Abstract
Objectives
Women of low socioeconomic status have been described as having suboptimal prenatal care, which in turn has been associated with poor pregnancy outcomes. Many types of conditional cash transfer (CCT) programmes have been developed, including programmes to improve prenatal care or smoking cessation during pregnancy, and their effects demonstrated. However, ethical critiques have included paternalism and lack of informed choice. Our objective was to determine if women and healthcare professionals (HPs) shared these concerns.
Design
Prospective qualitative research.
Setting
We included economically disadvantaged women, as defined by health insurance data, who participated in the French NAITRE randomised trial assessing a CCT programme during prenatal follow-up to improve pregnancy outcomes. The HP worked in some maternities participating in this trial.
Participants
26 women, 14 who received CCT and 12 who did not, mostly unemployed (20/26), and - 7 HPs.
Interventions
We conducted a multicentre cross-sectional qualitative study among women and HPs who participated in the NAITRE Study to assess their views on CCT. The women were interviewed after childbirth.
Results
Women did not perceive CCT negatively. They did not mention feeling stigmatised. They described CCT as a significant source of aid for women with limited financial resources. HP described the CCT in less positive terms, for example, expressing concern about discussing cash transfer at their first medical consultation with women. Though they emphasised ethical concerns about the basis of the trial, they recognised the importance of evaluating CCT.
Conclusions
In France, a high-income country where prenatal follow-up is free, HPs were concerned that the CCT programme would change their relationship with patients and wondered if it was the best use of funding. However, women who received a cash incentive said they did not feel stigmatised and indicated that these payments helped them prepare for their baby’s birth.
Trial registration number
Keywords: ethics (see medical ethics), reproductive medicine, health policy, organisation of health services
Strengths and limitations of this study.
This is the first study to assess the perception of conditional cash transfer (CCT) among women (of different backgrounds and pregnancy follow-up) and among healthcare professionals (HPs) who participated in a prenatal CCT trial implemented in France.
This study shows for the first time that the use of CCT is not perceived, by those for whom they are intended, as a stigmatising or infantilising approach.
Many women either did not respond to our request or refused to answer our questions, which may limit the representativeness of our data.
Women who did not adhere to prenatal follow-up are under-represented in our study and our findings may not adequately describe their perspective.
It was even more difficult to interview HPs, notably those who refused to evaluate the CCT programme, and although their opposition to the principle of CCT was expressed, the strength of this opposition may have been underestimated.
Introduction
Follow-up for missed antenatal care visits is inadequate for women who are homeless, those in vulnerable housing, those without funds or the means to attend healthcare appointments,1 2 and those who have difficulty arranging for childcare.3 4 In turn, poor follow-up, often defined by the completion of less than 80% or 50% of planned antenatal follow-up visits according to national guidelines, doubles a woman’s risk of severe maternal and neonatal morbidity.5 6 On the contrary, adequate antenatal follow-up reduces risk. This is why the WHO recommends at least eight antenatal appointments,7 and France recommends seven.6 During these visits, healthcare professionals (HPs) promote healthy behaviour like smoking cessation,8 and may diagnose pregnancy-related diseases like gestational diabetes9 or hypertension10 before they can cause serious harm. Since limited access to socioeconomic resources raises a woman’s risk for adverse maternal outcomes in pregnancy,11 improving follow-up in this population is essential.
Improving the health of pregnant women and their newborns who have limited access to socioeconomic resources (henceforth referred to as ‘underserved women’) depends on removing financial barriers to care. But even in systems where prenatal care is fully covered, as it is the case in France, underserved women have a lower adherence to prenatal care.5 6 A recent systematic review reported that all identified interventions aimed at improving prenatal and postnatal care in women with migrant and refugee backgrounds living in high-income countries, mostly based on community care with none considering conditional cash transfer (CCT), were acceptable to women and improved access, but few provided evidence of improved perinatal outcomes.12 An intensive nurse home-visiting programme, assessed in a Medicaid-eligible population, failed to reduce adverse birth outcomes.13 Among the interventions designed to improve access and attendance to health services or healthy behaviours are financial incentives.14 15 Financial incentives have helped women to make more use of appropriate prescription contraceptives,16 increase their use of health facilities17 and to stop smoking.18 One form of financial incentive is the CCT programme, which rewards people for attending antenatal and/or postnatal care.
For the most part, CCT programmes have been assessed, or implemented, in low-income to middle-income countries (LMICs). CCT programmes have encouraged women to attend prenatal and antenatal care19–21 and paediatric follow-up, and to send their children to school.22 23 In LMICs, these programmes are associated with better health outcomes,21 24 25 and may even reduce maternal mortality.25 A 2015 Cochrane review underlined the public health need for properly conducted randomised trials assessing incentives to collect sufficient data on maternal and neonatal outcomes.26 In high-income European countries, CCT programmes are rare, but some initiatives have targeted specific illnesses, especially mental illnesses,27 and behaviours like smoking during pregnancy,18 28 suggesting that financial incentives can increase medication compliance or smoking cessation.
The ethics of CCT programmes have been called into question;29 for example, opponents of economic incentive schemes argue that they can undermine freedom and dignity, discriminate against and disempower men and women, and create power imbalances between programme providers and recipients.29 30 CCT programmes have also been described as a poor use of resources.31 However, even when healthcare is covered, poverty may still hamper a family’s ability to care for their children, and CCT could increase attendance because it meets other material needs. We were aware of these potential criticisms when we designed the NAITRE Study, a French randomised clinical trial that adds CCT to usual prenatal care for underserved pregnant women identified according to their health insurance affiliation, either universal medical coverage (Couverture Médicale Universelle (Universal Medical Coverage), CMU) for people with no income from work or income below a certain threshold, or state medical welfare (Aide Médicale d’Etat (State Medical Coverage), AME) which offers a limited basket of care for undocumented migrants who have been on French territory for more than 3 months (NAITRE; at data analysis stage).32 In our case, the ethics committee expressed concern that CCT would stigmatise women and disrupt the relationship of trust between pregnant women and their HP by putting economic interests before health interests. To address these legitimate ethical concerns, we conducted a qualitative survey during the first year of recruitment in the NAITRE Study.32 Results were to be presented to the independent Data and Safety Monitoring Committee, so that the study could be stopped if the qualitative assessment showed that women felt degraded or stigmatised.
Considering that the care relationship is a two-way street, the negative perception of a CCT programme by HPs could also have a paradoxical negative effect on the quality of antenatal care. We thus also included HPs in our qualitative survey.
Results of the interviews with women and HPs are presented here.
Methods
Design of the study
In France, health authority guidelines recommend seven antenatal visits and three ultrasound exams as the highest standard of care.33 NAITRE is a pragmatic multicentre, open-label cluster-randomised trial with a parallel arm design. NAITRE was designed to determine if adding CCT to usual antenatal care would raise attendance to antenatal visits to meet the threshold for superiority compared with usual antenatal care only (control arm): a one-third drop in negative maternal-fetal outcomes (going from 18% to 14% of deliveries before 37 weeks of amenorrhoea or birth weight bellow 2500 g at term). The clusters were 2-month periods for each of the centres where all women in the same participating centre received the same intervention, either a CCT in addition to usual antenatal care (intervention group) or just usual antenatal care (control group). Each centre was therefore control or intervention, and the rotation of periods (control or intervention) within the same centre was determined by random sampling. NAITRE’s full protocol is available elsewhere.32
At their first prenatal consultation at participating antenatal centres, NAITRE invited women to take part in the trial. If they attended more than one prenatal consultation, women in the intervention group were given a €30 (roughly at parity with US$, US$31) incentive for each of up to six scheduled consultations (no more than one consultation per month). At each participating centre, women were randomly allotted to either the control or the intervention group. Women in the intervention group received a ‘debit card’ they could use at any store, but which they could not use to withdraw cash from cash dispensers or to do on-line payments. The card was issued with a €0 balance, and €30 were transferred after each qualifying antenatal visit. Women in the control group received the standard of care (seven identical scheduled visits without payment).
The randomised trial was designed to assess whether the CCT programme was likely to improve pregnancy outcomes.
Women who participated in the qualitative survey were eligible for a €40 gift after completing the interview, regardless of their inclusion group in the randomised trial. These face-to-face or phone interviews were designed to elicit the viewpoints of participants in antenatal care, purposely selecting women with a range of antenatal care and pregnancy outcomes. We selected women in both the intervention and control groups. We also interviewed HPs (obstetricians and midwives), including some who had refused to participate in the NAITRE Study or who worked at centres that had refused.
Participants and sample selection
Women were eligible for the NAITRE Trial if they were (1) Pregnant, (2) 18 years or older, (3) Attended their first pregnancy consultation in a participating centre before the end of their 26th week of amenorrhea, and, (4) Enrolled in health insurance for low-income families (CMU) or were undocumented immigrants (AME). We excluded women (1) Whose language skills were insufficient to understand the study or (2) Who were under legal protection. To ensure our qualitative study’s sample reflected a wide range of women’s experiences, we chose women 3–6 months postdelivery based on their group of intervention, their adherence or non-adherence to planned antenatal care, their parity status (primiparous and multiparous women), their age and the level and kind of complications they did or did not have during pregnancy or childbirth. We did not survey woman whose babies died or had been diagnosed with a severe medical condition at birth because we thought these women might not be prepared to discuss or to accurately recall their experiences during pregnancy.
In the first wave (figure 1), we recruited from three including centres to broaden the representation of underserved women included in the NAITRE Trial (rural/urban, former industrial area, or area with high proportion of immigrants). In the second wave of recruitment (figure 2) we then asked our data manager to select women from any of the including centres who met specific criteria (primiparous, non-adherent) to balance our sample. We sought the perspectives of women with diverse backgrounds that might influence their perception of medical follow-up. We contacted women by phone to inform them about the qualitative study and to invite them to participate. We did not set a target size for our sample, and instead continued interviewing until we reached saturation of data and concepts. Saturation is defined as the point at which little or no new relevant codes and/or categories are found in data, when questions (interviews) begin to be repeated with no further understanding or contribution to the phenomenon under study (here, women’s perceptions on CCT), its dimensions, nuances, or variability.34
Figure 1.
Flow diagram of women’s inclusion during the first wave in the three initial centres.
Figure 2.
Flow diagram of women’s inclusion during the second wave when the catch-up area was extended to recruit more women who were not fully compliant with antenatal follow-up.
We gathered data on determinants of medical follow-up during pregnancy from semistructured individual interviews (SSII) held at the hospital where women had been followed up during pregnancy, or at their home; a few SSIs were conducted by telephone. The interviewer used an interview guide designed to encourage women to describe situations that highlighted their habits, thoughts, and feelings about pregnancy and their medical care.
We asked women in the intervention group how they felt about the CCT incentive and if, and how, it helped them. We asked women in the control group: ‘What would you think if women were offered economic compensation every time they consulted during their pregnancy?’ Participants were encouraged to describe their health practices during pregnancy, visits, specific events during pregnancy, and any factors that encouraged or discouraged their adherence to scheduled prenatal care visits.
To capture the ethical and pragmatic factors that influenced HP decisions to participate in the NAITRE Trial,32 we invited HPs by email, asking them to participate in a face-to-face qualitative SSII. HPs were divided into three groups: (1) Those who worked in an including centre and agreed to participate; (2) Those who worked in an including centre but declined to participate; and (3) Those who worked in a centre that declined to participate in the NAITRE Trial. Interviewers used a second interview guide to encourage HPs to describe factors that influenced their decision to participate or not in the NAITRE Trial. We asked them to describe potential facilitators or barriers to implementing and scaling up CCT, if it were proven to be effective, and to tell us if and how they thought it could be integrated into standard practice.
Data collection
Two hospital-based health sociology researchers (AG-M, a female PhD and NM-B, a male PhD) collected the data and facilitated the interviews conducted between May 2017 and July 2019. Both sociologists are clinical researchers trained to conduct qualitative interviews. They used either the face-to-face guide for interviewing participating women or for interviewing HPS.
We took a grounded theory approach in writing our interview guides. They were designed to help interviewers lead subjects through a series of topics designed to elicit data we would analyse to meet our study objectives. The researchers selected the topics and the clinicians validated them. We tested the guides and tuned them in preliminary interviews to ensure that interviewees understood the open-ended questions and that they elicited useful information. We then edited the two guides. The guide for women in the intervention group, who received CCT, focused on eliciting descriptions of their experience of CCT. The guide for women in the control group elicited their thoughts about a CTT programme. All women were asked to describe their experience of and thoughts about their follow-up. It is important to note that while the perception of stigma was one of our questions, the term ’stigma' was deliberately not specifically used in our discussion guide. Interview guides are provided as online supplemental material.
bmjopen-2022-067066supp001.pdf (351.1KB, pdf)
We recorded and fully transcribed the audio of all interviews. We attached field notes to interview transcripts and indicated if the woman’s husband, or partner, was present during the interview, and whether the interviewer through his presence affected the woman’s responses during the interview. We collected data until interviews no longer added new concepts (theoretical saturation) and we had enough data to achieve our research objectives.
Data analysis
We initiated qualitative data analysis during data collection and immediately began analysing the raw data to identify themes. Two sociologists independently coded the interview transcripts. No software was used to analyse the data. We took an inductive approach to identifying patterns, highlighting topics that repeatedly emerged, characterising them, and then organising them into themes that we reviewed and discussed to reduce the likelihood of personal bias and to ensure analytical robustness.34 We then checked the themes we compared and identified against the available literature to ensure our characterisations were complete and accurate. Finally, we sorted themes into larger categories to identify patterns in our findings.
Patient and public involvement
Patients were not involved in the design, the recruitment to and conduct of the study.
Study participants were informed they could have access to the general results of the study by contacting the HP, obstetrician or midwife, who included them into the study.
More widely, the general population will be informed about our findings, both from the qualitative study and the randomised clinical trial, by dedicated communication through the general press and social media.
Results
Inclusion results
Between February and September 2017, we contacted 44 women participants in NAITRE from one of three including centres (Besançon University Hospital, Lille University Hospital, and Robert Debré Hospital in Paris). Of the 44 contacted, we included 22 (figure 1); only one was non-observant. To reach CCT non-adherent women, we extended our search to all the participating centres of the NAITRE Study and selected 23 patients (18 who were non-adherent); of these, we included 4 (figure 2). In total, we selected 67 patients, of whom we included 26 (38.8%): 14 (53.8%) had received cash payments from the programme (table 1).
Table 1.
Distribution of respondent groups
| Intervention | Control | Total | |
| Besançon | 3 | 3 | 6 |
| Paris | 4 | 6 | 10 |
| Lille | 5 | 1 | 6 |
| Toulouse | 2 | – | 2 |
| Dreux | – | 2 | 2 |
| Total | 14 | 12 | 26 |
Paris, Lille and Toulouse are large cities with an urban population and a strong immigrant presence. Besançon is a medium-sized city and Dreux a small-sized city. Both have a more rural population and a lower immigration population.
A professional interpreter was used for three interviews and the husbands served as interpreters for two interviews. Husbands were present at five of the SSIs. Interviews lasted 28 min on average. The general characteristics of the 26 women are presented in table 2.
Table 2.
Characteristics of the women
| n=26 | |
| Average age, years | 31 |
| Median age, years | 30 |
| Primiparous | 6 |
| Multiparous with at least one child at home | 20 |
| Marital status | |
| Lives alone | 2 |
| Lives with a spouse | 24 |
| Precarious housing | |
| Yes | 4 |
| No | 22 |
| Professional situation | |
| Craftsmen, salespersons, business owners | 2 |
| Students (including internship) | 2 |
| Employees | 2 |
| Unemployed (job seeker, housewife, …) | 20 |
The NAITRE team compiled a list of HPs at the 15 units that had been contacted for the study and recruited from this list. We sent an email to department heads at 25 centres to solicit interviews (figure 3); of these centres, 6 agreed to participate. Ultimately, four physicians and three midwives were interviewed. The physicians and one midwife had included patients in the NAITRE Study. Two midwives did not include patients: one worked at a participating centre but had refused to include patients, and one worked at a centre that refused to participate. To protect confidentiality, we do not specify the centres where the HPs worked. These interviews lasted 20 min on average.
Figure 3.
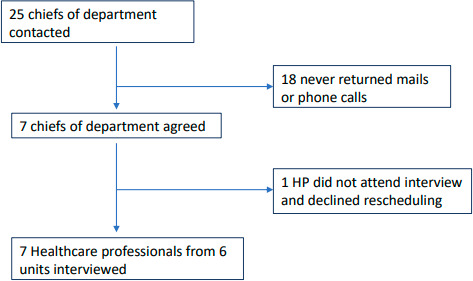
Flow diagram of healthcare professionals’ (HPs’) inclusion.
Women’s perceptions of the CCT initiative
From surprise…to use
The 14 women who received CCT as part of the NAITRE Trial said they were surprised to be offered it and found it unusual for the French health system. Their astonishment was not accompanied by negative perception; these underserved women expressed positive feelings about the CCT. They decided how to spend the CCT; most women used it to buy essential items for the baby (n=13), some also paid for transport to the hospital when travel fees were a problem (n=3) or used it to add to the family budget (n=12). These underserved women clearly indicated that CCT improved their family’s well-being and they were glad to receive the money. Three women said they had saved the incentives to buy expensive equipment like a changing table or baby gate (table 3).
Table 3.
Relevant verbatim
| Women’s perception of the CCT initiative | Healthcare professionals’ perception of the CCT initiative |
|
Surprise
Patient: At first, I thought it was weird, because I thought to myself why are they giving me money because I'm pregnant, I don't know…, I found it a little… I'd never seen it so I didn't know how to take it actually. On the one hand, I'm happy because it’s not… how do you say, it’s nice to have 30 € to buy something, but on the other hand I didn't understand, I thought why? But hey. Good thing—money for her and child Interviewer: What did you think of this proposal when the doctor told you about it? Patient: Well, I thought it was good. Because I've never had a credit card before, that was good. I buy, I feel like I have a card to buy what I want from time to time, it wasn't huge but it was good. Patient: And I thought it was interesting and 1 day I came home and said to my husband, "Yes, frankly that’s a good system they set up because I realise that there are people, it’s true that financially they can't and it’s really a plus”, because when you're pregnant you really want to eat special things and I realise that it helps to feel good during pregnancy and I honestly thought it was great. When I knew she was a girl, when I was sure (laughing), then I started buying things for her. Like the things you need in the hospital, for example, the first pack of diapers, wipes, cotton, things like that. I told one of my friends about it "and you know that now they do programmes and such for people who have the RSA or CMU, they give 30 €, honestly it’s so good and everything " because I know I have friends they didn't eat during their pregnancy, it was tough sometimes. Group control Patient: Uh, no, that’s a bad idea. Interviewer: Yes? Patient: I don't think that’s such a good idea, actually. Interviewer: What bothers you about it? Patient: To pay a person to go for the consultation. Interviewer: Yes? Patient: But wait, it’s her baby, it’s her baby plus it’s herself, it’s her health and the health of the baby you're carrying. Importance of follow-up Patients: With the examinations, the follow-up, they detected, they gave me the necessary check-up and I had no problems apart from the diagnosis of pregnancy at 28 weeks it was very good again, because back home, in A(Country), I didn't do a screening for gestational diabetes and I even think, maybe I had gestational diabetes there that I didn't know, because the girl was born at 3.8 kg. Maybe I developed gestational diabetes that I didn't even know I had. |
Financial aspect
Caregiver: I told myself that… not to mix up the medical follow-up, we'll say and then the financial aspect of things. I don't know, there was something in that that shocked me. For me, you have to separate the two, and the fact that in the end the medical staff validates the fact that, that there is behind a financial side, frankly for me they mixed the genres and that’s it. Non-equality between patients What also happened is that we have patients who know each other, who come from the same neighbourhood or the same area, and I have already had patients who said “but I don't understand, a friend of mine received money, I have nothing, what’s going on?” So twice, it’s not that big a deal, but there were two, so when this patient told me about it, I kind of avoided the question and said “but the study is ending”, well, I don't know what I said anymore to try to… Research At the time, we were just starting to set up studies in the department, because we didn't have a lot of clinical research until then, we had one or two studies in progress. And then, well, as we had practitioners that were really dedicated to obstetrics we were able to set up a little more, so we were motivated to do, to help with clinical studies. It’s not that anymore. Other ways to improve follow-up We have set up specific consultations, over a period longer than 1 hour, where we try to build trust and work in a multidisciplinary way, that is, in conjunction with social workers, the PMI (Protection Maternelle et Infantile – Women and Child Protective Services), charities if necessary, and so on. And generally, setting up these consultations finally helps to build patients' trust and to see them more regularly and in a way…, well, they come. They come for the consultation. |
CCT, conditional cash transfer; CMU, Couverture Médicale Universelle.
Women in the non-intervention group, who had no knowledge of the CCT while they were part of the NAITRE intervention trial as per the study design, were also surprised to hear about the CCT during prenatal follow-up. Two of them indicated they would have refused the offer. They stressed the importance of pregnancy follow-up, regardless of the existence of a financial incentive: ‘I think the priority should be the health of the baby, so come to the consultations’ or ‘People come for the treatment, not for the payment’. The husband of one woman participating in the qualitative survey had told her to refuse the €40 payment for participation in the survey, but all other women, including those who said they would have refused the CCT, accepted payment for the interview. All the women, even those who said they would have refused CCT payments, agreed that a cash incentive could be very helpful to ‘low-income women’, particularly to help them prepare for their baby’s arrival. None of the women found it easy to name an amount that would encourage women to attend consultations, saying it depended on a family’s situation. They also pointed out that payments of any amount would help the family.
A more noticeable hesitancy among husbands
Two husbands clearly objected to the payments, but no woman did. The first husband (intervention group), refused to let his wife use the CCT money received during pregnancy follow-up and declined the €40 compensation for the qualitative survey. He explained that he feared that it would give the medical staff the right to experiment on his unborn children. He took the CCT card and forbade his wife to use it. The second husband (control group) refused the compensation we offered at the end of the interview, saying he did not need the money and had only come to provide us with information.
The importance of follow-up
Whether or not they were financially compensated during the study, all women claimed that receiving a CCT would not have changed their behaviour and that they would have attended the consultations anyway. Only one woman (non-adherent, in the intervention group) said she had not needed medical follow-up during pregnancy. Thirteen women recognised that medical follow-up was important because they had experienced health issues during previous pregnancies (8 women) and/or because they felt that follow-up would protect their infant and improve their own health and that of their baby (13 women).
HPs’ perceptions of the CCT initiative
Of the HPs we interviewed, two had refused to include patients into the NAITRE Trial: one HP was from a participating centre and the other from a non-participating centre. HPs who had agreed to participate (n=6) viewed CCT differently than those who refused (n=2). Those who chose not to participate in NAITRE said they were mainly discouraged by the principle of CCT and made two arguments against compensation.
Money and care are not compatible
First, they felt the CCT programme was ethically problematic since it required them to discuss money and validate payments with patients. They felt it would change their relationship with patients and were not comfortable talking about money even though the NAITRE coordinating centre was responsible for wiring the cash to the woman’s payment card. They did not actually have to discuss money with women during their antenatal follow-up. They thought that talking about money with underserved patients violated the principles of justice and equality and felt that financial aspects should never interfere with medical care, for example, by putting financial interests before health considerations. HPs (n=5) also thought that patients might feel stigmatised if they were offered CCT.
The practical difficulties of the study design: an ethical concern
Their second ethical concern was relative to the design of the study, which used cluster randomisation. Since the clusters were 2-month periods within each of the participating centres, women from both groups, that is, those being offered CCT and those followed up normally, would be in the waiting room at the same time, where they could potentially discuss CCT. HPs were concerned that women from the control group would complain about the unfairness of the situation. But HPs reported only one case where a woman from the control group heard about the CCT; she did not request money. Overall, HPs were more concerned about the ethical, or more precisely moral, issues raised by economic incentives than the conceptual principles behind them.
Some participating HPs (n=3) were concerned that it might not be ethical to offer women money for attending consultations, but said these concerns were not serious enough to make them refuse to participate in the assessment of a CCT programme because they wanted to help us find out if it could be effective. All HPs had practical objections to the programme, for example, that the cost may too high for the health system to enable large-scale implementation. None of the HPs interviewed thought that a CCT would convince reluctant women to adhere to pregnancy follow-up. Instead of CCT, they suggested other interventions that they thought would be more likely to attract and retain women in care, including early follow-up, regular calls and comprehensive individualised care.
Discussion
All women who received the CCT intervention considered it positively and spent the money on their children and families. Most (13/14, 93%) women in the intervention group affirmed that they controlled the cash payments and that the money did not go to their husbands. None of the women who received the intervention said they felt stigmatised by the payments. Women who did not receive the intervention were surprised that CCT was an option, but most felt it could help women in need. It is possible to assume a selection bias, that is, that if only women who perceived the NAITRE Trial as non-stigmatising agreed to participate, then the trial may have included only women who did not perceive the incentives as stigmatising.
However, this risk of bias seems very unlikely for two reasons: (1) Only 130 women out of the 3917 approached (3.3%) refused to participate in the NAITRE Study, which would confirm our hypothesis that the incentives are not perceived as stigmatising for almost all of the women concerned, and (2) The women in the control group, included in the NAITRE Trial and in the qualitative study, were not aware of the economic incentives, so it is impossible that the recruitment in this group was biased.
On the other hand, even HPs who thought it was worth testing the CCT programme were concerned about the ethics of payment. They felt it broke the principle of equality and could stigmatise participating women. From a practical point of view, all the HPs said that, in the underfunded French health system, money would be better spent on other interventions to attract and retain women in prenatal care.
The women in the intervention group indicated that CCT had improved their lives and the lives of their babies, which is consistent with the results of studies from other countries.35–37 Our results are also in agreement with those of other studies that have shown that underserved women mainly use CCT income for everyday child-related expenses. Although all the women in the control group were surprised that women could be paid to attend their consultations, and although some found it inappropriate, they welcomed the principle of CCT during their interview and said they would spend this money on their children. This suggests that exposure to a CCT programme has the potential to reverse initial negative views.
HPs recognise randomised trials as the norm for evaluating drug efficacy, but have more difficulty accepting them for evaluating economic or social interventions,35 36 which may explain why HPs were very concerned about the breach of equality by paying only participants in the intervention group. HPs expressed concerns that the CCT programme would stigmatise unserved women,38 whereas the women themselves felt differently. They all reported benefiting, both themselves and their children, from the payments during pregnancy. Thus, this discrepancy between the views of women and HPs highlights that HPs project the fear of stigmatising underserved women without a sound rationale.
HPs may need to be persuaded that beneficiaries of CCT view it as a simple redistribution of wealth to those in need and that they do not feel stigmatised by it. Without the adherence of HPs, it is difficult to evaluate new economic and social approaches to help patients during pregnancy and early motherhood, especially underserved women. We may need to work proactively to change managerial paradigms,39 develop new health organisations, and work with HPs and patients to co-construct and adapt follow-up procedures.40 41
None of the HPs interviewed thought that the CCT programme was the best way to ensure that patients attended antenatal care as recommended. Even before the COVID-19 pandemic, HPs claimed that offering patients CCT was a poor allocation of resources which could be used more effectively, for example, by funding dedicated units and hiring more doctors and midwives to follow-up with women who missed appointments. Their views are consistent with published research showing that the most effective interventions are those that make it easier for patients to navigate the healthcare system during pregnancy.42–44 However, a study by Salam et al 45 found that in some situations CCT programmes are more likely to change women’s behaviour than other interventions.
Women who received CCT in the NAITRE Trial, for which the data are currently being analysed, were unanimous in stating that the payments did not change their attitudes towards, or their participation in, antenatal care. We cannot ascertain the accuracy of this claim before the NAITRE Study provides empirical evidence on the effectiveness of the programme. If attendance is the same in the intervention and control groups, we will know payments in these amounts do not encourage women to attend antenatal care visits. If a higher proportion of women in the CCT programme had antenatal care as recommended, this could indicate that the women surveyed felt social pressure to deny, or were unaware of, the effect CCT had on their behaviour.
Our study has some limitations
The main limitation of our study is the under-representation of women who did not adhere to antenatal care, limiting our ability to accurately describe their perspective. A selection bias is also possible. Indeed, because we mainly conducted this qualitative study in university hospitals, women who experienced complications in previous pregnancies, and who may thus have a better perception of the usefulness of antenatal care, may be over-represented. We were unable to interview women who gave birth without any follow-up during pregnancy because they were not included in the NAITRE Trial. Our finding that financial incentives do not create stigma cannot be generalised to the most marginalised women (those without any social security coverage, the homeless) as they were not included in the NAITRE Trial, in which all women had documented low socioeconomic status and had access to dedicated health insurance (CMU or AME). Our sample may be biased by only including women who are willing to publicly acknowledge their economic difficulties. Finally, we started interviewing HPs only 2 years after we started including women, so many of the physicians who had refused to participate in NAITRE had moved away and we were unable to reach them. It is therefore possible that we underestimated the extent to which HPs opposed the principle of CCT, even if it is already unambiguously illustrated here.
The difficulties we encountered in reaching women, particularly those who did not adhere to follow-up recommendations, may reflect that social and economic vulnerability that make these marginalised women even less likely to interact with the healthcare system than their more resourceful peers. Interventions that send HPs into the field to interact with these women in their daily lives and offer them incentives like CCT may help them overcome economic barriers to medical care. To provide high-quality antenatal and postnatal care, we need to reach women who do not attend appointments, but the use of mobile or connected health tools may not extend to women who are the most disconnected from the health system.
Conclusion
Although our results are promising, larger studies will be needed to determine the benefits of CCT for women of low socioeconomic status and to determine if CCT is cost-effective. The results of the NAITRE clinical trial, and the subsequent medico-economic evaluation, should answer these questions.
In France, where prenatal follow-up is fully covered, physicians and midwives questioned the ethics of using a CCT programme to improve medical follow-up during pregnancy. At the same time, the women who received a cash incentive as part of the NAITRE Study said they did not feel stigmatised and used these payments to prepare for their baby’s birth.
Even if clinical benefit and efficiency were to be demonstrated by the NAITRE Trial, the practical aspects of implementing economic incentives and their benefit over the long term remain unresolved.
Supplementary Material
Footnotes
Twitter: @mbardou
Collaborators: CHU Besançon : Dr Astrid ECKMAN-LACROIX, Dr Aude BOURTEMBOURG, Dr Claire TOUBIN; CHRU Brest, Pr Philippe MERVIEL, Mme Danièle ADDES, Mme Véronique UGUEN, Mme Cleo TOURBOT, Dr Caroline LELIEVRE, Dr Jacob HANNIGSBERG, Dr Christophe TREMOUILHAC, Dr Anne-Hélène SALIOU, Dr Aurelie DERRIEU, Dr Stephanie AUGET, Dr Anne LEGOURRIEREC, Mme Frédérique FALCHIER; CHU Lyon - Hôpital Femmes-Mères-Enfants, Pr Muriel DORET, Mme Anne LEROUX, Mme Julie FORT-JACQUIER, Mme Marion SERCLERAT, Mme Nathalie LAURENCEAU, Mme Audrey RENOULEAU; CHRU Lille, Pr Damien SUBTIL, Mme Eliane CATTEAU, Pr Philippe DERUELLE; AP-HM Hôpital Nord, Pr Xavier CARCOPINO-TUSOLI, Dr Nathalie LESAVRE, Dr Julie BLANC, Dr Candice RONIN, Dr Laurence PIECHON, Dr Séverine PUPPO, Dr Fanny GRECO, Mme Sandrine PETTAZZONI, Mme Muriel ATHLANI, Mme Amina DESVIGNES, Mme Annie PETITEAU, Mme Amina EL YAAKOUBI, Mme Valérie BECHADERGUE, Mme Valérie VAUGIRARD; AP-HP Bicêtre, Dr Marie-Emmanuelle Neveu, Dr Caroline GEYL, Dr Elodie DEBRAS, Pr Marie-Victoire SENAT, Pr Hervé FERNANDEZ, Dr Claire COLMANT, Dr Marie HOULLIER, Mme Myriam VIRLOUET, Dr Elise THELLIER; AP-HP Robert Debré, Pr Thomas SCHMITZ, Mme Marion MIR, Mme Yasmina BEJAOUI, Mme Hélène LE CORNU, Mme Lauriane NIKEL, Mme Elodie GUSTAVE; CHU Saint-Etienne, Pr Céline CHAULEUR, Dr Amandine STADLER, Dr Ahmad MEHDI, Dr Tiphaine BARJAT, Dr Suzanne LIMA, Dr Thomas CORSINI, Dr Anne GENOD, Dr Charlotte VERMESCH, Dr Cécile FANGET, Dr Marianne PERROT, Mme Manuela MUNOZ, Mme Sylvie PITAVAL, Mme Fanny MAGAND, Mme Françoise BALDI, Mme Stephanie BRET, Mme Anne-Lise VERDIER; CHU Tours, Pr Franck PERROTIN, Dr Christelle DENIS, Dr Carine ARLICOT, Dr Caroline DIGUISTO, Dr Jérôme POTIN, Dr Stéphanie CHRETIEN, Dr Julie PATERNOTTE, Dr Nathalie TRIGNOL, Dr Élisabeth BLIN, Dr Camille MATHIEU, Dr Anne DUBREUIL, Mme Anne VIALLON PELLETIER, Mme Catherine GUERIN, Dr Chloé ARTHUIS; CHU Toulouse, Pr Christophe VAYSSIERES, Pr Olivier PARANT, Dr Marion GROUSSOLLES, Mme Maria DENIS, M Mathieu MORIN; Saint-Etienne Cabinet de SF libérales, Mme Marie-Thérèse BAVOUX, Mme Juliette PELLOUX; CH Tourcoing, Dr Anne-Claire JAMBON, Mme Madeleine SANTRAINE, Mme Veronique LEBUFFE; CH Sambre-Avesnois, Mme Pascale BROUX, Dr Thierry DZUKOU, Dr Magloire GNANSOUNOU, Dr Didier HUBERT, Dr Claire DJAZET, Dr Ludivine DESTOOP, Mme Marine DERUE; CH Saint-Quentin, Dr Pierrick THERET, Mme Dominique DELZENNE, Mme Stéphanie DAUSSINDr Céline BROCHOT, Dr Alice FRAISSINET, Dr Mélanie VANNERUM; CH de Chartres, Dr Cyril FARAGUET, Mme Laurence LANDAIS, Dr Mariana RADU, Mme Anne ROUGET, Dr Sena AL SUDANI; CH Jura Sud - Lons-Le Saunier, Dr Bernard GUILLON, Mme Estelle WUCHER, Mme Véronique SELVA, Dr Sandrine REVIRON, Dr Francis SCHWETTERLÉ, Dr Cécile CHASSANDE, Dr Véronique GRANDIN, Dr Eliane KRTOLIZA, Dr Patrick BECHER; CHU BORDEAUX, Pr Loïc SENTILHES, Pr Dominique DALLAY, Dr Marie SARRAU, Dr Clémence HOUSSIN, Dr Claire LECOQ, Dr Elsa LUTRINGER, Dr Denis ROUX, Dr Noémie BERGE, Dr Frédéric COATLEVEN, Dr Clémentine BARBIER; CH Dreux, Dr Claude VIRTOS, Mme Anne HERON, Mme Audrey FARINA-BRACQUART, Mme Marie-Paule CURTET, Mme Evelyne LEFEBURE, Mme Marie-Hélène LE DOUARIN; CH Cambrais, Dr Hassan AL RAYES; CH Firminy, Dr Émilie MAGNE, Mme Nathalie DESTAMPES; CH SALON, Dr Émilie RICARD, Mme Pascale GHEZZI, Mme Catherine GUILLEN, Mme Fanny ALAZARD, Mme Marie-Thé CAMPANARO, Mme Florence MOJARD, Dr Magalie DAVID-REYNARD, Mme Patricia FUMA, Dr Remy DE MONTGOLFIER, Mme Capucine NEEL; CHU ANGERS, Dr Guillaume LEGENDRE, Pr Philippe GILLARD, Mme Isabelle ANDRE, Mme Sylvie NORDSTROM; CH VALENCIENNES, Dr Brigitte GUIONNET; AP-HP Louis-Mourier, Pr Olivier PICONE, Pr Laurent MANDELBROT, Dr Catherine CRENN HEBERT, Dr Jeanne SIBIUDE, Dr Chloé DUSSAUX, Mme Karine ACHAINTRE, Mme Anne WAGNER, Mme martine WERVEAKE, Mme Eloïse DE GOUVILLE, Mme George THERESIN, Mme Marie Pierre COUETOUX; CENTRE MUNICIPAL DE SANTÉ Les Pavillons Sous-Bois, Dr Lydia Caillaud; CH VALENCE, Dr Marie-Pierre FERNANDEZ, Mme Sabrina BOTTET, M Alain ALMODOVAR, Mme Elisa ETIENNE, Mme Véronique GUITERAS, Mme Angélique TORRES, Mme N. ROCHE, Mme Myriam NASSEF, Mme Christine ABEL-FAURE, Mme Marie LOUVET, Mme Carole ETTORI, Mme Karine CHEMIN; CH LA ROCHE SUR YON, Dr Guillaume DUCARME, Dr Valérie BONNENFANT-MEZERAY, Mme Laurence SZEZOT-RENAUDEAU, Mme Marie-Pierre BERTE, Mme Elodie NETIER-HERAULT, Mme Stéphanie MANSON-GALLONE; CH TOULON - Hôpital Sainte Musse, Dr Franck MAUVIEL, Mme Nathalie AGOSTINI, Mme Marine MAZEAUD, Dr Jean-Claude DAUSSET, Mme Isabelle DE MURCIA, Mme Emilie ALLIOT, Mme Anne-Marie BES, Mme Magali BIFERI Magali; AP-HM - La Conception, Dr Hélène HECKENROTH, Dr Sophie MORANGE, Mme Gersende CHIUOT, Dr Audrey GNISCI, Mme Annie ALLEGRE, Mme Laetitia LECQ, Dr Eva BALENBOIS, Dr Claire TOURETTE, Dr Aude FIGARELLA, Dr Dio ANDRIAMANJAY; CH DRAGUIGNAN, Dr Pauline VIGNOLES; CH AUCH, Mme Catherine CAZELLES, Dr Véronique LEJEUNE SAADA; CH EAUBONNE (S.Veil), Mme Benafsheh KASHANI, Dr Vincent VILLEFRANQUE, Mme Isabelle CHEVALIER, Mme Muriel TERRIERES, Mme Audrey COINTEMENT, Mme Valérie BENHAÏM, Mme Najat LINDOUNE; CH DENAIN, Mme Anne-Sophie MAISONNEUVE, M Frédéric DAUBERCY; CH TARBES, Dr Guilia MENCATTINI; CH SAUMUR, Dr Vanessa COMBAUD, Dr Isabelle MOYA; Hôp St Joseph - MARSEILLE, M Xavier-Côme DONATO, Dr Raoul DESBRIERE; CH du CHINONAIS, Mme Marie LAFON, Mme Véronique BAUDET.
Contributors: MB designed the full research programme, obtained funding, and drafted and revised the manuscript. AG-M and NM-B designed the interview grids, and conducted and analysed the interviews. PD, CV, AE-L, ED, TS reviewed the manuscript. MB acts as guarantor of this article.
Funding: The study obtained funding from the French Ministry for Health in December 2014 (PREPS-14-0173) and the study is promoted by Dijon Bourgogne University Hospital (France).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Contributor Information
the NAITRE Study group:
Astrid Eckman-Lacroix, Aude Bourtembourg, Claire Toubin, Philippe Merviel, Danièle Addes, Véronique Uguen, Cleo Tourbot, Caroline Lelievre, Jacob Hannigsberg, Christophe Tremouilhac, Anne-Hélène Saliou, Aurelie Derrieu, Stephanie Auget, Anne Legourrierec, Frédérique Falchier, Muriel Doret, Anne Leroux, Julie Fort-Jacquier, Marion Serclerat, Nathalie Laurenceau, Audrey Renouleau, Damien Subtil, Eliane Catteau, Philippe Deruelle, Xavier Carcopino-Tusoli, Nathalie Lesavre, Julie Blanc, Candice Ronin, Laurence Piechon, Séverine Puppo, Fanny Greco, Sandrine Pettazzoni, Muriel Athlani, Amina Desvignes, Annie Petiteau, Amina El Yaakoubi, Valérie Bechadergue, Valérie Vaugirard, Marie-Emmanuelle Neveu, Caroline Geyl, Elodie Debras, Marie-Victoire Senat, Hervé Fernandez, Claire Colmant, Marie Houllier, Myriam Virlouet, Elise Thellier, Thomas Schmitz, Marion Mir, Yasmina Bejaoui, Hélène Le Cornu, Lauriane Nikel, Elodie Gustave, Céline Chauleur, Amandine Stadler, Ahmad Mehdi, Tiphaine Barjat, Suzanne Lima, Thomas Corsini, Anne Genod, Charlotte Vermesch, Cécile Fanget, Marianne Perrot, Manuela Munoz, Sylvie Pitaval, Fanny Magand, Françoise Baldi, Stephanie Bret, Anne-Lise Verdier, Franck Perrotin, Christelle Denis, Carine Arlicot, Caroline Diguisto, Jérôme Potin, Stéphanie Chretien, Julie Paternotte, Nathalie Trignol, Élisabeth Blin, Camille Mathieu, Anne Dubreuil, Anne Viallon Pelletier, Catherine Guerin, Chloé Arthuis, Christophe Vayssieres, Olivier Parant, Marion Groussolles, Maria Denis, M Mathieu Morin, Marie-Thérèse Bavoux, Juliette Pelloux, Anne-Claire Jambon, Madeleine Santraine, Veronique Lebuffe, Pascale Broux, Thierry Dzukou, Magloire Gnansounou, Didier Hubert, Claire Djazet, Ludivine Destoop, Marine Derue, Pierrick Theret, Dominique Delzenne, Stéphanie Daussin, Céline Brochot, Alice Fraissinet, Mélanie Vannerum, Cyril Faraguet, Laurence Landais, Mariana Radu, Anne Rouget, Sena Al Sudani, Bernard Guillon, Estelle Wucher, Véronique Selva, Sandrine Reviron, Francis Schwetterlé, Cécile Chassande, Véronique Grandin, Eliane Krtoliza, Patrick Becher, Loïc Sentilhes, Dominique Dallay, Marie Sarrau, Clémence Houssin, Claire Lecoq, Elsa Lutringer, Denis Roux, Noémie Berge, Frédéric Coatleven, Clémentine Barbier, Claude Virtos, Anne Heron, Audrey Farina-Bracquart, Marie-Paule Curtet, Evelyne Lefebure, Marie-Hélène Le Douarin, Hassan Al Rayes, Émilie Magne, Nathalie Destampes, Émilie Ricard, Pascale Ghezzi, Catherine Guillen, Fanny Alazard, Marie-Thé Campanaro, Florence Mojard, Magalie David-Reynard, Patricia Fuma, Remy De Montgolfier, Capucine Neel, Guillaume Legendre, Philippe Gillard, Isabelle Andre, Sylvie Nordstrom, Brigitte Guionnet, Olivier Picone, Laurent Mandelbrot, Catherine Crenn Hebert, Jeanne Sibiude, Chloé Dussaux, Karine Achaintre, Anne Wagner, Martine Werveake, Eloïse De Gouville, George Theresin, Marie Pierre Couetoux, Lydia Caillaud, Marie-Pierre Fernandez, Sabrina Bottet, M Alain Almodovar, Elisa Etienne, Véronique Guiteras, Angélique Torres, N. Roche, Myriam Nassef, Christine Abel-Faure, Marie Louvet, Carole Ettori, Karine Chemin, Guillaume Ducarme, Valérie Bonnenfant-Mezeray, Laurence Szezot-Renaudeau, Marie-Pierre Berte, Elodie Netier-Herault, Stéphanie Manson-Gallone, Franck Mauviel, Nathalie Agostini, Marine Mazeaud, Jean-Claude Dausset, Isabelle De Murcia, Emilie Alliot, Anne-Marie Bes, Magali Biferi Magali, Hélène Heckenroth, Sophie Morange, Gersende Chiuot, Audrey Gnisci, Annie Allegre, Laetitia Lecq, Eva Balenbois, Claire Tourette, Aude Figarella, Dio Andriamanjay, Pauline Vignoles, Catherine Cazelles, Véronique Lejeune Saada, Benafsheh Kashani, Vincent Villefranque, Isabelle Chevalier, Muriel Terrieres, Audrey Cointement, Valérie Benhaïm, Najat Lindoune, Anne-Sophie Maisonneuve, M Frédéric Daubercy, Guilia Mencattini, Vanessa Combaud, Isabelle Moya, Xavier-Côme Donato, Raoul Desbriere, Marie Lafon, and Véronique Baudet
Data availability statement
Data are available upon reasonable request. Data, such as interview verbatims and analyses, can be made available uponreasonable request.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
Ethics approval
This study involves human participants and was approved by the Dijon Ethics Committee (CPP Est-1) on 18 September 2014, and the protocol was amended thereafter. Participants gave informed consent to participate in the study before taking part.
References
- 1. Blondel B, Lelong N, Kermarrec M, et al. Trends in perinatal health in france from 1995 to 2010. results from the French national perinatal surveys. J Gynecol Obstet Biol Reprod (Paris) 2012;41:e1–15. 10.1016/j.jgyn.2012.04.014 [DOI] [PubMed] [Google Scholar]
- 2. Yadav AK, Jena PK, Sahni B, et al. Comparative study on maternal healthcare services utilisation in selected empowered action group states of India. Health Soc Care Community 2021;29:1948–59. 10.1111/hsc.13309 [DOI] [PubMed] [Google Scholar]
- 3. Brugier C, Morel O, Ricbourg A, et al. Impact of precariousness on quality of prenatal detection: lariboisière hospital experience in Paris. J Gynecol Obstet Biol Reprod (Paris) 2012;41:454–9. 10.1016/j.jgyn.2012.02.004 [DOI] [PubMed] [Google Scholar]
- 4. Larrañaga I, Santa-Marina L, Molinuevo A, et al. Poor mothers, unhealthy children: the transmission of health inequalities in the INMA study, Spain. Eur J Public Health 2019;29:568–74. 10.1093/eurpub/cky239 [DOI] [PubMed] [Google Scholar]
- 5. Gonthier C, Estellat C, Deneux-Tharaux C, et al. Association between maternal social deprivation and prenatal care utilization: the precare cohort study. BMC Pregnancy Childbirth 2017;17:126. 10.1186/s12884-017-1310-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Linard M, Blondel B, Estellat C, et al. Association between inadequate antenatal care utilisation and severe perinatal and maternal morbidity: an analysis in the precare cohort. BJOG 2018;125:587–95. 10.1111/1471-0528.14794 [DOI] [PubMed] [Google Scholar]
- 7. WHO . New guidelines on antenatal care for a positive pregnancy experience. 2016. Available: https://www.who.int/news/item/07-11-2016-new-guidelines-on-antenatal-care-for-a-positive-pregnancy-experience [PubMed]
- 8. Passey ME, Adams C, Paul C, et al. Improving implementation of smoking cessation guidelines in pregnancy care: development of an intervention to address system, maternity service leader and clinician factors. Implement Sci Commun 2021;2:128. 10.1186/s43058-021-00235-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Relph S, Patel T, Delaney L, et al. Adverse pregnancy outcomes in women with diabetes-related microvascular disease and risks of disease progression in pregnancy: a systematic review and meta-analysis. PLoS Med 2021;18:e1003856. 10.1371/journal.pmed.1003856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moresi S, Martino C, Salvi S, et al. Perinatal outcome in gestational hypertension: which role for developing preeclampsia. A population-based cohort study. Eur J Obstet Gynecol Reprod Biol 2020;251:218–22. 10.1016/j.ejogrb.2020.05.064 [DOI] [PubMed] [Google Scholar]
- 11. Crone MR, Luurssen-Masurel N, Bruinsma-van Zwicht BS, et al. Pregnant women at increased risk of adverse perinatal outcomes: a combination of less healthy behaviors and adverse psychosocial and socio-economic circumstances. Prev Med 2019;127:105817. 10.1016/j.ypmed.2019.105817 [DOI] [PubMed] [Google Scholar]
- 12. Rogers HJ, Hogan L, Coates D, et al. Responding to the health needs of women from migrant and refugee backgrounds-models of maternity and postpartum care in high-income countries: a systematic scoping review. Health Soc Care Community 2020;28:1343–65. 10.1111/hsc.12950 [DOI] [PubMed] [Google Scholar]
- 13. McConnell MA, Rokicki S, Ayers S, et al. Effect of an intensive nurse home visiting program on adverse birth outcomes in a medicaid-eligible population: a randomized clinical trial. JAMA 2022;328:27–37. 10.1001/jama.2022.9703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vlaev I, King D, Darzi A, et al. Changing health behaviors using financial incentives: a review from behavioral economics. BMC Public Health 2019;19:1059. 10.1186/s12889-019-7407-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Washio Y, Atreyapurapu S, Hayashi Y, et al. Systematic review on use of health incentives in U.S. to change maternal health behavior. Prev Med 2021;145:106442. 10.1016/j.ypmed.2021.106442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Heil SH, Hand DJ, Sigmon SC, et al. Using behavioral economic theory to increase use of effective contraceptives among opioid-maintained women at risk of unintended pregnancy. Prev Med 2016;92:62–7. 10.1016/j.ypmed.2016.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Edmond KM, Foshanji AI, Naziri M, et al. Conditional cash transfers to improve use of health facilities by mothers and newborns in conflict affected countries, a prospective population based intervention study from Afghanistan. BMC Pregnancy Childbirth 2019;19:193. 10.1186/s12884-019-2327-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Berlin I, Berlin N, Malecot M, et al. Financial incentives for smoking cessation in pregnancy: multicentre randomised controlled trial. BMJ 2021;375:e065217. 10.1136/bmj-2021-065217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Glassman A, Duran D, Fleisher L, et al. Impact of conditional cash transfers on maternal and newborn health. J Health Popul Nutr 2013;31:48–66. [PubMed] [Google Scholar]
- 20. Ezenwaka U, Manzano A, Onyedinma C, et al. Influence of conditional cash transfers on the uptake of maternal and child health services in Nigeria: insights from a mixed-methods study. Front Public Health 2021;9:670534. 10.3389/fpubh.2021.670534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vanhuyse F, Stirrup O, Odhiambo A, et al. Effectiveness of conditional cash transfers (afya credits incentive) to retain women in the continuum of care during pregnancy, birth and the postnatal period in Kenya: a cluster-randomised trial. BMJ Open 2022;12:e055921. 10.1136/bmjopen-2021-055921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Baird SJ, Garfein RS, McIntosh CT, et al. Effect of a cash transfer programme for schooling on prevalence of HIV and herpes simplex type 2 in Malawi: a cluster randomised trial. Lancet 2012;379:1320–9. 10.1016/S0140-6736(11)61709-1 [DOI] [PubMed] [Google Scholar]
- 23. Neves JA, Vasconcelos F de AG de, Machado ML, et al. The Brazilian cash transfer program (bolsa família): a tool for reducing inequalities and achieving social rights in Brazil. Glob Public Health 2022;17:26–42. 10.1080/17441692.2020.1850828 [DOI] [PubMed] [Google Scholar]
- 24. Yotebieng M, Thirumurthy H, Moracco KE, et al. Conditional cash transfers and uptake of and retention in prevention of mother-to-child HIV transmission care: a randomised controlled trial. Lancet HIV 2016;3:e85–93. 10.1016/S2352-3018(15)00247-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rasella D, Alves FJO, Rebouças P, et al. Long-term impact of a conditional cash transfer programme on maternal mortality: a nationwide analysis of Brazilian longitudinal data. BMC Med 2021;19:127. 10.1186/s12916-021-01994-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Till SR, Everetts D, Haas DM. Incentives for increasing prenatal care use by women in order to improve maternal and neonatal outcomes. Cochrane Database Syst Rev 2015;2015:CD009916. 10.1002/14651858.CD009916.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Noordraven EL, Wierdsma AI, Blanken P, et al. Financial incentives for improving adherence to maintenance treatment in patients with psychotic disorders (money for medication): a multicentre, open-label, randomised controlled trial. Lancet Psychiatry 2017;4:199–207. 10.1016/S2215-0366(17)30045-7 [DOI] [PubMed] [Google Scholar]
- 28. Cahill K, Hartmann-Boyce J, Perera R. Incentives for smoking cessation. Cochrane Database Syst Rev 2015;5:CD004307. 10.1002/14651858.CD004307.pub5 [DOI] [PubMed] [Google Scholar]
- 29. Scheel IB, Scheel AE, Fretheim A. The moral perils of conditional cash transfer programmes and their significance for policy: a meta-ethnography of the ethical debate. Health Policy Plan 2020;35:718–34. 10.1093/heapol/czaa014 [DOI] [PubMed] [Google Scholar]
- 30. Oliver A, Brown LD. A consideration of user financial incentives to address health inequalities. J Health Polit Policy Law 2012;37:201–26. 10.1215/03616878-1538602 [DOI] [PubMed] [Google Scholar]
- 31. Rawlings LB. Evaluating the impact of conditional cash transfer programs. The World Bank Research Observer 2005;20:29–55. 10.1093/wbro/lki001 [DOI] [Google Scholar]
- 32. Bardou M, Crépon B, Bertaux A-C, et al. NAITRE study on the impact of conditional cash transfer on poor pregnancy outcomes in underprivileged women: protocol for a nationwide pragmatic cluster-randomised superiority clinical trial in France. BMJ Open 2017;7:e017321. 10.1136/bmjopen-2017-017321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Grossesse: le programme de suivi et la première consultation. 2022. Available: https://wwwamelifr/cote-d-or/assure/sante/themes/grossesse/grossesse-programme-de-suivi-et-premiere-consultation
- 34. Hennink M, Kaiser BN. Sample sizes for saturation in qualitative research: a systematic review of empirical tests. Soc Sci Med 2022;292:114523. 10.1016/j.socscimed.2021.114523 [DOI] [PubMed] [Google Scholar]
- 35. Tonguet-Papucci A, Houngbe F, Lompo P, et al. Beneficiaries’ perceptions and reported use of unconditional cash transfers intended to prevent acute malnutrition in children in poor rural communities in burkina faso: qualitative results from the MAM’out randomized controlled trial. BMC Public Health 2017;17:527. 10.1186/s12889-017-4453-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hjelm L, Handa S, de Hoop J, et al. Poverty and perceived stress: evidence from two unconditional cash transfer programs in Zambia. Soc Sci Med 2017;177:110–7. 10.1016/j.socscimed.2017.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Oduenyi C, Ordu V, Okoli U. Assessing the operational effectiveness of a maternal and child health (MCH) conditional cash transfer pilot programme in Nigeria. BMC Pregnancy Childbirth 2019;19:298. 10.1186/s12884-019-2418-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bantebya Kyomuhendo G. Indignity in cash transfers: the senior citizen’s grant in Uganda. The International Journal of Social Quality 2016;6:71–88. 10.3167/IJSQ.2016.060206 [DOI] [Google Scholar]
- 39. Forde I, Rasanathan K, Krech R. Cash transfer schemes and the health sector: making the case for greater involvement. Bull World Health Organ 2012;90:551–3. 10.2471/BLT.11.097733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Skovdal M, Mushati P, Robertson L, et al. Social acceptability and perceived impact of a community-led cash transfer programme in Zimbabwe. BMC Public Health 2013;13:342. 10.1186/1471-2458-13-342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jukić T, Pevcin P, Benčina J, et al. Collaborative innovation in public administration: theoretical background and research trends of co-production and co-creation. Administrative Sciences 2019;9:90. 10.3390/admsci9040090 [DOI] [Google Scholar]
- 42. Balaam MC, Thomson G. Building capacity and wellbeing in vulnerable/marginalised mothers: a qualitative study. Women Birth 2018;31:e341–7. 10.1016/j.wombi.2017.12.010 [DOI] [PubMed] [Google Scholar]
- 43. Austad K, Juarez M, Shryer H, et al. Obstetric care navigation: results of a quality improvement project to provide accompaniment to women for facility-based maternity care in rural Guatemala. BMJ Qual Saf 2020;29:169–78. 10.1136/bmjqs-2019-009524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Coleman J, Black V, Thorson AE, et al. Evaluating the effect of maternal mhealth text messages on uptake of maternal and child health care services in South Africa: a multicentre cohort intervention study. Reprod Health 2020;17:160. 10.1186/s12978-020-01017-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Salam RA, Lassi ZS, Das JK, et al. Evidence from district level inputs to improve quality of care for maternal and newborn health: interventions and findings. Reprod Health 2014;11 Suppl 2:S3. 10.1186/1742-4755-11-S2-S3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-067066supp001.pdf (351.1KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Data, such as interview verbatims and analyses, can be made available uponreasonable request.




