Abstract
Background
During pregnancy, fetal growth causes an increase in the total number of rapidly dividing cells, which leads to increased requirements for folate. Inadequate folate intake leads to a decrease in serum folate concentration, resulting in a decrease in erythrocyte folate concentration, a rise in homocysteine concentration, and megaloblastic changes in the bone marrow and other tissues with rapidly dividing cells
Objectives
To assess the effectiveness of oral folic acid supplementation alone or with other micronutrients versus no folic acid (placebo or same micronutrients but no folic acid) during pregnancy on haematological and biochemical parameters during pregnancy and on pregnancy outcomes.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (31 December 2012) and we contacted major organisations working in micronutrient supplementation, including UNICEF Nutrition Section, World Health Organization (WHO) Maternal and Reproductive Health, WHO Nutrition Division, and National Center on Birth defects and Developmnetal Disabilities, US Centers for Disease Control and Prevention (CDC).
Selection criteria
All randomised, cluster‐randomised and cross‐over controlled trials evaluating supplementation of folic acid alone or with other micronutrients versus no folic acid (placebo or same micronutrients but no folic acid) in pregnancy.
Data collection and analysis
Two review authors independently assessed trials for inclusion, assessed risk of bias and extracted data. Data were checked for accuracy.
Main results
Thirty‐one trials involving 17,771 women are included in this review. This review found that folic acid supplementation has no impact on pregnancy outcomes such as preterm birth (risk ratio (RR) 1.01, 95% confidence interval (CI) 0.73 to 1.38; three studies, 2959 participants), and stillbirths/neonatal deaths (RR 1.33, 95% CI 0.96 to 1.85; three studies, 3110 participants). However, improvements were seen in the mean birthweight (mean difference (MD) 135.75, 95% CI 47.85 to 223.68). On the other hand, the review found no impact on improving pre‐delivery anaemia (average RR 0.62, 95% CI 0.35 to 1.10; eight studies, 4149 participants; random‐effects), mean pre‐delivery haemoglobin level (MD ‐0.03, 95% CI ‐0.25 to 0.19; 12 studies, 1806 participants), mean pre‐delivery serum folate levels (standardised mean difference (SMD) 2.03, 95% CI 0.80 to 3.27; eight studies, 1250 participants; random‐effects), and mean pre‐delivery red cell folate levels (SMD 1.59, 95% CI ‐0.07 to 3.26; four studies, 427 participants; random‐effects). However, a significant reduction was seen in the incidence of megaloblastic anaemia (RR 0.21, 95% CI 0.11 to 0.38, four studies, 3839 participants).
Authors' conclusions
We found no conclusive evidence of benefit of folic acid supplementation during pregnancy on pregnancy outcomes.
Plain language summary
Folic acid supplementation in pregnancy
Folate is a naturally occurring vitamin while folic aid is the synthetic replacement of folate used in most supplements and in fortified foods. Folate is essential as its deficiency can be caused by poor dietary intake, genetic factors or the interaction between genetic factors and the environment. Women with sickle cell disease and those women in areas where malaria is endemic have a greater need for folate and in these areas anaemia can be a major health problem during pregnancy. Women need more folate in pregnancy to meet their need for extra blood and to meet the growing baby's need for blood. Without adequate folate intake in a mother's diet, she can become anaemic and this can contribute to her baby being small, anaemic and born too early (preterm birth). Folic acid supplementation taken before conception can reduce the chance of the baby having neural tube defects. This review looked to see if taking folic acid supplements during pregnancy could reduce the chance of the baby being born too early and of low birthweight and to see its impact on the mother’s blood (hematological values), folate levels and on pregnancy complications.
The review authors found 31 trials (involving 17,771 women) that looked at the impact of providing folic acid supplementation during pregnancy. The data showed that taking folate during pregnancy was not associated with reducing the chance of preterm births, stillbirths, neonatal deaths, low birthweight babies, pre‐delivery anaemia in the mother or low pre‐delivery red cell folate, although pre‐delivery serum levels were improved. The review also did not show any impact of folate supplementation on improving mean birthweight and the mother’s mean haemoglobin levels during pregnancy compared with taking a placebo. However, the review showed some benefit in indicators of folate status in the mother. The evidence provided so far from these trials did not find conclusive results for any overall benefit of folic acid supplementation during pregnancy.
Most of the studies were conducted over 30 to 45 years ago.
Background
Description of the intervention
Folate is a generic term for both the endogenous form of the vitamin occurring naturally in food and the synthetic form found in supplements and fortified foods (Bailey 1995). It should be noted, however, that folate is a naturally occurring vitamin while folic aid is the synthetic replacement of folate used in most supplements and in fortified foods. Humans are fully dependent on dietary sources or dietary supplements and microorganisms in their intestinal tract for their folate supply. Folate derivatives are essential for the synthesis of nucleic acid, amino acids, cell division, tissue growth, and DNA methylation (Krishnaswamy 2001; Morrison 1998; Scholl 2000).
Inadequate folate intake leads to a decrease in serum folate concentration, resulting in a decrease in erythrocyte (red blood cell) folate concentration, a rise in homocysteine (Hcy) concentration, and megaloblastic changes in the bone marrow and other tissues with rapidly dividing cells (Dietary Ref 1998; Willoughby 1968). During pregnancy, fetal growth causes an increase in the total number of rapidly dividing cells, which leads to increased requirements for folate (Bailey 1995). With inadequate folic acid intake, concentrations of folate in maternal serum, plasma, and red blood cells decrease from the fifth month of pregnancy onwards (Açkurt 1995; Bates 1986). If inadequate folate intake is sustained during pregnancy, megaloblastic anaemia (a blood disorder characterised by anaemia, with red blood cells that are larger than normal and cell contents that are not completely developed) occurs (Willoughby 1968). Folate concentrations continue to decrease for several weeks after pregnancy (Bruinse 1995; Smith 1983), and by the second to third month postpartum, a third of all mothers can have subnormal concentrations of folate in serum and red blood cells (Açkurt 1995). Possible causes for the decline in blood folate during pregnancy include increased folate demand for growth of the fetus due to an increase in the number of rapidly dividing cells (Bailey 1995) and growth of uteroplacental organs, decreased folate absorption, low folate intake, hormonal influence on folate metabolism as a physiologic response to pregnancy (Chanarin 1969), and dilution of folate due to blood volume expansion (Bruinse 1995). Folate demands may be further increased in women with sickle cell disease and women living in areas where malaria is endemic (Lawson 1988); in these areas, anaemia in pregnancy is a major health problem. Increased folate catabolism and urinary folate excretion (Fleming 1972; Landon 1971) may also contribute to increased folate needs in pregnancy (Caudill 1998; Gregory 2001b; Higgins 2000; McPartlin 1993), but the findings are controversial. As a consequence of folate deficiency, Hcy accumulates in the serum and is found to be associated with an increased risk in cardiovascular disease (Refsum 2008), late pregnancy complications such as pre‐eclampsia (Makedos 2007; Patrick 2004; Tamura 2006), and neural tube defects around the time of conception (De Benoist 2008).
The recommended folate intake for pregnant women is 400 µg/day (Food and Nutrition Board 1970). It was revised in 1999 after evaluating its bioavailability from food and synthetic folate, and the recommendation was increased to 450 µg (600 DFEs/day (dietary folate equivalent)) (Institute of Medicine 2000). It should be noted that as per NICE guidelines, this amount of folic acid when supplemented to pregnant women (and those intending to become pregnant), before conception and throughout the first 12 weeks, reduces the risk of having a baby with a neural tube defect (NICE 2008). However, the Food and Nutrition Board of the Institute of Medicine have suggested that an increased folate intake might delay the diagnosis of vitamin B‐12 deficiency by correcting the anaemia, or even exacerbate its neurologic and neuropsychiatric effects (Food and Nutrition Board 1998; Herbert 1997; Rush 1994). Further research is still needed in this area.
How the intervention might work
The relationship between pregnancy outcome and maternal blood folate concentrations, folate intake and hyperhomocysteinaemia cannot be ignored (Smits 2001). Plasma total homocysteine (tHcy) is regulated by folate status (Selhub 1993), and hyperhomocysteinaemia is linked to vaso‐occlusive disease (Green 1995). Impaired placental perfusion due to hyperhomocysteinaemia is implicated in having a negative effect on pregnancy outcome, as are inadequate folate intake and low serum folate concentrations (Scholl 2000). Folate has long been used as a supplement in combination with iron during pregnancy, largely on the basis of haematological benefits (Fleming 1968), although deficiency has also been associated with pregnancy complications and congenital malformations (Scholl 2000). Periconceptional supplementation with folic acid, three months before and early in pregnancy is recommended (Czeizel 1992; MRC 1991), and has been shown to reduce the risk of neural tube defects by almost three‐quarters (De‐Regil 2010). Although still unproven, folic acid supplementation has also been suggested to help prevent other fetal malformations such as congenital heart defects (Botto 1996; Czeizel 1993; Czeizel 1996; Shaw 1995), urinary tract anomalies (Li 1995), limb defects (Czeizel 1993), oro‐facial clefts (Czeizel 1993; Li 1995; Shaw 1995), and pyloric stenosis (Shaw 1995).
Why it is important to do this review
The role of folate deficiency in increasing the risk of spontaneous abortion and birth outcomes such as low birthweight, preterm birth, and perinatal mortality is unclear (Bukowski 2009; Scholl 2000). Hence, the aim of this review is to assess the effect of folic acid supplementation alone in pregnant women on haematological and biochemical parameters, adverse events during pregnancy, and on pregnancy outcomes. We did not assess periconceptional folic acid supplementation, or supplementation of folic acid along with iron during pregnancy and with other micronutrients, as these have been addressed by other reviews (Haider 2006; De‐Regil 2010; Pena‐Rosas 2006).
Objectives
To assess the effectiveness of oral folic acid supplementation alone or with other micronutrients versus no folic acid (placebo or same micronutrients but no folic acid) during pregnancy on haematological and biochemical parameters during pregnancy and on pregnancy outcomes.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised or quasi‐randomised controlled trials of folic acid supplementation alone or with other micronutrients versus no folic acid (placebo or same micronutrients but no folic acid).
Types of participants
We included pregnant women of any age and parity.
Types of interventions
Folic acid alone versus no treatment/placebo (no folic acid)
Folic acid+ iron versus iron (no folic acid)
Folic acid + other vitamins and minerals versus other vitamins and minerals (but no folic acid)
We excluded studies that supplemented folic acid in the form of fortification or home fortification alone or in combination with other micronutrients. We also excluded studies in which women were supplemented during periconception.
Types of outcome measures
Primary outcomes
Maternal outcomes
Pre‐delivery anaemia (less than 10 g/dL haemoglobin or haematocrit below 30%
Mean pre‐delivery haemoglobin level
Low pre‐delivery serum folate (less than 3 mg/L or 7 nmol/L or 3 ng/mL)
Mean pre‐delivery serum folate level
Low pre‐delivery red cell folate (less than 100 mg/L or 300 nmol/L or 140 ng/mL)
Mean pre‐delivery red cell folate
Pregnancy outcome
Preterm birth (delivery before 37 weeks of gestation)
Infant outcome
Low birthweight (birthweight less than 2500 g)
Secondary outcomes
Miscarriage (loss of pregnancy before 22 weeks of gestation)
Perinatal mortality ‐ includes stillbirth (deaths after 22 weeks of gestation) and mortality in the first seven days of life
Pre‐eclampsia‐ defined as blood pressure of > 140 mmHg systolic or > 90 mmHg diastolic after 20 weeks of gestation, and proteinuria of more than 0.3 g in 24 hours
Respiratory disease in child
Allergic disease in child
Megaloblastic anaemia
Hyperhomocysteinaemia (more than 16 micromol/L)
Search methods for identification of studies
Electronic searches
We contacted the Trials Search Co‐ordinator to search the Cochrane Pregnancy and Childbirth Group’s Trials Register (31 December 2012)
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of EMBASE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and EMBASE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
Searching other resources
For identification of ongoing or unpublished studies, we contacted major organisations working in micronutrient supplementation, including UNICEF Nutrition Section, World Health Organization (WHO) Maternal and Reproductive Health, WHO Nutrition Division, and National Center on Birth defects and Developmnetal Disabilities, US Centers for Disease Control and Prevention (CDC).
We did not apply any language restrictions.
Data collection and analysis
Selection of studies
Two review authors, Zohra Lassi (ZSL) and Rehana Salam (RAS), independently assessed for inclusion all the potential studies we identified as a result of the search strategy. We resolved any disagreement through discussion and, if required, we consulted the third review author, Zulfiqar Bhutta (ZAB)
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors (RAS and ZL) extracted the data using the agreed form. We resolved discrepancies through discussion and, if required, we consulted the third review author. Data were entered into ReviewManager software (RevMan 2011) and checked for accuracy.
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors (ZSL and RAS) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreement was resolved by discussion or by involving a third assessor (ZAB).
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it produced comparable groups. We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal the allocation sequence in sufficient detail and determine whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we re‐included missing data in the analyses which we undertook. We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting bias
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it was clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest were reported incompletely and so could not be used; study failed to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it was likely to impact on the findings.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference if outcomes were measured in the same way between trials. We used the standardised mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomised trials
We included cluster‐randomised/quasi‐randomised trials in the analyses along with individually‐randomised trials. We incorporated the data of cluster‐randomised/quasi‐randomised trials using generic inverse variance method in which logarithms of risk ratio estimates were used along with the standard error of the logarithms of risk ratio estimates.
Cross‐over trials
We also looked for any cross‐over trials on this topic, and such trials were deemed eligible for inclusion, However, we did not find any eligible cross‐over trials.
Dealing with missing data
We noted levels of attrition for included studies. We also planned to explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis. For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the T², I² and Chi² statistics. We regarded heterogeneity as substantial if the I² was greater than 30% and either T² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
If there were 10 or more studies in the meta‐analysis, we investigated reporting biases (such as publication bias) using funnel plots. We assessed funnel plot asymmetry visually, If asymmetry was suggested by a visual assessment, we performed exploratory analyses to investigate it.
Mostly studies were old and we suspected reporting bias, therefore, we attempted to contact study authors, where possible, asking them to provide missing outcome data.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2011). We used fixed‐effect Mantel‐Hanzel meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. trials were examining the same intervention, and the trials’ populations and methods were judged to be sufficiently similar. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average range of possible treatment effects and we discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we did not combine trials.
If we used random‐effects analyses, the results were presented as the average treatment effect with 95% confidence intervals, and the estimates of T² and I².
Subgroup analysis and investigation of heterogeneity
We planned to carry out subgroup analyses based on following factors.
• Different doses of folate used (< 400 μg and > 400 μg) • Different durations of folate supplementation • Haemoglobin level of participants • Co‐interventions
Not all included studies mentioned the baseline haemoglobin levels of participants and since duration and start of folic acid supplementation in women during pregnancy varied, we, therefore, did not carry out these subgroup analyses. However, subgroup analyses were carried out on studies in which iron was additionally provided with folic acid. We also performed subgroup analyses on the dosage of folic acid.
We also reported the outcomes based on how the outcome was defined in the individual study.
We assessed subgroup differences by the interaction tests available within RevMan (RevMan 2011). We reported the results of subgroup analyses quoting the χ² statistic and the P value, and the interaction test I² value.
Sensitivity analysis
We did not perform sensitivity analyses as studies were old and of mediocre quality.
Results
Description of studies
Results of the search
A total of 94 trial reports were considered for inclusion into this review, finally 31 studies involving 17,771 women were included in this review (Figure 1).
1.
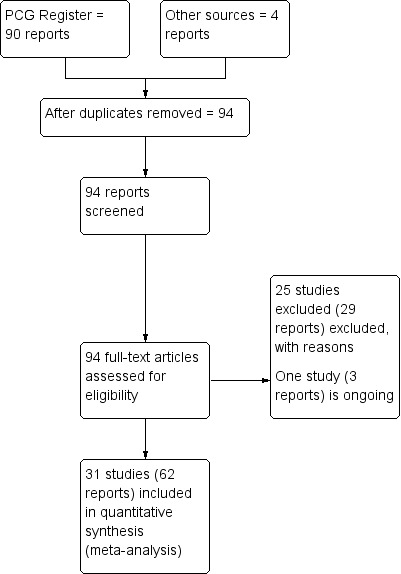
Study flow diagram.
Included studies
Thirty‐one studies have been included in this review. The majority of these studies were quite old and were conducted during the 1960s (Castren 1968; Chanarin 1965; Chanarin 1968; Chisholm 1966; Dawson 1962; Edelstein 1968; Fleming 1968; Hibbard 1969a; Menon 1962; Metz 1965; Willoughby 1967); the 1970s (Balmelli 1974; Batu 1976; Baumslag 1970; Fletcher 1971; Giles 1971; Iyengar 1975; Rae 1970; Rolschau 1979; Trigg 1976; Weil 1977), and the 1980s (Blot 1981; Harrison 1985; Lira 1989; Roth 1980; Srisupandit 1983; Tchernia 1982; Pack 1980). Three studies were published in 2005 (Charles 2005; Christian 2003; Decsi 2005), however, Charles 2005 re‐analysed data that were collected in 1966. Seven studies (Chanarin 1965; Christian 2003; Dawson 1962; Decsi 2005; Hibbard 1969a; Metz 1965; Pack 1980) were were not included in the meta‐analyses because they either did not mention their standard deviations/standard errors; or they reported the rise or fall in the haematological and biochemical levels.
Most of the outcomes were defined in the same way across different trials except for preterm birth, pre‐delivery anaemia, and low birthweight which were defined differently, however, we still included them and they were presented in subgroup according to their defined cut‐offs (Refer to Table 1). The majority of the studies were conducted in Europe (Balmelli 1974; Blot 1981; Castren 1968; Chanarin 1965; Chanarin 1968; Charles 2005; Chisholm 1966; Dawson 1962; Decsi 2005; Fletcher 1971; Hibbard 1969a; Rae 1970; Rolschau 1979; Tchernia 1982; Trigg 1976; Weil 1977; Willoughby 1967), Africa (Baumslag 1970; Edelstein 1968; Fleming 1968; Harrison 1985; Metz 1965) and Asia (Batu 1976; Christian 2003; Iyengar 1975; Menon 1962; Srisupandit 1983). One study was conducted in South America (Lira 1989), one in Australia (Giles 1971) and one in New Zealand (Pack 1980). One study (Roth 1980) did not mention the setting. The time for initiation of supplementation varied from 8th week of pregnancy till three days postpartum. Most of the studies supplemented women with folic acid in combination with iron (Balmelli 1974; Batu 1976; Baumslag 1970; Blot 1981; Castren 1968; Chanarin 1965; Chanarin 1968; Chisholm 1966; Christian 2003; Edelstein 1968; Fletcher 1971; Giles 1971; Harrison 1985; Iyengar 1975; Lira 1989; Menon 1962; Metz 1965; Rae 1970; Rolschau 1979; Roth 1980; Srisupandit 1983; Tchernia 1982; Trigg 1976; Weil 1977; Willoughby 1967) however, only a few compared folic acid alone with placebo (Charles 2005; Chisholm 1966; Decsi 2005; Fleming 1968; Pack 1980).
1. Outcomes definition.
| Study | Preterm delivery | Low birthweight | Perinatal mortality | Miscarriage | Pre‐eclampsia | Respiratory disease in child | Allergic disease in child | Pre‐delivery anaemia | Low pre‐delivery serum folate | Low pre‐delivery red cell folate | Megaloblastic anaemia | Hyperhomocystenaemia |
| Balmelli 1974 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | < 4 µg/L | < 150 µg/L | ‐ | ‐ |
| Batu 1976 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | < 10 g/dL | ‐ | ‐ | ‐ | |
| Baumslag 1970 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Blot 1981 | < 38 weeks | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Castren 1968 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Chanarin 1965 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Chanarin 1968 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Charles 2005 | ‐ | < 2500 g | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Chisholm 1966 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | < 11 g/dL | < 2.1 mµg/mL | ‐ | ‐ | ‐ |
| Dawson 1962 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Decsi 2005 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Edelstein 1968 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Fleming 1968 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Fleming 1968 | 36‐38 weeks | < 2500 g | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Fletcher 1971 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | < 11 g/100 mL | < 2.5 ng/mL | ‐ | ‐ | ‐ |
| Giles 1971 | ‐ | < 2400 g | ‐ | ‐ | ‐ | ‐ | < 10 g/100 mL | ‐ | ‐ | ‐ | ‐ | |
| Harrison 1985 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Iyengar 1975 | ‐ | < 2500 g | ‐ | ‐ | ‐ | ‐ | ‐ | < 10.5 g/dL | ‐ | ‐ | ‐ | ‐ |
| Lira 1989 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Menon 1962; | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | < 10.5 g% | ‐ | ‐ | ‐ | |
| Metz 1965 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Pack 1980 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Rae 1970; | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Rolschau 1979; | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Roth 1980; | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | < 4 µ/L | < 150 µ/L | ‐ | |
| Srisupandit 1983; | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Tchernia 1982; | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Trigg 1976; | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Weil 1977; | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Willoughby 1967 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | < 10 g/dL | ‐ | ‐ | < 10 g/100 mL | ‐ |
Please refer to the Characteristics of included studies table for more details.
Excluded studies
A total of 25 studies were excluded from the review as they did not satisfy the inclusion criteria. Hamilton 1973 was not a randomised controlled trial. There were four studies in which folic acid was given in combination with other micronutrients compared with a no supplement group (Bjerre 1967; Ma 2008; Wang 2012; Zeng 2008). Similarly, Giles 1960 compared the intervention group with historical controls; Gregory 2001 compared pregnant women with non pregnant women; Khanna 1977 evaluated the therapeutic use of folic acid in women with anaemia; and there were a few studies in which the association of folic acid supplementation was observed, with breast cancer, fetal apoptosis (Klinger 2006), congenital anomalies (Ulrich 1999) and with malaria when given with sulphadoxine pyrimethamine (Ouma 2006). We excluded studies in which therapy of iron and folic acid was compared with no therapy at all (Taylor 1979; Taylor 1981). We also excluded studies in which folic acid was given in a fortification form (Colman 1974; Colman 1975). We excluded studies that compared the duration of folic acid supplements (Ellison 2004; Polatti 1992), and different dosage of folic acid supplements (Hekmatdoost 2011; Hibbard 1969; Manizheh 2009). Trials were also excluded that were in the form of published abstracts only and had insufficient information to extract (Hague 1998; Kristoffersen 1979; Melli 2008; Thomson 1982). Also, one study in which results from three trials were re analysed was excluded (Tchernia 1982a).
Please refer to Characteristics of excluded studies table for more details.
Risk of bias in included studies
Most of the studies were conducted over 30 to 45 years ago, and we found poor subjective and objective compliance with random allocation, adequate concealment and blinding. Bias and confounding thus seem to us the likely explanation for our findings.
Figure 2 and Figure 3 provide a graphical summary of the results of risk of bias for the included studies.
2.
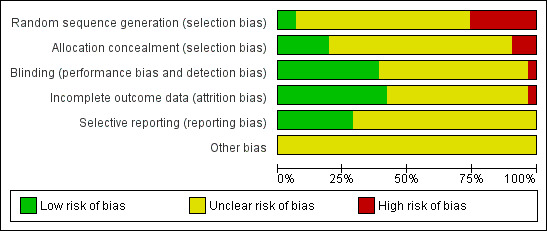
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
3.
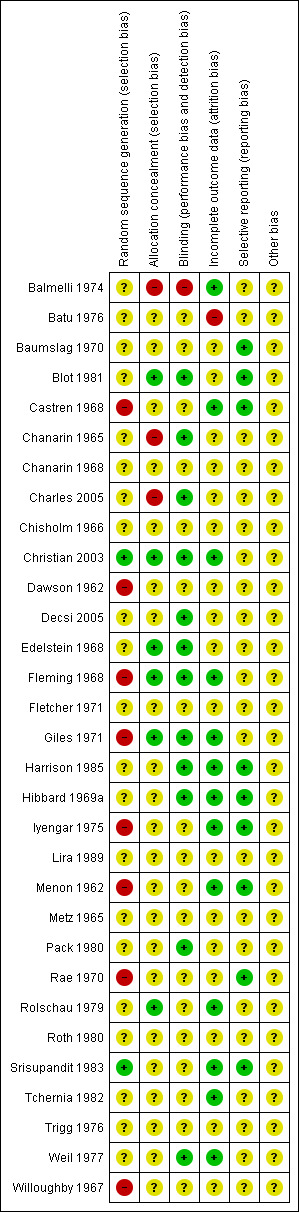
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
Sequence generation and adequate allocation concealment was a problem in almost all the studies and control of selection bias at entry was often difficult to assess as many authors stated that women were 'randomly allocated' without actually describing the technique, still there were studies that managed to report the methods of allocation concealment adequately (Blot 1981; Edelstein 1968; Fleming 1968; Giles 1971; Rolschau 1979).
Blinding
Blinding was the another issue which was rarely discussed in depth, and only few reported them adequately including Blot 1981; Edelstein 1968; Fleming 1968; Giles 1971; Harrison 1985; Weil 1977.
Incomplete outcome data
Mostly studies provided insufficient information regarding attrition rates, which meant we were unable to make any judgment. There were only a few studies that discussed their exclusion and attrition rates and reported their reasons. (Balmelli 1974; Batu 1976; Blot 1981Castren 1968; Fleming 1968; Giles 1971Harrison 1985; Iyengar 1975; Srisupandit 1983; Tchernia 1982).
Selective reporting
Again, studies provided insufficient information, which limited us from making any judgment (Balmelli 1974; Blot 1981; Castren 1968; Harrison 1985; Iyengar 1975; Srisupandit 1983).
Other potential sources of bias
No other bias was identified but we had insufficient information available to fully assess this 'Risk of bias' domain. Consequently, we assessed all included studies as being at 'unclear' risk of other bias.
Effects of interventions
a. Clinical measures of untoward events during pregnancy and of pregnancy outcome
Preterm birth
None of the included studies reported preterm birth in accordance with our definition of the outcome. We found two studies, of which one defined it as birth of a baby between 36 to 38 weeks, and another defined it as birth before 38 weeks of pregnancy. We pooled them both to look for an association with folic acid supplementation in pregnancy. Our analysis showed that administration of folic acid supplementation during pregnancy has no impact on reducing preterm birth (risk ratio (RR) 1.01, 95% confidence interval (CI) 0.73 to 1.38; three studies, 2959 participants (Analysis 1.1)).
1.1. Analysis.
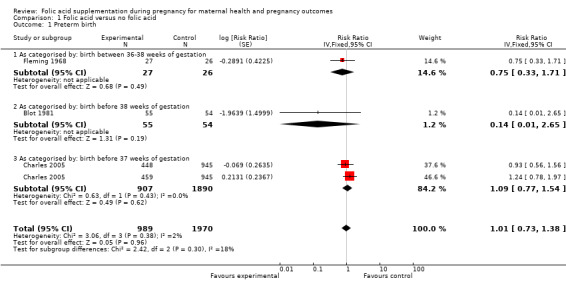
Comparison 1 Folic acid versus no folic acid, Outcome 1 Preterm birth.
Stillbirths/neonatal deaths
None of the included studies reported perinatal mortality. However, three studies reported stillbirth and neonatal mortality as a composite outcome, hence we pooled them to obtain data for perinatal mortality. Folic acid supplementation during pregnancy did not show any impact on reducing stillbirths/neonatal deaths (RR 1.33, 95% CI 0.96 to 1.85; three studies, 3110 participants (Analysis 1.2)).
1.2. Analysis.
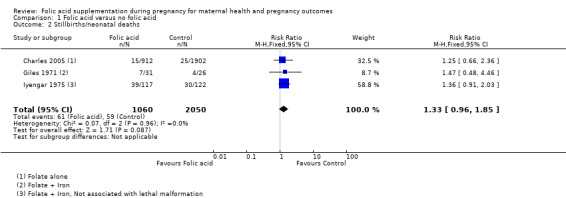
Comparison 1 Folic acid versus no folic acid, Outcome 2 Stillbirths/neonatal deaths.
Birthweight
Folic acid supplementation during pregnancy did not show any impact on reducing low birthweight (less than 2500 g) (RR 0.83, 95% CI 0.66 to 1.04; four studies, 3113 participants (Analysis 1.3)).
1.3. Analysis.
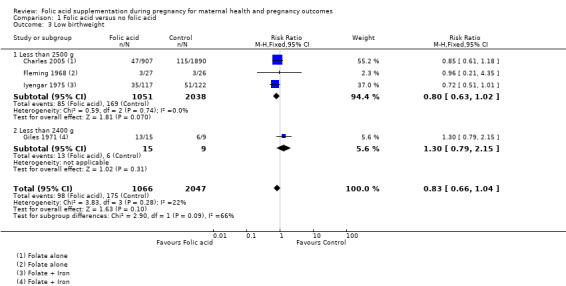
Comparison 1 Folic acid versus no folic acid, Outcome 3 Low birthweight.
We also attempted to look at the impact of folic acid supplementation during pregnancy on mean birthweight (g) of newborns and found no association (mean difference (MD) 104.96 g, 95% CI ‐0.25.50 g to 235.41 g; five studies, 774 participants; random‐effects, T² = 21694.29, I² = 90% (Analysis 1.4)). All the studies pooled for this outcome compared folic acid + iron versus iron alone.
1.4. Analysis.
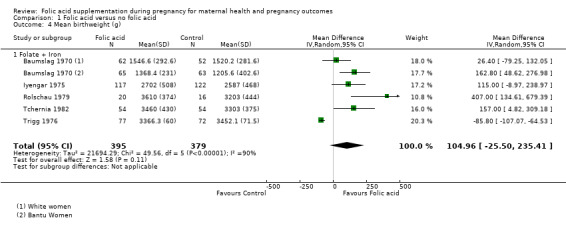
Comparison 1 Folic acid versus no folic acid, Outcome 4 Mean birthweight (g).
The standard errors for Trigg 1976 were very small as compared to the other trials for being plausible, therefore, we conducted a sensitivity analysis after removing this study. Heterogeneity was reduced from 90% to 50% (MD 135.76, 95% CI 47.85 to 223.68; four studies, 625 participants; random‐effects, T² = 4841.10, I² = 50% (Analysis 1.5)
1.5. Analysis.
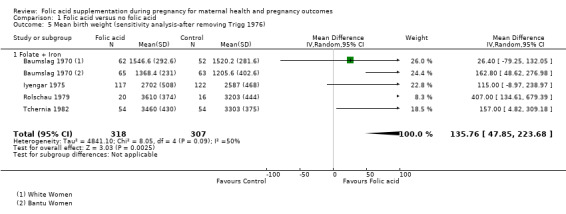
Comparison 1 Folic acid versus no folic acid, Outcome 5 Mean birth weight (sensitivity analysis‐after removing Trigg 1976).
Outcomes not reported in the included studies
The included studies did not report on the impact of folic acid supplementation on miscarriage, pre‐eclampsia, respiratory disease or allergic disease in children.
b. Haematological and biochemical parameters
Pre‐delivery anaemia
The included studies used different definitions of anaemia. Eight studies reported pre‐delivery anaemia as an outcome, but only two studies used our definition of anaemia. We included all studies reporting anaemia but pooled them separately according to the definition of anaemia used. Folic acid supplementation did not show any impact on reducing pre‐delivery anaemia (any cut‐off point) (average RR 0.62, 95% CI 0.35 to 1.10; eight studies, 4149 participants; random‐effects, T² = 0.51, I² = 90% (Analysis 1.6)). When studies were separately pooled according to the definition described in the earlier section of this review, we found that supplementation had no impact on reducing anaemia (haemoglobin less than 10 g/dL) (average RR 0.35, 95% CI 0.05 to 2.42; two studies, 2448 participants; random‐effects, T² = 1.86, I² = 97% (Analysis 1.6)).
1.6. Analysis.
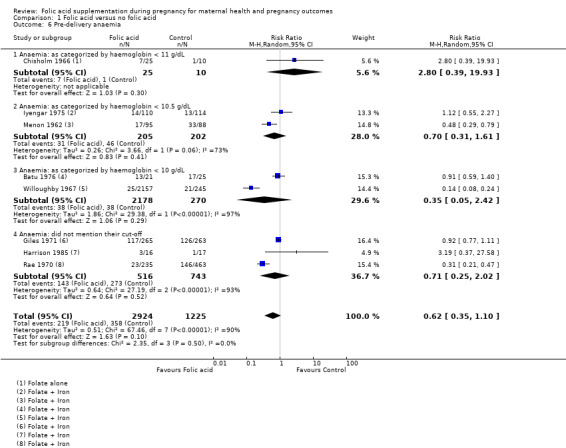
Comparison 1 Folic acid versus no folic acid, Outcome 6 Pre‐delivery anaemia.
We also looked at the impact of folic acid supplementation in pregnancy on mean pre‐delivery haemoglobin level, and found no difference in the mean haemoglobin concentration among those in the intervention arm compared with those in the placebo arm (MD ‐0.03, 95% CI ‐0.25 to 0.19; 12 studies, 1806 participants; random‐effects, T² = 0.12, I² = 95% (Analysis 1.7)). All the studies pooled for this outcome compared folic acid + iron versus iron alone.
1.7. Analysis.
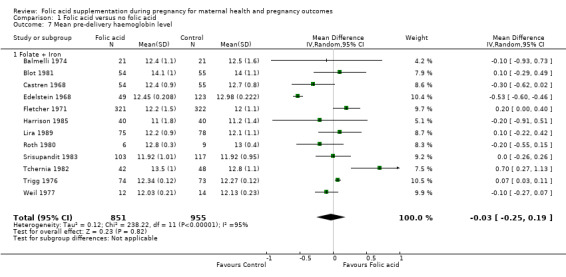
Comparison 1 Folic acid versus no folic acid, Outcome 7 Mean pre‐delivery haemoglobin level.
With regard to subgroup analysis based on dosage of folic acid supplementation, we found no differences on improving haemoglobin concentrations and the interaction test was insignificant (Chi² = 1.18, df = 1 (P = 0.28), I² = 15.1%). Analysis 1.8
1.8. Analysis.
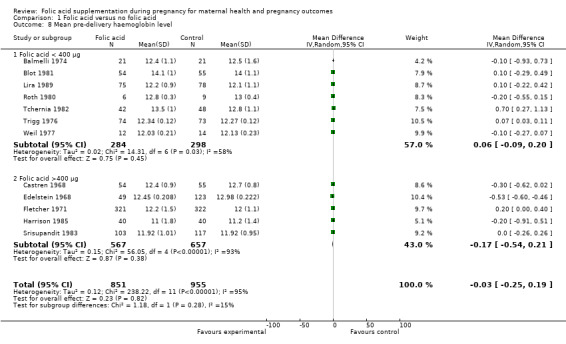
Comparison 1 Folic acid versus no folic acid, Outcome 8 Mean pre‐delivery haemoglobin level.
We also ran a funnel plot to assess the publication bias and we found studies were equally distributed on each side except for two outliers Figure 4.
4.
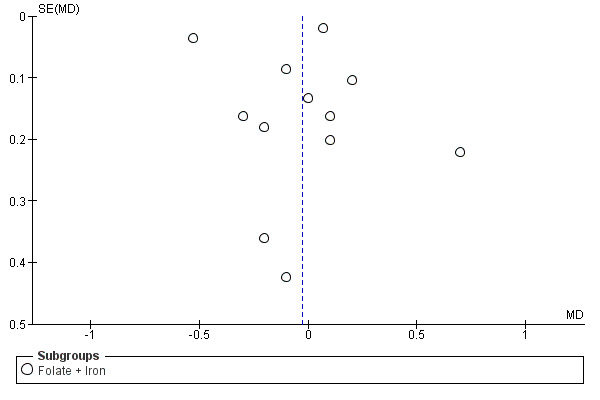
Funnel plot of comparison: 1 Folic acid versus no folic acid, outcome: 1.7 Mean pre‐delivery haemoglobin level.
Pre‐delivery serum folate
Folic acid supplementation in pregnancy showed a reduction in the incidence of low pre‐delivery serum folate by 62% (RR 0.38, 95% CI 0.25 to 0.59; two studies, 696 participants (Analysis 1.11)).
1.11. Analysis.
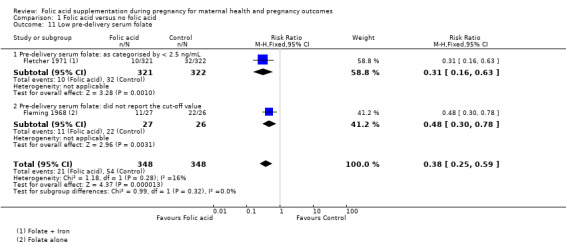
Comparison 1 Folic acid versus no folic acid, Outcome 11 Low pre‐delivery serum folate.
We found non‐significantly higher mean pre‐delivery serum folate levels among those in the folic acid supplementation arm compared with those in the placebo arm (standardised mean difference (SMD) 2.03, 95% CI 0.80 to 3.27; eight studies, 1250 participants; random‐effects, T² = 2.96, I² = 98% (Analysis 1.9)). All the studies pooled for this outcome compared folic acid + iron versus iron alone.
1.9. Analysis.
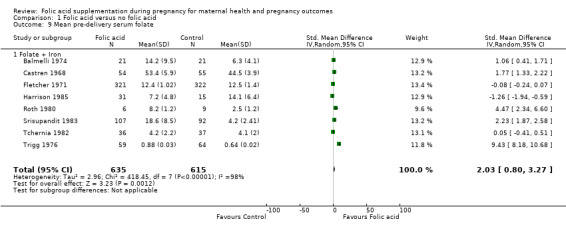
Comparison 1 Folic acid versus no folic acid, Outcome 9 Mean pre‐delivery serum folate.
For subgroup analysis based on dosage of folic acid supplementation, we found significant improvements in mean serum folate concentration when the dose was less than 400 μg (SMD 3.70, 95% CI: 0.28 to 7.11, four studies n = 253, random effects, I² = 99%), however, no impact was seen of folic acid > 400 μg (SMD 0.68, 95% CI: ‐0.75 to 2.10, four studies n = 997, random effects, I² = 98%) Analysis 1.10. The interaction test for the overall estimate was not significant (Chi² P value = 0.11, I² = 61%) suggesting no difference between groups.
1.10. Analysis.
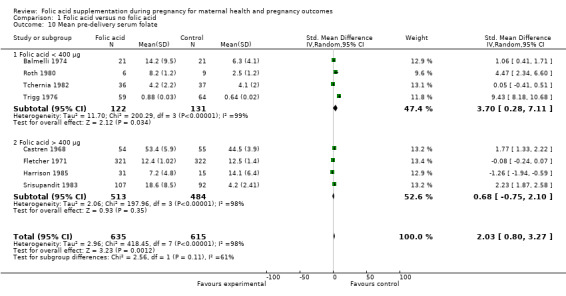
Comparison 1 Folic acid versus no folic acid, Outcome 10 Mean pre‐delivery serum folate.
Pre‐delivery red cell folate
None of the included studies reported data for pre‐delivery red cell folate deficiency status. However, mean red cell folate levels were reported in four studies. Folic acid supplementation during pregnancy did not show any impact on reducing mean pre‐delivery red cell folate levels (SMD 1.59, 95% CI ‐0.07 to 3.26; four studies, 427 participants; random‐effects, T² = 2.79, I² = 97% (Analysis 1.12)). All the studies pooled for this outcome compared folic acid + iron versus iron alone.
1.12. Analysis.

Comparison 1 Folic acid versus no folic acid, Outcome 12 Mean red cell folate.
Megaloblastic anaemia
Folic acid supplementation during pregnancy significantly reduced the incidence of megaloblastic anaemia by 79% (RR 0.21, 95% CI 0.11 to 0.38; four studies, 3839 women (Analysis 1.13)).
1.13. Analysis.
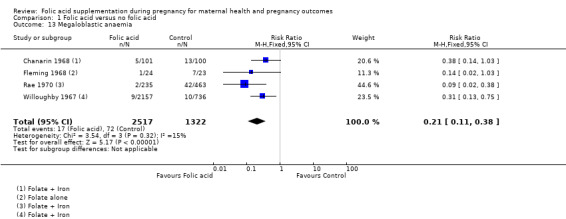
Comparison 1 Folic acid versus no folic acid, Outcome 13 Megaloblastic anaemia.
Outcomes not reported in the included studies
The included studies did not report on the impact of folic acid supplementation on hyperhomocysteinaemia, respiratory disease and allergic disease in the child.
Discussion
Summary of main results
From our meta‐analysis of randomised controlled trials on folic acid supplementation, we found no evidence of an effect of supplements on preterm birth, stillbirth/neonatal death, mean birthweight/low birthweight, low pre‐delivery haemoglobin and serum red cell folate. However, we found a risk reduction on low pre‐delivery serum folate and megaloblastic anaemia.
Quality of the evidence
First, all the included studies were conducted over 30 to 45 years ago, and we found poor subjective and objective compliance with random allocation, adequate concealment and blinding. Bias and confounding thus seem to be the likely explanation for our findings.
Second, for combining studies, it is important that the outcome measures are comparable. Of note, trials included in this analysis reported outcomes quite differently from each other. This could have resulted in higher risk of bias due to selective reporting in these trials. However, we pooled them separately, wherever possible, to minimise this bias.
Potential biases in the review process
We undertook a systematic, thorough search of the literature to identify all studies meeting the inclusion criteria and we are confident that the included trials met the set criteria. Study selection and data extraction were carried out in duplicate and independently and we reached consensus by discussing any discrepancies. A protocol was published for this review. All the analyses were specified a priori, with the exception of a post hoc analysis of the different cut‐off values for biochemistry markers.
Agreements and disagreements with other studies or reviews
Previous observational studies have suggested that higher folate status in pregnancy is associated with higher birthweight, higher placental weight, and prolonged gestation (Goldenberg 1992; Neggers 1997; Tamura 1992). Preconception folic acid supplementation has also shown effects on decreasing preterm births (Bukowski 2009). However, the findings from this review are inconclusive.
A review on folic acid supplementation during pregnancy by Charles et al (Charles 2005b) that included results from large randomised controlled trials found no conclusive evidence of benefit for folic acid supplementation in pregnant women. An earlier version of this Cochrane review also reached the same conclusion (Mahomed 1997).
Authors' conclusions
Implications for practice.
Our meta‐analysis of folic acid supplementation in pregnancy included 31 studies and provided non‐conclusive evidence of folic acid supplementation for pregnant women on pregnancy outcomes except for improvement in mean birthweight. A reduction in the risk of megaloblastic anaemia and improvement in folate levels, however, has been noted with folic acid supplementation against supplementation with placebo but the limitation to this finding is the few number of studies reporting the outcome.
Implications for research.
More well‐designed, large scale randomised controlled trials are needed to establish the benefit of folic acid supplementation during pregnancy. Researchers of future trials should also make efforts to describe the participants in more detail before enrolment and should undertake long‐term follow‐up of the participants and their children in order to study the long‐term effects of folic acid supplementation. Bias should also be reduced by adequate randomisation and allocation concealment of the assignment of intervention by achieving blinding of the participants, providers and the outcome assessors and by minimising loss to follow‐up of the participants, in order to produce trials of adequate methodological quality.
History
Protocol first published: Issue 1, 2008 Review first published: Issue 3, 2013
| Date | Event | Description |
|---|---|---|
| 16 February 2010 | Amended | Author contact details edited. |
| 20 September 2008 | Amended | Converted to new review format. |
Notes
This review has been developed to update the previously published review, 'Folate supplementation in pregnancy' , which was withdrawn from publication in Issue 3, 2006, of The Cochrane Library because it was out of date. SeeOther published versions of this review.
Acknowledgements
We thank Kate Barton and Rebecca Gainey as translators of Lira 1989; Elena Intra as translator of Polatti 1992; Alison Ledward as translator of Weil 1977, Austin Anderson Leirvik as translator of Tchernia 1982 and Caroline Summers as translator of Balmelli 1974 and Roth 1980.
As part of the pre‐publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team), a member of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
Data and analyses
Comparison 1. Folic acid versus no folic acid.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Preterm birth | 3 | 2959 | Risk Ratio (Fixed, 95% CI) | 1.01 [0.73, 1.38] |
| 1.1 As categorised by: birth between 36‐38 weeks of gestation | 1 | 53 | Risk Ratio (Fixed, 95% CI) | 0.75 [0.33, 1.71] |
| 1.2 As categorised by: birth before 38 weeks of gestation | 1 | 109 | Risk Ratio (Fixed, 95% CI) | 0.14 [0.01, 2.65] |
| 1.3 As categorised by: birth before 37 weeks of gestation | 1 | 2797 | Risk Ratio (Fixed, 95% CI) | 1.09 [0.77, 1.54] |
| 2 Stillbirths/neonatal deaths | 3 | 3110 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.96, 1.85] |
| 3 Low birthweight | 4 | 3113 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.66, 1.04] |
| 3.1 Less than 2500 g | 3 | 3089 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.63, 1.02] |
| 3.2 Less than 2400 g | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.3 [0.79, 2.15] |
| 4 Mean birthweight (g) | 5 | 774 | Mean Difference (IV, Random, 95% CI) | 104.96 [‐25.50, 235.41] |
| 4.1 Folate + Iron | 5 | 774 | Mean Difference (IV, Random, 95% CI) | 104.96 [‐25.50, 235.41] |
| 5 Mean birth weight (sensitivity analysis‐after removing Trigg 1976) | 4 | 625 | Mean Difference (IV, Random, 95% CI) | 135.76 [47.85, 223.68] |
| 5.1 Folate + Iron | 4 | 625 | Mean Difference (IV, Random, 95% CI) | 135.76 [47.85, 223.68] |
| 6 Pre‐delivery anaemia | 8 | 4149 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.35, 1.10] |
| 6.1 Anaemia: as categorized by haemoglobin < 11 g/dL | 1 | 35 | Risk Ratio (M‐H, Random, 95% CI) | 2.8 [0.39, 19.93] |
| 6.2 Anaemia: as categorized by haemoglobin < 10.5 g/dL | 2 | 407 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.31, 1.61] |
| 6.3 Anaemia: as categorized by haemoglobin < 10 g/dL | 2 | 2448 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.05, 2.42] |
| 6.4 Anaemia: did not mention their cut‐off | 3 | 1259 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.25, 2.02] |
| 7 Mean pre‐delivery haemoglobin level | 12 | 1806 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.25, 0.19] |
| 7.1 Folate + Iron | 12 | 1806 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.25, 0.19] |
| 8 Mean pre‐delivery haemoglobin level | 12 | 1806 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.25, 0.19] |
| 8.1 Folic acid < 400 µg | 7 | 582 | Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.09, 0.20] |
| 8.2 Folic acid >400 µg | 5 | 1224 | Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.54, 0.21] |
| 9 Mean pre‐delivery serum folate | 8 | 1250 | Std. Mean Difference (IV, Random, 95% CI) | 2.03 [0.80, 3.27] |
| 9.1 Folate + Iron | 8 | 1250 | Std. Mean Difference (IV, Random, 95% CI) | 2.03 [0.80, 3.27] |
| 10 Mean pre‐delivery serum folate | 8 | 1250 | Std. Mean Difference (IV, Random, 95% CI) | 2.03 [0.80, 3.27] |
| 10.1 Folic acid < 400 µg | 4 | 253 | Std. Mean Difference (IV, Random, 95% CI) | 3.70 [0.28, 7.11] |
| 10.2 Folic acid > 400 µg | 4 | 997 | Std. Mean Difference (IV, Random, 95% CI) | 0.68 [‐0.75, 2.10] |
| 11 Low pre‐delivery serum folate | 2 | 696 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.25, 0.59] |
| 11.1 Pre‐delivery serum folate: as categorised by < 2.5 ng/mL | 1 | 643 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.16, 0.63] |
| 11.2 Pre‐delivery serum folate: did not report the cut‐off value | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.30, 0.78] |
| 12 Mean red cell folate | 4 | 427 | Std. Mean Difference (IV, Random, 95% CI) | 1.59 [‐0.07, 3.26] |
| 12.1 Folate + Iron | 4 | 427 | Std. Mean Difference (IV, Random, 95% CI) | 1.59 [‐0.07, 3.26] |
| 13 Megaloblastic anaemia | 4 | 3839 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.11, 0.38] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Balmelli 1974.
| Methods | It was a RCT in which women were randomised into 2 groups and recruited from Clinic for Female Medicine at the University of Bern (Switzerland). Average age for iron group was 27.8 years while for Iron + folic acid group was 26.9 years. Measurement were taken over the period of 12 weeks. Blood samples were taken at monthly intervals. | |
| Participants | Pregnant women between 20‐25 weeks of pregnancy (n = 42). | |
| Interventions | Group 1: ferrous sulphate 125 mg + vitamin B12 100 µg (n = 21). Group 2: ferrous sulphate 125 mg + folic acid 100 µg + vitamin B12 100 µg (n = 21). |
|
| Outcomes | Pre‐delivery haemoglobin level (n = 42), serum folate level (n = 42). | |
| Notes | All the women were the residents of Switzerland for longer than a year. Participants were restricted to patients with a haemoglobin level between 10‐12 g%, suspected abnormal pregnancies or patients suffering from intercurrent illness were excluded from study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Adequate sequence generation was not described in the text. |
| Allocation concealment (selection bias) | High risk | Quote " women were randomised into two groups". Comment: probably not done. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Treatment was not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for exclusion were described. Attrition (21%) with reasons were mentioned in the study. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. |
| Other bias | Unclear risk | No other bias identified but insufficient information available to fully assess this 'Risk of bias' domain. |
Batu 1976.
| Methods | This RCT was conducted on Burmese women. Women were randomly placed into the treatment groups. Venous blood was collected before the commencement of treatment, near full term (38th to 40th week), and 4 to 7 weeks after birth. | |
| Participants | Women attending antenatal clinic in Rangoon for their antenatal visit (n = 96). | |
| Interventions | Group1: iron 60 mg (n = 30). Group2: iron 60 mg+ folic acid 5 mg (n = 25). Group3: placebo (n = 22). Group4: folic acid 5 mg (n = 19). |
|
| Outcomes | Pre‐delivery haemoglobin level (n = 46). | |
| Notes | For this review we compared group 2 with group 1 and group 3 with group 4. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "women were randomly placed to one of four treatment regimens". |
| Allocation concealment (selection bias) | Unclear risk | The methods used for allocation concealment was not stated in the text. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding was not described in the text. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Exclusion number and reasons were not described the text, while attrition (69%) was given with reasons. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make any judgement. |
| Other bias | Unclear risk | No other bias identified but insufficient information available to fully assess this 'Risk of bias' domain. |
Baumslag 1970.
| Methods | This was a randomised trial conducted on all pregnant women who were attending antenatal clinics at the Baragwanath and South Rand Hospitals, Johannesburg (South Africa). Pregnant women were allocated into 3 interventions groups based on random numbers. | |
| Participants | All pregnant women attending antenatal clinics at Baragwanath and South Rand Hospitals, Johannesburg (n = 355). | |
| Interventions | Group 1 received 200 mg of iron by mouth (n = 115). Group 2 received 5 mg of folic acid daily by mouth in addition to the iron (n = 127). Group 3 received 50 µg of vitamin B12 by mouth in addition to the folic acid and iron (n = 113). |
|
| Outcomes | Birthweight was measured. | |
| Notes | Birthweight was analysed separately for Bantu participants and white participants. In the white participants supplementation was started after the 24th week, while supplementation in Bantu participants was started after 28th week. We compared the data of group 1 with group 2 only. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were allocated by random numbers to three groups". |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Insufficient information about blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information about exclusion and attrition. |
| Selective reporting (reporting bias) | Low risk | The study has mentioned data on all outcome measure mentioned as objective. |
| Other bias | Unclear risk | No other bias identified but insufficient information available to fully assess this 'Risk of bias' domain. |
Blot 1981.
| Methods | This was a RCT conducted on women coming for antenatal examination in Paris. Each women was given a bottle containing iron or a combination thereof with folic acid. The 2 groups of women were totally comparable on their baseline characteristics. | |
| Participants | All women attending for the compulsory antenatal examination at the end of 6th month of pregnancy (n = 109). | |
| Interventions | Group1: iron 105 mg (n = 55). Group2: iron 105 mg + folic acid 350 mg (n = 54). |
|
| Outcomes | Pre‐delivery haemoglobin levels (n = 109). | |
| Notes | All women were given ascorbic acid 500 mg. Study population was generally from upper social class which may lead to underestimation of nutritional deficiencies. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were given bottle of 90 tablets, contained either iron or the combination of iron with folic acid". Comment: probably not done. |
| Allocation concealment (selection bias) | Low risk | Bottle of tablets without the awareness of intervention type was given to patients. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Neither the patient nor the obstetrician was aware of the nature of treatment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data on exclusion with its reason were not described in the text. Attrition (45.5%) with reasons were reported. |
| Selective reporting (reporting bias) | Low risk | Study appears to be free of selective reporting. |
| Other bias | Unclear risk | No other bias identified but insufficient information available to fully assess this 'Risk of bias' domain. |
Castren 1968.
| Methods | This RCT was conducted on pregnant women coming to the Maternity Centre of Turku (Finland). 63 women in each groups were started on prophylactic intervention and control treatment. Blood samples were studied 3 times: first before the institution of therapy in the 10th to 20th week of pregnancy, second in the 21st to 30th week, and third at the end of pregnancy in the 31st to 40th week. | |
| Participants | Healthy pregnant women who at the time of examination at the centre had shown no signs of anaemia (n = 126). | |
| Interventions | Group 1 comprised of 63 women started on 200 mg of ferrous sulphate and (n = 63). Group 2 was started on 200 mg of ferrous sulphate and 3 mg of folic acid (n = 63). |
|
| Outcomes | Pre‐delivery haemoglobin level (n = 109), pre‐delivery serum folate (n = 109). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "series of patients were collected from maternity centers of Turku and then the series was divided into two groups". Comment: probably not done. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Insufficient information about blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Number of pregnant women excluded was not mentioned nor its reasons. Attrition (14%) was mentioned along with its reasons. |
| Selective reporting (reporting bias) | Low risk | Study appears to be free of selective reporting. |
| Other bias | Unclear risk | No other bias identified but insufficient information available to fully assess this 'Risk of bias' domain. |
Chanarin 1965.
| Methods | The RCT was conducted on pregnant women coming to antenatal clinics at Saint Mary Hospital, London. | |
| Participants | Pregnant women coming to antenatal clinic (n = 144). | |
| Interventions | Women were allocated to 1 of the following 3 groups. Group 1: ferrous fumarate 100 mg (n = 50). Group 2: ferrous fumarate with 10 µg folic acid (n = 52). Group 3: lactose (n = 42). Subjects were asked to take 1 throughout pregnancy. |
|
| Outcomes | Mean urinary excretion (n = 144), mean haemoglobin (n = 144). | |
| Notes | For this review, group 1 was compared with group 2. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "women were allocated at random to one of the three groups". |
| Allocation concealment (selection bias) | High risk | Insufficient information about allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Glaxo Laboratories supplied these drugs with green, blue or red labels and the precise contents of each batch being unknown to us during the trials". Comment: investigators blinded, it seem from the available information that it was a single blinded study. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information. |
| Other bias | Unclear risk | No other bias identified but insufficient information available to fully assess this 'Risk of bias' domain. |
Chanarin 1968.
| Methods | This was a RCT in which women attending the antenatal clinic at St. Mary's hospital (London) took part in the study. | |
| Participants | 206 women took part in this study. They all were less than 16 weeks of pregnancy. Women were given 1 g of IV Iron dextran as 4 250 mg doses at weekly intervals. At the 20th week they were assigned in to groups (n = 206). | |
| Interventions | Ferrous fumarate 260 mg (n = 101). Ferrous fumarate 260 mg and 100 µg folic acid (n = 105). |
|
| Outcomes | Changes in haemoglobin (n = 206), serum iron (n = 206), serum folate (n = 206) and red cell folate levels (n = 206). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Allotted to one of the two groups". |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit any judgement. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "The survey being conducted as a blind trial". Comment: probably not done. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit any judgment. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit any judgment. |
| Other bias | Unclear risk | No other bias identified but insufficient information available to fully assess this 'Risk of bias' domain. |
Charles 2005.
| Methods | This is a RCT in which during the period June 1966 to June 1967, women (resident of Aberdeen city, Scotland) were identified as potentially eligible to enter into this study to examine the effect of folic acid supplementation on pregnancy outcome. | |
| Participants | All pregnant women booking for antenatal care under 30 weeks' gestation (n = 2928). | |
| Interventions | Women were assigned into 3 groups. Group 1: folic acid 200 µg daily doses (n = 466). Group 2: folic acid 5 mg daily doses (n = 485). Group 3: placebo (n = 1977). |
|
| Outcomes | Birthweight, placental weight, gestational age at delivery, placenta praevia, pre‐eclampsia, fetal abnormality and stillbirth or neonatal deaths (n = 2819). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomised". |
| Allocation concealment (selection bias) | High risk | Quote: "The tablets were kept in numbered drawers and distributed in sequence; during the first 2 weeks of recruitment, the tablets were not ready for distribution and 109 patients recruited at this time received no treatment ad were therefore not eligible for randomisation". Comment: probably not done. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "the study was a double blinded so neither the trial author, nor the patient knew the code to the tablets they were receiving". Comment: probably done. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. |
| Other bias | Unclear risk | No other bias identified but insufficient information available to fully assess this 'Risk of bias' domain. |
Chisholm 1966.
| Methods | Women attending the antenatal clinic at their first visit before the 28th week of pregnancy were asked to participate in a randomised clinical trial to investigate the best method of preventing anaemia during pregnancy in Oxford (UK). | |
| Participants | Women who had haemoglobin level less than 11 g per 100 mL and serum iron of less than 60 µg per 100 mL were not included in the trial and were treated immediately (n = 542). | |
| Interventions | Half of the patient treated with ferrous gluconate (300 mg) 3 times daily (n = 183) and half with placebo tablets (n = 177). These groups were again divided into 3 groups; 1 group was given 500 µg (n = 61), or a high dose of 5 mg folic acid (n = 62) or a placebo (n = 59). | |
| Outcomes | Mean haemoglobin level (360), red cell folate level and folate levels (360). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Random allocation of women to one of the 6 treatment groups". |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Bottles containing the tablets were numbered by random selection". Comment: .insufficient information to permit judgement. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "code was not known while the patients were still on trial". Comment: participants were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. |
| Other bias | Unclear risk | No other bias identified but insufficient information available to fully assess this 'Risk of bias' domain. |
Christian 2003.
| Methods | The study was a cluster‐randomised, double‐blind trial that featured an active control group and was conducted in the rural plains district of Sarlahi, Nepal. | |
| Participants | 4926 pregnant women and their 4130 infants in rural Nepal. | |
| Interventions | In addition to vitamin A (1000 g retinol equivalents), the intervention groups received either:
The control group received vitamin A only (n = 1051). |
|
| Outcomes | Perinatal deaths, Infant deaths, neonatal deaths. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was done in blocks of 5 within each village development community by the senior study investigators, who drew numbered chips from a hat." Comment: Probably done. |
| Allocation concealment (selection bias) | Low risk | Quote: "The supplements, which were of identical shape, size, and color, arrived in Nepal in opaque, sealed, and labelled bottles coded 1–5. The code allocation was kept locked at the Johns Hopkins University, Baltimore." Comment: Probably done. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "The investigators, field staff, and participants were blinded to the codes throughout the study." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | < 0.5% in all arms combined. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. |
| Other bias | Unclear risk | No other bias identified but insufficient information available to fully assess this 'Risk of bias' domain. |
Dawson 1962.
| Methods | Patients attending antenatal clinic were selected for this RCT in Crumpsall Hospital, Manchester. | |
| Participants | Women attending antenatal clinic and were at or before 28 weeks of pregnancy were selected (n = 144). | |
| Interventions | Women were assigned to receive intervention (folic acid 15 mg ) (n = 63) or control group (n = 81). | |
| Outcomes | Prepartum and postpartum haemoglobin levels. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Women were allotted a group in order in which they were booked". Comment: probably not done. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Insufficient information to permit judgment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgment. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgment. |
| Other bias | Unclear risk | No other bias identified but insufficient information available to fully assess this 'Risk of bias' domain. |
Decsi 2005.
| Methods | This is a placebo‐controlled, randomised, double‐blind trial on expecting mothers living in Germany, Hungary and Spain. | |
| Participants | Expectant women from the 20th week of gestation (n = 312). | |
| Interventions | Women received either: Group A: 500 mg Docosahexaenoic Acid (DHA) (n = 77) Group B: or 400 mg Methyltetrahydofolate (5‐MTHF) (n = 80) Group C: or placebo (n = 80) Group D: or the combination of 500 mg DHA and 400 mg 5‐MTHF (n = 75). |
|
| Outcomes | Contribution of docosahexaenoic acid (DHA) to the fatty acids of erythrocyte phophatidylcholine (PC) and phosphatidylethanolamine (PE) lipids at delivery (n = 312). | |
| Notes | For this review, we compared group B with group C. and group A with group D. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "....randomized,..." Comment: insufficient information to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "....double blind,..." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgment. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgment. |
| Other bias | Unclear risk | No other bias identified but insufficient information available to fully assess this 'Risk of bias' domain. |
Edelstein 1968.
| Methods | Patients were Bantu (Johannesberg, South Africa) attending Baragwanath Hospital were randomly allocated to 1 of the 2 groups in this RCT. | |
| Participants | Pregnant women (n = 396). | |
| Interventions | Group1: iron 200 mg (n = 235). Group 2: iron 200 mg + folic acid 5 mg (n = 89). Group3: iron 200 mg, folic acid 5 mg + vit B12 50 µg (n = 72). |
|
| Outcomes | Pre‐delivery haemoglobin levels (n = 172), postpartum haemoglobin levels (n = 291), pre‐delivery folate levels (n = 211), postpartum folate levels (n = 291). | |
| Notes | Their diet largely contains maize. For this review we only compared group 1 with group 2. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "pregnant patients were randomly allocated to one of the two groups". |
| Allocation concealment (selection bias) | Low risk | All tables were dispensed at identical gelatin capsules. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The type of supplementation was not known to the participants or the laboratory staff. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Exclusion and attrition (or reasons) were not reported. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. |
| Other bias | Unclear risk | No other bias identified but insufficient information available to fully assess this 'Risk of bias' domain. |
Fleming 1968.
| Methods | The RCT was conducted in Nigeria. Alternate women were allotted to 2 groups in the order in which they attended the clinic. | |
| Participants | Women with primigravida less than 26 weeks' pregnant with PCV 27% or more, and who had not received any treatment (n = 53). | |
| Interventions | Group 1: lactose based tab (n = 26), group 2: folic acid 5 mg (n = 27). | |
| Outcomes | Premature births (n = 53), folate deficiency (n = 53). | |
| Notes | All the women received antimalarials and iron supplements. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "alternate patients were allotted to group A or group B in the order in which they attended the clinic". Comment: probably not done. |
| Allocation concealment (selection bias) | Low risk | Tablets for both the groups were coloured in the same manner. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The identity of the tablets was not known to investigators until after the completion of the trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Number of exclusions were not mentioned (nor the reasons). Numbers of attrition (28%) were described but their reasons were not given in the text. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. |
| Other bias | Unclear risk | No other bias identified but insufficient information available to fully assess this 'Risk of bias' domain. |
Fletcher 1971.
| Methods | This RCT was conducted on the women living in London. Participants were ascribed at random to 2 treatment groups. | |
| Participants | Pregnant women booked for antenatal clinic (n = 643). | |
| Interventions | Group1: ferrous sulphate 200 mg (n = 322). Group 2: ferrous sulphate 200 mg + folic acid 5 mg (n = 321). |
|
| Outcomes | Pre‐eclampsia (n = 643), serum folate levels (n = 643). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "the subjects were ascribed at random to two treatment groups by instructing each patient to take one tablet daily". |
| Allocation concealment (selection bias) | Unclear risk | The methods used for allocation concealment was not stated in the text. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The methods used for blinding was not stated in the text. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number of exclusions and attritions (along with their reasons) were not reported. |
| Selective reporting (reporting bias) | Unclear risk | The study appears to be free of selective reporting. |
| Other bias | Unclear risk | No other bias identified but insufficient information available to fully assess this 'Risk of bias' domain. |
Giles 1971.
| Methods | Double‐blind controlled trial conducted on patients coming for their antenatal visits at Royal Women's Hospital, Melbourne. Women were allotted to the groups based on the order they were presented. Loss to follow‐up was between 10% to 20%. | |
| Participants | Pregnant women (n = 620). | |
| Interventions | Group 1 (folic acid ‐ Tiger) ferrous sulphate 200 mg (n = 308). Group 2 (folic acid ‐ Lion) folic acid 5 mg (n = 312). |
|
| Outcomes | Low pre‐delivery anaemia (n = 620), birthweight (n = 620), neonatal deaths (n = 620). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "members of each group were numbered consequently in the order in which they presented". Comment: probably not done. |
| Allocation concealment (selection bias) | Low risk | Quote: "the pharmacist, after consulting a list of random numbers, dispensed either folic acid‐tiger or folic acid‐lion from the two large stock bottles. these tablets looked identical, and the dispensing pharmacist did not know which was the placebo". Comment: probably done. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "a double‐blind control trial". Comment: probably done. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Exclusion data with their reasons were not reported in the study. Attrition (15%) along with reasons were reported. |
| Selective reporting (reporting bias) | Unclear risk | The study appears to be free of selective reporting. |
| Other bias | Unclear risk | No other bias identified but insufficient information available to fully assess this 'Risk of bias' domain. |
Harrison 1985.
| Methods | Double‐blind study. The identity of the tables was not known to researcher before the analysis of the study data and women were randomised to 1 of the 5 groups. Conducted in Nigeria. | |
| Participants | Pregnant women of 8 to 24 weeks of pregnancy (n = 69). | |
| Interventions | Group A: placebo only (n = 10). Group B: single dose of chloroquine 600 mg and followed by prognamil 100 mg (n = 18). Group C: ferrous sulphate 60 mg (n = 12). Group D: folic acid 1 mg (n = 10). Group E: ferrous sulphate 60 mg + folic acid 1 mg (n = 9). |
|
| Outcomes | Red cell folate (µg/L) levels, serum folate levels (n = 69). | |
| Notes | For this review group E was compared with group A. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "women were randomised in different treatment groups". |
| Allocation concealment (selection bias) | Unclear risk | The methods used for allocation concealment was not given in the text. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "the medication was double blind, the identity if the tablets not being known to the researchers before the analysis of the data". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Exclusion and attrition were mentioned together (70%) along with their reasons. |
| Selective reporting (reporting bias) | Low risk | The study has mentioned data on all outcome measures mentioned as objectives. |
| Other bias | Unclear risk | No other bias identified but insufficient information available to fully assess this 'Risk of bias' domain. |
Hibbard 1969a.
| Methods | This was a triple‐blind study conducted in Mill Road, maternity hospital, Liverpool, UK. The patients were divided into three groups. | |
| Participants | Pregnant women (before 20 weeks) with defective folate metabolism and excessive FIGLU excretion or low serum folate level (< 2 ng/mL) were admitted in the trial (n = 69). | |
| Interventions | Treatment groups were daily folic acid 500 µg (n = 27), 0.5 mg folic acid (n = 26), and placebo (n = 26). | |
| Outcomes | Serum folate levels and severe anaemia. | |
| Notes | Each group received 60 elemental iron within the trial medicine. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were divided into three groups" |
| Allocation concealment (selection bias) | Unclear risk | Quote" patients were allocated in consecutive numbers" ; "code was not known whilst the trial was in progress" Comment: Not enough information to permit judgement. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "...gelatin coated capsule of identical appearance..." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Approximately 19% were lost to follow‐up and folate levels were also checked in the final non‐attendees. |
| Selective reporting (reporting bias) | Low risk | The study has reported data on outcome measures mentioned as objectives. |
| Other bias | Unclear risk | No other bias identified but insufficient information available to fully assess this 'Risk of bias' domain. |
Iyengar 1975.
| Methods | This RCT was conducted in Niloufer Hospital, Hyderabad (India). Women were alternatively assigned to treatment groups. Groups were matched on parity and height. The women were followed at monthly intervals until 32 weeks of gestation, once every 2 weeks until 36 weeks, and at weekly intervals thereafter until delivery. | |
| Participants | Pregnant women between 20 and 28 weeks of gestation (n = 288). | |
| Interventions | Group 1: no supplement (n = 52), Group 1: iron 60 mg (n = 96), Group 2: iron 60 mg + folic acid 500 µg (n = 134). | |
| Outcomes | Birthweight (n = 230). | |
| Notes | Pregant women belonging to low income less than Rs. 3000/‐ per month. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "alternate subjects received either of therapy". Comment: probably not done. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement regarding allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement regarding blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Exclusion data with their reasons were not reported in the study. Attrition (> 20%) along with reasons were reported. |
| Selective reporting (reporting bias) | Low risk | Study seems to be free from selective reporting. |
| Other bias | Unclear risk | No other bias identified but insufficient information available to fully assess this 'Risk of bias' domain. |
Lira 1989.
| Methods | This is a RCT in which all pregnant women attending the outpatient obstetrics clinic at the Catholic University’s Clinical Hospital (Chile). | |
| Participants | Women with less than 16 weeks of pregnancy were selected (n = 153). | |
| Interventions | Treatment group received a preparation containing equal quantities of iron and folic acid, 350 µg (plus 500 mg of ascorbic acid) (n =78 and 75). | |
| Outcomes | Haemoglobin (g/dL), haematocrit (%), serum iron (µg/dL), transferrin (µg/dL), transferrin saturation (%), serum folate (ng/mL), red cell folate (ng/mL), plasmatic volume (mL) (n = 153). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Women were divided into 2 groups randomly". |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. |
| Other bias | Unclear risk | No other bias identified but insufficient information available to fully assess this 'Risk of bias' domain. |
Menon 1962.
| Methods | This is a RCT conducted in Inida, in which women were allotted to each group in the order they were registered. | |
| Participants | Pregnant women between 16 weeks and 24 weeks whose haemoglobin level was at or above 10.5 gm% (14.5 gm = 100%) (n = 273). | |
| Interventions | Group 1 was given 5 g of ferrous sulphate (n = 88). Group 2 was given 5 mg of folic acid (n = 90). Group 3 was given 5 g of ferrous sulphate and 5 mg of folic acid (n = 90). |
|
| Outcomes | Fall in pre‐delivery haemoglobin level (n = 273). | |
| Notes | All the women were given multivitamin along with these treatments. In this review we compared group 1 with group 3. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "subjects were allotted to each group in the order in which they registered". Comment: probably not done. |
| Allocation concealment (selection bias) | Unclear risk | Insuffient information on allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Insuffient information to make any judgment regarding blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Exclusion data with their reasons were not reported in the study. Attrition (> 20%) along with the reasons were reported. |
| Selective reporting (reporting bias) | Low risk | Study seemed to be free from selective reporting. |
| Other bias | Unclear risk | No other bias identified but insufficient information available to fully assess this 'Risk of bias' domain. |
Metz 1965.
| Methods | This RCT was conducted at the antenatal clinic at South Rand Hospital, Johannesburg, South Africa. | |
| Participants | A total of I75 pregnant white women attending the clinics were selected. | |
| Interventions | Group 1: 200 mg of iron orally daily, either as ferrous sulphate or ferrous fumarate (n = 57). Group 2 iron as in group 1 together with 5 mg of folic acid orally daily (n = 60). Group 3 vitamin B12 orally (daily in addition to iron and folic acid as in group 2 (n = 58). |
|
| Outcomes | Urinaru FIGLU (n = 175), serum folate activity, serum vitamin B12 concentration, serum haemoglobin and haematocrit (n = 175). | |
| Notes | In this review group 1 was compared with group 2. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomly allocated to one of three groups...". |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "All tablets were dispensed in identical gelatin capsules". Comment: insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. |
| Other bias | Unclear risk | No other bias identified but insufficient information available to fully assess this 'Risk of bias' domain. |
Pack 1980.
| Methods | This is a double‐blind study from NewZealand. | |
| Participants | Pregnant women in 4th and 8th months of gestation were enrolled (n = 30). | |
| Interventions | Group A: received placebo mouth wash and tablets (n = 10). Group B: received placebo mouth wash and 5 mg folate tablets (n = 10). Group C: folic acid mouth wash and placebo tablets (n = 10). |
|
| Outcomes | Correlation between gingival index and plaque index (n = 30). | |
| Notes | In this review, we compared group B with group A. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "thirty women were randomly divided in 3 roups of 10". Comment: insufficient information on sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Insuffient information on allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double blind". Comment: probably done. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insuffient information on outcome data. |
| Selective reporting (reporting bias) | Unclear risk | Insuffient information to make any judgment. |
| Other bias | Unclear risk | No other bias identified but insufficient information available to fully assess this 'Risk of bias' domain. |
Rae 1970.
| Methods | This was a RCT. Randomisation was done according to a day of week on which women coming for antenatal clinic in Liverpool (UK). Samples of venous blood were taken from patients in both groups at the first visit to the antenatal clinic, at the 32nd and 36th weeks of pregnancy, and during the first 3 days of puerperium. | |
| Participants | Pregnant women coming for antenatal visits (n = 698). | |
| Interventions | Pregnant women coming on Monday was assigned were prescribed ferrous gluconate 200 mg, while those coming in Tuesday were given same dose of ferrous gluconate in addition to a folic acid 5 mg. Monday group (n = 463) and Tuesday group (n = 235), total (n = 698). | |
| Outcomes | Low pre‐delivery anaemia (< 10.9 g/dL) (n = 698), megaloblastic anaemia (n = 698). | |
| Notes | Patients with a haemoglobin concentration of 10.9 g/dL or more were classified 'not anaemic', and those with a haemoglobin concentration of under 10.9 g/dL were classified 'normoblastic' or 'megaloblastic' according to the marrow smear. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "patients were allocated at random to one or other of the two groups, depending on which day of the week they attended the antenatal clinic". Comment: probably not done. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement. |
| Selective reporting (reporting bias) | Low risk | The study has mentioned data on all outcome measures mentioned as objectives. |
| Other bias | Unclear risk | No other bias identified but insufficient information available to fully assess this 'Risk of bias' domain. |
Rolschau 1979.
| Methods | This was a controlled trial conducted on women attending antenatal clinic of Odense University Hopsital. The criteria for selecting women was Danish birth and a normal pregnancy. Women were matched on parity, tobacco consumption, pre‐pregnant weight, housing condition and age. | |
| Participants | Pregnant women in 21 to 25 week of gestation (n = 36). | |
| Interventions | Group1: iron 250 mg (n = 16), group 2: iron 250 mg + folic acid 5 mg (n = 20). | |
| Outcomes | Birthweight. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Subjects were allotted to two groups". |
| Allocation concealment (selection bias) | Low risk | Quote: 'groups were supplied similar tablets". Comment: probably done. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Insufficient information to make any judgement. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Number excluded nor its reasons mentioned. Attrition (10%) was reported but reasons were not described. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make any judgement. |
| Other bias | Unclear risk | No other bias identified but insufficient information available to fully assess this 'Risk of bias' domain. |
Roth 1980.
| Methods | This is a RCT conducted during August 1976 ‐ September 1977. | |
| Participants | 23 pregnant women were selected. | |
| Interventions | Group A (11 patients): 1 x Tardyferon‐Fol tablet per day (80 mg iron sulphate, 80 mg mucoproteose, 350 µg folic acid) during pregnancy. Group B (12 patients): 1 x Tardyferon tablet per day (80 mg iron sulphate, 80 mg mucoproteose, no folic acid content) during pregnancy. Group A: 5 dropouts. Group B: 3 dropouts. Reasons for dropouts included irregular intake of the medication (3), change of residence (2), premature birth (2) and 1 unexplained failure to attend the final check‐up. |
|
| Outcomes | Haemoglobin level, red cell folate levels and serum folate levels (n = 23). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomised". Comment: insufficient information to make any judgement. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The tablets used in treatment were identical in appearance". Comment: insufficient information to make any judgement. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "Yes". Comment: insufficient information to make any judgement. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to make any judgement. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make any judgement. |
| Other bias | Unclear risk | No other bias identified but insufficient information available to fully assess this 'Risk of bias' domain. |
Srisupandit 1983.
| Methods | This is a RCT in which women were allocated using random number table. | |
| Participants | Pregnant women attending antenatal clinic of Siraj hospital Thailand (n = 329). | |
| Interventions | Group1: iron 60 mg (n = 109), Group 2: iron 180 mg (n = 117), Group 3: iron 180 mg + folic acid 5 mg (n = 103). | |
| Outcomes | Pre‐delivery haemoglobin level, serum folate levels, red cell folate levels. | |
| Notes | For this review we compared group 2 with group 3. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "subjects were allocated supplementation by using random table and divided into three groups". Comment: probably done. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Exclusion and attrition were reported in a single figure (18%) with their reasons. |
| Selective reporting (reporting bias) | Low risk | The study seems to be free from selective reporting. |
| Other bias | Unclear risk | No other bias identified but insufficient information available to fully assess this 'Risk of bias' domain. |
Tchernia 1982.
| Methods | This is a RCT on pregnant women attending the antenatal clinic and obstetric department of a hospital located in the outskirts of Paris. | |
| Participants | Pregnant women (n = 200). | |
| Interventions | Group 1: 105 mg iron (n = 100), group 2: 105 mg iron + folic acid 350 µg (n = 100). | |
| Outcomes | Birthweight, birth defects (n = 200). | |
| Notes | 3 studies conducted in the actual trial, we for this review only focused on above mentioned intervention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "women selected at random". |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Number excluded not reported nor its reason. Reasons for attrition (> 20%) were not reported. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. |
| Other bias | Unclear risk | No other bias identified but insufficient information available to fully assess this 'Risk of bias' domain. |
Trigg 1976.
| Methods | It was a controlled trial conducted in London. | |
| Participants | Pregnant women (n = 158). | |
| Interventions | Group 1: ferrous sulphate 50 mg (n = 76 women). Group 2: ferrous sulphate 50 mg + folic acid 0.05 mg (n = 82 women). |
|
| Outcomes | Pre‐delivery haemoglobin level, pre‐delivery serum folate level (ng/mL), serum folate level (ng/mL), birthweight (n = 158). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomly allocated to one of the two treatments". |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Insufficient information about the blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information about the exclusion and attrition. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit any judgement. |
| Other bias | Unclear risk | No other bias identified but insufficient information available to fully assess this 'Risk of bias' domain. |
Weil 1977.
| Methods | Double‐blind trial, patients were randomly allocated in 2 groups. Initially 31 patients were recruited in the trial, but during the trial 1 participant from each group was excluded. | |
| Participants | Pregnant women of 20 weeks of gestation who were attending clinic affiliated to University of Basel, Switzerland (n = 29). | |
| Interventions | Group 1: ferrous sulphate 80 mg (n = 15), group 2: ferrous sulphate 80 mg + folic acid 350 µg (n = 14). | |
| Outcomes | Pre‐delivery haemoglobin levels (n = 29). | |
| Notes | Women who were already taking multi‐vitamin containing folic acid prior to commencement of trial were excluded. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients were randomised and divided into 2 groups. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Number excluded were not reported while their reasons were described. Attrition (6%) was reported but reasons were not given. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. |
| Other bias | Unclear risk | No other bias identified but insufficient information available to fully assess this 'Risk of bias' domain. |
Willoughby 1967.
| Methods | For a period of 2 years (August 1964 to August 1966) every patient attending the antenatal clinic was randomly allocated to one of 5 prophylactic treatment groups in this RCT which was conducted in Glasgow (Scotland). | |
| Participants | Pregnant women attending clinic for antenatal visit (n = 3599). | |
| Interventions |
Group 1: iron (mg/day) = 0, folic acid (µg/day) = 0 (n = 706). Group 2: iron (mg/day) = 105, folic acid (µg/day) = 0 (n = 736). Group 3: iron (mg/day) = 105, folic acid (µg/day) = 100 (n = 716). Group 4: iron (mg/day) = 105, folic acid (µg/day) = 300 (n = 715). Group 5: iron (mg/day) = 105, folic acid (µg/day) = 450 (n = 726). |
|
| Outcomes | Pre‐delivery anaemia (n = 3599), megaloblastic anaemia (n = 3599). | |
| Notes | Patients with haemoglobin levels below 10 g/dL at their first attendance were excluded from the trial. We compared group 3, 4 and 5 with group 2. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: " patients attending the antenatal clinic were randomly allocated one of the five prophylactic groups". Comment: probably not done. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Insufficient information about blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number of pregnant women excluded and attrition rate (n = 0) were not mentioned nor their reasons. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit any judgment. |
| Other bias | Unclear risk | No other bias identified but insufficient information available to fully assess this 'Risk of bias' domain. |
FIGLU: formiminoglutamic acid IV: intravenous PCV: packed cell volume RCT: randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bjerre 1967 | Folic acid was supplemented in combination with other micronutrients versus placebo. |
| Colman 1974 | Study was to assess efficacy of folic acid supplementation in the form of food fortification. |
| Colman 1975 | Study was to assess efficacy of folic acid supplementation in the form of food fortification. |
| Ellison 2004 | Both the intervention and control group received folic acid supplementation, and groups were compared on duration of folic acid therapy. One group was given supplementation till 16 weeks of pregnancy and the other group received it till end of pregnancy. |
| Giles 1960 | There was no randomisation and intervention participants were compared with historical controls. |
| Gregory 2001 | Folate in pregnant women was compared with non‐pregnant controls. |
| Hague 1998 | Published abstract of a protocol, however, study was abandoned without completion. |
| Hamilton 1973 | Nothing has been mentioned about randomisation nor the word is used. It seems this is not a randomised controlled trial. |
| Hekmatdoost 2011 | This study compares 1: 5 methyltetrahydrofolate with folic acid. |
| Hibbard 1969 | Folic acid 1.5 mg was compared with folic acid 15 mg. |
| Khanna 1977 | Included patients were already receiving iron and then therapeutic folic acid was added among women who were found to have anaemia. |
| Klinger 2006 | Effect on folic acid on placental apoptosis was assessed. |
| Kristoffersen 1979 | Only a published abstract with insufficient information was available. |
| Ma 2008 | Folic acid and iron was given along with retinol and riboflavin. |
| Manizheh 2009 | High‐dose of folate was compared with low‐dose folate. |
| Melli 2008 | Onlt published abstract with insufficient information was available. |
| Ouma 2006 | Association of folic acid supplementation and sulfadoxine pyrimethamine was observed. |
| Polatti 1992 | Both the intervention and control groups received folic acid supplementation. Assessed the effectiveness of folic acid when given from 12 week of pregnancy compared with given from 20 weeks of pregnancy. |
| Taylor 1979 | Experimental group was given Iron and folate and were compared with no therapy group. |
| Taylor 1981 | Experimental group was given Iron and folate and were compared with no therapy group. |
| Tchernia 1982a | In this paper they have reviewed and re‐analysed the results of 3 different trials. |
| Thomson 1982 | Published abstract with insufficient information was only available. |
| Ulrich 1999 | Half of the participants were selected and allotted to the group based on randomisation, while other half of the study population was selected from the hospitals who delivered during the study period. Effect of folic acid was observed on development of congential anomalies. |
| Wang 2012 | This study compares folic acid, iron‐folic acid or multi‐micronutrients. Effect of folic acid alone cannot be determined. |
| Zeng 2008 | Both the intervention and control group were given folic acid and the intervention group also received iron. |
Characteristics of ongoing studies [ordered by study ID]
Wen 2012.
| Trial name or title | High dose folic acid supplementation throughout pregnancy for pre‐eclampsia prevention (FACT) |
| Methods | Double blind (participant, caregiver, investigator, outcomes assessor) randomised intervention trial with parallel assignment at Ottawa Hospital Research Institute |
| Participants | Pregnant women at high risk of developing pre‐eclampsia |
| Interventions | Folic acid 4 mg, folic acid 1.0 mg x 4 tablets will be taken daily by oral administration. The majority of women in the study will routinely take 1.0 mg folic acid in a prenatal vitamin supplement, as recommended by their primary obstetrical provider; the study requirements do not require that participants change their practice. Therefore the actual total daily dose may be up to 5.1 mg of folic acid |
| Outcomes | Pre‐eclampsia, Preterm birth, Stillbirth, Abortion |
| Starting date | April 2011 |
| Contact information | Contact: Mark Walker, MD; Contact: Shi Wu Wen, PhD |
| Notes | Recruiting |
Differences between protocol and review
Outcome measures have been separated into 'Primary' and 'Secondary' outcomes.
We have added two additional outcomes: respiratory disease in the child; allergic disease in the child.
Contributions of authors
Zohra Lassi entered the data, created the comparisons, conducted the analyses and wrote the text of the review under the guidance of Dr Zulfiqar Bhutta. The draft protocol was written by Dr Batool Haider (BAH) who also designed the eligibility and data extraction forms. Rehana Salam (RAS) took part in assisting with data analysis.
Sources of support
Internal sources
The Aga Khan University, Pakistan.
External sources
No sources of support supplied
Declarations of interest
None known.
New
References
References to studies included in this review
Balmelli 1974 {published data only}
- Balmelli GP, Huser HJ. Folic acid deficiency in pregnant women in Switzerland [Zur Frage des Folsäuremangels bei Schwangeren in der Schweiz]. Schweizerische Medizinische Wochenschrift 1974;104(10):351‐6. [PubMed] [Google Scholar]
Batu 1976 {published data only}
- Batu AT, Toe T, Pe H, Nyunt KK. A prophylactic trial of iron and folic acid supplements in pregnant Burmese women. Israel Journal of Medical Sciences 1976;12:1410‐7. [PubMed] [Google Scholar]
Baumslag 1970 {published data only}
- Baumslag N, Edelstein T, Metz J. Reduction of incidence of prematurity by folic acid supplementation in pregnancy. British Medical Journal 1970;1:16‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Blot 1981 {published data only}
- Blot I, Papiernik E, Kaltwasser JP, Werner E, Tchernia G. Influence of routine administration of folic acid and iron during pregnancy. Gynecologic and Obstetric Investigation 1981;12:294‐304. [DOI] [PubMed] [Google Scholar]
Castren 1968 {published data only}
- Castren O, Levanto A, Rauramo L, Ruponen S. Preventive iron and folic acid therapy in pregnancy. Annales Chirurgiae et Gynaecologiae Fenniae 1968;57:382‐6. [PubMed] [Google Scholar]
Chanarin 1965 {published data only}
- Chanarin I, Rothman D, Berry V. Iron deficiency and its relation to folic‐acid status in pregnancy: results of a clinical trial. British Medical Journal 1965;1:480‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chanarin 1968 {published data only}
- Chanarin I, Rothman D, Ward A, Perry J. Folate status and requirement in pregnancy. British Medical Journal 1968;2:390‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Charles 2005 {published data only}
- Charles D, Ness AR, Campbell D, Smith GD, Hall MH. Taking folate in pregnancy and risk of maternal breast cancer. BMJ 2004;329(7479):1375‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles DHM, Ness AR, Campbell D, Smith GD, Whitely E, Hall MH. Folic acid supplements in pregnancy and birth outcome: re‐analysis of a large randomised controlled trial and update of Cochrane review. Pediatric and Perinatal Epidemiology 2005;19:112‐24. [DOI] [PubMed] [Google Scholar]
- Hall M. Assessment of the effects of folic acid deficiency in pregnancy. [MD thesis]. University of Aberdeen, 1970. [Google Scholar]
- Hall MH. Folic acid deficiency and abruptio placentae. Journal of Obstetric and Gynaecology 1972;79:222‐5. [DOI] [PubMed] [Google Scholar]
- Hall MH, Pirani BB, Campbell D. The cause of the fall in serum folate in normal pregnancy. British Journal of Obstetrics and Gynaecology 1976;83:132‐6. [DOI] [PubMed] [Google Scholar]
Chisholm 1966 {published data only}
- Chisholm M. A controlled clinical trial of prophylactic folic acid and iron in pregnancy. Journal of Obstetrics and Gynaecology of the British Commonwealth 1966;73:191‐6. [DOI] [PubMed] [Google Scholar]
Christian 2003 {published data only}
- Christian P, Darmstadt GL, Wu L, Khatry SK, Leclerq SC, Katz J, et al. The effect of maternal micronutrient supplementation on early neonatal morbidity in rural Nepal: a randomised, controlled, community trial. Archives of Disease in Childhood. Fetal and Neonatal Edition 2008;93(8):660‐4. [DOI] [PubMed] [Google Scholar]
- Christian P, Jiang T, Khatry SK, LeClerq SC, Shrestha SR, West Jr KP. Antenatal supplementation with micronutrients and biochemical indicators of status and subclinical infection in rural Nepal. American Journal of Clinical Nutrition 2006;83:788‐94. [DOI] [PubMed] [Google Scholar]
- Christian P, Khatry SK, Katz J, Pradhan EK, LeClerq SC, Shrestha SR, et al. Effects of alternative maternal micronutrient supplements on low birth weight in rural Nepal: double blind randomised community trial. BMJ 2003;326(7389):571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian P, Khatry SK, LeClerq SC, Dali SM. Effects of prenatal micronutrient supplementation on complications of labor and delivery and puerperal morbidity in rural Nepal. International Journal of Gynecology & Obstetrics 2009;106(1):3‐7. [DOI] [PubMed] [Google Scholar]
- Christian P, Murray‐Kolb LE, Khatry SK, Katz J, Schaefer BA, Cole PM, et al. Prenatal micronutrient supplementation and intellectual and motor function in early school‐aged children in Nepal. JAMA 2010;304(24):2716‐23. [DOI] [PubMed] [Google Scholar]
- Christian P, Shrestha J, LeClerq SC, Khatry SK, Jiang T, Wagner T, et al. Supplementation with micronutrients in addition to iron and folic acid does not further improve the hematologic status of pregnant women in rural Nepal. Journal of Nutrition 2003;133(11):3492‐8. [DOI] [PubMed] [Google Scholar]
- Christian P, Stewart CP, LeClerq SC, Wu L, Katz J, West KPJ, et al. Antenatal and postnatal iron supplementation and childhood mortality in rural Nepal: a prospective follow‐up in a randomized, controlled community trial. American Journal of Epidemiology 2009;170(9):1127‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian P, West KP, Khatry SK, Leclerq SC, Pradhan EK, Katz J, et al. Effects of maternal micronutrient supplementation on fetal loss and infant mortality: a cluster‐randomized trial in Nepal. American Journal of Clinical Nutrition 2003;78(6):1194‐202. [DOI] [PubMed] [Google Scholar]
- Katz J, Christian P, Dominici F, Zeger SL. Treatment effects of maternal micronutrient supplementation vary by percentiles of the birth weight distribution in rural Nepal. Journal of Nutrition 2006;136(5):1389‐94. [DOI] [PubMed] [Google Scholar]
- Nanayakkara‐Bind A, Schulze K, Wu L, Le SC, Khatry SK, Christian P. Effects of antenatal micronutrient supplementation on cortisol and erythropoietin in pregnant Nepalese women. FASEB Journal 2011;25:779.15. [Google Scholar]
- Stewart CP, Christian P, LeClerq SC, West KPJ, Khatry SK. Antenatal supplementation with folic acid + iron + zinc improves linear growth and reduces peripheral adiposity in school‐age children in rural Nepal. American Journal of Clinical Nutrition 2009;90(1):132‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart CP, Christian P, Schulze KJ, Arguello M, Leclerq SC, Khatry SK, et al. Low maternal vitamin B‐12 status is associated with offspring insulin resistance regardless of antenatal micronutrient supplementation in rural Nepal. Journal of Nutrition 2011;141(10):1912‐7. [DOI] [PubMed] [Google Scholar]
- Stewart CP, Christian P, Schulze KJ, Leclerq SC, West KPJ, Khatry SK. Antenatal micronutrient supplementation reduces metabolic syndrome in 6‐ to 8‐year‐old children in rural Nepal. Journal of Nutrition 2009;139(8):1575‐81. [DOI] [PubMed] [Google Scholar]
Dawson 1962 {published data only}
- Dawson DW, More JRS, Aird DC. Prevention of megaloblastic anaemia in pregnancy by folic acid. Lancet 1962;2:1015‐20. [DOI] [PubMed] [Google Scholar]
Decsi 2005 {published data only}
- Broekaert I, Campoy C, Iznaola C, Hoffman B, Mueller‐Felber W, Koletzko BV. Visual evoked potentials in infants after dietary supply of docosahexaenoic acid and 5‐methyl‐tetrahydrofolate during pregnancy. Journal of Pediatric Gastroenterology and Nutrition 2004;39(Suppl 1):S33. [Google Scholar]
- Campoy C, Escolano‐Margarit MV, Ramos R, Parrilla‐Roure M, Csabi G, Beyer J, et al. Effects of prenatal fish‐oil and 5‐methyltetrahydrofolate supplementation on cognitive development of children at 6.5 y of age. American Journal of Clinical Nutrition 2011;94(6 Suppl):1880S‐8S. [DOI] [PubMed] [Google Scholar]
- Campoy C, Marchal G, Decsi T, Cruz M, Szabo E, Demmelmair H, et al. Spanish pregnant women's plasma phospholipids LC‐PUFAs concentrations and its influence on their newborns. Journal of Pediatric Gastroenterology and Nutrition 2004;39(Suppl 1):S11. [Google Scholar]
- Decsi T, Campoy C, Koletzko B. Effect of n‐3 polyunsaturated fatty acid supplementation in pregnancy: the nuheal trial. Advances in Experimental Medicine & Biology 2005;569:109‐13. [DOI] [PubMed] [Google Scholar]
- Demmelmair H, Klingler M, Campoy C, Decsi T, Koletzko B. Low eicosapentaenoic acid concentrations in fish oil supplements do not influence the arachidonic acid contents in placental lipids. Journal of Pediatric Gastroenterology and Nutrition 2004;39(Suppl 1):S11. [Google Scholar]
- Demmelmair H, Klingler M, Campoy C, Diaz J, Decsi T, Veszpremi B, et al. The influence of habitual diet and increased docosahexaenoic acid intake during pregnancy on the fatty acid composition of individual placental lipids [abstract]. Journal of Pediatric Gastroenterology & Nutrition 2005;40(5):622‐3. [Google Scholar]
- Dolz V, Campoy C, Molloy A, Scott J, Marchal G, Decsi T, et al. Homocysteine, folate & methylenetetrahydrofolate reductase (MTHFR) 677 ‐ T poly‐morphism in Spanish pregnant woman and in their offspring [abstract]. Journal of Pediatric Gastroenterology & Nutrition 2005;40(5):623‐4. [Google Scholar]
- Franke C, Demmelmair H, Decsi T, Campoy C, Cruz M, Molina‐Font JA, et al. Influence of fish oil or folate supplementation on the time course of plasma redox markers during pregnancy. British Journal of Nutrition 2010;103(11):1648‐56. [DOI] [PubMed] [Google Scholar]
- Krauss‐Etschmann S, Shadid R, Campoy C, Hoster E, Demmelmair H, iménez M, et al. Nutrition and Health Lifestyle (NUHEAL) Study Group. Effects of fish‐oil and folate supplementation of pregnant women on maternal and fetal plasma concentrations of docosahexaenoic acid and eicosapentaenioc acid: a European randomised multicenter trial. American Journal of Clinical Nutrition 2007;85(5):1392‐400. [DOI] [PubMed] [Google Scholar]
Edelstein 1968 {published data only}
- Edelstein T, Stevens K, Baumslag N, Metz J. Folic acid and vitamin B12 supplementation during pregnancy in a population subsisting on a sub‐optimal diet. Journal of Obstetrics and Gynaecology of the British Commonwealth 1968;75:133‐7. [DOI] [PubMed] [Google Scholar]
Fleming 1968 {published data only}
- Fleming AF, Hendrickse JP, Allan NC. The prevention of megaloblastic anaemia in pregnancy in Nigeria. Journal of Obstetrics and Gynaecology of the British Commonwealth 1968;75(4):425‐32. [DOI] [PubMed] [Google Scholar]
Fletcher 1971 {published data only}
- Fletcher J, Gurr A, Fellingham FR, Prankerd TAJ, Brant HA, Menzies DN. The value of folic acid supplements in pregnancy. Journal of Obstetrics and Gynaecology of the British Empire 1971;78:781‐5. [DOI] [PubMed] [Google Scholar]
Giles 1971 {published data only}
- Giles PF, Harcourt AG, Whiteside MG. The effect of prescribing folic acid during pregnancy on birthweight and duration of pregnancy. A double blind trial. Medical Journal of Australia 1971;2:17‐21. [PubMed] [Google Scholar]
Harrison 1985 {published data only}
- Fleming AF, Ghatoura GBS, Harrison KA, Briggs ND, Dunn DT. The prevention of anaemia in pregnancy in primigravidae in the guinea savanna of Nigeria. Annals of Tropical Medicine and Parasitology 1986;80:211‐33. [DOI] [PubMed] [Google Scholar]
- Fleming AF, Ludwig H, Thomsen K. Anaemia in pregnancy in the Guinea Savanna of Nigeria. Gynecology and Obstetrics. Berlin: Springer‐Verlag, 1986:122‐4.
- Harrison KA, Fleming AF, Briggs ND, Rossiter CE. Child‐bearing, health and social priorities: a survey of 22,774 consecutive hospital births in Zaria, Northern Nigeria. 5. Growth during pregnancy in Nigerian teenage primigravidae. British Journal of Obstetrics and Gynaecology 1985;92(5):32‐9. [PubMed] [Google Scholar]
Hibbard 1969a {published data only}
- Hibbard BM, Hibbard ED. The prophylaxis of folate deficiency in pregnancy. Acta Obstetricia et Gynecologica Scandinavica 1969;48:339‐48. [DOI] [PubMed] [Google Scholar]
Iyengar 1975 {published data only}
- Iyengar L. Effect of folic acid supplementation on birth weight of infants. American Journal of Obstetrics and Gynaecology 1974;122:332‐6. [DOI] [PubMed] [Google Scholar]
- Iyengar L. Folic acid requirements of Indian pregnant women. American Journal of Obstetrics and Gynecology 1971;111(1):13‐6. [DOI] [PubMed] [Google Scholar]
- Iyengar L, Apte SV. Prophylaxis of anemia in pregnancy. American Journal of Clinical Nutrition 1970;23(6):725‐30. [DOI] [PubMed] [Google Scholar]
Lira 1989 {published data only}
- Lira P, Barrena N, Foradori A, Gormaz G, Grebe G. Folate deficiency in pregnancy: effect of supplementary folic acid [Deficienca de folatos en el embarazo: efecto de una suplementacion con acido folico]. Sangre 1989;34(1):24‐7. [PubMed] [Google Scholar]
Menon 1962 {published data only}
- Menon MKK, Rajan L. Prophylaxis of anaemia in pregnancy. Journal of Obstetrics and Gynaecology of India 1962;12:382‐9. [Google Scholar]
Metz 1965 {published data only}
- Metz J, Festenstein H, Welch P. Effect of folic acid and vitamin B12 supplementation on test of folate and Vitamin B12 nutrition in pregnancy. American Journal of Clinical Nutrition 1965;16:472‐9. [DOI] [PubMed] [Google Scholar]
Pack 1980 {published data only}
- Pack ARC, Thomson ME. Effects of folic acid (FA) supplementation on gingivitis in pregnancy. Journal of Dental Research 1980;59(D1):1777. [Google Scholar]
- Pack ARC, Thomson ME. Effects of topical and systemic folic acid supplementation on gingivitis in pregnancy. Journal of Clinical Periodontology 1980;7:402‐14. [DOI] [PubMed] [Google Scholar]
Rae 1970 {published data only}
- Rae PG, Robb PM. Megaloblastic anaemia of pregnancy: a clinical and laboratory study with particular reference to the total and labile serum folate levels. Journal of Clinical Pathology 1970;23:379‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rolschau 1979 {published data only}
- Rolschau J, Date J, Kristoffersen K. Folic acid supplement and intrauterine growth. Acta Obstetricia et Gynecologica Scandinavica 1979;58:343‐6. [DOI] [PubMed] [Google Scholar]
Roth 1980 {published data only}
- Roth F, Mauracher E. Folic acid treatment during pregnancy [Folsauresubstitution bei schwangeren]. Geburtshilfe und Frauenheilkunde 1980;40:253‐8. [DOI] [PubMed] [Google Scholar]
Srisupandit 1983 {published data only}
- Srisupandit S, Pootrakul P, Areekul S, Neungton S, Mokkaves J, Kiriwat O, et al. A prophylactic supplementation of iron and folate in pregnancy. Southeast Asian Journal of Tropical Medicine and Public Health 1983;14:317‐23. [PubMed] [Google Scholar]
Tchernia 1982 {published and unpublished data}
- Tchernia G, Blot I, Rey A, Kaltwasser JP, Zittoun J, Papiernik E. Maternal folate status, birthweight and gestational age. Developmental Pharmacology Therapeutics 1982;4:58‐65. [PubMed] [Google Scholar]
Trigg 1976 {published data only}
- Trigg KH, Rendall EJC, Johnson A, Fellingham FR, Prankerd TAJ. Folate supplements during pregnancy. Journal of the Royal College of General Practitioners 1976;26:228‐30. [PMC free article] [PubMed] [Google Scholar]
Weil 1977 {published data only}
- Weil A, Mauracher E. Folic acid and pregnancy, a real problem? [Acide folique et gravidité, problème réel?]. Schweizer Medizinische Wochenschrift 1977;107:1943‐7. [PubMed] [Google Scholar]
Willoughby 1967 {published data only}
- Willoughby ML. An investigation of folic acid requirements in pregnancy. II. British Journal of Hematology 1967;13(4):503‐9. [DOI] [PubMed] [Google Scholar]
- Willoughby ML, Jewell FJ. Investigation of folic acid requirements in pregnancy. British Medical Journal 1966;2(5529):1568‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby MLN, Jewell FG. Folate status throughout pregnancy and in postpartum period. British Medical Journal 1968;4:356‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Bjerre 1967 {published data only}
- Bjerre B. Study of the haematological effect of prophylactic folic acid medicamentation in pregnancy. Acta Obstetricia et Gynecologica Scandinavica 1967;46(4):71‐85. [DOI] [PubMed] [Google Scholar]
Colman 1974 {published data only}
- Colman N, Barker M, Green R, Metz J. Prevention of folate deficiency in pregnancy by food fortification. American Journal Clinical Nutrition 1974;27:339‐44. [DOI] [PubMed] [Google Scholar]
Colman 1975 {published data only}
- Colman N, Larsen JV, Barker M, Barker EA, Green R, Metz J. Prevention of folate deficiency by food fortification. III. Effect in pregnant subjects of varying amounts of added folic acid. American Journal of Clinical Nutrition 1975;28:465‐70. [DOI] [PubMed] [Google Scholar]
Ellison 2004 {published data only}
- Ellison J, Clark P, Walker ID, Greer IA. Effect of supplementation with folic acid throughout pregnancy on plasma homocysteine concentration. Thrombosis Research 2004;114:25‐7. [DOI] [PubMed] [Google Scholar]
Giles 1960 {published data only}
- Giles C, Burton H. Observations on prevention and diagnosis of anaemia in pregnancy. British Medical Journal 1960;2:636‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gregory 2001 {published data only}
- Gregory JF 3rd, Caudill MA, Opalko FJ, Bailey LB. Kinetics of folate turnover in pregnant women (second trimester) and nonpregnant controls during folic acid supplementation: stable‐isotopic labeling of plasma folate, urinary folate and folate catabolites shows subtle effects of pregnancy on turnover of folate pools. Journal of Nutrition 2001;131(7):1928‐37. [DOI] [PubMed] [Google Scholar]
Hague 1998 {published data only}
- Hague B, Crowther C, Robinson J, Dekker G, Odendaal H. Prevention of recurrent pre‐eclampsia by folic acid supplementation in women with hyperhomocysteinaemia: proposal for a randomized trial. Netherlands Journal of Medicine 1998;52:S24. [Google Scholar]
- Hague B, Dekker G, Crowther C, Robinson J, Odendaal H. The HOPE (hyperhomocysteinaemia in pre‐eclampsia) trial: prevention of recurrent pre‐eclampsia by folic acid supplementation in women with hyperhomocysteinemia. Hypertension in Pregnancy 2000;19(Suppl 1):Abstract no P10. [Google Scholar]
Hamilton 1973 {published data only}
- Hamilton PJS, Gebbie DAM. Antenatal clinics as trial units in evaluating treatment of anaemia in pregnancy. The use and abuse of drugs and chemicals in Tropical Africa. Proceedings of the 1973 Annual Scientific Conference of the East African Medical Research Council. Nairobi, Kenya.: Nairobi: East African Literature Bureau, 1973:463‐6.
Hekmatdoost 2011 {published data only}
- Hekmatdoost A. Comparison of the effect of folic acid and 5‐methyltetrahydrofolate (5MTHF) on serum folate and homocysteine levels, and abortion rates in women suffering from recurrent abortion. IRCT Iranian Registry of Clinical Trials (www.irct.ir) (accessed 8 July 2011).
Hibbard 1969 {published data only}
- Hibbard BM, Hibbard ED. The treatment of folate deficiency in pregnancy. Acta Obstetricia et Gynecologica Scandinavica 1969;48:349‐56. [DOI] [PubMed] [Google Scholar]
Khanna 1977 {published data only}
- Khanna S, Dube B, Kumar S. Anaemia of pregnancy in northern India. Nature and therapeutic follow‐up. Tropical & Geographical Medicine 1977;29(1):24‐8. [PubMed] [Google Scholar]
Klinger 2006 {published data only}
- Klingler M, Blaschitz A, Campoy C, Cano A, Molloy AM, Scott JM, et al. The effect of docosahexaenoic avid and folic acid supplementation on placental apoptosis and proliferation. British Journal of Nutrition 2006;96(1):182‐90. [DOI] [PubMed] [Google Scholar]
Kristoffersen 1979 {published data only}
- Kristoffersen K, Rolschau J, Date JV, Honore E. The influence of folic acid supplement on intrauterine growth [abstract]. 9th World Congress of Gynecology and Obstetrics;Tokyo, Japan; 1979 October 26‐31. 1979:242.
Ma 2008 {published data only}
- Ma AG, Schouten EG, Zhang FZ, Kok FJ, Yang F, Jiang DC, et al. Retinol and riboflavin supplementation decreases the prevalence of anaemia in Chinese pregnant women taking iron and folic acid supplements. Journal of Nutrition 2008;138(10):1946‐50. [DOI] [PubMed] [Google Scholar]
Manizheh 2009 {published data only}
- Manizheh SM, Mandana S, Hassan A, Amir GH, Mahlisha KS, Morteza G. Comparison study on the effect of prenatal administration of high dose and low dose folic acid. Saudi Medical Journal 2009;30(1):88‐97. [PubMed] [Google Scholar]
Melli 2008 {published data only}
- Melli MS, Shojaiee M, Sheshvan MK. Prenatal administration of high dose and low dose of folic acid on maternal plasma homocysteine concentration and its relationship to the development of preeclampsia. Journal of Maternal‐Fetal and Neonatal Medicine 2008;21(Suppl 1):82. [Google Scholar]
Ouma 2006 {published data only}
- Ouma P, Parise ME, Hamel MJ, ter Kuile FO, Otieno K, Ayisi JG, et al. A randomised controlled trial of folate supplementation when treating malaria in pregnancy with sulfadoxine‐pyrimethamine. PLoS Clinical Trials 2006;1(6):e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eijk AM, Ouma PO, Williamson J, ter Kuile FO, Parise M, Otieno K, et al. Plasma folate level and high‐dose folate supplementation predict sulfadoxine‐primethamine treatment failure in pregnant women in Western Kenya who have uncomplicated malaria. Journal of Infectious Diseases 2008;198(10):1550‐3. [DOI] [PubMed] [Google Scholar]
Polatti 1992 {published data only}
- Polatti F, Perotti F, Nappi R, Prandi P. Calcium folinate in pregnancy: therapeutic efficacy [Calcio folinato in gravidanza]. Minerva Ginecologica 1992;44:33‐7. [PubMed] [Google Scholar]
Taylor 1979 {published data only}
- Taylor DJ, Lind T. Red cell mass during and after normal delivery. British Journal of Obstetrics and Gynaecology 1979;86:364‐70. [DOI] [PubMed] [Google Scholar]
Taylor 1981 {published data only}
- Taylor DJ, Lind T. Pueperal haematological indices. British Journal of Obstetrics and Gynaecology 1981;88:601‐6. [DOI] [PubMed] [Google Scholar]
Tchernia 1982a {published data only}
- Tchernia G, Blot I, Rey A, Papiernik E. Maternal iron and folate deficiencies. Incidence on the newborn. Annales de Pediatrie 1982;29:276‐80. [PubMed] [Google Scholar]
Thomson 1982 {published data only}
- Thomson ME, Pack ARC. Effects of extended systemic and topical folate supplementation on gingivitis of pregnancy. Journal of Clinical Periodontology 1982;9:275‐80. [DOI] [PubMed] [Google Scholar]
- Thomson ME, Pack ARC. Systemic folic acid (F.A.) supplementation and effects of pregnancy gingivitis. Journal of Dental Research 1981;60:1072. [Google Scholar]
Ulrich 1999 {published data only}
- Ulrich M, Kristoffersen K, Rolschau J, Grinsted P, Schaumburg E, Foged N. The influence of folic acid supplement on the outcome of pregnancies in the county of Funen in Denmark. Part II. Congenital anomalies. A randomised study. European Journal of Obstetrics & Gynecology and Reproductive Biology 1999;87(2):111‐3. [PubMed] [Google Scholar]
Wang 2012 {published data only}
- Wang W, Yan H, Zeng L, Cheng Y, Wang D, Li Q. No effect of maternal micronutrient supplementation on early childhood growth in rural western China: 30 month follow‐up evaluation of a double blind, cluster randomized controlled trial. European Journal of Clinical Nutrition 2012;66(2):261‐8. [DOI] [PubMed] [Google Scholar]
Zeng 2008 {published data only}
- Zeng L, Dibley MJ, Cheng Y, Dang S, Chang S, Kong L, et al. Impact of micronutrient supplementation during pregnancy on birth weight, duration of gestation, and perinatal mortality in rural western China: double blind cluster randomised controlled trial. BMJ 2008;337:a2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L, Yan H, Cheng Y, Dang S, Dibley MJ. Adherence and costs of micronutrient supplementation in pregnancy in a double‐blind, randomized, controlled trial in rural western China. Food and Nutrition Bulletin 2009;30(4):S480‐7. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Wen 2012 {published data only}
- Wen SW. Effect of folic acid supplementation in pregnancy on pre‐eclampsia ‐ folic acid clinical trial (FACT). Current Controlled Trials (http://www.current‐trials.com) (accessed 8 July 2011).
- Wen SW, Champagne J, Rennicks R, Walker M. Effect of folic acid supplementation in pregnancy on preeclampsia ‐ Folic acid clinical trial (FACT). Pregnancy Hypertension 2012;2(3):180. [DOI] [PubMed] [Google Scholar]
- Wen SW, Walker M. Effect of folic acid supplementation in pregnancy on preeclampsia. Pregnancy Hypertension 2010;1(Suppl 1):S28. [DOI] [PubMed] [Google Scholar]
Additional references
Açkurt 1995
- Açkurt F, Wetherilt H, Löker M, Hacibekiro M. Biochemical assessment of nutritional status in pre‐ and post‐natal Turkish women and outcome of pregnancy. European Journal of Clinical Nutrition 1995;49:613‐22. [PubMed] [Google Scholar]
Bailey 1995
- Bailey LB. Folate requirements and dietary recommendations. In: Bailey LB editor(s). Folate in Health and Disease. New York: Marcel Dekker, 1995:123‐52. [Google Scholar]
Bates 1986
- Bates CJ, Fuller NJ, Prentice AM. Folate status during pregnancy and lactation in a West African rural community. Human Nutrition. Clinical Nutrition 1986;40:3‐13. [PubMed] [Google Scholar]
Botto 1996
- Botto LD, Khoury MJ, Mulinare J, Erickson JD. Periconceptional multivitamin use and the occurrence of conotruncal heart defects: results from a population based, case‐control study. Pediatrics 1996;98(5):911‐7. [PubMed] [Google Scholar]
Bruinse 1995
- Bruinse HW, Berg H. Changes of some vitamin levels during and after normal pregnancy. European Journal of Obstetrics & Gynecology and Reproductive Biology 1995;61:31‐7. [DOI] [PubMed] [Google Scholar]
Bukowski 2009
- Bukowski R, Malone FD, Porter FT, Nyberg DA, Comstock CH, Hankins GDV, et al. Preconception folate supplementation and the risk of spontaneous preterm birth: prospective cohort study. PLoS Medicine 2009;6(5):e1000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
Caudill 1998
- Caudill MA, Gregory JF III, Hutson AD, Bailey LB. Folate catabolism in pregnant and nonpregnant women with controlled folate intakes. Journal of Nutrition 1998;128(2):204‐8. [DOI] [PubMed] [Google Scholar]
Chanarin 1969
- Chanarin I. The Megaloblastic Anemias. London, United Kingdom: Blackwell Scientific, 1969:786‐829. [Google Scholar]
Charles 2005b
- Charles DHM, Ness AR, Campbell D, Smith GD, Whitely E, Hall MH. Folic acid supplements in pregnancy and birth outcome: re‐analysis of a large randomised controlled trial and update of Cochrane review. Pediatric and Perinatal Epidemiology 2005;19:112‐24. [DOI] [PubMed] [Google Scholar]
Czeizel 1992
- Czeizel AE, Dudas I. Prevention of the first occurrence of neural‐tube defects by periconceptional vitamin supplementation. New England Journal of Medicine 1992;327(26):1832‐5. [DOI] [PubMed] [Google Scholar]
Czeizel 1993
- Czeizel AE. Prevention of congenital abnormalities by periconceptional multivitamin supplementation. BMJ 1993;306(6893):1645‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Czeizel 1996
- Czeizel AE. Reduction of urinary tract and cardiovascular defects by periconceptional multivitamin supplementation. American Journal of Medical Genetics 1996;62(2):179‐83. [DOI] [PubMed] [Google Scholar]
De Benoist 2008
- Benoist B. Conclusions of a WHO Technical Consultation on folate and vitamin B12 deficiencies. Food Nutrition Bulletin 2008;29(Suppl 2):S238‐S244. [DOI] [PubMed] [Google Scholar]
De‐Regil 2010
- De‐Regil LM, Fernández‐Gaxiola AC, Dowswell T, Peña‐Rosas JP. Effects and safety of periconceptional folate supplementation for preventing birth defects. Cochrane Database of Systematic Reviews 2010, Issue 10. [DOI: 10.1002/14651858.CD007950.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dietary Ref 1998
- Anonymous. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic acid, Biotin and Choline. Institute of Medicine, 1998. [PubMed] [Google Scholar]
Fleming 1972
- Fleming AF. Urinary excretion of folate in pregnancy. Journal of Obstetrics and Gynaecology of the British Commonwealth 1972;79:916‐20. [DOI] [PubMed] [Google Scholar]
Food and Nutrition Board 1970
- Food, Nutrition Board. National Research Council. Maternal Nutrition and the Course of Pregnancy. Washington, DC: National Academy of Sciences, 1970. [Google Scholar]
Food and Nutrition Board 1998
- Food, Nutrition Board. Institute of Medicine. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin and Choline. Washington, DC: National Academy Press, 1998. [PubMed] [Google Scholar]
Goldenberg 1992
- Goldenberg RL, Tamura T, Cliver SP, Cutter GR, Hoffman HJ, Copper RL. Serum folate and fetal growth retardation: a matter of compliance?. Obstetrics and Gynecology 1992;79:719‐22. [PubMed] [Google Scholar]
Green 1995
- Green R, Jacobsen DW. Clinical implications of hyperhomocysteinemia. In: Bailey LB editor(s). Folate in Health and Disease. Newyork (NY): Marcel Dekker, 1995:75‐122. [Google Scholar]
Gregory 2001b
- Gregory JF III, Caudill MA, Opalko J, Bailey LB. Kinetics of folate turnover in pregnant women (second trimester) and nonpregnant controls during folic acid supplementation: stable‐isotopic labelling of plasma folate, urinary folate and folate catabolites shows subtle effects of pregnancy on turnover of folate pools. Journal of Nutrition 2001;131:1928‐37. [DOI] [PubMed] [Google Scholar]
Haider 2006
- Haider BA, Bhutta ZA. Multiple‐micronutrient supplementation for women during pregnancy. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD004905.pub2] [DOI] [PubMed] [Google Scholar]
Herbert 1997
- Herbert V, Bigaoutte J. Call for endorsement of a petition to the Food and Drug Administration to always add vitamin B‐12 to any folate fortification or supplement. American Journal of Clinical Nutrition 1997;65(2):572‐3. [DOI] [PubMed] [Google Scholar]
Higgins 2000
- Higgins JR, Quinlivan EP, McPartlin J, Scott JM, Weir DG, Darling MRN. The relationship between increased folate catabolism and the increased requirement for folate in pregnancy. BJOG: an international journal of obstetrics and gynaecology 2000;107:1149‐54. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Institute of Medicine 2000
- Institute of Medicine. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin and Choline. Washington, DC: National Academy Press, 2000:196‐305. [PubMed] [Google Scholar]
Krishnaswamy 2001
- Krishnaswamy K, Nair KM. Importance of folate in human nutrition. British Journal of Nutrition 2001;85:S115‐S124. [DOI] [PubMed] [Google Scholar]
Landon 1971
- Landon MJ, Hytten FE. The excretion of folate in pregnancy. Journal of Obstetrics and Gynaecology of the British Commonwealth 1971;78:769‐75. [DOI] [PubMed] [Google Scholar]
Lawson 1988
- Lawson JB. Anaemia in pregnancy. In: Lawson JB, Stewart DB editor(s). Obstetrics and Gynaecology in the Tropics and Developing Countries. London: Edward Arnold, 1988:76‐8. [Google Scholar]
Li 1995
- Li DK, Daling JR, Mueller BA, Hickik DE, Fantel AG, Weiss NS. Periconceptional multivitamin use in relation to the risk of congenital urinary tract anomalies. Epidemiology 1995;6(3):212‐8. [DOI] [PubMed] [Google Scholar]
Makedos 2007
- Makedos G, Papanicolaou A, Hitoglou A, Kalogiannidis I, Makedos A, Vrazioti V, et al. Homocysteine, folic acid and B12 serum levels in pregnancy complicated with preeclampsia. Archives of Gynecology and Obstetrics 2007;275(2):121–4. [DOI] [PubMed] [Google Scholar]
McPartlin 1993
- McPartlin J, Halligan A, Scott JM, Darling M, Weir DG. Accelerated folate breakdown in pregnancy. Lancet 1993;341(8838):148‐9. [DOI] [PubMed] [Google Scholar]
Morrison 1998
- Morrison K, Papapetrou C, Hol FA, Mariman EC, Lynch SA, Burn J, et al. Susceptibility to spina bifida; an association study of five candidate genes. Annals of Human Genetics 1998;62(5):379‐96. [DOI] [PubMed] [Google Scholar]
MRC 1991
- MRC Vitamin Study Research Group. Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. Lancet 1991;338(8760):131‐7. [PubMed] [Google Scholar]
Neggers 1997
- Neggers YH, Goldenberg RL, Tamura T, Cliver SP, Hoffman HJ. The relationship between maternal dietary intake and infant birthweight. Acta Obstetricia et Gynecologica Scandinavica 1997;165:71‐5. [PubMed] [Google Scholar]
NICE 2008
- National Institute for Health and Clinical Excellence. NICE public health guidance 11: Improving the nutrition of pregnant and breastfeeding mothers and children in low‐income households. London: NICE, 2011 July. [Google Scholar]
Patrick 2004
- Patrick TE, Powers RW, Daftary AR, Ness RB, Roberts JM. Homocysteine and folic acid are inversely related in black women with preeclampsia. Hypertension 2004;43(6):1279–82. [DOI] [PubMed] [Google Scholar]
Pena‐Rosas 2006
- Pena‐Rosas JP, Viteri FE. Effects of routine oral iron supplementation with or without folic acid for women during pregnancy. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD004736.pub2] [DOI] [PubMed] [Google Scholar]
Refsum 2008
- Refsum H, Smith AD. Are we ready for mandatory fortification withvitamin B‐12?. American Journal of Clinical Nutrition 2008;88(2):253–4. [DOI] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Rush 1994
- Rush D. Periconceptional folate and neural tube defect. American Journal of Clinical Nutrition 1994;59(Suppl 1):511S‐515S. [DOI] [PubMed] [Google Scholar]
Scholl 2000
- Scholl TO, Johnson WG. Folic acid: influence on the outcome of pregnancy. American Journal of Clinical Nutrition 2000;71:1295‐303. [DOI] [PubMed] [Google Scholar]
Selhub 1993
- Selhub J, Jacques PF, Wilson PWF, Rush D, Rosenberg IH. Vitamin status and intake as primary determinants of homocysteinemia in an elderly population. JAMA 1993;270:2693‐8. [DOI] [PubMed] [Google Scholar]
Shaw 1995
- Shaw GM, Lammer EJ, Wasserman CR, O'Malley CD, Tolarova MM. Risks of orofacial clefts in children born to women using multivitamins containing folic acid periconceptionally. Lancet 1995;346(8972):393‐6. [DOI] [PubMed] [Google Scholar]
Smith 1983
- Smith AM, Picciano MF, Deering RH. Folate supplementation during lactation: maternal folate status, human milk folate content, and their relationship to infant folate status. Journal of Pediatric Gastroenterology and Nutrition 1983;2:622‐8. [PubMed] [Google Scholar]
Smits 2001
- Smits LJM, Essed GGM. Short interpregnancy intervals and unfavourable pregnancy outcome: role of folate depletion. Lancet 2001;358:2074‐7. [DOI] [PubMed] [Google Scholar]
Tamura 1992
- Tamura T, Goldenberg RL, Freeberg LE, Cliver SP, Cutter GR, Hoffman HJ. Maternal serum folate and zinc concentrations and their relationships to pregnancy outcome. American Journal of Clinical Nutrition 1992;56:365‐70. [DOI] [PubMed] [Google Scholar]
Tamura 2006
- Tamura T, Picciano MF. Folate and human reproduction. American Journal of Clinical Nutrition 2006;83(5):93‐1016. [DOI] [PubMed] [Google Scholar]
Willoughby 1968
- Willoughby MLN. Folate status throughout pregnancy and in post partum period. British Medical Journal 1968;4:356‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Mahomed 1995
- Mahomed K. Routine folate supplementation in pregnancy. [revised 28 April 1993]. In: Enkin MW, Keirse MJNC, Renfrew MJ, Neilson JP, Crowther C (eds.) Pregnancy and Childbirth Module. In: The Cochrane Pregnancy and Childbirth Database [database on disk and CDROM]. The Cochrane Collaboration; Issue 2, Oxford: Update Software; 1995.
Mahomed 1997
- Mahomed K. Folate supplementation in pregnancy. Cochrane Database of Systematic Reviews 1996, Issue 3. [DOI: 10.1002/14651858.CD000183.pub2] [DOI] [PubMed] [Google Scholar]


