Abstract
Background and Aims
Tofacitinib is an oral small molecule Janus kinase [JAK] inhibitor for the treatment of ulcerative colitis. We report an integrated summary of tofacitinib safety [exposure: ≤7.8 years] from the global clinical programme.
Methods
Patients receiving tofacitinib 5 or 10 mg twice daily [BID] from completed phase [P]2/3 placebo-controlled studies, an open-label, long-term extension study [final data cut-off: August 24, 2020], and interim analysis of a P3b/4 study (interim data cut-off: February 20, 2020; Overall plus P3b/4 [2020] Cohort) were included. Proportions with adverse events [AEs] and serious AEs, and incidence rates [IRs; unique patients with events/100 patient-years] for deaths and AEs of special interest [AESI] were evaluated. Opportunistic infections, malignancies, major adverse cardiovascular events [MACE] and gastrointestinal perforations were adjudicated.
Results
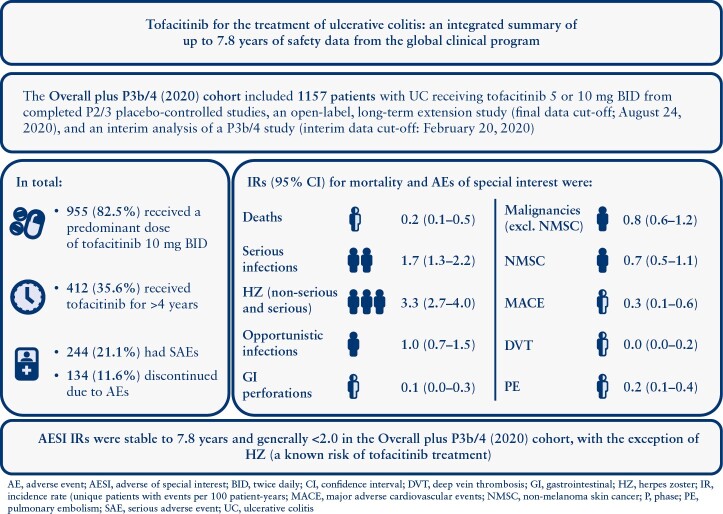
In total, 1157 patients received one or more dose of tofacitinib (mean duration: 946.9 days); 955/1157 [83%] received a predominant dose of 10 mg BID; 412/1157 [35.6%] received tofacitinib for >4 years; 992/1157 [85.7%] had AEs, 244/1157 [21.1%] had serious AEs and 134/1157 (11.6%) discontinued use due to AEs. IRs [95% confidence intervals] for all tofacitinib doses were: deaths, 0.23 [0.09–0.46]; serious infections, 1.69 [1.26–2.21]; herpes zoster [non-serious and serious], 3.30 [2.67–4.04]; opportunistic infections, 1.03 [0.70–1.46]; malignancies (excluding non-melanoma skin cancer [NMSC]), 0.84 [0.55–1.24]; NMSC, 0.73 [0.45–1.10]; MACE, 0.29 [0.13–0.55]; deep vein thrombosis, 0.03 [0.00–0.18]; pulmonary embolism, 0.19 [0.07–0.42]; gastrointestinal perforations, 0.10 [0.02–0.28].
Conclusions
AESI IRs were stable to 7.8 years and generally <2.0 in the Overall plus P3b/4 [2020] Cohort, with the exception of herpes zoster [a known risk of tofacitinib treatment]. ClinicalTrials.gov:NCT00787202;NCT01465763;NCT01458951;NCT01458574;NCT01470612;NCT03281304
JCC Topic/keyword selection: 3. Clinical trials
Keywords: Ulcerative colitis, tofacitinib, safety
An infographic plain language summary of this paper is available at: [10.25454/pfizer.figshare.20585331]
Graphical Abstract
Graphical Abstract.
1. Introduction
Tofacitinib is an oral, small molecule Janus kinase [JAK] inhibitor for the treatment of ulcerative colitis [UC]. The efficacy and safety of tofacitinib for the treatment of moderately to severely active UC were evaluated previously in a phase 2 induction study,1 two identical phase 3 induction studies [OCTAVE Induction 1 and 2],2 and a phase 3 maintenance study [OCTAVE Sustain],2 and have been further evaluated in an open-label, long-term extension [OLE] study [OCTAVE Open]3 and a phase 3b/4 study [RIVETING].4
Assessment of long-term safety is important to further characterize the safety profile of medicines for long-term use, and treatment with immunomodulators can result in adverse events [AEs] of special interest with a long latency period, such as malignancies or cardiovascular [CV] complications.5,6 Therefore, evaluation of the long-term safety profile of tofacitinib in patients with UC is important. Previously, an analysis of data pooled from phase 2/3/OLE studies in the tofacitinib UC clinical programme was carried out, which included data with up to 4.4 years (1612.8 patient-years [PY]) of exposure.7 Except for a higher incidence of herpes zoster [HZ] infection, the safety profile of tofacitinib in patients with UC appeared similar to that reported for other UC therapies.8 Since the previous analysis, data for a substantial number of additional PY of tofacitinib exposure have been accrued, including final data from the completed OLE OCTAVE Open study and the 6-month interim analysis of the phase 3b/4 RIVETING study. Here, we report an updated integrated summary of tofacitinib safety using cumulative experience throughout the global tofacitinib UC clinical programme to date with up to 7.8 years [2999.7 PY] of tofacitinib treatment exposure, which includes an additional 3.4 years of observation and 1386.9 PY of exposure, compared with the previous analysis.7
2. Methods
2.1 Patient populations and study designs
Data were pooled from patients with UC in the global tofacitinib UC clinical programme, which included an 8-week, phase 2 induction study [NCT00787202],1 two identical, 8-week, phase 3 induction studies [OCTAVE Induction 1 and 2; NCT01465763 and NCT01458951],2 a 52-week, phase 3 maintenance study [OCTAVE Sustain; NCT01458574],2 an OLE study [OCTAVE Open; NCT01470612]3 and a phase 3b/4 study [RIVETING; NCT03281304].4 Full design details of all studies have been published previously,1–4,7 and are summarised in the Supplementary Methods and Supplementary Figure 1.
2.2 Analysis cohorts
Safety data were analysed for the Overall plus P3b/4 [2020] Cohort, which comprised all patients who received one or more dose of tofacitinib 5 or 10 mg twice daily [BID] in any phase 2/3/OLE or phase 3b/4 study, and includes final data from OCTAVE Open [final data cut-off: August 24, 2020], plus data from the 6-month interim analysis of the RIVETING study [interim data cut-off: February 20, 2020; total tofacitinib exposure ≤7.8 years; Supplementary Figure 1].
For contextualisation of data in the Overall plus P3b/4 [2020] Cohort, data from three cohorts included in a previous integrated analysis (Induction Cohort, Maintenance Cohort, and Overall Cohort, which included data from OCTAVE Open as of December 16, 2016; total tofacitinib exposure ≤4.4 years; hereafter defined as the Overall [Dec 2016] Cohort) are also shown, as defined in the Supplementary Methods.7
2.3 Assessment of safety
The proportions of patients with AEs, serious AEs and discontinuations due to AEs were calculated for patients in all cohorts. Serious AEs are defined in the Supplementary Methods. AEs of special interest included serious infections, HZ [non-serious and serious], opportunistic infections, malignancies (excluding non-melanoma skin cancer [NMSC]), NMSC, major adverse CV events [MACE], venous thromboembolic events (VTEs; including deep vein thrombosis [DVT] and pulmonary embolism [PE]) and gastrointestinal [GI] perforations. Potential opportunistic infections, malignancies, MACE, VTEs and GI perforations were reviewed by blinded independent adjudication committees. Patients with a serious infection [defined as an infection that met the criteria to be classified as a serious AE, or that required parenteral antimicrobial therapy or hospitalisation] were required to discontinue the studies [with appropriate follow-up]. Disseminated or multidermatomal HZ events were considered to be HZ-opportunistic infections. Additional details can be found in the Supplementary Methods.
The proportions of patients in the Overall plus P3b/4 [2020] Cohort receiving lipid-lowering agents at baseline were reported, along with the proportions who began receiving lipid-lowering agents or had an increase in their dose of lipid-lowering agents during the programme. The proportions of patients and incidence rates (IRs; 95% confidence intervals [CIs]) were calculated for creatine kinase elevation, anaemia, lymphopaenia, neutropaenia, acute renal failure and rhabdomyolysis [defined in the Supplementary Methods].
2.4 Statistical analyses
Baseline demographics and disease characteristics were summarised descriptively for all cohorts. As doses could be switched across studies and during OCTAVE Open and RIVETING, tofacitinib doses in the Overall [Dec 2016] and Overall plus P3b/4 [2020] Cohorts were categorised based on the average daily dose of tofacitinib received: a predominant dose [PD] of tofacitinib 5 mg BID was defined as an average total daily dose of tofacitinib <15 mg, and a PD of tofacitinib 10 mg BID was defined as an average total daily dose of tofacitinib ≥15 mg.
For all cohorts, proportions and IRs [unique patients with events per 100 PY of exposure] along with 95% CIs were calculated for deaths and AEs of special interest. For deaths, malignancies [excluding NMSC], NMSC and MACE, calculation of IRs included all events; for other AEs of special interest, events occurring >28 days after the last dose of study treatment were not included in the calculation of IRs. Additional details are described in the Supplementary Methods.
Cox proportional regression models were used to assess the association of demographic and clinical factors with the risk of AEs of special interest. Models were applied to the Overall plus P3b/4 [2020] Cohort and excluded any time periods and events experienced while receiving placebo. If multiple continuous covariates were highly correlated, then only one was retained in the model to avoid problems with collinearity. The modelling approach for baseline characteristics was first applied to univariate models to identify individual risk factors with a statistically significant relationship to each AE; factors with p < 0.10 were then included in a stepwise multivariable model. The final model included all factors from the stepwise model with p < 0.05. No adjustment of p values was made for multiple comparisons. Additional details of the Cox proportional regression models, including all covariates, are described in the Supplementary Methods.
2.5 Ethical statement
All studies were conducted in accordance with the Declaration of Helsinki, International Council on Harmonization Guidelines for Good Clinical Practice and local regulations. Participating institutions provided Institutional Review Board approval prior to participation. All patients provided written informed consent.
3. Results
3.1 Patient demographics and baseline disease characteristics
Patient demographics and disease characteristics were generally similar among the treatment groups in each cohort [Table 1]. In total, 1157 patients receiving one or more dose of tofacitinib 5 or 10 mg BID were included in this analysis; 955/1157 [82.5%] patients received PD tofacitinib 10 mg BID [total exposure: 783.1 and 2216.6 PY with PD tofacitinib 5 and 10 mg BID, respectively]; 412/1157 [35.6%] patients had received tofacitinib for >4 years [Supplementary Table 1]. Total tofacitinib exposure was 2999.7 PY for all patients receiving tofacitinib in the Overall plus P3b/4 [2020] Cohort. Across cohorts and treatment groups, the majority of patients were male, White, aged <65 years, had a body mass index [BMI] <25 kg/m2 and had never smoked; mean disease duration ranged from 8.2 to 8.8 years, and the majority of patients had previously received immunosuppressants. Across treatment groups and cohorts, 40.6–51.0% of patients were receiving corticosteroids at baseline, and 41.6–54.1% had prior tumour necrosis factor inhibitor [TNFi] failure [Table 1].
Table 1.
Baseline demographics and disease characteristics in the tofacitinib UC clinical programme, by cohort
| Induction Cohorta | Maintenance Cohorta | Overall [Dec 2016] and Overall plus P3b/4 [2020] Cohortsa,b | ||||||
|---|---|---|---|---|---|---|---|---|
| Placebo [N = 282; 44.8 PY] |
Tofacitinib 10 mg BID [N = 938; 156.2 PY] | Placebo [N = 198; 100.4 PY] |
Tofacitinib 5 mg BID [N = 198; 146.2 PY] |
Tofacitinib 10 mg BID [N = 196; 154.3 PY] |
PD tofacitinib 5 mg BID [N = 202; 783.1 PY]c |
PD tofacitinib 10 mg BID [N = 955: 2216.6 PY]c |
Tofacitinib All [N = 1157: 2999.7 PY]c |
|
| Treatment duration [days], mean [SD] (range) |
57.9 [13.7] (7–80) | 60.8 [11.0] (1–96) | 185.1 [127.9] (14–382) | 269.7 [125.1] (22–420) | 287.4 [123.1] (1–399) | 1415.8 [789.8] (52–2850) | 847.7 [848.6] (1–2758) | 946.9 [865.6] (1–2850)c |
| Age [years], mean [SD] | 41.4 [14.4] | 41.3 [13.8] | 43.4 [14.0] | 41.9 [13.7] | 43.0 [14.4] | 44.5 [14.5] | 40.6 [13.7] | 41.3 [13.9] |
| Age ≥65 years, n [%] | 21 [7.4] | 62 [6.6] | 18 [9.1] | 13 [6.6] | 17 [8.7] | 18 [8.9] | 59 [6.2] | 77 [6.7] |
| Sex [male] | 155 [55.0] | 557 [59.4] | 116 [58.6] | 103 [52.0] | 110 [56.1] | 116 [57.4] | 563 [60.0] | 679 [58.7] |
| Weight [kg], mean [SD] | 73.2 [16.3]d | 73.7 [16.7] | 76.2 [16.7] | 73.4 [17.8] | 74.6 [15.2] | 74.1 [16.6] | 73.3 [16.8] | 73.5 [16.7] |
| BMI [kg/m2], n [%] | ||||||||
| <25 | 158 [56.2]d | 549 [58.5] | 100 [50.5] | 111 [56.1] | 103 [52.6] | 121 [59.9] | 560 [58.7] | 681 [58.9] |
| ≥25–<30 | 84 [29.9]d | 260 [27.7] | 65 [32.8] | 61 [30.8] | 61 [31.1] | 53 [26.2] | 263 [27.6] | 316 [27.3] |
| >30 | 39 [13.9]d | 129 [13.8] | 33 [16.7] | 26 [13.1] | 32 [16.3] | 28 [13.9] | 131 [13.7] | 159 [13.8] |
| Race, n [%] | ||||||||
| White | 229 [81.2] | 756 [80.6] | 155 [78.3] | 164 [82.8] | 153 [78.1] | 160 [79.2] | 767 [80.3] | 927 [80.1] |
| Black | 4 [1.4] | 6 [0.6] | 3 [1.5] | 2 [1.0] | 0 [0.0] | 1 [0.5] | 9 [0.9] | 10 [0.9] |
| Asian | 28 [9.9] | 114 [12.2] | 26 [13.1] | 23 [11.6] | 25 [12.8] | 28 [13.9] | 116 [12.1] | 144 [12.4] |
| Other | 11 [3.9] | 36 [3.8] | 9 [4.5] | 5 [2.5] | 9 [4.6] | 9 [4.5] | 33 [3.5] | 42 [3.6] |
| Geographical region, n [%] | ||||||||
| Asia | 26 [9.2] | 95 [10.1] | 20 [10.1] | 22 [11.1] | 21 [10.7] | 26 [12.9] | 97 [10.2] | 123 [10.6] |
| Eastern Europee | 90 [31.9] | 283 [30.2] | 57 [28.8] | 66 [33.3] | 63 [32.1] | 73 [36.1] | 269 [28.2] | 342 [29.6] |
| Western Europef | 79 [28.0] | 281 [30.0] | 55 [27.8] | 47 [23.7] | 57 [29.1] | 45 [22.3] | 299 [31.3] | 344 [29.7] |
| North America | 53 [18.8] | 187 [19.9] | 45 [22.7] | 39 [19.7] | 44 [22.4] | 39 [19.3] | 202 [21.2] | 241 [20.8] |
| Rest of the World | 34 [12.1] | 92 [9.8] | 21 [10.6] | 24 [12.1] | 11 [5.6] | 19 [9.4] | 88 [9.2] | 107 [9.2] |
| Smoking status, n [%] | ||||||||
| Never smoked | 195 [69.1] | 593 [63.2] | 113 [57.1] | 142 [71.7] | 127 [64.8] | 139 [68.8] | 601 [62.9] | 740 [64.0] |
| Current smoker | 11 [3.9] | 48 [5.1] | 12 [6.1] | 7 [3.5] | 6 [3.1] | 5 [2.5] | 54 [5.7] | 59 [5.1] |
| Ex-smoker | 76 [27.0] | 296 [31.6] | 73 [36.9] | 49 [24.7] | 63 [32.1] | 58 [28.7] | 299 [31.3] | 357 [30.9] |
| Disease duration [years], mean [SD] | 8.2 [6.8] | 8.2 [7.0] | 8.8 [7.5] | 8.3 [7.2] | 8.7 [7.0] | 8.3 [6.5] | 8.2 [7.1] | 8.2 [7.0] |
| Total Mayo score at baseline, mean [SD] | 8.9 [1.5] | 9.0 [1.5] | 3.3 [1.8] | 3.3 [1.8] | 3.4 [1.8] | 7.8 [2.5] | 8.8 [1.8] | 8.6 [2.0]d |
| History of diabetes mellitus, n [%] | 9 [3.2] | 42 [4.5] | 10 [5.1] | 8 [4.0] | 7 [3.6] | 10 [5.0] | 38 [4.0] | 48 [4.1] |
| History of hypertension, n [%] | 35 [12.4] | 132 [14.1] | 32 [16.2] | 24 [12.1] | 29 [14.8] | 31 [15.3] | 130 [13.6] | 161 [13.9] |
| Prior immunosuppressant use, n [%] | 160 [68.4]d | 683 [75.5]d | 134 [67.7] | 149 [75.3] | 144 [73.5] | 701 [76.0] | 137 [67.8] | 838 [74.6]d,h |
| Prior TNFi exposure, n [%] | 130 [55.6]d | 488 [53.9]d | 92 [46.5] | 90 [45.5] | 100 [51.0] | 91 [45.0] | 521 [56.5] | 612 [54.4]d,h |
| Prior TNFi failure, n [%] | 124 [53.0]d | 465 [51.4]d | 89 [44.9] | 83 [41.9] | 92 [46.9] | 84 [41.6] | 499 [54.1] | 583 [51.9]d,h |
| Corticosteroid use at baseline, n [%]g | 127 [45.0] | 430 [45.8] | 100 [50.5] | 101 [51.0] | 86 [43.9] | 82 [40.6] | 441 [46.2] | 523 [45.2]h |
| Corticosteroid dose at baseline [mg/day], mean [SD]i | 16.9 [6.2]d | 16.0 [6.4]d | 15.9 [6.2]d | 14.9 [6.2]d | 14.5 [5.9]d | 15.0 [6.1] | 16.1 [6.4] | 16.0 [6.3]d |
aData for the Induction, Maintenance and Overall [Dec 2016] Cohorts were previously reported by Sandborn et al.7
bThe patient population was the same for the Overall [Dec 2016] and Overall plus P3b/4 [2020] Cohorts because patients could enter RIVETING if they had received tofacitinib 10 mg BID for two or more consecutive years in OCTAVE Open, were in stable remission for ≥6 months, and had not received corticosteroids for UC for ≥4 weeks prior to baseline; the previously reported Overall [Dec 2016] Cohort includes data from OCTAVE Open up to December 16, 2016 [≤4.4 years of exposure] and the Overall plus P3b/4 [2020] Cohort includes final data from OCTAVE Open [final data cut-off: August 24, 2020], and data from RIVETING [interim data cut-off: February 20, 2020; ≤7.8 years of exposure].
cIn the Overall [Dec 2016] Cohort [≤4.4 years of exposure], N = 201 and N = 956 for PD tofacitinib 5 mg BID and PD tofacitinib 10 mg BID, respectively; total exposure with all tofacitinib doses was 1612.8 PY and mean [SD (range)] treatment duration was 509.1 [389.8 (1–1606)] days.
dDenominators for these characteristics vary due to data not being available for all patients.
eCroatia, Czechia, Estonia, Hungary, Latvia, Poland, Romania, Russia, Serbia, Slovakia and Ukraine.
fAustria, Belgium, Denmark, France, Germany, Israel, Italy, Netherlands, Spain and UK.
gCorticosteroid tapering was mandatory during OCTAVE Sustain and OCTAVE Open, and corticosteroids were not permitted during RIVETING.
hData are from Day 1, the start of active tofacitinib treatment in the tofacitinib UC clinical programme.
iBased on prednisone equivalent total daily dose [excludes medications such as budesonide and beclomethasone].
BID, twice daily; BMI, body mass index; N, total number of patients in a treatment group; n, number of patients with each characteristic; PD, predominant dose; PY, patient-years; SD, standard deviation; TNFi, tumour necrosis factor inhibitor; UC, ulcerative colitis.
3.2 Incidence of AEs and serious AEs in the tofacitinib UC clinical programme, by cohort
In the Induction and Maintenance Cohorts, the proportions of patients with AEs, serious AEs and discontinuations due to AEs were generally similar across treatment groups.
In the Overall [Dec 2016] Cohort, there were a total of 950/1157 [82.1%] patients with AEs and 169/1157 [14.6%] with serious AEs [Table 2]. In the Overall plus P3b/4 [2020] Cohort, 992/1157 [85.7%] and 244/1157 [21.1%] patients receiving tofacitinib experienced AEs and serious AEs, respectively. The most frequently reported AE was worsening UC [all tofacitinib doses, 26.5%], followed by nasopharyngitis [all tofacitinib doses, 22.0%; Table 2]. The most frequently occurring serious AE reported in all cohorts was worsening UC.
Table 2.
Proportions of patients with AEs, serious AEs and most frequently reported AEs,a by cohort
| Induction Cohort [8 weeks]b | Maintenance Cohort [52 weeks]b | Overall [Dec 2016] Cohort [≤4.4 years]b,c | Overall plus P3b/4 [2020] Cohort [≤7.8 years]d | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Placebo [N = 282] | Tofacitinib 10 mg BID [N = 938] | Placebo [N = 198] | Tofacitinib 5 mg BID [N = 198] | Tofacitinib 10 mg BID [N = 196] | Tofacitinib All [N = 1157] | PD tofacitinib 5 mg BID [N = 202] | PD tofacitinib 10 mg BID [N = 955] | Tofacitinib All [N = 1157] | |
| AEs | |||||||||
| Patients with AEs, n [%] | 155 [55.0] | 515 [54.9] | 149 [75.3] | 143 [72.2] | 156 [79.6] | 950 [82.1] | 189 [93.6] | 803 [84.1] | 992 [85.7] |
| Patients with serious AEs, n [%] | 18 [6.4] | 36 [3.8] | 13 [6.6] | 10 [5.1] | 11 [5.6] | 169 [14.6] | 44 [21.8] | 200 [20.9] | 244 [21.1] |
| Discontinuations due to AEs, n [%] | 14 [5.0] | 36 [3.8] | 37 [18.7] | 18 [9.1] | 19 [9.7] | 75 [7.9] | 24 [11.9] | 110 [11.5] | 134 [11.6] |
| Most frequently reported AEs, n [%]a | |||||||||
| Worsening UC | 20 [7.1] | 26 [2.8] | 71 [35.9] | 36 [18.2] | 29 [14.8] | 224 [19.4] | 49 [24.3] | 258 [27.0] | 307 [26.5] |
| Nasopharyngitis | 14 [5.0] | 56 [6.0] | 11 [5.6] | 19 [9.6] | 27 [13.8] | 211 [18.2] | 59 [29.2] | 196 [20.5] | 255 [22.0] |
| Arthralgia | 12 [4.3] | 27 [2.9] | 19 [9.6] | 17 [8.6] | 17 [8.7] | 122 [10.5] | 32 [15.8] | 117 [12.3] | 149 [12.9] |
| Headache | 19 [6.7] | 73 [7.8] | 12 [6.1] | 17 [8.6] | 6 [3.1] | 121 [10.5] | 34 [16.8] | 114 [11.9] | 148 [12.8] |
| Blood creatine phosphokinase increased | 3 [1.1] | 25 [2.7] | 4 [2.0] | 6 [3.0] | 13 [6.6] | 115 [9.9] | 30 [14.9] | 108 [11.3] | 138 [11.9] |
| Upper respiratory tract infection | 6 [2.1] | 26 [2.8] | 7 [3.5] | 13 [6.6] | 12 [6.1] | 103 [8.9] | 24 [11.9] | 113 [11.8] | 137 [11.8] |
| Influenza | 3 [1.1] | 9 [1.0] | 7 [3.5] | 4 [2.0] | 7 [3.6] | 67 [5.8] | 21 [10.4] | 84 [8.8] | 105 [9.1] |
| Hypertension | 1 [0.4] | 9 [1.0] | 1 [0.5] | 4 [2.0] | 4 [2.0] | 33 [2.9] | 26 [12.9] | 41 [4.3] | 67 [5.8] |
For the Induction and Maintenance Cohorts, events that occurred >28 days after the last dose of the study drug were excluded; for the Overall [Dec 2016] and Overall plus P3b/4 [2020] Cohorts, all events, including those outside the 28-day risk period, were included.
aData are reported for all AEs by preferred term, which occurred in ≥10% of patients in a treatment group in the Overall plus P3b/4 [2020] Cohort.
bData for the Induction, Maintenance and Overall [Dec 2016] Cohorts were previously reported by Sandborn et al.7
cThe previously reported Overall [Dec 2016] Cohort included data from OCTAVE Open up to December 2016 [≤4.4 years of exposure].
dThe Overall plus P3b/4 [2020] Cohort includes final data from OCTAVE Open [final data cut-off: August 24, 2020], and data from RIVETING [interim data cut-off: February 20, 2020; ≤7.8 years of exposure].
AE, adverse event; BID, twice daily; N, number of patients treated in the treatment group; n, number of unique patients in the specified category; PD, predominant dose; UC, ulcerative colitis.
3.3 Incidence of deaths in the tofacitinib UC clinical programme, by cohort
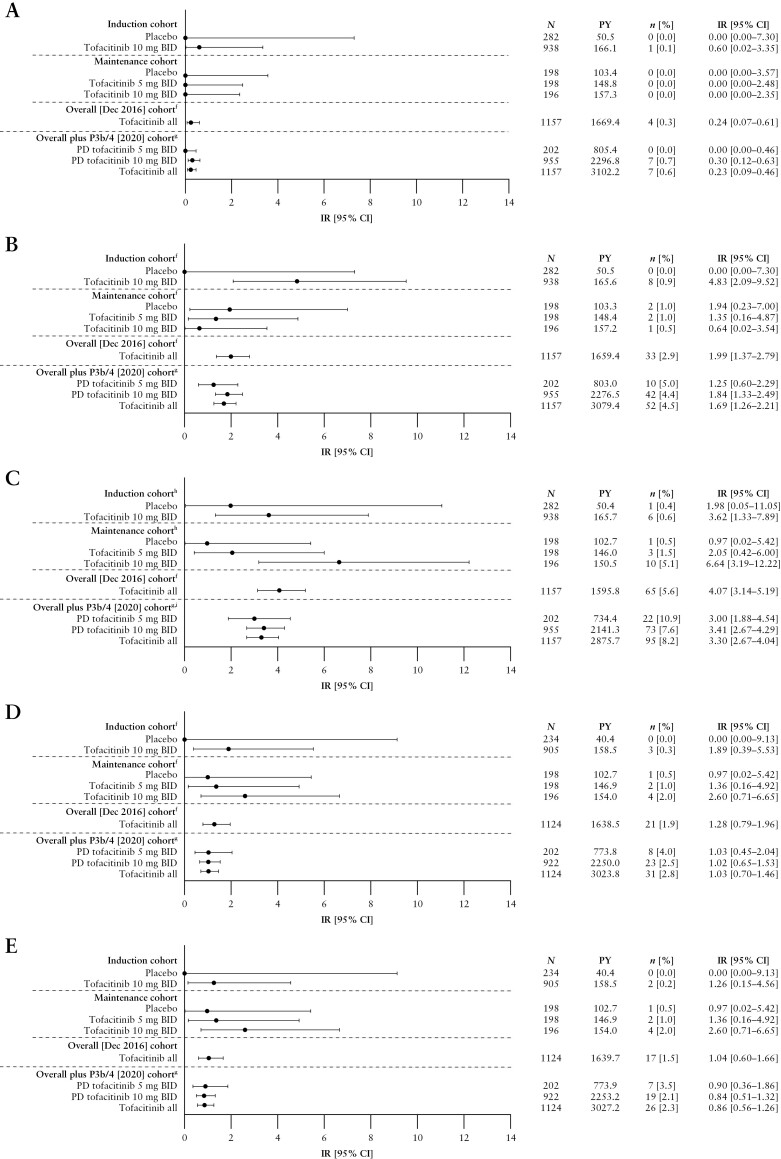
Deaths in the Induction and Maintenance Cohorts are shown in Figure 1A. In total, four deaths were reported in the Overall [Dec 2016] Cohort [IR 0.24, 95% CI 0.07–0.61], and seven deaths were reported in the Overall plus P3b/4 [2020] Cohort [IR 0.23, 95% CI 0.09–0.46]; Figure 1A]. Of these, one patient who had been receiving tofacitinib 10 mg BID experienced cardiac arrest on Day 1725; this patient also had malignant lung cancer. Six deaths occurred in patients receiving PD tofacitinib 10 mg BID (one case of aortic dissection after 31 days of treatment; one case of hepatic angiosarcoma after 187 days of treatment; one case of acute myeloid leukaemia after 347 days of treatment; one case of PE after 378 days of treatment [this patient also had cholangiocarcinoma metastasised to the peritoneum]; one case of malignant melanoma after 1359 days of treatment; and one case of metastatic adenocarcinoma after 2124 days of treatment).
Figure 1.
Incidence of [A] deaths,a [B] serious infections,b,c [C] HZ [non-serious and serious],b [D] opportunistic infections,b,d,e and [E] HZ opportunistic infections,b,d by cohort.
aFor the Induction and Maintenance Cohorts, events that occurred >28 days after the last dose of the study drug were excluded; for the Overall [Dec 2016] and Overall plus P3b/4 [2020] Cohorts, all events, including those outside the 28-day risk period, were included.
bEvents that occurred >28 days after the last dose of the study drug were excluded.
cDefined as any infection AE that requires hospitalization or parenteral antimicrobials, or meets other criteria that require the infection to be classified as a serious AE.
dAdjudicated events; for the Overall plus P3b/4 [2020] Cohort, N = 922 and N = 1124 for the PD tofacitinib 10 mg BID and tofacitinib all groups, respectively [excludes phase 2 study data, as the phase 2 induction study took place prior to the establishment of the adjudication committees].
eExcludes tuberculosis and HZ with two adjacent dermatomes.
fIncludes data previously reported by Sandborn et al.7; the previously reported Overall [Dec 2016] Cohort included data from OCTAVE Open up to December 2016 [≤4.4 years of exposure].
gThe Overall plus P3b/4 [2020] Cohort includes final data from OCTAVE Open [final data cut-off: August 24, 2020], and data from RIVETING [interim data cut-off: February 20, 2020; ≤7.8 years of exposure].
hIncludes data previously reported by Winthrop et al.10
iIncludes data previously reported by Winthrop et al.9
AE, adverse event; BID, twice daily; CI, confidence interval; HZ, herpes zoster; IR, incidence rate [unique patients with events/100 PY of exposure]; N, number of patients treated in the treatment group; n, number of unique patients with a particular AE; PD, predominant dose; PY, patient-years.
3.4 Incidence of serious infections in the tofacitinib UC clinical programme, by cohort
Serious infections in the Induction and Maintenance Cohorts are shown in Figure 1B. IRs for serious infections with all tofacitinib doses were 1.99 [95% CI 1.37–2.79] in the Overall [Dec 2016] Cohort and 1.69 [95% CI 1.26–2.21] in the Overall plus P3b/4 [2020] Cohort [Figure 1B]. In total, serious infections were reported in 52 patients in the Overall plus P3b/4 [2020] Cohort; 10/52 and 42/52 serious infections occurred in patients receiving PD tofacitinib 5 and 10 mg BID, respectively. In the Overall plus P3b/4 [2020] Cohort, the following serious infection event terms were reported more than once: serious HZ [eight events; reported in seven patients], anal abscess [four events; reported in four patients], appendicitis [three events; reported in three patients], upper respiratory tract infection [three events; reported in three patients], urinary tract infection [three events; reported in three patients], Clostridioides difficile infection [two events; reported in two patients] and influenza [two events; reported in two patients]. No serious infections resulted in death.
The results of univariate analyses of risk factors for serious infections in the Overall plus P3b/4 [2020] Cohort are shown in Supplementary Table 2. Higher BMI was associated with an increased risk of serious infections in the Overall plus P3b/4 [2020] Cohort in multivariable analysis [Supplementary Figure 2A].
3.5 Incidence of HZ in the tofacitinib UC clinical programme, by cohort
The incidence of HZ [non-serious and serious] in all cohorts has been reported previously.7,9 HZ rates in the Induction and Maintenance Cohorts are shown in Figure 1C. IR for HZ in the Overall [Dec 2016] Cohort was 4.07 [95% CI 3.14–5.19]. In the Overall plus P3b/4 [2020] Cohort, HZ was reported in 95 patients [22/95 and 73/95 in patients receiving PD tofacitinib 5 and 10 mg BID, respectively]; the overall IR for HZ was 3.30 [95% CI 2.67–4.04; Figure 1C]. In total, seven [0.6%] patients had serious HZ infection [of whom one patient was receiving PD tofacitinib 5 mg BID, and six patients were receiving PD tofacitinib 10 mg BID].
A previous analysis of risk factors for HZ, using data from the Overall plus P3b/4 [2020] Cohort, identified older age, lower body weight, geographical region [North America vs Europe] and prior TNFi failure as significant risk factors for HZ.9
3.6 Incidence of opportunistic infections in the tofacitinib UC clinical programme, by cohort
Opportunistic infections in the Induction and Maintenance Cohorts are detailed in Figure 1D. IRs for opportunistic infections with all tofacitinib doses were 1.28 [95% CI 0.79–1.96] in the Overall [Dec 2016] Cohort and 1.03 [95% CI 0.70–1.46; Figure 1D] in the Overall plus P3b/4 [2020] Cohort. In the Overall plus P3b/4 [2020] Cohort, opportunistic infections were reported in 31 patients, 23 of whom were receiving PD tofacitinib 10 mg BID. In total, 26 of the 31 opportunistic infections were HZ [IR 0.86, 95% CI 0.56–1.26; Figure 1E]. Non-HZ opportunistic infections included one case of pulmonary mycosis in a patient receiving PD tofacitinib 5 mg BID, and two cases of cytomegalovirus infection [one case of cytomegalovirus hepatitis and one case of histoplasmosis] in patients receiving PD tofacitinib 10 mg BID.
The results of univariate analyses of risk factors for opportunistic infections in the Overall plus P3b/4 [2020] Cohort are shown in Supplementary Table 3. Lower body weight, lower baseline absolute neutrophil count [ANC], history of diabetes and prior TNFi failure were significant risk factors for opportunistic infections in multivariable analysis [Supplementary Figure 2B].
3.7 Incidence of malignancies in the tofacitinib UC clinical programme, by cohort
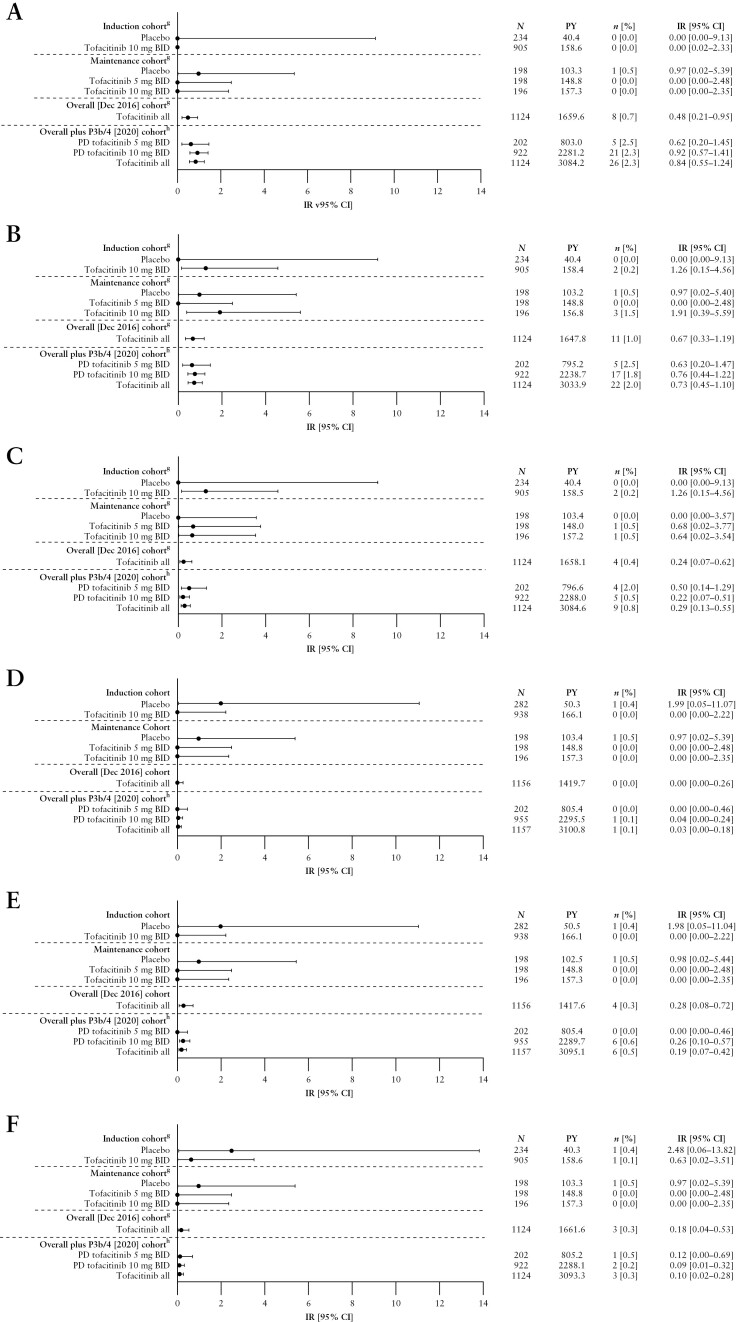
Malignancies [excluding NMSC] and NMSC in the Induction and Maintenance Cohorts are detailed in Figure 2A and B, respectively. Malignancies [excluding NMSC] were reported in eight [0.7%] patients in the Overall [Dec 2016] Cohort [IR 0.48, 95% CI 0.21–0.95] and 26 [2.3%] patients in the Overall plus P3b/4 [2020] Cohort [IR 0.84, 95% CI 0.55–1.24; Figure 2A]. In total, 21 of the 26 patients who experienced malignancies [excluding NMSC] in the Overall plus P3b/4 [2020] Cohort were receiving PD tofacitinib 10 mg BID. The most frequently reported malignancy [excluding NMSC] was colorectal cancer, which occurred in four patients—all were receiving PD tofacitinib 10 mg BID [Supplementary Table 4]; the duration of UC was ≥10 years for three of the four patients. Other malignancies [excluding NMSC] included one case each of acute myeloid leukaemia, Bowen’s disease, diffuse large B-cell lymphoma, Epstein-Barr virus-associated lymphoma, essential thrombocythaemia, hepatic angiosarcoma, leiomyosarcoma, oesophageal adenocarcinoma, renal cell carcinoma, penile dysplasia and vulvar cancer; two cases of cervical dysplasia, cholangiocarcinoma, lung cancer and malignant melanoma; and three cases of breast cancer. Additional details of malignancy events and incidence in the Overall plus P3b/4 [2020] Cohort are shown in Supplementary Table 4.
Figure 2.
Incidence of [A] malignancies [excluding NMSC],a,b,c [B] NMSC,a,b,c [C] MACE,a,b,c [D] DVT,d,e [E] PEd,e and [F] GI perforations,b,c,d,f by cohort.
aFor the Maintenance Cohort, events that occurred >28 days after the last dose of the study drug were excluded; for the Overall [Dec 2016] and Overall plus P3b/4 [2020] Cohort, all events, including those outside the 28-day risk period, were included.
bAdjudicated events.
cFor the Overall plus P3b/4 [2020] Cohort, N = 922 and N = 1124 for the PD tofacitinib 10 mg BID and tofacitinib all groups, respectively [excludes phase 2 study data, as the phase 2 induction study took place prior to the establishment of the adjudication committees].
dEvents that occurred >28 days after the last dose of the study drug were excluded.
eVTEs included adjudicated events from OCTAVE Open and RIVETING plus selected events from phase 2/3 studies from the Narrow Standardized MedDRA query for embolic and thrombotic events.
fGI perforation excludes preferred terms of pilonidal cyst, perirectal abscess, rectal abscess, anal abscess, perineal abscess and any preferred terms containing the term fistula.
gIncludes data previously reported by Sandborn et al.7; the previously reported Overall [Dec 2016] Cohort included data from OCTAVE Open up to December 2016 [≤4.4 years of exposure].
hThe Overall plus P3b/4 [2020] Cohort includes final data from OCTAVE Open [final data cut-off: August 24, 2020], and data from RIVETING [interim data cut-off: February 20, 2020; ≤7.8 years of exposure].
AE, adverse event; BID, twice daily; CI, confidence interval; DVT, deep vein thrombosis; GI, gastrointestinal; IR, incidence rate [unique patients with events/100 PY of exposure]; MACE, major adverse cardiovascular events; N, number of patients treated in the treatment group; n, number of unique patients with a particular AE; MedDRA, Medical Dictionary for Regulatory Activities; NMSC, non-melanoma skin cancer; PD, predominant dose; PE, pulmonary embolism; PY, patient-years; VTE, venous thromboembolic event.
NMSC was reported in 11 patients in the Overall [Dec 2016] Cohort [IR 0.67, 95% CI 0.33–1.19], and 22 patients in the Overall plus P3b/4 [2020] Cohort [IR 0.73, 95% 0.45–1.10; Figure 2B]. Of the 22 patients with NMSC, five and 17 were receiving PD tofacitinib 5 and 10 mg BID, respectively. There were 16 patients with basal cell carcinoma [four patients receiving PD tofacitinib 5 mg BID; 12 patients receiving PD tofacitinib 10 mg BID], and 11 patients with squamous cell carcinoma [two patients receiving tofacitinib 5 mg BID; nine patients receiving tofacitinib 10 mg BID] [Supplementary Table 4]. Five patients experienced both basal cell carcinoma and squamous cell carcinoma [one patient receiving PD tofacitinib 5 mg BID and four patients receiving PD tofacitinib 10 mg BID].
The results of univariate analyses of risk factors for malignancies [excluding NMSC] and NMSC in the Overall plus P3b/4 [2020] Cohort are shown in Supplementary Tables 5 and 6, respectively. In multivariable analysis, disease duration and history of NMSC were significant risk factors for malignancies [excluding NMSC] [Supplementary Figure 2C], and older age and history of NMSC were significant risk factors for NMSC [Supplementary Figure 2D].
3.8 Incidence of MACE in the tofacitinib UC clinical programme, by cohort
MACE in the Induction and Maintenance Cohorts are detailed in Figure 2C. There were four and nine patients with MACE in the Overall [Dec 2016] and Overall plus P3b/4 [2020] Cohorts, respectively. IRs for MACE with all tofacitinib doses were 0.24 [95% CI 0.07–0.62] in the Overall [Dec 2016] Cohort and 0.29 [95% CI 0.13–0.55] in the Overall plus P3b/4 [2020] Cohort [Figure 2C]. Additional details of MACE incidence in the Overall plus P3b/4 [2020] Cohort are shown in Supplementary Tables 7 and 8. MACE in the Overall plus P3b/4 [2020] Cohort included one case of acute coronary syndrome, one case of acute myocardial infarction, one case of cerebellar haemorrhage, one case of myocardial infarction and one case of cerebrovascular accident [patients receiving tofacitinib 5 mg BID at the time of the event]; and one case of haemorrhagic stroke, one case of aortic dissection, one case of cardiac arrest and one case of cerebrovascular accident [patients receiving tofacitinib 10 mg BID at the time of the event]. All patients who experienced MACE had CV risk factors [four patients were smokers or ex-smokers; two patients had a BMI > 30 kg/m2; eight patients had a medical history of CV events; Supplementary Table 8].
The results of univariate analyses of risk factors for MACE in the Overall plus P3b/4 [2020] Cohort are shown in Supplementary Table 9. Older age was a significant risk factor for MACE in multivariable analysis [Supplementary Figure 2E].
In the Overall plus P3b/4 [2020] Cohort, 74 [6.4%] patients were receiving lipid-lowering agents at baseline; 90 [7.8%] patients began receiving lipid-lowering agents, and 22 [1.9%] patients had an increase in their dose of lipid-lowering agents [Supplementary Table 10].
3.9 Incidence of VTEs in the tofacitinib UC clinical programme, by cohort
DVT and PE in the Induction and Maintenance Cohorts are detailed in Figure 2D and E, respectively. In the Overall [Dec 2016] Cohort, with all tofacitinib doses, there were no patients with DVT and four [0.3%] patients with PE [IR 0.28, 95% CI 0.08–0.72]. In the Overall plus P3b/4 [2020] Cohort, with all tofacitinib doses, there was one patient with DVT [IR 0.03, 95% CI 0.00–0.18] and six patients with PE [IR 0.19, 95% CI 0.07–0.42]; all were receiving tofacitinib 10 mg BID [Figure 2D and E]. Additional details of thromboembolic events and incidence in the Overall plus P3b/4 [2020] Cohort are shown in Supplementary Tables 11 and 12. The patient with DVT was diagnosed following a long-haul flight and had been treated for an infected leg wound sustained in a recent motorcycle accident. Patients with PE had the following notable medical history: one patient had a history of prior DVT and PE; one patient had a history of phlebothrombosis and stroke; one patient was receiving oral contraceptives for dysfunctional uterine bleeding; one patient had cholangiocarcinoma and metastases to the peritoneum [PE was the cause of death]; and two patients had no prior risk factors for PE.
The results of univariate analyses of risk factors for VTEs in the Overall plus P3b/4 [2020] Cohort are shown in Supplementary Table 13. In multivariable analysis, race was the only covariate that significantly increased the risk of VTEs in the Overall plus P3b/4 [2020] Cohort [with Black patients at a higher risk of VTEs vs White patients; Supplementary Figure 2F].
3.10 Incidence of GI perforations in the tofacitinib UC clinical programme, by cohort
GI perforations in the Induction and Maintenance Cohorts are detailed in Figure 2F. There were three patients with GI perforations in the Overall [Dec 2016] Cohort [IR 0.18, 95% CI 0.04–0.53], and three patients with GI perforations in the Overall plus P3b/4 [2020] Cohort [IR 0.10, 95% CI 0.02–0.28; Figure 2F]; two patients were receiving tofacitinib 10 mg BID [one case each of intestinal perforation and GI perforation], and one patient was receiving tofacitinib 5 mg BID [complicated appendicitis].
The results of univariate analyses of risk factors for GI perforations in the Overall plus P3b/4 [2020] Cohort are shown in Supplementary Table 14. Higher baseline high-density lipoprotein [HDL] was associated with a reduced risk of GI perforations in the Overall plus P3b/4 [2020] Cohort in multivariable analysis [Supplementary Figure 2G], although patient numbers were low [n = 3].
3.11 Laboratory abnormalities in the Overall plus P3b/4 [2020] Cohort
Laboratory abnormalities reported in patients in the Overall plus P3b/4 [2020] Cohort are detailed in Table 3. Across treatment groups, in patients with UC, the most frequent laboratory abnormality reported was creatine kinase elevation [IR 5.06, 95% CI 4.25–5.98], followed by anaemia [IR 2.66, 95% CI 2.10–3.32] and lymphopaenia [IR 1.46, 95% CI 1.06–1.96]. The laboratory abnormality associated with the most discontinuations was lymphopaenia [ten patients in total; 8/10 were receiving PD tofacitinib 10 mg BID].
Table 3.
Laboratory abnormalities in the Overall plus P3b/4 [2020] Cohort
| PD tofacitinib 5 mg BID [N = 202] | PD tofacitinib 10 mg BID [N = 955] | Tofacitinib All [N = 1157] | |
|---|---|---|---|
| Creatine kinase elevation, n [%] | 30 [14.9] | 108 [11.7] | 138 [12.3] |
| IR [95% CI]a | 4.16 [2.81–5.94] | 5.39 [4.42–6.50] | 5.06 [4.25–5.98] |
| Discontinuations due to creatine kinase elevation, n [%]b | 1 [3.3] | 2 [1.9] | 3 [2.2] |
| Anemia, n [%] IR [95% CI]c |
12 [5.9] 1.55 [0.80–2.71] |
66 [6.9] 3.06 [2.37–3.89] |
78 [6.7] 2.66[2.10–3.32] |
| Discontinuations due to anaemia, n [%]b | 1 [8.3] | 5 [7.2] | 6 [7.4] |
| Lymphopenia, n [%] IR [95% CI]d |
12 [5.9] 1.53 [0.79–2.68] |
32 [3.4] 1.43 [0.98–2.02] |
44 [3.8] 1.46 [1.06–1.96] |
| Discontinuations due to lymphopaenia, n [%]b | 2 [16.7] | 8 [25.0] | 10[22.7] |
| Neutropaenia, n [%] IR [95% CI]e |
5 [2.5] 0.63 [0.20–1.47] |
5 [0.5] 0.22 [0.07–0.51] |
10 [0.9] 0.33 [0.16–0.60] |
| Discontinuations due to neutropaenia, n [%]b | 0 [0.0] | 1 [20.0] | 1 [10.0] |
| Acute renal failure, n [%] IR [95% CI]c |
1 [0.5] 0.12 [0.00–0.69] |
9 [0.9] 0.40 [0.18–0.76] |
10 [0.9] 0.33 [0.16–0.60] |
| Discontinuations due to acute renal failure, n [%]b | 0 [0.0] | 1 [11.1] | 1 [10.0] |
| Rhabdomyolysis, n [%] IR [95% CI]c |
0 [0.0] 0.00 [0.00–0.46] |
0 [0.0] 0.00 [0.00–0.16] |
0 [0.0] 0.00 [0.00–0.12] |
| Discontinuations due to rhabdomyolysis, n [%]b | 0 [0.0] | 0 [0.0] | 0 [0.0] |
aCreatine kinase elevation included any event coded to the MedDRA preferred term blood creatine phosphokinase increased; Phase 2 study data were not included in evaluation of creatine kinase elevation; N = 922 and N = 1124 for the PD tofacitinib 10 mg BID and tofacitinib all groups, respectively.
bFor discontinuations due to laboratory abnormalities, proportions were calculated based on the number of patients with an abnormality in a treatment group.
cLaboratory abnormalities of anaemia, acute renal failure and rhabdomyolysis were analysed based on events coded to the SMQs of haematopoietic erythropaenia, acute renal failure and rhabdomyolysis/myopathy, respectively.
dLymphopaenia included any event coded to the preferred terms lymphopaenia and lymphocytopaenia neonatal, B lymphocyte count decreased, lymphocyte count decreased or T-lymphocyte count decreased.
eNeutropaenia included any event coded to the higher-level term neutropaenia, or the preferred terms granulocyte count decreased or neutrophil count decreased.
BID, twice daily; CI, confidence interval; IR, incidence rate [unique patients with events/100 PY of exposure]; MedDRA, Medical Dictionary for Regulatory Activities; N, number of patients treated in the treatment group; n, number of unique patients in each category; PD, predominant dose; PY, patient-years; SMQ, Standardized MedDRA Query.
4. Discussion
This integrated analysis of safety data from all phase 2/3/OLE and phase 3b/4 studies in the global tofacitinib UC clinical programme included data with up to 7.8 years and 2999.7 PY of tofacitinib exposure and represents the most comprehensive analysis of tofacitinib safety in patients with UC to date. In the Induction and Maintenance Cohorts, the proportions of patients who experienced AEs, serious AEs and discontinuations due to AEs were generally similar in patients receiving placebo or tofacitinib. With the exception of HZ, IRs for AEs of special interest were generally <2.0 in the Overall plus P3b/4 [2020] Cohort. In addition, the IRs for deaths and AEs of special interest with all tofacitinib doses in the Overall plus P3b/4 [2020] Cohort were generally similar to the IRs previously reported in the Overall [Dec 2016] Cohort (data ≤4.4 years; total exposure 1612.8 PY),7 which includes an additional 3.4 years of observation and 1386.9 PY of exposure, thus demonstrating that the safety profile of tofacitinib remained consistent with increased extent and length of exposure. The most frequent laboratory abnormality was creatine kinase elevation. Elevated creatine kinase levels are associated with clinically significant AEs such as rhabdomyolysis;11 however, there were no cases of rhabdomyolysis in this analysis. Lymphopaenia was the laboratory abnormality associated with the most discontinuations.
With the exception of HZ, the IRs for AEs of special interest reported in this analysis are generally consistent with the IRs reported for other UC treatments, including biological therapies.12 A population-based descriptive study of real-world data found that the IR for serious infections with any TNFi was 3.33 [95% 2.73–4.02],12 which was higher than the IR for serious infections reported here for the Overall plus P3b/4 [2020] Cohort. Higher BMI was a risk factor for serious infections in the Overall plus P3b/4 [2020] Cohort. In addition, a previous analysis reported that rates of serious infections were numerically higher in patients from OCTAVE Induction 1 and 2 with BMI ≥30 kg/m2 at baseline, compared with those with BMI < 30 kg/m2 at baseline. This trend was not observed in patients from OCTAVE Sustain, although interpretation of these findings was limited by low patient numbers.13 BMI ≥30 kg/m2 is an indicator of obesity,14 which has been shown to be associated with infectious disease in the general population,15 and in patients with inflammatory bowel disease [IBD].16 However, a previous analysis of patients with IBD receiving biological therapy reported that obesity was not associated with an increased risk of serious infections.17
In the Induction Cohort, the IR for HZ was numerically higher in patients receiving tofacitinib 10 mg BID compared with placebo. In the Maintenance Cohort, IRs for HZ [non-serious and serious] were numerically higher with both tofacitinib doses than with placebo. IRs for HZ in the Overall [Dec 2016]7 and Overall plus P3b/4 [2020]9 Cohorts were generally similar between predominant tofacitinib doses, and IRs remained stable over time. In the Overall plus P3b/4 [2020] Cohort, the IR for HZ [3.30, 95% CI 2.67–4.04] was lower than the IR for HZ previously reported with all tofacitinib doses in the Overall [Dec 2016] Cohort,7 but higher than the IR for HZ with any TNFi in a previous real-world analysis [1.77, 95% CI 1.34–2.29].12 Increased rates of HZ with tofacitinib and other JAK inhibitors vs TNFi have also been reported in previous analyses of data from clinical trials and real-world data for patients with immune-mediated inflammatory diseases including UC.18–22 A previous analysis of HZ risk in the Overall plus P3b/4 [2020] Cohort identified older age and prior TNFi failure as significant risk factors for HZ [as previously identified], as well as lower weight and geographical region [North America vs Europe].9 In the PD tofacitinib 10 mg BID or tofacitinib all groups, IRs for HZ in the Overall plus P3b/4 [2020] Cohort were numerically higher in patients with vs without baseline corticosteroid use; however, baseline corticosteroid use was not a significant risk factor for HZ. In the USA, use of non-live, adjuvant recombinant subunit zoster vaccine (RZV) is recommended for those receiving low-dose immunomodulatory therapy (e.g. prednisone <20 mg/day or equivalent; or inhaled or topical corticosteroids).23 RZV has the potential to impact the future risk of HZ in patients with UC receiving JAK inhibitors, and the clinical efficacy and safety of RZV are currently being evaluated in patients with UC receiving tofacitinib.24
In the Overall plus P3b/4 [2020] Cohort, the majority of opportunistic infections were HZ, and non-HZ opportunistic infections were infrequent. Risk factors for opportunistic infections in the Overall plus P3b/4 [2020] Cohort were lower body weight, lower baseline ANC, history of diabetes and prior TNFi failure. It is possible that some patients with lower body weight may have been suffering from malnutrition, which has been identified as a risk factor for opportunistic infections in patients with IBD.25 In contrast to these findings, a previous analysis of infection risk reported that a higher ANC was associated with an increased risk of infections in patients with IBD and in the general population.16 Diabetes has been identified as a risk factor for infections in patients with IBD,26 as has use of TNFi.25,26
In the Overall plus P3b/4 [2020] Cohort, 26 patients had malignancies [excluding NMSC; IR 0.84, 95% CI 0.55–1.24], and 22 patients had NMSC [IR 0.73, 95% CI 0.45–1.10]. There were four patients with colorectal cancer [all receiving PD tofacitinib 10 mg BID]. The IRs for malignancies [excluding NMSC] and NMSC reported here for tofacitinib were similar to those previously reported for TNFi in an analysis of real-world data (IR 0.63, 95% CI 0.43–0.90 and IR 1.69, 95% CI 1.35–2.10 for malignancies [excluding NMSC] and NMSC, respectively).12 In this analysis, risk factors for malignancies [excluding NMSC] in the Overall plus P3b/4 [2020] Cohort were longer disease duration and history of NMSC, and risk factors for NMSC were older age and history of NMSC. Patients with long-standing IBD have an increased risk of malignancies, including colorectal cancer,5,27 and older age was previously identified as a risk factor for NMSC in an analysis of data from the Overall Cohort [May 2019 interim data cut; up to 6.8 years of exposure],28 and also in an analysis of patients with IBD receiving thiopurines.29 History of NMSC was identified as a risk factor for subsequent malignancy in an analysis of data from the general population.30
Nine patients experienced MACE in this analysis [IR 0.29, 95% CI 0.13–0.55]. The MACE IR reported here for tofacitinib was similar to that previously reported for TNFi in patients with UC in an analysis of real-world data [IR 0.51, 95% CI 0.31–0.79].12
Previously, a prospective, head-to-head safety trial of tofacitinib 5 or 10 mg BID vs TNFi in patients with rheumatoid arthritis [RA] aged ≥50 years ≥1 additional CV risk factor was carried out (ORAL Surveillance [NCT02092467]).31 Evaluation of the co-primary endpoints demonstrated that rates of MACE and malignancies [excluding NMSC] were higher with tofacitinib vs TNFi, as were rates of key secondary endpoints [mortality, serious infections and VTEs].31 These findings resulted in regulatory safety communications and updates to the product label including US Prescribing Information and Boxed Warning.32,33 Risk factors for MACE and malignancies [excluding NMSC] in ORAL Surveillance included age ≥65 years and smoking status [with ever smokers at higher risk than never smokers].34,35 To enrich for CV risk and ensure enough events accrued, patients in ORAL Surveillance had to be aged ≥50 years with one or more additional CV risk factor. Patients enrolled in the UC clinical programme were generally younger than in ORAL Surveillance and were less likely to have smoked previously. While older age was a risk factor for MACE in patients with UC, this covariate was not associated with an increased risk of malignancy, and smoking status was not a risk factor for either endpoint in this analysis, although the numbers of patients with these events in which to evaluate risk factors were low in the UC programme.
A previous analysis of data from the Overall Cohort [up to 6.1 years of exposure] reported five patients with VTEs (one with DVT [IR 0.04, 95% CI 0.00–0.23] and four with PE [IR 0.16, 95% CI 0.04–0.41]).36 In this analysis [up to 7.8 years of exposure], there were seven patients with VTEs in the Overall plus P3b/4 [2020] Cohort (one with DVT [IR 0.03, 95% CI 0.00–0.18] and six with PE [IR 0.19, 95% CI 0.07–0.42]). Black patients were at a greater risk of VTEs than White patients. The IRs for DVT and PE reported in this analysis were consistent with those previously reported in patients with UC (IR 0.07–0.30 and 0.04–0.20, respectively),37–39 and were also consistent with the results of an analysis of safety in 12 410 patients with RA, psoriatic arthritis or psoriasis included in global tofacitinib development programmes, and observational data from the US Corrona registries for each disease.40 This previous cross-indication analysis reported that the incidence of VTEs was elevated in patients with vs without baseline CV or VTE risk factors.40 In this analysis, Black patients had a higher risk of VTEs than White patients, and this is consistent with findings in the general population,41 although the number of patients with VTEs in this analysis was low.
Previously, tofacitinib treatment was associated with generally reversible increases in serum lipids in patients with UC, but changes in lipid profiles were not associated with long-term increases in the risk of CV events.42 In this analysis, 6.4% of patients in the Overall plus P3b/4 [2020] Cohort were receiving lipid-lowering agents at baseline; 1.9% of patients had an increase in their dose of lipid-lowering agents during the programme, and 7.8% of patients began receiving lipid-lowering agents during the programme. Elevated HDL at baseline was associated with a significantly reduced risk of GI perforations, although patient numbers with GI perforations were low. Previously, GI perforations were reported in three patients in the Overall [Dec 2016] Cohort,7 and no additional GI perforations were observed with longer tofacitinib exposure in the Overall plus P3b/4 [2020] Cohort. To date, no GI perforations have been reported in patients with UC following treatment with upadacitinib [JAK1 inhibitor] in phase 3 studies after 8 weeks of treatment,43,44 although additional analyses will be required to fully evaluate the safety of upadacitinib in patients with UC.
Following drug approval, post-marketing studies are an important aspect of monitoring the safety of a drug, especially in a real-world setting. A recent post-marketing study conducted over a 27-month period after first marketing authorisation of tofacitinib for UC in May 2018, and included data derived from spontaneous reports from healthcare professionals, patients and regulatory authorities, solicited reports from market research programme patient support groups, and previously published reports.45 The types of AEs and reporting rates of AEs were consistent with the known safety profile of tofacitinib.45 In addition, a recent meta-analysis of real-world data reported that the safety profile of tofacitinib was comparable to that reported previously in clinical trials.46 Alongside the current analysis of all available clinical trial data to date, these findings suggest that the safety profile of tofacitinib in patients with UC has been consistent over time.
This integrated safety analysis has several limitations: only patients who initially participated in the OCTAVE clinical trials were included. In addition, due to study design, the majority [82.5%] of patients in the Overall plus P3b/4 [2020] Cohort received PD tofacitinib 10 mg BID, which limited between-group comparisons. Also, patients were permitted to switch tofacitinib dose during the OCTAVE Open and RIVETING studies, and therefore dose-dependency of IRs for AEs of special interest could not be firmly concluded in the Overall [Dec 2016] and Overall plus P3b/4 [2020] Cohorts. Moreover, compared with ORAL Surveillance, there was a relatively lower number of patients with high-risk profiles, and unlike ORAL Surveillance, the studies included in the UC clinical programme were not designed to be of sufficient size and duration to evaluate rare events or those with long latency. Therefore, the risk of malignancies and MACE in patients with UC remains an important consideration.
In conclusion, this analysis of data from the global tofacitinib UC clinical programme included an additional 3.4 years of observation and 1386.9 PY of exposure, compared with reported safety data,7 and the results were consistent with the known safety profile of tofacitinib. IRs for AEs of special interest were generally similar between dose groups, although dose-dependency could not be fully evaluated. IRs for AEs of special interest were similar to those reported in prior Overall Cohort analyses,7 and have remained stable over an extended period [up to 7.8 years; total exposure 2999.7 PY] with the inclusion of final data from OCTAVE Open and an interim analysis of data from the phase 3b/4 RIVETING study. These safety findings inform on the long-term use of tofacitinib 5 or 10 mg BID in patients with moderately to severely active UC.
Supplementary Material
Acknowledgements
The authors would like to thank the patients, investigators and study teams who were involved in the tofacitinib UC clinical programme. Medical writing support, under the direction of the authors, was provided by Anthony G. McCluskey, PhD, CMC Connect, a division of IPG Health Medical Communications, and was funded by Pfizer Inc, New York, New York, USA, in accordance with Good Publication Practice [GPP 2022] guidelines [Ann Intern Med 2022;175:1298–304].
Plain language summary An infographic plain language summary of this paper is available at: [https://pfizer.figshare.com/articles/journal_contribution/Plain_language_summary_for_the_manuscript_Tofacitinib_for_the_Treatment_of_Ulcerative_Colitis_An_Integrated_Summary_of_up_to_7_8_Years_of_Safety_Data_from_the_Global_Clinical_Programme/21652322]
Contributor Information
William J Sandborn, Division of Gastroenterology, University of California San Diego, La Jolla, CA, USA.
Geert R D’Haens, Department of Gastroenterology, Amsterdam University Medical Centres, Amsterdam, The Netherlands.
Bruce E Sands, Dr. Henry D. Janowitz Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Remo Panaccione, Division of Gastroenterology and Hepatology, Department of Medicine, University of Calgary, Calgary, AB, Canada.
Siew C Ng, Institute of Digestive Disease, Department of Medicine and Therapeutics, LKS Institute of Health Science, Chinese University of Hong Kong, Hong Kong.
Nervin Lawendy, Pfizer Inc, Collegeville, PA, USA.
Nicole Kulisek, Pfizer Inc, Collegeville, PA, USA.
Irene Modesto, Pfizer Inc, New York, NY, USA.
Xiang Guo, Pfizer Inc, Collegeville, PA, USA.
Rajiv Mundayat, Pfizer Inc, New York, NY, USA.
Chinyu Su, Pfizer Inc, Collegeville, PA, USA.
Ivana Vranic, Pfizer Ltd, Tadworth, Surrey, UK.
Julian Panés, Department of Gastroenterology, Hospital Clínic de Barcelona, IDIBAPS, CIBERehd, Barcelona, Spain.
Conference
Some of the data in this manuscript were presented previously (17th Congress of the European Crohn’s and Colitis Organisation [ECCO]; February 16–19, 2022). The manuscript is not under consideration elsewhere.
Funding
These studies were sponsored by Pfizer. Medical writing support was funded by Pfizer Inc.
Conflict of Interest
WJS has received research grants from AbbVie, Abivax, Arena Pharmaceuticals, Boehringer Ingelheim, Celgene, Genentech, Gilead Sciences, GlaxoSmithKline, Janssen, Eli Lilly, Pfizer Inc, Prometheus Biosciences, Seres Therapeutics, Shire, Takeda and Theravance Biopharma; consulting fees from AbbVie, Abivax, Admirx, Alfasigma, Alimentiv [previously Robarts Clinical Trials, owned by Alimentiv Health Trust], Alivio Therapeutics, Allakos, Amgen, Applied Molecular Transport, Arena Pharmaceuticals, Bausch Health [Salix], Beigene, Bellatrix Pharmaceuticals, Boehringer Ingelheim, Boston Pharmaceuticals, Bristol-Myers Squibb, Celgene, Celltrion, Cellularity, Cosmo Pharmaceuticals, Escalier Biosciences, Equillium, Forbion, Genentech/Roche, Gilead Sciences, Glenmark Pharmaceuticals, Gossamer Bio, Immunic [Vital Therapies], Index Pharmaceuticals, Intact Therapeutics, Janssen, Kyverna Therapeutics, Landos Biopharma, Eli Lilly, Oppilan Pharma, Otsuka, Pandion Therapeutics, Pfizer Inc, Progenity, Prometheus Biosciences, Prometheus Laboratories, Protagonists Therapeutics, Provention Bio, Reistone Biopharma, Seres Therapeutics, Shanghai Pharma Biotherapeutics, Shire, Shoreline Biosciences, Sublimity Therapeutics, Surrozen, Takeda, Theravance Biopharma, Thetis Pharmaceuticals, Tillotts Pharma, UCB, Vendata Biosciences, Ventyx Biosciences, Vimalan Biosciences, Vivelix Pharmaceuticals, Vivreon Biosciences and Zealand Pharma; is a shareholder of Allakos, BeiGene, Gossamer Bio, Oppilan Pharma, Prometheus Biosciences, Prometheus Laboratories Progenity, Shoreline Biosciences, Ventyx Biosciences, Vimalan Biosciences and Vivreon Biosciences; and is an employee of Shoreline Biosciences. GRD’H has been an advisor for AbbVie, Ablynx, Amakem, AM Pharma, Avaxia, Biogen, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Celltrion, Cosmo, Covidien, Dr. Falk Pharma, Engene, Ferring Pharmaceuticals, Galapagos, Gilead Sciences, GlaxoSmithKline, Hospira, Johnson & Johnson, Medimetrics, Millennium/Takeda, Mitsubishi Pharma, MSD, Mundipharma, Novo Nordisk, Pfizer Inc, Prometheus Laboratories/Nestlé, Receptos, Robarts Clinical Trials, Salix, Sandoz, Setpoint, Shire, Teva, TiGenix, Tillotts, Topivert, Versant and Vifor; and has received speaker fees from AbbVie, Ferring Pharmaceuticals, Johnson & Johnson, Millennium/Takeda, MSD, Mundipharma, Norgine, Pfizer Inc, Shire, Tillotts and Vifor. BES has received research grants from Arena Pharmaceuticals, Celgene, Theravance Biopharma R&D and personal fees from 4D Pharma, AbbVie, Allergan, Amgen, Abivax, Arena Pharmaceuticals, AstraZeneca, Baxalta Bioscience India, Boehringer-Ingelheim, Boston Pharmaceuticals, Capella Bioscience, Celgene, Celltrion Healthcare, Ferring Pharmaceuticals, Genentech, Gilead Sciences, GlaxoSmithKline, Hoffmann-La Roche, Immunic, InDex Pharmaceuticals, Inotrem, Ironwood Pharmaceuticals, Janssen, Johnson & Johnson, Kallyope, Eli Lilly, Morphic Therapeutic, Oppilan Pharma, OSE Immunotherapeutics, Otsuka, Palatin Technologies, Pfizer Inc, Progenity, Prometheus Biosciences IBD, Prometheus Laboratories, Protagonist Therapeutics, Redhill Biopharma, Rheos Medicines, Salix Pharmaceuticals, Seres Therapeutics, Shire, Sienna Biopharmaceuticals, Surrozen, Takeda, TARGET RWE, Theravance Biopharma R&D, USWM Enterprises, Viela Bio, Vivelix Pharmaceuticals, Ventyx Biosciences and is a shareholder of Ventyx Biosciences and Vivante Health; and gets non-financial support from Eli Lilly, Pfizer Inc and Takeda. RP has received consulting fees from AI4GI, AbbVie, Amgen, Arena Pharmaceuticals, Atlantic Healthcare, BioBalance, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Coronado Biosciences, Cosmo Technologies, Eagle, Eisai Medical Research, Elan, Eli Lilly, EnGene, Ferring Pharmaceuticals, Genentech, Gilead Sciences, Given Imaging, GlaxoSmithKline, Innomar, Janssen, Lycera, Meda, Merck & Co., Merck Research Laboratories, Novo Nordisk, PDL Biopharma, Pfizer Inc, Prometheus Laboratories, Protagonist, Receptos, Robarts Clinical Trials, Sandoz, Sanofi Genzyme, Satisfai Health, Shire, Sigmoid Pharma, Specialty Rx, Sublimity, Takeda and TherAdvance; and has acted as a reviewer for AI4GI, AbbVie, Amgen, Arena Pharmaceuticals, Atlantic Healthcare, BioBalance, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Coronado Biosciences, Cosmo Technologies, Eagle, Eisai Medical Research, Elan, Eli Lilly, EnGene, Ferring Pharmaceuticals, Genentech, Gilead Sciences, Given Imaging, GlaxoSmithKline, Innomar, Janssen, Lycera, Meda, Merck & Co., Merck Research Laboratories, Novo Nordisk, PDL Biopharma, Pfizer Inc, Prometheus Laboratories, Protagonist, Receptos, Robarts Clinical Trials, Sandoz, Sanofi Genzyme, Satisfai Health, Shire, Sigmoid Pharma, Specialty Rx, Sublimity, Takeda and TherAdvance. SCN has received research grants from AbbVie, Ferring Pharmaceuticals, and Olympus; speakers fees from AbbVie, Ferring Pharmaceuticals, Janssen, Menarini, Takeda, Tillotts and Pfizer Inc; is a scientific co-founder of GenieBiome Ltd; and is a director of Microbiota I Center. NL, NK, IM, XG, RM, CS and IV are employees and shareholders of Pfizer Inc. JP has received research grants from AbbVie and Pfizer Inc; and personal fees from AbbVie, Arena, Athos, Boehringer Ingelheim, Celgene, Celltrion, Ferring Pharmaceuticals, Galapagos, Genentech/Roche, GlaxoSmithKline, Immunic, Janssen, Mirum, Morphic, Nestlé, Origo, Pandion, Pfizer Inc, Progenity, Takeda, Theravance Biopharma and Wassermann.
Authors’ Contributions
All authors contributed to [1] the conception and design of the study, acquisition of data, or analysis and interpretation of data; [2] drafting the article or revising it critically for important intellectual content; and [3] approval of the final version for submission.
Data Availability Statement
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
References
- 1. Sandborn WJ, Ghosh S, Panes J, et al. ; Study A3921063 Investigators. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N Engl J Med 2012;367:616–24. [DOI] [PubMed] [Google Scholar]
- 2. Sandborn WJ, Su C, Sands BE, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2017;376:1723–36. [DOI] [PubMed] [Google Scholar]
- 3. Sandborn WJ, Lawendy N, Danese S, et al. Safety and efficacy of tofacitinib for treatment of ulcerative colitis: final analysis of OCTAVE Open, an open-label, long-term extension study with up to 7.0 years of treatment. Aliment Pharmacol Ther 2022;55:464–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vermeire S, Su C, Lawendy N, et al. Outcomes of tofacitinib dose reduction in patients with ulcerative colitis in stable remission from the randomised RIVETING trial. J Crohns Colitis 2021;15:1130–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Greuter T, Vavricka S, König AO, Beaugerie L, Scharl M; Swiss IBDnet, an official working group of the Swiss Society of Gastroenterology. Malignancies in inflammatory bowel disease. Digestion 2020;101:136–45. [DOI] [PubMed] [Google Scholar]
- 6. Bunu DM, Timofte CE, Ciocoiu M, et al. Cardiovascular manifestations of inflammatory bowel disease: pathogenesis, diagnosis, and preventive strategies. Gastroenterol Res Pract 2019;2019:3012509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sandborn WJ, Panes J, D’Haens GR, et al. Safety of tofacitinib for treatment of ulcerative colitis, based on 4.4 years of data from global clinical trials. Clin Gastroenterol Hepatol 2019;17:1541–50. [DOI] [PubMed] [Google Scholar]
- 8. Curtis JR, Regueiro M, Yun H, et al. Tofacitinib treatment safety in moderate to severe ulcerative colitis: comparison of observational population cohort data from the IBM MarketScan® administrative claims database with tofacitinib trial data. Inflamm Bowel Dis 2020;27:1394–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Winthrop KL, Vermeire S, Long MD, et al. Long-term risk of herpes zoster infection in patients with ulcerative colitis receiving tofacitinib. Inflamm Bowel Dis 2022. doi: 10.1093/ibd/izac063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Winthrop KL, Melmed GY, Vermeire S, et al. Herpes zoster infection in patients with ulcerative colitis receiving tofacitinib. Inflamm Bowel Dis 2018;24:2258–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bagley WH, Yang H, Shah KH.. Rhabdomyolysis. Intern Emerg Med 2007;2:210–8. [DOI] [PubMed] [Google Scholar]
- 12. Curtis JR, Regueiro M, Yun H, et al. Tofacitinib treatment safety in moderate to severe ulcerative colitis: comparison of observational population cohort data from the IBM MarketScan® administrative claims database with tofacitinib trial data. Inflamm Bowel Dis 2021;27:1394–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Farraye FA, Qazi T, Kotze PG, et al. The impact of body mass index on efficacy and safety in the tofacitinib OCTAVE ulcerative colitis clinical programme. Aliment Pharmacol Ther 2021;54:429–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. World Health Organisation. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser 2000;894:i-xii, 1–253. [PubMed] [Google Scholar]
- 15. Huttunen R, Syrjänen J.. Obesity and the risk and outcome of infection. Int J Obes (Lond) 2013;37:333–40. [DOI] [PubMed] [Google Scholar]
- 16. Irving PM, de Lusignan S, Tang D, Nijher M, Barrett K.. Risk of common infections in people with inflammatory bowel disease in primary care: a population-based cohort study. BMJ Open Gastroenterol 2021;8:e000573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Singh S, Heien HC, Sangaralingham L, Shah ND, Sandborn WJ.. Obesity is not associated with an increased risk of serious infections in biologic-treated patients with inflammatory bowel diseases. Clin Transl Gastroenterol 2021;12:e00380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Olivera PA, Lasa JS, Bonovas S, Danese S, Peyrin-Biroulet L.. Safety of Janus kinase inhibitors in patients with inflammatory bowel diseases or other immune-mediated diseases: a systematic review and meta-analysis. Gastroenterology 2020;158:1554–1573.e12. [DOI] [PubMed] [Google Scholar]
- 19. Curtis JR, Xie F, Yun H, Bernatsky S, Winthrop KL.. Real-world comparative risks of herpes virus infections in tofacitinib and biologic-treated patients with rheumatoid arthritis. Ann Rheum Dis 2016;75:1843–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Winthrop KL. The emerging safety profile of JAK inhibitors in rheumatic disease. Nat Rev Rheumatol 2017;13:320. [DOI] [PubMed] [Google Scholar]
- 21. Conaghan PG, Mysler E, Tanaka Y, et al. Upadacitinib in rheumatoid arthritis: a benefit–risk assessment across a phase III program. Drug Saf 2021;44:515–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Winthrop K, Buch MH, Curtis J, et al. POS0092 Herpes zoster in the filgotinib rheumatoid arthritis program [abstract]. Ann Rheum Dis 2021;80(Suppl 1):255–256. POS0092. [Google Scholar]
- 23. Dooling KL, Guo A, Patel M, et al. Recommendations of the Advisory Committee on Immunization Practices for use of herpes zoster vaccines. MMRW Morb Mortal Wkly Rep 2018;67:103–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. ClinicalTrials.gov. Shingrix vaccine in patients with moderate to severe ulcerative colitis on tofacitinib . 2018. https://clinicaltrials.gov/ct2/show/NCT03591770 Accessed July 30, 2021.
- 25. Rahier JF, Magro F, Abreu C, et al. Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis 2014;8:443–68. [DOI] [PubMed] [Google Scholar]
- 26. Ananthakrishnan AN, Cagan A, Cai T, et al. Diabetes and the risk of infections with immunomodulator therapy in inflammatory bowel diseases. Aliment Pharmacol Ther 2015;41:1141–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Herszényi L, Barabás L, Miheller P, Tulassay Z.. Colorectal cancer in patients with inflammatory bowel disease: the true impact of the risk. Dig Dis 2015;33:52–7. [DOI] [PubMed] [Google Scholar]
- 28. Sands BE, Long MD, Reinisch W, et al. Tofacitinib for the treatment of ulcerative colitis: analysis of nonmelanoma skin cancer rates from the ulcerative colitis clinical program. Inflamm Bowel Dis 2022;28:234–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Peyrin-Biroulet L, Khosrotehrani K, Carrat F, et al. ; Cesame Study Group. Increased risk for nonmelanoma skin cancers in patients who receive thiopurines for inflammatory bowel disease. Gastroenterology 2011;141:1621–28.e1. [DOI] [PubMed] [Google Scholar]
- 30. Chen J, Ruczinski I, Jorgensen TJ, et al. Nonmelanoma skin cancer and risk for subsequent malignancy. J Natl Cancer Inst 2008;100:1215–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ytterberg SR, Bhatt DL, Mikuls TR, et al. ; ORAL Surveillance Investigators. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med 2022;386:316–26. [DOI] [PubMed] [Google Scholar]
- 32. Pfizer Inc. Xeljanz® (tofacitinib): highlights of prescribing information . 2020. http://labeling.pfizer.com/ShowLabeling.aspx?id=959 Accessed October 14, 2021.
- 33. European Medicines Agency. Xeljanz (tofacitinib): summary of product characteristics . 2022. https://www.ema.europa.eu/en/documents/product-information/xeljanz-epar-product-information_en.pdf Accessed June 2, 2022.
- 34. Charles-Schoeman C, Buch M, Dougados M, et al. Risk factors for major adverse cardiovascular events in patients aged ≥ 50 years with RA and ≥ 1 additional cardiovascular risk factor: results from a Phase 3b/4 randomized safety study of tofacitinib vs TNF inhibitors [abstract]. Arthritis Rheumatol 2021;73(suppl 19):0958. [Google Scholar]
- 35. Curtis J, Yamaoka K, Chen Y-H, et al. Malignancies in patients aged ≥ 50 years with RA and ≥ 1 additional cardiovascular risk factor: results from a Phase 3b/4 randomized safety study of tofacitinib vs TNF inhibitors [abstract]. Arthritis Rheumatol 2021;73:1940. [Google Scholar]
- 36. Sandborn WJ, Panés J, Sands BE, et al. Venous thromboembolic events in the tofacitinib ulcerative colitis clinical development programme. Aliment Pharmacol Ther 2019;50:1068–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Weng MT, Park SH, Matsuoka K, et al. Incidence and risk factor analysis of thromboembolic events in East Asian patients with inflammatory bowel disease, a multinational collaborative study. Inflamm Bowel Dis 2018;24:1791–800. [DOI] [PubMed] [Google Scholar]
- 38. Kappelman MD, Horvath-Puho E, Sandler RS, et al. Thromboembolic risk among Danish children and adults with inflammatory bowel diseases: a population-based nationwide study. Gut 2011;60:937–43. [DOI] [PubMed] [Google Scholar]
- 39. Bernstein CN, Blanchard JF, Houston DS, Wajda A.. The incidence of deep venous thrombosis and pulmonary embolism among patients with inflammatory bowel disease: a population-based cohort study. Thromb Haemost 2001;85:430–4. [PubMed] [Google Scholar]
- 40. Mease P, Charles-Schoeman C, Cohen S, et al. Incidence of venous and arterial thromboembolic events reported in the tofacitinib rheumatoid arthritis, psoriasis and psoriatic arthritis development programmes and from real-world data. Ann Rheum Dis 2020;79:1400–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zakai NA, McClure LA, Judd SE, et al. Racial and regional differences in venous thromboembolism in the United States in 3 cohorts. Circulation 2014;129:1502–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sands BE, Taub PR, Armuzzi A, et al. Tofacitinib treatment is associated with modest and reversible increases in serum lipids in patients with ulcerative colitis. Clin Gastroenterol Hepatol 2020;18:123–132.e3. [DOI] [PubMed] [Google Scholar]
- 43. Danese S, Vermeire S, Zhou W, et al. Efficacy and safety of upadacitinib induction therapy in patients with moderately to severely active ulcerative colitis: results from the phase 3 U-ACHIEVE study [abstract]. J Crohns Colitis 2021;15(Suppl 1):S022–S024: OP24. [Google Scholar]
- 44. Vermeire S, Danese S, Zhou W, et al. Efficacy and safety of upadacitinib as induction therapy in patients with moderately to severely active ulcerative colitis: results from Phase 3 U-ACCOMPLISH study [abstract]. Am J Gastroenterol 2021;116:S357S357: S771–S357. [Google Scholar]
- 45. Rubin DT, Modesto I, Vermeire S, et al. Worldwide post-marketing safety surveillance experience with tofacitinib in ulcerative colitis. Aliment Pharmacol Ther 2021;55:302–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lucaciu LA, Constantine-Cooke N, Plevris N, et al. Real-world experience with tofacitinib in ulcerative colitis: a systematic review and meta-analysis. Therap Adv Gastroenterol 2021;14:17562848211064004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.