Abstract
Background and Aims
Previous studies conducted in Europe suggested that darvadstrocel, a suspension of expanded, allogeneic, adipose-derived, mesenchymal stem cells, is safe and effective for treatment-refractory complex perianal fistulas in patients with Crohn’s disease. The aim of this study was to evaluate the efficacy and safety of darvadstrocel for the treatment of complex perianal fistulas in Japanese adults with Crohn’s disease.
Methods
This is a phase 3, open-label, single-arm study conducted at nine sites in Japan. Adult patients with non-active or mildly active Crohn’s disease and complex perianal fistulas received a single 24-mL intralesional injection of darvadstrocel [120 × 106 cells]. The primary endpoint was combined remission (clinically confirmed closure of all treated external openings that were draining at screening, and absence of collections >2 cm, [confirmed by magnetic resonance imaging] among treated fistulas) at Week 24.
Results
Between March 6, 2019 and February 1, 2021, 22 patients received darvadstrocel and completed the 52-week follow-up. The proportion of patients achieving combined remission at Week 24 was 59.1% (95% confidence interval [CI], 38.5–79.6). The effect was maintained at Week 52, with 68.2% [95% CI, 48.7–87.6] of patients achieving combined remission. Treatment-related adverse events included: one [4.5%] patient with worsening of Crohn’s disease and diarrhoea, and one [4.5%] patient with blood bilirubin increase. No new safety findings were identified in this study.
Conclusions
The efficacy and tolerability of darvadstrocel in Japanese adult patients with treatment-refractory complex perianal fistulas in Crohn’s disease were similar to those observed in the previous European study. ClinicalTrials.gov number, NCT03706456.
Keywords: Darvadstrocel, Crohn’s disease, perianal fistula
Graphical Abstract
Graphical Abstract.
1. Introduction
Crohn’s disease [CD] is a chronic and relapsing inflammatory bowel disease affecting the entire gastrointestinal tract.1 The highest prevalence rates [>300 per 100 000 people] have been reported in North America and Europe,2 and the prevalence of CD among Japanese people is increasing.3 In an analysis of a nationwide survey in Japan, the estimated number of patients with CD in 2015 was 70 700.4
One of the most frequent complications of CD is perianal fistulas.5 The prevalence of perianal fistulas in patients with CD ranges from 11% to 38%.6 Approximately one-quarter of patients with CD are estimated to develop perianal fistulas within two decades after diagnosis,7 and up to 80% of these are classified as complex perianal fistulas, ie, fistulas at a high location, that may have multiple external openings, and that are associated with perianal abscess, rectovaginal fistula, proctitis, or anorectal stricture.8,9 Complex perianal fistulas are associated with high morbidity and decreased quality of life.8,10 In a study of patients with CD in Japan, the prevalence of anal fistula/abscess was higher than that in Western countries and observed in 64% of patients, 58% being classified as complex perianal fistulas.11 A trans-ethnic association study identified heterogeneity in genetic variant risk for inflammatory bowel disease between European and non-European [eg, East Asian including Japanese] populations.12
Complex perianal fistulas represent a significant burden for patients with CD and a challenge for physicians/surgeons to treat.1,8 Most patients require surgical and palliative treatment. Conventional fistulotomy is contraindicated in high and complex fistulas due to the high rate of recurrence, impaired wound healing, and risk of damage to the anal sphincter muscle with subsequent faecal incontinence.13 Surgeons often prefer non-cutting loose setons, which are left in place for varied lengths of time for symptomatic relief, inflammation reduction, and reduction of abscess recurrence,6,14 however, these setons may subsequently deteriorate patients’ quality of life. Moreover, non-cutting loose setons are not a curative treatment for fistula closure. According to the current guideline of the European Crohn’s and Colitis Organisation [ECCO], surgical treatment is combined with medical measures such as antibiotics, thiopurines, and/or biologic therapies.15 Patients with refractory perianal fistula may require treatment with immunosuppressants, which might have side effects. Despite the available medical and surgical options, not all patients achieve long-term remission.16,17 Only 36% of patients achieved fistula closure after 54 weeks of treatment with the anti-tumour necrosis factor alpha [anti-TNFα] monoclonal antibody, infliximab17—the only biologic that has been approved in Japan for the treatment of CD with external fistula.3 However, increased risk of infection is a concern with anti-TNFα.17 The results from several studies show that combined medical and surgical management leads to perianal fistula healing in only 50% of cases.18 There is, therefore, an unmet medical need for alternative treatment options that have improved efficacy.
Recently there has been an increasing interest in the mesenchymal stem cell [MSC]-based therapeutic approach for complex perianal fistulas in patients with CD. In vivo studies have demonstrated therapeutic effects of MSCs in inflammation and autoimmune-related conditions, whereby MSCs have modulatory effects on immune cells and macrophages. However, the full mechanism of action of MSCs is still not fully understood. In a recent in vitro study, it was found that adipose tissue-derived MSCs modulated the differentiation of myeloid cells toward an anti-inflammatory and reparative profile. This modulatory effect was mediated mainly by prostaglandin E2 and, to a lesser extent, interleukin-6 [IL-6].19
A recent meta-analysis10 evaluated the efficacy and safety of MSCs for complex perianal fistulas and showed that the adipose-derived MSC group achieved a higher healing rate than that of the control group, who had received intralesional injections of fibrin glue or saline solution [odds ratio = 2.29; 95% CI, 1.38–3.79; p = 0.001]. These results indicate that MSC therapy alone promotes tissue repair and is efficacious for complex perianal fistulas.10
Darvadstrocel is a suspension of allogeneic, expanded, adipose-derived mesenchymal stem cells [eASCs] which has been approved in Europe for the treatment of complex perianal fistulas that have shown an inadequate response to at least one conventional or biologic therapy in adult patients with non-active/mildly active luminal CD.20 This approval was based on the pivotal ADMIRE-CD study, a randomised, double-blind, controlled, phase 3 trial performed in Europe and Israel.9 The results demonstrated statistically significant superiority for darvadstrocel versus the control for combined remission at 24 weeks [50% versus 34%; p = 0.024]9 and the effect was maintained at Week 52 [56% versus 39%; p = 0.010].21
In this article, we relate the results of a phase 3, multicentre, open-label, uncontrolled study investigating the efficacy and safety of darvadstrocel for treatment of active complex perianal fistulas in Japanese adults with non-active/mildly active luminal CD.
2. Methods
2.1. Study design
This is an ongoing phase 3, multicentre, open-label, uncontrolled study at nine sites in Japan [ClinicalTrials.gov number: NCT03706456], designed to evaluate the efficacy and safety of darvadstrocel for the treatment of complex perianal fistulas in adult Japanese patients with CD. The design of this study is similar to that of the ADMIRE-CD study except that this is an open-label, single-arm study.9,21
The study consisted of a screening period [with a screening visit and a preparation visit, at 5 and 3 weeks prior to the treatment visit, respectively], a treatment visit [administration of study product], a follow-up period [from Week 2 to Week 52 post-treatment], and a long-term follow-up period [from Week 52 to Week 156 post-treatment] [Supplementary Figure 1]. The patients signed an informed consent form at the screening visit before proceeding to the preparation and treatment visits. The follow-up periods and visits were determined to evaluate the long-term safety of darvadstrocel in Japanese patients, based on actual clinical practice [differences in follow-up period and visits between this study and the ADMIRE-CD study are available in Supplementary Figure 1]. The study adopted an add-on design, in which continuation of baseline treatment for CD, eg, biologics and immunosuppressants, was allowed. This study has been conducted in compliance with the Declaration of Helsinki, the International Conference on Harmonisation Harmonised Tripartite Guideline for Good Clinical Practice, and all applicable local regulations. All authors had access to the study data and reviewed and approved the final manuscript.
At the time of writing, the assessments up to 52 weeks were completed and the long-term follow-up study [up to 156 weeks] was ongoing under the same phase 3 study protocol. In this manuscript, we report results at the end of 52 weeks [data cut-off date February 1, 2021; last visit for the last patient at Week 52].
2.2. Study population
Eligible patients were adults aged ≥ 18 years who had non-active or mildly active CD for at least 6 months, defined as Crohn’s Disease Activity Index [CDAI] ≤ 220, and had active complex perianal fistulas defined as one of the following: [1] a fistula with a high location [above the dentate line], including inter-sphincteric, trans-sphincteric, extra-sphincteric, or supra-sphincteric fistulas; [2] at least two external openings; [3] associated fluid collections. The diagnosis of CD was made according to the diagnostic criteria for CD issued by Research Group for Intractable Inflammatory Bowel Disease Designated as Specified Disease by the Ministry of Health, Labor and Welfare of Japan.22 The fistulas had to have a maximum of two internal and three external openings and had to have been draining for at least 6 weeks prior to screening.
Eligible patients had fistulas that were refractory [inadequate response, loss of response, or intolerance] to at least one of the following treatments: antibiotics, immunosuppressants, or biologics [anti-TNFs, anti-integrin, or anti-IL-12/23]. Patients with fistulas that were refractory only to antibiotics represented < 25% of the total study population.
Patients were excluded if they had: rectovaginal, rectourethral, or bladder fistulas; anorectal stenosis; active proctitis, diverting stomas, or an abscess or collection >2 cm diameter; or a malignant tumour [including patients who had a history of any type of malignant tumour]. Patients with an active luminal lesion in the lower part of the rectum, observed by rectosigmoidoscopy, those who had not received previous treatment for perianal fistulising CD [ie, antibiotics, immunosuppressants, or biologics], and those who underwent previous surgery other than drainage or seton placement for the active fistula, were excluded from the study. Patients were not eligible if they had received systemic steroids within the previous 4 weeks.
2.3. Procedures
After the patients had signed written informed consent, rectosigmoidoscopy was performed to evaluate the Simple Endoscopic Score for Crohn’s Disease [SES-CD] before the preparation visit if the patient did not already have an SES-CD score available. At the preparation visit, patients underwent examination under anaesthesia, fistula curettage, and seton placement [-Day 21 to -Day 14]. At the treatment visit [Day 1], seton[s] were removed, all fistulas were curetted, and internal opening[s] were sutured. After the curettage, all patients received antibiotic treatment (ciprofloxacin and/or metronidazole [unless intolerance to these drugs was observed or these drugs were contraindicated]) for at least 7 days. All patients received 24 mL of darvadstrocel, a cell suspension containing 120 × 106 cells [eASC; Cx601] as a single intralesional dose. The first half of the dose was injected into a site adjacent to the sutured internal opening[s] through the anal canal, and the other half was injected into the tract wall along the fistula through the external opening[s], resulting in several micro-blebs forming inside the tract wall.
During the study, patients could be treated with antibiotics for no more than 4 weeks. Immunosuppressants and biologics [anti-TNF, anti-integrin, anti-IL-12/23] were maintained at stable doses throughout the study. The following treatments were permitted as rescue therapy: a steroid course starting at a dose of 40 mg or less [as prednisolone or equivalent, 9 mg for budesonide] and tapered over 12 weeks, or central venous nutrition/enteral total nutrition.
Fistula closures were clinically assessed at Weeks 2, 4, 8, 16, 24, 40, and 52, and were radiologically assessed by magnetic resonance imaging [MRI] at Weeks 24 and 52.
2.4. Endpoints
The endpoints were defined in the same way as they were in the ADMIRE-CD study,9 to enable comparison. The primary endpoint was the proportion of patients with combined remission at Week 24, defined as the clinically confirmed closure of all treated external openings that were draining at the screening, and the absence of collections >2 cm of the treated fistulas, confirmed by a central MRI assessment. Clinical assessment of closure was defined as the absence of draining upon gentle finger compression.23
Secondary efficacy endpoints at Weeks 24 and 52 included response, clinical remission, time to combined remission, time to clinical remission, time to response, and proportion of patients who experienced relapse among those who had combined remission at Week 24 [see Supplementary Table 1 for definition of terms]. Combined remission at Week 52 was also assessed. Changes in Perianal Disease Activity Index [PDAI],24 CDAI,25 and Van Assche26 scores were assessed. The PDAI score24 is an evaluation consisting of five clinical elements on a 5-point scale each: fistula discharge, pain/restriction of activities, restriction of sexual activities, type of perianal disease, and degree of induration. The score ranges 0–20 and a higher score indicates a higher disease activity. The Van Assche score26 is a quantitative evaluation that consists of six elements, the assessment requiring MRI: the number, location, and extension of fistula tracts, hyperintensity on T2-weighted images, presence or absence of collections [cavities >3 mm in diameter], and the rectal wall involvement. The score ranges 0–22 and a higher score indicates more complex and anatomically severe perianal fistulas.
Safety endpoints included treatment-emergent adverse events [TEAEs], including: serious TEAEs, TEAEs and serious TEAEs related to study treatment, intensity of TEAEs, intensity of study treatment-related TEAEs, TEAEs leading to discontinuation, TEAEs of special interest, product malfunction-related TEAEs, TEAEs over time, and deaths. TEAEs of special interest included ectopic tissue formation, hypersensitivity, transmission of infectious agents, immunogenicity, alloimmune reactions, medication errors, and tumorigenicity.
2.5. Statistical analysis
The planned sample size was 20 patients, which allowed for at least a 94% probability of detecting combined remission in ≥ 35% of the patients, assuming that 50% of patients achieved combined remission by Week 24, as was seen in the ADMIRE-CD study.9
Descriptive statistics were used to analyse the data. Primary and secondary endpoints were analysed in the intention-to-treat [ITT] population, which included all patients enrolled in the treatment period. Analyses were also performed using the modified intention-to-treat [mITT] population, which included all patients who received the study treatment and whose primary efficacy endpoint was evaluable, and the per protocol set [PPS], which included all patients who had no major protocol deviations. The proportions of patients achieving primary and secondary endpoints were presented as percentages of patients with two-sided 95% confidence intervals [CIs]. Subgroup analyses were conducted to assess the impact of sex, duration of CD, and number of internal/external openings on combined remission. Owing to the small sample size and insufficient statistical power, statistical significance between subgroups was not assessed.
TEAEs were analysed in the safety analysis set, defined as all patients who received the study treatment. TEAEs were coded with the Medical Dictionary for Regulatory Activities [MedDRA] by system organ class and preferred terms.
3. Results
3.1. Study population
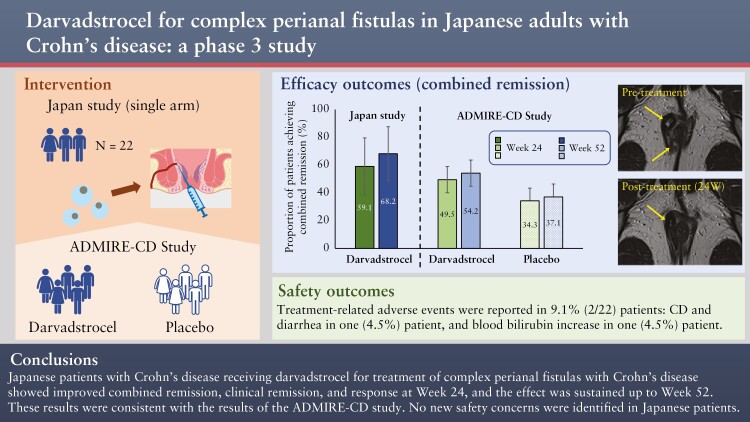
Between March 6, 2019 and February 1, 2021, of the 26 patients assessed for eligibility, 22 patients received darvadstrocel and completed the 52-week follow-up [Figure 1]. Between Week 25 and Week 52, two patients discontinued the study because of lack of efficacy. For the 22 patients, the mean (standard deviation [SD]) time in the study was 49.6 [7.1] weeks. All 22 patients were included in the safety analysis set, ITT, and mITT populations. Of these, 19 patients [86.4%] were included in the PPS; three patients were excluded due to deviations of concomitant medication.
Figure 1.
Patient disposition; *two patients violated excluded medications; one patient violated rescue medications.
3.2. Baseline demographic and clinical characteristics
In the ITT population, the mean [SD] age of the patients was 36.4 [10.4] years, 63.6% were male, and 72.7% had never smoked [Table 1]. Patients had CD for a mean [SD] of 11.3 [6.6] years. The mean [SD] total score of the SES-CD was 1.4 [2.0]. All patients were receiving concomitant medications: nine [40.9%] patients were receiving biologics only [infliximab, n = 6; adalimumab, n = 1; ustekinumab, n = 2], two [9.1%] were receiving immunosuppressants only [azathioprine, n = 2], and seven [31.8%] patients were receiving both biologics and immunosuppressants [infliximab + azathioprine, n = 5; adalimumab + azathioprine, n = 2] [Table 1]. Concomitant medications also included antibiotics as follows: cefpodoxime proxetil [n = 8, 36.4%], cefmetazole sodium [n = 4, 18.2%], ciprofloxacin [n = 3, 13.6%], flomoxef sodium [n = 3, 13.6%], and ampicillin sodium [sulbactam sodium] [n = 1, 4.5%]. None of the patients received metronidazole concomitantly [Table 1].
Table 1.
Demographic and other baseline characteristics.
| Parameter | Valuea [n = 22] |
|---|---|
| Age [years], mean [SD] | 36.4 [10.4] |
| Male, n [%] | 14 [63.6] |
| Weight [kg], mean [SD] | 68.3 [23.1] |
| Duration of Crohn’s disease [years], mean [SD] | 11.3 [6.6] |
| SES-CD total score, mean [SD] | 1.4 [2.0] |
| CDAI score, mean [SD] | 113.6 [64.7] |
| Smoking classification, n [%] | |
| Never smoked | 16 [72.7] |
| Current smoker | 2 [9.1] |
| Ex-smoker | 4 [18.2] |
| Concomitant medications | |
| Biologics only | 9 [40.9] |
| Infliximab | 6 [27.3] |
| Adalimumab | 1 [4.5] |
| Ustekinumab | 2 [9.1] |
| Immunosuppressants only | 2 [9.1] |
| Azathioprine | 2 [9.1] |
| Biologics + immunosuppressants | 7 [31.8] |
| Infliximab + azathioprine | 5 [22.7] |
| Adalimumab + azathioprine | 2 [9.1] |
| No biologics or immunosuppressants | 4 [18.2] |
| Antibiotics | |
| Cefpodoxime proxetil | 8 [36.4] |
| Cefmetazole sodium | 4 [18.2] |
| Ciprofloxacin | 3 [13.6] |
| Flomoxef sodium | 3 [13.6] |
| Ampicillin sodium [sulbactam sodium] | 1 [4.5] |
| Not using antibiotics | 3 [13.6] |
| Number of internal openings at baseline, n [%] | |
| 1 | 16 [72.7] |
| 2 | 6 [27.3] |
| Number of external openings at baseline, n [%] | |
| 1 | 3 [13.6] |
| 2 | 14 [63.6] |
| 3 | 5 [22.7] |
| Topography at baseline, n [%] | |
| 1 internal + 1 external | 3 [13.6] |
| 1 internal + 2 external | 11 [50.0] |
| 1 internal + 3 external | 2 [9.1] |
| 2 internal + 1 external | 0 |
| 2 internal + 2 external | 3 [13.6] |
| 2 internal + 3 external | 3 [13.6] |
aData are number [%] unless otherwise indicated. Data from this table were collected at screening.
CDAI, Crohn’s Disease Activity Index; SD, standard deviation; SES-CD, Simple Endoscopic Score for Crohn’s Disease.
At baseline, the mean [SD] CDAI, PDAI total, and Van Assche scores were 113.6 [64.7], 4.8 [2.2], and 14.5 [4.0], respectively [Tables 1 and 2].
Table 2.
Summary of efficacy findings.
| Endpoint | Point estimate | 95% CI | |
|---|---|---|---|
| n = 22 | |||
| Combined remissiona | |||
| Week 24 | 59.1 | [38.5–79.6] | |
| Week 52 | 68.2 | [48.7–87.6] | |
| Weeks 24 and 52 | 50.0 | [29.1–70.9] | |
| Clinical remissionb | |||
| Week 24 | 59.1 | [38.5–79.6] | |
| Week 52 | 72.7 | [54.1–91.3] | |
| Responsec | |||
| Week 24 | 81.8 | [65.7–97.9] | |
| Week 52 | 90.9 | [78.9–100.0] | |
| Relapsed | |||
| Week 24e | 25.0 | [3.8–46.2] | |
| Week 52f | 23.1 | [0.2–46.0] | |
| Time to combined remission [weeks], median | 25.1 | [23.6–52.1] | |
| Time to clinical remission [weeks], median | 3.8 | [2.0–15.6] | |
| Time to response [weeks], median | 2.6 | [1.7–4.3] | |
| Baseline | Change from baseline to Week 24 | Change from baseline to Week 52 | |
|---|---|---|---|
| PDAI score, mean [SD] | 4.8 [2.2] | −2.4 [2.2] | −2.8 [2.6] |
| CDAI scoreg, mean [SD] | 94.3 [60.0] | −5.2 [47.5] | −20.9 [51.3] |
| Van Assche score, mean [SD] | 14.5 [4.0] | −1.7 [4.4] | −2.2 [4.9] |
Data are % [95% CI] unless otherwise indicated.
CDAI, Crohn’s Disease Activity Index; CI, confidence interval; PDAI, Perianal Disease Activity Index; SD, standard deviation.
aDefined as the clinically confirmed closure of all treated external openings that were draining at the screening upon gentle finger compression and the absence of collections >2 cm diameter in the treated fistulas confirmed by the central MRI assessment
bDefined as the clinically confirmed closure of all treated external openings that were draining at the screening despite gentle finger compression.
cDefined as the clinically confirmed closure of at least 50% of all treated external openings that were draining at the screening despite gentle finger compression.
dDefined as the clinically confirmed reopening of any of the treated external openings with active drainage, or the development of a collection >2 cm in the treated fistulas confirmed by central magnetic resonance imaging [MRI] assessment.
eIn patients with clinical remission at previous visit; darvadstrocel [n = 16].
fIn patients with combined remission at Week 24; darvadstrocel [n = 13].
gData are collected at Day 1 [refer to Supplementary Figure 1].
The majority of patients [72.7%] had one internal opening, whereas the remaining [27.3%] had two internal openings. The most common distribution was one internal opening and two external openings [50%; Table 1]. The internal openings were sutured in all patients [n = 22] prior to the darvadstrocel treatment.
3.3. Efficacy
The primary endpoint of combined remission at Week 24 in the ITT population was achieved in 59.1% of patients [13/22; 95% CI, 38.5–79.6] [Table 2]. The effect was also observed at Week 52; with 68.2% [15/22; 95% CI, 48.7–87.6] of patients achieving combined remission. Of the 13 patients who achieved combined remission at Week 24, 11 [84.6%] patients were still in remission at Week 52, and combined remission was newly observed in four patients.
In the ITT population, 16 [72.7%] patients achieved clinical remission [95% CI, 54.1–91.3] and 20 [90.9%] had a response [95% CI, 78.9–100.0] at Week 52. Similar results were observed at Week 24. Among patients who achieved combined remission at Week 24, three patients [23.1%] experienced relapse at Week 52. The median [95% CI] time to obtain combined remission, clinical remission, and response by Week 52 was 25.1 [23.6–52.1], 3.8 [2.0–15.6], and 2.6 [1.7–4.3] weeks, respectively.
The mean changes [SD] in PDAI total score from baseline to Weeks 24 and 52 were -2.4 [2.2] and -2.8 [2.6], respectively, and individual PDAI subscores followed a similar trend [Table 2 and Supplementary Figure 2]. The PDAI scores and clinical remission and response rates improved over time. The clinical remission rate was 50.0% at Week 2, 59.1% from Week 4 to Week 24, 63.6% at Week 40, and 72.7% at Week 52. The response rate was 68.2% at Week 2, 68.2% at Week 4, 90.9% at Week 24, and 86.4% at Weeks 40 and 52. The mean [SD] PDAI total score was 4.8 [2.2], 3.1 [2.4], 2.4 [2.9], and 2.1 [2.1] at baseline and Weeks 4, 24, and 52, respectively [Supplementary Figure 3]. The mean changes [SD] in CDAI scores from baseline to Weeks 24 and 52 were -5.2 [47.5] and -20.9 [51.3], and those for Van Assche scores were -1.7 [4.4] and -2.2 [4.9], respectively [Table 2].
A subgroup analysis assessing combined remission by duration of CD showed differences between subgroups. Ten [90.9%] patients with a disease duration of ≤ 9.7 years achieved remission at Week 52 versus one [9.1%] patient with a disease duration of >9.7 years; however, remission at Week 24 was similar irrespective of disease duration category. Subgroups based on sex and the number of internal/external openings showed no apparent differences between categories for remission at Week 24 and Week 52 [Supplementary Table 2]. No clear relationship was observed between pre-existing allo-sensitisation (presence of anti-human leukocyte antigens [HLA] antibody) or donor-specific antibody [DSA] status and clinical efficacy of darvadstrocel up to Week 52 [data not shown].
The objective MRI assessments at Week 24 showed no patient with >2 cm collection. At Week 52, only one patient was observed to have collection >2 cm. Supplementary Figure 4 shows representative baseline and post-treatment MRI data. From these images, suppression of the inflammation and decrease in infected granulation tissue resulting in complete or partial closure of the fistula tract and scarring could be presumed.
3.4. Safety
Over the 52-week study period, 90.9% [20/22] of patients experienced TEAEs [Table 3]. The most commonly reported TEAEs [in ≥ 5% patients] were proctalgia [27.3%], nasopharyngitis [22.7%], anal fistula [18.2%], acrochordon [9.1%], CD [9.1%], and nausea [9.1%]. Treatment-related TEAEs reported in 9.1% [2/22] patients consisted of diarrhoea [‘worsening of diarrhoea’ was originally reported by an investigator as a non-MedDRA term] and CD [‘worsening of CD’ was also originally reported, but the date of onset was different from ‘worsening of diarrhoea’] in one patient [4.5%], and blood bilirubin increase in one patient [4.5%]. Blood bilirubin increase was observed 47 weeks after darvadstrocel treatment and was moderate in intensity, and the investigator considered darvadstrocel treatment to be a possible cause. Most TEAEs were mild [54.5%] or moderate [27.3%] in intensity. Severe TEAEs were reported in two patients [9.1%]; however, none was considered treatment-related. No TEAE that affected anal function was reported. There was no clear increasing trend in the incidence of TEAEs over time [data not shown].
Table 3.
Overview of TEAEs up to Week 52 [safety population].
| Event | n [%] [n = 22] |
|---|---|
| TEAEs | 20 [90.9] |
| Not related | 18 [81.8] |
| Related | 2 [9.1] |
| Mild | 12 [54.5] |
| Moderate | 6 [27.3] |
| Severe | 2 [9.1] |
| Leading to study discontinuation | 0 |
| Serious TEAEs | 4 [18.2] |
| Not related | 3 [13.6] |
| Related | 1 [4.5] |
| Leading to study discontinuation | 0 |
| Deaths | 0 |
| TEAEs in ≥ 5% patients | |
| Proctalgia | 6 [27.3] |
| Nasopharyngitis | 5 [22.7] |
| Anal fistula | 4 [18.2] |
| Crohn’s disease | 2 [9.1] |
| Nausea | 2 [9.1] |
| Acrochordon | 2 [9.1] |
| Treatment-related AEs | |
| Diarrhoea | 1 [4.5] |
| Blood bilirubin increased | 1 [4.5] |
| Treatment-related serious AE | |
| Crohn’s disease | 1 [4.5] |
AE, adverse event; TEAE, treatment-emergent adverse event.
The incidence of serious TEAEs was reported in four patients [18.2%], and treatment-related serious TEAE [CD] was observed in one patient. Serious TEAEs considered not related to the treatment were intestinal obstruction, intestinal anastomosis complication, urinary calculus, and tubulointerstitial nephritis. No deaths or TEAEs leading to study discontinuation and no TEAEs of special interest were reported up to 52 weeks of the study period. No clinically relevant findings with respect to clinical laboratory tests [including serum chemistry, haematology, and urinalysis], vital signs, or physical examinations were observed.
At Week 52, in the subgroup of patients with pre-existing allo-sensitisation [presence of anti-HLA antibody at baseline], the incidence of TEAEs was 77.8% [7/9] and 100% [7/7 patients] in presence or absence of DSA, respectively. In the subgroup of naïve [absence of anti-HLA antibody] patients, incidence of TEAEs was 100.0% [3/3 patients] and 100.0% [3/3 patients] in the presence or absence of DSA, respectively. No clear relationship between pre-existing sensitisation or DSA status and incidences of TEAEs was observed.
4. Discussion
This current phase 3, multicentre, open-label, uncontrolled study demonstrated the sustained effect of darvadstrocel, allogeneic eASCs, on combined remission and clinical remission and response [closure of external openings] up to 52 weeks, as well as improvement in PDAI, CDAI, and Van Assche scores in Japanese patients with CD and perianal fistulas that were refractory to medication. The darvadstrocel treatment also had a favourable safety profile, with no TEAEs leading to discontinuation and no deaths.
Current treatment for complex perianal fistulas in Japan includes surgical approaches by seton placement and stepwise medical approaches with antibiotics, immunomodulators, and/or anti-TNFα agents [eg, infliximab, adalimumab].1,27 To date, infliximab has the most evidence among biologics to support treatment of perianal lesion in patients with CD.17,28 However, it has been reported that after stopping anti-TNFα treatment, 60% to 70% of patients experience relapse and only a few patients experience long-term remission.9 Surgical options are limited by the potential complication of faecal incontinence. Conventional fistulotomy involves the risk of invasion of the anal sphincter muscle, and repeated procedures may necessitate colostomy. Therefore, there remains an unmet need for alternative treatments for perianal fistulising CD.
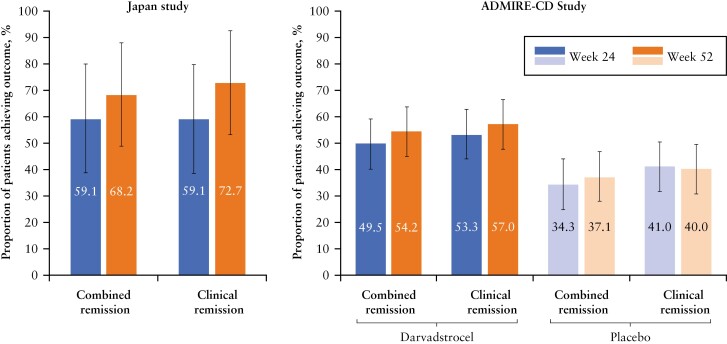
This study was designed to enable comparison of the results with those of the ADMIRE-CD study. The characteristics of the patients in both studies were generally similar, and results from this study are consistent with the previous findings in the ADMIRE-CD study which showed that 54.2% of patients treated with a single dose of darvadstrocel achieved combined remission at Week 52 [versus 37.1% of patients in the placebo arm; Figure 2].9,21 Our study demonstrated that almost 60% [13/22] of patients achieved combined remission at Week 24, on the basis of the primary efficacy endpoint combining both clinical and radiological evaluations. An even higher proportion [68%] of patients [15/22 patients] achieved combined remission at Week 52. Of 13 patients achieving combined remission at Week 24, 11 [84.6%] achieved combined remission at Week 52; thus, four patients who had not attained combined remission at Week 24 achieved combined remission at Week 52.
Figure 2.
Proportion of patients achieving combined remission and clinical remission at Week 24 and Week 52 in the darvadstrocel Japan study and ADMIRE-CD study. The data for the ADMIRE-CD study are from the intention-to-treat population [data on file]. Error bars represent 95% confidence interval [CI]. Combined remission is defined as the clinically confirmed closure of all treated external openings that were draining at the screening despite gentle finger compression, and the absence of collections >2 cm in the treated fistulas confirmed by the central magnetic resonance imaging [MRI] assessment. Clinical remission is defined as the clinically confirmed closure of all treated external openings that were draining at the screening despite gentle finger compression.
Clinical remission at Week 52 in the ITT population was 72.7% in the current study compared with 57.0% of darvadstrocel-treated patients in the ADMIRE-CD [and 40.0% of patients in the placebo arm] [Figure 2]. The time to clinical remission and response was shorter in the current Japan phase 3 study [3.8 and 2.6 weeks, respectively] than it was in the ADMIRE-CD study [6.7 and 6.3 weeks, respectively], which further suggests the effectiveness of darvadstrocel.9 The patients in our study had refractory complex fistulas that did not adequately respond to existing medical treatments [eg, biologics]. Our study patients treated with darvadstrocel also presented with clinical remission by Week 24 [59%], which was maintained through Week 52 [73%] [Table 2]. In addition to clinical remission, the PDAI score showed improvement over time [Supplementary Figure 2]. Moreover, the PDAI score >4 suggests active perianal disease with an accuracy of 87%.29 The mean [SD] PDAI scores at Week 24 and Week 52 were 2.4 [2.9] and 2.1 [2.1], respectively, suggesting that darvadstrocel treatment in this study has shown increased healing of fistulas which require no further medical or surgical treatment. These results suggested that darvadstrocel treatment demonstrated modest and progressive improvement up to 52 weeks and had similar efficacy to that reported in the ADMIRE-CD study.
The observed sustainable improvement following darvadstrocel treatment was in line with the results of the ADMIRE-CD study, which reported 57% and 61% of clinical remission by Week 24 and Week 52, respectively, in the mITT group [versus placebo, 43% by Week 24 and 42% by Week 52]. Our results are also similar to the real-world analysis from an observational post-marketing European study [INSPIRE] in darvadstrocel-treated patients with CD and complex perianal fistulas. This real-world analysis found clinical remission in 65% of darvadstrocel-treated patients at 24 weeks.30
In the past two decades, the use of cell therapies, including MSCs, in humans has attracted attention and provided promising results. In patients with perianal fistula in CD, numerous clinical trials have been conducted and reported complete cure rates in the range of 46% to 90% after injection of autologous or allogeneic adipose-derived mesenchymal stem cells [ASCs].31 However, trials with autologous ASCs are limited by their varied study designs, small patient numbers, and varied definitions of response. In theory, autologous cells are the ideal choice for many kinds of cell therapy for histocompatibility reasons.31 However, the immunomodulatory and secretome potency of autologous MSCs varies between donors32 and the source of the MSCs can be limited because of the time course required for ex vivo expansion. The ECCO 2019 guidelines grade the use of allogeneic ASC therapy at a higher level of evidence than that of autologous ASC therapy [level of evidence 2 versus 5].33 ASCs are noted to be an effective and safe approach for treating complex perianal fistula, although there is a need for more research in patient selection, optimal mode of delivery, and dose and frequency of injections.33
In this study, allogeneic eASCs were used rather than autologous eASCs. Allogeneic MSCs have some potential for an immune rejection and cannot be considered completely immune privileged.34 Although 12 [54.5%] patients were DSA-positive in the presence or absence of anti-HLA antibody at baseline, our results showed no clear relationship between pre-existing sensitisation or DSA status and the incidence of TEAEs. Darvadstrocel is prepared from donors in Western countries. Considering the difference in antigenicity between Japanese and non-Japanese populations, it should be noted that darvadstrocel did not cause any prominent TEAEs related to injection, such as hypersensitivity reactions.
The mechanism of action of darvadstrocel in human studies is not fully understood. However, preclinical studies have demonstrated that allogeneic eASCs exert an immunomodulatory action in the presence of inflammatory mediators, eg. inhibition of T lymphocyte function, increase in regulatory T cells, reduced production of pro-inflammatory cytokines [eg, interferon γ and TNFα], and increased production of anti-inflammatory cytokines [eg, IL-10].9 It is well known that MSCs can promote tissue repair by producing various growth factors, cytokines, and extracellular matrices35,36 and facilitating proliferation and migration of different cells involved in wound healing.37 However, the immunological effect of MSCs which triggers in reverting consolidated fibrosis is yet to be clarified.38
In the present study, none of the analysed patients [n = 22] had internal openings that failed to suture, which might have contributed to the outcome. Mean [SD] Van Assche MRI activity score decreased 2.2 [4.9] from 14.5 [4.0] at baseline to 11.2 [5.3] at Week 52 [Table 2]. Consistently, reduced disease activity, in terms of suppression of inflammation and reduced infection of the granulation tissue, was assumed on the basis of representative MRIs [T2-weighted, fast spin echo]. The resulting complete or partial closure of the fistula tracts indicated the effect of the darvadstrocel treatment [Supplementary Figure 4]. To provide optimal treatment to patients with complex perianal fistulas, there is a need for more evidence, including MRI studies from individual cases receiving darvadstrocel treatment. Several factors may be associated with the outcome of fistula healing, such as the distance between the anal verge and the internal opening, length and number of fistula tracts, and complexity of suturing of internal opening[s]. In addition, the majority of the patients from this study were receiving concomitant medications including biologics and/or immunosuppressants which are standard treatments for CD. Thus, the add-on effect of darvadstrocel rather than the stand-alone effect might have reflected the outcomes for the healing of perianal fistulas. Darvadstrocel obtained marketing authorisation approval in the European Union in March 201839 and in Japan in September 202140 as the first allogeneic stem cell therapy for complex perianal fistulas in adult patients with non-active or mildly active luminal CD, when fistulas have shown an inadequate response to at least one conventional or biologic therapy. Therefore, darvadstrocel may be used for second-line or third-line treatment for perianal fistulas in clinical practice.
The results of our subgroup analysis suggested that shorter disease duration of CD might be associated with better treatment outcome, because patients with disease duration of ≤ 9.7 years had increased remission versus those having a disease duration of >9.7 years at Week 52. Further investigations with large sample sizes are warranted to elucidate a link between treatment effect and duration of CD.
This study demonstrated that darvadstrocel is safe and no new safety concerns were identified in Japanese patients. These results are consistent with the findings of the ADMIRE-CD study.9
A strength of this study is the consistency of the study design with the ADMIRE-CD study, which allows extrapolation of the findings to Japanese patients. The study, however, had a few limitations. First, this was an open-label study with no comparator; thus, the relatively high proportion of patients achieving the study outcomes [Figure 2] needs to be cautiously interpreted. Second, the study included a small number of patients [n = 22] and was not powered to detect statistical differences among subgroups. Hence, the results of this study and the subgroup analysis should be interpreted with caution. Furthermore, the study results are provided up to Week 52, with long-term safety follow-up of the patients to Week 156 under way.
Thus, darvadstrocel represents a novel treatment of complex perianal fistulas that have shown an inadequate response to one or more conventional or biologic therapies in patients with non-active/mildly active luminal CD.41
In conclusion, Japanese patients treated with darvadstrocel for complex perianal fistulas showed improved combined remission, clinical remission, and response at Week 24, and the effect was sustained up to Week 52. These results were consistent with the results of the ADMIRE-CD study.9 Additionally, no new safety concerns were identified in Japanese patients.
Supplementary Material
Acknowledgements
The authors thank the study participants, the investigators, Akira Sugita [Yokohama Municipal Citizen’s Hospital], Hiroki Ikeuchi [Hyogo College of Medicine], Daijiro Higashi [Fukuoka University Chikushi Hospital], Toru Kono [Sapporo Higashi Tokushukai Hospital], Akihiko Ohta [Ieda Hospital], Yoshiki Okita [Mie University Graduate School of Medicine], Ken-Ichi Takahashi [Tohoku Rosai Hospital], and staff at the study sites for their invaluable contribution to this study.
Contributor Information
Satomi Furukawa, Department of Coloproctology, JCHO Tokyo Yamate Medical Center, Shinjuku, Tokyo, Japan.
Tsunekazu Mizushima, Department of Therapeutics for Inflammatory Bowel Diseases, Osaka University Graduate School of Medicine, Suita, Osaka, Japan.
Ryo Nakaya, Takeda Development Center, Takeda Pharmaceutical Company Limited, Osaka, Japan.
Mari Shibata, Takeda Development Center, Takeda Pharmaceutical Company Limited, Osaka, Japan.
Takayoshi Yamaguchi, Takeda Development Center, Takeda Pharmaceutical Company Limited, Osaka, Japan.
Kenji Watanabe, Center for Inflammatory Bowel Disease, Division of Internal Medicine, Hyogo College of Medicine, Nishinomiya, Hyogo, Japan.
Kitaro Futami, Department of Surgery, Center for Clinical Medical Research, Fukuoka University Chikushi Hospital, Chikushino, Fukuoka, Japan.
Funding
This work was supported by Takeda Pharmaceutical Company Limited. The sponsor assisted with designing the study and supported on data collection, analysis, and interpretation. Medical writing assistance was provided by Aisling O’Keeffe, Mittal Makhija, and Mie Yamamoto of MIMS Co Ltd, sponsored by Takeda, in compliance with Good Publication Practice 3 ethical guidelines [Battisti et al., Ann Intern Med 2015;163:461–4].
Conflict of Interest
SF receives honoraria from Takeda Pharmaceutical and AbbVie. TM receives an honorarium from Takeda Pharmaceutical and research grants from Takeda Pharmaceutical, Kaken Pharmaceutical, Taiho Pharmaceutical, Sanofi, Chugai Pharmaceutical, Astellas Pharma, Shionogi, Mitsubishi Tanabe Pharma, Yakult Honsha, Bayer AG, Daiichi Sankyo, EA Pharma, Eli Lilly, and MSD, and the Department of Therapeutics for Inflammatory Bowel Diseases, Osaka University Graduate School of Medicine is supported by an unrestricted grant from Kinshukai Medical Corporation. RN, MS, and TY are current employees of Takeda Pharmaceutical. KW receives honoraria from AbbVie Japan, Mitsubishi Tanabe Pharma, EA Pharma, Takeda Pharmaceutical, Kyorin Pharmaceutical, Mochida Pharmaceutical, Janssen Pharmaceutical, Pfizer Japan, and Kissei Pharmaceutical. KW also receives grants including consigned/joint research expenses, scholarship donations, and course affiliations from EA Pharma, Takeda Pharmaceutical, AbbVie Japan, Astellas Pharma, Zeria Pharmaceutical, Kyorin Pharmaceutical, Mitsubishi Tanabe Pharma, JIMRO, Otsuka Pharmaceutical, Asahi Kasei Medical, and Mochida Pharmaceutical. KF receives honoraria from Takeda Pharmaceutical, Janssen Pharmaceutical, Mitsubishi Tanabe Pharma, AbbVie, and Kyorin Pharmaceutical. KF also receives research grants from Takeda Pharmaceutical.
Author Contributions
SF: concept: support, investigation: supporting, methodology: support; writing: review and editing: equal. TM; concept: support; investigation: support; methodology: support; writing: review and editing: equal. RN: formal analysis: lead; writing: review and editing: equal. MS: methodology: support; writing: review and editing: equal. TY: concept: lead; data curation: equal; methodology: lead; project administration: lead; writing:review and editing: equal. KW: concept: support; methodology: support; supervision: lead; writing: review and editing: equal]. KF: concept: support; investigation: lead; methodology: support; supervision: support; writing: review and editing: equal.
Data Availability
The datasets, including the redacted study protocol, redacted statistical analysis plan, and individual participants’ data supporting the results of the study, will be made available after the publication of the final study results within 3 months from initial request to researchers who provide a methodologically sound proposal. The data will be provided after their de-identification, in compliance with applicable privacy laws, data protection, and requirements for consent and anonymisation.
References
- 1. Panes J, Reinisch W, Rupniewska E, et al. Burden and outcomes for complex perianal fistulas in Crohn’s disease: systematic review. World J Gastroenterol 2018;24:4821–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Molodecky NA, Soon IS, Rabi DM, Ghali WA, Kaplan GG.. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012;42:46–56. [DOI] [PubMed] [Google Scholar]
- 3. Okabayashi S, Kobayashi T, Hibi T.. Inflammatory bowel disease in Japan: is it similar to or different from Westerns? J Anus Rectum Colon 2020;4:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Murakami Y, Nishiwaki Y, Oba MS, et al. Estimated prevalence of ulcerative colitis and Crohn’s disease in Japan in 2014: an analysis of a nationwide survey. J Gastroenterol 2019;54:1070–7. [DOI] [PubMed] [Google Scholar]
- 5. Mizushima T, Ota M, Fujitani Y, Kanauchi Y, Iwakiri R.. Diagnostic features of perianal fistula in patients with Crohn’s disease: analysis of a Japanese Claims Database. Crohns Colitis 360 2021;3:otab055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sica GS, Di Carlo S, Tema G, et al. Treatment of peri-anal fistula in Crohn’s disease. World J Gastroenterol 2014;20:13205–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schwartz DA, LoftusTremaine EVWJ, Panaccione R, Harmsen WS, Zinsmeister AR, Sandborn WJ.. The natural history of fistulizing Crohn’s disease in Olmsted County, Minnesota. Gastroenterology 2002;122:875–80. [DOI] [PubMed] [Google Scholar]
- 8. Aguilera-Castro L, Ferre-Aracil C, Garcia-Garcia-de-Paredes A, Rodriguez-de-Santiago E, Lopez-Sanroman A.. Management of complex perianal Crohn’s disease. Ann Gastroenterol 2017;30:33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Panés J, García-Olmo D, Van Assche G, et al. Expanded allogeneic adipose-derived mesenchymal stem cells [Cx601] for complex perianal fistulas in Crohn’s disease: a phase 3 randomised, double-blind controlled trial. Lancet 2016;388:1281–90. [DOI] [PubMed] [Google Scholar]
- 10. Cheng F, Huang Z, Li Z.. Efficacy and safety of mesenchymal stem cells in treatment of complex perianal fistulas: a meta-analysis. Stem Cells Int 2020;2020:8816737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Futami K, Higashi D, Egawa Y, Ishibashi Y, Maekawa T.. [Treatment for perianal Crohn’s disease]. Nihon Rinsho 2012;70:437–42. [PubMed] [Google Scholar]
- 12. Liu JZ, van Sommeren S, Huang H, et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet 2015;47:979–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Buchmann P, Keighley MR, Allan RN, Thompson H, Alexander-Williams J.. Natural history of perianal Crohn’s disease. Ten year follow-up: a plea for conservatism. Am J Surg 1980;140:642–4. [DOI] [PubMed] [Google Scholar]
- 14. Hermann J, Eder P, Banasiewicz T, Matysiak K, Łykowska-Szuber L.. Current management of anal fistulas in Crohn’s disease. Prz Gastroenterol 2015;10:83–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Van Assche G, Dignass A, Reinisch W, et al. The second European evidence-based consensus on the diagnosis and management of Crohn’s disease: special situations. J Crohns Colitis 2010;4:63–101. [DOI] [PubMed] [Google Scholar]
- 16. Molendijk I, Nuij VJAA, van der Meulen-de Jong AE, van der Woude CJ.. Disappointing durable remission rates in complex Crohn’s disease fistula. Inflamm Bowel Dis 2014;20:2022–8. [DOI] [PubMed] [Google Scholar]
- 17. Sands BE, Anderson FH, Bernstein CN, et al. Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med 2004;350:876–85. [DOI] [PubMed] [Google Scholar]
- 18. Kotze PG, Shen B, Lightner A, et al. Modern management of perianal fistulas in Crohn’s disease: future directions. Gut 2018;67:1181–94. [DOI] [PubMed] [Google Scholar]
- 19. Ortiz-Virumbrales M, Menta R, Pérez LM, et al. Human adipose mesenchymal stem cells modulate myeloid cells toward an anti-inflammatory and reparative phenotype: role of IL-6 and PGE2. Stem Cell Res Ther 2020;11:462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Meng ZW, Baumgart DC.. Darvadstrocel for the treatment of perianal fistulas in Crohn’s disease. Expert Rev Gastroenterol Hepatol 2020;14:405–10. [DOI] [PubMed] [Google Scholar]
- 21. Panés J, García-Olmo D, Van Assche G, et al. Long-term efficacy and safety of stem cell therapy [Cx601] for complex perianal fistulas in patients with Crohn’s disease. Gastroenterology 2018;154:1334–42.e4. [DOI] [PubMed] [Google Scholar]
- 22. Research Group for Intractable Inflammatory Bowel Disease Designated as Specified Disease by the Ministry of Health, Labour and Welfare of Japan. Diagnostic Criteria and Treatment Guidelines for Ulcerative Colitis and Crohn’s Disease. 2021. http://www.ibdjapan.org/pdf/doc01.pdf Accessed September 5, 2021.
- 23. Present DH, Rutgeerts P, Targan S, et al. Infliximab for the treatment of fistulas in patients with Crohn’s disease. N Engl J Med 1999;340:1398–405. [DOI] [PubMed] [Google Scholar]
- 24. Irvine EJ. Usual therapy improves perianal Crohn’s disease as measured by a new disease activity index. McMaster IBD Study Group. J Clin Gastroenterol 1995;20:27–32. [PubMed] [Google Scholar]
- 25. Best WR, Becktel JM, Singleton JW, Kern F.. Development of a Crohn’s disease activity index. National Cooperative Crohn’s Disease Study. Gastroenterology 1976;70:439–44. [PubMed] [Google Scholar]
- 26. Van Assche G, Vanbeckevoort D, Bielen D, et al. Magnetic resonance imaging of the effects of infliximab on perianal fistulizing Crohn’s disease. Am J Gastroenterol 2003;98:332–9. [DOI] [PubMed] [Google Scholar]
- 27. Wasmann KA, de Groof JE, Stellingwerf ME, et al. Treatment of perianal fistulas in Crohn’s Disease, seton versus anti-TNF versus surgical closure following anti-TNF [PISA]: a randomised controlled trial. J Crohns Colitis 2020;14:1049–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Panés J, Rimola J.. Perianal fistulizing Crohn’s disease: pathogenesis, diagnosis and therapy. Nat Rev Gastroenterol Hepatol 2017;14:652–64. [DOI] [PubMed] [Google Scholar]
- 29. Losco A, Viganò C, Conte D, Cesana BM, Basilisco G.. Assessing the activity of perianal Crohn’s disease: comparison of clinical indices and computer-assisted anal ultrasound. Inflamm Bowel Dis 2009;15:742–9. [DOI] [PubMed] [Google Scholar]
- 30. Zmora O, Baumgart DC, Faubion W, et al. P603 INSPIRE: 6-month interim analysis from an observational post-marketing registry on the effectiveness and safety of darvadstrocel in patients with Crohn’s disease and complex perianal fistulas. J Crohns Colitis 2022;16:i536–i7. [Google Scholar]
- 31. Buscail E, Le Cosquer G, Gross F, et al. Adipose-derived stem cells in the treatment of perianal fistulas in Crohn’s disease: rationale, clinical results and perspectives. Int J Mol Sci 2021;22:9967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. François M, Romieu-Mourez R, Li M, Galipeau J.. Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol Ther 2012;20:187–95. [DOI] [PubMed] [Google Scholar]
- 33. Adamina M, Bonovas S, Raine T, et al. ECCO guidelines on therapeutics in Crohn’s disease: surgical treatment. J Crohns Colitis 2020;14:155–68. [DOI] [PubMed] [Google Scholar]
- 34. Ankrum JA, Ong JF, Karp JM.. Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol 2014;32:252–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Na YK, Ban JJ, Lee M, Im W, Kim M.. Wound healing potential of adipose tissue stem cell extract. Biochem Biophys Res Commun 2017;485:30–4. [DOI] [PubMed] [Google Scholar]
- 36. Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999;284:143–7. [DOI] [PubMed] [Google Scholar]
- 37. Hu MS, Borrelli MR, Lorenz HP, Longaker MT, Wan DC.. Mesenchymal stromal cells and cutaneous wound healing: a comprehensive review of the background, role, and therapeutic potential. Stem Cells International 2018;2018:6901983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Krampera M, Le Blanc K.. Mesenchymal stromal cells: putative microenvironmental modulators become cell therapy. Cell Stem Cell 2021;28:1708–25. [DOI] [PubMed] [Google Scholar]
- 39. Alofisel. EPAR - Product Information. https://www.ema.europa.eu/documents/product-information/alofisel-epar-product-information_en.pdfAccessed July 25, 2022.
- 40. Japan Pharmaceuticals and Medical Devices Agency. Products Approved in FY 2021: regenerative Medical Products. 2021. https://www.pmda.go.jp/files/000246141.pdf Accessed October 3, 2022.
- 41. Scott LJ. Darvadstrocel: a review in treatment-refractory complex perianal fistulas in Crohn’s disease. BioDrugs 2018;32:627–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets, including the redacted study protocol, redacted statistical analysis plan, and individual participants’ data supporting the results of the study, will be made available after the publication of the final study results within 3 months from initial request to researchers who provide a methodologically sound proposal. The data will be provided after their de-identification, in compliance with applicable privacy laws, data protection, and requirements for consent and anonymisation.