Abstract
Activity-based probes are small molecules that covalently bind to the active site of a protease in an activity-dependent manner. We synthesized and characterized two fluorescent activity-based probes that target serine proteases with trypsin-like or elastase-like activity. We assessed the selectivity and potency of these probes against recombinant enzymes and demonstrated that while they are efficacious at labeling active proteases in complex protein mixtures in vitro, they are less valuable for in vivo studies. We used these probes to evaluate serine protease activity in two mouse models of acute inflammation, including pancreatitis and colitis. As anticipated, the activity of trypsin-like proteases was increased during pancreatitis. Levels of elastase-like proteases were low in pancreatic lysates and colonic luminal fluids, whether healthy or inflamed. Exogenously added recombinant neutrophil elastase was inhibited upon incubation with these samples, an effect that was augmented in inflamed samples compared to controls. These data suggest that endogenous inhibitors and elastase-degrading proteases are upregulated during inflammation.
Keywords: Fluorescent probes, Activity-based probes, Trypsin, Elastase, Protease, Pancreatitis, Colitis, Inflammation, Protease inhibitors, Diphenylphosphonate
Proteases are enzymes that cleave proteins to mediate critical processes during normal physiology and disease. Covalent activity-based probes (ABPs) are unique tools that can measure the activity of specific proteases within complex protein mixtures.1–4 Probes incorporating biotin or click chemistry groups can be used for biochemical assessment of activity and for identification of probe targets after affinity purification. Fluorescent ABPs allow for shorter biochemical assays, as labeling can be detected directly in the gel without the need for click reactions, membrane transfer or streptavidin binding. Furthermore, fluorescent ABPs can be used to visualize protease activity in situ, by whole animal or tissue imaging or fluorescent microscopy. Fluorescent ABPs for cysteine proteases have been well characterized and are efficacious in vivo.5–13 Despite the critical importance of serine proteases in physiological and pathophysiological processes, ABPs for these enzymes are scarce, and they have not been extensively characterized in complex biological fluids or in animal models.14–22 We therefore aimed to synthesize and characterize fluorescent ABPs that can detect serine proteases that are activated during inflammation, namely trypsin- and elastase-like proteases.23–28
There are few electrophilic warheads that react with serine proteases. This is largely owing to the fact that the hydroxyl group in the active site is not as nucleophilic as the thiol group of cysteine proteases. Most of the reported warheads react broadly with serine hydrolases (fluorophosphonates,29 β-lactams,30 β-lactones31), and some also bind to lipases (sulfonyl fluorides32,33). Phosphonates,14,17 phosphoramidates,21 and 4-chloroisocoumarins19,20,22 are more specific for serine proteases. For our fluorescent ABPs, we elected to use a diphenylphosphonate (DPP) moiety based on its reported stability in aqueous environments and the irreversible nature of its binding to the serine protease hydroxyl group.14,16,17,34 Whether fluorescent DPP probes are suitable for in vivo use has not previously been reported.
We based our design on two existing biotin probes with DPP warheads. Biotin-Pro-Lys-DPP targeted trypsin-like proteases, including trypsin, tryptase, plasmin and thrombin.17 Biotin-Val-DPP14 was reported to label elastase-like proteases. In both cases, we replaced the biotin tag and PEG spacer with a Cy5 tag to generate Cy5-PK-DPP and Cy5-V-DPP (Scheme 1). Cy5 is a near-infrared fluorophore that is well suited for in vivo imaging.7,8
Scheme 1.
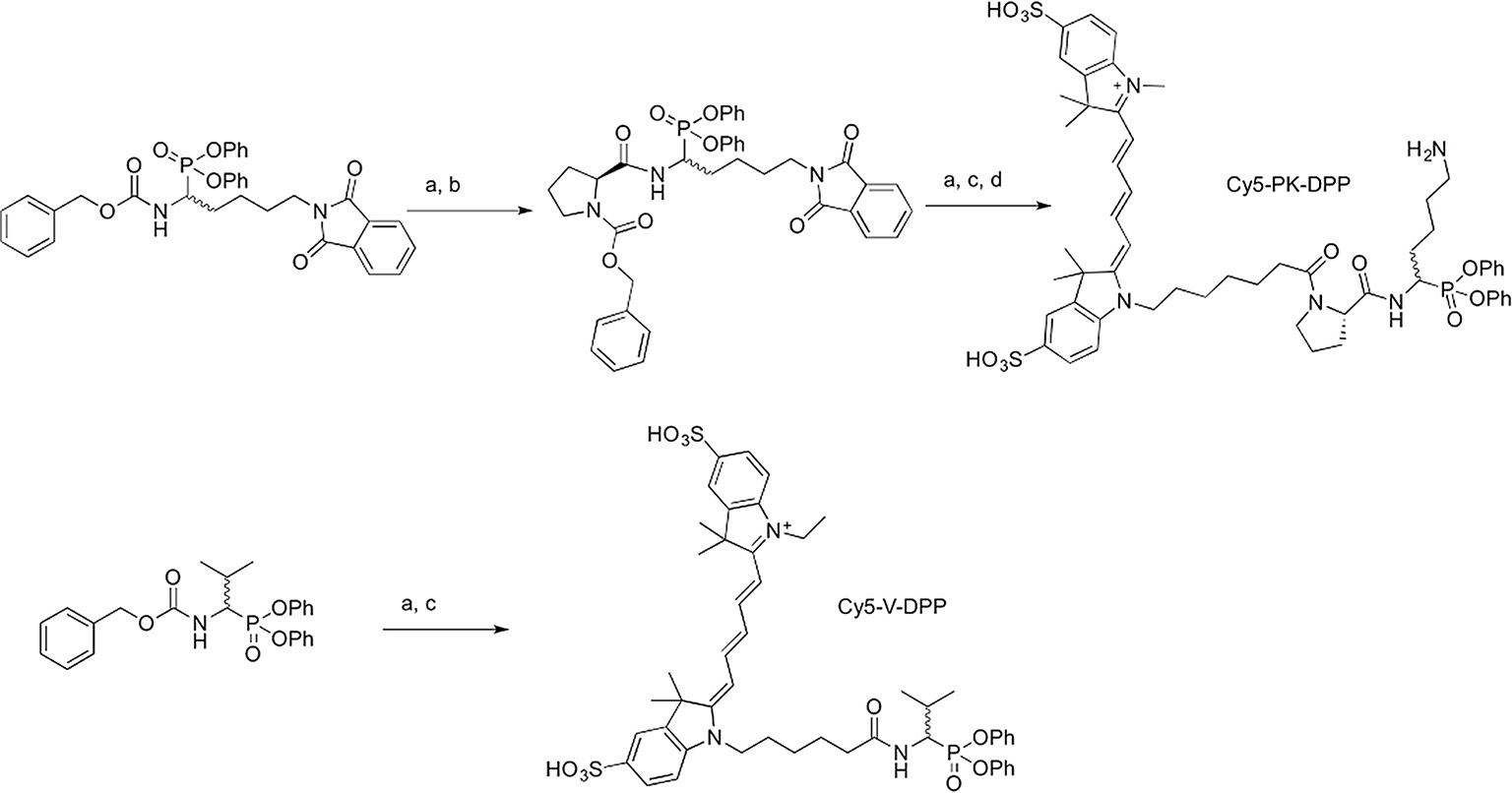
Synthesis of probes. Reagents and conditions: (a) 10% Pd/C, H2, MeOH, 2–6 h; (b) Cbz-Pro-OH, HOBt, EDCl, Et3N, MeCN, 15 h; (c) sulfonated Cy5 carboxylic acid, HCTU or PyBOP, Et3N, 5 h-overnight; (d) 30% N2H4H2O, MeCN/H2O, 15 h.
For the synthesis of Cy5-PK-DPP (Scheme 1), diphenyl benzyloxycarbonylamino-(4-phthalimidobutyl)methanephosphonate was prepared from 5-amino-1-pentanol by phthalamide protection, Swern oxidation and Birum-Oleksyszyn condensation, following the procedures described by Joossens et al.35 Benzylcarbamate (Z) deprotection by hydrogenation was followed by amide coupling to Z-protected proline in the presence of HOBt and EDCI, providing a Z-protected intermediate that could be purified by silica gel chromatography. Deprotection of the Z group, again by hydrogenation, followed by HCTU-mediated amide coupling to sulfonated Cy5 carboxylic acid provided a fluorescent intermediate, which was purified by preparative HPLC. The phthalamide group was then removed by treatment with hydrazine to provide Cy5-PK-DPP, which was isolated in pure form following further preparative HPLC (Fig. S1).
An analogous synthetic approach was applied for the preparation of Cy5-V-DPP (Scheme 1). The Birium-Oleksyszyn condensation was performed with isobutylaldehyde to provide diphenyl benzyloxycarbonylaminoisopropyl-methanephosphonate. Deprotection of the Z group was performed by hydrogenation, and the crude amine was coupled to sulfonated Cy5 carboxylic acid in the presence of PyBOP, providing pure Cy5-V-DPP after preparative HPLC (Fig. S2).
We first tested the ability of the fluorescent ABPs to label key target proteases. The probes were incubated with recombinant proteases, followed by fluorescent SDS-PAGE analysis. Cy5-PK-DPP labeled recombinant trypsin in a concentration-dependent manner (Fig. 1A, B). Cy5-V-DPP labeled recombinant neutrophil elastase (NE) and proteinase 3 (PR-3).
Fig. 1.
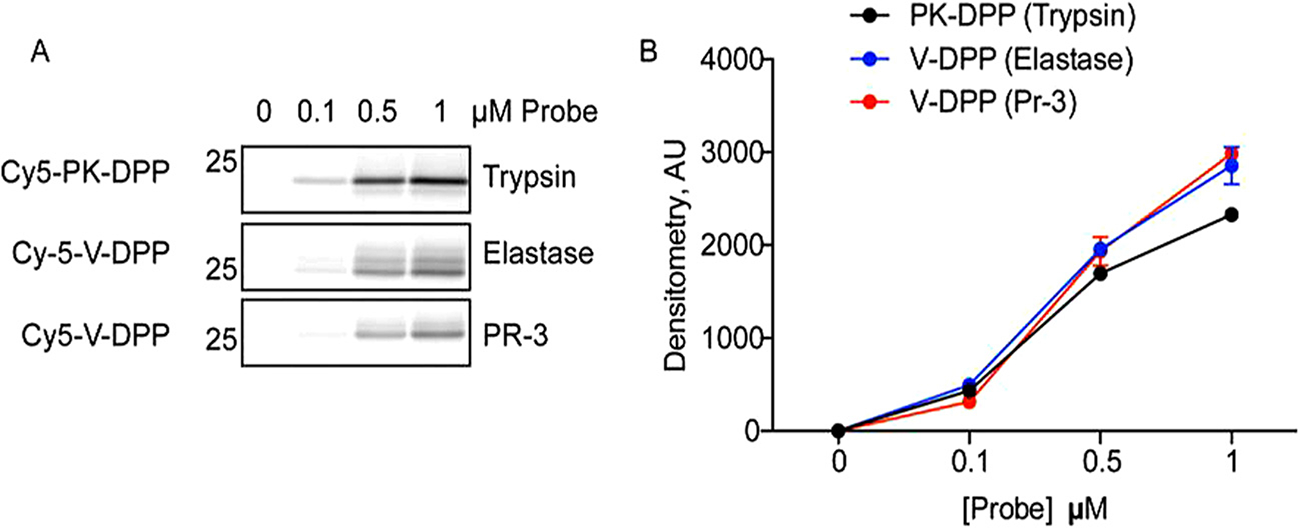
Concentration-dependent labeling of recombinant serine proteases. A) Recombinant trypsin, elastase, and proteinase-3 were incubated with increasing doses of Cy5-PK-DPP or Cy5-V-DPP. Proteins were resolved by SDS-PAGE and in-gel fluorescence was measured to detect protease labeling. B) Protease labeling in A was quantified using densitometry (n = 3).
Next, the selectivity of the probes against a panel of recombinant serine and cysteine proteases was examined (Fig. 2). Equal amounts of each recombinant protease were labeled with 1 μM probe, followed by fluorescent SDS-PAGE analysis. Cy5-PK-DPP strongly targeted trypsin, thrombin and plasmin, but was unreactive towards chymotrypsin, NE, and PR-3. This is consistent with the specificity of the biotin-tagged version of this probe.17 Like Biotin-PEG-succinyl-V-DPP14, Cy5-V-DPP strongly labeled NE. It also labeled PR-3, albeit to a lesser extent. Very low interaction with trypsin, chymotrypsin, and thrombin was observed. As the DPP warhead is selective for serine proteases, neither of the probes reacted with the cysteine protease cathepsin S.
Fig. 2.
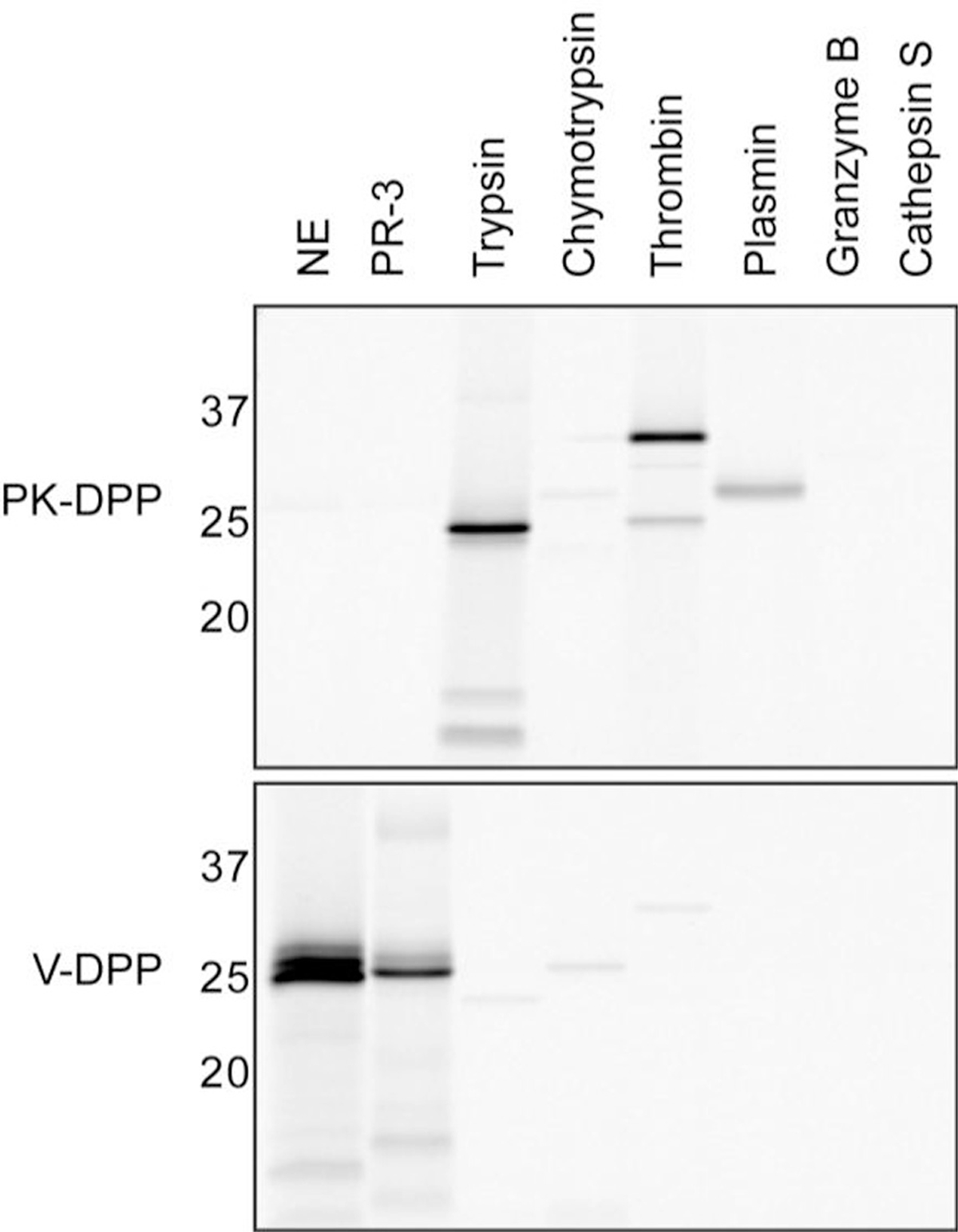
Selectivity of activity-based probes. Cy5-PK-DPP or Cy5-V-DPP incubated with a panel of recombinant serine proteases (elastase, proteinase-3, trypsin, chymotrypsin, thrombin, plasmin, and granzyme B) and one cysteine protease (cathepsin S). Proteins were resolved by SDS-PAGE and protease labeling was detected by measuring in-gel fluorescence.
Having demonstrated that these probes can detect the activity of purified serine proteases, we then examined their efficacy in complex protein mixtures (Fig. 3A). Bone marrow cells were extracted from healthy mice, lysed, and incubated with the probes. Cy5-PK-DPP labeling was observed at both 1 and 10 μM. Cy5-V-DPP labeled one protease, but only at 10 μM. In an immunoprecipitation assay with a NE-specific antibody, we confirmed that this band was NE (Fig. 3B). Cy5-V-DPP also labeled a high molecular weight species that did not immunopreciptate with the NE antibody (Fig. 3A, B). Based on its size, this may be a serum protein that binds to the probe non-specifically.
Fig. 3.
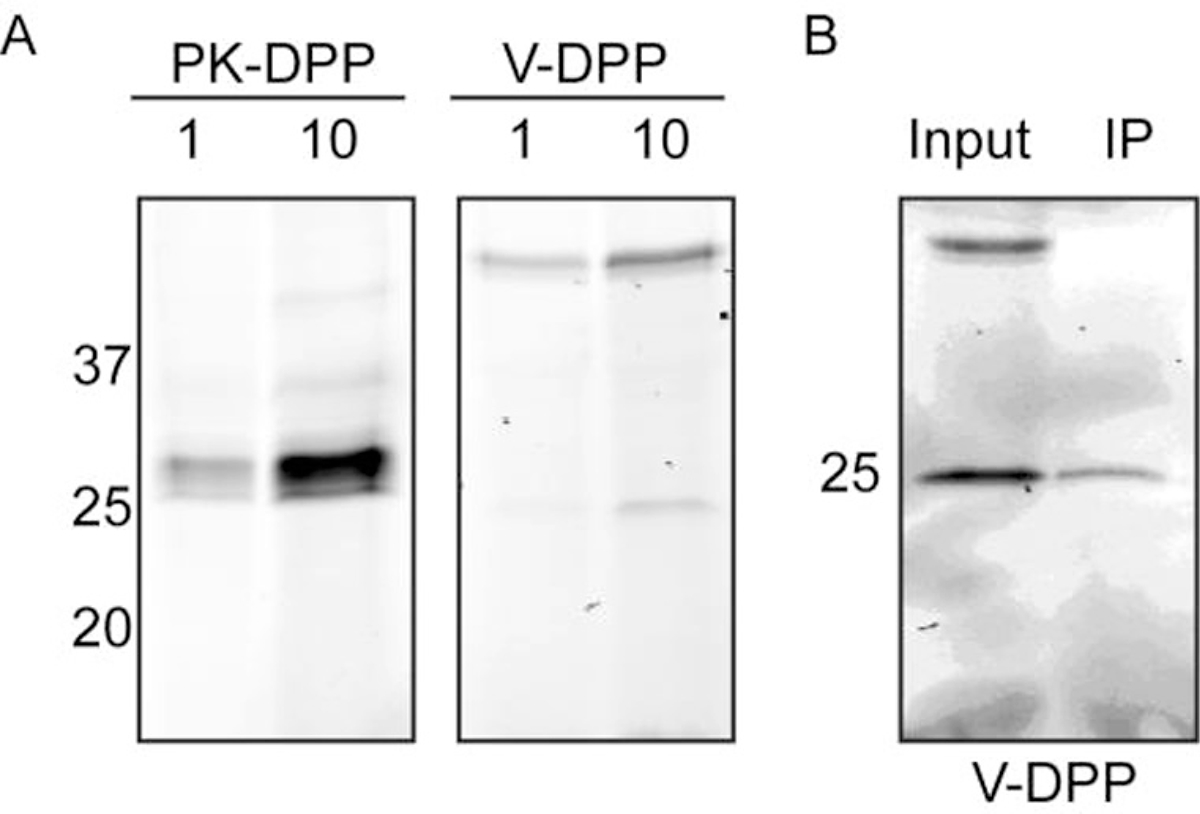
ABP labeling in complex protein mixtures. A) Bone marrow cells were isolated, lysed, and incubated with Cy5-PK-DPP or Cy5-V-DPP at 1 or 10 μM. Proteins were resolved by SDS-PAGE and protease labeling was detected by measuring in-gel fluorescence. B) Immunoprecipitation of Cy5-V-DPP-labeled lysates in A with an elastase-specific antibody, followed by SDS-PAGE and in-gel fluorescence detection.
Next, we analyzed serine protease activity in a mouse model of pancreatitis in which multiple proteases become activated. Pancreatic lysates were prepared from normal mice and those with acute pancreatitis induced by the secretogogue caerulein. As expected, Cy5-PK-DPP exhibited increased labeling of trypsin-like proteases in the 25-KDa region in the inflamed pancreas (Fig. 4A). As in the bone marrow lysate, a high molecular weight species was detected with the Cy5-V-DPP probe, though no NE labeling was observed.
Fig. 4.
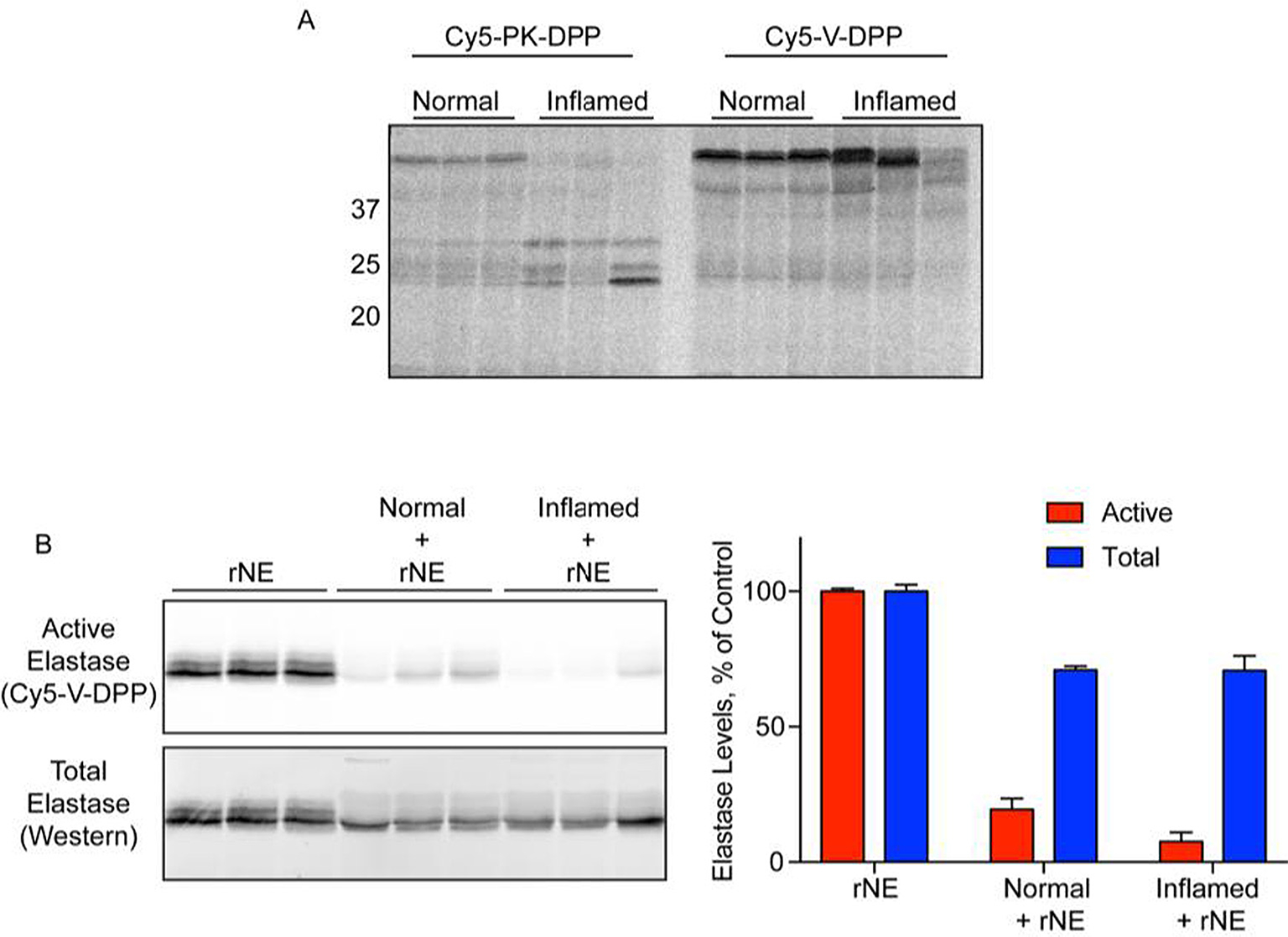
Ex vivo labeling of protease activation during acute pancreatitis. A) Lysates were prepared from normal and inflamed pancreas and incubated with Cy5-PK-DPP or Cy5-V-DPP (10 μM). Proteins were resolved by SDS-PAGE and protease labeling was detected by in-gel fluorescence. B) Recombinant neutrophil elastase was incubated with Cy5-V-DPP (1 μM), either alone (rNE) or in the presence of pancreas lysates (normal/inflamed + rNE). Elastase activity was detected by in-gel fluorescence (top/red). Total elastase levels were then measured by western blot (bottom/blue). Bands were quantified by densitometry. (Activity: rNE v Norm p = 4.1e-5; rNE v Infl p = 1.4e-5; Norm v Infl p = 0.09. Total: rNE v Norm p = 0.0005; rNE v Infl p = 0.008; Norm v Infl p = 0.97. Norm Act v Tot p = 0.0003; Infl Act v Tot p = 0.0006).
To confirm that Cy5-V-DPP is capable of detecting NE activity in pancreatic lysates, we spiked recombinant NE (rNE) into pancreas lysates from normal and inflamed mice. The spiked rNE was successfully detected by Cy5-V-DPP. Compared to samples containing rNE alone, however, the labeling in the spiked samples was greatly reduced (Fig. 4B). The reduction of rNE activity was greater in inflamed pancreas lysates than in the normal pancreas samples, though this did not reach significance due to sample variability (p = 0.09). These results suggest that elastase activity is somehow suppressed in pancreatic lysates, and that these effects may be increased during pancreatitis. To investigate this further, we examined total rNE levels in the reactions by Western blot. The rNE that was spiked into pancreatic lysates was less abundant despite the initial addition of equal amounts. Furthermore, the higher-molecular weight forms of elastase were no longer present, suggesting degradation by pancreatic proteases. The activity was significantly lower than the total amount of elastase present (when normalized as a percentage or rNE alone), suggesting that endogenous inhibitors contribute to the reduced activity in addition degradation.
We also examined serine protease activity in a mouse model of acute colitis (Fig. 5A). Using Cy5-PK-DPP, we observed strong labeling of two trypsin-like proteases in luminal colon fluids. There was considerable variation in the activation of these proteases in both the normal and inflamed groups and thus no difference between the two groups. Minimal labeling was observed with the Cy5-V-DPP probe. As we observed in the pancreas lysates, rNE lost activity upon incubation with luminal fluids of normal mice, and this effect was significantly exacerbated in fluids from mice with colitis (Fig. 5B). Western blotting for elastase revealed a reduction in total rNE levels upon incubation with luminal fluids. This effect was more apparent when rNE was pre-incubated with the luminal fluids for 10 min prior to Cy5-V-DPP addition (Fig. 5C). The reduction in activity was also confirmed using the AAPV-AMC fluorogenic substrate probe, an independent measure of elastase activity (Fig. 5D). These data suggest the presence of elastase-degrading proteases as well as endogenous inhibitors in luminal fluids. Thus, elastase degrading/inhibiting factors are likely to be upregulated in diverse inflammatory settings. Serpin B1, elafin, and secretory leukocyte protease inhibitor (SLPI) are endogenous inhibitors of elastase. All three inhibitors were reported to be increased in colonic biopsies from patients with ulcerative colitis, which supports our hypothesis.28,36 Trypsin-like proteases in both pancreatic lysates and luminal fluids are likely responsible for elastase degradation.
Fig. 5.
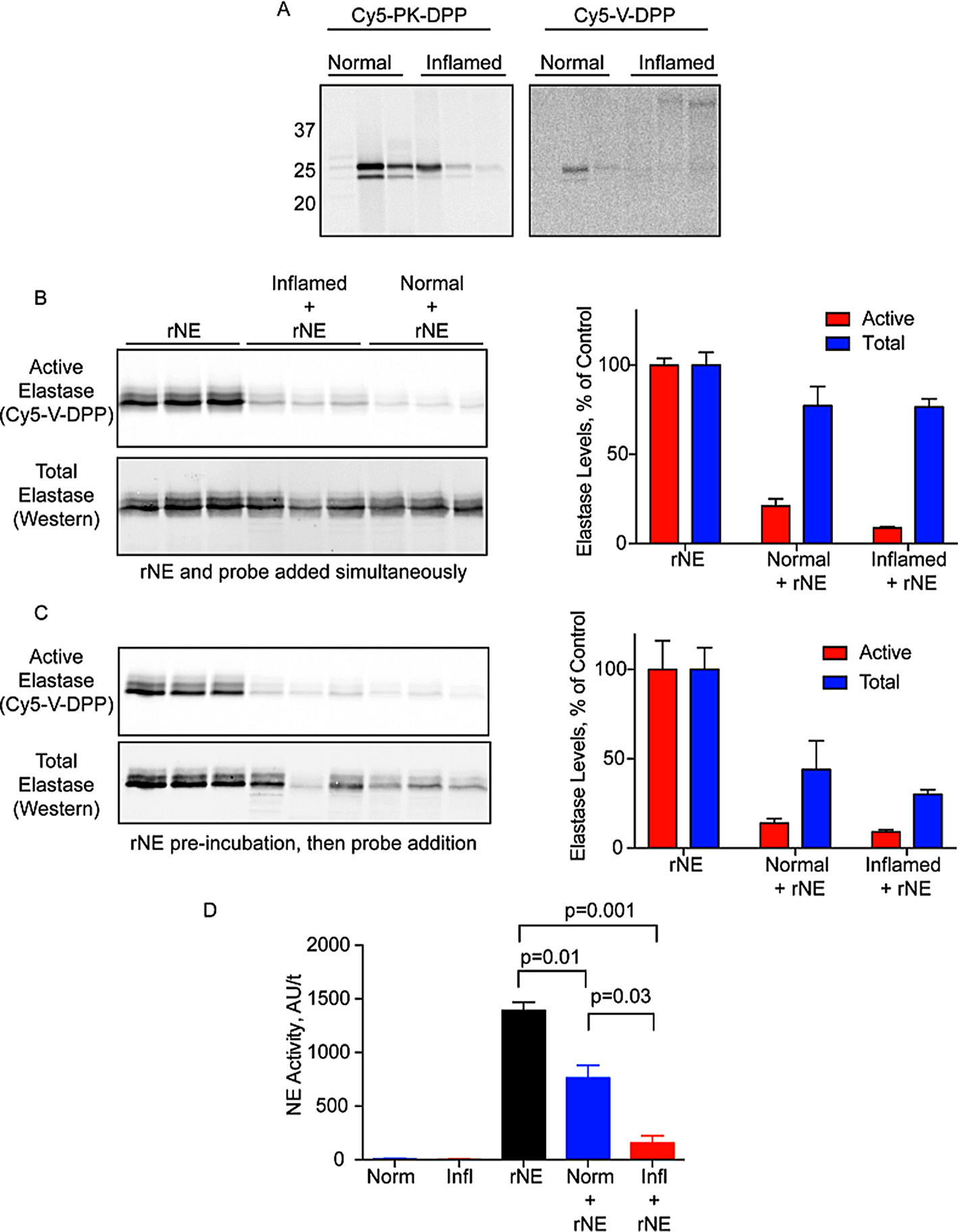
Ex vivo labeling of protease activation during acute colitis. A) Luminal fluids were collected from normal and inflamed colons and incubated with Cy5-PK-DPP or Cy5-V-DPP (1 μM). Proteins were resolved by SDS-PAGE and protease labeling was detected by measuring in-gel fluorescence. B) Recombinant neutrophil elastase was incubated with Cy5-V-DPP, either alone (rNE) or in the presence of luminal fluids (normal/inflamed + rNE). Elastase activity was then detected by in-gel fluorescence (top/red) followed by western blotting for total elastase (bottom/blue) Bands were quantified by densitometry. (Activity: rNE v Norm p = 0.0001; rNE v Infl p = 1.7e-5; Norm v Infl p = 0.03. Total: rNE v Norm p = 0.15; rNE v Infl p = 0.05; Norm v Infl p = 0.96. Norm Act v Tot p = 0.008; Infl Act v Tot p = 0.0001). C) Samples were treated as in B except rNE was preinbuated with the samples for 10 min prior to Cy5-V-DPP addition. (Activity: rNE v Norm p = 0.006; rNE v Infl p = 0.005; Norm v Infl p = 0.16. Total: rNE v Norm p = 0.05; rNE v Infl p = 0.005; Norm v Infl p = 0.43. Norm Act v Tot p = 0.13; Infl Act v Tot p = 0.002). D) Measurement of NE activity in luminal fluids of normal and inflamed mice in the presence and absence of rNE using the fluorogenic substrate probe AAPV-AMC (n = 3 mice per condition).
It is possible that tissue lysis resulted in a disruption of the compartmentalization of elastase, its inhibitors and degrading proteases in both of these models. For this reason, it is most preferable to analyze protease activity in its native environment without manipulation of the tissue. Thus, we aimed to monitor serine protease activity in vivo. We administered the probes to mice with pancreatitis and healthy controls by intravenous injection. After allowing the probes to circulate for 5 h, the pancreata were removed and imaged for Cy5 fluorescence (Fig. 6A). With both probes, the Cy5 signal in the inflamed pancreas was significantly increased compared to normal controls (Fig. 6A, B). To determine whether the fluorescent signal was due to specific binding of proteases, we analyzed the tissues biochemically (Fig. 6C). However, no protease labeling was observed with either probe. Some autofluorescent background signal was observed in control samples to which no probe had been added. Cy5-V-DPP showed an increase in a high molecular weight protein in the pancreatitis tissues. Its size suggests non-specific binding to a serum protein. The lack of clear labeling in the 25–30 kDa range suggests that the increase in fluorescent signal in the whole pancreas was due to non-specific accumulation of the probes. This may be due to increased blood flow to the inflamed pancreas or less clearance of unbound probe. While it is obvious that the probes do arrive at the pancreas, the lack of protease labeling indicates that the probes are not stable enough to detect in vivo protease activity.
Fig. 6.
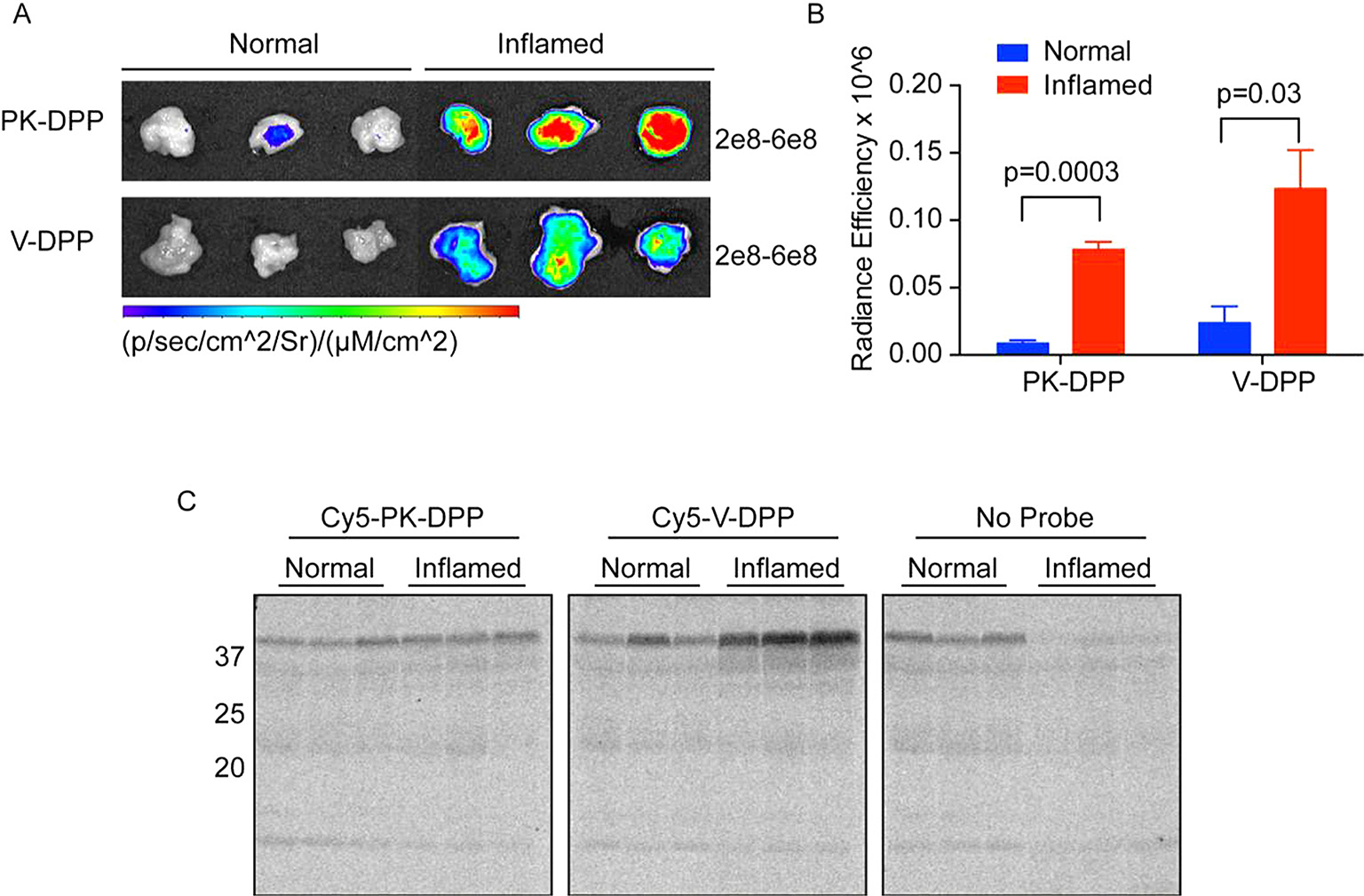
In vivo imaging of pancreatitis with ABPs. A) Healthy mice or those with acute pancreatitis were injected with Cy5-PK-DPP, Cy5-V-DPP, or vehicle control (No Probe). After 5 h, pancreata were removed and imaged for Cy5 fluorescence. Gain settings are indicated to the right of each image series. B) Quantification of absolute levels of Cy5 fluorescence measured in A with background subtracted (no-probe control). C) Biochemical analysis of protease labeling in the tissues from A. Pancreata were lysed and proteins were resolved by SDS-PAGE. Protease labeling was detected by measuring in-gel fluorescence. Note autofluorescent background bands in the no-probe control.
In summary, we have synthesized two fluorescent ABPs for serine proteases and assessed their utility in vitro and in vivo. The fluorescent Cy5 moiety allows for rapid, in-gel assessment of protease labeling without the need for membrane transfer and subsequent detection of biotin with tagged streptavidin conjugates. Both probes efficiently labeled serine proteases, either purified or in complex protein mixtures. As anticipated, the activity of trypsin-like proteases was increased during pancreatitis. Neutrophil elastase activity, on the other hand, was not strongly detected in the pancreas and colonic luminal fluids, whether healthy or inflamed. Using exogenously added elastase, we have provided evidence that elastase-degrading proteases and inhibitors may be upregulated during inflammation.
The in vivo efficacy of these probes was less desirable, showing non-specific binding to serum proteins, but no specific protease labeling. Thus, these probes are suitable for rapid ex vivo monitoring of serine protease activity. They will be extremely powerful tools, especially in combination with their biotinylated analogs, which are essential for affinity purification and proteomic identification of the specific probe targets.
Labeling proteases with ABPs
For the ABP dose curve experiment, recombinant proteases were diluted in 20 μL of phosphate-buffered saline: trypsin (100 ng), neutrophil elastase (1 μg), proteinase-3 (1 μg). The indicated ABP was added from 100x DMSO stocks to yield final concentrations of 0, 0.1, 0.5, and 1 μM with 1% DMSO. Samples were incubated for 30 min at 37 °C and then solubilized with 4× sample buffer (40% glycerol, 200 mM Tris-Cl [pH 6.8], 8% SDS, 0.04% bromophenol blue, 5% beta-mercaptoethanol). Proteins were resolved on a 15% SDS-PAGE gel. To detect probe-labeled proteins, the gel was scanned for Cy5 fluorescence using a Typhoon flatbed laser scanner (GE Healthcare). For the protease panel, 500 ng of the indicated protease was labeled and analyzed as above with 1) μM of each probe: chymotrypsin, neutrophil elastase (Elastin Products Company); trypsin, plasmin, proteinase-3, thrombin (Sigma); granzyme B (R&D); cathepsin S (gift from Medivir). Bone marrow was obtained by flushing femurs and tibias of normal mice with PBS. Cells were sonicated in PBS, and 60 μg of total protein (as measured by BCA assay) were analyzed as above with the indicated probe at 1 and 10 μM.
Immunoprecipitation
Probe-labeled lysates were divided into input and immunoprecipitation (IP) samples (100 μg each). The pulldown sample was diluted in 500 μL IP buffer (PBS [pH 7.4], 1 mM EDTA, 0.5% NP-40) along with 5 μL sheep anti-mouse neutrophil elastase/ELA2 antibody (R&D). Protein A/G beads (40 μl slurry) were washed with IP buffer and then added to the sample. Tubes were rocked overnight at 4 °C. Beads were washed four times with IP buffer and once with 0.9% sodium chloride. After the last wash, all liquid was removed and proteins were released from the beads by boiling in 2× sample buffer (20 μl). Supernatants were then analyzed by fluorescent SDS-PAGE as above.
Acute pancreatitis model
All animal experiments were approved by the Animal Ethics Committee of Monash University. Eight-week old male C57BL/6 J mice were given 12 hourly injections of caerulein (50 μg/kg in PBS) or PBS.12 After the ninth injection, the indicated ABP or vehicle control was administered intravenously (20 nmol dissolved in 100 μl 20% DMSO/PBS). Five hours later, pancreata were removed and imaged for Cy5 fluorescence using an IVIS Spectrum (Perkin Elmer). Radiance efficiency was determined using Living Image software. Pancreata were lysed by sonication in PBS, and supernatants were cleared by centrifugation. Total protein (100 μg) was analyzed by fluorescent SDS-PAGE analysis as above. Alternatively, lysates from the mice that did not receive an ABP injection (no probe controls) were analyzed ex vivo as above. Cy5-PK-DPP and Cy5-V-DPP were used at 10 μM.
Acute colitis model
Eight-week old male C57BL/6 J mice were administered 3% dextran sodium sulfate in the drinking water for 7 days. Colons were excised and flushed with 1 mL cold PBS. Supernatants were cleared by centrifugation. Total protein (50 μg) was labeled with Cy5-PK-DPP or Cy5-V-DPP and analyzed by fluorescent SDS-PAGE as above. Alternatively, elastase activity was measured with the fluorogenic substrate probe MeOSucc-Ala-Ala-Pro-Val-7-amino-4-methylcoumarin (Bachem, AAPV-AMC). Protein (50 μg) was incubated in the presence and absence of recombinant neutrophil elastase (1 μg) in a 96-well format. AAPV-AMC (150 μM final) was added and fluorescence was measured at 10-s intervals over 30 min at 360/440 nm excitation/emission. Slopes were determined and reported.
Cy5-V-DPP stability test
Recombinant neutrophil elastase (rNE; 1 or 10 μg as indicated) was aliquoted alone or spiked into lysates prepared from normal or inflamed pancreata or luminal fluids (100 μg total protein) followed by immediate addition of Cy5-V-DPP for 30 min at 37 °C or addition after 10 min. All samples were then analyzed using fluorescent SDS-PAGE as above and then analyzed for total elastase levels by western blot (R&D AF4517; 1:1000) and donkey anti-sheep-IR800 (LiCOR; 1:10,000). Blots were scanned on an Odyssey Imaging System (LiCOR).
Statistics
All experiments were performed in triplicate using at least 3 biological replicates. Data are reported as means ± SEM. Statistical significance was determined by comparing 2 groups using the Student’s t test, and p values of less than 0.05 were considered significant.
Supplementary Material
Acknowledgements
LEM is supported by an Early Career Fellowship from the National Health and Medical Research Council (NHMRC, Australia, GNT1091636). BG is the recipient of an Australian Research Council (ARC) Future Fellowship (FT130100838). NB is supported by NHMRC and ARC Centre of Excellence in Convergent Bio-Nano Science and Technology. Work in the authors’ laboratory is funded in part by Takeda Pharmaceuticals Inc.
Abbreviations:
- ABP
activity-based probe
- DPP
diphenylphosphonate
- NE
neutrophil elastase
- PR-3
proteinase 3
Footnotes
A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.bmcl.2016.11.064.
References
- 1.Edgington LE, Verdoes M, Bogyo M. Curr Opin Chem Biol. 2011;15:798–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanman LE, Bogyo M. Annu Rev Biochem. 2014;83:249–273. [DOI] [PubMed] [Google Scholar]
- 3.Serim S, Haedke U, Verhelst SHL. ChemMedChem. 2012;7:1146–1159. [DOI] [PubMed] [Google Scholar]
- 4.Haedke U, Küttler EV, Vosyka O, Yang Y, Verhelst SH. Curr Opin Chem Biol. 2013;17:102–109. [DOI] [PubMed] [Google Scholar]
- 5.Edgington LE, van Raam BJ, Verdoes M, Wierschem C, Salvesen GS, Bogyo M. Chem Biol. 2012;19:340–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edgington-Mitchell LE, Rautela J, Duivenvoorden HM, et al. Oncotarget. 2015;6:27008–27022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edgington LE, Verdoes M, Ortega A, et al. J Am Chem Soc. 2013;135:174–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verdoes M, Oresic Bender K, Segal E, et al. J Am Chem Soc. 2013;135:14726–14730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verdoes M, Edgington LE, Scheeren FA, et al. Chem Biol. 2012;19:619–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Segal E, Prestwood TR, van der Linden WA, et al. Chem Biol. 2015;22:148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oresic Bender K, Ofori L, van der Linden WA, et al. J Am Chem Soc. 2015;137:4771–4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edgington-Mitchell LE, Wartmann T, Fleming AK, et al. Am J Physiol Gastrointest Liver Physiol. 2016;311:G548–G560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lyo V, Cattaruzza F, Kim TN, et al. Am J Physiol Gastrointest Liver Physiol. 2012;303:G894–G903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilmore BF, Quinn DJ, Duff T, Cathcart GR, Scott CJ, Walker B. Bioconjug Chem. 2009;20:2098–2105. [DOI] [PubMed] [Google Scholar]
- 15.Serim S, Baer P, Verhelst SHL. Org Biomol Chem. 2015;13:2293–2299. [DOI] [PubMed] [Google Scholar]
- 16.Serim S, Mayer SV, Verhelst SHL. Org Biomol Chem. 2013;11:5714–5718. [DOI] [PubMed] [Google Scholar]
- 17.Pan Z, Jeffery DA, Chehade K, et al. Bioorg Med Chem Lett. 2006;16:2882–2885. [DOI] [PubMed] [Google Scholar]
- 18.Kasperkiewicz P, Poreba M, Snipas SJ, et al. Proc Natl Acad Sci. 2014;111:2518–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kam CM, Abuelyaman AS, Li Z, Hudig D, Powers JC. Bioconjug Chem. 1993;4:560–567. [DOI] [PubMed] [Google Scholar]
- 20.Winkler U, Allison NJ, Woodard SL, et al. Mol Immunol. 1996;33:615–623. [DOI] [PubMed] [Google Scholar]
- 21.Haedke UR, Frommel SC, Hansen F, et al. ChemBioChem. 2014;15:1106–1110. [DOI] [PubMed] [Google Scholar]
- 22.Haedke U, Götz M, Baer P, Verhelst SHL. Bioorg Med Chem Lett. 2012;20:633–640. [DOI] [PubMed] [Google Scholar]
- 23.Novovic S, Andersen AM, Nord M, et al. Scand J Clin Lab Invest. 2013;73:485–493. [DOI] [PubMed] [Google Scholar]
- 24.Ceppa EP, Lyo V, Grady EF, et al. Am J Physiol Gastrointest Liver Physiol. 2011;300:G1033–G1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao J, Liu Q. Inflammation. 2013;36:1348–1356. [DOI] [PubMed] [Google Scholar]
- 26.Grady T, Saluja A, Kaiser A, Steer M. Am J Physiol. 1996;271:G20–G26. [DOI] [PubMed] [Google Scholar]
- 27.Motta JP, Magne L, Descamps D, et al. Gastroenterology. 2011;140:1272–1282. [DOI] [PubMed] [Google Scholar]
- 28.Schmid M, Fellermann K, Fritz P, Wiedow O, Stange EF, Wehkamp J. J Leukoc Biol. 2007;81:907–915. [DOI] [PubMed] [Google Scholar]
- 29.Patricelli MP, Giang DK, Stamp LM, Burbaum JJ. Proteomics. 2001;1:1067–1071. [DOI] [PubMed] [Google Scholar]
- 30.Zuhl AM, Mohr JT, Bachovchin DA, et al. J Am Chem Soc. 2012;134:5068–5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Böttcher T, Sieber SA. Angew Chem Int Ed. 2008;47:4600–4603. [DOI] [PubMed] [Google Scholar]
- 32.Yan X, Luo Y, Zhang Z, et al. Angew Chem Int Ed. 2012;51:3358–3363. [DOI] [PubMed] [Google Scholar]
- 33.Tam J, Henault M, Li L, Wang Z, Partridge AW, Melnyk RA. Anal Biochem. 2011;414:254–260. [DOI] [PubMed] [Google Scholar]
- 34.Powers JC, Asgian JL, Ekici ÖD, James KE. Chem Rev. 2002;102:4639–4750. [DOI] [PubMed] [Google Scholar]
- 35.Joossens J, Van der Veken P, Lambeir AM, Augustyns K, Haemers A. J Med Chem. 2004;47:2411–2413. [DOI] [PubMed] [Google Scholar]
- 36.Uchiyama K, Naito Y, Takagi T, et al. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1163–G1170. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


