Abstract
Objective
We aim to test whether leukocyte telomere length (LTL) is causally associated with the risk of bipolar disorder (BD) using the Mendelian randomization (MR) method.
Methods
Results of a genome-wide association study (GWAS) conducted with 472,174 individuals of European descent were used to screen for single-nucleotide polymorphisms (SNPs) related with LTL traits. Summary-level data for BD (7,647 cases and 27,303 controls) were obtained from UK Biobank. An inverse-variance-weighted (IVW) method was employed as the primary MR analysis. Sensitivity analyses were conducted via MR-Egger, maximum likelihood, MR-pleiotropy residual sum outlier (MR-PRESSO), and MR-robust adjusted profile score (MR-RAPS) methods. Finally, the MR Steiger test was utilized to validate the hypothesized relationship between exposure and outcome.
Results
Two-sample MR analysis revealed inverse relationships between genetically predicted LTL and BD risk (IVW OR [odds ratio] = 0.800, 95% CI [0.647–0.989] P = 0.039). Genetically predicted LTL exhibits a consistent connection with BD across five MR methods. Sensitivity analyses showed that the genetically determined effect of LTL on BD was stable and reliable. Furthermore, the MR Steiger test demonstrated that LTL was causal for BD rather than the opposite (P < 0.001).
Conclusion
Our findings show that genetically determined LTL reduces the risk of BD. More research is required to clarify the mechanisms underlying this apparent causal connection. In addition, these findings may be useful for developing strategies for the prevention and treatment of BD.
Keywords: Leukocyte telomere length, Bipolar disorder, Mendelian randomization, Genome-wide association study, Single-nucleotide polymorphisms, Spinal stenosis
Introduction
Bipolar disorder (BD) is a severe neuropsychiatric condition characterized by recurrent periods of mania and depression that impair cognition, perception, emotion, and social interaction. The cause of BD is unknown, although genetic, neurochemical, and structural abnormalities, as well as stress, may contribute (McIntyre et al., 2020). BD is more prevalent in persons with a family history of BD and those suffering from depression, anxiety problems, or substance use issues (Bauer, 2022). BD is a significant public health issue and a large contributor to the global burden of illness due to its lifetime frequency of 1–2%, increased morbidity and mortality, beginning in young adulthood, and typically chronic course (Stahl et al., 2019). According to Fries et al. (2020), BD is a disease that accelerates aging in both clinical and molecular features. The causes of the illness and any associated effective therapy approaches are still unknown despite the considerable effort being put into examining the mechanisms underlying BD. Because of this, it is becoming more important to find people who are likely to get BD and could benefit from early preventive methods.
There is evidence that certain mental illnesses are linked to a faster rate of biological aging, either at the organismal or even the cellular level (Lindqvist et al., 2015). The telomere length (TL) is an emerging indicator of cellular aging that is frequently tested in leukocytes (as LTL). Telomeres are areas of repeating nucleotide sequences at the end of eukaryotic chromosomes that play a crucial function in chromosomal integrity. LTL is known as the ”molecular clock” related to the senescence of cells and organisms (Vaiserman & Krasnienkov, 2020). Epidemiological studies have shown evidence that shortened LTL is linked to several psychiatric disorders, including major depressive disorder (Monroy-Jaramillo, Dyukova & Walss-Bass, 2018; Pisanu et al., 2020a; Pisanu et al., 2020b; Wang et al., 2017), anxiety (Monroy-Jaramillo, Dyukova & Walss-Bass, 2018; Wang et al., 2017), schizophrenia (Ayora et al., 2022; Wolkowitz et al., 2017). The occurrence of BD may be closely related to these diseases, which may all be affected by shortened LTL. As a result, we speculate that the change of LTL may be closely related to the occurrence of BD. Observational study results indicate connections between LTL and BD (Ferensztajn-Rochowiak et al., 2021; Huang et al., 2018; Joo et al., 2021). Despite being helpful, these observational studies are vulnerable to confounding factors, which can lead to inaccurate causal conclusions (Grimes & Schulz, 2002). Hence, randomized controlled trials are required to demonstrate the validity of the relationships found in observational studies.
Randomized studies on LTL are challenging to conduct because of the need for large sample sizes and extensive follow-up periods. Because of this, establishing a causal link between LTL and diseases might be difficult. To establish whether the alleged connection between LTL and diseases is a causal one, an effective method must be identified. Mendelian randomization (MR) provides the opportunity to solve this issue explicitly (Do et al., 2013; Emdin, Khera & Kathiresan, 2017; Frikke-Schmidt et al., 2008; Voight et al., 2012). Since genetic mutations are intrinsic and unaffected by environmental circumstances, the MR research approach employing SNP as an instrumental variable can effectively control the interference of confounding variables, similar to a randomized controlled trial. In addition, genetic variation can have an effect on outcomes, but outcomes cannot have an impact on genes; hence, there is no possibility of inferring reverse causality. MR relies on the following assumptions: the genetic instrument should be highly related to the exposure but not confounders. The genetic variant should solely influence the outcome via the risk factor (Emdin, Khera & Kathiresan, 2017). Hence, establishing causality is possible with a genetic instrument that meets all MR assumptions.
Therefore, this study used a two-sample MR analysis and a variety of sensitivity analyses to investigate the causal link between LTL and BD risk. The discovered causal links between LTL and BD risk will help progress research into early preventive or diagnosis measures.
Methods
Overall study design
In this study, we utilized the two-sample MR method to evaluate the causal relationship of LTL with the risk of BD. The two-sample MR analyses used summary-level data from IEU Open GWAS (https://gwas.mrcieu.ac.uk/), namely LTL (472,174 individuals, ieu-b-4879) and BD (34,950 individuals, ebi-a-GCST003724). The relevant ethics board authorized the initial GWAS, and all participants provided informed consent.
Assumptions of the Mendelian randomization study
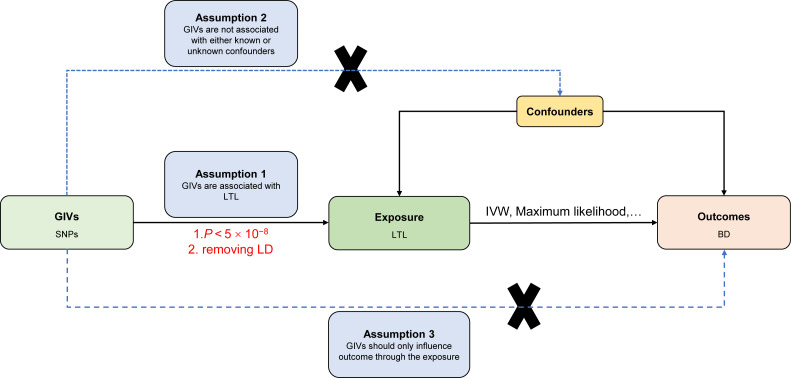
The current MR study must acknowledge and accept these three key assumptions: (1) Genetic instrument variables (GIVs) should be strongly associated with LTL. (2) The GIVs must not be associated with confounders that may affect the relationship between LTL and BD. (3) The GIVs should only influence the BD via LTL (horizontal pleiotropy does not exist) (Davey Smith & Hemani, 2014; Smith & Ebrahim, 2003). Figure 1 illustrates the MR study’s assumptions and its overall design.
Figure 1. Directed acyclic graph of the MR framework investigating the causal relationship between LTL and BD.
Instrumental variable assumptions: (1) Genetic instrument variables (GIVs) should be strongly associated with LTL. (2) The GIVs must not be associated with confounders that may play a role in the relationship between LTL and BD. (3) The GIVs should only influence the BD via LTL. SNPs, single-nucleotide polymorphisms; LTL, leukocyte telomere length; BD, bipolar disorder; IVW, inverse-variance-weighted; MR, Mendelian randomization.
Two-sample MR
SNP selection
After obtaining the GWAS summary-level data for LTL, we performed several quality control measures to decide which instrumental SNPs could be included in subsequent MR analyses. First, genome-wide significant SNPs associated with LTL (P < 5 × 10−8) were obtained. Second, it was critical to establish that none of the LTL-related instrumental SNPs were in linkage disequilibrium (LD). In this study, the LD between SNPs was determined by employing the clumping method (r2 < 0.001, window size = 10,000 kb) on European samples obtained from the 1000 Genomes Project. Among the pairs of SNPs where the level of LD r2 exceeds the stated threshold (r2 = 0.001), only the SNP with the lowest P value is retained. Moreover, the F statistic was calculated according to previous studies (Pierce, Ahsan & Vanderweele, 2011; Wu et al., 2020) to estimate the instrument strength. When the F statistic for the instrument-exposure correlation was significantly higher than 10, it indicated a low probability of weak instrumental variable bias (Davies, Holmes & Davey Smith , 2018).
Primary analyses
The effect of LTL on BD risk was estimated using the inverse-variance-weighted (IVW) model, with LTL serving as the exposure and BD as the outcome. The IVW method combines Wald estimates for each SNP using a meta-analysis approach to determine the overall estimates of the impact of the exposure on the result (Burgess, Butterworth & Thompson, 2013).
Sensitivity analyses
To test the robustness of primary analysis, four methods were used, including MR-Egger (Bowden, Davey Smith & Burgess, 2015), Maximum likelihood (Xue, Shen & Pan, 2021), MR-pleiotropy residual sum outlier (MR-PRESSO) (Verbanck et al, 2018), and robust adjusted profile score (MR-RAPS) methods (Cui & Tian, 2021) to test the reliability and stability of the results. In short, MR-Egger recalculates the IVW causal estimates while taking the intercept out of the equation. The maximum likelihood approach assumes that each SNP’s effect on the outcome is the same; therefore, it may provide more robust results when the measurement error exists. MR-PRESSO is an approach for detecting and correcting outliers in linear IVW data. MR-RAPS accounts for the measurement error in SNP-exposure effects and is impartial in the presence of numerous weak instruments and robust to systematic and idiosyncratic pleiotropy. However, each of the above methods has some advantages and disadvantages. For example, the IVW method is the most powerful resilient approach when all variants are valid instrumental variables (IVs), as it is at its most effective when using only valid IVs. The efficiency of MR-Egger procedures is drastically lower (Burgess et al., 2020). In addition, to estimate consistently, the MR-Egger method requires the InSIDE (Instrument Strength Independent of Direct Effect) assumption. MR-RAPS works best when pleiotropic effects are actually normally distributed about zero. The MR-PRESSO is useful when there are few genetic variants with heterogeneous ratio estimates, but it is less valuable when there are many mildly pleiotropic variants or when the average pleiotropic effect of non-outliers is not zero. Due to variances in analysis platforms, experimental circumstances, inclusion populations, and SNPs, the assessment of causal effects may be impacted by heterogeneity in two-sample MR analyses. This study, therefore, examined the IVW and MR-Egger estimations for heterogeneity (Hemani et al., 2018). The heterogeneities were measured using the Cochran Q statistic; a P value (P-het) > 0.05 indicated no heterogeneity in the included instrumental variables. Hence the influence of heterogeneity on the assessment of causal effects could be disregarded. If there was heterogeneity, the random-effects model was employed to determine the effect size (Julian et al., 2021; Li et al., 2022). In addition, it is essential to determine if pleiotropy occurs in the MR causal inference. It is possible to evaluate pleiotropy using the Egger model’s intercept statistically; departures from 0 suggest the presence of directional pleiotropy (Burgess & Thompson, 2017). MR-PRESSO method was also employed to determine if the pleiotropy existed (Verbanck et al, 2018). Pleiotropy is unlikely in the causal analysis if P > 0.05. The MR Steiger test was performed to confirm the directionality of the exposure’s effect on the outcome, and P < 0.05 was considered statistically significant. Finally, according to a prior investigation (Brion, Shakhbazov & Visscher, 2013), the statistical power of our MR findings was assessed.
All MR studies were carried out in R (version 4.1.2; R Core Team, 2021) using the TwoSampleMR package (version 0.5.6) (Hemani et al., 2018).
Results
All 133 distinct genetic variations connected to LTL were accessible in the summary statistics for BD. The F statistic for these SNPs exceeded 10 (range, 29.86–1628.82; mean, 119.94) for LTL, showing that there is little chance of weak-instrument bias (Fig. S1) (Baumeister et al., 2021). File S1 contains information on these SNPs in great detail.
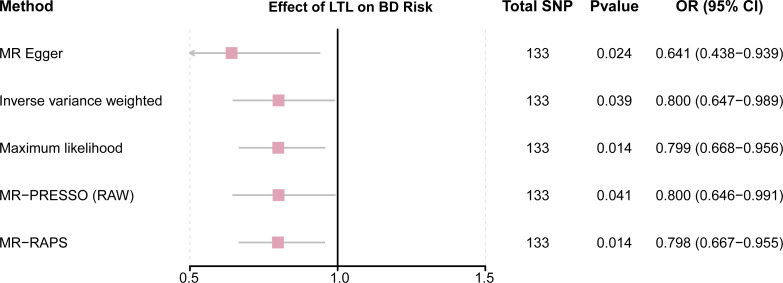
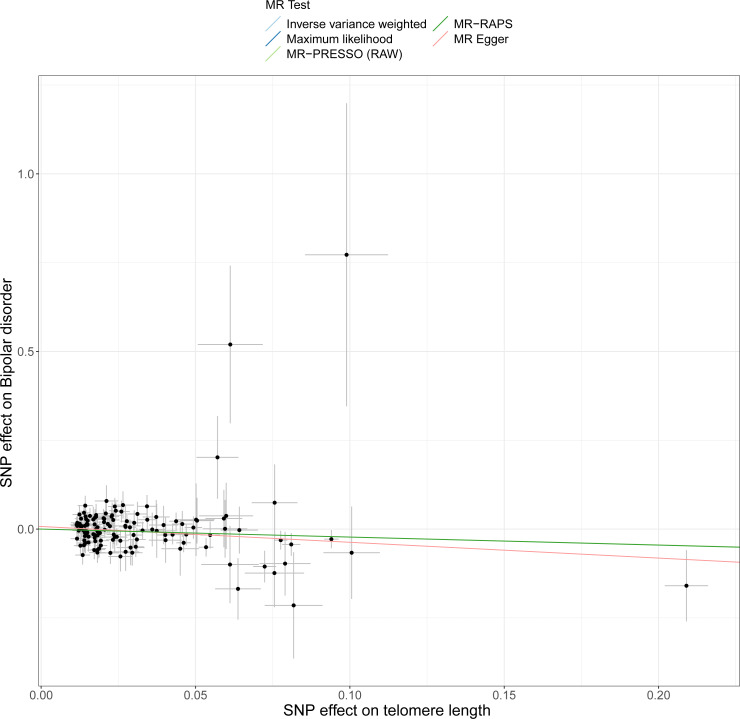
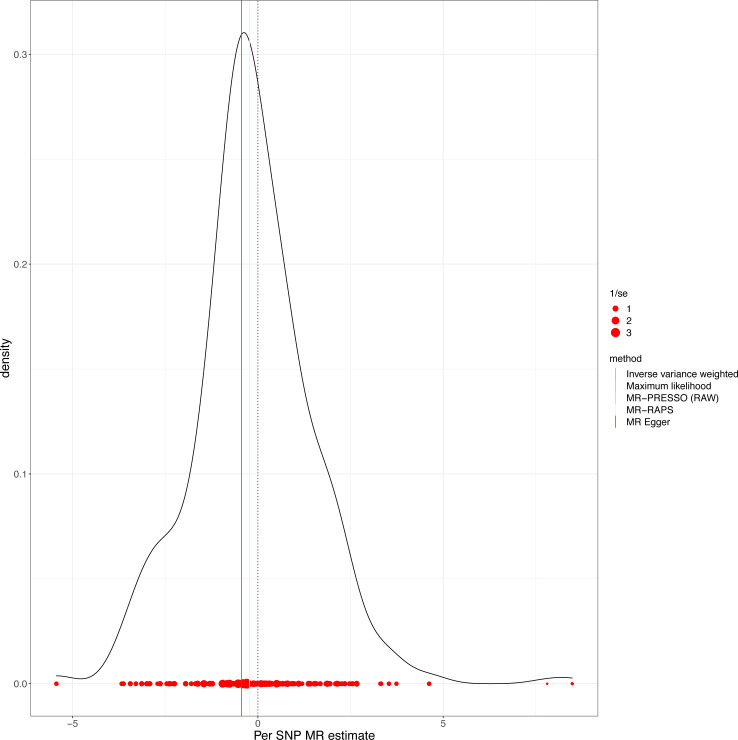
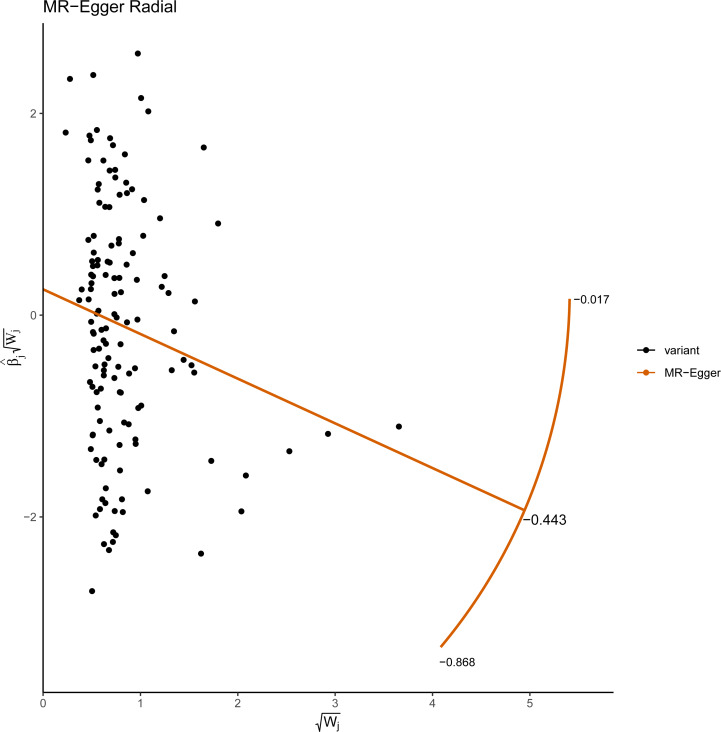
This study demonstrated that genetically determined LTL has a negative association with BD risk; the OR was 0.800 (95% CI [0.647–0.989]; P = 0.039) in the IVW analysis (Table 1) (Fig. 2). Subsequent heterogeneity study results demonstrated substantial heterogeneity among the GIVs (P-het = 0.001), so we utilized the random-effects model to directly estimate the aforementioned MR effect size. Genetically predicted LTL showed a broadly consistent association with BD across the different MR methods (Table 1). The scatter plots also revealed that the slopes of the results among LTL and BD assessed by various methodologies are all negative, and the steady correlation pattern demonstrates that our study results are pretty dependable (Fig. 3). Additionally, density plots also show that the predicted effect values of most SNPs fall within a rather narrow range, indicating the absence of significant heterogeneity in our research (Fig. 4). The intercept term estimated from MR-Egger was centered at the origin (P-intercept = 0.173), indicating that the results were unaffected by the directional pleiotropy. No outlier SNP was identified that resulted in enhanced pleiotropy in the overall MR estimate by MR-PRESSO analysis and MR-Egger test (Fig. 5). Moreover, even though the SNPs explained 3.26% of the variance of LTL, there was 83.6% power to detect the causal association between LTL and BD. The MR Steiger test was used to validate the causal assumption of LTL and BD, and the results proved that LTL’s influence on BD was the proper causal direction (P < 0.001).
Table 1. MR results of LTL on risk of BD.
| Exposure | Method | No. of SNPs | OR (95% CI) | P | P-het | P-intercept |
|---|---|---|---|---|---|---|
| MR Egger | 133 | 0.641 (0.438–0.939) | 0.024 | 0.001584 | 0.173404 | |
| IVW | 133 | 0.800 (0.647–0.989) | 0.039 | 0.001255 | ||
| LTL | Maximum likelihood | 133 | 0.799 (0.668–0.956) | 0.014 | ||
| MR-PRESSO (RAW) | 133 | 0.800 (0.646–0.991) | 0.041 | |||
| MR-RAPS | 133 | 0.798 (0.667–0.955) | 0.014 |
Notes.
- MR
- Mendelian randomization
- LTL
- leukocyte telomere length
- BD
- bipolar disorder
- IVW
- inverse variance weighted
- MR-PRESSO
- Mendelian randomization-pleiotropy residual sum outlier
- MR-RAPS
- MR-robust adjusted profile score
- OR
- odds ratio
- P-het
- P value for heterogeneity using Cochran Q test
- P-intercept
- P value for MR-Egger intercept
- SNP
- single-nucleotide polymorphism
Figure 2. Forest plot to visualize causal effects of variation in LTL on BD.
Presented odds ratios (OR) and confidence intervals (CI) correspond to the effects of LTL on BD. The results of MR analyses using various analysis methods (MR–Egger, maximum likelihood, MR-PRESSO, MR–RAPS, IVW) are presented for comparison. Total single-nucleotide polymorphism (SNP) indicates the number of genetic variants used as instruments for MR analysis.
Figure 3. Scatter plots of LTL with the risk of BD.
Scatter plot demonstrating the effect of each LTL-associated SNP on BD on the log-odds scale. The slopes of each line represent the causal association for each method.
Figure 4. MR density plots to visualize the overall heterogeneity of MR estimates for the effect of LTL on BD.
MR, Mendelian randomization; SNP, single-nucleotide polymorphism.
Figure 5. Radial plots of the MR-Egger test analyzed the outlier SNPs.
The X axis represents the square root of the actual weight that every SNP gets in the IVW analysis. The Y axis represents each SNP’s ratio estimate multiplied by the same square-root weight.
Discussion
In the present study, we estimated the causative influence of LTL on the risk of BD using the MR method. We observed that genetically determined LTL was negatively associated with BD.
Telomeres are nucleoprotein structures that are present at the ends of each chromosome arm and help to keep the genome stable. Gene abnormalities that are connected with telomere maintenance in humans have been linked to a variety of germline and somatic degenerative illnesses, including dyskeratosis congenital and ulcerative colitis (Calado et al., 2009). In addition, telomere dysfunction has become one of the molecular hallmarks of cellular aging (Lopez-Otin et al., 2013). Therefore, TL may be related to many aging-related diseases. For example, Haycock et al. (2014) found that LTL was linked to a lower risk of coronary heart disease, even when traditional vascular risk factors were taken into account. Pousa et al. (2021) reported an inverse association between TL and distress-related mental disorders (including traumatic stress disorder, anxiety disorder, and depression). Interestingly, Roberts et al. (2014) indicated that both long and short TL may play a role in the pathogenesis of amnestic mild cognitive impairment (aMCI) and may be markers of increased risk of aMCI. In addition, according to the findings of Jebaraj and colleagues, chronic lymphocytic leukemia is characterized by short telomeres, which are linked to a poor prognosis, genomic complexity, and clonal evolution (Jebaraj et al., 2019).
In addition to the results of observational studies, there are many studies using MR methods that also show a causal association between telomere length (TL) and many diseases. For example, a comprehensive MR analysis by The Telomeres Mendelian Randomization Collaboration (2017) showed the strongest positive association between genetically predicted longer TL and a variety of cancers. There were also many MR studies reporting the relationship between TL and neurological diseases, including amyotrophic lateral sclerosis (Xia et al., 2021), Alzheimer’s disease (Gao et al., 2019; Rodriguez-Fernandez et al., 2022; Yu et al., 2021), multiple sclerosis (Liao et al., 2022; Shu, Li & Zhu, 2022). But there are also some MR studies showing that telomere length is not associated with some neurological traits, such as depression (Wium-Andersen et al., 2017), and Parkinson’s disease (Chen & Zhan, 2021).
Currently, observational studies exist on the association of TL with BD. Rizzo and colleagues revealed evidence of accelerated aging in BD in the form of shorter telomeres (a marker of cellular aging) (Rizzo et al., 2013). In addition, Huang et al. (2018) found BD patients had shorter LTLs than controls. Ferensztajn-Rochowiak et al. (2021) also found that BD patients had significantly shorter TL compared with the control group. In addition to observational studies, an MR analysis enrolling 131 patients with BD and 336 controls conducted by Pisanu et al. (2020a) and Pisanu et al. (2020b) indicated that there was no association between genetically determined LTL and BD risk. Nevertheless, Pisanu’s study had a limited number of participants, which is a significant limitation that has an impact on the outcomes of the MR analysis (Davies, Holmes & Davey Smith , 2018). Besides this, to our knowledge, there were no large-sample MR analyses to explore the causal relationship between telomere length and BD. Consequently, it is crucial to reevaluate whether it will produce different conclusions if more extensive GWAS datasets become available. In line with previous studies, utilizing the MR analysis based on the largest LTL and BD-related datasets, we found a negative association between LTL and BD risk. At the same time, to ensure the reliability of the results, we performed a series of sensitivity analyses. The results show strong consistency of our findings across methods. Taken together, these data indicate that LTL is a substantial protective factor for BD. In addition, LTL may be an essential indicator for predicting BD risk.
MR studies must satisfy three basic assumptions (Davies, Holmes & Davey Smith, 2018). In this study, we evaluated the veracity of these assumptions in various methods. We primarily assessed the relevance assumption through P-value (P < 5 × 10−8) and LD analyses. In addition, we also use the F statistic to rule out the presence of weak instrumental variables. Horizontal pleiotropy cannot exist due to the nature of the exclusion restriction assumption. Hence, many sensitivity analyses were performed to examine the impact of horizontal pleiotropy on our MR study. The estimates between LTL and BD were generally consistent throughout IVW and sensitivity analysis, making the conclusion trustworthy. Finally, it is challenging to ensure that the independence assumption is not violated due to the presence of confounders that cannot be assessed or are unknown from prior knowledge. There is a possibility that the link between a GIV and an outcome is confounded in certain samples due to a hidden population structure (Davies, Holmes & Davey Smith , 2018). Consequently, the exposure and outcome datasets utilized in this investigation were all sourced from European populations, thereby avoiding the confounding effects of diverse populations on causal analyses. However, it remains to be determined whether additional confounding factors influence the association between LTL and BD risk.
Despite the fact that two-sample MR is an excellent method for making causal inferences among exposures and outcomes employing summary statistics, we should proceed with caution due to a number of limitations. First, our study was conducted using European populations, which limits its ability to be applied to a larger group. Second, it is also possible that additional factors, such as other disease states, confound our results, but it is hard to avoid. In addition, in our work, telomere length was detected in leucocytes, and whether it also be able actually to reflect the telomere length of other organ tissues is unclear. Lastly, even though that a number of sensitivity analyses were done to investigate violations of exchangeability and exclusion limitation criteria, those assumptions remain unverifiable.
The large sample size is one of the strengths of this study. In addition, to our knowledge, no MR evaluating the link between LTL and BD has been done. MR research had advantages over conventional observational studies, such as the reduction of residual confounding risk. In addition, we performed a series of sensitivity assessments to verify the stability and reliability of the MR analysis results. As a consequence, we were able to provide novel insights that may help clarify the role of LTL in BD occurrence.
In summary, using the MR method, we found that LTL was a negative causal factor for BD risk. More research is required to establish how this potential cause-and-effect relationship works. Clinically, BD may be predicted by detecting LTL. In addition, because genetic variants cause the effect of LTL on BD is lifelong. Therefore, our findings are helpful in developing strategies for treating BD.
Supplemental Information
The F statistic of these SNPs was greater than 10 (range, 29.86–1628.82; mean, 119.94) for LTL.
All 133 distinct genetic variations connected to telomere length were accessible in the summary statistics for bipolar disorder. Further Mendelian randomization analysis demonstrated that genetically determined telomere length has an inverse relationship with bipolar disorder. Created with Biorender.com.
Abbreviations
- LTL
leukocyte telomere length
- BD
bipolar disorder
- MR
mendelian randomization
- SNPs
single-nucleotide polymorphisms
- GWAS
genome-wide association study
- IVW
inverse-variance-weighted
- MR-PRESSO
Mendelian randomization-pleiotropy residual sum outlier
- MR-RAPS
MR-robust adjusted profile score
- OR
odds ratio
- GIVs
genetic instrument variables
- LD
linkage disequilibrium
- aMCI
amnestic mild cognitive impairment
Funding Statement
This work was supported by grants from the National Key R&D Program of China (2019YFA0802600), the National Natural Science Foundation of China (81974244), the Hainan Province Clinical Medical Center (QWYH202175), the Research and Cultivation Fund of Hainan Medical University (HYPY2020015), and the Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX21-2972). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Bangbei Wan, Email: 939313612@qq.com.
Miao Sun, Email: miaosunsuda@163.com.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Likui Lu conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft.
Hongtao Zeng conceived and designed the experiments, authored or reviewed drafts of the article, and approved the final draft.
Bangbei Wan conceived and designed the experiments, authored or reviewed drafts of the article, and approved the final draft.
Miao Sun conceived and designed the experiments, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft.
Data Availability
The following information was supplied regarding data availability:
The raw data is available in the Supplemental Files.
References
- Ayora et al. (2022).Ayora M, Fraguas D, Abregu-Crespo R, Recio S, Blasco MA, Moises A, Derevyanko Aksinya, Arango Celso, Diaz-Caneja CM. Leukocyte telomere length in patients with schizophrenia and related disorders: a meta-analysis of case-control studies. Molecular Psychiatry. 2022;27(7):2968–2975. doi: 10.1038/s41380-022-01541-7. [DOI] [PubMed] [Google Scholar]
- Bauer (2022).Bauer MS. Bipolar disorder. Annals of Internal Medicine. 2022;175(7):ITC97–ITC112. doi: 10.7326/AITC202207190. [DOI] [PubMed] [Google Scholar]
- Baumeister et al. (2021).Baumeister SE, Nolde M, Alayash Z, Leitzmann M, Baurecht H, Meisinger C. Cannabis use does not impact on type 2 diabetes: a two-sample Mendelian randomization study. Addiction Biology. 2021;26(6):e13020. doi: 10.1111/adb.13020. [DOI] [PubMed] [Google Scholar]
- Bowden, Davey Smith & Burgess (2015).Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. International Journal of Epidemiology. 2015;44(2):512–525. doi: 10.1093/ije/dyv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brion, Shakhbazov & Visscher (2013).Brion MJ, Shakhbazov K, Visscher PM. Calculating statistical power in Mendelian randomization studies. International Journal of Epidemiology. 2013;42(5):1497–1501. doi: 10.1093/ije/dyt179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess, Butterworth & Thompson (2013).Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genetic Epidemiology. 2013;37(7):658–665. doi: 10.1002/gepi.21758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess et al. (2020).Burgess S, Foley CN, Allara E, Staley JR, Howson JMM. A robust and efficient method for Mendelian randomization with hundreds of genetic variants. Nature Communications. 2020;11(1):376. doi: 10.1038/s41467-019-14156-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess & Thompson (2017).Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. European Journal of Epidemiology. 2017;32(5):377–389. doi: 10.1007/s10654-017-0255-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calado et al. (2009).Calado RT, Yewdell WT, Wilkerson KL, Regal JA, Kajigaya S, Stratakis CA, Young NS. Sex hormones, acting on the TERT gene, increase telomerase activity in human primary hematopoietic cells. Blood. 2009;114(11):2236–2243. doi: 10.1182/blood-2008-09-178871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen & Zhan (2021).Chen R, Zhan Y. Association between telomere length and Parkinson’s disease: a Mendelian randomization study. Neurobiology of Aging. 2021;97:144 e149-144 e111. doi: 10.1016/j.neurobiolaging.2020.07.019. [DOI] [PubMed] [Google Scholar]
- Cui & Tian (2021).Cui Z, Tian Y. Using genetic variants to evaluate the causal effect of serum vitamin D concentration on COVID-19 susceptibility, severity and hospitalization traits: a Mendelian randomization study. Journal of Translational Medicine. 2021;19(1):300. doi: 10.1186/s12967-021-02973-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey Smith & Hemani (2014).Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Human Molecular Genetics. 2014;23(R1):R89–R98. doi: 10.1093/hmg/ddu328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies, Holmes & Davey Smith (2018).Davies NM, Holmes MV, Smith Davey G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ. 2018;362:k601. doi: 10.1136/bmj.k601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do et al. (2013).Do R, Willer CJ, Schmidt EM, Sengupta S, Gao C, Peloso GMC, Gustafsson SC, Kanoni SC, Ganna AC, Chen JC, Buchkovich MLC, Mora SC, Beckmann JSC, Bragg-Gresham JLC, Chang HYC, Demirkan AC, Hertog HMDC, Donnelly LAC, Ehret GBC, Esko TC, Feitosa MFC, Ferreira TC, Fischer KC, Fontanillas PC, Fraser RMC, Freitag DFC, Gurdasani DC, Heikkilä KC, Hyppönen EC, Isaacs AC, Jackson AUC, Johansson AC, Johnson TC, Kaakinen MC, Kettunen JC, Kleber MEC, Li XHC, Luan JAC, Lyytikäinen LPC, Magnusson PKEC, Mangino MC, Mihailov EC, Montasser MEC, Müller-Nurasyid MC, Nolte IMC, O’Connell JRC, Palmer CDC, Perola MC, Petersen AKC, Sanna SC, Saxena RC, Service SKC, Shah SC, Shungin DC, Sidore CC, Song CC, Strawbridge RJC, Surakka IC, Tanaka TC, Teslovich TMC, Thorleifsson GC, Herik EGVDC, Voight BFC, Volcik KAC, Waite LLC, Wong AC, Wu YC, Zhang WHC, Absherv DC, Asiki GC, Barroso IC, Been LFC, Bolton JLC, Bonnycastle LLC, Brambilla PC, Burnett MSC, Cesana GC, Dimitriou MC, Doney ASFC, Döring AC, Elliott PC, Epstein SEC, Eyjolfsson GIC, Gigante BC, Goodarzi MOC, Grallert HC, Gravito MLC, Groves CJC, Hallmans GC, Hartikainen ALC, Hayward CC, Hernandez DC, Hicks AAC, Holm HC, Hung YJC, Illig TC, Jones MRC, Kaleebu PC, Kastelein JJPC, Khaw KTC, Kim EC, Klopp NC, Komulainen PC, Kumari MC, Langenberg CC, Lehtimäki TC, Lin SYC, Lindström JC, Loos RJFC, Mach FC, McArdle WLC, Meisinger CC, Mitchell BDC, Müller GC, Nagaraja RC, Narisu NC, Nieminen TVMC, Nsubuga RNC, Olafsson IC, Ong KKC, Palotie AC, Papamarkou TC, Pomilla CC, Pouta AC, Rader DJC, Reilly MPC, Ridker PMC, Rivadeneira FC, Rudan IC, Ruokonen AC, Samani NC, Scharnagl HC, Seeley JC, Silander KC, Stančáková AC, Stirrups KC, Swift AJC, Tiret LC, Uitterlinden AGC, Pelt LJVC, Vedantam SC, Wainwright NC, Wijmenga CC, Wild SHC, Willemsen GC, Wilsgaard TC, Wilson JFC, Young EHC, Zhao JHC, Adair LSC, Arveiler DC, Assimes TLC, Bandinelli SC, Bennett FC, Bochud MC, Boehm BOC, Boomsma DIC, Borecki IBC, Bornstein SRC, Bovet PC, Burnier MC, Campbell HC, Chakravarti AC, Chambers JCC, Chen YDIC, Collins FSC, Cooper RSC, Danesh JC, Dedoussis GC, Faire UDC, Feranil ABC, Ferriéres JC, Ferrucci LC, Freimer NBC, Gieger CC, Groop LCC, Gudnason VC, Gyllensten UC, Hamsten AC, Harris TBC, Hingorani AC, Hirschhorn JAC, Hofman AC, Hovingh GKC, Hsiung CAC, Humphries SEC, Hunt SCC, Hveem KC, Iribarren CC, Järvelin MRC, Jula AC, Kähönen MC, Kaprio JC, Kesäniemi AC, Kivimaki MC, Kooner JSC, Koudstaal PJC, Krauss RMC, Kuh DC, Kuusisto JC, Kyvik KOC, Laakso MC, Lakka TAC, Lind LC, Lindgren CMC, Martin NGC, März WC, McCarthy MIC, McKenzie CAC, Meneton PC, Metspalu AC, Moilanen LC, Morris ADC, Munroe PBC, Njølstad IC, Pedersen NLC, Power CC, Pramstaller PPC, Price JFC, Psaty BMC, Quertermous TC, Rauramaa RC, Saleheen DC, Salomaa VC, Sanghera DKC, Saramies JC, Schwarz PEHC, Sheu WHHC, Shuldiner ARC, Siegbahn AC, Spector TDC, Stefansson KC, Strachan DPC, Tayo BOC, Tremoli EC, Tuomilehto JC, Uusitupa MC, Duijn CMVC, Vollenweider PC, Wallentin LC, Wareham NJC, Whitfield JBC, Wolffenbuttel BHRC, Altshuler DC, Ordovas JMC, Boerwinkle EC, Palmer CNAC, Thorsteinsdottir UC, Chasman DIC, Rotter JIC, Franks PWC, Ripatti SC, Cupples LAC, Sandhu MSC, Rich SSC, Boehnke MC, Deloukas PC, Mohlke KLC, Ingelsson EC, Abecasis GRC, Daly MJC, Neale BMC, Kathiresan S. Common variants associated with plasma triglycerides and risk for coronary artery disease. Nature Genetics. 2013;45(11):1345–1352. doi: 10.1038/ng.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emdin, Khera & Kathiresan (2017).Emdin CA, Khera AV, Kathiresan S. Mendelian randomization. JAMA. 2017;318(19):1925–1926. doi: 10.1001/jama.2017.17219. [DOI] [PubMed] [Google Scholar]
- Ferensztajn-Rochowiak et al. (2021).Ferensztajn-Rochowiak E, Kurczewska E, Rubis B, Lulkiewicz M, Holysz H, Rybakowski F, Rybakowski JK. Decreased leucocyte telomere length in male patients with chronic bipolar disorder: lack of effect of long-term lithium treatment. Acta Neuropsychiatry. 2021;33(6):299–306. doi: 10.1017/neu.2021.20. [DOI] [PubMed] [Google Scholar]
- Fries et al. (2020).Fries GR, Zamzow MJ, Andrews T, Pink O, Scaini G, Quevedo J. Accelerated aging in bipolar disorder: a comprehensive review of molecular findings and their clinical implications. Neuroscience & Biobehavioral Reviews. 2020;112:107–116. doi: 10.1016/j.neubiorev.2020.01.035. [DOI] [PubMed] [Google Scholar]
- Frikke-Schmidt et al. (2008).Frikke-Schmidt R, Nordestgaard BG, Stene MC, Sethi AA, Remaley AT, Schnohr P, Grande P, Tybjaerg-Hansen A. Association of loss-of-function mutations in the ABCA1 gene with high-density lipoprotein cholesterol levels and risk of ischemic heart disease. JAMA. 2008;299(21):2524–2532. doi: 10.1001/jama.299.21.2524. [DOI] [PubMed] [Google Scholar]
- Gao et al. (2019).Gao K, Wei C, Zhu J, Wang X, Chen G, Luo Y, Zhang D, Yue W, Yu H. Exploring the causal pathway from telomere length to Alzheimer’s disease: an update mendelian randomization study. Frontiers in Psychiatry. 2019;10:843. doi: 10.3389/fpsyt.2019.00843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes & Schulz (2002).Grimes DA, Schulz KF. Bias and causal associations in observational research. Lancet. 2002;3599302:248–252. doi: 10.1016/S0140-6736(02)07451-2. [DOI] [PubMed] [Google Scholar]
- Haycock et al. (2014).Haycock PC, Heydon EE, Kaptoge S, Butterworth AS, Thompson A, Willeit P. Leucocyte telomere length and risk of cardiovascular disease: systematic review and meta-analysis. BMJ. 2014;349:g4227. doi: 10.1136/bmj.g4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemani et al. (2018).Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, Laurin C, Burgess S, Bowden J, Langdon R, Tan VY, Yarmolinsky J, Shihab HA, Timpson NJ, Evans DM, Relton C, Martin RM, Smith GD, Gaunt TR, Haycock PC. The MR-Base platform supports systematic causal inference across the human phenome. Elife. 2018;7:e34408. doi: 10.7554/eLife.34408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang et al. (2018).Huang YC, Wang LJ, Tseng PT, Hung CF, Lin PY. Leukocyte telomere length in patients with bipolar disorder: an updated meta-analysis and subgroup analysis by mood status. Psychiatry Research. 2018;270:41–49. doi: 10.1016/j.psychres.2018.09.035. [DOI] [PubMed] [Google Scholar]
- Jebaraj et al. (2019).Jebaraj BMC, Tausch E, Landau DA, Bahlo J, Robrecht S, Taylor-Weiner AN, Stilgenbauer S, Bloehdorn J, Scheffold A, Mertens D, Böttcher S, Kneba M, Jäger U, Zenz T, Wenger MK, Fingerle-Rowson G, Wendtner Clemens, Fink A-M, Wu CJ, Eichhorst B, Fischer K, Hallek M, Döhner H. Short telomeres are associated with inferior outcome, genomic complexity, and clonal evolution in chronic lymphocytic leukemia. Leukemia. 2019;33(9):2183–2194. doi: 10.1038/s41375-019-0446-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo et al. (2021).Joo EJ, Ahn YM, Park M, Kim SA. Significant shortening of leukocyte telomere length in Korean patients with bipolar disorder 1. Clinical Psychopharmacology and Neuroscience. 2021;19(3):559–563. doi: 10.9758/cpn.2021.19.3.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian et al. (2021).Julian TH, Glascow N, Barry ADF, Moll T, Harvey C, Klimentidis YC, Newell M, Zhang S, Snyder MP, Cooper-Knock J, Shaw PJ. Physical exercise is a risk factor for amyotrophic lateral sclerosis: convergent evidence from Mendelian randomisation, transcriptomics and risk genotypes. EBioMedicine. 2021;68:103397. doi: 10.1016/j.ebiom.2021.103397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2022).Li R, Chen Y, Zhao A, Huang L, Long Z, Kang W, Yin Y, Tong S, Guo Y, Li S. Exploring genetic association of insomnia with allergic disease and asthma: a bidirectional Mendelian randomization study. Respiratory Research. 2022;23(1):84. doi: 10.1186/s12931-022-02009-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao et al. (2022).Liao Q, He J, Tian FF, Bi FF, Huang K. A causal relationship between leukocyte telomere length and multiple sclerosis: a Mendelian randomization study. Frontiers in Immunology. 2022;13:922922. doi: 10.3389/fimmu.2022.922922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist et al. (2015).Lindqvist D, Epel ES, Mellon SH, Penninx BW, Revesz D, Verhoeven JE, Reus VI, Lin J, Mahan L, Hough CM, Rosser R, Bersani FS, Blackburn EH, Wolkowitz OM. Psychiatric disorders and leukocyte telomere length: underlying mechanisms linking mental illness with cellular aging. Neuroscience & Biobehavioral Reviews. 2015;55:333–364. doi: 10.1016/j.neubiorev.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Otin et al. (2013).Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre et al. (2020).McIntyre RS, Berk M, Brietzke E, Goldstein BI, Lopez-Jaramillo C, Kessing LV, Malhi GS, Nierenberg AA, Rosenblat JD, Majeed A, Vieta E, Vinberg M, Young AH, Mansur RB. Bipolar disorders. Lancet. 2020;396(10265):1841–1856. doi: 10.1016/S0140-6736(20)31544-0. [DOI] [PubMed] [Google Scholar]
- Monroy-Jaramillo, Dyukova & Walss-Bass (2018).Monroy-Jaramillo N, Dyukova E, Walss-Bass C. Telomere length in psychiatric disorders: is it more than an ageing marker? The World Journal of Biological Psychiatry. 2018;19(sup2):S2–S20. doi: 10.1080/15622975.2016.1273550. [DOI] [PubMed] [Google Scholar]
- Pierce, Ahsan & Vanderweele (2011).Pierce BL, Ahsan H, Vanderweele TJ. Power and instrument strength requirements for Mendelian randomization studies using multiple genetic variants. International Journal of Epidemiology. 2011;40(3):740–752. doi: 10.1093/ije/dyq151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisanu et al. (2020a).Pisanu C, Congiu D, Manchia M, Caria P, Cocco C, Dettori T, Frau DV, Manca E, Meloni A, Nieddu M, Noli B, Pinna F, Robledo R, Sogos V, Ferri GL, Carpiniello B, Vanni R, Bocchetta A, Severino G, Ardau R, Chillotti C, Zompo MD, Squassina A. Differences in telomere length between patients with bipolar disorder and controls are influenced by lithium treatment. Pharmacogenomics. 2020a;21(8):533–540. doi: 10.2217/pgs-2020-0028. [DOI] [PubMed] [Google Scholar]
- Pisanu et al. (2020b).Pisanu C, Tsermpini EE, Skokou M, Kordou Z, Gourzis P, Assimakopoulos K, Congiu D, Meloni A, Balasopoulos D, Patrinos GP, Squassina A. Leukocyte telomere length is reduced in patients with major depressive disorder. Drug Development Research. 2020b;81(3):268–273. doi: 10.1002/ddr.21612. [DOI] [PubMed] [Google Scholar]
- Pousa et al. (2021).Pousa PA, Souza RM, Melo PHM, Correa BHM, Mendonca TSC, Simoes ESAC, Miranda DM. Telomere shortening and psychiatric disorders: a systematic review. Cells. 2021;10(6):1423. doi: 10.3390/cells10061423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2021).R Core Team . Version 4.1.2. R Foundation for Statistical Computing; Vienna: 2021. [Google Scholar]
- Rizzo et al. (2013).Rizzo LB, Do Prado CH, Grassi-Oliveira R, Wieck A, Correa BL, Teixeira AL, Bauer ME. Immunosenescence is associated with human cytomegalovirus and shortened telomeres in type I bipolar disorder. Bipolar Disorders. 2013;15(8):832–838. doi: 10.1111/bdi.12121. [DOI] [PubMed] [Google Scholar]
- Roberts et al. (2014).Roberts RO, Boardman LA, Cha RH, Pankratz VS, Johnson RA, Druliner BR, Christianson TJH, Roberts LR, Petersen RC. Short and long telomeres increase risk of amnestic mild cognitive impairment. Mechanisms of Ageing and Development. 2014;141-142:64–69. doi: 10.1016/j.mad.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Fernandez et al. (2022).Rodriguez-Fernandez B, Gispert JD, Guigo R, Navarro A, Vilor-Tejedor N, Crous-Bou M. Genetically predicted telomere length and its relationship with neurodegenerative diseases and life expectancy. Computational and Structural Biotechnology Journal. 2022;20:4251–4256. doi: 10.1016/j.csbj.2022.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu, Li & Zhu (2022).Shu MJ, Li J, Zhu YC. Genetically predicted telomere length and multiple sclerosis. Multiple Sclerosis and Related Disorders. 2022;60:103731. doi: 10.1016/j.msard.2022.103731. [DOI] [PubMed] [Google Scholar]
- Smith & Ebrahim (2003).Smith GD, Ebrahim S. ’Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? International Journal of Epidemiology. 2003;32(1):1–22. doi: 10.1093/ije/dyg070. [DOI] [PubMed] [Google Scholar]
- Stahl et al. (2019).Stahl EA, Breen G, Forstner AJ, McQuillin A, Ripke S, Trubetskoy V, Mattheisen M, Wang Y, Coleman JRI, Gaspar HA, Leeuw CA, Steinberg S, Pavlides JMW, Trzaskowski M, Byrne EM, Pers TH, Holmans PA, Richards AL, Abbott L, Agerbo E, Akil H, Albani D, Alliey-Rodriguez N, Als TD, Anjorin A, Antilla V, Awasthi S, Badner JA, Bækvad-Hansen M, Barchas JD, Bass N, Bauer M, Belliveau R, Bergen SE, Pedersen CB, Bøen E, Boks MP, Boocock J, Budde M, Bunney W, Burmeister M, Bybjerg-Grauholm J, Byerley W, Casas M, Cerrato F, Cervantes P, Chambert K, Charney AW, Chen D, Churchhouse C, Clarke TK, Coryell W, Craig DW, Cruceanu C, Curtis D, Czerski PM, Dale AM, Jong S, Degenhardt F, Del-Favero J, DePaulo JR, Djurovic S, Dobbyn AL, Dumont A, Elvsåshagen T, Escott-Price V, Fan CC, Fischer SB, Flickinger M, Foroud TM, Forty L, Frank J, Fraser C, Freimer NB, Frisén L, Gade K, Gage D, Garnham J, Giambartolomei C, Pedersen MG, Goldstein J, Gordon SD, Gordon-Smith K, Green EK, Green MJ, Greenwood TA, Grove J, Guan W, Guzman-Parra J, Hamshere ML, Hautzinger M, Heilbronner U, Herms S, Hipolito M, Hoffmann P, Holland D, Huckins L, Jamain S, Johnson JS, Juréus A, Kandaswamy R, Karlsson R, Kennedy JL, Kittel-Schneider S, Knowles JA, Kogevinas M, Koller AC, Kupka R, Lavebratt C, Lawrence J, Lawson WB, Leber M, Lee PH, Levy SE, Li JZ, Liu C, Lucae S, Maaser A, MacIntyre DJ, Mahon PB, Maier W, Martinsson L, McCarroll S, McGuffin P, McInnis MG, McKay JD, Medeiros H, Medland SE, Meng F, Milani L, Montgomery GW, Morris DW, Mühleisen TW, Mullins N, Nguyen H, Nievergelt CM, Adolfsson AN, Nwulia EA, O’Donovan C, Loohuis LMO, Ori APS, Oruc L, Ösby U, Perlis RH, Perry A, Pfennig A, Potash JB, Purcell SM, Regeer EJ, Reif A, Reinbold CS, Rice JP, Rivas F, Rivera M, Roussos P, Ruderfer DM, Ryu E, Sánchez-Mora C, Schatzberg AF, Scheftner WA, Schork NJ, Weickert CS, Shehktman T, Shilling PD, Sigurdsson E, Slaney C, Smeland OB, Sobell JL, Hansen CS, Spijker AT, Clair DS, Steffens M, Strauss JS, Streit F, Strohmaier J, Szelinger S, Thompson RC, Thorgeirsson TE, Treutlein J, Vedder H, Wang W, Watson SJ, Weickert TW, Witt SH, Xi S, Xu W, Young AH, Zandi P, Zhang P, Zöllner S, eQTLGen C, BIOS C, Adolfsson R, Agartz I, Alda M, Backlund L, Baune BT, Bellivier F, Berrettini WH, Biernacka JM, Blackwood DHR, Boehnke M, Børglum AD, Corvin A, Craddock N, Daly MJ, Dannlowski U, Esko T, Etain B, Frye M, Fullerton JM, Gershon ES, Gill M, Goes F, Grigoroiu-Serbanescu M, Hauser J, Hougaard DM, Hultman CM, Jones I, Jones LA, Kahn RS, Kirov G, Landén M, Leboyer M, Lewis CM, Li QS, Lissowska J, Martin NG, Mayoral F, McElroy SL, McIntosh AM, McMahon FJ, Melle I, Metspalu A, Mitchell PB, Morken G, Mors O, Mortensen PB, Müller-Myhsok B, Myers RM, Neale BM, Nimgaonkar V, Nordentoft M, Nöthen MM, O’Donovan MC, Oedegaard KJ, Owen MJ, Paciga SA, Pato C, Pato MT, Posthuma D, Ramos-Quiroga JA, Ribasés M, Rietschel M, Rouleau GA, Schalling M, Schofield PR, Schulze TG, Serretti A, Smoller JW, Stefansson H, Stefansson K, Stordal E, Sullivan PF, Turecki G, Vaaler AE, Vieta E, Vincent JB, Werge T, Nurnberger JI, Wray NR, Florio AD, Edenberg HJ, Cichon S, Ophoff RA, Scott LJ, Andreassen OA, Kelsoe J, Sklar P, Bipolar Disorder Working Group of the Psychiatric Genomics C Genome-wide association study identifies 30 loci associated with bipolar disorder. Nature Genetics. 2019;51(5):793–803. doi: 10.1038/s41588-019-0397-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Telomeres Mendelian Randomization Collaboration (2017).The Telomeres Mendelian Randomization Collaboration Association between telomere length and risk of cancer and non-neoplastic diseases: a Mendelian randomization study. JAMA Oncology. 2017;3(5):636–651. doi: 10.1001/jamaoncol.2016.5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaiserman & Krasnienkov (2020).Vaiserman A, Krasnienkov D. Telomere length as a marker of biological age: state-of-the-art, open issues, and future perspectives. Frontiers in Genetics. 2020;11:630186. doi: 10.3389/fgene.2020.630186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbanck et al (2018).Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nature Genetics. 2018;50(5):693–698. doi: 10.1038/s41588-018-0099-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voight et al. (2012).Voight BF, Peloso GM, Orho-Melander M, Frikke-Schmidt R, Barbalic M, Jensen MK, Hindy G, Hólm H, Ding EL, Johnson T, Schunkert H, Samani NJ, Clarke R, Hopewell JC, Thompson JF, Li M, Thorleifsson G, Newton-Cheh C, Musunuru K, Pirruccello JP, Saleheen D, Chen L, Stewart AFR, Schillert A, Thorsteinsdottir U, Thorgeirsson G, Anand S, Engert JC, Morgan T, Spertus J, Stoll M, Berger K, Martinelli N, Girelli D, McKeown PP, Patterson CC, Epstein SE, Devaney J, Burnett M, Mooser V, Ripatti S, Surakka I, Nieminen MS, Sinisalo J, Lokki M, Perola M, Havulinna A, Faire U, Gigante B, Ingelsson E, Zeller T, Wild P, Bakker PIW, Klungel OH, Zee AM, Peters BJM, Boer A, Grobbee DE, Kamphuisen PW, Deneer VHM, Elbers CC, Onland-Moret NC, Hofker MH, Wijmenga C, Verschuren WMM, Boer JMA, Schouw YT, Rasheed A, Frossard P, Demissie S, Willer C, Do R, Ordovas JM, Abecasis GR, Boehnke M, Mohlke KL, Daly MJ, Guiducci C, Burtt NP, Surti A, Gonzalez E, Purcell S, Gabriel S, Marrugat J, Peden J, Erdmann J, Diemert P, Willenborg C, König IR, Fischer M, Hengstenberg C, Ziegler A, Buysschaert I, Lambrechts D, Werf FV, Fox KA, Mokhtari NEE, Rubin D, Schrezenmeir J, Schreiber S, Schäfer A, Danesh J, Blankenberg S, Roberts R, McPherson R, Watkins H, Hall AS, Overvad K, Rimm E, Boerwinkle E, Tybjaerg-Hansen A, Cupples LA, Reilly MP, Melander O, Mannucci PM, Ardissino D, Siscovick D, Elosua R, Stefansson K, O’Donnell CJ, Salomaa V, Rader DJ, Peltonen L, Schwartz SM, Altshuler D, Kathiresan S. Plasma HDL cholesterol and risk of myocardial infarction: a Mendelian randomisation study. Lancet. 2012;3809841:572–580. doi: 10.1016/S0140-6736(12)60312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2017).Wang X, Sundquist K, Hedelius A, Palmer K, Memon AA, Sundquist J. Leukocyte telomere length and depression, anxiety and stress and adjustment disorders in primary health care patients. BMC Psychiatry. 2017;17(1):148. doi: 10.1186/s12888-017-1308-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wium-Andersen et al. (2017).Wium-Andersen MK, Orsted DD, Rode L, Bojesen SE, Nordestgaard BG. Telomere length and depression: prospective cohort study and Mendelian randomisation study in 67,306 individuals. British Journal of Psychiatry. 2017;210(1):31–38. doi: 10.1192/bjp.bp.115.178798. [DOI] [PubMed] [Google Scholar]
- Wolkowitz et al. (2017).Wolkowitz OM, Jeste DV, Martin AS, Lin J, Daly RE, Reuter C, Kraemer H. Leukocyte telomere length: effects of schizophrenia, age, and gender. Journal of Psychiatric Research. 2017;85:42–48. doi: 10.1016/j.jpsychires.2016.10.015. [DOI] [PubMed] [Google Scholar]
- Wu et al. (2020).Wu F, Huang Y, Hu J, Shao Z. Mendelian randomization study of inflammatory bowel disease and bone mineral density. BMC Medicine. 2020;18(1):312. doi: 10.1186/s12916-020-01778-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia et al. (2021).Xia K, Zhang L, Zhang G, Wang Y, Huang T, Fan D. Leukocyte telomere length and amyotrophic lateral sclerosis: a Mendelian randomization study. Orphanet Journal of Rare Diseases. 2021;16(1):508. doi: 10.1186/s13023-021-02135-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue, Shen & Pan (2021).Xue H, Shen X, Pan W. Constrained maximum likelihood-based Mendelian randomization robust to both correlated and uncorrelated pleiotropic effects. The American Journal of Human Genetics. 2021;108(7):1251–1269. doi: 10.1016/j.ajhg.2021.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu et al. (2021).Yu G, Lu L, Ma Z, Wu S. Genetically predicted telomere length and its relationship with Alzheimer’s disease. Frontiers in Genetics. 2021;12:595864. doi: 10.3389/fgene.2021.595864. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The F statistic of these SNPs was greater than 10 (range, 29.86–1628.82; mean, 119.94) for LTL.
All 133 distinct genetic variations connected to telomere length were accessible in the summary statistics for bipolar disorder. Further Mendelian randomization analysis demonstrated that genetically determined telomere length has an inverse relationship with bipolar disorder. Created with Biorender.com.
Data Availability Statement
The following information was supplied regarding data availability:
The raw data is available in the Supplemental Files.