Summary:
RORγt+ regulatory T (Treg) cells are critical toward maintaining gut immune tolerance. In recent studies published in Nature, Kedmi et al., Lyu et al. and Akagbosu et al. describe MHCII+RORγt+ antigen-presenting cells that mediate RORγt+ Treg cell differentiation but propose disparate identities for these cells.
Treg cells are critical in maintaining immune tolerance and organismal health. Although Treg cells generated in the thymus under the influence of medullary thymic epithelial cells (mTEC) are important in the maintenance of tolerance to self-antigens, Treg cells induced in the periphery (pTreg cells) by antigen presenting cells (APCs) are critical in mediating tolerance to exogenous antigens. In example, the gut microbiota induces pTreg cells that express the transcription factor RORγt, and which maintain intestinal health by regulating tolerance to commensals and dietary antigens (Abdel-Gadir et al., 2019; Ohnmacht et al., 2015; Sefik et al., 2015). CD103+ dendritic cells (DCs) is suggested to induce gut pTreg cell differentiation (Coombes et al., 2007; Sun et al., 2007). However, the specific APCs involved in RORγt+ Treg cell differentiation have not been definitively identified. In recently published studies in Nature, three independent reports identify MHCII+RORγt+ APCs, distinct from DCs, as regulators of RORγt+ Treg cell differentiation (CITE). However, these studies reach different conclusions about the precise identity of those cells.
In one report (Kedmi et al., 2022), Kedmi et al transferred naïve Helicobacter hepaticus specific T cells to H. hepaticus-colonized WT or CD11cCreH2-Ab1fl/fl recipient mice. Analysis of H. hepaticus-specific T cells revealed that lack of MHCII in CD11c+ cells resulted in failure to induce Treg cells and conversely led to the induction of pathogenic Th17 cells. Since CD11c+ DCs are known to induce Treg cell differentiation the authors tested the role of DCs by depleting conventional type 1 and type 2 DCs (cDC1 and cDC2). Remarkably depletion of cDC1 or cDC2 cells did not affect pTreg cell differentiation, suggesting that a novel CD11c expressing APC drives the differentiation of microbiota-specific pTreg cells in the gut. By employing a combination of fate mapping and cellular indexing of transcriptomes and epitopes by sequencing (CITE seq) analysis, the authors identified two CD11c+ lineage cell subsets that were RORγt+MHCII+: innate lymphoid cells type 3 (ILC3) and the recently identified Aire+RORγt+ Janus cells (JC), that had the potential to mediate antigen presenting functions (Yamano et al., 2019). Transfer of H. hepaticus- specific T cells to RorcCre H2-Ab1fl/fl mice resulted in failed H. hepaticus -specific Treg cell differentiation. Instead, the transferred cells adopted an inflammatory signature characterized by increased in Th1 and Th17 cells. Furthermore, and given the role of TGFβ1 signaling in pTreg cell differentiation, the authors showed that targeting integrin αvβ8, which is required for latent TGFβ1 release from cell surfaces, resulted in a marked reduction in RORγt+ pTreg cell differentiation and a concomitant increase in Th1 and Th17 cells. The authors thus conclude that ILC3s and/or JC cells are the APCs that regulate the differentiation of RORγt+ Treg cells in the gut.
In a second report, Lyu et al performed single cell (sc)RNA sequencing from the MLN using RorcGFP mice. RORγt+ Treg cells prominently featured among the T cell clusters, while the CD3− clusters were dominated by ILC3s or ILC3-like cells including lymphoid tissue inducer (Lti)-like ILC3. Additionally, the authors found 2 clusters of cells that were identified by the expression of Aire and which resembled extra-thymic Aire expressing cells (eTACs). While the analysis does not account for eTACs that do not express Aire, ILC3s and eTACs expressed MHC II (Lyu et al., 2022). A previous study from the same group had reported that ILC3-dependent antigen presentation suppressed microbiota driven colitis through a process termed as intestinal selection (Hepworth et al., 2013). Hence the authors investigated the influence of ILC3s in Treg cell differentiation. RORγt+ Treg cells frequency was abrogated in RorcCreH2-Ab1fl/fl mice, while deletion of MHCII in CD4+ T cells, ILC2, T-bet+ ILC3 or DCs had no impact on RORγt+ Treg cell differentiation in the gut. This suggests that ILC3 and or eTACs were the key cell type regulating RORγt+ Treg cell differentiation. Similarly, deletion of MHCII or Rorc or Aire in Aire expressing eTACs did not perturb the differentiation of RORγt+ Treg cells, pointing to ILC3 cells are the relevant RORγt+ APCs.
Distinct bacteria in the gut regulate specific T cell responses. For example, Segmented Filamentous Bacteria (SFB) are known to induce Th17 cell responses, while H. hepaticus drives the differentiation of RORγt+ Treg cells under homeostasis (Xu et al., 2018). To test the APC functions of ILC3 in regulating microbiota-specific T cell responses, Lyu et al. transferred congenic naïve SFB-specific and H. hepaticus-specific T cells into RorcCreH2-Ab1fl/fl mice harboring SFB, which promote Th17 cell responses, and colonized with H. hepaticus, which promote RORγt+ Treg Treg cells. Analysis of donor T cells revealed that SFB-specific Th17 cell differentiation was robust in the recipient mice, there was a complete abrogation of H. hepaticus-specific RORγt+Treg cell differentiation in RorcCreH2-Ab1fl/fl mice relative to littermate controls. Mechanistic studies using RorcCreItgavfl/fl mice indicated that the integrin αv on ILC3 was critical for the differentiation of microbiota specific RORγt+ Treg cell. The authors extended their studies to investigate ILC3 and RORγt+ Treg cells in human IBD. Both populations were reduced in tandem within the inflamed relative to non-inflamed tissues, implicating a potential role for the disruption of this axis in disease pathogenesis.
In contrast to the above two studies, a different conclusion was arrived at by Akagbosu et al (Akagbosu et al., 2022), who used novel mouse genetic lines to uncover the identify of APCs that regulate RORγt+ Treg cell differentiation. Akagbosu et al, analyzed the RORγt Treg cell responses at 3 weeks of age in RorcCreH2-Ab1fl/fl mice, and found a severe deficiency of RORγt+ Treg cells in the gut. To identify the relevant APCs, the authors developed a novel RorcVenusCre-Ert2 mouse line that allowed for temporal manipulation of RORγt cells. In contrast, the authors found that sustained deletion of MHCII in adult RorcVenusCre-Ert2H2-Ab1fl/fl did not alter the already established RORγt+ Treg cell pool, indicating a key role for RORγt+ APC in the development of peripheral Treg cell early in life. To identify the RORγt+ APC regulating early life tolerance, the authors performed scRNA/ATAC sequencing of CD45+Lin−RORγt(Venus)+MHCII+ cells isolated from the MLN of 2-week-old RorcVenusCre-Ert2 mice. Unsupervised clustering revealed 2 major cell types: one belonging to ILCs including ILC3, Lti cells, ILC3p, and NCR+ILC3s, the second cell type distinguished based on a combination of epithelial and DC associated transcription factors. The authors referred to these non ILC3s RORγt+APCs as Thetis cells (TC) due to their hybrid phenotype between mTECs and DCs. Gene expression analysis of the TC cluster indicated that TCs comprised 4 distinct subsets: TCI and TCIII were identified as Aire+ (also referred to as Janus cells/eTACs); TCII and IV were identified as Aire−.
To determine the ontogeny of TCs the authors used cell lineage tracing analyses to demonstrate that TC cells are not derived from DC, ILC or adaptive lymphoid precursors. Furthermore, by deleting MHCII using a cre recombinase driven by Rora (RoraCreH2Ab-1fl/fl) whose expression is uniquely present in ILCs and not in TCs, the authors could demonstrate that ILC3s were dispensable for the regulation of early life peripheral tolerance.
Akagbosu et al next examined postnatal TC development from weeks 1 to 6 after birth, finding that TCs were abundantly present in the MLN in the first two weeks of life and declined rapidly thereafter. Notably, TCs - specifically subset IV - was enriched for a Treg-inducing module that included IL2, Tgfb1 and the TGFβ1 activating integrins Itgav and Itgb8. Differential comparison between TCs and ILC3 indicated that ILC3 did not express Itgb8. Accordingly, mice lacking Itgb8 in RORγt+ cells were associated with a defect in the differentiation of RORγt+ Treg cell at 3 weeks of age. Finally, the authors identified a cluster of human cells expressing the signature TC genes, thus suggesting the presence of putative human TCs. The putative human TCs were enriched within fetal samples and were almost exclusively present within the MLN.
Altogether Akagbosu et al have identified previously uncharacterized cell type(s) that regulate gastrointestinal tolerance by governing the differentiation of RORγt+ Treg cells early in life. Interestingly, the decline in TCs (around the weaning period) coincides with the exposure to a panoply of commensal and dietary antigens that are distinct from the those that TCs are exposed to early in life. Moreover, the immune system is continuously exposed to exotic dietary and commensal antigens throughout life. Importantly, RORγt+ Treg cell differentiation can be induced at any stage in life. For example, commensals such as Clostridia and Bacteroidetes introduced into adult germ-free mice can effectively induce RORγt+ Treg cell differentiation (Abdel-Gadir et al., 2019; Sefik et al., 2015). These results suggest that whereas TCs may set the threshold for early life tolerance, different RORγt+ APCs such as ILC3s may regulate tolerance to de novo antigens at different developmental stages. In this regard, it is important to note that both Kedmi et al and Lyu et al performed their microbiota-specific T cell transfer studies in adult mice to demonstrate a role for ILC3s in RORγt+ Treg cell differentiation. The specific roles of the respective RORγt+ APCs in peripheral tolerance and their role during development will require future investigations. Nevertheless, together these studies offer a new paradigm in our understanding of intestinal health and the potential to target the RORγt+ APCs in gut inflammatory settings to restore tolerance
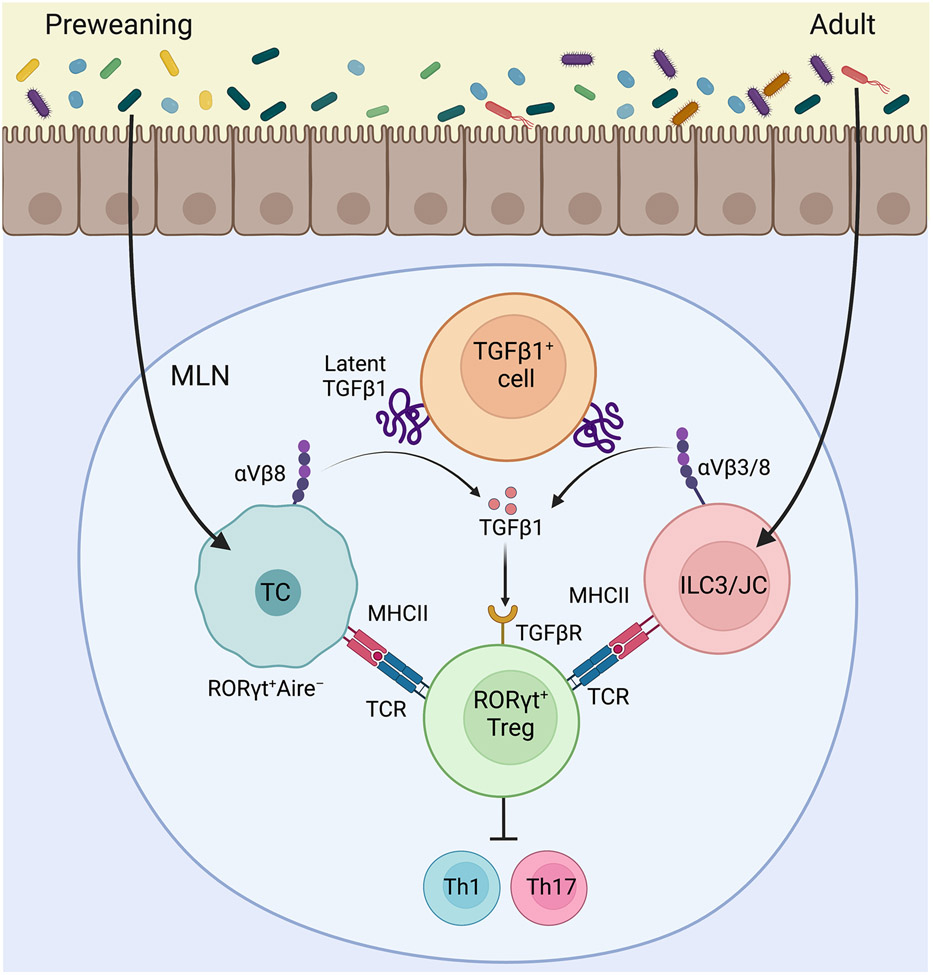
Fig. 1: Regulation of Intestinal tolerance by RORγt+ APCs:
Akagbosu et al demonstrated that Thetis Cells (TC) present commensal antigens and in concert process active TGFβ1 through αVβ8 integrin to induce the differentiation of RORγt+ Treg cells early in life. RORγt+ Treg cells stably persist through adult life and promote tolerance by suppressing inflammatory response to the microbiota. Kedmi et al and Lyu et al showed that commensal antigens are processed by RORγt+ ILC3s (and for Kedimi et al possibly JCs as well) to induce RORγt+ Treg cells also by aTGFβ1-αVβ3/8 integrin dependent mechanism. ILC3-dependent tolerance may become prominent with age to suppress inflammatory responses to colitogenic commensals. The schematics were prepared using BioRender.
Acknowledgments
This work was supported by NIH NIAID grants 5R01AI126915 to T.A.C.
Footnotes
The authors declare no conflict of interest.
References:
- Abdel-Gadir A, Stephen-Victor E, Gerber GK, Noval Rivas M, Wang S, Harb H, Wang L, Li N, Crestani E, Spielman S, et al. (2019). Microbiota therapy acts via a regulatory T cell MyD88/RORgammat pathway to suppress food allergy. Nat Med 25, 1164–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akagbosu B, Tayyebi Z, Shibu G, Paucar Iza YA, Deep D, Parisotto YF, Fisher L, Pasolli HA, Thevin V, Elmentaite R, et al. (2022). Novel antigen presenting cell imparts Treg-dependent tolerance to gut microbiota. Nature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, and Powrie F (2007). A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med 204, 1757–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepworth MR, Monticelli LA, Fung TC, Ziegler CG, Grunberg S, Sinha R, Mantegazza AR, Ma HL, Crawford A, Angelosanto JM, et al. (2013). Innate lymphoid cells regulate CD4+ T-cell responses to intestinal commensal bacteria. Nature 498, 113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedmi R, Najar TA, Mesa KR, Grayson A, Kroehling L, Hao Y, Hao S, Pokrovskii M, Xu M, Talbot J, et al. (2022). A RORγt+ cell instructs gut microbiota-specific Treg cell differentiation. Nature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu M, Suzuki H, Kang L, Gaspal F, Zhou W, Goc J, Zhou L, Zhou J, Zhang W, Artis D, et al. (2022). ILC3s select microbiota-specific regulatory T cells to establish tolerance in the gut. Nature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnmacht C, Park JH, Cording S, Wing JB, Atarashi K, Obata Y, Gaboriau-Routhiau V, Marques R, Dulauroy S, Fedoseeva M, et al. (2015). MUCOSAL IMMUNOLOGY. The microbiota regulates type 2 immunity through RORgammat(+) T cells. Science 349, 989–993. [DOI] [PubMed] [Google Scholar]
- Sefik E, Geva-Zatorsky N, Oh S, Konnikova L, Zemmour D, McGuire AM, Burzyn D, Ortiz-Lopez A, Lobera M, Yang J, et al. (2015). MUCOSAL IMMUNOLOGY. Individual intestinal symbionts induce a distinct population of RORgamma(+) regulatory T cells. Science 349, 993–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, and Belkaid Y (2007). Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med 204, 1775–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Pokrovskii M, Ding Y, Yi R, Au C, Harrison OJ, Galan C, Belkaid Y, Bonneau R, and Littman DR (2018). c-MAF-dependent regulatory T cells mediate immunological tolerance to a gut pathobiont. Nature 554, 373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamano T, Dobes J, Voboril M, Steinert M, Brabec T, Zietara N, Dobesova M, Ohnmacht C, Laan M, Peterson P, et al. (2019). Aire-expressing ILC3-like cells in the lymph node display potent APC features. J Exp Med 216, 1027–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]