Abstract
Background
Dyslipidemia may be an important modifiable risk factor contributing to the increased cardiovascular risk in inflammatory bowel disease (IBD). The lipid metabolism is subject to both systemic inflammation and drug therapy; however, it is unclear if this effect is drug-class dependent. Our aim was to assess lipid changes after IBD induction therapy and evaluate associated factors with a particular focus on drug class and disease activity.
Methods
In this prospective study, consecutive IBD patients starting systemic therapy (eg, corticosteroids, thiopurines, methotrexate, anti-TNF-α agents, vedolizumab, ustekinumab, and tofacitinib) were included. Primary outcomes were changes in total cholesterol, high density lipoprotein cholesterol (HDL-c), low-density lipoprotein cholesterol (LDL-c), and triglycerides at week 10.
Results
One hundred ninety-eight IBD patients (107 women [54%], median age 36 years; interquartile range [IQR], 27-47) were included: 137 Crohn’s disease (67%), 61 ulcerative colitis (29%), and 8 IBD-unclassified (4%). Median C-reactive protein and fecal calprotectin at baseline were 5.1 mg/L (IQR, 1.6-12.0) and 1040 ug/g (IQR, 383-1800), respectively. Relative increases in total cholesterol, HDL-c, and LDL-c were significant after prednisone (+26%, +31%, +12%) and tofacitinib therapy (+20%, +25%, +26%), respectively. Results remained after adjusting for concomitant corticosteroids, cholestyramine, and PSC diagnosis. Changes in clinical scores were inversely correlated with total cholesterol changes (R −186, P = .014), as was CRP with total cholesterol and LDL-c (R −0.292 and R −0.259, P < .001). No correlation was found with FCP. Lipid changes remained after adjusting for age and CRP.
Conclusions
Prednisone and tofacitinib induction therapy significantly increase serum lipid levels, whereas no changes were observed in other drug classes. The observations seem drug-specific inasmuch as adjustment for systemic inflammation did not alter the results.
Keywords: corticosteroids, immunomodulators, biologics, tofacitinib, lipids
Key Messages.
What is already known? The lipid metabolism in IBD is subject to both systemic inflammation and drug therapy.
What is new here? Increase in lipid levels seem drug-specific, as prednisone and tofacitinib induction therapy significantly increase serum lipid levels, whereas no changes are observed in immunomodulators or biologics.
How can this study help patient care? The results prompt further research to assess the long-term consequences of these drug-induced lipid increases on cardiovascular risk in IBD patients.
Introduction
Inflammatory bowel disease (IBD) is a proatherogenic condition associated with increased risk of cardiovascular disease compared with the general population.1,2 However, since traditional risk factors do not capture full cardiovascular risk in IBD, risk assessment models may underestimate the cardiovascular risk.3,4 In the general population, dyslipidemia is one of the most important modifiable risk factors for cardiovascular disease through promoting atherosclerosis. In particular, the association between total cholesterol and low density lipoprotein cholesterol (LDL-c) with cardiovascular morbidity and mortality is robust, even in young patients, with an estimated low 10-year cardiovascular risk.5,6 Unlike the general population, the role of lipid levels in cardiovascular outcomes in IBD is not well established and therefore subject to research. Paradoxically, patients with active IBD show reduced levels of total cholesterol and LDL-c but not high density lipoprotein cholesterol (HDL-c) and triglycerides compared with patients in disease remission and the general population.7,8 This might be explained by the reverse association between systemic inflammation and lipid levels, as well as the intestinal malabsorption of cholesterol and fat.9 Drug therapy to treat intestinal inflammation might significantly modify circulating lipid levels through both pathways. The magnitude of the effect of currently used IBD drug therapies on lipid levels is largely unknown. In IBD, elevations of lipid levels have been systematically studied and consistently reported in corticosteroids and tofacitinib.10 The lipid-modulating effect of tumor necrosis factor alpha (TNF-α) is derived from small studies with conflicting results; this effect is yet unknown for other IBD drugs such as immunomodulators and newer biologicals.11,12 These data are needed to evaluate whether lipid changes are drug-specific or a more general consequence of improved control of systemic and/or intestinal inflammation. In drug-specific lipid changes, long-term consequences of these drugs need to be explored to guide screening and management. Therefore, the aim of the current study was to evaluate lipid changes after induction therapy with various drugs for IBD.
Materials and Methods
Study Design and Population
In this single-center, prospective cohort study, all consecutive IBD patients 17 years and older who started systemic drug therapy between April 2019 and March 2021 were enrolled after informed consent. In the Netherlands, corticosteroids, thiopurines, methotrexate, infliximab, adalimumab, vedolizumab, ustekinumab, and tofacitinib are registered as IBD therapies (Supplementary Table 1). Exclusion criteria were (1) the use of lipid-lowering drugs, with the exception of cholestyramine on stable dose throughout the study; (2) supplements containing plant sterols/stanols or cholestin; (3) pregnancy; and (4) a history of liver transplantation.
Outcome Measures
Primary outcomes were lipid changes after induction therapy measured by serum levels of total cholesterol, HDL-c, LDL-c, triglycerides, apolipoprotein B (apo B), and lipoprotein(a) (lp[a]) at baseline and at 10 weeks (scope ±2 weeks) after start of treatment. Secondary outcomes included (1) changes in other lipid markers (apolipoprotein B and lipoprotein[a]); (2) atherogenic lipid indices (LDL-c/HDL-c ratio and total cholesterol/HDL-c ratio); and (3) associations between lipid changes and therapy response. Clinical activity scores used were the Harvey Bradshaw Index (HBI) and Simple Clinical Colitis Activity Index (SCCAI). Inflammatory parameters collected were C-reactive protein (CRP) and fecal calprotectin (FCP), with normal values defined as < 10 mg/L and < 250 ug/g, respectively. All lipid levels were measured on nonfasting samples using standard laboratory techniques.13 Samples were centrifuged for 5 minutes on 3000 g. Rochec/Hitachi cobas c systems were used for quantitative determination of lipid parameters. Total cholesterol, HDL-c, LDL-c, and triglycerides were executed with homogeneous enzymatic colorimetric assays. Apolipoprotein B and lipoprotein(a) were executed with immunoturbidimetric assays. Active inflammation was defined as HBI>5, SCCAI>5 and/or fecal calprotectin >250 ug/g. Covariates measured were patient and disease characteristics (ie, age, sex, body mass index, smoking state, IBD diagnosis, Montreal classification, disease duration, surgical history; and concomitant use, type, and dose of corticosteroids and cholestyramine).
Statistical Analysis
Patient and disease characteristics were described as frequencies (%), mean (standard deviation) and median (interquartile range [IQR]) depending on type and distribution of data. Lipid levels at baseline and after induction therapy were compared within each treatment group using 1-sample Wilcoxon signed rank test. Within the corticosteroid group, stratification was performed on type of drug (ie, budesonide vs prednisone). Waterfall plots were used to graphically illustrate the intraindividual variation in response. In all other groups, sensitivity analyses were performed, excluding patients on concomitant corticosteroids and cholestyramine or those diagnosed with primary sclerosing cholangitis. Spearman correlation test was performed to assess the association between changes in lipid levels and markers of disease activity. Linear regression models were applied to calculate lipid changes (mean, standard error [SE]) corrected for factors shown to influence drug-induced lipid changes in IBD (ie, age and CRP changes).10 A 2-sided P value < .05 was considered statistically significant for all tests. Data were analyzed in IBM SPSS Statistics for Windows (version 24.0).
Power Calculation
Based on data from 905 subjects participating in the OCTAVE trials with mean baseline HDL-c level of 1.5 mmol/L and LDL-c level of 2.6 mmol/L, relative changes of ≥20% (0.3 mmol/L) in HDL-c and ≥15% in LDL-c (0.4 mmol/L) were considered clinically relevant.14 Following a 2-sided, 1-sample t test with significance level 0.05, a sample size of 20 participants per treatment group reaches ≥80% power to detect changes in relevant ranges. The power calculation was performed in R Core Team (2018).
Ethical Considerations
The institutional medical ethics committee of the Erasmus Medical Center approved the protocol (MEC-2019-0073). The study was publicly registered at the Netherlands Trial Register, part of the Dutch Cochrane Centre (NL9844). The study was conducted in compliance with the Declaration of Helsinki.
Results
Baseline Characteristics
Inclusion flowchart and baseline patient characteristics and lipid levels are displayed in Supplementary Figure 1 and Table 1. In total, 198 patients were included: 107 women (54%), with a median age of 36 years (IQR, 27-47). The majority had Crohn’s disease (n = 133, 67%) and presented with active disease (n = 184, 93%). Median follow-up was 9.7 weeks (range 8.0-11.9). Seven patients were diagnosed with primary sclerosing cholangitis (3.5%). Twenty-nine patients started corticosteroids (20 budesonide and 9 prednisone), 27 thiopurines, 10 methotrexate, 21 infliximab, 30 adalimumab, 28 vedolizumab, 35 ustekinumab, and 18 tofacitinib. At baseline, 42 patients (21%) were on concomitant corticosteroids with a median dose of budesonide 6 mg (IQR, 6-–9) and prednisone 20 mg (IQR, 16-–30); in 29/42 patients (69%) corticosteroids were tapered until discontinuation during follow-up. Nine patients were on stable dose cholestyramin (4.5%). The distribution of lipid levels at baseline was similar between the drug therapy groups.
Table 1.
Baseline characteristics of total population.
| Total (n = 198) | CORT (n = 29) | THIO (n = 27) | MTX (n = 10) | IFX (n = 21) | ADA (n = 30) | VEDO (n = 28) | UST (n = 35) | TOFA (n = 18) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Men | N(%) | 91 (46.0) | 12 (41.4) | 9 (33.3) | 2 (20.0) | 13 (61.9) | 15 (50.0) | 9 (32.1) | 20 (57.1) | 11 (61.1) |
| Age, years | Md(IQR) | 36 (27–47) | 36 (29–48) | 30 (24–35) | 38 (31–62) | 33 (24–52) | 36 (25–47) | 43 (36–56) | 36 (27–46) | 46 (34–52) |
| Body mass index, kg/m2 | Md(IQR) | 23.2 (21.0–26.3) | 22.1 (20.4–24.4) | 23.6 (21.2–27.7) | 23.1 (20.5–31.4) | 23.7 (19.8–26.5) | 23.6 (21.0–26.6) | 23.4 (20.6–26.8) | 23.5 (21.3–26.3) | 23.4 (22.1–24.4) |
| Active smokers | N(%) | 34 (17.2) | 7 (24.1) | 6 (22.2) | 1 (10.0) | 5 (23.8) | 3 (10.0) | 4 (14.8) | 8 (22.9) | NA |
| Crohn’s disease | N(%) | 133 (67.2) | 20 (69.0) | 13 (48.1) | 9 (90.0) | 16 (76.2) | 23 (76.7) | 22 (78.6) | 30 (85.7) | NA |
| Age at diagnosis | N(%) | |||||||||
| <17 years | 28 (21.1) | 4 (20.0) | 2 (15.4) | 2 (22.2) | 3 (18.8) | 7 (30.4) | 2 (9.1) | 8 (26.7) | NA | |
| 17–40 years | 87 (65.4) | 14 (70.0) | 10 (76.9) | 4 (44.4) | 9 (56.3) | 13 (56.5) | 18 (81.8) | 19 (63.3) | NA | |
| >40 years | 18 (13.5) | 2 (10.0) | 1 (7.7) | 3 (33.3) | 4 (25.0) | 3 (13.0) | 2 (9.1) | 3 (10.0) | NA | |
| Disease location | N(%) | |||||||||
| Ileum | 35 (26.3) | 2 (10.0) | 3 (23.1) | 4 (44.4) | 3 (18.8) | 8 (34.8) | 8 (36.4) | 7 (23.3) | NA | |
| Colon | 32 (24.1) | 8 (40.0) | 2 (15.4) | 2 (22.2) | 1 (6.3) | 5 (16.7) | 6 (27.3) | 8 (26.7) | NA | |
| Ileum and colon | 66 (49.6) | 10 (50.0) | 8 (61.5) | 3 (33.3) | 12 (75.0) | 10 (33.3) | 8 (36.4) | 15 (50.0) | NA | |
| Proximal | 17 (12.8) | 1 (5.0) | 1 (7.7) | 1 (11.1) | 1 (6.3) | 3 (13.0) | 4 (18.2) | 6 (20.0) | NA | |
| Disease behavior | N(%) | |||||||||
| Luminal | 63 (47.4) | 11 (55.0) | 6 (46.2) | 5 (55.6) | 6 (37.5) | 12 (52.2) | 9 (40.9) | 14 (46.7) | NA | |
| Stricturing | 56 (42.1) | 8 (40.0) | 5 (38.5) | 3 (33.3) | 7 (43.8) | 7 (30.4) | 13 (59.1) | 13 (43.4) | NA | |
| Penetrating | 14 (10.5) | 1 (5.0) | 2 (15.4) | 1 (11.1) | 3 (18.8) | 4 (17.4) | NA | 3 (10.0) | NA | |
| Perianal | 31 (23.3) | 6 (30.0) | 2 (15.4) | 1 (11.1) | 4 (25.0) | 3 (13.0) | 5 (22.7) | 10 (33.3) | NA | |
| IBD-unclassified | N(%) | 8 (4.0) | NA | 6 (3.7) | NA | NA | NA | NA | NA | 2 (11.1) |
| Ulcerative colitis | N(%) | 57 (28.8) | 9 (31.0) | 8 (29.6) | 1 (10.0) | 5 (23.8) | 7 (23.3) | 6 (21.4) | 5 (14.3) | 16 (88.9) |
| Disease extent | N(%) | |||||||||
| Proctitis | 3 (4.6) | NA | 2 (14.3) | NA | NA | NA | NA | 1 (16.7) | NA | |
| Left-sided | 28 (43.1) | 5 (55.6) | 8 (57.1) | NA | 1 (20.0) | 3 (42.9) | 3 (50.0) | 2 (33.3) | 6 (33.3) | |
| Pancolitis | 35 (53.8) | 4 (44.4) | 4 (28.6) | 1 (100) | 4 (80.0) | 4 (57.1) | 3 (50.0) | 3 (50.0) | 12 (66.7) | |
| Disease duration, years | Md(IQR) | 12 (6–19) | 16 (5–25) | 24 (21–28) | 15 (6–21) | 9 (5–15) | 12 (6–16) | 18 (12–21) | 13 (8–22) | 13 (7–21) |
| Intestinal resection | N(%) | 71 (35.9) | 11 (37.9) | 4 (14.8) | 5 (50.0) | 8 (38.1) | 12 (40.0) | 15 (53.6) | 16 (45.7) | NA |
| Ileocecal | 43 (62.3) | 8 (27.6) | 3 (11.1) | 4 (40.0) | 5 (23.8) | 9 (30.0) | 11 (39.3) | 12 (34.3) | NA | |
| Ileocolonic/colectomy | 26 (37.7) | 3 (10.3) | 1 (3.7) | 1 (10.0) | 3 (14.3) | 3 (10.) | 4 (14.3) | 4 (11.4) | NA | |
| Primary sclerosing cholangitis | N(%) | 8 (4.0) | NA | NA | NA | 1 (4.8) | 2 (6.7) | NA | 3 (8.6) | 2 (11.1) |
| Steroid use at baseline | N(%) | 42 (21.2) | NA | 7 (25.9) | 3 (30.0) | 4 (19.0) | 8 (26.7) | 7 (25.0) | 6 (17.1) | 7 (38.9) |
| Budesonide | N(%) | 26 (61.9) | NA | 5 | 2 | 2 | 4 | 7 | 5 | 1 |
| Dose, mg/day | Md(IQR) | 6 (6–9) | NA | 6 (6–7.5) | 7.5 (6–7.5) | 6 (NA) | 9 (6.7–9) | 6 (6–9) | 9 (6–9) | 9 (NA) |
| Prednisone | N(%) | 16 (38.1) | NA | 2 | 1 | 2 | 4 | NA | 1 | 6 |
| Dose, mg/day | Md(IQR) | 20 (16–30) | NA | 23 (20–25) | 40 (NA) | 15 (10–15) | 16 (20–28) | NA | 25 (NA) | 20 (15–33) |
| Cholestyramin use | N(%) | 9 (4.5) | NA | 1 (3.7) | NA | 1 (4.8) | 2 (6.7) | 3 (10.7) | 2 (5.7) | NA |
| Clinical disease activity | Md(IQR) | |||||||||
| HBI | 8 (6–12) | 9 (7–13) | 8 (6–8) | 7 (6–23) | 9 (6–12) | 8 (6–12) | 9 (7–13) | 8 (5–12) | NA | |
| SCCAI | 8 (6–11) | 10 (6–15) | 7 (5–9) | 6 (NA) | 8 (6–9) | 9 (6–13) | 6 (5–9) | 10 (9–14) | 9 (7–12) | |
| Inflammatory parameters | ||||||||||
| CRP assessment | N(%) | 180 | 28 | 25 | 9 | 18 | 28 | 24 | 31 | 17 |
| CRP, mg/L | Md(IQR) | 5.1 (1.6–12.0) | 3.3 (1.3–6.6) | 4.7 (1.1–8.8) | 1.6 (0.4–11) | 7.0 (2.8–18.3) | 4.7 (1.7–17) | 3.1 (1.8–11) | 6.6 (3.4–17) | 8.1 (5.3–12) |
| FCP assessment | N(%) | 21 | 12 | 4 | 14 | 22 | 23 | 24 | 18 | |
| FCP, ug/g | Md(IQR) | 1040 (383–1800) | 1159 (501–1800) | 1082 (340–1800) | 329 (190–1490) | 600 (211–1800) | 871 (402–1800) | 942 (303–1235) | 1235 (427–1800) | 1800 (935–1800) |
| Lipid profile, nonfasting | Md(IQR) | |||||||||
| Total cholesterol, mmol/L | 4.2 (3.5–4.9) | 4.0 (3.6–4.7) | 4.1 (3.3–5.1) | 4.2 (3.0–5.2) | 3.8 (3.1–4.7) | 4.3 (3.7–4.7) | 4.4 (3.5–5.1) | 3.9 (3.0–4.6) | 4.6 (4.0–5.0) | |
| HDL-c, mmol/L | 1.3 (1.1–1.6) | 1.2 (1.1–1.6) | 1.2 (1.1–1.4) | 1.4 (1.1–2.0) | 1.2 (1.0–1.5) | 1.3 (1.0–1.5) | 1.3 (1.1–1.6) | 1.3 (1.1–1.5) | 1.7 (1.3–2.1) | |
| LDL-c, mmol/L | 2.4 (1.9–3.1) | 2.4 (1.9–3.0) | 2.5 (1.9–3.1) | 2.4 (1.6–3.3) | 2.2 (1.5–2.8) | 2.5 (2.2–3.0) | 2.6 (2.0–3.3) | 2.1 (1.7–3.0) | 2.4 (2.1–3.4) | |
| Triglycerides, mmol/L | 1.3 (0.9–1.9) | 1.2 (1.0–1.8) | 1.3 (0.9–1.8) | 1.4 (1.3–1.7) | 1.1 (0.7–1.9) | 1.3 (1.0–2.0) | 1.5 (1.0–3.4) | 1.2 (0.9–1.8) | 1.0 (0.7–1.6) | |
| Apolipoprotein B, g/L | 0.7 (0.6–0.9) | 0.7 (0.6–0.9) | 0.8 (0.6–0.9) | 0.8 (0.6–1.0) | 0.8 (0.7–0.9) | 0.9 (0.7–1.1) | 0.7 (0.6–0.9) | 0.7 (0.7–0.9) | ||
| Lp(a), nmol/L | 36 (15–100) | 36 (15–128) | 32 (15–80) | 26 (11–54) | 53 (13–177) | 33 (14–80) | 36 (14–97) | 36 (17–111) | 50 (26–97) | |
| Atherogenic index | Md(IQR) | |||||||||
| LDL-c/HDL-c ratio | 1.7 (1.4–2.3) | 2.2 (1.3–2.6) | 1.8 (1.6–2.8) | 1.7 (1.4–2.2) | 1.7 (1.2–2.4) | 1.9 (1.5–2.5) | 2.1 (1.5–2.7) | 1.9 (1.3–2.3) | 1.4 (1.2–2.5) | |
| Total cholesterol/HDL-c ratio | 3.2 (2.7–4.0) | 3.5 (2.6–3.8) | 3.1 (2.6–4.3) | 3.3 (2.5–3.7) | 2.9 (2.2–3.8) | 3.2 (2.7–3.7) | 3.4 (2.5–4.1) | 3.0 (2.4–3.7) | 2.7 (2.2–3.7) |
Abbreviations: Md, median; IQR, interquartile range; HBI, Harvey Bradshaw Index; SCCAI, Simple Clinical Colitis Activity Index; CRP, C-reactive protein; FCP, fecal calprotectin; HDL-c, high density lipoprotein cholesterol; LDL-c, low density lipoprotein cholesterol; Lp(a), lipoprotein a; CORT, corticosteroids; THIO, thiopurines; MTX, methotrexate; IFX, infliximab; ADA, adalimumab; VEDO, vedolizumab; UST, ustekinumab; TOFA, tofacitinib; NA, not applicable
Lipid Changes
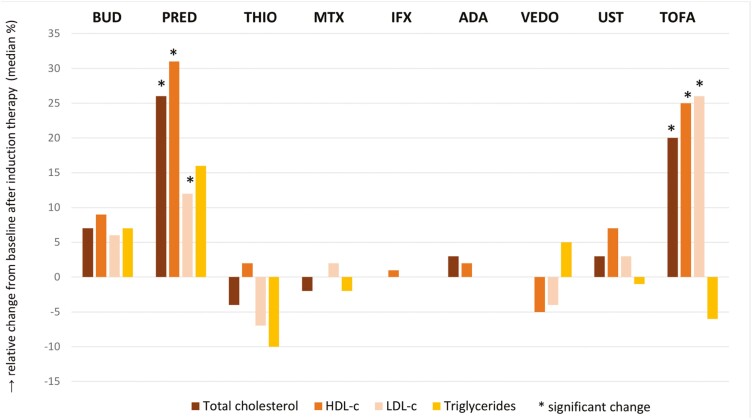
Mean lipid changes after induction therapy are shown in Table 2 and Figure 1. Treatment with corticosteroids increased total cholesterol and HDL-c levels significantly (+0.40 mmol/L; IQR, −0.10 to 0.85; +8%, P = .009; and + 0.19 mmol/L; IQR, −0.02 to 0.34; +15%, P = .002, respectively). Tofacitinib increased total cholesterol, HDL-c, and LDL-c levels (+0.90 mmol/L; IQR, 0.38-1.43; +20%, P = .002; +0.41 mmol/L; IQR, −0.14 to 0.71; +25%, P = .028; and +0.73 mmol/L; IQR, 0.05-1.15; +26%, P = .002, respectively). After treatment with thiopurine, a significant decrease in lp(a) levels was observed. No significant lipid changes were observed after treatment with methotrexate, thiopurines, vedolizumab, or ustekinumab.
Table 2.
Absolute and relative changes of lipid levels after induction therapy.
| Total cholesterol, mmol/L | HDL-c, mmol/L | LDL-c, mmol/L | Triglycerides, mmol/L | Apolipoprotein B, g/L | Lp(a), nmol/L | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median (IQR) | % | P | Median (IQR) | % | P | Median (IQR) | % | P | Median (IQR) | % | P | Median (IQR) | % | P | Median (IQR) | % | P | |
| CORT (n = 29) | +0.40 (−0.10 to 0.85) | +8 | 0.009 | +0.19 (−0.02 to 0.34) | +15 | 0.002 | +0.19 (−0.19 to 0.50) | +6 | ns | +0.12 (−0.25 to 0.12) | +9 | ns | _0.05 (−0.03 to 0.12) | +6 | ns | −2.0 (−12 to 4) | −4 | ns |
| BUD (n = 20) | +0.30 (-0.25 to 0.48) | +7 | ns | +0.11 (−0.01 to 0.32) | +9 | ns | +0.15 (−0.32 to 0.50) | +6 | ns | +0.09 (−0.29 to 0.20) | +7 | ns | −0.05 (−0.04 to 0.12) | +7 | ns | −1 (−11 to 5) | −1 | ns |
| PRED (n = 9) | +0.90 (0.20 to 1.25) | +26 | 0.024 | +0.30 (-0.07 to 0.66) | +31 | 0.022 | +0.30 (0.04 to 0.50) | +12 | 0.048 | +0.20 (−0.04 to 0.98) | +16 | ns | −0.20 (−0.02 to 0.13) | +3 | ns | −5 (−16 to 1) | −16 | 0.042 |
| THIO (n = 27) | −0.18 (−0.53 to 0.15) | −4 | ns | +0.03 (−0.13 to 0.12) | +2 | ns | −0.12 (−0.35 to 0.18) | −7 | ns | −0.11 (−0.40 to 0.23) | −10 | ns | −0.04 (−0.15 to 0.02) | −3 | ns | −6 (−27 to 0) | −15 | 0.003 |
| MTX (n = 10) | −0.10 (−0.65 to 0.20) | −2 | ns | 0 (−0.19 to 0.05) | 0 | ns | +0.03 (−0.51 to 0.21) | +1 | ns | −0.27 (−0.36 to 0.48) | −2 | ns | −0.02 (−0.13 to 0.01) | −2 | ns | −0.5 (−6 to 2) | −1 | ns |
| IFX (n = 21) | 0 (−0.20 to 0.45) | 0 | ns | +0.01 (−0.1 to 0.15) | +1 | ns | 0 (−0.11 to 0.41) | 0 | ns | 0.00 (−0.28 to 0.41) | 0 | ns | 0.00 (−0.08 to 0.03) | 0 | ns | 0 (−28 to 0.5) | 0 | ns |
| ADA (n = 30) | +0.10 (−0.23 to 0.33) | +3 | ns | +0.03 (−0.08 to 0.18) | +2 | ns | +0.01 (−0.56 to 0.58) | 0 | ns | 0 (−0.20 to 0.22) | 0 | ns | −0.01 (−0.09 to 0.03) | 0 | ns | −2 (−20 to 3) | −10 | ns |
| VEDO (n = 28) | 0 (−0.40 to 0.20) | 0 | ns | −0.07 (−0.23 to 0.09) | −5 | ns | −0.12 (−0.32 to 0.37) | −4 | ns | +0.06 (−0.36 to 0.44) | +5 | ns | −0.04 (−0.10 to 0.04) | −5 | ns | 0 (−6 to 2) | 0 | ns |
| UST (n = 35) | +0.10 (−0.30 to 0.40) | +3 | ns | +0.10 (−0.02 to 0.20) | +7 | ns | +0.07 (−0.15 to 0.18) | +3 | ns | +0.02 (−0.31 to 0.24) | −1 | ns | −0.03 (−0.07 to 0.07) | −4 | ns | 0 (−9 to 8) | 0 | ns |
| TOFA (n = 18) | +0.90 (0.38 to 1.43) | +20 | 0.002 | +0.41 (−0.14 to 0.71) | +25 | 0.028 | +0.73 (0.05 to 1.15) | +26 | 0.002 | -0.09 (−0.55 to 0.51) | −6 | ns | +0.03 (−0.09 to 0.15) | −5 | ns | −0.05 (−4 to 3) | −1 | ns |
Figure 1.
Relative lipid changes from baseline after induction therapy. Abbreviations: BUD, budesonide; PRED, prednisone; THIO, thiopurines; MTX, methotrexate; IFX, infliximab; ADA, adalimumab; VEDO, vedolizumab; UST, ustekinumab; TOFA, tofacitinib; HDL-c, high density lipoprotein cholesterol; LDL-c, low density lipoprotein cholesterol.
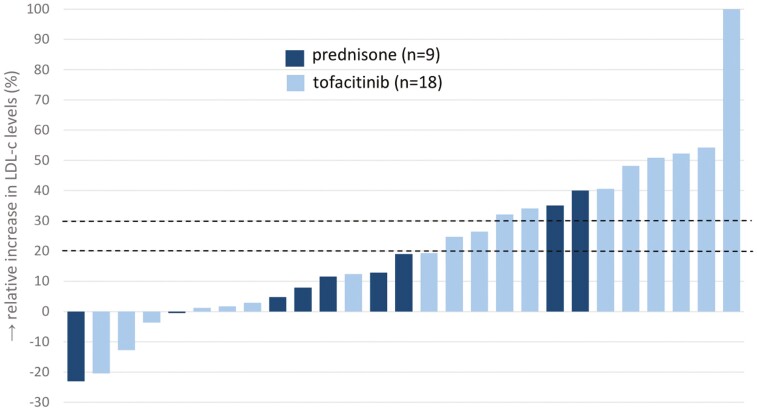
Within the corticosteroid group, overall changes seemed to be mainly contributed by the subgroup of prednisone users (total cholesterol, +0.90 mmol/L; IQR, 0.20-1.2; +26%; P = .024; HDL-c, +0.30; IQR, −0.07-0.66; +31%, P = .022; and LDL-c, +0.30; IQR, 0.04-0.50; +12%, P = .048, respectively) in contrast to the modest changes observed for budesonide (total cholesterol, +0.30 mmol/L; IQR, −0.25 to 0.48; +7%; HDL-c, +0.11; IQR, −0.01 to 0.32; +9%; and LDL-c, +0.15; IQR, −0.32 to 0.50; +6%, respectively). At individual patient level, the effect of prednisone and tofacitinib on lipid levels varied, with 44% and 37% of patients exceeding the 20% and 30% thresholds of LDL-c increase (Figure 2). Atherogenic indices decreased significantly after start of prednisone (LDL-c/HDL-c ratio, −0.32; IQR, −0.63 to 0.09; P = .047; and total cholesterol/HDL-c ratio, −0.33; IQR, −0.88 to 0.19; P = .049, respectively) (Supplementary Table 3).
Figure 2.
Distribution of relative changes in LDL-c for individual patients after 10-week treatment with prednisone or tofacitinib.
Sensitivity Analyses and Linear Regression Models
Exclusion of patients using concomitant corticosteroids or cholestyramine and those diagnosed with primary sclerosing cholangitis did not significantly affect observed total cholesterol changes (Supplementary Table 2). Within the tofacitinib group, a potential additive effect of corticosteroid exposure was observed on median total cholesterol changes (tofacitinib monotherapy, +0.90 mmol/L; IQR, 0.38-1.43); combination therapy, +1.10 mmol/L; IQR, 0.60-1.70, respectively).
Clinical disease activity scores, CRP levels, and FCP levels at baseline and after induction therapy were available in 198 patients (100%), 163 patients (82.3%), and 104 patients (52.5%), respectively. Changes in clinical disease activity scores inversely correlated with total cholesterol changes (R −0.186, P = .014) and changes in CRP with changes in both total cholesterol and LDL-c (R −0.292, P < .001; and R −0.259, P = .001, respectively; Table 3, Supplementary Figure 2). No correlation was found between FCP and lipid changes. Correction for age and CRP changes did not modify observed lipid changes in any therapy group (Supplementary Table 4).
Table 3.
Correlation between lipid changes and clinical scores and inflammatory markers
| Clinical Score (n = 198) | CRP (n = 163) | FCP (n = 104) | ||||
|---|---|---|---|---|---|---|
| R | P | R | P | R | P | |
| Total cholesterol | −0.166 | 0.028 | −0.282 | < .001 | + 0.023 | ns |
| HDL-c | −0.064 | ns | −0.031 | ns | + 0.081 | ns |
| LDL-c | −0.094 | ns | −0.254 | .001 | + 0.045 | ns |
| Triglycerides | −0.047 | ns | −0.060 | ns | + 0.040 | ns |
| Apo B | −0.030 | ns | −0.119 | ns | + 0.171 | ns |
| Lp(a) | −0.088 | ns | + 0.149 | ns | + 0.089 | ns |
Abbreviations: R, correlation coefficient; P, significance value; ns, not significant; CRP, C-reactive protein; FCP, fecal calprotectin. Clinical scores: Harvey-Bradshaw Index for Crohn’s disease and Simple Clinical Colitis Activity Index for ulcerative colitis.
Discussion
Inflammatory bowel disease is associated with an increased risk of arterial cardiovascular disease. However, the etiology of this association is insufficiently explored, including the nature and cause of lipid changes during the stepwise progression of atherosclerosis. In this study, we observed that prednisone and tofacitinib are associated with significant increases in total cholesterol, HDL-c, and LDL-c levels; although for other systemic IBD drugs, no lipid changes were observed. Changes in total cholesterol within therapy groups inversely correlated with—but were not significantly modified by—changes in CRP, as a marker of systemic inflammation.
In general, corticosteroids are well-known for their adverse effect on lipid levels.15 This study adds to existing data in providing IBD-specific data, which is important because in active IBD, intestinal inflammation also plays a role in lipid absorption.16 The observed LDL-c and HDL-c elevation following prednisone treatment can be explained by an increase of very low density lipoprotein (VLDL) synthesis and lipoprotein lipase activity.17 In contrast to prednisone, budesonide has a neutral effect on lipid levels. This so-called second generation glucocorticosteroids undergo a high first-pass metabolism (80% to 90% inactivation by the liver), resulting in minimal systemic absorption, reduced bioavailability, and low incidence rates of endocrine-related adverse events.18,19
Our findings are similar to previous tofacitinib studies that reported modest increases in lipid levels after induction therapy.20–22 Lipoprotein kinetics studies in rheumatoid arthritis and psoriasis showed that tofacitinib reduced cholesterol ester catabolism, resulting in lipid level increases.23,24 These 2 pathways could explain the observed additive effect of combination therapy with tofacitinib and prednisone on the observed increase of lipid levels. The decrease in atherogenic indices was observed only after prednisone induction and results from a relatively higher increase of HDL-c levels compared with levels after tofacitinib induction. This observation might be explained by the net effect of the previously mentioned prednisone-induced changes in the HDL-c biogenesis and metabolism. However, a postulated benefit of a decrease of atherogenic indices is presumably offset by the increase in LDL-c and triglyceride levels and other cardiovascular side effects of prednisone, such as insulin resistance and hypertension.
Literature on the effect of TNF-α antagonists on lipid levels is conflicting. A recent meta-analysis of our group showed no significant changes after induction and maintenance treatment with TNF-α antagonists.21 In contrast with our results, 2 studies on infliximab reported increases in total cholesterol and HDL-c levels over time in IBD patients.11,25,26 Similarly, lipid increases induced by anti-TNF-α are reported in patients with rheumatoid arthritis.23 The contrasting results seem not to arise from clinical or methodological differences.
The concurrent decline in clinical scores and CRP levels and increase in total cholesterol levels are in line with previous observations made in IBD.27 Disease severity has been linked to the degree of lipid reduction in various inflammatory and infectious diseases.7 Since active inflammation might mask hyperlipidemia, these findings highlight the importance of interpretation of lipid levels in the context of inflammatory activity in IBD patients. The direct biological link between lipids and intestinal inflammation (ie, potential disturbed intestinal lipid uptake) is not yet fully understood. No correlation between lipids and fecal calprotectin was observed, which might be explained by missing data in 50% of patients and/or by the fact that fecal calprotectin is not an accurate marker of disease extent and severity. The observed differences in lipid-modulating effect between drug classes suggest drug-specific effects on top of control of inflammation. Assessment of cholesterol and lipoprotein kinetics during therapy are needed to provide insight in pathophysiological mechanisms underlying the observed differences between registered IBD drugs. This will also help to further understand the link between lipids and cardiovascular risk in IBD.
Further studies are required to assess long-term effects of IBD drugs on lipid levels. In addition, the impact of short-term lipid changes on the long-term consequence of cardiovascular events needs further consideration. Corticosteroid exposure has been associated with an increased risk of heart failure and ischemic heart disease, although confounding by severe IBD has not been refuted.28–30 However, long-term prednisone exposure is merely of historical interest inasmuch as maintenance of prednisone is no longer a valid therapy for IBD. Data on a possible cumulative effect of repeated course of prednisone are lacking. Additional data are required for the long-term consequence of tofacitinib-induced transient vs persistent lipid changes. According to the tofacitinib registration trials and long-term extension data, lipid increases fluctuate but overall stabilize during maintenance therapy; and they reverse to baseline values after therapy cessation (ie, re-randomization to placebo).20 Recently, a Food and Drug Administration (FDA) warning was launched after reports on an increased incidence of myocardial infarction in tofacitinib-treated rheumatoid arthritis patients (50 years and oder).31 For IBD, data on cardiovascular endpoints from long-term real-world cohorts are lacking.
To our knowledge, we are the first to study the short-term lipid-modulating effects of various IBD drugs, including thiopurines, methotrexate, vedolizumab, and ustekinumab. Further strengths of the present study are the inclusion of all consecutive patients and the single-center design, which ascertains homogeneity in clinical practice and data collection, including laboratory techniques to improve between-class comparison. Limitations are the potential selection bias by disease severity (eg, higher levels of FCP in ustekinumab and tofacitinib groups) and treatment indication (eg, prior drug exposure, coexistence of extraintestinal manifestations), despite the unselected inclusion of IBD patients to minimize potential confounding. An effect of methotrexate on lipid levels cannot be refuted due to the small sample size as a consequence of the low prescription rate in this relatively young population of IBD patients. Moreover, statistical power was insufficient to stratify for IBD diagnosis, disease extent, and history of intestinal surgery. Further studies are required to address these associations. Moreover, no data were collected on body weight, diet, and physical activity over the course of the study, hindering adjustment for these corelated factors.
Conclusion
To conclude, prednisone and tofacitinib induction therapy significantly increase total cholesterol, HDL-c, and LDL-c levels. These lipid changes are not observed after induction therapy with thiopurines, methotrexate, infliximab, adalimumab, vedolizumab, and ustekinumab. Although an inverse correlation between lipid levels and systemic inflammation exists, the observed lipid changes are drug-specific and occur independent of control of systemic inflammation. Studies on long-term consequences of lipid changes associated with tofacitinib exposure are required.
Supplementary Material
Acknowledgments
Authors would like to thank all patients and treating physicians for their participation and contribution to this study.
Contributor Information
Jasmijn A M Sleutjes, Department of Gastroenterology and Hepatology, Erasmus Medical Center, Rotterdam, the Netherlands.
Jeanine E Roeters van Lennep, Department of Internal Medicine, Erasmus Medical Center, Rotterdam, the Netherlands.
C Janneke van der Woude, Department of Gastroenterology and Hepatology, Erasmus Medical Center, Rotterdam, the Netherlands.
Annemarie C de Vries, Department of Gastroenterology and Hepatology, Erasmus Medical Center, Rotterdam, the Netherlands.
Author Contributions
J.S., J.R., and A.V. contributed to the design of the study. J.S. collected and analyzed the data. The submitted manuscript was co-written by J.R., C.W., and A.V. All authors have approved the final version of this manuscript.
Funding
There was no financial support for this work.
Conflicts of Interest
A.C. de Vries: MLDS, Tramedico (research funding), Jansen, Takeda, and Abbvie (advisory board)
C.J. van der Woude: Celltrion, Abbvie, and Takeda (advisory board)
J.E. Roeters van Lennep and J.A.M. Sleutjes: nothing to declare.
Data Availability
The data underlying this article are available in the article and in its online supplementary material. Individual patient data will be shared on reasonable request.
References
- 1. Sun HH, Tian F.. Inflammatory bowel disease and cardiovascular disease incidence and mortality: a meta-analysis. Eur J Prev Cardiol. 2018;25(15):1623–1631. [DOI] [PubMed] [Google Scholar]
- 2. Chen Y, Wang X.. Increased risk of stroke among patients with inflammatory bowel disease: a PRISMA-compliant meta-analysis. Brain Behav. 2021;11(6):e02159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aarestrup J, Jess T, Kobylecki CJ, Nordestgaard BG, Allin KH.. Cardiovascular risk profile among patients with inflammatory bowel disease: a population-based study of more than 100 000 individuals. J Crohns Colitis. 2019;13(3):319–323. [DOI] [PubMed] [Google Scholar]
- 4. Biondi RB, Salmazo PS, Bazan SGZ, Hueb JC, de Paiva SAR, Sassaki LY.. Cardiovascular risk in individuals with inflammatory bowel disease. Clin Exp Gastroenterol. 2020;13:107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stamler J, Wentworth D, Neaton JD.. Is relationship between serum cholesterol and risk of premature death from coronary heart disease continuous and graded? Findings in 356,222 primary screenees of the Multiple Risk Factor Intervention Trial (MRFIT). JAMA. 1986;256(20):2823–2828. [PubMed] [Google Scholar]
- 6. Abdullah SM, Defina LF, Leonard D, et al. . Long-term association of low-density lipoprotein cholesterol with cardiovascular mortality in individuals at low 10-year risk of atherosclerotic cardiovascular disease. Circulation. 2018;138(21):2315–2325. [DOI] [PubMed] [Google Scholar]
- 7. Agouridis AP, Elisaf M, Milionis HJ.. An overview of lipid abnormalities in patients with inflammatory bowel disease. Ann Gastroenterol. 2011;24(3):181–187. [PMC free article] [PubMed] [Google Scholar]
- 8. Koutroumpakis E, Ramos-Rivers C, Regueiro M, et al. . Association between long-term lipid profiles and disease severity in a large cohort of patients with inflammatory bowel disease. Dig Dis Sci. 2016;61(3):865–871. [DOI] [PubMed] [Google Scholar]
- 9. Romanato G, Scarpa M, Angriman I, et al. . Plasma lipids and inflammation in active inflammatory bowel diseases. Aliment Pharmacol Ther. 2009;29(3):298–307. [DOI] [PubMed] [Google Scholar]
- 10. Sleutjes JAM, Roeters van Lennep JE, Boersma E, et al. . Systematic review with meta-analysis: effect of inflammatory bowel disease therapy on lipid levels. Aliment Pharmacol Ther. 2021;54(8):999–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Koutroubakis IE, Oustamanolakis P, Malliaraki N, et al. . Effects of tumor necrosis factor alpha inhibition with infliximab on lipid levels and insulin resistance in patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2009;21(3):283–288. [DOI] [PubMed] [Google Scholar]
- 12. Kalkan Ç, Karakaya F, Törüner M, Çetinkaya H, Soykan I.. Anti-TNF-α agents and serum lipids in inflammatory bowel diseases. Clin Res Hepatol Gastroenterol. 2016;40(4):e46–e47. [DOI] [PubMed] [Google Scholar]
- 13. Visseren FLJ, Mach F, Smulders YM, et al. . 2021 ESC guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021;42(34):3227–3337. [DOI] [PubMed] [Google Scholar]
- 14. Feagan BG, Ha CY, Taub PR, et al. . P676 correlation of lipid levels with reduction in inflammation in patients with ulcerative colitis: data from the tofacitinib OCTAVE clinical trials. J Crohns Colitis. 2018;12(suppl_1):S453–S454. [Google Scholar]
- 15. Ross IL, Marais AD.. The influence of glucocorticoids on lipid and lipoprotein metabolism and atherosclerosis. S Afr Med J. 2014;104(10):671–674. [DOI] [PubMed] [Google Scholar]
- 16. Uchiyama K, Kishi H, Komatsu W, Nagao M, Ohhira S, Kobashi G.. Lipid and bile acid dysmetabolism in Crohn’s disease. J Immunol Res. 2018;2018:7270486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ettinger WH, Klinefelter HF, Kwiterovitch PO.. Effect of short-term, low-dose corticosteroids on plasma lipoprotein lipids. Atherosclerosis. 1987;63(2-3):167–172. [DOI] [PubMed] [Google Scholar]
- 18. O’Donnell S, O’Morain CA.. Therapeutic benefits of budesonide in gastroenterology. Ther Adv Chronic Dis. 2010;1(4):177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lichtenstein GR, Bengtsson B, Hapten-White L, Rutgeerts P.. Oral budesonide for maintenance of remission of Crohn’s disease: a pooled safety analysis. Aliment Pharmacol Ther. 2009;29(6):643–653. [DOI] [PubMed] [Google Scholar]
- 20. Sands BE, Taub PR, Armuzzi A, et al. . Tofacitinib treatment is associated with modest and reversible increases in serum lipids in patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2020;18(1):123–132.e3. [DOI] [PubMed] [Google Scholar]
- 21. Biemans VBC, Sleutjes JAM, de Vries AC, et al. . Tofacitinib for ulcerative colitis: results of the prospective Dutch Initiative on Crohn and Colitis (ICC) registry. Aliment Pharmacol Ther. 2020;51(9):880–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Honap S, Chee D, Chapman TP, et al. . Real-world effectiveness of tofacitinib for moderate to severe ulcerative colitis: a multicentre UK experience. J Crohns Colitis. 2020;14(10):1385–1393. [DOI] [PubMed] [Google Scholar]
- 23. Charles-Schoeman C, Fleischmann R, Davignon J, et al. . Potential mechanisms leading to the abnormal lipid profile in patients with rheumatoid arthritis vs healthy volunteers and reversal by tofacitinib. Arthritis Rheumatol. 2015;67(3):616–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wolk R, Armstrong EJ, Hansen PR, et al. . Effect of tofacitinib on lipid levels and lipid-related parameters in patients with moderate to severe psoriasis. J Clin Lipidol. 2017;11(5):1243–1256. [DOI] [PubMed] [Google Scholar]
- 25. Parmentier-Decrucq E, Duhamel A, Ernst O, et al. . Effects of infliximab therapy on abdominal fat and metabolic profile in patients with Crohn’s disease. Inflamm Bowel Dis. 2009;15(10):1476–1484. [DOI] [PubMed] [Google Scholar]
- 26. van Sijl AM, Peters MJ, Knol DL, et al. . The effect of TNF-alpha blocking therapy on lipid levels in rheumatoid arthritis: a meta-analysis. Semin Arthritis Rheum. 2011;41(3):393–400. [DOI] [PubMed] [Google Scholar]
- 27. Sands BE, Colombel JF, Ha C, et al. . Lipid profiles in patients with ulcerative colitis receiving tofacitinib-implications for cardiovascular risk and patient management. Inflamm Bowel Dis. 2021;27(6):797–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Aniwan S, Pardi DS, Tremaine WJ, Loftus EV Jr.. Increased risk of acute myocardial infarction and heart failure in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2018;16(10):1607–1615.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rungoe C, Basit S, Ranthe MF, Wohlfahrt J, Langholz E, Jess T.. Risk of ischaemic heart disease in patients with inflammatory bowel disease: a nationwide Danish cohort study. Gut. 2013;62(5):689–694. [DOI] [PubMed] [Google Scholar]
- 30. Pujades-Rodriguez M, Morgan AW, Cubbon RM, Wu J.. Dose-dependent oral glucocorticoid cardiovascular risks in people with immune-mediated inflammatory diseases: a population-based cohort study. PLoS Med. 2020;17(12):e1003432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. FDA Drug Safety Communication. Initial safety trial results find increased risk of serious heart-related problems and cancer with arthritis and ulcerative colitis medicine Xeljanz. 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material. Individual patient data will be shared on reasonable request.