Abstract
Objective
To develop a score assessing the probability of relapse in granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA).
Methods
Long-term follow-up data from GPA and MPA patients included in five consecutive randomised controlled trials were pooled. Patient characteristics at diagnosis were entered into a competing-risks model, with relapse as the event of interest and death the competing event. Univariate and multivariate analyses were computed to identify variables associated with relapse and build a score, which was then validated in an independent cohort of GPA or MPA patients.
Results
Data collected from 427 patients (203 GPA, 224 MPA) at diagnosis were included. Mean±SD follow-up was 80.6±51.3 months; 207 (48.5%) patients experienced ≥1 relapse. Relapse risk was associated with proteinase 3 (PR3) positivity (HR=1.81 (95% CI 1.28 to 2.57); p<0.001), age ≤75 years (HR=1.89 (95% CI 1.15 to 3.13); p=0.012) and estimated glomerular filtration rate (eGFR) ≥30 mL/min/1.73 m² (HR=1.67 (95% CI 1.18 to 2.33); p=0.004) at diagnosis. A score, the French Vasculitis Study Group Relapse Score (FRS), from 0 to 3 points was modelised: 1 point each for PR3-antineutrophil cytoplasmic antibody positivity, eGFR ≥30 mL/min/1.73 m² and age ≤75 years. In the validation cohort of 209 patients, the 5-year relapse risk was 8% for a FRS of 0, 30% for 1, 48% for 2 and 76% for 3.
Conclusion
The FRS can be used at diagnosis to assess the relapse risk in patients with GPA or MPA. Its value for tailoring the duration of maintenance therapy should be evaluated in future prospective trials.
Keywords: Systemic vasculitis, Granulomatosis with polyangiitis, Rituximab
WHAT IS ALREADY KNOWN ON THIS TOPIC.
Granulomatosis with polyangiitis and microscopic polyangiitis are two antineutrophil cytoplasmic antibody (ANCA)-associated vasculitides (AAV) in which relapse is common. There is a lack of criteria to predict the risk of relapse in AAV.
WHAT THIS STUDY ADDS
We identified three factors (PR3-ANCA, age ≤75 years and estimated glomerular filtration rate ≥30 mL/min/1.73 m²) associated with a higher risk of relapse of AAV. These factors were combined to form a score, ranging from 0 to 3 points (1 point for each factor) that predicts the risk of relapse in AAV.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This score could, therefore, help clinicians tailor the duration of maintenance therapy in AAV, provided it is evaluated and validated in future prospective trials.
Introduction
Granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA) are the two main antineutrophil cytoplasmic antibody (ANCA)-associated vasculitides (AAV).1 GPA and MPA differ in their pathogenesis, genetics and serotypes, but patients with these two types of vasculitis share many clinical features and currently receive similar treatments.2–7 A therapeutic strategy combining glucocorticoids (GC) and cyclophosphamide or rituximab to induce remission has dramatically improved survival of AAV in the past decades.5–10
Despite this improvement, maintaining remission in patients with GPA or MPA remains challenging. Relapse occurs in 13.7%–44% of cases at 18–36 months depending on the duration of follow-up, patient characteristics and maintenance treatment.2 4 5 10 Maintenance therapy with low-dose preemptive rituximab has significantly decreased the risk of relapse in comparison with azathioprine,11 but relapses still occur after rituximab discontinuation. Long-term follow-up of the MAINRITSAN 1 trial showed that relapse-free survival was 58% at 60 months in patients treated with rituximab 500 mg every 6 months for 18 months.12 Increasing the duration of azathioprine maintenance therapy from 24 to 48 months has been shown to decrease the risk of relapse and improve renal outcome.13
However, adverse events were more frequent in the group of patients receiving 48 months of maintenance treatment, suggesting that prolonged maintenance therapy is probably not suitable for every GPA or MPA patient.13 The MAINRITSAN 3 trial recently confirmed that extended therapy with biannual rituximab infusions over 18 months also significantly decreases the frequency of AAV relapse without increasing the number of severe adverse events. Although prolonged treatment with rituximab had a good safety profile during the follow-up period of the pivotal clinical trials,5 9 11 12 14 data available since the SARS-Cov2 pandemic have shown that rituximab increases morbidity and mortality in these patients when infected with SARS-Cov215 16 because it increases the risk of severe forms of COVID-19 and because it decreases the quality of the postvaccine humoral immune response to SARS-Cov2.17 18
We do not yet have sufficient knowledge to predict flares or relapses. We also lack reliable indicators that could be used to tailor treatment in order to minimise immunosuppression in those less likely to experience relapse. Such markers could also be used to monitor patients more likely to experience relapse while maintaining higher levels of immunosuppressive therapy. It is therefore essential to better identify the factors associated with the risk of relapse in order to take a further step towards personalised management. To date, studies have mainly identified kidney failure as a protective factor against relapse, whereas anti-proteinase 3 (PR3) antibodies and cardiovascular involvement have been associated with an increased risk of relapse.19–22
The objective of this study was to identify baseline clinical and biological patient characteristics associated with the probability of relapse during follow-up in order to develop a relapse prediction score. This score could help clinicians determine whether patients in remission after induction therapy should receive extended maintenance treatment.
Patients and methods
Patients and definitions
Patient and disease characteristics at diagnosis and long-term follow-up data were pooled from five consecutive prospective trials conducted by the French Vasculitis Study Group (FVSG) involving GPA and MPA patients (CHUSPAN I, CHUSPAN II, CORTAGE, MAINRITSAN and WEGENT).4 10–12 23–27 All trials included GPA and MPA patients fulfilling the revised Chapel Hill Consensus Conference nomenclature.1 All patients were included at AAV diagnosis, except in the MAINRITSAN and WEGENT studies in which patients were included at the start of maintenance therapy. Thereby, GPA and MPA patients included in these studies after a last flare which was already a relapse and for whom data at diagnosis of AAV were missing, were excluded from this study. Patients with eosinophilic GPA (EGPA) or polyarteritis nodosa (PAN) included in the CHUSPAN I, CHUSPAN II and CORTAGE studies were also excluded from the analysis.
Data collection
Development cohort
Patients were assessed at baseline for manifestations of GPA and MPA in each organ system using the Birmingham Activity Score (BVAS)28 and were treated according to the protocol of the CHUSPAN I, CHUSPAN II, WEGENT, MAINRITSAN and CORTAGE trials. Then, patients were prospectively monitored routinely in extended monitoring studies with data reported on relapses, treatments, vasculitis-induced damage and vital status. Follow-up data on ANCA were not collected in this study since we were only interested in the effect of clinico-biological characteristics at diagnosis on subsequent relapse risk.
Validation cohort
The validation cohort included AAV patients aged ≥65 years from the FVSG registry database.29 Patients were included if they had a new diagnosis of GPA or MPA made after 2000, according to the revised Chapel Hill Consensus Conference,1 and if they were either followed up for at least 6 months and/or were deceased. Duplicates with the development cohort were removed from the validation cohort. All patients enrolled in the validation cohort were drawn from the FVSG registry and thus provided written informed consent.
Definitions
Remission was defined as the absence of disease activity attributable to GPA or MPA manifestations for ≥3 consecutive months, corresponding to BVAS=0, not requiring being off or on a specified GC dose. Relapse was defined as the recurrence and/or appearance of ≥1 new vasculitis manifestation(s) after remission lasting ≥3 months.30
Statistical analyses
Continuous variables are expressed as means ±SD and categorical variables as numbers (%). Statistical analyses were computed using SAS V.9.4 (SAS Institute) and R software using the TimeROC package.31
In AAV, death and relapse are not independent events. Conventional statistical methods for time-to-event analysis assume that competing risks are absent, meaning that incidence (assuming independent competing risks) of relapse is estimated in a population where no one dies, which may not realistically reflect relapse risk in a population.32 Thus, we applied a Fine-Gray subdistribution hazard model to model the subdistribution hazard of AAV relapse with the death being considered as the competing event. The proportional hazards assumption was not realistic for all data, insofar as the effectiveness of the treatments differs from one trial to the other. To account for this heterogeneity of treatment effect on relapse risk, we perform a stratified analysis, using The STRATA statement of SAS PHREG procedure.33 The trial arm (three arms for CHUSPAN I, and two arms each for CHUSPAN II, CORTAGE, MAINRITSAN and WEGENT)4 10–12 23–27 was used to determine the strata levels. Times to relapse and/or death were calculated from treatment onset.
The candidate variables to be entered in the score were: sex, diagnosis (MPA vs GPA), ANCA status (positive vs negative), PR3-ANCA status (positive vs negative), MPO-ANCA status (positive vs negative), fever, myalgias, arthralgias, ear, nose and throat (ENT) involvement, pulmonary involvement, asthma, alveolar haemorrhage, pulmonary nodules, kidney involvement, proteinuria >0.2 g/day, haematuria, skin lesions, neurological involvement, peripheral nervous system involvement, mononeuritis multiplex, peripheral polyneuropathy, central nervous system involvement, cardiac involvement, pericarditis, specific cardiomyopathy, gangrene, gastrointestinal involvement, abdominal pain, severe abdominal involvement, digestive haemorrhage, ophthalmologic symptoms, serum creatinine, estimated glomerular filtration rate (eGFR) (CKD-EPI), five factor score (1996 and 2011 versions).
We developed the score using the following steps:
Step 1
The relapse sub-HRs and corresponding CIs and p values were obtained for all candidate variables, separately, using a bivariate Fine-Gray model stratified on the treatment arm. All candidate variables were categorical except for age and GFR, for which the optimal threshold was determined according to clinical relevance and spline regression.
Step 2
A multivariate Fine-Gray model was constructed using a manual backward selection procedure including all variables influencing the relapse sub-HR with a p≤0.2 in the last step. All covariates with a significant (p<0.05) effect on relapse subhazard were kept in the final model (the list of included variables is provided in online supplemental file.
rmdopen-2022-002953supp001.pdf (53.1KB, pdf)
Step 3
Two score calculations have been proposed from the final model. In the first, estimated regression coefficients were multiplied by 10 and rounded to the nearest integer.34 The resultant integer represents the ponderation for the presence of the given risk factor. Reference levels of the categorical variable were assigned a score of zero. In the second, we developed a simple score for which the weighting was 1 (for variables increasing the risk of relapse) or −1 (for variable protecting against relapse). The relapse score was obtained by summing the weights of the risk factors present in both scoring systems.
Step 4
Then, the discriminative ability of simple and complex scores to predict relapse at 24, 36, 48, 60, 72 and 84 months after a diagnosis of vasculitis were investigated. For this purpose, we estimated the area under the ROC curve (AUC) for relapse prediction as a function of the score value at each of these time points using the procedure described by Blanche et al.31 This step allowed us to choose the best weighting system for scoring.
Step 5
Finally, the score was validated in an independent cohort of 209 patients with GPA or MPA using a Fine-Gray model with the score value as the independent variable. The cumulative incidence function of relapse was estimated and compared between the different categories of score value. Finally, the AUC of the score to predict relapse at different times was calculated to check the discrimination of the score.
Results
Studied population
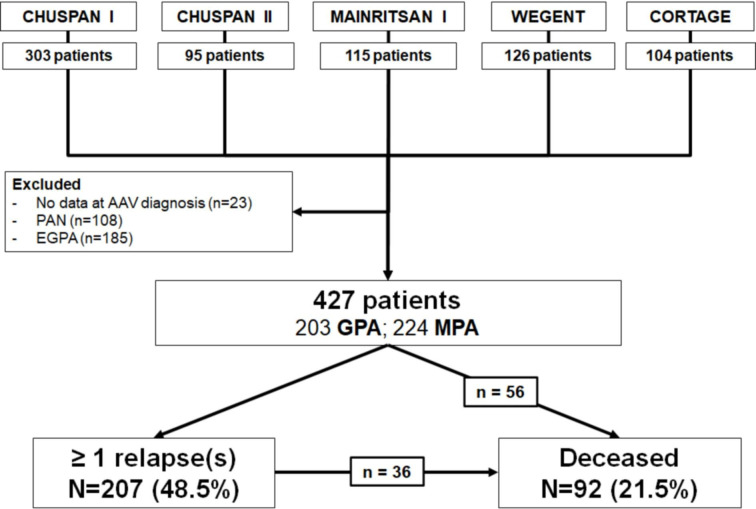
Patients with PAN (n=108), EGPA (n=185) and those with missing data at diagnosis of AAV (n=23) were not considered in this study. Finally, a total of 427 patients included at diagnosis of AAV (203 GPA and 224 MPA) were analysed in the development cohort. Mean follow-up was 80.6±51.3 months; 207 (48.5%) patients experienced at least one relapse during follow-up. Of the 92 (21.5%) that died, 56 had no previous relapse and 36 did have a relapse (figure 1). The characteristics of the clinical trials in which these 427 patients were included are summarised in table 1.
Figure 1.
Flow chart of the study (development cohort). AAV, ANCA-associated vasculitides; EGPA, eosinophilic GPA; GPA, granulomatosis with polyangiitis; MPA, microscopic polyangiitis; PAN, polyarteritis nodosa.
Table 1.
Summary of eligibility and treatment regimen in the included trials
| Trial | Included disease stage | Age (years) at diagnosis, mean±SD | Induction treatment | Maintenance treatment | No of included patients* (MPA/GPA) | Follow-up (months), mean±SD | Outcome relapse: n (%) deceased: n (%) |
| CHUSPAN I (FFS=0) |
|
57.4±15.0 | GC | GC | 61 (61/0) | 97.8±40.3 | 35/61 (57) 8/61 (13) |
| CHUSPAN I (FFS≥1) |
|
60.3±15.4 | GC+IV CYC (6 vs 12 pulses) | GC | 40 (40/0) | 99.9±77.9 | 19/40 (48) 25/40 (63) |
| CHUSPAN II | MPA, PAN, EGPA | 72.7±11.6 | GC+PLA vs GC+AZA | GC+PLA vs GC+AZA | 25 (25/0) | 23.1±4.8 | 9/25 (36) 0/25 (0) |
| CORTAGE | MPA, GPA, PAN, EGPA | 75.8±6.3 |
|
|
83 (47/36) | 31.0±16.5 | 22/83 (27) 21/83 (25) |
| MAINRITSAN I | MPA, GPA | 55.1±13.4 | GC+RTX or GC+IV CYC | GC+RTX vs GC+AZA | 92 (21/71) | 65.4±9.2 | 47/92 (51) 4/92 (4) |
| WEGENT | MPA, GPA | 58.5±12.9 | GC+IV CYC | GC+AZA vs GC+MTX |
126 (30/96) | 121.4±114.3 | 75/126 (60) 34/126 (27) |
*Number of patients included in the development cohort of this study
AZA, azathioprine; EGPA, eosinophilic GPA; FFS, five factor score; GC, glucocorticoids; GPA, granulomatosis with polyangiitis; IV CYC, intravenous cyclophosphamide; MPA, microscopic polyangiitis; MTX, methotrexate; PAN, polyarteritis nodosa; PLA, placebo; RTX, rituximab.
Baseline characteristics of the development cohort
The main characteristics of the patients at AAV diagnosis are summarised in table 2. At diagnosis, mean age was 62.0±14.7 years, mean serum creatinine level was 184.0±186.8 µmol/L and GFR, as estimated by Chronic Kidney Disease EPIdemiology collaboration (CKD-EPI), was 56.6±35.0 mL/min/1.73 m². Maintenance therapy included immunosuppressive drugs (methotrexate, azathioprine or rituximab) in 301 patients (70%).
Table 2.
Description of the two cohorts of AAV patients at diagnosis
| Development cohort (n=427) | Validation cohort (n=209) | |
| Follow-up (months) | 80.6±51.3 | 38.1±19.9 |
| Age at diagnosis (years) | 62.0±14.7 | 78.1±5.8 |
| Age at diagnosis >75 years | 74 (17%) | 168 (80%) |
| Sex (F/M) | 209/218 | 122/87 |
| Diagnosis | ||
| GPA | 203 (48%) | 102 (49%) |
| MPA | 224 (52%) | 105 (50%) |
| Unclassified | 0 | 2 (1%) |
| Clinical characteristics | ||
| Weight loss >2 kg | 279 (65%) | 88/204 (43%) |
| Arthralgia or myalgia | 283 (66%) | 82/206 (40%) |
| ENT signs | 216 (51%) | 81 (39%) |
| Pulmonary signs | 256 (60%) | 96 (46%) |
| Alveolar haemorrhage | 57 (13%) | 33 (16%) |
| Pulmonary nodules | 116 (27%) | 37 (18%) |
| Kidney involvement | 302 (71%) | 140 (67%) |
| Proteinuria >0.2 g/day | 254 (60%) | 105/129 (81%) |
| Haematuria | 212 (50%) | 115/195 (59%) |
| Skin lesions | 173 (41%) | 38 (28%) |
| Neurological involvement | 194 (46%) | 54 (26%) |
| Peripheral nervous system | 157 (37%) | 43 (21%) |
| Central nervous system | 22 (5%) | 10 (5%) |
| Cardiovascular involvement | 59 (14%) | 12 (6%) |
| Pericarditis | 26 (6%) | 3 (1%) |
| Cardiomyopathy | 16 (4%) | 1 (0.5%) |
| Gastrointestinal symptoms | 72 (17%) | 3 (1%) |
| Ophthalmologic symptoms | 71 (17%) | 9 (4%) |
| Biology | ||
| Serum creatinine level (µmol/L) | 184.0±186.8 | 226±210 |
| Glomerular filtration rate (CKD-EPI, mL/min/1.73 m²) | 56.6±35.0 | 46.9±36.2 |
| ANCA immunofluorescence, n (%)* | 365 (85%) | 204 (98%) |
| ANCA ELISA, n (%) | ||
| Negative | 76 (18%) | 9 (4%) |
| MPO-ANCA | 184 (43%) | 131 (63%)† |
| PR3-ANCA | 167 (39%) | 74 (35%)† |
| Induction treatment | ||
| Glucocorticoids | 427 (100%) | 208 (99%) |
| Plasma exchange | 0 | 24/208 (12%) |
| Immunosuppressant | 350 (82%) | 178 (85%) |
| IV cyclophosphamide | 341 (80%) | 125 (60%) |
| Rituximab | 0 | 53 (25%) |
| Other | 9 (2%)‡ | 10 (5%) |
| Maintenance treatment | ||
| Immunosuppressant | 301 (70%) | 168 (80%) |
| Azathioprine | 184 (43%) | 81 (39%) |
| Methotrexate | 66 (15%) | 15 (7%) |
| Rituximab | 46 (11%) | 84 (40%) |
| Other | 5 (1%) | 10 (5%) |
Continuous variables are expressed by mean±SD. Categorical variables are expressed by number (percentage).
*As defined by immunofluorescence. **5 patients had both anti MPO and anti PR3 antibodies.
†5 patients had both anti MPO and anti PR3 antibodies.
‡These patients were all included in the AZA arm of the CHUSPAN II trial.
AAV, ANCA-associated vasculitides; ANCA, antineutrophil cytoplasmic antibody; ENT, ear nose and throat; GPA, granulomatosis with polyangiitis; MPA, microscopic polyangiitis.
Baseline characteristics of the validation cohort
Characteristics of the 209 patients (102 GPA, 105 MPA and 2 unclassified AAV) of the validation cohort are detailed in table 2. Mean age at diagnosis was 78.1±5.8 years, 178 (85%) patients received immunosuppressants as induction therapy and 190 (91%) received immunosuppressants during maintenance therapy. After a mean follow-up of 38.1±19.9 months, 44 (21%) patients relapsed and 14 (7%) died.
Factors associated with relapse
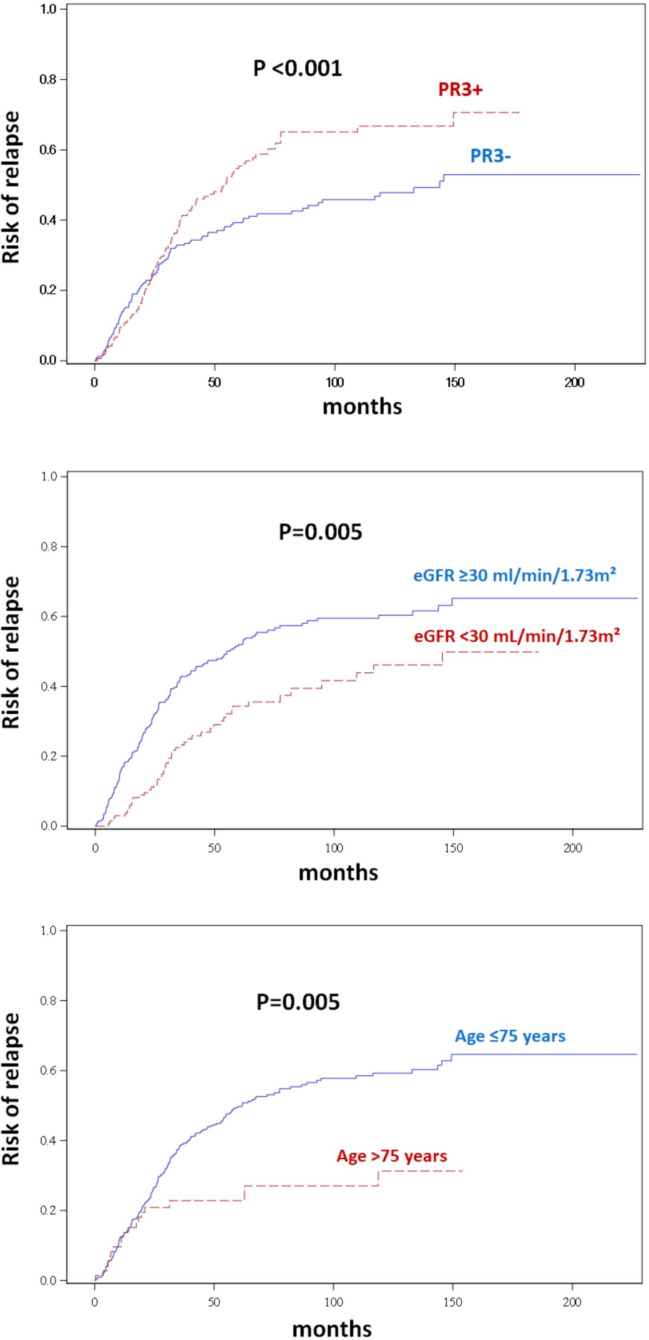
Results of univariate and multivariate analyses are summarised in table 3 and figure 2. A higher risk of relapse was independently associated with PR3-ANCA (HR=1.81 (95% CI 1.28 to 2.57); p<0.001), age ≤75 years (HR=1.89 (95% CI 1.15 to 3.13); p=0.012) and eGFR (CKD-EPI) ≥30 mL/min/1.73 m² (HR=1.67 (95% CI 1.18 to 2.33); p=0.004) at AAV diagnosis.
Table 3.
Factors associated with the occurrence of relapse in the development cohort
| Univariate | Multivariate model | |||
| HR | P value | HR | P value | |
| Age ≤60 years | 1.05 | 0.702 | ||
| Age ≤65 years | 1.52 | 0.010 | ||
| Age ≤70 years | 1.59 | 0.009 | ||
| Age ≤75 years | 2.04 | 0.005 | 1.89 | 0.012 |
| Sex (M vs F) | 1.01 | 0.938 | ||
| MPA (vs GPA) | 0.55 | 0.003 | * | * |
| Fever | 1.05 | 0.714 | ||
| Weight loss ≥2 kg | 0.93 | 0.619 | ||
| Arthralgia | 1.11 | 0.479 | ||
| Myalgia | 1.14 | 0.373 | ||
| ENT signs | 1.21 | 0.237 | ||
| Skin lesions | 0.93 | 0.649 | ||
| Pulmonary signs | 1.04 | 0.806 | ||
| Alveolar haemorrhage | 1.24 | 0.317 | ||
| Pulmonary nodules | 1.48 | 0.018 | * | * |
| Kidney involvement | 0.95 | 0.803 | ||
| Proteinuria >0.2 g/day | 0.98 | 0.891 | ||
| Haematuria | 0.92 | 0.633 | ||
| eGFR ≥30 mL/min/1.73 m² | 1.61 | 0.005 | 1.67 | 0.004 |
| Creatinine blood level >200 µmol/L | 0.62 | 0.010 | ||
| Cardiac involvement | 1.13 | 0.537 | ||
| Pericarditis | 1.62 | 0.060 | * | * |
| Specific myocardiopathy | 0.69 | 0.385 | ||
| Gastrointestinal symptoms | 0.89 | 0.547 | ||
| Peripheral nervous system involvement | 0.95 | 0.711 | ||
| Central nervous system involvement | 0.87 | 0.679 | ||
| Ophthalmological symptoms | 1.26 | 0.168 | * | * |
| Positive ANCA (any) | 1.37 | 0.181 | ||
| PR3-ANCA | 1.85 | <0.001 | 1.81 | <0.001 |
| MPO-ANCA | 0.67 | 0.010 | * | * |
*Excluded in the backward procedure.
eGFR, estimated glomerular filtration rate; ENT, ear nose and throat; GPA, granulomatosis with polyangiitis; MPA, microscopic polyangiitis.
Figure 2.
Risk of relapse depending on age, eGFR (CKD-EPI) and PR3 positivity in the development cohort. Curves are showing the risk of relapse (Fine-Gray model) with stratification on the group of treatment. CKD-EPI, Chronic Kidney Disease EPIdemiology collaboration; eGFR, estimated glomerular filtration rate.
Score predicting AAV relapse
Multivariate analysis was used to build a predictive score for relapse. AAV treatment (induction and maintenance) was taken into account via stratification on the randomisation arm in each therapeutic trial. For the first model, each item independently associated with the occurrence of relapse was weighted based on its HR. This first version of the score (complex score) varied from −11 to +6 points with 6 possible states. Simplification of this complex score was evaluated by grouping=strata (online supplemental figure 1). Therefore, a simplified version of the score, entitled the FVSG Relapse Score (FRS), was modelised as follow: 1 point for PR3-ANCA, 1 point for eGFR (CKD-EPI) ≥30 mL/min/1.73 m² and 1 point for age ≤75 years at AAV diagnosis for each item. Thus, the FRS varied from 0 to 3 points. Comparison of the AUC assessing the sensitivity and specificity of the complex score versus the FRS in the development cohort showed that the performance of these two scores was comparable (online supplemental table 1). We; therefore, retained the simple version of the score (FRS) for further analysis.
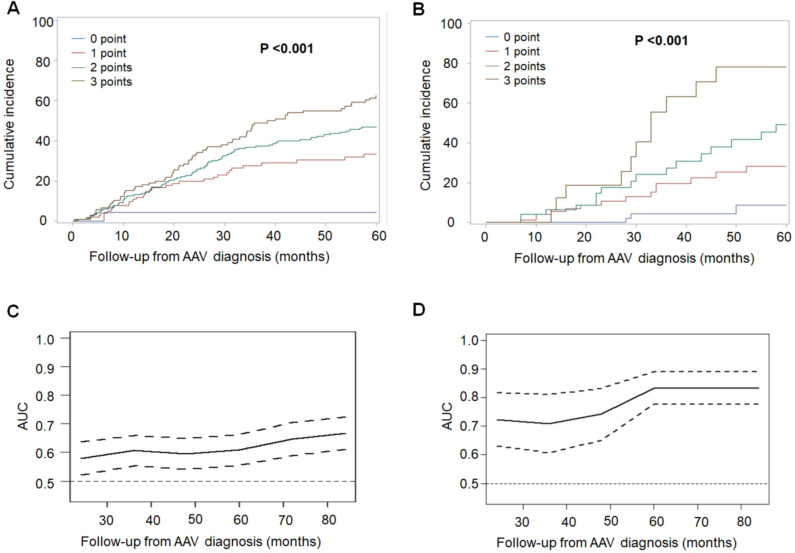
In the development cohort, the FRS was 0 for 23 (5.4%) patients, 1 for 104 (24.4%) patients, 2 for 195 (45.7%) patients and 3 for 105 (24.6%) patients. The risk of relapse as a function of score value at different time points after diagnosis of AAV is detailed in table 4 and illustrated in figure 3.
Table 4.
Risk of relapse (cumulative incidence function) in each cohort depending on the FRS at AAV diagnosis
| FRS | Development cohort (n=427) | Validation cohort (n=209) | ||||
| Risk of relapse 2 years after diagnosis | Risk of relapse 3 years after diagnosis | Risk of relapse 5 years after diagnosis | Risk of relapse 2 years after diagnosis | Risk of relapse 3 years after diagnosis | Risk of relapse 5 years after diagnosis | |
| 0 point | 2 (0.4–13.8) | 3 (0.6–20.4) | 4 (0.6–25.8) | 2 (0.6–6.0) | 5 (1.5–14.4) | 8 (2.3–26.8) |
| 1 point | 18 (13.1–24.2) | 27 (19.8–37.0) | 31 (23.5–41.3) | 8 (4.1–15.9) | 19 (11.2–31.2) | 30 (19.8–47.0) |
| 2 points | 25 (19.4–31.3) | 37 (30.6–43.8) | 42 (36.1–47.9) | 14 (7.4–26.4) | 31 (20.2–47.4) | 48 (33.0–69.1) |
| 3 points | 34 (28.5–41.1) | 49 (41.0–58.6) | 55 (47.6–63.2) | 29 (17.3–47.0) | 56 ((39.8–79.0) | 76 (58.2–100) |
Relapse score=age ≤75 years (1 point) + positive PR3-ANCA (1 point) + eGFR (CKD-EPI) ≥30 mL/min/1.73 m² (1 point). Results are expressed by % (CI at 95%).
AAV, ANCA-associated vasculitides; FRS, FVSG Relapse Score.
Figure 3.
(A, B) Risk of relapse (cumulative incidence function) depending on the French Vasculitis Study Group Relapse Score at AAV diagnosis in the development cohort (A) and the validation cohort (B). (C, D) Area under the ROC curve (AUC) evaluating sensitivity and specificity of the score to predict the occurrence of relapse across the follow-up from AAV diagnosis in the development cohort (C) and the validation cohort (D). Full line is the AUC and dotted lines show lower and higher limits of the CI at 95%. AAV, ANCA-associated vasculitides.
FRS in the validation cohort
In the validation cohort, the FRS was 0 in 59 (28.2%) patients, 1 in 83 (39.7%) patients, 2 in 49 (23.5%) patients and 3 in 18 (8.6%) patients. The risk of relapse as a function of score at the time of diagnosis of AAV in the validation cohort is detailed in table 4 and illustrated in figure 3. The risk of relapse 5 years after diagnosis of AAV was 8% for a FRS of 0 points, 30% for 1 point, 48% for 2 points and 76% for 3 points (table 4).
In the GPA patients (n=102/209), 16 patients had a 0-point FRS, 33 had a 1-point FRS, 36 had a 2-point FRS and 17 had a 3-point FRS. Among the patients with MPA (n=105/209), 43 patients had a 0-point FRS, 48 patients a 1-point FRS, 13 patients a 2-point FRS and only 1 patient a 3-point FRS. The risk of relapse at 3 and 5 years according to the FRS for each subgroup is shown in table 5. It varied significantly with FRS in both subgroups, especially at 5 years of follow-up: MPA (p=0.009), GPA (p=0.007) (table 5).
Table 5.
analysis of the risk of relapse according to the FRS in MPA and GPA patients (validation cohort)
| FRS | MPA (n=105) | GPA (n=102) | ||||
| Risk of relapse 3 years after diagnosis | Risk of relapse 5 years after diagnosis | P value | Risk of relapse 3 years after diagnosis | Risk of relapse 5 years after diagnosis | P value | |
| 0 point | 5.7 (1.0–16.9) | 11.2 (2.4–27.7) | 0.009 | 0* | 0* | 0.007 |
| 1 point | 6.6 (1.1–19.6) | 17.1 (4.8–35.9) | 39.7 (18.4–60.5) | 46.4 (22.5–67.4) | ||
| 2 points | 16.1 (2.3–41.4) | 28.1 (5.3–57.7) | 31.8 (14.4–50.8) | 56.1 (32.3–74.4) | ||
| 3 points | † | † | 60.0 (26.8–82.0) | 76.4 (37.7–92.8) | ||
FRS = age ≤75 years (1 point) + positive PR3-ANCA (1 point) + eGFR (CKD-EPI) ≥30 mL/min/1.73m² (1 point). Results are expressed by % (confidence interval at 95%). FRS: FVSG Relapse Score. P is the result of Fine-Gray tests comparing the risk of relapse according to the value of the FRS.
*No relapse occurred in this subgroup during follow-up.
†It was not possible to estimate the risk of relapse for the three-point FRS in the subgroup of patients with MPA because only one patient was included in this subgroup.
eGFR, estimated glomerular filtration rate; FRS, FVSG Relapse Score; GPA, granulomatosis with polyangiitis; MPA, microscopic polyangiitis.
Specificity and sensitivity of the FRS
AUC the ROC curves analysing the sensitivity and specificity of the score for predicting relapse throughout follow-up after AAV diagnosis showed that the performance of the FRS was better in the validation cohort than in the development cohort, increasing over time to 0.71 (0.61–0.81) at 36 months and 0.83 (0.78–0.89) at 60 months after AAV diagnosis (figure 3C, D).
Discussion
This study provides a simple score, entitled FRS, which can be calculated at the time of diagnosis of GPA or MPA to predict the risk of relapse during follow-up and which may ultimately allow clinicians to tailor the duration of maintenance therapy for these vasculitides. The FRS is composed of three variables and ranges from 0 to 3 points: positivity of PR3-ANCA, GFR ≥30 mL/min/1.73 m² and age ≤75 years (1 point each). The higher the FRS, the greater the risk of relapse. Thus, we can consider that the risk is very low for a FRS of 0, moderate for a FRS of 1 and high for a FRS of 2 or 3.
As previously reported,19–22 35 we found that the presence of PR3-ANCA at diagnosis was associated with an increased risk of relapse, whereas kidney failure reduced it. However, unlike previous studies,19 21 22 we did not find that cardiovascular involvement was associated with an increased risk of relapse, which may be related to the fact that this type of AAV involvement was not frequent in our development cohort (only 14% of cases). Another study36 had also shown that having PR3 ANCA was associated with an increased risk of relapse in two independent cohorts. The authors also found that the presence of lung involvement was associated with an increased risk of relapse, although this was not the case in our study. This discrepancy can be explained in several ways: (1) less than 50% of patients received maintenance immunosuppressive therapy in the study of Pagnoux et al (compared with 70% in the development cohort and 80% in the validation cohort of our study), (2) the statistical model used in this study did not take into account competing risks (death vs relapse) and (3) and because there is a very significant overlap between pulmonary manifestations and the presence of PR3 ANCA, but PR3 ANCA has a greater impact on the risk of relapse.
By contrast, we were able to identify that an age ≤75 years at AAV diagnosis was independently associated with an increased risk of relapse, which was also confirmed by the FVSG in a recently published study.29 It is likely that this finding was not previously identified because patients aged over 80 years were not included in European Vasculitis Society (EUVAS) therapeutic trials,19 which was not the case in the present work since the CORTAGE study, which was specifically designed for elderly individuals, enrolled 83 patients.10 Age could also explain why the performance of the FRS was better in the validation cohort than in the development cohort since the mean age at diagnosis of the validation cohort was 78 years. This result is very important since age is one of the most important prognostic factors for severe infections, which are currently the most frequent cause of death within 1 year after AAV diagnosis and the third most common after 1 year of follow-up.37–39 Therefore, our results suggest that the oldest patients, who are most at risk of infection and least at risk of relapse, should not be overtreated. However, we have also shown that although AAV patients >75 years have a lower relapse risk than patients aged 65–75 years despite a lower probability of having received maintenance therapy, they still benefit from such treatment regimen.29
The FRS is not applicable to EGPA and PAN patients, who were not included in this study. In PAN, the risk of relapse is lower.40 Regarding EGPA, even if it is also classified as an AAV,1 this vasculitis has notable differences and it is not treated exactly the same as GPA and MPA, including an approach that increasingly targets eosinophils.41 42
Estimation of the risk of relapse in the subgroup of MPA or GPA patients in the validation cohort showed that for the same FRS, the risk of relapse was higher in GPA than in MPA, although the CI was wide due to the small number of patients, and remained significantly predictive of relapse in both vasculitides. After 5 years of follow-up, none of the GPA patients with an FRS of 0 points had relapsed, 3/4 of those with an FRS of 3 points had relapsed, and about one in two patients with an initial intermediate FRS (1 or 2 points) had relapsed.
One of the weaknesses of our study is that most patients were treated before the widespread use of rituximab, which changed the treatment of GPA and MPA patients in a major way.43 In the validation cohort, 84 patients received rituximab maintenance therapy and of these only 8 relapsed during follow-up.44 Thus, it was not possible to reliably assess the performance of our score in the subgroup of patients who received maintenance therapy with rituximab. In a recent study including GPA and MPA patients treated with rituximab in induction and maintenance, age, presence of ANCA and ENT signs (which are associated with PR3-ANCA) were among the variables included in a predictive model for the risk of relapse after rituximab discontinuation, which is consistent with our results.45 The long-term follow-up data of the MAINRITSAN 1 trial also identified the importance of PR3-ANCA and the persistence of this marker after 1 year of maintenance therapy in predicting the occurrence of relapse after discontinuation of rituximab.12 The importance of the ANCA status during follow-up was confirmed in an independent cohort of GPA patients.46 However, these recent data and the fact that our analyses were stratified on the treatment arm of each trial, one of which included rituximab maintenance therapy (MAINRITSAN 1), suggests that our score may work in patients receiving maintenance therapy with rituximab. In any case, it will be necessary to validate the FRS in a larger and more recent cohort of patients, in whom RTX is the main used agent for induction and maintenance, as per current guidelines.
Our study has many strengths: the reliability of the data (data from prospective therapeutic trials), the use of a Fine-Gray model to distinguish between two competitive risks (death and relapse), the analysis stratified on the induction and maintenance treatment (which influences the progression of the disease), and its validation in an independent cohort, which allowed us to show that the FRS reliably predicts the risk of relapse and that its level of performance increases with time.
In summary, we propose a simple score, entitled FRS, ranging from 0 to 3 points (PR3-ANCA (1 point), age≤75 years (1 point) and GFR >30 mL/min/1.73 m² (1 point)), which may be used at diagnosis to predict the risk of relapse in patients with GPA or MPA. The value of the FRS for tailoring maintenance treatment for AAVs deserves to be validated in future prospective trials, with the aim of providing patients with appropriate personalised management.
Acknowledgments
We thank Suzanne Rankin for her help in reviewing the English. We thank Abderrahmane Bourredjem and Christine Binquet for their help in analyzing data. We thank all FVSG members who included patients (listed in the appendix).
Footnotes
Twitter: @Maxime_Samson21
Collaborators: French Vasculitis Study Group.
Contributors: MS and LG were the principal investigators and take primary responsibility for the paper. MS, ST, PC, CP, PC, AK, LM, BT, XP and LG recruited the patients. MS, HD, ST, BT, XP and LG contributed to data interpretation. HD did the statistical analysis. MS, BT, XP and LG drafted the manuscript. All authors revised the manuscript for important intellectual content and approved the final version of the article. Guarantor: MS.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: MS: Roche-Chugai, Vifor Pharma, Novartis, Boerhinger Ingelheim (consulting), Novartis (research grant).
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Patients enrolled in the development cohort provided signed informed consent at time they were included in five consecutive prospective trials conducted by the French Vasculitis Study Group (FVSG) (CHUSPAN I, CHUSPAN II, CORTAGE, MAINRITSAN and WEGENT). These trials were conducted according to the Declaration of Helsinki and subsequent amendments. In addition, all patients enrolled in the validation cohort were drawn from the French Vasculitis Study Group registry and thus provided written informed consent. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies. Participants gave informed consent to participate in the study before taking part.
References
- 1.Jennette JC, Falk RJ, Bacon PA, et al. 2012 revised international chapel Hill consensus conference Nomenclature of vasculitides. Arthritis Rheum 2013;65:1–11. 10.1002/art.37715 [DOI] [PubMed] [Google Scholar]
- 2.Jayne D, Rasmussen N, Andrassy K, et al. A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N Engl J Med 2003;349:36–44. 10.1056/NEJMoa020286 [DOI] [PubMed] [Google Scholar]
- 3.Lyons PA, Rayner TF, Trivedi S, et al. Genetically distinct subsets within ANCA-associated vasculitis. N Engl J Med 2012;367:214–23. 10.1056/NEJMoa1108735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pagnoux C, Mahr A, Hamidou MA, et al. Azathioprine or methotrexate maintenance for ANCA-associated vasculitis. N Engl J Med 2008;359:2790–803. 10.1056/NEJMoa0802311 [DOI] [PubMed] [Google Scholar]
- 5.Stone JH, Merkel PA, Spiera R, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med 2010;363:221–32. 10.1056/NEJMoa0909905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Groot K, Harper L, Jayne DRW, et al. Pulse versus daily oral cyclophosphamide for induction of remission in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized trial. Ann Intern Med 2009;150:670–80. 10.7326/0003-4819-150-10-200905190-00004 [DOI] [PubMed] [Google Scholar]
- 7.Jones RB, Tervaert JWC, Hauser T, et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med 2010;363:211–20. 10.1056/NEJMoa0909169 [DOI] [PubMed] [Google Scholar]
- 8.Holle JU, Gross WL, Latza U, et al. Improved outcome in 445 patients with Wegener’s granulomatosis in a German vasculitis center over four decades. Arthritis Rheum 2011;63:257–66. 10.1002/art.27763 [DOI] [PubMed] [Google Scholar]
- 9.Specks U, Merkel PA, Seo P, et al. Efficacy of remission-induction regimens for ANCA-associated vasculitis. N Engl J Med 2013;369:417–27. 10.1056/NEJMoa1213277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pagnoux C, Quéméneur T, Ninet J, et al. Treatment of systemic necrotizing vasculitides in patients aged sixty-five years or older: results of a multicenter, open-label, randomized controlled trial of corticosteroid and cyclophosphamide-based induction therapy. Arthritis Rheumatol 2015;67:1117–27. 10.1002/art.39011 [DOI] [PubMed] [Google Scholar]
- 11.Guillevin L, Pagnoux C, Karras A, et al. Rituximab versus azathioprine for maintenance in ANCA-associated vasculitis. N Engl J Med 2014;371:1771–80. 10.1056/NEJMoa1404231 [DOI] [PubMed] [Google Scholar]
- 12.Terrier B, Pagnoux C, Perrodeau É, et al. Long-Term efficacy of remission-maintenance regimens for ANCA-associated vasculitides. Ann Rheum Dis 2018;77:1150–6. 10.1136/annrheumdis-2017-212768 [DOI] [PubMed] [Google Scholar]
- 13.Karras A, Pagnoux C, Haubitz M, et al. Randomised controlled trial of prolonged treatment in the remission phase of ANCA-associated vasculitis. Ann Rheum Dis 2017;76:1662–8. 10.1136/annrheumdis-2017-211123 [DOI] [PubMed] [Google Scholar]
- 14.Charles P, Perrodeau É, Samson M, et al. Long-Term rituximab use to maintain remission of antineutrophil cytoplasmic antibody-associated vasculitis: a randomized trial. Ann Intern Med 2020;173:179–87. 10.7326/M19-3827 [DOI] [PubMed] [Google Scholar]
- 15.Strangfeld A, Schäfer M, Gianfrancesco MA, et al. Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 global rheumatology alliance physician-reported registry. Ann Rheum Dis 2021;80:930–42. 10.1136/annrheumdis-2020-219498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.FAI2R /SFR/SNFMI/SOFREMIP/CRI/IMIDIATE consortium and contributors . Severity of COVID-19 and survival in patients with rheumatic and inflammatory diseases: data from the french RMD COVID-19 cohort of 694 patients. Ann Rheum Dis 2021;80:527–38. 10.1136/annrheumdis-2020-218310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gavriatopoulou M, Terpos E, Ntanasis-Stathopoulos I, et al. Poor neutralizing antibody responses in 106 patients with WM after vaccination against SARS-cov-2: a prospective study. Blood Adv 2021;5:4398–405. 10.1182/bloodadvances.2021005444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Puéchal X, Cottin V, Faguer S, et al. French vasculitis study group recommendations for the management of COVID-19 vaccination and prophylaxis in patients with systemic vasculitis. Presse Med 2022;51:104107. 10.1016/j.lpm.2021.104107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walsh M, Flossmann O, Berden A, et al. Risk factors for relapse of antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum 2012;64:542–8. 10.1002/art.33361 [DOI] [PubMed] [Google Scholar]
- 20.Li Z-Y, Chang D-Y, Zhao M-H, et al. Predictors of treatment resistance and relapse in antineutrophil cytoplasmic antibody-associated vasculitis: a study of 439 cases in a single Chinese center. Arthritis Rheumatol 2014;66:1920–6. 10.1002/art.38621 [DOI] [PubMed] [Google Scholar]
- 21.Pierrot-Deseilligny Despujol C, Pouchot J, Pagnoux C, et al. Predictors at diagnosis of a first Wegener’s granulomatosis relapse after obtaining complete remission. Rheumatology (Oxford) 2010;49:2181–90. 10.1093/rheumatology/keq244 [DOI] [PubMed] [Google Scholar]
- 22.King C, Druce KL, Nightingale P, et al. Predicting relapse in anti-neutrophil cytoplasmic antibody-associated vasculitis: a systematic review and meta-analysis. Rheumatol Adv Pract 2021;5:rkab018. 10.1093/rap/rkab018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Puéchal X, Pagnoux C, Baron G, et al. Adding azathioprine to remission-induction glucocorticoids for eosinophilic granulomatosis with polyangiitis (Churg-Strauss), microscopic polyangiitis, or polyarteritis nodosa without poor prognosis factors: a randomized, controlled trial. Arthritis Rheumatol 2017;69:2175–86. 10.1002/art.40205 [DOI] [PubMed] [Google Scholar]
- 24.Puéchal X, Pagnoux C, Perrodeau É, et al. Long-Term outcomes among participants in the WEGENT trial of remission-maintenance therapy for granulomatosis with polyangiitis (Wegener’s) or microscopic polyangiitis. Arthritis Rheumatol 2016;68:690–701. 10.1002/art.39450 [DOI] [PubMed] [Google Scholar]
- 25.Samson M, Puéchal X, Devilliers H, et al. Long-term follow-up of a randomized trial on 118 patients with polyarteritis nodosa or microscopic polyangiitis without poor-prognosis factors. Autoimmun Rev 2014;13:197–205. 10.1016/j.autrev.2013.10.001 [DOI] [PubMed] [Google Scholar]
- 26.Samson M, Puéchal X, Devilliers H, et al. Long-term outcomes of 118 patients with eosinophilic granulomatosis with polyangiitis (churg-strauss syndrome) enrolled in two prospective trials. J Autoimmun 2013;43:60–9. 10.1016/j.jaut.2013.03.003 [DOI] [PubMed] [Google Scholar]
- 27.Samson M, Puéchal X, Mouthon L, et al. Microscopic polyangiitis and non-HBV polyarteritis nodosa with poor-prognosis factors: 10-year results of the prospective CHUSPAN trial. Clin Exp Rheumatol 2017;35 Suppl 103:176–84. [PubMed] [Google Scholar]
- 28.Luqmani RA, Bacon PA, Moots RJ, et al. Birmingham vasculitis activity score (BVAS) in systemic necrotizing vasculitis. QJM 1994;87:671–8. [PubMed] [Google Scholar]
- 29.Thietart S, Beinse G, Smets P, et al. Patients of 75 years and over with ANCA-associated vasculitis have a lower relapse risk than younger patients: a multicentre cohort study. J Intern Med 2022;291:350–63. 10.1111/joim.13417 [DOI] [PubMed] [Google Scholar]
- 30.Hellmich B, Flossmann O, Gross WL, et al. EULAR recommendations for conducting clinical studies and/or clinical trials in systemic vasculitis: focus on anti-neutrophil cytoplasm antibody-associated vasculitis. Ann Rheum Dis 2007;66:605–17. 10.1136/ard.2006.062711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blanche P, Dartigues JF, Jacqmin-Gadda H. Estimating and comparing time-dependent areas under receiver operating characteristic curves for censored event times with competing risks. Stat Med 2013;32:5381–97. 10.1002/sim.5958 [DOI] [PubMed] [Google Scholar]
- 32.Austin PC, Lee DS, D’Agostino RB, et al. Developing points-based risk-scoring systems in the presence of competing risks. Stat Med 2016;35:4056–72. 10.1002/sim.6994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wenink MH, Santegoets KCM, Roelofs MF, et al. The inhibitory Fc gamma IIb receptor dampens TLR4-mediated immune responses and is selectively up-regulated on dendritic cells from rheumatoid arthritis patients with quiescent disease. J Immunol 2009;183:4509–20. 10.4049/jimmunol.0900153 [DOI] [PubMed] [Google Scholar]
- 34.Sullivan LM, Massaro JM, D’Agostino RB. Presentation of multivariate data for clinical use: the Framingham study risk score functions. Stat Med 2004;23:1631–60. 10.1002/sim.1742 [DOI] [PubMed] [Google Scholar]
- 35.Mahr A, Girard T, Agher R, et al. Analysis of factors predictive of survival based on 49 patients with systemic Wegener’s granulomatosis and prospective follow-up. Rheumatology (Oxford) 2001;40:492–8. 10.1093/rheumatology/40.5.492 [DOI] [PubMed] [Google Scholar]
- 36.Pagnoux C, Hogan SL, Chin H, et al. Predictors of treatment resistance and relapse in antineutrophil cytoplasmic antibody-associated small-vessel vasculitis: comparison of two independent cohorts. Arthritis Rheum 2008;58:2908–18. 10.1002/art.23800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wallace ZS, Fu X, Harkness T, et al. All-Cause and cause-specific mortality in ANCA-associated vasculitis: overall and according to ANCA type. Rheumatology (Oxford) 2020;59:2308–15. 10.1093/rheumatology/kez589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Flossmann O, Berden A, de Groot K, et al. Long-Term patient survival in ANCA-associated vasculitis. Ann Rheum Dis 2011;70:488–94. 10.1136/ard.2010.137778 [DOI] [PubMed] [Google Scholar]
- 39.Rathmann J, Jayne D, Segelmark M, et al. Incidence and predictors of severe infections in ANCA-associated vasculitis: a population-based cohort study. Rheumatology (Oxford) 2021;60:2745–54. 10.1093/rheumatology/keaa699 [DOI] [PubMed] [Google Scholar]
- 40.Pagnoux C, Seror R, Henegar C, et al. Clinical features and outcomes in 348 patients with polyarteritis nodosa: a systematic retrospective study of patients diagnosed between 1963 and 2005 and entered into the French vasculitis Study Group database. Arthritis Rheum 2010;62:616–26. 10.1002/art.27240 [DOI] [PubMed] [Google Scholar]
- 41.Bettiol A, Urban ML, Bello F, et al. Sequential rituximab and mepolizumab in eosinophilic granulomatosis with polyangiitis (EGPA): a European multicentre observational study. Ann Rheum Dis 2022;81:1769–72. 10.1136/ard-2022-222776 [DOI] [PubMed] [Google Scholar]
- 42.Wechsler ME, Akuthota P, Jayne D, et al. Mepolizumab or placebo for eosinophilic granulomatosis with polyangiitis. N Engl J Med 2017;376:1921–32. 10.1056/NEJMoa1702079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kitching AR, Anders H-J, Basu N, et al. Anca-Associated vasculitis. Nat Rev Dis Primers 2020;6:71. 10.1038/s41572-020-0204-y [DOI] [PubMed] [Google Scholar]
- 44.Thietart S, Karras A, Augusto J-F, et al. Evaluation of rituximab for induction and maintenance therapy in patients 75 years and older with antineutrophil cytoplasmic antibody-associated vasculitis. JAMA Netw Open 2022;5:e2220925. 10.1001/jamanetworkopen.2022.20925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McClure ME, Zhu Y, Smith RM, et al. Long-Term maintenance rituximab for ANCA-associated vasculitis: relapse and infection prediction models. Rheumatology (Oxford) 2021;60:1491–501. 10.1093/rheumatology/keaa541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cho LK, Carette S, Pagnoux C. ANCA status and renal parameters at month 12 post-diagnosis can help predict subsequent relapses in patients with granulomatosis with polyangiitis. Semin Arthritis Rheum 2021;51:1011–5. 10.1016/j.semarthrit.2021.07.013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2022-002953supp001.pdf (53.1KB, pdf)
Data Availability Statement
Data are available on reasonable request.