Abstract
Purpose
The Renal cell cancer: Lifestyle, prognosis and quality of life (ReLife) study is set up to obtain insight into the association of patient and tumour characteristics, lifestyle habits and circulating biomarkers with body composition features in patients with localised renal cell cancer (RCC). Further, it aims to assess the association of body composition features, lifestyle habits and circulating biomarkers with clinical outcomes, including health-related quality of life.
Participants
The ReLife study is a multicentre prospective cohort study involving 368 patients with newly diagnosed stages I–III RCC recruited from January 2018 to June 2021 from 18 hospitals in the Netherlands. At 3 months, 1 year and 2 years after treatment, participants fill out a general questionnaire and questionnaires about their lifestyle habits (eg, diet, physical activity, smoking and alcohol consumption), medical history and health-related quality of life. At all three time points, patients wear an accelerometer and have blood samples taken. CT scans for body composition analysis are being collected. Permission is asked for collection of tumour samples. Information about disease characteristics, treatment of the primary tumour and clinical outcomes is being collected from medical records by the Netherlands Cancer Registry.
Findings to date
A total of 836 invited patients were eligible and 368 patients were willing to participate and were included (response rate 44%). The mean age of patients was 62.5±9.0 years and 70% was male. The majority had stage I (65%) disease and were treated with radical nephrectomy (57%). Data collection at 3 months and 1 years after treatment have been finalised.
Future plans
Data collection at 2 years after treatment is expected to be finalised in June 2023 and longitudinal clinical data will continue to be collected. Results of studies based on this cohort are important to develop personalised evidence-based lifestyle advice for patients with localised RCC to enable them to get more control over their disease course.
Keywords: NUTRITION & DIETETICS, Kidney tumours, Epidemiology, PREVENTIVE MEDICINE
STRENGTHS AND LIMITATIONS OF THIS STUDY.
The Renal cell cancer: Lifestyle, prognosis, and quality of life study is the first population-based prospective cohort study on lifestyle-related factors and clinical outcomes in patients with localised RCC worldwide.
Comprehensive data on lifestyle-related factors and quality of life are collected at 3 months, 1 year and 2 years after treatment.
Both self-reported and objective data on body composition and physical activity are collected.
A limitation is that power for survival analyses is likely to be insufficient and future pooling with other studies may be required.
Introduction
Incidence rates of kidney cancer are increasing,1 which is partly explained by the increased use of diagnostic imaging but also by the increased prevalence of obesity.2 The worldwide number of new kidney cancer cases was estimated to be over 430 000 in 2020.3 In the Netherlands, over 2700 new cases with kidney cancer were diagnosed in 2019.4 More than 90% of kidney cancers are renal cell cancers (RCC).5 Of all patients with RCC, about 70%–80% are diagnosed with localised disease (stages I–III) and about 20%–30% with advanced or metastatic disease (stage IV).2 Almost all patients with RCC with localised disease are treated with partial or radical nephrectomy.6 Despite this treatment, 20%–30% of patients with localised disease will have a relapse or develop metastatic RCC during follow-up.7 Five-year relative survival rates are approximately 90% (stage I and II), 65% (stage III) and 12% (stage IV).2
Classical prognostic factors for localised RCC include anatomical (eg, tumour, node, metastases (TNM) classification), histological (eg, tumour grade and histological subtype), clinical (eg, performance status and certain blood values) and molecular features (eg, BAP1 and PBRM1 mutations), but the combination of these features does not have sufficient predictive accuracy.8 In order to provide tailored treatment and follow-up care, the identification of additional prognostic factors that predict the expected clinical course in each individual patient is subject of active scientific research.
Nowadays, more than 60% of patients with RCC are overweight or obese at diagnosis (body mass index (BMI) ≥25 kg/m2).9 A meta-analysis of prospective observational studies showed a 24% increased risk of RCC for men and a 34% increased risk for women per 5 kg/m2 increase in BMI.10 It is estimated that about 17% and 24% of RCC cases are attributable to overweight in the Netherlands and in the UK, respectively.11 12 Paradoxically, meta-analyses on BMI and survival suggest that patients with RCC who were overweight or obese at diagnosis have a significantly better overall, cancer-specific and recurrence-free survival compared with normal weight patients.13 14 The higher risk but better prognosis with higher BMI is counterintuitive. Possibly, body composition explains part of this paradox.
Body composition refers to the content of fat, lean tissue and bone in the human body. The amount and distribution of these tissues may be independent of BMI; subjects with similar BMI may have different amounts of visceral adipose tissue (VAT), subcutaneous adipose tissue (SAT), skeletal muscle (SM) and intermuscular adipose tissue (IMAT). Cross-sectional areas and mean radiodensity of these tissues can be assessed by analysis of CT scans at the level of the third lumbar vertebra (L3), using established Hounsfield Unit thresholds for each tissue. Cross-sectional total adipose tissue (TAT) and SM areas at L3 are linearly related to body TAT and SM mass.15–17
High VAT mass, low SM index (SMI (SM/height2)) and low SM radiodensity (SMD) have been associated with adverse postoperative18 and survival outcomes19–21 in several cancer types. In our meta-analysis, we showed that low SMI and low SMD are also associated with increased overall mortality in patients with metastatic RCC.22 No meta-analysis could be performed for localised RCC due to the limited number of studies and heterogeneity in body composition parameters and outcomes.22 Studies also suggested an association of low versus high SMI with higher overall and cancer-specific mortality23 and of lower SMD with higher overall mortality.24 Other studies found that low versus high VAT was associated with a higher risk of recurrence,25 cancer-specific26 27 and overall mortality.24
Body composition is known to differ by age, gender and race.28 29 Studies on the association of tumour characteristics with body composition features are inconsistent30 31 and studies on the association of lifestyle habits and circulating biomarkers with body composition parameters are not available in patients with RCC. Smoking has been associated with increased RCC risk and RCC-specific mortality.32 Studies on dietary factors and physical activity are inconsistent for RCC risk33 and hardly available for clinical outcomes, including health-related quality of life (HRQoL). Some studies suggest that circulating biomarkers (eg, adiponectin, leptin and C-reactive protein) are associated with tumour size,34 invasion, progression or metastasis34–36 and survival37 38 in patients with RCC, but results are inconsistent.
Thus, the association of patient and tumour characteristics, lifestyle habits and circulating biomarkers with body composition features in patients with localised RCC needs to be clarified. Further, there is a clear need to obtain more insight in body composition features and lifestyle habits and their relation with clinical outcomes in patients with localised RCC. This information is important to develop personalised evidence-based lifestyle advice for patients with localised RCC to improve their clinical outcomes. Therefore, the objectives of this study are to evaluate (1) the association of patient and tumour characteristics, lifestyle habits and circulating biomarkers with body composition features and (2) the association of body composition features, lifestyle habits and circulating biomarkers with clinical outcomes, including postoperative outcomes (eg, complications and length of hospital stay), recurrence, progression, survival and HRQoL.
Cohort description
Setting
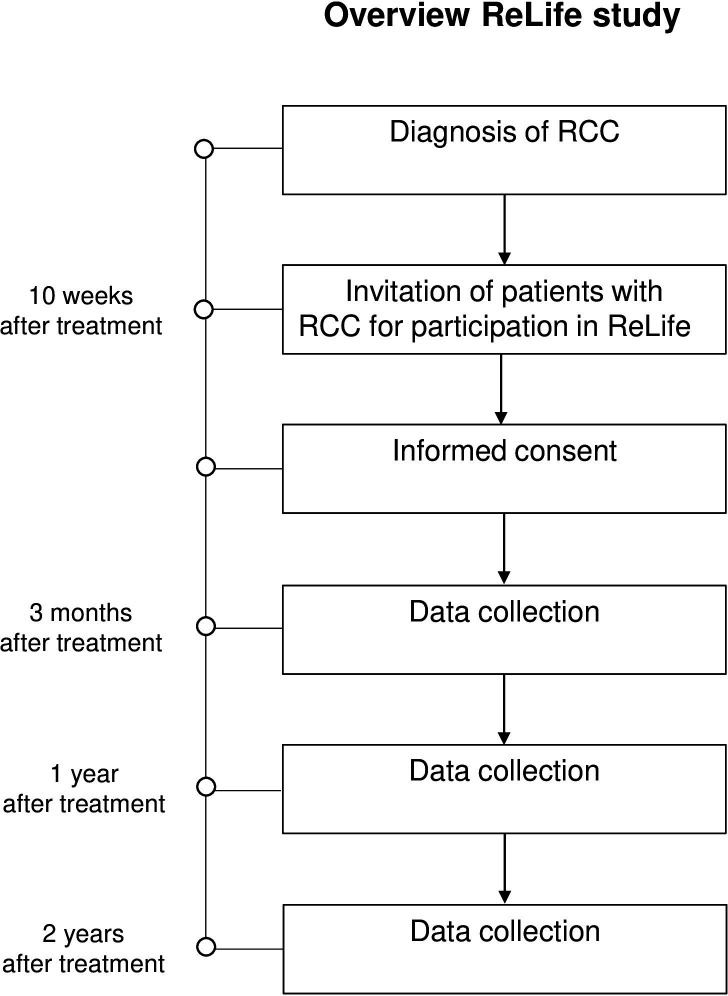
The Renal cell cancer: Lifestyle, prognosis and quality of life (ReLife) study is a prospective cohort study involving patients with newly diagnosed pathologically confirmed primary stages I–III RCC. Patients were recruited in 18 hospitals in the East, South and Central parts of the Netherlands. Before the start of the study, permission was asked from all urologists of the participating hospitals to select and invite eligible patients from the Netherlands Cancer Registry (NCR), held by the Netherlands Comprehensive Cancer Organisation (IKNL). Once every 2 weeks, newly diagnosed patients were identified by IKNL personnel using notification lists of the Pathological Anatomical National Automate Archive (PALGA foundation) in the Netherlands. Approximately 10 weeks after treatment (surgery or ablation), patients were invited by IKNL personnel on behalf of their urologist to participate in this study (figure 1). Patients who agreed to participate provided a written informed consent. Enrolment started in January 2018 and ended in June 2021 and collection of follow-up data is still ongoing.
Figure 1.
Timeline and study design of the ReLife study. ReLife, Renal cell cancer: Lifestyle, prognosis and quality of life.
Patient and public involvement
Four patient representatives were asked for feedback on the grant proposal and one patient representative was involved in the design and set-up phase of the study. Patients were not involved in the conduction of this research, but will be involved in the reporting and dissemination plans regarding information provision to patients. Results from the study will be communicated to participants and urologists from the participating hospitals through the study website (www.radboudumc.nl/trials/relife), through newsletters and through the website of the patient society. Results will be submitted for publication in peer-reviewed journals and presented at relevant (inter)national conferences.
Participants
Eligible participants were men and women between 18 and 75 years old who were newly diagnosed with a histologically confirmed primary stages I–III RCC tumour and who underwent a (partial) nephrectomy or ablation. Patients had to have sufficient command of the Dutch language since all study materials and questionnaires were only available in Dutch. Patients with a previous diagnosis of cancer in the 5 years before RCC diagnosis and those with a lymph node metastasis or distant metastasis were not eligible.
Data collection and management
Questionnaires
Participants are asked to complete self-administered web-based or paper-and-pencil-based questionnaires at 3 months, 1 year and 2 years after treatment (figure 1, table 1). Web-based questionnaires are collected using Castor EDC. Follow-up telephone calls are made to non-responding participants and to respondents whose questionnaires have missing items.
Table 1.
Overview of data collection in ReLife at the three time points after treatment
| Measures | 3 months | 1 year | 2 years | |
| Questionnaires | ||||
| Sociodemographic data | Date of birth, gender, country of birth of participant, father, mother, race, living situation, marital status, highest level of education and working history | X | ||
| Anthropometry | Height at baseline, weight 2 years before diagnosis, weight loss 3–6 months before diagnosis and average weight during adult life | X | ||
| Current body weight and waist and hip circumference | X | X | X | |
| Lifestyle | Current and past smoking behaviour, including dose and duration, alcohol consumption, (reasons for) changes in eating habits and mobility | X | X | X |
| SQUASH (29) | X | X | X | |
| Frequency and amount of alcohol consumption during week and weekend days (32–34) | X | X | X | |
| Changes in eating habits and reasons for/type of changes | X | X | ||
| Medical history | Previously diagnosed with cancer and family history of cancer | X | ||
| Comorbidities, medication use and dietary supplement use | X | X | X | |
| Diet | 163-item Food Frequency Questionnaire | X | X | X |
| HRQoL | EORTC QLQ-C3044 | X | X | X |
| Accelerometer | ||||
| Habitual physical (in)activity and sedentary behaviour | X | X | X | |
| Blood | ||||
| EDTA whole blood for DNA isolation | X | |||
| EDTA plasma and serum | X | X | X | |
| Tissue | ||||
| Formalin-fixed paraffin-embedded tissue of the primary tumour | X* | |||
| CT scan | ||||
| Diagnostic CT scan | X | |||
| Follow-up CT scans | X† | X† | ||
| Clinical data | ||||
| Disease characteristics and treatment | X | X | X | |
| Postoperative outcomes, recurrence and progression | X | X | X |
*To date only permission, no actual collection.
†Dependent on availability.
EORTC-QLQ-C30, European Organisation for Research and Treatment of Cancer quality of life questionnaire; HRQoL, health-related quality of life; ReLife, Renal cell cancer: Lifestyle, prognosis and quality of life; SQUASH, short questionnaire to assess health-enhancing physical activity.
The general questionnaire at 3 months contains questions on demographics (age, sex, ethnicity, education, living situation, occupation and marital status) and personal and family history of cancer. All questionnaires collect information about height, body weight, amount and frequency of alcohol consumption during weekdays and weekend days, smoking habits, comorbidities and the use of dietary supplements and medication. Information on smoking habits is collected in detail, including age or date of starting and stopping smoking, number of cigarettes smoked per day and duration of smoking. Information about habitual physical activity is collected by using the validated short questionnaire to assess health-enhancing physical activity (SQUASH).39 The SQUASH questionnaire assesses the average time, that is, number of days per week and hours and minutes per day, spent in commuting activities, leisure time activities, household activities and activities at work in a normal week in the past month. At all three time points, patients are also asked to measure and report their waist and hip circumference.
Habitual dietary intake is collected at all three time points using a 163-item validated and reproducible self-administered food frequency questionnaire that was developed by Wageningen University.40–42 The questionnaire contains questions about the frequency of consumption of food products and the portion size during the previous month. Frequency and portion size of consumed food products are multiplied to obtain their intake in grams per day. Nutrient intake is calculated using the Dutch Food Composition Table NEVO 2011.43
HRQoL is assessed at all three time points with the validated European Organisation for Research and Treatment of Cancer quality of life questionnaire (EORTC QLQ-C30).44 The EORTC QLQ-C30 contains five function scales (physical, role, cognitive, emotional and social functioning), three symptom scales (fatigue, nausea, pain and vomiting) and six single items (dyspnoea, insomnia, loss of appetite, constipation, diarrhoea and financial impact), all scored from 1 (not at all) to 4 (very much) and a global health status scale which ranges from 1 (very poor) to 7 (excellent). All scores will be linearly transformed to a 0–100 scale.
Accelerometer
Habitual physical (in)activity is objectively measured at all three time points using the activPAL physical activity monitor (PAL Technologies, Glasgow, UK). This accelerometer has shown to be an accurate tool for measuring daily physical activity levels.45 Participants are asked to wear the device continuously on the front right thigh for 7 consecutive days. Data are uploaded using the activPAL software.
Blood samples
Non-fasting blood samples are collected at all three time points. At 3 months, 10 mL EDTA whole blood (for DNA isolation), 10 mL EDTA plasma and 8.5 mL serum are collected. At the other two time points, 10 mL EDTA plasma and 8.5 mL serum are collected. All blood samples are collected, processed and stored at −80 °C locally in the participating hospitals according to a standard protocol before transportation on dry ice to the Radboud Biobank. The blood samples are stored in the Radboud Biobank at −80 °C for future analyses of genetic and other biomarkers. Analysis of adiponectin, leptin, C-reactive protein, and interleukin 6 by the Laboratory for Experimental Internal Medicine of Radboudumc using commercially available ELISAs is planned.
Tumour samples
From all patients, permission for collection of tumour specimens is requested for future assessment of tumour characteristics (eg, tumour necrosis) and acquired genetic alterations (eg, in the BAP1 or PBRM1 genes).6 Formalin-fixed paraffin-embedded tumour blocks can be identified by using the PALGA foundation and retrieved using the Dutch National Tissuebank Portal (DNTP) from the local pathology laboratories.
CT scans
CT scans are retrieved from medical records of all patients for the assessment of body composition. Diagnostic CT scans are available from almost all patients with RCC as they are used for diagnosis and staging of the disease. If available, follow-up CT scans are collected as well. From these CT scans, cross-sectional areas (cm2) and mean radiodensity of SM, VAT, SAT and IMAT are quantified at the landmark level of the third lumbar vertebra (L3).
Clinical data
Information about disease characteristics and treatment for the initial tumour and subsequent recurrences is collected from the medical records by data managers of the NCR. Information about tumour characteristics includes incidence date, clinical TNM and post-surgical TNM stage, Fuhrman grade and morphology. With respect to therapy, information is collected on type of treatment (type of nephrectomy and type of ablation), operation time, blood loss, complications (Clavien-Dindo classification) and length of hospital stay. Furthermore, data on performance status (eg, World Health Organisation performance status and American Society of Anaesthesiologists score) are collected.
Data on clinical outcomes, that is, recurrence and progression with dates of diagnosis, stage and Fuhrman grade, and survival, are also collected. We will continue to collect further information on these clinical outcomes in the future to evaluate their association with body composition features, lifestyle habits and circulating biomarkers.
Power calculation and data analyses
The power calculation of this study is based on our initial research question, that is, the cross-sectional association of patient and tumour characteristics, lifestyle habits and circulating concentrations of biomarkers with body composition features. With 368 patients, we will have sufficient power (≥80%) to detect a multiple correlation coefficient of 0.30 (Cohen’s f2=0.10 for patient and tumour characteristics, dietary and lifestyle habits, and circulating concentrations of biomarkers with body composition features), corresponding to a small (f2=0.02) to medium (f2=0.15) effect size.46 This power calculation is based on 276 stages I–III patients (assuming 75% available and analyzable CT scans), 19 predictor variables, and 3 body composition features as outcome variables (cross-sectional area and radiodensity of SM and cross-sectional area of VAT). For the power calculation, we correct for multiplicity (three body composition features) by using the Bonferroni corrected α of 0.05/3.
Patient characteristics were described using means and SD, medians and IQR, or total numbers and percentages where appropriate. Differences in sociodemographic and clinical characteristics between participants and non-participants were evaluated with χ2 tests. Two-sided p values <0.05 were considered statistically significant. Multiple linear regression analyses will be used to estimate the cross-sectional association of patient and tumour characteristics, lifestyle habits and biomarkers with body composition features. Longitudinal associations of body composition features, lifestyle habits and biomarkers with HRQoL will be assessed using linear mixed models. Logistic regression and Cox proportional hazard analyses will be used to estimate the association of body composition features, lifestyle habits and biomarkers with other clinical outcomes. All statistical analyses will be conducted in R.
Findings to date
Characteristics of study participants
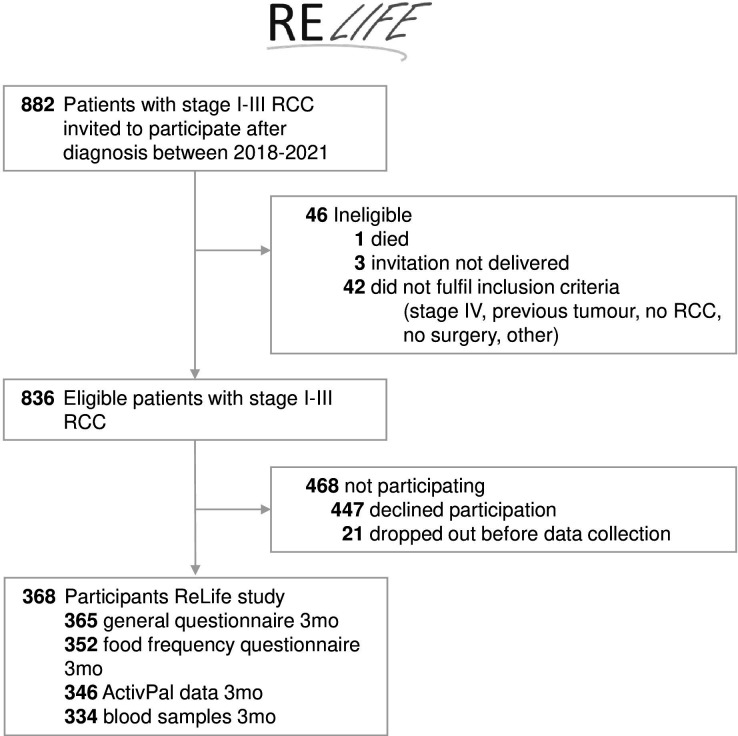
From January 2018 to June 2021, 882 patients diagnosed with stage I–III RCC were invited to participate. Recruitment was paused between 16 March and 18 May 2020 due to COVID-19 measures. In total, 836 patients were eligible and 368 patients agreed to participate and filled out the first or second questionnaires (response rate 44%) (figure 2). The median time between time of treatment and time of the 3 months’ questionnaire completion was 13 weeks (IQR: 12–14 weeks). The number of questionnaires, ActivPal measurements and blood samples available at 3 months is also shown in figure 2.
Figure 2.
Flowchart of the ReLife study. ReLife, Renal cell cancer: Lifestyle, prognosis and quality of life.
In table 2, the baseline characteristics of the cohort are presented. The mean age of patients was 62.4±9.0 years and 70% was male. Most patients had stage I (65%) and Fuhrman grade 2 (50%) disease. The majority was treated with radical (57%) or partial nephrectomy (42%). The majority of participants were overweight (44%) or obese (25%) and 50% were former smokers. Compared with non-participants, participants were less likely to be male but were comparable with respect to age, tumour stage, tumour grade, morphology and type of treatment (table 3).
Table 2.
Baseline characteristics of 368 patients with RCC included in the ReLife study
| Age at diagnosis (years) | 62.4±9.0 |
| Sex | |
| Male | 257 (70) |
| Female | 111 (30) |
| Race | |
| White | 356 (97) |
| Black | 1 (0.3) |
| Asian | 3 (1) |
| Other | 5 (1) |
| Missing | 3 (1) |
| Educational level* | |
| Low | 151 (41) |
| Medium | 115 (31) |
| High | 98 (27) |
| Missing | 4 (1) |
| Paid occupation | |
| Yes | 170 (46) |
| No | 195 (53) |
| Missing | 3 (1) |
| Living situation | |
| Alone | 48 (13) |
| With partner | 228 (62) |
| With partner and kids | 81 (22) |
| Alone, but with kids | 8 (2) |
| Missing | 3 (1) |
| BMI (kg/m2) | 27.6±4.7 |
| BMI (kg/m2) | |
| Underweight (≤18.5) | 1 (0.3) |
| Normal weight (18.5–25) | 110 (30) |
| Overweight (25-≤30) | 163 (44) |
| Obese (>30) | 91 (25) |
| Missing | 3 (1) |
| Waist circumference (cm)† | 101.2±12.1 |
| Hip circumference (cm)† | 102.1±9.5 |
| Cigarette smoking status | |
| Current | 43 (12) |
| Former | 185 (50) |
| Never | 137 (37) |
| Missing | 3 (1) |
| Alcohol consumption (g/day) | |
| 0 | 104 (28) |
| >0–10 | 145 (39) |
| >10 | 101 (27) |
| Missing | 18 (5) |
| Total moderate-to-vigorous physical activity (min/week) | |
| <75 | 27 (7) |
| 75–150 | 142 (39) |
| ≥150 | 193 (52) |
| Missing | 6 (2) |
| Tumour stage | |
| I | 238 (65) |
| II | 55 (15) |
| III | 75 (20) |
| Fuhrman grade | |
| 1 | 49 (13) |
| 2 | 185 (50) |
| 3 | 67 (18) |
| 4 | 23 (6) |
| Unknown | 44 (12) |
| Treatment | |
| Radical nephrectomy | 210 (57) |
| Partial nephrectomy | 152 (41) |
| Ablation‡ | 6 (2) |
| Comorbidities | |
| 0 | 54 (15) |
| 1 | 85 (23) |
| ≥2 | 226 (61) |
| Missing | 3 (1) |
Values are mean±SD or n (%).
*Low (primary, secondary and vocational education), medium (intermediate vocational education, higher general secondary education and pre-university education) and high (university of vocational education and university).
†Values for eight participants were missing.
‡Other treatment consists of cryoablation (n=2), radiofrequency ablation (n=3) and microwave ablation (n=1).
BMI, body mass index; RCC, renal cell cancer; ReLife, Renal cell cancer: Lifestyle, prognosis and quality of life.
Table 3.
Comparison of demographic and clinical characteristics of 368 patients with RCC included in the ReLife study and 468 invited non-participants
| Participants | Non-participants | P value* | |
| N | 368 | 468 | |
| Age category (years) | |||
| 18–44 | 14 (4) | 28 (6) | 0.34 |
| 45–64 | 180 (49) | 218 (47) | |
| 65–75 | 174 (47) | 222 (47) | |
| Sex | |||
| Male | 257 (70) | 360 (77) | 0.02 |
| Female | 111 (30) | 108 (23) | |
| Tumour stage | |||
| I | 238 (65) | 298 (64) | 0.51 |
| II | 55 (15) | 61 (13) | |
| III | 75 (20) | 109 (23) | |
| Fuhrman grade | |||
| 1 | 49 (13) | 86 (18) | 0.32 |
| 2 | 185 (50) | 219 (47) | |
| 3 | 67 (18) | 74 (16) | |
| 4 | 23 (6) | 33 (7) | |
| Unknown | 44 (12) | 56 (12) | |
| Morphology | 0.97 | ||
| Clear cell renal tumour | 260 (71) | 338 (72) | |
| Papillary renal tumour | 48 (13) | 58 (12) | |
| Chromophobe renal tumour | 25 (7) | 30 (6) | |
| Other† | 35 (9) | 42 (9) | |
| Treatment | |||
| Radical nephrectomy | 210 (57) | 272 (58) | 0.76 |
| Partial nephrectomy | 152 (41) | 191 (41) | |
| Ablation‡ | 6 (2) | 5 (1) |
Values are n (%).
*From χ2 test.
†Other morphology consists of adenocarcinoma with mixed subtypes (n=4 and n=5), eosinophilic solid and cystic renal cell carcinoma (n=1 and n=0), renal cell carcinoma not otherwise specified (n=28 and n=29), sarcomatoid renal cell carcinoma (n=2 and n=6), collecting duct carcinoma (n=0 and n=1) and clear cell papillary renal cell tumour (n=0 and n=1) for participants and non-participants, respectively.
‡Other treatment consists of cryoablation (n=2 and n=2), radiofrequency ablation (n=3 and n=2) and microwave ablation (n=1 and n=1) for participants and non-participants, respectively.
RCC, renal cell cancer; ReLife, Renal cell cancer: Lifestyle, prognosis and quality of life.
Future plans
We have already started and will first continue to work on the statistical analyses and writing of manuscripts addressing our main study objectives, that is, (1) the association of patient and tumour characteristics, lifestyle habits and circulating biomarkers with body composition features and (2) the association of body composition features, lifestyle habits and circulating biomarkers with clinical outcomes, including postoperative outcomes (eg, complications and length of hospital stay) and HRQoL. Statistical analyses for recurrence, progression and survival will be conducted once follow-up is more mature or pooling with similar cohorts becomes possible.
Strengths and limitations
To the best of our knowledge, the ReLife study is the first population-based prospective longitudinal study on lifestyle-related factors and clinical outcomes in patients with localised RCC worldwide. Comprehensive data on lifestyle-related factors and HRQoL are collected at 3 months, 1 year and 2 years after treatment. Besides questionnaire data on lifestyle-related factors, also objective data on body composition and physical activity are collected. Data on sociodemographic variables and comorbidity are available as well. Information on several clinical outcomes is collected, including postoperative outcomes (eg, complications and length of hospital stay), recurrence, progression, survival and HRQoL. Moreover, blood samples are collected to measure lifestyle-related, disease-related and genetic biomarkers. Permission is available from participants to use their tumour tissue blocks for assessment of tumour characteristics and acquired genetic alterations.
However, there are also some limitations to this study. As is the case for all longitudinal studies, participants may drop out during the course of the study, potentially leading to selection bias. Some variables have missing values which will be addressed using multiple imputation when applicable. No information on lifestyle-related factors and HRQoL after the 2 years follow-up measurement is available. Power for survival analyses is likely to be insufficient and future pooling with other studies may be necessary. Lastly, we did not use RCC-specific measures of HRQoL in our study.
Results that can be obtained from this study are important to develop personalised evidence-based lifestyle advice for patients with localised RCC to improve their clinical outcomes.
Supplementary Material
Acknowledgments
We are grateful to all the patients who participate in this study and we thank the following hospitals for their involvement in recruitment for the ReLife study: Amphia Ziekenhuis, Breda/Oosterhout (DKE van der Schoot); Ziekenhuis Bernhoven, Uden (AQHJ Niemer); Canisius-Wilhelmina Ziekenhuis, Nijmegen (DM Somford); Catharina Ziekenhuis, Eindhoven (WA Scheepens); Elisabeth-TweeSteden Ziekenhuis, Tilburg/Waalwijk (PJM Kil and BP Wijsman); Elkerliek Ziekenhuis, Helmond (PJ van Hest); Gelre Ziekenhuizen, Apeldoorn/Zutphen (DM Bochove-Overgaauw); Jeroen Bosch Ziekenhuis, ‘s-Hertogenbosch (S van der Meer); Maasziekenhuis Pantein, Boxmeer (E van Boven); Maxima Medisch Centrum, Veldhoven/Eindhoven (LMCL Fossion and K de Laet); Meander Medisch Centrum, Amersfoort (FS van Rey); Radboudumc, Nijmegen; Rijnstate, Arnhem/Velp/Zevenaar (GAHJ Smits); Slingeland Ziekenhuis, Doetinchem (ADH Geboers); St Jansdal Ziekenhuis, Harderwijk (WJ Kniestedt); UMC Utrecht (RP Meijer); Ziekenhuis Gelderse Vallei, Ede (MDH Kortleve) and Ziekenhuisgroep Twente, Almelo/Hengelo (S Stomps). In addition, we thank Ms Ivy Beeren, Ms Monique Eijgenberger, Ms Jolanda van Haren and Ms Ursula Oldenhof for their assistance in data collection. We also thank the data managers of the Netherlands Cancer Registry held by the Netherlands Comprehensive Cancer Organisation (IKNL) for inviting patients and collecting the clinical data.
Footnotes
Collaborators: The ReLife study group is open for collaborations with national and international colleagues. Any person interested in collaborating on the ReLife study or in getting access to ReLife data for data analyses can contact the corresponding author. Requests for data will be discussed and decided by the ReLife study group and will require a Data Transfer Agreement.
Contributors: AV, EK, JPMS, JSFM, KKHA and LALMK contributed to the conception and design of the study. AV provides overall study management and coordinates the project. JPMS contributed to data collection. AV and JSFM drafted the manuscript. All authors have critically read and revised the manuscript and approved the final version of the manuscript. AV is the guarantor of the study.
Funding: This project is funded by the Dutch Cancer Society (KUN 2015-7948). Sponsors were not involved in the study design nor will they be in the collection, analysis and interpretation of data, or in the publications that will result from this study.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Contributor Information
Collaborators: The ReLife study group, DKE van derSchoot, AQHJ Niemer, DM Somford, WA Scheepens, PJM Kil, BP Wijsman, PJ van Hest, DM Bochove-Overgaauw, S van derMeer, E van Boven, LMCL Fossion, K de Laet, FS van Rey, GAHJ Smits, ADH Geboers, WJ Kniestedt, RP Meijer, MDH Kortleve, and S Stomps
Data availability statement
Data are available upon reasonable request. Data and material are not yet available since data collection has not been completed yet. After completion of data collection, data will be made available by the corresponding author upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by Committee for Human Research region Arnhem-Nijmegen (CMO 2016-3078). Participants gave informed consent to participate in the study before taking part.
References
- 1.Capitanio U, Bensalah K, Bex A, et al. Epidemiology of renal cell carcinoma. Eur Urol 2019;75:74–84. 10.1016/j.eururo.2018.08.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van de Schans SAM, Aben KKH, Mulders PFA, et al. Modest improvement in 20 years of kidney cancer care in the Netherlands. Eur J Cancer 2012;48:1822–30. 10.1016/j.ejca.2012.01.033 [DOI] [PubMed] [Google Scholar]
- 3.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–49. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 4.Netherlands Cancer Registry (NCR), Netherlands Comprehensive Cancer Organisation (IKNL). 2023. Available: www.iknl.nl/en/ncr/ncr-data-figures
- 5.Chow WH, Dong LM, Devesa SS. Epidemiology and risk factors for kidney cancer. Nat Rev Urol 2010;7:245–57. 10.1038/nrurol.2010.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ljungberg B, Albiges L, Abu-Ghanem Y, et al. European association of urology guidelines on renal cell carcinoma: the 2022 update. Eur Urol 2022;82:399–410. 10.1016/j.eururo.2022.03.006 [DOI] [PubMed] [Google Scholar]
- 7.Dabestani S, Marconi L, Kuusk T, et al. Follow-up after curative treatment of localised renal cell carcinoma. World J Urol 2018;36:1953–9. 10.1007/s00345-018-2338-z [DOI] [PubMed] [Google Scholar]
- 8.Volpe A, Patard JJ. Prognostic factors in renal cell carcinoma. World J Urol 2010;28:319–27. 10.1007/s00345-010-0540-8 [DOI] [PubMed] [Google Scholar]
- 9.Laguna MP, Algaba F, Cadeddu J, et al. Current patterns of presentation and treatment of renal masses: a clinical research office of the endourological Society prospective study. J Endourol 2014;28:861–70. 10.1089/end.2013.0724 [DOI] [PubMed] [Google Scholar]
- 10.Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 2008;371:569–78. 10.1016/S0140-6736(08)60269-X [DOI] [PubMed] [Google Scholar]
- 11.Parkin DM, Boyd L. 8. cancers attributable to overweight and obesity in the UK in 2010. Br J Cancer 2011;105 Suppl 2:S34–7. 10.1038/bjc.2011.481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lanting CI, de Vroome EMM, Elias SG, et al. Contribution of lifestyle factors to cancer: secondary analysis of Dutch data over 2010 and a projection for 2020. Ned Tijdschr Geneeskd 2014;159:A8085. [PubMed] [Google Scholar]
- 13.Choi Y, Park B, Jeong BC, et al. Body mass index and survival in patients with renal cell carcinoma: a clinical-based cohort and meta-analysis. Int J Cancer 2013;132:625–34. 10.1002/ijc.27639 [DOI] [PubMed] [Google Scholar]
- 14.Kim LH, Doan P, He Y, et al. A systematic review and meta-analysis of the significance of body mass index on kidney cancer outcomes. J Urol 2021;205:346–55. 10.1097/JU.0000000000001377 [DOI] [PubMed] [Google Scholar]
- 15.Mourtzakis M, Prado CMM, Lieffers JR, et al. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 2008;33:997–1006. 10.1139/H08-075 [DOI] [PubMed] [Google Scholar]
- 16.Shen W, Punyanitya M, Wang Z, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol (1985) 2004;97:2333–8. 10.1152/japplphysiol.00744.2004 [DOI] [PubMed] [Google Scholar]
- 17.Shen W, Punyanitya M, Wang Z, et al. Visceral adipose tissue: relations between single-slice areas and total volume. Am J Clin Nutr 2004;80:271–8. 10.1093/ajcn/80.2.271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weerink LBM, van der Hoorn A, van Leeuwen BL, et al. Low skeletal muscle mass and postoperative morbidity in surgical oncology: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle 2020;11:636–49. 10.1002/jcsm.12529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiao J, Mazurak VC, Olobatuyi TA, et al. Visceral adiposity and cancer survival: a review of imaging studies. Eur J Cancer Care (Engl) 2018;27:e12611. 10.1111/ecc.12611 [DOI] [PubMed] [Google Scholar]
- 20.Shachar SS, Williams GR, Muss HB, et al. Prognostic value of sarcopenia in adults with solid tumours: a meta-analysis and systematic review. Eur J Cancer 2016;57:58–67. 10.1016/j.ejca.2015.12.030 [DOI] [PubMed] [Google Scholar]
- 21.Aleixo GFP, Shachar SS, Nyrop KA, et al. Myosteatosis and prognosis in cancer: systematic review and meta-analysis. Crit Rev Oncol Hematol 2020;145:102839. 10.1016/j.critrevonc.2019.102839 [DOI] [PubMed] [Google Scholar]
- 22.Vrieling A, Kampman E, Knijnenburg NC, et al. Body composition in relation to clinical outcomes in renal cell cancer: a systematic review and meta-analysis. Eur Urol Focus 2018;4:420–34. 10.1016/j.euf.2016.11.009 [DOI] [PubMed] [Google Scholar]
- 23.Psutka SP, Boorjian SA, Moynagh MR, et al. Decreased skeletal muscle mass is associated with an increased risk of mortality after radical nephrectomy for localized renal cell cancer. J Urol 2016;195:270–6. 10.1016/j.juro.2015.08.072 [DOI] [PubMed] [Google Scholar]
- 24.Maurits JSF, Sedelaar JPM, Mulders PFA, et al. Skeletal muscle radiodensity and visceral adipose tissue index are associated with survival in renal cell cancer - a multicenter population-based cohort study. Clin Nutr 2022;41:131–43. 10.1016/j.clnu.2021.11.012 [DOI] [PubMed] [Google Scholar]
- 25.Kaneko G, Miyajima A, Yuge K, et al. Visceral obesity is associated with better recurrence-free survival after curative surgery for Japanese patients with localized clear cell renal cell carcinoma. Jpn J Clin Oncol 2015;45:210–6. 10.1093/jjco/hyu193 [DOI] [PubMed] [Google Scholar]
- 26.Naya Y, Zenbutsu S, Araki K, et al. Influence of visceral obesity on oncologic outcome in patients with renal cell carcinoma. Urol Int 2010;85:30–6. 10.1159/000318988 [DOI] [PubMed] [Google Scholar]
- 27.Lee HW, Jeong BC, Seo SI, et al. Prognostic significance of visceral obesity in patients with advanced renal cell carcinoma undergoing nephrectomy. Int J Urol 2015;22:455–61. 10.1111/iju.12716 [DOI] [PubMed] [Google Scholar]
- 28.Bredella MA. Sex differences in body composition. Adv Exp Med Biol 2017;1043:9–27. 10.1007/978-3-319-70178-3_2 [DOI] [PubMed] [Google Scholar]
- 29.Wulan SN, Westerterp KR, Plasqui G. Ethnic differences in body composition and the associated metabolic profile: a comparative study between Asians and Caucasians. Maturitas 2010;65:315–9. 10.1016/j.maturitas.2009.12.012 [DOI] [PubMed] [Google Scholar]
- 30.Keehn A, Srivastava A, Maiman R, et al. The relationship between visceral obesity and the clinicopathologic features of patients with small renal masses. J Endourol 2015;29:372–6. 10.1089/end.2014.0512 [DOI] [PubMed] [Google Scholar]
- 31.Guo H, Zhao W, Wang A, et al. The value of sex-specific abdominal visceral fat as measured via CT as a predictor of clear renal cell carcinoma T stage. Adipocyte 2021;10:285–92. 10.1080/21623945.2021.1924957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cumberbatch MG, Rota M, Catto JWF, et al. The role of tobacco smoke in bladder and kidney carcinogenesis: a comparison of exposures and meta-analysis of incidence and mortality risks. Eur Urol 2016;70:458–66. 10.1016/j.eururo.2015.06.042 [DOI] [PubMed] [Google Scholar]
- 33.Al-Bayati O, Hasan A, Pruthi D, et al. Systematic review of modifiable risk factors for kidney cancer. Urol Oncol 2019;37:359–71. 10.1016/j.urolonc.2018.12.008 [DOI] [PubMed] [Google Scholar]
- 34.Pinthus JH, Kleinmann N, Tisdale B, et al. Lower plasma adiponectin levels are associated with larger tumor size and metastasis in clear-cell carcinoma of the kidney. Eur Urol 2008;54:866–73. 10.1016/j.eururo.2008.02.044 [DOI] [PubMed] [Google Scholar]
- 35.Horiguchi A, Ito K, Sumitomo M, et al. Decreased serum adiponectin levels in patients with metastatic renal cell carcinoma. Jpn J Clin Oncol 2008;38:106–11. 10.1093/jjco/hym158 [DOI] [PubMed] [Google Scholar]
- 36.Horiguchi A, Sumitomo M, Asakuma J, et al. Increased serum leptin levels and over expression of leptin receptors are associated with the invasion and progression of renal cell carcinoma. J Urol 2006;176:1631–5. 10.1016/j.juro.2006.06.039 [DOI] [PubMed] [Google Scholar]
- 37.Steffens S, Köhler A, Rudolph R, et al. Validation of CRP as prognostic marker for renal cell carcinoma in a large series of patients. BMC Cancer 2012;12:399. 10.1186/1471-2407-12-399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Martino M, Leitner CV, Hofbauer SL, et al. Serum adiponectin predicts cancer-specific survival of patients with renal cell carcinoma. Eur Urol Focus 2016;2:197–203. 10.1016/j.euf.2015.06.012 [DOI] [PubMed] [Google Scholar]
- 39.Wendel-Vos GCW, Schuit AJ, Saris WHM, et al. Reproducibility and relative validity of the short questionnaire to assess health-enhancing physical activity. J Clin Epidemiol 2003;56:1163–9. 10.1016/s0895-4356(03)00220-8 [DOI] [PubMed] [Google Scholar]
- 40.de Vries JHM, de Groot LCPGM, van Staveren WA. Dietary assessment in elderly people: experiences gained from studies in the Netherlands. Eur J Clin Nutr 2009;63 Suppl 1:S69–74. 10.1038/ejcn.2008.68 [DOI] [PubMed] [Google Scholar]
- 41.Siebelink E, Geelen A, de Vries JHM. Self-reported energy intake by FFQ compared with actual energy intake to maintain body weight in 516 adults. Br J Nutr 2011;106:274–81. 10.1017/S0007114511000067 [DOI] [PubMed] [Google Scholar]
- 42.Streppel MT, de Vries JHM, Meijboom S, et al. Relative validity of the food frequency questionnaire used to assess dietary intake in the Leiden longevity study. Nutr J 2013;12:75. 10.1186/1475-2891-12-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.NEVO . NEVO-tabel: dutch food composition database. Den Haag: RIVM/Voedingscentrum, 2011. [Google Scholar]
- 44.Aaronson NK, Ahmedzai S, Bergman B, et al. The European organization for research and treatment of cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365–76. 10.1093/jnci/85.5.365 [DOI] [PubMed] [Google Scholar]
- 45.Godfrey A, Culhane KM, Lyons GM. Comparison of the performance of the activpal professional physical activity logger to a discrete accelerometer-based activity monitor. Med Eng Phys 2007;29:930–4. 10.1016/j.medengphy.2006.10.001 [DOI] [PubMed] [Google Scholar]
- 46.Faul F, Erdfelder E, Buchner A, et al. Statistical power analyses using G*power 3.1: tests for correlation and regression analyses. Behav Res Methods 2009;41:1149–60. 10.3758/BRM.41.4.1149 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request. Data and material are not yet available since data collection has not been completed yet. After completion of data collection, data will be made available by the corresponding author upon reasonable request.