Abstract
Background
Aspergillus fumigatus is an opportunistic fungal pathogen, which is commonly found in lungs and rarely causes infections in mediastinum. Mediastinal Aspergillus abscess is a serious infectious condition, and is characterized by difficult diagnosis due to its clinical manifestations being nonspecific.
Case Presentation
Here, we report a case of a mediastinal Aspergillus fumigatus abscess in an immunocompetent patient. The patient was a 45-year-old woman who presented with a 20-day history of sore throat without any underlying diseases. Chest computed tomography (CT) showed a mass in the anterior superior mediastinum. Metagenomic next-generation sequencing (mNGS) identified Aspergillus fumigatus sequences in endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) tissue, indicating the mediastinal Aspergillus fumigatus infection of this patient. The following mediastinal biopsy histological analysis and tissue fungi culture also suggested Aspergillus fumigatus infection, confirming the mNGS detection. The patient was diagnosed with mediastinal aspergillosis caused by Aspergillus fumigatus. After timely voriconazole treatment, the patient was discharged with good condition.
Conclusion
Our study presented a rare case with mediastinal Aspergillus fumigatus abscess in an immunocompetent patient. As a new clinical diagnostic method, mNGS could assist timely diagnosis and precise treatment of Aspergillus infection.
Keywords: mediastinal abscess, Aspergillus fumigatus, metagenomic next-generation sequencing, diagnosis, case report
Introduction
Invasive aspergillosis is a life-threatening infectious disease associated with high mortality in immunocompromised hosts;1 Aspergillus fumigatus is an opportunistic fungal pathogen, and Aspergillus fumigatus infection usually occurs in the lungs. However, mediastinal Aspergillus fumigatus infection is rare, especially in immunocompetent hosts.2 The gold standard for diagnosing invasive aspergillosis is histopathological evidence or a positive culture result;3 however, these methods are time consuming, and culture tests exhibit low sensitivity for Aspergillus.4 In addition, the clinical presentation of patients with Aspergillus infection is variable and non-specific. Aspergillus fumigatus infection is a difficult diagnosis to establish and prove. At present, diagnostic tools using metagenomic next-generation sequencing (mNGS) have become the focus of clinical study of aspergillosis.
mNGS can theoretically detect any nucleic acids from pathogenic microorganisms, and has been widely applied in clinical infectious disease pathogen diagnosis.5 It has been reported that the use of mNGS improves the diagnosis of invasive fungal infection.6 Moreover, mNGS provides relatively high precise and fast detection of pathogens, enabling the prompt and accurate treatment of infection disease. Here, through mNGS and endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) biopsy co-diagnosis, we report a case of mediastinal Aspergillus fumigatus abscess in an immunocompetent patient.
Case Presentation
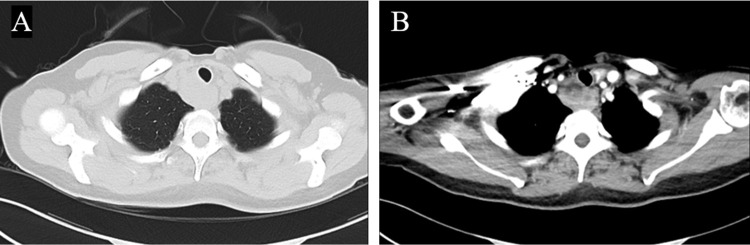
A 45-year-old female patient was admitted to the First Affiliated Hospital of Guangxi Medical University for a sore throat lasting for 20 days on September 8, 2022. She was a teacher and had no underlying diseases. Computed tomography (CT) examination showed a space-occupying lesion. The primary lesion had not yet been determined, and the patient was admitted to our hospital. The contrast-enhanced chest CT examination showed mixed nodules in the anterior segment of the right upper lobe, inflammation of posterior segment, and middle of the right upper lobe and inferior lingual segment of left upper lobe, and mediastinal space-occupying lesions and multiple lymphadenopathy (Figure 1). The mediastinal space-occupying lesion was suspected to be a tumor, so ultrasound-guided mediastinal mass puncture was performed in the daytime operating room of our hospital.
Figure 1.
Contrast-enhanced chest CT of the patient on September 1, 2022. Space-occupying lesion of anterior upper mediastinum is shown. A low-density necrosis area can be seen on enhanced scan, which is separated from the surrounding esophagus. (A) Lung view. (B) Mediastinal view.
On admission, the patient’s laboratory results were as follows: hemoglobin (HGB) was 106.60g/L, the CD4+ lymphocyte count was 549/µL, the serum high sensitivity C-reactive protein levels were 2.65mg/L (normal 0–1mg/L), serum albumin was 37.6g/L (normal 40–55g/L), glycoprotein antigen 199 54.0U/mL (normal 0–37U/mL), serum glycosylated HGB was normal, and β-(1,3)-D-glucan (BDG) and galactomannan (GM) were negative. Liver and renal functions were normal. The human immunodeficiency virus antibody test was negative. The patient’s mediastinal lesion was located near the main trachea, and infection was not ruled out, and EBUS-TBNA was performed. EBUS-TBNA showed an extratracheal pressure stenosis and unspecified echo mass at the 4–6 o’clock position in the 5cm trachea under the glottis (Figure 2). The patient’s mediastinal mass tissue was sent for mNGS (KingMed Diagnostics, Nanning, China). DNA extraction was performed with the JianShi universal DNA/RNA extraction kit, and subsequently an Illumina Next 550 sequencer was used for a single-end 76-base pair sequencing strategy. A DNA library was constructed, and subsequently Burrows-Wheeler Aligner (BWA) software and the NCBI database were used for analysis. Within 24 h after receipt of the sample, the mNGS results reported Aspergillus fumigatus infection. The mNGS detected 301 sequences that could be mapped to Aspergillus fumigatus in a total of 28,759,120 sequences, and the coverage was 0.12%, making up 97.80% of the total microbe sequences (Figure 3). Tissue from EBUS-TBNA mass puncture tissue was sent for pathological examination, and 5 days after receipt of the sample, the histopathological results showed large number of neutrophil cell infiltrations and Aspergillus hyphae (Figure 4), and periodic acid-Schiff (PAS) staining of specimens was positive. Microscopic examination of EBUS-TBNA mass tissue cytological smear showed no cancer cells. Fourteen days after receipt of the sample, the EBUS-TBNA tissue fungi culture (the culture media medium was CHROMagar medium) suggested growth of Aspergillus fumigatus, and bacterial culture showed no bacteria growth. No fungi sequences were found in mNGS of the bronchoalveolar lavage (BAL) fluid. BAL-BDG and -GM were negative. Subsequently, the histopathology of ultrasound-guided mediastinal mass puncture tissue results also showed inflammatory cell infiltration and Aspergillus hyphae (Figure 4). PAS staining of specimens was positive, and the mass was considered aspergillosis with suppuration. Considering that the patient had eating disorder, gastroscopy was performed. An esophageal mass with stenosis was found, and gastroscopic biopsy of the esophageal submucosal mass tissue revealed esophageal aspergillosis. The patient was diagnosed as having mediastinal abscess with Aspergillus fumigatus infection and treated with voriconazole (0.2 g, IV infusion, q.d.) beginning September 13. Her symptoms improved, and she was discharged on September 18 on continued oral voriconazole (0.2 g, twice a day) antifungal therapy. One month after discharge, the patient was followed up by telephone. The patient was generally in good condition, and the liver and kidney functions and blood routine tests were normal. The patient is still being followed-up.
Figure 2.
EBUS-TBNA showed (A) extratracheal pressure stenosis and (B) an unspecified echo mass at the 4–6 o’clock position in the 5cm trachea under the glottis.
Figure 3.
EBUS-TBNA tissue mNGS results in the patient. (A) The mNGS results showed that the coverage of Aspergillus fumigatus was 0.12%. (B) 97.8% of the microbial DNA sequences were Aspergillus fumigatus.
Figure 4.
The histopathology of the mediastinal specimen obtained from the mediastinal mass. Hematoxylin & eosin (H&E) staining showed many neutrophil cell infiltration and Aspergillus hyphae, along with the hyphae with regular septation and branching at a acute angle (black arrows). (A) H&E staining of ultrasound-guided mediastinal mass puncture tissue; (B) H&E staining of EBUS-TBNA tissue; (C and D) Histiocyte smear of EBUS-TBNA tissue.
Discussion and Conclusions
Aspergillus fumigatus species are ubiquitous in nature, and they can colonize the human upper respiratory tract. Human infection occurs mostly through inhalation. Invasive aspergillosis is associated with high mortality in immunocompromised individuals.7 Mediastinum involvement with Aspergillus species is rare, especially in immunocompetent hosts. We systematically reviewed the literatures in the PubMed database using the term “mediastinal Aspergillus”. A total of 17 articles, including 19 case reports with mediastinal Aspergillus abscess were identified, which shows that the disease is rare (Table 1). Of the 19 patients with mediastinal Aspergillus infection, 10 were male and 7 were female; age ranged from 11 to 76 years; 52.6% (10 out of 19 cases) occurred after mediastinal surgery; of the 19 patients, 9 had immunosuppressed status; the majority of patients (14 of 19) had underlying diseases. The radiological features in the majority of cases were mediastinal mass; voriconazole treatment was the main targeted antimicrobial therapy. The prognosis was good in most cases; two patients died. Five patients were diagnosed by culture, eight by biopsy histological analysis, and six by culture and biopsy histological analysis. Our patient is the first case to report of a mediastinal Aspergillus fumigatus abscess detected by mNGS, and confirmed by biopsy histological analysis and tissue fungi culture.
Table 1.
Summary of Cases with Mediastinal Aspergillus Abscess
| Publication Year | Gender | Age | Immunosuppressed Status | Underlying Diseases | Radiological Features | Diagnostic Test | Targeted Antimicrobial Therapy | Prognosis |
|---|---|---|---|---|---|---|---|---|
| 200022 | NR | 11 | No | Klinefelter’s syndrome | A left cervical and mediastinal mass | Biopsy and culture | Itraconazole | Recovered |
| 200423 | M | 61 | Tacrolimus, mycophenolate mofetil, and prednisone. | Chronic obstructive pulmonary disease and heart transplantation | Haziness of the mediastinal fat planes | Culture | Voriconazole | Recovered |
| 200524 | F | 51 | Cyclosporin, prednisone, and mycophenolate mofetil | Heart transplantation | Sternal osteomyelitis and a latero-aortic abscess | Culture | Amphotericin B and caspofungin | Recovered |
| 200525 | F | 30 | Tacrolimus, prednisone, and azathioprine | Lung transplantation | A subcarinal mediastinal mass | Biopsy | Voriconazole | Recovered |
| 200826 | F | 57 | Chemotherapy | Acute myelomonocytic leukemia | Hypodense lesions suggestive of mediastinal abscesses | Biopsy | Caspofungin and amphotericin B | Recovered |
| 201227 | F | 47 | No | No | A large right-sided mediastinal mass encasing her superior vena cava | Biopsy | No antifungal | Died |
| 201528 | M | 68 | Yes | Orthotopic heart transplantation | A large paratracheal mass compressing the trachea | Biopsy and culture | Voriconazole, micafungin, and amphotericin B | Recovered |
| 201528 | M | 52 | Yes | Acquired immunodeficiency syndrome | Wall thickening and stenosis of the right mainstem and bronchus intermedius | Biopsy | Voriconazole and micafungin | Recovered |
| 201528 | M | 63 | Yes | Chronic lymphoid leukemia | Soft tissue infiltration with narrowing of the subglottic trachea | Culture | Voriconazole, micafungin, and amphotericin B | Recovered |
| 201529 | F | 55 | Yes | Heart transplantation, cardiomyopathy hypertension, dyslipidemia, and coronary artery disease | NR | Biopsy | Posaconazole and voriconazole | Recovered |
| 201530 | NR | 11 | No | No | A mass in the anterior mediastinum | Cultures | Voriconazole | Recovered |
| 201631 | F | 42 | No | Open-heart surgeries for mitral and aortic valve replacements | NR | Biopsy | Amphotericin B, voriconazole, and caspofungin | Recovered |
| 201732 | M | 70 | No | Non-ischaemic dilated cardiomyopathy | A mass centred within the junction of the superior vena cava and right atrium | Cultures | Voriconazole and caspofungin | Recovered |
| 201733 | F | 18 | No | No | A conglomerate undefined mass | Biopsy and culture | Itraconazole | Recovered |
| 201734 | M | 29 | No | No | Homogeneous poorly enhancing soft tissue indenting left atrium | Biopsy | Voriconazole | Died |
| 201835 | M | 28 | No | No | A mass centred in the anterior and middle mediastinum, more on the right side. | Biopsy and culture | Voriconazole | Recovered |
| 201936 | M | 50 | Tacrolimus and prednisone | Heart transplant surgery | A mediastinum fat infiltration | Biopsy | Voriconazole | Recovered |
| 202137 | M | 74 | No | Hypertension | Sternal disruption, and mediastinal fluid collection | Biopsy | Voriconazole | Recovered |
| 202238 | M | 76 | No | Stanford type A aortic dissection | Fluid collection surrounding the prosthetic ascending aorta | Culture | Antifungal therapy | Recovered |
| Present | F | 45 | No | No | A mass in anterior superior mediastinum | mNGS, biopsy, and culture | Voriconazole | Recovered |
Abbreviations: M, Male; F, Female; NR, No report.
Histopathological examination or a positive culture result of a biopsy is the gold standard to prove the diagnosis of invasive aspergillosis. However, histopathological examination is time consuming, and culture sensitivity is low. The BDG test has been validated for the diagnosis of invasive fungal disease. Detection of GM is widely used for the diagnosis of Aspergillus fumigatus infection. According to one report, the sensitivity rate of GM serum samples identifying aspergillosis was 65.71%, and the specificity rate was 78.12%.8 BAL-GM is a sensitive and specific test for the diagnosis of invasive aspergillosis.9 The efficiency of BAL-GM for diagnosing aspergillosis was relatively high in one study, with 60–100% sensitivity and 68–100% specificity.10 However, GM is not specific for Aspergillus and is also affected by anti-infective drug treatment. Aspergillus PCR improves the diagnostic accuracy, but could lead to false-negative and false-positive results.11,12 The most important disadvantage of Aspergillus PCR is lack of standardization and poor performance in non-neutropenic patients.13 Aspergillus PCR has low sensitivity and specificity in patients treated with antifungal therapy.14 mNGS may circumvent the disadvantages of culture and the GM test because mNGS is unbiased test and detect any nucleic acids from pathogenic microorganisms. It has been reported that mNGS has a sensitivity rate of 95.59% and a specificity rate of 84.6% for the diagnosis of pulmonary invasive fungal disease.15 Therefore, mNGS is particularly useful in the diagnosis of Aspergillus infection.
mNGS can comprehensively analyze pathogens, enabling universal pathogen detection of bacteria, viruses, fungi, and parasites in a single run; this has great advantages in diagnosis of mixed fungal infections. In addition, mNGS greatly reduces time to the diagnosis; the traditional cultivation method takes at least 2–5 days, or even longer for fungi. Furthermore, the results of mNGS are less affected by prior antibiotic exposure.16 In our case, the serum and BAL-GM of the patient were negative, Aspergillus fumigatus was detected by EBUS-TBNA tissue mNGS, and subsequently validated by histopathological examination and tissue fungi culture. This indicates that mNGS can provide the precise pathogenic diagnosis. Invasive fungal infection is associated with high mortality, and early diagnosis and precise antifungal strategy are crucial to improving clinical outcomes. The mNGS results in our patients were obtained within 24 hours, while the culture took 14 days and the histopathological results took 5 days. Our patient was initially suspected to have a tumor, but the results of mNGS showed Aspergillus fumigatus infection. The patient was treated with antifungals immediately, and her symptoms quickly resolved. Therefore, mNGS can shorten turnround time for pathogenic diagnosis and help select the precise treatment.
Tissue samples obtained through invasive operations procedures, such as transbronchial lung biopsy (TBLB) and EBUS-TBNA, are recommended for analysis by mNGS.17 EBUS-TBNA is conducted under ultrasound guidance and can directly obtain pathological tissues, therefore enhancing the positivity rate of mNGS analysis in pathogen detection. It has been reported that the specificity of mNGS for pathogen diagnosis is higher in TBLB tissue samples than in BAL.18 However, histopathological examination or tissue culture of tissue samples have their own limitations. mNGS for tissue samples can improve the sensitivity of pathogen diagnosis, enhancing the diagnosis rate of pathogens and enabling early initiation of targeted treatment.
mNGS has advantages in the diagnosis of Aspergillus infection, but there are many challenges for its routine use in the clinic. Aspergillus is widely distributed and it is difficult to differentiate colonization from infection.17 Although a tissue sample is considered aseptic, there may be contaminants from surgery or laboratory reagents. Therefore, tissue sample collection and laboratory processing require strict aseptic procedures as well as nucleic acid-free standard operating procedures and appropriate controls. Also, Aspergillus has a large genome and a thick cell wall, so efficiency of nucleic acid extraction is low. Even after its wall is broken, the number of sequences detected is still less than that of other microorganisms. Another challenge is that there are currently no standards for the basic performance and parameters of mNGS. Therefore, the results of mNGS should be considered together within the clinical context, including underlying disease, medication history, radiographic evidence, BDG, GM, culture, and immune status.
This report describes a case of a mediastinal abscess caused by Aspergillus fumigatus in an immunocompetent patient. Previous studies have shown that Aspergillus fumigatus, which is mostly contracted through inhalation, can cause life-threatening lung disease in immunocompromised individuals.19,20 In the clinic, the outcome of inhaled Aspergillus infection depends on the balance between host and Aspergillus. In the immunocompromised host, it often leads to invasive aspergillosis, while in the immunocompetent host, host immune cells, such as macrophages and neutrophils, can efficiently clear Aspergillus spores and prevent infection.20 Invasive aspergillosis is seen in patients with risk factors, such as receipt of a solid organ transplant, recent history of neutropenia, and hematologic malignancy.21 In our case, the absence of past medical history and leukocytopenia did not lead to suspicion of a fungal infection. Eventually, the biopsy specimen from the EBUS-TBNA showed abundant fungal hyphae, leading to a conclusive diagnosis of invasive mediastinal aspergillosis.
The mediastinum is closed and is not susceptible to infection by foreign pathogens. Aspergillus fumigatus infection mainly occurs in lungs of immunocompromised hosts. In our case, the patient was an immunocompetent host. Aspergillus fumigatus becomes colonized in the human upper respiratory tract, mediastinal aspergillosis may result from a primary Aspergillus airway infection and spread to the adjacent mediastinal structure. The imaging evidence showed that the lesion location was adjacent to the trachea. Aspergillus easily invades blood vessels and may cause mediastinal abscess through blood-borne infection. This patient also had esophageal aspergillosis, and Aspergillus may invade the mediastinum through the esophagus. Therefore, gastroscopy should be performed for patients with mediastinal fungal infection to determine whether they have esophageal aspergillosis.
In conclusion, our study presents a rare case with mediastinal Aspergillus fumigatus abscess in an immunocompetent patient detected by mNGS, and the patient recovered after antifungal treatment. mNGS, as a new clinical diagnostic method, could assist in the timely diagnosis of Aspergillus infection and in identifying its precise treatment.
Funding Statement
This work was supported by National Natural Science Foundation of China (No. 82260023, 82104499, 82160783, and 81760743); the Natural Science Foundation of Guangxi Province (No. 2022GXNSFAA035646); the Key Research Program of Guangxi Science and Technology Department (No. AB21196010); the Health and Family Planning Commission of Guangxi Zhuang Autonomous Region, self-funded projects (No. Z20200825); Guangxi Health Commission Key Lab of Fungi and Mycosis Research and Prevention (No. ZZH2020004); The First Affiliated Hospital of Guangxi Medical University Provincial and Ministerial Key Laboratory Cultivation Project: Guangxi Key Laboratory of Tropical Fungi and Mycosis Research (No. YYZS2020006); Guangxi Medical and Health Suitable Technology Development and Popularization Application Project (No. S2019090); the First Affiliated Hospital of Guangxi Medical University Clinical Research Climbing Program Youth Science and Technology Morning Star Program (No. 201903032); Advanced Innovation Teams and Xinghu Scholars Program of Guangxi Medical University.
Data Sharing Statement
The data are available from the corresponding author on reasonable request.
Ethics Approval and Consent for Publication
This study was approved by the Medical Ethics Committee of the First Affiliated Hospital of Guangxi Medical University (NO. 2022-E367-01). Written informed consent was provided by the patient for the publication of the case details and images. Details of the case can be published without institutional approval.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Wusheng Deng, Yun Jiang, Jiaoxia Qin and Gang Chen are co-first authors for this study. The authors report no conflicts of interest in this work.
References
- 1.Lian XH, Scott-Thomas A, Lewis JG, et al. Monoclonal antibodies and invasive Aspergillosis: diagnostic and therapeutic perspectives. Int J Mol Sci. 2022;23(10):5563. doi: 10.3390/ijms23105563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borges A, Ferreira L, Pacheco R, Fonseca I. Invasive aspergillosis of the skull base in an immunocompetent patient: a diagnostic challenge. BMJ Case Rep. 2021;14(11):e245517. doi: 10.1136/bcr-2021-245517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Danion F, Rouzaud C, Duréault A, et al. Why are so many cases of invasive aspergillosis missed? Med Mycol. 2019;57(Supplement_2):S94–S103. doi: 10.1093/mmy/myy081 [DOI] [PubMed] [Google Scholar]
- 4.Son HJ, Song JS, Choi S, et al. A comparison of histomorphologic diagnosis with culture- and immunohistochemistry-based diagnosis of invasive aspergillosis and mucormycosis. Infect Dis. 2020;52(4):279–283. doi: 10.1080/23744235.2020.1716063 [DOI] [PubMed] [Google Scholar]
- 5.Chen Z, Cheng H, Cai Z, et al. Identification of microbiome etiology associated with drug resistance in pleural empyema. Front Cell Infect Microbiol. 2021;11:637018. doi: 10.3389/fcimb.2021.637018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang L, Song J, Wang Y, Feng J. Metagenomic next-generation sequencing for pulmonary fungal infection diagnosis: lung biopsy versus bronchoalveolar lavage fluid. Infect Drug Resist. 2021;14:4333–4359. doi: 10.2147/IDR.S333818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nguyen PT, Wacker T, Brown AJP, Dantas AD, Shekhova E. Understanding the role of nitronate monooxygenases in virulence of the human fungal pathogen Aspergillus fumigatus. J Fungi. 2022;8(7):736. doi: 10.3390/jof8070736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang C, You Z, Fu J, et al. Application of metagenomic next-generation sequencing in the diagnosis of pulmonary invasive fungal disease. Front Cell Infect Microbiol. 2022;12:949505. doi: 10.3389/fcimb.2022.949505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo YL, Chen YQ, Wang K, Qin SM, Wu C, Kong JL. Accuracy of BAL galactomannan in diagnosing invasive aspergillosis: a bivariate metaanalysis and systematic review. Chest. 2010;138(4):817–824. doi: 10.1378/chest.10-0488 [DOI] [PubMed] [Google Scholar]
- 10.Miceli MH, Kauffman CA. Aspergillus galactomannan for diagnosing invasive Aspergillosis. J Am Med Assoc. 2017;318(12):1175–1176. doi: 10.1001/jama.2017.10661 [DOI] [PubMed] [Google Scholar]
- 11.Morton CO, White PL, Barnes RA, et al. Determining the analytical specificity of PCR-based assays for the diagnosis of IA: what is Aspergillus? Med Mycol. 2017;55(4):402–413. doi: 10.1093/mmy/myw093 [DOI] [PubMed] [Google Scholar]
- 12.Bretagne S. Primary diagnostic approaches of invasive aspergillosis--molecular testing. Med Mycol. 2011;49(Suppl S1):S48–S53. doi: 10.3109/13693786.2010.508186 [DOI] [PubMed] [Google Scholar]
- 13.Egger M, Jenks JD, Hoenigl M, Prattes J. Blood Aspergillus PCR: the good, the bad, and the ugly. J Fungi. 2020;6(1):1. doi: 10.3390/jof6010018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Springer J, Lackner M, Nachbaur D, et al. Prospective multicentre PCR-based Aspergillus DNA screening in high-risk patients with and without primary antifungal mould prophylaxis. Clin Microbiol Infect. 2016;22(1):80–86. doi: 10.1016/j.cmi.2015.09.009 [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Song Z, Wang E, et al. Potential antifungal targets based on histones post-translational modifications against invasive aspergillosis. Front Microbiol. 2022;13:980615. doi: 10.3389/fmicb.2022.980615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miao Q, Ma Y, Wang Q, et al. Microbiological diagnostic performance of metagenomic next-generation sequencing when applied to clinical practice. Clin Infect Dis. 2018;67(suppl_2):S231–S240. doi: 10.1093/cid/ciy693 [DOI] [PubMed] [Google Scholar]
- 17.Li N, Cai Q, Miao Q, Song Z, Fang Y, Hu B. High-throughput metagenomics for identification of pathogens in the clinical settings. Small Methods. 2021;5(1):2000792. doi: 10.1002/smtd.202000792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu N, Kan J, Cao W, et al. Metagenomic next-generation sequencing diagnosis of peripheral pulmonary infectious lesions through virtual navigation, radial EBUS, ultrathin bronchoscopy, and ROSE. J Int Med Res. 2019;47(10):4878–4885. doi: 10.1177/0300060519866953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Latgé JP, Chamilos G. Aspergillus fumigatus and Aspergillosis in 2019. Clin Microbiol Rev. 2019;33(1). doi: 10.1128/CMR.00140-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van de Veerdonk FL, Gresnigt MS, Romani L, Netea MG, Latgé JP. Aspergillus fumigatus morphology and dynamic host interactions. Nat Rev Microbiol. 2017;15(11):661–674. doi: 10.1038/nrmicro.2017.90 [DOI] [PubMed] [Google Scholar]
- 21.Donnelly JP, Chen SC, Kauffman CA, et al. Revision and update of the consensus definitions of invasive fungal disease from the European Organization For Research And Treatment Of Cancer and the Mycoses Study Group education and research consortium. Clin Infect Dis. 2020;71(6):1367–1376. doi: 10.1093/cid/ciz1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chalumeau M, Adamsbaum C, Raymond J, Iniguez JL, Gendrel D. Mediastinal aspergilloma ten years after thoracic surgery. Pediatr Infect Dis J. 2000;19(7):662–664. doi: 10.1097/00006454-200007000-00020 [DOI] [PubMed] [Google Scholar]
- 23.Levin T, Suh B, Beltramo D, Samuel R. Aspergillus mediastinitis following orthotopic heart transplantation: case report and review of the literature. Transpl Infect Dis. 2004;6(3):129–131. doi: 10.1111/j.1399-3062.2004.00064.x [DOI] [PubMed] [Google Scholar]
- 24.Forestier E, Remy V, Lesens O, et al. A case of Aspergillus mediastinitis after heart transplantation successfully treated with liposomal amphotericin B, caspofungin and voriconazole. Eur J Clin Microbiol Infect Dis. 2005;24(5):347–349. doi: 10.1007/s10096-005-1327-5 [DOI] [PubMed] [Google Scholar]
- 25.Shlobin OA, Dropulic LK, Orens JB, et al. Mediastinal mass due to Aspergillus fumigatus after lung transplantation: a case report. J Heart Lung Transplant. 2005;24(11):1991–1994. doi: 10.1016/j.healun.2005.02.020 [DOI] [PubMed] [Google Scholar]
- 26.Sendino O, Pellisé M, Ghita G, Solé M, Rimola J, Ginès A. Aspergillus mediastinitis diagnosed by EUS-guided FNA. Gastrointest Endosc. 2008;67(1):153;commentary 154. doi: 10.1016/j.gie.2007.07.029 [DOI] [PubMed] [Google Scholar]
- 27.Shakoor MT, Ayub S, Ayub Z, Mahmood F. Fulminant invasive aspergillosis of the mediastinum in an immunocompetent host: a case report. J Med Case Rep. 2012;6(1):311. doi: 10.1186/1752-1947-6-311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Argento AC, Wolfe CR, Wahidi MM, Shofer SL, Mahmood K. Bronchomediastinal fistula caused by endobronchial aspergilloma. Ann Am Thorac Soc. 2015;12(1):91–95. doi: 10.1513/AnnalsATS.201406-247BC [DOI] [PubMed] [Google Scholar]
- 29.El-Sayed Ahmed MM, Almanfi A, Aftab M, Singh SK, Mallidi HR, Frazier OH. Aspergillus mediastinitis after orthotopic heart transplantation: a case report. Tex Heart Inst J. 2015;42(5):468–470. doi: 10.14503/THIJ-14-4732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agarwal S, Das A, Gochhait D, Trehan A, Srinivasan R, Singh M. Aspergillus mediastinitis presenting as superior mediastinal syndrome in an immunocompetent child. Pediatr Infect Dis J. 2015;34(2):221–222. doi: 10.1097/INF.0000000000000518 [DOI] [PubMed] [Google Scholar]
- 31.Caballero MJ, Mongardon N, Haouache H, et al. Aspergillus mediastinitis after cardiac surgery. Int J Infect Dis. 2016;44:16–19. doi: 10.1016/j.ijid.2016.01.014 [DOI] [PubMed] [Google Scholar]
- 32.Farid S, AbuSaleh O, Aburjania N, Sohail MR. Postsurgical mediastinal aspergilloma masquerading as malignancy. BMJ Case Rep. 2017;2017:bcr–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mustafa SF, Khan HA, Fatimi SH. Extensive mediastinal aspergillosis presenting with dyspnoea and cardiac tamponade symptoms. Hong Kong Med J. 2017;23(2):202–203. doi: 10.12809/hkmj154790 [DOI] [PubMed] [Google Scholar]
- 34.Kartik M, Kanala A, Sunilnadikuda RSM, Prakasham PS. Invasive mediastinal Aspergillosis in immunocompetent male with invasion of left atrium and hilar structures. Indian J Crit Care Med. 2017;21(6):408–411. doi: 10.4103/ijccm.IJCCM_18_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rahim Y, Memon A, Khan JA. Invasive mediastinal aspergillosis presenting as superior vena cava syndrome in an immunocompetent patient. BMJ Case Rep. 2018;2018. doi: 10.1136/bcr-2018-225614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caron J, Conti M, Pietro R, et al. Atypical presentation of Aspergillus mediastinitis infection in a heart transplant patient: the importance of combined medical and surgical treatment. Exp Clin Transplant. 2019;17(5):695–698. doi: 10.6002/ect.2018.0185 [DOI] [PubMed] [Google Scholar]
- 37.Monteiro OMC, Higa Júnior MG, Palhares MA, et al. Case of Aspergillus mediastinitis after coronary artery bypass surgery: a case report and literature review. Am J Case Rep. 2021;22:e933193. doi: 10.12659/AJCR.933193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giammarino AT, Sarmiento IC, Scheinerman S, et al. Robotic-assisted closed-chest management of a fungal-infected prosthetic aortic graft: a case report. J Med Case Rep. 2022;16(1):186. doi: 10.1186/s13256-022-03380-0 [DOI] [PMC free article] [PubMed] [Google Scholar]