Abstract
Background:
Perioperative management of patients with hip fracture patients receiving oral anticoagulants requires navigating the risks associated with surgical delay and perioperative hemostasis. The aim of this systematic review and meta-analysis was to evaluate the effect of expedited-surgery protocols on time to surgery and perioperative outcomes in anticoagulant-treated patients with hip fracture.
Methods:
We searched MEDLINE, Embase and CENTRAL from inception to May 5, 2020, to identify English-language studies reporting outcomes after expedited hip fracture surgery in patients receiving vitamin K antagonists (VKAs) or direct oral anticoagulants (DOACs) before hospital admission. We performed a meta-analysis using Mantel–Haenszel weighting for dichotomous variables and inverse variance weighting for continuous variables.
Results:
Among the 4253 citations identified, 14 studies were included. In the 6 studies eligible for meta-analysis, compared to hip fracture surgery before implementation of a VKA-reversal protocol, surgery after implementation of such a protocol was associated with a significant reduction in time to surgery (mean difference 45.31 h, 95% confidence interval [CI] 15.81 h to 74.80 h). Expedited surgery (within 48 h) in patients who received DOACs preoperatively was not associated with increased surgical duration (mean difference −7.29 min, 95% CI −22.5 min to 7.95 min) or 30-day mortality (odds ratio [OR] 1.30, 95% CI 0.49 to 3.89) compared to patients who did not receive anticoagulants (control patients). However, expedited surgery in DOAC-treated patients was associated with an increased blood transfusion risk compared to control patients (OR 0.58, 95% CI 0.36 to 0.96).
Conclusion:
Implementing a VKA-reversal protocol for patients with hip fracture is effective in decreasing time to surgery, without an increased bleeding risk. Performing hip fracture surgery within 48 hours in DOAC-treated patients is also safe, with a small increase in blood transfusion risk.
Abstract
Contexte:
La prise en charge périopératoire de patients sous anticoagulothérapie ayant subi une fracture de la hanche nécessite la gestion des risques associés aux délais chirurgicaux et à l’hémostase périopératoire. La présente revue systématique et métaanalyse a pour objectif d’évaluer les effets de protocoles chirurgicaux accélérés sur le délai d’accès à la chirurgie et les issues périopératoires chez ces patients.
Méthodes:
Nous avons interrogé les bases MEDLINE, Embase et CENTRAL de leur création au 5 mai 2020 pour recenser les études publiées en anglais qui traitaient des issues d’opérations accélérées pour une fracture de la hanche chez des patients sous antivitamine K (AVK) ou anticoagulants oraux directs (AOD) avant leur hospitalisation. La méta-analyse a été effectuée au moyen de la méthode de Mantel–Haenszel pour les variables dichotomiques et de la pondération par l’inverse de la variance pour les variables continues.
Résultats:
À partir des 4253 citations recensées, 14 études ont été retenues. Dans les 6 études répondant aux critères de la méta-analyse, l’opération pour une fracture de la hanche après mise en oeuvre d’un protocole de désanticoagulation pour l’AVK a été associée à une réduction significative de l’intervalle préchirurgical (différence moyenne 45,31 h, intervalle de confiance [IC] de 95 % 15,81 h à 74,80 h), comparativement à la même intervention avant la mise en place du protocole. La chirurgie accélérée (dans les 48 h) chez les patients sous AOD avant l’opération n’a pas été associée à un allongement de la durée de l’intervention (différence moyenne −7,29 min, IC de 95 % −22,5 min à 7,95 min) ni à une augmentation de la mortalité dans les 30 jours (rapport de cotes [RC] 1,30, IC de 95 % 0,49 à 3,89), comparativement aux patients qui ne recevaient pas d’anticoagulothérapie (groupe témoin). Cependant, la chirurgie accélérée chez les patients sous AOD a été associée à un risque accru de besoin de transfusion sanguine, comparativement au groupe témoin (RC 0,58, IC de 95 % 0,36 à 0,96).
Conclusion:
La mise en place d’un protocole de désanticoagulation pour l’AVK chez les patients ayant subi une fracture de la hanche réduit efficacement l’intervalle préchirurgical sans augmentation du risque de saignement. Il est également sécuritaire d’effectuer une opération pour une fracture de la hanche dans les 48 heures chez les patients sous AOD, quoique l’intervention soit associée à un risque légèrement accru de transfusion sanguine.
Each year, 340 000 people are admitted with acute hip fracture in the United States, accruing an estimated US$9.8 billion in health care costs.1 Although oral anticoagulants (OACs), including vitamin K antagonists (VKAs) and direct oral anticoagulants (DOACs), are highly effective in preventing and treating thromboembolic conditions,2 OAC use is most prevalent among adults more than 65 years of age, a vulnerable population with a lifetime risk of having an osteoporotic hip fracture greater than 20%.3 The prevalence of patients with hip fracture receiving VKAs and DOACs has been reported to be 10.3% and 9.1%, respectively.4,5 With the rising trend in OAC uptake, specifically DOACs, with expanding indications for primary and secondary thromboprophylaxis,6 the impact of OACs at the time of hip fracture will become increasingly relevant.
The perioperative management of OAC-treated patients with hip fracture requires navigating the delicate competing risks associated with surgical delay and perioperative hemostasis. On one hand, a delay to hip fracture surgery has consistently been shown to increase postoperative morbidity and all-cause mortality.7,8 On the other hand, there is concern that a residual degree of anticoagulant effect exists within the first 24–48 hours after DOAC intake that may impair perioperative hemostasis.9
Although effective VKA-reversal agents, including vitamin K, fresh frozen plasma and prothrombin complex concentrates (PCCs), are readily available,10 an antidote for DOACs did not exist until recently, with the advent of idarucizumab and andexanet alfa for the reversal of direct thrombin and factor Xa inhibitors, respectively.11 However, these novel antidotes are expensive: reversal of factor Xa inhibition with andexanet alfa is associated with a 3.9-fold higher cost per patient compared to conventional reversal agents such as PCCs.12 Furthermore, not reversing oral anticoagulation before hip fracture surgery may be safe and expedite time to surgery. Meinig and colleagues13 found that blood transfusion rates were similar between patients in whom oral anticoagulation was not reversed and those who had anticoagulant reversal before hip fracture surgery. Nonetheless, the impact of rapid OAC reversal protocols13–15 and expediting hip fracture surgery (within 48 h of admission) on outcomes in anticoagulant-treated patients with hip fracture has not been systematically evaluated.
Given the uncertainty in the current literature on the impact of OAC reversal and expedited surgery on clinical outcomes in anticoagulant-treated patients with hip fracture, we performed a systematic review and meta-analysis to evaluate the effect of expedited surgical protocols on time to surgery, perioperative outcomes and thromboembolic complications in this population.
Methods
We performed this systematic review using a predetermined study protocol registered with the International Prospective register of systematic reviews (PROSPERO) (CRD42020155306). The review is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines.
Search strategy and study selection
In collaboration with a medical librarian, we performed a systematic literature search of Embase, MEDLINE and the Cochrane Central Register of Controlled Trials (CENTRAL) from inception to May 5, 2020 (Appendix 1, available at canjsurg.ca/lookup/doi/10.1503/cjs.010021/tab-related-content). We retrieved grey literature by accessing ClinicalTrials.gov and ClinicalStudyResults.org, contacting study authors for unavailable articles, and forward and backward searching of references from publications included in the review. We compiled the studies using Covidence and deleted duplicates. Two authors (Y.X., D.Y.) screened titles and abstracts independently based on predetermined inclusion and exclusion criteria, and performed full-text review of retained articles. Conflicts were resolved by a consensus decision between the 2 reviewers or with a third author (P.S.), if required.
Inclusion and exclusion criteria
Study eligibility included randomized controlled trials, prospective/retrospective cohort studies, cross-sectional studies and case–control studies, including meeting abstracts. Studies were included if they focused on patients with hip fracture receiving a VKA (warfarin, phenprocoumon or acenocoumarol) or DOAC (dabigatran, rivaroxaban, apixaban or edoxaban) at the time of hip fracture who were treated after a predetermined anticoagulant-reversal protocol or underwent expedited surgery within 48 hours without anticoagulant reversal. Case reports, reviews, letters to the editor, commentaries, and studies or abstracts not available in English were excluded.
Data extraction and quality assessment
Data were extracted by 2 authors (Y.X., D.Y.) working independently. A standardized data extraction form was used to record author, publication date, study design, number of patients, patient demographic characteristics, anticoagulant-reversal protocol and outcomes of interest. The primary outcome was time to surgery. Secondary outcomes included length of stay, postoperative venous thromboembolism (VTE), postoperative red blood cell transfusion and change in hemoglobin level, surgical duration and 30-day mortality. Postoperative VTE included symptomatic deep vein thrombosis and pulmonary embolism. Asymptomatic deep vein thrombosis detected with screening ultrasonography was excluded. Risk of bias was assessed and classified as low, high or unclear with the use of components of the Newcastle–Ottawa Scale,16 which was used in a prior meta-analysis involving anticoagulant therapy.17
Data synthesis and analysis
We performed a meta-analysis for all comparative studies that reported on 3 or more studies by pooling the results using Review Manager 5.3 (Cochrane Collaboration). Continuous data were reported as means and standard deviations (SDs), and dichotomous outcomes as number of events or odds ratios (ORs). We performed statistical analysis by comparing mean differences of 2 or more studies when results were collected with the use of similar measures. For continuous data reported as median and interquartile range, we approximated the sample mean and SD using an established estimation method for performing meta-analysis.18 If the SD value was not specified or given, we imputed from comparable studies, as per Cochrane manual guidelines.19 Studies that did not include enough data for a sample mean and SD were not included in the meta-analysis.
We pooled random-effects models for unadjusted/crude study estimates using Mantel–Haenszel weighting for dichotomous variable analysis and inverse variance weighting for continuous variable analysis. Data were reported as mean difference with 95% confidence intervals (CIs). When meta-analysis was not possible, we summarized results using descriptive statistics. We assessed heterogeneity between studies using the I2 statistic, with values of 75% or greater indicating substantial heterogeneity. For all analyses, p values < 0.05 were considered statistically significant.
To approach our objectives, we included 2 predefined sets of analyses. In the first set of analyses, we compared differences in surgical outcomes before and after implementation of expedited-surgery protocols in anticoagulant-treated patients. In the second set of analyses, we included studies that compared surgical outcomes between VKA- and DOAC-treated patients who received an expedited-surgery protocol and patients who were not anticoagulant treated at the time of their hip fracture (control group).
Results
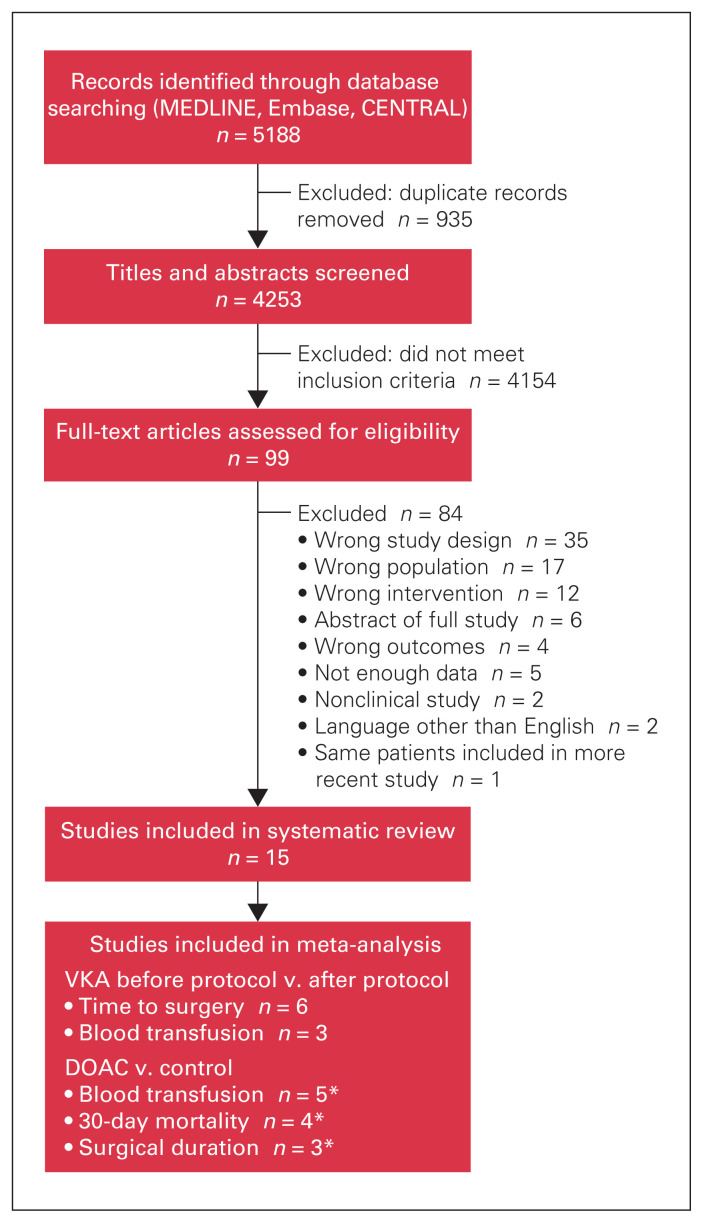
A total of 5188 records were identified through database searching (Figure 1). After duplicates were removed, 4253 abstracts underwent title and abstract screening, of which 4154 were excluded because they did not meet the inclusion criteria. The remaining 99 studies underwent full-text review, and 14 were determined to be eligible and were included in the systematic review.14,15,20–31 The year of publication ranged from 2010 to 2019.
Fig. 1.
Flow diagram showing study selection. DOAC = direct oral anticoagulant; VKA = vitamin K antagonist. *Schermann and colleagues20 grouped patients into hemiarthroplasty and closed reduction internal fixation cohorts.
We divided the review into studies that included patients who received VKAs and those who received DOACs. Eleven studies included VKA-treated patients (247 before VKA-reversal protocol, 516 after VKA-reversal protocol and 1023 control patients) (Table 1). Although no study assessed the efficacy of standardized DOAC-reversal protocols by means of a pre-/postimplementation methodology, in 4 studies, DOAC-treated patients who underwent hip fracture surgery within 48 hours were compared to patients who did not receive anticoagulants (Table 2).
Table 1.
Study characteristics and baseline demographic characteristics of patients with hip fracture who received a vitamin K antagonist before or after implementation of a vitamin K antagonist–reversal protocol, and of patients who did not receive an anticoagulant (control)
| Study | Study design | Inclusion criteria | Reversal protocol | No. of patients | Age, mean ± SD (range), yr | Female sex, no. of patients | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| Before protocol | After protocol | Control | Before protocol | After protocol | Control | Before protocol | After protocol | Control | ||||
| Schuetze et al.,29 2019 | Retrospective cohort | Inter- or subtrochanteric hip fracture | Surgery within 24 h; reversal not mentioned | — | 25 | 146 | — | NR | NR | — | NR | NR |
|
| ||||||||||||
| Mattisson et al.,15 2018 | Retrospective case–control | Inter- or subtrochanteric hip fracture, > 60 yr of age | Surgery within 24 h; vitamin K and PCCs until INR < 1.5 | — | 99 | 99 | — | 86.0 ± 7.4 | 86.0 ± 7.1 | — | 69 | 69 |
|
| ||||||||||||
| Moores et al.,26 2018 | Retrospective case–control | Hip fracture | Vitamin K on admission and every 6 h until INR < 1.7 | 46 | 48 | — | 80.5 ± 7.6 | 81.2 ± 8.0 | — | 33 | 32 | — |
|
| ||||||||||||
| Ng et al.,28 2019 | Case series | Hip fracture, INR > 1.5 | PCCs until INR ≤ 1.4 | — | 33 | — | — | 81.0 ± 7 | — | — | 19 | — |
|
| ||||||||||||
| Diament et al.,21 2015 | Prospective cohort study | Hip fracture | Vitamin K on admission and every 12 h until INR ≤ 1.5 | 80 | 42 | 403 | 82.1 (64–100) | 83.3 (72–95) | 81.7 (61–100) | NR | 25 | NR |
|
| ||||||||||||
| Ahmed et al.,23 2014 | Retrospective cohort | Hip fracture | Vitamin K on admission, repeat if INR > 1.5 after 24 h | 27 | 40 | — | 81.0 ± 5.9 | 81.8 ± 8.9 | — | 14 | 26 | — |
|
| ||||||||||||
| Buecking et al.,24 2014 | ||||||||||||
|
| ||||||||||||
| PCCs* | Prospective cohort | Proximal femur fracture, > 60 yr of age | Pre: vitamin K on admission and every 12 h until INR < 1.5 Post: vitamin K + PCCs |
50 | 12 | — | 81.0 ± 7.0 | 81.0 ± 6.0 | — | 30 | 8 | — |
|
| ||||||||||||
| Vitamin K ± PCCs | Prospective cohort | Proximal femur fracture, > 60 yr of age | Vitamin K on admission and every 12 h until INR < 1.5; PCCs to accelerate reversal | — | 62 | 340 | — | 81.0 ± 7.0 | 81.0 ± 8.0 | — | 38 | 255 |
|
| ||||||||||||
| Tal et al.,30 2013 | Retrospective cohort | Hip fracture | Vitamin K on admission | 10 | 11 | 35 | 82.2 ± 5.2 | 81.9 ± 8.9 | 80.1 ± 6.5 | 6 | 10 | 22 |
|
| ||||||||||||
| Ashouri et al.,22 2011 | Retrospective cohort | Femoral neck fracture | Vitamin K or FFP on admission | 16 | 41 | — | NR | NR | — | NR | NR | — |
|
| ||||||||||||
| Vitale et al.,31 2011 | Retrospective cohort | Hip fracture, INR > 1.5 | Vitamin K or FFP on admission | 23 | 70 | — | 78.7 ± 10.5 | 81.9 ± 8.9 | — | 14 | 44 | — |
|
| ||||||||||||
| Bhatia et al.,25 2010 | Retrospective cohort | Femoral neck fracture, INR > 1.5 | Vitamin K on admission | 45 | 45 | — | 71.3 | 74.1 | — | 29 | 33 | — |
|
| ||||||||||||
| Weighted total, no. (%) | — | — | — | 247 | 516 | 1023 | 74.1 | 75.0 | 81.9 | 96 (63.6) | 296 (65.8) | 346 (73.0) |
FFP = fresh frozen plasma; INR = International Normalized Ratio; NR = not recorded; PCC = prothrombin complex concentrate; SD = standard deviation.
Not included in total calculations as it was a subgroup analysis.
Table 2.
Study characteristics and baseline demographic characteristics of patients with hip fracture who received a direct oral anticoagulant before or after implementation of a vitamin K antagonist–reversal protocol, and of patients who did not receive an anticoagulant (control)
| Study | Study design | Inclusion criteria | Reversal protocol | No. of patients | Age, mean ± SD (range), yr | Female sex, no. of patients | |||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Control | DOAC | Control | DOAC | Control | DOAC | ||||
| Schermann et al.,20 2019 | |||||||||
|
| |||||||||
| CRIF | Retrospective cohort | Proximal hip fracture | 24–36 h delay: rivaroxaban, apixaban; 12–24 h delay: dabigatran | 977 | 60 | 82.7 ± 8 | 86.1 ± 5.7 | 762 | 44 |
|
| |||||||||
| HA | Retrospective cohort | Proximal hip fracture | 24–36 h delay: rivaroxaban, apixaban; 12–24 h delay: dabigatran | 489 | 29 | 82.8 ± 7.6 | 86.2 ± 7.1 | 335 | 16 |
|
| |||||||||
| Schuetze et al.,29 2019 | Retrospective cohort | Inter- or subtrochanteric hip fracture | Surgery within 24 h of admission, no reversal | 146 | 52 | NR | NR | NR | NR |
|
| |||||||||
| Franklin et al.,14 2018 | Retrospective cohort | Hip fracture, 60–89 yr of age | Surgery within 48 h of admission, no reversal | 76 | 19 | NR | NR | 32 | 8 |
|
| |||||||||
| Mullins et al.,27 2018 | Retrospective cohort | Hip fracture | No time delay to surgery, no reversal | 62 | 63 | 85 (66–100) | NR | NR | 47 |
|
| |||||||||
| Weighted total, no. (%) | NA | NA | NA | 1750 | 223 | 82.8 | 86.1 | 1542 (73.2) | 171 (67.3) |
CRIF = closed reduction internal fixation; DOAC = direct oral anticoagulant; HA = hemiarthroplasty; NA = not available; NR = not recorded; SD = standard deviation.
The weighted mean age of patients included before implementation of a VKA-reversal protocol, those included after implementation of a VKA-reversal protocol and control patients was 74.1 years, 75.0 years and 81.9 years, respectively (Table 1). The corresponding proportions of female patients were 63.6% (n = 96), 65.8% (n = 296) and 73.0% (n = 346). In the studies that included patients who received DOACs and control patients, the weighted mean age was 86.1 years and 82.8 years, respectively (Table 2); 67.3% (171) and 73.2% (1542), respectively, were female.
Study quality
Of the 14 included studies, 13 were retrospective studies,14,15,20–23, 25–31 and 2 were prospective cohort studies.21,24 The quality of the included studies was variable: 5 studies received a score of 6,14,22,23,25,28 5 studies received a score of 7, 20,21,24,29,30 and 4 studies received a score of 815,26,27,31 (maximum possible score 9). The majority of studies were deficient in adjusting key demographic characteristics such as age, sex and marital status (Appendix 1, Supplemental Table S1).
Time to surgery with expedited-surgery protocols
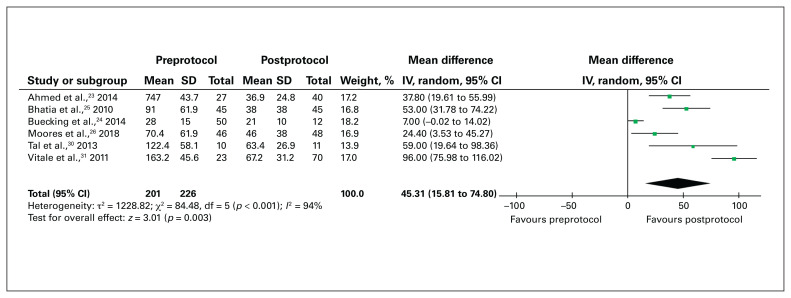
The mean time to surgery for VKA-treated patients without an established VKA-reversal protocol, those with a VKA-reversal protocol and control patients was 71.4 hours, 35.5 hours and 19.6 hours, respectively (Table 3). In the 6 studies eligible for meta-analysis, compared to hip fracture surgery before implementation of a VKA-reversal protocol, surgery after implementation of such a protocol was associated with a significant reduction in time to surgery (mean difference 45.31 h, 95% CI 15.81 h to 74.80 h) (Figure 2).
Table 3.
Perioperative surgical outcomes for patients who received or did not receive a vitamin K antagonist
| Study | Admission INR, mean ± SD (range) | Time to surgery, mean ± SD (range), h | Length of stay, mean ± SD (range), d | Deep vein thrombosis, no. of patients | Pulmonary embolism, no. of patients | Postoperative venous thromboembolism, no. of patients | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
||||||||||||
| Before protocol | After protocol | Before protocol | After protocol | Control | Before protocol | After protocol | Control | Before protocol | After protocol | Control | Before protocol | After protocol | Control | Before protocol | After protocol | Control | |
| Schuetze et al.,29 2019 | — | NR | — | 10.0 (4.3–23.8) | 8.2 (1.2–23.9) | — | NR | NR | — | NR | NR | — | NR | NR | — | NR | NR |
|
| |||||||||||||||||
| Mattisson et al.,15 2018 | — | 2.5 (0.6) | — | 16.0 (4.8) | 14.0 (5.6) | — | 4.9 (2.6) | 4.9 (2.6) | — | 0 | 0 | — | 0 | 0 | — | 0 | 0 |
|
| |||||||||||||||||
| Moores et al.,29 2019 | 2.6 (0.9) | 2.5 (1.0) | 70.4 (61.9) | 46.0 (38.0) | — | 17.3 (14.2) | 22.4 (39.7) | — | NR | NR | — | NR | NR | — | NR | NR | — |
|
| |||||||||||||||||
| Ng et al.,28 2019 | — | 3.1 (1.5) | 32.5 (23.2) | — | — | 11 (2–62)* | — | — | 1 | — | — | 0 | — | — | 1 | — | |
|
| |||||||||||||||||
| Diament et al.,21 2015 | — | 2.5 (1.5–4.4) | 53.7 (1.7–128.0) | 37.6 (14.7–71.8) | 28.4 (5.0–286.4) | 16.7 (2.0–65.0) | 15.8 (8.0–44.0) | 14.1 (2.0–67.0) | — | 0 | — | — | 0 | — | — | 0 | — |
|
| |||||||||||||||||
| Ahmed et al.,23 2014 | 3.4 (3.6) | 3.3 (2.6) | 74.7 (43.7) | 36.9 (12.6) | — | 24.4 (14.8) | 22.1 (14.1) | — | NR | NR | — | NR | NR | — | NR | NR | — |
|
| |||||||||||||||||
| Buecking et al.,24 2014 | |||||||||||||||||
|
| |||||||||||||||||
| PCCs† | 2.0 (0.7) | 2.3 (0.6) | 28.0 (15.0) | 21.0 (10.0) | — | NR | NR | — | 0 | 0 | — | 0 | 0 | — | 0 | 0 | — |
|
| |||||||||||||||||
| Vitamin K ± PCCs | — | 2.1 (0.7) | 27.0 (14.0) | 16.0 (12.0) | — | 15.0 (6.0) | 13.0 (6.0) | — | 0 | 1 | — | 0 | 2 | — | 0 | 3 | |
|
| |||||||||||||||||
| Tal et al.,30 2013 | 2.7 (0.7) | 2.4 (0.7) | 122.4 (58.1) | 63.4 (26.9) | 16.6 (19.9) | 13.2 (4.9) | 9.4 (1.9) | 7.3 (2.4) | NR | NR | NR | NR | NR | NR | NR | NR | NR |
|
| |||||||||||||||||
| Ashouri et al.,22 2011 | NR | NR | 74.7 (43.7) | 36.9 (12.6) | — | 24.4 (14.8) | 22.1 (14.1) | — | NR | NR | — | NR | NR | — | NR | NR | — |
|
| |||||||||||||||||
| Vitale et al.,31 2011 | 1.9 (0.7) | 2.6 (1.1) | 163.2 (45.6) | 67.2 (31.2) | — | NR | NR | — | 0 | 2 | — | 0 | 1 | — | 0 | 3 | — |
|
| |||||||||||||||||
| Bhatia et al.,25 2010 | 2.9 (1.5–6.5) | 2.5 (1.9–7.1) | 91 | 38 | — | NR | NR | — | NR | NR | — | NR | NR | — | NR | NR | — |
|
| |||||||||||||||||
| Weighted total, no. (%) | 2.6 | 2.6 | 71.4 | 35.5 | 19.6 | 19.2 | 15.3 | 11.5 | 0 (0.0) | 3 (0.9) | 1 (0.2) | 0 (0.0) | 1 (0.3) | 2 (0.5) | 0 (0.0) | 4 (1.3) | 3 (0.7) |
INR = International Normalized Ratio; NR = not recorded; PCC = prothrombin complex concentrate; SD = standard deviation.
Reported as median and range (not included in weighted total calculation).
Not included in total calculations as it was a subgroup analysis.
Fig. 2.
Time from admission to surgery for patients with hip fracture included before implementation of a vitamin-K-antagonist–reversal protocol (preprotocol) compared to after implementation of the protocol (postprotocol). CI = confidence interval; IV = inverse variance weighting; SD = standard deviation.
No studies involving DOAC-treated patients compared time to surgery before and after an expedited-surgery protocol. In the 3 studies in which the authors reported mean time to surgery for DOAC-treated patients who had expedited surgery and compared them to control patients, the mean time to surgery was 29.3 hours and 30.2 hours, respectively (Table 4).
Table 4.
Perioperative surgical outcomes for patients who received or did not receive a direct oral anticoagulant
| Study | Time to surgery, mean ± SD (range), h | Surgical duration, mean ± SD,* h | Change in postoperative hemoglobin level, mean ± SD (range), g/L | Change in postoperative hemoglobin level, mean, % | pRBC transfusion, no. of patients | 30-day mortality, no. of patients | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
|||||||
| Control | DOAC | Control | DOAC | Control | DOAC | Control | DOAC | Control | DOAC | Control | DOAC | |
| Schermann et al.,20 2019 | ||||||||||||
|
| ||||||||||||
| CRIF | 31.2 ± 22.2 | 40.2 ± 26.9 | 110.7 ± 48.9 | — | NR | NR | 24.0 | 22.6 | 77 | 5 | 43 | 4 |
|
| ||||||||||||
| HA | 36.3 ± 25.8 | 42.3 ± 27.3 | 126.2 ± 39.8 | — | NR | NR | 21.0 | 21.7 | 36 | 3 | 30 | 2 |
|
| ||||||||||||
| Schuetze et al.,29 2019 | 8.2 (1.3–23.9) | 9.5 (2.3–24.0) | NR | NR | NR | NR | NR | NR | 24 | 20 | NR | NR |
|
| ||||||||||||
| Franklin et al.,14 2018 | 21.4 ± 12.4 | 28.9 ± 11.8 | 93.5 ± 44.2 | 82.7 ± 37.9 | 30 ± 2.0 | 32 ± 1.4 | NR | NR | 6 | 25 | 12 | 1 |
|
| ||||||||||||
| Mullins et al.,27 2018 | 19 (3–44)* | 19 (7–64)* | NR | NR | 23 (1–47) | 23 (0–49) | NR | NR | 6 | 11 | 5 | 1 |
|
| ||||||||||||
| Weighted total, no. (%) | 30.2† | 29.3† | 114.8 | 120.9 | 29.2 | 29.3 | 23.0 | 22.3 | 149 (8.6) | 64 (29.1) | 90 (5.6) | 8 (4.7) |
CRIF = closed reduction internal fixation; DOAC = direct oral anticoagulant; HA = hemiarthroplasty; NR = not recorded; pRBC = packed red blood cells; SD = standard deviation.
Reported as median and range.
Weighted mean does not include the study by Mullins and colleagues.27
Blood loss and transfusion
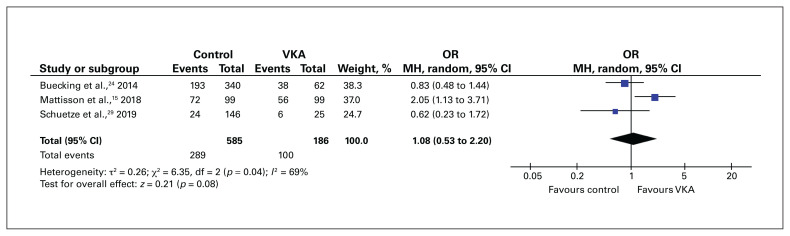
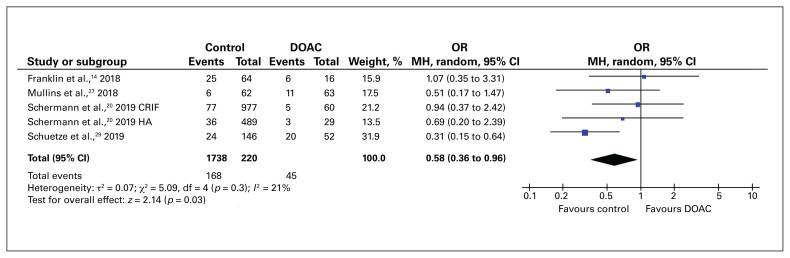
The rate of blood transfusion was compared between patients who followed a VKA-reversal protocol and control patients in 3 studies. We did not identify an increase in transfusion requirements among VKA-treated patients who underwent expedited surgery compared to control patients (OR 1.08, 95% CI 0.53 to 2.20) (Figure 3). In contrast, the rate of blood transfusion was significantly higher for DOAC-treated patients who underwent surgery within 48 hours than for control patients (OR 0.58, 95% CI 0.36 to 0.96) (Figure 4).
Fig. 3.
Blood transfusion rate for patients with hip fracture who received a vitamin K antagonist (VKA) compared to those who did not receive an anticoagulant (control). CI = confidence interval; MH = Mantel–Haenszel weighting; OR = odds ratio; SD = standard deviation.
Fig. 4.
Blood transfusion rate for patients with hip fracture who received a direct oral anticoagulant (DOAC) compared to those who did not receive an anticoagulant (control). CI = confidence interval; CRIF = closed reduction internal fixation; HA = hemiarthroplasty; MH = Mantel–Haenszel weighting; OR = odds ratio.
Surgical duration
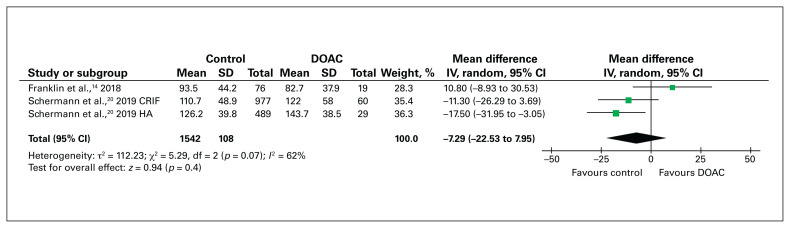
Mattisson and colleagues15 compared surgical duration between patients with a VKA-reversal protocol and control patients, and found no significant difference (65 min v. 64 min; p = 0.8). Similarly, in the 2 studies comparing surgical duration between DOAC-treated patients who underwent surgery within 48 hours and control patients, there was no significant difference in the mean duration of surgery between the 2 groups (mean difference −7.29 min, 95% CI −22.5 min to 7.95 min) (Figure 5).14,20
Fig. 5.
Mean difference in duration of surgery (minutes) for patients with hip fracture who received a direct oral anticoagulant (DOAC) compared to those who did not receive an anticoagulant (control). CI = confidence interval; CRIF = closed reduction internal fixation; HA = hemiarthroplasty; IV = inverse variance weighting; SD = standard deviation.
Length of acute hospital stay
In 8 studies, the length of acute hospital stay before a VKA-reversal protocol (n = 179), after a VKA-reversal protocol (n = 376) and control patients (n = 877) was recorded. The length of stay was 19.2 days, 15.3 days and 9.8 days, respectively.
Venous thromboembolism
Six studies reported on postoperative VTE in VKA-treated patients. In the 2 studies involving VKA-treated patients who did not follow an expedited-surgery protocol, there were no cases of VTE.22,29 In contrast, the overall rate of VTE among VKA-treated patients who underwent expedited surgery (n = 318) was 1.3% (n = 4) (Table 3). The rate of VTE in control patients was 0.7% (n = 3). Postoperative VTE rates were not reported in any of the studies involving DOAC-treated patients.
Thirty-day mortality
Two studies reported on 30-day mortality in VKA-treated patients. Moores and colleagues26 found a 30-day mortality rate of 6.2% in patients who followed a VKA-reversal protocol (vitamin K every 6 h until the International Normalized Ratio [INR] was < 1.7), compared to 15.2% among patients before implementation of a VKA-reversal protocol. Mattisson and colleagues15 reported that patients who underwent early warfarin reversal and surgery within 24 hours did not show a higher 30-day mortality rate than control patients (9.1% v. 7.1%; p = 0.8).
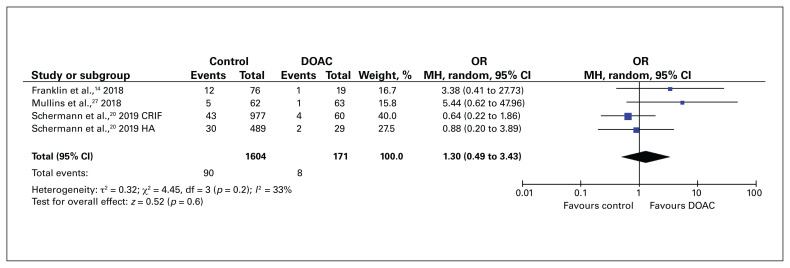
In the 3 studies that reported on 30-day mortality rate in DOAC-treated patients,14,20,27 expedited surgery (within 48 h) was not associated with an increase in 30-day mortality compared to control patients (OR 1.30, 95% CI 0.49 to 3.89) (Figure 6).
Fig. 6.
Thirty-day mortality rate for patients with hip fracture who received a direct oral anticoagulant (DOAC) compared to those who did not receive an anticoagulant (control). CI = confidence interval; CRIF = closed reduction internal fixation; HA = hemiarthroplasty; MH = Mantel–Haenszel weighting; OR = odds ratio; SD = standard deviation.
Discussion
In this meta-analysis involving 3821 patients with hip fracture across 14 studies, we found that implementation of a VKA-reversal protocol significantly decreased the time to surgery among those treated with VKA. In addition, there was no significant difference in blood transfusion rates between patients who underwent VKA reversal and patient who did not receive anticoagulants. Among studies in which outcomes were compared between DOAC-treated patients and control patients, undergoing hip fracture surgery within 48 hours of admission without DOAC reversal was not associated with longer surgical duration or a higher 30-day mortality rate, despite a higher rate of perioperative blood transfusion in the former.
We observed a reduction in surgical delay of more than 36 hours among VKA-treated patients after implementation of a VKA-reversal protocol before surgery. Furthermore, patients who received nonprotocolized perioperative VKA management, an established risk factor for postdischarge VTE,32 had a longer hospital stay than those who followed a VKA-reversal protocol. In most studies in which VKA reversal was studied, vitamin K was used; concurrent PCCs were used in a limited number of studies. Notably, all 3 cases of postoperative VTE occurred with protocols that actively used PCCs or fresh frozen plasma.28,31 Based on the pharmaco-dynamic profile of vitamin K in restoring coagulation factors,33 contemporary antithrombotic guidelines recommend parenteral vitamin K, without adjunctive plasma or PCCs, if surgical management can be delayed by 6–8 hours from the time of hospital admission.34 Nonetheless, INR reversal is among the most commonly cited barriers to timely surgery in VKA-treated patients with hip fracture.13,35,36 Our data support the implementation of preoperative VKA-reversal protocols using reversal agents including vitamin K or PCCs as an effective way of achieving consistent and appropriate warfarin reversal. In addition, timely internal medicine consultation should be performed when a high risk of thromboembolic complications is expected.35 Owing to the heterogeneity of current VKA-reversal protocols in the literature, further research is required to determine the most cost-effective method that allows for patients to receive timely hip fracture surgery while maintaining a low rate of perioperative bleeding and thrombotic complications.
Among DOAC-treated patients who underwent expedited surgery (within 48 h), we observed an increased risk of perioperative transfusion compared to patients who did not receive anticoagulants. However, no difference in transfusion rates was observed among VKA-treated patients. This is related to the fact that VKA-reversal agents are readily available, and VKA in VKA-treated patients is reversed before hip fracture surgery. In contrast, expedited surgery in DOAC-treated patients does not always involve reversal. Schuetze and colleagues29 included only patients with interor subtrochanteric hip fractures, which were treated with cephalomedullary nail fixation rather than arthroplasty procedures.37 As cephalomedullary nail fixation is associated with higher surgical blood loss than arthroplasty,38 any underlying hemostatic impairment is likely amplified in this population. In a recent study by Lott and colleagues,39 no significant differences in duration of surgery, estimated blood loss or transfusion requirements were shown between DOAC-treated patients who underwent hip fracture surgery within 48 hours of presentation and those whose surgery was delayed more than 48 hours. Furthermore, among the included studies, rates of DOAC reversal were lower than rates of VKA reversal, with most protocols calling for no reversal before surgery within 24 hours of hospital admission. Although this imbalance in risk of transfusion did not translate to increased risk of 30-day mortality, the role of emerging DOAC-reversal agents may be relevant in maintaining perioperative hemostasis and decreasing blood transfusion rates in DOAC-treated patients undergoing hip fracture surgery.
Limitations
This study has several strengths. The comprehensive search strategy summarized the existing published literature on expedited-surgery protocols for both VKA- and DOAC-treated patients with hip fracture. Statistical heterogeneity was low to moderate for most outcomes assessed. For VKA-treated patients, reporting baseline INR allowed for interpretation of potential confounding on effect estimates. In addition, we selected studies in which the mean time to surgery among DOAC-treated patients was within 48 hours of admission, consistent with the American Academy of Orthopaedic Surgeons evidence-based clinical practice guideline recommendations.40
There are limitations to our study. Patients’ comorbidity profiles varied across included studies. Owing to the heterogeneity of presentation of comorbidities between studies, we were unable to assess how potential differences in comorbidities may have contributed to the study outcomes. Most studies of VKA-treated patients did not include descriptions of postoperative thromboprophylaxis or how VKA was restarted after surgery, which may have affected postoperative VTE rates. Finally, the majority of included studies were retrospective, entailing a risk of selection bias toward including anticoagulant-treated patients who were medically fit for expedited surgery.
Conclusion
Implementing a VKA-reversal protocol in patients with hip fracture is effective in decreasing the time to surgery without an associated increase in bleeding risk. Furthermore, performing hip fracture surgery within 48 hours of presentation in DOAC-treated patients is likely safe, as no differences in duration of surgery or 30-day mortality were observed between these patients and those who did not receive anticoagulants despite an increased rate of red blood cell transfusion among the former. Although reversal agents may not be necessary for all patients receiving DOACs, those with inter- or subtrochanteric hip fractures receiving cephalomedullary nail fixation may benefit from DOAC reversal, in order to decrease transfusion risk. As evidenced by the significant decrease in time to surgery with reversal protocols in VKA-treated patients, there is an urgent priority to evaluate and establish expedited-surgery protocols for DOAC-treated patients with hip fracture, to decrease the morbidity and mortality associated with surgical delay in this population.
Supplementary Material
Footnotes
Competing interests: Marc Carrier reports honoraria for speakers bureaus and support for attending meetings and/or travel from AbbVie, AngioDynamics, Anthos Therapeutics, AstraZeneca, Bayer, EOCI Pharmacomm, LEO Pharma, Pfizer, Sanofi, Servier Laboratories and Valeo Pharma. He has participated on data safety monitoring boards for the TRIM-Line study and the LIMIT Trial. He is president of Thrombosis Canada, was chair of the Thrombosis Canada Scientific Subcommittee, Scientific and Standardization Committee, International Society on Thrombosis and Haemostasis in 2019, and was a member of the Guideline Panel of the American Society of Hematology in 2015–2021. Prism Schneider sits on the Scientific Advisory Council, Osteoporosis Canada and is chair of the Research Committee of the reimplementation of VKA Orthopaedic Trauma Association. No other competing interests were declared.
Presented at the virtual 2021 Canadian Orthopaedic Association Annual Meeting, June 17, 2021
Contributors: D. You, Y. Xu and P. Schneider designed the study. D. You and H. Krzyzaniak acquired the data, which D. You, H. Krzyzaniak, R. Korley, M. Carrier and P. Schneider analyzed. D. You and H. Krzyzaniak wrote the manuscript, which D. You, Y. Xu, R. Korley, M. Carrier and P. Schneider critically revised. All authors gave final approval of the article to be published.
References
- 1.Bhandari M, Swiontkowski M. Management of acute hip fracture. N Engl J Med 2017;377:2053–62. [DOI] [PubMed] [Google Scholar]
- 2.Schulman S. Care of patients receiving long-term anticoagulant therapy. N Engl J Med 2003;349:675–83. [DOI] [PubMed] [Google Scholar]
- 3.Kanis JA, Johnell O, Oden A, et al. Long-term risk of osteoporotic fracture in Malmö. Osteoporos Int 2000;11:669–74. [DOI] [PubMed] [Google Scholar]
- 4.Tarrant SM, Catanach MJ, Sarrami M, et al. Direct oral anticoagulants and timing of hip fracture surgery. J Clin Med 2020;9:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papachristos IV, Giannoudis PV. Proximal femur fractures in patients taking anticoagulants. EFORT Open Rev 2020;5:700–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weitz JI, Semchuk W, Turpie AGG, et al. Trends in prescribing oral anticoagulants in Canada, 2008–2014. Clin Ther 2015;37:2506–2514.e4. [DOI] [PubMed] [Google Scholar]
- 7.Pincus D, Ravi B, Wasserstein D, et al. Association between wait time and 30-day mortality in adults undergoing hip fracture surgery. JAMA 2017;318:1994–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simunovic N, Devereaux PJ, Sprague S, et al. Effect of early surgery after hip fracture on mortality and complications: systematic review and meta-analysis. CMAJ 2010;182:1609–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pernod G, Albaladejo P, Godier A, et al. Working Group on Perioperative Haemostasis. Management of major bleeding complications and emergency surgery in patients on long-term treatment with direct oral anticoagulants, thrombin or factor-Xa inhibitors: proposals of the Working Group on Perioperative Haemostasis (GIHP) – March 2013. Arch Cardiovasc Dis 2013;106:382–93. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein JN, Refaai MA, Milling TJ, et al. Four-factor prothrombin complex concentrate versus plasma for rapid vitamin K antagonist reversal in patients needing urgent surgical or invasive interventions: a phase 3b, open-label, non-inferiority, randomised trial. Lancet 2015;385:2077–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siegal DM. What we have learned about direct oral anticoagulant reversal. Hematol Am Soc Hematol Educ Program 2019;2019:198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frontera JA, Bhatt P, Lalchan R, et al. Cost comparison of andexanet versus prothrombin complex concentrates for direct factor Xa inhibitor reversal after hemorrhage. J Thromb Thrombolysis 2020;49: 121–31. [DOI] [PubMed] [Google Scholar]
- 13.Meinig R, Jarvis S, Orlando A, et al. Is anticoagulation reversal necessary prior to surgical treatment of geriatric hip fractures? J Clin Orthop Trauma 2020;11:S93–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franklin NA, Ali AH, Hurley RK, et al. Outcomes of early surgical intervention in geriatric proximal femur fractures among patients receiving direct oral anticoagulation. J Orthop Trauma 2018; 32: 269–73. [DOI] [PubMed] [Google Scholar]
- 15.Mattisson L, Lapidus LJ, Enocson A. Is fast reversal and early surgery (within 24 h) in patients on warfarin medication with trochanteric hip fractures safe? A case–control study. BMC Musculoskelet Disord 2018;19:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wells G, Shea B, O’Connell D, et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [Internet]. 2014. Available: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed 2021 May 1).
- 17.Khan F, Rahman A, Carrier M, et al. Long term risk of symptomatic recurrent venous thromboembolism after discontinuation of anticoagulant treatment for first unprovoked venous thromboembolism event: systematic review and meta-analysis. BMJ 2019; 366: 14363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins J, Li T, Deeks J. Choosing effect measures and computing estimates of effect. In: Higgins JPT, Thomas J, Chandler J, et al., editors. Cochrane handbook for systematic reviews of interventions. 2nd ed. Version 6.0. The Cochrane Collaboration; 2019. [Google Scholar]
- 20.Schermann H, Gurel R, Gold A, et al. Safety of urgent hip fracture surgery protocol under influence of direct oral anticoagulation medications. Injury 2019;50:398–402. [DOI] [PubMed] [Google Scholar]
- 21.Diament M, MacLeod K, O’Hare J, et al. “Early trigger” intravenous vitamin K. Geriatr Orthop Surg Rehabil 2015;6:263–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ashouri F, Al-Jundi W, Patel A, et al. Management of warfarin anticoagulation in patients with fractured neck of femur. ISRN Hematol 2011;2011:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahmed I, Khan MA, Nayak V, et al. An evidence-based warfarin management protocol reduces surgical delay in hip fracture patients. J Orthop Traumatol 2014;15:21–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buecking B, Eschbach D, Bliemel C, et al. Effectiveness of vitamin K in anticoagulation reversal for hip fracture surgery — a prospective observational study. Thromb Res 2014;133:42–7. [DOI] [PubMed] [Google Scholar]
- 25.Bhatia M, Talawadekar G, Parihar S, et al. An audit of the role of vitamin K in the reversal of International Normalised Ratio (INR) in patients undergoing surgery for hip fracture. Ann R Coll Surg Engl 2010;92:473–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moores TS, Chatterton BD, Walker MJ, et al. Standardised warfarin reversal expedites time to theatre for fractured neck of femur surgery and improves mortality rates: a matched cohort study. Adv Orthop 2018;2018:4791214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mullins B, Akehurst H, Slattery D, et al. Should surgery be delayed in patients taking direct oral anticoagulants who suffer a hip fracture? A retrospective, case-controlled observational study at a UK major trauma centre. BMJ Open 2018;8:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ng R, Shabani-Rad MT. Results of Octaplex for reversal of warfarin anticoagulation in patients with hip fracture. Can J Surg 2019;62:14–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schuetze K, Eickhoff A, Dehner C, et al. Impact of oral anticoagulation on proximal femur fractures treated within 24 h — a retrospective chart review. Injury 2019;50:2040–4. [DOI] [PubMed] [Google Scholar]
- 30.Tal A, Rubin G, Rozen N. Treatment with vitamin K in hip fracture patients receiving warfarin. Isr Med Assoc J 2013;15:348–51. [PubMed] [Google Scholar]
- 31.Vitale MA, Vanbeek C, Spivack JH, et al. Pharmacologic reversal of warfarin-associated coagulopathy in geriatric patients with hip fractures: a retrospective study of thromboembolic events, postoperative complications, and time to surgery. Geriatr Orthop Surg Rehabil 2011; 2:128–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petersen PB, Jørgensen CC, Kehlet H; Lundbeck Foundation Centre for Fast-track Hip Knee Replacement Collaborative Group. Venous thromboembolism despite ongoing prophylaxis after fast-track hip and knee arthroplasty: a prospective multicenter study of 34,397 procedures. Thromb Haemost 2019; 119:1877–85. [DOI] [PubMed] [Google Scholar]
- 33.Dezee KJ, Shimeall WT, Douglas KM, et al. Treatment of excessive anticoagulation with phytonadione (vitamin K): a meta-analysis. Arch Intern Med 2006;166:391–7. [DOI] [PubMed] [Google Scholar]
- 34.Keeling D, Tait RC, Watson H; British Committee of Standards for Haematology. Peri-operative management of anticoagulation and antiplatelet therapy. Br J Haematol 2016;175:602–13. [DOI] [PubMed] [Google Scholar]
- 35.Burton A, Davis CM, Boateng H, et al. A multidisciplinary approach to expedite surgical hip fracture care. Geriatr Orthop Surg Rehabil 2020;11:215145931989864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Belotti LMB, Bartoli S, Trombetti S, et al. Factors influencing surgical delay after hip fracture in hospitals of Emilia Romagna Region, Italy: a multilevel analysis. Hip Int 2013;23:15–21. [DOI] [PubMed] [Google Scholar]
- 37.Bateman L, Vuppala S, Porada P, et al. Medical management in the acute hip fracture patient: a comprehensive review for the internist. Ochsner J 2012;12:101–10. [PMC free article] [PubMed] [Google Scholar]
- 38.Foss NB, Kehlet H. Hidden blood loss after surgery for hip fracture. J Bone Joint Surg Br 2006;88:1053–9. [DOI] [PubMed] [Google Scholar]
- 39.Lott A, Haglin J, Belayneh R, et al. Surgical delay is not warranted for patients with hip fractures receiving non-warfarin anticoagulants. Orthopedics 2019;42:E331–5. [DOI] [PubMed] [Google Scholar]
- 40.Management of hip fractures in older adults: evidence-based clinical practice guideline. Rosemont (IL): American Academy of Orthopaedic Surgeons; 2021. Available: https://www.aaos.org/globalassets/quality-and-practice-resources/hip-fractures-in-the-elderly/hipfxcpg.pdf (accessed 2021 May 1). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.