Abstract
Objective
Antiphospholipid syndrome (APS) is defined by the association of thromboembolic and/or obstetrical clinical manifestations and the presence of antiphospholipid antibodies. The objective of our study was to evaluate the impact of the triple-positive profile in a cohort of 204 APS patients.
Methods
We conducted a retrospective study, including patients with primary or secondary APS, meeting the Sydney criteria with at least one thrombotic and/or obstetrical complication. Clinical characteristics and the risk of relapse (defined by the occurrence of a new thrombotic event and/or a new adverse obstetrical event) between triple-positive and non-triple-positive APS patients were compared.
Results
204 patients were included in our study, 68 were triple-positive and 136 were single or double positive. 122 patients (59.8%) had primary APS. 67 patients (32.8%) had obstetrical APS, with a higher rate among triple-positive patients (45.6% vs 26.5%, p=0.010), and 170 patients (83.3%) had thrombotic APS, without difference between triple-positive and others. Thrombotic events were more often venous (56.4%) than arterial (37.7%). Triple-positive patients had more placental complications than others (17.6% vs 2.9%, p=0.001) and more non-criteria events (48.5% vs 25.7%, p=0.002). Among non-criteria events, there was a higher frequency of Sneddon syndrome in triple-positive patients (7.4% vs 0.7%, p=0.028). The relapse rate was higher in triple-positive patients than in others (63.2% vs 39,7%, p=0002). In multivariate analysis, the triple-positive profile was associated with a higher risk of relapse (HR 1.63; 95% CI 1.04 to 2.55; p=0.031).
Conclusion
The triple-positivity is associated with a higher risk of relapse and obstetrical complications.
Keywords: antiphospholipid syndrome; antibodies, anticardiolipin; autoimmune diseases
WHAT IS ALREADY KNOWN ON THIS TOPIC
Triple-positivity is associated with a higher risk of relapse and obstetrical complications.
WHAT THIS STUDY ADDS
Triple-positive antiphospholipid syndrome (APS) had significantly more frequently obstetrical features, non-criteria manifestations and positive antinuclear autoantibodies, with a younger age at diagnosis.
Triple-positive APS had higher rates of relapse and tend to have more frequently catastrophic APS.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The stratification of APS therapies and overall risk on the status of antiphospholipid antibodies triple positivity could be relevant and further studies are needed.
Introduction
Antiphospholipid syndrome (APS) is defined by a combination of at least one clinical feature characterised by vascular thrombosis (venous and/or arterial) or pregnancy morbidity and presence of antiphospholipid antibodies (aPL), namely lupus anticoagulant (LA), anticardiolipin antibodies (aCL) and/or anti-ß2 glycoprotein-I antibodies.1 In addition to APS classification criteria, there is a great variety of non-criteria clinical manifestations related to aPL, but the real prevalence of most of them is unknown.2–4
Patients with positive antibodies for LA, ACL and antiβ2-GPI antibodies are said to be triple-positive APS. The triple-positive profile appears to be the most important factor of thrombosis, with a relative risk of 33, compared with a relative risk of 4 and 3 for LA and anti-β2GPI respectively.5 The triple-positivity also appears to be a risk factor for catastrophic APS (CAPS).6 Triple-positive profile in obstetric APS appears to be associated with obstetric complications including fetal loss, low neonatal weight and stillbirth.7–15 Few data described the overall prognosis, relapse and long-term outcome of a triple-positive APS.
The aims of this study were to assess the clinical and laboratory characteristics of triple-positive APS patients, the impact of triple positivity on the risk of relapse and the management of these patients.
Patients and methods
We conducted a retrospective, descriptive study, including primary and secondary APS patients (defined by Sydney criteria) from 2012 to 2019 and from four French University Centres, from internal medicine department. Patients with another hereditary or acquired thrombophilia (antithrombin, protein C or protein S deficiencies; factor V Leiden and prothrombin G20210A mutations; hyperhomocysteinaemia) were excluded (online supplemental figure 1).
rmdopen-2022-002534supp001.pdf (866.9KB, pdf)
Data collection
For each patient, demographic, laboratory and clinical data, in particular thromboembolic events and pregnancy morbidity, were collected through a dedicated case report form. Obstetrical complications were defined according to the Sydney criteria (1 or more unexplained deaths of morphologically normal fetus beyond the 10th week of gestation (WG), or 1 or more premature births before 34 WG, or 3 or more unexplained consecutive spontaneous abortions before 10 WG). Relapse was defined by the occurrence of a new thrombotic event and/or a new adverse obstetrical event (as defined in Sydney criteria). Data about the associated autoimmune diseases (systemic lupus erythematosus (SLE) or other connective tissue disease) were collected. Cardiovascular risk factors were recorded as follows: diabetes mellitus, chronic hypertension, hypercholesterolaemia, obesity and smoking habit.
Laboratory tests
LA was determined according to updated guidelines of the International Society on Thrombosis and Hemostasis using a diluted Russell viper venom time kit by Instrumentation Laboratory; results of mixing tests are expressed as the ratio of diluted Russell viper venom time of 1:1 mixing of patient and normal pooled plasma, divided by diluted Russell viper venom time of pooled normal plasma (normal value 1.2).16 17 ACL and anti-β2GP1 IgG and M isotypes were measured by commercial ELISA and were considered positive when values exceeded the cut-off value calculated using the 99th percentile of normal age-matched and sex-matched controls. Non-criteria APL antibodies were recorded if available: anti-PS/PT IgG and M, anti-PE IgG and M, anti-annexin V.
Statistical analysis
Descriptive statistics are reported as appropriate: categorical data are expressed as frequencies (percentage); continuous data are reported as mean with SD. Clinical characteristics of triple positive (TP) patients and non-TP (NTP) patients were compared with Student’s t-test for continuous variables and χ2 for qualitative variables. Kaplan-Meier survival analysis was used to determine the relapse-free survival (ie, survival without the occurrence of thrombotic event or adverse obstetrical event). HRs and 95% CI for the risk of relapse were assessed with Cox proportional hazard regression models. Clinically relevant variables associated with p<0.10 in univariate models were selected in the multivariate model. Models were first fitted on the overall population, and only among primary APS. Statistical significance was considered for a p value of 0.05. All analyses were performed using R software, V.3.4.2 (R Fundation for Statistical Computing, Vienna, Austria).
Results
Patients’ characteristics
A total of 204 APS patients were included in our study, with a mean age at diagnosis of 41.5 (17.5) years and 156 (76.5%) were females. Among them, 136 (67%) were single or double positive (NTP APS) and 68 (33%) were TP APS.
One hundred and twenty-two patients had primary APS (59.8%) and 82 patients (40.2%) had APS associated with another systemic autoimmune disease. The most common associated systemic autoimmune diseases were SLE (31%), Sjögren’s syndrome (4.4%), systemic sclerosis (4.4%) and rheumatoid arthritis (2%). 27 TP patients (39.7%) had APS associated with SLE whereas 37 NTP patients (27.2%) (p=0.098). The analysis of aPL isotypes showed that NTP patients had ACL antibodies in 87/134 (64.9%) vs 68/68 TP patients (p<0.001), with ACL IgG subtypes in 57/134 (42.5%) NTP patients vs 56/67 (83.6%) TP patients (p<0.001), and ACL IgM subtypes in 43/133 (32.3%) NTP patients vs 31/66 (46.7%) TP patients (p=0.063). NTP patients had positive B2GP1 antibodies in 43/131 (32.8%) vs 68/68 TP patients (p<0.001), with IgG subtypes in 22/131 (16.8%) NTP patients vs 54/67 (80.6%) TP patients (p<0.001), and IgM subtypes in 27/131 (20.6%) NTP patients vs 34/67 (50.7%) TP patients (p<0.001). LA was positive in 57/125 (45.6%) NTP patients vs 68/68 (100%) TP patients (p<0.001). Antinuclear antibodies were positive in 13/101 (13%) NTP patients vs 20/56 (35.7%) TP patients and anti-SSA and anti-SSB types did not differ between NTP and TP patients, respectively, 20/86 (23.3%) and 13/58 (22.4%). Concerning cardiovascular risk factors, 61/145 patients (42%) had chronic hypertension, 32/142 (22.5%) had dyslipidaemia and 32/125 (25.6%) were current smokers, 35% were overweight or obese and 13.8% were diabetic.
Thrombotic, obstetrical and non-criteria manifestations in triple-positive APS
Of the 68 triple-positive patients with primary and associated APS, 57 (83.8%) were women. The mean age at first thrombotic or obstetrical event was significantly younger in triple-positive patients (34.6 years vs 44.9 years; p<0.001) (table 1). Among cardiovascular risk factors, only higher smoking rates were noted in triple-positive patients (38.5% vs 19.8%; p=0.046).
Table 1.
APS patients’ characteristics and comparison of triple negative with triple positive patients
| Features | All patients | Non-triple-positive patients | Triple-positive patients | Triple-positive primary APS |
| (n=204) | (n=136) | (n=68) | (n=37) | |
| Female gender (%) | 156 (76.5) | 99 (72.8) | 57 (83.8) | 31 (83.7) |
| Age at diagnosis (years) | 41.5 (17.5) | 44.9 (18.3) | 34.6 (14.0)* | 37.8 (13.9) |
| Follow-up (years) | 10.6 (10.1) | 9.3 (9.8) | 13.2 (11.4)* | 11.9 (11.1) |
| Associated disease | ||||
| SLE (%) | 64 (31.4) | 37 (27.2) | 27 (39.7) | – |
| Sjögren (%) | 9 (4.4) | 7 (5.1) | 2 (2.9) | – |
| Systemic sclerosis (%) | 9 (4.4) | 7 (5.1) | 2 (2.9) | – |
| Obstetrical APS (%) | 67 (32.8) | 36 (26.5) | 31 (45.6)* | 17 (45.9) |
| Thrombotic APS (%) | 170 (83.3) | 114 (83.8) | 56 (82.4) | 31 (83.8) |
| Cardiovascular risk factors | ||||
| Chronic hypertension (%) | 61/145 (42.1) | 43/98 (43.9) | 18/47 (38.3) | 12/30 (40) |
| Dyslipidaemia (%) | 32/142 (22.5) | 23/95 (24.2) | 9/47 (19.1) | 6/30 (20) |
| Smoking (%) | 32/125 (25.6) | 17/86 (19.8) | 15/39 (38.5)* | 8/25 (32) |
| Diabetes mellitus (%) | 18/130 (13.8) | 12/88 (13.6) | 6/42 (14.3) | 3/27 (11.1) |
| Overweight/obesity (%) | 41/116 (35) | 24/78 (30.8) | 17/38 (44.7) | 12/24 (50) |
| Obstetrical events | ||||
| Recurrent miscarriage (%) | 22 (10.8) | 15 (11.0) | 7 (10.3) | 3 (8.1) |
| Stillbirth (%) | 26 (12.7) | 14 (10.3) | 12 (17.6) | 6 (16.2) |
| Prematurity (%) | 12 (5.9) | 2 (1.5) | 10 (14.7)* | 2 (5.4) |
| IUGR (%) | 8 (3.9) | 5 (3.7) | 3 (4.4) | 1 (2.7) |
| HELLP syndrome/pre-eclampsia (%) | 16 (7.8) | 4 (2.9) | 12 (17.6)* | 8 (21.6) |
| Thrombotic events | ||||
| VTE (%) | 115 (56.4) | 78 (57.4) | 37 (54.4) | 20 (54.1) |
| Deep Venous thrombosis (%) | 92 (45.1) | 61 (44.9) | 31 (45.6) | 15 (40.5) |
| PE (%) | 41 (20.1) | 31 (22.8) | 10 (14.7) | 6 (16.2) |
| Arterial thromboses (%) | 77 (37.7) | 48 (35.3) | 29 (42.6) | 14 (37.8) |
| Stroke (%) | 49 (24) | 31 (22.8) | 18 (26.5) | 8 (21.6) |
| CAPS | 5 (2.5) | 1 (0.7) | 4 (5.9)‡ | 2 (5.4) |
| Non-criteria manifestations (%) | 68 (33.3) | 35 (25.7) | 33 (48.5)* | 14 (37.8) |
| ITP (%) | 24 (11.8) | 9 (6.6) | 15 (22.1)* | 8 (21.6) |
| Haemolytic anaemia (%) | 10 (4.9) | 5 (3.7) | 5 (7.4) | 1 (2.7) |
| Thrombotic microangiopathy (%) | 10 (4.9) | 5 (3.7) | 5 (7.4) | 2 (5.4) |
| Cardiac valve abnormalities (%) Sneddon (%) | 9 (4.4) | 5 (3.7) | 4 (5.9) | 1 (2.7) |
| Livedo (%) | 7 (3.4) | 6 (4.4) | 1 (1.5) | 0 |
| Sneddon (%) | 6 (2.9) | 1 (0.7) | 5 (7.4)* | 3 (8.1) |
| Migraine (%) | 11 (5.4) | 9 (6.6) | 2 (2.9) | 1 (2.7) |
| Outcome | ||||
| All relapses (%) | 97 (47.5) | 54/136 (39.7) | 43/68 (63.2)* | 22 (59.5) |
| Thrombotic relapses (%) | 74 (36.3) | 44 (32.4) | 30 (44.1) | 16 (43.2) |
| Obstetrical relapses (%) | 30 (14.7) | 12 (8.8) | 18 (26.5)* | 8 (21.6) |
| Death (%) | 21 (10.3) | 17 (12.5) | 4 (5.9) | 3 (8.1) |
| Therapies | ||||
| DOA or VKA (%) | 156/187 (83.4) | 96/123 (78.0) | 60/64 (93.8) | 34/35 (97.1) |
| Antiplatelet therapy (%) | 94/188 (50) | 61/127 (48) | 33/61 (54) | 16/33 (48.5) |
| Hydroxychloroquine (%) | 91/194 (46.9) | 49/129 (38) | 42/65 (64.6)* | 15/35 (42.9) |
| Corticosteroids (%) | 70/173 (40) | 44/120 (36.7) | 26/53 (49.1) | 9/33 (27.3) |
| Laboratory data | ||||
| Antinuclear antibodies | 33 (16) | 13 (13) | 20 (36)* | 13 (35) |
| Anti-β2GP1 antibodies (%) | 111/199 (55.6) | 43/131 (32.8) | 68/68 (100)* | 37/37 (100) |
| Anti-cardiolipin antibodies (%) | 155/202 (76.7) | 87/134 (64.9) | 68/68 (100)* | 37/37 (100) |
| LA (%) | 124/193 (64.2) | 57/125 (45.6) | 68/68 (100)* | 37/37 (100) |
| Non-criteria antibodies (%) | 26/204 (12.7) | 14/136 (10.3) | 12/68 (17.6) | 8 (21.6) |
*p<0.05 triple-positive APS (primary and associated) versus triple-negative APS.
‡p=0.07.
APS, antiphospholipid syndrome; CAPS, catastrophic APS; DOA, direct oral anticoagulants; ITP, immune thrombocytopaenia; IUGR, intrauterine growth retardation; LA, lupus anticoagulant; PE, pulmonary embolism; SLE, systemic lupus erythematosus; HELLP syndrome, low platelets, haemolysis and elevated liver enzymes; VKA, vitamine K antagonists; VTE, venous thrombolic embolism.
Of these 68 triple-positive patients, 31 (54.3%) had obstetric APS, which was significantly higher than in NTP patients (54.3% vs 36.3%; p=0.010). Triple-positive patients had significantly more pre-eclampsia and placental abruption than NTP patients (17.6% vs 2.9%, p=0.001), as well as a higher rate of preterm births (14.7% vs 1.5%, p=0.001). Other obstetric complications (spontaneous miscarriage, intrauterine growth retardation and stillbirth) did not differ between the two groups.
Thrombotic manifestations of APS occurred in 170 patients (83.3%), with no difference in the prevalence of thrombotic APS between TP and NTP patients. Thrombotic events were more often venous (56.4%) than arterial (37.7%), mostly strokes (24%). Five patients presented a CAPS and among them, four were triple-positive and one was not triple-positive (4/68 (5.9%) vs 1/143 (0.7%, p=0.078).
Regarding non-criteria features, 68 patients (33.3%) had at least one such event and the rate of these non-criteria was significantly higher in triple-positive patients (48.5% vs 25.7%, p=0.002). The most frequent manifestations were autoimmune cytopenias, including immunological thrombocytopaenic purpura (ITP), which occurred in 24 patients (11.8%) and autoimmune haemolytic anaemia in 10 patients (4.9%). Triple-positive patients had more ITP than other patients (22.1% vs 6.6%; p=0.003). There was also a higher frequency of Sneddon syndrome in triple-positive patients (7.4% vs 0.7%, p=0.028). There was no difference in the frequency of Libman-Sacks endocarditis (table 1). The rate of non-criteria antibodies did not differ between the two groups (10.3% vs 17.6%; p=0.207). Positive antinuclear antibodies were more common in triple-positive patients (72.7% vs 53.3%; p=0.018).
Thrombotic, obstetrical and non-criteria manifestations in primary APS patients
Of the 122 patients with primary APS, 37 were triple-positive (38.3%) with 31 (83.7%) women. Of these, 17 patients (54.8%) had obstetric APS, which was significantly higher than NTP primary APS (54.8% vs 37.3%, p=0.016). Triple-positive patients had significantly more preeclampsia than NTP primary APS (21.6% vs 2.4%, p=0.001).
One hundred and four primary APS patients (85.2%) presented with thrombotic APS, with no difference between triple-positive and NTP patients. Thrombotic events were more often venous (55.7%) than arterial (36.1%), mostly strokes (24.6%). Two patients presented a CAPS, both of them were triple-positive.
Regarding non-criteria events, 33 patients (27%) had at least one such event, and the rate was significantly higher in triple-positive patients (37.8% vs 22.4%, p=0.0122). The most frequent manifestations were autoimmune cytopenias, including ITP, which occurred in 13 patients (10.7% and were more frequent in triple-positive patients (21.6% vs 5.9%, p=0.023). The diagnosis of ITP was made with a platelet count of less than 100×109/L, with isolated thrombocytopaenia without any other aetiologies after usual screening of thrombocytopaenia. A peripheral blood smear had be assessed to rule out pseudothrombocytopaenia and morphological alterations associated with hereditary thrombopathies.
Management and outcome of triple-positive APS
A total of 156 patients (83.4%) received therapeutic anticoagulation during the follow-up, and triple-positive patients were more often anticoagulated than others (93.8% vs 78%, p=0.011). Antiplatelet therapy was initiated in 94 patients (50%), with no difference between the two groups. Concerning immunomodulating treatments, 91 patients (46.9%) received hydroxychloroquine (HCQ) during their follow-up and HCQ use was significantly higher in triple-positive patients (64.6% vs 38%; p=0.001).
The median follow-up was 9.3 years, and was significantly longer in triple-positive patients (13.2 years vs 9.3 years; p=0.011). Ninety-seven patients (47.5%) presented at least one thrombotic or obstetrical relapse, and the relapse rate was significantly higher in triple-positive patients (63.2% vs 39.7%; p=0.002). Among the relapses, 30 were obstetrical and 74 were thrombotic events, with higher rates of obstetrical relapses in triple-positive patients. During follow-up, 21 patients (10.3%) died, without significant difference between the two groups.
Relapse risk factors
In univariable analysis, the factors associated with relapse were the following: aPL triple-positivity (HR 1.77; 95% CI 1.17 to 2.68; p=0.007), venous APS (HR 1.74; 95% CI 1.13 to 2.69; p=0.013), a history of premature birth (HR 2.47; 95% CI 1.24 to 4.93; p=0.010), anti-β2GP1 antibody (HR 1.70; 95% CI 1.09 to 2.64; p=0.018) and LA (HR 1.59; 95% CI 1.01 to 2.50; p=0.046). The non-criteria manifestations of APS tended to be associated with a higher risk of relapse (HR 1.49; 95% CI 1.00 to 2.23; p=0.052).
In multivariable analysis, the only factors independently associated with the risk of relapse were triple-positive profile (HR 1.63; 95% CI 1.04 to 2.55; p=0.031), venous APS (HR 2.05; 95% CI 1.30 to 3.23; p=0.002) and previous premature delivery (HR 2.33; 95% CI 1.10 to 4.92; p=0.027) (figure 1).
Figure 1.
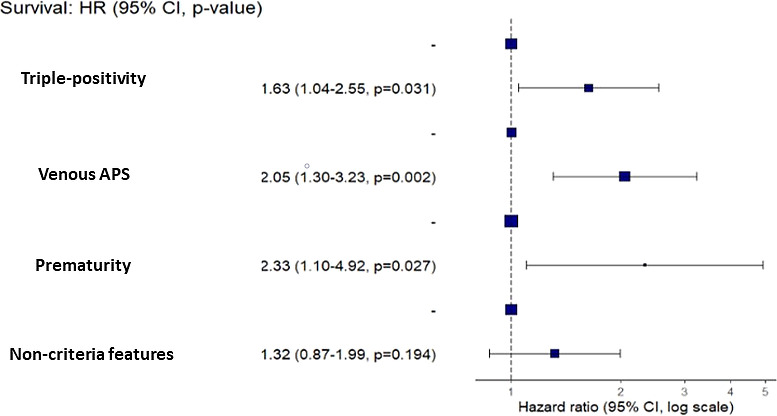
Risk factors associated with relapse in multivariate analysis.
Relapse-free survival was significantly decreased in triple-positive patients at 6 years (3.75–11.7) vs 14 years (8–27.4) (log rank=0.007) (figure 2A). Considering the 122 patients with primary APS, the relapse-free survival was also significantly decreased in triple-positive patients (6 years vs 14 years; log rank <0.001) (figure 2B).
Figure 2.
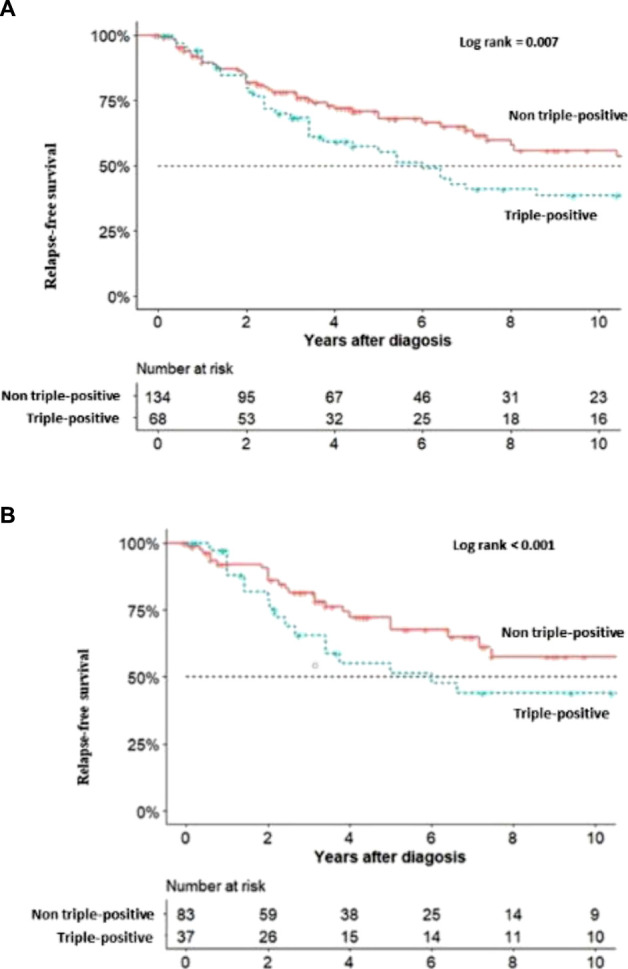
(A) Relapse-free survival in triple-positive and non-triple positive patients. (B) Relapse-free survival in triple-positive and non-triple-positive primary APS. APS, antiphospholipid syndrome.
Discussion
Our study highlighted several messages concerning APS triple-positive patients. The prevalence of triple-positive APS was 33%, which is consistent with the literature.18–20
Triple-positive APS had significantly more clinical features, which distinguish them from NTP patients. These patients had more frequently obstetrical features, non-criteria manifestations and positive antinuclear autoantibodies, with a younger age at diagnosis. They were also prescribed more frequently long-term anticoagulation and immunomodulatory therapies, and despite these medications, they had higher rates of relapse and tended to have more frequently CAPS.
One significant finding of our study is the impact of triple-positivity on the outcomes and the management of APS. The triple-positive profile was associated with a significant increase in relapse rates (63.2% vs 39.7%) in this cohort with a long-term follow-up of more than 10 years, however, triple-positive patients were followed up for longer. This relapse rate was also higher than in another study with a shorter follow-up and a lower number of patients (73). Even the therapies were all analysed the patients compliance is lacking. Specifically, the time in therapeutic range in patients treated with AVK could not be analysed in this retrospective study and constitutes a limitation for the analyse of relapse. Another important limitation is higher rates of triple positivity in obstetrical APS than previous studies, which could be correlated with less frequent early miscarriages in our cohort. In thrombotic triple-positive APS, the cumulative incidence of deep venous thrombosis was 37% at 10 years.21 Only one study previously assessed the risk factors of relapse, and found male sex and combined risk factors for deep venous thrombosis (DVT) (oestrogen, immobilisation, pregnancy, neoplasia and DVT family history) predictive for DVT. Here, we demonstrated that triple positivity was an independent and strong factor of obstetrical relapses. Non-criteria features in primary APS have been recently reported to be more associated with triple positivity and constitute another prognostic factor, similar to this cohort.22
The impact of therapies complementary to anticoagulant and aspirin could be an important issue for this particular subtype of APS patients. Previous studies assessed the use of complementary therapies in APS patients.21 23–28 HCQ was used in 194 high-risk patients with a history of unexplained fetal loss or serious maternal or fetal complications, and was found to be effective in increasing the live birth rate to 84%.28 Another study of 196 pregnancies in 156 women, 54% of whom had a triple-positive profile, investigated the benefit of additional treatments, most often intravenous immunoglobulins, in addition to conventional treatment with aspirin and low-molecular-weight heparin on pregnancy outcomes. The treatment increased the live birth rate only in patients with a history of thrombosis and triple-positive profile.29 The pathophysiology of obstetric complications is complex, and factors predictive of obstetric morbidity could be useful in patients’ management. The PROMISSE study showed that there is an imbalance in angiogenic factors during pregnancy, and in particular a significant increase in soluble fms-like tyrosine kinase-1 (sflt1), an anti-angiogenic factor, associated with the risk of obstetric complications in patients with lupus and/or APS.30 The interest of HCQ on the occurrence of thrombosis was also recently evaluated in a prospective randomised open-label study in 50 patients with primary APS.31 The use of HCQ appeared to reduce the risk of thrombosis, without however reaching significance in multivariable analysis. The HYDROSAPL study is designed to evaluate the value of combining HCQ with conventional treatment in obstetric APS excluding isolated early miscarriages. The BBQ study is the first European study to demonstrate the effectiveness of HCQ in preventing recurrent miscarriage.
Several limitations should be arised, which could limit the definite conclusion from this study. Despite retrospective design, among cardiovascular risk factors, only higher smoking rates were noted in triple-positive patients, which could be a confounding factor. Another limit is the lack of analysis of the treatments, including INR level at the time of the event, which can affect the rate of recurrences.
Conclusion
Triple-positive APS patients constitute a particular subtype of APS with a high risk of relapse and a need of additional therapies. The stratification of APS therapies and overall risk on the status of aPL triple positivity could be relevant and further studies are awarded.
Acknowledgments
The abstract was presented at EULAR 2022: http://dx.doi.org/10.1136/annrheumdis-2022-eular.993.
Footnotes
Contributors: All authors have made substantial contributions to the conception or design of the work: Conceptualisation CL, LR, AM and OF; Data curation CL, LR, SD, CDM and FM; Formal analysis CL, YN and AM; Methodology AM and VP; Supervision AM, JJB, ER, GG, IE, SD, CDM, VP, CJ and FM; Validation OF, AM, SD and CDM; Writing original draft CL and AM; Writing review and Editing CL and AM. AM is fully responsible of all data presented in this paper.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
Ethics approval
Ethical committee was not needed for this observational study according to Helsinki law and the French institutional committee and patients gave non-opposition consent.
References
- 1.Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006;4:295–306. 10.1111/j.1538-7836.2006.01753.x [DOI] [PubMed] [Google Scholar]
- 2.Cervera R, Piette J-C, Font J, et al. Antiphospholipid syndrome: clinical and immunologic manifestations and patterns of disease expression in a cohort of 1,000 patients. Arthritis Rheum 2002;46:1019–27. 10.1002/art.10187 [DOI] [PubMed] [Google Scholar]
- 3.Duarte-García A, Pham MM, Crowson CS, et al. The epidemiology of antiphospholipid syndrome: a population-based study. Arthritis Rheumatol 2019;71:1545–52. 10.1002/art.40901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cervera R, Serrano R, Pons-Estel GJ, et al. Morbidity and mortality in the antiphospholipid syndrome during a 10-year period: a multicentre prospective study of 1000 patients. Ann Rheum Dis 2015;74:1011–8. 10.1136/annrheumdis-2013-204838 [DOI] [PubMed] [Google Scholar]
- 5.Pengo V, Biasiolo A, Pegoraro C, et al. Antibody profiles for the diagnosis of antiphospholipid syndrome. Thromb Haemost 2005;93:1147–52. 10.1160/TH04-12-0839 [DOI] [PubMed] [Google Scholar]
- 6.Ruffatti A, De Silvestro G, Marson P, et al. Catastrophic antiphospholipid syndrome: lessons from 14 cases successfully treated in a single center. A narrative report. J Autoimmun 2018;93:124–30. 10.1016/j.jaut.2018.07.001 [DOI] [PubMed] [Google Scholar]
- 7.De Carolis S, Tabacco S, Rizzo F, et al. Antiphospholipid syndrome: an update on risk factors for pregnancy outcome. Autoimmun Rev 2018;17:956–66. 10.1016/j.autrev.2018.03.018 [DOI] [PubMed] [Google Scholar]
- 8.Alijotas-Reig J, Ferrer-Oliveras R, Ruffatti A, et al. The european registry on obstetric antiphospholipid syndrome (EUROAPS): a survey of 247 consecutive cases. Autoimmun Rev 2015;14:387–95. 10.1016/j.autrev.2014.12.010 [DOI] [PubMed] [Google Scholar]
- 9.Latino JO, Udry S, Aranda FM, et al. Pregnancy failure in patients with obstetric antiphospholipid syndrome with conventional treatment: the influence of a triple positive antibody profile. Lupus 2017;26:983–8. 10.1177/0961203317692432 [DOI] [PubMed] [Google Scholar]
- 10.De Carolis S, Botta A, Santucci S, et al. Complementemia and obstetric outcome in pregnancy with antiphospholipid syndrome. Lupus 2012;21:776–8. 10.1177/0961203312444172 [DOI] [PubMed] [Google Scholar]
- 11.Rezk M, Dawood R, Badr H. Maternal and fetal outcome in women with antiphospholipid syndrome: a three-year observational study. J Matern Fetal Neonatal Med 2016;29:4015–9. 10.3109/14767058.2016.1152254 [DOI] [PubMed] [Google Scholar]
- 12.Ruffatti A, Tonello M, Cavazzana A, et al. Laboratory classification categories and pregnancy outcome in patients with primary antiphospholipid syndrome prescribed antithrombotic therapy. Thromb Res 2009;123:482–7. 10.1016/j.thromres.2008.03.012 [DOI] [PubMed] [Google Scholar]
- 13.Belhocine M, Coutte L, Martin Silva N, et al. Intrauterine fetal deaths related to antiphospholipid syndrome: a descriptive study of 65 women. Arthritis Res Ther 2018;20:249. 10.1186/s13075-018-1745-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lazzaroni M-G, Fredi M, Andreoli L, et al. Triple antiphospholipid (APL) antibodies positivity is associated with pregnancy complications in APL carriers: a multicenter study on 62 pregnancies. Front Immunol 2019;10:1948. 10.3389/fimmu.2019.01948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saccone G, Berghella V, Maruotti GM, et al. Antiphospholipid antibody profile based obstetric outcomes of primary antiphospholipid syndrome: the PREGNANTS study. Am J Obstet Gynecol 2017;216:525. 10.1016/j.ajog.2017.01.026 [DOI] [PubMed] [Google Scholar]
- 16.Pengo V, Biasiolo A, Gresele P, et al. Survey of lupus anticoagulant diagnosis by central evaluation of positive plasma samples. J Thromb Haemost 2007;5:925–30. 10.1111/j.1538-7836.2007.02454.x [DOI] [PubMed] [Google Scholar]
- 17.Pengo V, Tripodi A, Reber G, et al. Update of the guidelines for lupus anticoagulant detection. Journal of Thrombosis and Haemostasis 2009;7:1737–40. 10.1111/j.1538-7836.2009.03555.x [DOI] [PubMed] [Google Scholar]
- 18.Grimaud F, Yelnik C, Pineton de Chambrun M, et al. Clinical and immunological features of antiphospholipid syndrome in the elderly: a retrospective national multicentre study. Rheumatology (Oxford) 2019;58:1006–10. 10.1093/rheumatology/key437 [DOI] [PubMed] [Google Scholar]
- 19.Jackson WG, Oromendia C, Unlu O, et al. Recurrent thrombosis in patients with antiphospholipid antibodies and arterial thrombosis on antithrombotic therapy. Blood Adv 2017;1:2320–4. 10.1182/bloodadvances.2017008185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sevim E, Zisa D, Andrade D, et al. Characteristics of patients with antiphospholipid antibody positivity in the APS ACTION international clinical database and repository. Arthritis Care Res (Hoboken) 2022;74:324–35. 10.1002/acr.24468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pengo V, Ruffatti A, Legnani C, et al. Incidence of a first thromboembolic event in asymptomatic carriers of high-risk antiphospholipid antibody profile: a multicenter prospective study. Blood 2011;118:4714–8. 10.1182/blood-2011-03-340232 [DOI] [PubMed] [Google Scholar]
- 22.Guédon AF, Catano J, Ricard L, et al. Non-criteria manifestations in primary antiphospholipid syndrome: a French multicenter retrospective cohort study. Arthritis Res Ther 2022;24:33. 10.1186/s13075-022-02726-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alijotas-Reig J, Esteve-Valverde E, Ferrer-Oliveras R, et al. The european registry on obstetric antiphospholipid syndrome (EUROAPS): a survey of 1000 consecutive cases. Autoimmun Rev 2019;18:406–14. 10.1016/j.autrev.2018.12.006 [DOI] [PubMed] [Google Scholar]
- 24.da Silva Saraiva S, de Moraes Mazetto B, Quinteiro Tobaldine L, et al. The impact of antibody profile in thrombosis associated with primary antiphospholipid syndrome. Am J Hematol 2017;92:1163–9. 10.1002/ajh.24875 [DOI] [PubMed] [Google Scholar]
- 25.Sciascia S, Radin M, Cecchi I, et al. Identifying phenotypes of patients with antiphospholipid antibodies: results from a cluster analysis in a large cohort of patients. Rheumatology (Oxford) 2021;60:1106–13. 10.1093/rheumatology/kez596 [DOI] [PubMed] [Google Scholar]
- 26.Ruffatti A, Calligaro A, Hoxha A, et al. Laboratory and clinical features of pregnant women with antiphospholipid syndrome and neonatal outcome. Arthritis Care Res (Hoboken) 2010;62:302–7. 10.1002/acr.20098 [DOI] [PubMed] [Google Scholar]
- 27.Ruffatti A, Tonello M, Visentin MS, et al. Risk factors for pregnancy failure in patients with anti-phospholipid syndrome treated with conventional therapies: a multicentre, case-control study. Rheumatology (Oxford) 2011;50:1684–9. 10.1093/rheumatology/ker139 [DOI] [PubMed] [Google Scholar]
- 28.Ruffatti A, Tonello M, Hoxha A, et al. Effect of additional treatments combined with conventional therapies in pregnant patients with high-risk antiphospholipid syndrome: a multicentre study. Thromb Haemost 2018;118:639–46. 10.1055/s-0038-1632388 [DOI] [PubMed] [Google Scholar]
- 29.Ruffatti A, Salvan E, Del Ross T, et al. Treatment strategies and pregnancy outcomes in antiphospholipid syndrome patients with thrombosis and triple antiphospholipid positivity. A European multicentre retrospective study. Thromb Haemost 2014;112:727–35. 10.1160/TH14-03-0191 [DOI] [PubMed] [Google Scholar]
- 30.Kim MY, Buyon JP, Guerra MM, et al. Angiogenic factor imbalance early in pregnancy predicts adverse outcomes in patients with lupus and antiphospholipid antibodies: results of the PROMISSE study. Am J Obstet Gynecol 2016;214:108. 10.1016/j.ajog.2015.09.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kravvariti E, Koutsogianni A, Samoli E, et al. The effect of hydroxychloroquine on thrombosis prevention and antiphospholipid antibody levels in primary antiphospholipid syndrome: a pilot open label randomized prospective study. Autoimmun Rev 2020;19:102491. 10.1016/j.autrev.2020.102491 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2022-002534supp001.pdf (866.9KB, pdf)
Data Availability Statement
Data are available on reasonable request.


