Abstract
Background and Aims
Whereas immediate postoperative treatment has shown effectiveness in reducing endoscopic postoperative recurrence [POR], evidence regarding the clinical benefit is limited. We compared rates of clinical POR in Crohn’s disease [CD] patients receiving immediate prophylactic treatment with rates in patients receiving endoscopy-driven treatment.
Methods
We retrospectively collected data from 376 consecutive CD patients who underwent an ileocaecal resection with anastomosis between 2007 and 2018 with at least 3 years of follow-up at three sites. Subsequently, high- and low-risk patients categorised by established guidelines, who underwent endoscopy within 12 months postoperatively, were grouped according to a prophylactic- or endoscopy-driven approach and compared for incidence and time till endoscopic and clinical POR.
Results
Prophylactic treatment reduced rates of and time till endoscopic POR within 1 year in high-risk (hazard ratio [HR] 0.48, 95% confidence interval [CI] 0.27-0.86, p = 0.04, number needed to treat [NNT] = 5) but not low-risk [HR 0.90, 95% CI 0.32-2.56, p = 0.85] patients. Conversely, no significant differences in clinical POR within 3 years between prophylactic- and endoscopy-driven low-risk [HR 1.17, 95% CI 0.41-3.29, p = 0.75] and high-risk patients were observed [HR 1.06, 95% CI 0.63-1.79, p = 0.82, NNT = 22]. However, a large numerical albeit not statistical significant difference in 3-year clinical POR [28.6% vs. 62.5%, p = 0.11] in a subset of high-risk patients with three or more ECCO-defined risk factors was observed, indicating a cumulative effect of having multiple risk factors.
Conclusion
Our observations favour step-up treatment guided by early endoscopic evaluation with prophylactic treatment reserved for carefully selected high-risk patients, in order to avoid potential overtreatment of a significant number of patients.
Keywords: Inflammatory bowel disease, prevention, postoperative recurrence, risk stratification
Graphical Abstract
Graphical Abstract.
1. Introduction
Despite a significant increase in medical treatment options for Crohn’s disease [CD], around a third of the patients still require bowel surgery within the first 10 years following diagnosis.1 Although surgery induces clinical and endoscopic remission, ~50% of patients develop recurrence of clinical symptoms within 3 years, typically preceded by endoscopic postoperative recurrence [POR].2 Many studies have sought to identify clinical characteristics that can distinguish patients at low or high probability of developing endoscopic POR. As a result, current guidelines2,3 suggest starting ‘immediate prophylactic treatment’ with metronidazole, immunomodulatory drugs, or anti-tumour necrosis factor [TNF] agents in high-risk patients, adhered to by the majority of gastroenterologists.4 Conversely, patients considered at low risk of developing endoscopic POR are advised to undergo step-up of treatment according to the presence of endoscopic POR determined within 6–12 months.2,3,5 The level of evidence supporting these recommendations is, however, of low quality, warranting further evaluation.
Whereas immediate prophylactic treatment has shown to be effective in reducing endoscopic POR,6 conclusive evidence on both the mid- and the long-term clinical benefit of prophylactic treatment is limited. Two prospective studies evaluated the benefit of early treatment according to clinical risk stratification at surgery. The POCER trial concluded superiority of endoscopy-driven step-up at 6 months over standard care alone in patients treated according to clinical risk,7 yet did not allow for direct comparison of prophylactic- vs. endoscopy-driven treatment as all high-risk patients received postoperative treatment. In a small study, Ferrante et al. randomised high-risk CD patients to prophylactic- or endoscopy-driven step-up with azathioprine treatment,8 but the trial was terminated prematurely due to slow inclusions leaving the question unanswered. In addition, the use of azathioprine as the only treatment option limits generalisability of this study to modern-day postoperative management with a more established role for and availability of biologics.6,9
Thus, the clinical benefit of immediate prophylactic- compared with an endoscopy-driven step-up of treatment with currently available treatment options remains to be shown. We therefore assessed the incidence of clinical POR in postoperative risk-stratified CD patients who started immediate prophylactic therapy compared with those who underwent step-up of treatment guided by endoscopic findings within the first year following surgery.
2. Methods
2.1. Patient selection
This study is a multicentre, retrospective cohort study performed in one academic [the Amsterdam UMC] and two non-academic [OLVG and Flevo] hospitals. We included adult Crohn’s disease patients who underwent a primary or re-do ileocolonic resection between January 2007 and September 2018 and had at least 3 years of follow-up data available. We excluded patients who remained symptomatic after surgery and those with a permanent ileostomy or with malignancy during follow-up. If at first a temporary ileostomy was constructed, follow-up started when continuity was restored.
Next, we risk-stratified patients according to the current ECCO guideline,3 with high risk defined as ≥ 1 of the following clinical characteristics: active smoking, prior intestinal surgery, penetrating disease at index surgery, perianal disease, or granulomas in the resection specimen. Due to the heterogeneity in location, plexus type, scoring of myenteric plexitis, and lack of data on intra-observer and inter-observer variability, we decided not to include myenteric plexitis as risk factor.10–13
We then selected only those with endoscopic evaluation within 12 months post-surgery, and subsequently divided the low- and high-risk patients into those who received immediate prophylactic metronidazole, immunomodulatory [ie, azathioprine, mercaptopurine, thioguanine, methotrexate] or biologic [ie, anti-TNF, vedolizumab, ustekinumab] therapy following surgery [prophylactic group], or those who received step-up of treatment according to endoscopic POR [endoscopy-driven group]. Both prophylactic- and endoscopy-driven step-up treatments were given at standard dosage. Patients who prophylactically used monotherapy with budesonide or 5-aminosalicylate [5-ASA] agents were excluded from this selection, as these treatments are not recommended as postoperative prophylaxis according to the current guidelines.2,3,5 Patients in the endoscopy-driven treatment group who reached the primary endpoint of clinical recurrence before their routine endoscopic evaluation within 12 months were retained for analysis in this group.
2.2. Clinical variables
Medical records were reviewed for the following clinical, surgical, and histopathological characteristics: gender, age at surgery, Crohn’s disease duration, Montreal classification at surgery, smoking behaviour, inflammatory bowel disease [IBD] medication 12 weeks prior to surgery, surgical history, American Society of Anesthesiologists [ASA] fitness classification, surgical indication, surgical access, type of anastomosis, temporary ileostomy, length of resected ileum, and presence of granulomas in the resection specimen.
We defined active smoking post-surgery as any number of cigarettes smoked at least weekly following surgery, and former smoking as an active smoker < 1 year before surgery who stopped smoking prior to or following surgery. Non-smokers were defined as patients who had never smoked or those who ceased smoking > 1 year before surgery and continued to be a non-smoker throughout follow-up. We reviewed all pathology reports for the presence of granulomas defined as at least one evident granuloma present in any of the intestinal slides, excluding slides of the lymphatic glands.
2.3. Endpoints
The primary endpoint of this study was the incidence of clinical POR during 3 years of follow-up in risk-stratified patients, comparing prophylactic treatment with initiation of treatment following endoscopic evaluation within 12 months. We defined clinical POR as the development of IBD-related symptoms [diarrhoea, fever, rectal bleeding, abdominal pain, or evident fatigue] objectively confirmed by elevated biomarker levels (C-reactive protein [CRP] ≥ 5mg/L or faecal calprotectin ≥ 250μg/g), colonoscopy [Rutgeerts score ≥ i2b14], radiological imaging [active small bowel or colon lesions confirmed by the local radiologist on ultrasound/CT/MRI], or the start, switch, or intensification of drug therapy. Secondary endpoints included the incidence and time till endoscopic POR within 12 months in risk-stratified patients receiving prophylactic vs. those without prophylactic treatment, as well as the time till clinical POR for both groups. All endoscopies were reviewed by a single, trained investigator [VJ], based on detailed endoscopy reports and high-quality images. Endoscopic POR was defined as a modified Rutgeerts score of ≥ i2b.14
2.4. Statistical analyses
Descriptive statistics were used to examine baseline characteristics of all included patients and separately for low- and high-risk patients assigned to the prophylactic- and endoscopy-guided groups. Differences in the distribution of clinical characteristics between the prophylactic- and endoscopy-driven groups were compared using the chi square or Mann–Whitney U test.
Next, the rate of clinical recurrence at 1, 2, and 3 years after surgery in both groups, with or without risk stratification and stratified by amount of risk factors, were compared using the chi square test. The time till clinical recurrence was calculated as the difference in days between surgery and the objectification of recurring IBD-related symptoms. Recurrence-free survival plots were created using Kaplan–Meier analysis with use of the log rank test to calculate statistical differences between both survival curves. P-values and confidence intervals were calculated around a 95% confidence interval. All data were analysed using IBM SPSS statistics [version 26].
2.5. Ethical considerations
This study was waived from review of the medical ethics board.
3. Results
3.1. Patient selection and clinical characteristics
Following the screening of 541 patients, we identified a total of 376 adult Crohn’s disease patients who met the inclusion criteria and whose characteristics are shown in Table 1.
Table 1.
Patient characteristics.
| Total n = 376 | Amsterdam UMC n = 239 | OLVG n = 104 | Flevo n = 33 | p-value | ||
|---|---|---|---|---|---|---|
| Clinical variables | ||||||
| Gender | Female, n [%] | 234 [62.2] | 147 [61.5] | 66 [63.5] | 21 [63.6] | 0.93 |
| Age [at surgery] | Median years[IQR] | 34 [26–46] | 34 [26–47] | 35 [26–47] | 28 [21–42] | 0.69 |
| Disease duration | Median years [IQR] | 5 [1–13] | 6 [2–14] | 3 [1–10] | 6 [1–10] | 0.14 |
| Age at diagnosis, | -≤16 years old [A1] | 45 [12.1] | 35 [14.8] | 5 [4.8] | 5 [19.2] | 0.02 |
| n [%]a | - 17–40 years old [A2] | 263 [71.7] | 169 [71.3] | 74 [71.2] | 20 [76.9] | 0.83 |
| -≥40 years old [A3] | 59 [16.1] | 33 [13.9] | 25 [24] | 1 [3.8] | 0.01 | |
| Disease location, n [%]b | - Ileal disease [L1] | 217 [58.8] | 137 [57.8] | 62 [59.6] | 18 [64.3] | 0.79 |
| - Colonic disease [L2] | 1 [0.3] | 1 [0.4] | – | – | 0.64 | |
| -Ileocolonic disease [L3] | 151 [40.9] | 99 [41.8] | 42 [40.4] | 10 [35.7] | 0.82 | |
| - Upper GI involvement [L4] | 37 [10.0] | 28 [11.9] | 8 [7.7] | 1 [3.6] | 0.25 | |
| Disease behaviour, n [%]c | - Non-stricturing/penetrating [B1] | 51 [14.0] | 25 [10.5] | 20 [19.2] | 6 [26.1] | 0.02 |
| -Stricturing [B2] | 175 [48.1] | 106 [44.7] | 57 [54.8] | 12 [52.2] | 0.21 | |
| - Penetrating [B3] | 138 [37.9] | 106 [44.7] | 27 [26.0] | 5 [21.7] | 0.00 | |
| - Perianal disease [P] | 96 [26.5] | 70 [29.5] | 21 [21.6] | 5 [17.9] | 0.19 | |
| Previous IBD related surgery, n [%] | - Appendectomy | 40[10.6] | 24 [10.0] | 11 [10.6] | 5 [15.2] | 0.67 |
| - ≥1 prior resection | 91 [24.2] | 65 [27.2] | 18 [17.3] | 8 [24.2] | 0.15 | |
| - ≥2 prior resections | 33 [8.8] | 27 [11.3] | 4 [3.8] | 2 [6.1] | 0.07 | |
| Smoking post-surgery, n [%]d | - Active smoker | 97 [27.1] | 66 [28.2] | 27 [27.3] | 4 [16.0] | 0.43 |
| - Non-smoker | 239 [66.8] | 156 [66.7] | 63 [63.6] | 20 [80.0] | 0.3 | |
| - Ceased smoking | 22 [6.1] | 12 [5.1] | 9 [9.1] | 1 [4.0] | 0.35 | |
| Treatment 12 weeks prior to surgery, n [%]e | - Immunomodulator only | 94 [25.1] | 50 [20.9] | 35 [34.3] | 9 [27.3] | 0.03 |
| - Anti-TNF only | 148 [39.6] | 90 [37.7] | 43 [42.2] | 15 [45.4] | 0.57 | |
| - Anti-TNF combination | 68 [18.2] | 41 [17.2] | 23 [22.5] | 4 [12.1] | 0.32 | |
| - Vedolizumab | 3 [0.8] | 2 [0.8] | – | 1 [3.0] | 0.24 | |
| - Ustekinumab | 2 [0.5] | 2 [0.8] | – | – | 0.41 | |
| - Corticosteroids | 129 [34.5] | 79 [33.1] | 41 [40.2] | 9 [27.3] | 0.29 | |
| Risk stratification | High risk, n [%] | 274 [72.9] | 195 [81.6] | 64 [61.5] | 15 [45.5] | 0.00 |
| Post-surgery prophylactic treatment | Yes, n [%] | 127 of 274 | 85 of 195 [43.6] | 37 of 64 [57.8] | 5 of 15 [33.3] | 0.08 |
| [46.4] | ||||||
| Endoscopic POR [Rs≥i2b] | - Within 1 year | 83 [40.9] | 59 [40.7] | 13 [33.3] | 11 [44.0] | 0.63 |
| - Within 3 years | 169 [57.3] | 113 [57.4] | 35 [55.6] | 21 [67.6] | 0.5 | |
| Clinical recurrence, n [%] | - Within 3 years | 157 [41.5] | 93 [38.9] | 45 [43.3] | 11 [33.3] | 0.16 |
| - Days till event | 360 [200–662] | 352 [200–620] | 399[214–809] | 352 [124–724] | 0.51 | |
| Surgical variables | ||||||
| ASA fitness grade, n [%]f | -1 | 59 [17.3] | 39 [16.6] | 12 [14.6] | 8 [32.0] | 0.12 |
| -2 | 267 [78.1] | 187 [79.6] | 62 [76.8] | 17 [68.0] | 0.39 | |
| -3 | 16 [4.7] | 9 [3.8] | 7 [8.5] | – | 0.08 | |
| Surgery indication, n [%]g | - Perforation | 84[22.9] | 66 [28.0] | 14 [14.0] | 4 [12.9] | 0.01 |
| - Stenosis | 206 [56.1] | 131 [55.5] | 59 [59.0] | 16 [51.6] | 0.81 | |
| - Refractory disease | 75 [20.4] | 38 [16.1] | 27 [27.0] | 10 [32.3] | 0.02 | |
| Type of anastomosis, n [%]h | - Side-to-side anastomosis [SSA] | 306 [91.1] | 199[91.3] | 78 [88.6] | 29 [96.7] | 0.41 |
| - End-to-side anastomosis [ESA] | 20 [6.0] | 15 [6.9] | 4 [4.5] | 1 [3.3] | 0.6 | |
| - End-to-end anastomosis [EEA] | 10 [3.0] | 4 [1.8] | 6 [6.8] | – | 0.04 | |
| - Extracorporeal anastomosis | 180 [57] | 124 [59.0] | 35 [47.9] | 21 [95.5] | 0.00 | |
| Surgical access, n [%]i | - Laparotomy | 91 [25.3] | 74 [31.8] | 16 [16.5] | 1 [3.3] | 0.00 |
| - Laparoscopic | 248 [68.9] | 155 [66.5] | 65 [67.0] | 28 [93.3] | 0.01 | |
| - Conversion | 21 [5.8] | 4 [1.7] | 16 [16.5] | 1 [3.3] | 0.00 | |
| Temporary ileostomyj | Yes, n [%] | 43 [11.4] | 23 [9.6] | 18 [17.3] | 2 [6.1] | 0.12 |
| Days reversal of ileostomy, median [IQR] or mean [± SD] | 151 [109–276] | 130 [72–218] | 184 [132–321] | 190 [±8.5] | 0.25 | |
| Length of resected ileumk | Cm, median [IQR] | 20 [13–30] | 20 [15–30] | 20 [13.5–30] | 15 [10–20] | 0.07 |
| Extended resection [≥50cm]l | Yes, n [%] | 26 [6.9] | 17 [7.1] | 9 [8.7] | – | 0.2 |
| Histology | ||||||
| Granulomas in resection specimenm | Yes, n [%] | 80 [27.4] | 59 [30.7] | 19 [24.7] | 2 [8.7] | 0.07 |
Values in bold are significant.
POR, postoperative recurrence; IBD, inflammatory bowel disease; Rs, Rutgeert’s score; ASA, American Society of Anesthesiologists; GI, gastrointestinal; IQR, interquartile range; SD, standard deviation; TNF, tumour necrosis factor; immunomodulator [azathioprine; 6-mercaptopurine; 6-thioguanine; methotrexate]; anti-TNF [infliximab; adalimumab or golimumab].
Missing variables, n [ %]: a, 9 [2.4%]; b, 7 [1.9%]; c, 12 [3.2%]; d, 18 [4.8%]; e, 2 [0.5%]; f, 39 [9%]; g, 11 [2.9%]; h, 40 [10.6%]; i, 16 [4.3%]; j, 199 [52.9%]; k, 33 [8.8%]; l, 33 [8.8%];m, 84 [22.3%].
The majority of the included patients did not smoke [66.8%], were female [62.2%], with a median age of 34 [26–46]. Most underwent a laparoscopic [68.9%] primary ileocaecal resection [75.8%] with side-to-side stapled anastomosis [91.1%] due to stenosis [56.1%]. A total of 221 [58.8%] patients were treated with a biologic within 12 weeks before surgery, indicating an overall therapy-refractory CD population [Table 1].
Main differences between the participating centres were seen for the distribution of age at diagnosis, disease behaviour at surgery, immunomodulatory treatment 12 weeks prior to surgery, surgical indication, type of anastomosis, and surgical access [Table 1].
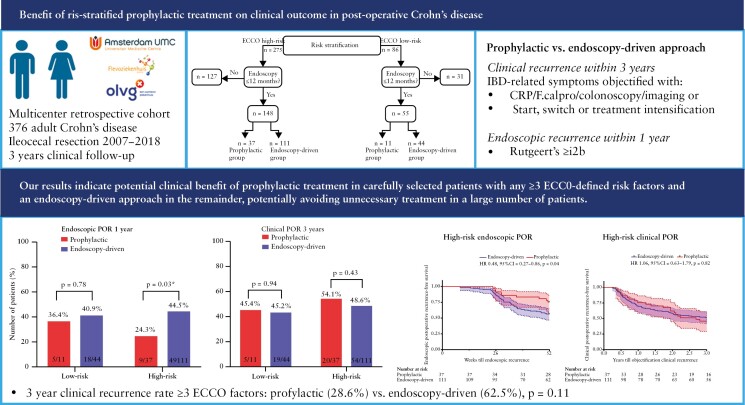
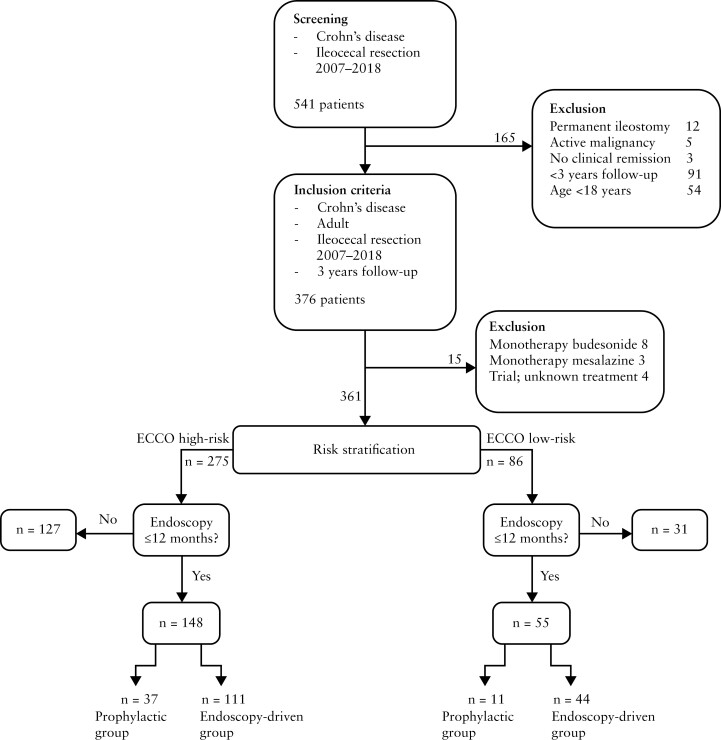
We next excluded 15 patients with monotherapy budesonide, mesalazine, or trial medication, and stratified patients into low- [n = 86] and high-risk [n = 275] according to risk of endoscopic POR [Figure 1]. Endoscopic evaluation in the first 12 months was observed for 64% [55 out of 86] in the low-risk group and 53.8% [148 out of 275] in the high-risk group. Out of the remaining 31 low-risk patients, 15 [48.4%] underwent endoscopic evaluation within 3 years, with a median time till endoscopy of 567 [506–845] days, showing endoscopic POR in 53.3%. Eighteen out of these 31 patients [58.1%] received prophylactic treatment and a total of nine [29%] patients reported clinical POR within 3 years. Similar observations were made for high-risk patients, where in the remaining 127 patients, 63 [49.6%] underwent endoscopic evaluation within 3 years, with a median time till endoscopy of 558 [407–834] days, 30 [49.2%] with endoscopic POR; 86 [67.7%] patients received prophylactic treatment and a total of 40 [31.5%] reported clinical POR within 3 years. Notably, 64 [23.3%] high-risk patients did not undergo endoscopic evaluation within 3 years, which is outside current guidelines.2,3,5 To ensure an unbiased comparison between prophylactic- and endoscopy-driven risk-stratified patients, we focused our subsequent analyses only on patients who underwent endoscopic evaluation within 12 months, distinguishing low- and high-risk patients into prophylactic- and endoscopy-driven treatment groups [Figure 1]. Clinical characteristics of the patients without endoscopic evaluation within 12 months are shown in Supplementary Table 1.
Figure 1.
Flowchart demonstrating the selection of patients.
Out of the 48 prophylactic treated patients [Table 2], 27 were treated with immunomodulatory monotherapy, 12 with biologic monotherapy, and nine with combination treatment. A larger proportion of the patients who were treated with biologic monotherapy or combination treatment had previously failed anti-TNF treatment compared with those receiving immunomodulator monotherapy [66.7% and 100% vs. 25.9%, respectively].
Table 2.
Clinical characteristics of prophylactic- and endoscopy-driven groups with endoscopy ≤ 12 months.
| Low-riskpatients [n = 55] | High-risk patients [n = 148] | ||||||
|---|---|---|---|---|---|---|---|
| Prophylactic n = 11 | Endoscopy-driven n = 44 | p-value | Prophylactic n = 37 | Endoscopy-driven n = 111 | p-value | ||
| Clinical variables | |||||||
| Gender | Female, n [%] | 6 [54.5] | 32 [72.7] | 0.25 | 22 [59.5] | 69 [62.2] | 0.77 |
| Age [at time of surgery] | Median years [IQR] | 28 [24–38] | 30 [23–48] | 1 | 33[24–47] | 33 [25–44] | 0.86 |
| Disease duration | Median years [IQR] | 2 [0–4] | 4 [1–7] | 0.17 | 9 [3–16] | 5 [1–13] | 0.03 |
| Age at diagnosis, n [%]a | -≤16 years old [A1] | – | 4 [9.1] | 0.22 | 10 [27.8] | 14 [12.7] | 0.03 |
| - 17–40 years old [A2] | 10 [90.9] | 28 [63.6] | 0.09 | 24 [66.7] | 84[76.4] | 0.25 | |
| -≥40 years old [A3] | 1 [9.1] | 10 [22.7] | 0.22 | 2 [5.6] | 12 [10.9] | 0.34 | |
| Disease location, n [%]b | - Ileal disease [L1] | 7 [63.6] | 31 [70.5] | 0.46 | 14 [38.9] | 64 [58.2] | 0.04 |
| - Colonic disease [L2] | – | – | – | 1 [2.8] | – | 0.09 | |
| - Ileocolonic disease [L3] | 4[36.4] | 11 [25.0] | 0.46 | 21 [58.3] | 46 [41.8] | 0.08 | |
| - Upper GI involvement [L4] | 1 [9.1] | 2 [4.5] | 0.63 | 6 [16.7] | 14 [12.7] | 0.55 | |
| Disease behaviour, n [%]c | - Non-stricturing/penetrating [B1] | 1 [9.1] | 15 [34.1] | 0.09 | 1 [2.8] | 11 [10.1] | 0.17 |
| - Stricturing [B2] | 9[81.8] | 25 [56.8] | 0.09 | 19 [52.8] | 42 [38.5] | 0.13 | |
| - Penetrating [B3] | – | – | – | 16 [44.4] | 56 [51.4] | 0.47 | |
| - Perianal disease [P] | – | – | – | 16 [45.7] | 27 [24.8] | 0.02 | |
| Previous IBD-related surgery, n [%] | - Appendectomy | 1 [9.1] | 2 [4.5] | 0.61 | 2 [5.4] | 8 [7.2] | 0.71 |
| - ≥1 prior resection | – | – | – | 15 [40.5] | 27 [24.3] | 0.06 | |
| - ≥2 prior resections | – | – | – | 7 [18.9] | 7 [6.3] | 0.02 | |
| Smoking post-surgery, n [%]d | - Active smoker | – | – | – | 8 [21.6] | 38 [35.2] | 0.13 |
| - Non-smoker | 9 [81.8] | 35 [79.5] | 0.51 | 28 [75.7] | 66 [61.1] | 0.11 | |
| - Ceased smoking | 2 [18.2] | 4 [9.1] | 0.51 | 1 [2.7] | 4 [3.6] | 0.77 | |
| Treatment 12 weeks prior to surgery, n [%] | - Immunomodulator only | 6 [54.5] | 16 [36.4] | 0.29 | 24 [64.9] | 28 [25.2] | 0.00 |
| - Anti-TNF only | 4 [36.4] | 16 [36.4] | 0.89 | 20 [54.1] | 36 [32.4] | 0.02 | |
| - Anti-TNF combination | 2[18.2] | 4 [9.1] | 0.46 | 12 [32.4] | 15 [13.5] | 0.01 | |
| - Vedolizumab | – | – | – | – | 2 [1.8] | 0.28 | |
| - Ustekinumab | – | – | – | 1 [2.7] | 1 [0.9] | 0.45 | |
| - Corticosteroids | 5 [45.5] | 20 [45.4] | 0.93 | 7 [18.9] | 32 [28.8] | 0.24 | |
| Surgical variables | |||||||
| ASA fitness grade, n [%]e | -1 | 2[18.2] | 10 [22.7] | 0.83 | 4 [10.8] | 20 [18.5] | 0.28 |
| -2 | 6 [54.5] | 31 [70.5] | 0.65 | 31 [83.8] | 85 [78.7] | 0.51 | |
| -3 | 1 [9.1] | – | 0.06 | 2 [5.4] | 3 [2.8] | 0.47 | |
| Surgery indication, n [%]f | - Perforation | – | – | – | 8 [22.2] | 36 [33.3] | 0.21 |
| - Stenosis | 10 [90.9] | 23 [52.3] | 0.01 | 23[63.9] | 54 [50.0] | 0.15 | |
| - Refractory disease | – | 20 [45.5] | 0.01 | 5 [13.9] | 18 [16.7] | 0.69 | |
| Type of anastomosis, n [%]g | - Side-to-side anastomosis [SSA] | 10 [90.9] | 42 [95.5] | 0.37 | 32 [91.4] | 90 [90.9] | 0.93 |
| - End-to-side anastomosis [ESA] | – | 1 [2.3] | 0.48 | 1 [2.9] | 6 [6.1] | 0.46 | |
| - End-to-end anastomosis [EEA] | 1 [9.1] | – | 0.08 | 2 [5.7] | 3 [3.0] | 0.49 | |
| - Extracorporeal | 8 [72.7] | 26 [59.1] | 0.45 | 17 [53.1] | 60 [60.6] | 0.46 | |
| Surgical accessh | - Laparotomy | 1 [9.1] | – | 0.07 | 12 [33.3] | 30 [27.3] | 0.49 |
| - Laparoscopic | 9 [81.8] | 44 [100] | 0.01 | 19 [52.8] | 78 [70.9] | 0.05 | |
| - Conversion | 1 [9.1] | – | 0.07 | 5 [13.9] | 2 [1.8] | 0.03 | |
| Temporary ileostomyi | Yes, n [%] | 5 [45.5] | – | 0.01 | 1 [4.5] | 14 [32.6] | 0.01 |
| Days reversal of ileostomy, median [IQR] | 140 [128–336] | – | – | – | 149 [90–237] | – | |
| Length of resected ileumj [cm] | Median [IQR] | 15 [8.5–25.5] | 20 [12–28] | 0.46 | 20 [10–38] | 20 [12.5–25] | 0.2 |
| Extended resectionk [≥50cm ileum resected] |
Yes, n [%] | – | 1 [2.3] | 0.52 | 4 [11.4] | 3 [2.9] | 0.04 |
| Histology | |||||||
| Granulomas in resection specimenl | Yes, n [%] | – | – | – | 9 [30.0] | 38 [40.4] | 0.31 |
Values in bold are significant.
IBD, inflammatory bowel disease; ASA, American Society of Anesthesiologists; GI, gastrointestinal; IQR, interquartile range; TNF, tumour necrosis factor; immunomodulator [azathioprine; 6-mercaptopurine; 6-thioguanine; methotrexate]; anti-TNF [infliximab; adalimumab or golimumab].
Missing variables low-risk, n [%]: a, 2[3.6%]; b, 2 [3.6%]; c, 2 [3.6%]; d, 5 [9.1%]; e, 5 [9.1%]; f, 2 [3.6%]; g, 13 [23.6%]; i, 29 [52.7%]; l, 15 [27.3%].
Missing variables high-risk, n [%]: a, 2 [1.4%]; b, 2 [1.4%]; c, 2 [1.4%]; d, 3 [2.0%]; e, 3 [2.0%]; f, 4 [2.7%]; g, 14 [9.5%]; h, 8 [5.4%]; i,83 [56.1%]; j, [%]; k, 8 [5.4%]; l, 24 [16.2%].
As expected, patients in the low-risk prophylactic- and endoscopy-driven groups did not have any guideline-defined risk factors for endoscopic recurrence, with overall comparable clinical characteristics [Table 2]. Interestingly, the prophylactic- vs. endoscopy-driven high-risk patients significantly differed in young age at diagnosis [27.8% vs. 12.7%, p = 0.03], disease duration [9 vs. 5 years, p = 0.03], and perianal disease phenotype [45.7% vs. 24.8%, p = 0.02], as well as previous immumodulator- [64.9% vs. 25.2%, p = 0.00], anti-TNF monotherapy [54.1% vs. 32.4%, p = 0.02], or combination treatment failure [32.4% vs. 13.5%, p = 0.01]. In addition, several surgical factors such as two or more prior resections [18.9% vs. 6.3%, p = 0.02], conversion procedure [13.9% vs. 1.8%, p = 0.03], or extensive ileal resection [11.4% vs. 2.9%, p = 0.04], significantly differed between both groups, respectively, indicating their importance for postoperative decision making in our cohort [Table 2].
3.2. Clinical recurrence
Without any risk stratification, clinical recurrence occurred in 41.5% [n = 156 out of 376] within 3 years of follow-up after a median of 329 [177–654] days with biomarker/imaging confirmation after a median of 360 [200–662] days.
In these 156 patients with clinical recurrence, symptoms that were most often reported were abdominal pain and diarrhoea, observed in respectively 130 [83.3%] and 91 [58.3%] patients.
Most symptoms were objectified as recurrent CD through a combination of endoscopy/radiological imaging and elevated levels of CRP/faecal calprotectin [35.3%] or through endoscopy/radiological imaging alone [31.4%]. Treatment was switched or intensified in 101 [64.7%] patients, 9.9% [10 out of 101] without objectification through CRP, faecal calprotectin, endoscopy, or radiological imaging. These 10 patients all started either methotrexate, infliximab, or adalimumab, and only one patient presented with persistent diarrhoea, refractory to symptomatic treatment, as the only symptom. All others reported a combination of persistent diarrhoea, abdominal pain, and fatigue.
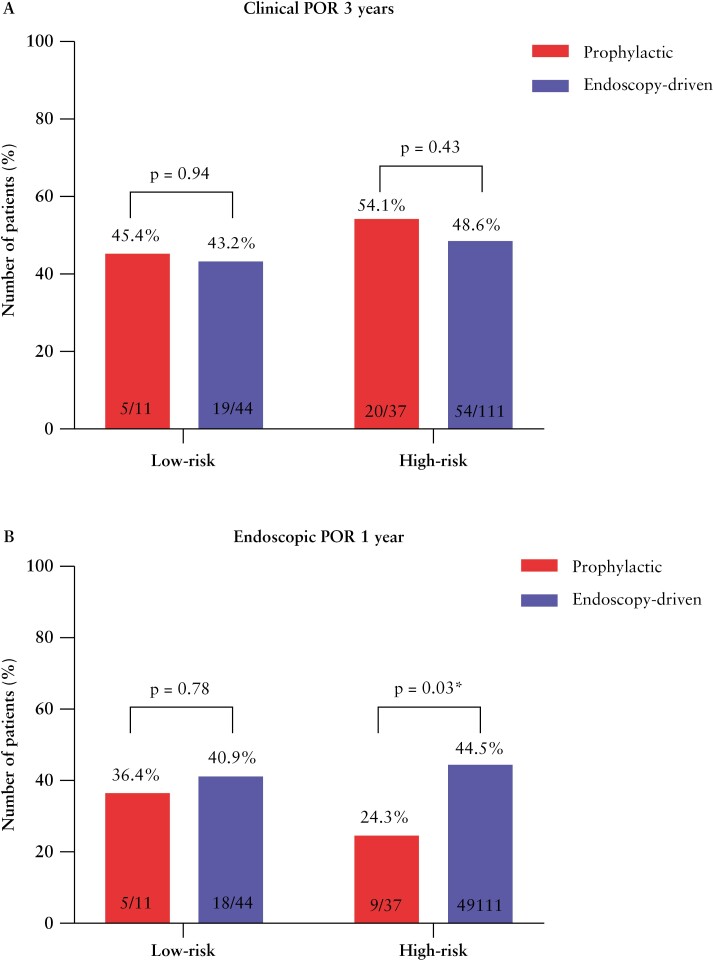
After categorisation into low and high risk with endoscopic evaluation in the first year following surgery, overall clinical POR was observed in 43.6% [n = 24 out of 55] and 50% [n = 74 out of 148] within 3 years of follow-up, respectively. We next compared the rates of clinical POR between the risk-stratified prophylactic- and endoscopy-driven patients in both groups. For both the low- and high-risk prophylactic-treated patients, the percentage of clinical recurrence was numerically higher at 3 years of follow-up [45.4% vs. 43.2% and 54.1% vs. 48.6%, respectively] when compared with endoscopy-driven patients, respectively. Despite this numerical difference, the incidence of clinical POR at 1 [p = 0.73], 2 [p = 0.22], and 3 [p = 0.94] years of follow-up did not significantly differ between low-risk prophylactic- and endoscopy-driven patients. Similar observations were made for the incidence of clinical POR at 1 [p = 0.19], 2 [p = 0.19], and 3 [p = 0.43] years between high-risk prophylactic- and endoscopy-driven patients [Table 3, Figure 2a].
Table 3.
Rates of endoscopic and clinical recurrence.
| Low-risk patients | High-risk patients | |||||
|---|---|---|---|---|---|---|
| Prophylactic n = 11 | Endoscopy-guided n = 44 | p-value | Prophylactic n = 37 | Endoscopy-guided n = 111 | p-value | |
| Endoscopic recurrence, n [%] | 4 [36.4] | 18 [40.9] | 0.78 | 9 [24.3] | 49 [44.5] | 0.03 |
| Modified Rutgeerts score, n [%] | 0.36 | 0.23 | ||||
| i0 | 2 [18.2] | 12 [27.3] | 12 [32.4] | 21 [19.1] | ||
| i1 | 3 [27.3] | 6 [13.6] | 4 [10.8] | 15 [13.6] | ||
| i2a | 2 [18.2] | 8 [18.2] | 12[32.4] | 25 [22.7] | ||
| i2b | 1 [9.1] | 12 [27.3] | 6 [16.2] | 27 [24.5] | ||
| i3 | - | 2 [4.5] | 1 [2.7] | 12 [10.9] | ||
| i4 | 3 [27.3] | 4 [9.4] | 2 [5.4] | 10 [9.1] | ||
| Clinical recurrence, n [%] | ||||||
| 1 year | 3 [27.3] | 13 [29.5] | 0.73 | 9 [24.3] | 34 [30.6] | 0.19 |
| 2 years | 5 [45.4] | 16 [36.4] | 0.22 | 14 [37.8] | 46 [41.4] | 0.19 |
| 3 years | 5 [45.4] | 19 [43.2] | 0.94 | 20 [54.1] | 54 [48.6] | 0.43 |
Values in bold are significant.
Figure 2.
Bar charts showing the rates of clinical POR within 3 years [a] and endoscopic POR within the first year following surgery [b] for the risk-stratified prophylactic in red and endoscopy-driven in blue. POR, postoperative recurrence.
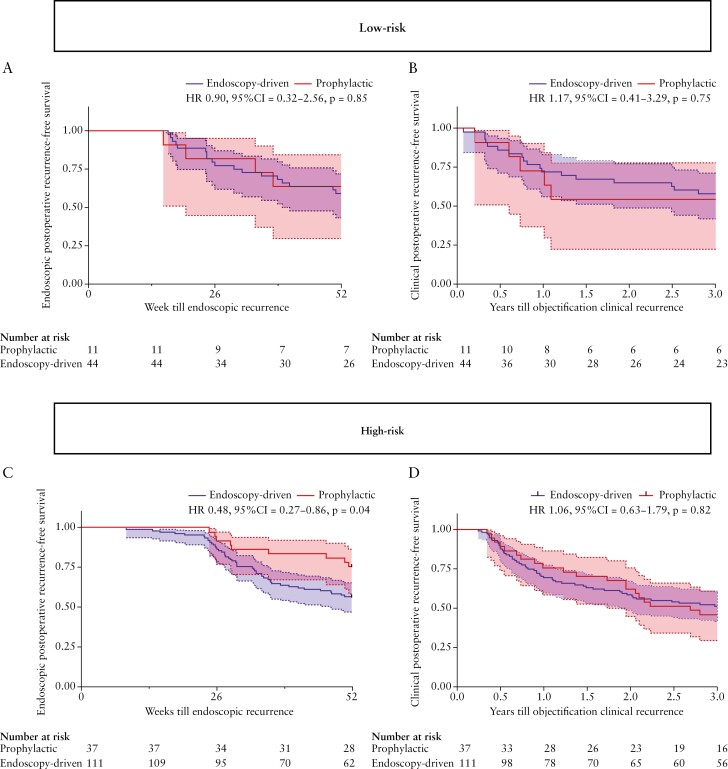
In addition, the time to clinical POR between the prophylactic- and endoscopy-driven treatment strategies for both low-risk [HR 1.17, 95% CI 0.41-3.29, p = 0.75] and high-risk [HR 1.06, 95% CI 0.63-1.79, p = 0.82] patients did not significantly differ during 3 years of follow-up [Figure 3b and d]. When combining both high- and low-risk patients together, we also found comparable rates [52.1% vs. 47.7%, p = 0.63, Supplementary Table 2] and time-to clinical POR within 3 years [HR 1.08, 95% CI 0.68-1.72, p = 0.73, Supplementary Figure 1]
Figure 3.
Kaplan–Meier survival plot showing the endoscopic- and clinical POR-free survival rates between the low-risk [3a and 3b] prophylactic- [n = 11] and endoscopy-driven [n = 44] as well as high-risk [3c and 3d] prophylactic- [n = 37]- and endoscopy-driven [n = 111] treatment strategies. Time till event is calculated as either weeks or years from surgery till objectified endoscopic and clinical POR, respectively. POR, postoperative recurrence.
Next, we stratified high-risk patients according to number of risk factors present, distinguishing into those with one [n = 148], two [n = 72], or three or more [n = 31] ECCO-defined risk factors and compared rates of clinical POR within 3 years between prophylactic- and endoscopy-driven patients. Doing so, we did not observe a significant difference in 3 year clinical POR between prophylactic- and endoscopy-driven patients with any two or more ECCO-defined risk factors [57.1% vs. 51%, p = 0.63]. Notably, comparing prophylactic- with endoscopy-driven treatment in patients with any three or more ECCO-defined risk factors [28.6% vs. 62.5%, p = 0.11] showed a large numerical difference, albeit not statistically significant.
3.3. Endoscopic recurrence
In total, 295 [78.5%] patients underwent endoscopic evaluation within 3 years, with a median time till the first endoscopic evaluation of 235 [181–382] days. Overall endoscopic POR rates were 40.9% [n = 83] and 57.3% [n = 169] at 1 and 3 years, respectively [Table 1].
We observed a significant reduction in endoscopic POR in high-risk patients receiving prophylactic treatment compared with those who remained untreated in the endoscopy-driven group [24.3% vs. 44.5%, p = 0.03], shown in Figure 2b. In low-risk patients, no significant difference was observed [36.4% vs. 40.9%, p = 0.78]. Furthermore, for low-risk patients, no significant difference in time to endoscopic POR could be observed [HR 0.90, 95% CI 0.32-2.56, p = 0.85] as opposed to a significant prolonged time till endoscopic POR in high-risk prophylactic patients [HR 0.48, 95% CI 0.27-0.86, p = 0.04], shown in Figure 3a and c, respectively. Additionally, although not significantly different [p = 0.23], a numerically higher percentage of patients in the endoscopy-driven group presented Rutgeert’s i3 [10.9% vs. 2.7%] or i4 [9.1% vs. 5.4%], indicating a more severe image of recurrence. Combining both low- and high-risk patients, discarding any form of risk-stratification, resulted in a similar significant decreased rate of [27.1% vs. 42.6%, p = 0.047, Supplementary Table 2] and prolonged time till endoscopic POR of prophylactic-treated patients, however not reaching statistical significance [HR 0.58, 95% CI 0.35-0.96, p = 0.06, Supplementary Figure 1a].
4. Discussion
In this multicentre retrospective cohort study, we observed lower rates of and longer time till endoscopic POR in prophylactic-treated compared with endoscopy-driven high-risk patients. Yet, although endoscopic POR rates were lower, both the incidence and the time till clinical POR did not significantly differ in the first 3 years of postoperative follow-up. Based on our data, we can conclude that the 6–2 months delay of treatment until an early endoscopic evaluation did not alter the probability of clinical POR. In fact, rates of overall endoscopic POR within 1 year [40% vs. 39.2%, respectively] and clinical POR at 3 years of follow-up [43.6% vs. 50%, respectively] were remarkably similar for low- and high-risk patients, questioning the utility of current risk stratification.15
Overall, we observed a 41.5% clinical POR rate within 3 years following surgery, in line with previous literature using similar definitions of clinical POR, with comparable follow-up time in the post-biologic era.8,16–18 Despite current guidelines and the decent adherence, it appears that there is room for improvement of postoperative management in Crohn’s disease patients. To do so, current guidelines2,3,5 suggest selecting patients at high risk of endoscopic POR to receive immediate prophylactic treatment, as opposed to low-risk patients undergoing endoscopic surveillance with treatment according to the presence of endoscopic POR.
Although a minority of patients were considered low risk in our cohort, our data did not show a significant difference in either endoscopic or clinical POR between the prophylactic- or endoscopy-driven groups, supporting guideline-advised endoscopic surveillance rather than prophylaxis in low-risk patients.
Interestingly, although most of our cohort [73.1%] would be classified as at high risk of developing endoscopic POR, only 44.7% of these patients received prophylactic treatment. Furthermore, comparing the distribution of clinical characteristics between high-risk prophylactic- and endoscopy-driven patients suggests that the choice of prophylactic postoperative treatment in these patients was driven by factors indicating a more aggressive phenotype, therapy refractoriness, or a complex surgical procedure. Arguably, these observations indicate an increased probability of endoscopic, and thus presumably clinical, POR19 in the high-risk prophylactic group, further supported by the recently published systematic review and meta-analysis of 37 studies [n = 4053 postoperative CD patients] from Ble et al.20 where the authors strongly associated endoscopic POR with subsequent clinical POR (pooled relative risk [RR] of 10.77 [95% CI 4.08-28.40]) in the total postoperative CD population.
However in our cohort, specifically focusing on the effect of prophylactic treatment compared with the 6–12 months ‘delayed’ treatment according to endoscopic POR, showed a reduced rate of endoscopic POR in high-risk prophylactic- compared with endoscopy-driven patients [24.3% vs. 44.5%, p = 0.03] within the first 12 months following surgery, with a number needed to treat [NNT] of five. Despite this difference in endoscopic POR within 12 months, at the end of the 3-year follow-up period, the difference in cumulative clinical recurrence-free survival between the high-risk endoscopy-driven and high-risk prophylactic groups was only 4.6%, with an NNT of 22. In other words, whereas five high-risk patients starting prophylactic treatment were needed to avoid one patient progressing to endoscopic POR within 12 months, 22 were needed to avoid clinical POR within 3 years. These results therefore indicate that whereas endoscopic POR strongly associates with clinical POR, delayed treatment in endoscopically objectified patients, rather than treatment of all clinically characterised high-risk patients, might be equally effective in preventing objectified clinical POR. In addition, surgically induced clinical remission up to 3 years was seen in 50% of all high-risk patients, who would have otherwise received prophylactic treatment.
Notably, the similarity in rates of clinical POR [54.1% vs. 48.6%, respectively], even though prophylactic-treated patients were more likely to have additional risk factors, implies a potential benefit of prophylaxis in carefully selected as opposed to all high-risk patients. In fact, in the subset of high-risk patients with three or more ECCO-defined risk factors, we observed a large numerically, yet not a statistically significant, different distribution in 3-year clinical POR between the prophylactic- and endoscopy-driven patients, indicating a cumulative effect of having multiple risk factors. Previous analyses from our own group of 142 Crohn’s disease patients without prophylactic treatment following surgery, also indicated a cumulative effect of having any three or more ECCO-defined risk factors on the occurrence of endoscopic POR [OR 4.87, 95% CI 1.30-18.29 p = 0.02].15 Our results therefore point towards prophylactic treatment in patients presenting any three or more ECCO-defined risk factors and an endoscopy-driven approach in the remainder, potentially avoiding unnecessary treatment in a large number of patients. However, this hypothesis needs to be replicated in a larger cohort of patients with multiple risk factors.
Previous research in cohorts without risk stratification has shown a similar benefit of prophylactic treatment on the occurrence of endoscopic POR.6,17 The PREVENT trial, a placebo-controlled, randomised trial in which post-surgical CD patients received either immediate infliximab or placebo, reported significantly lower rates of endoscopic POR in infliximab-treated patients [p ≤ 0.001]. More recently, Rivière et al. published data from a large [n = 365] cohort of post-surgery CD patients with at least 3 years of follow-up. In this retrospective study, 74 [20%] patients received immediate prophylactic treatment, 51 [14%] received thiopurines and 20 [5%] anti-TNF agents.17 The authors reported lower rates of endoscopic POR in patients who were treated prophylactically compared with those without prophylactic treatment [59% vs. 72%, p = 0.02].
The effect of prophylactic treatment on clinical POR in patients without risk stratification has, however, been conflicting. While data from two large placebo-controlled trials report a non-significant effect of prophylactic mercaptopurine21 or infliximab6 on clinical POR, previous data from Hanauer et al.,22 as well as the recent retrospective data from Rivière et al.,17 point toward significantly reduced rates of clinical POR following postoperative thiopurine treatment.
Our results, both with and without risk stratification, add to this body of evidence, suggesting little beneficial effect of prophylactic treatment in all high-risk patients on mid-term clinical POR rates.
Strengths of this study include the use of a large cohort of postoperative CD patients in the post biologic era having at least 3 years of follow-up. In addition, the inclusion of patients from both an academic as well as two non-academic centres make these results well generalisable to the total post-surgery IBD population.
There are however several limitations to address. First of all, due to the retrospective nature of our study, we were not able to use a validated clinical score such as the Crohn’s Disease Activity Index [CDAI] score to define and quantify clinical recurrence.8,21 However, our definition combined recurring clinical symptoms with objective parameters [CRP, faecal calprotectin, endoscopy/imaging, or treatment escalation], limiting the chance of recurring symptoms as a result of post-surgical complications or irritable bowel syndrome. Also, all available endoscopy reports and high-quality images were reviewed for endoscopic POR, using the modified Rutgeert’s score, by a single investigator [VJ]. However, reproducibility of this score has been reported as suboptimal related to the type of anastomosis used, with a potential overestimation of Rutgeert’s i2b in side-to-side anastomoses.23 Considering that in 91% of our cohort, a side-to-side anastomosis was constructed, we could have overestimated the rate of endoscopic POR. Furthermore, no other endoscopic indices besides the Rutgeert’s score (such as the Simple Endoscopic Score of Severity-CD [SES-CD] or The CD Endoscopic Index of Severity [CDEIS] scores) were retrieved, yet endoscopy reports/images were reviewed for colonic CD involved as a potential driver of recurrent clinical symptoms. Consequently, only 1 or one showed colonic involvement without anastomotic recurrence [Rs i1, CRP > 5, persistent diarrhoea]. We are therefore confident that the majority of the patients with clinical POR, objectified with endoscopy, owe this to disease activity in the anastomotic area.
Second, while selection of only those patients that underwent routine endoscopic evaluation within 12 months resulted in a more equal comparison of both strategies, we acknowledge a potential selection bias, filtering out postoperative CD patients with a milder phenotype and possibly underestimating the total effect of prophylactic treatment on clinical POR.
Third, insufficient data on disease progression, the small number of patients with repeat surgery in 3 years [n = 4], and the relatively small sample size of sub-stratified high-risk prophylactic patients, limited power to detect a significant difference between the prophylactic- and endoscopy-driven strategies for these outcomes. As lower rates of endoscopic POR are potentially more beneficial in preventing long-term development of disease complications such as strictures or perforation and need of repeat surgery, additional studies with longer follow-up duration are needed.
Fourth, we hypothesise that in some specific situations [ie, ongoing luminal inflammation elsewhere or perianal fistulising disease], immediate prophylactic therapy might be beneficial; however, due to the relatively low number of patients in these subgroups, we were unable to address these hypotheses.
5. Conclusion
In summary, our results suggest that postoperative prophylactic treatment of all high-risk patients does not reduce rates of nor time till clinical POR in CD patients, despite reduced rates of endoscopic recurrence. We therefore suggest towards the use of early endoscopic evaluation and treatment according to the presence of endoscopic recurrence, reserving prophylactic treatment for carefully selected high-risk patients in order to avoid potential over-treatment.
The data that support the findings of this study are available from the corresponding author, VJ, upon reasonable request.
Supplementary Material
Acknowledgments
The authors thank Dr Aart Mookhoek for histology consultations.
Contributor Information
Vincent Joustra, Department of Gastroenterology and Hepatology, Amsterdam University Medical Centers, AGEM University of Amsterdam, Amsterdam, The Netherlands.
Joris van Sabben, Department of Gastroenterology and Hepatology, Amsterdam University Medical Centers, AGEM University of Amsterdam, Amsterdam, The Netherlands.
Eline van der does de Willebois, Department of Surgery, Amsterdam University Medical Centers, AGEM University of Amsterdam, Amsterdam, The Netherlands.
Marjolijn Duijvestein, Department of Gastroenterology and Hepatology, Radboud University Medical Centre, Nijmegen, The Netherlands.
Nanne de Boer, Department of Gastroenterology and Hepatology, Amsterdam Gastroenterology Endocrinology Metabolism [AGEM] Research Institute, Amsterdam University Medical Centre, Amsterdam, The Netherlands.
Jeroen Jansen, Department of Gastroenterology, Onze Lieve Vrouwe Gasthuis, Amsterdam, The Netherlands.
Jarmila van der Bilt, Department of Surgery, Amsterdam University Medical Centers, AGEM University of Amsterdam, Amsterdam, The Netherlands.
Wytze Lameris, Department of Surgery, Amsterdam University Medical Centers, AGEM University of Amsterdam, Amsterdam, The Netherlands.
Willem Bemelman, Department of Surgery, Amsterdam University Medical Centers, AGEM University of Amsterdam, Amsterdam, The Netherlands.
Christianne Buskens, Department of Surgery, Amsterdam University Medical Centers, AGEM University of Amsterdam, Amsterdam, The Netherlands.
Geert D’Haens, Department of Gastroenterology and Hepatology, Amsterdam University Medical Centers, AGEM University of Amsterdam, Amsterdam, The Netherlands.
Funding
The authors received no financial support for research, authorship, and/or publication of this article.
Conflict of Interest
VJ, JS, EW MD, JJ, JB, AM, WL, WB, CB, GD: none to declare. NdB has served as a speaker for AbbVie and MSD and has served as consultant and/or principal investigator for TEVA Pharma BV and Takeda. He has received [unrestricted] research grant from Dr Falk, TEVA Pharma BV, MLDS, and Takeda, all outside the submitted work.
Author Contributions
Concept: VW, JS, EW, MD, GD. Methodology: VW, JS, MD, GD. Formal analysis: VW. Data acquisition: VW, JS, EW. Writing original draft: VW. Writing review and editing: VW, JS, EW, MD, NB, JJ, JB, WL, WB, CB, GD. Approval of final manuscript: all authors.
References
- 1. Tsai L, Ma C, Dulai PS, et al. Contemporary risk of surgery in patients with ulcerative colitis and Crohn’s disease: a meta-analysis of population-based cohorts. Clin Gastroenterol Hepatol 2021;19:2031–45.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019;68:s1–s106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gionchetti P, Dignass A, Danese S, et al. Third European Evidence-based consensus on the diagnosis and management of Crohn’s disease 2016. Part 2: surgical management and special situations. J Crohns Colitis 2017;11:135–49. [DOI] [PubMed] [Google Scholar]
- 4. Dragoni G, Ding N, Gecse KB, et al. The prevention and management of Crohn’s disease postoperative recurrence: results from the Y-ECCO/ClinCom 2019 Survey. Eur J Gastroenterol Hepatol 2020;32:1062–6. [DOI] [PubMed] [Google Scholar]
- 5. Nguyen GC, LoftusHirano EVI, Falck-Ytter Y, Singh S, Sultan S.. American Gastroenterological Association Institute Guideline on the Management of Crohn’s Disease After Surgical Resection. Gastroenterology 2017;152:271–5. [DOI] [PubMed] [Google Scholar]
- 6. Regueiro M, Feagan BG, Zou B, et al. Infliximab reduces endoscopic, but not clinical, recurrence of Crohn’s disease after ileocolonic resection. Gastroenterology 2016;150:1568–78. [DOI] [PubMed] [Google Scholar]
- 7. De Cruz P, Kamm MA, Hamilton AL, et al. Crohn’s disease management after intestinal resection: a randomised trial. Lancet 2015;385:1406–17. [DOI] [PubMed] [Google Scholar]
- 8. Ferrante M, Papamichael K, Duricova D, et al. Systematic versus endoscopy-driven treatment with azathioprine to prevent postoperative ileal Crohn’s disease recurrence. J Crohns Colitis 2015;9:617–24. [DOI] [PubMed] [Google Scholar]
- 9. Yamada A, Komaki Y, Patel N, et al. The use of vedolizumab in preventing postoperative recurrence of Crohn’s disease. Inflamm Bowel Dis 2018;24:502–9. [DOI] [PubMed] [Google Scholar]
- 10. Ferrante M, de Hertogh G, Hlavaty T, et al. The value of myenteric plexitis to predict early postoperative Crohn’s disease recurrence. Gastroenterology 2006;130:1595–606. [DOI] [PubMed] [Google Scholar]
- 11. Decousus S, Boucher AL, Joubert J, et al. Myenteric plexitis is a risk factor for endoscopic and clinical postoperative recurrence after ileocolonic resection in Crohn’s disease. Dig Liver Dis 2016;48:753–8. [DOI] [PubMed] [Google Scholar]
- 12. Misteli H, Koh CE, Wang LM, Mortensen NJ, George B, Guy R.. Myenteric plexitis at the proximal resection margin is a predictive marker for surgical recurrence of ileocaecal Crohn’s disease. Colorectal Dis 2015;17:304–10. [DOI] [PubMed] [Google Scholar]
- 13. Nakao S, Itabashi M, Yamamoto T, Okamoto T.. Predictive value of myenteric and submucosal plexitis for postoperative Crohn’s disease recurrence. J Anus Rectum Colon 2017;1:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ma C, Gecse KB, Duijvestein M, et al. Reliability of endoscopic evaluation of postoperative recurrent Crohn’s disease. Clin Gastroenterol Hepatol 2020;18:2139–41.e2. [DOI] [PubMed] [Google Scholar]
- 15. Joustra V, Duijvestein M, Mookhoek A, et al. Natural history and risk stratification of recurrent Crohn’s disease after ileocolonic resection: a multicenter retrospective cohort study. Inflamm Bowel Dis 2022;28:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hammoudi N, Auzolle C, Tran Minh ML, et al. Postoperative endoscopic recurrence on the neoterminal ileum but not on the anastomosis is mainly driving long-term outcomes in Crohn’s disease. Am J Gastroenterol 2020;115:1084–93. [DOI] [PubMed] [Google Scholar]
- 17. Riviere P, Vermeire S, Irles-Depe M, et al. Rates of postoperative recurrence of Crohn’s disease and effects of immunosuppressive and biologic therapies. Clin Gastroenterol Hepatol 2021;19:713–20. [DOI] [PubMed] [Google Scholar]
- 18. Navaratne L, Hurndall KH, Richardson DM, et al. Risk factors for symptomatic anastomotic postoperative recurrence following ileo-colic resection in Crohn’s disease. Colorectal Dis 2021;23:1184–92. [DOI] [PubMed] [Google Scholar]
- 19. Rutgeerts P, Geboes K, Vantrappen G, Beyls J, Kerremans R, Hiele M.. Predictability of the postoperative course of Crohn’s disease. Gastroenterology 1990;99:956–63. [DOI] [PubMed] [Google Scholar]
- 20. Ble A, Renzulli C, Cenci F, et al. The relationship between endoscopic and clinical recurrence in postoperative Crohn’s disease: a systematic review and meta-analysis. J Crohns Colitis 2022;16:490–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mowat C, Arnott I, Cahill A, et al. Mercaptopurine versus placebo to prevent recurrence of Crohn’s disease after surgical resection [TOPPIC]: a multicentre, double-blind, randomised controlled trial. Lancet Gastroenterol Hepatol 2016;1:273–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hanauer SB, Korelitz BI, Rutgeerts P, et al. Postoperative maintenance of Crohn’s disease remission with 6-mercaptopurine, mesalamine, or placebo: a 2-year trial. Gastroenterology 2004;127:723–9. [DOI] [PubMed] [Google Scholar]
- 23. Riviere P, Bislenghi G, Vermeire S, et al. Postoperative Crohn’s disease recurrence: time to adapt endoscopic recurrence scores to the leading surgical techniques. Clin Gastroenterol Hepatol 2022;20:1201–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.