 1. Cells were washed and plated on semi-solid LB plates without rhamnose. Plates were incubated for 24–48 hr at 37°C. For isolates that grew on LB without rhamnose, algU was Sanger sequenced.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank Cai Tao and Maria Alexandra Ledesma for technical assistance; and Joe J. Harrison for critical discussions of this work. MCS, AAK, DR, and BT are supported by the Cystic Fibrosis Foundation (TSENG19I0). MCS and EKC are supported by University of Nevada Las Vegas doctoral graduate research assistantships. EAC and PAJ are supported by the National Institutes of Health (R01GM114450 and K22AI127473, respectively). PAJ is also supported by the Cystic Fibrosis Foundation (JORTH17F5).
Funding information
Cystic Fibrosis Foundation, Grant/Award Number: JORTH17F5 and TSENG19I0; National Institute of General Medical Sciences, Grant/Award Number: R01GM114450; National Institute of Allergy and Infectious Diseases, Grant/Award Number: K22AI127473
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicts of interest to disclose.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the Supporting Information section.
REFERENCES
- Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M et al. (2006) Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: The Keio collection. Molecular Systems Biology, 2, 2006‒0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher JC, Yu H, Mudd MH & Deretic V (1997) Mucoid Pseudomonas aeruginosa in cystic fibrosis: Characterization of muc mutations in clinical isolates and analysis of clearance in a mouse model of respiratory infection. Infection and Immunity, 65, 3838–3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron DE, Urbach JM & Mekalanos JJ (2008) A defined transposon mutant library and its use in identifying motility genes in Vibrio cholerae. Proceedings of the National Academy of Sciences of the United States of America, 105, 8736–8741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campagne S, Marsh ME, Capitani G, Vorholt JA & Allain FH (2014) Structural basis for -10 promoter element melting by environmentally induced sigma factors. Nature Structural & Molecular Biology, 21, 269–276. [DOI] [PubMed] [Google Scholar]
- Candido Cacador N, Paulino da Costa Capizzani, C., Gomes Monteiro Marin Torres, L.A., Galetti, R., Ciofu, O., da Costa Darini, A.L., & Hoiby, N. (2018) Adaptation of Pseudomonas aeruginosa to the chronic phenotype by mutations in the algTmucABD operon in isolates from Brazilian cystic fibrosis patients. PLoS One, 13, e0208013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KH, Kumar A & Schweizer HP (2006) A 10-min method for preparation of highly electrocompetent Pseudomonas aeruginosa cells: Application for DNA fragment transfer between chromosomes and plasmid transformation. Journal of Microbiol Methods, 64, 391–397. [DOI] [PubMed] [Google Scholar]
- Ciofu O, Lee B, Johannesson M, Hermansen NO, Meyer P & Hoiby N (2008) Investigation of the algT operon sequence in mucoid and non-mucoid Pseudomonas aeruginosa isolates from 115 Scandinavian patients with cystic fibrosis and in 88 in vitro non-mucoid revertants. Microbiology, 154, 103–113. [DOI] [PubMed] [Google Scholar]
- Colvin KM, Irie Y, Tart CS, Urbano R, Whitney JC, Ryder C et al. (2012) The Pel and Psl polysaccharides provide Pseudomonas aeruginosa structural redundancy within the biofilm matrix. Environmental Microbiology, 14, 1913–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross AR, Raghuram V, Wang Z, Dey D & Goldberg JB (2020) Overproduction of the AlgT sigma factor is lethal to mucoid Pseudomonas aeruginosa. Journal of Bacteriology, 202, e00445–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cystic Fibrosis Foundation Patient Registry. (2019) 2018 annual data report. Cystic Fibrosis Foundation [Google Scholar]
- Dainese E, Rodrigue S, Delogu G, Provvedi R, Laflamme L, Brzezinski R et al. (2006) Posttranslational regulation of Mycobacterium tuberculosis extracytoplasmic-function sigma factor sigma L and roles in virulence and in global regulation of gene expression. Infection and Immunity, 74, 2457–2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damron FH & Goldberg JB (2012) Proteolytic regulation of alginate overproduction in Pseudomonas aeruginosa. Molecular Microbiology, 84, 595–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damron FH, Qiu D & Yu HD (2009) The Pseudomonas aeruginosa sensor kinase KinB negatively controls alginate production through AlgW-dependent MucA proteolysis. Journal of Bacteriology, 191, 2285–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deretic V, Gill JF & Chakrabarty AM (1987) Gene algD coding for GDPmannose dehydrogenase is transcriptionally activated in mucoid Pseudomonas aeruginosa. Journal of Bacteriology, 169, 351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries CA & Ohman DE (1994) Mucoid-to-nonmucoid conversion in alginate-producing Pseudomonas aeruginosa often results from spontaneous mutations in algT, encoding a putative alternate sigma factor, and shows evidence for autoregulation. Journal of Bacteriology, 176, 6677–6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas TA, Brennan S, Gard S, Berry L, Gangell C, Stick SM et al. (2009) Acquisition and eradication of P. aeruginosa in young children with cystic fibrosis. European Respiratory Journal, 33, 305–311. [DOI] [PubMed] [Google Scholar]
- Emerson J, Rosenfeld M, McNamara S, Ramsey B & Gibson RL (2002) Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatric Pulmonology, 34, 91–100. [DOI] [PubMed] [Google Scholar]
- Farrell PM, Collins J, Broderick LS, Rock MJ, Li Z, Kosorok MR et al. (2009) Association between mucoid pseudomonas infection and bronchiectasis in children with cystic fibrosis. Radiology, 252, 534–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firoved AM & Deretic V (2003) Microarray analysis of global gene expression in mucoid Pseudomonas aeruginosa. Journal of Bacteriology, 185, 1071–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher LA, Shendure J & Manoil C (2011) Genome-scale identification of resistance functions in Pseudomonas aeruginosa using Tn-seq. Mbio, 2, e00315–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govan JR & Deretic V (1996) Microbial pathogenesis in cystic fibrosis: Mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiological Reviews, 60, 539–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin JE, Gawronski JD, Dejesus MA, Ioerger TR, Akerley BJ & Sassetti CM (2011) High-resolution phenotypic profiling defines genes essential for mycobacterial growth and cholesterol catabolism. PLoS Pathogens, 7, e1002251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmann TC, Deutschbauer AM & Lindow SE (2019) Genome-wide identification of Pseudomonas syringae genes required for fitness during colonization of the leaf surface and apoplast. Proceedings of the National Academy of Sciences of the United States of America, 116, 18900–18910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry RL, Mellis CM & Petrovic L (1992) Mucoid Pseudomonas aeruginosa is a marker of poor survival in cystic fibrosis. Pediatric Pulmonology, 12, 158–161. [DOI] [PubMed] [Google Scholar]
- Hershberger CD, Ye RW, Parsek MR, Xie ZD & Chakrabarty AM (1995) The algT (algU) gene of Pseudomonas aeruginosa, a key regulator involved in alginate biosynthesis, encodes an alternative sigma factor (sigma E). Proceedings of the National Academy of Sciences of the United States of America, 92, 7941–7945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hmelo LR, Borlee BR, Almblad H, Love ME, Randall TE, Tseng BS et al. (2015) Precision-engineering the Pseudomonas aeruginosa genome with two-step allelic exchange. Nature Protocols, 10, 1820–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsburgh MJ & Moir A (1999) sigmaM, an ECF RNA polymerase sigma factor of Bacillus subtilis 168, is essential for growth and survival in high concentrations of salt. Molecular Microbiology, 32, 41–50. [DOI] [PubMed] [Google Scholar]
- Huang H, Shao X, Xie Y, Wang T, Zhang Y, Wang X et al. (2019) An integrated genomic regulatory network of virulence-related transcriptional factors in Pseudomonas aeruginosa. Nature Communications, 10, 2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intile PJ, Diaz MR, Urbanowski ML, Wolfgang MC & Yahr TL (2014) The AlgZR two-component system recalibrates the RsmAYZ posttranscriptional regulatory system to inhibit expression of the Pseudomonas aeruginosa type III secretion system. Journal of Bacteriology, 196, 357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AK, Fulcher NB, Balzer GJ, Urbanowski ML, Pritchett CL, Schurr MJ et al. (2010) Activation of the Pseudomonas aeruginosa AlgU regulon through mucA mutation inhibits cyclic AMP/Vfr signaling. Journal of Bacteriology, 192, 5709–5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CJ, Newsom D, Kelly B, Irie Y, Jennings LK, Xu B et al. (2014) ChIP-Seq and RNA-Seq reveal an AmrZ-mediated mechanism for cyclic di-GMP synthesis and biofilm development by Pseudomonas aeruginosa. PLoS Pathogens, 10, e1003984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong W, Zhao J, Kang H, Zhu M, Zhou T, Deng X & et al. (2015) ChIP-seq reveals the global regulator AlgR mediating cyclic di-GMP synthesis in Pseudomonas aeruginosa. Nucleic Acids Research, 43, 8268–8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo BM, Kritikos G, Farelli JD, Todor H, Tong K, Kimsey H et al. (2017) Construction and analysis of two genome-scale deletion libraries for Bacillus subtilis. Cell Systems, 4(291–305), e297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SA, Gallagher LA, Thongdee M, Staudinger BJ, Lippman S, Singh PK et al. (2015) General and condition-specific essential functions of Pseudomonas aeruginosa. Proceedings of the National Academy of Sciences USA, 112, 5189–5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech AJ, Sprinkle A, Wood L, Wozniak DJ & Ohman DE (2008) The NtrC family regulator AlgB, which controls alginate biosynthesis in mucoid Pseudomonas aeruginosa, binds directly to the algD promoter. Journal of Bacteriology, 190, 581–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Lou X, Xu Y, Teng X, Liu R, Zhang Q et al. (2019) Structural basis for the recognition of MucA by MucB and AlgU in Pseudomonas aeruginosa. The FEBS Journal, 286, 4982–4994. [DOI] [PubMed] [Google Scholar]
- Li Z, Kosorok MR, Farrell PM, Laxova A, West SE, Green CG et al. (2005) Longitudinal development of mucoid Pseudomonas aeruginosa infection and lung disease progression in children with cystic fibrosis. JAMA, 293, 581–588. [DOI] [PubMed] [Google Scholar]
- Liberati NT, Urbach JM, Miyata S, Lee DG, Drenkard E, Wu G et al. (2006) An ordered, nonredundant library of Pseudomonas aeruginosa strain PA14 transposon insertion mutants. Proceedings of the National Academy of Sciences USA, 103, 2833–2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DW, Schurr MJ, Mudd MH, Govan JR, Holloway BW & Deretic V (1993) Mechanism of conversion to mucoidy in Pseudomonas aeruginosa infecting cystic fibrosis patients. Proceedings of the National Academy of Sciences USA, 90, 8377–8381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DW, Schurr MJ, Yu H & Deretic V (1994) Analysis of promoters controlled by the putative sigma factor AlgU regulating conversion to mucoidy in Pseudomonas aeruginosa: Relationship to sigma E and stress response. Journal of Bacteriology, 176, 6688–6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathee K, McPherson CJ & Ohman DE (1997) Posttranslational control of the algT (algU)-encoded sigma22 for expression of the alginate regulon in Pseudomonas aeruginosa and localization of its antagonist proteins MucA and MucB (AlgN). Journal of Bacteriology, 179, 3711–3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez R, Gutierrez A, Reyes J & Marquez-Magana L (2012) The extracytoplasmic function sigma factor SigY is important for efficient maintenance of the Spbeta prophage that encodes sublancin in Bacillus subtilis. DNA and Cell Biology, 31, 946–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon GM, Armstrong DS, Carzino R, Carlin JB, Olinsky A, Robertson CF et al. (2001) Clinical outcome after early Pseudomonas aeruginosa infection in cystic fibrosis. Journal of Pediatrics, 138, 699–704. [DOI] [PubMed] [Google Scholar]
- Panmanee W, Su S, Schurr MJ, Lau GW, Zhu X, Ren Z et al. (2019) The anti-sigma factor MucA of Pseudomonas aeruginosa: Dramatic differences of a mucA22 vs. a DeltamucA mutant in anaerobic acidified nitrite sensitivity of planktonic and biofilm bacteria in vitro and during chronic murine lung infection. PLoS One, 14, e0216401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parad RB, Gerard CJ, Zurakowski D, Nichols DP & Pier GB (1999) Pulmonary outcome in cystic fibrosis is influenced primarily by mucoid Pseudomonas aeruginosa infection and immune status and only modestly by genotype. Infection and Immunity, 67, 4744–4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen SS, Hoiby N, Espersen F & Koch C (1992) Role of alginate in infection with mucoid Pseudomonas aeruginosa in cystic fibrosis. Thorax, 47, 6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchett CL, Little AS, Okkotsu Y, Frisk A, Cody WL, Covey CR et al. (2015) Expression analysis of the Pseudomonas aeruginosa AlgZR two-component regulatory system. Journal of Bacteriology, 197, 736–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulcrano G, Iula DV, Raia V, Rossano F & Catania MR (2012) Different mutations in mucA gene of Pseudomonas aeruginosa mucoid strains in cystic fibrosis patients and their effect on algU gene expression. New Microbiologica, 35, 295–305. [PubMed] [Google Scholar]
- Qiu D, Eisinger VM, Head NE, Pier GB & Yu HD (2008) ClpXP proteases positively regulate alginate overexpression and mucoid conversion in Pseudomonas aeruginosa. Microbiology, 154, 2119–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu D, Eisinger VM, Rowen DW & Yu HD (2007) Regulated proteolysis controls mucoid conversion in Pseudomonas aeruginosa. Proceedings of the National Academy of Sciences USA, 104, 8107–8112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sautter R, Ramos D, Schneper L, Ciofu O, Wassermann T, Koh CL et al. (2012) A complex multilevel attack on Pseudomonas aeruginosa algT/U expression and algT/U activity results in the loss of alginate production. Gene, 498, 242–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz S, Eckweiler D, Bielecka A, Nicolai T, Franke R, Dotsch A et al. (2015) Elucidation of sigma factor-associated networks in Pseudomonas aeruginosa reveals a modular architecture with limited and function-specific crosstalk. PLoS Pathogens, 11, e1004744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurr MJ, Martin DW, Mudd MH & Deretic V (1994) Gene cluster controlling conversion to alginate-overproducing phenotype in Pseudomonas aeruginosa: Functional analysis in a heterologous host and role in the instability of mucoidy. Journal of Bacteriology, 176, 3375–3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurr MJ, Yu H, Martinez-Salazar JM, Boucher JC & Deretic V (1996) Control of AlgU, a member of the sigma E-like family of stress sigma factors, by the negative regulators MucA and MucB and Pseudomonas aeruginosa conversion to mucoidy in cystic fibrosis. Journal of Bacteriology, 178, 4997–5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skurnik D, Roux D, Aschard H, Cattoir V, Yoder-Himes D, Lory S et al. (2013) A comprehensive analysis of in vitro and in vivo genetic fitness of Pseudomonas aeruginosa using high-throughput sequencing of transposon libraries. PLoS Pathogens, 9, e1003582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strateva T, Petrova G & Mitov I (2010) Antimicrobial activity of tobramycin against respiratory cystic fibrosis Pseudomonas aeruginosa isolates from Bulgaria. Journal of Chemotherapy, 22, 378–383. [DOI] [PubMed] [Google Scholar]
- Turner KH, Wessel AK, Palmer GC, Murray JL & Whiteley M (2015) Essential genome of Pseudomonas aeruginosa in cystic fibrosis sputum. Proceedings of the National Academy of Sciences USA, 112, 4110–4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel HJ & Bonner DM (1956) Acetylornithinase of Escherichia coli: Partial purification and some properties. Journal of Biological Chemistry, 218, 97–106. [PubMed] [Google Scholar]
- Wood LF, Leech AJ & Ohman DE (2006) Cell wall-inhibitory antibiotics activate the alginate biosynthesis operon in Pseudomonas aeruginosa: Roles of sigma (AlgT) and the AlgW and Prc proteases. Molecular Microbiology, 62, 412–426. [DOI] [PubMed] [Google Scholar]
- Wood LF & Ohman DE (2009) Use of cell wall stress to characterize sigma 22 (AlgT/U) activation by regulated proteolysis and its regulon in Pseudomonas aeruginosa. Molecular Microbiology, 72, 183–201. [DOI] [PubMed] [Google Scholar]
- Wozniak DJ & Ohman DE (1994) Transcriptional analysis of the Pseudomonas aeruginosa genes algR, algB, and algD reveals a hierarchy of alginate gene expression which is modulated by algT. Journal of Bacteriology, 176, 6007–6014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak DJ, Sprinkle AB & Baynham PJ (2003) Control of Pseudomonas aeruginosa algZ expression by the alternative sigma factor AlgT. Journal of Bacteriology, 185, 7297–7300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie ZD, Hershberger CD, Shankar S, Ye RW & Chakrabarty AM (1996) Sigma factor-anti-sigma factor interaction in alginate synthesis: Inhibition of AlgT by MucA. Journal of Bacteriology, 178, 4990–4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Withers TR, Wang X & Yu HD (2013) Evidence for sigma factor competition in the regulation of alginate production by Pseudomonas aeruginosa. PLoS One, 8, e72329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Schurr MJ & Deretic V (1995) Functional equivalence of Escherichia coli sigma E and Pseudomonas aeruginosa AlgU: E. coli rpoE restores mucoidy and reduces sensitivity to reactive oxygen intermediates in algU mutants of P. aeruginosa. Journal of Bacteriology, 177, 3259–3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Roistacher DM & Helmann JD (2019a) Deciphering the essentiality and function of the anti-sigma(M) factors in Bacillus subtilis. Molecular Microbiology, 112, 482–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Sachla AJ & Helmann JD (2019b) Mutations of the Bacillus subtilis YidC1 (SpoIIIJ) insertase alleviate stress associated with sigmaM-dependent membrane protein overproduction. PLoS Genetics, 15, e1008263. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


